-
湖库是我国许多城市的重要饮用水水源地[1]。然而,我国35个城市水源水库中有80%的水体存在饮用水嗅味问题[2],其中由致嗅化合物2-甲基异莰醇(2-methylisoborneol,2-MIB)导致的水体土霉味问题居多[3-7]。由于该物质嗅阈值极低[5](4~16 ng·L−1),且很难通过常规水处理工艺去除[8],饮用水中残留的痕量2-MIB物质容易引发消费者对水质的担忧,威胁城市供水安全[4]。水源地嗅味问题已经成为我国供水行业中重点关注的问题之一[9]。
2-MIB主要由丝状蓝藻生长代谢产生,这类蓝藻包括颤藻属(Oscillatoria)、席藻属(Phormidium)、浮颤藻属(Planktothrix)以及伪鱼腥藻属(Pseudanabaena)等[9]。丝状蓝藻导致的2-MIB嗅味问题可最早追溯到1983年,由于水源水库底泥附着生长的颤藻引发美国加利福尼亚州3个供水系统出现嗅味问题[10];1991年,MARTIN等[11]在密西西比某池塘中采集到一株产2-MIB的颤藻;1998年,日本学者SUGIURA等[12]在Kasumigaura湖中分离出4株丝状产嗅蓝藻,包括2株席藻、1株颤藻和1株鞘丝藻,发现这些藻种生物量与水体土霉味之间存在正相关关系。
近10年来,我国关于水源地嗅味问题的报道越来越多。北京密云水库每年9—10月局部区域出现浮颤藻生长导致水体嗅味问题[7];上海市新建水源地青草沙水库由于丝状蓝藻爆发导致季节性嗅味问题[13];天津于桥水库、苏州东太湖湖滨水库、上海金泽水库及银川某化工园区水源水库等均存在由于不同种属的丝状产嗅蓝藻生长或爆发导致的嗅味问题[14]。
大部分水源地中2-MIB由丝状蓝藻产生,由于丝状蓝藻种类较多、形态相近,不同藻种具有不同的产2-MIB特征,甚至同一藻种在不同外部环境条件下也存在产嗅差异。传统方法主要依托显微镜镜检通过藻细胞形态进行藻种计数。然而,从细胞形态上无法区分产嗅丝状藻种与非产嗅丝状藻种,因此,无法特异性检测水体中的产嗅藻;此外显微镜镜检前处理耗时长,检测效率低,准确度与精确度较低[7]。致嗅物质2-MIB可基于气相色谱-质谱联用(GC-MS)方法[15]分析,但因仪器昂贵、操作要求高、运行维护成本高,大部分水源管理机构不具备该条件,且该方法只能测定水样中已产生的2-MIB物质浓度,无法提前预警,也不能鉴别产生来源。因此,有必要构建一种能特异性检测水体2-MIB产生潜力的方法。
由于丝状藻代谢产生2-MIB受相关功能基因控制,可通过实时荧光定量PCR(real-time quantitative polymerase chain reaction, qPCR)方法构建特异性检测2-MIB合成功能基因的方法。关于2-MIB的合成途径最初在放线菌中被发现[16],主要由SCO7701基因与SCO7700基因调控;随后,GIGLIO等[17]发现蓝藻中2-MIB的生物合成机制为GPP的甲基化和甲基GPP的环化反应,分别由GPPMT基因和2-MIBS基因调控。根据序列相似性分析,蓝藻和放线菌的2-MIB相关基因具有相对较高的相似度和同源性[18]。
本研究通过设计针对蓝藻中2-MIB功能基因的特异性引物,构建实时定量PCR方法,并采用实际水体检验方法的可行性,实现水源中丝状蓝藻的致嗅功能基因定量检测,进而评估水体产嗅潜力与产嗅风险,为水源嗅味问题的监测与预警提供实时、快速、科学的方法。
全文HTML
-
所用藻种均为产2-MIB蓝藻,分别为FACHB-1277(Pseudanabaena sp., 伪鱼腥藻)、FACHB-1375(Planktothrix sp., 浮颤藻),购自中国科学院淡水藻种库。藻类培养使用BG11培养基,扩大培养后用于DNA提取及致嗅物质检测等目的。
-
取500 mL藻培养液将藻细胞过滤富集至1.2 μm滤膜(RTTP04700,1.2 µm 聚碳酸酯膜,IsoporeTM, 美国)上;随后将滤膜剪切破碎后使用FastDNA ®SPIN Kit for Soil 试剂盒(6560-200,MPBio,美国)提取样品DNA。
-
采用已有研究[19]中的2-MIB引物(MIB3313F/MIB4226R和MIB3324F/MIB4050R)可以有效扩增FACHB-1277与FACHB-1375样品中2-MIB功能基因,验证了实验藻种含MIB功能基因。但由于两对引物所扩增得到的2-MIB基因片段较长,分别为913 bp与726 bp,不能用于定量PCR扩增。进一步检验了已有研究中报道的定量PCR引物MIBS-RTF/ MIBS-RTR[19],发现不能扩增本研究中实验藻种的2-MIB功能基因。因此,本研究基于NCBI已公开的2-MIB功能基因数据库,设计2-MIB功能基因的特异性引物(表1),基因引物均由生工生物工程(上海)股份有限公司合成。将所设计的引物与NCBI中已有2-MIB基因数据库比对验证引物可行性与特异性;并采用普通PCR法测试相关引物,使用1.2 %琼脂糖凝胶电泳方法检验了引物的特异性。
-
普通PCR反应体系为:2×PCR Taq MasterMix with dye (RR005A,TAKARA Bio公司,日本)12.5 μL,上下游引物各0.8 μL,模板DNA 2.0 μL,ddH2O 8.9 μL。普通PCR扩增程序为:高保真酶活化阶段95 ℃,持续5 min;高温变性阶段95 ℃,持续30 s;低温退火阶段64 ℃,持续 45 s;延伸阶段72 ℃,持续30 s;高温变性、低温退火、延伸阶段共45个循环。
qPCR反应体系为:TB GreenTM Premix Ex TaqTM (RR820,TAKARA Bio公司,日本)12.5 μL,上下游引物MIBF/MIBR各0.5 μL,模板DNA 2.0 μL,ddH2O 9.5 μL。qPCR扩增程序为:高温变性95 ℃,持续10 s;低温退火64 ℃,持续20 s;延伸阶段72 ℃,持续30 s;反应共40个循环;另外,溶解曲线设置条件为由65 ℃升温至97 ℃,速率为2.2 ℃·s−1。
-
基于引物MIBF与MIBR扩增FACHB-1277 DNA模板,经测序后根据序列信息合成2-MIB功能基因质粒,浓度为359 ng·μL−1(由生工生物工程(上海)股份有限公司合成)。根据式(1)计算质粒拷贝数。
式中:N为基因拷贝数浓度,copies·μL−1;NA为阿伏伽德罗常数;c为核酸质量浓度,ng·μL−1;L为碱基长度,bp;MW为单位碱基平均摩尔质量。
由式(1)计算得到质粒拷贝数为8.44×1011copies·μL−1。按10倍进行梯度稀释,得到7个浓度水平,以此为DNA模板,按照1.4节提及的优化体系与运行参数进行qPCR扩增。同一浓度梯度质粒DNA为模板的反应孔作3组平行实验,将得到的Cq值与2-MIB基因量的对数构建线性模型,绘制标准曲线。
-
配制了9个不同浓度产嗅藻的培养样品,包括FACHB-1277伪鱼腥藻-1 (S01)、FACHB-1277伪鱼腥藻-2 (S02)、FACHB-1277伪鱼腥藻-3 (S03)、FACHB-1375浮颤藻-1 (S04)、FACHB-1375浮颤藻-2 (S05)、FACHB-1375浮颤藻-3 (S06)、青草沙分离的Pseudanabaena sp. 01 (S10)、青草沙分离的Pseudanabaena sp. 02 (S11)、青草沙分离的Pseudanabaena sp. 03 (S12)。采集了12个南水北调工程的环境样品,包括S07~S09、S13~S21。对这21组样品分别进行了定量PCR方法检测与气相色谱-质谱联用的2-MIB物质浓度分析[15, 20-22],每个样品重复分析3次。
1.1. 实验材料
1.2. 培养藻种及环境样品DNA提取方法
1.3. 引物设计与特异性验证
1.4. 普通PCR与qPCR扩增条件
1.5. 标准曲线的建立
1.6. 实际样品测试
-
实验测试了已有研究[19]的2-MIB基因相关引物,发现MIB3313F/MIB4226R、MIB3324F/MIB4050R两对引物能有效扩增模拟培养藻种FACHB-1277与FACHB-1375。然而,目标基因片段过长,因此,无法用于定量PCR扩增。已有研究[19]的引物不能有效扩增本实验的藻种,这与2-MIB功能基因序列具有一定的可变性有关。因此,采用MIB3313F/MIB4226R引物扩增了FACHB-1277 DNA,并通过测序获得2-MIB功能基因序列,序列NCBI登记号为MN869917。基于该序列信息设计了MIBF/MIBR引物(见表1)。经测试发现,这对引物能有效扩增2-MIB功能基因,PCR产物长度为389 bp,可用于qPCR方法。MIBF/MIBR NCBI比对结果见表2。
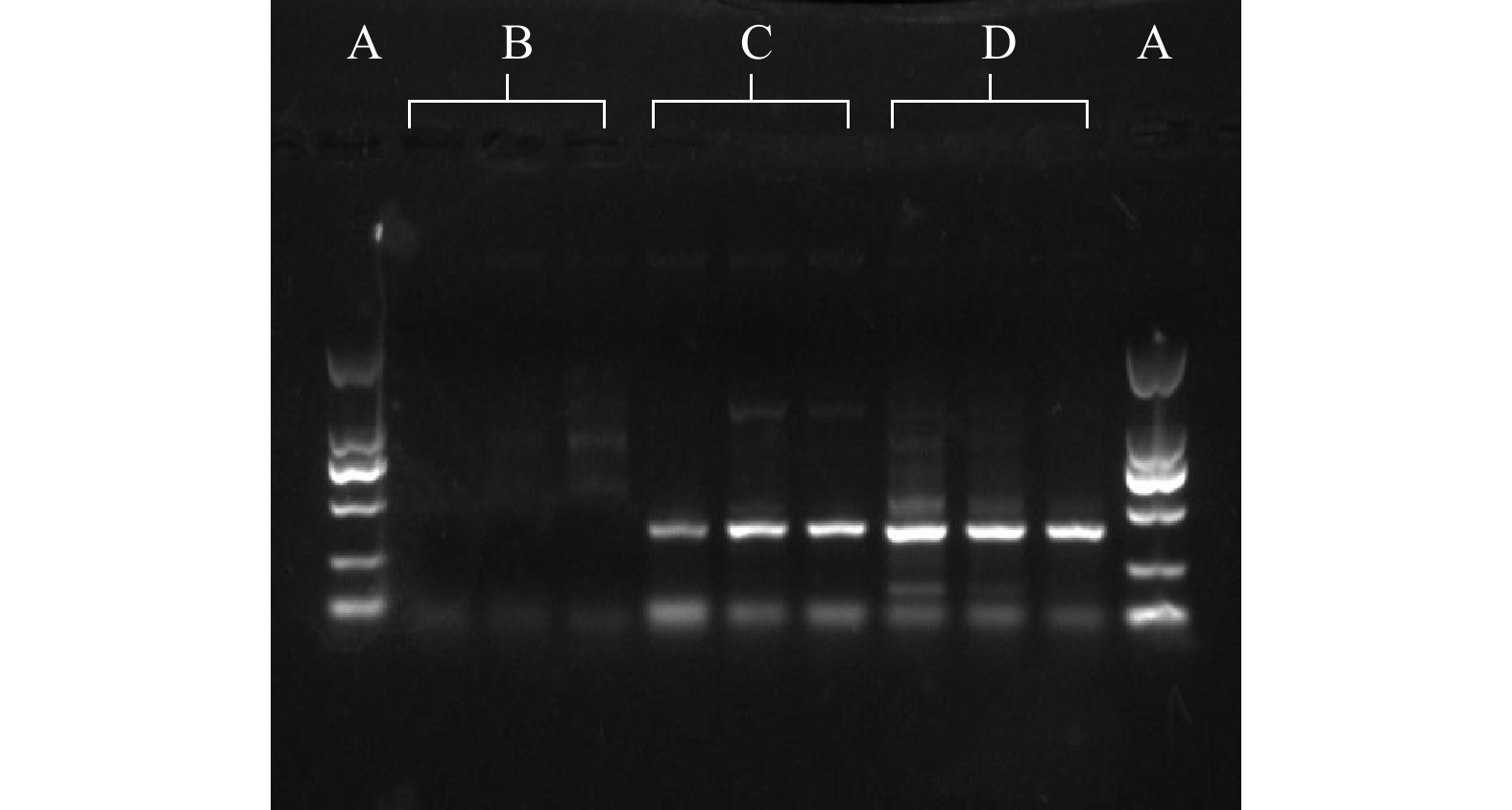
为验证MIBF/MIBR引物的可行性,比对了引物序列与NCBI基因数据库中所有蓝藻中MIB合成功能基因结果,发现这对引物能匹配18条MIB功能基因序列(截至目前发表了23条)。由结果分析可知:不能匹配的基因序列(19~23)是由于这些序列仅为部分MIB基因序列,未包含MIBF/MIBR引物段序列;而这对引物仅能匹配NCBI中已发表的MIB功能基因。进一步采用实际藻种进行普通PCR扩增实验,产物条带介于250 bp至500 bp之间,验证了MIBF/MIBR引物的可行性,结果如图1所示。
-
将1.5节合成的2-MIB功能基因质粒(拷贝数8.44×1011copies·μL−1)作为标准品进行定量PCR测试,基于结果构建循环次数Cq值与2-MIB基因拷贝数的对数构建线性模型,绘制标准曲线如图2所示。标准曲线公式见式(2)。
式中:Cq为循环次数;Cg为2-MIB基因丰度,copies·L−1。图2表明两者符合线性关系(R2=0.999 5,P<0.01),检测限为8.44×102 copies·L−1,说明该方法能满足2-MIB功能基因检测要求。
-
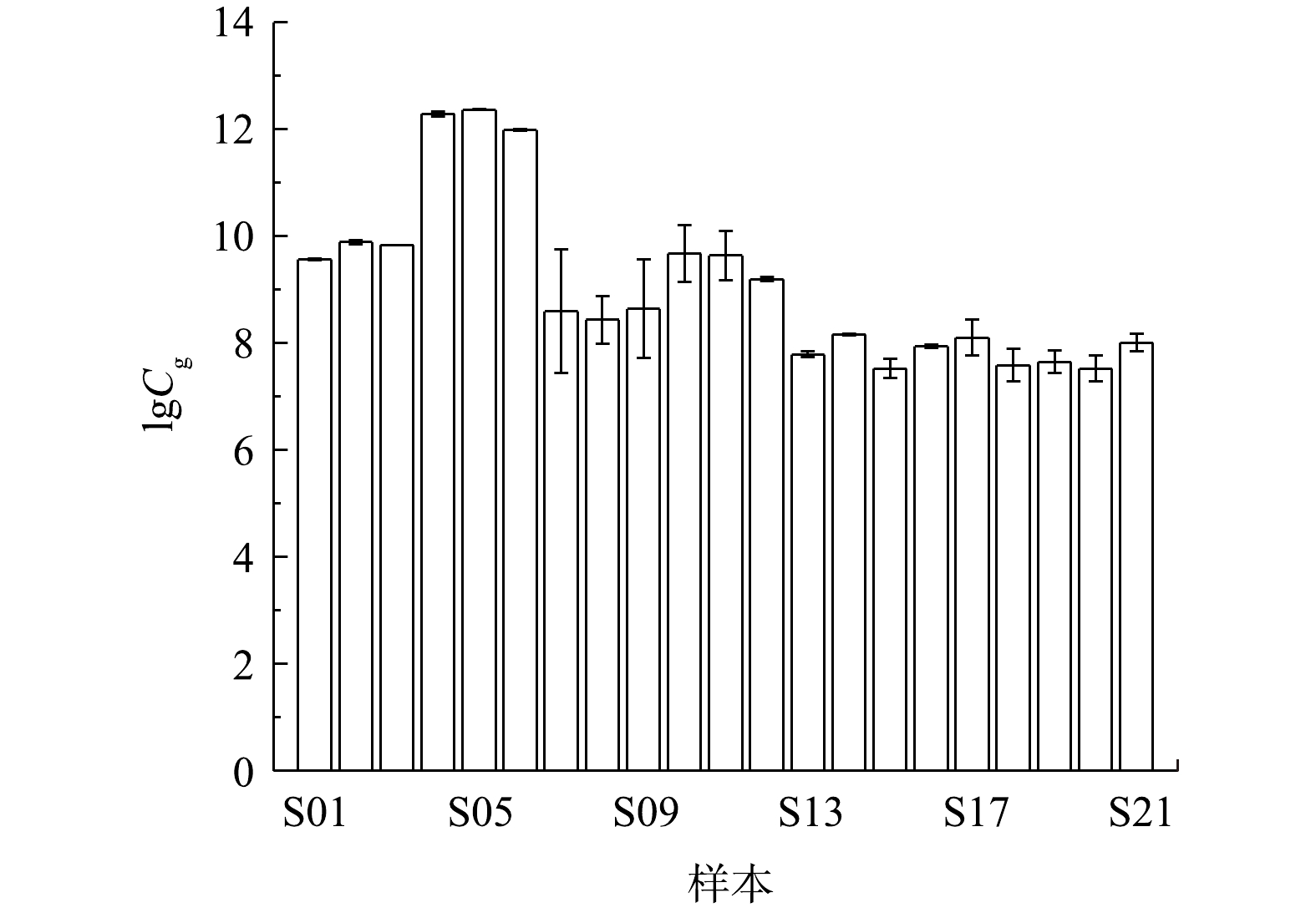
图3为12个环境水体及9个不同浓度产嗅藻的培养样品的测试结果。由图3可知,该方法能够检测出环境水体及培养样品中的2-MIB功能基因,得到的环境样品浓度为2.09×107 ~1.94×1010 copies·L−1。其中,实验室培养的FACHB-1375 Planktothrix sp.浮颤藻样品2-MIB功能基因丰度最高,实际环境样品中2-MIB功能基因丰度水平相近,最低为于桥水库样品,相应基因丰度为2.09×107 copies·L−1。此外,各水样3组重复样品检测结果十分接近,表明该方法的重现性较好。
-
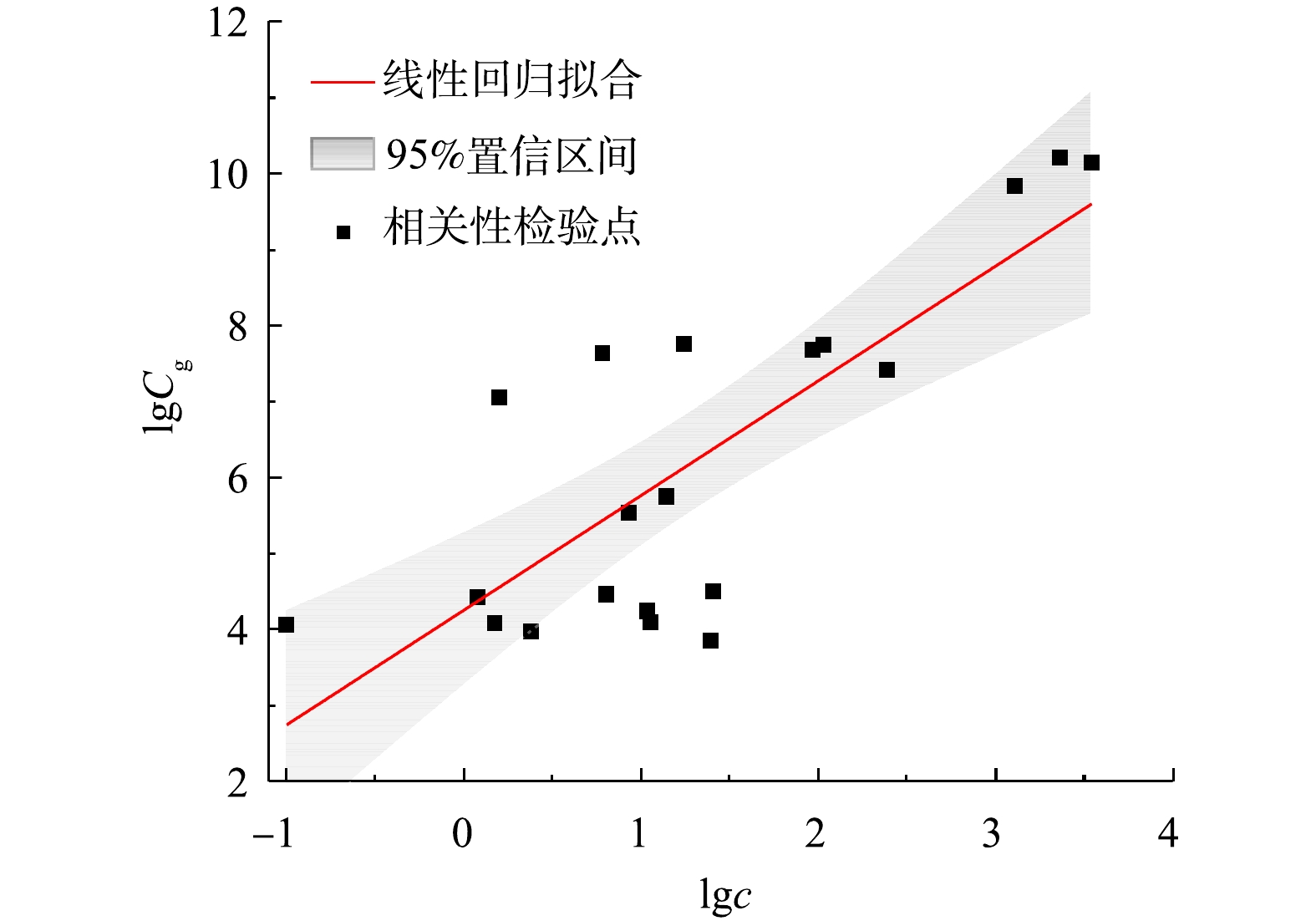
为验证方法的可靠性,用GC-MS方法检测了上述21个样品中2-MIB浓度[7]。对比分析2种方法的结果发现其符合线性关系(R2=0.63,P<0.01),表明定量PCR方法可以表征水体中2-MIB浓度(见图4)。式(3)为2-MIB浓度与功能基因之间的定量关系模型,可用于利用定量PCR结果评估水体中2-MIB浓度水平。
式中:Cg为2-MIB基因丰度,copies·L−1;c为2-MIB浓度,ng·L−1。
-
水源地中藻类生长产生的嗅味问题已成为供水行业最为关注的问题。在我国,由于丝状蓝藻生长代谢2-MIB导致的水体土霉味问题尤为普遍,确认2-MIB的产生来源能提升水源地嗅味管理及防控水平。实时荧光定量PCR(qPCR)对基因定量的检测方法已广泛应用于检测蓝藻或有毒蓝藻总量中。该方法具有快速高效、灵敏度高、检出限低、特异性好和高重复性高等优点。目前,已有少量基于qPCR方法的检测水体中产土臭素(geosmin)微生物生物总量的方法[23],但是用于含2-MIB功能基因微生物生物总量的检测应用还较少。
本研究中构建的定量PCR方法,能直接定量检测水体中的2-MIB功能基因,避免了传统方法基于形态学无法区分产嗅藻与非产嗅藻的缺陷。相比于采用气相色谱-质谱联用(GC-MS)的仪器分析方法,定量PCR方法能同时检测多达96个样品,具有通量高、速度快等特点。此外,该方法检测限低,能最低检测到8.44×102 copies·L−1个2-MIB功能基因拷贝数。该结果与基于定量PCR方法检测水体中土臭素功能基因的结果一致[7]。
然而,由于该方法存在一些缺陷,限制了其推广应用。大部分分子生物学方法是以目标生物DNA为主要研究对象,而蓝藻具有细胞壁,DNA的提取难度与误差增加。SU等[7]发现DNA提取是影响检测样品中土嗅素功能基因准确度的最主要步骤,与本研究的结论类似。DNA提取过程的不确定显著降低了结果的准确性。此外,由于产2-MIB的蓝藻并未完全界定,不同产2-MIB藻种的基因序列不完全相同,加上当前有关2-MIB的功能基因研究很少,NCBI中的MIB基因序列仍在不断完善中。因此,本研究中所设计的引物可能无法扩增之后新发现的产嗅藻2-MIB功能基因,正如以往引物不能有效扩增本研究涉及的产嗅藻种一样。随着DNA提取方法的不断改进,以及2-MIB功能基因库序列信息的完善,基于定量PCR的水源2-MIB风险评估方法具有广泛的应用前景。
基于此,本课题组还将此方法应用于上海市、厦门市的水源水库中,旨在考察该方法是否能快速、有效地核算出水体的产嗅潜力,达到水体嗅味问题预警的要求,为水源地的水质管理提供十分重要的工具与技术支持。
2.1. 引物设计与特异性验证结果分析
2.2. 定量PCR标准曲线的构建
2.3. 实际样品2-MIB基因丰度测试结果
2.4. 2-MIB监测方法的对比与验证
2.5. 2-MIB的定量PCR监测方法的应用前景
-
1)建立了检测水体中2-MIB功能基因的定量PCR方法,该方法能克服传统方法无法区分产嗅藻与非产嗅藻的缺点,且满足检测要求(R2=0.999 5,P<0.01)。
2)通过采集我国不同地区的实际水源样品,对比验证了定量PCR方法与仪器分析方法结果的一致性(R2=0.63,P<0.01),说明本研究提出基于定量PCR评估水体嗅味风险的方法具有可行性。
3)基于qPCR的产嗅基因丰度检测代替了繁琐、重现性低的显微镜看藻、数藻过程。快速、准确有效地检测水体中致嗅功能基因的丰度,能起到预警作用,为水源地的水质管理提供十分重要的工具与技术支持。



 DownLoad:
DownLoad: