-
当前,随着工业化进程的加快推进,工业排放的二氧化硫造成的一系列环境问题日益严重,给国民的健康和经济带来了巨大的挑战,更难以满足人民群众对优美生态环境的需要[1-3]。据报道[4-5],2019年度SO2的排放要比往年下降3%,其中京津冀和周边城市、长三角区域、汾渭平原区域等,都属于传统资源产业聚集的地方,是煤炭、钢铁等的主要产地,而钢铁、煤炭等的大量使用必然会产生SO2,因此,应将这些地区作为重点管理区域,集中开展“控硫”整治。
为了控制二氧化硫的超标排放,我国已展开了多种脱硫技术的研发和应用。目前常见的烟气脱硫技术按工艺特性分为干法、半干法和湿法[6-7]。其中,湿法脱硫具有初期投资费用高、系统复杂、设备占地面积大、消耗能源多、对设备有严重的腐蚀、产生的脱硫产物可对管道堵塞,且有新的污染物—脱硫废水的产生等问题[8-9],而干法烟气脱硫技术具有操作简单、初期运行投资低、腐蚀小等优点,因此,它将是未来控制SO2排放的大势所趋,而脱硫效率较低始终制约着该技术的发展,制备高性能的脱硫剂和确定较优脱硫条件对提高干法烟气脱硫效率起至关重要的作用[10-12]。
干法烟气脱硫中水的作用必不可少,在脱硫塔内水有2个重要作用[13-15]:首先,水可以起到增加烟气湿度的目的,形成必要的反应条件;其次,由于入口烟气温度较高,反应能力较弱,须创造较低温度的脱硫环境,但此部分作用较弱,所以本实验中加入水量的多少用相对湿度来衡量。在脱硫塔内,相对湿度越大,反应效率越高。但随着相对湿度的不断提高,过多的水分会引起脱硫塔的阻塞和黏壁,甚至造成脱硫塔停止运行,不利于烟气的净化排放,所以合适的相对湿度在干法烟气脱硫中尤其重要[16]。
本研究利用自建的干法烟气脱硫反应实验台,探讨了相对湿度对干法烟气脱硫效率的影响,并验证了钙基脱硫剂在干法脱硫中的可行性,最后得到了较优的相对湿度,为干法脱硫条件提供参考。
全文HTML
-
实验所用原料为生石灰,来源于四川江油某石灰石矿厂,通过X射线荧光光谱分析仪(XRF)测得生石灰中钙所占比例为89%。实验前期,经过颚式破碎机将原料粒度破碎为3~5 mm,比表面积为5 m2·g−1。
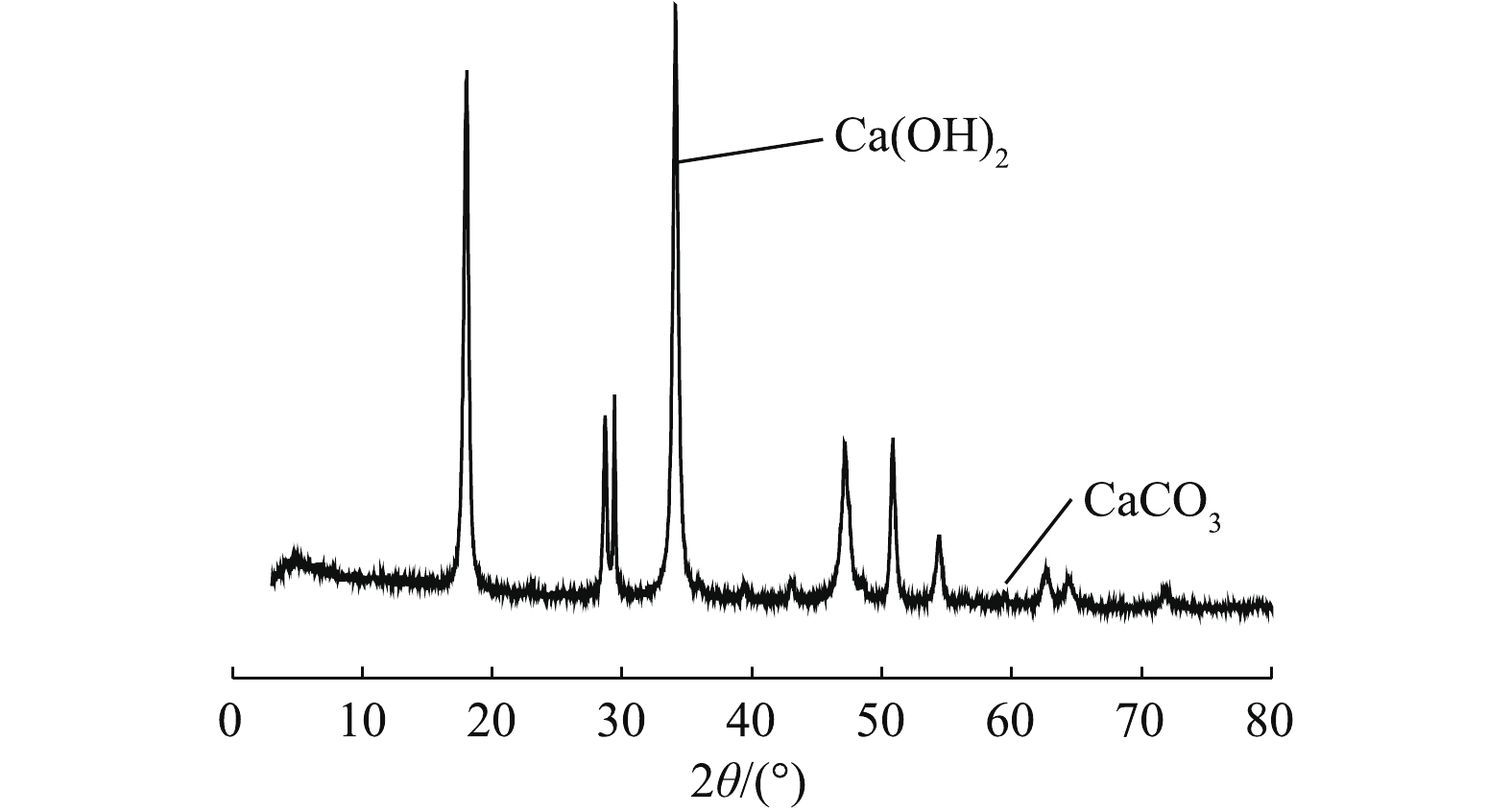
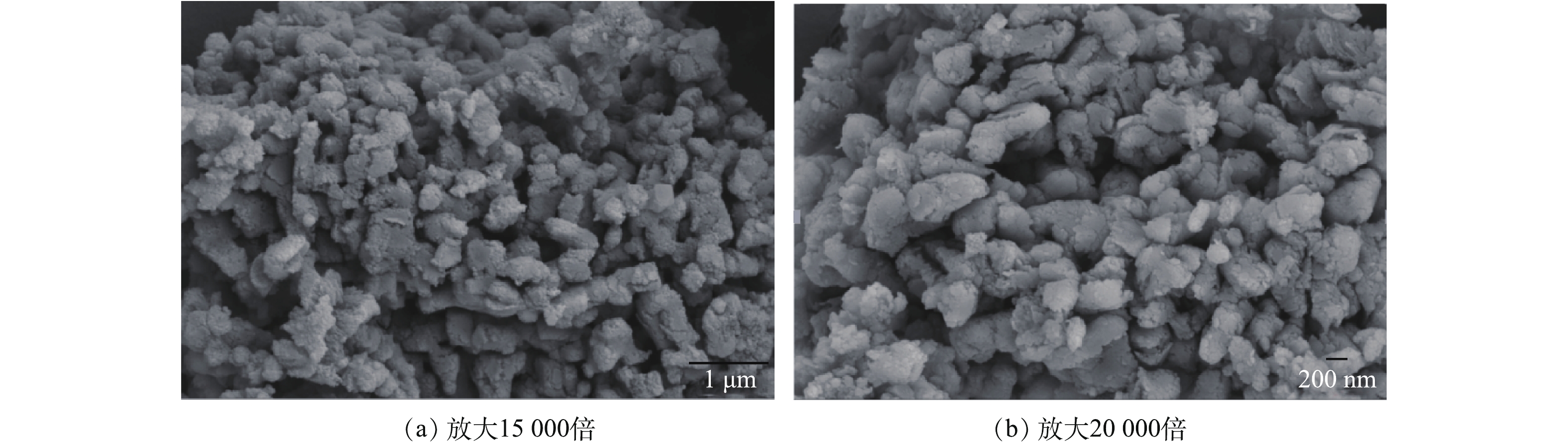
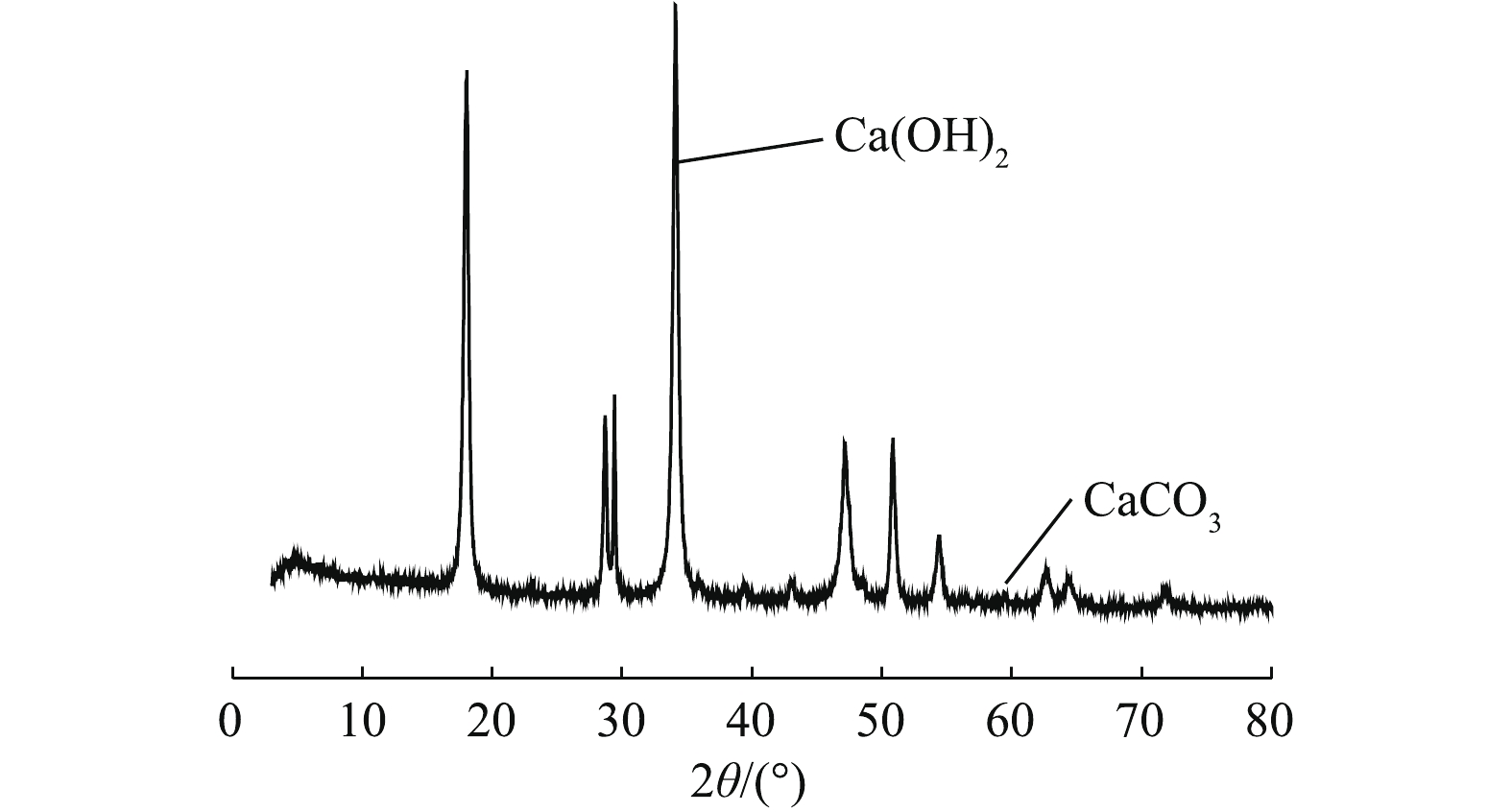
将破碎后的生石灰原料经过蒸汽动能磨消化处理,得到的产物作为本实验所用脱硫剂。与常规消化脱硫剂相比较,其具有粒度细、分布均匀、无明显大颗粒、流动性好等特点。其粒度为d10=0.992 μm,d50=2.873 μm,d90=8.013 μm,堆密度为0.284 g·cm−3,比表面积为24.34 m2·g−1,比常规消石灰比表面积12~14 m2·g−1增大近1倍。通过扫描电镜分析其内部结构,发现物料内部类似蜂窝状结构,饱含多孔的蓬松结构,SEM分析如图1所示。通过XRD及EDTA滴定法分析发现,脱硫剂中生石灰被全部消化,无氧化钙存在,XRD分析结果如图2所示。
-
众多研究结果[17-21]表明,在绝对干燥的条件下,Ca(OH)2和SO2几乎不发生化学反应,而随着相对湿度的增加,反应速率逐渐升高。本实验中,控制钙硫比、SO2浓度、烟气温度和气流速度保持恒定,通过改变脱硫塔内的相对湿度,来研究脱硫效率,并确定较优的相对湿度。
实验采用道尔顿的分压定律,通过用水浴锅的温度来控制相对湿度的变化,水浴锅中的水维持恒定的温度,气体通过气流控制器在水中鼓泡,使之达到饱和,获得必要的湿度。实验中水浴锅中的水温控制为30、50、70和80 ℃。相对湿度计算方法[22]见式(1)。
式中:H为绝对湿度,g·kg−1;ε为相对湿度;P0为湿空气总压,Pa;P为同温度下水的饱和蒸汽压,Pa。
根据SO2前后出口浓度计算脱硫率[23],计算方法见式(2)。
式中:
η 为脱硫效率;C0为初始二氧化硫浓度,本实验中取C0=850 mg·m−3;Ct为脱硫塔后端的二氧化硫浓度,mg·m−3。 -
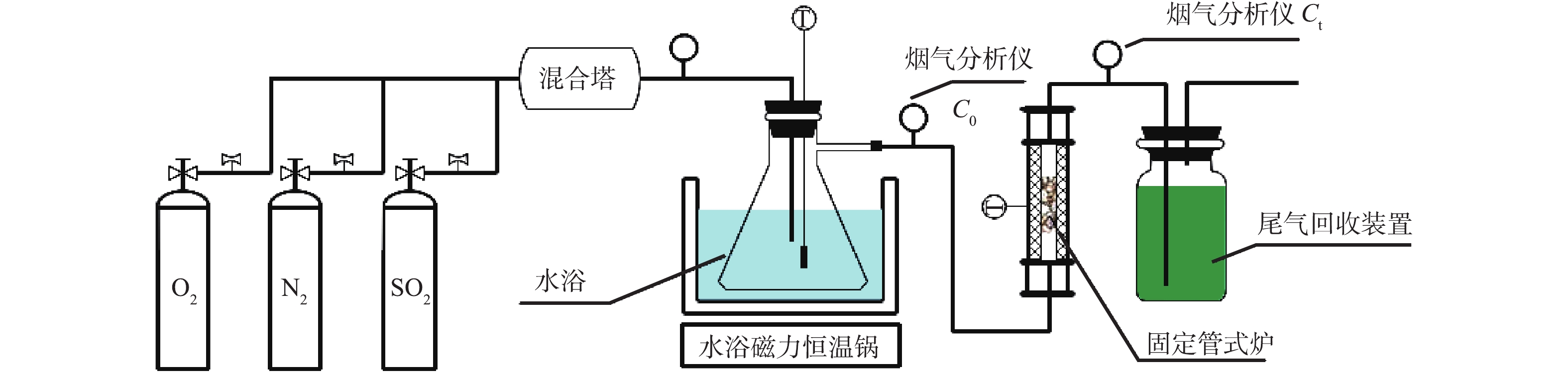
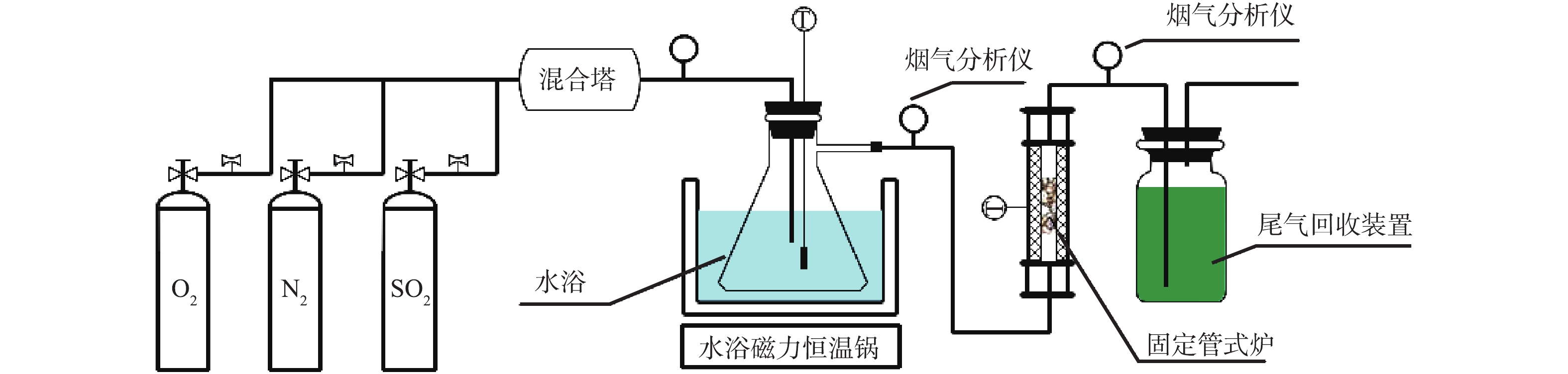
烟气脱硫实验装置如图3所示。装置主要由模拟气源装置、气体混合装置、烟气分析处理装置、烟气反应装置和尾气回收装置等5部分组成。模拟气源装置主要包括N2、O2、SO2等,调节烟气流量至700 mL·min−1,SO2浓度为850 mg·m−3,炉内温度为100 ℃,流速为2.4 m·s−1,氧气为12%(据中国环境科学研究院提供的数据分析:脱硫塔内气体中O2的含量为11.2%~18.5%)[24-25]。气体混合装置主要由烟气智能混合塔、管道、保温装置和水浴磁力恒温锅等组成。烟气分析处理装置主要为粉尘/水过滤系统和烟气分析仪等,烟气分析仪用来测试前后端反应系统SO2的浓度值。烟气反应装置主要包括过滤层(含菁青石)、脱硫剂和固定管式炉等。尾气回收装置表示的是装有NaOH水溶液的集气瓶,脱硫塔内没有被吸收的SO2在集气瓶内与NaOH发生反应,以达到烟气净化的目的。
实验步骤主要包括3部分:首先,将脱硫剂放置在菁青石上,并用脱硫剂专用垫层3M纸包裹,每次脱硫剂质量为5 g(为防止物料漏出,在脱硫剂下面布有玻纤滤纸);然后,将各气瓶后端连接气体流量计,其流量大小通过混合塔前端的截止阀来控制,并用转子流量计二次测量,矫正后进入混合塔;最后,在反应装置末端,接尾气回收系统,未完全反应的烟气经NaOH溶液吸收后排空。由于SO2会在水中溶解,所以实验开始前须在常温条件下的水浴瓶中溶解4 h,使其达到饱和状态。
-
实验参数:SO2浓度为850 mg·m−3,床层温度(T)为100 ℃,停留时间为2 s,相对湿度为0、5%、15%、30%,45%。
实验采用SO2智能烟气分析仪,反应每隔20 min,记录1组二氧化硫出口浓度,直至二氧化硫出口浓度大于500 mg·m−3(穿透浓度)后,记录脱硫剂总的运行时间(穿透时间)。根据式(2)和文献中的方法[26]计算出脱硫剂的脱硫效率和总固硫量,以此评价脱硫剂的脱硫性能。脱硫剂的固硫量计算方法见式(3)。
式中:
φ(t) 为脱硫剂在相同条件下总的固硫量,mg;C0和Ct为脱硫剂在反应前后端的SO2浓度,mg·m−3;t为烟气反应时间,min;V为烟气的体积流量,m3·min−1。
1.1. 实验原料
1.2. 实验原理
1.3. 实验装置及步骤
1.4. 实验条件
-
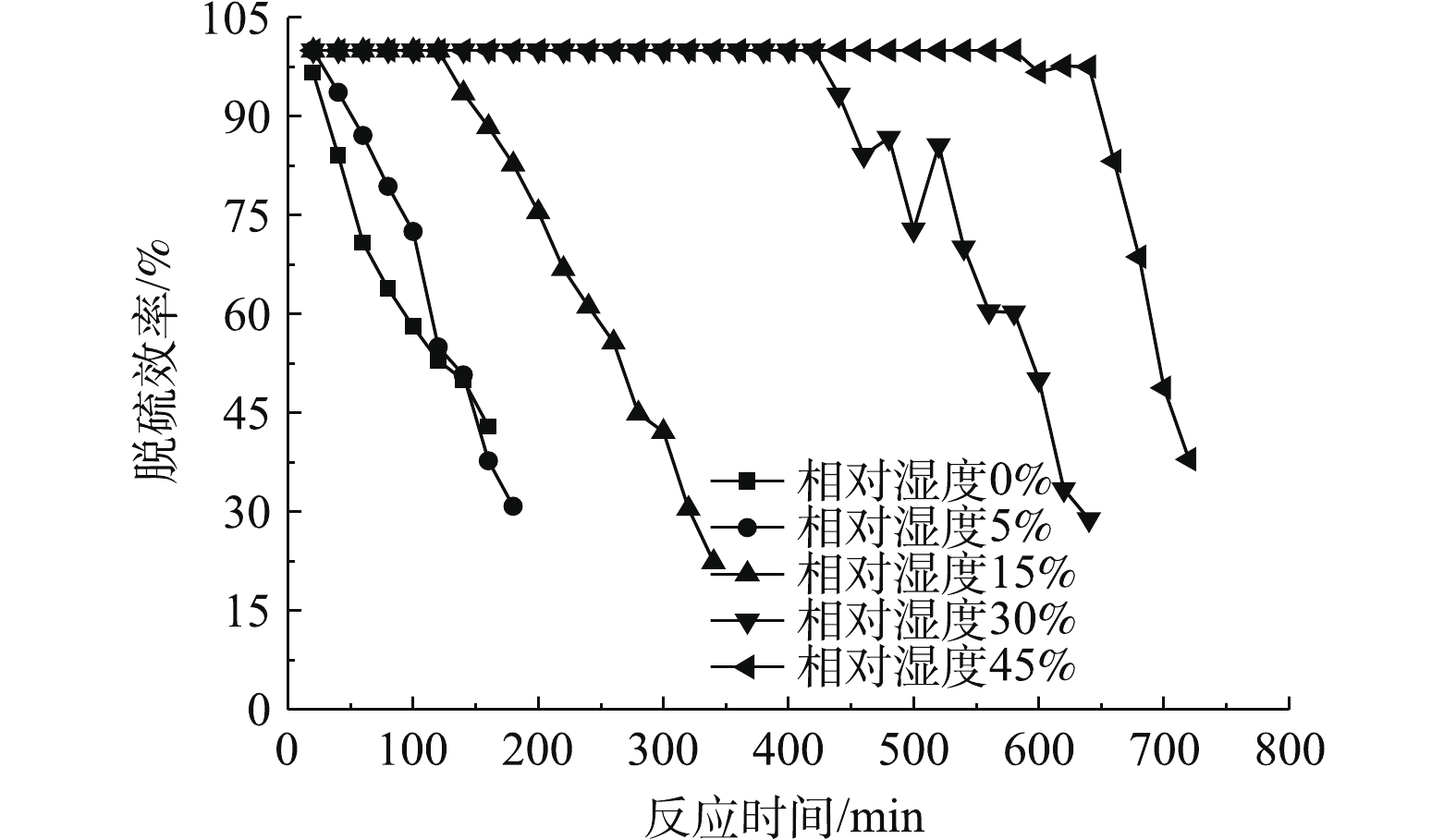
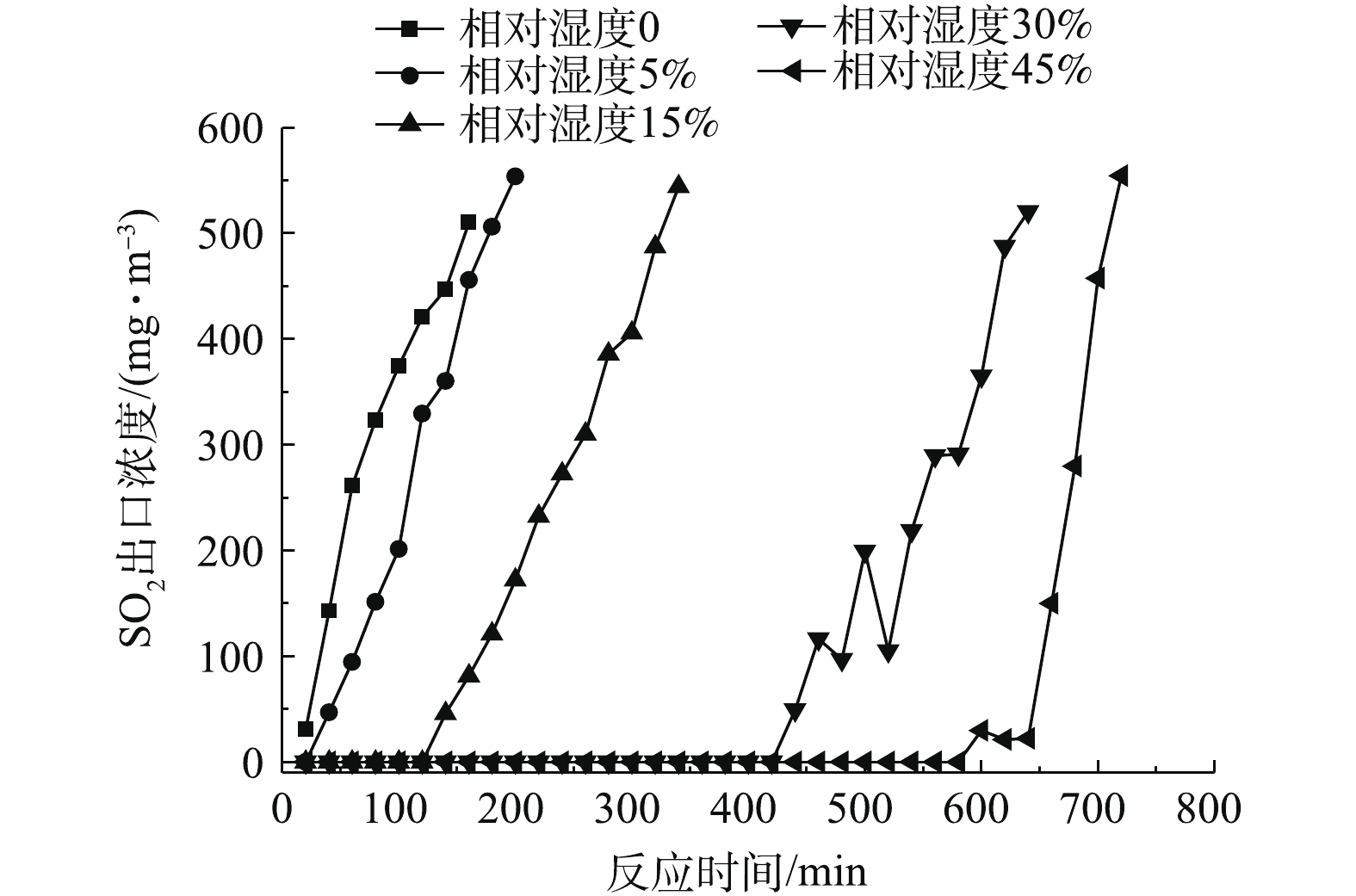
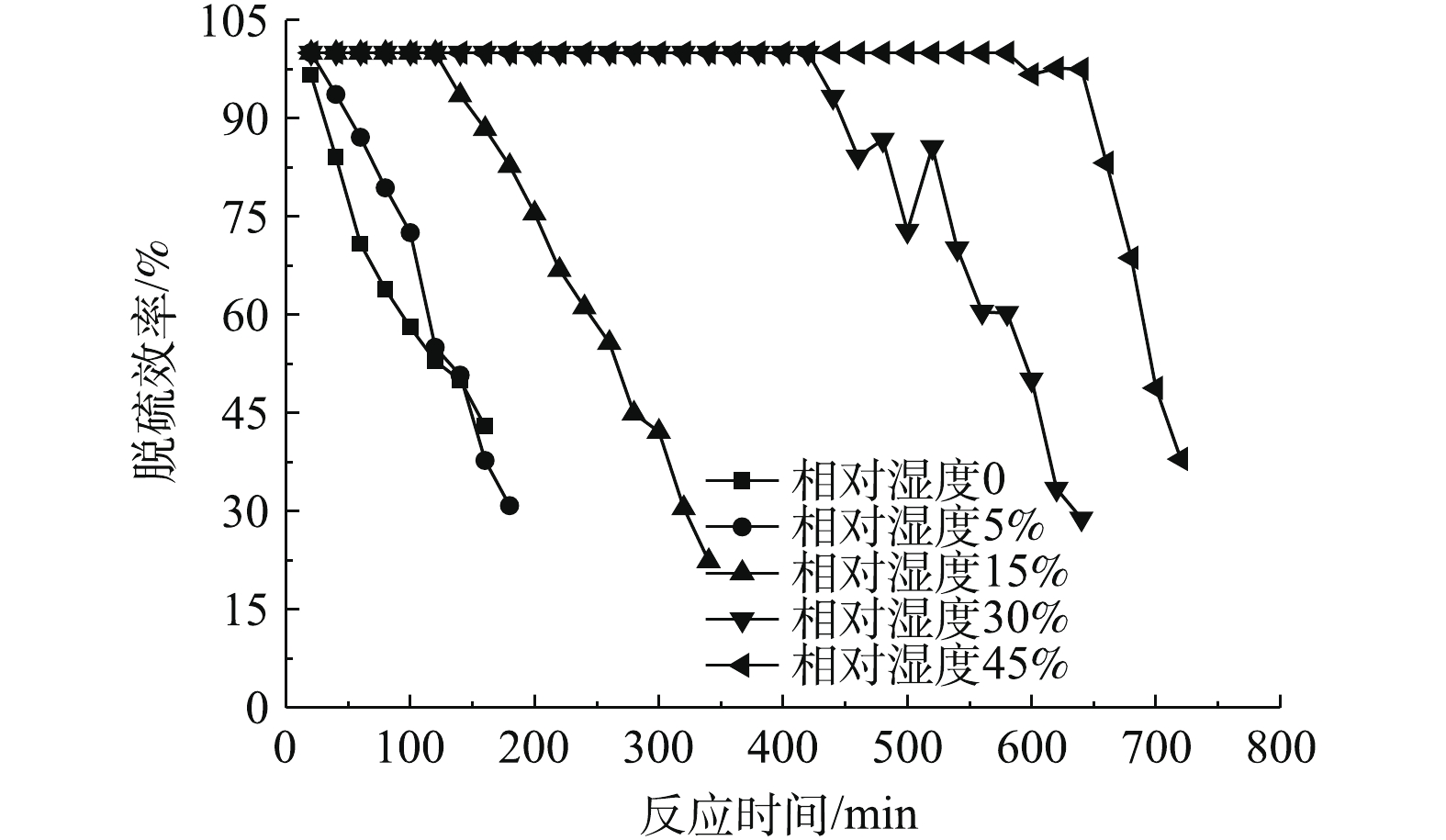
图4反映了相对湿度为0、5%、15%、30%、45%时,二氧化硫出口浓度随运行时间的变化情况。图5反映了脱硫剂的脱硫效率与运行时间的关系。可以看出,当相对湿度从0变化到45%时,脱硫剂的总反应时间不断增加,同时随着反应时间的增加,二氧化硫出口浓度不断升高,脱硫效率逐渐降低。
由图4可以看出,相对湿度对SO2出口浓度的影响比较显著。当相对湿度为0,总反应时间为160 min时,SO2出口浓度为510.3 mg·m−3,超过穿透浓度,将该段时间定义为脱硫剂穿透时间,那么相对湿度为0的烟气穿透时间就是160 min。当反应时间在20 min时,可以满足国家大部分地区SO2排放浓度低于35 mg·m−3的要求[5]。结合图5可以发现,当反应20 min时,由于脱硫剂本身含有微量水且本身孔隙发达,此时脱硫率可达96.57%;反应时间在160 min时,脱硫效率降至42.92%。相对湿度从0提高到5%时,烟气穿透时间增加并不明显,由160 min增加到180 min,反应20 min的脱硫效率从96.57%增至100%,足以满足35 mg·m−3的排放标准。当相对湿度为15%时,达到100%脱硫效率的反应为120 min,烟气穿透时间为340 min,浓度不低于35 mg·m−3的反应时间为140 min。当相对湿度为30%时,达到100%脱硫效率的反应时间为420 min,烟气穿透时间为640 min,浓度不低于35 mg·m−3的反应时间为440 min。当相对湿度为45%时,达到100%脱硫效率的反应时间580 min,烟气穿透时间为720 min,浓度不低于35 mg·m−3的反应时间为640 min。由图5还可以看出,随着相对湿度的增大,烟气穿透速率也缓慢增加,这是由于相对湿度增加,脱硫剂在反应床内部的松装密度变小,蓬松度减小,烟气穿透时间减小。
-
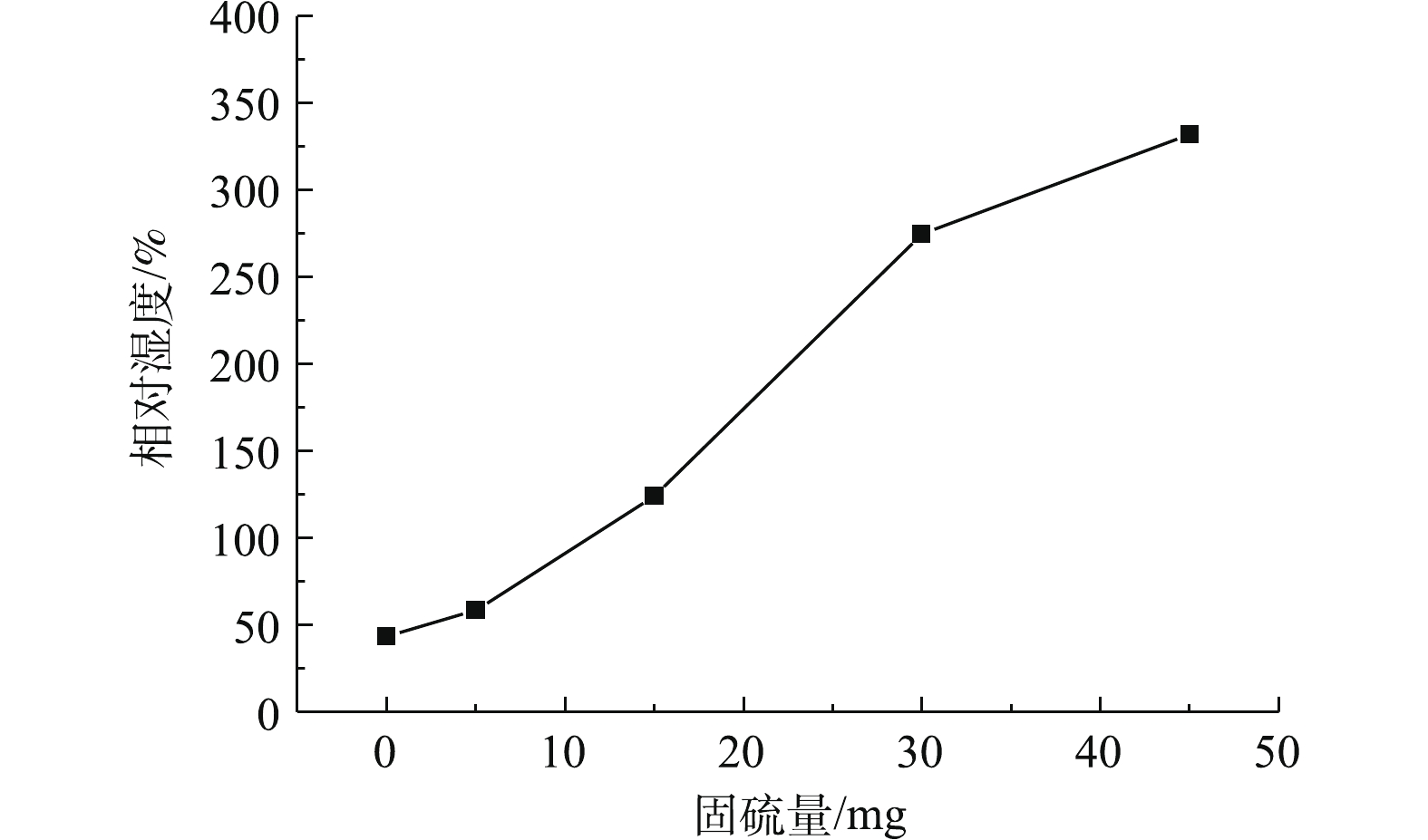
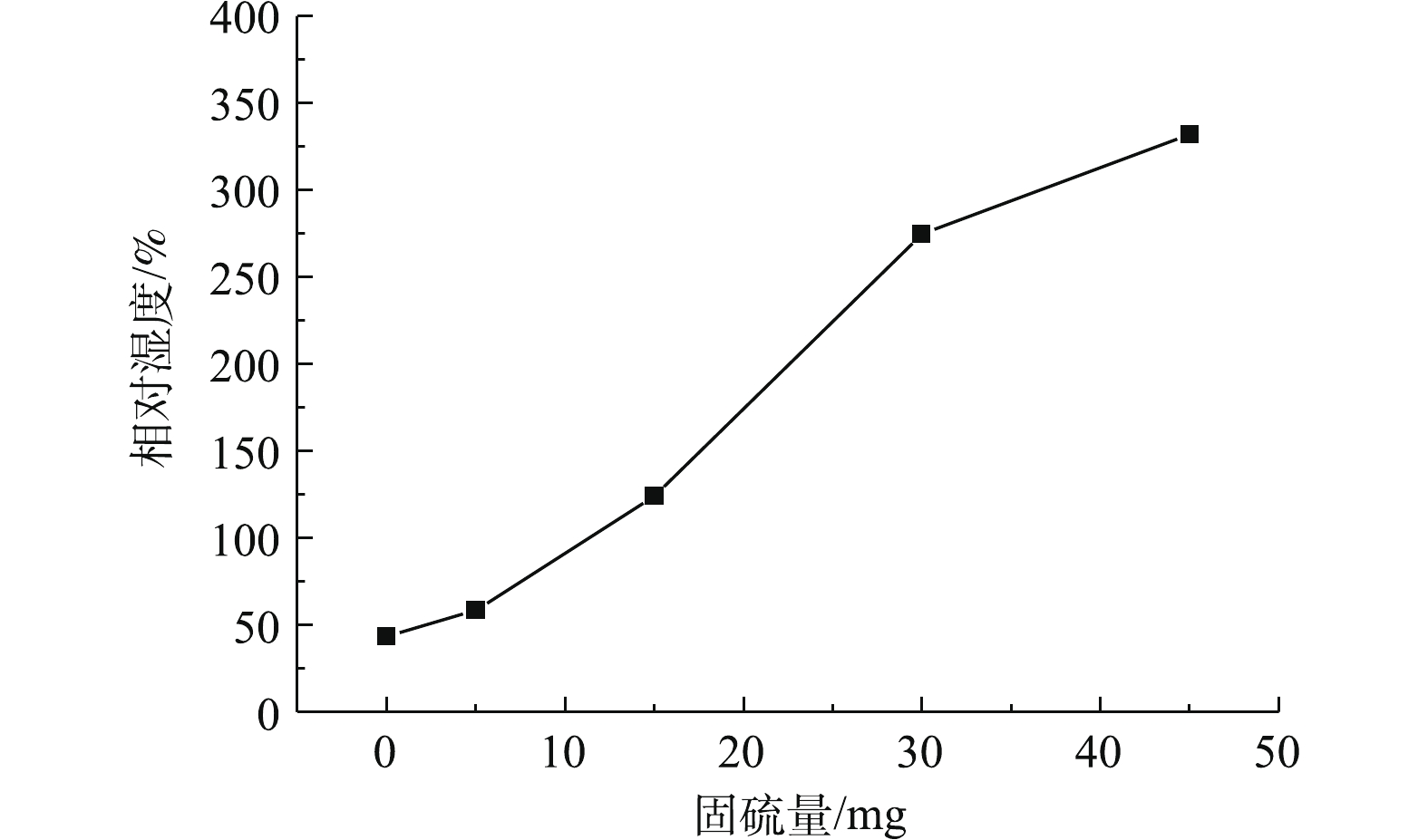
根据式(3),用origin软件计算得出相对湿度在0、5%、15%、30%、45%的脱硫剂总的SO2固硫量,结果如图6所示。在本实验中,相对湿度为0、5%、15%、30%和45%,总固硫量分别为43.37、58.29、124.05、274.58和332.09 mg。当相对湿度升高时,总固硫量也升高,相对湿度从0增加至45%,固硫量增大7.6倍,这是由于含湿脱硫剂的硫盐化反应速率明显高于干燥脱硫剂。随着反应炉内相对湿度的不断增加,脱硫剂表面越湿润,气相SO2在颗粒表面的溶解及液相中的离解反应都起到了相应的促进作用,
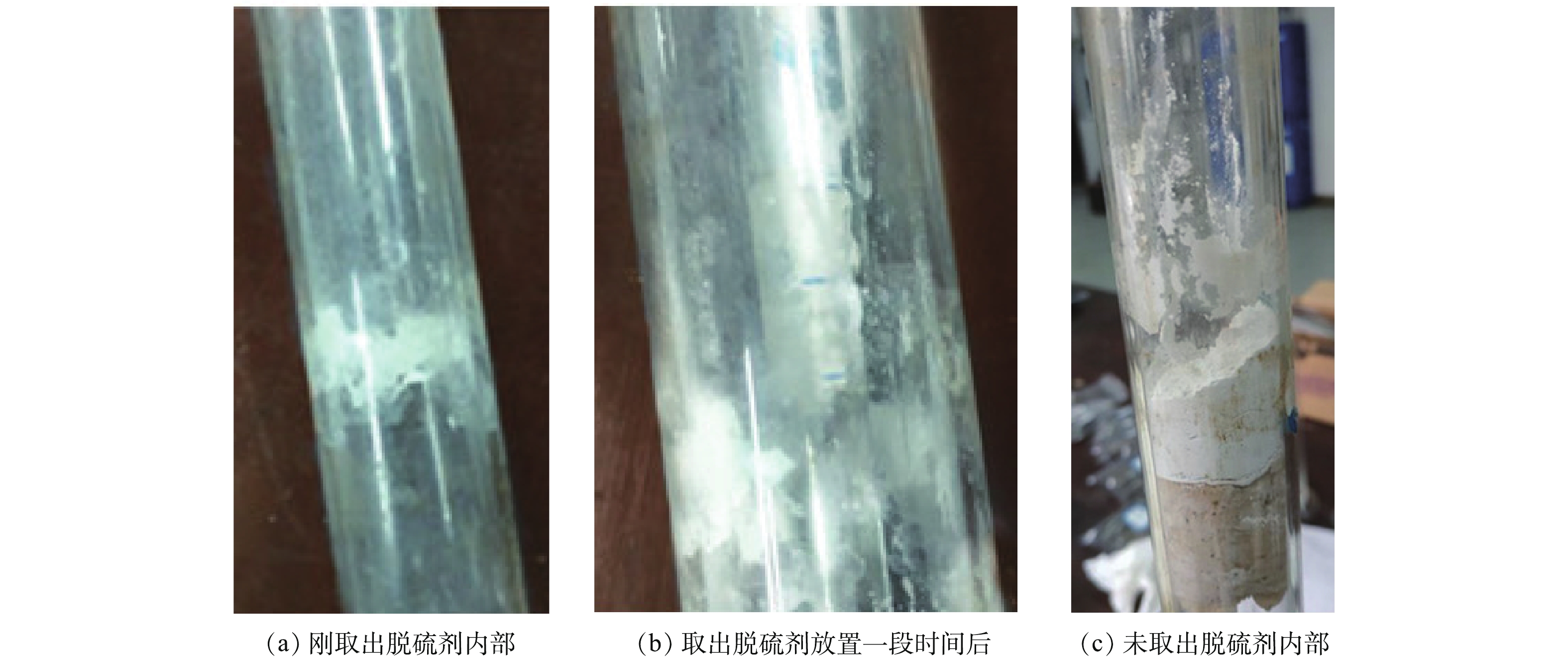
HSO−3 、SO2−3 与Ca2+等离子的扩散速率越大,Ca(OH)2、H2O与SO2等接触的可能性就越高,继而增加了脱硫剂的脱硫效率。因此,在工业应用中,当脱硫塔内部不出现黏壁等现象并有利于烟气排放的情况时,尽量增大脱硫塔内的相对湿度,以提高脱硫效率[17, 20]。本次干法烟气脱硫实验条件下,由于在相对湿度45%时,出现略微黏壁现象,如图7所示,此时相对湿度过高,脱硫剂、烟气与H2O反应后,有剩余的水分且此部分水不能完全蒸发,脱硫产物中部分呈半干状态。因此,为了保证烟气脱硫的稳定进行,相对湿度宜选择45%。
2.1. SO2出口浓度和脱硫效率对脱硫剂脱硫效果的影响
2.2. SO2固硫量对脱硫剂脱硫效果的影响
-
1)随着相对湿度的升高,烟气穿透时间增加,烟气脱硫效率增大,因此,在工业应用中,在保证脱硫塔内部不出现黏壁现象时,为增加脱硫剂的脱硫效率,可以提高脱硫塔内的相对湿度。
2)在本实验条件下,钙基脱硫剂在相对湿度为45%时脱硫效率较优,此时可以达到100%脱硫效率的运行时间为580 min,总反应时间为720 min,浓度不低于35 mg·m−3的运行时间为640 min,总固硫量为332.09 mg。
3)本实验采用道尔顿的分压定律来计算相对湿度,但是此种方式忽略了烟气带入水本身温度的问题,建议下一阶段对此问题展开更加细致的研究。




 下载:
下载: