-
亚硝化反应是短程脱氮、自养型脱氮的重要氮转化过程之一。自养型脱氮工艺较传统工艺具有显著优势,可节约曝气能耗25%和碳源消耗100%[1-2]。近年来,以厌氧氨氧化工艺为核心的自养型脱氮工艺在低氨氮废水处理方面得到初步研究。实现稳定的亚硝化反应是限制自养型脱氮工艺在低氨氮废水处理中应用的重要因素之一。亚硝化反应首先将氨氮通过氨氧化细菌(ammonia oxidizing bacteria, AOB)在好氧条件氧化为亚硝酸盐氮,为实现亚硝酸盐累积,需要抑制亚硝酸盐氧化细菌(nitrite oxidizing bacteria, NOB),将亚硝酸盐氮进一步氧化为硝酸盐氮。
在低氨氮废水处理方面,尤其城镇生活污水,较难实现亚硝酸盐积累。这是由于较低的氨氮浓度难以形成游离氨或者游离亚硝酸盐等NOB抑制因子,并且在DO较低的条件下,NOB的抑制效果不稳定,同时也抑制了AOB的反应速率[3]。WANG等[4]的研究表明,DO为0.5 mg·L−1并控制SRT为8 d时,可促进亚硝酸盐累积。JARDIN等[5]的研究表明,采用间歇曝气调控,有利于亚硝化反应,这是由于AOB在缺氧-好氧交替环境可快速恢复活性,而NOB活性恢复较慢。
羟胺(NH2OH)和肼(N2H4)分别为氨氧化反应与厌氧氨氧化反应的中间产物[6]。投加NH2OH和N2H4有利于NOB的抑制和亚硝酸盐的累积[7-8],但由于其影响效果不一,缺乏NH2OH和N2H4促进亚硝化的对比研究,且影响机制尚不清晰。因此,本研究首先开展了NH2OH和N2H4对硝化反应影响的平行实验,对比分析了NH2OH和N2H4对氨氧化与亚硝酸盐氧化反应的影响;在此基础上,选择处理效果较好的NH2OH开展污泥驯化实验,明确了低氨氮废水亚硝化的快速启动方法及其潜在微生物学机制,为低氨氮废水亚硝化的快速启动提供参考。
HTML
-
1) NH2OH、N2H4对比实验。为考察NH2OH、N2H4对亚硝化的影响,分别采用3个处理,包括空白对照(CK)、NH2OH和N2H4,开展平行实验。采用有效容积为1.5 L的烧杯,通过加热板控制温度30 ℃。采用空气压缩机、气体流量计和曝气头分别对3个处理组进行曝气并气体流量控制。为促进亚硝酸盐积累,控制DO在0.5~1.0 mg·L−1。CK组、NH2OH组和N2H4组污泥浓度分别为0.92、1.02和0.96 g·L−1。NH2OH以NH2OH·HCl的形式投加,N2H4以N2H4·H2SO4的形式投加。考虑到2种物质在水溶液中易氧化的特性,实验开始时,直接称取19.86 mg NH2OH·HCl和18.57 mg N2H4·H2SO4,分别溶于少量去离子水后,再进行投加。控制体系内部NH2OH和N2H4的初始浓度为2 mg·L−1。
2)亚硝化快速启动实验。通过对比实验,考案NH2OH的投加对促进亚硝化反应的影响效果。本阶段采用序批式运行方式,每周期开始时投加NH2OH。圆柱形反应器有效容积为25 L,顶部安装搅拌器,底部配置微孔曝气盘,采用空气压缩机、气体流量计控制曝气量。污泥驯化的单个周期为5 h,包括进水5 min、曝气4 h,沉淀50 min、排水5 min,每个周期交换体积为15 L,即交换比为60%。实验过程中NH2OH以NH2OH·HCl的形式投加,称量NH2OH·HCl的质量为248.3 mg,溶于少量去离子水后再进行投加,NH2OH初始浓度为2 mg·L−1,每周期投加1次NH2OH。
-
进水均采用模拟废水,
NH+4 -N采用(NH4)2SO4配置,矿物元素(7.2 mg·L−1 KH2PO4、0.03 mg·L−1 CaCl2、0.07 mg·L−1 MgSO4·7H2O)、微量元素的投加参照已有研究[9]的投加量。批次实验与亚硝化启动阶段接种污泥采用北京某城市污水处理厂CASS段活性污泥,由于进水水质差异尤其有机物浓度对菌群结构产生较大影响,在开展亚硝化驯化实验前,以低氨氮模拟废水,在连续流反应器中维持DO在 1.0~1.2 mg·L−1条件运行43 d,该过程发生全程硝化反应。在此基础上,开展NH2OH驯化实验,进而用于揭示NH2OH投加对亚硝化反应的促进及对菌群结构的影响。 -
在NH2OH投加促进亚硝化快速启动实验前,与驯化第9天各取污泥混合液5 mL,在10 000 r·min−1离心10 min,弃上清液,使用试剂盒Fast DNA Spin Kit for Soil(MP, Biomedicals, USA) 提取DNA。
-
采用Illumina Miseq平台(Illumina, USA)测序分析,测序数据经优化后,样品经均一化后均含有32 107条序列。有效序列采用Ribosomal Database Project (RDP)进行物种分类。
-
采用纳氏试剂比色法测定
NH+4 -N;采用N-(1-萘基)-乙二胺光度法测定NO−2 -N;采用紫外分光光度法测定NO−3 -N;采用重量法测定SS和VSS;采用WTW型便携式pH测定仪测定pH和ORP。通过OriginPro 9.0(OriginLab, USA)完成制图,采用OriginPro 9.0(OriginLab, USA)对Monod方程进行曲线拟合。
亚硝酸盐氮积累率(nitrate accumulation rate, NAR)按式(1)计算。
式中:η为亚硝酸盐积累率;C(
NO−2 -Neff)和C(NO−3 -Neff)分别为出水亚硝酸盐氮和硝酸盐氮浓度,mg·L−1。氨氮与亚氮氧化反应采用Monod方程,根据式(2)进行最大比反应速率(Vm)与底物半饱和常数Ks模拟。
式中:V为单位污泥质量浓度的反应速率,kg·(kg·d)−1;S为氨氮或亚氮底物浓度,mg·L−1;Vm为单位污泥质量浓度的最大反应速率,kg·(kg·d)−1;Ks为底物半饱和常数,mg·L−1。
1.1. 实验装置
1.2. 实验废水与接种污泥
1.3. DNA提取
1.4. 高通量测序与OUT分类
1.5. 水质测定方法与数据分析方法
-
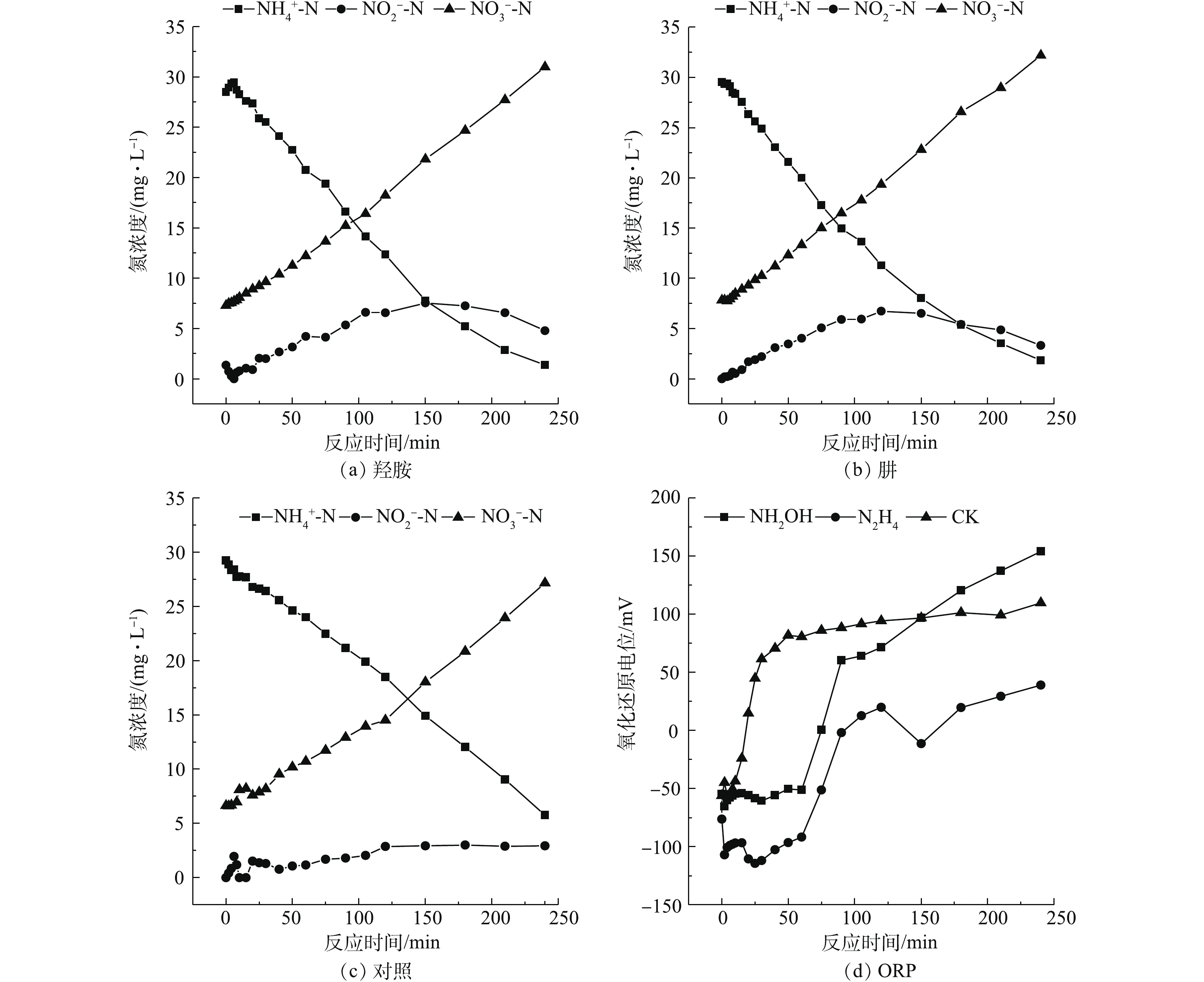
氨氮和亚氮的氧化过程如图1所示。随着反应的进行,2组实验的氨氮浓度逐渐下降,硝酸盐浓度逐渐上升。实验结束时,3组
NO−3 -N的生成量基本相同,但相比于空白对照组,NH2OH组和N2H4的NH+4 -N剩余量都在5 mg·L−1以下,接近1 mg·L−1;而空白对照组的NH+4 -N剩余量>5 mg·L−1,这说明投加NH2OH和N2H4有利于氨氧化反应。从NO−2 -N积累量来看,在实验进行到150 min时,NH2OH组和N2H4组的NO−2 -N浓度逐步提升,此时NAR分别达到25.67%和22.19%,而对照组的NO−2 -N浓度维持在较低水平,这表明NH2OH和N2H4的投加抑制NOB且促进NO−2 -N的积累。在150 min后,NH2OH组与N2H4组的NO−2 -N开始下降,这可能是由于NH2OH和N2H4的降解所致,使NOB的活性逐步恢复。在150 min时,NH2OH组的NAR略高于N2H4组。对实验过程中ORP进行了实时监测,结果如图1(d)所示。实验初期,CK组、NH2OH组、N2H4组的Eh分别为−56.1、−54.6、−76.3 mV,随着氨氮的逐步氧化,在第60分钟,CK组的Eh上升至80.5 mV,而NH2OH组和N2H4组的Eh分别为−51.2 mV和−91.8 mV,直至实验结束后,3组实验的Eh分别为109.6、153.9、39 mV。这是因为NH2OH与N2H4都是强还原剂,尽管在好氧条件仍能保持较低Eh水平,并维持约60 min。MA等[10]指出NOB从还原条件转变为氧化条件的适应期更长。30~90 min的还原条件有利于抑制NOB的代谢[11]。NH2OH组Eh在60 min后逐步升高,这可能由于NH2OH是氨氧化过程中间产物,AOB将NH2OH氧化为亚硝酸盐氮,因此,NH2OH的降解可能快于N2H4的降解。
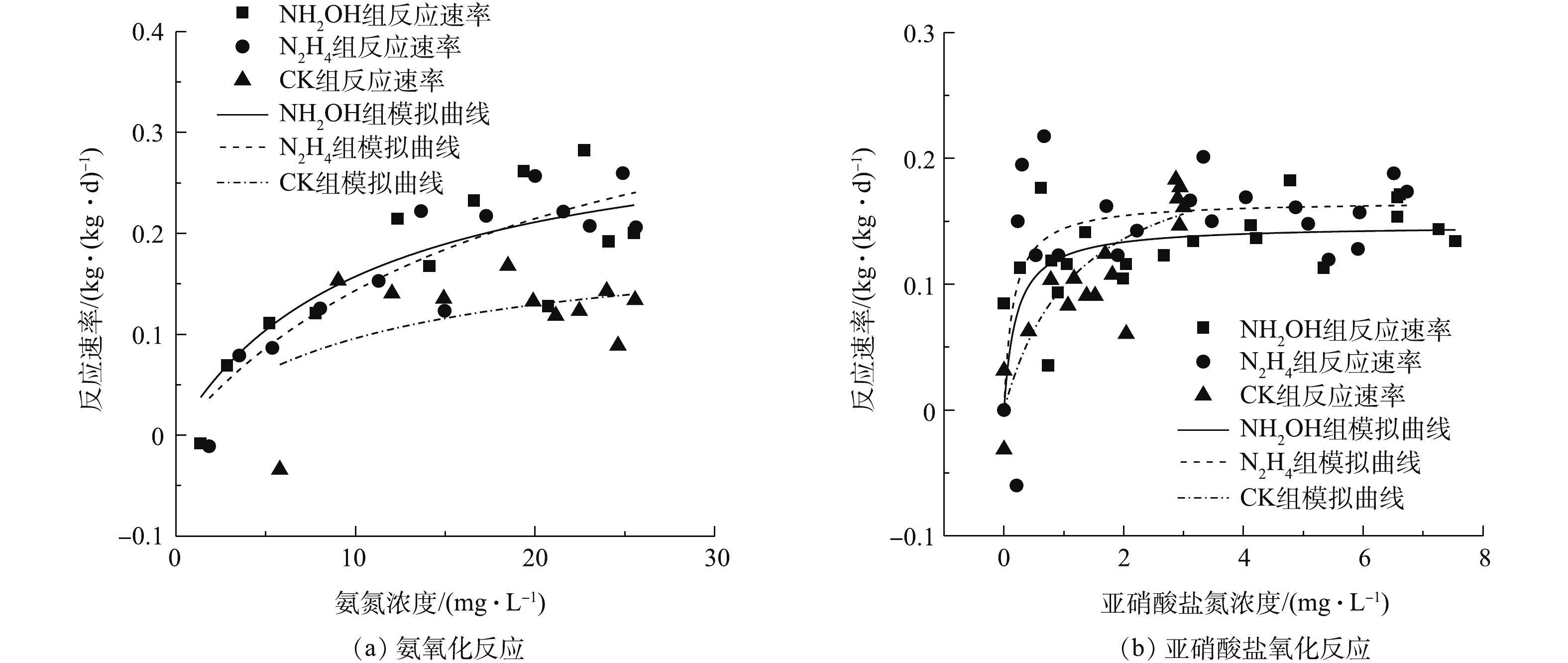
根据莫诺方程对反应动力学进行模拟,结果如图2所示。NH2OH、N2H4与CK组最大氨氧化速率分别为0.32、0.42和0.20 kg·(kg·d)−1。在氨氮浓度低于20 mg L−1时,NH2OH组氨氧化速率高于N2H4组,这主要由于NH2OH组氨氮半饱和常数较低(NH2OH与N2H4分别为10.40 mg·L−1和19.64 mg·L−1)。NH2OH、N2H4与CK组亚硝酸盐氮最大氧化速率分别为0.14、0.16和0.22 kg·(kg·d)−1,NH2OH组亚硝酸盐氮氧化速率低于N2H4组。因此,在处理低氨氮废水方面,NH2OH投加比N2H4更有利于亚硝酸盐积累,故在后续驯化实验中采用NH2OH投加考察了其对亚硝化的影响及菌群分布、关键功能细菌的响应。
-
采用序批式运行方式考察NH2OH投加对污泥驯化促进亚硝化的快速启动。实验过程中,每周期初期投加NH2OH,使体系内浓度为2 mg·L−1。尽管有研究采用较高浓度NH2OH(5~10 mg·L−1)促进亚硝酸盐累积[12],但由于高浓度NH2OH易引起污泥絮体破碎[13],本研究采用较低剂量(2 mg·L−1)考察了NH2OH对亚硝化的驯化效果。
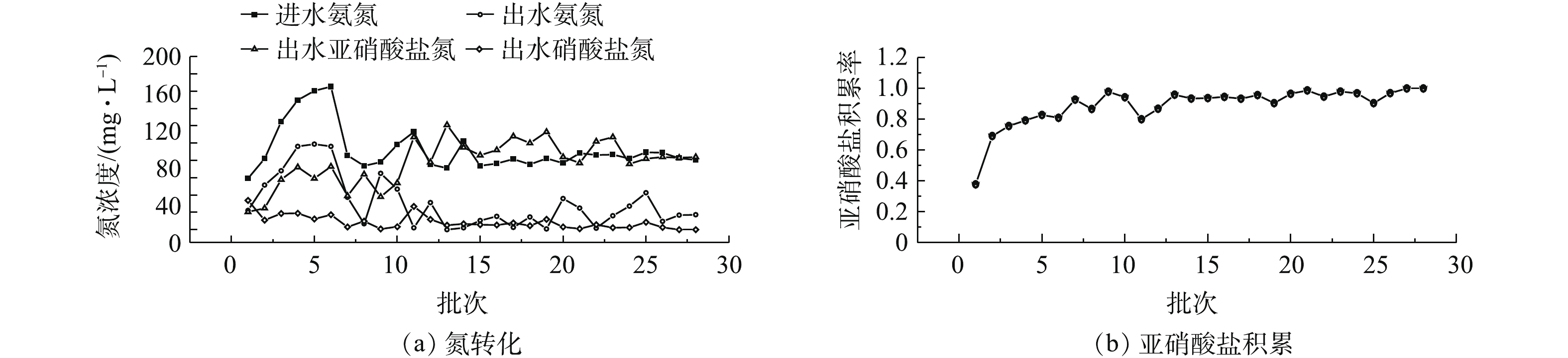
进、出水氮浓度与NAR结果如图3所示。投加NH2OH后,
NO−2 -N表现出快速积累。初次投加NH2OH,经5 h反应,出水NO−2 -N浓度为20.5 mg·L−1,NAR为37.8%。说明投加NH2OH之后,NOB的活性被迅速抑制。随着驯化周期的增加,出水NO−2 -N浓度迅速升高,NO−3 -N浓度明显下降,NAR迅速增加,氨氧化反应未受明显影响。在第7个周期末,亚硝酸盐积累率达到90%以上。在第27个周期,出水NO−3 -N浓度几乎为0,亚硝酸盐积累率达到100%。这表明NH2OH的投加对NOB活性的抑制获得较好效果,证明了投加NH2OH快速启动亚硝化的可行性。经过28个周期驯化后,进水NH+4 -N几乎全部转化成NO−2 -N,亚硝酸盐积累率从37.8%上升至100%,亚硝化快速启动完成。经过低浓度NH2OH(2 mg·L−1)驯化后的污泥可以有效抑制NOB活性,降低NO−2 -N向NO−3 -N的氧化速率。这可能是因为NH2OH抑制亚硝酸盐氧化还原酶的合成,从而抑制了NOB的生长[14]。 -
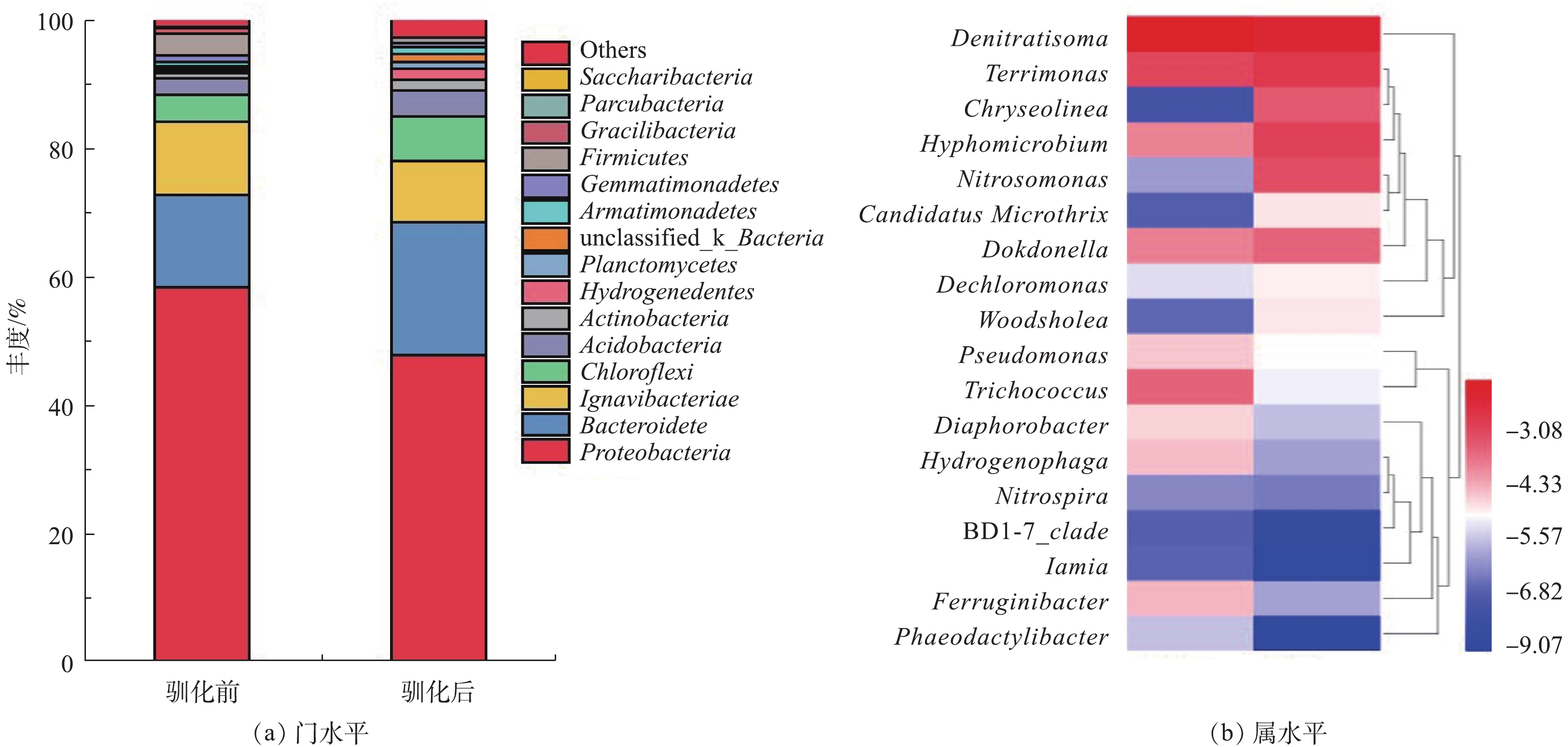
经过NH2OH投加处理后,微生物群落alpha多样性指数的变化如表1所示。通过9 d的驯化处理后,菌群多样性表现出升高趋势。NH2OH处理前后污泥混合液微生物群落结构变化如图4所示。门水平结构变化结果表明,变形菌门(Proteobacteria)、Ignavibacteriae的丰度明显降低,而拟杆菌门(Bacteroidetes)和绿弯菌门(Chloroflexi)的丰度有所升高。关键菌属的丰度的变化如图4(b)所示,Denitratisoma、Terrimonas、Hyphomicrobium、Nitrosomonas、Candidatus Microthrix、Dokdonella、Dechloromonas和Woodshloea丰度在NH2OH驯化后得到升高,而Pseudomnas、Trichococcus、Diaphorobacteria、Hydrogenophaga、Nitrospira和Ferruginibacter等的丰度明显降低。Denitratisoma、Hyphomicrobium、Dechloromonas和Pseudomnas是活性污泥系统中重要的反硝化细菌[15]。
为说明NH2OH对氨氧化与亚硝酸氧化关键功能微生物的影响,分析高通量测序结果发现,Nitrosomonas是主要AOB,Nitropira是主要NOB,而已有研究中关注较多的NOB菌属Nitrobacter未检出。由于在较低DO条件运行,NOB主要以Nitropira形式存在。JUBANY等[16]指出,Nitropira对DO具有较强亲和性,在较低DO条件下发生亚硝酸盐氧化反应。NH2OH驯化前,AOB与NOB丰度丰度相似(表1),分别为0.265%和0.206%;经过9 d的羟胺驯化, AOB丰度有大幅提高、NOB丰度稍有降低,分别为4.451%和0.178%。AOB/NOB丰度比为25。AOB与NOB丰度的差异有利于实现亚硝酸盐累积。由于NH2OH是氨氧化反应的中间产物,其被AOB利用促进AOB细菌的增殖,此外,由于NH2OH对NOB活性的抑制作用,进而对NOB丰度产生一定的削减。
2.1. NH2OH、N2H4投加对亚硝化影响的对比
2.2. 亚硝化快速启动
2.3. NH2OH投加对微生物群落结构的影响
-
1)通过NH2OH、N2H4组与空白对照组实验对比发现,NH2OH与N2H4单次投加均可实现亚硝酸盐累积。NH2OH与N2H4投加可维持较低Eh环境(−50~−100 mV)约60 min,这有利于NOB抑制。在低氨氮浓度(<20 mg·L−1)条件下,NH2OH投加可获得较大的氨氧化速率和较小的亚硝酸盐氧化速率。因此,在污泥驯化实验中,采用NH2OH投加考察对亚硝化反应的快速启动效果。
2)采用NH2OH投加(2 mg·L−1)研究亚硝化的快速启动,经过9 d的驯化,实现亚硝酸盐积累率100%,微生物群落多样性略有增加,AOB大幅增加且NOB丰度稍有降低,AOB/NOB丰度比为25,可实现亚硝化的快速启动。






 DownLoad:
DownLoad:
