-
铬(Cr)及其化合物常用于采矿、电镀等行业中,而在水体中主要以Cr(Ⅲ)和Cr(Ⅵ)存在[1-2]。 相较于Cr(Ⅲ),Cr(Ⅵ)具有可吸附性低、生物毒性强的特点,如未经处理排放到水体中,会严重威胁我国水生态环境和人民生命健康[3-4]。目前,关于Cr(Ⅵ)的处理方法主要包括物理法[5-6]和化学法[7-8]。物理法主要是采用吸附材料(活性炭、生物炭、高分子聚合物)将Cr(Ⅵ)从水相转移至吸附材料中,该方法虽具有操作方便、材料廉价易得等优势,但却存在二次污染风险[9],需进行二次处理。而化学法主要是通过加入一定数量的还原剂,从而将Cr(Ⅵ)还原转化为毒性相对较低的Cr(Ⅲ),再通过调节水体的pH值,使Cr(Ⅲ)生成沉淀物质,实现水体和Cr(Ⅲ)的分离,达到去除Cr(Ⅵ)的目的,该方法是目前应用最为广泛的方法。
零价铁(Fe)具有还原性高、环境友好等优势,常用于水体重金属去除,但也存在颗粒易聚集、表面易氧化等缺点[10-11]。四氧化三铁(Fe3O4)是立方反尖晶石,由O2-与Fe2+、Fe3+亚晶格紧密堆积组成。其中,Fe(Ⅲ)([Fe3+]tetra)占据四面体阳离子位,Fe(Ⅱ)([Fe2+]octa)和Fe(Ⅲ)([Fe3+]octa)占据正八面体阳离子位,这使得Fe3O4电子可自由转移[12]。近几年来,Fe/Fe3O4被广泛应用于污染物催化氧化、机械脱卤过程中。一方面Fe可促进Fe(Ⅱ)/Fe(Ⅲ)的循环;另一方面Fe3O4可促进将电子传递给污染物的过程。在基于过一硫酸盐的催化氧化过程中,与Fe和Fe3O4相比,Fe/Fe3O4具有更高的催化效率和活性物种产率[13]。
机械球磨是利用磨球和物料碰撞摩擦,使得反应活化能降低、有利于固体污染物的降解。在机械球磨脱卤过程中,Fe/Fe3O4作为助磨剂可高效去除六氯苯、全氟辛烷磺酸等污染物[14-16],且在Fe/Fe3O4质量为3:7时效果最佳。由于Fe/Fe3O4具有较高的电子传递效率,将其作为助磨剂时可降低物料比(即降解单位质量污染物所需的助磨剂量),但仍面临着助磨剂尾料资源化利用的难题[16-21]。然而,研磨的Fe/Fe3O4具有良好的Cr(Ⅵ)还原性能。目前,有研究报道手动研磨的Fe和Fe3O4混合物可将Cr(Ⅵ)还原为Cr(Ⅲ),究其原因是Fe和[Fe3+]octa发生反应生成[Fe2+]octa[8]。因此,将机械球磨后的Fe/Fe3O4尾料用于Cr(Ⅵ)的还原去除,是促进尾料资源化利用的有效方式。
因此,本研究基于Fe/Fe3O4尾料资源化利用和高性能Cr(Ⅵ)还原材料开发的双重考虑,通过球磨模拟制备Fe/Fe3O4材料,研究球磨Fe/Fe3O4材料对Cr(Ⅵ)的还原性能以及其强化机理;探究最佳球磨条件(球磨氛围和时间)及还原条件(球磨材料投加量和初始pH),为研究Fe/Fe3O4尾料资源化利用以及机械球磨Fe/Fe3O4对Cr(Ⅵ)还原性能的影响提供重要数据支撑。
-
Fe、Fe3O4、H2SO4和H3PO4等试剂采购于国药集团化学试剂有限公司。丙酮、K2Cr2O7、邻菲罗啉、二苯碳酰二肼和NH2OH·HCl等试剂采购于上海麦克林生化科技股份有限公司。Pulverisette 7行星式球磨机(德国飞驰仪器公司)的球磨罐体为不锈钢材质(体积为80 mL)。
-
采用Fe和Fe3O4共球磨制备球磨Fe/Fe3O4材料(质量比为3:7,转速为600 r·min‒1)。所有Cr(Ⅵ)还原实验全部都在棕色锥形瓶(300 mL)中进行:先加入Cr2K2O7溶液(100 mL),然后加入定量球磨材料后进行搅拌(200 r·min‒1、25 ℃),定时取样1 mL进行分析。为确保实验结果的精确性,上述实验均重复操作两次以上。
-
检测Cr(Ⅵ)的质量浓度:取1 mL样品置于50 mL玻璃比色管中,用蒸馏水稀释至标线位置;然后依次加入H2SO4(50%,0.5 mL)和H3PO4(50%,0.5 mL)后进行混匀;再加入2 mL显色液(将0.2 g二苯碳酰二肼溶于50 mL丙酮中)后进行混匀;静置15 min后,用分光光度计测定样品溶液在540 nm处的吸光度。配制Cr(Ⅵ)标准溶液,绘制标准曲线(1~50 mg·L−1)。
检测Fe(Ⅱ)的质量浓度:取1 mL样品置于50 mL玻璃比色管中,用蒸馏水稀释至标线位置;依次加入5 mL乙酸-乙酸铵缓冲液(10 mmol·L−1)和0.5 mL邻菲罗啉溶液(5%)后混匀;静置15 min后,用分光光度计测定样品溶液在510 nm处的吸光度。配制Fe(Ⅱ)标准溶液,绘制标准曲线(2.5~100 mg·L−1)。
检测总铁的质量浓度:取1 mL样品置于50 mL玻璃比色管中,用蒸馏水稀释至标线位置;依次加入1 mL盐酸羟胺(10%)、0.5 mL邻菲罗啉溶液(5%)和5 mL醋酸盐缓冲液(10 mmol·L−1);静置15 min后,用分光光度计测定样品溶液在510 nm处的吸光度。配制总铁标准溶液,绘制标准曲线(2.5~100 mg·L−1)。总铁质量浓度减去Fe(II) 质量浓度即为Fe(III) 质量浓度。
材料表征:元素表征采用K-Alpha型X射线光电子能谱分析仪(X-ray photoelectron spectroscopy,XPS,Thermo Fisher Scientific)。以Al Kα为激发源,工作电压为12 kV,灯丝电流为6 mA;全谱扫描通能为100 eV,步长为1 eV,窄谱扫描通能为50 eV,步长为0.1 eV。晶型表征采用Ultima Ⅵ型X射线衍射光谱分析仪(X-Ray diffraction,XRD,Rigku)。以Cu-Kα 为测试靶,管电压为60 kV,电流为55 mA,扫描范围(2θ)为10°~80°。
-
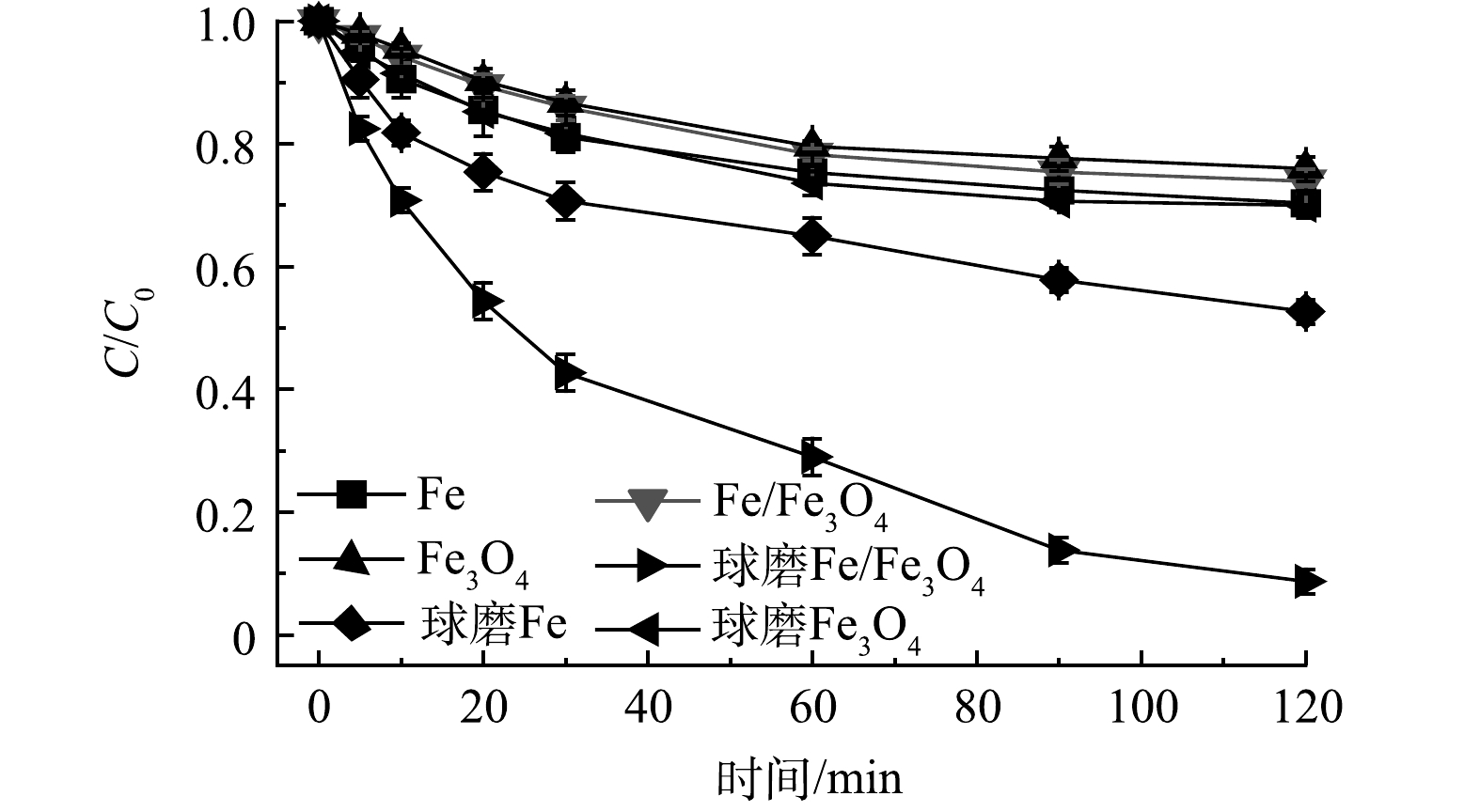
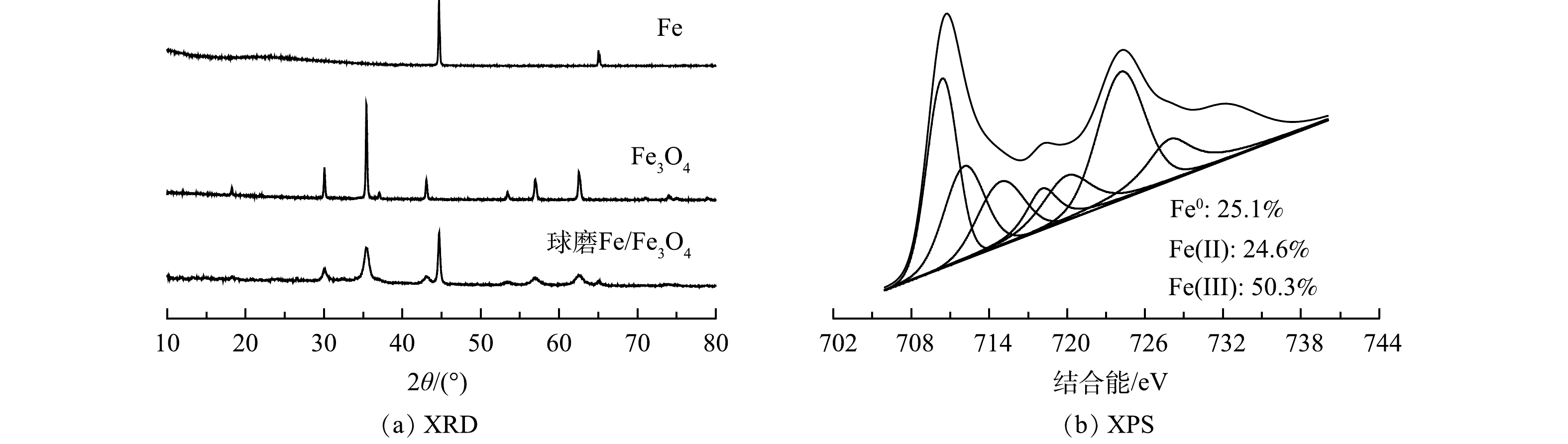
图1表示Fe、Fe3O4和Fe/Fe3O4材料的Cr(Ⅵ)还原性能状况。实验条件:Fe、Fe3O4和Fe/Fe3O4材料投加量为2.0 g·L−1,球磨氛围为空气,球磨时间为60 min,Cr(Ⅵ) 质量浓度为10 mg·L−1,初始 pH为3.0。可以发现,未球磨Fe、Fe3O4、Fe/Fe3O4材料对Cr(Ⅵ)的去除效果较差,反应120 min后,Cr(Ⅵ)去除率分别为29.7%、24.0%、26.1%。此时,Fe/Fe3O4的Cr(Ⅵ)去除效果高于Fe3O4,但低于Fe,说明Fe与Fe3O4之间的反应较弱。球磨Fe、Fe3O4、Fe/Fe3O4材料对Cr(Ⅵ)的去除效果均有提升,相比未球磨的材料,去除率分别增加了17.6%、6.0%、65.2%。对于Fe,机械球磨可去除其表面钝化层[22-23];而对于Fe/Fe3O4,机械球磨可强化Fe与Fe3O4之间的反应[8,16]。球磨Fe/Fe3O4的稳定性结果表明,在120 min 时 Cr(Ⅵ)的去除率在第1、2次循环中分别为41.3%和26.9%,再次球磨后,Cr(VI)的去除率可恢复到>90%。
-
球磨Fe/Fe3O4材料的晶型和元素表征结果如图2所示。由图2(a)中 XRD结果可见,球磨后Fe和Fe3O4晶体减少;XPS结果表明 Fe0含量有所减小,而Fe(II)含量有所增加(图2(b))。该结果可能是由以下2点原因所致:一方面,Fe与含氧物质(空气中的氧或Fe3O4释放的氧)在高温下反应生成Fe(II)(式(1));另一方面,Fe与Fe3O4发生氧化还原反应生成Fe(II)(式(2))。Fe(Ⅱ)含量增加可加速Cr(VI)的还原(式(3))。然而,HU等报道球磨Fe/Fe3O4材料具有较小的电化学阻抗,Fe3O4作为导体可加速Fe释放的电子向污染物传递[13,16]。因此,机械球磨也可能强化Fe与Fe3O4的界面接触,加速Fe释放的电子向Cr(Ⅳ)传递,从而促进Cr(Ⅳ)的还原(式(4)和式(5))。
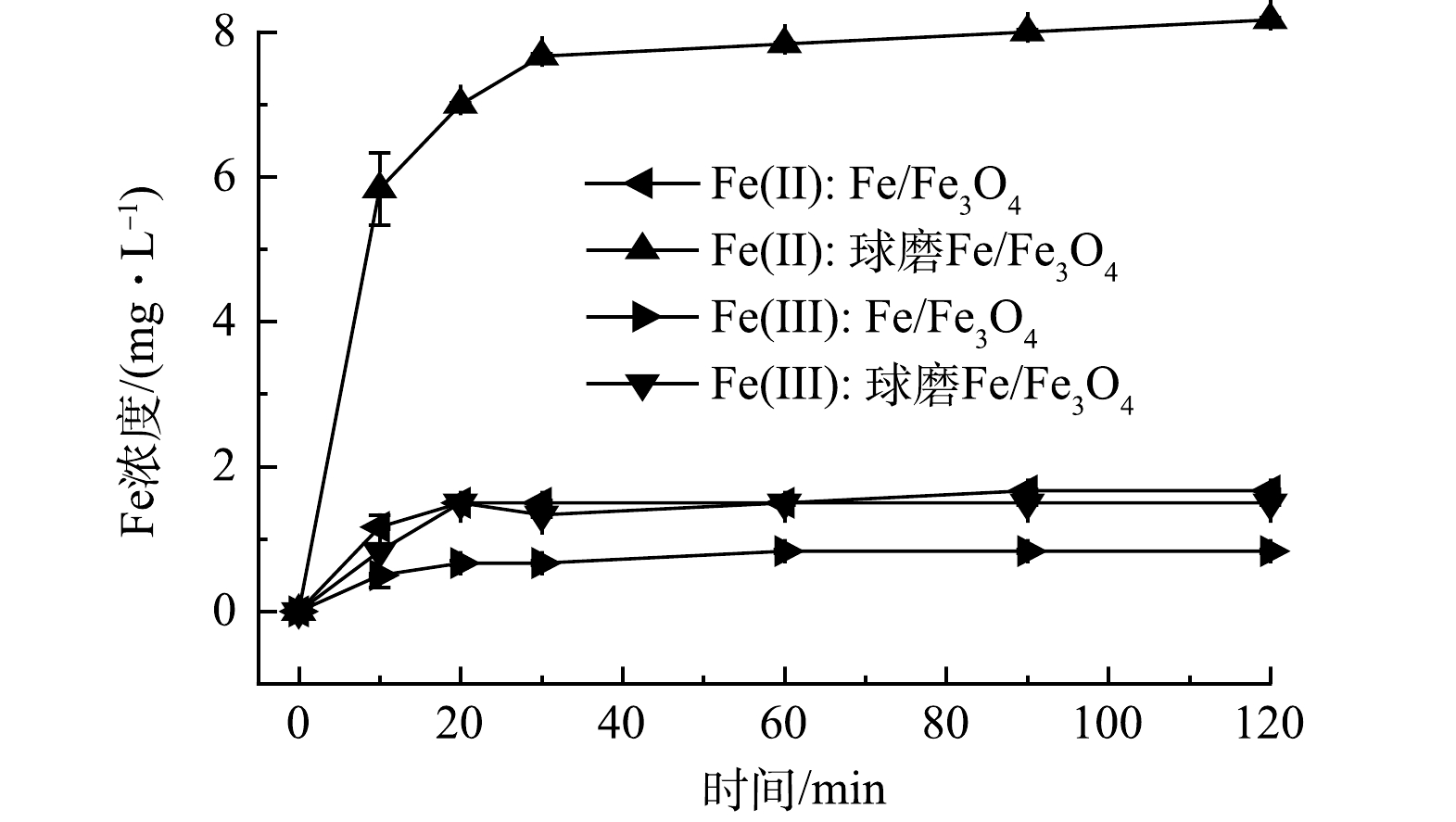
本研究探索了Fe(Ⅱ)和Fe(Ⅲ)在Fe/Fe3O4样品Cr(Ⅵ) 还原过程中的生成情况。实验条件:Fe/Fe3O4投加量为2.0 g·L−1,空气氛围下球磨时间为60 min,Cr(Ⅵ) 质量浓度为10 mg·L−1,初始 pH为3.0。实验表明球磨还原Cr(Ⅵ)过程中,Fe/Fe3O4样品Fe(Ⅱ)和Fe(Ⅲ)的生成量较高,分别稳定在8.2 mg·L−1和1.5 mg·L−1(图3)。如图4(b)所示,采用球磨Fe/Fe3O4还原Cr(Ⅵ)时,反应液pH大幅上升。这是由于H+参与了Cr(Ⅵ)还原反应(式(3))。上述结果说明,机械球磨促进了Fe(Ⅱ)生成,随后与Cr(Ⅵ)反应后转化为Fe(Ⅲ)。
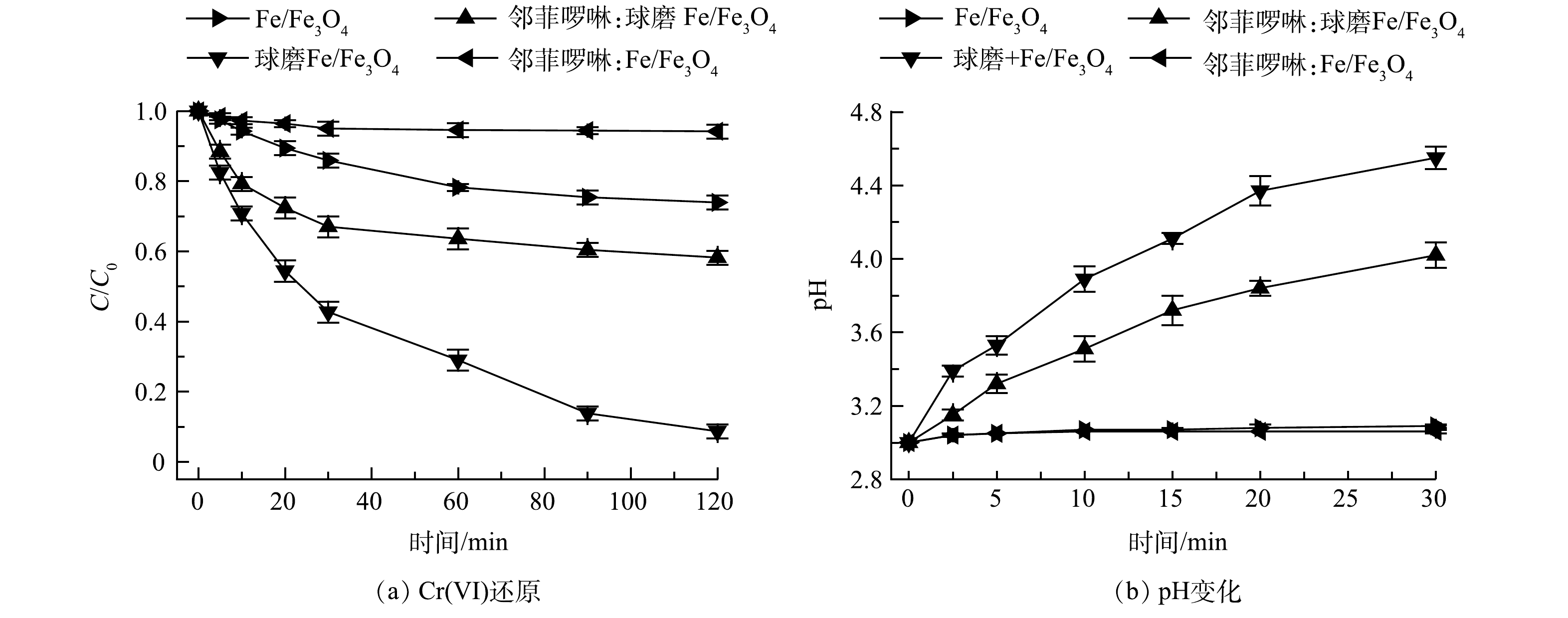
SHAO等[24]报道表明邻菲啰啉可以快速淬灭Fe(Ⅱ),阻断Fe(Ⅱ)与Cr(Ⅵ)之间的氧化还原反应。因此,本研究通过加入过量邻菲啰啉作为Fe(Ⅱ) 的淬灭剂,分析Fe(Ⅱ)在球磨Fe/Fe3O4还原Cr(Ⅵ)过程中的作用。结果表明(图4(a)),加入邻菲啰啉后, Fe/Fe3O4对Cr(Ⅵ)的还原性能有所下降。经过120 min反应后,未球磨Fe/Fe3O4对Cr(Ⅵ) 的去除率由26.1%降至6.8%,而球磨Fe/Fe3O4对Cr(Ⅵ) 的去除率由91.3%降至41.8%。该结果表明,Fe(Ⅱ)在Fe/Fe3O4还原Cr(Ⅵ)过程中具有重要作用,而机械球磨可强化Fe(Ⅱ)的生成。值得注意的是,当Fe(Ⅱ)被淬灭后,与未球磨Fe/Fe3O4材料相比,球磨Fe/Fe3O4对Cr(Ⅵ)的去除率仍然较高,反应液pH上升幅度仍然较大,这说明机械球磨除了强化Fe(Ⅱ)的生成之外,还强化了Fe与Fe3O4界面的电子传递。Fe释放出电子,Fe3O4作为导体将电子传递给Cr(Ⅵ),从而加速了Cr(Ⅵ)还原(式(4)和式(5))。
-
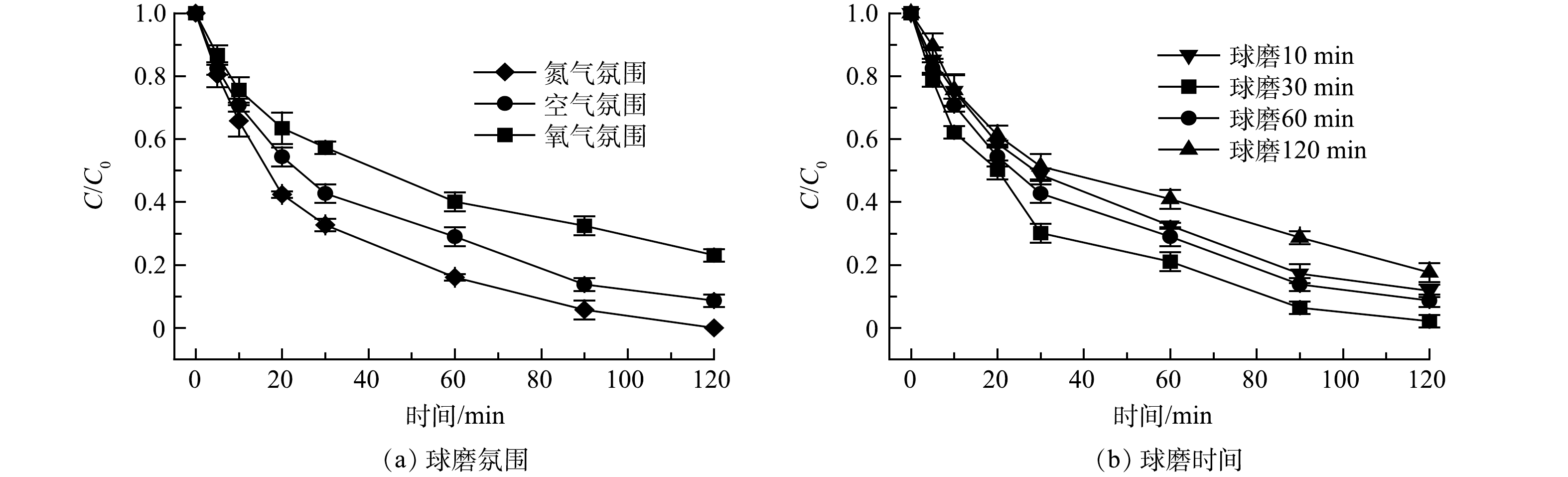
本研究探索了球磨氛围和球磨时间对Fe/Fe3O4材料还原性能可能产生的影响。图5(a)反映了球磨氛围的影响,实验条件:球磨Fe/Fe3O4投加量为2.0 g·L−1,球磨时间为60 min,Cr(Ⅵ) 质量浓度为10 mg·L−1,初始 pH为3.0。球磨Fe/Fe3O4在不同球磨氛围下的还原性能依次为氮气> 空气> 氧气,反应120 min 后, Cr(Ⅵ)去除率分别为100%、91.3%和76.9%。如前文所述,可以通过2条途径生成Fe(Ⅱ):一是Fe与Fe3O4表面反应生成Fe(Ⅱ);二是Fe与O2反应生成Fe(Ⅱ)。其中,Fe与Fe3O4的反应是Fe(Ⅱ)生成的主要途径[16]。在富氧条件下进行球磨时,过量的氧气会将生成的Fe(Ⅱ)进一步氧化为Fe(Ⅲ),从而降低球磨Fe/Fe3O4对Cr(Ⅵ) 的还原性能[21,25]。
图5(b)反映了球磨时间Cr(Ⅵ)还原性能的影响。实验条件:球磨Fe/Fe3O4投加量为2.0 g·L−1,球磨氛围为空气,Cr(Ⅵ) 质量浓度为10 mg·L−1,初始 pH为3.0。球磨时间增加,球磨Fe/Fe3O4的还原效果呈现先增强后减弱趋势。当球磨时间由10 min增加至30、60、120 min后,经过120 min 反应,Cr(Ⅵ)去除率分别为88.2%、97.9%、91.3%、82.4%。说明过长时间的球磨会导致Fe和Fe(Ⅱ)的过度氧化,不利于球磨Fe/Fe3O4对Cr(Ⅵ)的有效还原[16]。综上所述,球磨Fe/Fe3O4材料的最佳球磨条件为:氮气氛围、球磨30 min。
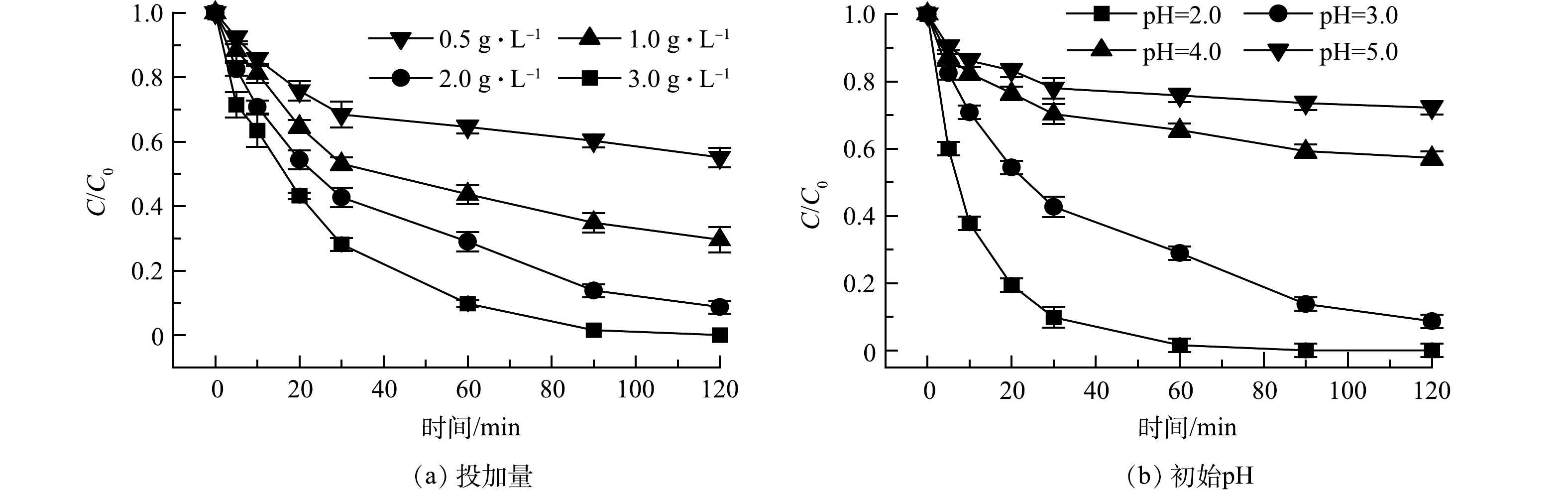
本研究探索了投加量和初始pH对球磨Fe/Fe3O4对Cr(Ⅵ)还原性能可能产生的影响。图6(a)为Fe/Fe3O4球磨材料投加量的影响,实验条件:空气氛围下球磨时间为60 min,Cr(Ⅵ) 质量浓度为10 mg·L−1,初始 pH依然设置为3.0。Cr(Ⅵ)的还原性能随着球磨Fe/Fe3O4材料投加量的增大而不断升高。当投加量为0.5、1.0、2.0、3.0 g时,经过120 min反应,Cr(Ⅵ)去除分别为44.9%、70.4%、91.3%、100%。球磨Fe/Fe3O4投加量的增加提供了更多的Fe和Fe(Ⅱ),从而可促进对Cr(Ⅵ)的还原。
图6(b)为初始pH对Cr(Ⅵ)还原性能的影响。实验条件为:球磨Fe/Fe3O4投加量为2.0 g·L−1,空气氛围下球磨时间为60 min,Cr(Ⅵ) 质量浓度为10 mg·L−1。球磨Fe/Fe3O4的还原性能随着初始pH的增加而不断降低。当初始pH为2.0、3.0、4.0和5.0时,经过120 min反应,Cr(Ⅵ)去除率分别为100%、91.3%、42.7%和27.8%。pH会影响溶液中铬的存在形态:当pH在2~4时,铬主要以Cr2O72‒和HCrO4‒存在;当pH在4~6时,铬主要以HCrO4‒和CrO42‒存在[26]。一方面,根据能斯特方程,在酸性条件下, Cr(Ⅵ)与Fe、Fe(Ⅱ)的反应具有较高的氧化还原电位和反应驱动力(ΔE)(式(4)和式(5));另一方面,高pH会降低反应液中Fe(Ⅱ) 质量浓度,从而不利于Cr(Ⅵ)的还原。
-
1)机械球磨可以有效强化Fe/Fe3O4的Cr(Ⅵ)还原性能。反应120 min后,Cr(Ⅵ)去除率从26.1%提高至91.3%。
2) Fe(Ⅱ)可显著促进Cr(Ⅵ)还原。机械球磨可强化Fe(Ⅱ)生成,促进Fe电子传递。
3) Fe/Fe3O4最佳球磨条件如下:氮气氛围,球磨30 min。过量氧气和过长球磨时间会削弱球磨Fe/Fe3O4的Cr(Ⅵ)还原性能。
4) Cr(Ⅵ)去除效果与球磨Fe/Fe3O4投加量呈正相关性,而与初始pH呈负相关性。
机械球磨强化铁/四氧化三铁对Cr(Ⅵ)的还原性能
Performance enhancement of Cr(Ⅵ) reduction for Fe/Fe3O4 by mechanical milling
-
摘要: Fe/Fe3O4作为机械球磨助磨剂可高效去除含卤污染物,但也面临着助磨剂尾料资源化利用的难题。机械球磨后的Fe/Fe3O4尾料具有良好的Cr(Ⅵ)还原性能。本研究基于尾料资源化利用和高性能材料开发的双重考虑,通过球磨模拟制备具有高Cr(Ⅵ)还原性能的Fe/Fe3O4材料。结果表明,机械球磨强化了Fe/Fe3O4材料的Cr(Ⅵ)还原性能,Cr(Ⅵ)去除率由26.1%提升至91.3%。机械球磨主要是通过促进Fe(Ⅱ)的生成和Fe释放电子的传递强化Cr(Ⅵ)还原。采用球磨Fe/Fe3O4还原Cr(Ⅵ)时,Fe(Ⅱ)的高生成量说明机械球磨促进了Fe(Ⅱ)的生成,而Fe(Ⅲ) 的高生成量和pH的大幅上升说明Fe(Ⅱ)转化为Fe(Ⅲ)。利用邻菲啰啉淬灭Fe(Ⅱ),使得球磨Fe/Fe3O4材料对Cr(Ⅵ) 的去除率降至41.8%,这表明Fe(Ⅱ)对Cr(Ⅵ) 还原起主要作用。过量的氧气和过长的球磨时间可削弱球磨Fe/Fe3O4的Cr(Ⅵ)还原性能,最佳球磨条件为氮气氛围、球磨30 min;Cr(Ⅵ)去除效果与球磨Fe/Fe3O4投加量呈正相关,而与初始pH呈负相关。Abstract: Fe/Fe3O4 as an additive for mechanical milling can efficiently degrade halogenated pollutants, but the reutilization of additive tailing is a problem. The Fe/Fe3O4 tailing has a good performance on Cr(Ⅵ) reduction. In this study, the Fe/Fe3O4 material with high performance of Cr(Ⅵ) reduction was prepared by mechanical milling simulation based on the dual consideration of tailing reutilization and material development. The results show that mechanical milling enhanced the reduction performance of Fe/Fe3O4, and the removal ratio of Cr(Ⅵ) increased from 26.1% to 91.3%. The enhancement of mechanical milling was primarily through the promotion of Fe(Ⅱ) generation and transfer of Fe-released electrons. When the milled Fe/Fe3O4 was used for Cr(Ⅵ) reduction, the high formation of Fe(Ⅱ) implies that the mechanical milling promoted its yield, and the high generation of Fe(Ⅲ) and rise of pH indicate the conversion of Fe(Ⅱ) to Fe(Ⅲ). After the quenching of Fe(Ⅱ) by phenanthroline, the removal ratio of Cr(Ⅵ) by the milled Fe/Fe3O4 decreased to 41.8%, indicating that Fe(Ⅱ) played a major role in Cr(Ⅵ) reduction, and the mechanical milling enhanced the transfer of electrons released from Fe. Excessive oxygen and milling time weakened the reduction performance of the milled Fe/Fe3O4, and the optimum conditions were nitrogen atmosphere and a milling time of 30 min. The removal ratio of Cr(Ⅵ) was positively correlated with the dosage of the milled Fe/Fe3O4 material, while was negatively correlated with the initial pH.
-
Key words:
- Fe /
- Fe3O4 /
- mechanical milling /
- Cr(Ⅵ) /
- reduction enhancement
-
氮氧化物指的是只由氮、氧两种元素组成的化合物。常见的氮氧化物(NOx)包括一氧化氮(NO)、二氧化氮(NO2)、一氧化二氮(N2O)和五氧化二氮(N2O5)等。作为空气污染物的NOx常指NO和NO2[1]。全球每年因人类活动向大气排放的NOx约5 300万t。NOx对自然环境的破坏力非常大,它是形成酸雨、光化学烟雾的重要物质,同时也是消耗臭氧的一个重要因子[2]。火力发电厂作为NOx的一个重要排放源,每年向大气中排放大量的NOx,随着我国环保政策的日益严格,NOx排放得到了严格控制。目前,国家大力推行火电厂超低改造,依据超低排放的要求,NOx排放标准为50 mg/m3[3]。根据生态环境部2018年数据显示,全国实现超低排放的煤电机组约8.1×109 kW,占全国煤电总装机容量的80%[4]。目前,火电厂对于NOx的脱除措施主要包括2种:选择性非催化还原法(Selective Non-Catalytic Reduction,SNCR)和选择性催化还原法(Selective Catalytic Reduction,SCR),由于SNCR脱硝技术脱硝效率较低,很多时候难以满足NOx的排放要求,大多数火电厂选择了脱硝效率更高的SCR脱硝技术来进行NOx的控制。
近年来,我国大力推行清洁能源发展,清洁能源发电量所占比例逐年升高,但是由于自然条件的限制,其存在发电量不稳定,变化较大的问题。社会用电量在一段时间内也存在着很大的变化。这些变化因素都需要火电机组进行负荷的调整。机组负荷的变化会引起烟气温度、氧含量和污染物含量等的变化,进而影响SCR脱硝系统的正常工作。本文以SCR脱硝系统为研究对象,研究机组不同负荷条件下SCR的脱硝效果。
1. 方法及设备
1.1 试验方法
试验选取5台燃煤机组,将5台机组按照#1机组、#2机组、#3机组、#4机组和#5机组的方式进行分类编号。5台机组燃烧的煤种主要以东胜和乌海烟煤为主,脱硝方式为SCR,脱硝还原剂均为液氨,催化剂均为(2+1)层模式,备用层均未投运。为保证测试结果仅与负荷调整有关,试验采取控制变量的方式,将各负荷段的测试试验集中在一定时间内完成,保证机组燃烧煤种和催化剂等条件不发生明显变化,保证机组负荷稳定。同时,各负荷段试验期间不进行燃烧调整和风量调整。试验主要测定5台机组A、B侧在不同负荷条件下SCR反应器进出口NOx、进出口温度、进出口氧含量、NH3/NOx摩尔比、氨逃逸浓度、脱硝效率以及SO2/SO3转化率。
采样点布置采用烟道断面网格法,进出口NOx、进出口温度、进出口氧含量,入口SO2以及氨逃逸浓度等采用便携式的仪器设备进行测定,每组测定10个平行样,每次测定3 min;SO3按照标准《石灰石-石膏湿法烟气脱硫装置性能验收试验规范:DL/T998—2016》[5]中的方法拼装仪器进行测定,每组测定3个平行样,每次测定30 min。用Excel 2003将NOx、SO2以及SO3浓度折算到标准状态、干基、6%O2时的浓度,同时对所有数据进行平均值计算。
1.2 试验设备仪器
试验分析的设备仪器包括便携式大流量低浓度烟尘自动测试仪(3012HD)、芬兰GASMT公司的便携式红外多阻气体分析仪(GASMET DX-4000)、德国testo公司的烟气分析仪(testo 350)、德国testo公司的单点烟气温度测量仪(testo 925)、德国M&C公司的顺磁氧量分析仪(PMA10)、加拿大Unisearch公司的便携式氨逃逸浓度分析仪以及《石灰石-石膏湿法烟气脱硫装置性能验收试验规范:DL/T998—2016》中的SO3采样设备等。
2. 结果与分析
2.1 氧含量与机组负荷的关系
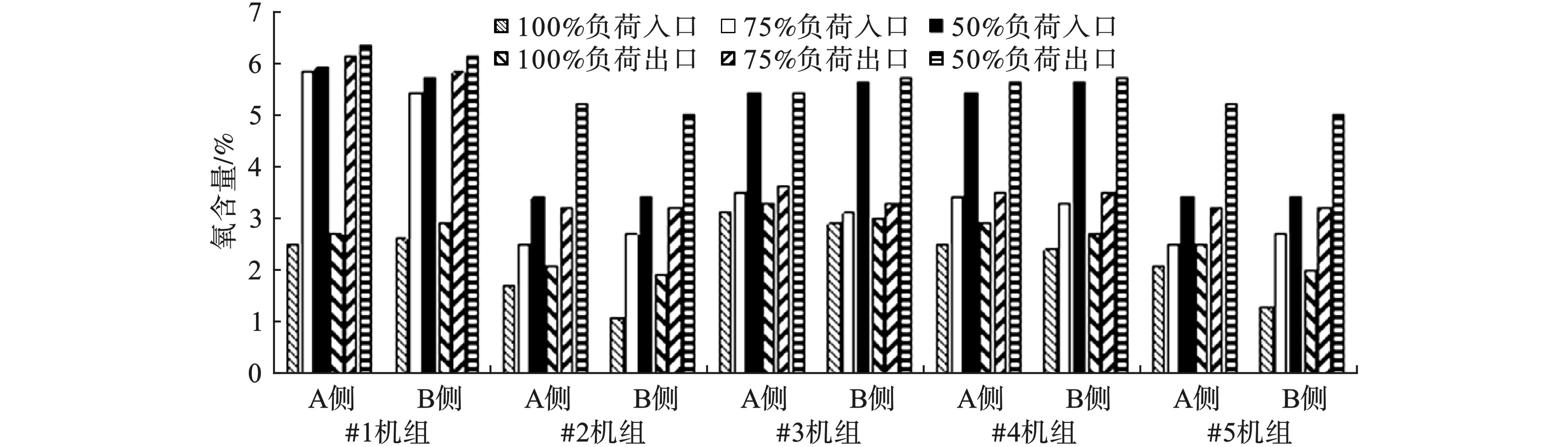
不同负荷条件下的氧含量变化见图1。5台机组SCR入口、出口氧含量均随负荷的降低而增加。
对于入口氧含量,负荷为50%时,最大值和最小值分别为5.9%和3.4%;负荷为75%时最大值和最小值分别为5.8%和2.5%;负荷为100%时最大值和最小值分别为3.1%和1.1%。50%负荷的入口氧含量平均是75%负荷的1.4倍,是100%负荷的2.1倍。
对于出口氧含量,负荷为50%时,最大值和最小值分别为6.3%和5.0%;负荷为75%时最大值和最小值分别为6.1%和3.2%;负荷为100%时最大值和最小值分别为3.3%和1.9%。50%负荷的出口氧含量平均是75%负荷的1.4倍,是100%负荷的2.1倍。
氧含量随着负荷的降低而升高。当负荷较高时,炉膛温度较高,炉膛内着火条件、煤粉与空气混合条件较好,燃烧稳定,最佳过量空气系数较低。而当锅炉低负荷运行时,锅炉燃烧所需的燃料减少,炉膛内火球较小,锅炉燃烧范围减少,锅炉炉膛温度降低,燃烧效率降低,这时需要加入更多的空气来维持燃烧中心的稳定,最佳过量空气系数较高。因此,高负荷时的最佳氧含量要低于低负荷时的最佳氧含量[6-7]。
2.2 温度与机组负荷的关系
不同负荷条件下温度的变化见图2a、2b。5台机组SCR入口,出口烟气温度均随负荷的降低而降低。
对于入口温度,负荷为50%时,最大值和最小值分别为340和278 ℃;负荷为75%时,最大值和最小值分别为356和287 ℃;负荷为100%,时最大值和最小值分别为370和313 ℃。100%负荷的入口温度平均比75%负荷高6.0%,比50%负荷平均高10.7%。
对于出口温度,负荷为50%时,最大值和最小值分别为337和275 ℃;负荷为75%时最大值和最小值分别为354和283 ℃;负荷为100%时,最大值和最小值分别为367和309 ℃。100%负荷的出口温度平均比75%负荷高6.1%,比50%负荷平均高10.9%。
烟气温度随负荷的降低而降低。当锅炉进行负荷调整时,燃料消耗量发生变化,锅炉内的温度场也会随之发生改变,炉内温度场的变化也必将会导致炉内辐射换热量的改变。但是,燃料消耗量引起的热量变化要大于炉内辐射换热量的变化,所以烟气温度变化主要由燃料消耗量来决定。当负荷降低时,燃料消耗量减少,烟气温度降低[8]。
2.3 NOx质量浓度与机组负荷的关系
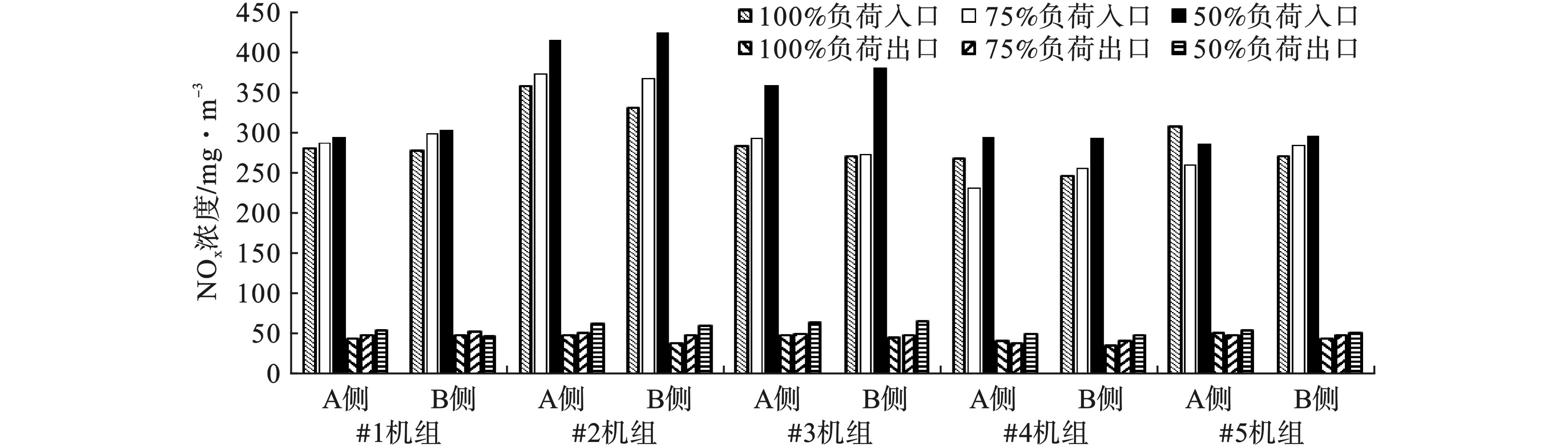
不同负荷条件下NOx质量浓度变化见图3。5台机组SCR入口、出口的NOx质量浓度随负荷的降低整体呈现出升高的趋势。
对于入口NOx质量浓度,负荷为50%时,入口NOx质量浓度最大值和最小值分别为423和285 mg/m3;负荷为75%时,入口NOx质量浓度最大值和最小值分别为372和231 mg/m3;负荷为100%时,入口NOx质量浓度最大值和最小值分别为358和246 mg/m3。50%负荷的入口NOx质量浓度平均比75%负荷的高14.4%,比100%负荷高15.5%。
对于出口NOx质量浓度,负荷为50%时,出口NOx质量浓度最大值和最小值分别为67和48 mg/m3;负荷为75%时,出口NOx质量浓度最大值和最小值分别为54和40 mg/m3;负荷为100%时,出口NOx质量浓度最大值和最小值分别为53和37 mg/m3。50%负荷的出口NOx质量浓度平均比75%负荷高17.1%,比100%负荷高24.2%。
负荷降低时,锅炉尾部烟道之后SCR反应器前的NOx质量浓度整体呈现了升高的趋势。根据NOx生成机理,燃料型NOx占总生成NOx的70~80%左右,热力型NOx占总生成NOx的15~25%[9]。炉膛氧含量和温度对燃料型与热力型NOx的生成都有影响,其中氧含量对燃料型NOx生成有更加显著的影响,而温度对热力型NOx生成有更明显的作用[10]。随着负荷的降低,氧含量升高,空气过剩系数增大,锅炉内燃烧区域出现氧过量的情况,会促进燃料型和热力型NOx的生成;负荷降低,温度也会降低,而温度降低主要对热力型NOx的生成有一定的抑制作用,但是热力型NOx所占比例较少,所以在负荷降低时总体表现为NOx质量浓度升高[11-13]。
2.4 NH3/NOx摩尔比、氨逃逸浓度以及脱硝效率与机组负荷的关系
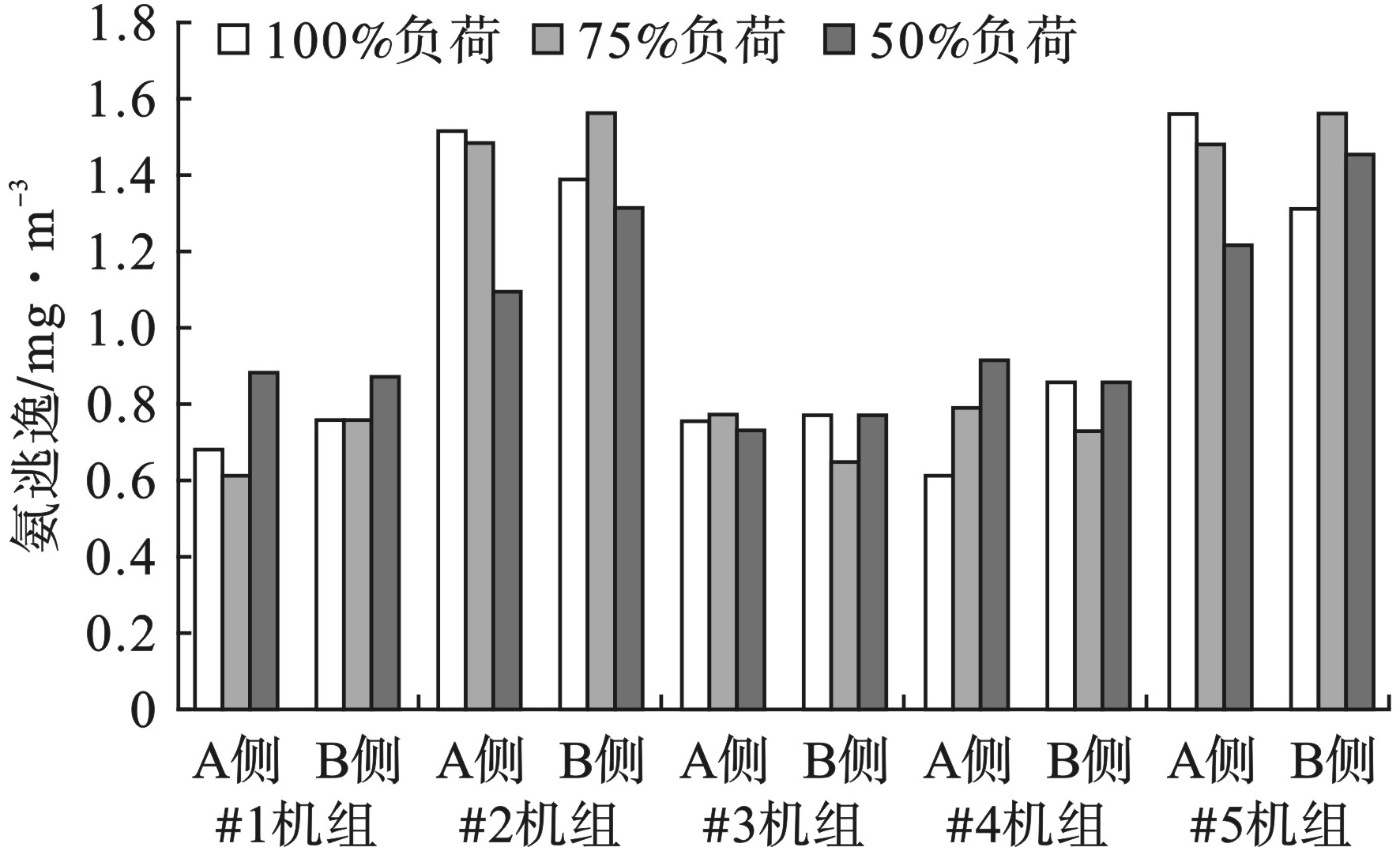
不同负荷条件下NH3/NOx摩尔比、氨逃逸浓度以及脱硝效率变化见图4~6。5台机组脱硝效率和NH3/NOx摩尔比整体呈现出随着负荷的降低而降低的趋势;氨逃逸浓度均在2.28 mg/m3以下,达到了设计要求。
对于NH3/NOx摩尔比,负荷为50%时,最大值和最小值分别为0.864和0.818;负荷为75%时最大值和最小值分别为0.878和0.826;负荷为100%时最大值和最小值分别为0.893和0.831。100%负荷的NH3/NOx摩尔比平均比75%负荷高1.0%,比50%负荷高1.6%。
对于氨逃逸浓度,负荷为50%时,最大值和最小值分别为1.45和0.73 mg/m3;负荷为75%时,最大值和最小值分别为1.56和0.61 mg/m3;负荷为100%时,最大值和最小值分别为1.56和0.61 mg/m3。100%负荷的氨逃逸浓度平均比75%负荷低2.0%,比50%负荷高1.1%。
对于脱硝效率,负荷为50%时,最大值和最小值分别为85.6%和80.7%;负荷为75%时,最大值和最小值分别为86.6%和81.2%;负荷为100%时,最大值和最小值分别为88.2%和82.4%。100%负荷的脱硝效率平均比75%负荷高1.1%,比50%负荷高1.5%。
SCR反应器一般布置在省煤器与空预器之间,SCR反应器中最重要的部分是催化剂,催化剂主要包括载体、活性成分以及助催化剂。影响脱硝效率的因素主要包括温度、烟气流速与停留时间、NH3/NOx摩尔比及氨逃逸浓度等。温度对脱硝效率的影响主要表现在当温度低于催化剂最适宜的温度,脱硝还原剂会发生很多副反应,减少对NOx还原作用,温度太高催化剂活性成分会形成多聚态晶体,多聚态晶体比表面积较小,减少了与NOx的接触面积,催化能力降低[14];对于烟气流速与停留时间,烟气流速低停留时间长,NOx与还原剂反应更充分,脱硝效率越高,反之则脱硝效率降低[15];NH3/NOx摩尔比和氨逃逸浓度对脱硝效率的作用体现在未达到最大脱硝效率之前,NH3/NOx摩尔比越大脱硝效率越高,氨逃逸浓度较低,当达到最大脱硝效率之后,NH3/NOx摩尔比增加,脱硝效率变化不大,而氨逃逸浓度会显著性增加,因此需要根据氨逃逸浓度确定最佳NH3/NOx摩尔比[16]。试验结果表明在保证NH3/NOx摩尔比和氨逃逸浓度较佳的情况下,负荷由100%降到50%,温度降低,脱硝效率降低,出口NOx质量浓度增加。烟气流速的降低,停留时间的增加,并没有使脱硝效率升高,表明在负荷调整过程中,温度对脱硝效率影响较大。
2.5 SO2/SO3转化率与机组负荷的关系
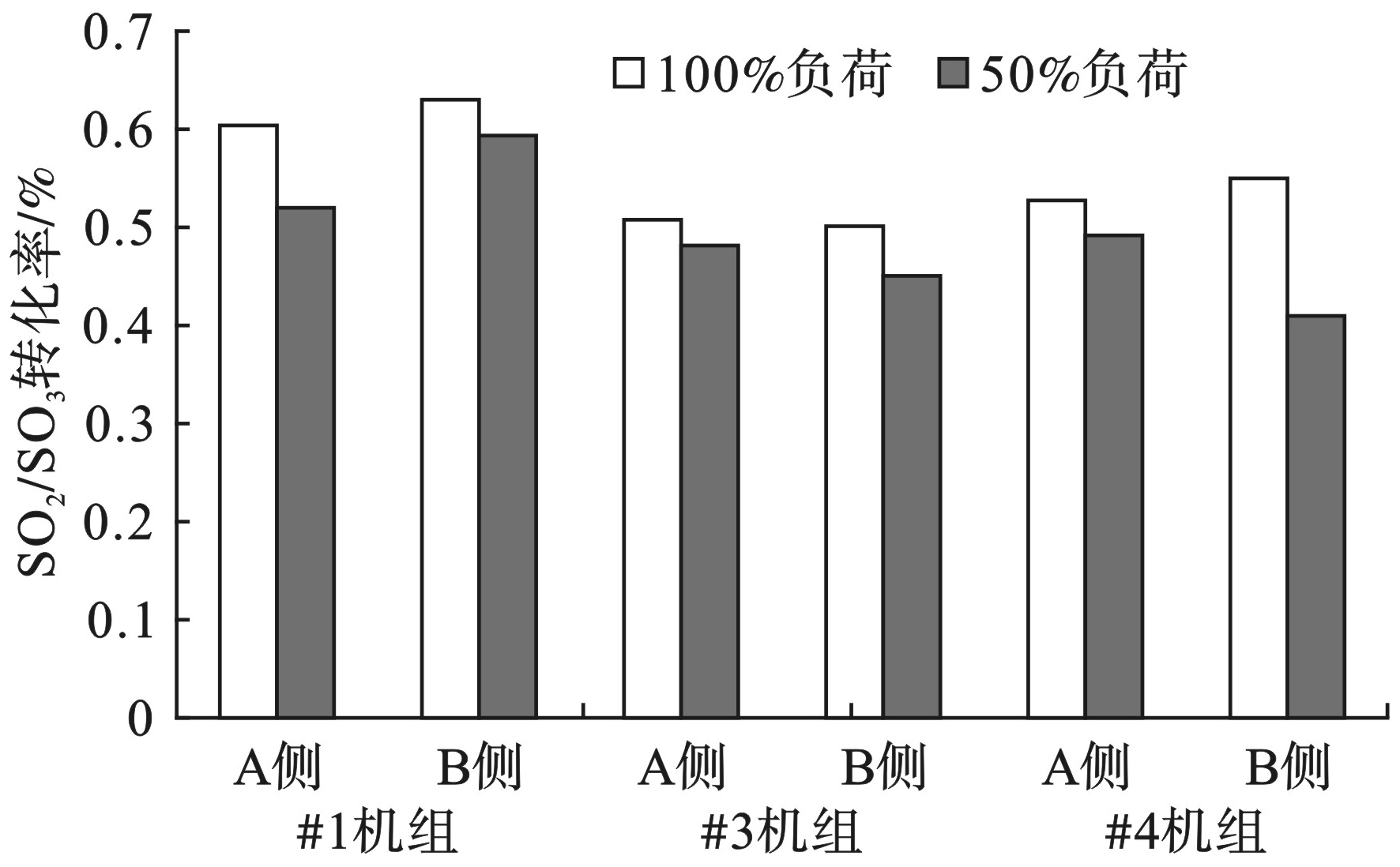
不同负荷条件下SO2/SO3转化率见图7。
此次试验进行了50%和100%两个负荷段3台机组SO2/SO3转化率的测定。测试结果显示,100%负荷的SO2/SO3转化率均大于50%负荷。负荷为50%时,最大值和最小值分别为0.59%和0.41%;负荷为100%时,最大值和最小值分别为0.63%和0.50%;100%负荷比50%负荷SO2/SO3转化率平均高出12.9%。
SO3与烟气中NH3反应生成的NH4HSO4和(NH4)2SO4会对下游的设备造成腐蚀、堵塞以及磨损,影响了机组的安全稳定运行。同时,硫酸雾气溶胶还是有色烟羽的主要成因,因此合理有效控制SO2/SO3转化率非常重要。影响SO2/SO3转化率的因素主要包括烟气温度以及催化剂中V2O5的含量。温度越高,SO2/SO3转化率越大,温度越低,SO2/SO3转化率越小;SO2/SO3转化率与催化剂V2O5含量呈线性关系,V2O5含量越大SO2/SO3转化率越高[17-19]。试验结果表明,在负荷降低时,温度降低,V2O5含量不变,SO2/SO3转化率降低。
3. 结论
1)机组进行负荷调整时,氧含量以及NOx质量浓度变化与负荷变化负相关,温度、脱硝效率以及SO2/SO3转化率则与负荷变化正相关。当负荷的降低,氧含量以及NOx质量浓度升高,温度、脱硝效率以及SO2/SO3转化率降低。
2)机组进行负荷调整时,氧含量和温度的变化都会对NOx的生成产生影响,同温度相比,氧含量变化对NOx生成量的影响更大。
3)影响催化剂活性的主要因素是温度,同时催化剂又是控制SCR技术脱硝效率以及SO2/SO3转化率的重要因素。进行负荷调整时,温度的变化影响催化剂的活性,催化剂影响脱硝效率以及SO2/SO3转化率。因此,温度对于脱硝效率以及SO2/SO3转化率有较为显著的影响。
-
-
[1] 万仲豪, 李孟, 张倩. 间苯胺改性磁性壳聚糖对六价铬的还原-吸附协同作用机制研究[J]. 环境科学学报, 2018, 38(8): 3118-3126. [2] 张力, 赵勇胜. 六价铬污染模拟含水层的注入型黄原胶凝胶阻截屏障试验研究[J]. 水文地质工程地质, 2023, 50(2): 171-177. [3] 张进德, 田磊, 裴圣良. 矿山水土污染与防治对策研究[J]. 水文地质工程地质, 2021, 48(2): 157-163. [4] 徐衍忠, 秦绪娜, 刘祥红, 等. 铬污染及其生态效应[J]. 环境科学与技术, 2002, 25: 8-9. [5] LIU Z G, ZHANG F S, WU J Z. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment[J]. Fuel, 2010, 89(2): 510-514. doi: 10.1016/j.fuel.2009.08.042 [6] 陈园园, 井琦, 任仲宇. 改性硅藻土负载纳米零价铁去除六价铬[J]. 应用化工, 2020, 49(3): 541-544. [7] MALLICK S, DASH S S, PARIDA K M. Adsorption of hexavalent chromium on manganese nodule leached residue obtained from NH3-SO2 leaching[J]. Journal of Colloid Interface Science, 2006, 297(2): 419-425. doi: 10.1016/j.jcis.2005.11.001 [8] COELHO F D S, ARDISSON J D, MOURA F C, et al. Potential application of highly reactive Fe(0)/Fe3O4 composites for the reduction of Cr(Ⅵ) environmental contaminants[J]. Chemosphere, 2008, 71: 90-96. doi: 10.1016/j.chemosphere.2007.10.016 [9] 平松, 杨茸茸, 吴雷, 等. 改性多孔兰炭末吸附处理模拟含铬废水[J]. 环境工程, 2023, 41(2): 7-15. [10] LI J X, ZHANG X Y, SUN Y K, et al. Advances in sulfidation of zerovalent iron for water decontamination[J]. Environmental Science & Technology, 2017, 51(23): 13533-13544. [11] WEI K, LI H, GU H, et al. Strained zero-valent iron for highly efficient heavy metal removal[J]. Advanced Functional Materials, 2022, 32(26): 2200498. doi: 10.1002/adfm.202200498 [12] RITTER M, WEISS W. Fe3O4(Ⅲ) surface structure determined by LEED crystallography[J]. Surface Science, 1999, 432: 81-94. doi: 10.1016/S0039-6028(99)00518-X [13] HU J, CHEN H, DONG H Y, et al. Transformation of iopamidol and atrazine by peroxymonosulfate under catalysis of a composite iron corrosion product (Fe/Fe3O4): Electron transfer, active species and reaction pathways[J]. Journal of Hazardous Materials, 2021, 403: 123553. doi: 10.1016/j.jhazmat.2020.123553 [14] HU J, HUANG Z Y, YU J M, Highly-effective mechanochemical destruction of hexachloroethane and hexachlorobenzene with Fe/Fe3O4 mixture as a novel additive. Science of the Total Environment. 2019, 659: 578−586. [15] 张震, 陈飞勇, 刘汝鹏, 等. 基于响应曲面法优化的臭氧/过硫酸盐/四氧化三铁工艺对结晶紫的降解[J]. 环境工程学报, 2023, 17(7): 2192-2204. [16] HU J, QIU Y F, GU B, et al. Enhancement mechanism of magnetite on the ball-milling destruction of perfluoro- octane sulfonate by iron [J]. Environmental Pollution, 2023: 121014. [17] ZHANG W, WANG H Z, HUANG J, et al. Acceleration and mechanistic studies of the mechanochemical dechlorination of HCB with iron powder and quartz sand[J]. Chemical Engineering Journal, 2014, 239: 185-191. doi: 10.1016/j.cej.2013.11.018 [18] DENG S S, KANG S G, FENG N N, et al. Mechanochemical mechanism of rapid dechlorination of hexachloro -benzene[J]. Journal of Hazardous Materials, 2017, 333: 116-127. doi: 10.1016/j.jhazmat.2017.03.022 [19] 王文豪. 零价金属及其复合物去除废水中Cr(Ⅵ)的效能与机理[D]. 重庆: 重庆大学, 2022. [20] 张文秋, 史晓国, 刘伟鑫. 湿式球磨法机械化学合成FeS2工艺[J]. 材料科学与工程学报, 2023, 41(3): 502-508. [21] 胡俊, 章献钊, 姚蕾, 等. 活性炭共球磨强化铁/四氧化三铁的六价铬还原性能[J]. 环境科学学报, 2023, 43(10): 116-122. [22] AMBIKA S, DEVASENA M. , NAMBI I M, Synthesis, characterization and performance of high energy ball milled meso-scale zero valent iron in Fenton reaction[J]. Journal of Environmental Management, 2016, 181: 84-855. [23] GU Y, WANG B, HE F, Mechanochemically sulfidated microscale zero valent iron: pathways, kinetics, mechanism, and efficiency of trichloroethylene dechlorination [J]. Environmental Science & Technology, 2017, 51(21): 12653-12662. [24] SHAO Q Q, XU C H, WANG Y H, et al. Dynamic interactions between sulfidated zerovalent iron and dissolved oxygen: Mechanistic insights for enhanced chromate removal[J]. Water Research, 2018, 135: 322-330. doi: 10.1016/j.watres.2018.02.030 [25] WANG W H, HU B B, WANG C, et al. Cr(Ⅵ) removal by micron-scale iron-carbon composite induced by ball milling: The role of activated carbon[J]. Chemical Engineering Journal, 2020, 389: 122633. doi: 10.1016/j.cej.2019.122633 [26] GAN C, LIU Y G, TAN X F, et al. Effect of porous zinc-biochar nanocomposites on Cr(Ⅵ) adsorption from aqueous solution[J]. RSC Advances, 2015, 5(44): 35107-35115. doi: 10.1039/C5RA04416B -






 下载:
下载: