-
芳香胺类化合物广泛应用于染料、杀虫剂、橡胶制品及树脂等化工产品的生产合成中。这些产品的降解及生产废水的排放都会导致芳香胺释放到环境中[1]。由于芳香胺类化合物具有生物毒性,会造成肝肾等器官的损伤及“三致效应”[2]。因此,芳香胺类化合物亟待得到有效处理。
芬顿高级氧化法是一种高效处理芳香胺废水的方法,H2O2与亚铁离子(Fe2+)反应产生的HO·具有较高的氧化电位,可有效降解芳香胺类化合物。但传统的芬顿反应必须在酸性条件下进行,且须持续不断地投加芬顿试剂(Fe2+、H2O2),导致其成本较高。AHMADI等[3]利用PAC@Fe(Ⅱ)Fe2(Ⅲ)O4做催化剂构建光/电芬顿反应,使苯胺的降解率可达到93.8%,但该过程同样须引入光照并持续投加H2O2。为此,SEKAR等[4]利用微生物好氧呼吸产生的H2O2及铁还原菌厌氧还原Fe3+产生的Fe2+构建芬顿反应,并通过厌氧-好氧循环来不断地产生Fe2+和H2O2。然而,微生物好氧呼吸产生的H2O2非常有限。据研究[5-6],真菌G. trabeum胞外分泌的2,5-二甲氧基-1,4-氢醌和4,5二甲氧基-1,2-氢醌与Fe3+反应,产生Fe2+和醌自由基,后者与氧气反应产生H2O2,从而构建芬顿反应以降解聚乙二醇。然而,真菌生长速率缓慢,且产生的氢醌浓度也非常有限。KEREM等[5]研究表明,在有氧条件下,厌氧生物还原后的醌化合物可以自氧化产生醌自由基,进而与氧气发生反应,生成H2O2。
基于上述研究,本研究采取外加醌类化合物的方法来提高H2O2的产生量,在厌氧条件下,醌类化合物能够作为氧化还原介体[7]提高Fe3+还原为Fe2+的速率,从而加快芬顿反应的构建;为使醌化合物能够重复利用,采用化学方法将蒽醌-2-磺酸(AQS)固定在大孔聚氨酯泡沫上,来构建固定化醌耦合生物驱动的芬顿反应,并研究了该反应对苯胺和难生物降解的磺酸萘胺的降解性能。
全文HTML
-
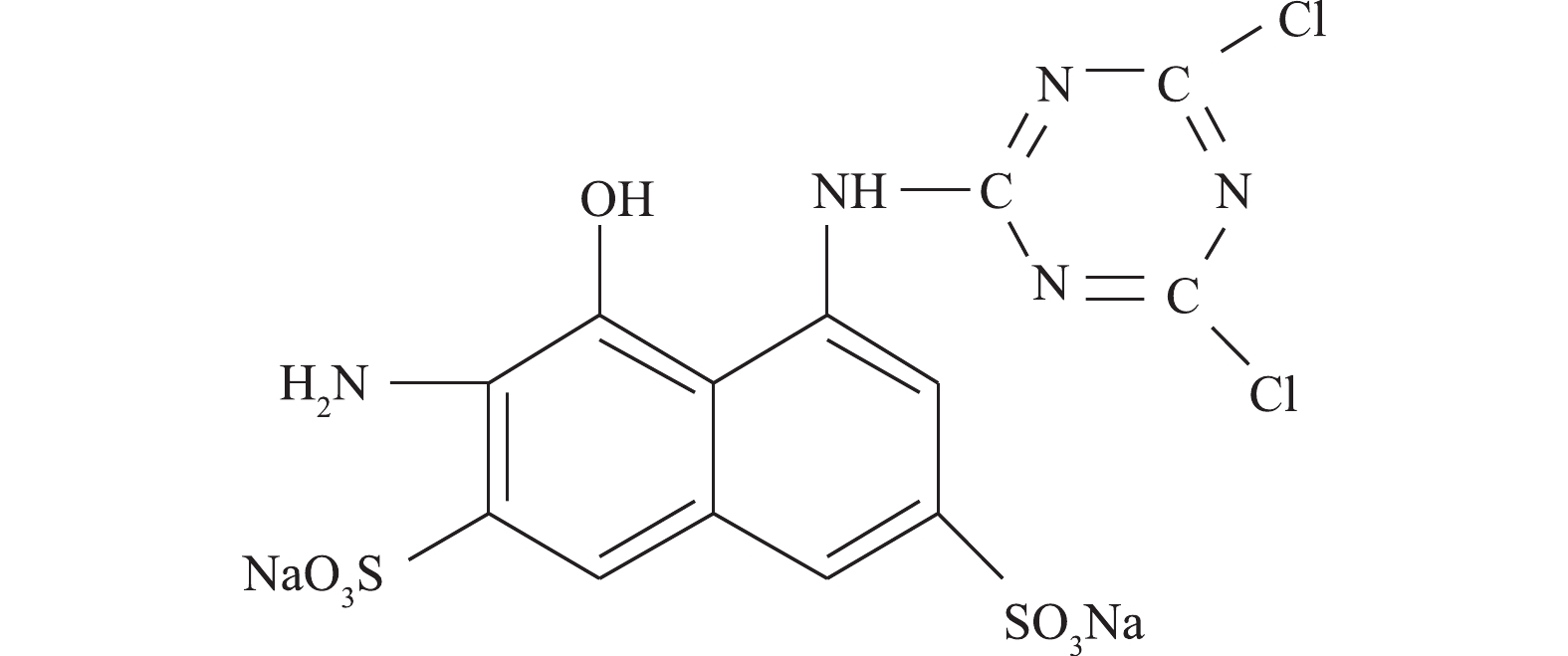
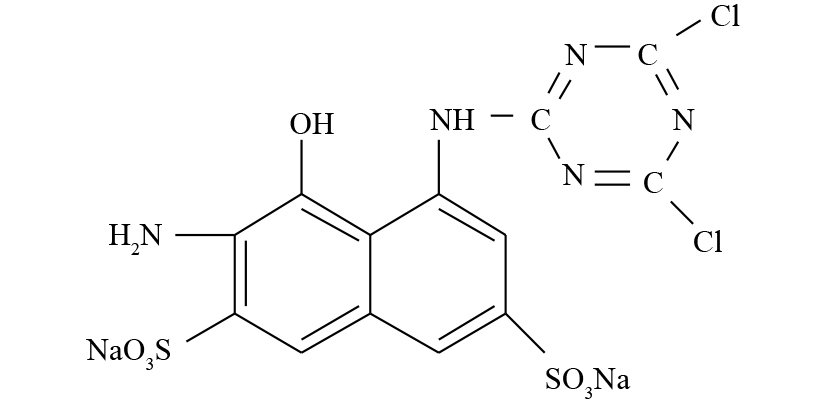
苯胺购自美仑生物科技公司;2-氨基-8-N-(4, 6-二氯-1, 3, 5-三嗪-2-基)氨基-1-萘酚-3,6-二磺酸钠盐(ADCTNDS)为本实验室制备提纯(结构式见图1);蒽醌-2-磺酸钠盐(AQS)购于美国Sigma有限公司;大孔聚氨酯泡沫(PUF)来自大连博多科技开发有限公司(1 cm × 1 cm × 1 cm),其比表面积为18 000 m2·m−3。液相色谱所用甲醇等均为色谱纯;其他试剂均为分析纯。实验用菌株Shewanella sp. ROs-106由本实验室分离纯化,活化培养基和无机盐培养基不变[8]。
厌氧培养基为无机盐培养基加乳酸钠。采用化学方法将AQS固定到泡沫材料上[9-10]。通过元素分析可知,制成的醌改性大孔泡沫(AQS-PUF)中醌含量为(0.047 5 ± 0.019 0) mmol·g−1 PUF。采用傅里叶红外漫反射光谱法研究AQS-PUF反应前后表面基团的变化。
-
紫外-可见分光光度仪(UV-5500,上海元析仪器有限公司);化学发光仪(MPI-B型,西安瑞迈分析仪器有限责任公司);高效液相色谱(SIL-20A,岛津公司);电子顺磁共振(A200,德国布鲁克仪器公司);元素分析仪(Vario EL cube,德国元素分析系统公司);傅里叶红外光谱仪(EQUINOX55,德国布鲁克仪器公司)。
-
1) 菌株的活化与培养。从−80 ℃冰箱中取出菌株RQs-106,常温解冻后接入灭菌后的20 mL LB培养基,在30 ℃,150 r·min−1条件下培养5 h后,按1%的接种量将菌液接入100 mL LB培养基中扩大培养,12 h后,高速离心(10 000 r·min−1,5 min,4 ℃),弃去上清液,然后用磷酸盐缓冲液将细胞洗涤3次,并悬浮在磷酸盐缓冲液中待用。
2) 生物蒽醌法产H2O2条件优化。在125 mL血清瓶中,加入100 mL厌氧培养基以及一定浓度游离态的AQS,丁基胶塞封口后曝氮气15 min,钳口铝盖密封并于121 ℃下灭菌20 min,冷却至室温,然后在厌氧箱中将菌株ROs-106加入到血清瓶中培养。定时用注射器取样,并使样品与空气充分反应,最后测量样品中H2O2浓度。分别研究AQS浓度(0~0.20 mmol·L−1)、生物量(0~0.22 g·L−1)和乳酸钠浓度(0~60 mmol·L−1)对反应体系中H2O2产生量的影响。
3) 柠檬酸铁浓度对苯胺降解的影响。分别向125 mL血清瓶中加入100 mL厌氧培养基(乳酸钠 40 mmol·L−1)、制备的AQS-PUF泡沫0.5 g(0.20 mmol·L−1 AQS)和不同浓度柠檬酸铁(0~6.0 mmol·L−1),曝气灭菌方法同上,待冷却至室温后,于厌氧箱中加入0.16 g·L−1生物量,每间隔1.5 h取样,测量样品中Fe2+浓度,以确定厌氧反应需要持续时间。当厌氧反应结束后,好氧反应开始,当体系中Fe2+浓度低于25%时,好氧反应结束,这段时间为好氧反应所需时间。厌氧-好氧反应时间确定后,于不同浓度柠檬酸铁(0~6.0 mmol·L−1)体系中,加入初始浓度为50 μmol·L−1的苯胺,开始厌氧-好氧循环反应。厌氧反应条件为反应开始前曝氮气20 min,将瓶口用胶塞密封,放入厌氧箱30 ℃培养;好氧反应条件为将胶塞换为透气棉塞,于30 ℃,150 r·min−1摇床中培养。在好氧反应开始和结束时取样并测定水中苯胺浓度,研究不同浓度柠檬酸铁对苯胺降解的影响。
4) 芬顿反应的构建和芳香胺的降解。向125 mL血清瓶中加入100 mL厌氧培养基(40 mmol·L−1乳酸钠、0.5 g AQS-PUF(0.20 mmol·L−1 AQS)和4.5 mmol·L−1柠檬酸铁),曝气灭菌方法同上,待冷却至室温后,于厌氧箱中加入0.16 g·L−1生物量和初始浓度为50 μmol·L−1的苯胺和ADCTNDS,开始厌氧-好氧循环(21 h/3 h)反应;同时,以无柠檬酸铁或者无生物量作为空白对照实验,其他反应条件相同。在好氧反应开始和结束时取样,测量样品中2种芳香胺的浓度。
-
细胞浓度采用分光光度法检测;H2O2浓度采用鲁米诺化学发光法[11]检测;Fe2+浓度采用Ferrozine方法检测;苯胺的检测方法为高效液相色谱法(分析条件:甲醇∶水 = 40∶60,UV检测波长为280 nm,色谱柱为Sapphire C18(尺寸:4.6 mm × 250 mm × 5 μm),流速为1.0 mL·min−1,柱温为40 ℃);ADCTNDS检测方法为高效液相色谱法(分析条件:甲醇∶乙酸铵水溶液(0.385 g·L−1) = 60∶40,检测波长为462 nm,流速为0.5 mL·min−1,其他测定条件同苯胺);反应体系中菌株RQs-106细胞数量采用细菌菌落计数法检测;体系中羟基自由基采用电子自旋共振法(以80 mmol·L−1二甲基吡咯啉氮氧化物(DMPO)捕捉水样中的HO·)检测。
1.1. 实验原料
1.2. 实验仪器
1.3. 实验方法
1.4. 分析方法
-
生物还原AQS为AH2QS的反应,通常较慢,而AH2QS与氧气接触产生H2O2的过程是化学反应过程,反应速率非常快,因此,AQS的厌氧还原过程是限速步骤。在这一过程中,AQS浓度、初始生物量和碳源浓度是影响H2O2产生量和产生速率的重要因素。因此,本研究以乳酸钠作为碳源,研究了AQS浓度、初始生物量和碳源浓度对H2O2产生量和产生速率的影响,结果如图2所示。
AQS作为产H2O2反应的主要反应物,其浓度对H2O2产量有直接影响[12]。考虑到固定化AQS浓度有限以及泡沫体积的影响,反应体系中添加的固定化AQS浓度最大为0.20 mmol·L−1。0~0.20 mmol·L−1 AQS浓度对H2O2产生量的影响如图2(a)所示,可以看出,反应体系中H2O2产量随AQS浓度增大而增大,当AQS浓度为0.20 mmol·L−1,H2O2的产生量最大。
图2(b)表明,随着初始生物量的增加,H2O2的产生量逐渐增高,当初始菌浓度为0.16 g·L−1时,在11 h内,生成了44.9 μmol·L−1 H2O2;但当生物量继续增加到0.22 g·L−1时,虽然在反应开始阶段H2O2的产生速率较快,但是之后速度明显降低,推测可能是大量的微生物代谢消耗了作为电子供体的乳酸钠,因此,最合适的生物量为0.16 g·L−1。
图2(c)表明,乳酸钠浓度为0~60 mmol·L−1时,随乳酸钠浓度增大,H2O2产生量也增大,60 mmol·L−1乳酸钠体系在9 h产生了55.0 μmol·L−1的H2O2;但在随后的反应中,H2O2浓度随反应时间大幅降低。相比较,40 mmol·L−1乳酸钠体系在9 h产生的53.4 μmol·L−1 H2O2与60 mmol·L−1体系产量相差不大且较为稳定,因此,选择乳酸钠浓度为40 mmol·L−1。
综上可知,本研究中用生物还原AQS的方法产生的H2O2为53 μmol·L−1左右,高于MCKINZI等[13]构建生物驱动的芬顿反应中H2O2的产生量30 μmol·L−1,且远小于会对细胞造成毒性的浓度(1 mmol·L−1)[14],因此,采用0.20 mmol·L−1 AQS、0.16 g·L−1生物量和40 mmol·L−1乳酸钠作为最优条件用于H2O2的制备。
-
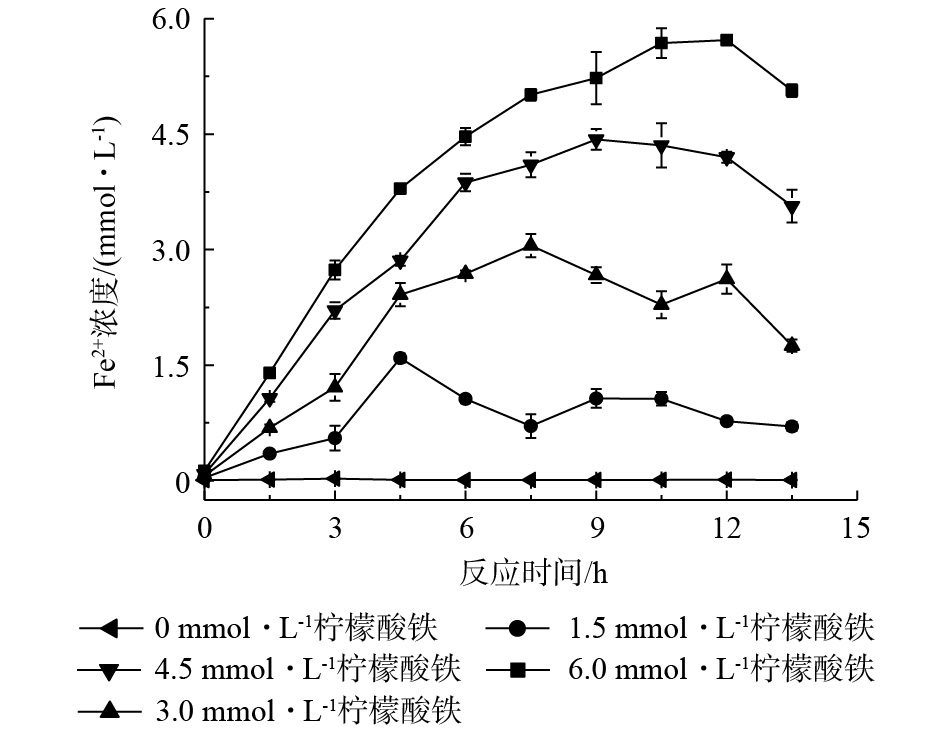
固定化AQS耦合生物驱动的芬顿反应的厌氧反应具体分为2个阶段。第1阶段为Fe2+的制备,菌株RQs-106作为铁还原菌,在厌氧条件下以乳酸钠为电子供体,将柠檬酸铁中的Fe3+还原为Fe2+,同时,AQS-PUF的存在能够加速这一反应过程[7]。由图3可以看出,当柠檬酸铁为0~6.0 mmol·L−1时,体系中Fe2+浓度达到最大所需的时间为12 h。第2阶段为氢醌的制备,Fe3+完全还原为Fe2+后,菌株RQs-106作为AQS还原菌,在厌氧条件下,以乳酸钠为电子供体,可以将AQS-PUF还原为AH2QS-PUF,这一阶段反应需要12 h(图2(c))。然后开始进行好氧反应,AH2QS-PUF自氧化产生H2O2和Fe2+构建芬顿反应,进而降解苯胺,结果如图4所示。可以看出,苯胺去除率随柠檬酸铁浓度的增大而增大,4次厌氧-好氧循环(24 h/3 h)后,在无柠檬酸铁的反应体系中,苯胺的去除率为12.8%;在无生物量的反应体系中,苯胺的去除率仅为7.6%,即泡沫对苯胺的吸附量占7.6%。由此可见,空白对照组中苯胺浓度的降低主要为泡沫材料对苯胺的吸附所致。当柠檬酸铁浓度为4.5 mmol·L−1和6.0 mmol·L−1时,反应体系中苯胺去除率可分别达到46.2%和45.3%。由此可见,4.5 mmol·L−1柠檬酸铁体系对苯胺的去除率最大。由于4.5 mmol·L−1柠檬酸铁体系中的Fe2+浓度在9 h达到最大,因此,厌氧反应第1阶段的反应时间设为9 h。从图2可知,第2阶段时间为12 h,在好氧阶段,当体系中Fe2+浓度低于总铁含量的25%时,好氧反应结束,这段时间为3 h。综上可知,在后续反应中,厌氧-好氧循环的时间为21 h/3 h。
-
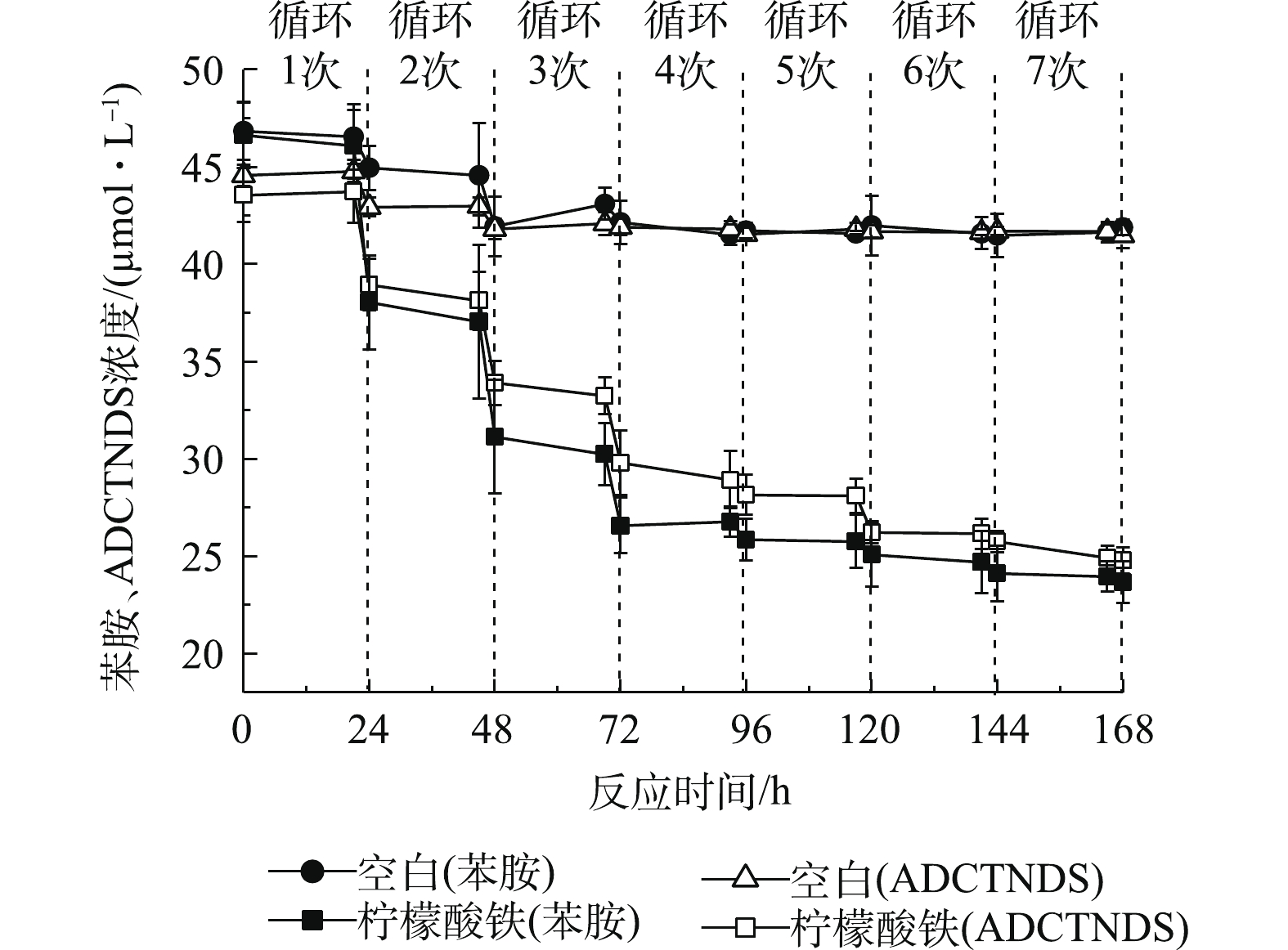
偶氮染料X-3B的脱色产物为苯胺和ADCTNDS。本研究以这2种芳香胺为模型化合物,苯胺稍溶于水,ADCTNDS含有磺酸基团,使其易溶于水,难于生物降解。据报道[15],芬顿反应的非特异性使其对多种芳香胺均有较好的降解效果,因此,须继续研究AQS-PUF耦合生物驱动的芬顿反应对这2种芳香胺的降解。在最适条件下,添加0.20 mmol·L−1固定化AQS、4.5 mmol·L−1柠檬酸铁、50 μmol·L−1的苯胺和ADCTNDS,结果如图5所示。可以看出,经过7次厌氧-好氧循环后,苯胺去除率为48.6%(无柠檬酸铁的反应体系中苯胺去除率仅为10.0%,其中,AQS-PUF对苯胺的吸附量占6.6%),ADCTNDS的去除率为43.3%(无柠檬酸铁的反应体系中ADCTNDS去除率仅为7.3%,其中,AQS-PUF对ADCTNDS的吸附量占4.3%)。其主要的降解反应发生在前4次循环中,苯胺和ADCTNDS在前4次循环中的去除率分别达到了44.6%和35.4%。在无柠檬酸铁体系中,对应的去除率分别为6.9%和6.8%。苯胺和ADCTNDS的去除率在后3次循环中有所下降。为了探究其原因,以无芳香胺体系作为空白对照,检测了7次循环体系中的Fe2+、H2O2和菌株RQs-106活细胞数目变化,结果如图6(a)所示。在7次循环反应过程中,厌氧阶段结束后产生的Fe2+的浓度均能维持在3.5~4.5 mmol·L−1,好氧阶段结束后,Fe2+浓度在芬顿反应和空气中O2氧化的作用下降低至0~1.5 mmol·L−1,芳香胺的存在对Fe2+的生成量和消耗量几乎没有影响。但是,在最后3次循环中,Fe2+的生成量和消耗量低于前4次的循环。由图6(b)可以看出,7次循环中AH2QS-PUF自氧化产生的H2O2浓度维持在25 μmol·L−1以上,最高可达42.9 μmol·L−1。与空白对照相比,有芳香胺存在的体系,H2O2的消耗量更大一些,这表明H2O2参与了芳香胺的降解。同样,在最后3次循环中,双氧水的产生量也低于前4次的循环。为了探究后3次循环苯胺去除率降低的原因,对反应体系中的生物量进行测量。在前4次循环中,虽然在厌氧结算体系内RQs-106出现死亡,但是好氧阶段细胞增长快速,活细胞数仅略低于初始细胞浓度水平;但是在后3次循环中,体系内的菌株RQs-106在厌氧期间大量死亡,且最后一次循环中的体系中活细胞数仅为4.2 × 106 mL−1,小于0 h的105 × 106 mL−1,这可能是由于在厌氧反应阶段须消耗大量电子供体乳酸钠还原Fe3+和AQS-PUF,最后3次循环中电子供体的不足,造成了菌体死亡,从而导致芳香胺去除率的降低。因此,可以通过每3次循环后向体系中添加乳酸钠来提高芳香胺的去除率。
采用自由基捕获剂DMPO对体系中的HO·进行测定,其结果如图7所示。相对于空白体系,添加柠檬酸铁的体系中检测到峰高比例为1∶2∶2∶1的HO·加合物特征峰[16],证明了体系中HO·的存在,由此可判定体系中发生了芬顿反应。但是体系中产生的HO·的氧化性没有选择性,可能会进攻材料结构以及AQS-PUF材料上共价键结合的AQS,从而造成材料的不可逆损失。因此,对反应前后的AQS-PUF进行了傅里叶红外漫反射检测。如图8(a)所示,反应前后泡沫骨架结构并未发生改变,且图8(b)表明了泡沫表面各基团特征峰也未发生明显变化,1 658 cm−1处的醌式结构特征峰未出现明显降低,可见芬顿反应对材料的影响极小,表明采用这种固定化的AQS-PUF用于H2O2浓度较低(0~50 μmol·L−1)的芬顿反应中是可行的。
上述研究表明,本研究成功构建了固定化醌耦合生物驱动的芬顿反应。其反应过程为:首先,固定化AQS在体系中作为氧化还原介体加快了Fe3+向Fe2+的还原;然后,固定化AQS在菌株RQs-106的作用下参与厌氧/好氧循环从而产生H2O2;最后,Fe2+与H2O2在好氧体系中发生芬顿反应,产生的HO·能够降解芳香胺。可见,固定化AQS的加入不仅提高了H2O2的产生量,并且该材料可以循环利用,使得AQS-PUF耦合生物驱动的芬顿反应在处理芳香胺类物质方面具有潜在的应用价值。
2.1. 生物蒽醌法产H2O2特性
2.2. 柠檬酸铁浓度对苯胺降解的影响
2.3. 芬顿反应对芳香胺物质的降解
-
1)在AQS-PUF耦合生物驱动的芬顿反应中,H2O2来自于AQS-PUF厌氧生物还原-好氧自氧化循环,Fe2+来自于AQS-PUF介导的柠檬酸铁厌氧生物还原。反应的最适条件为:0.20 mmol·L−1固定化AQS、0.16 g·L−1生物量、40 mmol·L−1乳酸钠、4.5 mmol·L−1柠檬酸铁。
2)在最适条件下,在厌氧-好氧(21 h/3 h)循环过程中,H2O2浓度最高可达42.9 μmol·L−1,循环7次后,对苯胺和ADCTNDS去除率分别可达48.6%和43.3%。
3)本研究中的芬顿反应对固定化AQS的影响很小,表明采用这种固定化的AQS用于H2O2浓度小于50 μmol·L−1的芬顿反应中是可行的。



 下载:
下载: