-
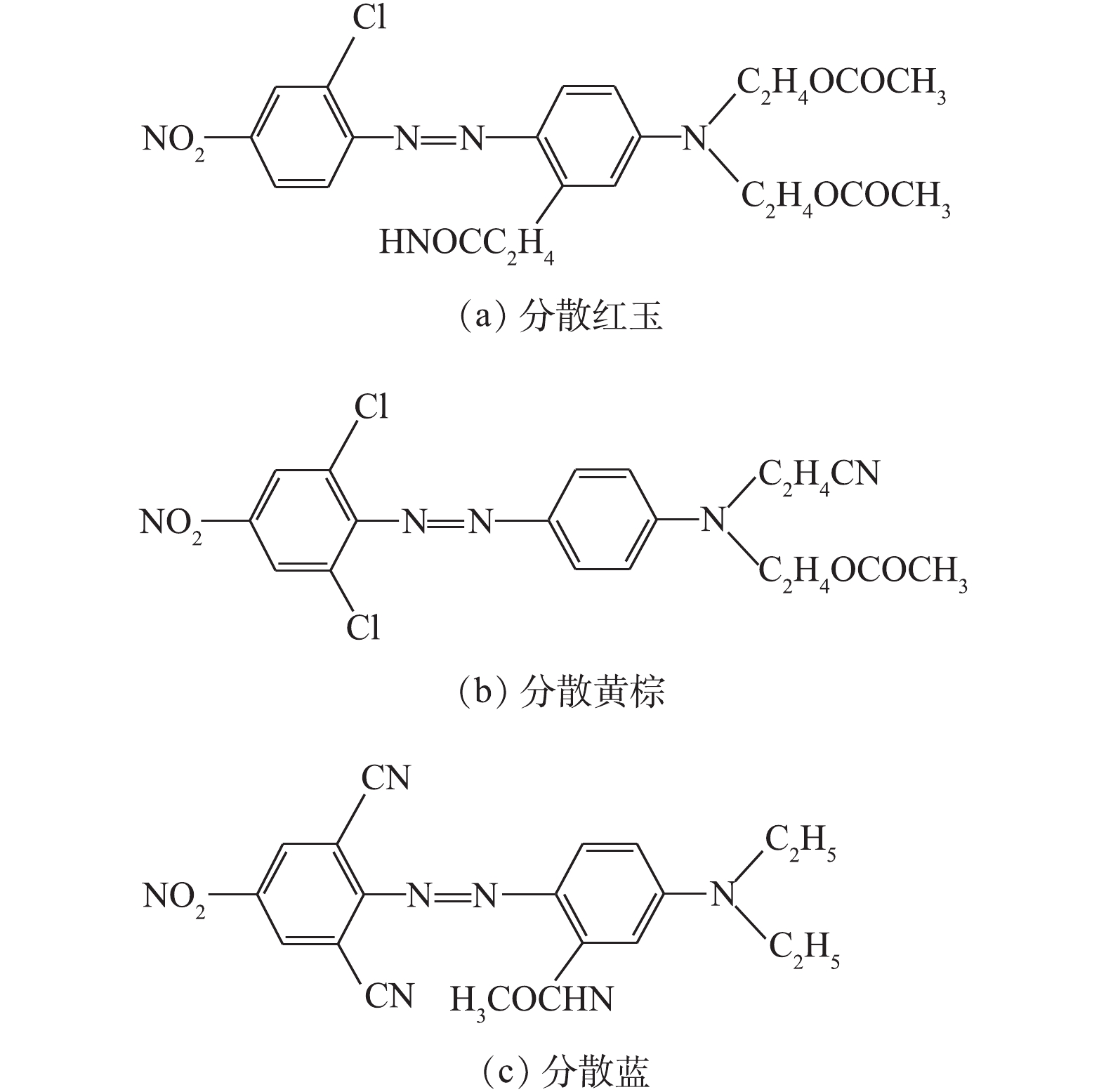
印染废水性质多变且排放量大、色度高、含大量难降解有机物,是较难处理的工业废水之一[1]。我国生产的染料多达550种[2],按性质分为直接染料、活性染料、酸性染料、分散染料等,其中分散染料应用范围较广[3-4],主要用于涤纶纤维的染色和印花,是近年来染料发展的重点之一[5]。我国分散染料的产量位居世界第一,约占我国染料总产量的50%[6]。分散染料溶解度低[7],按分子结构主要分为偶氮类、蒽醌类和杂环类,其中偶氮类产量占分散染料的70%以上[8],分散染料颜色主要为红、黄、蓝3种。
印染废水的处理工艺主要有混凝、光催化、电化学氧化、膜分离等[9],其中混凝因具有投资运行成本低和操作管理便捷等优点,已被广泛应用于实际印染废水的处理中[10]。由于染料种类庞大,目前,针对所有种类染料的混凝去除机理还没有被一一揭示,即使是相对容易去除的分散染料,对其机理的研究认识也还未完全明确和统一。
有研究[11-12]认为,分散染料易被混凝去除的主要原因和机理是其溶解度较低所致。也有研究[13-14]表明,分散染料被去除的主要机理是通过混凝剂吸附作用完成的,由于分散染料是非离子型染料,在溶液中难电离出离子,带电较少,表现为憎水性,因此,易通过被颗粒物或混凝剂吸附形成絮体从而沉淀去除。然而有研究[15-16]通过测定溶液中分散染料的Zeta电位发现,尽管分散染料难电离出离子,但在溶液中,其颗粒表面仍带较多的负电荷,因此,易被电中和能力强的混凝剂去除,同时,混凝剂的吸附架桥作用也可以进一步提高染料的去除率。还有部分研究[17-18]通过在染料中投加高分子混凝剂,利用混凝剂的吸附架桥能力去除分散染料。
综上可知,目前,对于分散染料的混凝去除机理认识仍不统一。因此,本研究以应用较为广泛、较有代表性的典型分散染料—分散红玉、分散黄棕、分散蓝作为去除对象,以常见无机盐(即阳离子电解质)AlCl3、FeCl3以及特殊无机盐CaCl2作为混凝剂,通过研究典型无机混凝剂与分散染料的直接作用,深入揭示分散染料与无机混凝剂的内在反应机理和作用本质,以丰富和完善混凝去除分散染料的作用机理,为相关复合混凝药剂或专效型混凝药剂的开发提供参考。
全文HTML
-
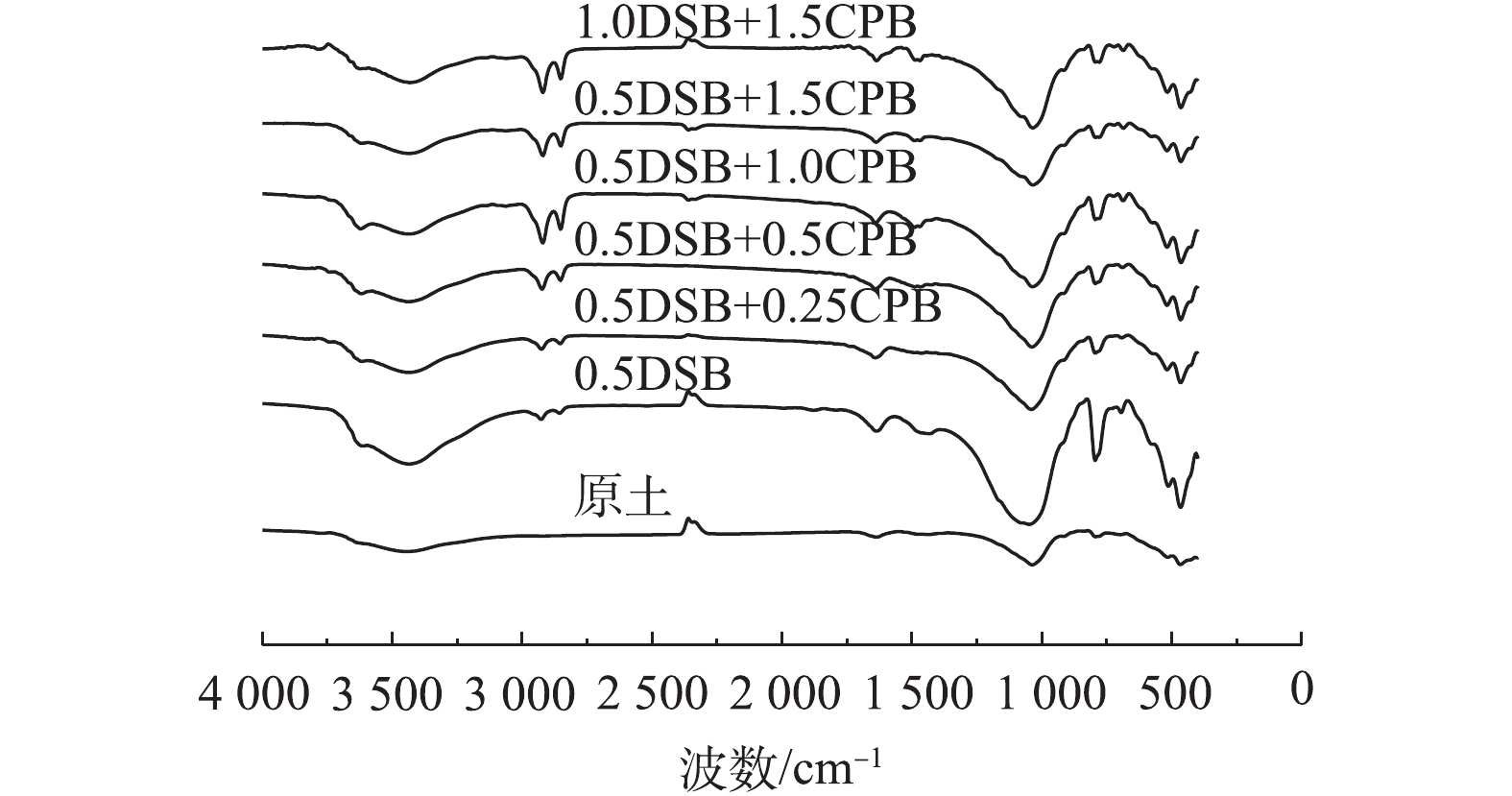
试剂:FeCl3·6H2O、AlCl3·6H2O、CaCl2均为AR级。分散红玉S-2GFL(disperse red dye, DR)、分散黄棕S-2RFL(disperse orange, DO)、分散蓝BBLS(disperse blue, DB)均为BS级,分子式分别为C22H24ClN5O7、C19H17Cl2N5O4、C20H19N7O3,其相对分子质量分别为505.93、450.29、405.41,其分子结构式如图1所示。实验用水均为去离子水。
仪器:ZR4-6六联搅拌器(深圳市中润水工业技术发展有限公司),日立U-2910紫外分光光度计,梅特勒-托利多pH计,真空干燥机(上海博迅实业有限公司DZF-6020),TOC测量仪器(德国耶拿Multi N/C 3100),粒度/Zeta电位分布分析仪(美国BROOKHAVEN NanoBrook 90 Plus PALS),傅里叶红外测量仪(德国布鲁克TENSOR 27),智能X射线衍射仪(日本理学SmartLab)。
-
称取一定量干燥的分散红玉(DR)、分散黄棕(DO)、分散蓝(DB)染料,充分溶解搅拌后,配制浓度为5 g·L−1的模拟染料废水储备液。染料使用液浓度为50 mg·L−1。
称取一定量的FeCl3·6H2O、AlCl3·6H2O和CaCl2,充分溶解搅拌后,配制一定浓度的储备液。
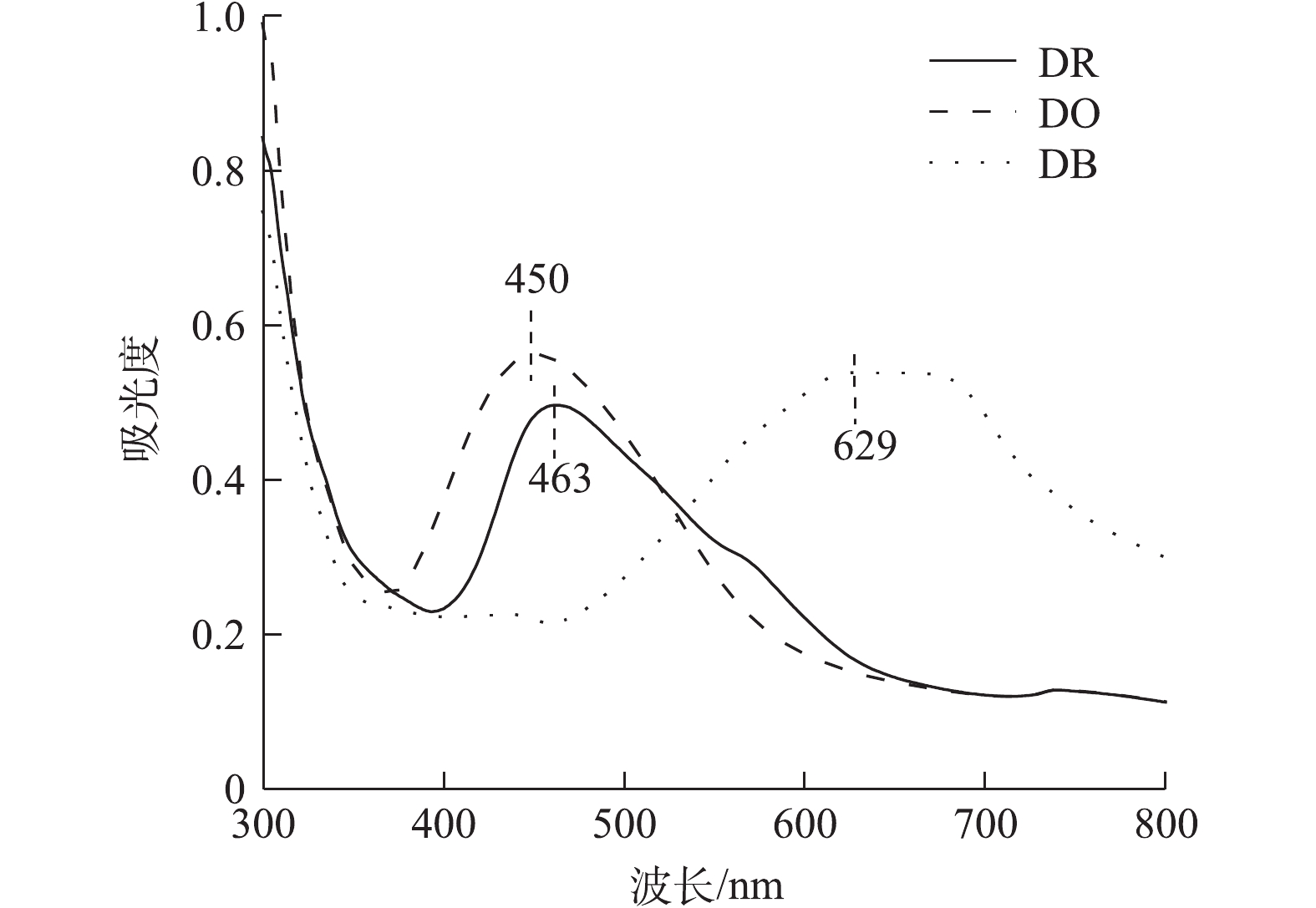
1)混凝实验。采用标准烧杯搅拌实验,混凝搅拌程序见表1。在混凝程序第1段结束、第2段开始前分别投加0.12 mmol·L−1 AlCl3、0.12 mmol·L−1 FeCl3、550 mmol·L−1 CaCl2,每组实验设3个平行。分散红玉、分散黄棕、分散蓝染料的紫外全波长扫描图谱见图2,最佳吸收峰分别位于463、450、629 nm处。静置30 min后,在距液面2 cm处取上清液进行吸光度、TOC、Zeta电位和粒径的测定。
2)单一pH对混凝效果的影响实验。混凝前,调节分散红玉、分散黄棕、分散蓝使用液的pH分别为1、2、3、4、5(混凝实验后,投加AlCl3、FeCl3的染料废水pH在此范围内,混凝前后pH的变化见表2),不投加混凝剂,其他条件保持与混凝实验一致,混凝程序见表1,每组实验设3个平行。静置结束后,在距液面2 cm处取上清液进行吸光度测定。
3)混凝剂投加顺序对混凝去除率的影响实验。改变混凝剂投加顺序为先向去离子水中分别投加0.12 mmol·L−1 AlCl3、0.12 mmol·L−1 FeCl3、550 mmol·L−1 CaCl2,调节pH至混凝实验中染料使用液的初始pH(6.35左右,具体见表2),每组实验设3个平行,混凝程序见表1,第2段程序开始前投加染料,使得染料废水浓度为50 mg·L−1。静置结束后,在距液面2 cm处,取上清液进行吸光度的测定。
1.1. 实验材料
1.2. 实验方法
-
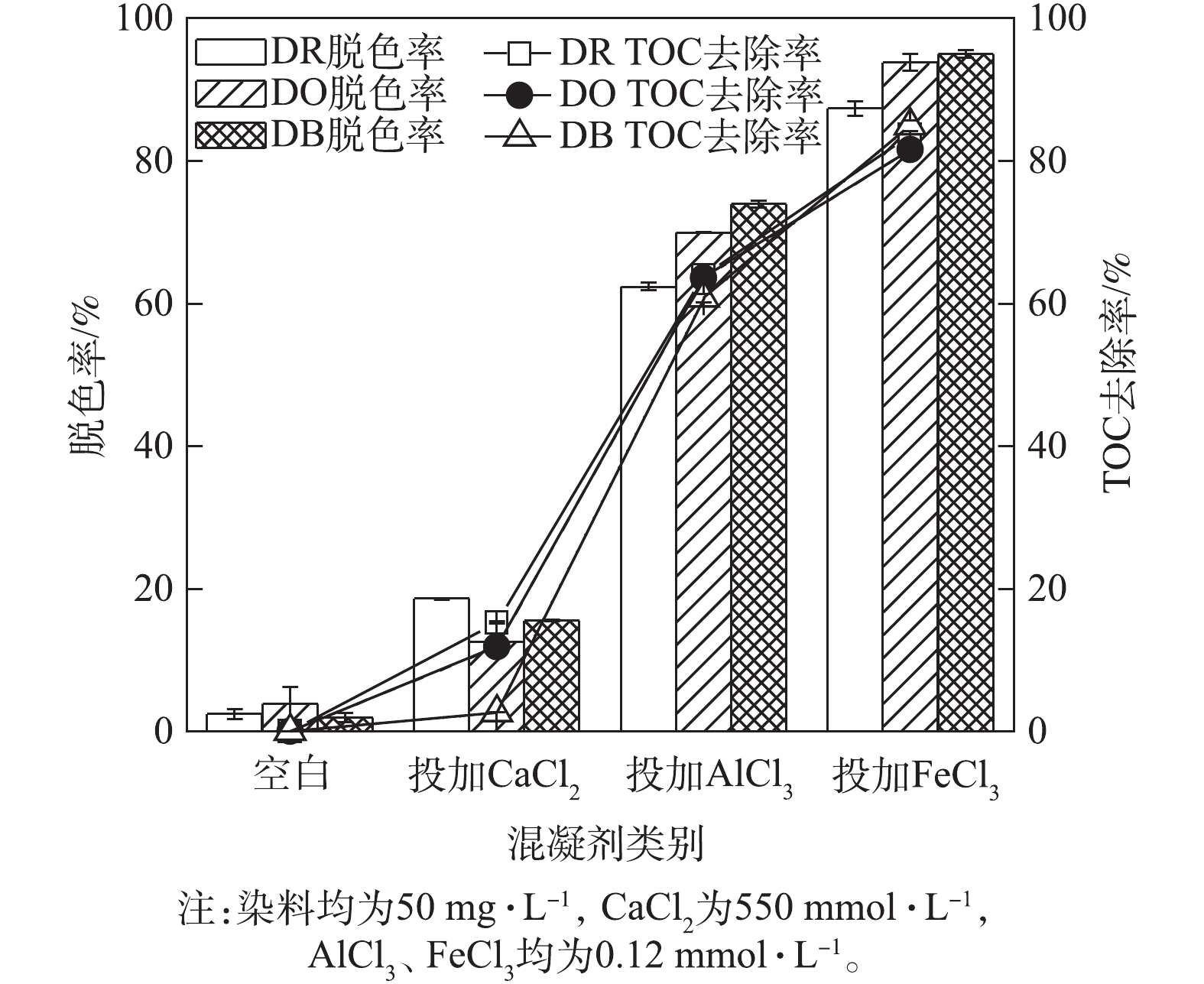
首先,对各分散染料进行基本的混凝实验研究,即了解当溶液体系颗粒Zeta电位接近等电点时各染料的去除情况。混凝实验前后不进行任何的pH调节。从实验现象来看,投加AlCl3、FeCl3均有矾花出现,上清液明显清澈,投加CaCl2的实验组无矾花出现,上清液没有明显的颜色变化。图3为混凝后上清液的脱色率和TOC的测定结果。投加AlCl3和FeCl3对分散蓝的去除率均较高,分别为73.94%、95.02%,但CaCl2对分散红玉的去除率仅为18.57%。TOC去除率与色度去除率(即脱色率)基本一致。
因此,在单纯染料溶液直接投加混凝剂且不调节pH情况下,混凝剂对染料的去除率依次为FeCl3>AlCl3>CaCl2,其中CaCl2对染料去除率远不及前两者。在任何混凝实验过程中,pH均会发生变化,因此,本实验中染料被去除的可能原因有2种:一是pH本身的变化可能导致染料的变性,从而使其被去除;二是混凝剂与染料的直接相互作用或相互结合。
-
对于混凝实验,无论先固定pH还是后固定pH,在混凝剂投入溶液及反应过程中,pH都会发生变化,因此,本研究探讨了单一pH的变化是否会直接导致染料的明显去除。表2为基本混凝实验(见2.1节)中混凝前后pH的变化情况。由表2可知,混凝前各分散染料溶液的pH在6.35左右,投加AlCl3后,pH降至4.40左右,投加FeCl3后,pH降至3.50左右,投加CaCl2后,pH在9.00左右,呈弱碱性。
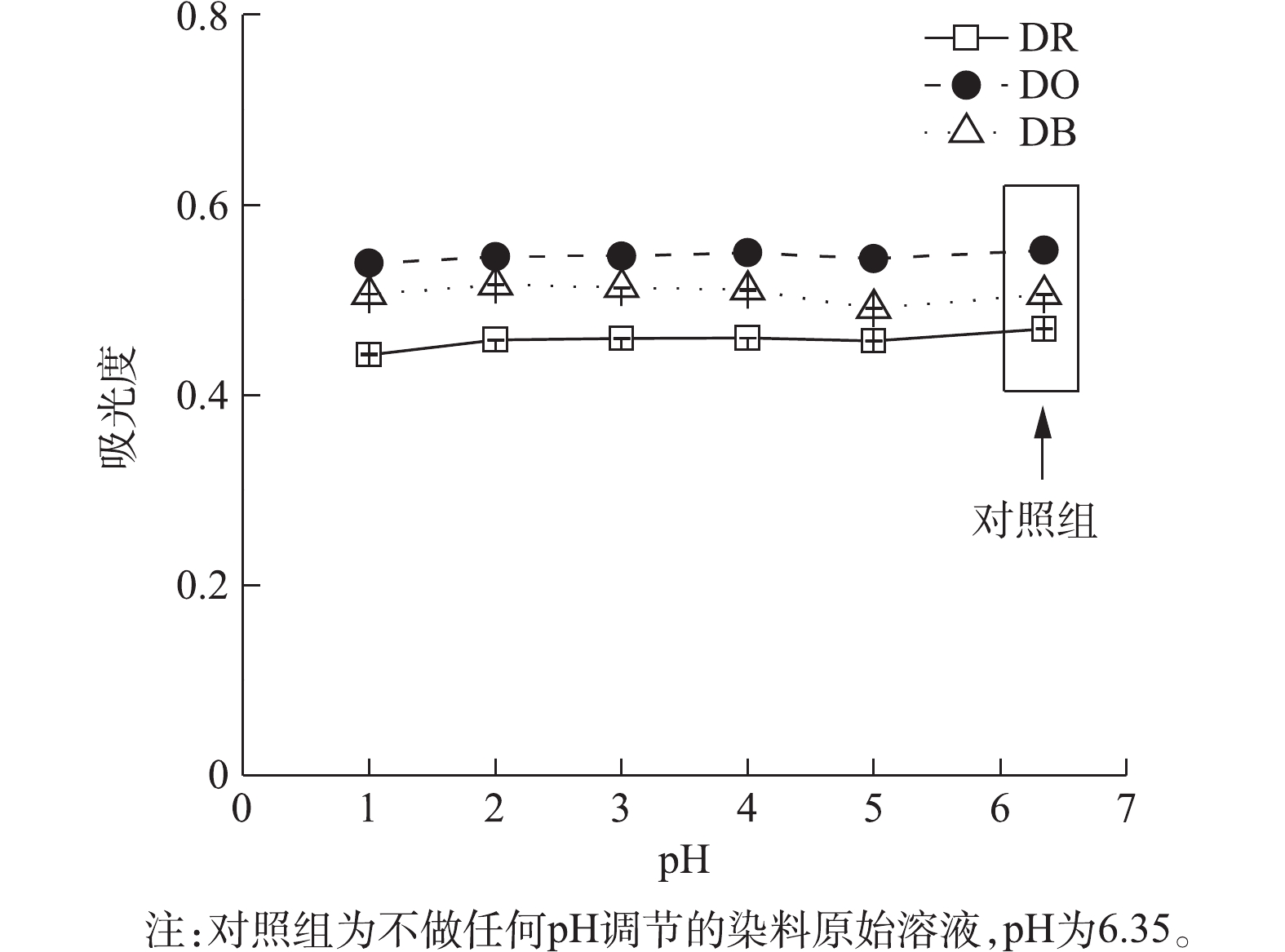
图4为仅调节pH,不投加混凝剂测得的上清液吸光度结果,其中各分散染料不做任何pH调节的原始溶液(即对照组)的pH在6.35左右。由图4可知,当pH为1~6.35时,分散红玉、分散黄棕和分散蓝的吸光度基本保持稳定,没有明显的下降和去除。基于对照组的吸光度计算色度去除率可知,单一pH变化引起的分散红玉去除率最大仅为5.79%,分散黄棕和分散蓝去除率最大仅为1.59%和1.85%,均接近或在实验系统误差5.00%范围之内。因此,单一pH的改变对50 mg·L−1的分散红玉、分散黄棕、分散蓝染料的去除影响基本可忽略不计。由此可见,溶液单独pH的变化基本不会引起分散染料的直接去除,虽然混凝反应过程中pH也在变化,但是染料的去除不是由pH变化引起的,而应是混凝剂与染料的相互作用导致的。
-
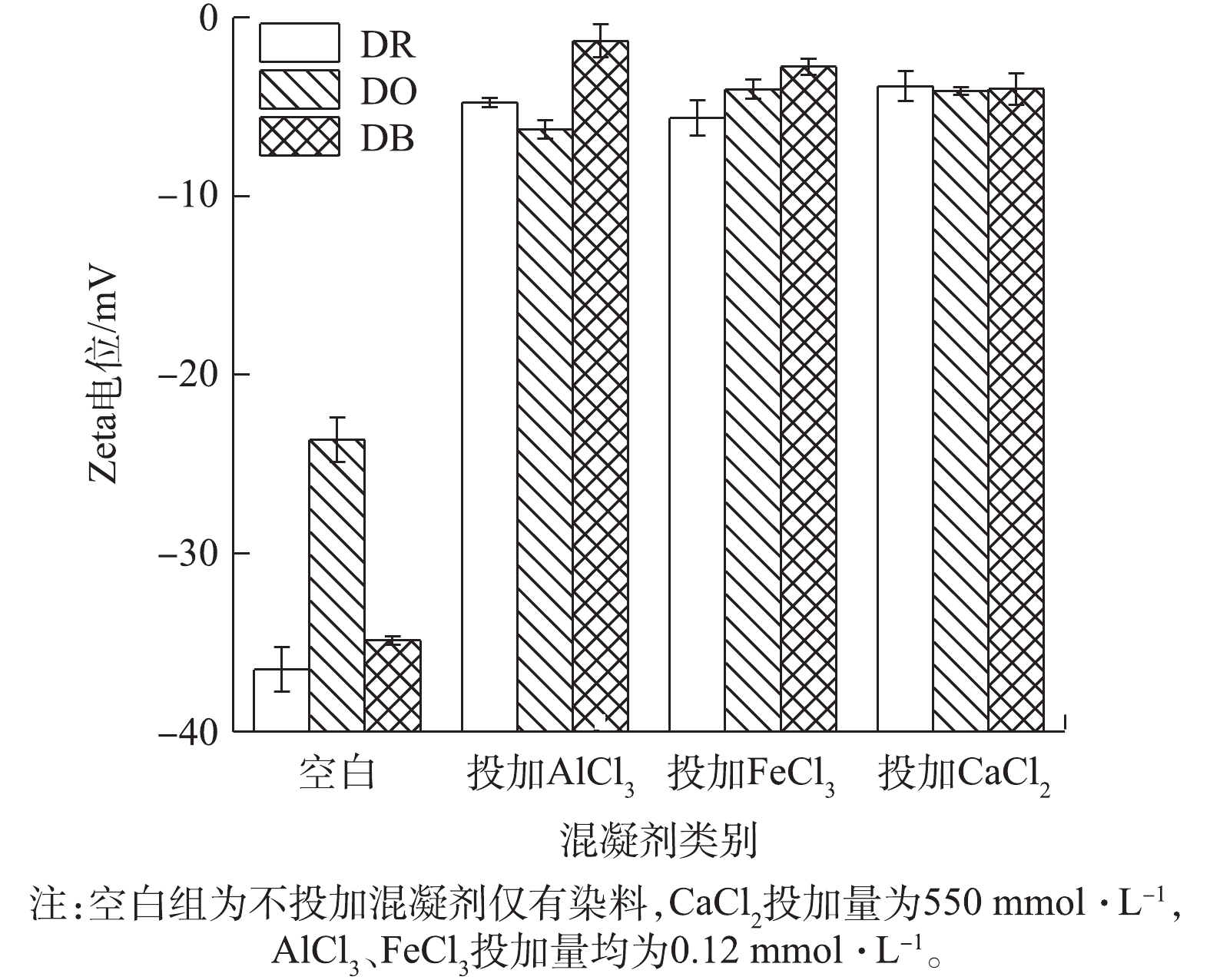
图5为混凝实验上清液的Zeta电位测定结果。可以看出,混凝前,3种独立染料溶液颗粒的Zeta电位平均值为−31.67 mV,各染料溶液平行3份,分别投加不同混凝剂,使混凝后溶液颗粒表面Zeta电位均接近0 mV,但结合各混凝剂对染料的平均去除率(ACl3, 68.75%; FeCl3, 92.09%; CaCl2, 15.54%)(图3)可知,CaCl2对染料的去除率最低。特别是对于分散红玉,投加CaCl2后溶液颗粒Zeta电位要比投加其他2种混凝剂更接近于0 mV,但CaCl2对分散红玉去除率(18.57%)仍远低于AlCl3和FeCl3对此染料的去除率(分别为62.37%和87.37%)。由此可见,CaCl2未能去除大部分染料,并非是因为其电中和能力弱于AlCl3和FeCl3,而是电中和作用并不是导致此类分散染料被直接混凝沉淀去除的主要机理或充分条件。
-
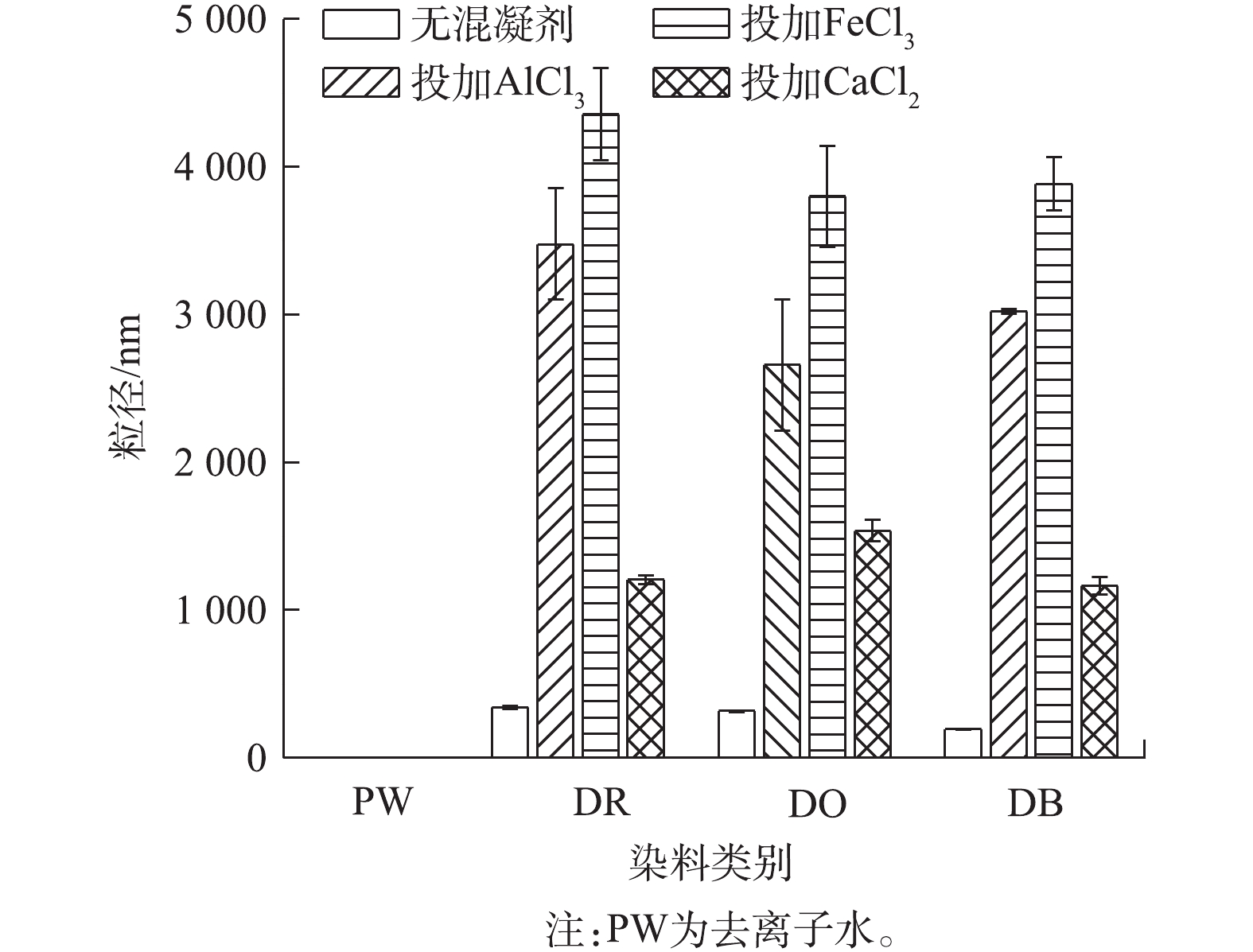
图6为不同条件下溶液颗粒的粒径测定结果。图6中PW实验组是去离子水中只投加混凝剂,测其粒径测定值,作为实验对照。首先,由图6可知,混凝剂单独溶于去离子水后,粒径仪的测定值为0 nm,说明混凝剂投加于去离子水后其本身不会引起粒径仪测定值的增加。其次,单纯染料溶液(无混凝剂投加组)的原始颗粒粒径值均相对较小,处于194~342 nm。而各染料溶液一旦加入混凝剂后,溶液中颗粒粒径均明显增大。对于3种染料,投加AlCl3和FeCl3后,形成的颗粒粒径较混凝前染料粒径增长了近10倍(如DO)或10倍以上(如DR和DB),投加CaCl2后,颗粒粒径也达到混凝前染料粒径的5倍左右。可见,染料和混凝剂结合后,产生的颗粒粒径远大于二者单独存在时的粒径,这说明混凝剂与染料发生了结合,但是单独从粒径的变化无法确定是物理吸附作用导致的结合还是化学作用导致的结合。
-
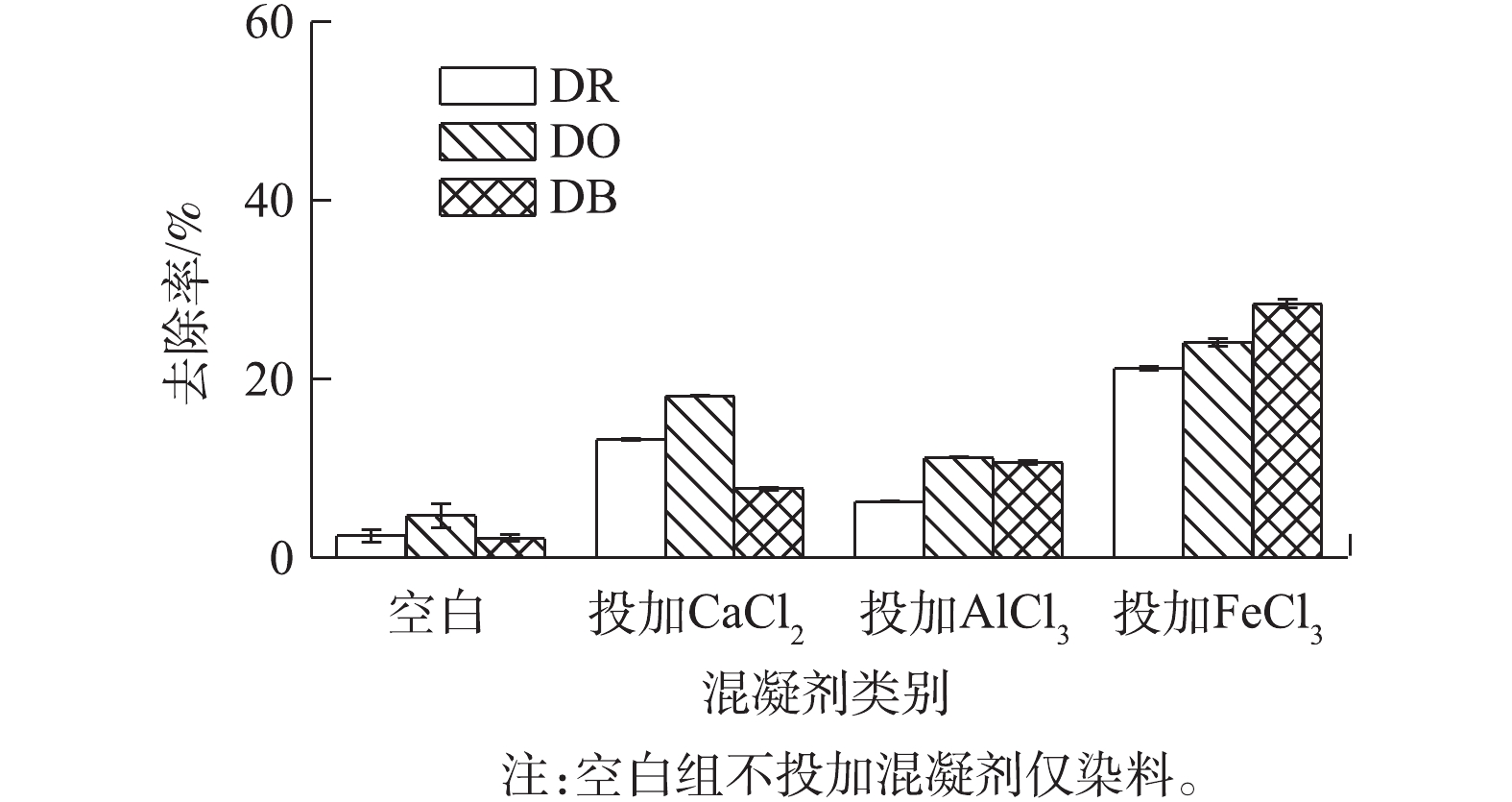
图7反映了改变混凝剂投加顺序对染料去除率影响的结果,即在去离子水中先投加混凝剂再投加染料的混凝实验结果,目的是先让混凝剂进入水中全部充分完成水解,然后考察其水解终产物是否可吸附去除较多的染料。由图7可知,改变AlCl3和FeCl3的投加顺序(先投加混凝剂再投加染料),对染料也有一定的去除率(AlCl3和FeCl3对3种染料的平均去除率分别为9.32%和24.52%),但是与正常混凝剂投加方式(先溶解染料后投加混凝剂)的去除率相比,有明显的降低。正常混凝剂投加方式下,FeCl3对3种染料的平均去除率为92.06%,AlCl3为68.75%(图3)。这说明AlCl3和FeCl3主要不是通过最终形成的水解产物吸附染料的方式去除染料的,即水解终产物的物理吸附对去除染料的贡献率不大。同时也说明混凝剂瞬间水解产生的形态具有较强的混凝效能,去除机制极有可能是混凝剂瞬间水解形态与染料直接发生化学结合,导致染料团聚而粒径增大。
改变CaCl2的投加顺序,与正常投加顺序的结果(图3)对比,没有太大差异。正常投药方式下,CaCl2对3种染料的平均去除率为15.54%,改变投加顺序后,CaCl2对3种染料的平均去除率为12.93%。这是因为CaCl2在室温条件下的水解率非常低[19],无论是先投加还是后投加,产生的中间水解形态本身就较少(大部分以游离态Ca2+形态存在),因此,对染料去除率均不高。其他少量Ca2+直接转为氢氧化物,该产物可能对染料具有一定的吸附作用,从而也对染料产生一定的去除效能。
总之,3种混凝剂最终水解产物通过吸附作用对染料的去除率最大不超过25%,由此可见,水解终产物的物理吸附不应是导致分散染料被混凝去除的主要原因。
-
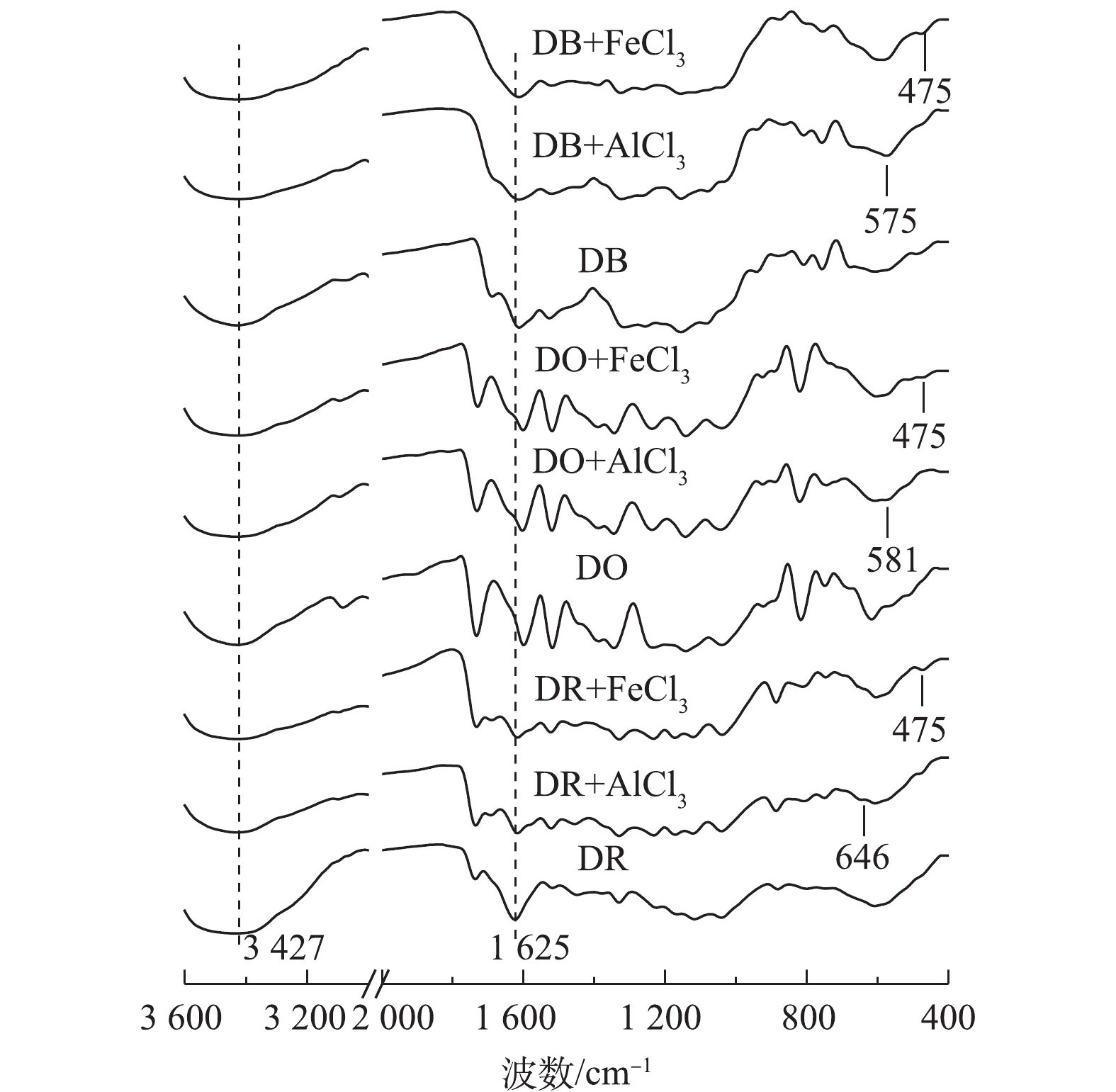
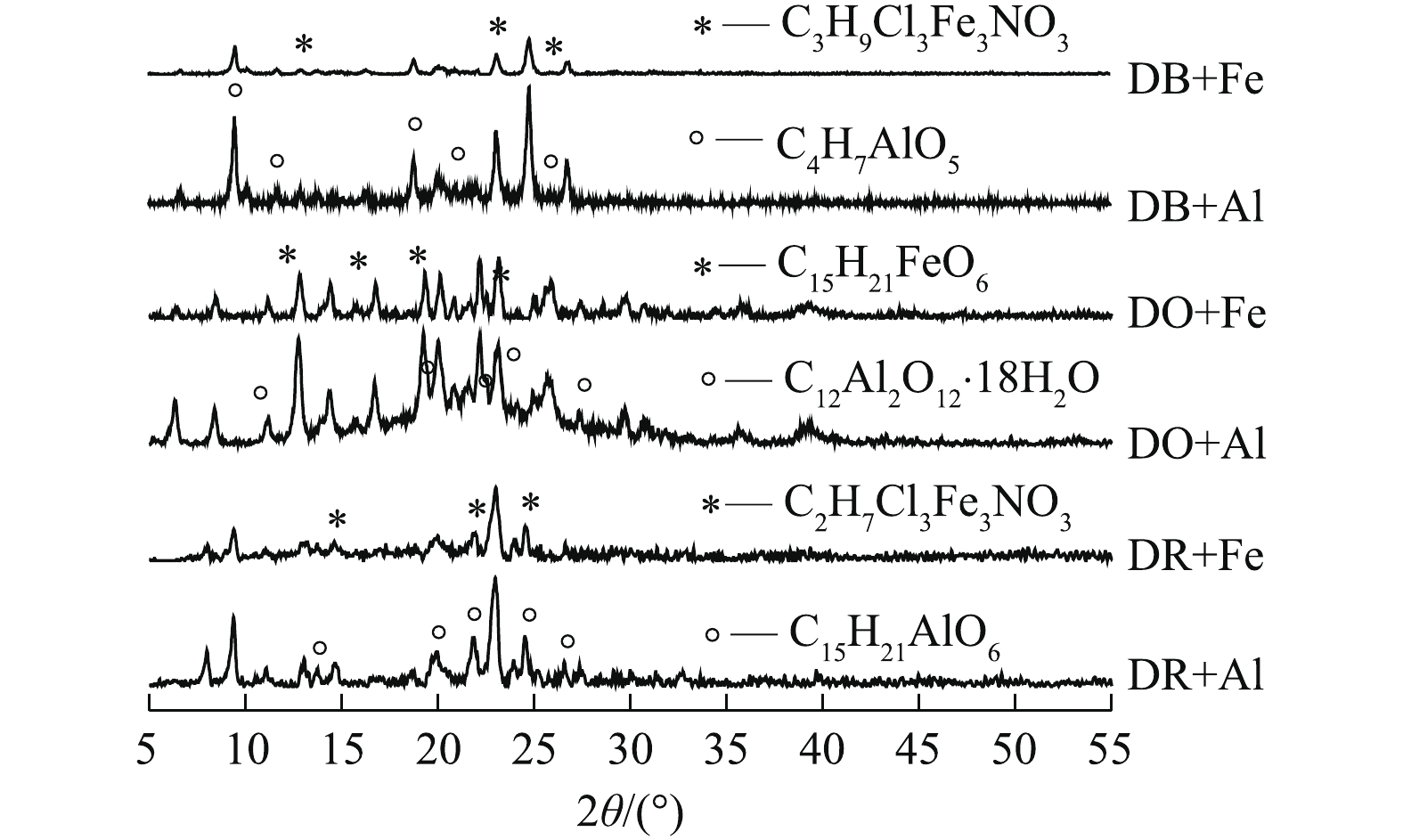
为进一步明确染料与混凝剂(AlCl3和FeCl3)结合的内在作用机理,对投加AlCl3、FeCl3后产生的沉淀物经离心、真空干燥等步骤收集后,测定其傅里叶红外光谱。图8分别给出了单独染料(分散红玉、分散黄棕和分散蓝)及其与AlCl3和FeCl3混凝反应形成的沉淀物的红外图谱。比较染料与沉淀物的图谱发现,沉淀物在3 427 cm−1的O—H伸缩振动峰[20]和1 625 cm−1的O—H弯曲振动峰[21]位置基本不变,2处峰强略微有减弱,这可能是由于AlCl3和FeCl3与染料结合导致氢键发生改变所致。比较染料(DR, DO, DB)与投加FeCl3形成的沉淀物(DR+FeCl3, DO+FeCl3, DB+FeCl3)光谱发现,沉淀物在475 cm−1出现Fe—O特征吸收峰[22-23],比较染料(DR, DO, DB)与投加AlCl3形成的沉淀物(DR+AlCl3, DO+AlCl3, DB+AlCl3)光谱发现,沉淀物在580 cm−1左右出现Al—O特征吸收峰[24-25]。这说明AlCl3和FeCl3发生了化学作用,生成了新的基团,这可能是AlCl3和FeCl3对3种染料去除率较高的主要原因。另外,染料与FeCl3形成的沉淀物产生的吸收峰峰强较染料与AlCl3形成的沉淀物吸收峰峰强更强,这个结果与混凝实验的结果(FeCl3对染料的去除率优于AlCl3)基本一致。
结合FT-IR结果,可以看出,染料与AlCl3形成的沉淀物中含有Al—O基团,与FeCl3形成的沉淀物中含有Fe—O基团,进一步通过XRD对沉淀物进行定性分析(图9)。与标准卡片比对后发现,投加AlCl3形成的沉淀物的分子式中都含有Al、O元素,投加FeCl3形成的沉淀物的分子式中都含有Fe、O元素,这表明沉淀物是AlCl3和FeCl3与染料聚合形成的新的化合物,而不是简单的混合物,这个结果与红外结果总体一致。
FT-IR和XRD表征结果共同验证了之前的推测,即这3种分散染料被AlCl3和FeCl3去除的主要机制应是染料与混凝剂发生了特定的化学结合作用。
2.1. 分散染料的基本混凝去除特征
2.2. 单一pH变化对染料去除率的影响
2.3. 染料Zeta电位变化与去除率的关系
2.4. 混凝前后染料粒径的变化
2.5. 单一吸附作用对染料去除率的影响
2.6. 沉淀物表征分析
-
1) Zeta电位的分析结果表明,CaCl2可达到甚至超过AlCl3和FeCl3的电中和能力,但对3种染料的平均去除率最低(15.54%),表明电中和作用不是导致分散染料被直接混凝沉淀去除的主要原因或充分条件。
2)各染料溶液投加3种混凝剂后,体系颗粒粒径明显增大,说明染料与混凝剂发生了某种结合作用。改变混凝剂投加顺序的实验结果表明,混凝剂最终水解产物对染料的吸附能力均较弱,这说明水解终产物的物理吸附不应是导致分散染料被直接混凝沉淀去除的主要原因。
3)沉淀物的FT-IR和XRD图谱进一步表明这3种分散染料被AlCl3和FeCl3去除的主要机制应是染料与混凝剂发生了特定的化学结合,生成了新的易沉降的共聚物。





 下载:
下载: