-
矿山开采、冶金、涂料、塑料以及电池制造业等都会产生镉废物、废渣以及镉废水,若不及时处理或处理不当,将会导致周边土壤和水体受到污染,严重情况下会造成环境失调、生态失衡等不良现象[1-4]。镉可通过食物链和呼吸系统等途径在人体蓄积,尤其是肾脏和肝,从而引发一系列的急慢性疾病,甚至癌症[3, 5]。因而,很有必要开展对含镉废水及镉污染地下水的修复与治理研究。
镉废水及镉污染地下水处理与修复的方法有生物降解法、化学沉淀法和吸附法等[6-7]。其中,吸附法由于成本低、去除效果好而得到广泛的应用[7]。吸附水中镉的主要吸附剂有羟基磷灰石、硅酸钙、聚甲醛脲醛树脂、生物炭等[6-11],但这些材料大都比较昂贵,或来源不便,或制作成本高。相比之下,我国钢渣累积量达1×109 t,具有来源广,经济实惠,且可利用成分多等优点,故将钢渣运用到地下水镉修复和镉废水处理中具有广泛的应用前景[12-15]。
此外,利用钢渣修复镉污染地下水或含镉废水的研究较少,且钢渣负载HAP(羟基磷灰石,hydroxyapatite)去除水中镉的相关报道也很少。因此,本研究将低碱度钢渣、低碱度钢渣负载HAP、高碱度钢渣和高碱度钢渣负载HAP 4种材料进行对比研究,分别从pH、反应时间和初始浓度等方面考察4种材料对镉的吸附效果,并结合SEM和XRD表征结果对镉的吸附机理进行探讨,以期为镉污染地下水修复和含镉废水处理提供参考。
全文HTML
-
磷酸(H3PO4)、四水硝酸钙(Ca(NO3)2·4H2O)、氨水(NH3·H2O)、氢氧化钠(NaOH)、硝酸(HNO3)、盐酸(HCl)、硫酸(H2SO4)、水合氯化镉(CdCl2·2.5 H2O)均为分析纯;实验用水为去离子水。
扫描电子显微镜(S4800型,日本 Hitachi 公司);X射线衍射仪(DX2700型,辽宁丹东浩元仪器有限公司);实验室纯水系统(Smart-Q30,上海和泰仪器有限公司);气浴恒温振荡器(SHZ-82A,金坛市荣华仪器制造有限公司);pH计(FE20,梅特勒-托利多仪器(上海)有限公司);电感耦合等离子体发射光谱仪(Agilent 5100 ICP-OES型,安捷伦科技(中国)有限公司)。
-
1)钢渣来源及预处理。低碱度钢渣和高碱度钢渣(均为100目)分别来自巩义市金丰净水材料有限公司和萍乡钢铁有限责任公司安源分公司。将2种钢渣分别用自来水反复清洗至水变清,去离子水反复冲洗3遍,再用10%的H2SO4溶液浸泡1 h,去离子水反复清洗干净,滤干放入恒温烘箱,80 ℃烘干24 h,取出冷却后,装入自封袋备用。
2)钢渣负载HAP的制备。量取0.25 mol·L−1的H3PO4溶液120 mL于烧杯中,并用氨水调节pH=10,加入50 g预处理过的钢渣,用电动搅拌器(425 r·min−1)搅拌均匀,后缓慢加入50 mL 1 mol·L−1的Ca(NO3)2·4H2O溶液,同时用氨水调节烧杯中的溶液pH=10[16],待搅拌30 min后,取出静置48 h,离心,倒掉上清液,去离子水反复清洗3~5次,取出固体放入恒温烘箱(80 ℃),烘干24 h,制得复合材料,用自封袋密封备用。
-
采用扫描电子显微镜(SEM)观察反应前后4种材料的形貌结构;采用X射线衍射仪(XRD)分析其反应前后的物质组成。
-
取2.031 7 g CdCl2·2.5H2O固体,加水溶解后定容于1 000 mL的容量瓶中,以制备1 g·L−1的Cd2+储备溶液,并根据需求稀释到一定浓度,以便静态实验使用。量取100 mL一定浓度的Cd2+溶液于锥形瓶中,后用1∶3的盐酸和氨水调节到指定的pH,再加入0.1 g材料,放入气浴恒温振荡器(180 r·min−1、温度(25±1) ℃),振荡到指定时间,取出静置10 min,再取其上清液离心,过滤,最后用电感耦合等离子体发射光谱仪(ICP-OES)测定剩余镉浓度。
镉的吸附量按式(1)计算。
式中:q为材料对Cd2+的吸附量,mg·g−1;C0为Cd2+初始浓度,mg·L−1;Ce为Cd2+平衡浓度,mg·L−1;V为Cd2+溶液体积,L;m为材料质量,g。
1.1. 试剂与仪器
1.2. 实验材料
1.3. 表征方法
1.4. 实验方法
-
在初始浓度为10 mg·L−1、pH为5的条件下,反应时间(0.5~30 h)对4种材料吸附水溶液中Cd2+的影响如图1所示。由图1可知,随着反应时间的增加,高碱度钢渣及其负载HAP 2种材料对Cd2+的吸附量均呈上升的趋势。高碱度钢渣负载HAP在反应前5 h内,吸附速率较快,5 h后吸附速率有所减低,24 h时达到吸附平衡,之后吸附量变化不大;对于高碱度钢渣,可能因成分含量的差异性,导致在12~21 h吸附量波动较大,在21 h时达到吸附平衡。而另外2种材料对Cd2+的吸附性能较差,低碱度钢渣及其负载HAP分别在起初的7 h和9 h内对Cd2+有微弱的吸附作用,但在7 h和9 h后便分别发生脱附现象。由此可知,2种钢渣负载了HAP后对Cd2+的吸附能力都有所增强,其中高碱度钢渣增强较为显著。将4种材料对Cd2+的吸附能力进行比较:高碱度钢渣负载HAP>高碱度钢渣>低碱度钢渣负载HAP>低碱度钢渣,但由于低碱度钢渣及其复合材料会自发地产生脱附现象,不宜用作Cd2+的吸附材料。故以下实验只采用高碱度钢渣及其复合材料作为吸附剂。
-
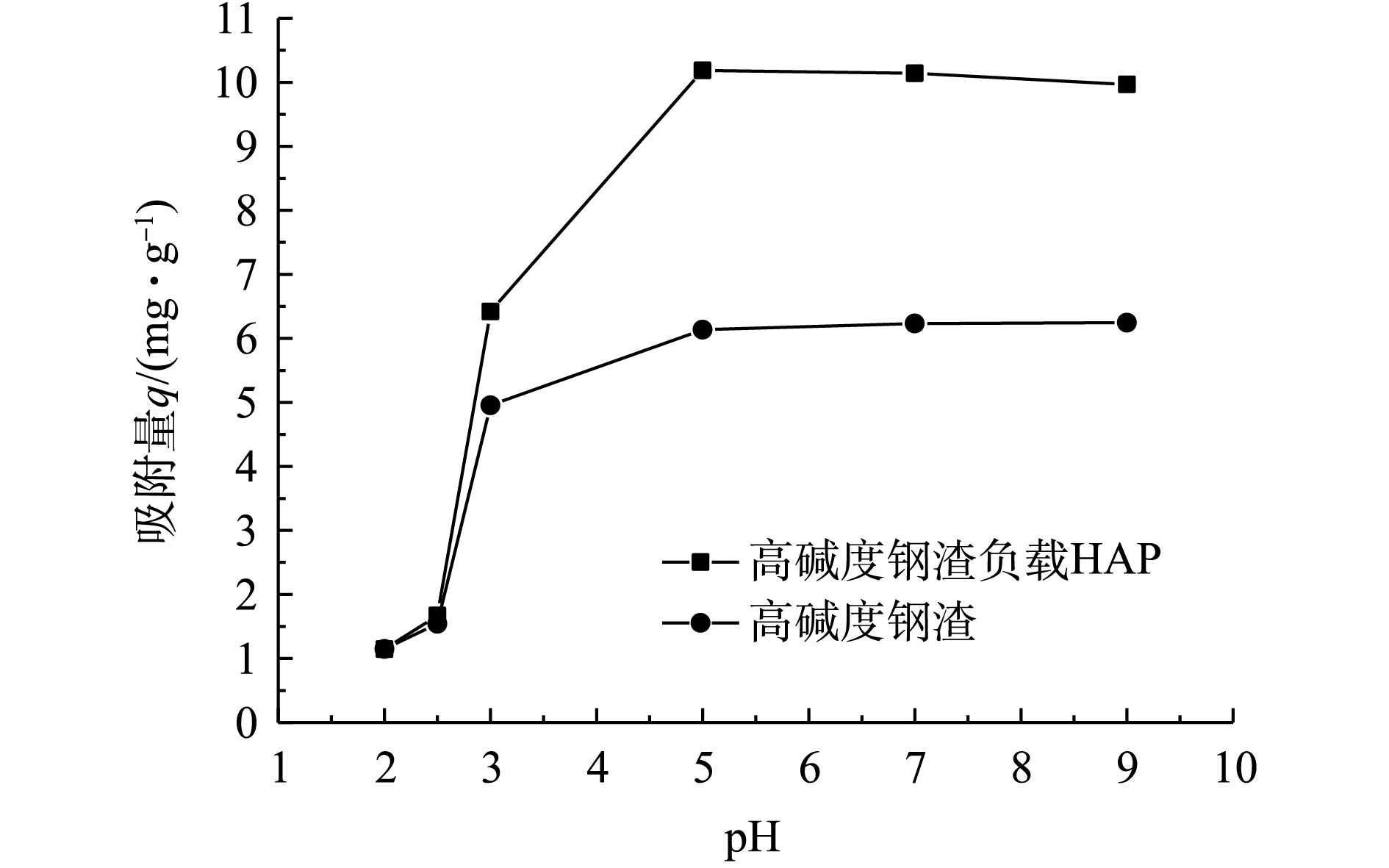
在初始浓度为10 mg·L−1、反应时间为24 h的条件下,pH(2 ~9)对高碱度钢渣及其负载HAP吸附Cd2+的影响如图2所示。由图2可知,pH对2种材料吸附Cd2+的影响较大,当pH<5时,随着pH的增大,吸附量快速增加;当pH=5时,吸附量最大;当pH>5时,吸附量几乎保持在最佳状态,说明2种材料在较广pH范围内对Cd2+均具有一定的吸附作用。但在pH<3时,2种材料对Cd2+的吸附量相差不大。这可能是因为强酸条件下,溶液中H+的含量大,可破坏HAP与钢渣的胶结结构,甚至破坏HAP(式(2))及钢渣本身的内部结构,从而使得HAP失去吸附能力或吸附能力较弱[17-19];再者,HAP表面的≡P—O—和≡Ca—OH会与水溶液中的H+发生质子化反应,而以≡P—OH和≡Ca—OH2+的形式存在,导致材料表面带正电荷或中性电荷而排斥吸附Cd2+[20-21]。随着pH的增加,H+含量较少,加上高碱度钢渣含有CaSO4、CaO、FeO和Fe3O4等组分[22-23],在反应过程中释放OH−,消耗掉部分H+,导致溶液的pH上升,利于Cd2+的去除[24-26]。
-
在pH=5、反应时间为24 h的条件下,初始浓度(1~35 mg·L−1)对高碱度钢渣及其负载HAP吸附Cd2+的影响如图3所示。由图3可知,随着初始浓度的增加,吸附量均呈上升的趋势。在C0<11.60 mg·L−1时,随着初始浓度的增加,吸附量快速增加,镉浓度的提高为吸附剂提供更多未吸附的Cd2+,使得吸附剂表面及内部孔隙与Cd2+充分接触,吸附速率增加,总吸附量提高;在C0>11.60 mg·L−1时,2种材料对Cd2+的吸附量变化不明显,说明在初始浓度为11.60 mg·L−1时,2种吸附材料对Cd2+的吸附接近饱和状态。将2种材料进行对比得出,高碱度钢渣负载HAP对Cd2+的吸附性能优于高碱度钢渣,最大吸附量分别为12.63 mg·g−1和7.65 mg·g−1,相比之下,提高了60.58%。
-
采用准一级[8-9]和准二级[11, 24]吸附动力学模型对高碱度钢渣及其负载HAP吸附Cd2+的吸附动力学特征进行拟合。
准一级吸附动力学模型见式(3)。
准二级吸附动力学模型见式(4)。
式中:qe为吸附平衡时的吸附量,mg·g−1;qt为反应时间t时的吸附量,mg·g−1;k1为准一级动力学的吸附速率常数,min−1;k2为准二级动力学模型的吸附速率常数,g·(mg·min)−1。
拟合结果(见表1)表明,2种材料对Cd2+的吸附动力学特征均符合准二级吸附动力学模型,高碱度钢渣及其负载HAP对Cd2+的吸附以化学吸附为主。
-
采用Freundlich[8-9]和Langmuir模型[11, 24]对镉初始浓度与平衡吸附量之间的关系进行拟合,拟合结果如表2所示,相应的表达式如式(5)和式(6)所示,且可通过分离系数RL(式(7))来描述吸附过程进行的难易程度。
Freundlich模型见式(5)。
Langmuir模型见式(6)。
分离系数RL的计算见式(7)。
式中:Kf为Freundlich的特征常数,L·g−1;n为Freundlich的吸附强度;qmax为饱和吸附量,mg·g−1;b为Langmuir的吸附常数,L·mg−1。
由表2可知,Langmuir 等温线模型对高碱度钢渣及其负载HAP吸附Cd2+的拟合结果最好,可决系数分别为0.990 8和0.999 7,说明高碱度钢渣及其负载HAP对Cd2+的吸附机制为吸附剂表面上的单层吸附。RL计算结果表明,2种材料都满足RL∈(0,1),属较易吸附[27-28]。
-
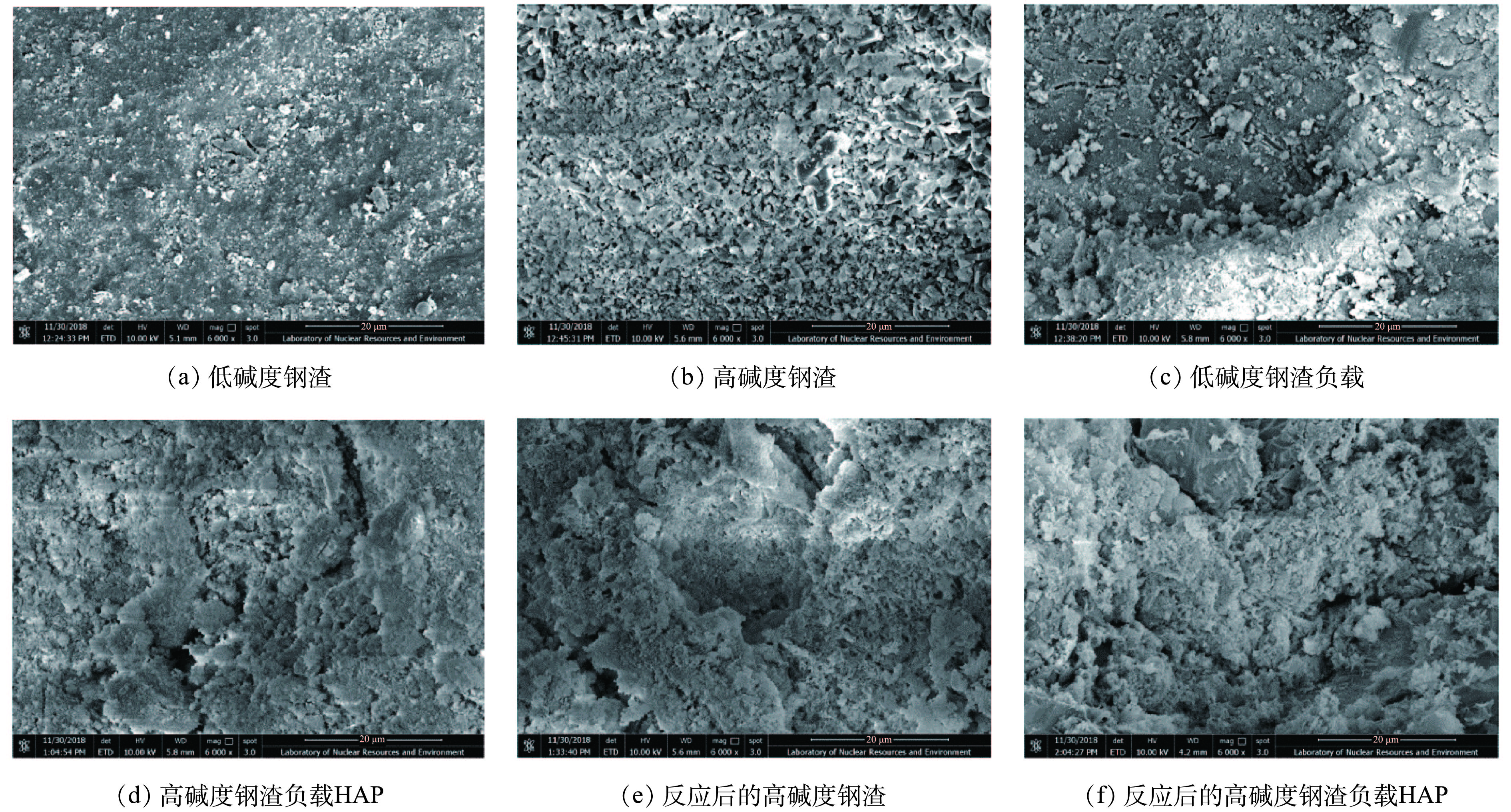
图4为钢渣及其负载HAP 4种材料吸附Cd2+前后的SEM图。对比图4(a)和图4(b)可知,低碱度钢渣表面较为平坦密实,孔隙极少;高碱度钢渣表面粗糙多孔,颗粒多呈不规则排列,这大大地增加了高碱度钢渣的比表面积,为HAP提供了较好的负载条件,也为高碱度钢渣吸附Cd2+提供了大量的吸附位点。由图4(a)与图4(c)、图4(b)与图4(d)可知,图4(c)上的颗粒物质较多,表明了低碱度钢渣表面负载了一定量的HAP;图4(d)表面含有大量的白色层状物,说明HAP成功地负载于高碱度钢渣上,且保持疏松多孔的特征[29-30]。对比图4(b)和图4(e)发现,高碱度钢渣的孔隙被填充,块状和短柱状颗粒被覆盖,表明高碱度钢渣成功地吸附了Cd2+;相对于图4(d),图4(f)上具有明显的绒状附着物,表明高碱度钢渣负载HAP表面存在大量的含镉物质;同时,也验证了负载HAP的高碱度钢渣对Cd2+的吸附能力优于未负载的高碱度钢渣。
-
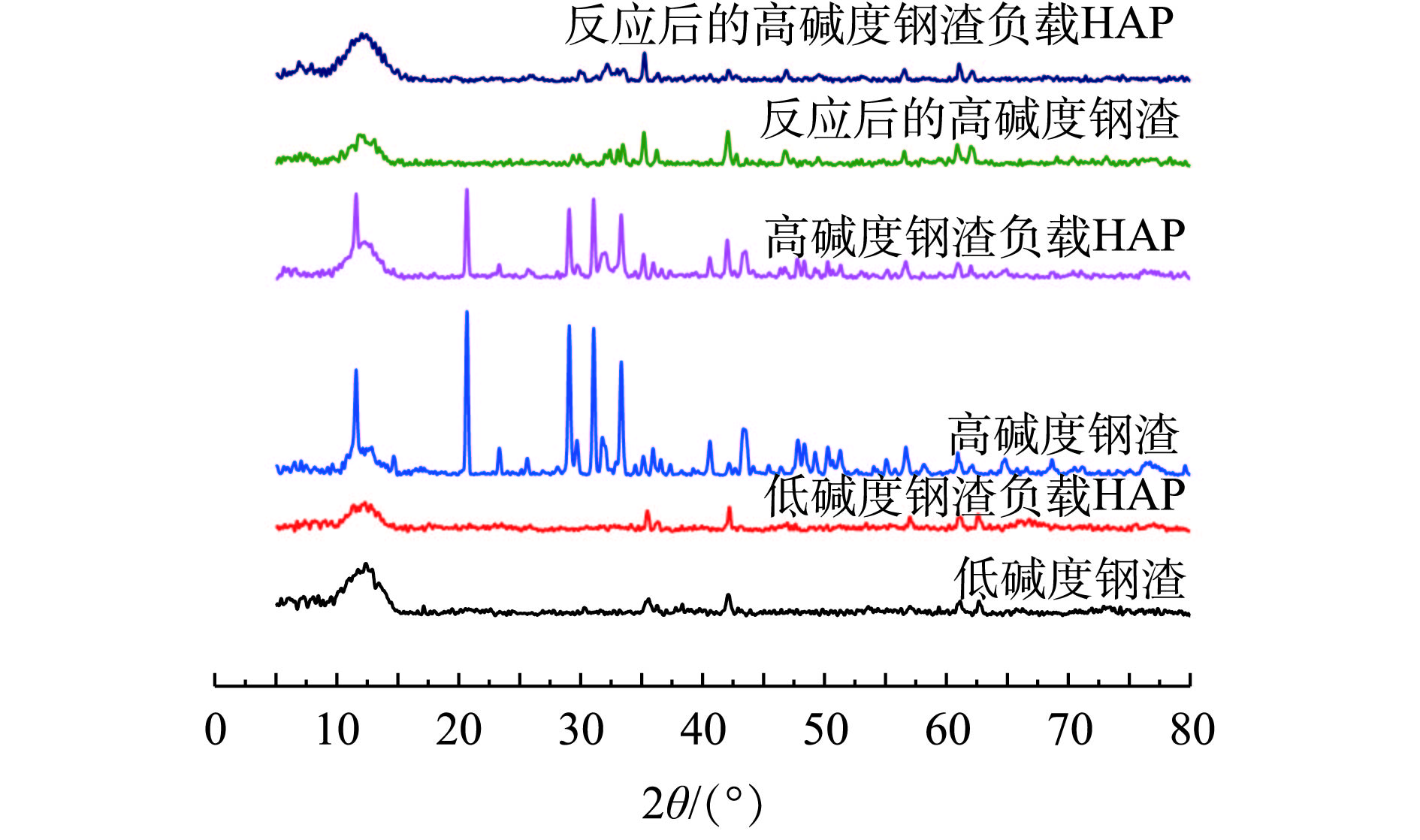
4种材料吸附前后的XRD图谱如图5所示,对应的主要矿物组成见表3。经过图谱和物相检索发现:钢渣衍射峰多且密集,成分十分复杂,主要以Si、O、Ca、Mg、Al、Fe等元素组成的矿物质为主;相对于低碱度钢渣,高碱度钢渣衍射峰较强,其磷酸(氢)钙矿物和硫酸钙矿物较多,表明了2种钢渣成分的差异性对吸附性能的影响较大;相对于低碱度钢渣,高碱度钢渣对HAP的负载能力较强。从图5可看出,负载HAP后的低碱度钢渣图谱中,除个别峰宽稍微变窄或增强外,其余衍射峰变化不明显,这说明低碱度钢渣可少量地负载HAP,但负载效果不佳;负载HAP后的高碱度钢渣部分衍射峰降低,个别衍射峰明显增强,这说明高碱度钢渣成功地负载了HAP,这进一步验证了SEM表征结果的可靠性。HAP对Cd2+具有较好的吸附性能,将其负载于高碱度钢渣上,可明显地提高材料对Cd2+的吸附能力,这与实验结果相符。而通过对反应后的高碱度钢渣及其负载HAP进行物相分析发现,Cd2+主要以Ca10-xCdx(PO4)6(OH)2、CdxH3-x(PO4)2、Cd(OH)2等形式存在,这说明高碱度钢渣及其负载HAP对Cd2+的吸附作用主要以离子交换作用和化学沉淀作用为主[20, 31],主要吸附机理如式(8)~式(12)所示。
2.1. 反应时间对材料吸附镉的影响
2.2. pH对材料吸附镉的影响
2.3. 初始浓度对材料吸附镉的影响
2.4. 高碱度钢渣及其复合材料吸附镉的机理分析
2.4.1. 吸附动力学特征
2.4.2. 吸附等温线模型
2.4.3. SEM
2.4.4. XRD
-
1)采用静态实验,从pH、反应时间和初始浓度等方面考察了低碱度钢渣、低碱度钢渣负载HAP、高碱度钢渣和高碱度钢渣负载HAP 4种材料对水溶液中Cd2+的吸附效果,结果显示,4种材料对Cd2+的吸附效果顺序为高碱度钢渣负载HAP>高碱度钢渣>低碱度钢渣负载HAP>低碱度钢渣。
2)高碱度钢渣及其负载HAP对Cd2+的最大吸附量分别为7.65 mg·g−1和12.63 mg·g−1,相比之下,提高了60.58%。
3)高碱度钢渣及其负载HAP对Cd2+的吸附过程均符合准二级吸附动力学模型和Langmuir吸附等温线模型,表明吸附过程主要为吸附剂表面上的单层化学吸附;且分离系数RL均在 0~1之间,为较易吸附。
4)SEM和XRD表征分析表明,高碱度钢渣及其负载HAP对Cd2+的吸附作用均以离子交换作用和化学沉淀作用为主。
5)钢渣碱度的差异性对其吸附镉的影响较大。高碱度钢渣资源丰富,可将其负载HAP后用于废水除镉或作为PRB介质材料用于地下水镉修复,以达到以废治废的目的。






 下载:
下载:

 百度学术
百度学术


