-
我国工业高速发展及城镇化进程,使得重金属造成的水污染日趋严重,成为了全球关注的环境问题之一[1]. 重金属不可被生物降解,易在生物体内累积并通过食物链放大,严重威胁人类健康及生态系统. 镉(Cd)、锌(Zn)、镍(Ni)是工业废水中最常见的重金属污染物[2]. Ni、Zn是生命体需要的微量元素,但高浓度Ni2+、Zn2+能引起呕吐、哮喘及中枢神经系统紊乱等中毒症状[3 − 4];Cd2+即使在较低浓度下也表现出较高生物毒性[5],长期接触Cd2+会导致慢性肺部疾病、骨骼畸形和肾功能衰减等问题[6]. 因此,高效去除水体中的以镉(Cd)、锌(Zn)、镍(Ni)为代表的重金属离子成为了亟待解决的问题,并在近年来受到了相关研究领域的广泛关注.
从水中去除重金属离子的方法包括吸附、化学沉淀/混凝、离子交换、膜技术和电化学方法等[7]. 随着纳米技术的发展,纳米材料在水处理中逐渐发挥重要作用. 其中,纳米零价铁(nanoscale zero-valent iron, nZVI)凭借比表面积大、还原活性高、适用面广、环境友好等特性被广泛用于水环境中的重金属去除[8]. nZVI的粒径在(20—100) nm范围,呈链状,合成后瞬间在表面生成铁(氢)氧化物,这使nZVI形成了独特“核-壳”结构[9]. nZVI在参与重金属去除过程中,外氧化壳层首先通过静电引力和表面络合作用吸附重金属离子,随后单质铁核可以充当电子供体还原被吸附的重金属离子,因此nZVI对重金属的去除可能涉及吸附和还原机制[10]. 但nZVI在实践应用中也呈现出一定的局限性,如易自发团聚,表面活性位点减少;极易被空气和水氧化,大大削弱其还原能力,导致活性降低. 为了解决上述问题,大量研究对nZVI的改性进行了探索[11 − 13],旨在进一步提升nZVI的稳定性、电子传递效率和去除的选择性.
研究发现,nZVI对磷酸盐具有很强的亲和力,能通过吸附、沉淀等作用高效去除水中PO43-[14 − 15]. 研究进一步表明,吸附在nZVI表面的PO43-能生成钝化层,磷酸基团的侧链质子抑制nZVI与氧和水的反应,从而对nZVI起到一定保护作用[16]. 因此,表面磷酸化能提高nZVI在水中的稳定性. 此外,磷酸盐能取代nZVI表面的羟基,与重金属形成三元配合物,进而增强其对重金属的配位能力[17]. 基于上述特性,表面磷酸化的nZVI(phosphorylated nanoscale zero-valent iron, P-nZVI)用于污染物去除已被广泛研究,如Zhang等[16]发现四聚磷酸盐改性nZVI对阿特拉津的降解过程中,四聚磷酸盐的存在抑制了质子还原,增强了分子氧活化,使阿特拉津的降解率提高955倍. Li等[18]的研究表明,磷酸化改性后,P-nZVI对Cr(Ⅵ)还原的电子选择性从6.1%提高到31.3%,去除效率提高了4倍,这是由于磷酸化修饰增强了对铬的吸附能力,进而促进其还原. 综上所述,nZVI的表面磷酸盐改性能提高对重金属离子的配位能力,同时表面磷酸盐抑制了nZVI被水和氧气氧化,在增强nZVI对重金属离子吸附能力的基础上有效提高了nZVI对吸附在表面的重金属的还原能力,其改性策略成本低,操作简单,效果显著. 但目前,P-nZVI对不同种类重金属的去除性能、机理的相关比较研究仍较少,因此,比较P-nZVI对常见重金属的去除能力和作用机制具有较大的研究价值.
本研究以KH2PO4为磷化剂,通过液相还原法制备磷化改性的nZVI,并且选择了Cd2+、Zn2+、Ni2+3种典型的重金属离子作为目标污染物. 由于Fe0对Cd2+、Zn2+、Ni2+具有不同的还原能力;Cd2+、Zn2+、Ni2+受pH影响的沉淀-溶解特性存在差异;同时,其与表面磷酸根的亲和力也不尽相同,因此磷酸化改性后的nZVI对于上述3种金属离子的去除特性可能存在差异. 本文在讨论P-nZVI去除水溶液中Cd2+、Zn2+、Ni2+效果的基础上,进一步研究了pH、干扰离子等影响因素的影响,并结合XRD、XPS、SEM、TEM等表征,讨论P-nZVI去除Cd2+、Zn2+、Ni2+的微观机理差异. 本研究旨在比较P-nZVI对不同重金属的去除能力、重金属去除过程中的影响因素和微观界面特征,为进一步深入探索P-nZVI在微界面上与重金属离子的作用机理提供一定的参考.
-
研究所用的nZVI、P-nZVI均采用NaBH4液相还原Fe3+法合成[19]. 根据先前的实验,P/Fe物质的量比在0.6左右P-nZVI去除效果较好,因此制备磷酸化修饰的nZVI时,需要将NaH2PO4以一定比例和NaBH4混合,确保P/Fe为0.6,并通过蠕动泵将混合溶液缓缓滴入三颈瓶中,其他步骤与nZVI的制备相同. 反应完成后,采用离心的方式收集nZVI并用去离子水和无水乙醇各洗涤3次,储存于无水乙醇中备用.
-
实验分别探究了P-nZVI吸附Cd2+、Zn2+、Ni2+的动力学特征、pH及干扰离子等因素对重金属去除的影响. 所有实验中P-nZVI的投加量均为0.5 g∙L−1,重金属离子的初始浓度为100 mg∙L−1. 在动力学实验中,首先配制一定量Cd2+、Zn2+、Ni2+溶液至三颈瓶中,调节反应初始pH为6±0.1,通氮20 min脱去溶液中O2,再加入适量P-nZVI,分别反应0(空白)、5、10、15、30、40、50、60、75、90、120 min取出少量溶液,过0.22 μm滤膜后测定离子浓度. 反应过程中,采用磁力搅拌器以250 r·min−1进行机械搅拌,使P-nZVI与目标离子充分接触. pH实验中,为防止Cd2+、Zn2+、Ni2+大量沉淀控制pH范围在2—8之间,使用HCl和NaOH调节反应pH分别为2±0.1、3±0.1、4±0.1、5±0.1、6±0.1、7±0.1、8±0.1,其他步骤同上. 为探究溶液中干扰离子对吸附的影响,在其他操作不变的情况下,控制反应pH为6±0.1,量取适量含有共存离子的溶液[HA、Na2SO4、NaHCO3、Mg(NO3)2、Ca(NO3)2]加入三颈瓶中,使共存离子浓度分别为0、10、50 mg∙L−1. 上述所有实验,控制实验温度为25 ℃.
-
采用电感耦合等离子体发射光谱仪(ICP-720 ES,安捷伦公司,美国)测定溶液中Cd2+、Zn2+、Ni2+浓度. 采用Zeta电位测定仪(Zetasizer Nano ZS90)测定P-nZVI的零电荷点,ASPS 2460气体分析仪测定材料孔径分布及比表面积. 为比较反应前后材料的微观形貌变化,采用场发射电子扫描显微镜(Nova naniSEM-450, FEI公司,美国)及球差校正扫描透射电子显微镜(TatanTMG2 60-300, FEI公司,美国)进行表征,并使用EDS能谱对材料的元素分布情况进行定性及半定量分析. 采用X射线衍射仪(D8 Advance,布鲁克公司,德国)及X射线光电子能谱仪(ESCALAB 250XI, 赛默飞, 美国)探测材料体相物质的晶体结构及表相化学组成、元素种类及价态,并使用MDI Jade 6软件对XRD结果进行比对分析、Advantage 5.948软件对XPS谱进行分峰拟合. 采用Origin 2021软件对所得数据进行绘图.
-
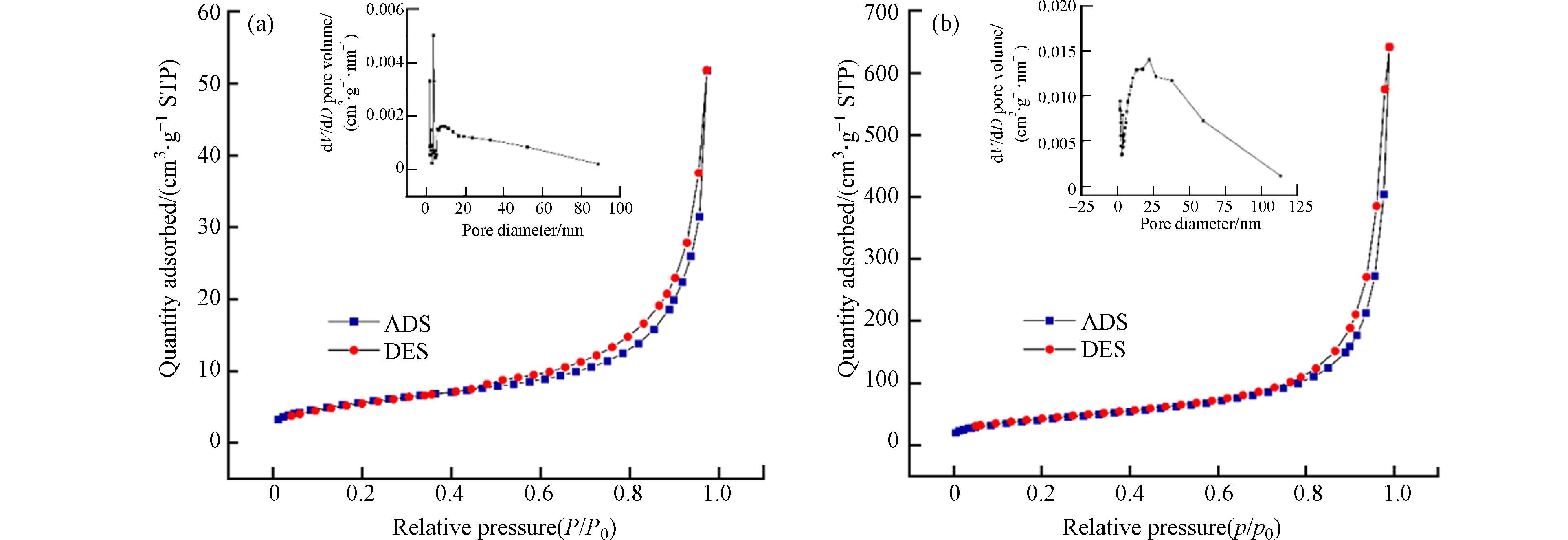
HAADF-STEM图直观反映出P-nZVI的壳-核结构特征及表层2 nm左右的无定形壳(图1a). Fe、O、P及Fe+O重叠的EDS元素分布图显示,Fe、O元素分别分布在颗粒内核与外壳,P元素均匀分布在颗粒表面(图1a). 磷酸基团的修饰虽不影响零价铁的“壳-核”结构,却使nZVI的球形轮廓稍有变形并出现不规则边缘;同时,P-nZVI的HAADF-STEM图揭示P-nZVI内部出现了明显的径向裂纹结构,该裂纹从外壳层延伸至铁芯内部. 这与Zhang等的研究结果一致[20],Zhang等指出在磷酸基团存在下,铁壳表面生成的磷酸铁物种阻碍颗粒的继续长大,并且铁芯生长和外壳层施加的阻力发生了对抗,导致P-nZVI最终生长成边缘缺陷、内部皲裂的不规则球状颗粒. BET测试也证实了这一现象,计算结果显示P-nZVI的比表面积为(159.27±1.01) m2∙g−1,远大于nZVI的比表面积(26.54±2.13) m2∙g−1;同时,P-nZVI的总孔容及孔径均值分别为(0.6093±0.0025) cm³·g−1、(21.22±0.19) nm,相较于nZVI均有所增加(图2、表1). 因此,裂纹结构显著增加了材料的比表面积,这有利于提供更多活性位点、促进吸附. 该结构还有利于污染物快速穿过氧化外壳层,提高Fe0的电子利用率[21].
-
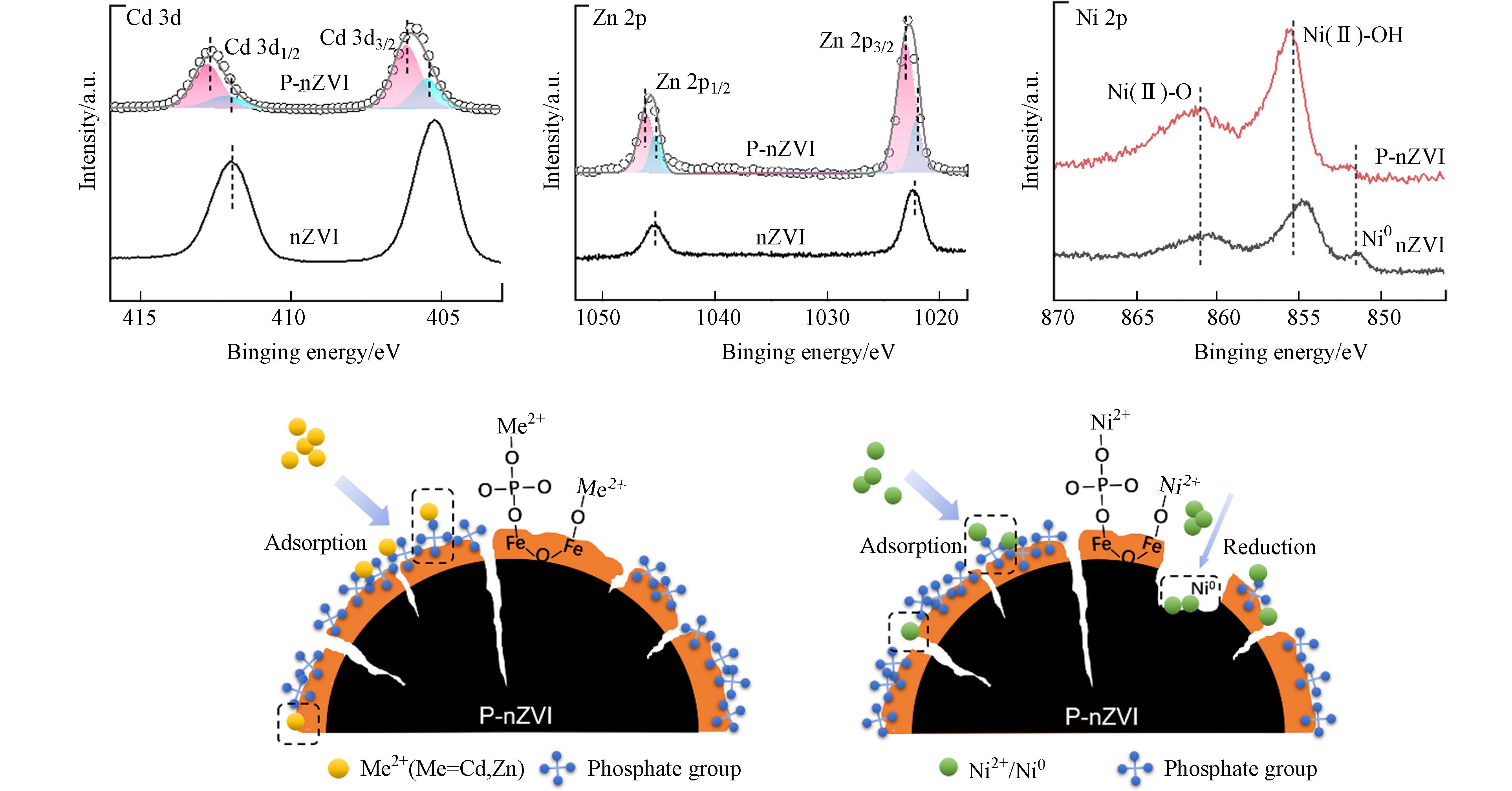
为进一步确定P-nZVI表面元素成分及化学性质,对P-nZVI进行了XPS分析(图1b)及不同pH下的Zeta电位测试(图1c). P 2p XPS谱图在132.54 eV、133.49 eV的特征峰分别归属于P 2p1/2、P 2p3/2[20],该结果证实了纳米铁表面的成功磷酸化. Fe 2p XPS谱图观察到P-nZVI在710.69 eV、713.76 eV附近分别出现Fe(Ⅱ)—O与Fe(Ⅲ)—O的特征峰[22],表明新鲜制备的P-nZVI表面出现一定程度的氧化,氧化层的形成主要来自于溶液中水及少量氧的腐蚀作用[23],这一现象与nZVI类似. 有趣的是,P-nZVI与nZVI的O 1s XPS谱存在明显区别. P-nZVI在结合能为529.83 eV、530.98 eV、532.48 eV附近的特征峰分别归属于O2-、OH-以及物理或化学吸附水[24]. 其中,P-nZVI的OH-光电子特征峰占比高达到75.67%,O2-的占比偏低,仅为12.59%(表2);nZVI的O2-与OH-光电子峰面积占比接近,分别为43.98%和53.08%,化学计量比接近1:1,表明nZVI表面铁氧化物以FeOOH为主(表2)[25]. 该对比表明,P-nZVI表面存在较高比例的 OH-,这主要来自于磷酸基团的贡献,表明nZVI表面被大量磷酸根包被. 磷酸盐可能主要以3种方式结合在nZVI表面(图1d):(1)nZVI的氧化铁外壳对磷酸基团发生静电吸附,该结合方式会受到pH的显著影响[26]. (2)PO43-与颗粒表面羟基脱水络合(方程式1),以单齿单核形式结合在nZVI表面[18,27]. (3)随着nZVI外壳的腐蚀,释放的Fe2+能与PO43-发生沉淀作用(方程式2),以表面沉积[26]的方式附着在颗粒上. Zeta电位测试的结果与预期一致,由于结合在nZVI表面的磷酸基团能提供一定负电荷,导致P-nZVI的IEP相较于nZVI大幅下降. Zhang等[20]通过DFT计算证实,磷酸基团周围负电量增加,因此P-nZVI表面能通过累积负电荷的方式增大对重金属阳离子的静电引力[28].
-
通过批量去除实验,对nZVI、P-nZVI去除Cd2+、Ni2+、Zn2+的动力学过程进行了对比研究. 结果表明,磷酸化修饰成功实现了对3种重金属离子的高效去除(图3a、b). 未经修饰的nZVI对Cd2+和Zn2+的去除率仅为45.4%、53.8%,并且去除率在达到平衡后的一定时间内出现波动,这是由于P-nZVI对金属离子的吸附不牢固,易出现解吸现象. 而P-nZVI对Cd2+、Zn2+的去除率相比nZVI有大幅提升,分别达到79.6%、90.6%. 这是由于P-nZVI表面形成了nZVI-PO43--金属阳离子三络合物,磷酸基团能快速富集并稳定结合重金属离子,不易造二次释放.
对比Ni2+的去除动力学,发现P-nZVI能加快Ni2+的去除,在15 min左右即去除了80%的Ni2+,并将去除率提高10%左右. 结合标准氧化还原电位可知,Ni2+/Ni的标准电位(E0 = − 0.23 V)高于Fe2+/Fe(E0 = − 0.44 V),因此Ni2+不仅可以被吸附固定,还能通过还原作用去除[29]. Fe0的电子转移被认为是整个反应过程的限速步[19],而P-nZVI的缺陷结构有利于Ni2+快速突破氧化外壳的反应屏障、“攻击”富含电子的铁核,促进还原过程并提升去除效率.
为了进一步认识P-nZVI去除Cd2+、Zn2+、Ni2+的反应过程,对3种重金属离子的反应动力学进行了评估,分别采用伪一级、伪二级动力学模型进行模拟,两种模型表达式如下:
式中,
qt (mg∙g−1)为t时刻材料对重金属离子的吸附量,k1 (min−1)为伪一级动力学模型的吸附速率常数,k2 (g·mg−1·min−1)为伪二级动力学模型的吸附速率常数. 图3c、d表明,P-nZVI对Cd2+、Zn2+、Ni2+的去除过程均更符合伪二级动力学模型,R2分别为0.9988、0.9992、0.9997.相较于活性炭、沸石等常见商用重金属去除材料,P-nZVI对上述3种重金属的去除能在短时间内达到相近的水平[30],但不同于这些材料的单一吸附作用,P-nZVI在与水及污染物相互作用中会不断产生高活性的新鲜表面,在一定程度上提升颗粒周边pH,从而能够通过吸附、沉淀、共沉淀等多种方式去除更多的重金属,同时该材料及其产物具有一定的磁性,便于分离回收,因此P-nZVI具有一定潜在应用价值.
-
环境因素对水体中重金属存在形式及迁移转化具有重要影响,其中,pH是影响重金属吸附行为的关键因素之一[31]. 由于Cd2+、Zn2+、Ni2+在碱性条件下均会大量沉淀,因此本实验控制溶液初始pH值在2—8范围内,探究pH对P-nZVI吸附3种重金属离子的影响,结果如图3e所示. 当pH值为2、3时,P-nZVI对重金属离子的吸附量较低;pH值提升至4时,吸附量增幅明显, 因为P-nZVI在偏酸性环境下会受到H+的腐蚀而大量溶解. 当pH值从4增加到8,P-nZVI对Cd2+、Zn2+、Ni2+的吸附量分别从(152.24±1.89) mg·g−1、(165.52±1.14) mg·g−1、(172.25±3.21) mg·g−1提升至(168.24±2.83) mg·g−1、(185.52±4.23) mg·g−1、(188.54±2.84) mg·g−1.
从表面化学的角度分析,由于P-nZVI的IEP为4.51(图1c),因此在低pH条件下,H+与重金属离子竞争P-nZVI表面的吸附位点,导致目标离子的吸附量较低. 随着pH升高,P-nZVI表面带明显负电,对金属阳离子的静电吸引大大增加,吸附量随之升高. 因此,在pH 7—8时重金属的去除效果最佳.
-
为进一步探究P-nZVI在实际水体中对Cd2+、Zn2+、Ni2+的去除情况,本研究选择了自然水体中最为常见的几种阴阳离子SO42-、HCO3-、Ca2+和Mg2+,以及广泛存在于自然水体中的天然有机物质腐殖酸HA(黄腐酸含量>90%)进行探究. 结果与预期一致(图3f-h),阳离子Mg2+和Ca2+对Cd2+、Zn2+、Ni2+的去除抑制作用明显,且随着浓度的增加,抑制作用将增强. 因为Mg2+和Ca2+与目标离子带有相同的电荷且离子水合半径接近,在反应过程中能与目标离子竞争P-nZVI表面结合位点,导致去除效率下降[32]. 而阴离子SO42-、HCO3-存在时,P-nZVI对目标离子的吸附几乎不受影响.
共存物质为HA时,目标重金属的去除率受到显著影响. 当HA浓度从0 mg·L−1增加到50 mg·L−1时,其对目标离子的去除效率至少下降20%. 这与HA表面丰富的官能基团有关,该表面特性使其具有较强的配位结合能力[33],在短时间极易占据大量活性位点,导致目标离子的解吸释放.
-
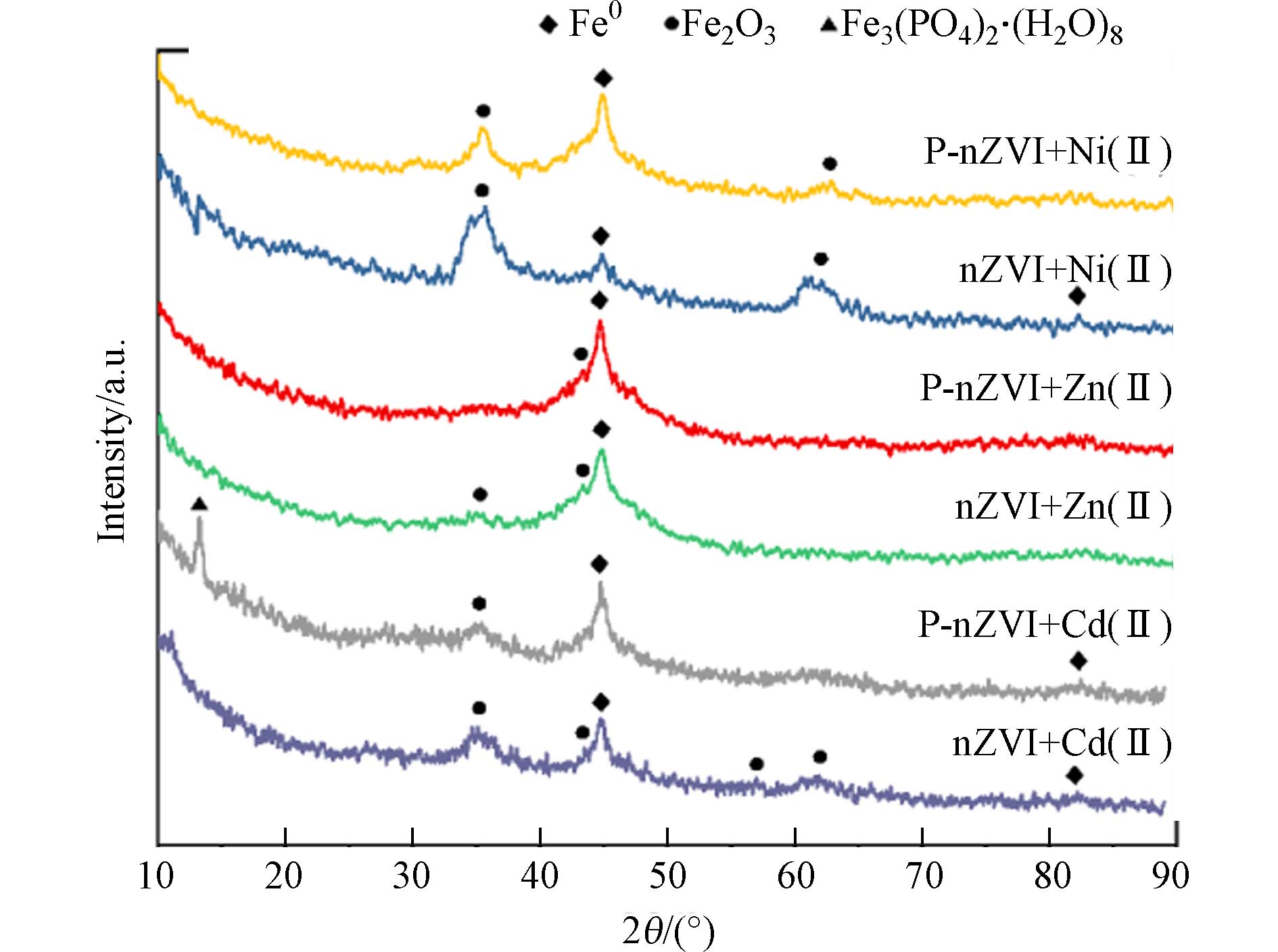
图4为P-nZVI、nZVI与Cd2+、Zn2+、Ni2+3种重金属离子反应2 h后的XRD图谱. 谱图显示,在2θ = 44.8°附近均出现了尖峰,经对比确认,为Fe0(110)晶面峰(PDF # 89-7194);在2θ = 35.5°附近出现的峰为Fe3O4(311)面的宽化衍射峰(PDF # 75-0033),说明P-nZVI、nZVI反应后均表现出一定程度的氧化. 其中,与Cd2+、Zn2+反应后,该氧化峰信号较弱,可能由于表面铁(氢)氧化物的结晶度较差或主要以无定形态存在[34];与Ni2+反应后,相对较强的氧化峰信号表明铁的氧化较明显,并且P-nZVI的氧化更加显著. 值得注意的是,P-nZVI与Cd2+反应后,在2θ = 13.2°处出现了尖峰,这来自于Fe3(PO4)2·8H2O(020)晶面的衍射(PDF # 83-2453),表明少量磷酸盐还可能进一步在nZVI表面生成具有一定结晶度的蓝矿石[26]. XRD图谱上并未反映出与Cd、Ni、Zn元素相关的晶面衍射峰,因此需要结合其他表征手段进一步分析.
-
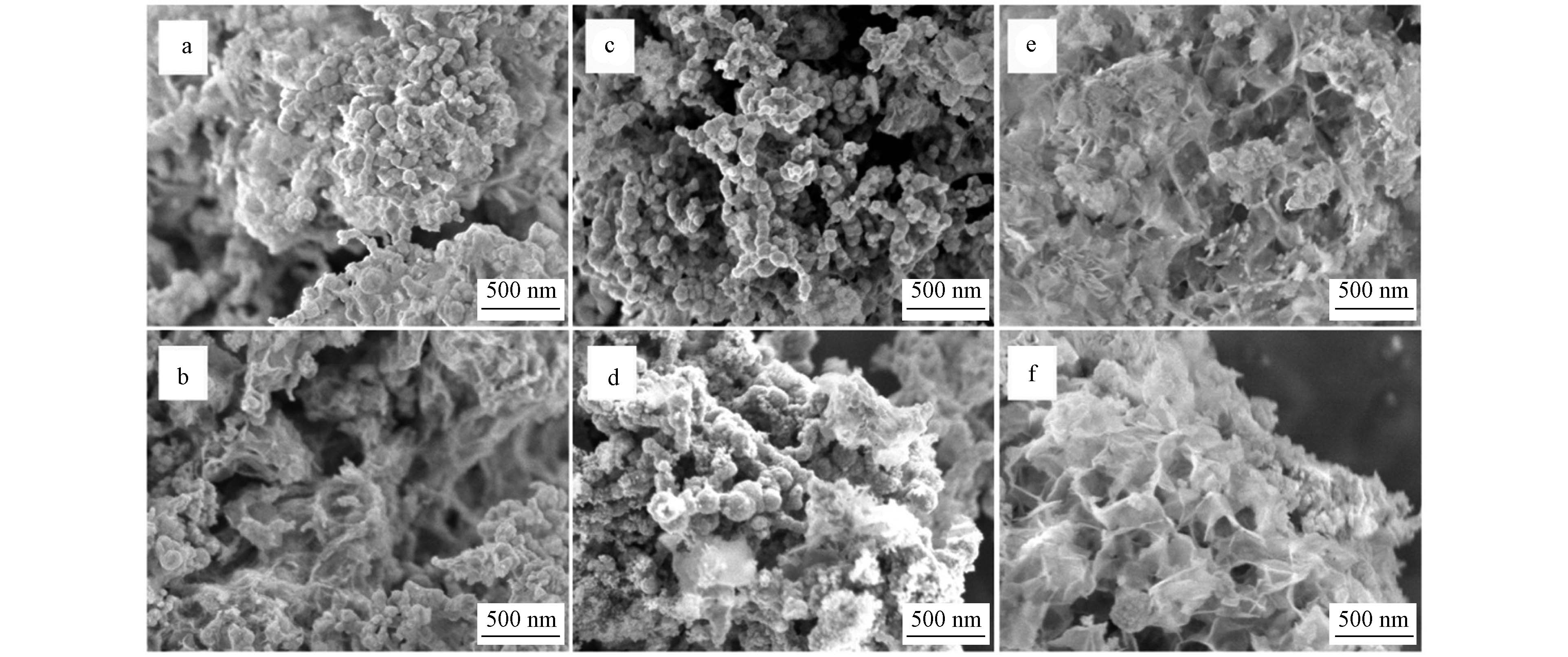
nZVI、P-nZVI去除Cd2+、Zn2+、Ni2+后的形貌对比如图5所示. P-nZVI去除Cd2+后(图5b)外表变为不规则球形并出现絮状沉淀,而nZVI与Cd2+反应未出现明显絮状沉淀. 该对比表明P-nZVI的去除能力强于nZVI,P-nZVI表面的高浓度磷酸盐环境促使Cd2+形成了Cd3(PO4)2界面沉淀(pKsp = 32.6)[35]. 与Cd2+类似,Zn2+反应后纳米铁的球形形貌仍然保持并出现少量絮状沉淀(图5c、5d). 但与Ni2+反应后,P-nZVI表面的球形则完全消失,外表被大量片层状结构及针状结构覆盖(图5e、5f). 结合上述XRD谱图,这再次证实P-nZVI对Ni2+的去除能力最强,在反应过程材料表面氧化明显,生成了结晶度低/无定形铁(氢)氧化物,根据外观结构推测其主要成分可能为FeOOH[36].
-
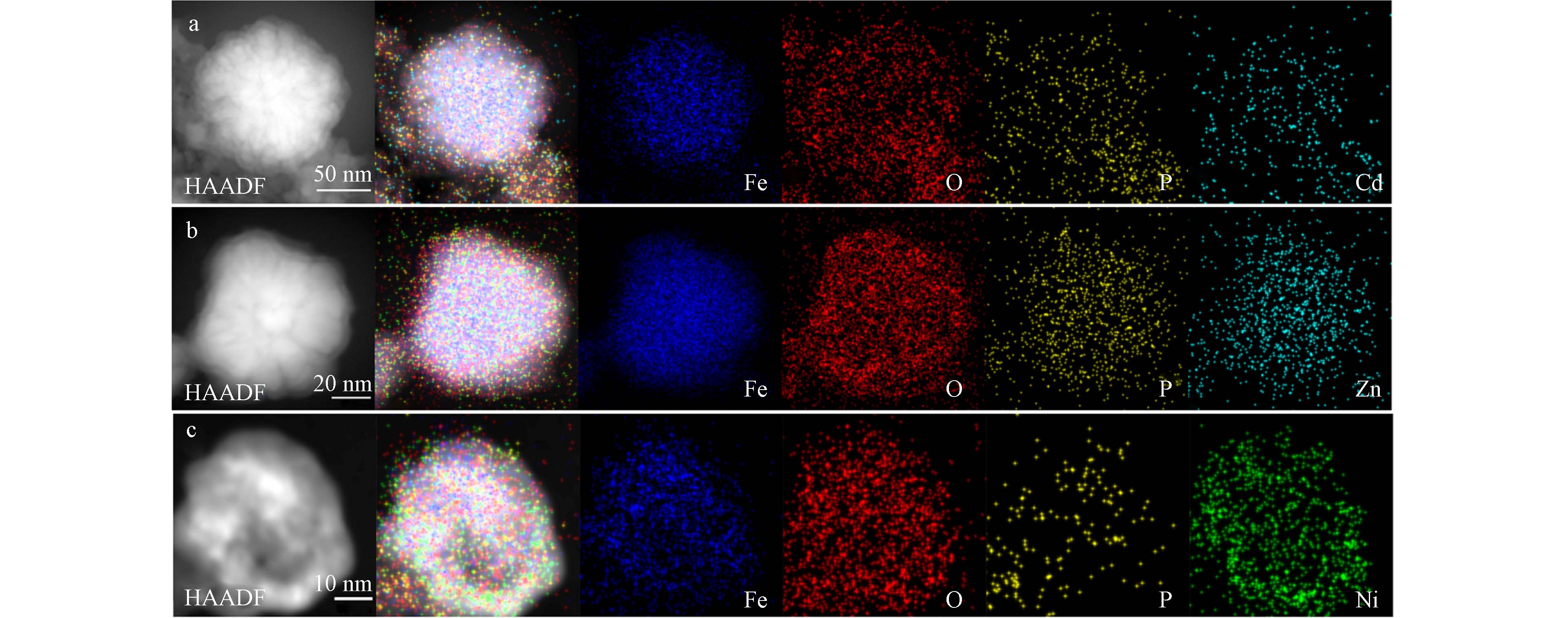
对P-nZVI反应后的单颗粒进行了STEM及EDS分析,以进一步对比微观界面的变化. 如图6a、6b所示,P-nZVI与Cd2+、Zn2+反应后,“壳-核”结构仍然保持,外形及内部结构没有明显改变,颗粒的裂纹及外部的氧化薄层清晰可见. 但EDS能谱的定量结果表明,反应后颗粒O原子相对丰度小幅增加(表3),这是由于溶液中O2、H2O对Fe0的腐蚀作用[23]. 根据Cd、Zn及P的EDS元素分布图,Cd2+、Zn2+与P元素的分布高度相关,均匀分布在外壳层,由此推测磷酸基团在Cd2+、Zn2+的去除过程中起到主导作用.
HAADF-STEM图显示,与Ni2+反应2 h后,P-nZVI颗粒的“壳-核”结构遭到严重破化,裂纹结构消失,图像明暗衬度的差异反映出铁芯已被明显腐蚀. Ni、Fe元素分布图表明Ni元素不仅分布在外壳层还深入铁核内部,颗粒中心的铁元素大量减少(图6c). 同时,反应后P-nZVI单颗粒中Ni原子相对丰度高于Cd、Zn,并且O原子比例升高至54.5%、Fe原子占比明显降低(表3),这进一步证明P-nZVI颗粒的快速氧化及铁离子的大量溶出[37]. 因此,P-nZVI与Ni2+的反应较为剧烈,这与SEM表征结果一致.
-
为进一步确定P-nZVI去除重金属的机理,采用XPS分析反应后的表面元素组成及价态变化. 如图7a所示,反应后的XPS谱图检测出了Cd、Zn、Ni元素的特征峰,证实重金属离子成功结合在P-nZVI表面.
Cd 3d与Zn 2p的XPS谱图显示,nZVI去除Cd2+后Cd 3d5/2结合能为405.28 eV,归因于Cd(Ⅱ)在nZVI表面的吸附;Zn2+2p3/2结合能更高,特征峰值在1022.38 eV附近,均与文献报道一致[38]. 与P-nZVI反应后,Cd 3d5/2特征峰稍稍左移,通过分峰分别得到峰值为405.5 eV及406.18 eV的两个峰,根据Cd2+所处的化学环境不同,将 405.5 eV处的峰分配给吸附在nZVI活性位点上的Cd2+,406.18 eV处的峰分配给吸附于磷酸基团上的Cd2+. 由于Cd2+/Cd(E0 = − 0.40 V)的标准电位非常接近Fe2+/Fe(E0 = − 0.44 V),因此在nZVI上Cd2+被还原为Cd0在热力学上是不利的[34,39],并且在pH≤8时Cd2+几乎不发生沉淀[10]. 由上推测,P-nZVI主要以两种不同吸附方式去除Cd2+:(1)直接吸附于nZVI的表面活性位点[40](2)与磷酸基团结合,形成三元络合物[41]. 由于吸附在磷酸基团位点的Cd2+特征峰面积远大于直接吸附在nZVI表面的特征峰面积,这归因于磷酸根对Cd2+较高的亲和力(
pKsp[Cd3(PO4)2] = 32.6),证实磷酸基团对Cd2+的吸附起到了主导作用. Zn2+的XPS结果与Cd2+类似,可通过同样的方式分峰并分配给不同吸附位点上的Zn2+. 由于Zn2+/Zn的E0 = − 0.7624 V 显著低于Fe2+/Fe, Zn2+在nZVI表面以吸附为主,并且Zn2+对PO43-也具有较高亲和力(pKsp[Zn3(PO4)2] = 32.04),因此Zn2+可能的去除机制与Cd2+相近,即优先吸附在磷酸根位点,少量直接与nZVI表面的铁(氢)氧化物结合[25](图7). Cd2+、Zn2+与nZVI的反应方程式可表达如下:Ni2+与P-nZVI反应后的XPS谱图显示,在851.89 eV、855.60 eV、861.25 eV附近出现了Ni0、Ni(Ⅱ)—O与Ni(Ⅱ)—OH的特征峰[42],其结合能相较于nZVI均略向左偏移. 同时,P-nZVI的Ni(Ⅱ)—O、Ni(Ⅱ)—OH特征峰信号明显强于Ni0,表明磷酸化修饰的纳米零价铁表面更倾向于吸附Ni2+,少量Ni2+被还原为Ni0. 而STEM及EDS元素分布图显示,P-nZVI的内部被严重腐蚀. 根据上述现象推测,P-nZVI首先通过三元络合作用将大量Ni2+吸附在表面,部分Ni2+能通过径向裂纹结构深入铁芯内部,发生较为剧烈的氧化还原反应. Zhang等[20]采用XPS刻蚀,详细比较了Ni2+在4 h内对nZVI及P-nZVI铁芯的腐蚀过程,结果证实P-nZVI的确大大提升了反应速率、促进腐蚀. 原因可作如下分析:浓度梯度被认为是污染物进入铁核速率的决定因素[43],虽然铁氧化物外壳及表面磷酸基团阻碍Ni2+与Fe0的直接接触,但由于P-nZVI表面负电荷量大,能比nZVI更迅速地通过静电引力作用将溶液中游离的Ni2+以物理、化学吸附结合在铁壳表面,较高的浓度梯度促进 Ni2+跨越P-nZVI氧化外壳层,向铁芯转移;其次,P-nZVI独特的径向裂纹及表面缺陷更有助于Ni2+向铁芯扩散,加快电子转移,提高Fe0的利用率(图7). 因此,P-nZVI的结构降低了Ni2+跨越铁氧化壳的阻碍,其反应方程式可表达如下:
-
本文以KH2PO4为磷化剂通过液相还原法制备出磷酸化修饰的纳米铁,并结合XRD、SEM、TEM、XPS等分析手段探究其对Cd2+、Zn2+、Ni2+的去除效果及微观作用机制. 结果表明,P-nZVI是一种表面缺陷、内部皲裂的不规则球状颗粒,其较大的比表面积及表面包被的磷酸基团为重金属的吸附提供了更多位点. 反应动力学表明,P-nZVI对Cd2+、Zn2+、Ni2+的去除效率均显著优于nZVI,2 h左右分别达到了79.6%、90.6%、92.6%. 其中,P-nZVI对Cd2+、Zn2+的去除以表面磷酸基团的吸附为主,形成了nZVI-PO43--金属阳离子三元络合物,使重金属离子结合牢固、不易解吸. 该吸附过程均可用准二级动力学描述. 而Ni2+的去除过程有所不同,其首先在P-nZVI的表面吸附作用下被富集,其次部分Ni2+通过径向裂纹深入铁芯并被快速还原,因此P-nZVI独特的裂纹结构能促进电子的转移. 综上,P-nZVI表面的磷酸化修饰及物理结构的缺陷,使其具有较好的重金属去除活性,本研究结果为开发简单实用的改性nZVI高效去除重金属的方法提供了一定可行性思路.
磷酸化纳米铁去除水中Cd2+、Zn2+、Ni2+的比较
Investigation on the removal of Cd2+, Zn2+, Ni2+ from water by phosphorylated nanoscale zero-valent iron
-
摘要: 重金属污染已成为全球关注的环境问题,镉、镍和锌是工业生产中常见的重金属污染. 纳米零价铁是重金属污染控制的重要环境功能材料,其改性优化工作也备受关注. 本文采用液相还原法在制备过程中添加KH2PO4合成磷酸化纳米铁(phosphorylated nanoscale zero-valent iron,P-nZVI),考察了磷酸化对纳米铁去除Cd2+、Zn2+、Ni2+的效果的影响,评估了磷酸化对抗pH、干扰离子影响的效果,并结合XRD、SEM、S/TEM、XPS等表征手段比较了P-nZVI去除3种重金属的作用机制. 研究表明,P-nZVI对Cd2+、Zn2+的去除效率均显著优于纳米零价铁(nanoscale zero-valent iron,nZVI),分别为79.6%、90.6%. 吸附过程以P-nZVI表面磷酸基团的吸附为主,均可用准二级动力学描述. Ni2+的去除包括吸附和还原作用,加剧了铁芯腐蚀,使其去除效率达到92.6%. 因此,磷酸化修饰能通过累积零价铁表面负电荷以加速吸附过程;裂纹结构能降低金属离子跨越氧化铁层的阻碍,促进氧化还原,提高Fe0利用率.Abstract: Heavy metal pollution has become an environmental issue raising global concern, and cadmium, nickel and zinc are common heavy metal pollution in industrial production. Nano zero-valent iron is an important environmental functional material for heavy metal pollution removal, and its modification as well as optimization have attracted much attention. In this study, we prepared phosphorylated nanoscale zero-valent iron (P-nZVI) by liquid-phase reduction using NaBH4, FeCl3∙6H2O in the presence of KH2PO4. The performances of P-nZVI on the removal of Cd2+, Zn2+, Ni2+were examined and the effects of initial solution pH and interfering ions were also investigated. Several characterization techniques were adopted to explore the morphology, structure and interface characteristics of P-nZVI including scanning electron microscopy (SEM), scanning transmission electron microscopy (STEM) integrated with energy-dispersive X-ray spectrometry (EDS), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). The results indicated that the removal efficiency of P-nZVI was significantly improved than that of nanoscale zero-valent iron(nZVI) for both Cd2+ and Zn2+ with 79.6% and 90.6%, respectively. The removal process was dominated by the adsorption of phosphate groups on the surface of P-nZVI, and both could be described by quasi-secondary kinetics. Unlike Cd2+ and Zn2+, removal of Ni2+ involved adsorption and reduction, with increased corrosion of internal iron core, resulting in a high removal efficiency of 92.6%. In summary, the advantages of phosphorylated zero-valent iron nanoparticles are described as follows: phosphate groups can accelerate the adsorption process resulting from more negative charge on the zero-valent iron surface; the cracked structure can reduce the barrier of metal ions across the iron oxide layer, promote e-transfer and improve utilization of Fe0.
-
Key words:
- nanoscale zero-valent iron /
- phosphorylation /
- heavy metal /
- adsorption /
- redox reaction.
-
我国工业高速发展及城镇化进程,使得重金属造成的水污染日趋严重,成为了全球关注的环境问题之一[1]. 重金属不可被生物降解,易在生物体内累积并通过食物链放大,严重威胁人类健康及生态系统. 镉(Cd)、锌(Zn)、镍(Ni)是工业废水中最常见的重金属污染物[2]. Ni、Zn是生命体需要的微量元素,但高浓度Ni2+、Zn2+能引起呕吐、哮喘及中枢神经系统紊乱等中毒症状[3 − 4];Cd2+即使在较低浓度下也表现出较高生物毒性[5],长期接触Cd2+会导致慢性肺部疾病、骨骼畸形和肾功能衰减等问题[6]. 因此,高效去除水体中的以镉(Cd)、锌(Zn)、镍(Ni)为代表的重金属离子成为了亟待解决的问题,并在近年来受到了相关研究领域的广泛关注.
从水中去除重金属离子的方法包括吸附、化学沉淀/混凝、离子交换、膜技术和电化学方法等[7]. 随着纳米技术的发展,纳米材料在水处理中逐渐发挥重要作用. 其中,纳米零价铁(nanoscale zero-valent iron, nZVI)凭借比表面积大、还原活性高、适用面广、环境友好等特性被广泛用于水环境中的重金属去除[8]. nZVI的粒径在(20—100) nm范围,呈链状,合成后瞬间在表面生成铁(氢)氧化物,这使nZVI形成了独特“核-壳”结构[9]. nZVI在参与重金属去除过程中,外氧化壳层首先通过静电引力和表面络合作用吸附重金属离子,随后单质铁核可以充当电子供体还原被吸附的重金属离子,因此nZVI对重金属的去除可能涉及吸附和还原机制[10]. 但nZVI在实践应用中也呈现出一定的局限性,如易自发团聚,表面活性位点减少;极易被空气和水氧化,大大削弱其还原能力,导致活性降低. 为了解决上述问题,大量研究对nZVI的改性进行了探索[11 − 13],旨在进一步提升nZVI的稳定性、电子传递效率和去除的选择性.
研究发现,nZVI对磷酸盐具有很强的亲和力,能通过吸附、沉淀等作用高效去除水中PO43-[14 − 15]. 研究进一步表明,吸附在nZVI表面的PO43-能生成钝化层,磷酸基团的侧链质子抑制nZVI与氧和水的反应,从而对nZVI起到一定保护作用[16]. 因此,表面磷酸化能提高nZVI在水中的稳定性. 此外,磷酸盐能取代nZVI表面的羟基,与重金属形成三元配合物,进而增强其对重金属的配位能力[17]. 基于上述特性,表面磷酸化的nZVI(phosphorylated nanoscale zero-valent iron, P-nZVI)用于污染物去除已被广泛研究,如Zhang等[16]发现四聚磷酸盐改性nZVI对阿特拉津的降解过程中,四聚磷酸盐的存在抑制了质子还原,增强了分子氧活化,使阿特拉津的降解率提高955倍. Li等[18]的研究表明,磷酸化改性后,P-nZVI对Cr(Ⅵ)还原的电子选择性从6.1%提高到31.3%,去除效率提高了4倍,这是由于磷酸化修饰增强了对铬的吸附能力,进而促进其还原. 综上所述,nZVI的表面磷酸盐改性能提高对重金属离子的配位能力,同时表面磷酸盐抑制了nZVI被水和氧气氧化,在增强nZVI对重金属离子吸附能力的基础上有效提高了nZVI对吸附在表面的重金属的还原能力,其改性策略成本低,操作简单,效果显著. 但目前,P-nZVI对不同种类重金属的去除性能、机理的相关比较研究仍较少,因此,比较P-nZVI对常见重金属的去除能力和作用机制具有较大的研究价值.
本研究以KH2PO4为磷化剂,通过液相还原法制备磷化改性的nZVI,并且选择了Cd2+、Zn2+、Ni2+3种典型的重金属离子作为目标污染物. 由于Fe0对Cd2+、Zn2+、Ni2+具有不同的还原能力;Cd2+、Zn2+、Ni2+受pH影响的沉淀-溶解特性存在差异;同时,其与表面磷酸根的亲和力也不尽相同,因此磷酸化改性后的nZVI对于上述3种金属离子的去除特性可能存在差异. 本文在讨论P-nZVI去除水溶液中Cd2+、Zn2+、Ni2+效果的基础上,进一步研究了pH、干扰离子等影响因素的影响,并结合XRD、XPS、SEM、TEM等表征,讨论P-nZVI去除Cd2+、Zn2+、Ni2+的微观机理差异. 本研究旨在比较P-nZVI对不同重金属的去除能力、重金属去除过程中的影响因素和微观界面特征,为进一步深入探索P-nZVI在微界面上与重金属离子的作用机理提供一定的参考.
1. 实验部分(Experimental section)
1.1 纳米零价铁及改性纳米零价铁的制备
研究所用的nZVI、P-nZVI均采用NaBH4液相还原Fe3+法合成[19]. 根据先前的实验,P/Fe物质的量比在0.6左右P-nZVI去除效果较好,因此制备磷酸化修饰的nZVI时,需要将NaH2PO4以一定比例和NaBH4混合,确保P/Fe为0.6,并通过蠕动泵将混合溶液缓缓滴入三颈瓶中,其他步骤与nZVI的制备相同. 反应完成后,采用离心的方式收集nZVI并用去离子水和无水乙醇各洗涤3次,储存于无水乙醇中备用.
1.2 批量去除实验
实验分别探究了P-nZVI吸附Cd2+、Zn2+、Ni2+的动力学特征、pH及干扰离子等因素对重金属去除的影响. 所有实验中P-nZVI的投加量均为0.5 g∙L−1,重金属离子的初始浓度为100 mg∙L−1. 在动力学实验中,首先配制一定量Cd2+、Zn2+、Ni2+溶液至三颈瓶中,调节反应初始pH为6±0.1,通氮20 min脱去溶液中O2,再加入适量P-nZVI,分别反应0(空白)、5、10、15、30、40、50、60、75、90、120 min取出少量溶液,过0.22 μm滤膜后测定离子浓度. 反应过程中,采用磁力搅拌器以250 r·min−1进行机械搅拌,使P-nZVI与目标离子充分接触. pH实验中,为防止Cd2+、Zn2+、Ni2+大量沉淀控制pH范围在2—8之间,使用HCl和NaOH调节反应pH分别为2±0.1、3±0.1、4±0.1、5±0.1、6±0.1、7±0.1、8±0.1,其他步骤同上. 为探究溶液中干扰离子对吸附的影响,在其他操作不变的情况下,控制反应pH为6±0.1,量取适量含有共存离子的溶液[HA、Na2SO4、NaHCO3、Mg(NO3)2、Ca(NO3)2]加入三颈瓶中,使共存离子浓度分别为0、10、50 mg∙L−1. 上述所有实验,控制实验温度为25 ℃.
1.3 分析方法
采用电感耦合等离子体发射光谱仪(ICP-720 ES,安捷伦公司,美国)测定溶液中Cd2+、Zn2+、Ni2+浓度. 采用Zeta电位测定仪(Zetasizer Nano ZS90)测定P-nZVI的零电荷点,ASPS 2460气体分析仪测定材料孔径分布及比表面积. 为比较反应前后材料的微观形貌变化,采用场发射电子扫描显微镜(Nova naniSEM-450, FEI公司,美国)及球差校正扫描透射电子显微镜(TatanTMG2 60-300, FEI公司,美国)进行表征,并使用EDS能谱对材料的元素分布情况进行定性及半定量分析. 采用X射线衍射仪(D8 Advance,布鲁克公司,德国)及X射线光电子能谱仪(ESCALAB 250XI, 赛默飞, 美国)探测材料体相物质的晶体结构及表相化学组成、元素种类及价态,并使用MDI Jade 6软件对XRD结果进行比对分析、Advantage 5.948软件对XPS谱进行分峰拟合. 采用Origin 2021软件对所得数据进行绘图.
2. 结果与讨论 (Results and discussion)
2.1 P-nZVI结构及界面性质
2.1.1 结构特征
HAADF-STEM图直观反映出P-nZVI的壳-核结构特征及表层2 nm左右的无定形壳(图1a). Fe、O、P及Fe+O重叠的EDS元素分布图显示,Fe、O元素分别分布在颗粒内核与外壳,P元素均匀分布在颗粒表面(图1a). 磷酸基团的修饰虽不影响零价铁的“壳-核”结构,却使nZVI的球形轮廓稍有变形并出现不规则边缘;同时,P-nZVI的HAADF-STEM图揭示P-nZVI内部出现了明显的径向裂纹结构,该裂纹从外壳层延伸至铁芯内部. 这与Zhang等的研究结果一致[20],Zhang等指出在磷酸基团存在下,铁壳表面生成的磷酸铁物种阻碍颗粒的继续长大,并且铁芯生长和外壳层施加的阻力发生了对抗,导致P-nZVI最终生长成边缘缺陷、内部皲裂的不规则球状颗粒. BET测试也证实了这一现象,计算结果显示P-nZVI的比表面积为(159.27±1.01) m2∙g−1,远大于nZVI的比表面积(26.54±2.13) m2∙g−1;同时,P-nZVI的总孔容及孔径均值分别为(0.6093±0.0025) cm³·g−1、(21.22±0.19) nm,相较于nZVI均有所增加(图2、表1). 因此,裂纹结构显著增加了材料的比表面积,这有利于提供更多活性位点、促进吸附. 该结构还有利于污染物快速穿过氧化外壳层,提高Fe0的电子利用率[21].
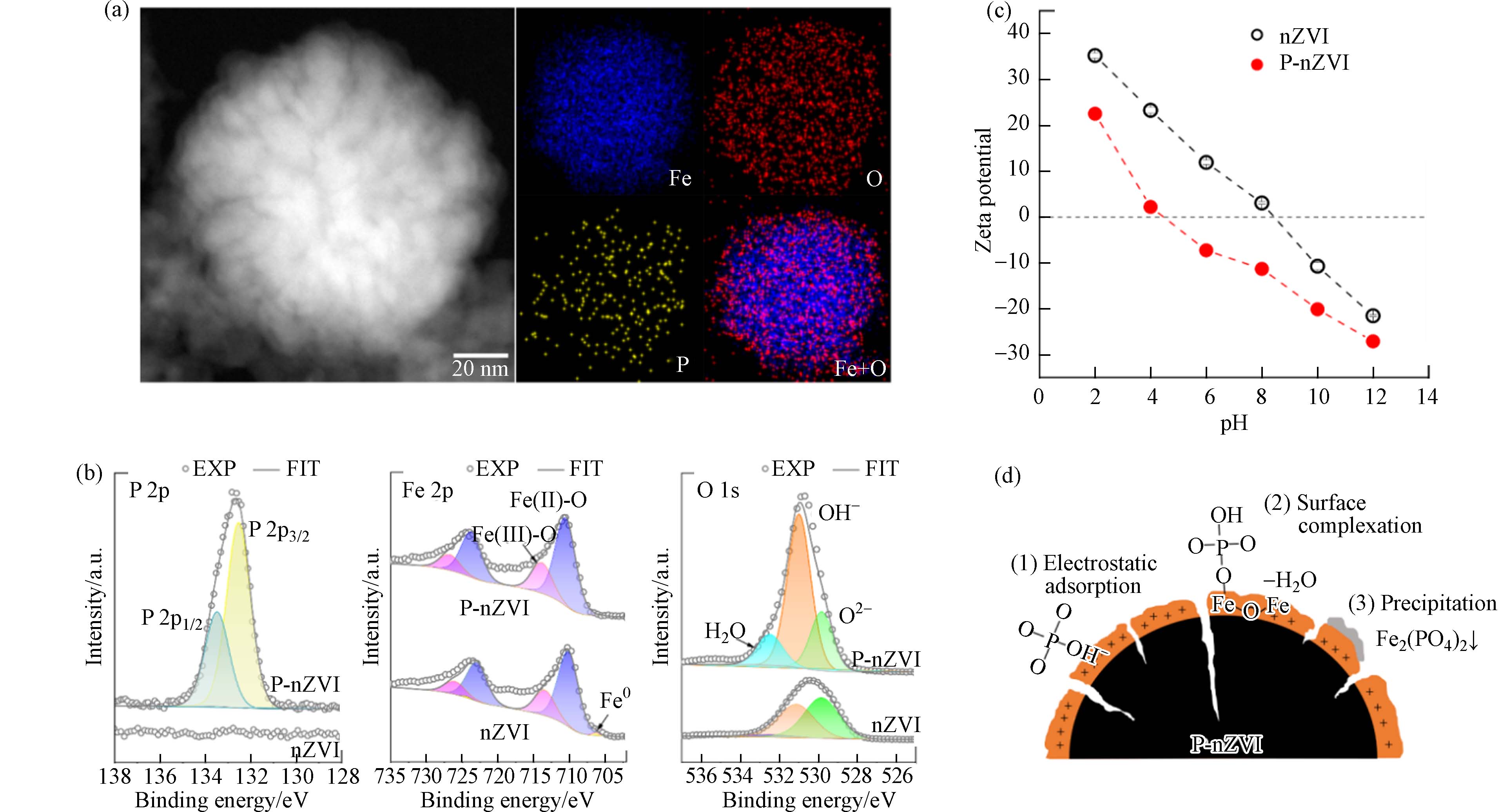 图 1 (a)P-nZVIHAADF-STEM图像和Fe、O、P、Fe+O的EDS 元素分布图;(b)P-nZVI的Fe 2p、O1s XPS谱图;(c)不同pH下P-nZVI、nZVI的Zeta电位图;(d)P-nZVI上磷酸基团的结合示意图Figure 1. (a)HAADF-STEM image of fresh P-nZVI as well as the corresponding elemental mapping of Fe, O, P and the overlapped mapping of Fe, O;(b)XPS survey spectra of P 2p, Fe 2p and O 1s of P-nZVI;(c)zeta potential of P-nZVI and nZVI at different pH;(d)schematic diagram of phosphate groups binding mode表 1 nZVI及P-nZVI的比表面积、孔容、孔径对比Table 1. Comparison of specific surface area, pore volume and pore size of nZVI and P-nZVI
图 1 (a)P-nZVIHAADF-STEM图像和Fe、O、P、Fe+O的EDS 元素分布图;(b)P-nZVI的Fe 2p、O1s XPS谱图;(c)不同pH下P-nZVI、nZVI的Zeta电位图;(d)P-nZVI上磷酸基团的结合示意图Figure 1. (a)HAADF-STEM image of fresh P-nZVI as well as the corresponding elemental mapping of Fe, O, P and the overlapped mapping of Fe, O;(b)XPS survey spectra of P 2p, Fe 2p and O 1s of P-nZVI;(c)zeta potential of P-nZVI and nZVI at different pH;(d)schematic diagram of phosphate groups binding mode表 1 nZVI及P-nZVI的比表面积、孔容、孔径对比Table 1. Comparison of specific surface area, pore volume and pore size of nZVI and P-nZVI比表面积/(m2·g−1)Surface area 孔容/(cm3·g−1)Pore volume 孔径/nmPore size nZVI 26.54±2.13 0.0759±0.0030 27.67±0.06 P-nZVI 159.27±1.01 0.6093±0.0025 21.22±0.19 2.1.2 表面化学性质
为进一步确定P-nZVI表面元素成分及化学性质,对P-nZVI进行了XPS分析(图1b)及不同pH下的Zeta电位测试(图1c). P 2p XPS谱图在132.54 eV、133.49 eV的特征峰分别归属于P 2p1/2、P 2p3/2[20],该结果证实了纳米铁表面的成功磷酸化. Fe 2p XPS谱图观察到P-nZVI在710.69 eV、713.76 eV附近分别出现Fe(Ⅱ)—O与Fe(Ⅲ)—O的特征峰[22],表明新鲜制备的P-nZVI表面出现一定程度的氧化,氧化层的形成主要来自于溶液中水及少量氧的腐蚀作用[23],这一现象与nZVI类似. 有趣的是,P-nZVI与nZVI的O 1s XPS谱存在明显区别. P-nZVI在结合能为529.83 eV、530.98 eV、532.48 eV附近的特征峰分别归属于O2-、OH-以及物理或化学吸附水[24]. 其中,P-nZVI的OH-光电子特征峰占比高达到75.67%,O2-的占比偏低,仅为12.59%(表2);nZVI的O2-与OH-光电子峰面积占比接近,分别为43.98%和53.08%,化学计量比接近1:1,表明nZVI表面铁氧化物以FeOOH为主(表2)[25]. 该对比表明,P-nZVI表面存在较高比例的 OH-,这主要来自于磷酸基团的贡献,表明nZVI表面被大量磷酸根包被. 磷酸盐可能主要以3种方式结合在nZVI表面(图1d):(1)nZVI的氧化铁外壳对磷酸基团发生静电吸附,该结合方式会受到pH的显著影响[26]. (2)PO43-与颗粒表面羟基脱水络合(方程式1),以单齿单核形式结合在nZVI表面[18,27]. (3)随着nZVI外壳的腐蚀,释放的Fe2+能与PO43-发生沉淀作用(方程式2),以表面沉积[26]的方式附着在颗粒上. Zeta电位测试的结果与预期一致,由于结合在nZVI表面的磷酸基团能提供一定负电荷,导致P-nZVI的IEP相较于nZVI大幅下降. Zhang等[20]通过DFT计算证实,磷酸基团周围负电量增加,因此P-nZVI表面能通过累积负电荷的方式增大对重金属阳离子的静电引力[28].
表 2 nZVI及P-nZVI的O1s XPS谱中O2-、OH-及H2O相对丰度(% at.)Table 2. Relative abundance of O2-, OH- and H2O in the O1s XPS spectra of nZVI and P-nZVIP-nZVI nZVI O2- 23.11 43.98 OH- 62.88 53.08 H2O 14.01 2.94 总计 100 stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) 2.2 重金属去除实验
2.2.1 反应动力学
通过批量去除实验,对nZVI、P-nZVI去除Cd2+、Ni2+、Zn2+的动力学过程进行了对比研究. 结果表明,磷酸化修饰成功实现了对3种重金属离子的高效去除(图3a、b). 未经修饰的nZVI对Cd2+和Zn2+的去除率仅为45.4%、53.8%,并且去除率在达到平衡后的一定时间内出现波动,这是由于P-nZVI对金属离子的吸附不牢固,易出现解吸现象. 而P-nZVI对Cd2+、Zn2+的去除率相比nZVI有大幅提升,分别达到79.6%、90.6%. 这是由于P-nZVI表面形成了nZVI-PO43--金属阳离子三络合物,磷酸基团能快速富集并稳定结合重金属离子,不易造二次释放.
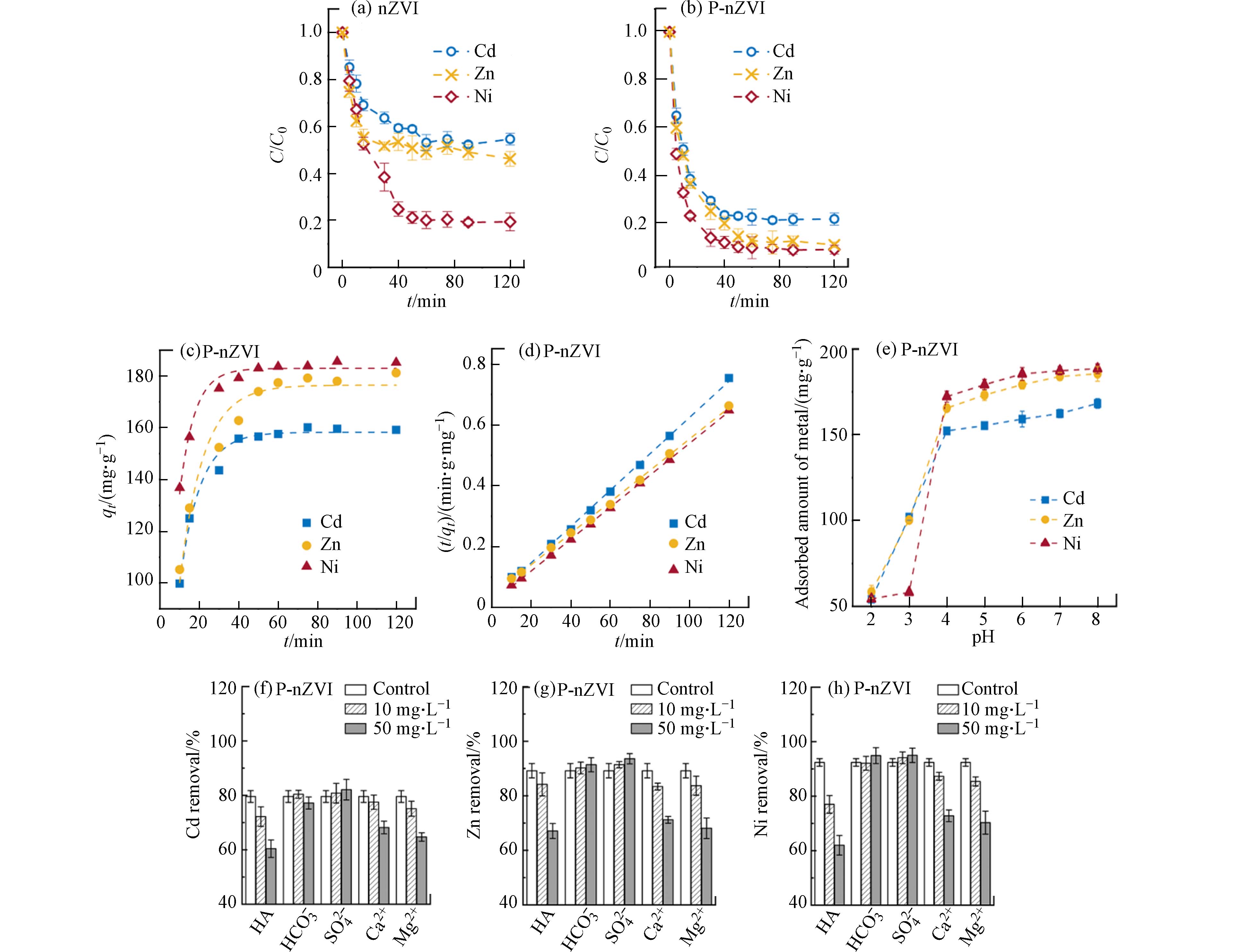 图 3 (a, b)反应动力学曲线;(c, d)伪一级、伪二级动力学拟合曲线;(e)pH的影响;(f, g, h)干扰离子的影响Figure 3. (a, b)Kinetic adsorption experiments of nZVI and P-nZVI;(c, d)fitted curves of pseudo-first-order kinetic model and pseudo-second-order kinetic model;(e)influence of different pH conditions;(f, g, h)influence of interfering ions
图 3 (a, b)反应动力学曲线;(c, d)伪一级、伪二级动力学拟合曲线;(e)pH的影响;(f, g, h)干扰离子的影响Figure 3. (a, b)Kinetic adsorption experiments of nZVI and P-nZVI;(c, d)fitted curves of pseudo-first-order kinetic model and pseudo-second-order kinetic model;(e)influence of different pH conditions;(f, g, h)influence of interfering ions对比Ni2+的去除动力学,发现P-nZVI能加快Ni2+的去除,在15 min左右即去除了80%的Ni2+,并将去除率提高10%左右. 结合标准氧化还原电位可知,Ni2+/Ni的标准电位(E0 = − 0.23 V)高于Fe2+/Fe(E0 = − 0.44 V),因此Ni2+不仅可以被吸附固定,还能通过还原作用去除[29]. Fe0的电子转移被认为是整个反应过程的限速步[19],而P-nZVI的缺陷结构有利于Ni2+快速突破氧化外壳的反应屏障、“攻击”富含电子的铁核,促进还原过程并提升去除效率.
为了进一步认识P-nZVI去除Cd2+、Zn2+、Ni2+的反应过程,对3种重金属离子的反应动力学进行了评估,分别采用伪一级、伪二级动力学模型进行模拟,两种模型表达式如下:
stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) 式中,
qt k1 k2 相较于活性炭、沸石等常见商用重金属去除材料,P-nZVI对上述3种重金属的去除能在短时间内达到相近的水平[30],但不同于这些材料的单一吸附作用,P-nZVI在与水及污染物相互作用中会不断产生高活性的新鲜表面,在一定程度上提升颗粒周边pH,从而能够通过吸附、沉淀、共沉淀等多种方式去除更多的重金属,同时该材料及其产物具有一定的磁性,便于分离回收,因此P-nZVI具有一定潜在应用价值.
2.2.2 pH的影响
环境因素对水体中重金属存在形式及迁移转化具有重要影响,其中,pH是影响重金属吸附行为的关键因素之一[31]. 由于Cd2+、Zn2+、Ni2+在碱性条件下均会大量沉淀,因此本实验控制溶液初始pH值在2—8范围内,探究pH对P-nZVI吸附3种重金属离子的影响,结果如图3e所示. 当pH值为2、3时,P-nZVI对重金属离子的吸附量较低;pH值提升至4时,吸附量增幅明显, 因为P-nZVI在偏酸性环境下会受到H+的腐蚀而大量溶解. 当pH值从4增加到8,P-nZVI对Cd2+、Zn2+、Ni2+的吸附量分别从(152.24±1.89) mg·g−1、(165.52±1.14) mg·g−1、(172.25±3.21) mg·g−1提升至(168.24±2.83) mg·g−1、(185.52±4.23) mg·g−1、(188.54±2.84) mg·g−1.
从表面化学的角度分析,由于P-nZVI的IEP为4.51(图1c),因此在低pH条件下,H+与重金属离子竞争P-nZVI表面的吸附位点,导致目标离子的吸附量较低. 随着pH升高,P-nZVI表面带明显负电,对金属阳离子的静电吸引大大增加,吸附量随之升高. 因此,在pH 7—8时重金属的去除效果最佳.
2.2.3 干扰离子的影响
为进一步探究P-nZVI在实际水体中对Cd2+、Zn2+、Ni2+的去除情况,本研究选择了自然水体中最为常见的几种阴阳离子SO42-、HCO3-、Ca2+和Mg2+,以及广泛存在于自然水体中的天然有机物质腐殖酸HA(黄腐酸含量>90%)进行探究. 结果与预期一致(图3f-h),阳离子Mg2+和Ca2+对Cd2+、Zn2+、Ni2+的去除抑制作用明显,且随着浓度的增加,抑制作用将增强. 因为Mg2+和Ca2+与目标离子带有相同的电荷且离子水合半径接近,在反应过程中能与目标离子竞争P-nZVI表面结合位点,导致去除效率下降[32]. 而阴离子SO42-、HCO3-存在时,P-nZVI对目标离子的吸附几乎不受影响.
共存物质为HA时,目标重金属的去除率受到显著影响. 当HA浓度从0 mg·L−1增加到50 mg·L−1时,其对目标离子的去除效率至少下降20%. 这与HA表面丰富的官能基团有关,该表面特性使其具有较强的配位结合能力[33],在短时间极易占据大量活性位点,导致目标离子的解吸释放.
2.3 机理研究
2.3.1 XRD
图4为P-nZVI、nZVI与Cd2+、Zn2+、Ni2+3种重金属离子反应2 h后的XRD图谱. 谱图显示,在2θ = 44.8°附近均出现了尖峰,经对比确认,为Fe0(110)晶面峰(PDF # 89-7194);在2θ = 35.5°附近出现的峰为Fe3O4(311)面的宽化衍射峰(PDF # 75-0033),说明P-nZVI、nZVI反应后均表现出一定程度的氧化. 其中,与Cd2+、Zn2+反应后,该氧化峰信号较弱,可能由于表面铁(氢)氧化物的结晶度较差或主要以无定形态存在[34];与Ni2+反应后,相对较强的氧化峰信号表明铁的氧化较明显,并且P-nZVI的氧化更加显著. 值得注意的是,P-nZVI与Cd2+反应后,在2θ = 13.2°处出现了尖峰,这来自于Fe3(PO4)2·8H2O(020)晶面的衍射(PDF # 83-2453),表明少量磷酸盐还可能进一步在nZVI表面生成具有一定结晶度的蓝矿石[26]. XRD图谱上并未反映出与Cd、Ni、Zn元素相关的晶面衍射峰,因此需要结合其他表征手段进一步分析.
2.3.2 SEM
nZVI、P-nZVI去除Cd2+、Zn2+、Ni2+后的形貌对比如图5所示. P-nZVI去除Cd2+后(图5b)外表变为不规则球形并出现絮状沉淀,而nZVI与Cd2+反应未出现明显絮状沉淀. 该对比表明P-nZVI的去除能力强于nZVI,P-nZVI表面的高浓度磷酸盐环境促使Cd2+形成了Cd3(PO4)2界面沉淀(pKsp = 32.6)[35]. 与Cd2+类似,Zn2+反应后纳米铁的球形形貌仍然保持并出现少量絮状沉淀(图5c、5d). 但与Ni2+反应后,P-nZVI表面的球形则完全消失,外表被大量片层状结构及针状结构覆盖(图5e、5f). 结合上述XRD谱图,这再次证实P-nZVI对Ni2+的去除能力最强,在反应过程材料表面氧化明显,生成了结晶度低/无定形铁(氢)氧化物,根据外观结构推测其主要成分可能为FeOOH[36].
2.3.3 TEM
对P-nZVI反应后的单颗粒进行了STEM及EDS分析,以进一步对比微观界面的变化. 如图6a、6b所示,P-nZVI与Cd2+、Zn2+反应后,“壳-核”结构仍然保持,外形及内部结构没有明显改变,颗粒的裂纹及外部的氧化薄层清晰可见. 但EDS能谱的定量结果表明,反应后颗粒O原子相对丰度小幅增加(表3),这是由于溶液中O2、H2O对Fe0的腐蚀作用[23]. 根据Cd、Zn及P的EDS元素分布图,Cd2+、Zn2+与P元素的分布高度相关,均匀分布在外壳层,由此推测磷酸基团在Cd2+、Zn2+的去除过程中起到主导作用.
HAADF-STEM图显示,与Ni2+反应2 h后,P-nZVI颗粒的“壳-核”结构遭到严重破化,裂纹结构消失,图像明暗衬度的差异反映出铁芯已被明显腐蚀. Ni、Fe元素分布图表明Ni元素不仅分布在外壳层还深入铁核内部,颗粒中心的铁元素大量减少(图6c). 同时,反应后P-nZVI单颗粒中Ni原子相对丰度高于Cd、Zn,并且O原子比例升高至54.5%、Fe原子占比明显降低(表3),这进一步证明P-nZVI颗粒的快速氧化及铁离子的大量溶出[37]. 因此,P-nZVI与Ni2+的反应较为剧烈,这与SEM表征结果一致.
表 3 P-nZVI及去除重金属后的EDS定量结果Table 3. Quantitative results of EDS before and after removal of heavy metalsP-nZVI 除Cd2+后After removal of Cd2+ 除Zn2+后After removal of Zn2+ 除Ni2+后After removal of Ni2+ Fe 85.68 63.55 76.01 28.22 O 13.83 34.37 21.90 54.50 P 0.49 1.45 1.13 0.62 目标污染物 — 0.64 0.95 16.55 总计 100 2.3.4 XPS
为进一步确定P-nZVI去除重金属的机理,采用XPS分析反应后的表面元素组成及价态变化. 如图7a所示,反应后的XPS谱图检测出了Cd、Zn、Ni元素的特征峰,证实重金属离子成功结合在P-nZVI表面.
Cd 3d与Zn 2p的XPS谱图显示,nZVI去除Cd2+后Cd 3d5/2结合能为405.28 eV,归因于Cd(Ⅱ)在nZVI表面的吸附;Zn2+2p3/2结合能更高,特征峰值在1022.38 eV附近,均与文献报道一致[38]. 与P-nZVI反应后,Cd 3d5/2特征峰稍稍左移,通过分峰分别得到峰值为405.5 eV及406.18 eV的两个峰,根据Cd2+所处的化学环境不同,将 405.5 eV处的峰分配给吸附在nZVI活性位点上的Cd2+,406.18 eV处的峰分配给吸附于磷酸基团上的Cd2+. 由于Cd2+/Cd(E0 = − 0.40 V)的标准电位非常接近Fe2+/Fe(E0 = − 0.44 V),因此在nZVI上Cd2+被还原为Cd0在热力学上是不利的[34,39],并且在pH≤8时Cd2+几乎不发生沉淀[10]. 由上推测,P-nZVI主要以两种不同吸附方式去除Cd2+:(1)直接吸附于nZVI的表面活性位点[40](2)与磷酸基团结合,形成三元络合物[41]. 由于吸附在磷酸基团位点的Cd2+特征峰面积远大于直接吸附在nZVI表面的特征峰面积,这归因于磷酸根对Cd2+较高的亲和力(
pKsp[Cd3(PO4)2] pKsp[Zn3(PO4)2] stringUtils.convertMath(!{formula.content}) stringUtils.convertMath(!{formula.content}) stringUtils.convertMath(!{formula.content}) Ni2+与P-nZVI反应后的XPS谱图显示,在851.89 eV、855.60 eV、861.25 eV附近出现了Ni0、Ni(Ⅱ)—O与Ni(Ⅱ)—OH的特征峰[42],其结合能相较于nZVI均略向左偏移. 同时,P-nZVI的Ni(Ⅱ)—O、Ni(Ⅱ)—OH特征峰信号明显强于Ni0,表明磷酸化修饰的纳米零价铁表面更倾向于吸附Ni2+,少量Ni2+被还原为Ni0. 而STEM及EDS元素分布图显示,P-nZVI的内部被严重腐蚀. 根据上述现象推测,P-nZVI首先通过三元络合作用将大量Ni2+吸附在表面,部分Ni2+能通过径向裂纹结构深入铁芯内部,发生较为剧烈的氧化还原反应. Zhang等[20]采用XPS刻蚀,详细比较了Ni2+在4 h内对nZVI及P-nZVI铁芯的腐蚀过程,结果证实P-nZVI的确大大提升了反应速率、促进腐蚀. 原因可作如下分析:浓度梯度被认为是污染物进入铁核速率的决定因素[43],虽然铁氧化物外壳及表面磷酸基团阻碍Ni2+与Fe0的直接接触,但由于P-nZVI表面负电荷量大,能比nZVI更迅速地通过静电引力作用将溶液中游离的Ni2+以物理、化学吸附结合在铁壳表面,较高的浓度梯度促进 Ni2+跨越P-nZVI氧化外壳层,向铁芯转移;其次,P-nZVI独特的径向裂纹及表面缺陷更有助于Ni2+向铁芯扩散,加快电子转移,提高Fe0的利用率(图7). 因此,P-nZVI的结构降低了Ni2+跨越铁氧化壳的阻碍,其反应方程式可表达如下:
stringUtils.convertMath(!{formula.content}) stringUtils.convertMath(!{formula.content}) stringUtils.convertMath(!{formula.content}) stringUtils.convertMath(!{formula.content}) 3. 结论(Conclusion)
本文以KH2PO4为磷化剂通过液相还原法制备出磷酸化修饰的纳米铁,并结合XRD、SEM、TEM、XPS等分析手段探究其对Cd2+、Zn2+、Ni2+的去除效果及微观作用机制. 结果表明,P-nZVI是一种表面缺陷、内部皲裂的不规则球状颗粒,其较大的比表面积及表面包被的磷酸基团为重金属的吸附提供了更多位点. 反应动力学表明,P-nZVI对Cd2+、Zn2+、Ni2+的去除效率均显著优于nZVI,2 h左右分别达到了79.6%、90.6%、92.6%. 其中,P-nZVI对Cd2+、Zn2+的去除以表面磷酸基团的吸附为主,形成了nZVI-PO43--金属阳离子三元络合物,使重金属离子结合牢固、不易解吸. 该吸附过程均可用准二级动力学描述. 而Ni2+的去除过程有所不同,其首先在P-nZVI的表面吸附作用下被富集,其次部分Ni2+通过径向裂纹深入铁芯并被快速还原,因此P-nZVI独特的裂纹结构能促进电子的转移. 综上,P-nZVI表面的磷酸化修饰及物理结构的缺陷,使其具有较好的重金属去除活性,本研究结果为开发简单实用的改性nZVI高效去除重金属的方法提供了一定可行性思路.
-
图 1 (a)P-nZVIHAADF-STEM图像和Fe、O、P、Fe+O的EDS 元素分布图;(b)P-nZVI的Fe 2p、O1s XPS谱图;(c)不同pH下P-nZVI、nZVI的Zeta电位图;(d)P-nZVI上磷酸基团的结合示意图
Figure 1. (a)HAADF-STEM image of fresh P-nZVI as well as the corresponding elemental mapping of Fe, O, P and the overlapped mapping of Fe, O;(b)XPS survey spectra of P 2p, Fe 2p and O 1s of P-nZVI;(c)zeta potential of P-nZVI and nZVI at different pH;(d)schematic diagram of phosphate groups binding mode
图 3 (a, b)反应动力学曲线;(c, d)伪一级、伪二级动力学拟合曲线;(e)pH的影响;(f, g, h)干扰离子的影响
Figure 3. (a, b)Kinetic adsorption experiments of nZVI and P-nZVI;(c, d)fitted curves of pseudo-first-order kinetic model and pseudo-second-order kinetic model;(e)influence of different pH conditions;(f, g, h)influence of interfering ions
表 1 nZVI及P-nZVI的比表面积、孔容、孔径对比
Table 1. Comparison of specific surface area, pore volume and pore size of nZVI and P-nZVI
比表面积/(m2·g−1)Surface area 孔容/(cm3·g−1)Pore volume 孔径/nmPore size nZVI 26.54±2.13 0.0759±0.0030 27.67±0.06 P-nZVI 159.27±1.01 0.6093±0.0025 21.22±0.19 表 2 nZVI及P-nZVI的O1s XPS谱中O2-、OH-及H2O相对丰度(% at.)
Table 2. Relative abundance of O2-, OH- and H2O in the O1s XPS spectra of nZVI and P-nZVI
P-nZVI nZVI O2- 23.11 43.98 OH- 62.88 53.08 H2O 14.01 2.94 总计 100 表 3 P-nZVI及去除重金属后的EDS定量结果
Table 3. Quantitative results of EDS before and after removal of heavy metals
P-nZVI 除Cd2+后After removal of Cd2+ 除Zn2+后After removal of Zn2+ 除Ni2+后After removal of Ni2+ Fe 85.68 63.55 76.01 28.22 O 13.83 34.37 21.90 54.50 P 0.49 1.45 1.13 0.62 目标污染物 — 0.64 0.95 16.55 总计 100 -
[1] PAITHANKAR J G, SAINI S, DWIVEDI S, et al. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction[J]. Chemosphere, 2021, 262: 128350. doi: 10.1016/j.chemosphere.2020.128350 [2] SHENG P X, TING Y P, CHEN J P, et al. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms[J]. Journal of Colloid and Interface Science, 2004, 275(1): 131-141. doi: 10.1016/j.jcis.2004.01.036 [3] BARCZAK M, SKWAREK E, JANUSZ W, et al. Functionalized SBA-15 organosilicas as sorbents of zinc(II) ions[J]. Applied Surface Science, 2010, 256(17): 5370-5375. doi: 10.1016/j.apsusc.2009.12.082 [4] ONG D C, KAN C C, PINGUL-ONG S M B, et al. Utilization of groundwater treatment plant (GWTP) sludge for nickel removal from aqueous solutions: Isotherm and kinetic studies[J]. Journal of Environmental Chemical Engineering, 2017, 5(6): 5746-5753. doi: 10.1016/j.jece.2017.10.046 [5] GUO X J, CHEN F H. Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater[J]. Environmental Science & Technology, 2005, 39(17): 6808-6818. [6] MOHAN D, SINGH K P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste[J]. Water Research, 2002, 36(9): 2304-2318. doi: 10.1016/S0043-1354(01)00447-X [7] BASHIR A, AHMAD MALIK L, AHAD S, et al. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods[J]. Environmental Chemistry Letters, 2019, 17(2): 729-754. doi: 10.1007/s10311-018-00828-y [8] ZHU W B, QIAO X, LING L. Improved cadmium removal induced by interaction of nanoscale zero-valent iron and microplastics debris[J]. Journal of Environmental Engineering, 2023, 149(6): 04023029. doi: 10.1061/JOEEDU.EEENG-7141 [9] 盛杰, 傅浩洋, 王伟, 等. 纳米零价铁的表征及改性研究进展[J]. 环境化学, 2020, 39(11): 2959-2978. doi: 10.7524/j.issn.0254-6108.2020070803 SHENG J, FU H Y, WANG W, et al. Research progress on the characterization and modification of nanoscale zero-valent iron for applications[J]. Environmental Chemistry, 2020, 39(11): 2959-2978 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020070803
[10] 黄潇月, 王伟, 凌岚, 等. 纳米零价铁与重金属的反应: “核-壳”结构在重金属去除中的作用[J]. 化学学报, 2017, 75(6): 529-537. doi: 10.6023/A17020051 HUANG X Y, WANG W, LING L, et al. Heavy metal-nZVI reactions: The core-shell structure and applications for heavy metal treatment[J]. Acta Chimica Sinica, 2017, 75(6): 529-537 (in Chinese). doi: 10.6023/A17020051
[11] 杜毅, 王向宇. 新型纳米零价铁的绿色合成和改性工艺研究进展[J]. 环境化学, 2016, 35(2): 337-347. doi: 10.7524/j.issn.0254-6108.2016.02.2015081702 DU Y, WANG X Y. Green synthesis and modification of nano zero-valent iron[J]. Environmental Chemistry, 2016, 35(2): 337-347 (in Chinese). doi: 10.7524/j.issn.0254-6108.2016.02.2015081702
[12] LI Q, JIANG Z, ZHENG J H, et al. Interaction of pyrite with zerovalent iron with superior reductive ability via Fe(ii) regeneration[J]. Environmental Science:Nano, 2022, 9(8): 2713-2725. doi: 10.1039/D2EN00349J [13] 汤晶, 汤琳, 冯浩朋, 等. 硫化纳米零价铁去除水体污染物的研究进展[J]. 化学学报, 2017, 75(6): 575-582. doi: 10.6023/A17020045 TANG J, TANG L, FENG H M, et al. Research progress of aqueous pollutants removal by sulfidated nanoscale zero-valent iron[J]. Acta Chimica Sinica, 2017, 75(6): 575-582 (in Chinese). doi: 10.6023/A17020045
[14] XU Q Y, LI W P, MA L, et al. Simultaneous removal of ammonia and phosphate using green synthesized iron oxide nanoparticles dispersed onto zeolite[J]. The Science of the Total Environment, 2020, 703: 135002. doi: 10.1016/j.scitotenv.2019.135002 [15] NRIAGU J O. Stability of vivianite and ion-pair formation in the system Fe3(PO4)2-H3PO4H3PO4-H2O[J]. Geochimica et Cosmochimica Acta, 1972, 36(4): 459-470. doi: 10.1016/0016-7037(72)90035-X [16] MU Y, AI Z H, ZHANG L Z. Phosphate shifted oxygen reduction pathway on Fe@Fe2O3 core-shell nanowires for enhanced reactive oxygen species generation and aerobic 4-chlorophenol degradation[J]. Environmental Science & Technology, 2017, 51(14): 8101-8109. [17] FU H Y, HE H F, ZHU R L, et al. Phosphate modified magnetite@ferrihydrite as an magnetic adsorbent for Cd(Ⅱ) removal from water, soil, and sediment[J]. The Science of the Total Environment, 2021, 764: 142846. doi: 10.1016/j.scitotenv.2020.142846 [18] LI M Q, MU Y, SHANG H, et al. Phosphate modification enables high efficiency and electron selectivity of nZVI toward Cr(Ⅵ) removal[J]. Applied Catalysis B:Environmental, 2020, 263: 118364. doi: 10.1016/j.apcatb.2019.118364 [19] LING L, PAN B C, ZHANG W X. Removal of selenium from water with nanoscale zero-valent iron: Mechanisms of intraparticle reduction of Se(IV)[J]. Water Research, 2015, 71: 274-281. doi: 10.1016/j.watres.2015.01.002 [20] LI M Q, SHANG H, LI H, et al. Kirkendall effect boosts phosphorylated nZVI for efficient heavy metal wastewater treatment[J]. Angewandte Chemie International Edition, 2021, 60(31): 17115-17122. doi: 10.1002/anie.202104586 [21] LING L, HUANG X Y, LI M R, et al. Mapping the reactions in a single zero-valent iron nanoparticle[J]. Environmental Science & Technology, 2017, 51(24): 14293-14300. [22] TANG L, FENG H P, TANG J, et al. Treatment of arsenic in acid wastewater and river sediment by Fe@Fe2O3 nanobunches: The effect of environmental conditions and reaction mechanism[J]. Water Research, 2017, 117: 175-186. doi: 10.1016/j.watres.2017.03.059 [23] 刘静, 刘爱荣, 张伟贤. 纳米零价铁及其在环境介质中氧化后性质演变研究进展[J]. 环境化学, 2014, 33(4): 576-583. doi: 10.7524/j.issn.0254-6108.2014.04.009 LIU J, LIU A R, ZHANG W X. Review on transformation of oxidized nanoscale zero valent iron in environment media[J]. Environmental Chemistry, 2014, 33(4): 576-583 (in Chinese). doi: 10.7524/j.issn.0254-6108.2014.04.009
[24] LI X Q, ELLIOTT D W, ZHANG W X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects[J]. Critical Reviews in Solid State and Materials Sciences, 2006, 31(4): 111-122. doi: 10.1080/10408430601057611 [25] LI X Q, ZHANG W X. Sequestration of metal cations with zerovalent iron Nanoparticles - A study with high resolution X-ray photoelectron spectroscopy (HR-XPS)[J]. The Journal of Physical Chemistry C, 2007, 111(19): 6939-6946. doi: 10.1021/jp0702189 [26] NAGOYA S, NAKAMICHI S, KAWASE Y. Mechanisms of phosphate removal from aqueous solution by zero-valent iron: A novel kinetic model for electrostatic adsorption, surface complexation and precipitation of phosphate under oxic conditions[J]. Separation and Purification Technology, 2019, 218: 120-129. doi: 10.1016/j.seppur.2019.02.042 [27] LIANG X L, LIN X J, WEI G L, et al. Competitive adsorption geometries for the arsenate As(V) and phosphate P(V) oxyanions on magnetite surfaces: Experiments and theory[J]. American Mineralogist, 2021, 106: 374-388. doi: 10.2138/am-2020-7350 [28] MATUSIK J, BAJDA T, MANECKI M. Immobilization of aqueous cadmium by addition of phosphates[J]. Journal of Hazardous Materials, 2008, 152(3): 1332-1339. doi: 10.1016/j.jhazmat.2007.08.010 [29] LI X Q, ZHANG W X. Iron nanoparticles: The core-shell structure and unique properties for Ni(II) sequestration[J]. Langmuir, 2006, 22(10): 4638-4642. doi: 10.1021/la060057k [30] SHRESTHA R, BAN S, DEVKOTA S, et al. Technological trends in heavy metals removal from industrial wastewater: A review[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105688. doi: 10.1016/j.jece.2021.105688 [31] LI M R, LING L. Visualizing dynamic environmental processes in liquid at nanoscale via liquid-phase electron microscopy[J]. ACS Nano, 2022, 16(10): 15503-15511. doi: 10.1021/acsnano.2c04246 [32] 白子昂, 陈瑞兴, 庞宏伟, 等. 硫化纳米零价铁对水中U(Ⅵ)的高效去除研究[J]. 化学学报, 2021, 79(10): 1265-1272. doi: 10.6023/A21060263 BAI Z A, CHEN R X, PANG H W, et al. Investigation on the efficient removal of U(Ⅵ) from water by sulfide nanoscale zero-valent iron[J]. Acta Chimica Sinica, 2021, 79(10): 1265-1272 (in Chinese). doi: 10.6023/A21060263
[33] LUO Y X, GAO B Y, YUE Q Y, et al. Application of enteromorpha polysaccharides as coagulant aid in the simultaneous removal of CuO nanoparticles and Cu2+: Effect of humic acid concentration[J]. Chemosphere, 2018, 204: 492-500. doi: 10.1016/j.chemosphere.2018.03.168 [34] LIANG L, LI X Q, GUO Y Q, et al. The removal of heavy metal cations by sulfidated nanoscale zero-valent iron (S-nZVI): The reaction mechanisms and the role of sulfur[J]. Journal of Hazardous Materials, 2021, 404: 124057. doi: 10.1016/j.jhazmat.2020.124057 [35] WANG K J, XING B S. Mutual effects of cadmium and phosphate on their adsorption and desorption by goethite[J]. Environmental Pollution, 2004, 127(1): 13-20. doi: 10.1016/S0269-7491(03)00262-8 [36] GUO H B, BARNARD A S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability[J]. Journal of Materials Chemistry A, 2013, 1(1): 27-42. doi: 10.1039/C2TA00523A [37] SONG M, HU X L, GU T H, et al. Nanocelluloses affixed nanoscale zero-valent iron (nZVI) for nickel removal: Synthesis, characterization and mechanisms[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107466. doi: 10.1016/j.jece.2022.107466 [38] BOSE D N, HEDGE M S, BASU S, et al. XPS investigation of CdTe surfaces: Effect of Ru modification[J]. Semiconductor Science and Technology, 1989, 4(10): 866-870. doi: 10.1088/0268-1242/4/10/006 [39] ZHANG Y L, LI Y T, DAI C M, et al. Sequestration of Cd(II) with nanoscale zero-valent iron (nZVI): Characterization and test in a two-stage system[J]. Chemical Engineering Journal, 2014, 244: 218-226. doi: 10.1016/j.cej.2014.01.061 [40] DONG H R, ZENG Y L, XIE Y K, et al. Single and combined removal of Cr(Ⅵ) and Cd(Ⅱ) by nanoscale zero-valent iron in the absence and presence of EDDS[J]. Water Science and Technology, 2017, 76(5/6): 1261-1271. [41] LIU J, ZHU R L, MA L Y, et al. Adsorption of phosphate and cadmium on iron (oxyhydr)oxides: A comparative study on ferrihydrite, goethite, and hematite[J]. Geoderma, 2021, 383: 114799. doi: 10.1016/j.geoderma.2020.114799 [42] McINTYRE N S, COOK M G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper[J]. Analytical Chemistry, 1975, 47(13): 2208-2213. doi: 10.1021/ac60363a034 [43] GU B, LIANG L, DICKEY M J, et al. Reductive precipitation of uranium(Ⅵ) by zero-valent iron[J]. Environmental Science & Technology, 1998, 32(21): 3366-3373. -






 下载:
下载: