-
近年来,工业化快速发展所引起的环境问题越来越严重,光催化技术作为解决环境问题的新技术得到广泛应用。光催化技术作为一种高效、安全的环境友好型环境净化技术,已成为现在最有前景的技术之一[1]。光催化是基于光催化剂在光照条件下具有的氧化还原能力而净化污染物[2]。二氧化钛(TiO2)由于其具有化学性质稳定、无毒、抗腐蚀性能强、价格低廉等优点,成为目前被广泛研究应用的半导体光催化材料,并应用于各类催化反应[3-5]。但是由于TiO2带隙较宽(锐钛矿型电位为3.2 eV),只能响应波长小于388 nm的紫外光,而太阳光中紫外线只占3%~5%,利用率低[6-8]。
大量的研究发现,TiO2的光催化活性取决于能带结构,而改变能带结构并扩展吸收光谱范围最有效的办法是掺杂金属和非金属元素[9]。其中掺杂非金属元素中研究最多的是有关氮掺杂的研究。目前报道的制备氮掺杂的方法主要包括溅射法[10]、脉冲激光沉积法[11]、溶胶凝胶法[12]和机械化学法[13]等。在这些方法中,溅射法和脉冲激光沉积法对于反应设备的要求较高,溶胶凝胶法和机械化学法对于反应条件的控制要求较高。相对于以上方法,高温煅烧法是可以通过控制煅烧氛围、温度及时间来制成光催化剂且易操作的方法。胡振华[14]通过采用改变TiO2前驱物的煅烧氛围,利用高纯氮气作为TiO2结晶的气氛,获得了禁带宽度小、颜色变化、具有可见光响应的锐钛矿相TiO2。
尽管国内外已有对制作掺氮二氧化钛的相关报道[15-16],但是煅烧氛围对氮掺杂二氧化钛的影响及其光催化性能的研究报道较少。本实验采用溶胶-凝胶法,以钛酸丁酯为钛源,以尿素为氮源制备N-TiO2前驱体,分别在空气和氮气氛围下煅烧,制备出N-TiO2光催化剂粉体;借助XRD、BET、TEM等手段对光催化剂进行表征,分析光催化剂的微观结构和比表面积等,选用甲基橙溶液为目标污染物,对光催化剂N-TiO2可见光降解性能进行评价,为相关的氮掺杂研究提供参考。
全文HTML
-
实验及主要试剂包括钛酸丁酯、无水乙醇、冰乙酸、尿素、甲基橙,均为分析纯。采用溶胶-凝胶法制得一定量TiO2粉体[17]。称取一定量的TiO2粉体于烧杯中,按照5∶1的氮钛比[18]加入一定浓度的尿素溶液,然后将烧杯放到磁力搅拌器中剧烈搅拌1 h,转移到超声波清洗器中,超声处理0.5 h,最后将烧杯放到温度为100 ℃的烘箱中,干燥12 h,取出研磨,得到掺氮的TiO2凝胶粉末。掺氮的TiO2凝胶粉末置于洁净的坩埚中,放入管式炉中,分别向管式炉(GSL-1100X,合肥料晶材料技术有限公司)中通入空气和氮气,以5 ℃·min−1的升热速率在温度为500 ℃下煅烧2 h,最终得到2种氛围下制得的掺氮TiO2光催化剂粉体,分别标记为N-TiO2(空气)和N-TiO2(N2)。
-
采用X射线衍射仪(XRD,D/MAX2500型,日本Rigaku)对光催化剂的晶型结构组成进行测定;采用透射电子显微镜(TEM,JEM-2100型,日本JEOM)对光催化剂的微观结构进行测定;采用紫外-可见漫反射光谱仪(UV-Vis DRS,UV-2550型,日本岛津)考察光催化剂的光的响应范围;采用比表面积测定仪(BET,NOVA2000型,美国康塔)测定光催化剂的比表面积;采用X射线光电子能谱仪(XPS,ESCALAB250,美国ThermoVG)测定光催化剂的表面元素及其化学状态;采用傅里叶转换红外线光谱分析仪(FTIR,FRONTIER,美国PE)测定光催化剂的表面官能团组成;采用扫描电子显微镜(SEM,S-4800I型,日本日立)配置的X射线能谱仪(EDS)对光催化剂中的主要元素组成及N元素分布性能进行测定。
-
取浓度为30 mg·L−1的甲基橙溶液100 mL置于烧杯中,然后向烧杯中分别加入2 g的N-TiO2(空气或N-TiO2(N2))粉体,在磁力搅拌条件下暗置一段时间,然后以装有紫外截止滤光片(UVCUT400,北京纽比特科技有限公司)和可见反射滤光片(VISREF780,北京纽比特科技有限公司)的氙光灯(HSX-F300,北京纽比特科技有限公司)为光源进行光催化反应。每隔一定时间取6 mL的上清液,用高速离心机(LG16C,北京雷勃尔医疗器械有限公司)离心约30 min,转速4 000 r·min−1,使用0.22 µm的微孔滤膜过滤器过滤,利用可见分光光度计(V-5000,上海元析仪器有限公司)测量样品中甲基橙在最大吸收波长(λmax=471 nm)下的吸光度,根据吸光度数值换算出净化效率,考察N-TiO2可见光催化降解甲基橙的性能。
1.1. 实验试剂及N-TiO2的制备方法
1.2. N-TiO2的表征
1.3. N-TiO2可见光催化活性评价
-
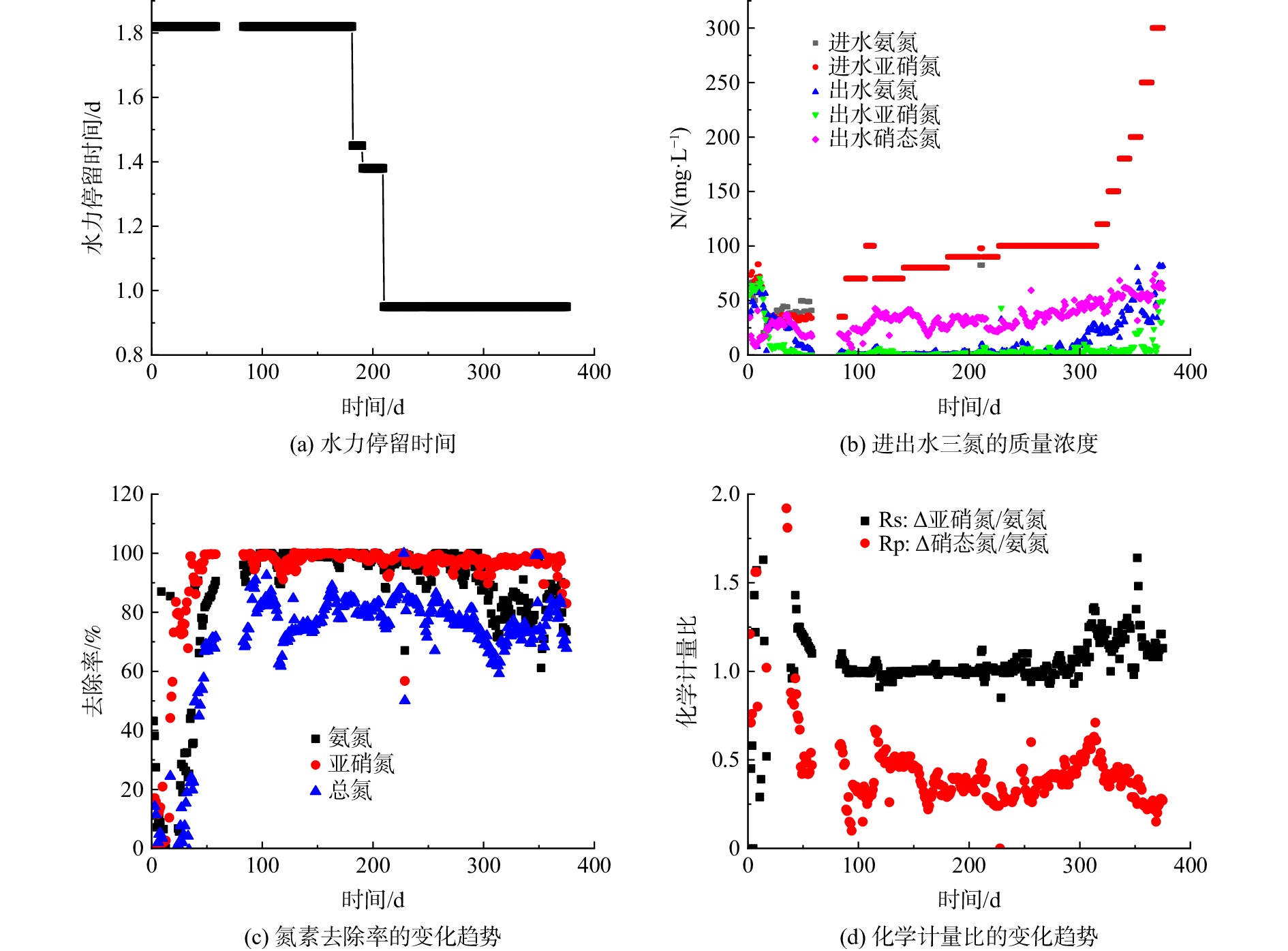
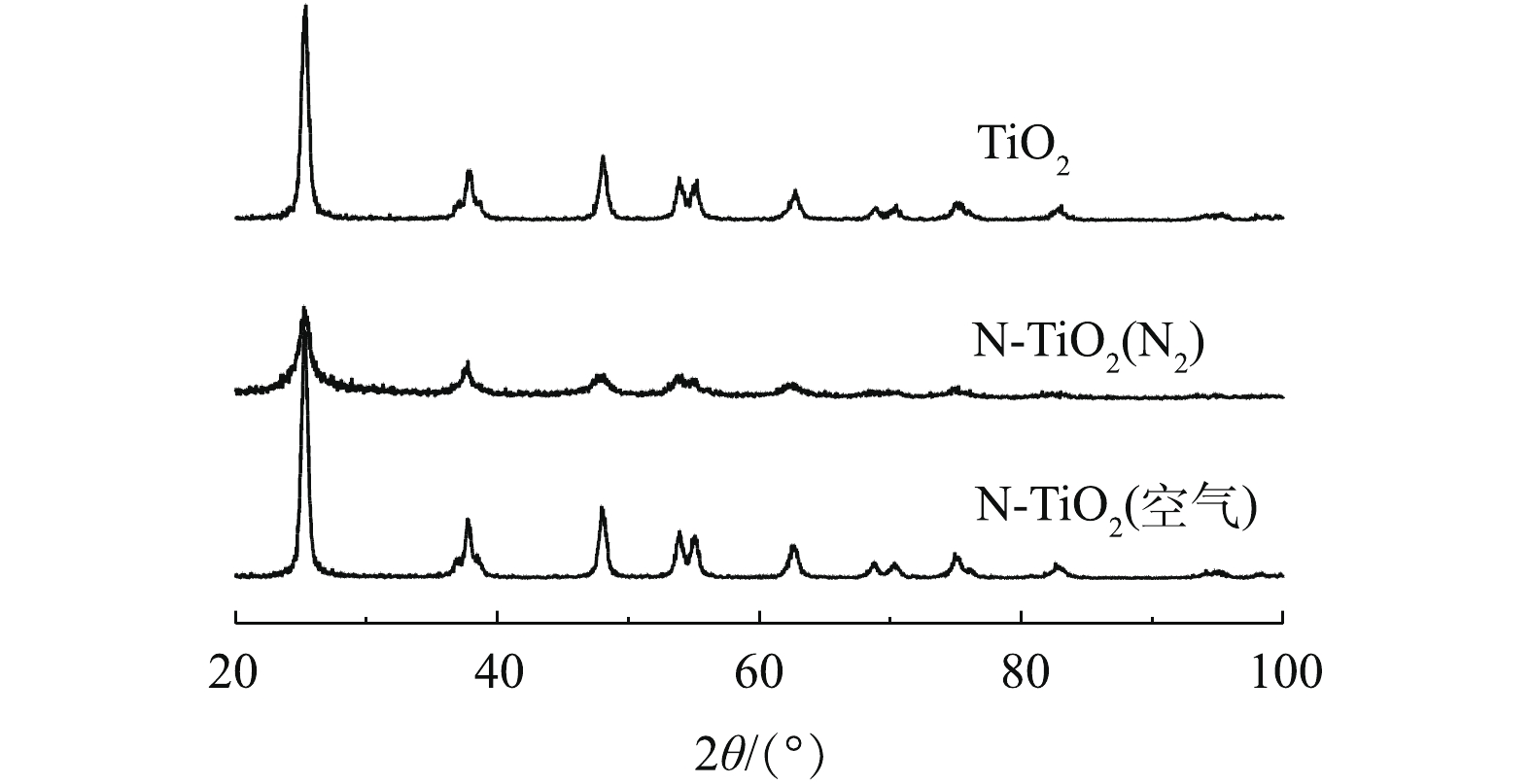
1) XRD的表征。由图1可看出,N-TiO2(空气)和N-TiO2(N2)2种光催化剂的特征峰出现位置相同,即二者均在2θ=25.3°、37.7°、47.9°、53.9°、55.2°位置处出现衍射峰,与TiO2锐钛矿相的(101)、(004)、(200)、(105)、(211)晶面相[19]一致,表明2种样品的晶型均是锐钛矿型,且掺入的N元素对TiO2原有的晶型组成没有影响。但N-TiO2(N2)的衍射峰较N-TiO2(空气)的峰高偏低,且峰形偏宽。
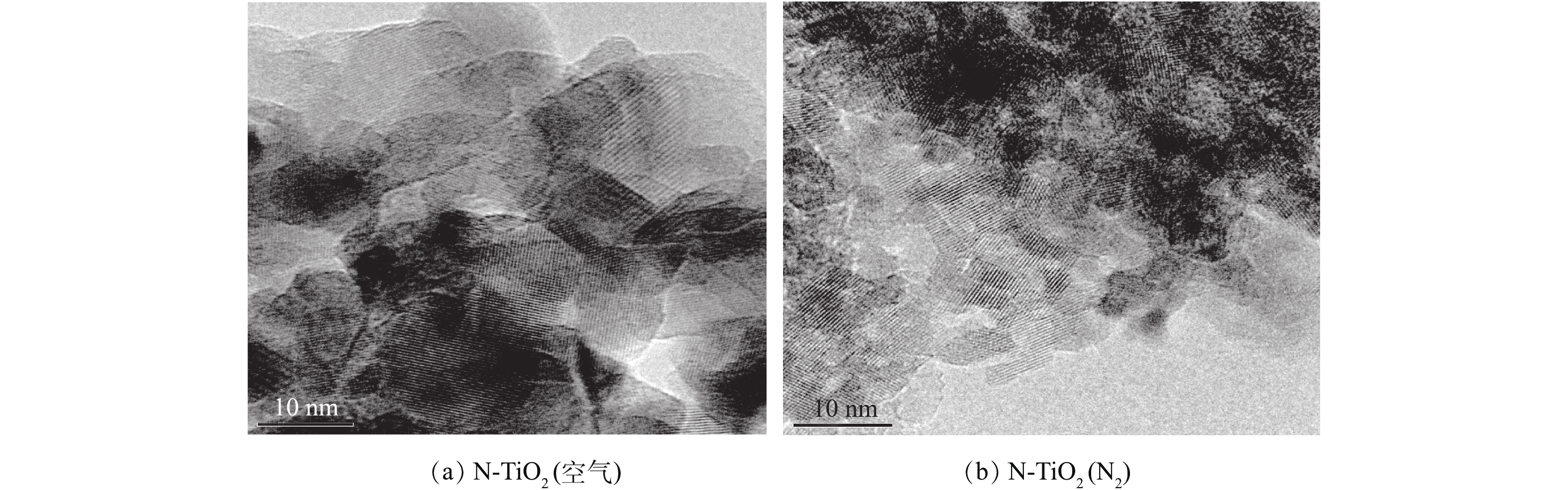
2) TEM表征及BET的测定。图2(a)和图2(b)分别为N-TiO2(空气)和N-TiO2(N2)光催化剂的TEM图。可以看出,N-TiO2(空气)和N-TiO2(N2)晶型结构均良好,颗粒大小均匀,且分散性好。相比而言,N-TiO2(N2)的晶粒尺寸明显小于N-TiO2(空气),这正是造成N-TiO2(N2)较N-TiO2(空气)的XRD特征峰形宽化的原因。此外,由BET分析仪测得,N-TiO2(N2)的比表面积为172. 269 m2·g−1,而N-TiO2(空气)的比表面积仅为76. 756 m2·g−1,前者为后者的2倍之多,且二者的比表面积均明显高于商业P25[20]。由此可见,N元素的掺入不仅使TiO2的原有晶粒尺寸变小,而且N2氛围下煅烧制得的N-TiO2较空气氛围下煅烧更能引起TiO2晶粒尺寸变小。
3) UV-vis DRS的测定。图3为N-TiO2和TiO2的紫外可见光漫反射图。可以看出,纯TiO2光催化剂仅在紫外区具有强吸收性,而N-TiO2(空气)和N-TiO2(N2) 2种光催化剂不仅在紫外区具有强吸收性,而且在可见光区也表现出良好的吸收性,且N-TiO2(N2)的吸收带扩展至800 nm左右,其可见光吸收性明显优于N-TiO2(空气)。
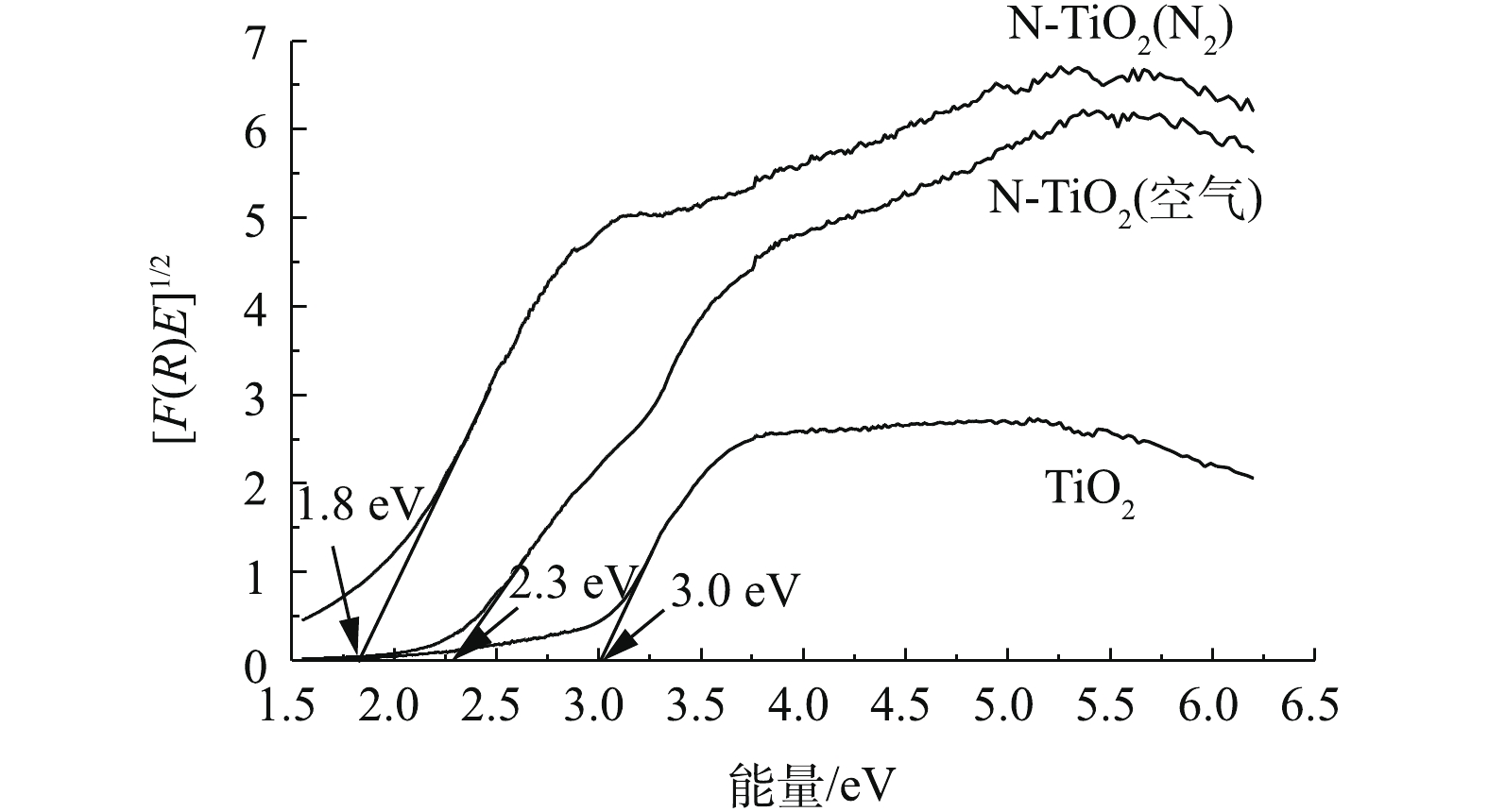
借助Kubelka-Munk函数绘制了[F(R)E]1/2与吸收光子能量E的关系曲线(见图4),用于估算TiO2,N-TiO2(空气)和N-TiO2(N2)的带隙能量。由图4可以看出,TiO2、N-TiO2(空气)和N-TiO2(N2)的带隙能量分别为3.0、2.3和1.8 eV。与TiO2相比,N-TiO2(空气)和N-TiO2(N2)的带隙能均变小,即禁带变窄,且N-TiO2(N2)的带隙能最小,大大低于纯TiO2的带隙能。
由此表明,N元素的掺入可有效降低TiO2的带隙能,从而提高TiO2对可见光的吸收响应性能。相比而言,N-TiO2制备过程中在N2氛围下煅烧处理较空气氛围下煅烧处理更有利于N元素的掺入,且对TiO2原有带隙能的改善效果更明显,更有利于提高TiO2对可见光的吸收响应性。
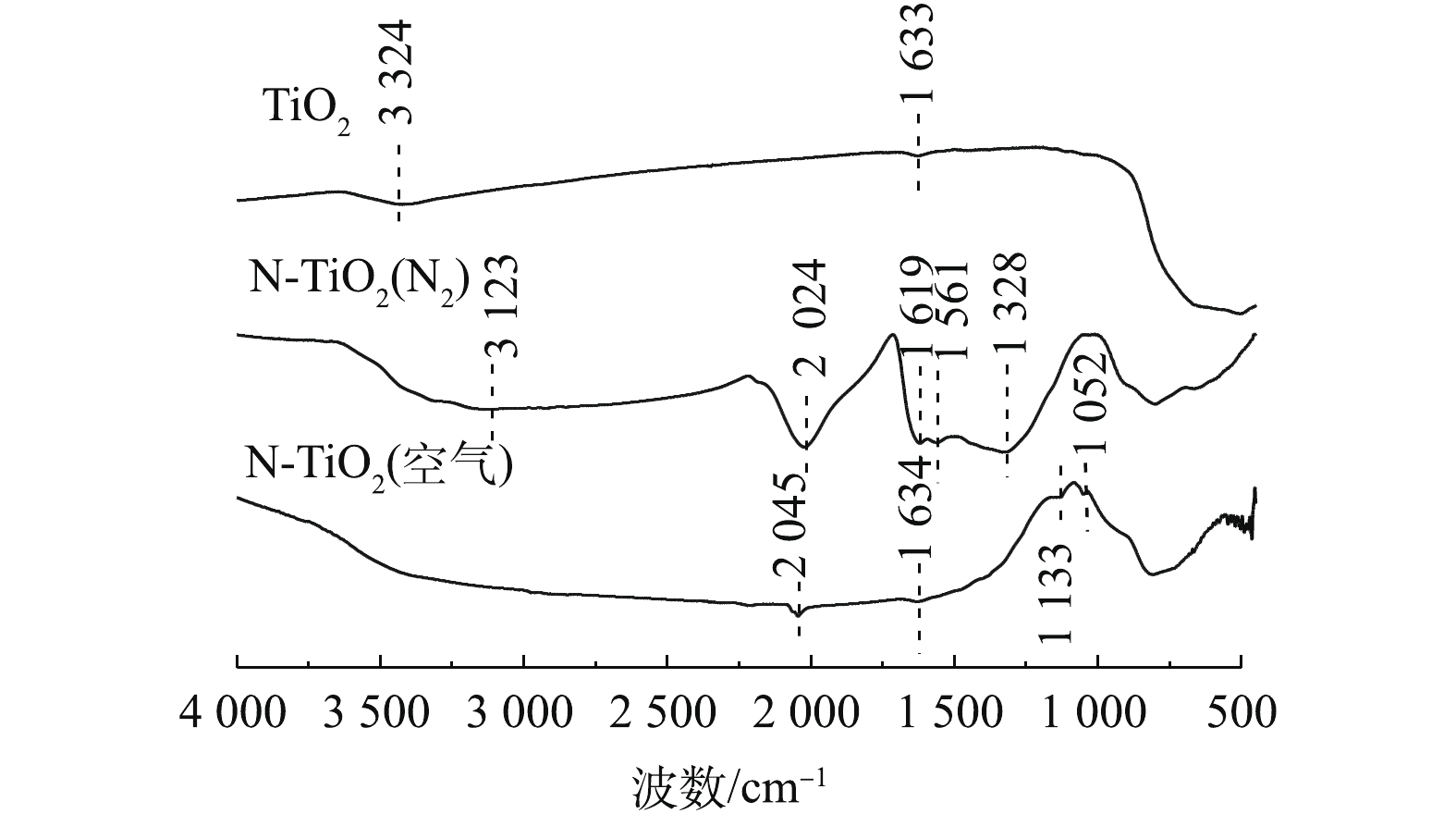
4) FT-IR表征。TiO2、N-TiO2(空气)和N-TiO2(N2)的表面官能团组成如图5所示。对于TiO2,3 424 cm−1和1 633 cm−1处的2个峰分别来自表面吸附水的O—H伸缩振动和O—H弯曲振动,而500~750 cm−1处的宽峰则归属于锐钛矿相的TiO2。与TiO2相比,在N-TiO2(N2)中,除了在1 619 cm−1处的峰归因于O—H之外,在3 123、2 024和1 328 cm−1处出现新峰,分别归属于N—H伸缩振动、C=O伸缩振动和C—H弯曲振动。在N-TiO2(空气)中,除了上述特征峰外,出现了1 052 cm−1的新峰,其归属于次硝酸盐(N2O2)2−,这是特征间质N的结构。此外,相比于锐钛矿相TiO2,N-TiO2(空气)和N-TiO2(N2)在500~830 cm−1处的宽峰发生一定程度的蓝移,其可能是由于N掺入到TiO2晶格中而引起的。
5) EDS的测定。通过EDS能谱分析可得出2种光催化剂中各组成元素的含量,测定结果如表1所示。由表1可知,N-TiO2(空气)中未检出N元素,其原因在于N-TiO2(空气)中掺入的N元素量太少,未达到仪器的响应范围。相比而言,N-TiO2(N2)中测得较高含量的N元素。结合N-TiO2(N2)光催化剂表面的N元素面分布图(如图6所示),可以看出,N元素在N-TiO2(N2)表面含量高且分散性好。由此表明,煅烧氛围对N元素在TiO2中的掺杂量有明显的影响,且在N2氛围中进行煅烧处理所得N-TiO2表面的N掺杂量要明显优于在空气氛围。
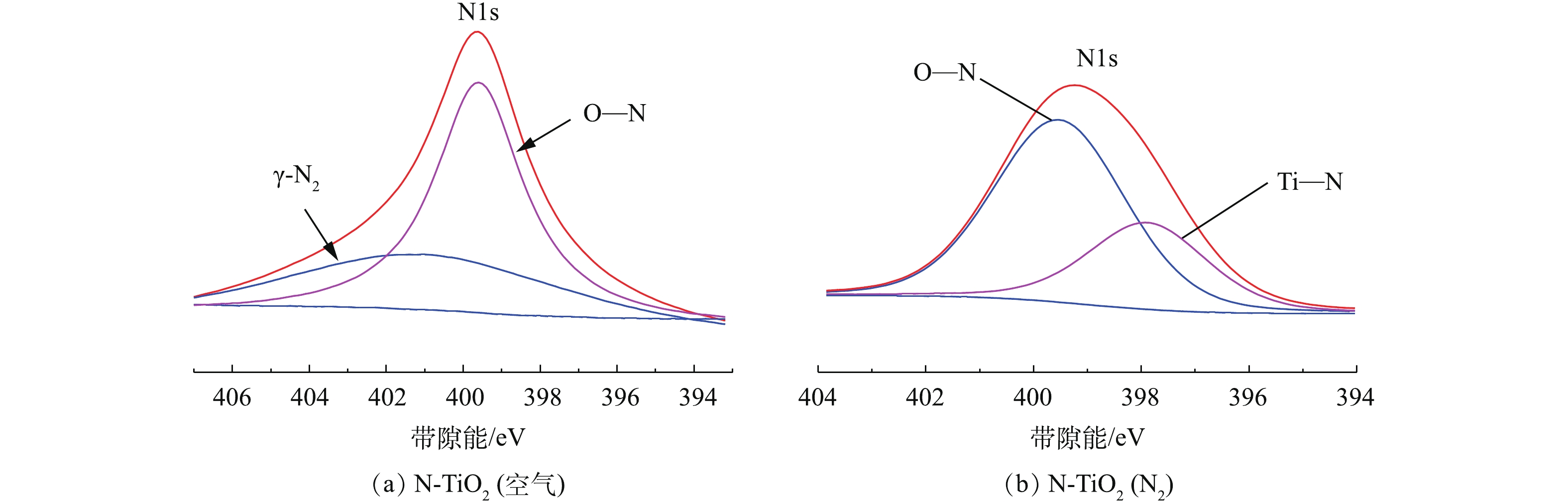
6) XPS分析。图7(a)和图7(b)分别为N-TiO2(空气)和N-TiO2(N2) 2种光催化剂的表面N元素的XPS图。由图7(a)可以看出,2个对称的吸收峰分别位于401.1 eV和399.6 eV,这表明在N-TiO2(空气)中,N1s存在2种形态,其中401.1 eV处的吸收峰是由于N原子通过化学吸附生成γ-N2并以分子形式存在于TiO2晶格内,而399.6 eV处的吸收峰则归属于Ti—O—N键当中的间隙氮。由图7(b)可以看出,2个对称的吸收峰分别位于399.5 eV和397.8 eV,这表明在N-TiO2(N2)中N1s存在2种形态,其中399.5 eV处的吸收峰可归属于Ti—O—N键当中的间隙氮,而397.8 eV处的吸收峰则归属于N—Ti—N键的吸收峰,这是由于N原子取代TiO2晶格中的O原子形成的。XPS结果也同时解释了N-TiO2(空气)和N-TiO2(N2) 在500~830 cm−1处TiO2特征峰发生一定程度的蓝移(见图5)的原因。由此可见,2种N-TiO2光催化剂中均成功掺杂N元素,但N元素掺入形态有所不同,N-TiO2(空气)中N元素的掺入形态主要为间隙氮和吸附态γ-N2分子,而N-TiO2(N2)中N元素的掺入形态主要为间隙氮和替代氮。
由XPS能谱分析可得出2种光催化剂中N元素含量,N-TiO2(空气)中N元素含量为1.46%,N-TiO2(N2)中N元素含量为17.13%。由此可知,N-TiO2(N2)的N元素含量明显高于N-TiO2(空气),这与EDS结果大致相同。
综上可以看出,与空气氛围相比,N-TiO2制备过程中在N2氛围下煅烧处理更有利于N元素以间隙氮和替代氮的方式掺入TiO2晶格中,且有效掺入量显著提高,从而大大提高了TiO2对可见光的吸收响应性。
-
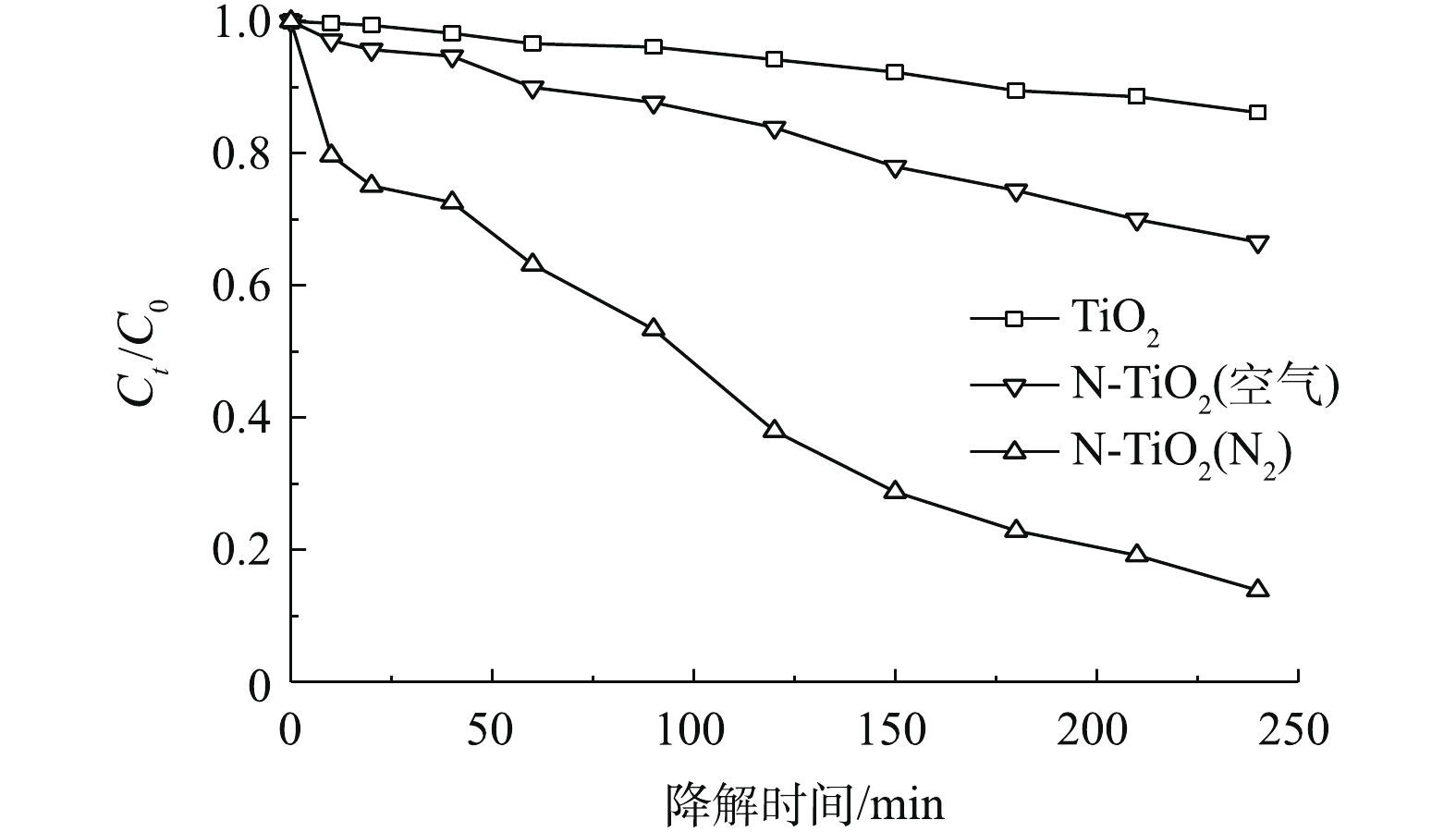
图8为可见光照射条件下TiO2、N-TiO2(空气)和N-TiO2(N2)对甲基橙溶液的光降解效果。可以看出,纯TiO2对甲基橙溶液的可见光降解能力较小,而N-TiO2(空气)和N-TiO2(N2)的可见光降解性能明显优于纯TiO2。随着光照时间的增加,N-TiO2(N2)对甲基橙溶液的可见光降解性能明显优于N-TiO2(空气),当可见光持续光照240 min时,N-TiO2(N2)对甲基橙溶液的光降解率为86.2%,而N-TiO2(空气)的光降解率为66.6%,前者比后者高近20%。由此可见,采用N元素对TiO2进行改性处理后,不仅扩展了TiO2对可见光的吸收性能,而且TiO2的可见光催化活性也得到了有效提升。此外,N2氛围下煅烧处理较空气氛围下煅烧更有利于N-TiO2可见光催化活性的提高。这与上述TEM、UV-vis DRS、EDS、XPS等表征结果具有很好的一致性。
-
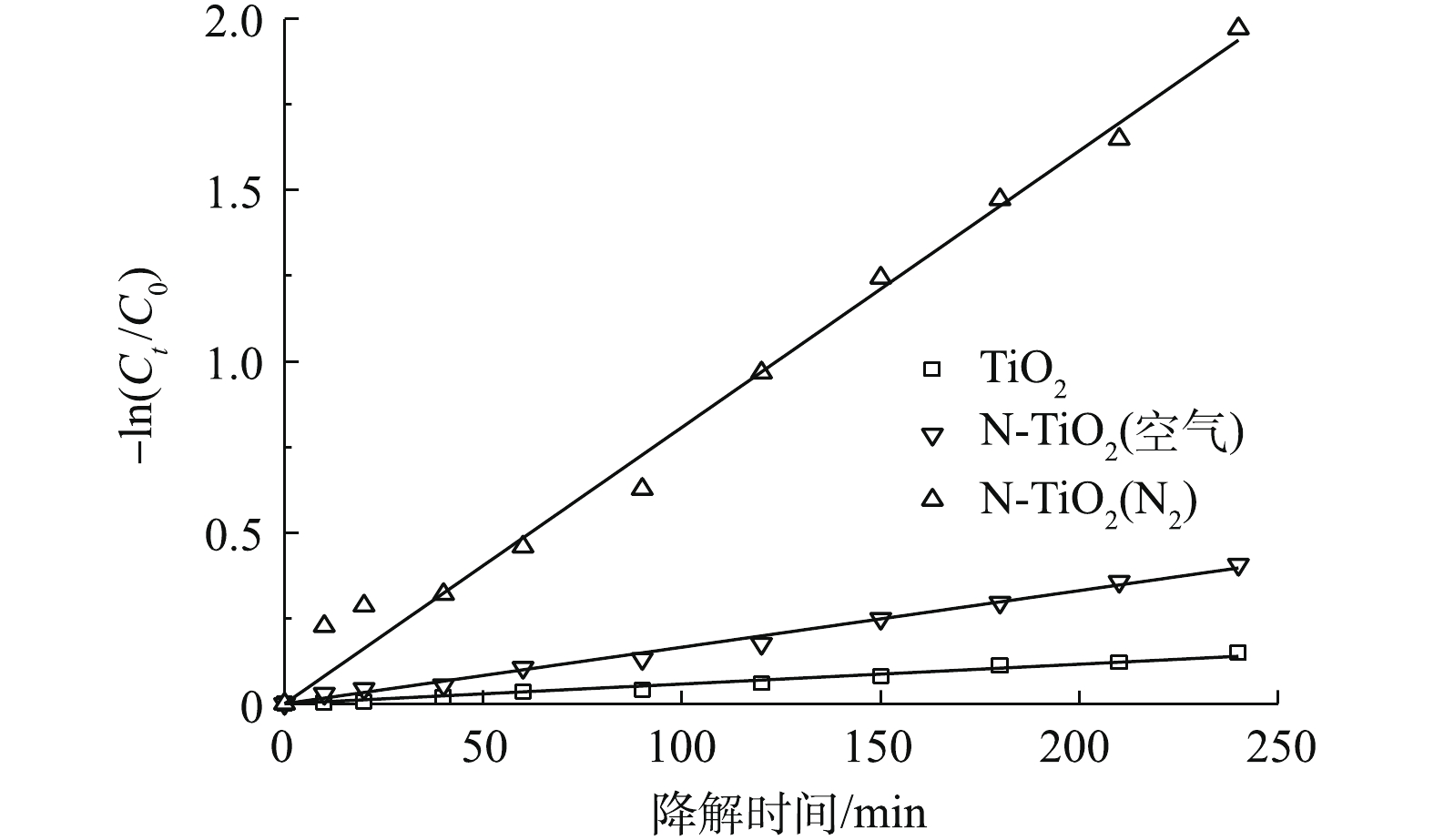
以-ln(Ct/C0)对光照时间t作图,如图9所示。其线性回归系数与表观速率常数见表2。
由图9和表2可以看出,−ln(Ct /C0)与t呈良好的线性关系,即满足光催化假一级动力学方程。
式中:t为降解时间,min;kapp为表观速率常数,min−1。
式(1)说明N-TiO2和 TiO2对甲基橙溶液的可见光降解性能符合Langmiur- Hinshewood动力学模型[21]。与TiO2相比,N-TiO2对应的表观速率常数kapp明显提高,是前者的2.8~13.9倍,说明N元素的掺入的确增强了TiO2在太阳光下的光催化活性。对比不同煅烧氛围下制得的N-TiO2的光催化性能,N-TiO2(N2)的表观速率常数kapp是N-TiO2(空气)的近5倍,前者的光降解速率大大高于后者。
2.1. 表征结果
2.2. N-TiO2可见光降解甲基橙溶液的性能
2.3. 光降解反应动力学分析
-
1)在空气和氮气氛围下煅烧制得的N-TiO2均成功掺入N元素且均为锐钛矿相型;N-TiO2(N2)较N-TiO2(空气)晶粒尺寸小、比表面积大、可见光响应性强和有效掺氮量高。
2)N-TiO2可见光催化性能较TiO2明显提高。N-TiO2对甲基橙溶液的可见光降解反应符合假一级动力学方程。N-TiO2(N2)的可见光降解性能优于N-TiO2(空气),其中N-TiO2(N2)对甲基橙溶液的可见光降解率最高可达86.2%。



















 下载:
下载: