-
自然界中,砷(As)常以硫化物/氧化物或铜、镍、铁等砷化物的形式存在. 接触高浓度的砷会对健康造成严重影响,国际癌症研究机构(IARC)和美国环境保护署(EPA)分别将砷列为“1”类和“A”类致癌物质,世界卫生组织(WHO)规定饮用水中砷的最高允许浓度为10 μg·L−1. 矿区尾矿不合理堆存[1]、地热与火山活动[2]、农业生产[3]等都会造成砷污染,严重影响生态环境和人类生命健康.
针对地表土壤和径流中的砷,人们开发出各种除砷方法与工艺,包括化学淋洗[4]、化学钝化/稳定[5]、植物修复[6]、微生物修复[7-8]、电化学法[9]、膜分离[10]、可渗透反应墙(PRB)[11]等,取得良好的脱砷和固砷效果. 其中,基于原位修复技术的PRB方法具有除砷效率高、成本低、使用时效长等特点,特别适用于受污染地下水的修复[11]. PRB是一个填充有反应活性材料的原位处理区,填充材料能够选择性滞留、转化或稳定化流经该墙体的污染组分,从而达到治理污染物的目的. PRB处理系统不占用地面空间,无需外加动力系统,检测与维护简单[12].
填充材料是PRB的反应主体,其拦截性能、寿命、稳定性、经济性、施工可行性、安全性、环保性等都是PRB工程技术的关键要素. PRB填充材料在工程应用前,需要从污染物特性和场地水文地质条件等出发,开展性能评价、除砷机理等实验研究确定其组成与性能[13].
本文在综述PRB除砷反应填料及其固砷机理的基础上,简要介绍除砷PRB填充材料的实验研究,并以PRB除砷工程/中试为例阐明PRB的除砷效果,以促进我国地表径流砷污染治理PRB材料和工程技术的开发.
-
黏土矿物、碳基材料、钙基材料、铁基材料和固体废弃物等是常见的除砷材料. 针对不同特征的砷污染水体,可选取合适的填充材料或将其进行改性、联用实现对水体砷及其伴生污染物的去除.
-
除砷功能较好的黏土矿物主要为含水层状铝/镁硅酸盐,其中的硅氧四面体和铝/镁氧八面体由氧桥键连接,晶格中的铝/镁金属离子常与铁发生类质同象置换. 黏土矿物孔结构复杂、比表面积大,可与溶液中的砷类化合物(As(Ⅲ)/As(Ⅴ)氧阴离子)发生物理和化学作用,如吸附、离子交换、静电作用等. 同时,黏土矿物来源广泛、价格低廉、绿色环保,在砷及其伴生污染物的处理方面具有较好的应用潜力. 然而,黏土矿物孔尺寸或层间距小,与砷及其伴生污染物作用后,容易堵塞孔隙,造成吸附容量下降,故增大黏土矿物孔尺寸或层间距的研究受到广泛关注.
1:1型层状黏土矿物有蛇纹石族、高岭石族等;2:1型包括滑石族、蒙皂石族、蛭石族、绿泥石族、海泡石族、坡缕石族等. 其中,蛇纹石、绿泥石等层间域呈现一定的惰性[14],改性较困难,其应用于废水处理较少见;而高岭土(主要成分为高岭石)和膨润土作为脱砷材料研究较多,两种材料的比表面积较大、离子交换能力较强、可溶性Al可与As反应生成沉淀[15],材料简单改性后的除砷性能可得到有效提升. 为强化黏土矿物的除砷效果,机械活化、热活化、酸/碱活化、金属负载或插层/柱撑等改性方法被广泛采用.
-
Arnamwong等[15]利用高岭土作为土壤改良剂来降低砷在稻谷中的含量,高岭土溶出的Al3+能形成Al(OH)3促进砷氧阴离子沉淀,同时,砷氧阴离子可与高岭土表面的氢氧根发生离子交换,形成Al-O-As氧化物沉淀,从而减少砷的溶出而被水稻吸收. 然而,部分黏土矿物的零电点(pHpzc)较低,表现出较高的阳离子吸附活性,而对砷氧阴离子显现出静电排斥,不利于其吸附于黏土矿物表面. 而经Fe2+、Fe3+、Al3+等阳离子预处理,可以增强黏土矿物对含氧阴离子的吸附亲和力.
Doušová等[16]将高岭土经Fe2+、Mn2+离子处理后,Fe(Ⅱ)/Mn(Ⅱ)物种先吸附于其表面,随后砷(As(Ⅲ/Ⅴ))氧阴离子自发聚集于水合Fe/Mn物种上. 虽然砷氧阴离子在改性高岭土(Fe/Mn-O@高岭土)上的平衡吸附时间长,但As-O@Fe/Mn-O@高岭土吸附产物稳定,砷脱除效果较好.
采用有机物插层方法可活化高岭土[17],但会在除砷时带入二次污染物. 而经过机械活化、热活化或酸/碱活化等处理,高岭土的除砷效果将有所提升,但目前研究较少.
-
膨润土由蒙脱石、高岭石、绿泥石等矿物组成,其中蒙脱石含量一般超过65%. 天然蒙脱石的吸附性能一般,常经过改性后才用作吸附剂[18].
由于铁基材料对砷氧化合物的亲和力强,膨润土/蒙脱石常用作铁物种的载体而应用于除砷领域. Shabani等[19]制备出四氧化三铁@膨润土纳米复合颗粒,其对水溶液中As和Cu均具有良好去除效果. Michael-Leo等[20]将零价铁负载于膨润土,显著增加砷酸根吸附位点,砷的去除转为化学吸附控制. Barakan等[21]发现,天然膨润土经Fe3+浸渍后,其多孔结构中分布大量Fe(Ⅲ)活性物种,增强了砷吸附能力,溶液中砷的吸附率可达99%,残余砷浓度低于WHO标准. Fe(Ⅲ)@膨润土吸附As(Ⅴ)的机理见图1[21],其表明,As(Ⅴ)在Fe(Ⅲ)@膨润土界面可形成稳定的双齿或单齿Fe-O-As络合物. Buzetzky等[22]发现,Fe3+离子交换改性钙基膨润土所得Fe(Ⅲ)@钙基膨润土对亚砷酸盐阴离子的吸附能力较弱. 蒙脱石层间距一般为1—2 nm,Fe3+浸渍后层间距略有增大,但Fe3+与(亚)砷酸根的反应会堵塞层间Fe3+的释放,使得Fe(Ⅲ)@膨润土吸附砷的容量降低.
将Al、Ti、Fe等的聚合羟基阳离子插层于黏土中则显著增大层间距,得到高比表面积的柱撑黏土[23],这些无机聚合羟基阳离子称为柱化剂. 王楠[23]等以聚合羟基Al-Fe复合物为柱化剂对蒙脱石进行柱撑改性,改性后的蒙脱石比表面积、微孔容、活性位点增加,对As(Ⅴ)的吸附能力明显增强. Na等[24]研究了钛柱撑蒙脱土对水溶液中砷酸根和亚砷酸根的吸附性能,发现多羟基钛离子进入层间,增大层间距,产生许多活性羟基,砷物种进入蒙脱石层间后与活性羟基发生络合作用而固定. 此外,氧化铁[25]、氧化锆[26]、氧化锰[25]柱撑蒙脱石的制备及对砷的吸附研究也有报道.
膨润土、高岭土[27]、沸石[28]等黏土材料能够作为PRB的填充材料用以去除水体中的多种污染物. PRB工程应用于实际的污染场地往往需要大量的填充材料,黏土材料来源广泛又廉价易得能够满足这一要求,(改性)黏土材料能够有效去除砷和其他多种污染物,同时绿色环保的黏土材料也能够减少对环境的影响.
-
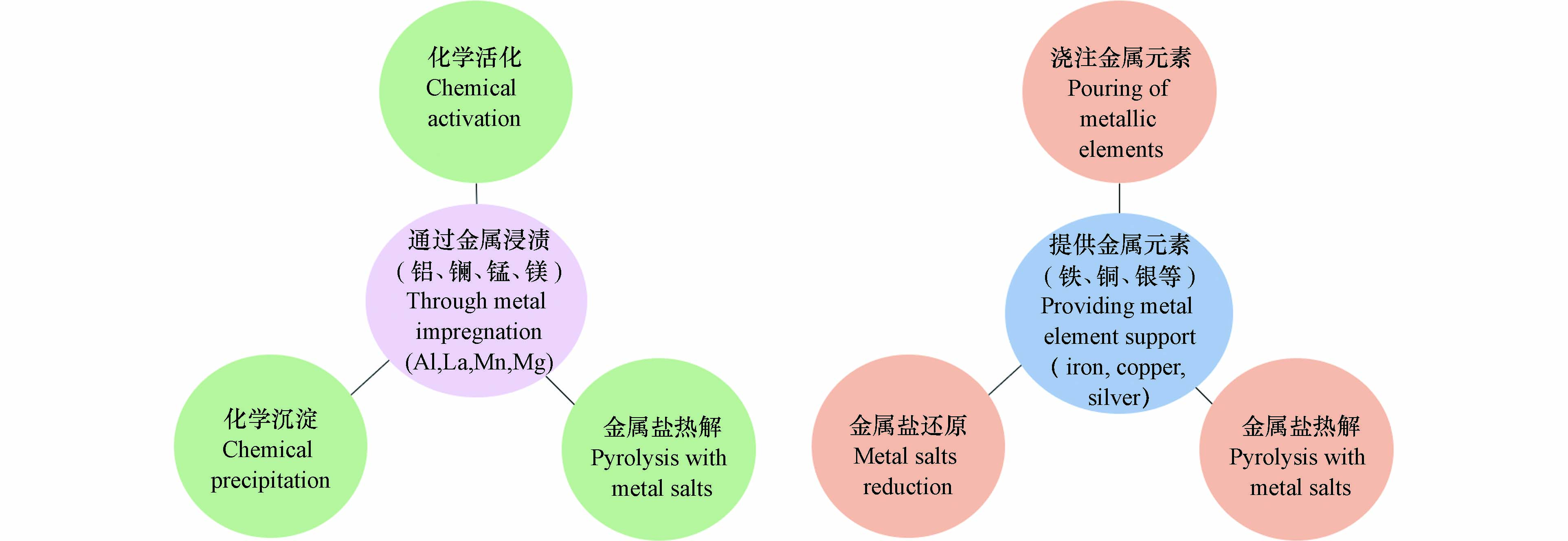
由于多孔、比表面积大、吸附位点多、绿色环保等特点,无机活性碳、有机生物碳、纳米碳纤维、碳纳米管、石墨烯/氧化石墨烯等碳基材料被广泛研究用于修复污染的土壤和水源[29]. 活性碳本身除砷效果不理想,一般需要负载Fe、Al、Ca、Mn等金属或其氧化物和氢氧化物才能用作PRB反应填料来修复水体. 生物碳材料来源广、廉价易得,且具有还原功能,能将硫酸盐还原成硫负离子沉降重金属离子,但大多数生物碳表面带负电,对砷等阴离子污染物的去除能力较弱[29]. 生物碳用于PRB除砷前,一般需要改性. Boni等[30]在研究中发现,仅增加松木生物碳使用量并不能有效提高砷的去除效率,但表面负载Fe、Mg、Ca、Al、Mn及其氧化物或氢氧化物(如图2所示)后,生物碳对砷酸根的吸附能力明显增强[31].
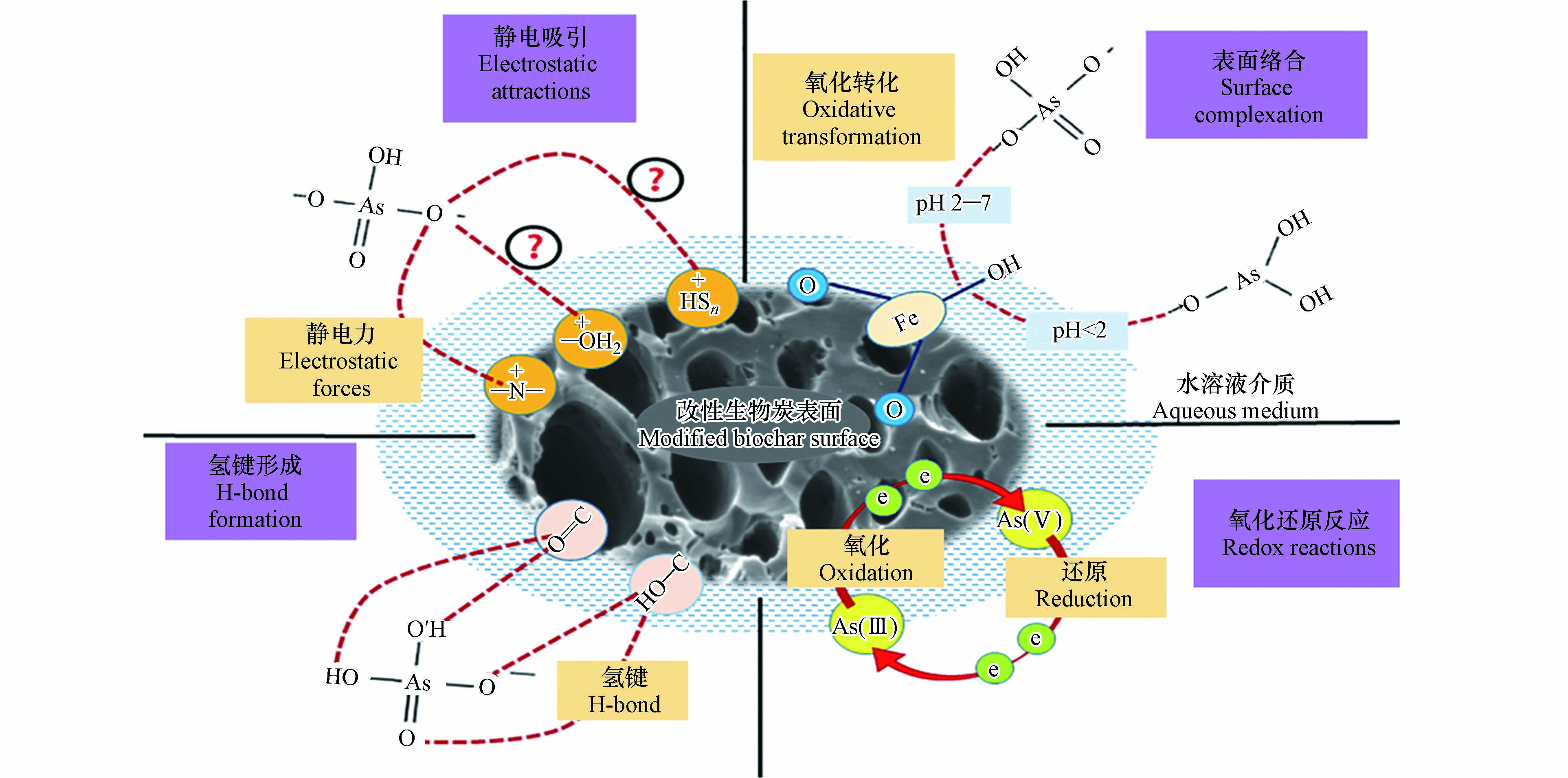
Alkurdi等[32]总结了生物碳及其改性材料在砷污水净化过程中的机理,见图3,包括吸附、化学氧化还原、化学沉淀、络合、静电吸引和离子交换等. Agrafioti等[33]采用稻壳、有机固废和污泥生物碳来固定As(Ⅴ),其中静电吸引起主要作用. Dong等[34]发现,含游离半醌自由基的生物碳,会参与As(Ⅲ)的氧化. Zhang等[35]将MgO物种负载于生物碳表面提高其零电点,进而增强与阴离子的静电引力;他们也制备出Mg/Al水滑石复合生物碳[36],用于阴离子的吸附与固定.
碳基材料与黏土材料一样,是一种可供选择的PRB填充材料[37-38],利用碳基材料的多孔、比表面积大、吸附位点多等特点通过改性后结合其他材料能够提高除砷能力,同时碳基材料来源广泛、可降解与绿色环保性能可以保证材料的有效应用.
-
含钙材料如石膏、磷石膏、石灰石等,广泛存在于自然界中,是廉价且较为有效的砷吸附载体,其除砷的主要机理是(亚)砷酸根阴离子与钙离子生成不溶性(亚)砷酸钙盐. 此外,石膏、磷石膏、脱硫石膏等还可用作胶凝材料,可将PRB填充材料胶凝成透水砖,便于填充材料的安装与更换.
石膏具有较大的砷吸附容量,在水溶液中易于与砷氧阴离子结合,起到拦截固化砷的作用. Chen等[39]以天然石膏为原料制备出CaSO4和CaSO4·2H2O晶须,发现它们与砷酸根和亚砷酸根阴离子的相互作用主要为吸附与表面溶解-沉淀. Jia等[40]研究了α-CaSO4·0.5H2O对砷的固定机理,发现砷酸根离子通过取代硫酸根而逐步从α-CaSO4·0.5H2O表界面扩展到体相中,从而去除水体中的砷.
方解石[41]、石灰石[42]、白云石[43]等碳酸钙矿物,是常见的PRB填充材料,可用于砷水体污染防治,还有调节地表径流pH的功能. Fukushi等[41]的研究表明单水方解石(CaCO3·H2O)对砷酸根的吸附能力强于方解石. Tatsuhara等[44]发现碳酸钙和部分风化的火山灰组成的中和-吸附系统可显著降低流入柱中流体中的砷浓度,其拦截机制为共沉淀和吸附反应.
富含硅酸钙的胶凝材料水泥则是合成的含钙材料,在与水、骨料和添加剂混合后,可制成具有一定力学强度、透水性能和净水性能的砖块,是潜在的PRB除砷固砷填充材料. Kundu等[45]将廉价普通硅酸盐水泥作为除砷吸附剂,除砷率大于95%. Tangviroon等[46]发现钙或镁基添加剂加入到粉质土壤-水泥混合物中能降低渗滤液中的砷浓度.
利用钙离子可与(亚)砷酸根阴离子生成不溶性(亚)砷酸钙盐的优势,钙基材料能够作为一种高效的除砷材料,但当以钙基材料作为PRB的填充材料时,可能会造成系统堵塞[47]、失活和失效[48]等问题,需要进行系统性的研究后才能够保证材料的有效应用.
-
与钙、镁、铝等的(亚)砷酸盐相比,(亚)砷酸铁盐的溶解度更低,溶解释放的砷也更少,能很好达到环境保护的要求. 与此同时,铁基材料资源丰富、价格低廉、绿色环保,将其用于固砷被广泛研究. 常见的PRB除砷铁基材料及其除砷效果列举于表1中,铁基除砷材料包括零价铁(ZVI)、含铁矿物、废铁材料、复合铁基材料等,都表现出优良的除砷效率.
-
零价铁能够有效去除水中的可溶性砷,是最常用的除砷PRB填料. Eljamal等[64]研究了As(Ⅴ)和As(Ⅲ)在ZVI上的吸附行为,发现As(Ⅴ)通过表面吸附和与Fe(Ⅲ)共沉淀而固定于ZVI表面,As(Ⅲ)也通过表面吸附或氧化为As(Ⅴ)而被ZVI固定,且As(Ⅲ)的去除速率高于As(Ⅴ). Njaramba等[65]发现,ZVI和ZVI-P(ZVI+浮石)反应床90 d内对砷的截留率均为100%,但ZVI反应床出现明显的堵塞现象,而ZVI-P未见堵塞. Wilopo等[66]以ZVI、堆肥、羊粪或木屑为活性材料,玻璃微珠或砾石为非活性材料,构建PRB实验柱去除亚砷酸根,结果表明,As(Ⅲ)主要吸附于含铁矿物,与铁生成沉淀或最终转化为砷酸铁而被固定;与ZVI柱相比,采用未灭菌的ZVI+羊粪混合柱,砷的固定效果更好.
-
除砷含铁矿物包括磁铁矿、菱铁矿、赤铁矿、褐铁矿和针铁矿等等. 其中,磁铁矿、菱铁矿、赤铁矿和褐铁矿在自然界中分布广、价格低. 然而,含铁矿物溶度积小,比表面积小,砷吸附容量低,为充分释放活性铁物种,可通过机械活化、热活化等方法增大比表面积.
磁铁矿的主要成分为Fe3O4. Shipley等[67]研究表明,磁铁矿纳米颗粒能有效去除溶液中的砷酸根和亚砷酸根. Liang等[68]的研究表明淀粉稳定的磁铁矿纳米颗粒对模拟砂壤土中砷酸根离子有较好的吸附和固定效果,不但显著降低溶出液中As(Ⅴ)的含量,也大大减弱土壤中As(Ⅴ)的可溶出性.
菱铁矿的主要成分为FeCO3. Guo等[69]研究表明,菱铁矿包覆石英砂和赤铁矿包覆石英砂都具有好的除砷性能,但高浓度HCO3-会抑制FeCO3的溶解,限制新的Fe(Ⅲ)氧化物的形成,除砷能力降低;而H2O2处理则提高了材料对砷的吸附能力. Zhao等[70]通过添加有机粘结剂、挤压造粒、煅烧等工艺制备出改性粒状天然菱铁矿(MGNS),与天然菱铁矿相比,MGNS对As(Ⅲ)具有更高的吸附速率和吸附容量,并认为MGNS对As(Ⅲ)的吸附和非均相氧化是其去除As(Ⅲ)的主要原因.
赤铁矿的主要成分为α-Fe2O3. Mamindy等[71]研究了赤铁矿、针铁矿、磁铁矿和零价铁对As(Ⅴ)的吸附性能,发现在自然pH值(pH 6—9)和初始砷浓度较高时,赤铁矿是最适宜的吸附剂. Wang等[72]通过热解天然赤铁矿和松木混合物来合成磁性生物碳,认为该材料表面的γ-Fe2O3是吸附砷物种的活性位点.
褐铁矿的化学组成为Fe2O3·nH2O,一般为针铁矿、水铁矿、纤铁矿、赤铁矿等矿物的混合物,天然褐铁矿较为疏松多孔,砷吸附位点丰富,是一种具有潜在砷修复能力的天然材料. 邵金秋等[73]对比研究了天然赤铁矿、天然褐铁矿、天然菱铁矿、天然钛铁矿、天然磁铁矿、Fe2O3、Fe3O4及铁锰双金属材料(FMBO)对As(Ⅲ)和As(Ⅴ)的吸附-解吸特性,发现天然含铁矿物中褐铁矿对砷的吸附效果最好,对As(Ⅲ)和As(Ⅴ)的吸附容量分别可达3.96 mg·g−1和2.99 mg·g−1. Yan等[59]研究表明,机械活化后褐铁矿粒度减小、比表面积增大,结晶度降低、表面活性位点增加,矿物相转变为非晶态氧化铁物质,对砷的吸附和稳定作用显著增加.
-
工业生产中产生大量的含铁废弃物,如炼钢副产物(废钢和炉渣)[74]、铜渣[62]和废铸铁[63]等,这些废弃物可作为PRB填料来固化砷. Ahn等[75]采用炼钢副产物(含有铁、铁氧化物、钙铁氧化物和氢氧化钙等成分)处理高砷渗滤液,结果表明,蒸发冷却器粉尘、含氧污泥、碱性氧炉渣和电除尘器粉尘等对As(Ⅴ)和As(Ⅲ)均有较好的去除效果. Li等[76]采用铜渣(含Fe2SiO4和Fe3O4)处理冶炼含砷酸性废水,发现对含砷8458 mg·L−1的废水,铜渣可去除98.8%的砷,吸附容量可达66.86 mg·g−1,其除砷机制主要为Fe-O-As共沉淀和核-壳原位封装,其中核为非晶态砷酸铁,壳为硅胶. Choi等[63]研究了废铸铁加工研磨粉尘(GPD)和铸铁丸(CIS)对水溶液中As(Ⅲ)和As(Ⅴ)的去除能力,实验得出,GPD比CIS对砷的吸附容量更大,这与其更高的比表面积和Fe含量有关. 此外,GPD和CIS对As(Ⅲ)的去除效果均优于As(Ⅴ),但两种材料在pH 3.0—10.5范围内随pH的升高除砷性能下降. Luukkonen等[77]表明高炉矿渣对矿山废水中的As(Ⅲ)具有一定去除能力.
-
零价铁可以在好氧和厌氧环境中与水反应生成Fe2+和Fe3+(见式(1)—(4))[78],根据氧化还原条件和pH值差异,生成的Fe2+和Fe3+可进一步转化为铁氧化物/氢氧化物的腐蚀产物,如磁铁矿、氢氧化亚铁等(见式(5)—(8))[79]. 砷物种则吸附于零价铁表面的腐蚀产物上,同时可与铁物种反应生成(亚)砷酸铁而被固化. 此外,零价铁的腐蚀产物(Fe2+等)可将As(Ⅲ)催化氧化为As(Ⅴ),进而提升固化效率.
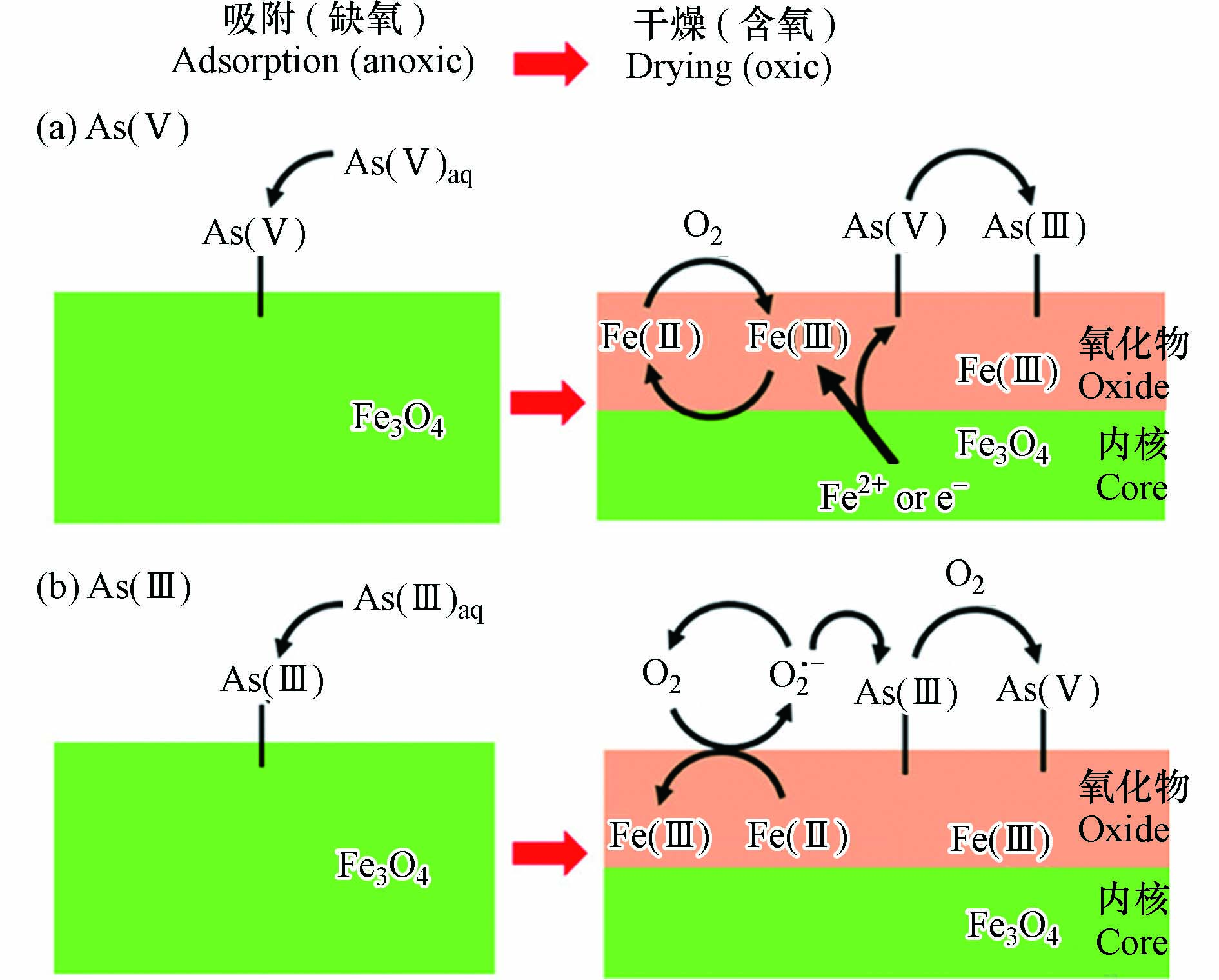
对于铁氧化物/氢氧化物,表面络合作用、静电作用和共沉淀过程都有助于砷氧阴离子的去除. Liu等[80]研究了磁铁矿纳米颗粒(MNP)对As(Ⅲ)和As(Ⅴ)的去除机理(见图4),在MNP表面,分别生成As(Ⅴ)的Fe-O-As双齿双核共角配合物和As(Ⅲ)的Fe-O-As三齿六核共角配合物,在含氧条件下,Fe3O4还可催化As(Ⅲ)转化为As(Ⅴ). Mamindy等[71]研究表明,赤铁矿、针铁矿和磁铁矿的零电点分别在pH 8.1、6.9和6.4,当溶液pH值低于矿物零电点时,这3种铁矿物通过静电吸引作用吸附砷氧阴离子. Park等[81]研究发现,酸性矿山废水中形成的施氏矿物和水铁矿,能与砷物种反应固化稳定化砷.
-
大宗固体废弃物,如煤矸石、粉煤灰、赤泥等,具有一定的除砷效果,若将其用于PRB工程,既可有效处理砷污染,又可实现固废利用. 煤矸石由SiO2、Al2O3和Fe2O3等组成. Kim等[82]发现,风化煤矸石对Cu、Cd、As等具有良好吸附性能,吸附容量分别为4.44、3.66、0.72 mg·g−1,去除率分别为99.8%、95.4%和71.0%. 粉煤灰是煤燃烧过程中排出的微小灰粒,其粒径一般在1—100 μm. 粉煤灰疏松多孔、比表面积大且活性位点多,具备良好的吸附特性. Li等[83]以富铁粉煤灰为原料,通过热水洗涤、酸化、碱化等过程制备高氧化铁粉煤灰吸附剂,其比表面积是原粉煤灰的22倍,对As(Ⅴ)的吸附容量达19.46 mg·g−1. 赤泥是氧化铝生产时排出的工业固体废弃物,大约每生产1 t氧化铝排放1.0—1.8 t赤泥,其组成包括SiO2、Al2O3、CaO和Fe2O3等. Altundoğan等[84]发现,酸活化促进赤泥中方钠石溶出,可提高其对As(Ⅴ)和As(Ⅲ)的吸附能力.
废钢、炉渣等含铁固废,粉煤灰、石膏、赤泥、矿山尾矿等大宗固废以及贝壳、植物壳/叶等生物废弃物也是较好的砷拦截材料,它们价格低廉,符合“以废治废”理念,可实现废物利用. 通过优化这些废弃物与其它填充材料的配比,可提高除砷效率,同时去除地下水中重金属等污染物. 需要引起注意的是,废弃物的选用不能增加新的污染源,尤其是不能将材料中的重金属等带入地下径流中再次造成污染.
-
确定目标污染物和场地特征后,需对拟选用的材料进行实验室研究,包括材料的批处理实验和动态柱实验. 批处理实验将所选取的填料加入到污染溶液,通过测量污染物的浓度变化,分析填料的吸附性能. 通过批处理实验初筛填料后,采用动态柱实验来模拟填充材料在PRB工程中应用的可行性,确定柱内污染物的流动状况与动态吸附模型,进而分析、模拟和预测真实PRB场地污染物截留状况.
-
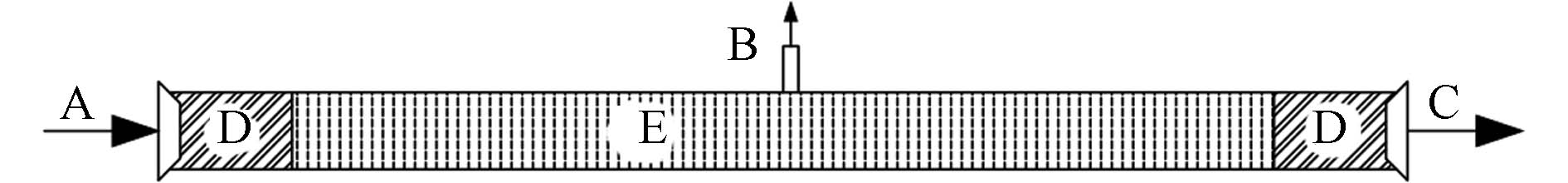
动态柱实验旨在模拟流体流动条件下,考察污染物种类、浓度、流速、渗透系数、孔隙度和环境条件等因素对PRB截留性能的影响. 动态柱吸附实验的主体装置为一根长柱,如图5所示,柱体内部放置填充材料,污水从柱一端流入,流经柱内填充材料后流出. 通常柱的侧端设置一定数量、一定间隔的取样孔,分析污染液流在柱体内部的浓度变化. 污染物流经柱反应器的保留时间以及材料在此时间内的除砷性能,是PRB反应器设计的主要依据.
-
污染物在土壤和地下水中一般呈羽状扩散,其流经PRB时的动力学较复杂,为较好的模拟PRB动态柱对污染物的吸附与拦截性能,通常采用如表2所示的模拟方法.
-
PRB除砷实验室研究较多,实际工程应用较少,下面简要介绍5个PRB除砷实例.
-
污染场地:美国蒙大拿州海伦娜附近的一个废铅冶炼厂,其地下水被砷、硒、铅、镉和锌等污染,砷的羽流已迁移到场地外,PRB工程主要是处理含中高浓度As(Ⅲ)和As(Ⅴ)的地下水.
PRB尺寸与材料:可渗透反应墙长9.1 m(垂直于地下水流动)、深14 m、宽1.8—2.4 m(平行于地下水流动),反应介质为零价铁颗粒,7.6 m厚(地下6.1—13.7 m),其上为6.1 m厚的砂层(地下0—6.1 m),共约174 t零价铁颗粒,初始孔隙度约50%.
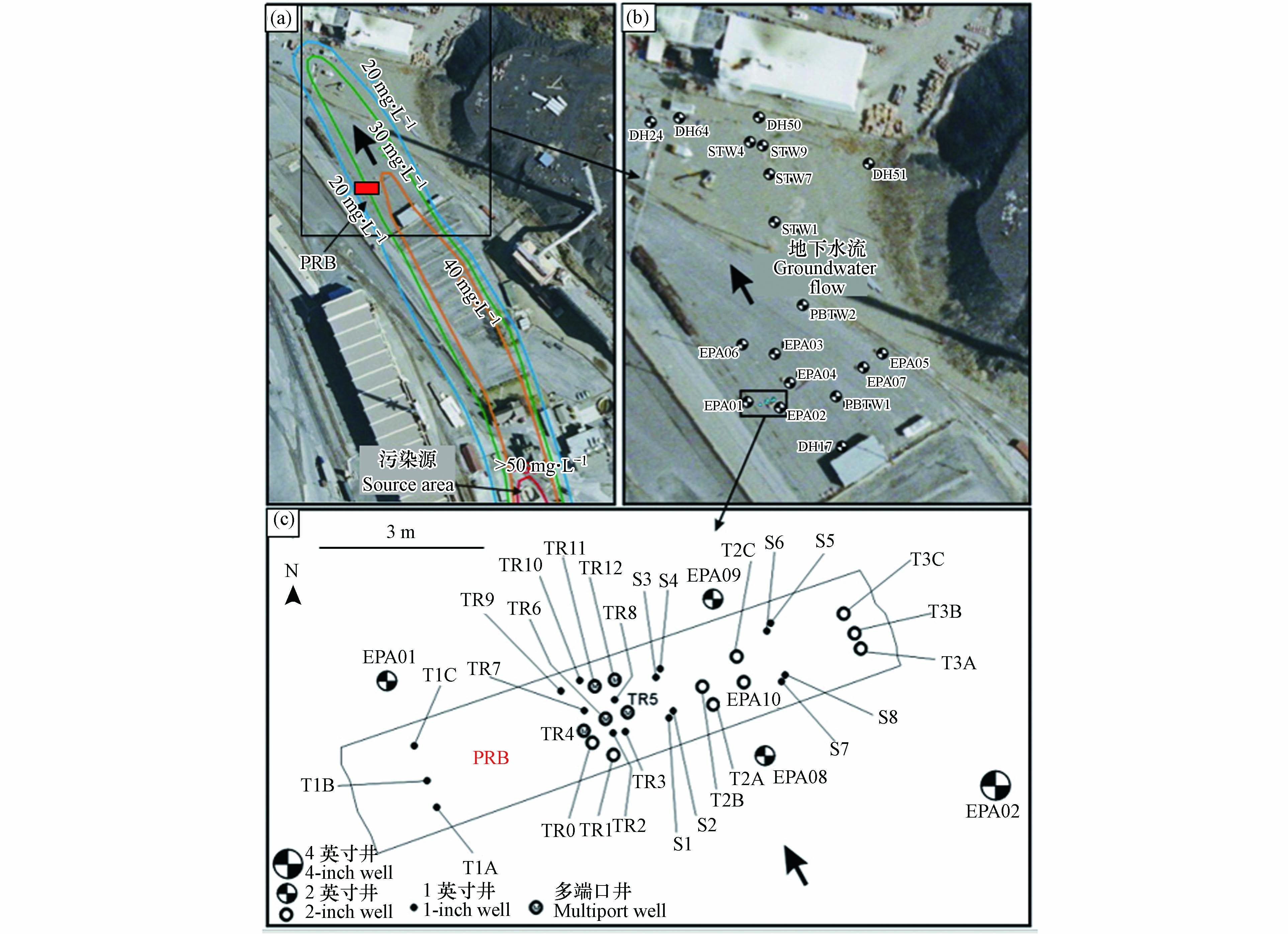
PRB监测:PRB的内部和周围安装由多级井组成的监测网络,如图6所示,地下水采样点约40个.
PRB运行与性能:运行第1个月、4个月、12个月、15个月和25个月的地下水样本分析结果表明,PRB实验区中11个样品砷浓度超过0.50 mg·L−1,62个样品砷浓度不高于0.50 mg·L−1,24个样品砷浓度不高于0.01 mg·L−1,在PRB内,砷浓度可降至2—0.01 mg·L−1. 运行2年后,PRB还能显著降低地下水中的砷浓度.
-
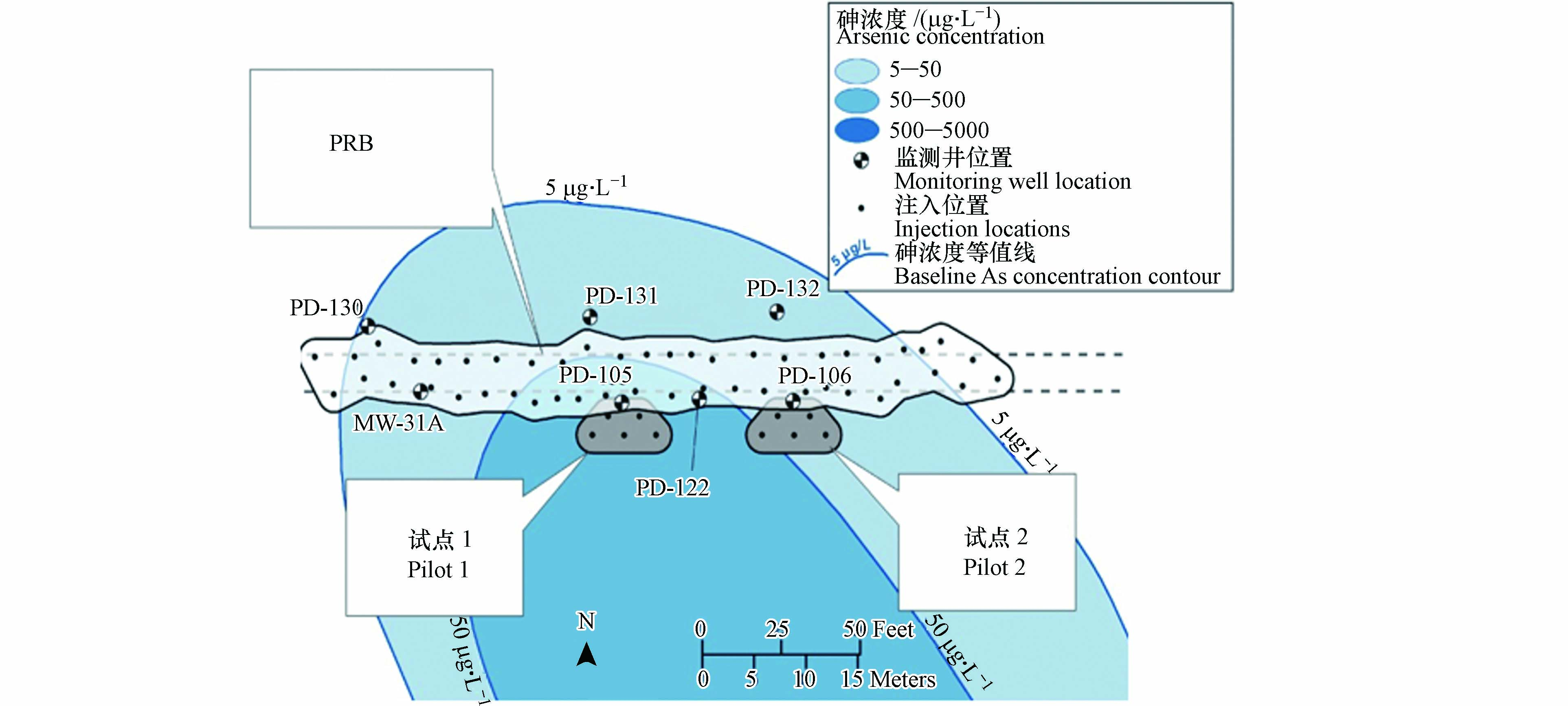
污染场地:华盛顿州塔科马附近湿地地区的溶解砷羽流前缘,砷来源为含砷量~2%的铜回转炉渣污染的木屑和土壤,地下水溶解砷约为5000 μg·L−1.
PRB尺寸与材料:PRB填料为商业化产品EHC-M(包含硫酸钾/镁盐、以植物纤维形式存在的亲水性有机碳、零价铁). 图7列出PRB的具体位置,2009年3月,进行了中试规模填料注入(试点1和试点2),两个中试PRB均长7.6 m,宽4.6 m,深3.0—6.7 m,填充材料质量分别为450 kg和1360 kg;2009年11月和12月,进行了全尺寸PRB规模填料注入,该PRB长67 m,宽6 m,深3.0—6.7 m,填充材料质量为8.7 t.
PRB监测:监测井网如图7所示,一排位于PRB前端(南部)监测井(MW-31A,PD-105,PD-122和PD-106),另一排位于后端(北部)的监测井(PD-130,PD-131和PD-132).
PRB运行与性能:实验结果表明,经过填充EHC-M材料的PRB处理后,地下水中总砷浓度不高于5 µg·L−1,97%的砷在PRB中被捕获,该PRB工程持续有效拦截砷的寿命约为2 年.
-
污染地点:南卡罗来纳州查尔斯顿的一个废磷肥制造厂,废弃黄铁矿逐渐氧化,长期产生和释放硫酸,同时将砷和重金属淋滤到地下水中. 受影响的地下水具有低pH的特点,直接进入场地边的潮汐沼泽.
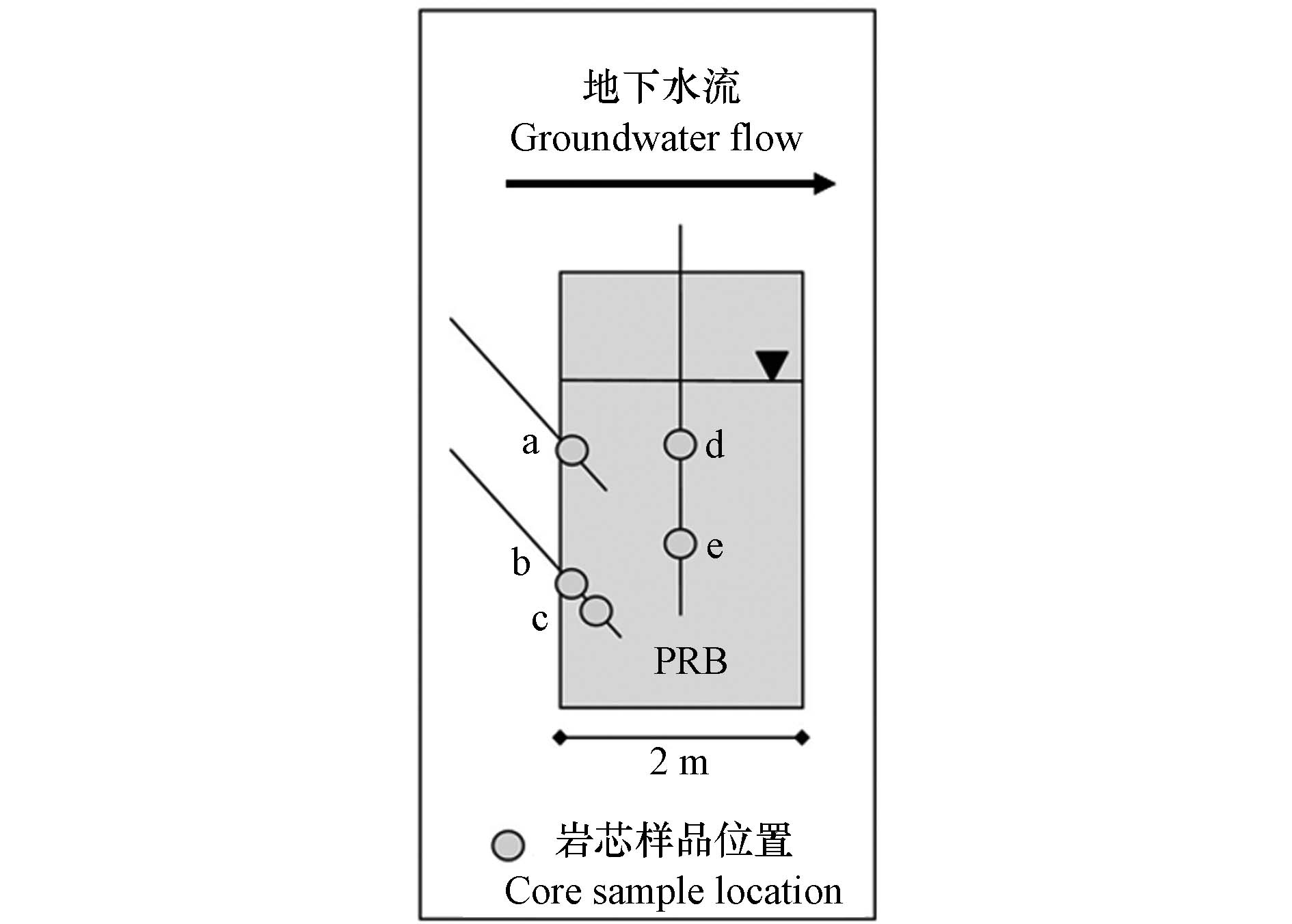
PRB尺寸与材料:PRB长约7.9 m,深4.1 m,宽1.8 m,安装在污染羽流必经的潮沼斜坡上. 填充材料由零价铁(20% V/V,确保砷的去除,并提供H2以维持硫酸盐的还原)、树叶堆肥(30% V/V,促进硫酸盐还原)、石灰石(5% V/V,缓冲pH值,建立微生物调节系统促进硫酸盐还原)和花岗岩(45% V/V,保持足够的渗透率)的混合物组成.
PRB监测:在PRB内和周围安装了压力计和监测井,通过上升和下降头段塞测试提供水力数据,同时从含水层和PRB采集垂直和倾斜岩芯样品(图8),分析砷和重金属元素含量.
PRB运行与性能:该PRB旨在微生物体系下调控硫酸盐还原和硫化物沉淀,促使砷和Pb、Cd、Ni、Zn等重金属离子的吸附与固化,从而去除地下水中的有害物质. PRB安装30个月后各有害物质在PRB中的平均垂直浓度为As<0.03 mg·L−1、Pb<0.003 mg·L−1、Cd<0.001 mg·L−1、Zn<0.23 mg·L−1、Ni<0.003 mg·L−1,PRB效果良好.
-
污染场地:湖南省郴州市邓家塘镇砷污染区,其土壤砷含量为23.41—827.09 mg·kg−1,平均值为130.01 mg·kg−1,废弃砷渣导致该地区的地表水和地下水均受到污染.
PRB尺寸与材料:4个PRB中试区,每个长1.1 m,宽0.5 m,深0.8 m,图9为PRB的位置图. PRB填料分别为粒度为0.076—0.08 mm的天然锰矿石、造粒锰矿石(天然锰矿、5%水泥和粒度为0.844 mm的5%活性碳混合成型造粒)、负载锰矿石(80%粒度为3—5 mm的碎石,16%锰矿,4%水泥)和混合锰矿石(95%天然锰矿与5%粒度为3—5 mm的金红石),天然锰矿石中锰和铁含量较高(MnO占45.46%,Fe2O3占23.72%).
PRB监测:每月采集1次水样,在每个监测点采集3个平行样品,用平均值分析砷浓度.
PRB运行与性能:从2009年6月15日至2010年5月15日,对于流入PRB中砷浓度为66—187 μg·L−1的地表水,各监测点的出水砷含量均低于10 μg·L−1,4种填料均有一定的除砷效果. 负载锰矿石吸附砷达到饱和后进行浸出试验,其浸出液中砷浓度小于1 μg·L−1.
-
污染地点:保加利亚Curilo铀矿,酸性矿井水含铜、锌、镉、铅、镍、钴等重金属、放射性元素、砷和硫酸盐.
PRB尺寸与材料:该酸性矿井水处理系统由碱化(铁和铝氢氧化物)石灰石(石灰石+碎石)排水沟/池(长1.0 m、宽1.7 m、深1.5 m)、PRB和天然湿地(阔叶香蒲和狭叶香蒲等植被和多样微生物群落)3个单元串联组成. 其中,PRB长8.0 m、宽1.7 m、深1.5 m,由可生物降解的固体有机基质(牛粪、植物堆肥、稻草)、粉碎的石灰石和浸透磷酸铵的沸石混合物填充,该PRB上生长着硫酸盐还原菌和其它代谢依赖微生物.
PRB监测:监测点位于上述3个处理单元的入口和出口以及PRB内部.
PRB运行与性能:通过碱性排水沟和PRB的总停留时间为30—300 h,此时污染物基本被清除,砷浓度由0.05—0.32 mg·L−1降至0.01 mg·L−1以下,pH由2.42—4.15升至6.22—7.74.
-
通过对5个除砷PRB工程应用的分析发现,不同尺寸大小(2.2.2中PRB超过1000 m3,而2.2.4中PRB不超多10 m3)的PRB都能有效拦截场地的主要污染液流,从而达到对场地砷污染的修复. 但地下/地表的(细小)污染支流难以通过PRB系统处理,进一步扩大PRB的尺寸也收效甚微且成本太高,而要解决这一问题,可以在PRB工程周围多点联用其他除砷技术,或者通过其他技术对砷污染物进行预处理以期达到对污染场地的完全修复.
案例中PRB工程的运行与监测较为简便,通常在PRB的内部与外部设置一定数量、一定深度的监测井分析周围整体水环境中的砷浓度变化,以便工程的监控与管理. 除砷PRB工程的使用周期长(2.2.1中有效期可超2年),后期若通过对材料的更换则可进一步延长PRB的有效期.
PRB工程的后续处理并未在上述案例中提到. 含砷沉淀物在PRB墙体内不断积累存在砷溶出的风险,现场工程更换材料需要尽可能的迁移含砷沉淀物;迁移出来的沉淀物需要及时有效的处理,要避免含砷沉淀物的再次堆积造成的污染;现场施工对生态环境的影响也需要得到监管,要评估全工程周期周围环境条件的变化、场地动植物的生长状况以及水溶液理化性质的变化等. 工程结束后,需要对地下的填料与地面设施进行处理,尽可能恢复原厂地的生态,或者是将PRB工程保留下来,实现工程应用与生态环境相结合.
-
黏土矿物、碳基材料、钙基材料、铁基材料和固体废弃物是目前广为研究的PRB除砷固砷材料. 由于通过砷酸铁固砷等类臭葱石化合物的溶度积低,砷物种释放到地下水的量少,可使溶液中砷浓度远低于WHO的排放标准,铁基材料是最具潜力的PRB除砷反应材料. 相对于零价铁,铁矿物和废铁材料量大价低,更具实际应用价值. 针对铁矿物和废铁材料的比表面积小、活性位点数少、砷吸附速率较慢、容量较低等缺点,为充分释放铁活性物种,可通过机械活化、热活化、化学活化等简单方法提高其吸附容量和反应速率,这是PRB除砷反应材料未来研究的重要方向之一.
PRB除砷是一种经济环保的原位修复技术,PRB能够有效地处理大型污染场地,应用于地下径流拦截污染物具有广阔的发展前景. 与此同时,PRB与物理拦截、微生物修复、植物修复或化学修复等技术联用,可获得更好的除砷去重金属效果. 此外,反应材料定期更换、工程的监测与维护可延长PRB的使用时间,实现对场地砷及其伴生污染物的长期拦截与修复.
Progress and prospect of arsenic removal by permeable reactive barrier
- Received Date: 04/09/2022
- Accepted Date: 25/10/2022
- Available Online: 27/03/2024
-
Key words:
- arsenic /
- permeable reactive barrier /
- solidification /
- iron-based materials /
- arsenic-removal mechanism.
Abstract: The arsenic pollution seriously affects the ecological environment and human health. The effective removal and prevention of arsenic pollution is one of the most urgent problems to be solved in the current industrial circles. The permeable reactive barrier (PRB) technology based on in-situ remediation technology, with its advantages of low cost, high efficiency, sustainability and simple process, has been the green, economical and efficient choice for removing and preventing arsenic pollution. As the filling material in PRB project is the main reactive body to remove arsenic pollution, the types of arsenic removal filling materials of PRB and their performance and mechanism of arsenic removal are summarized, including clay minerals, carbon-based materials, calcium-based materials, iron-based materials, solid waste, etc. Of all these materials, the iron-based materials have obvious advantages over others in adsorption ability towards arsenic species. Iron-based materials such as zero-valent iron, iron-containing minerals and iron-containing waste have been widely used in arsenic pollution remediation, due to their advantages of low cost, easy availability and high arsenic removal efficiency, and have been the main research direction of arsenic removal material in PRB in the future. Therefore, the laboratory research and model simulation of PRB filling materials are briefly introduced, including several pilot or project examples of PRB for arsenic removal, illustration of the related operation and monitoring process with treatment effect on arsenic pollution. On this basis, we put forward the development prospects and challenges of PRB technology for arsenic removal filling materials and engineering applications, and we hope that this paper can contribute to the development and progress of PRB technology for arsenic pollution control in overland runoff.







 DownLoad:
DownLoad:
