-
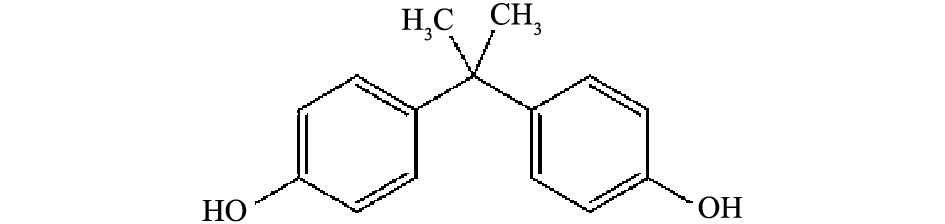
化工、制药及个人护肤品等行业产生的一些新兴有机污染物如内分泌干扰物、药物、杀虫剂等通过各种途径进入到了天然水体当中,这些被污染的水体普遍具有毒性大、新型污染物众多和难生物降解等特点[1-2]。双酚A(bisphenol A, BPA)是内分泌干扰物中的一种,分子结构如图1所示。其被广泛用于聚碳酸酯、环氧树脂、抗氧剂、增塑剂、油漆、农药等方面[3]。有研究[4-5]表明,BPA具有雌激素作用,摄取低浓度就能破坏人体的内分泌系统,造成不育、畸胎等,对人体的危害是持续性、累积和不可逆的,对家畜和野生动物的健康也会产生极大的影响。因此,寻求一种经济高效的解决BPA污染的方法具有重大的现实意义。
近年来,对BPA的去除方法主要有物理、生物及高级氧化法。传统的物理去除法如膜技术、离子交换以及活性炭吸附等仅能实现污染物的相转移,需要进一步后续处理[6]。生物法对含有生物毒性的酚类、醛类、酸类降解去除时会表现出很大的局限性[7-9]。高级氧化法如光催化[3]、电催化[10]、Fenton-类芬顿[11]和超声氧化[12]等对BPA的降解去除具有降解彻底、反应速度快、二次污染小等特点。但光、电催化、超声氧化等仍处于实验室阶段,工程应用难度大,Fenton法在水处理中有较多应用,但存在出水水质偏黄、pH局限于3左右、含铁污泥需要进一步处理等缺点[13]。本研究提出了以硫酸铜为催化剂的类芬顿法,主要考察了催化剂、H2O2用量、反应温度、BPA初始浓度和pH对BPA去除效果的影响,以期为处理此类有机废水提供技术参考。
全文HTML
-
BPA(C15H16O2、Adamas试剂有限公司)、香豆素(C9H6O2、Adamas试剂有限公司)、7-羟基香豆素(C9H6O3、Acros)、氯化铜(CuCl2·2H2O、广东省化学试剂工程技术研究开发中心)、硫酸亚铁(FeSO4·7H2O)、硫酸铁(Fe2(SO4)3)、硫酸锰(MnSO4·H2O)均购自成都市科隆化学品有限公司,硝酸铜(Cu(NO3)2·3H2O)、硫酸铜(CuSO4·5H2O)、二氧化钛(TiO2)购自成都市科龙化工试剂厂,双氧水(H2O2、30%)购自重庆川东化工(集团)有限公司,以上均为分析纯。紫外可见分光光度仪(UV-2550、日本岛津)、恒温水浴振荡器(SHA-C、金坛市易晨仪器制造有限公司)、离心机(TGL20M-Ⅱ、金坛市城西春兰实验仪器厂)、总有机碳(TOC)分析仪(TOC-VCPN、日本岛津)、荧光分光光度仪(F-7100、日本HITACHI)、pH计(FE20、瑞士梅特勒)。
-
在放置于恒温水浴振荡器的具塞锥形瓶中,进行BPA模拟废水的类芬顿催化氧化去除反应。向锥形瓶中加入一定量的由蒸馏水和BPA配成的模拟BPA废水溶液,然后分别加入一定量的硫酸铜催化剂和质量分数为30%的H2O2,之后在120 r·min−1的转速下反应。取不同反应时间条件下的反应液,经离心分离后,取其上清液分别测定TOC和吸光度值。
-
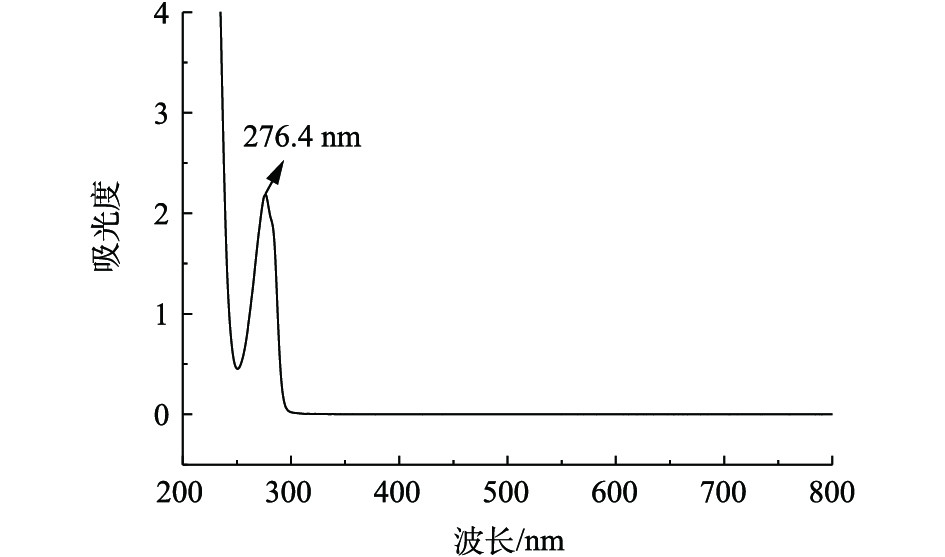
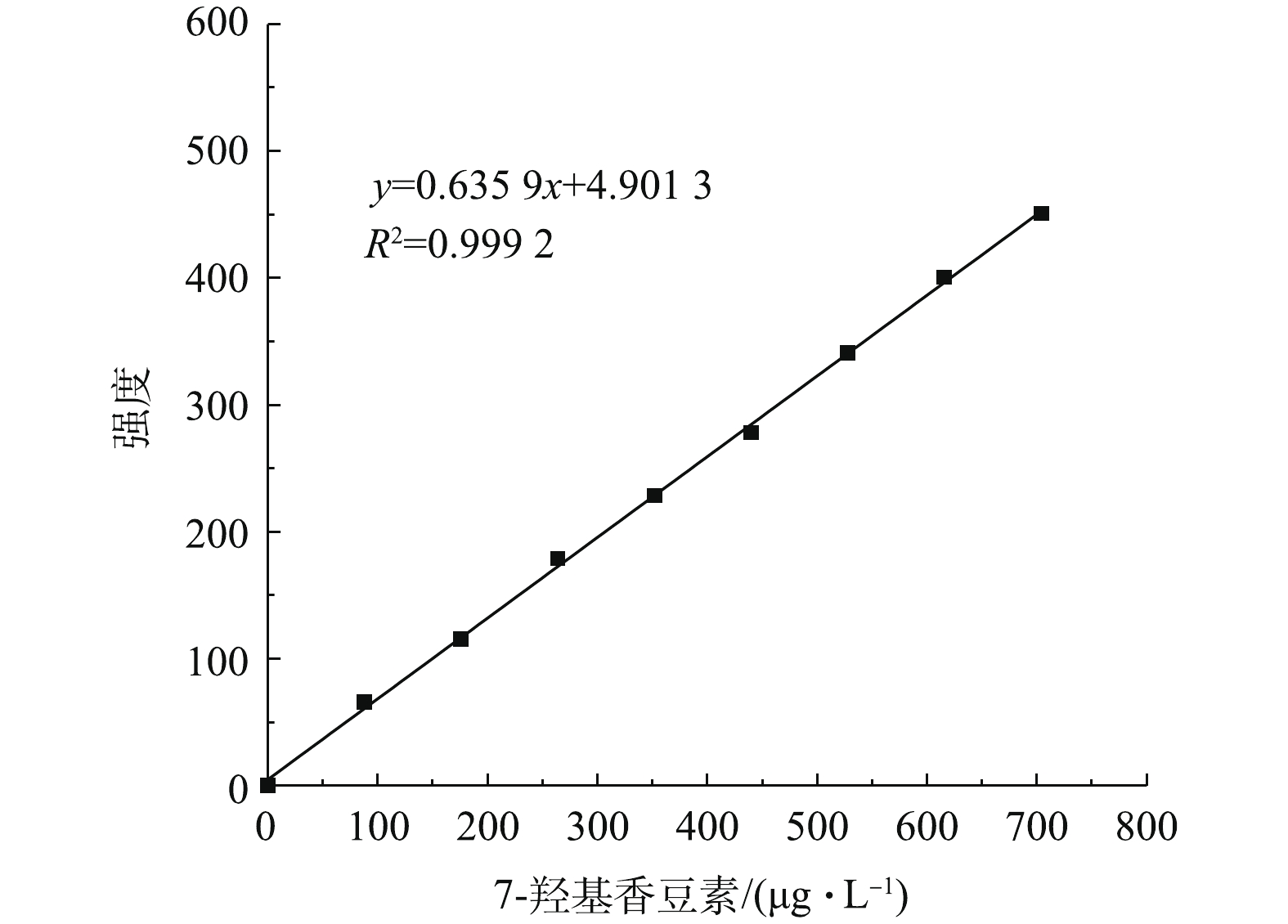
BPA的浓度可通过紫外-可见分光光度法建立的BPA浓度标准曲线来计算,其特征吸收波长为276.4 nm,全波长扫描结果见图2。总有机碳由TOC仪测定。H2O2浓度由钛盐光度法[14]测定。反应过程中产生的 · OH由香豆素捕获,通过荧光分光光度仪定量检测反应过程中产生的7-羟基香豆素,从而推算 · OH浓度[15]。
1.1. 实验原料与仪器
1.2. 实验方法
1.3. 分析方法
-
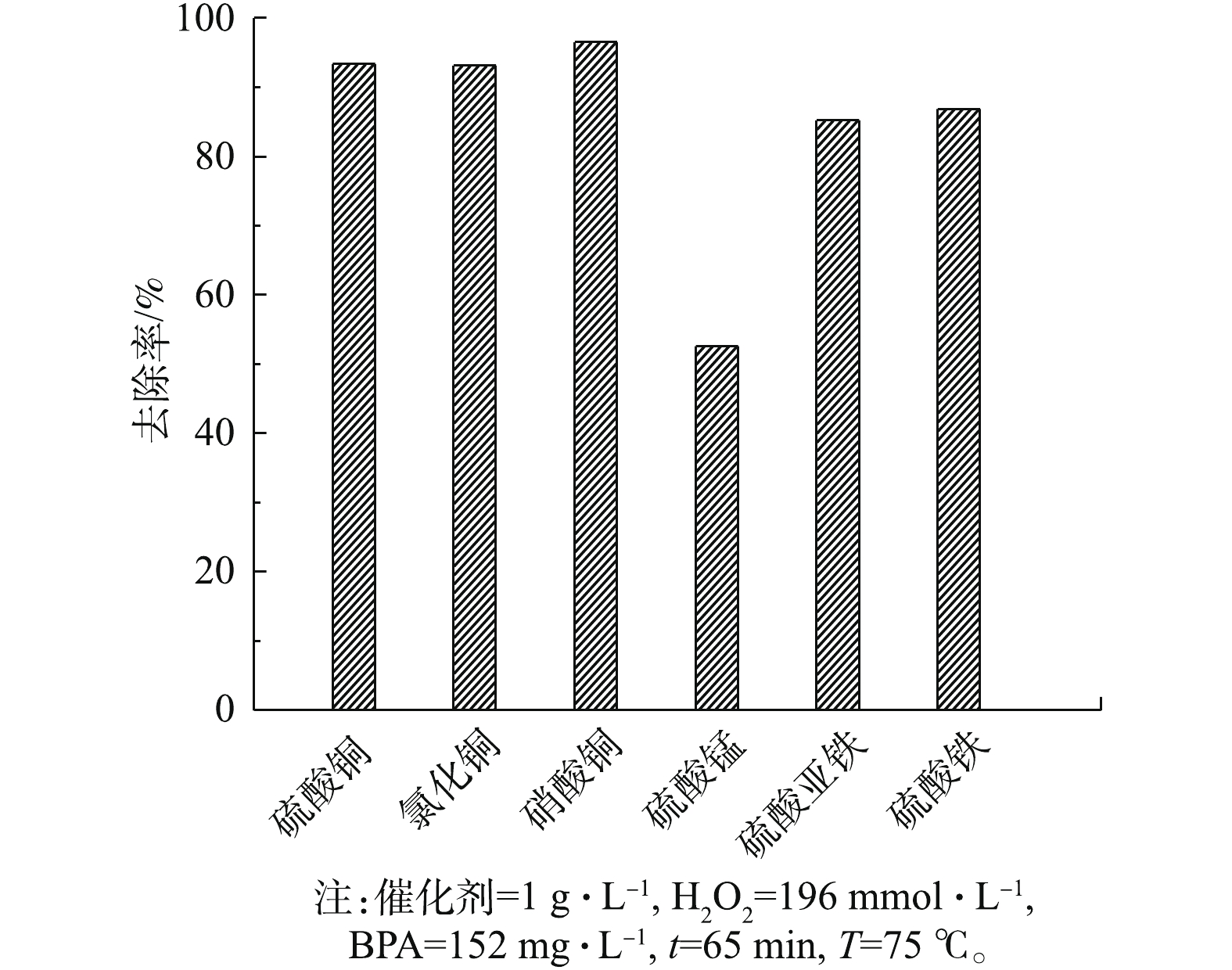
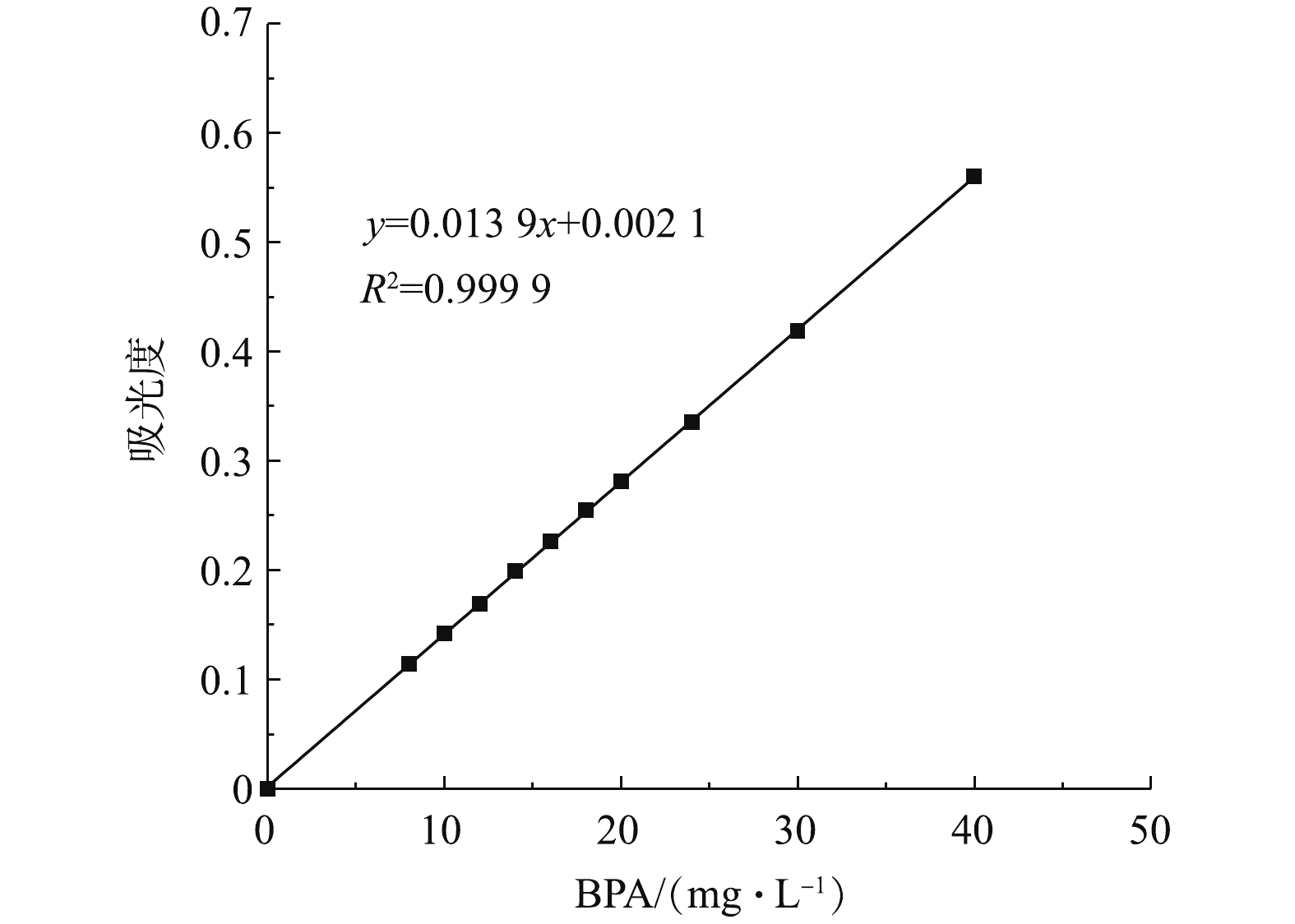
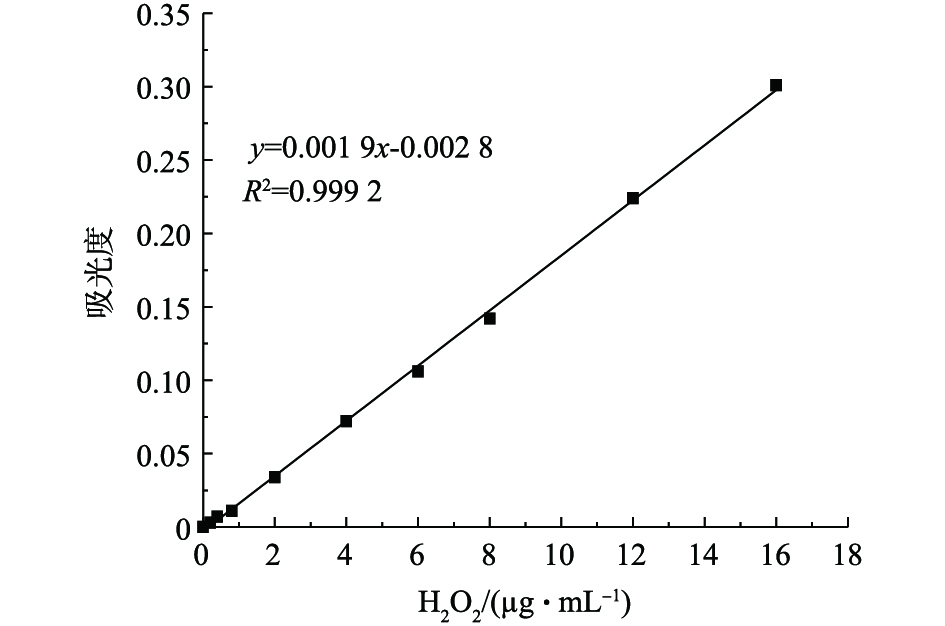
为了筛选成本低廉、效果较好的催化剂,主要考察了过渡金属化合物在类芬顿反应体系中对BPA的去除效果,结果如图3所示。图4和图5分别为BPA和H2O2的浓度标准曲线。由图3可知,硫酸铜、氯化铜和硝酸铜3种铜类化合物对BPA的去除效果相差不大,在65 min内,去除率均超过了95%。硫酸锰对BPA的去除效果最差,去除率仅有52.6%。硫酸亚铁和硫酸铁对BPA的去除率分别为85.4%和86.9%,效果相对较差,但反应后水质呈褐黄色、浑浊,在离心分离后,水质依然偏黄,沉淀3 d后水质才较澄清。从实验结果来看,3类含铜化合物的效果较好,由于氯化铜在溶液中含有氯离子,对水质有二次污染,硝酸铜存在硝酸根离子,在酸性条件下有一定的氧化能力,为此,本研究选择硫酸铜作为评价类芬顿催化氧化去除能力的最佳催化剂。
-
分别考察了BPA溶液挥发、单独H2O2氧化和单独催化剂对BPA去除效果的影响。结果表明,在反应2 h后,BPA溶液的挥发、单独H2O2的氧化和单独催化剂对去除率的贡献分别为8.9%、8.5%和8.6%,由图3可知,3种铜类化合物在65 min内对BPA的去除率均超过了95%,这说明催化剂和双氧水的协同作用是类芬顿法去除BPA模拟废水的关键。
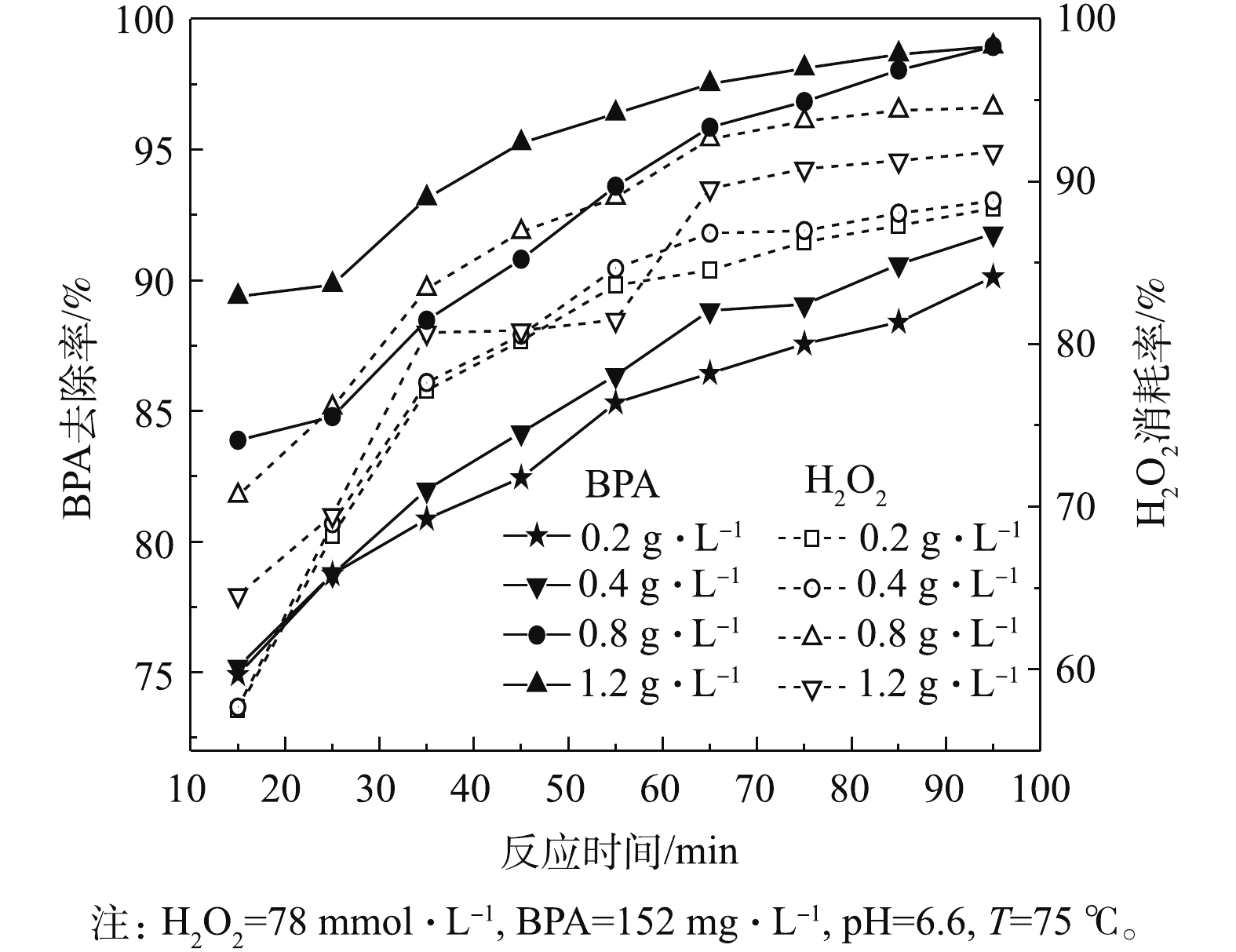
催化剂用量对BPA去除效果的影响结果如图6所示。由图6可知:当催化剂用量在0.4 g·L−1以下时,反应15 min后,BPA的去除率约为73.5%;在反应65 min后,达到约90%。当催化剂用量为0.8 g·L−1时,去除效果最好;在反应65 min和95 min后,BPA的去除率分别为95.4%和96.6%,比在其他条件下的BPA去除率均提高了约4%,TOC去除率分别为83.6%和85.9%。当超过最佳催化剂用量时,BPA去除率稍有下降。这可能归因于在较合适催化剂用量条件下,反应体系中产生的 · OH和其消耗达到一个平衡,当催化剂用量继续增大时,其会催化H2O2产生大量的 · OH,导致其自身的消耗反应加速,最终生成H2O、
HO−2 和O2而被消耗掉[16];另一方面,过量的 · OH与H2O2会反应生成具有抑制 · OH能力的HO−2 ,导致类芬顿体系去除效果降低[17]。 -
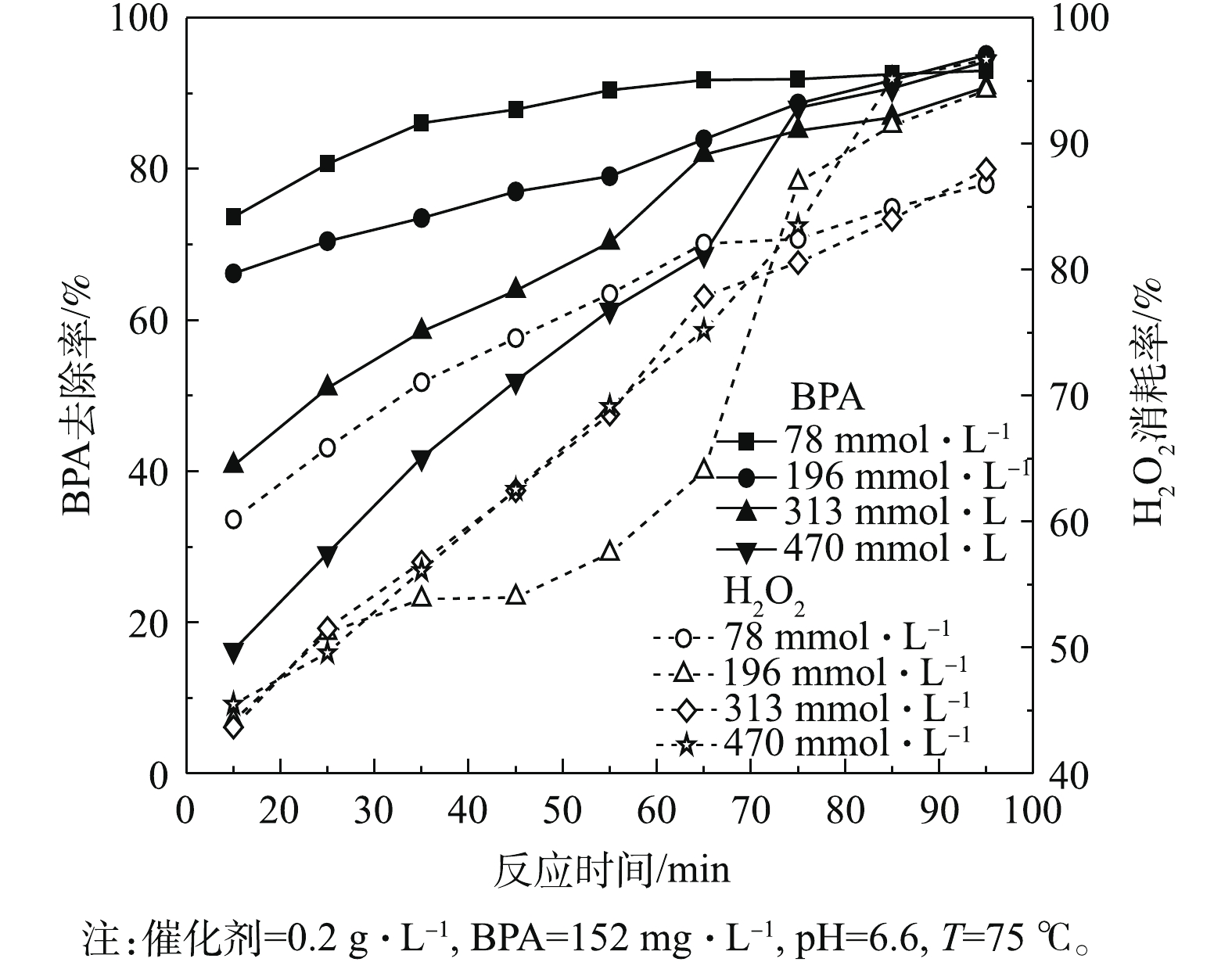
H2O2的理论需求量计算方程如式(1)所示。
196 mmol·L−1为H2O2理论需求量的12.4倍。由图7可知,当H2O2用量为78 mmol·L−1时,BPA的去除率最高,在反应65 min后,BPA的去除率为91.8%,H2O2的消耗率为82.1%。随着H2O2用量的增加,BPA的去除率下降,当H2O2用量增加到470 mmol·L−1时,在反应65 min后,BPA的去除率下降到68.8%。这说明H2O2用量对BPA的去除效果具有显著的影响。当H2O2过量时,会导致类芬顿反应对BPA的去除效果大幅下降,这可归因于 · OH与过量的H2O2发生了自身消耗反应[16,18]。
-
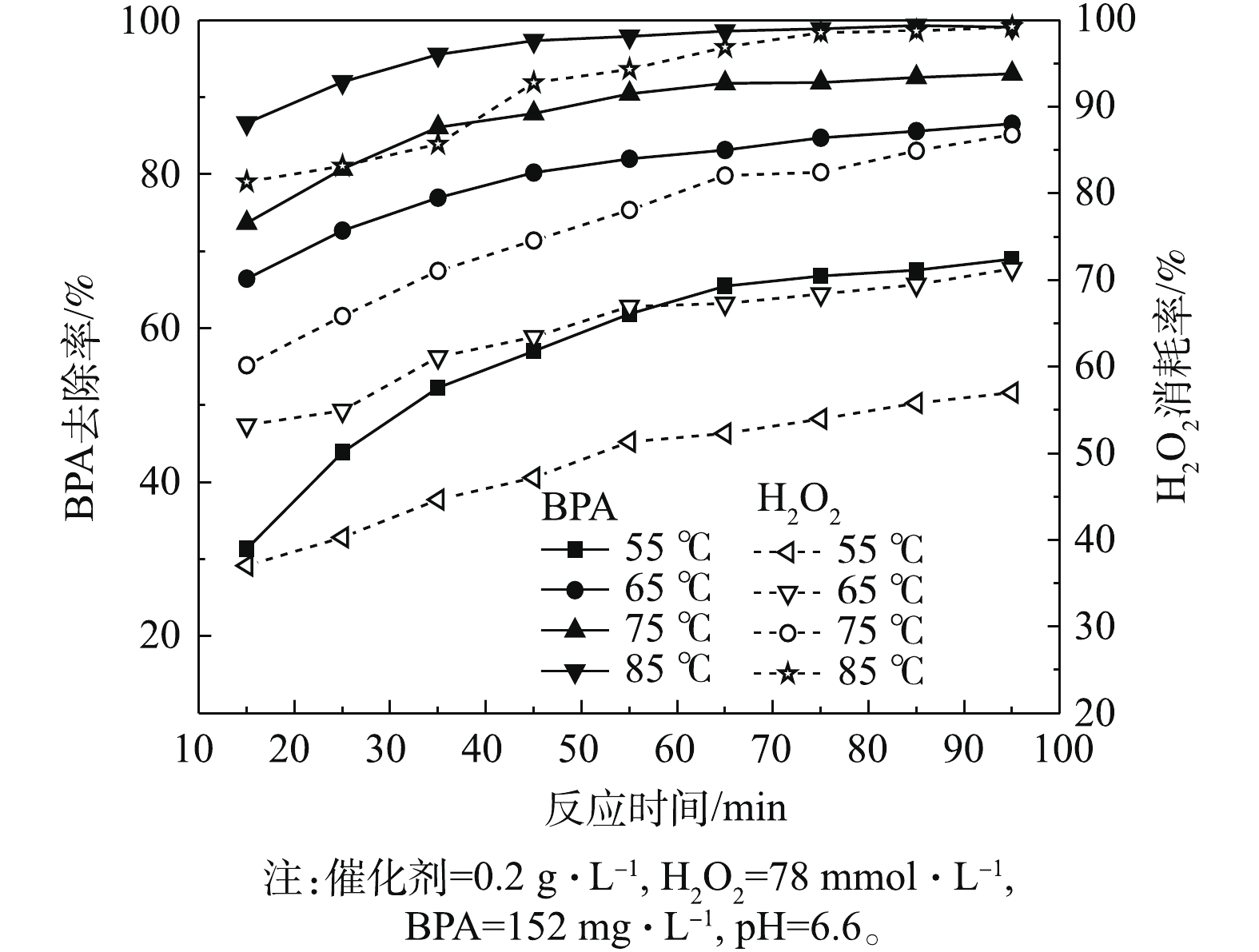
反应温度对BPA去除效果的影响如图8所示。由图8可知,反应温度对BPA的去除率影响很大,当反应温度从55 ℃升高至85 ℃时,反应15 min后,BPA的去除率从31.3%升高到86.6%,H2O2的消耗率从37%升高到81.4%。当反应65 min后,在55 、65 、75 和85 ℃下,BPA的去除率分别为65.5%、83.1%、91.8%和98.6%,H2O2的消耗率分别为52.3%、67.3%、82.1%和96.9%。由此可知,反应温度对BPA的去除和H2O2的消耗影响较大。通过观察在不同温度下溶液颜色的变化可知:在55 ℃时,溶液呈浅紫红色;在65 ℃时,在前25 min内,溶液先呈浅紫红色,后变为透明;在85 ℃时,溶液呈透明状态。这可归因于随着反应温度的升高,H2O2活化导致 · OH的生成量增加,同时提高了反应物分子间发生有效碰撞的概率,缩短了催化诱导期[19],加速了反应过程。由于更高的温度,会加速H2O2分解[20],综合考虑,本研究选择75 ℃为最佳反应温度。
-
BPA初始浓度对BPA去除效果的影响如图9所示。由图9可知,BPA初始浓度对去除效果影响较大。在反应65 min后,当BPA浓度从40.1 mg·L−1增加到331 mg·L−1时,BPA的去除率从97.4%下降到91.9%,这说明随着BPA浓度的增加,对 · OH的需求也有所增加,导致在相同催化剂、H2O2浓度和反应时间内,产生的强氧化性 · OH不足以氧化足够多的BPA。由图9可知,BPA浓度对H2O2消耗率的影响显著,在反应65 min后,H2O2消耗率从96.7%下降到82.7%,可归因于BPA具有双苯环结构且浓度较高,会导致空间位阻效应增强[21],阻碍了Cu2+与H2O2的充分接触,导致其分解变慢,体系产生 · OH的速率下降。
-
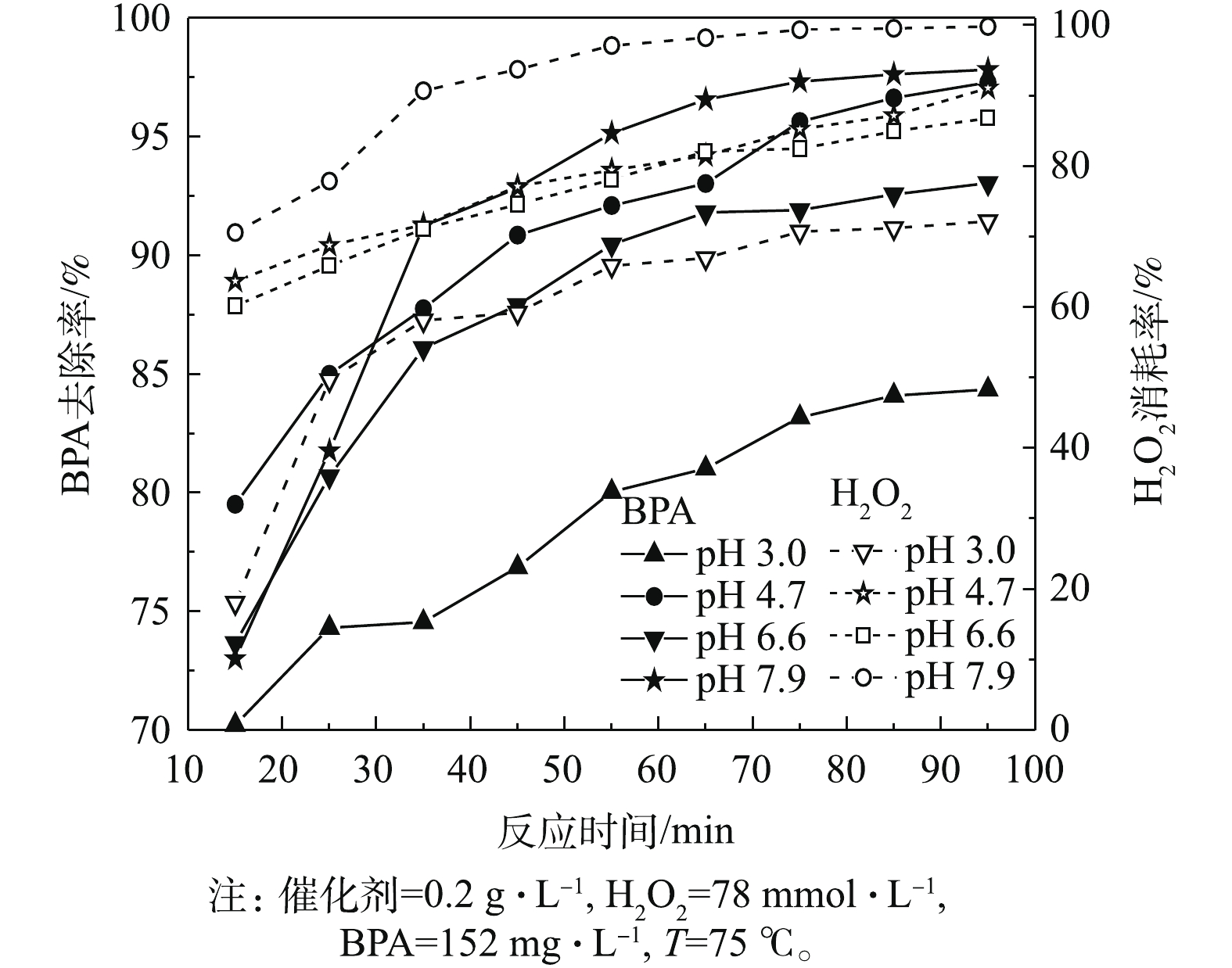
初始pH对BPA去除效果的影响如图10所示。由图10可知,在pH为3.0~7.9,反应一定时间后,BPA的去除率均比较高。在早期反应阶段,BPA的去除率有小幅波动,反应15 min后,分别为70.2%、79.5%、73.7%和73%,H2O2的消耗率差距较大,分别为18%、63.4%、60.2%和70.6%。这说明在早期反应阶段,pH对BPA的去除有一定影响,尤其是在弱酸条件下,可归因于弱酸性条件下H2O2的分解受到一定的抑制[22]。随着反应的进行,体系pH可能升高,H2O2的分解抑制解除;当反应65 min后,除了在pH=3.0的条件下,对应的BPA去除率偏低外,其他条件下对应的BPA去除率均在91%以上,在pH=7.9时,BPA的去除率可达到96.6%,H2O2的消耗率达到最高值98.1%。
另外本研究考察了在其他pH条件下,BPA在类芬顿反应体系中的去除效果。结果表明,在pH=1.6和pH=2.0的条件下,BPA几乎没有被去除,此时H2O2的消耗率分别仅有4.9%和11.3%,这说明在强酸条件下,不利于H2O2分解为强氧化性 · OH。有研究[23]表明,过渡金属离子在溶液pH发生变化时,其价态可能发生变化,变为水合氧化物沉淀或变为带正电荷的多羟基配合物聚合体,从而易吸附
HO−2 ,降低其活度,抑制H2O2的分解。在pH=10.1时,当反应65 min后,BPA的去除率仍然高达86.6%,H2O2消耗率达到100%;在pH=11.4和pH=12.0的条件下,当反应65 min后,BPA几乎没有去除,但H2O2完全消耗完,且观察到溶液均有红褐色沉淀生成。说明在强碱性条件下,H2O2极不稳定,容易形成HO−2 [17],而HO−2 是一种亲核试剂,易引发H2O2分解产生游离基,而Cu2+也更容易与OH−发生沉淀反应,故在强碱性条件下不利于类芬顿反应的进行。从pH对BPA去除效果来看,采用硫酸铜作为催化剂的类芬顿法,相比Fenton法,一般需要在pH 2~4条件下反应,具有更宽的pH适应范围,故其优势更为显著。 -
考察反应过程中pH和羟基自由基浓度的变化,可为后续进一步考察降解机理和路径提供依据。另外随着反应的进行,反应体系pH的变化,是否会超出催化剂的pH适应范围,需要进一步分析。通过考察羟基自由基浓度的变化来反映其是否参与了氧化反应。 · OH浓度通过荧光法间接测定,经全谱荧光扫描确定:香豆素的最大激发波长为277 nm,最大发射波长为392 nm;7-羟基香豆素的最大激发波长为321 nm,最大发射波长为452.6 nm。图11为7-羟基香豆素的标准滴定曲线。
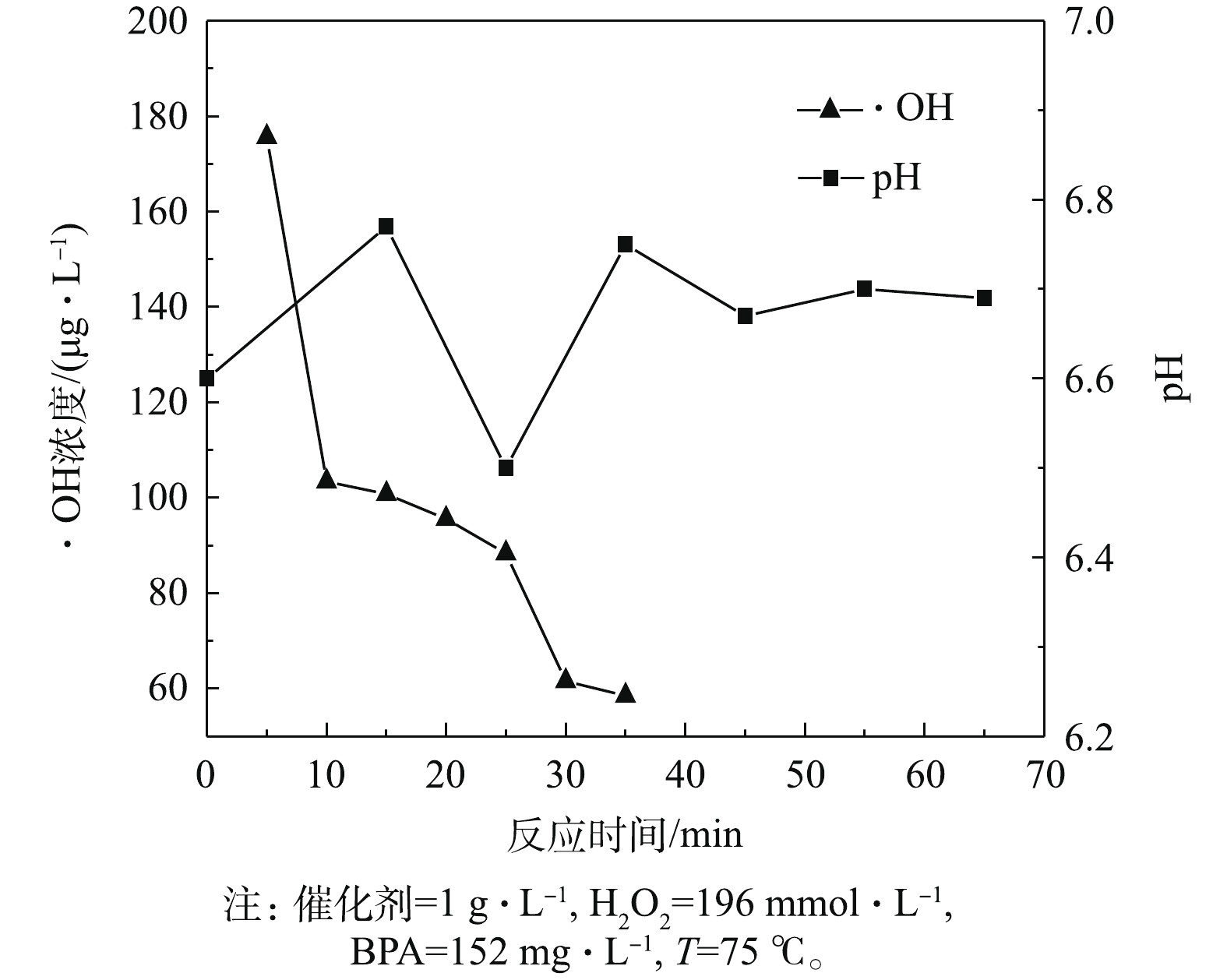
图12为反应过程中pH和 · OH浓度的变化情况。由图12可知,在硫酸铜类芬顿反应体系中,pH在早期升高后,又有小幅的下降,这可能是因为在此过程中生成了酸性中间产物。当这些中间产物转化为其他物质后,pH又逐渐升高,但升高幅度并不大,体系pH维持在6.7左右,比初始pH稍高。这表明尽管体系pH发生了变化,但催化剂依然在pH适应范围内,同时说明BPA在硫酸铜类芬顿反应体系中的结构受到破坏,可能有弱碱性中间产物生成。此外,在类芬顿反应体系中,随着反应时间的延长,体系中 · OH的浓度呈逐渐下降趋势,这说明大量的 · OH参与了BPA的氧化降解去除反应。
2.1. 催化剂的筛选
2.2. 催化剂用量对BPA去除效果的影响
2.3. H2O2用量对BPA去除效果的影响
2.4. 反应温度对BPA去除效果的影响
2.5. BPA初始浓度对BPA去除效果的影响
2.6. 初始pH对BPA去除效果的影响
2.7. 反应过程中pH和羟基自由基浓度的变化
-
1)在早期反应阶段,提高催化剂用量有利于反应的快速进行,但反应后期,受催化剂用量影响不大;H2O2用量对BPA的去除影响较大,随着H2O2用量的增大,去除率明显降低;随着反应温度的升高BPA的去除率增大;BPA的去除随浓度的增加有一定的下降。反应过程中溶液的pH变化不大。
2)在催化剂用量为0.8 g·L−1、H2O2为78 mmol·L−1、BPA为152 mg·L−1和反应温度为75 ℃的条件下,反应65 min后,BPA和TOC的去除率分别为95.4%和85.9%。
3)以硫酸铜为催化剂,采用类芬顿法对BPA进行催化氧化去除,相比Fenton法一般需要在pH 2~4条件下反应更具优势,其具有更宽的pH适应性,可以在pH=3.0~10.1的条件下反应,无需调节反应液的pH,具有一定的应用前景。



 下载:
下载: