-
挥发性有机污染物(VOCs)来源广泛、组分复杂,对环境和人体均有一定危害[1-2],因此,近年来,对VOCs的减排与控制备受关注,相关标准逐渐明确、严格,已有许多针对VOCs处理技术的研究。现有的VOCs处理技术包括吸附法、吸收法、燃烧法、膜分离处理法、生物法、光催化降解和等离子体法[3]。但针对实际中产生的低浓度VOCs废气,前6种技术存在运行费用高、设备性能要求高、涉及产物复杂等劣势。而作为近几年新兴的低温等离子体技术(NTP)在净化低浓度VOCs时,具有反应迅速、启停便捷、设备简单[4-5]等优势,极具应用潜力。但是单一的NTP技术依然存在能耗较高、副产物难以避免等问题[6-7]。因此,研究者将吸附、等离子体氧化和催化耦合于一体,从而产生了吸附-等离子体催化氧化技术。填充的介质阻挡放电反应器可以实现3种技术的有效结合,已有研究表明,间歇的吸附存储-等离子体催化技术不仅可以提高能量效率[8-9],而且还可以有效降低副产物的排放[10-11]。
在吸附-等离子体催化氧化技术中,催化剂的作用至关重要,不仅要有较好的吸附性能,还要有较好的等离子体催化氧化性能。锰基催化剂在协同低温等离子体降解不同挥发性有机物时表现出较优的氧化性能。郝翰[12]在石墨烯上通过电化学沉积法负载Mn3O4耦合介质阻挡放电来氧化降解甲苯,获得了较好的甲苯降解率以及CO2选择性,并有效控制了O3和NOx等副产物的产生;LYULYUKIN等[13]利用电晕放电联合TiO2来氧化丙酮和乙醇,发现负载MnOx的催化剂不仅可以抑制副产物的生成,还促进了丙酮和乙醇的深度氧化,提高了CO2选择性;ODA等[14]的研究结果显示,MnO2负载在氧化铝小球上,在低温等离子体氧化降解TCE时起到了积极的作用;向东等[15]的研究表明,介质阻挡放电与MnOx/SBA-15催化剂对正己醛氧化降解表现出了良好的协同效应,去除率最高可达99%。但目前针对等离子体联合催化技术用于氧化降解乙酸乙酯的研究还不深入,而乙酸乙酯是汽车制造、制药、电子制造等行业的代表性污染物[16],更是包装印刷行业VOC排放最为显著的复合膜干复工艺的主要污染物[17]。因此,针对低温等离子体联合锰基催化剂净化乙酸乙酯的研究有待于进一步深入。
本研究以13X和γ-Al2O3为载体,负载MnOx并联合低温等离子体,氧化降解吸附态乙酸乙酯,以COx产率、CO2选择性以及副产物的生成量为评价指标,探究了不同载体或催化剂对乙酸乙酯的氧化性能及反应动力学的影响,为DBD降解挥发性有机物系统中催化剂的优化及其应用提供参考。
全文HTML
-
实验以13X和γ-Al2O3(直径为3~5 mm,上海有新催化剂厂)为载体,Mn(NO3)2溶液为前驱体,采用等体积浸渍法制备MnOx/13X和MnOx/γ-Al2O3 2种锰基催化剂。制备条件:缓慢滴加一定量的前驱体溶液于载体上,然后静置过夜,干燥箱烘6 h(105 ℃),于马弗炉中焙烧3 h(500 ℃)。
催化剂的比表面积采用比表面积及孔径分析仪(BET)(Micromeritics ASAP 2460, USA)分析。样品在250 ℃下进行脱气预处理4 h,在−196 ℃下,进行N2吸附-脱附实验。样品形貌利用日本电子JSM-6510LV型扫描电子显微镜分析。测定样品前,在20 mA 工作电流下喷金100 s,对样品进行预处理,扫描工作电压为15 kV。采用美国热电K-Alpha X射线光电子能谱仪(XPS)对催化剂表面的元素组成、含量进行测定,光源为Al Kα(1 486.6 eV)射线,能谱采用C1s的标准结合能284.5 eV进行校正,采用XPS peak4.1软件对谱图进行分峰拟合。
-
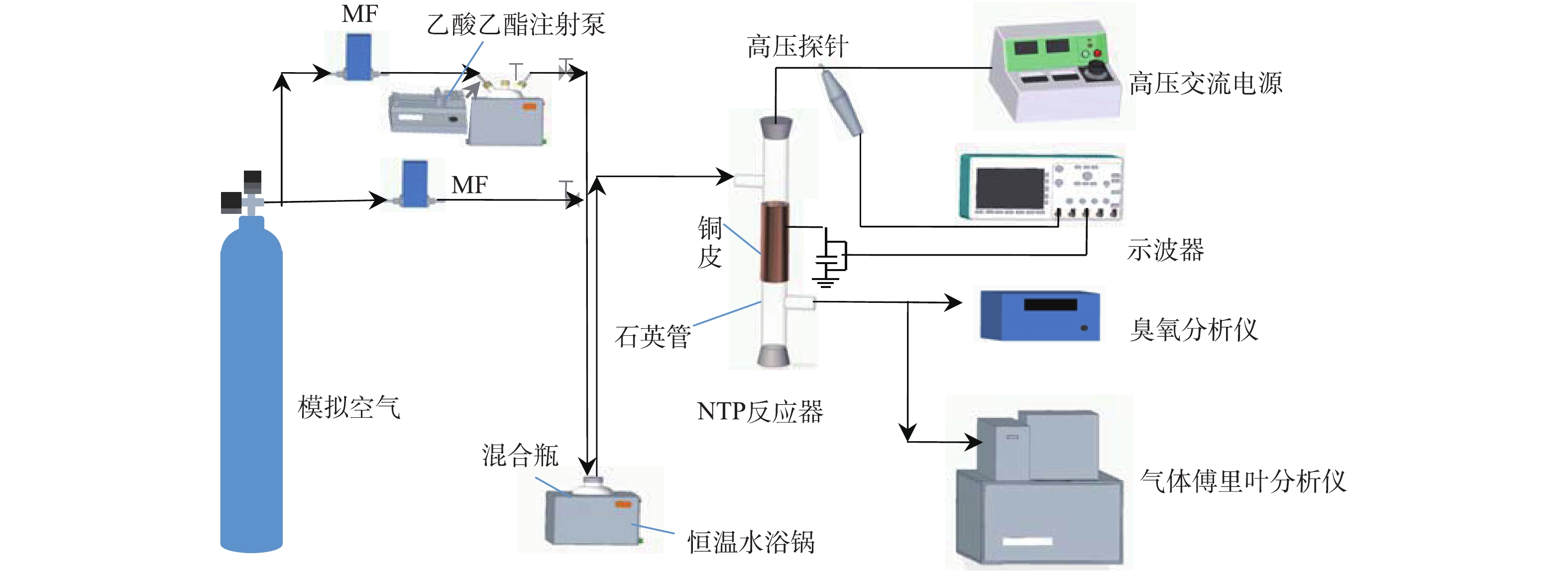
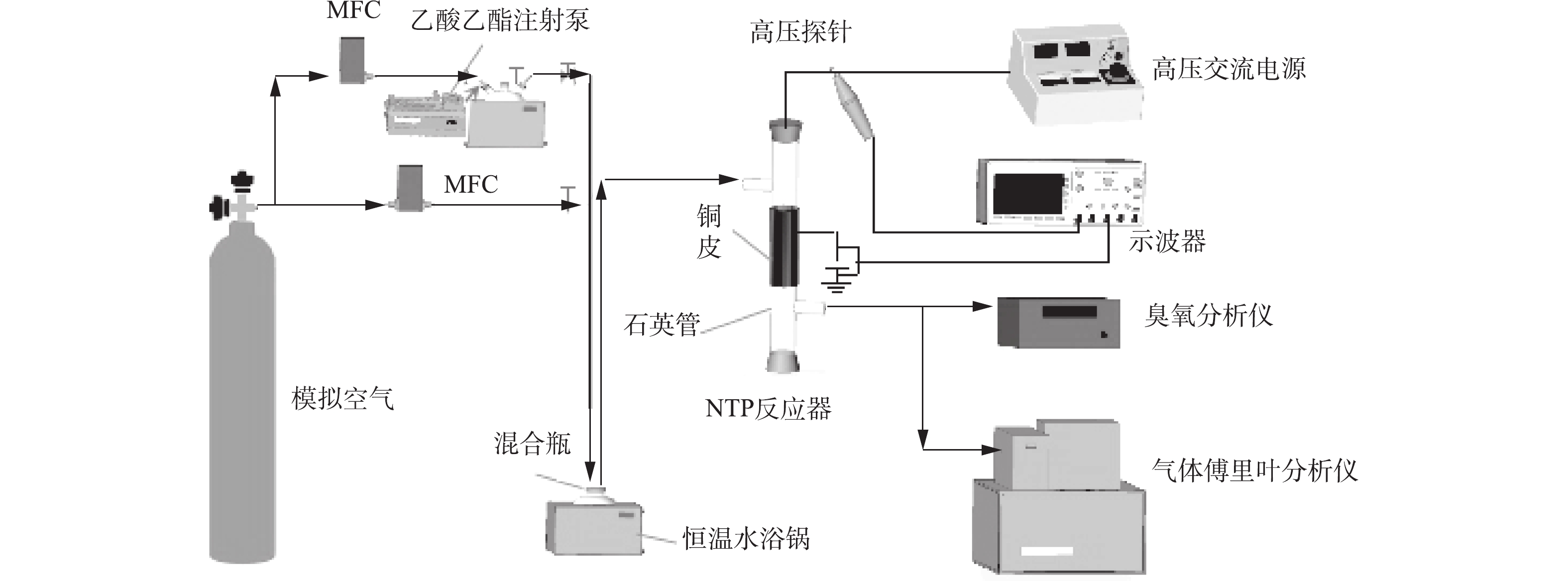
实验系统主要由配气装置、等离子体反应器和测试系统组成,实验系统流程如图1所示。配气系统包含模拟空气(79%N2、21%O2)、质量流量计、注射泵、恒温水浴锅和缓冲混合瓶,用于产生1 L·min−1、1 571 mg·m−3的乙酸乙酯。反应装置为自制的线管式反应器,材质为石英玻璃(内径=21.5 mm),内电极为不锈钢丝(直径=0.8 mm),外电极为100 mm长的铜皮,缠绕在反应器外壁,接地。采用50 Hz交流高压电源(GJTK-0.01/30K,上海南罡电除尘器有限公司),电压为22 kV,电压-电流波形见图2。实验产生的CO2、CO、N2O和乙酸乙酯浓度均采用傅里叶变换红外光谱仪(Nicolet Antaris IGS,Thermo Scientific Company)分析,O3浓度由臭氧检测仪(2B Technologies Model 106-M)测得。
本实验分为吸附和放电2个阶段:1)在吸附阶段,乙酸乙酯吸附存储在填充的催化剂表面,此过程不放电;2)在放电阶段,以1 L·min−1的空气为放电背景气,利用放电产生的活性粒子,将吸附态乙酸乙酯氧化降解。
-
实验中乙酸乙酯氧化的评价指标为COx产率和CO2选择性,计算方法见式(1)和式(2)。
式中:R为COx产率;S为CO2选择性;
nC4H8O2 为乙酸乙酯的初始吸附量,mmol;nCO2 和nCO分别为等离子体氧化降解阶段反应器出口的CO2和CO的量,mmol。
1.1. 催化剂的制备与表征
1.2. 实验系统
1.3. 性能评价指标
-
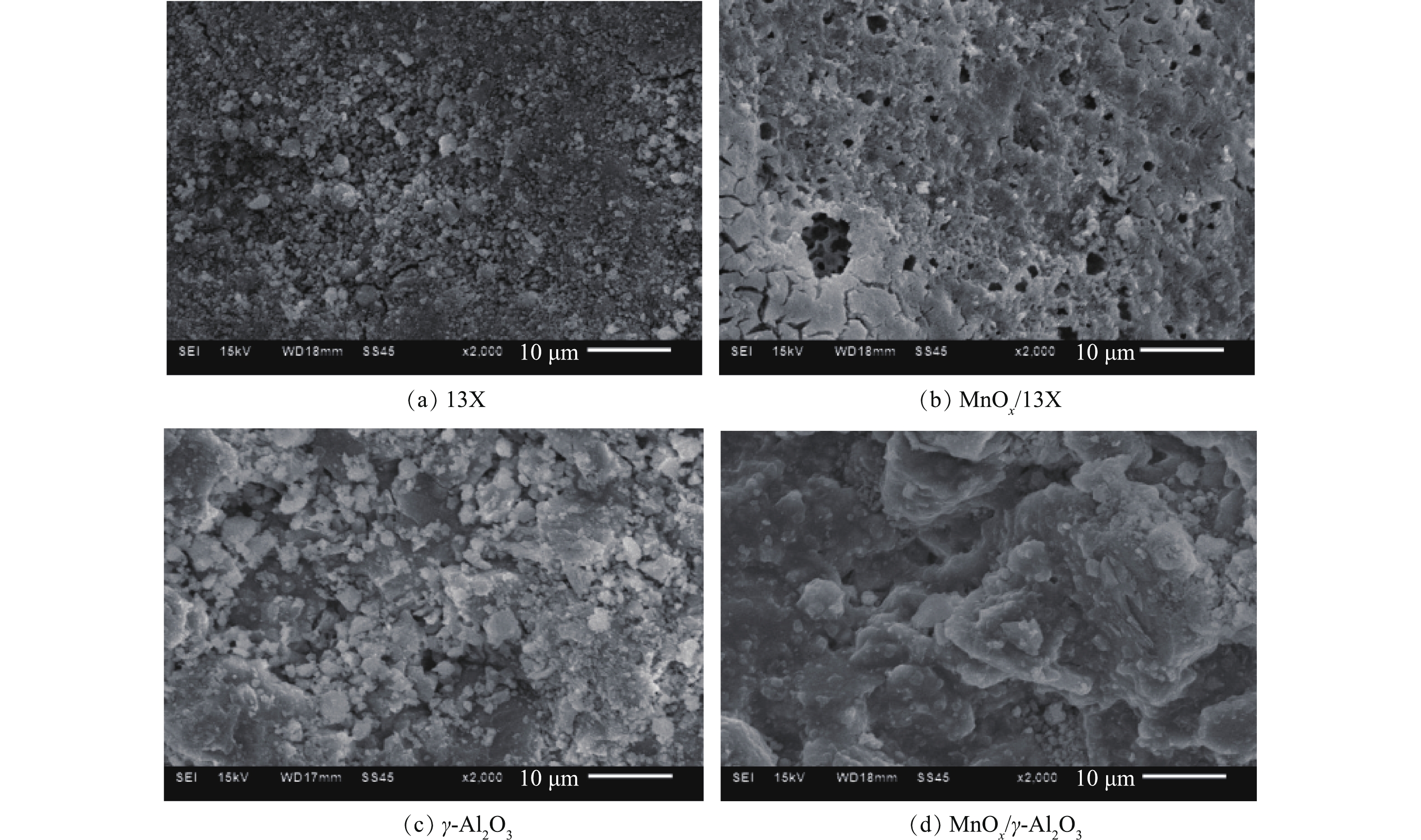
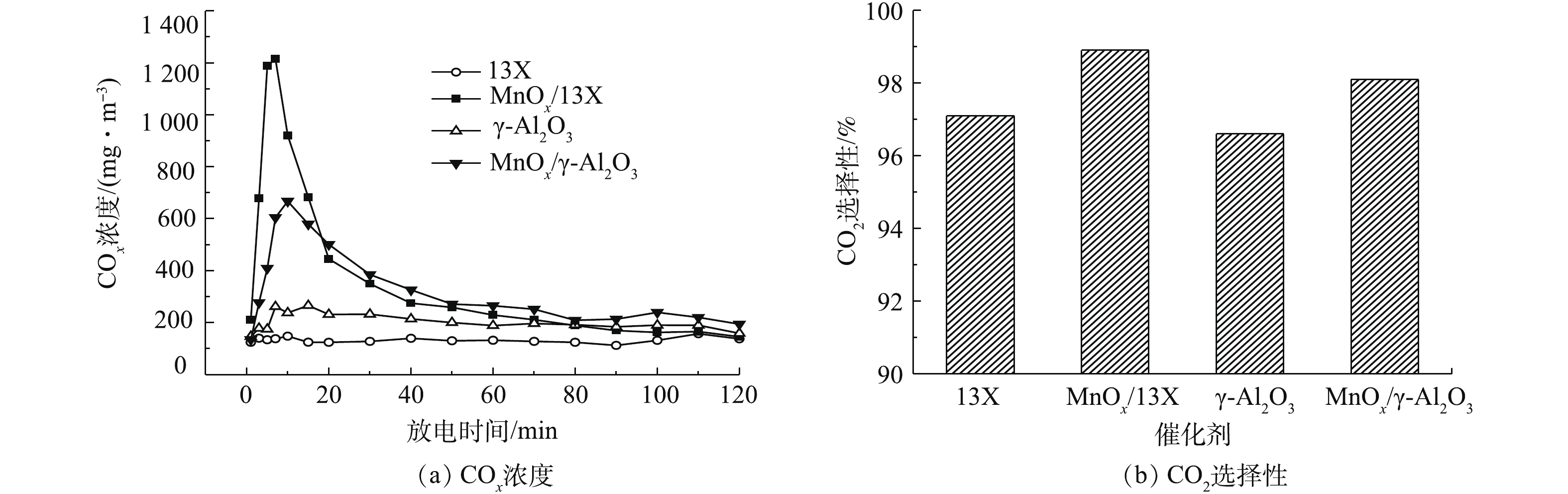
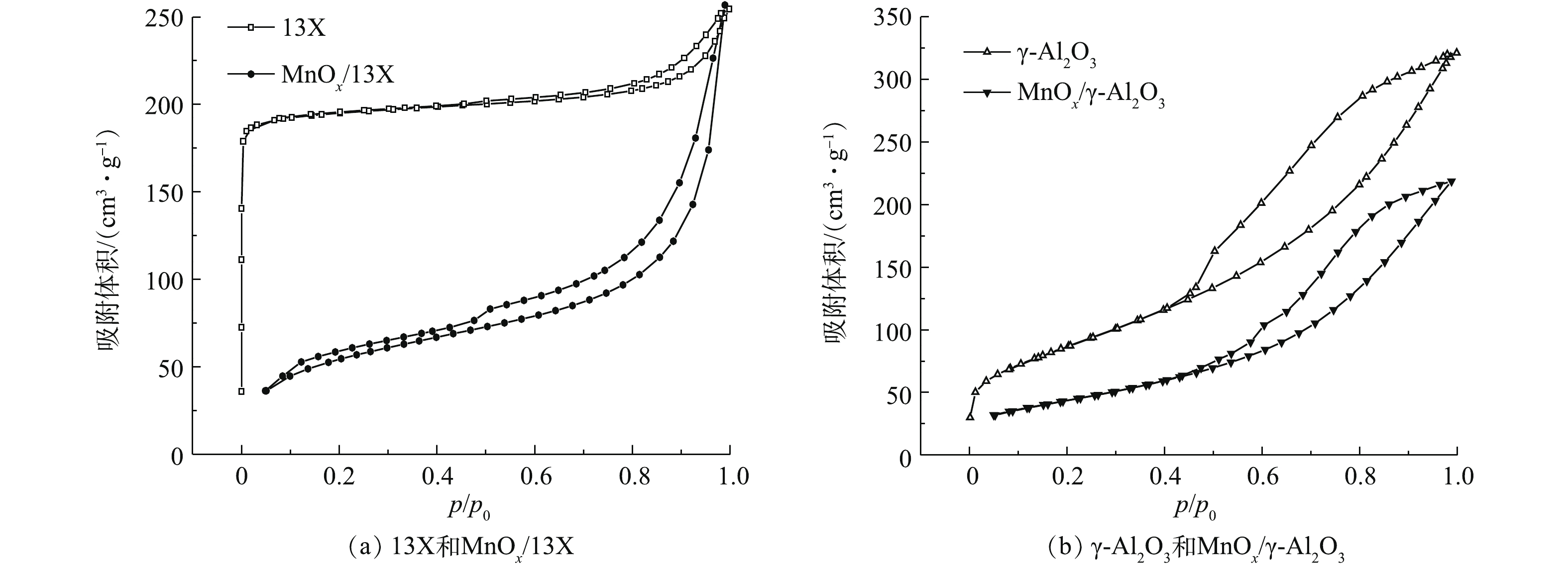
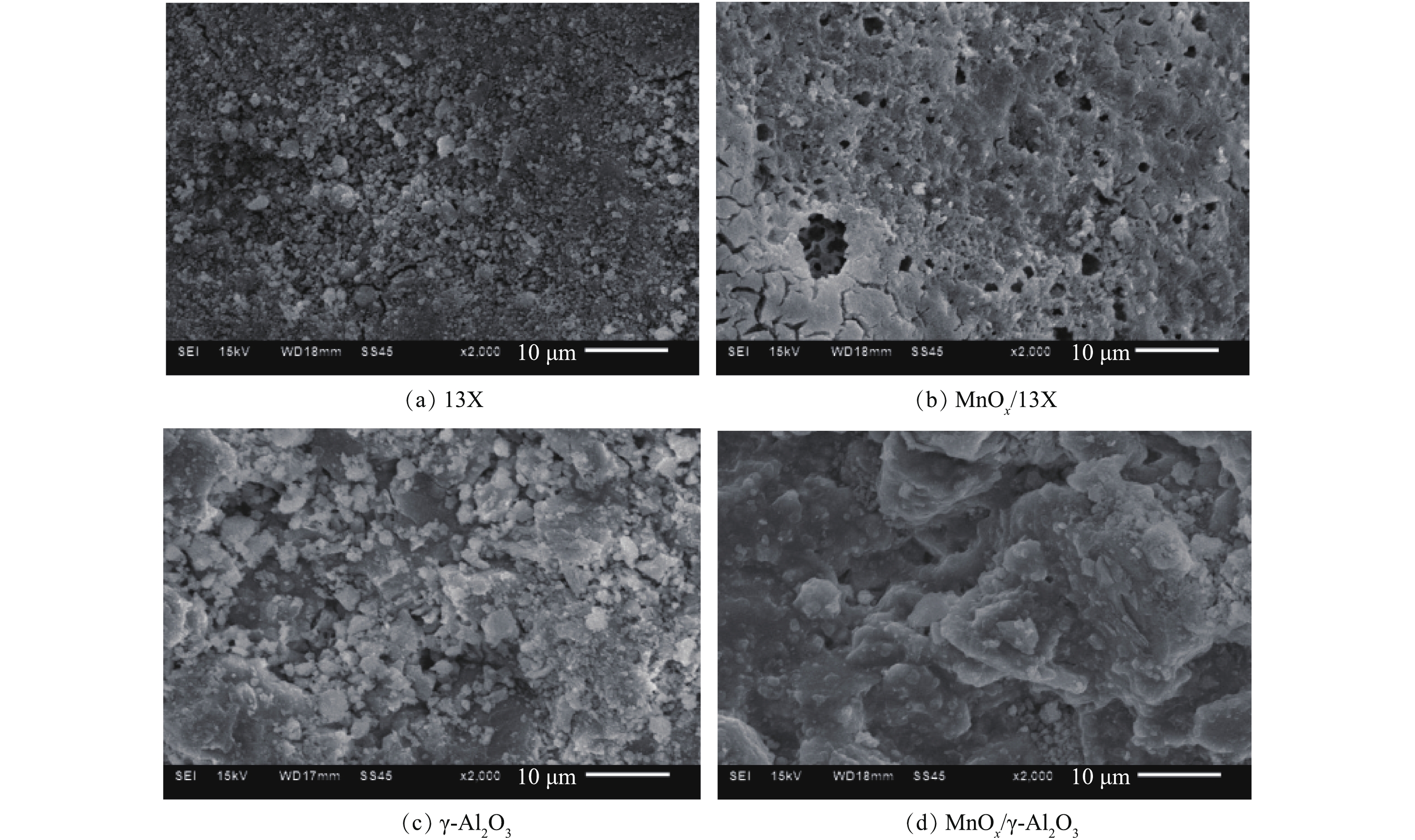
图3反映了催化剂的 COx浓度和CO2选择性。由图3(a)可以看出,COx浓度随放电时间的延长先是急剧上升,然后缓慢降低,这是由于在放电初始时,催化剂表面乙酸乙酯的吸附量最多,大部分乙酸乙酯很快被氧化降解,产生了高浓度COx;但随着放电的进行,催化剂表面吸附的乙酸乙酯越来越少,所以反应器出口的COx也随之降低。不论是13X还是γ-Al2O3,负载MnOx之后,COx浓度明显升高。放电120 min后,MnOx/13X和MnOx/γ-Al2O3对乙酸乙酯的COx产率分别为61.5%和59%,比13X和γ-Al2O3相应高出36.3%和29%。图3(b)表明,负载MnOx之后,CO2选择性也提高了1.8%和1.5%,因为MnOx对O3有着极强的分解能力,O3分解生成的O活性物种进一步将CO氧化为CO2[18]。以上结果表明,MnOx的负载有效促进了乙酸乙酯的深度降解,为探究其原因,采用BET和SEM对催化剂进行表征。图4为催化剂的N2吸附-解吸等温线,可以看出,13X的吸附等温线属于典型的I型等温线,说明其主要以微孔吸附为主,在较低压力下,吸附量急剧增加,发生微孔填充。而γ-Al2O3属于Ⅳ型吸附曲线,在较高压力下,吸附质发生毛细管凝聚,可观察到滞后现象,这种现象与孔的形状及大小有关。催化剂的物理化学性质如表1所示。负载MnOx后,一方面使催化剂的孔容减少、平均孔径增大,导致比表面积减小[19-20],不利于乙酸乙酯的等离子体氧化;另一方面,MnOx的负载增加了催化反应活性中心,可大大提高乙酸乙酯氧化。由于促进作用远大于比表面积减少带来的不利影响,负载MnOx后,乙酸乙酯的降解效果显著提高。图5为不同催化剂的SEM扫描电镜图。可以看出,13X表面粗糙,孔隙多,而γ-Al2O3表面呈块状结构,孔隙少。负载MnOx后,催化剂表面变得平整,还会出现少量裂缝和大孔,说明MnOx的负载造成了一定的刻蚀,扩充了孔径,这与BET所测的负载后平均孔径变大的结果相吻合。
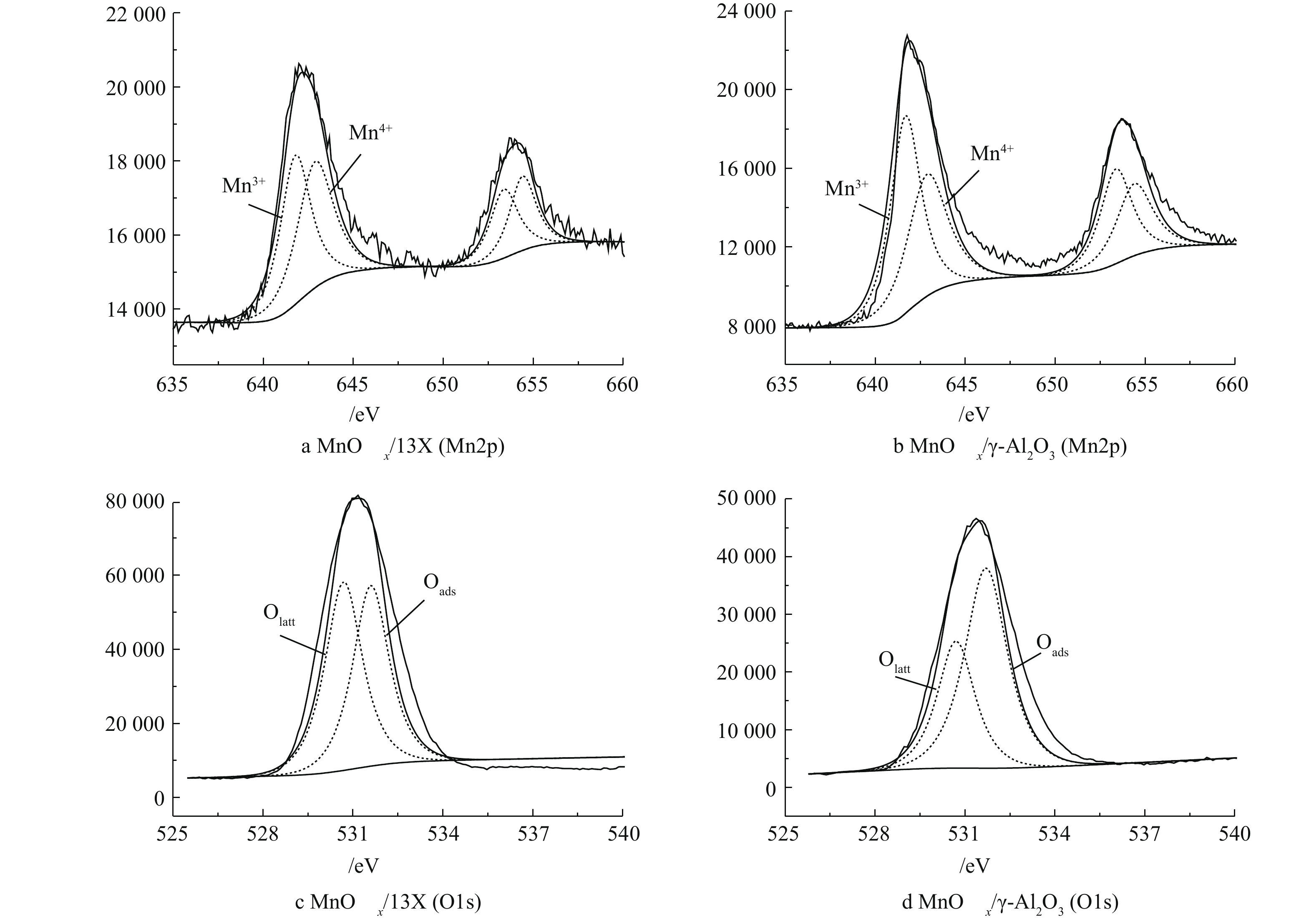
填充γ-Al2O3的COx产率比13X高出13%左右(图3(a)),这可能是由于γ-Al2O3介电常数比13X高[21-22],在相同的外加电压条件下,增强了电场强度,相应提高了电场中高能电子的数量,产生更多的活性粒子[23],将吸附态乙酸乙酯氧化降解成为CO2和CO。但值得注意的是,尽管MnOx/γ-Al2O3的介电常数比MnOx/13X的高,MnOx/13X的COx产率却比MnOx/γ-Al2O3高,这说明除了材料的介电常数,还有其他重要因素影响着低温等离子体催化降解吸附态乙酸乙酯。由表1可以看出,MnOx/13X的比表面积大于MnOx/γ-Al2O3的比表面积,有更多的活性位点,同时污染物的停留时间更长,有利于乙酸乙酯的氧化降解,这可能是MnOx/13X的COx产率比MnOx/γ-Al2O3高的原因之一。另外,在图6(a)和图6(b)中,Mn2p的XPS谱图显示,MnOx/13X上的Mn4+(结合能在(642.7±0.5) eV的拟合峰)含量比MnOx/γ-Al2O3高(表1),而较高的Mn4+含量有利于有机物的氧化。MnOx/13X和MnOx/γ-Al2O3的O1s(图6(c)和图6(d))的XPS谱图显示,MnOx/13X上的晶格氧Olatt(结合能在(530.5±0.5) eV)含量也远高于MnOx/γ-Al2O3(表1),而晶格氧含量越高,越有利于有机物的催化氧化[24-25]。以上结果表明,填充材料的介电性质、比表面积、Mn4+的含量以及晶格氧的含量均对吸附态乙酸乙酯的氧化起着非常重要的作用。
-
图7反映了 副产物O3和N2O浓度随放电时间的变化情况。由图7(a)可以看出,O3浓度随放电时间的延长在不断上升,这是因为随着放电时间的推移,有机中间产物的累积覆盖了活性位点,抑制了O3在催化剂表面的分解[26]。填充负载型催化剂MnOx/13X和MnOx/γ-Al2O3的反应器出口的O3浓度大大降低,这是由于MnOx的负载有助于对O3的分解[27-28],如式(3)~式(5)所示,减少副产物的同时形成了高活性O·,从而促进了污染物的降解。
此外,MnOx的负载略微增加了催化剂的平均孔径(表1),使得O3的扩散阻力减弱,臭氧更容易迁移到催化剂孔道内参与反应[29]。图7(b)显示,无论负载MnOx与否,γ-Al2O3作为催化剂时产生的N2O浓度均高于13X,这是由于γ-Al2O3的介电常数大,填充后反应器内放电场强增加,产生更多高能电子,与N2碰撞,使其处于不稳定的激发态N2(A),进一步被氧化为N2O[30],反应见式(6)和式(7)。
MnOx的负载对N2O的产生影响不大,说明MnOx的负载对材料的介电形式和放电影响不大,对产生的N2O几乎没有分解能力。
2.1. 不同锰基催化剂的氧化性能
2.2. 不同锰基催化剂的副产物和N2O
-
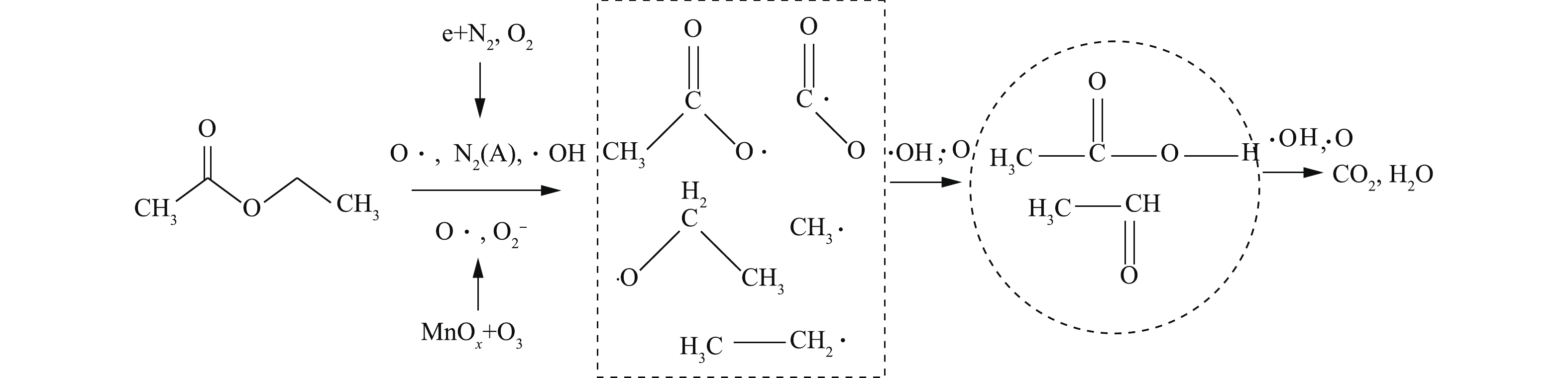
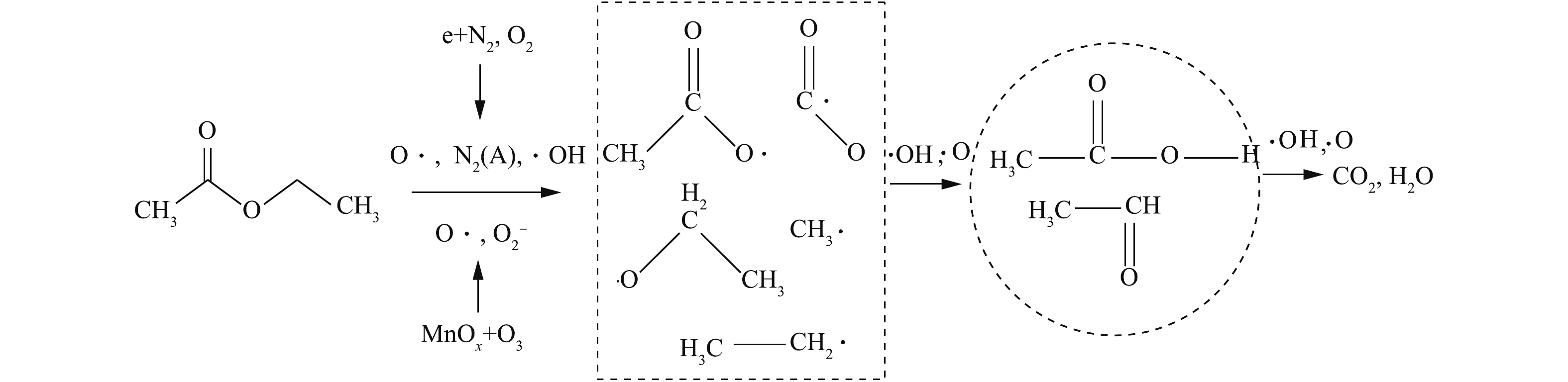
在实验中,吸附态乙酸乙酯的降解路径主要分为2个部分:1)等离子体在空气气氛下的放电过程中会产生羟基自由基、O·、
O−2 、电子和N2(A)等多种活性粒子,直接与乙酸乙酯发生碰撞;2)催化剂表面的活性组分MnOx分解O3,形成的活性基团或MnOx直接与乙酸乙酯生成CH3·、CH3CO·、CH3COO·、CH3CH2COO·[31],从而进一步氧化成乙酸、乙醛等中间产物[32],然后再矿化为COx和H2O。反应过程如图8所示。总反应方程式可简化为式(8)的形式。假设在一定运行条件下,活性粒子的浓度视为恒定,则吸附态乙酸乙酯的瞬时矿化速率方程见式(9)。
式中:t为反应时间,min;k为总反应速率常数;b为反应级数;n为乙酸乙酯的吸附量,mmol。由于n在放电过程中无法直接测定,故采用矿化产物COx(CO2和CO)的量来间接计算,计算方法如式(10)所示。
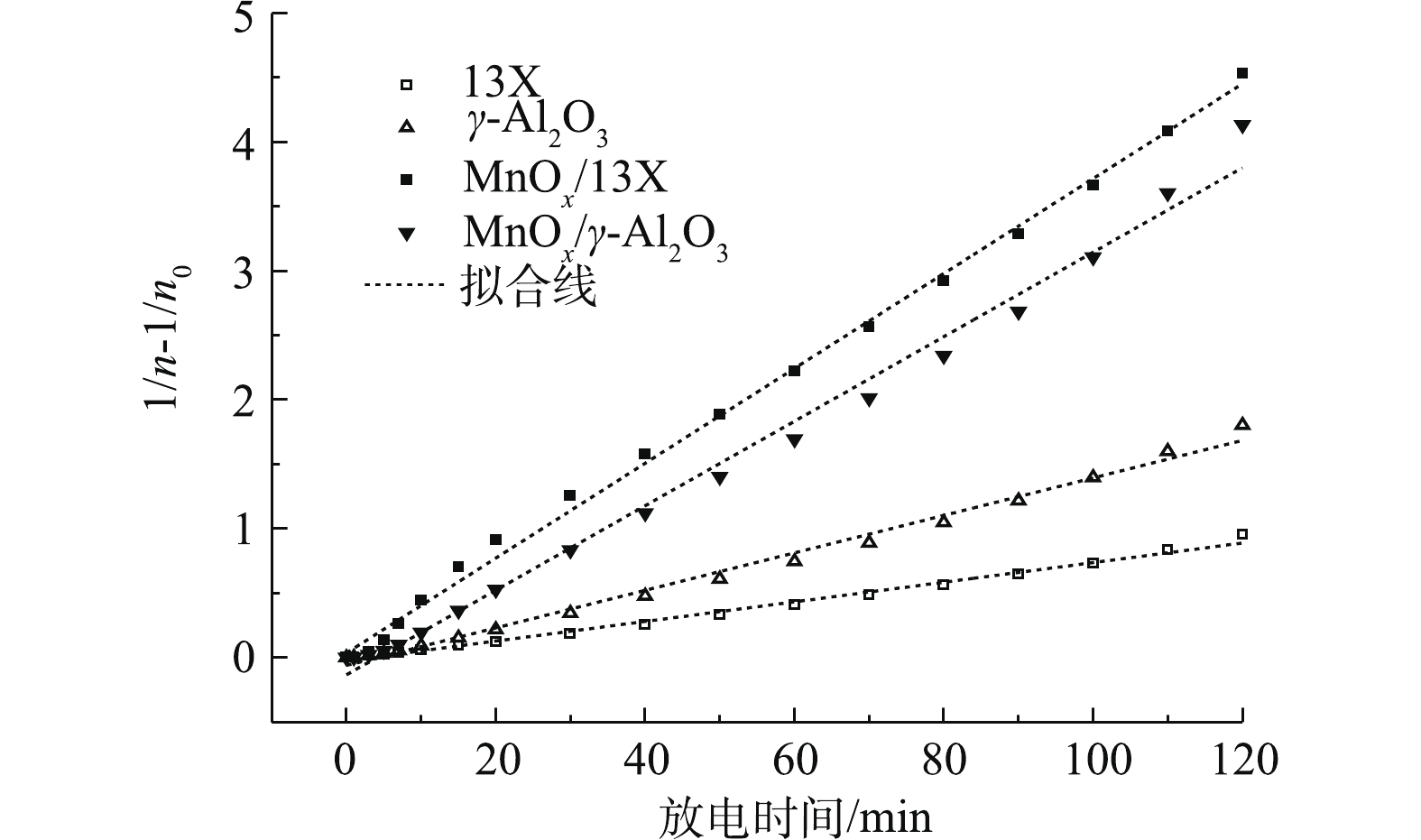
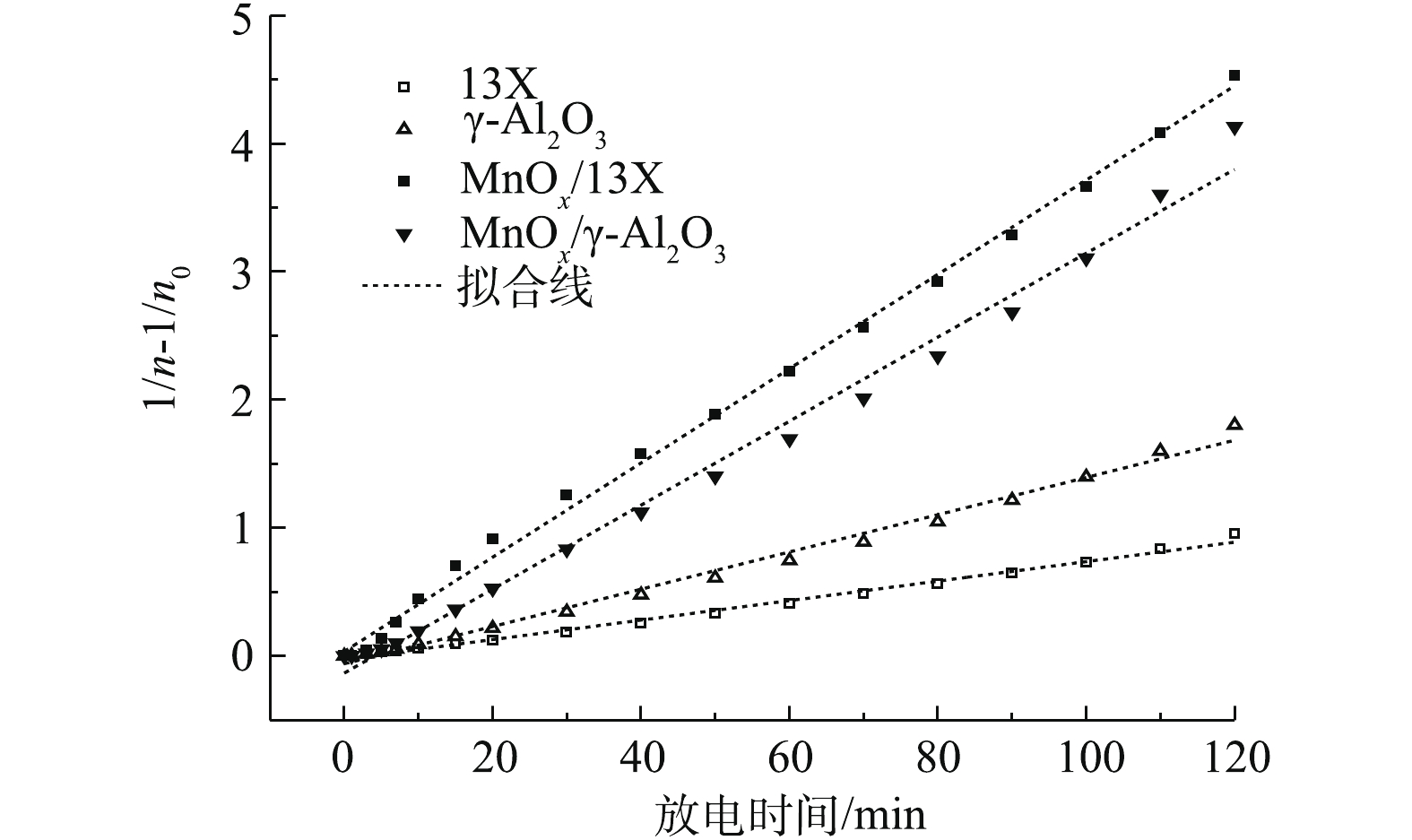
拟合结果如图9所示,不同催化剂(13X、γ-Al2O3、MnOx/13X、MnOx/γ-Al2O3)矿化吸附态乙酸乙酯的过程均符合二级动力学模型(b=2,可决系数都在0.99以上,如表2所示),说明矿化速率与乙酸乙酯吸附量的平方成正比。不同催化剂的反应速率常数k不同,由表2可知,MnOx/13X的总反应速率最大。另外,值得注意的是,催化剂总反应速率常数k的大小顺序(表2)与COx产率的大小顺序一致(图3(a))。
-
1)相比于13X和γ-Al2O3,MnOx/13X、MnOx/γ-Al2O3将COx产率分别提高了36.3%和29%,同时CO2选择性相应上升到98.9%和98.1%。从表征结果看出,COx产率和CO2选择性的提高主要是由于MnOx的负载增加了等离子体催化反应的活性中心数量。
2) MnOx与13X协同后效果更佳,XPS的表征显示,MnOx/13X上的Mn4+和晶格氧含量更高,更有利于乙酸乙酯的降解。
3) MnOx的负载可以有效降低O3,但对N2O的产生并无显著影响。
4) DBD降解吸附态乙酸乙酯符合二级反应动力学模型,不同催化剂的总反应速率常数k与COx产率大小顺序一致,产率大小顺序为MnOx/13X>MnOx/γ-Al2O3>γ-Al2O3>13X。



 下载:
下载: