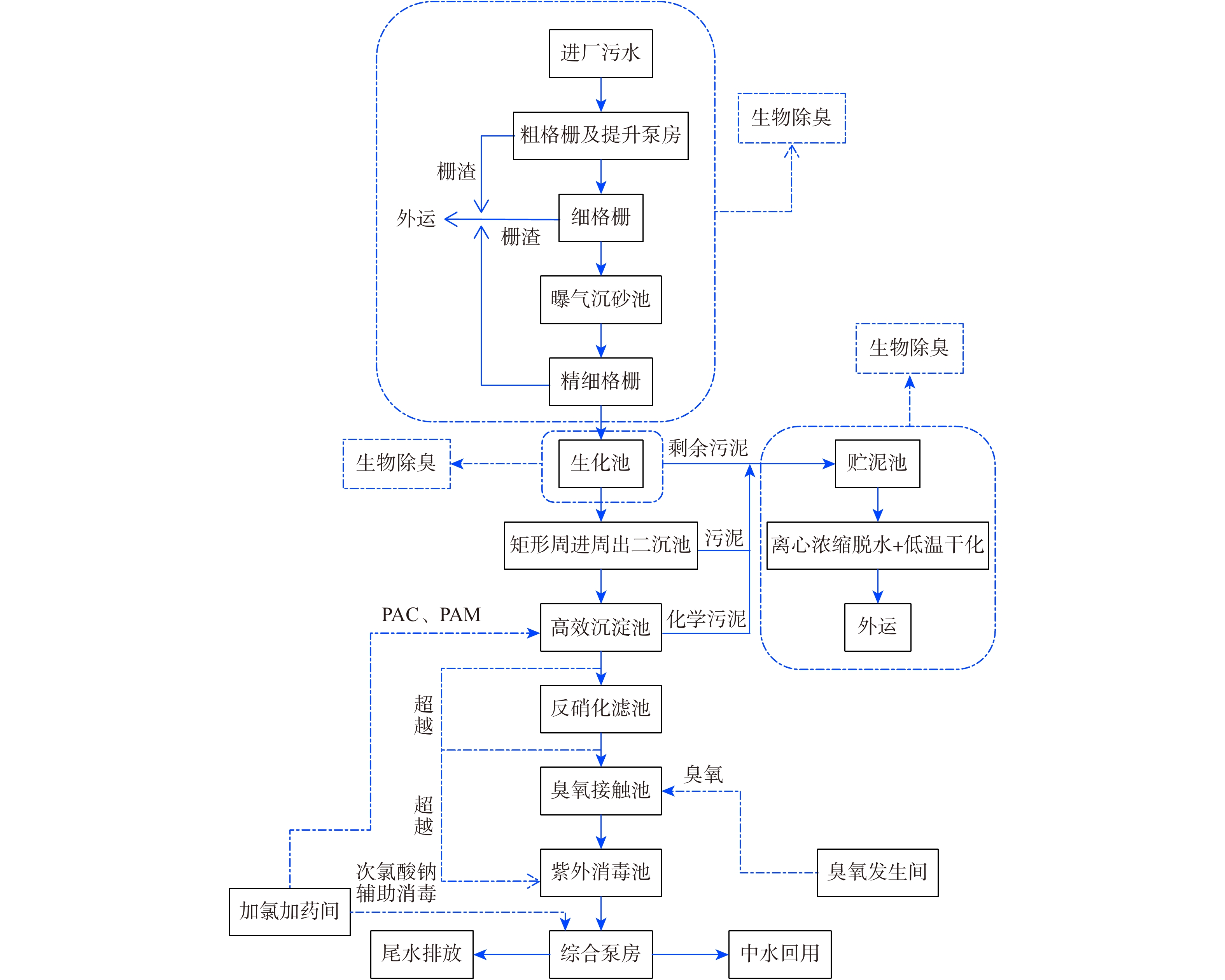
-
在我国,农药被大量生产和广泛使用,随之产生了严重的环境污染问题。有机磷杀虫剂是一类常用农药,在我国地表水和地下水中常被检出[1-4]。乐果作为一种典型的有机磷类农药,不仅污染土壤、水体,而且对动植物和人类的健康也造成潜在危害,因此,去除饮用水中的微量污染物乐果具有重要的现实意义。
传统的饮用水处理流程包括预氧化、混凝/沉淀、过滤、消毒等工艺,难以去除水中微量的乐果[5]。有些研究采用臭氧氧化法[6-7]、纳米TiO2光催化氧化法[8-9]、芬顿(Fenton)氧化法[10-11]、超声波降解法[12-13]等技术去除水中的乐果,虽有一定的效果,但这些方法在实际应用中仍存在一些局限性,如降解效率低、光量子产率低、催化剂难再生、能耗大等,无法高效、经济地对水中有机磷农药进行去除[14]。近年来,有研究[15]发现,真空紫外(VUV)及其组合工艺对水中微量有机污染物具有高效的去除能力,且与其他高级氧化工艺(AOPs)相比,具有低能耗、低成本等优点[16],因而日益受到研究者的关注。采用此法降解全氟辛酸、农药涕灭威、甲草胺、氯烯酮等[17-18],均取得较好的效果。
本研究将VUV/UV新型光源辐照与饮用水常用消毒剂Cl2相结合,构建了VUV/UV/Cl2工艺,考察其对饮用水中乐果的去除效果,以期为饮用水中难降解微量污染物的高效去除提供参考。
全文HTML
-
乐果(分析纯)购自美国Sigma-Aldrich公司;乙腈(ACN,色谱纯)购自比利时Fisher Scientific公司;次氯酸钠(NaOCl)溶液、磷酸二氢钠(NaH2PO4)、氢氧化钠(NaOH)、硝酸钠(NaNO3)、氯化钠(NaCl)、碳酸氢钠(NaHCO3)、硫酸钠(Na2SO4)、亚硫酸氢钠(NaHSO3)等试剂(均为分析纯)和邻苯二甲酸氢钾(C8H5KO4,优级纯)均购自北京国药集团化学试剂有限公司;天然有机物(NOM)购自天津津科精细化工研究所;所有溶液都使用由Milli-Q设备(Advantage A10, Millipore)制备的超纯水配制。
-
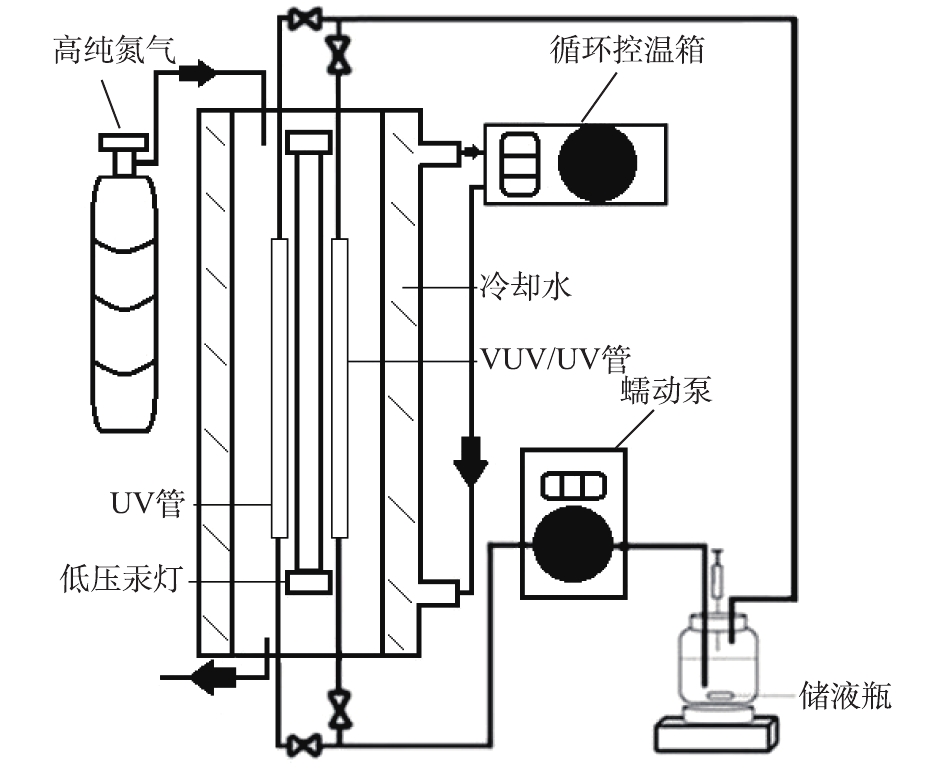
实验所用VUV/UV细管流反应装置[19]如图1所示。装置的主体部分是一个圆柱体双层石英玻璃光反应器。低压汞灯置于反应器内部,功率为8 W,可同时发射VUV(185 nm)和UV(254 nm)光。UV管仅能透过UV,管内水样只受到UV辐射;而VUV/UV管能同时透过2种波长的紫外光,管内水样可以受到VUV/UV组合辐射。采用化学剂量法分别测定了UV辐照强度(以尿苷和阿特拉津为感光剂)和VUV辐照强度(以甲醇为反应物),其值分别为14.5 mW·cm−2和1.75 mW·cm−2[20]。冷却水通过2层石英玻璃之间的外室循环,以控制光反应器的温度,确保稳定的VUV/UV输出。高纯氮气通入反应腔体以排出空气,避免内部空气吸收VUV产生臭氧。蠕动泵将水样连续注入装置辐射部分(即VUV/UV和UV细管)。
整个反应装置的温度控制在(25 ± 1) ℃,溶液pH用5 mmol·L−1磷酸盐缓冲液控制。每次实验开始前,低压汞灯都先预热10 min;反应开始后,在预定的时间间隔取样分析。所有实验均平行2次,所得结果的相对百分比误差(RPD)都在10%以内。
-
乐果浓度通过高效液相色谱(HPLC,Agilent 1200)进行测定,采用紫外二极管阵列检测器(DAD),检测波长210 nm,色谱柱为安捷伦C18柱(150 mm × 2.1 mm,3 μm),柱温40 ℃,每次进样量100 μL。流动相为1∶1的ACN和H2O的混合液,流速1 mL·min−1。在色谱图上,乐果的出峰时间约为3.5 min。
总有机碳(TOC)采用TOC分析仪(TOC-VCPH,Shimadzu)测定。余氯/总氯通过Hach水质分析仪(DR 6000)测定,采用USEPA DPD方法(Method 8167),测量范围为0.02~2.00 mg·L−1。
1.1. 实验材料
1.2. 实验装置和反应条件
1.3. 检测方法
-
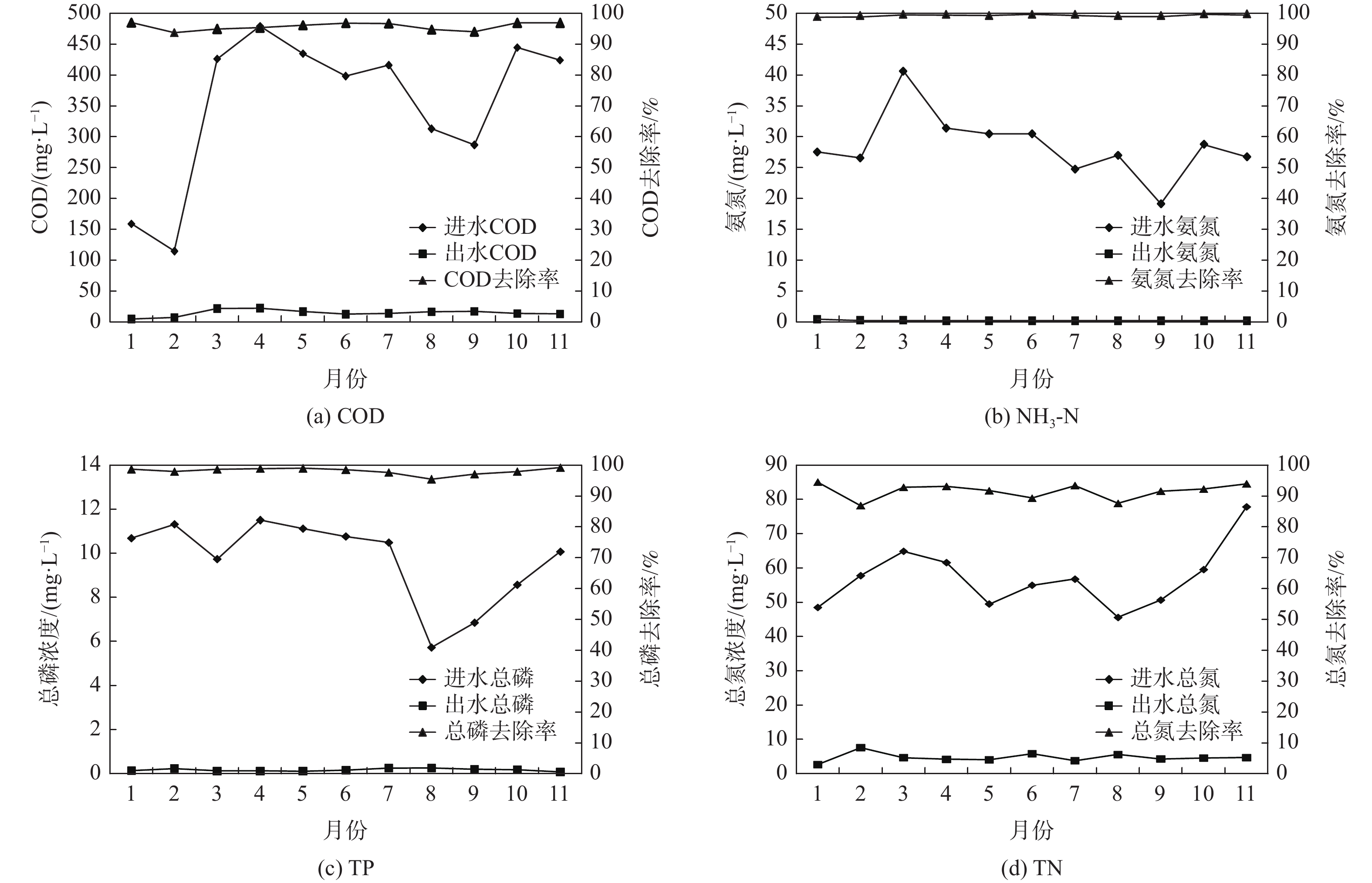
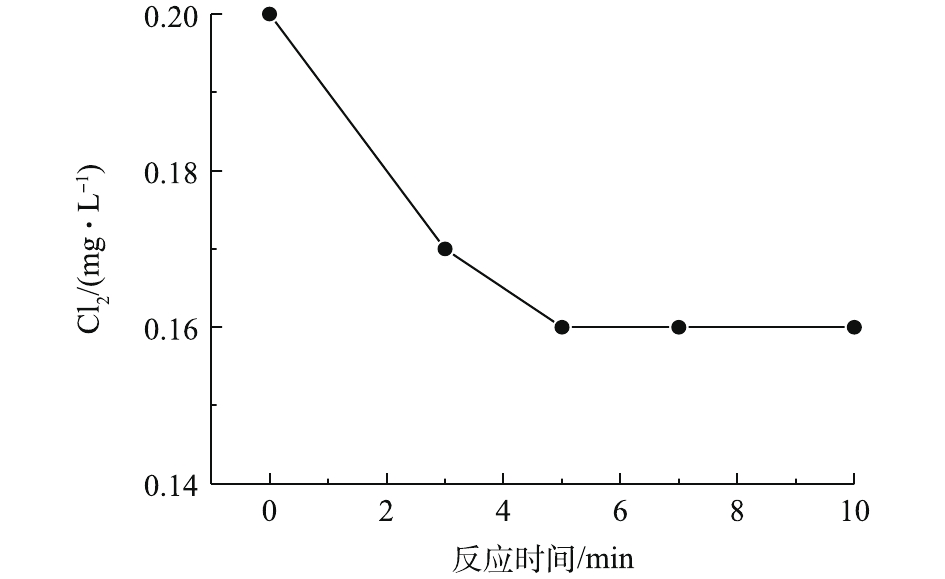
首先,在pH为7.0、乐果初始浓度为5.0 mg·L−1、Cl2投加量为0.2 mg·L−1(通过投加一定浓度的NaOCl溶液得到)的反应条件下,考察样品中Cl2浓度的变化。由图2可见,前5 min内,Cl2浓度快速下降,这主要是因为Cl2(即HOCl/OCl−)能够通过氧化、加成和亲电取代等作用与乐果反应[21];随着乐果中的活性基团被消耗,5 min后,Cl2浓度基本稳定在0.16 mg·L−1左右。该实验结果说明,反应过程中始终有Cl2的存在,如果外加VUV/UV辐照,可以形成VUV/UV/Cl2工艺。
-
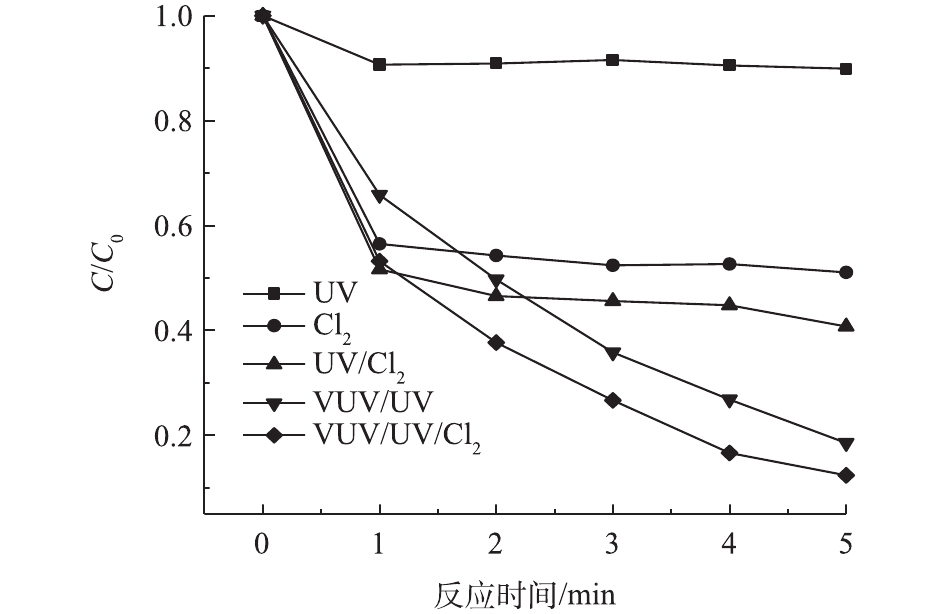
乐果在UV、Cl2、UV/Cl2、VUV/UV和VUV/UV/Cl2 5种处理工艺中的降解情况如图3所示。结果表明,直接UV光降解对乐果的去除作用十分有限,在整个反应时间(5 min)内,仅去除10%左右。乐果在Cl2作用下表现出瞬时的降解效果,在前1 min内,就去除了43.5%,但后续反应缓慢,5 min后,其去除率仅为49.0%。UV/Cl2对乐果的降解效率略优于Cl2,降解趋势也类似。相比之下,VUV/UV对乐果的降解效率有明显的提高,且表现出持续降解作用,这是由于水吸收VUV光子后发生裂解,能持续生成HO·和H·[22];HO·具有非常强的氧化能力,从而能够快速去除乐果。在VUV/UV辐照下投加Cl2,使得HO·被更有效地利用,形成较长寿命的次级自由基·OCl[23],可进一步提高乐果的降解速率。因此,乐果在5种处理工艺中的降解效率依次为VUV/UV/Cl2 > VUV/UV > UV/Cl2 > Cl2 > UV。
-
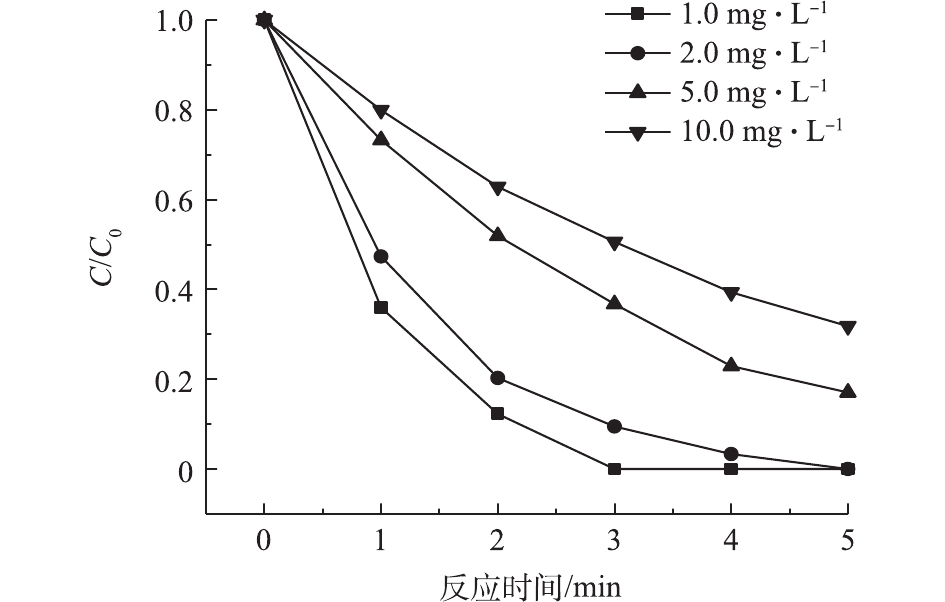
常温(25 ℃)下,乐果在水中的溶解度可达39 g·L−1,属易溶物质。为此,考察了不同较高初始浓度的乐果在VUV/UV/Cl2工艺中的降解情况,结果如图4所示。乐果的降解速率随着其初始浓度的增加而降低;经过5 min的反应,初始浓度为1.0、2.0、5.0、10.0 mg·L−1的乐果去除率分别为100%、100%、82.9%和68.2%。由前述结果可知,UV或VUV直接光降解对乐果的去除作用很小,因此,乐果的降解主要依靠VUV光解水产生的HO·和投加Cl2后产生的氯自由基。当VUV/UV的辐照强度一定时,高活性自由基在水中处于一种低浓度的准平衡状态[24],因此,当乐果的初始浓度增加时,其降解效率自然会下降。
-
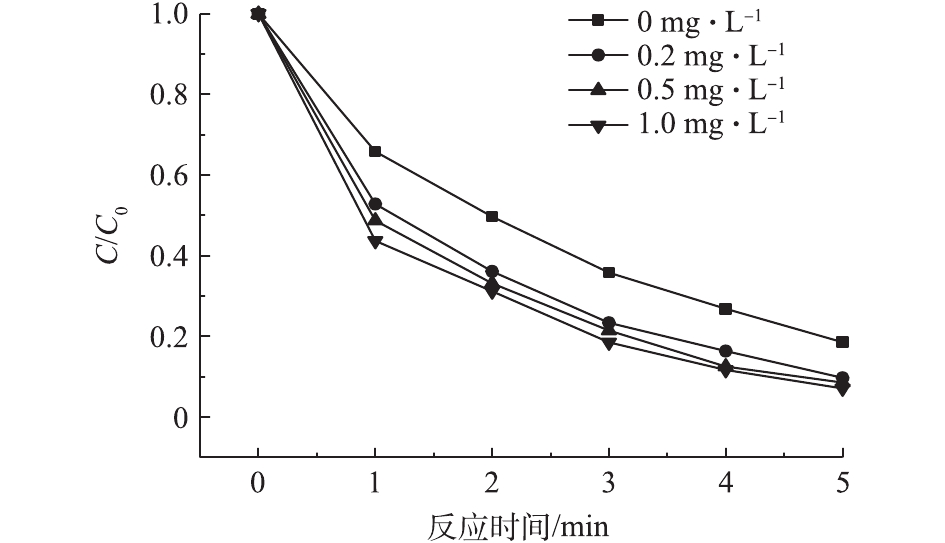
在pH为7.0、乐果初始浓度为5 mg·L−1的条件下,当Cl2投加量分别为0、0.2、0.5、1.0 mg·L−1时,VUV/UV/Cl2工艺对乐果的降解效果如图5所示。随着Cl2投加量的增加,乐果的降解率有一定的提升。当Cl2投加量从0 mg·L−1增加到1.0 mg·L−1时,在5 min内,乐果的去除率从81.5%增加到92.9%,这与反应体系中HOCl的量子产率有关;随着Cl2投加量的增加,HOCl的量子产率增加[25],从而提高了乐果的降解速率。由图5还可看出,当Cl2投加量大于0.2 mg·L−1时,降解效率提升幅度很有限。
-
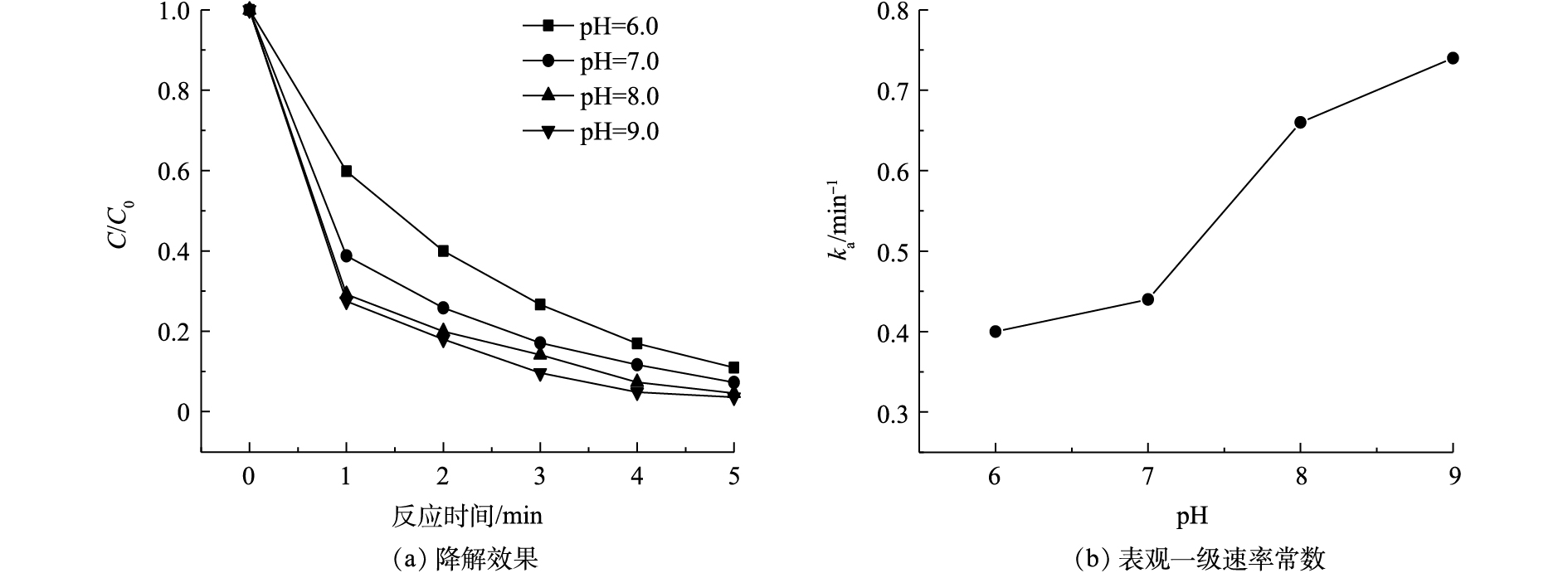
在乐果初始浓度为5 mg·L−1、Cl2投加量为0.2 mg·L−1的条件下,pH对VUV/UV/Cl2工艺降解乐果的影响如图6(a)所示。乐果的降解速率随着pH的升高而增大,在pH为9.0时,经过5 min的反应,乐果的去除率可达96.4%。乐果在水中的离解常数(pKa)分别为−0.44和16.6[26],因此,在本研究pH范围内,乐果为中性分子,不受溶液pH的影响。由于常温下HOCl的pKa = 7.5,所以碱性条件更有利于OCl−的生成(见式(1)),从而产生更多的高活性自由基HO·和Cl·[23, 27-28](见式(2)~式(4))。
这些高活性自由基对乐果的降解起到促进作用,因此,当pH从6.0升高到9.0时,乐果的表观一级降解速率常数(ka)从0.40 min−1逐渐上升到0.74 min−1,如图6(b)所示。
-
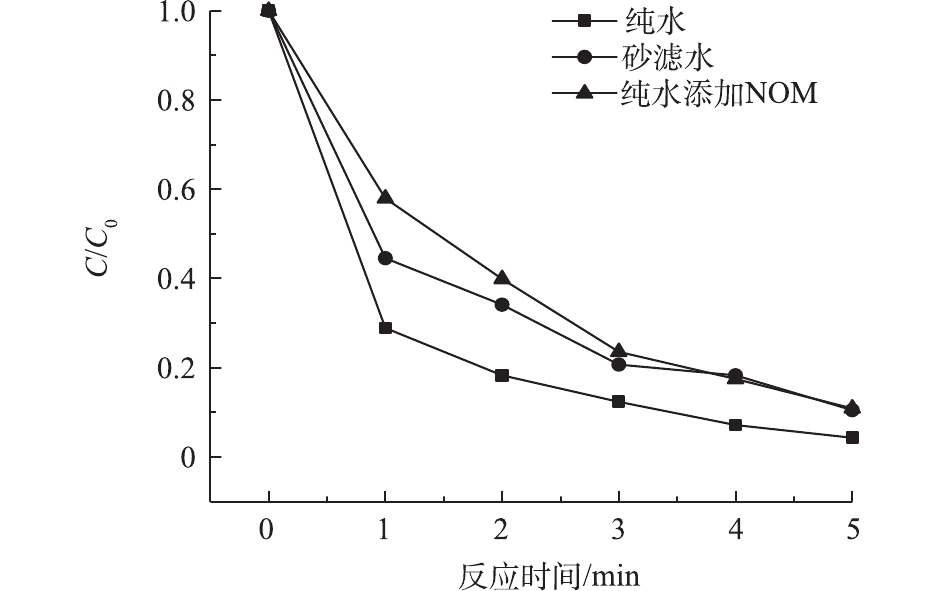
NOM具有复杂的化学结构和较强的还原性[29],通常会竞争HO·,从而抑制目标污染物的降解。在乐果初始浓度为5 mg·L−1、Cl2投加量为0.2 mg·L−1、pH为7.0的条件下,对比了乐果在纯水、北京某自来水厂砂滤水(pH = 7.08、TOC = 3.2 mg·L−1)、添加3.0 mg·L−1 NOM的纯水3种体系中的降解效率。图7表明,在VUV/UV与Cl2的协同作用下,即使水中的背景有机物产生一定的干扰,乐果的降解效率依然十分显著。
-
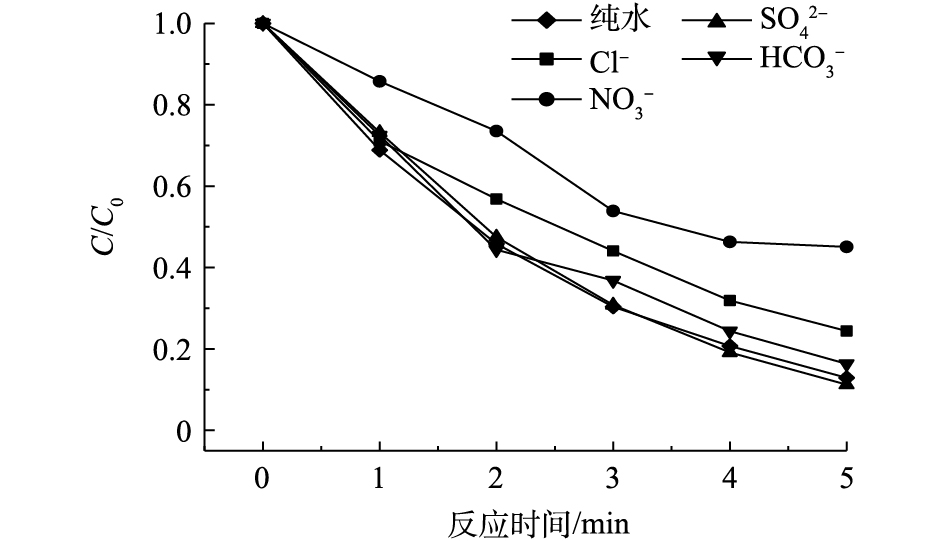
无机阴离子在水源水中普遍存在。在pH为7.0、乐果初始浓度为5 mg·L−1、Cl2投加量为0.2 mg·L−1的条件下,分别添加100 mg·L−1的
NO−3 、Cl−、HCO−3 和SO2−4 ,考察其对VUV/UV/Cl2降解乐果的影响。由图8可见,在没有添加任何无机阴离子的纯水中,乐果的去除率可达87.1%。NO−3 可以捕获HO·,也可通过VUV/UV辐照分解产生NO−2 来捕获HO·[30] (见式(5)~式(7))。这些次生的NO3·和H
NO−3 ·的氧化能力都较弱,因此,NO−3 的存在对乐果的降解起到了较大的抑制作用,反应结束时,乐果的去除率仅为54.9%。同样,Cl‒也可通过捕获HO· (见式(8))来抑制乐果的降解。
因此,在Cl‒存在情况下,乐果的去除率也有所降低(75.6%)。
HCO−3 作为一种常见的HO·捕获剂,在VUV/UV/Cl2降解乐果过程中,仅产生轻微的抑制作用。相比之下,SO2−4 因其捕获HO·的反应非常缓慢[31],对乐果的降解无抑制作用。在SO2−4 存在的情况下,乐果的去除率达到88.8%,与纯水中乐果的去除率几乎相同。
2.1. 暗反应过程中Cl2的衰减
2.2. 乐果在不同处理工艺中的降解效率对比
2.3. 乐果初始浓度的影响
2.4. Cl2投加量的影响
2.5. pH的影响
2.6. 水中共存NOM的影响
2.7. 水中共存无机阴离子的影响
-
1)VUV/UV/Cl2工艺对乐果的降解效率最高,明显优于UV、Cl2、UV/Cl2和VUV/UV。VUV/UV/Cl2工艺对乐果的降解速率随着乐果初始浓度的增加而减小,随着Cl2投加量或pH的增加而增大。
2)在VUV/UV/Cl2工艺中,水中共存NOM对乐果的降解有一定的抑制作用,但并不明显。水中共存无机阴离子
NO−3 、Cl−和HCO−3 对乐果的降解有抑制作用,依次为NO−3 > Cl− >HCO−3 ,而SO2−4 无抑制作用。

















 下载:
下载: