锰氧化物八面体分子筛活化过一硫酸氢盐降解酸性橙7
Heterogeneous activation of peroxymonosulfate by cryptomelane type manganese oxide for Acid Orange 7 degradation in aqueous solutions
-
摘要: 采用无溶剂法制备了氧化锰八面体分子筛(OMS-2),通过XRD、FT-IR、SEM、TEM及N2吸附-脱附等温线等对其结构进行了表征,并以典型难降解偶氮染料酸性橙7(AO7)为目标污染物,考察了OMS-2活化过一硫酸氢盐(PMS)降解AO7的性能。研究结果发现,无溶剂法制备的OMS-2呈纳米棒状,为典型的锰钾矿型结构,比表面积达到129 m2·g-1,平均粒径10.5 nm左右。OMS-2催化剂能够高效催化PMS产生活性自由基降解偶氮染料,反应10 min内可使AO7几乎完全脱色,重复使用10次均能保持较高的催化稳定性;脱色降解后染料分子中的共轭体系和芳香环结构被破坏。Abstract: Cryptomelane-type manganese oxide, OMS-2, was prepared by a solvent-free process, and characterized by X-ray diffraction, scanning electron microscopy, N2 adsorption/desorption isotherms, and fourier transform infrared spectroscopy. The activity and stability of the catalyst were tested for degradation of Acid Orange 7 (AO7) in the presence of peroxymonosulfate. OMS-2 exhibited the typical cryptomelane phase with a nanorod morphology, and had a surface area of 129 m2·g-1 and crystallite size of 11 nm. The catalyst was quite active for AO7 degradation, with production of sulfate radicals;AO7 was completely decolorized within 10 min due to the destruction of its conjugate structure and naphthaquinone ring. The catalyst also exhibited a high stability over ten cycles.
-
Key words:
- cryptomelane type manganese oxide /
- peroxymonosulfate /
- Acid Orange 7 /
- degradation
-
铬(Cr)在金属表面处理、电镀、制革等领域有着广泛运用,在环境中主要以+6、+3价态形式存在。相比于Cr(Ⅲ),Cr(Ⅵ)更具危险性,对皮肤和呼吸道有强烈刺激作用,造成细胞毒性的同时还可诱发肝癌和肺癌[1]。以HCrO4−、CrO42-、Cr2O72- 3种形式存在的Cr(Ⅵ)均具有较高的溶解度和较强的迁移能力,容易在人体富集[2]。由于Cr(Ⅵ)具有极强的环境危害,相关污染事故频发,美国环保署将其列为优先污染物,我国将其列入5种重点重金属污染物名单。我国黄土高原和三门峡盆地的地下水中Cr(Ⅵ)浓度是GB/T 14848-2017《地下水质量标准》Ⅲ类标准的3.84~7.30倍[3-4],而湖南某污染场地地下水Cr(Ⅵ)的最高浓度可达Ⅲ类标准的2 180倍[5],此外,白云湖、东山湖、宿鸭湖等地表水也发现了不同程度的Cr(Ⅵ)污染,污染水平与当地工业发展相关,存在严重的生态风险[6]。因此,有必要研发安全、高效去除水体中Cr(Ⅵ)的方法。
纳米零价铁(nanoscale zero-valent iron, nZVI)比表面积大,其反应活性是普通微米或毫米级铁粉的数十倍甚至上百倍[7],可作为还原剂将Cr(Ⅵ)转化为毒性低、易沉淀、迁移能力差的Cr(Ⅲ),再通过控制pH即可将Cr(Ⅲ)从水中脱除[8]。但nZVI的缺点是易氧化或钝化、易团聚、电子选择性差,严重限制了在实践中的应用,因此,近年来关于nZVI的研究集中在使用改性手段克服上述缺陷,提高nZVI的修复性能[9]。由于活性炭具有丰富的孔隙结构和较大的比表面积,可为nZVI提供大量附着位点,因此,可减小nZVI颗粒间团聚趋势。此外,活性炭本身具有良好的吸附性能,也可通过吸附作用去除Cr(Ⅵ)[10]。聚天冬氨酸(polyaspartic acid, PASP)是一种绿色高分子材料,缓释性能良好,可通过静电排斥和空间位阻的双重作用,进一步降低nZVI粒径。作为缓释层,PASP一方面可起到有效的保护作用,提升材料抗氧化性能,另一方面可提高nZVI的电子选择性,提升使用性能[11]。
本研究使用PASP作为缓释剂,活性炭为载体,制备了缓释型nZVI-C复合材料。通过一系列表征、正交和吸附实验,明确了复合材料最佳制备条件,证实了复合材料对水中Cr(Ⅵ)具有优秀的去除效果,可为废水和天然水体中的Cr(Ⅵ)污染修复提供参考。
1. 材料与方法
1.1 实验试剂与仪器
实验所需试剂主要包括:七水合硫酸亚铁(FeSO4·7H2O)、无水乙醇(CH3CH2OH)、聚乙二醇(HO(CH2CH2O)nH)、硼氢化钾(KBH4)、重铬酸钾(K2Cr2O7)、氢氧化钠(NaOH)、硫酸(H2SO4)、盐酸(HCl)均为分析纯;PASP((C4H7NO4)x)为优级纯;二苯碳酰二肼(C13H14N4O)为色谱纯。高纯氮气(N2,99.999%)。本研究使用的黑色颗粒状活性炭购自天津科密欧试剂厂,纯度为定制分析纯。
实验所需仪器主要包括:X射线衍射仪(SmartLab(9),日本理学株式会社);扫描电子显微镜(SUPRATM55,德国蔡司股份有限公司);傅里叶变换红外光谱仪(MTN-
5800 ,日本理学株式会社);X射线光电子能谱(ESCALAB 250XI,美国赛默飞世尔科技有限公司);孔径比表面积分析仪(SSA-4300 ,北京众力挽生物科技有限公司)。1.2 材料制备方法
nZVI制备:将2.78 g的FeSO4·7H2O溶于20 mL水,同80 mL乙醇和3 g聚乙二醇一起加入三口烧瓶中,搅拌转速为700 r·min−1。在合成过程中持续通入氮气以保持无氧环境,通气20 min后将100 mL的pH=8,浓度为0.04 mol·L−1的KBH4溶液从恒压漏斗中滴入三口烧瓶,滴速控制为2滴·s−1。滴加完毕后继续搅拌50 min,反应机理如式(1)所示。
stringUtils.convertMath(!{formula.content}) (1) 复合材料制备:与制备nZVI的方法类似,在制备过程中使用PASP的乙醇溶液代替聚乙二醇,即在80 mL的乙醇中预先溶解0.5 g的PASP,并同活性炭加入三口烧瓶中。
1.3 最佳制备条件筛选
通过设计正交实验,研究4个因素(碳粒径、铁碳比、醇水体积比、PASP浓度)对nZVI去除Cr(Ⅵ)性能的影响,并通过极差和方差分析得出最佳制备条件和主要影响因素。每个因素设置3个水平,碳粒径设置60、100和200目;铁碳比设置2∶1、1∶2和1∶4;PASP质量百分比设置0.01%、0.1%和0.5%;醇水体积比设置4∶1、1∶1和3∶7。其中,醇与水体积比的设置参考了预实验结果,选取了醇水体积比为1:1,以及大于或小于1∶1时的最佳比例,探究3种比例下材料制备效果的差异。在不设空列时,可选取L9(34)正交表,如表1所示。选取对100 mg·L−1的Cr(Ⅵ)溶液的去除率作为评价标准,该浓度为电镀废水中的Cr(Ⅵ)相关浓度[12]。将9种不同条件下制得的复合材料加入含Cr(Ⅵ)溶液的离心管,溶液体积为40 mL,C-PASP-nZVI投加量为1.25 g·L−1,pH=2,放置在25 ℃的恒温振荡器中以220 r·min−1振荡24 h后取样过滤。
表 1 正交实验表Table 1. Orthogonal experimental table实验号 碳粒径/目 铁碳比 PASP质量百分比/% 醇水体积比 1 60 2:1 0.01 4:1 2 60 1:2 0.10 1:1 3 60 1:4 0.50 3:7 4 100 2:1 0.10 3:7 5 100 1:2 0.50 4:1 6 100 1:4 0.01 1:1 7 200 2:1 0.50 1:1 8 200 1:2 0.01 3:7 9 200 1:4 0.10 4:1 1.4 材料表征
采用X射线衍射仪(XRD)分析晶体结构,将图像经MDI Jade 6软件平滑处理;扫描电子显微镜(SEM)用于观测材料表面形貌;傅里叶变换红外光谱仪(FTIR)用于分析材料表面物质结构以及PASP稳定nZVI颗粒机制;使用X射线光电子能谱(XPS)对材料表面元素价态进行分析,使用Avantage软件进行分峰拟合;孔径比表面积分析仪(BET)用于分析不同材料的比表面积。
1.5 材料性能测试及影响因素分析
本实验中Cr(Ⅵ)的质量浓度均使用二苯炭酰二肼分光光度法,酸性条件下使用紫外可见分光光度计(732,中国)在540 nm波长下测定。对比C-PASP-nZVI与nZVI、活性炭去除Cr(Ⅵ)的效果时,分别向含有Cr(Ⅵ)溶液的离心管中加入一定量的C-PASP-nZVI、nZVI、活性炭,其中C-PASP-nZVI投加量为1.25 g·L−1,nZVI、活性炭的投加量依正交实验结果中的最佳铁碳比而定。溶液体积不变,pH=2,Cr(Ⅵ)质量浓度设置为150 mg·L−1,放置在25 ℃恒温振荡器中以220 r·min−1振荡。分别在5、10、30、60、120、240、360、480、600、720 min时用注射器过滤取样。
C-PASP-nZVI投加量、污染物质量浓度和初始pH可能影响对Cr(Ⅵ)的去除效果。在探究C-PASP-nZVI投加量对去除Cr(Ⅵ)的影响时,分别取0.01、0.03、0.05 g材料加入含Cr(Ⅵ)溶液离心管,溶液体积为40 mL,Cr(Ⅵ)质量浓度为150 mg·L−1,pH=2,放置在25 ℃恒温振荡器中以220 r·min−1振荡。分别在5、10、30、60、120、240、360、480、600、720 min时用注射器过滤取样。
探究污染物浓度对去除Cr(Ⅵ)的影响时,溶液体积不变,Cr(Ⅵ)质量浓度分别设置为100、150、200 mg·L−1,C-PASP-nZVI投加量为1.25 g·L−1,pH=2,在相同条件下振荡并在相同时间间隔用注射器过滤取样。
探究pH对去除Cr(Ⅵ)的影响时,分别配置pH=2、4、6的Cr(Ⅵ)溶液,此处pH梯度的选择参考了实际工程中电镀废水的初始pH区间[13]。溶液体积不变,C-PASP-nZVI投加量为1.25 g·L−1,在相同条件下振荡并在相同时间间隔用注射器过滤取样。
1.6 吸附动力学实验
实验后进行吸附动力学研究,以Cr(Ⅵ)质量浓度的变化来表征反应速率v的准一级(式(2))和准二级(式(3))反应动力学方程拟合分析吸附过程。
stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) 式中:t为反应时间,min;C0和Ct分别表示初始和反应时间为t时液相中Cr(Ⅵ)的质量浓度,mg·L−1;k1为准一级吸附动力学速率常数,min−1;k2为准二级吸附动力学速率常数,g·(mg·min)−1。
2. 结果与讨论
2.1 最佳制备条件
对正交实验结果进行极差分析,如表2所示,表中不同的K值表示该代表因素的某水平下的去除率和,例如K1(碳粒径)表示碳粒径为60目时,对应去除率的和为295.860,K2(碳粒径)则表示100目时的去除率之和,以此类推。K值越大,说明该因素在该水平下对应的去除率越高。通过选择各因素最大K值所对应的水平,即可得到最佳制备条件。由表2中的极差R值可见,碳粒径是对去除率的影响最大的因素,这可能是因为碳粒径的变化直接影响活性炭的吸附能力,从而影响负载活性炭上nZVI颗粒与Cr(Ⅵ)的接触概率。对去除率的影响顺序为碳粒径>PASP浓度>铁碳比>醇水体积比。最佳制备条件为:碳粒径为200目,铁碳比为1∶2,PASP浓度为0.5%,醇水体积比为4∶1。
表 2 极差分析表Table 2. Range analysis table控制因素 K1 K2 K3 极差R 最优水平 碳粒径 295.860 271.878 296.308 8.143 200目 铁碳比 276.111 296.113 291.822 6.667 1∶2 PASP浓度 294.243 274.526 295.277 6.917 0.5% 醇水体积比 294.199 293.824 276.023 6.059 4∶1 对实验结果进行方差分析可以判断各因素对实验指标的影响是否显著,弥补极差分析的不足。方差分析结果如表3所示,碳粒径、铁碳比、PASP浓度、醇水体积比对C-PASP-nZVI材料去除Cr(Ⅵ)的性能均有非常显著影响。
表 3 方差分析表Table 3. Analysis of variance table变异来源 偏差平方和 自由度 方差 F比 F分布 显著水平 碳粒径 390.795 2 195.397 20.557 F0.10(2,18)=2.62F0.05(2,18)=3.55F0.01(2,18)=6.01 P<0.01 铁碳比 221.783 2 110.892 11.666 P<0.01 PASP浓度 273.517 2 136.759 14.388 P<0.01 醇水体积比 215.718 2 107.859 11.347 P<0.01 误差e 171.093 18 9.505 碳粒径的变化会直接导致比表面积的改变,这一方面会影响其对Cr(Ⅵ)的吸附性能进而影响去除率;另一方面也会导致可供nZVI颗粒附着点位数量的变化,影响负载活性炭上nZVI颗粒与Cr(Ⅵ)的接触几率。因此,碳粒径和铁碳比对C-PASP-nZVI材料去除Cr(Ⅵ)性能有显著影响。在合成过程中,PASP可通过空间位阻与静电排斥的双重作用稳定纳米铁颗粒,而在与污染物的反应过程中又具有一定的缓释作用,可减少nZVI颗粒与非目标物质的反应概率[14]。该过程解释了PASP浓度对Cr(Ⅵ)去除率的显著影响。醇水体积比也会影响吸附性能,是因为在nZVI合成过程中,添加适量可与水互溶的醇类可将nZVI颗粒表面吸附的水分子替换为相应的醇分子,有利于减少颗粒间的团聚[15]。另一方面,醇类的介电常数小于水,在一定范围内,反应溶液的介电常数越低,nZVI颗粒尺寸越小,而过高的醇水体积比也会影响材料的合成效果,因此选取最佳的醇水体积比对提高材料的吸附性能至关重要[16]。
2.2 材料表征
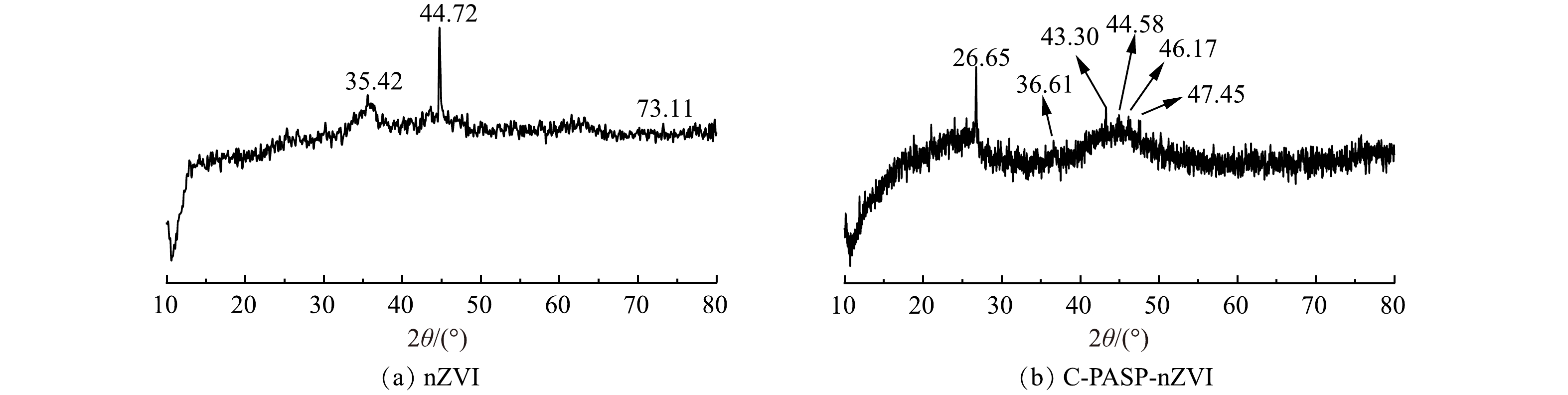
1) XRD分析。图1所示为本实验制备的nZVI和C-PASP-nZVI颗粒的XRD图谱。由图1(a)可看出,当扫描衍射角度(2θ)在10°~80°时,衍射峰对应Fe3O4的330晶面衍射(35.42°)、Fe0的110晶界衍射(44.72°)、FeOOH的042晶面衍射(73.11°)或FeO的042晶界。Fe0的110晶界衍射峰可表明nZVI成功合成。nZVI是典型核壳结构,在铁核周围存在一层铁氧化物层[17]。由XRD图谱可见,本实验中nZVI颗粒表面的铁氧化物层可能由Fe3O4、FeOOH和FeO构成。图1(b)中的衍射峰分别对应C的004晶界衍射(26.65°)、FeOOH的111晶界衍射(36.61°)、C的101晶界衍射(43.30°)、Fe0的110晶界衍射(44.58°)、C的104晶界衍射(46.17°)、C的103晶界衍射(47.45°)。C与Fe0的相关晶界衍射峰的出现表明nZVI已成功负载于活性炭上。对比nZVI颗粒的XRD图,Fe0的110晶界衍射峰变得相当微弱且峰型弥散,说明经活性炭及PASP改性的nZVI呈现微晶态,复合材料由小晶粒组成且结晶度较差[18]。
2) SEM分析。图2为本实验3种材料的SEM图。由图2(a)可看出,200目活性炭具有丰富的孔隙结构,可为nZVI和Cr(Ⅵ)提供较多的吸附位点。由图2(b)可看出,nZVI呈球形,颗粒间因为磁性、范德华力、表面能过高等原因相互吸引并团聚,呈现树枝般链状结构,颗粒平均粒径为105.4 nm。由图2(c)可知,nZVI吸附在活性炭表面,有效降低了nZVI的团聚,复合材料的粒径相比nZVI有所减小,改性符合预期效果。
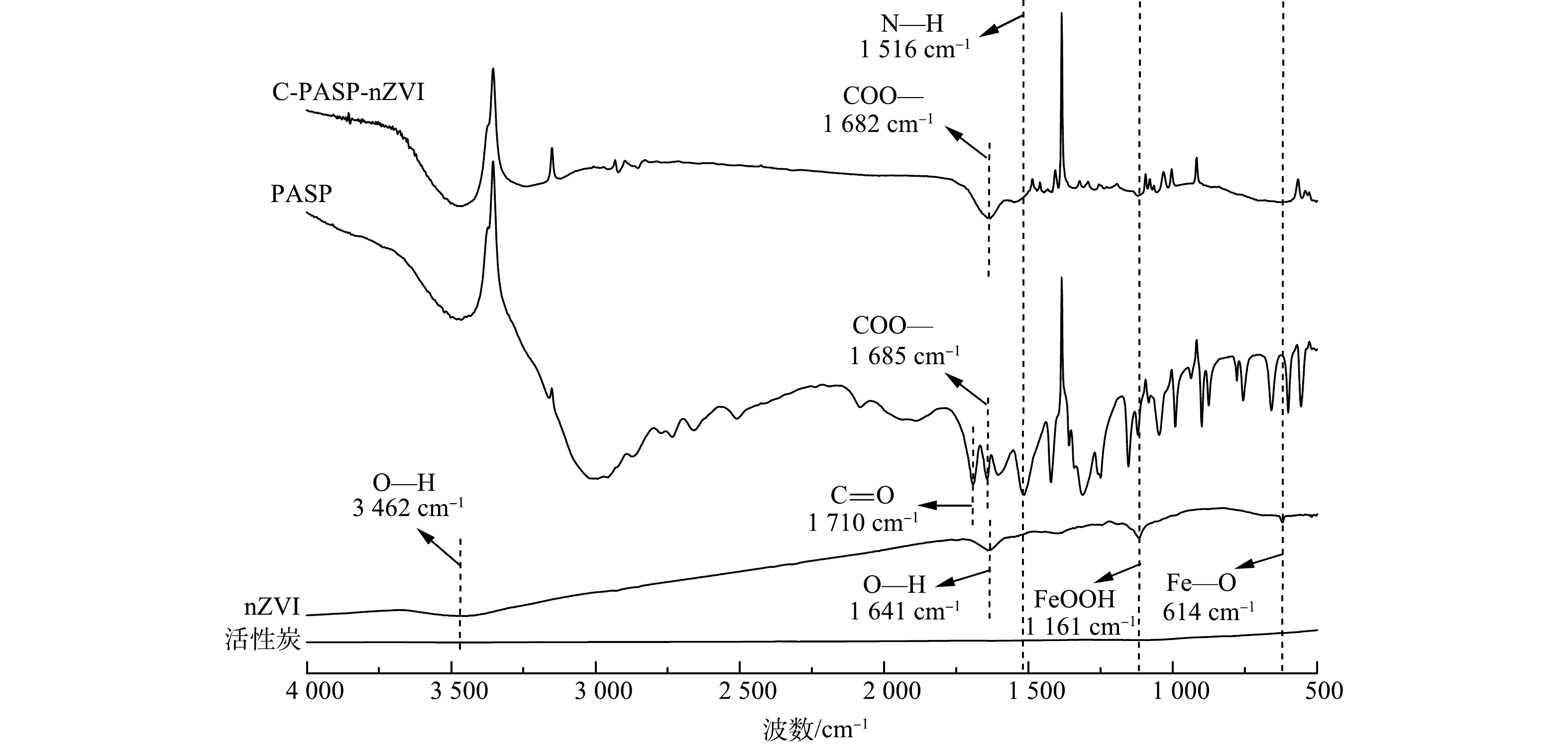
3) FTIR分析。图3为3种材料和PASP的傅里叶变换红外图谱。通过对比可发现,指纹区1 000~1 500 cm−1处频繁出现的各峰、1 516 cm−1处的N—H的变形振动峰、1 682 cm−1处的羧酸根反对称伸缩振动峰(对应PASP的1 685处的吸收峰)在C-PASP-nZVI及PASP上出现而未在nZVI上出现,可推断PASP已成功结合于材料表面。nZVI在614 cm−1处的宽吸收带由Fe—O键引起,1 161 cm−1处是FeOOH的特征峰,1 641 cm−1处的吸收峰由样品中的O—H键引起,3 462 cm−1处的吸收峰是O—H的伸缩振动,这与SINGH等[19]的研究结果相似,说明nZVI表面的铁氧化物层中含有FeOOH。PASP是天冬氨酸的氨基与羧基缩水而成的聚氨基酸类物质,分子式为(C4H7NO4)x。PASP中的羧基与金属间可以以双齿桥联、单齿螯合、双齿螯合3种方式结合[20]。由图3可知,C-PASP-nZVI在1 710 cm−1处未出现C=O的吸收峰,样品的羧酸根反对称吸收峰与对称吸收峰位置之差为259 cm−1,而作为稳定剂,PASP的吸收峰之差为264 cm−1,二者数值接近,因此,PASP与nZVI间以双齿桥联的方式结合。
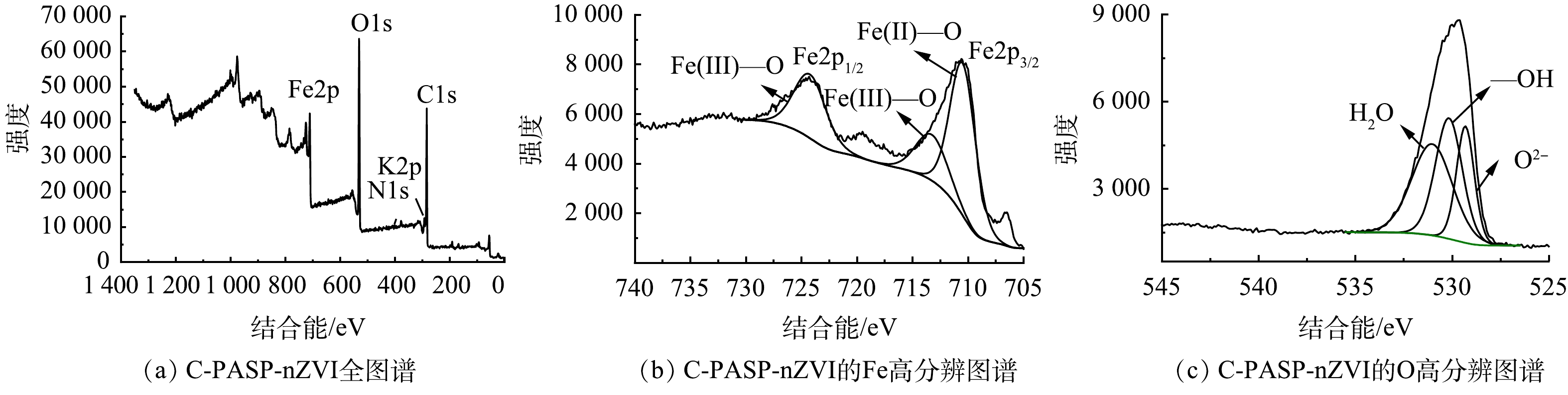
4) XPS分析。图4(a)中出现了Fe2p、O1s、C1s、N1s、K2p衍射峰。其中K元素的出现可能是因为材料合成过程中表面残留了KBH4;N元素的出现证实PASP存在于材料表面,这与FTIR的分析结果一致。对Fe和O元素进行高分辨扫描,图4(b)中没有出现Fe0的特征谱线,但XRD的测试结果已经证实了C-PASP-nZVI中Fe0的存在。这可能是因为XPS的探测深度有限,只有靠近表面的电子无能量损失,一般而言金属和金属氧化物的探测深度仅有2 nm左右,本研究中PASP覆于材料表面,使得位于更深处的Fe0的光电子无法逸出[21]。由图4(c)可见,O的高分辨扫描中529.1 eV处的特征峰对应O2-,531.8 eV处的特征峰对应-OH,532.1 eV处对应H2O。其中531.8 eV处的特征峰归因于nZVI表面铁氧化物层中的FeOOH,与FTIR的分析结果一致,表明成功合成了C-PASP-nZVI。
5) BET分析。通过BET分析测得200目活性炭、nZVI、C-PASP-nZVI的比表面积分别为503.5、30.0、213.5 m2·g−1。可见200目活性炭可为nZVI颗粒提供大量的吸附位点,与活性炭的SEM检测结果一致,本研究中制备的nZVI比表面积在常规范围内[22]。活性炭与nZVI的复合显著提高了nZVI的比表面积,而对比活性炭本身有大幅下降。由图2中的SEM表征结果可知,这归因于nZVI往往附着于活性炭表面,因此,复合材料比表面积介于nZVI与活性炭之间。
2.3 复合材料性能测试
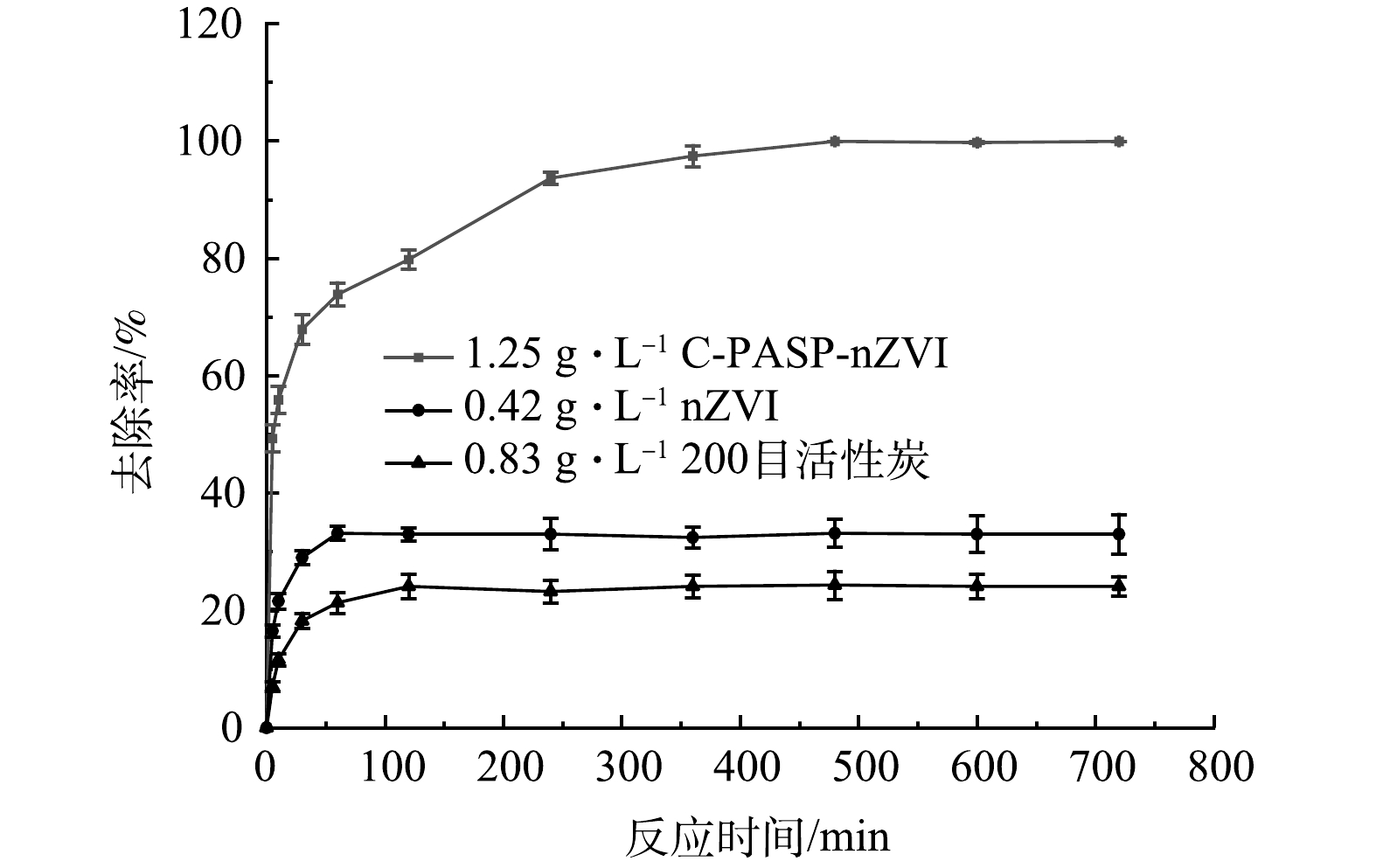
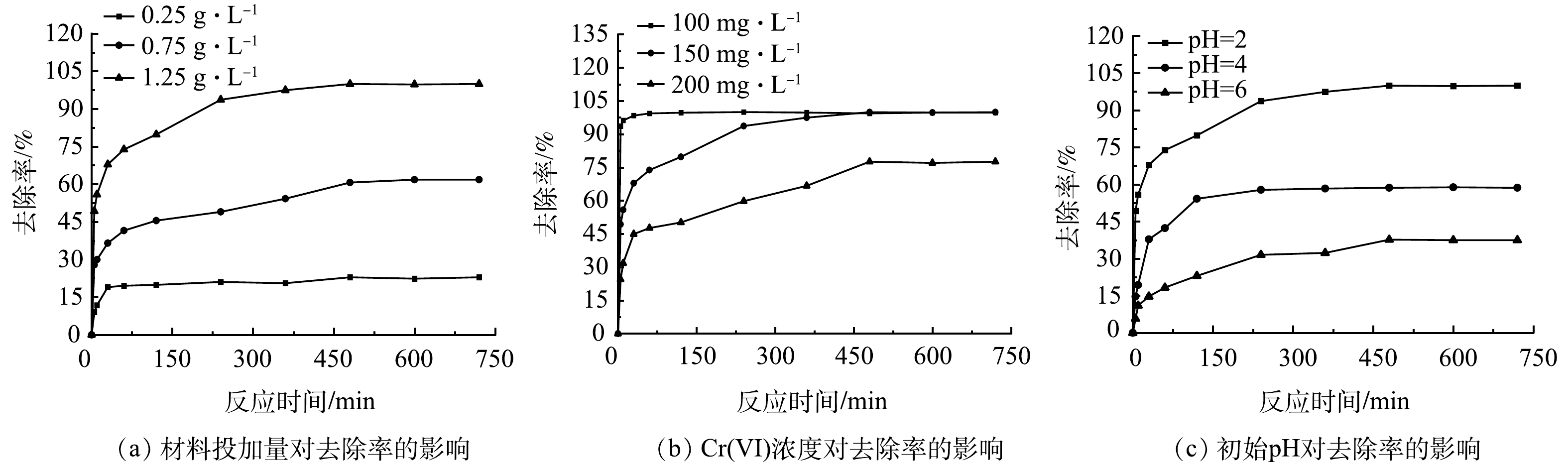
3种吸附材料对Cr(Ⅵ)的去除效果如图5所示。可以看出,200目活性炭及nZVI对Cr(Ⅵ)都有一定的去除效果,活性炭去除率最高为24.1%,而本研究中单独的PASP溶液不能去除Cr(Ⅵ),说明C-PASP-nZVI去除Cr(Ⅵ)的过程是活性炭上负载的nZVI颗粒起主导作用。活性炭与nZVI最高去除率的加和为57.0%,显著低于C-PASP-nZVI材料99.9%的去除率。说明PASP与活性炭对nZVI颗粒起到了很好的协同保护作用,有效提升了C-PASP-nZVI去除Cr(Ⅵ)的性能。使用化学法制作工艺生产的nZVI已被用于实地污染修复,该方法材料成本约为50元·kg−1[23]。另外,考虑本研究中的复合材料制作方法和活性炭、PASP成本,与单独的nZVI相比,C-PASP-nZVI可用1.3倍左右的预算成本取得1.75倍的修复效果,具有良好的经济和环境效益[24-25]。由图5可知,nZVI对与Cr(Ⅵ)的反应在70 min左右达到平衡,而C-PASP-nZVI对Cr(Ⅵ)的平衡时间在470 min左右。一方面,这是因为200目活性炭也有一定的吸附作用且反应平衡时间大于nZVI;另一方面,PASP具有良好的缓释性能,反应初期有相当一部分被PASP包裹的nZVI颗粒并未立即暴露在表面,而是随着反应的进行逐渐裸露出来,因此,达到反应平衡所需时间大幅延长。以上结果说明PASP起到了预期的缓释作用,有助于提高C-PASP-nZVI的吸附容量,显著提升对Cr(Ⅵ)的去除效果。
2.4 复合材料对Cr(Ⅵ)去除的影响因素
1) C-PASP-nZVI投加量对去除率的影响。修复材料的投加量是污染物去除过程中需要考虑的重要因素。由图6(a)可知,Cr(Ⅵ)去除率随着C-PASP-nZVI的投加量的增加而升高,这是因为吸附活性位点随吸附剂投加量增加而增多。此外,反应平衡时间也随C-PASP-nZVI投加量的增加而延长。当C-PASP-nZVI投加量较低时无法去除全部Cr(Ⅵ),Fe0在较短时间内就达到吸附平衡,随着投加量的增加,可与Cr(Ⅵ)反应的Fe0增加,反应平衡时间随之延长。随着投加量由0.25 g·L−1增至1.25 g·L−1,C-PASP-nZVI对Cr(Ⅵ)的吸附量也由133.4 mg·g−1降至120 mg·g−1。这是因为一方面随着C-PASP-nZVI投加量的提高,吸附点位相对于Cr(Ⅵ)而言变得过量,从Fe0传递出来的电子并未全部被Cr(Ⅵ)接受,而是传递给溶液中的H+或O2,从而造成去除效果的下降[26]。另一方面,随着反应的进行,Cr(Ⅵ)被还原成Cr(Ⅲ)并与Fe(Ⅲ)形成共沉淀,沉积在材料表面阻碍Fe0的电子转移,从而造成处理能力的下降[27]。
2)污染物浓度对去除率的影响。由图6(b)可知,随着Cr(Ⅵ)质量浓度的上升,固定浓度的C-PASP-nZVI对Cr(Ⅵ)去除率逐渐降低。Cr(Ⅵ)质量浓度为100 mg·L−1时,浓度为1.25 g·L−1的C-PASP-nZVI可迅速将溶液中的Cr(Ⅵ)去除,Cr(Ⅵ)质量浓度升至150 mg·L−1时反应平衡时间变长但Cr(Ⅵ)的去除率变化不大。Cr(Ⅵ)质量浓度继续增长至200 mg·L−1时,Cr(Ⅵ)的最高去除率降至77.0%,说明固定浓度的修复材料污染物去除能力有限。另一方面,Cr(Ⅵ)质量浓度从100 mg·L−1增加至200 mg·L−1时,C-PASP-nZVI的去除量由80 mg·g−1增至123.2 mg·g−1,表明在一定范围内,污染物浓度的升高有助于电子从铁核向材料表面迁移并传递给溶液中的Cr(Ⅵ)[28]。
3)初始pH对去除率的影响。pH的改变会影响水体理化性质,同时影响nZVI在水中的腐蚀,因此是影响材料修复效果的重要因素[29]。由图6(c)可见随着pH的升高,去除效果显著变差。pH=4时去除率约为58%,pH=6时将至37%左右。这是因为pH较低时,C-PASP-nZVI表面更容易荷正电,此时带负电荷的Cr2O72-更容易被吸引,增大了接受电子的概率从而促使Cr(Ⅵ)被还原为Cr(Ⅲ),pH较高时则相反[30]。pH过高还不利于Fe0的腐蚀,从而抑制了Cr(Ⅵ)还原[31]。此外,较高pH下溶液中的Cr(Ⅲ)与Fe(Ⅲ)将会在材料表面形成氢氧化物钝化层,阻碍nZVI内部的电子向外转移[32]。
2.5 吸附动力学
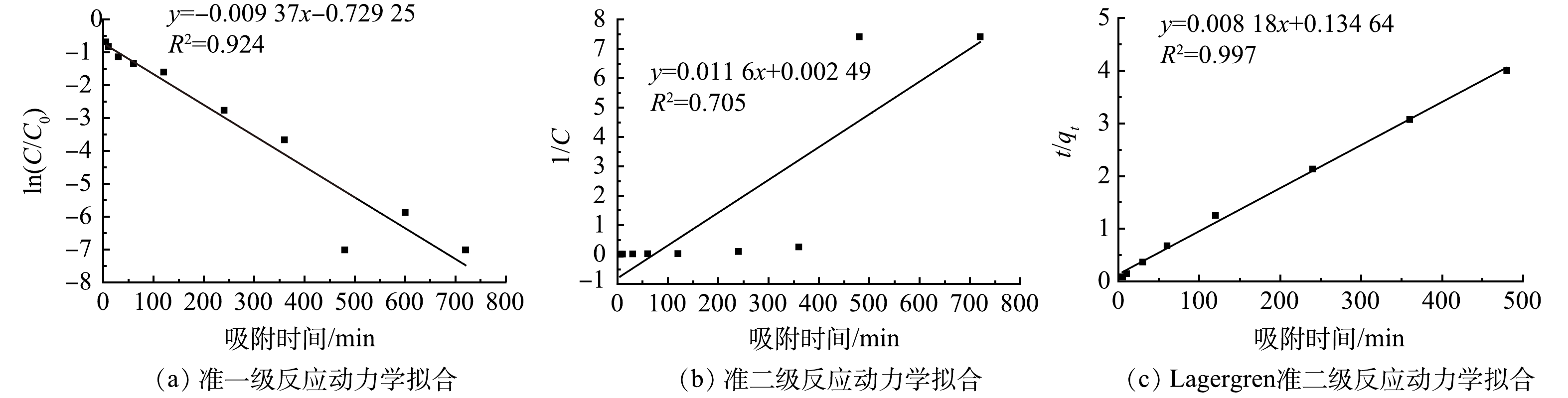
由以上研究结果,选定复合材料去除效果最好的条件并做反应动力学研究,即pH=2,C-PASP-nZVI投加量=1.25 g·L−1,Cr(Ⅵ)质量浓度=150 mg·L−1。以Cr(Ⅵ)质量浓度的变化来表征反应速率的准一级反应动力学模型和准二级反应动力学模型对动力学过程进行拟合,结果分别如图7(a)和图7(b)所示。对比可知,ln(C/C0)对t有较强的线性相关性(R2=0.924),但拟合的回归方程未经过原点,说明吸附在整个反应过程中有着极为重要的作用[33]。因此,采用Lagergren准二级吸附动力学模型进行拟合,如图7(c)所示,可见t/qt与t具有很好的线性相关性(R2>0.99)。反应动力学过程高度符合Lagergren准二级模型,表明化学吸附在C-PASP-nZVI对Cr(Ⅵ)的去除过程中占主导作用[34]。
3. 结论
1)在C-PASP-nZVI材料制备过程中,碳粒径对材料的吸附性能影响最大,铁碳比、PASP浓度、醇水体积比对吸附性能也均有明显的影响,最佳制备条件为:碳粒径为200目,铁碳比为1∶2,PASP质量百分比为0.5%,醇水体积比为4∶1。
2)以活性炭为基底负载nZVI,并以PASP为缓释剂制得的复合材料呈微晶态,活性炭有效缓解了nZVI的团聚,复合材料粒径显著减小,比表面积相对nZVI显著提升。PASP与nZVI间以双齿桥联的方式结合。
3)活性炭与PASP对nZVI颗粒起到了很好的协同保护作用,活性炭增加了nZVI的吸附位点,显著提高了吸附容量,PASP对nZVI的缓释可延长反应平衡时间,减少了Fe0传递出来的电子与非目标物质结合的概率,进而提升了修复效果。
4)提高C-PASP-nZVI投加量、降低溶液pH是促进Cr(Ⅵ)去除的有效手段,Cr(Ⅵ)质量浓度=150 mg·L−1,C-PASP-nZVI投加量=1.25 g·L−1,pH=2时去除率高达99.91%。反应动力学过程符合Lagergren准二级模型,说明C-PASP-nZVI对Cr(Ⅵ)的去除过程以化学吸附为主导。
-
[1] 张宇峰, 滕洁, 张雪英, 等. 印染废水处理技术的研究进展. 工业水处理,2003, 23(4): 23-27 ZHANG Yufeng, TENG Jie, ZHANG Xueying, et al. Progress of the researches on dyeing wastewater treatment techniques. Industrial Water Treatment, 2003, 23(4): 23-27(in Chinese) [2] 钟恒, 曹文彪, 熊重铎, 等. 铈离子光催化降解茜素绿的性能和机理. 环境工程学报, 2014,8(2): 448-452 ZHNG Heng, CAO Wenbian, XIONG Zhongduo, et al. Activity and mechanism of photocatalytic degradaion of Alizan Green by cerium ions. Chinese Journal of Environmental Engineering, 2014,8(2): 448-452(in Chinese) [3] LUO M., LYU L., DENG G., et al. The mechanism of bound hydroxyl radical formation and degradation pathway of Acid Orange II in Fenton-like Co2+-HCO3- system. Applied Catalysis A: Genera, 2014, 469: 198-205 [4] KHAN J. A., HE X, KHAN H. M., et al. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82-/Fe2+ and UV/HSO5-/Fe2+ processes: A comparative study. Chemical Engineering Journal, 2013, 218: 376-383 [5] NFODZO P., CHOI H. Triclosan decomposition by sulfate radicals: Effects of oxidant and metal doses. Chemical Engineering Journal, 2011, 174(2/3): 629-634 [6] ZHANG J., SHAO X., SHI C., et al. Decolorization of Acid Orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon. Chemical Engineering Journal, 2013, 232: 259-265 [7] CHEN X., CHEN J., QIAO X., et al. Performance of nano-Co3O4/peroxymonosulfate system: Kinetics and mechanism study using Acid Orange 7 as a model compound. Applied Catalysis B:Environmental, 2008, 80(1/2): 116-121 [8] SHI P., SU R., ZHU S., et al. Supported cobalt oxide on graphene oxide: Highly efficient catalysts for the removal of Orange II from water. Journal of Hazardous Materials, 2012, 229: 331-339 [9] JI F., LI C., WE Xi. Efficient performance of porous Fe2O3 in heterogeneous activation of peroxymonosulfate for decolorization of Rhodamine B. Chemical Engineering Journal, 2013, 231: 434-440 [10] LIU J., ZHAO Z., SHAO P., et al. Activation of peroxymonosulfate with magnetic Fe3O4-MnO2 core-shell nanocomposites for 4-chlorophenol degradation. Chemical Engineering Journal, 2015, 262: 854-861 [11] ZHANG T., ZHU H., CROU J. P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environmental Science & Technology, 2013,47(6): 2784-2791 [12] SAPUTRA E., MUHAMMAD S., SUN H., et al. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Applied Catalysis B:Environmental, 2013, 142-143: 729-735 [13] CHEN X., SHEN Y., SUIB S. L., et al. Catalytic decomposition of 2-propanol over different metal-cation-doped OMS-2 materials. Journal of Catalysis, 2001, 197(2): 292-302 [14] KUMAR R., SITHAMBARAM S., SUIB S. L. Cyclohexane oxidation catalyzed by manganese oxide octahedral molecular sieves: Effect of acidity of the catalyst. Journal of Catalysis, 2009, 262(2): 304-313 [15] YANG Y., HUANG J., ZHANG S., et al. Catalytic removal of gaseous HCBz on Cu doped OMS: Effect of Cu location on catalytic performance. Applied Catalysis B: Environmental, 2014, 150: 167-178 [16] 刘雪松,鲁继青,王晓霞,等.OMS-2的制备及其负载PdO对CO氧化的催化活性. 催化学报, 2010, 3(2): 181-185 LIU Xuesong, LU Jiqing, WANG Xiaoxia, et al. Preparation of manganese oxide octahedral molecular sieve and catalytic activity of its supported PdO for CO oxidation. Chinese Journal of Catalysis, 2010, 3(2): 181-185(in Chinese) [17] LUO S., DUAN L., SUN B., et al. Manganese oxide octahedral molecular sieve (OMS-2) as an effective catalyst for degradation of organic dyes in aqueous solutions in the presence of peroxymonosulfate. Applied Catalysis B:Environmental, 2015, 164: 92-99 [18] DUAN L., SUN B., WEI M., et al. Catalytic degradation of Acid Orange 7 by manganese oxide octahedral molecular sieves with peroxymonosulfate under visible light irradiation. Journal of Hazardous Materials, 2015, 285: 356-365 [19] 王燕彩, 刘昕, 宁平, 等. 制备方法对氧化锰八面体分子筛的NH3选择性催化还原NOx性能的影响. 燃料化学学报, 2014, 42(11): 1357-136 WANG Yancai, LIU Xin, NING Ping, et al. Effect of preparation methods on selective catalytic reduction of NOx with NH3 over manganese oxide octahedral molecular sieves. Journal of Fuel Chemistry and Technology, 2014, 42(11): 1357-136 [20] DING Y., SHEN X., SITHAMBARAM S., et al. Synthesis and catalytic activity of cryptomelane-type manganese dioxide nanomaterials produced by a novel solvent-free method. Chemistry of Materials, 2006, 17(21): 5382-5389 [21] 余林, 孙明, 余坚, 等. 锰八面体分子筛的合成、表征及其对二甲醚燃烧的催化性能. 催化学报, 2008, 29(11): 1127-1132 YU Lin, SUN Ming, YU Jian, et al. Synthesis and characterization of manganese oxide octahedral molecular sieve catalyt for DME combustion. Chinese Journal of Catalysis, 2008, 29(11): 1127-1132(in Chinese) 期刊类型引用(6)
1. 沈悦,曹鸿健,刘晓恬,廖用开,蔡超. OMS-2活化过硫酸盐降解有机污染物. 中国环境科学. 2025(04): 1939-1950 .  百度学术
百度学术
2. 许一凡,陈思,鱼涛,陈帅,闫凤平,屈撑囤. 基于PMS活化的类Fenton高级氧化技术在水处理中的应用. 石油化工应用. 2024(04): 1-8 .  百度学术
百度学术
3. 李向奎,肖河,游少鸿,何慧军,李洁月,黄宏伟,俞果,蒋萍萍. CoMn@AC活化过一硫酸氢盐降解水中罗丹明B. 环境工程. 2022(04): 14-21 .  百度学术
百度学术
4. 宋珍霞,蔡长青,赵晓宇,邾文婷. ZIF-67吸附AO_7的动力学特性研究. 安全与环境学报. 2020(05): 1871-1878 .  百度学术
百度学术
5. 何若南,徐润,牛传峰. 锰氧八面体分子筛的制备及应用研究进展. 化工进展. 2018(S1): 125-132 .  百度学术
百度学术
6. 徐浩,张静,张古承,叶倩,周冠宇,杨富花. 钴锰双金属氧化物催化过硫酸氢钾降解酸性橙7的研究. 中国环境科学. 2017(08): 2963-2969 .  百度学术
百度学术
其他类型引用(8)
-

 点击查看大图
点击查看大图
计量
- 文章访问数: 1820
- HTML全文浏览数: 1450
- PDF下载数: 463
- 施引文献: 14





 DownLoad:
DownLoad: