-
土壤是人类赖以生存的物质基础和不可或缺的自然资源,随着工业化、城镇化的加快推进,土壤重金属污染问题也日益突出。铅(Pb)作为土壤累积率最高的重金属元素,来源广泛且生态危害大,“三废”的排放、农药化肥的不合理施用、矿山开采及冶炼活动等都会造成土壤Pb污染,其中尤以铅锌矿的开采及冶炼过程最为严重[1]。进入土壤中的Pb在各粒级颗粒中的分布具有不均一性,其会优先依附于小颗粒土壤[2],粒径越细,对重金属的富集能力越强[3]。同时,细小的土壤颗粒在胶体共迁移的作用下更容易发生迁移,造成其他环境介质的污染[4-5]。另外,受土壤理化性质的影响,进入土壤中Pb的化学活性分布也具有高度不均一性,进而导致生物对Pb的吸收效率存在较大差异,因此,对生物体的影响程度也明显不同[6]。
重金属污染土壤的修复技术类型多样,不同修复方法的适用范围和修复效果也各不相同。其中,原位化学钝化法在修复时间和经济成本上能更好地满足重金属污染土壤的修复要求,其钝化效果及机理得到广泛研究。有机物料、石灰和磷酸盐来源广泛、价格低廉,且对重金属有着良好的钝化效果[7-8]。但上述3种钝化剂的钝化修复机理有很大差异,有机物料修复机理较复杂,其本身可以通过吸附、络合/螯合、氧化还原等方式降低重金属有效性,又可以通过影响土壤理化性质及土壤微生物的丰度与活性以间接减轻重金属的毒害性[9]。石灰与磷酸盐的钝化机理较单一,石灰主要通过提高土壤pH以促进Pb2+形成氢氧化铅及碳酸铅沉淀而降低其有效性[10];磷酸盐可以与Pb形成磷酸盐沉淀,并且当土壤中存有Cl−、F−等卤素离子时,可以形成非常稳定的磷铅矿类物质[11]。但土壤不是一个均质体,不同成分在土壤中的分布并不均匀[12]。土壤颗粒作为土壤最基本的组成部分,积极参与生态系统的地球化学过程,不同粒级土壤组分上存在不同的土壤固相组成,导致很多特定的反应或现象只出现在特定的粒径范围之内[13],因此,在研究钝化剂修复效果的同时,也应考虑重金属在土壤中不同粒级间的转化富集。虽然关于Pb污染土壤的化学钝化修复已有大量报道,但绝大多数研究均把土壤作为均质体,而钝化剂添加后对不同粒级土壤中Pb的迁移转化的相关研究较少,钝化剂修复Pb污染土壤的内在微观机制仍不明晰,钝化处理后的土壤对其他环境介质的影响尚未探究。
本研究以外源Pb污染的贵州黄壤为研究对象,以羊厩肥、石灰、磷酸盐为钝化材料,通过室内钝化培养实验,比较了不同钝化剂对外源Pb污染土壤的钝化修复效果,分析了不同钝化剂在不同添加量下在各个粒级土壤中对Pb的富集状况和形态分布的影响,探讨了不同钝化剂对Pb在不同粒级土壤中迁移转化规律的影响,以期为羊厩肥、石灰、磷酸盐在Pb污染土壤修复中的高效利用及修复后土壤的潜在生态风险管控提供参考。
-
供试土壤采自贵阳市花溪区某菜地(106°39′48″E,26°21′20″N),采样深度为0~20 cm,pH为6.46,田间持水量为30.40%,阳离子交换量为31.30 cmol·kg−1,有机质含量为36.89 g·kg−1,全氮含量为2.20 g·kg−1,全磷含量为0.58 g·kg−1,速效磷含量为41.32 mg·kg−1,全钾含量为12.46 g·kg−1,Pb含量为84.58 mg·kg−1。将采集的土壤于室内风干后过2 mm筛,以分析纯级Pb(NO3)2溶液为铅源加入到供试土壤中,使土壤Pb含量达到2 000 mg·kg−1,利用称重法加入超纯水,使土壤保持60%的田间持水量,60 d后风干,磨细过2 mm筛,制成模拟Pb污染土壤。
供试钝化剂分别为羊厩肥、石灰(Ca(OH)2)、磷酸盐(Ca(H2PO4)2·H2O)。其中,羊厩肥采自贵州修文某山羊养殖场,于自然条件下腐熟半年后,风干磨细过2 mm筛备用,其Pb含量为128.81 mg·kg−1;石灰及磷酸盐试剂均为分析纯。
-
钝化实验设置3组,共12个处理,每个处理重复3次。依次对照空白处理(CK)添加,羊厩肥添加量为1%、2%、5%、10%(GM1、GM2、GM5、GM10);石灰添加量为1%、2%、5%、10%(L1、L2、L5、L10);磷酸盐添加量为1%、2%、5%、10%(P1、P2、P5、P10)。取300 g模拟Pb污染土壤置于500 mL塑料烧杯中,按上述比例将土壤与钝化剂充分混匀,于室温下保持60%田间持水量,钝化培养45 d后取样,自然风干后磨细备用。
-
参照STEMMER等[14]和武天云等[15]的研究方法,首先对过2 mm筛的土样进行超声处理,再利用湿筛法分离出粗砂粒(2~0.2 mm),最后根据Stokes公式,使用离心法依次分离得到细沙粒(0.2~0.02 mm)、粉粒(0.02~0.002 mm)和黏粒(<0.002 mm)。为确保粒径分离的准确性,分离完成后,立即使用激光粒度仪(Mastersizer2000,英国Malvern公司)对各粒级土壤的粒径分布进行验证。将所得的各粒径土壤于50 ℃下烘干,磨细过0.149 mm筛备用。
-
土壤理化性质测定参照文献中的方法[16],其中土壤pH使用超纯水浸提(水土比为2.5∶1),pH计(PHS-3C+,上海仪电科学仪器股份有限公司)测定;土壤有机质(SOM)采用重铬酸钾容量法测定;速效磷(Olsen-P)及水溶性磷(CaCl2-P)[17]分别采用pH为8.50的0.50 mol·L−1 NaHCO3及0.01 mol·L−1 CaCl2提取,全自动间断化学分析仪(CleverChem200+,德国DeChem-Tech Gmbh公司)测定。Pb全量[18]采用HCl-HNO3-HF-HClO4消解;植物有效态Pb(DTPA-Pb)[19]使用二乙烯三胺五乙酸浸提;土壤中各形态Pb的含量分布采用改进的BCR连续提取法[20]处理;均使用原子吸收光谱仪(GGX-800,北京海光仪器有限公司)测定。
-
本研究所有实验数据均使用Excel 2016进行整理及计算,以平均值±标准差的形式作为分析结果;单因素方差分析(One-way ANONA)、Dancan多重比较法(差异性水平为P<0.05)及相关矩阵分析(Pearson)采用SPSS 22.0进行处理;实验数据绘图使用Origin 2017完成。
-
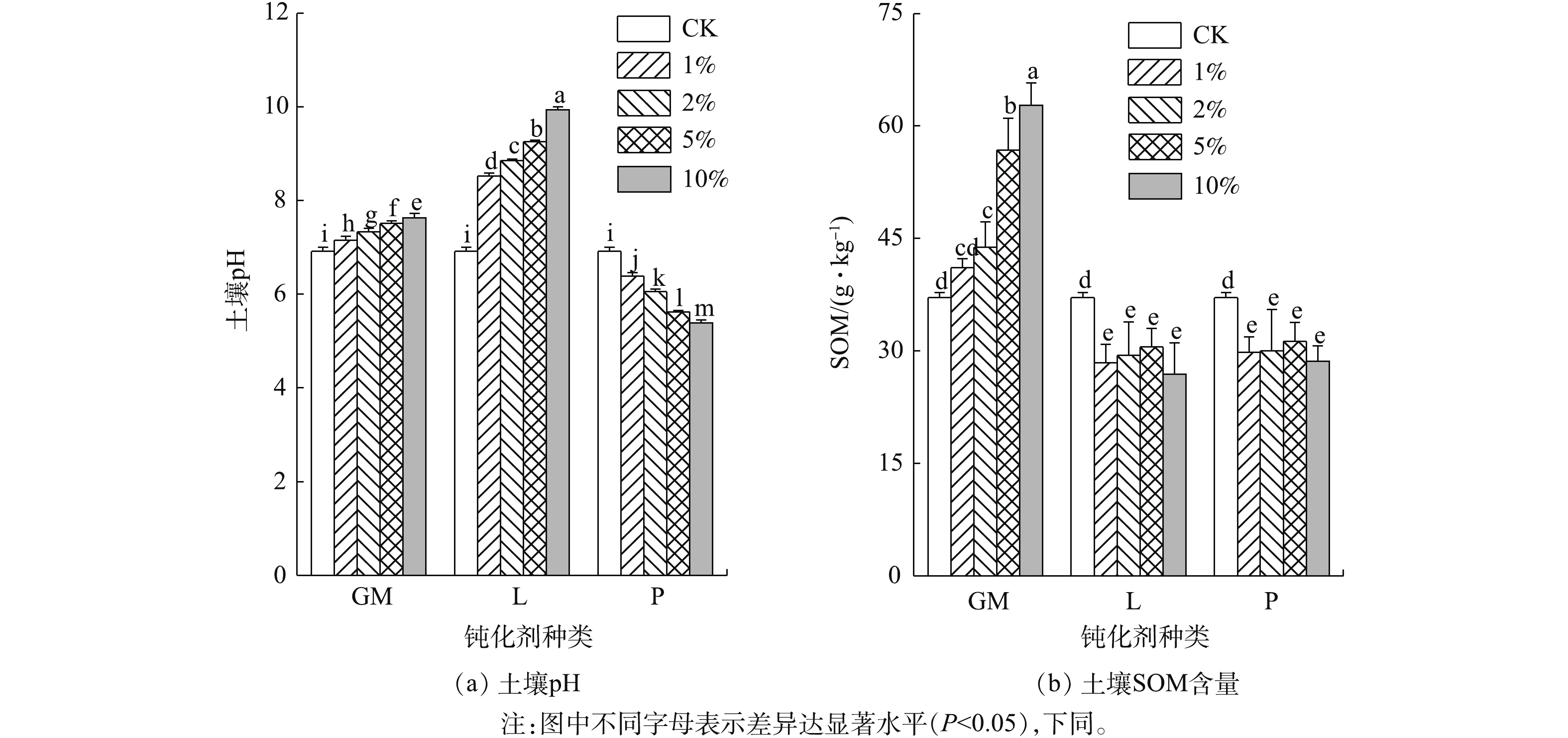
土壤pH与SOM是影响土壤中重金属有效态的重要因素。其中,pH可影响土壤中重金属的溶解-沉淀、吸附-解吸等反应过程,进而影响土壤中重金属的活性[21]。不同钝化剂处理下土壤pH及SOM含量见图1。由图1可知,羊厩肥添加可显著提升土壤pH,与CK相比,各添加量处理组分别提升了pH 0.22、0.43、0.59、0.72个单位,这可能是因为羊厩肥本身含有大量的盐基离子,致使土壤溶液的离子强度与阳离子交换量升高[22];石灰作为碱性钝化剂,对土壤pH有着显著的影响,所有石灰处理组的土壤pH均显著高于空白处理,石灰用量越大,pH增幅越高;磷酸盐施入土壤后可与土壤水溶液发生水解反应生成H3PO4,各添加量分别使土壤pH降低了0.54、0.85、1.25、1.48个单位,均达到显著水平。
SOM可强烈吸附重金属,与金属离子生成螯合物,且SOM具有还原性,能降低水溶态重金属的比例,减轻重金属的毒性[23]。与CK相比,羊厩肥添加均显著提升了SOM含量,GM10提升的幅度最大,为14.88 g·kg−1,GM1提升的幅度最小,为2.30 g·kg−1;石灰及磷酸盐处理均不同程度地降低了SOM的含量,与CK相比,均达到显著相关水平,这可能是由于石灰和磷酸盐的添加改善了土壤微生物的生存条件,使含碳有机物的转化速度加快,从而促进了SOM分解[24]。
磷是植物生长发育所必需的营养元素,土壤磷的存在形式多样,不同赋存形态的磷的移动性和植物可利用性也有很大差异。其中Olsen-P可直接被植物吸收,而CaCl2-P则被认为是土壤磷可被淋溶损失的部分[25]。图2为不同钝化剂处理对土壤Olsen-P和CaCl2-P含量的影响。由图2可知,3种钝化剂处理均显著提高了土壤Olsen-P含量,其中P10对Olsen-P提高量最大,为145.78 mg·kg−1,GM1最小,为24.93 mg·kg−1。羊厩肥既可直接供给磷素,又能促进相关酶和微生物的活性,进而增加Olsen-P含量[26];石灰可促使铁铝氧化物对磷的释放,以增加Olsen-P含量Olsen-P含量[27];磷酸盐属于易溶性磷肥,故对Olsen-P含量影响最大。对CaCl2-P而言,磷酸盐极大地提高了土壤中CaCl2-P的含量,各处理与CK间的差异均达到显著水平;羊厩肥与石灰虽提高了土壤中CaCl2-P的含量,但GM1、GM2、L1、L2和L10间的差异并不显著。
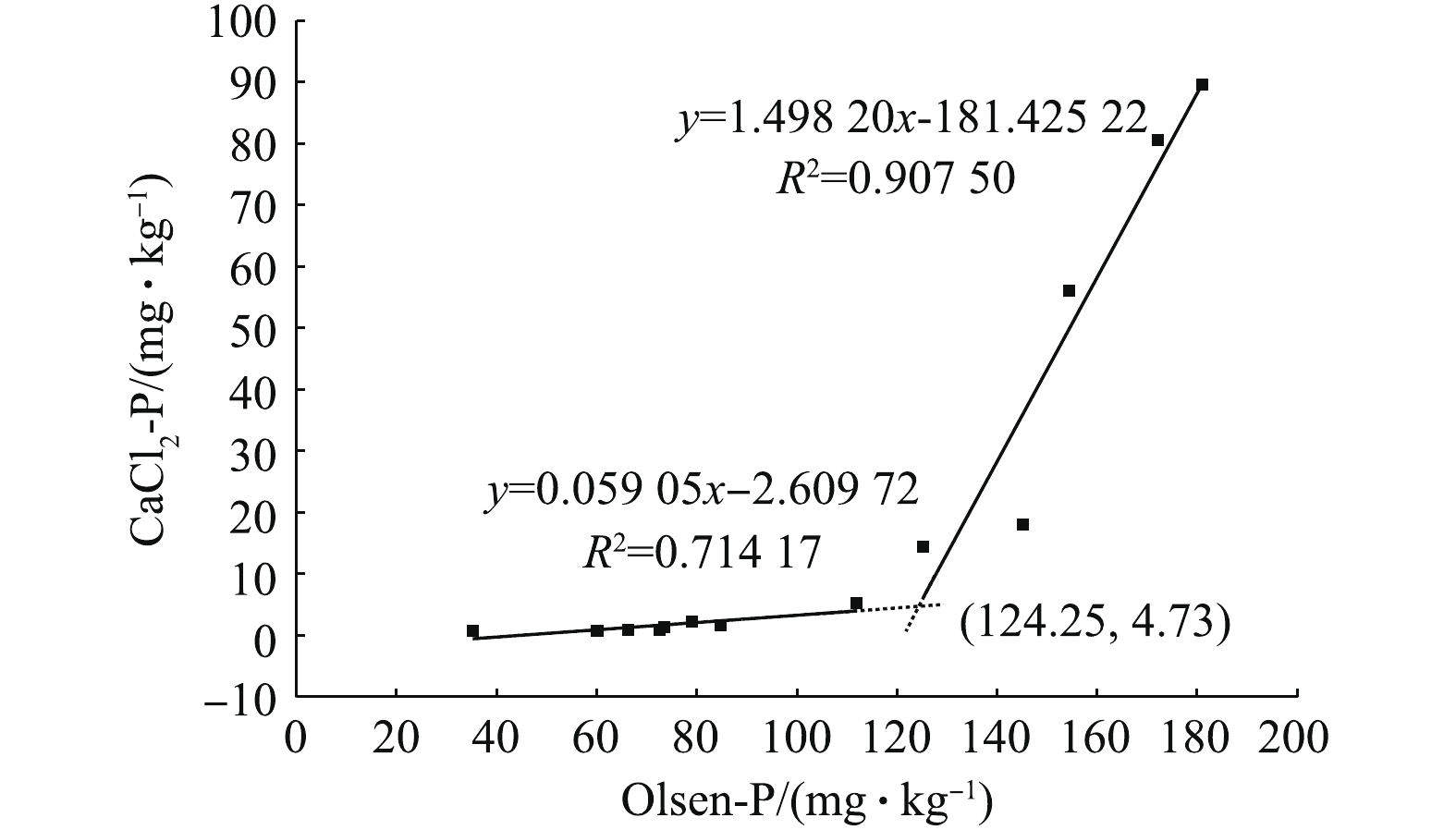
磷可降低土壤重金属的有效性,但土壤在磷素含量过高时,磷会通过淋溶/渗漏的形式向地表或地下水迁移,造成水体富营养化[28]。因此,在保证钝化修复效果的同时,避免其他营养元素的污染也是钝化修复亟待解决的问题。HESKETH等[29]提出的土壤磷淋溶临界值模型可有效评估不同处理对地下水体可能产生的影响。本研究中不同钝化剂处理后土壤Olsen-P与CaCl2-P的关系曲线如图3所示。2条直线相交点即为土壤磷淋溶临界点。结果表明,当土壤Olsen-P含量大于124.25 mg·kg−1时,土壤磷就会发生淋溶,即GM10及所有磷酸盐处理均会造成土壤磷素淋溶现象,使地下水产生富营养化的风险。
-
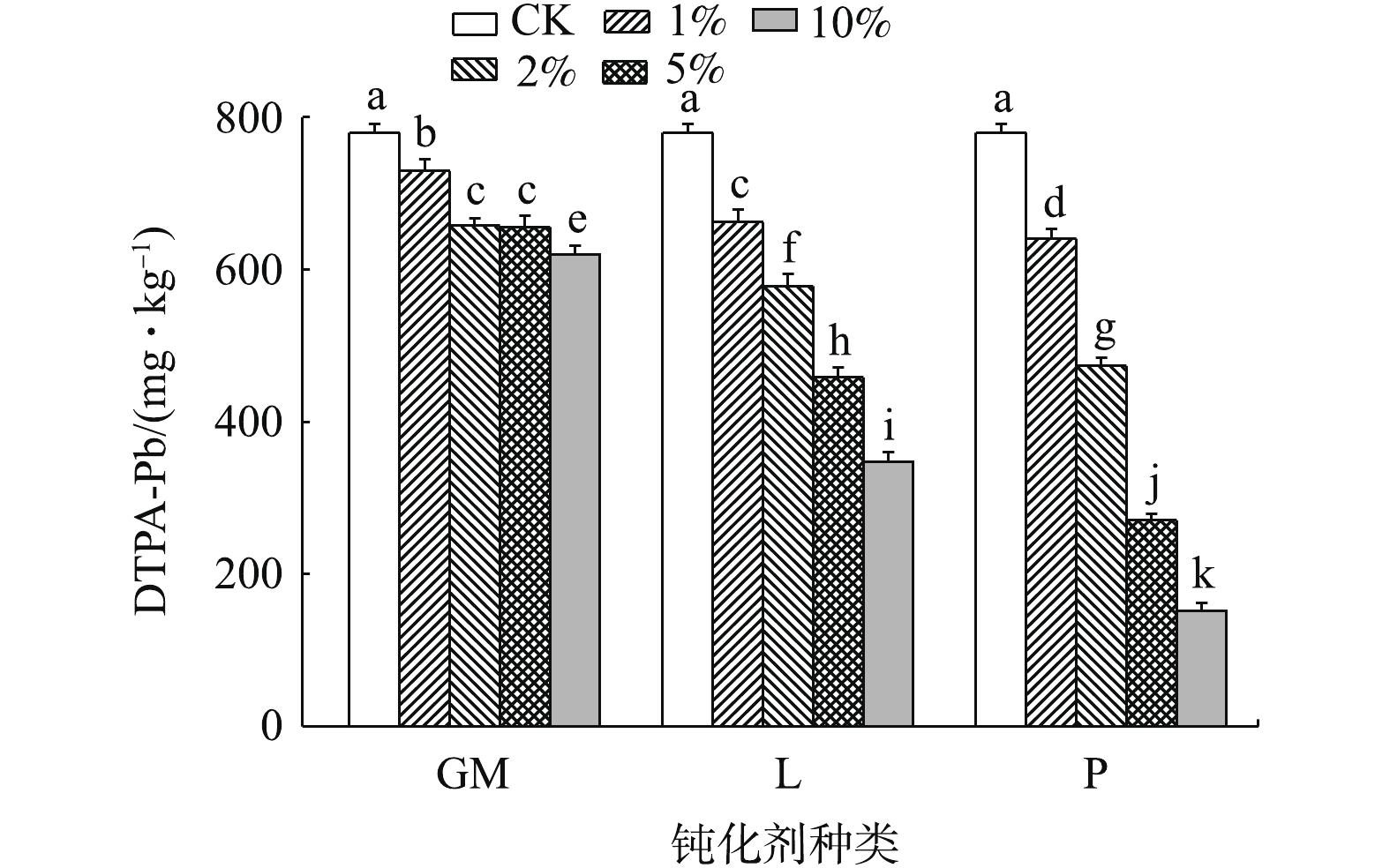
DTPA提取态重金属可直接被植物吸收利用,能直观反映土壤重金属对植物的毒害性,因此,有效降低土壤DTPA提取态重金属含量,对于评估原位钝化修复效果至关重要[30]。由图4可知,3种钝化剂在不同添加量下均显著降低了土壤DTPA-Pb含量,钝化效果随钝化剂添加量的增加而增强,但GM2与GM5之间差异不显著;在所有处理中,GM1降低幅度最小,为6.51%;P10降幅最大,高达80.53%。当钝化剂添加量相同时,对Pb的钝化效果为磷酸盐>石灰>羊厩肥,这与已有研究结果一致:ZENG等[31]通过对比分析氢氧化铅与磷酸铅的稳定性差异认为磷酸盐的钝化效果优于碱性材料;代允超等[32]研究发现酸性土壤添加石灰对于重金属的钝化效果优于有机物料。
土壤不同形态重金属对生态环境的危害程度有所差异,钝化剂可通过改变重金属的赋存形态,以降低其对人体和生态环境的危害,因此,可以用形态分级方案来评价重金属污染土壤的钝化效果[33]。图5为不同钝化处理对土壤Pb形态分布的影响。由图5可知,与CK相比,羊厩肥与磷酸盐均降低了土壤中弱酸提取态Pb含量,降幅与添加量成正比,其中P1降幅最小为0.64%,P10降幅最大为7.05%。石灰处理均提高了土壤中弱酸提取态Pb含量,L5的增幅达4.51%,这可能是由于石灰处理增加了土壤中氢氧化铅和碳酸铅的含量。石灰添加可提高土壤pH,高pH会促进土壤中碳酸盐化合物和金属氢氧化物的生成[34],而上述2种物质在酸性条件极易分解[35]。各处理均降低了土壤中可还原态Pb含量,羊厩肥低添加量降幅适中而高添加量降幅较小,各处理降幅分别为2.47%、7.63%、10.52%和17.07%;石灰低添加量降幅最大但L10降幅适中,分别为8.12%、9.44%、16.49%和20.83%;磷酸盐与石灰相反,低添加量降幅较小而P10降幅最大,各添加量降幅分别为1.74%、4.83%、17.32%和29.52%。不同处理均提高了土壤可氧化态Pb含量,羊厩肥、石灰、磷酸盐在各添加量下增幅分别为1.17%~5.44%、4.22%~14.19%、2.35%~17.89%。对于残渣态Pb而言,羊厩肥与磷酸盐处理均不同程度的增加了其含量,其中P10增幅最大为18.69%,而石灰处理对土壤残渣态Pb含量基本无影响。
以上结果表明,羊厩肥与磷酸盐主要是通过将弱酸提取态与可还原态Pb转化为可氧化态与残渣态Pb,以降低土壤中Pb的活性;石灰处理大幅度降低了可还原态Pb含量,但是却被转化为弱酸提取态与可氧化态,残渣态含量基本无变化,虽然降低了土壤中Pb的活性,但其钝化效果的稳定性与长效性须进一步验证。
-
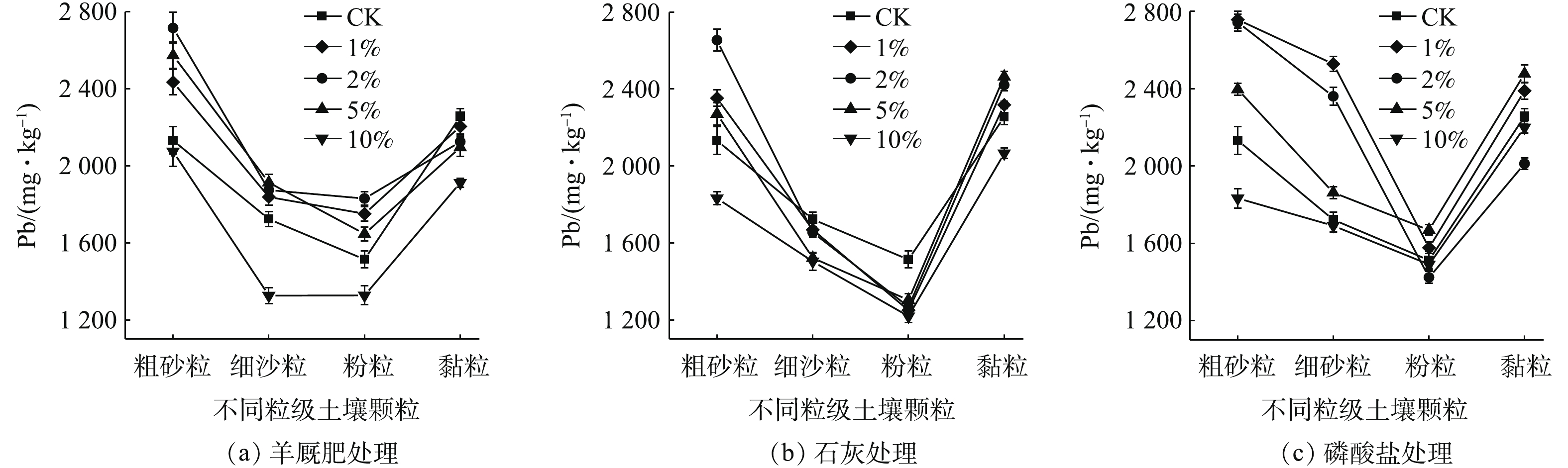
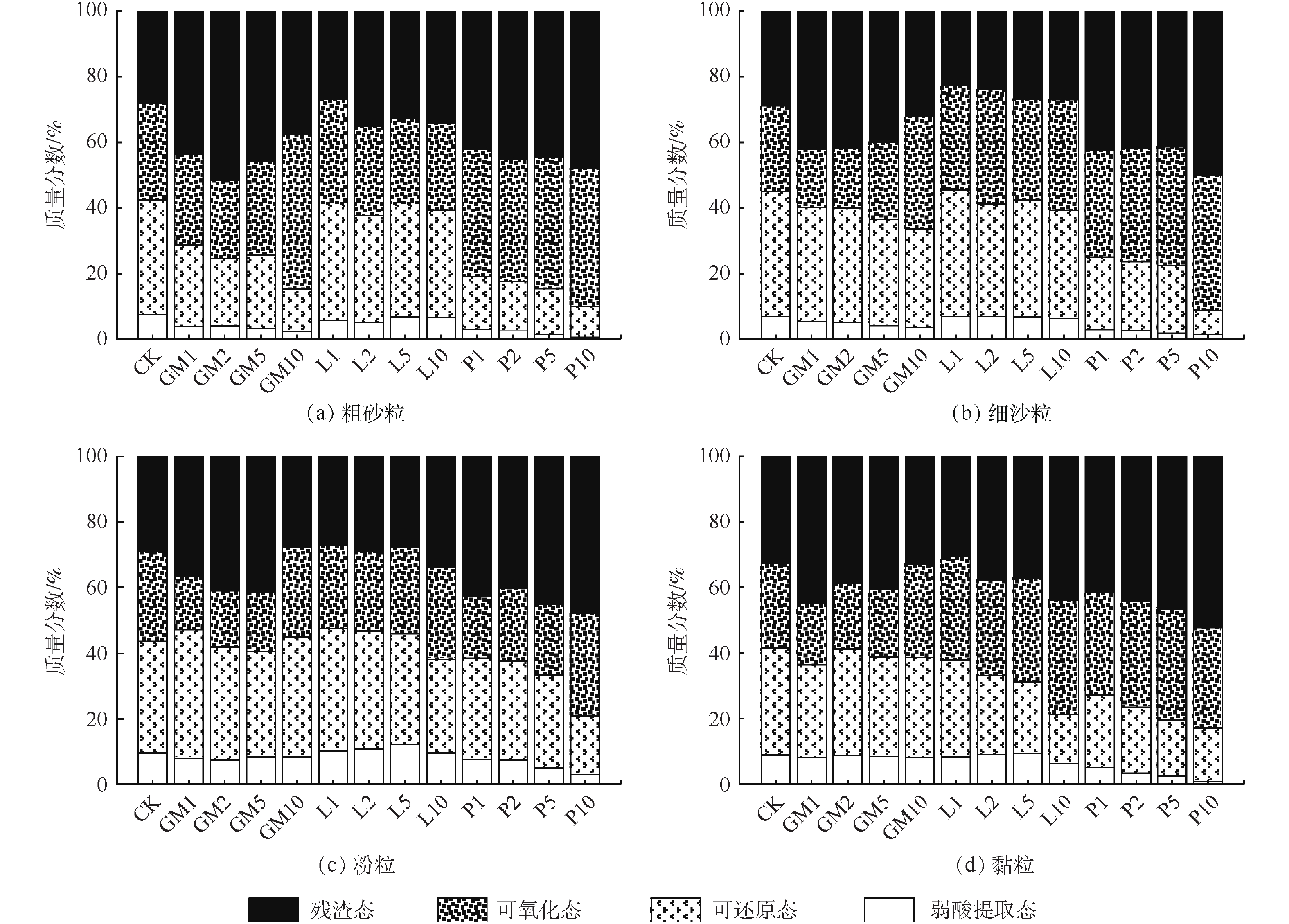
图6为不同钝化剂种类及添加量下各粒级颗粒中Pb的含量分布情况。由图6可知,Pb含量在土壤颗粒中呈双峰分布趋势,即粗砂粒和黏粒中的Pb含量较高,而细沙粒和粉粒中的Pb含量较低。黏粒比表面积较大,且黏土矿物和铁锰/铁铝氧化物含量较高,对Pb的吸附能力较强;粗砂粒富集Pb的原因较复杂,李恋卿等[36]认为这是由于有机质与其他物质通过复合作用而富集在团聚后的粗颗粒表面的结果,而QIAN等[37]则认为是粗砂粒中含有对重金属固持能力较强的粗矿物或重矿物。3种钝化剂10%添加量处理均降低了各粒级颗粒中Pb含量,这可能是因为钝化剂添加量较大,导致钝化剂在不同粒级颗粒中大量富集,而钝化剂本身Pb含量较低,进而使各粒级颗粒中Pb含量降低。1%、2%、5%羊厩肥添加量均不同程度提高了粗砂粒、细沙粒、粉粒中Pb含量,其中GM2处理对粗砂粒和黏粒中Pb增加量最大,分别为583.30 mg·kg−1和317.00 mg·kg−1,GM5对细沙粒中Pb含量增加量最大,为190.29 mg·kg−1;GM1、GM2和GM5均降低了黏粒中Pb含量,降低量分别为52.56、132.29和160.85 mg·kg−1。这可能是由于羊厩肥显著提高了土壤SOM量,但SOM在各粒级颗粒中的活性有所差异,砂粒中的活性最强,粉粒次之,黏粒最差[38],活性不同对污染物的吸附能力也有所差异,一般来说,活性越强,对污染物的吸附能力越好。与CK相比,1%、2%、5%石灰添加量均增加了粗砂粒中Pb含量,增加量分别为22.16、522.09和138.64 mg·kg−1,同时增加了黏粒中Pb含量,增加量与添加量成正比,L5处理增加量为208.32 mg·kg−1;石灰1%、2%、5%添加量却降低了细沙粒和粉粒中Pb含量。这可能是由于石灰添加增加了土壤颗粒表面负电荷,并生成了CaCO3和Ca(OH)2胶结物质引起的,黏粒比表面积大,持有的负电荷数量最高,因此,加大了对Pb2+的吸附,粗砂粒中的胶结物质与Pb2+进行交换吸附,以增加其Pb固持量[39-40]。相比于CK,磷酸盐增大了粗砂粒与细沙粒对Pb的富集,而对粉粒和黏粒中Pb含量影响无明显规律。
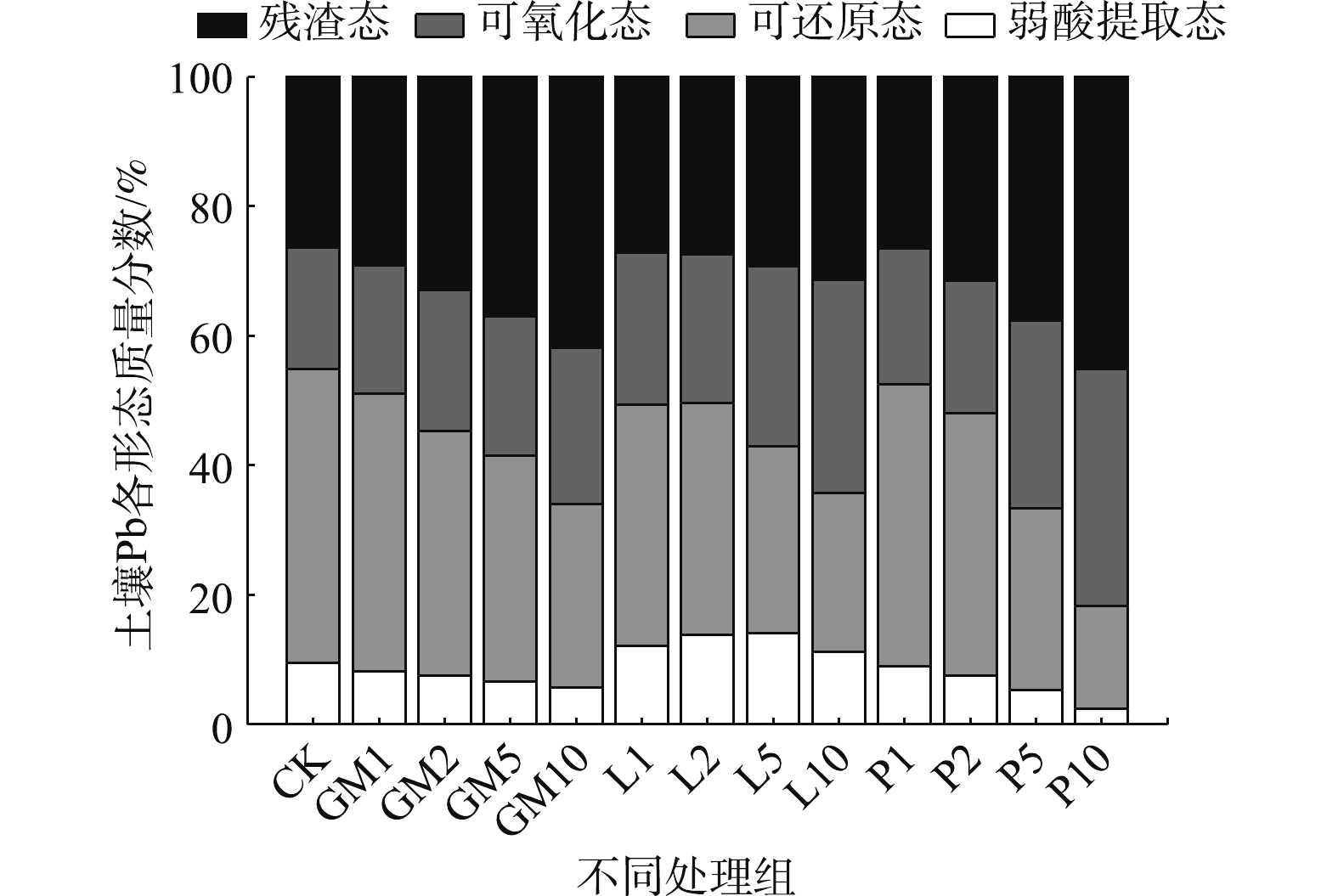
钝化剂添加不仅会影响Pb在各粒级颗粒中的再分配,还可能改变各粒级颗粒中Pb的形态分布,进而影响土壤Pb的有效性。由图7可知,CK处理下各粒级颗粒中Pb形态分布并无明显差异,表明外源Pb进入土壤后在各粒级颗粒中的分布无明显区别[41]。羊厩肥对粗砂粒中各形态Pb所占比例影响最大;而石灰对黏粒中Pb形态占比影响最大;磷酸盐处理下各粒级颗粒中Pb形态分布均由高毒性形态向低毒性形态转化的表现,这可能是由于易溶性磷酸盐与其他矿物晶体形成的光滑涂层,其可将Pb2+包缚在土壤颗粒表面所致[33]。
-
为了解土壤与各粒级颗粒Pb形态含量间可能存在的关系,对土壤与各粒级颗粒中Pb形态含量间做Pearson相关矩阵分析,其中易利用态为弱酸提取态与可还原态之和,难利用态为可氧化态与残渣态之和[42]。由表1可知,土壤DTPA-Pb含量与细沙粒、粉粒和黏粒中易利用态Pb含量均呈显著正相关关系,相关系数值排序为黏粒>细沙粒>粉粒,这说明土壤中植物有效态Pb含量可能来源于上述3部分中易利用态Pb。土壤弱酸提取态有着最高的生物毒性,各粒级颗粒中弱酸提取态含量均与其达到显著正相关关系,说明土壤弱酸提取态与各粒级弱酸提取态Pb有着密切的关系,本研究中土壤弱酸提取态Pb含量较低,在土壤DTPA-Pb中占比较小,残渣态在土壤中活性最低,因此,土壤DTPA-Pb含量主要受可还原态与可氧化态Pb控制[43]。土壤可还原态Pb含量与细沙粒、粉粒和黏粒中的可还原态呈极显著正相关,还与粉粒和黏粒中的可氧化态含量达到了显著负相关关系,表明钝化剂主要通过将细沙粒、粉粒和黏粒中的可还原态Pb转化为粉粒和黏粒中的可氧化态Pb,来降低土壤Pb的毒害性。
-
1) 3种钝化剂在不同添加量下均能显著降低外源Pb污染土壤中DTPA-Pb含量,在所有处理中,GM1对DTPA-Pb含量降低幅度最小,为6.51%,P10降幅最大,高达80.53%,3种钝化剂在相同添加量下对外源Pb的钝化效果为磷酸盐>石灰>羊厩肥。形态分布显示,羊厩肥和磷酸盐通过将弱酸提取态与可还原态Pb转化为可氧化态与残渣态Pb,以降低土壤外源Pb有效性,石灰通过将可还原态Pb转化为可氧化态Pb,以降低其有效性。
2) 3种钝化剂均不同程度地提高了土壤Olsen-P含量,其中羊厩肥、石灰和磷酸盐处理下Olsen-P含量分别为60.12~145.12、66.30~84.72、125.29~180.98 mg·kg−1。土壤磷淋溶临界值模型显示,当土壤Olsen-P含量大于124.25 mg·kg−1时,土壤磷素就会向地下水淋溶。
3) 3种钝化剂对各粒级颗粒中Pb含量及形态分布的影响有所差异。羊厩肥可增加粗砂粒、细沙粒、粉粒中Pb含量,降低黏粒中Pb含量,对各粒级颗粒中Pb形态分布均有影响但粗砂粒影响最大。石灰会增加粗砂粒和黏粒中Pb含量,降低细沙粒和粉粒中Pb含量,并对黏粒中Pb形态分布产生较大影响。磷酸盐提高粗砂粒与细沙粒对Pb的富集而对粉粒和黏粒中Pb含量影响无明显规律,在磷酸盐作用下各粒级颗粒均明显出现高毒性向低毒性形态转化的现象。
4)相关性分析表明,土壤DTPA-Pb主要来源于细沙粒、粉粒和黏粒中易利用态Pb,钝化剂主要通过将细沙粒、粉粒和黏粒中的可还原态Pb转化为粉粒和黏粒中的可氧化态Pb,以降低土壤Pb的毒害性。
不同钝化剂对外源铅在土壤中的钝化效果及粒径分布的影响
Effects of different passivators on the immobilization effect and particle-size distribution of exogenous lead in soil
-
摘要: 通过室内钝化培养实验比较了羊厩肥、石灰和磷酸盐对土壤外源Pb的钝化效果和钝化修复后Pb的粒径分布特征。结果表明:3种钝化剂均能显著降低土壤中DTPA-Pb的含量,且钝化效果与钝化剂添加量成正比;磷酸盐对外源Pb钝化效果最好,P10处理下土壤中DTPA-Pb的含量降幅达80.53%,羊厩肥钝化效果最差,GM1对DTPA-Pb含量的降幅为6.51%;羊厩肥与磷酸盐将弱酸提取态Pb和可还原态Pb转变为活性更低的可氧化态Pb和残渣态Pb,以降低其活性,石灰将可还原态Pb转化为可氧化态Pb,以降低其活性;3种钝化剂添加均会提升土壤Olsen-P的含量。土壤磷淋溶临界值模型显示,当土壤Olsen-P含量>124.25 mg·kg−1时,会发生磷素淋溶现象;Pb在土壤粗砂粒、细沙粒、粉粒和黏粒中的含量差别很大,但赋存形态无明显差异,钝化剂添加会影响外源Pb在各粒级颗粒中的富集及形态分布。相关性分析结果表明,钝化剂主要通过将细沙粒、粉粒和黏粒中的可还原态Pb转化为粉粒和黏粒中的可氧化态Pb来降低土壤Pb的毒害性。研究结果可为3种钝化剂在Pb污染土壤修复中的高效利用及修复后土壤的潜在生态风险管控提供参考。Abstract: In order to compare the differences of immobilization effects of goat manure, lime and phosphate, and the characteristic of exogenous Pb distributions in soil particle-size fractions after remediation, exogenous Pb-contaminated soil incubation experiments were conducted. The results showed that three types of amendments could significantly reduce the DTPA-Pb contents in soil, and the immobilization effect was proportional to the dosages of amendments. Among three amendments, phosphate has the best immobilization performance on exogenous Pb, and the contents of DTPA-Pb in P10 treated soil decreased by 80.53%; while the immobilization effect of goat manure was the worst, and the DTPA-Pb contents in GM1 treated soil decreased by 6.51%. Goat manure and phosphate could convert the weak acid extractable fraction and reducible fraction of Pb into the oxidizable and residual fractions so as to reduce the soil available Pb, while lime could convert the reducible fraction into oxidizable fraction. The Olsen-P content of soil increased with the addition of these three amendments. The soil phosphorus leaching critical value model showed that when the Olsen-P contents were over 124.25 mg·kg−1, phosphorus leaching into the groundwater occurred. There was a large difference of Pb contents in coarse sand, fine sand, silt and clay, yet there was no significant difference regarding Pb speciation distribution. The addition of amendments affected the enrichment and speciation distribution of exogenous Pb in each soil particle-size fractions. The correlation analysis showed that toxicity of soil Pb was reduced by amendments mainly through converting the reducible fraction of Pb in the fine sand, the silt and the clay into the oxidizable fraction in the silt and the clay. This work provides theoretical support for the efficient use of three amendments on Pb contaminated soil and the potential ecological risk control afterwards.
-
土壤是人类赖以生存的物质基础和不可或缺的自然资源,随着工业化、城镇化的加快推进,土壤重金属污染问题也日益突出。铅(Pb)作为土壤累积率最高的重金属元素,来源广泛且生态危害大,“三废”的排放、农药化肥的不合理施用、矿山开采及冶炼活动等都会造成土壤Pb污染,其中尤以铅锌矿的开采及冶炼过程最为严重[1]。进入土壤中的Pb在各粒级颗粒中的分布具有不均一性,其会优先依附于小颗粒土壤[2],粒径越细,对重金属的富集能力越强[3]。同时,细小的土壤颗粒在胶体共迁移的作用下更容易发生迁移,造成其他环境介质的污染[4-5]。另外,受土壤理化性质的影响,进入土壤中Pb的化学活性分布也具有高度不均一性,进而导致生物对Pb的吸收效率存在较大差异,因此,对生物体的影响程度也明显不同[6]。
重金属污染土壤的修复技术类型多样,不同修复方法的适用范围和修复效果也各不相同。其中,原位化学钝化法在修复时间和经济成本上能更好地满足重金属污染土壤的修复要求,其钝化效果及机理得到广泛研究。有机物料、石灰和磷酸盐来源广泛、价格低廉,且对重金属有着良好的钝化效果[7-8]。但上述3种钝化剂的钝化修复机理有很大差异,有机物料修复机理较复杂,其本身可以通过吸附、络合/螯合、氧化还原等方式降低重金属有效性,又可以通过影响土壤理化性质及土壤微生物的丰度与活性以间接减轻重金属的毒害性[9]。石灰与磷酸盐的钝化机理较单一,石灰主要通过提高土壤pH以促进Pb2+形成氢氧化铅及碳酸铅沉淀而降低其有效性[10];磷酸盐可以与Pb形成磷酸盐沉淀,并且当土壤中存有Cl−、F−等卤素离子时,可以形成非常稳定的磷铅矿类物质[11]。但土壤不是一个均质体,不同成分在土壤中的分布并不均匀[12]。土壤颗粒作为土壤最基本的组成部分,积极参与生态系统的地球化学过程,不同粒级土壤组分上存在不同的土壤固相组成,导致很多特定的反应或现象只出现在特定的粒径范围之内[13],因此,在研究钝化剂修复效果的同时,也应考虑重金属在土壤中不同粒级间的转化富集。虽然关于Pb污染土壤的化学钝化修复已有大量报道,但绝大多数研究均把土壤作为均质体,而钝化剂添加后对不同粒级土壤中Pb的迁移转化的相关研究较少,钝化剂修复Pb污染土壤的内在微观机制仍不明晰,钝化处理后的土壤对其他环境介质的影响尚未探究。
本研究以外源Pb污染的贵州黄壤为研究对象,以羊厩肥、石灰、磷酸盐为钝化材料,通过室内钝化培养实验,比较了不同钝化剂对外源Pb污染土壤的钝化修复效果,分析了不同钝化剂在不同添加量下在各个粒级土壤中对Pb的富集状况和形态分布的影响,探讨了不同钝化剂对Pb在不同粒级土壤中迁移转化规律的影响,以期为羊厩肥、石灰、磷酸盐在Pb污染土壤修复中的高效利用及修复后土壤的潜在生态风险管控提供参考。
1. 材料与方法
1.1 供试材料
供试土壤采自贵阳市花溪区某菜地(106°39′48″E,26°21′20″N),采样深度为0~20 cm,pH为6.46,田间持水量为30.40%,阳离子交换量为31.30 cmol·kg−1,有机质含量为36.89 g·kg−1,全氮含量为2.20 g·kg−1,全磷含量为0.58 g·kg−1,速效磷含量为41.32 mg·kg−1,全钾含量为12.46 g·kg−1,Pb含量为84.58 mg·kg−1。将采集的土壤于室内风干后过2 mm筛,以分析纯级Pb(NO3)2溶液为铅源加入到供试土壤中,使土壤Pb含量达到2 000 mg·kg−1,利用称重法加入超纯水,使土壤保持60%的田间持水量,60 d后风干,磨细过2 mm筛,制成模拟Pb污染土壤。
供试钝化剂分别为羊厩肥、石灰(Ca(OH)2)、磷酸盐(Ca(H2PO4)2·H2O)。其中,羊厩肥采自贵州修文某山羊养殖场,于自然条件下腐熟半年后,风干磨细过2 mm筛备用,其Pb含量为128.81 mg·kg−1;石灰及磷酸盐试剂均为分析纯。
1.2 实验设计
钝化实验设置3组,共12个处理,每个处理重复3次。依次对照空白处理(CK)添加,羊厩肥添加量为1%、2%、5%、10%(GM1、GM2、GM5、GM10);石灰添加量为1%、2%、5%、10%(L1、L2、L5、L10);磷酸盐添加量为1%、2%、5%、10%(P1、P2、P5、P10)。取300 g模拟Pb污染土壤置于500 mL塑料烧杯中,按上述比例将土壤与钝化剂充分混匀,于室温下保持60%田间持水量,钝化培养45 d后取样,自然风干后磨细备用。
1.3 不同土壤粒级分离
参照STEMMER等[14]和武天云等[15]的研究方法,首先对过2 mm筛的土样进行超声处理,再利用湿筛法分离出粗砂粒(2~0.2 mm),最后根据Stokes公式,使用离心法依次分离得到细沙粒(0.2~0.02 mm)、粉粒(0.02~0.002 mm)和黏粒(<0.002 mm)。为确保粒径分离的准确性,分离完成后,立即使用激光粒度仪(Mastersizer2000,英国Malvern公司)对各粒级土壤的粒径分布进行验证。将所得的各粒径土壤于50 ℃下烘干,磨细过0.149 mm筛备用。
1.4 分析方法
土壤理化性质测定参照文献中的方法[16],其中土壤pH使用超纯水浸提(水土比为2.5∶1),pH计(PHS-3C+,上海仪电科学仪器股份有限公司)测定;土壤有机质(SOM)采用重铬酸钾容量法测定;速效磷(Olsen-P)及水溶性磷(CaCl2-P)[17]分别采用pH为8.50的0.50 mol·L−1 NaHCO3及0.01 mol·L−1 CaCl2提取,全自动间断化学分析仪(CleverChem200+,德国DeChem-Tech Gmbh公司)测定。Pb全量[18]采用HCl-HNO3-HF-HClO4消解;植物有效态Pb(DTPA-Pb)[19]使用二乙烯三胺五乙酸浸提;土壤中各形态Pb的含量分布采用改进的BCR连续提取法[20]处理;均使用原子吸收光谱仪(GGX-800,北京海光仪器有限公司)测定。
1.5 数据处理
本研究所有实验数据均使用Excel 2016进行整理及计算,以平均值±标准差的形式作为分析结果;单因素方差分析(One-way ANONA)、Dancan多重比较法(差异性水平为P<0.05)及相关矩阵分析(Pearson)采用SPSS 22.0进行处理;实验数据绘图使用Origin 2017完成。
2. 结果与讨论
2.1 钝化剂添加对土壤理化性质的影响
土壤pH与SOM是影响土壤中重金属有效态的重要因素。其中,pH可影响土壤中重金属的溶解-沉淀、吸附-解吸等反应过程,进而影响土壤中重金属的活性[21]。不同钝化剂处理下土壤pH及SOM含量见图1。由图1可知,羊厩肥添加可显著提升土壤pH,与CK相比,各添加量处理组分别提升了pH 0.22、0.43、0.59、0.72个单位,这可能是因为羊厩肥本身含有大量的盐基离子,致使土壤溶液的离子强度与阳离子交换量升高[22];石灰作为碱性钝化剂,对土壤pH有着显著的影响,所有石灰处理组的土壤pH均显著高于空白处理,石灰用量越大,pH增幅越高;磷酸盐施入土壤后可与土壤水溶液发生水解反应生成H3PO4,各添加量分别使土壤pH降低了0.54、0.85、1.25、1.48个单位,均达到显著水平。
SOM可强烈吸附重金属,与金属离子生成螯合物,且SOM具有还原性,能降低水溶态重金属的比例,减轻重金属的毒性[23]。与CK相比,羊厩肥添加均显著提升了SOM含量,GM10提升的幅度最大,为14.88 g·kg−1,GM1提升的幅度最小,为2.30 g·kg−1;石灰及磷酸盐处理均不同程度地降低了SOM的含量,与CK相比,均达到显著相关水平,这可能是由于石灰和磷酸盐的添加改善了土壤微生物的生存条件,使含碳有机物的转化速度加快,从而促进了SOM分解[24]。
磷是植物生长发育所必需的营养元素,土壤磷的存在形式多样,不同赋存形态的磷的移动性和植物可利用性也有很大差异。其中Olsen-P可直接被植物吸收,而CaCl2-P则被认为是土壤磷可被淋溶损失的部分[25]。图2为不同钝化剂处理对土壤Olsen-P和CaCl2-P含量的影响。由图2可知,3种钝化剂处理均显著提高了土壤Olsen-P含量,其中P10对Olsen-P提高量最大,为145.78 mg·kg−1,GM1最小,为24.93 mg·kg−1。羊厩肥既可直接供给磷素,又能促进相关酶和微生物的活性,进而增加Olsen-P含量[26];石灰可促使铁铝氧化物对磷的释放,以增加Olsen-P含量Olsen-P含量[27];磷酸盐属于易溶性磷肥,故对Olsen-P含量影响最大。对CaCl2-P而言,磷酸盐极大地提高了土壤中CaCl2-P的含量,各处理与CK间的差异均达到显著水平;羊厩肥与石灰虽提高了土壤中CaCl2-P的含量,但GM1、GM2、L1、L2和L10间的差异并不显著。
磷可降低土壤重金属的有效性,但土壤在磷素含量过高时,磷会通过淋溶/渗漏的形式向地表或地下水迁移,造成水体富营养化[28]。因此,在保证钝化修复效果的同时,避免其他营养元素的污染也是钝化修复亟待解决的问题。HESKETH等[29]提出的土壤磷淋溶临界值模型可有效评估不同处理对地下水体可能产生的影响。本研究中不同钝化剂处理后土壤Olsen-P与CaCl2-P的关系曲线如图3所示。2条直线相交点即为土壤磷淋溶临界点。结果表明,当土壤Olsen-P含量大于124.25 mg·kg−1时,土壤磷就会发生淋溶,即GM10及所有磷酸盐处理均会造成土壤磷素淋溶现象,使地下水产生富营养化的风险。
2.2 钝化剂添加对土壤Pb形态的影响
DTPA提取态重金属可直接被植物吸收利用,能直观反映土壤重金属对植物的毒害性,因此,有效降低土壤DTPA提取态重金属含量,对于评估原位钝化修复效果至关重要[30]。由图4可知,3种钝化剂在不同添加量下均显著降低了土壤DTPA-Pb含量,钝化效果随钝化剂添加量的增加而增强,但GM2与GM5之间差异不显著;在所有处理中,GM1降低幅度最小,为6.51%;P10降幅最大,高达80.53%。当钝化剂添加量相同时,对Pb的钝化效果为磷酸盐>石灰>羊厩肥,这与已有研究结果一致:ZENG等[31]通过对比分析氢氧化铅与磷酸铅的稳定性差异认为磷酸盐的钝化效果优于碱性材料;代允超等[32]研究发现酸性土壤添加石灰对于重金属的钝化效果优于有机物料。
土壤不同形态重金属对生态环境的危害程度有所差异,钝化剂可通过改变重金属的赋存形态,以降低其对人体和生态环境的危害,因此,可以用形态分级方案来评价重金属污染土壤的钝化效果[33]。图5为不同钝化处理对土壤Pb形态分布的影响。由图5可知,与CK相比,羊厩肥与磷酸盐均降低了土壤中弱酸提取态Pb含量,降幅与添加量成正比,其中P1降幅最小为0.64%,P10降幅最大为7.05%。石灰处理均提高了土壤中弱酸提取态Pb含量,L5的增幅达4.51%,这可能是由于石灰处理增加了土壤中氢氧化铅和碳酸铅的含量。石灰添加可提高土壤pH,高pH会促进土壤中碳酸盐化合物和金属氢氧化物的生成[34],而上述2种物质在酸性条件极易分解[35]。各处理均降低了土壤中可还原态Pb含量,羊厩肥低添加量降幅适中而高添加量降幅较小,各处理降幅分别为2.47%、7.63%、10.52%和17.07%;石灰低添加量降幅最大但L10降幅适中,分别为8.12%、9.44%、16.49%和20.83%;磷酸盐与石灰相反,低添加量降幅较小而P10降幅最大,各添加量降幅分别为1.74%、4.83%、17.32%和29.52%。不同处理均提高了土壤可氧化态Pb含量,羊厩肥、石灰、磷酸盐在各添加量下增幅分别为1.17%~5.44%、4.22%~14.19%、2.35%~17.89%。对于残渣态Pb而言,羊厩肥与磷酸盐处理均不同程度的增加了其含量,其中P10增幅最大为18.69%,而石灰处理对土壤残渣态Pb含量基本无影响。
以上结果表明,羊厩肥与磷酸盐主要是通过将弱酸提取态与可还原态Pb转化为可氧化态与残渣态Pb,以降低土壤中Pb的活性;石灰处理大幅度降低了可还原态Pb含量,但是却被转化为弱酸提取态与可氧化态,残渣态含量基本无变化,虽然降低了土壤中Pb的活性,但其钝化效果的稳定性与长效性须进一步验证。
2.3 钝化剂添加对不同粒级土壤颗粒中Pb的影响
图6为不同钝化剂种类及添加量下各粒级颗粒中Pb的含量分布情况。由图6可知,Pb含量在土壤颗粒中呈双峰分布趋势,即粗砂粒和黏粒中的Pb含量较高,而细沙粒和粉粒中的Pb含量较低。黏粒比表面积较大,且黏土矿物和铁锰/铁铝氧化物含量较高,对Pb的吸附能力较强;粗砂粒富集Pb的原因较复杂,李恋卿等[36]认为这是由于有机质与其他物质通过复合作用而富集在团聚后的粗颗粒表面的结果,而QIAN等[37]则认为是粗砂粒中含有对重金属固持能力较强的粗矿物或重矿物。3种钝化剂10%添加量处理均降低了各粒级颗粒中Pb含量,这可能是因为钝化剂添加量较大,导致钝化剂在不同粒级颗粒中大量富集,而钝化剂本身Pb含量较低,进而使各粒级颗粒中Pb含量降低。1%、2%、5%羊厩肥添加量均不同程度提高了粗砂粒、细沙粒、粉粒中Pb含量,其中GM2处理对粗砂粒和黏粒中Pb增加量最大,分别为583.30 mg·kg−1和317.00 mg·kg−1,GM5对细沙粒中Pb含量增加量最大,为190.29 mg·kg−1;GM1、GM2和GM5均降低了黏粒中Pb含量,降低量分别为52.56、132.29和160.85 mg·kg−1。这可能是由于羊厩肥显著提高了土壤SOM量,但SOM在各粒级颗粒中的活性有所差异,砂粒中的活性最强,粉粒次之,黏粒最差[38],活性不同对污染物的吸附能力也有所差异,一般来说,活性越强,对污染物的吸附能力越好。与CK相比,1%、2%、5%石灰添加量均增加了粗砂粒中Pb含量,增加量分别为22.16、522.09和138.64 mg·kg−1,同时增加了黏粒中Pb含量,增加量与添加量成正比,L5处理增加量为208.32 mg·kg−1;石灰1%、2%、5%添加量却降低了细沙粒和粉粒中Pb含量。这可能是由于石灰添加增加了土壤颗粒表面负电荷,并生成了CaCO3和Ca(OH)2胶结物质引起的,黏粒比表面积大,持有的负电荷数量最高,因此,加大了对Pb2+的吸附,粗砂粒中的胶结物质与Pb2+进行交换吸附,以增加其Pb固持量[39-40]。相比于CK,磷酸盐增大了粗砂粒与细沙粒对Pb的富集,而对粉粒和黏粒中Pb含量影响无明显规律。
钝化剂添加不仅会影响Pb在各粒级颗粒中的再分配,还可能改变各粒级颗粒中Pb的形态分布,进而影响土壤Pb的有效性。由图7可知,CK处理下各粒级颗粒中Pb形态分布并无明显差异,表明外源Pb进入土壤后在各粒级颗粒中的分布无明显区别[41]。羊厩肥对粗砂粒中各形态Pb所占比例影响最大;而石灰对黏粒中Pb形态占比影响最大;磷酸盐处理下各粒级颗粒中Pb形态分布均由高毒性形态向低毒性形态转化的表现,这可能是由于易溶性磷酸盐与其他矿物晶体形成的光滑涂层,其可将Pb2+包缚在土壤颗粒表面所致[33]。
2.4 土壤与各粒级颗粒间Pb各形态含量相关分析
为了解土壤与各粒级颗粒Pb形态含量间可能存在的关系,对土壤与各粒级颗粒中Pb形态含量间做Pearson相关矩阵分析,其中易利用态为弱酸提取态与可还原态之和,难利用态为可氧化态与残渣态之和[42]。由表1可知,土壤DTPA-Pb含量与细沙粒、粉粒和黏粒中易利用态Pb含量均呈显著正相关关系,相关系数值排序为黏粒>细沙粒>粉粒,这说明土壤中植物有效态Pb含量可能来源于上述3部分中易利用态Pb。土壤弱酸提取态有着最高的生物毒性,各粒级颗粒中弱酸提取态含量均与其达到显著正相关关系,说明土壤弱酸提取态与各粒级弱酸提取态Pb有着密切的关系,本研究中土壤弱酸提取态Pb含量较低,在土壤DTPA-Pb中占比较小,残渣态在土壤中活性最低,因此,土壤DTPA-Pb含量主要受可还原态与可氧化态Pb控制[43]。土壤可还原态Pb含量与细沙粒、粉粒和黏粒中的可还原态呈极显著正相关,还与粉粒和黏粒中的可氧化态含量达到了显著负相关关系,表明钝化剂主要通过将细沙粒、粉粒和黏粒中的可还原态Pb转化为粉粒和黏粒中的可氧化态Pb,来降低土壤Pb的毒害性。
表 1 土壤与各粒级颗粒中Pb形态含量间的相关系数(n=13)Table 1. Correlation coefficients between Pb speciation concentrations in soil and in soil particle-size fractions(n=13)粒级 Pb形态 DTPA-Pb 弱酸提取态 可还原态 可氧化态 残渣态 粗砂粒 弱酸提取态 0.564* 0.890** 0.263 −0.473 −0.581* 可还原态 0.525 0.935** 0.234 −0.368 −0.498 可氧化态 −0.061 −0.369 0.238 0.013 0.354 残渣态 0.065 −0.305 0.475 −0.137 0.460 易利用态 0.537 0.935** 0.241 −0.390 −0.517 难利用态 0.017 −0.391 0.447 −0.090 0.492 细沙粒 弱酸提取态 0.642* 0.876** 0.318 −0.500 −0.438 可还原态 0.842** 0.702** 0.727** −0.751** −0.148 可氧化态 −0.524 −0.241 −0.206 0.309 −0.147 残渣态 −0.182 −0.575* 0.312 0.035 0.425 易利用态 0.828** 0.751** 0.673* −0.725** −0.203 难利用态 −0.360 −0.500 0.120 0.115 0.223 粉粒 弱酸提取态 0.723** 0.790** 0.428 −0.631* −0.335 可还原态 0.766** 0.222 0.765** −0.700** 0.316 可氧化态 −0.581* −0.470 −0.590* 0.485 −0.118 残渣态 −0.250 −0.649* 0.240 0.099 0.454 易利用态 0.817** 0.378 0.745** −0.740** 0.84 难利用态 −0.427 −0.774** 0.041 0.251 0.398 粘粒 弱酸提取态 0.720** 0.765** 0.317 −0.434 −0.216 可还原态 0.882** 0.354 0.692** −0.495 0.309 可氧化态 −0.671* 0.171 −0.606* 0.504 −0.738** 残渣态 −0.701** −0.396 −0.435 0.429 −0.047 易利用态 0.882** 0.522 0.604* −0.506 0.143 难利用态 −0.826** −0.172 −0.614* 0.555* −0.426 注:*为P<0.05,显著;**为P<0.01,显著。 3. 结论
1) 3种钝化剂在不同添加量下均能显著降低外源Pb污染土壤中DTPA-Pb含量,在所有处理中,GM1对DTPA-Pb含量降低幅度最小,为6.51%,P10降幅最大,高达80.53%,3种钝化剂在相同添加量下对外源Pb的钝化效果为磷酸盐>石灰>羊厩肥。形态分布显示,羊厩肥和磷酸盐通过将弱酸提取态与可还原态Pb转化为可氧化态与残渣态Pb,以降低土壤外源Pb有效性,石灰通过将可还原态Pb转化为可氧化态Pb,以降低其有效性。
2) 3种钝化剂均不同程度地提高了土壤Olsen-P含量,其中羊厩肥、石灰和磷酸盐处理下Olsen-P含量分别为60.12~145.12、66.30~84.72、125.29~180.98 mg·kg−1。土壤磷淋溶临界值模型显示,当土壤Olsen-P含量大于124.25 mg·kg−1时,土壤磷素就会向地下水淋溶。
3) 3种钝化剂对各粒级颗粒中Pb含量及形态分布的影响有所差异。羊厩肥可增加粗砂粒、细沙粒、粉粒中Pb含量,降低黏粒中Pb含量,对各粒级颗粒中Pb形态分布均有影响但粗砂粒影响最大。石灰会增加粗砂粒和黏粒中Pb含量,降低细沙粒和粉粒中Pb含量,并对黏粒中Pb形态分布产生较大影响。磷酸盐提高粗砂粒与细沙粒对Pb的富集而对粉粒和黏粒中Pb含量影响无明显规律,在磷酸盐作用下各粒级颗粒均明显出现高毒性向低毒性形态转化的现象。
4)相关性分析表明,土壤DTPA-Pb主要来源于细沙粒、粉粒和黏粒中易利用态Pb,钝化剂主要通过将细沙粒、粉粒和黏粒中的可还原态Pb转化为粉粒和黏粒中的可氧化态Pb,以降低土壤Pb的毒害性。
-
表 1 土壤与各粒级颗粒中Pb形态含量间的相关系数(n=13)
Table 1. Correlation coefficients between Pb speciation concentrations in soil and in soil particle-size fractions(n=13)
粒级 Pb形态 DTPA-Pb 弱酸提取态 可还原态 可氧化态 残渣态 粗砂粒 弱酸提取态 0.564* 0.890** 0.263 −0.473 −0.581* 可还原态 0.525 0.935** 0.234 −0.368 −0.498 可氧化态 −0.061 −0.369 0.238 0.013 0.354 残渣态 0.065 −0.305 0.475 −0.137 0.460 易利用态 0.537 0.935** 0.241 −0.390 −0.517 难利用态 0.017 −0.391 0.447 −0.090 0.492 细沙粒 弱酸提取态 0.642* 0.876** 0.318 −0.500 −0.438 可还原态 0.842** 0.702** 0.727** −0.751** −0.148 可氧化态 −0.524 −0.241 −0.206 0.309 −0.147 残渣态 −0.182 −0.575* 0.312 0.035 0.425 易利用态 0.828** 0.751** 0.673* −0.725** −0.203 难利用态 −0.360 −0.500 0.120 0.115 0.223 粉粒 弱酸提取态 0.723** 0.790** 0.428 −0.631* −0.335 可还原态 0.766** 0.222 0.765** −0.700** 0.316 可氧化态 −0.581* −0.470 −0.590* 0.485 −0.118 残渣态 −0.250 −0.649* 0.240 0.099 0.454 易利用态 0.817** 0.378 0.745** −0.740** 0.84 难利用态 −0.427 −0.774** 0.041 0.251 0.398 粘粒 弱酸提取态 0.720** 0.765** 0.317 −0.434 −0.216 可还原态 0.882** 0.354 0.692** −0.495 0.309 可氧化态 −0.671* 0.171 −0.606* 0.504 −0.738** 残渣态 −0.701** −0.396 −0.435 0.429 −0.047 易利用态 0.882** 0.522 0.604* −0.506 0.143 难利用态 −0.826** −0.172 −0.614* 0.555* −0.426 注:*为P<0.05,显著;**为P<0.01,显著。 -
[1] VENKATACHALAM P, JAYALAKSHMI N, GEETHA N, et al. Accumulation efficiency, genotoxicity and antioxidant defense mechanisms in medicinal plant Acalypha indica L. under lead stress[J]. Chemosphere, 2017, 171: 544-553. doi: 10.1016/j.chemosphere.2016.12.092 [2] 代豫杰, 郭建英, 董智, 等. 不同沙生灌木下土壤颗粒及重金属空间分布特征[J]. 环境科学, 2017, 38(11): 4809-4818. [3] LIU G, WANG J, LIU X, et al. Partitioning and geochemical fractions of heavy metals from geogenic and anthropogenic sources in various soil particle size fractions[J]. Geoderma, 2018, 312: 104-113. doi: 10.1016/j.geoderma.2017.10.013 [4] HU X, ZHANG Y, DING Z, et al. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China[J]. Atmospheric Environment, 2012, 57: 146-152. [5] HU S P, CHEN X C, SHI J Y, et al. Particle-facilitated lead and arsenic transport in abandoned mine sites soil influenced by simulated acid rain[J]. Chemosphere, 2008, 71(11): 2091-2097. doi: 10.1016/j.chemosphere.2008.01.024 [6] CALMANO W, FÖRSTNER U. Chemical extraction of heavy metals in polluted river sediments in central Europe[J]. Science of the Total Environment, 1983, 28(1/2/3): 77-88. [7] BOLAN N, KUNHIKRISHNAN A, THANGARAJAN R, et al. Remediation of heavy metal(loid)s contaminated soils: To mobilize or to immobilize?[J]. Journal of Hazardous Materials, 2014, 266(4): 141-166. [8] 王济, 李丁, 宣斌, 等. 有机物料对土壤Pb有效态作用机理及影响效果研究进展[J]. 贵州师范大学学报(自然科学版), 2018, 36(2): 107-113. [9] 王立群, 罗磊, 马义兵, 等. 重金属污染土壤原位钝化修复研究进展[J]. 应用生态学报, 2009, 20(5): 1214-1222. [10] HALE B, EVANS L, LAMBERT R. Effects of cement or lime on Cd, Co, Cu, Ni, Pb, Sb and Zn mobility in field-contaminated and aged soils[J]. Journal of Hazardous Materials, 2012, 199-200: 119-127. doi: 10.1016/j.jhazmat.2011.10.065 [11] DU Y J, WEI M L, REDDY K R, et al. New phosphate-based binder for stabilization of soils contaminated with heavy metals: Leaching, strength and microstructure characterization[J]. Journal of Environmental Management, 2014, 146: 179-188. doi: 10.1016/j.jenvman.2014.07.035 [12] CÁRDENAS J P, SANTIAGO A, TARQUIS A M, et al. Soil porous system as heterogeneous complex network[J]. Geoderma, 2010, 160(1): 13-21. doi: 10.1016/j.geoderma.2010.04.024 [13] ZHANG Z Y, HUANG L, LIU F, et al. Characteristics of clay minerals in soil particles of two Alfisols in China[J]. Applied Clay Science, 2016, 120: 51-60. doi: 10.1016/j.clay.2015.11.018 [14] STEMMER M, GERZABEK M H, KANDELER E. Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication[J]. Soil Biology and Biochemistry, 1998, 30(1): 9-17. doi: 10.1016/S0038-0717(97)00093-X [15] 武天云, 李凤民, 钱佩源, 等. 利用离心法进行土壤颗粒分级[J]. 应用生态学报, 2004, 15(3): 477-481. doi: 10.3321/j.issn:1001-9332.2004.03.024 [16] 鲍士旦. 土壤农化分析[M]. 3版. 北京: 中国农业出版社, 2000. [17] HOUBA V J G, NOVOZAMSKY I, LEXMOND T M, et al. Applicability of 0.01 M CaCl2 as a single extraction solution for the assessment of the nutrient status of soils and other diagnostic purposes[J]. Communications in Soil Science and Plant Analysis, 1990, 21(19/20): 2281-2290. [18] 中华人民共和国国家环境保护局, 中国国家技术监督局. 土壤质量铅、镉的测定: GB/T 17140-1997[S]. 北京: 中国环境科学出版社, 1997. [19] 中华人民共和国生态环境部. 土壤8种有效态元素的测定: HJ 804-2016[S]. 北京: 中国环境科学出版社, 2016. [20] NEMATI K, BAKAR N K A, ABAS M R, et al. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia[J]. Journal of Hazardous Materials, 2011, 192(1): 402-410. [21] 陈杰, 张晶, 王鑫, 等. 不同物料对污染土壤中铅的钝化[J]. 农业环境科学学报, 2015, 34(9): 1674-1678. doi: 10.11654/jaes.2015.09.007 [22] 原梦云, 商和平, 李洋, 等. 畜禽粪便有机肥中Cu, Zn在水稻土中的有效性动态变化和形态归趋[J]. 环境科学学报, 2016, 36(7): 2613-2623. [23] 周宇杰, 赵文, 罗春岩, 等. 有机肥对铅在土壤中形态分配的影响[J]. 环境化学, 2018, 37(3): 534-543. doi: 10.7524/j.issn.0254-6108.2017070801 [24] HE Y B, HUANG D Y, ZHU Q H, et al. A three-season field study on the in-situ remediation of Cd-contaminated paddy soil using lime, two industrial by-products, and a low-Cd-accumulation rice cultivar[J]. Ecotoxicology and Environmental Safety, 2017, 136: 135-141. doi: 10.1016/j.ecoenv.2016.11.005 [25] 李学平, 石孝均, 刘萍, 等. 紫色土磷素流失的环境风险评估-土壤磷的" 临界值”[J]. 土壤通报, 2011, 42(5): 1153-1158. [26] CHUNG H, PARK M, MADHAIYAN M, et al. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea[J]. Soil Biology and Biochemistry, 2005, 37(10): 1970-1974. doi: 10.1016/j.soilbio.2005.02.025 [27] 廖萍. 石灰和稻草还田对双季水稻产量和土壤性状的互作效应[D]. 南昌: 江西农业大学, 2017. [28] 刘利花, 杨淑英, 吕家珑. 长期不同施肥土壤中磷淋溶" 阈值”研究[J]. 西北农林科技大学学报(自然科学版), 2003, 31(3): 123-126. doi: 10.3321/j.issn:1671-9387.2003.03.028 [29] HESKETH N, BROOKES P C. Development of an indicator for risk of phosphorus leaching[J]. Journal of Environmental Quality, 2000, 29(1): 105-110. [30] STANISLAWSKA-GLUBIAK E, KORZENIOWSKA J, KOCON A. Effect of peat on the accumulation and translocation of heavy metals by maize grown in contaminated soils[J]. Environmental Science and Pollution Research, 2015, 22(6): 4706-4714. doi: 10.1007/s11356-014-3706-x [31] ZENG G, WAN J, HUANG D, et al. Precipitation, adsorption and rhizosphere effect: the mechanisms for phosphate-induced Pb immobilization in soils: A review[J]. Journal of Hazardous Materials, 2017, 339: 354-367. doi: 10.1016/j.jhazmat.2017.05.038 [32] 代允超, 吕家珑, 曹莹菲, 等. 石灰和有机质对不同性质镉污染土壤中镉有效性的影响[J]. 农业环境科学学报, 2014, 33(3): 514-519. doi: 10.11654/jaes.2014.03.017 [33] 赵庆圆, 李小明, 杨麒, 等. 磷酸盐、腐殖酸与粉煤灰联合钝化处理模拟铅镉污染土壤[J]. 环境科学, 2018, 39(1): 389-398. [34] 王力, 杨亚提, 王爽, 等. 陕西省采矿业污染农田土壤中Cd、Pb的释放特征[J]. 西北农林科技大学学报(自然科学版), 2015, 43(7): 192-200. [35] BASTA N T, GRADWOHL R, SNETHEN K L, et al. Chemical immobilization of lead, zinc, and cadmium in smelter-contaminated soils using biosolids and rock phosphate[J]. Journal of Environmental Quality, 2001, 30(4): 1222-1230. doi: 10.2134/jeq2001.3041222x [36] 李恋卿, 潘根兴, 张平究, 等. 太湖地区水稻土颗粒中重金属元素的分布及其对环境变化的响应[J]. 环境科学学报, 2001, 21(5): 607-612. doi: 10.3321/j.issn:0253-2468.2001.05.020 [37] QIAN J, SHAN X Q, WANG Z J, et al. Distribution and plant availability of heavy metals in different particle-size fractions of soil[J]. Science of the Total Environment, 1996, 187(2): 131-141. doi: 10.1016/0048-9697(96)05134-0 [38] 刘慧云, 鲜青松, 刘琛, 等. 生物质炭对紫色土耕地土壤中溶解性有机物含量和组成特征的影响[J]. 农业环境科学学报, 2017, 36(4): 718-726. doi: 10.11654/jaes.2016-1538 [39] 崔俊义, 马友华, 陈亮妹, 等. 原位钝化-低积累品种联合修复镉污染农田研究[J]. 环境科学与技术, 2018, 41(7): 77-83. [40] 鲁秀国, 过依婷. 重金属污染土壤钝化修复技术研究[J]. 应用化工, 2018, 47(7): 1473-1477. doi: 10.3969/j.issn.1671-3206.2018.07.038 [41] LI H, JI H, SHI C, et al. Distribution of heavy metals and metalloids in bulk and particle size fractions of soils from coal-mine brownfield and implications on human health[J]. Chemosphere, 2017, 172: 505-515. doi: 10.1016/j.chemosphere.2017.01.021 [42] 闫家普, 丁效东, 崔良, 等. 不同改良剂及其组合对土壤镉形态和理化性质的影响[J]. 农业环境科学学报, 2018, 37(9): 1842-1849. doi: 10.11654/jaes.2018-0187 [43] ZHU Y G, CHEN S B, YANG J C. Effects of soil amendments on lead uptake by two vegetable crops from a lead-contaminated soil from Anhui, China[J]. Environment International, 2004, 30(3): 351-356. doi: 10.1016/j.envint.2003.07.001 -




 下载:
下载: