-
抗生素因其能够治疗疾病,促进牲畜生长和水产养殖而得到普遍使用. 土霉素(OTC)是一种四环素类抗生素,具有广谱抗病原微生物作用,被广泛应用于畜禽养殖. 但生物体只能代谢掉少量的OTC,约75%的OTC被排放到水环境中,从而致使其在水环境中的积累并造成潜在危害[1 − 2]. 例如过量的抗生素残留会增加水系统和土壤中细菌的耐药性,导致抗生素耐药性基因的产生和传播[3]. 因此开发有效的抗生素去除技术具有重要意义.
常见的抗生素去除方法有吸附法[4 − 6]、膜处理法[7 − 8]和生物法[9 − 10]等. 高级氧化技术(AOPs)是通过活化过氧化氢(H2O2)、过硫酸盐(PS)等氧化剂产生活性物种去除难降解有机物的工艺,因其氧化能力强、操作简便而被认为是最有前途的废水净化技术之一. 与其他氧化剂相比,过硫酸盐具有更好的化学稳定性、更低的价格以及方便储存和运输等特点[11]. 通过活化PS产生硫酸根自由基(SO4·−)的高级氧化技术(SR-AOPs)被广泛应用于水中难降解有机化合物的去除[12 − 14]. 相较于活化H2O2产生的·OH,SO4·−是一种高活性物质,具有更高的氧化电位(2.6—3.1 V,·OH为1.9—2.7 V),更长的半衰期(30—40 μs,·OH为20 ns)和更宽的pH值范围(4—9),且对难降解污染物具有更高的去除效率[15 − 17].
PS可通过热量、超声波、紫外线和过渡金属等方式活化[17 − 19]. 其中金属基催化剂具有高活性、能耗小、成本低和易实际应用的优点[20 − 21]. 近年来,金属有机骨架(MOF)作为拥有框架结构和金属位点的金属和非金属结合的材料,在活化PS降解有机物的研究中得到越来越多的关注[22 − 24],其中铁基MOF具有丰富的纳米孔,较大的比表面积,良好的热稳定性和化学稳定性,是一种环境友好型材料[25 − 26]. 在众多铁基MOF中,由对苯二甲酸和三价铁组成的八面体聚合物MIL-88B(Fe)是一种结构优良的铁基MOF材料,被认为是活化PS的潜在催化剂[27]. 由于MOF材料的水稳定性较差,常通过高温煅烧制备MOF衍生物. MOF衍生的多孔碳材料不仅能够保持原有的结构和形貌,而且继承了MOF前驱体的高比表面积和丰富的孔隙结构,还具备更高的活性和稳定性,扩大了适用范围[28]. 因此可通过碳化处理MOF前驱物提高其结构稳定性和催化性能.
该文以制备MOF前驱体的氯化铁和二氨基对苯二甲酸为原料,通过溶剂热和高温裂解制备了Fe/NC. 利用扫描电子显微镜(scanning electron microscope,SEM)、能量色散光谱(Energy dispersive Spectrometer,EDS)、N2吸附/解吸技术(Brunauer-Emmett-Teller,BET)、X射线衍射仪技术(X-ray diffraction,XRD)、拉曼光谱(Raman spectra)和X射线光电子能谱技术(X-ray photoelectron spectroscopy,XPS)对材料的结构和形貌进行表征. 探究了Fe/NC催化剂对PS活化降解OTC的性能. 采用单一变量法探究Fe/NC活化PS体系中催化剂投加量、溶液初始pH和PS浓度对OTC降解的影响. 对Fe/NC/PS体系进行淬灭反应实验,探究OTC降解过程中体系内主要存在的活性物质,进一步推测反应机理;通过无机阴离子和自然有机物对降解的影响及循环实验,探讨Fe/NC活化PS降解抗生素的适用性及稳定性,为Fe/NC在活化SR-AOPs体系降解抗生素领域应用提供科学依据.
-
主要试剂:土霉素(OTC,C22H24O9N2,97%)、三氯化铁(FeCl3·6H2O,99%,分析纯)、二氨基对苯二甲酸(NH2-H2BDC,C8H7NO4,98%)、盐酸(HCl,分析纯)、氢氧化钠(NaOH,分析纯)、氯化钠(NaCl、分析纯)、无水碳酸钠(Na2CO3、分析纯)、L-组氨酸(L-His,C6H9N3O2,99%,分析纯)购于上海阿拉丁生化科技股份有限公司. 过硫酸钠(PS,Na2S2O8,99%,分析纯)、叔丁醇(TBA,C4H10O,99%,分析纯)、对苯醌(p-BQ,C2H6O,99%)、磷酸二氢钾(NaH2PO4·2H2O,分析纯)、腐殖酸(HA,90%,分析纯)购于上海麦克林生化有限公司. N,N-二甲基甲酰胺(DMF,C3H7NO,分析纯)、无水乙醇(EtOH,CH3CH2OH,分析纯)购于天津市大茂化学试剂厂. 实验中所有溶液均使用去离子水配制.
主要仪器:KABML-602A型管式炉(天津玛福尔科技有限公司)、BY-400C型离心机(北京白洋医疗器械有限公司)、FD-1-50型烘箱(北京博医康实验仪器有限公司)、SHA-CA型恒温振荡箱(常州市伟嘉仪器制造有限公司)、YS0410型超声波清洗机(深圳云奕科技股份有限公司)、NB-1M型磁力搅拌器(苏州九联科技有限公司)、UV765型紫外-可见分光光度计(上海精密科学仪器有限公司)、PHS-3C型pH计(上海仪电科学仪器股份有限公司)、FA2104N型电子分析天平(上海菁华科技仪器有限公司)、JSM-6460LV型扫描电子显微镜(日本电子株式会社)、Thermo escalab 250Xi型X射线光电子能谱仪(美国热电)、BRUKER D8 ADVANCE型X射线粉末衍射仪(德国布鲁克)、Renishaw inVia型激光共聚焦拉曼光谱仪(英国雷尼绍).
-
称取1.6218 g(6 mmol)FeCl3·6H2O和1.0869 g(6 mmol)NH2-H2BDC分别溶解在30 mL DMF中,超声10 min后,转移至100 mL聚四氟乙烯反应釜中混合,磁力搅拌30 min后,将反应釜密封后置入预热到120 ℃的烘箱中溶剂热12 h. 待冷却至室温后取出反应釜内衬,将产物转移至离心管中,在11000 r·min−1下进行固液分离,用DMF和乙醇交替洗涤所得固体产物3—4次,随后放入鼓风干燥箱60 ℃下烘干12 h,研磨后可得MIL-88B-NH2. 将盛放MIL-88B-NH2粉末的石英舟置于管式炉中,在氮气氛围下,以2 ℃·min−1的速率分别升温至700、800、900 ℃,保持2 h,待降至室温后取出,用1 mol·L−1 HCl酸洗以去除不稳定物质,烘干后制得的材料分别记为Fe/NC-700、Fe/NC-800和Fe/NC-900.
样品的形貌特征通过SEM结合EDS在高分辨率微区观察;BET用于检测样品的比表面积、孔径和孔尺寸等信息;样品的结构特性和结晶情况由Cu Kα辐射的XRD分析其衍射图谱,获得材料的成分、材料内部原子或分子的结构或形态等信息;通过拉曼光谱在652 nm的激发波长下探测样品的缺陷和石墨化结构;利用XPS对材料进行表面分析,使用X射线辐射样品,使原子或分子的内层电子或价电子受激发射出来,测量光电子的能量和数量,获得待测物组成,测定电子的结合能来鉴定分析样品表面的化学性质及组成.
-
将一定量的催化剂和PS添加到含有30 mL 20 mg·L−1 OTC的50 mL锥形瓶中. 将锥形瓶用盖子密封,放于恒温震荡箱中,在(25±1)℃,180 r·min−1的转速下进行降解反应实验. 在固定的时间间隔取1 mL样品,经0.22 µm的膜过滤后置于预先加入1 mL乙醇的离心管中. 吸附实验与上述步骤基本相同,区别在于吸附实验中没有PS的投加步骤.
在影响因素实验中,采用单一变量法,探究催化剂投加量(0.1—0.5 g·L−1)、初始pH(2—10)和PS浓度(1—5 mmol·L−1)对抗生素降解效果的影响. 用一定量Na2CO3、KH2PO4、NaCl和HA分别溶于污染物溶液中模拟自然水体中的无机阴离子和有机物,并添加催化剂进行实验,以探究催化剂在水环境中的适应性和稳定性. 在循环试验中,催化剂重复使用若干次. 每次用去离子水清洗使用后的催化剂,并在80 ℃下干燥以进行后续循环实验. 反应前投加淬灭剂进行淬灭实验,EtOH可以掩蔽溶液中的·OH和SO4·−,TBA单独掩蔽·OH, p-BQ用于淬灭·O2−,L-His用于掩蔽1O2,通过掩蔽剂对抗生素去除效果的影响,推测降解机理和主要活性物质.
通过紫外分光光度计在356 nm波长处测定OTC的剩余浓度. 抗生素的降解效率计算方法如公式(1):
式中,R为抗生素去除率,%;C0为抗生素初始浓度,mg·L−1;Ct为特定时刻抗生素剩余浓度,mg·L−1. 所有实验均重复3次,以提高精密度.
-
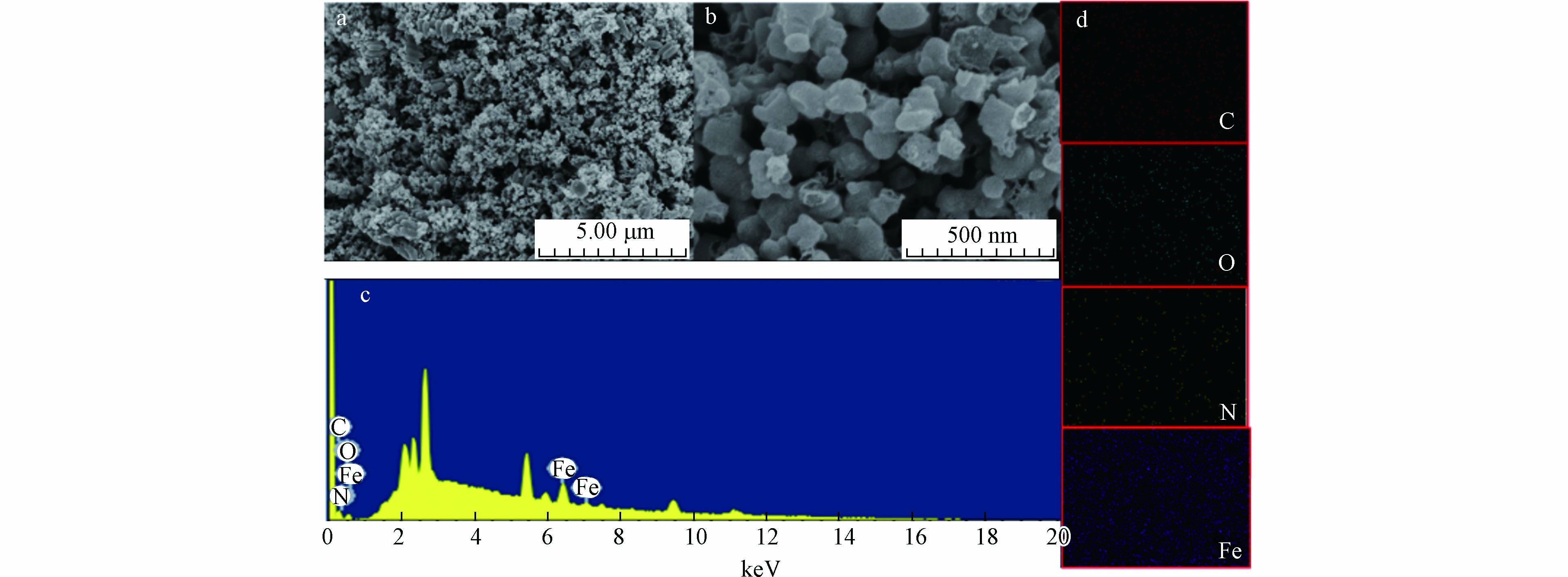
图1为Fe/CN-900的形貌图和表面元素的面扫图像. 从图1(b)中可以看到,部分两头锥形中间八面棱柱形的多面体,为制备过程中产生的一些尺寸较大的MOF,其形貌与文献中所体现的一致[29],表明该研究中MIL-88B-NH2的制备方法可行. 同时,在制备过程中添加的含氮有机配体,使MOF的结构孔隙率增大,有利于催化过程中PS与活性位点的接触. 在图1(a)中可以看到,Fe/NC-900呈不规则多孔形状,这是由于热解温度较高,MOF的结构遭到一定程度的破坏[30]. 材料表面元素面扫结果如图1(c)和表1所示,Fe/NC-900中各元素所占比例分别为56.89%(C)>17.45%(O)>14.65%(Fe)>11.01%(N),表明Fe/NC材料的成功制备. 表2中描述了Fe/NC在热解温度为700、800、900 ℃下产物的比表面积、孔体积和孔径尺寸. 可以看出催化剂的比表面积随温度升高而上升,Fe/NC-900的比表面积达到429.54 m2·g−1. 虽然Fe/NC-900的孔径尺寸(3.80 nm)小于Fe/NC-700的孔径尺寸(5.60 nm),但Fe/NC-900的孔体积最大(0.41 cm2·g−1).
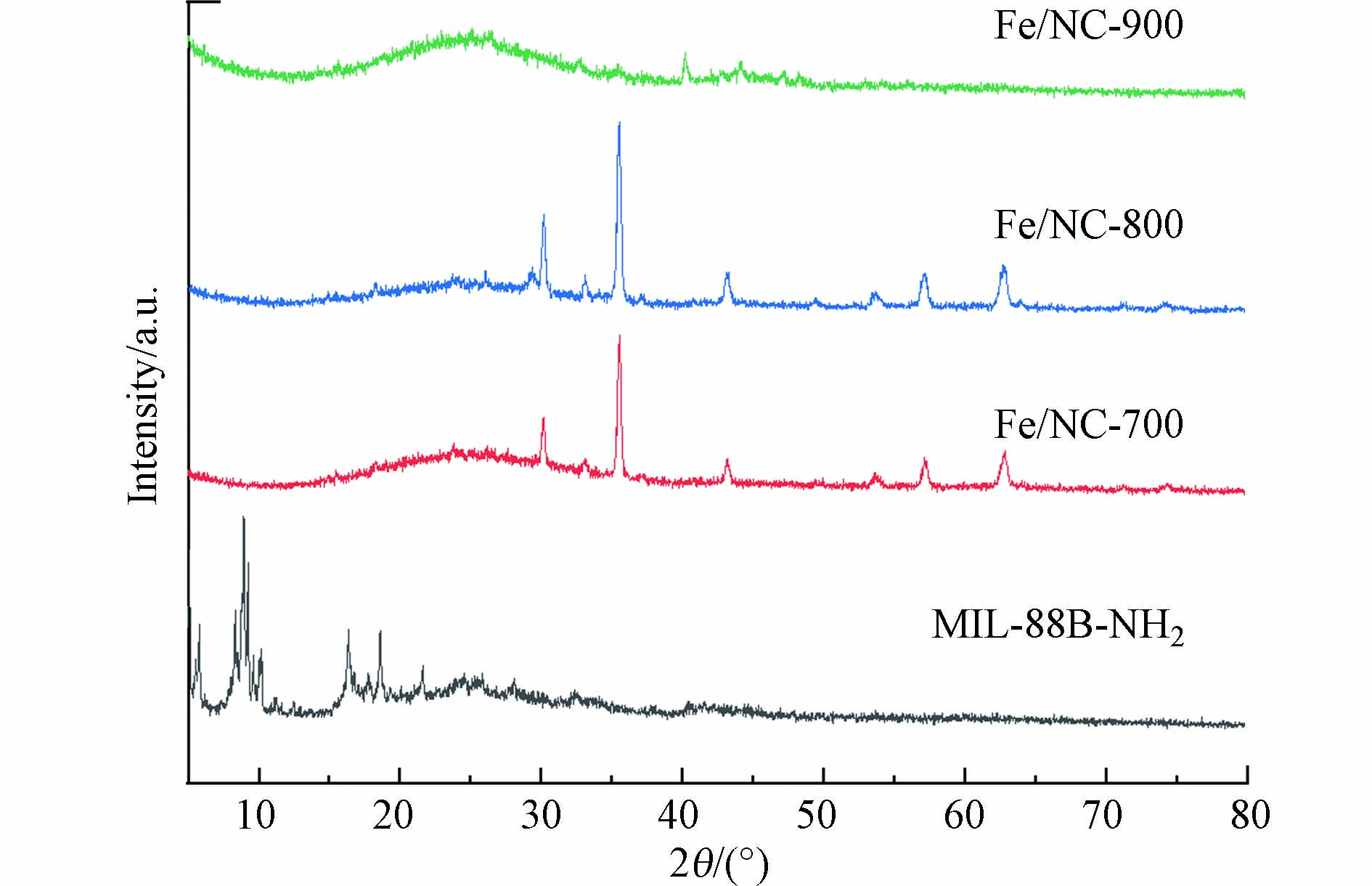
制备的MIL-88B-NH2碳化材料和原始NH2-MIL-88B(Fe)的XRD图谱如图2所示. MIL-88B-NH2的特征衍射峰出现在约9.2°(002),10.3°(101),13.1°(102),16.7°(103),18.5°(200),19.1°(201),20.7°(202),26.3°(204)、29.5°(302),这些峰都与根据结晶学信息文件(CIF)(剑桥结晶学数据中心(CCDOX)647646)建立的MIL-88B-NH2结构的模拟XRD图谱一致[31]. 表明成功合成了MIL-88B-NH2结构的纯产物. Fe/NC-700和Fe/NC-800的XRD图谱显示出相同的特征峰,这些峰的位置与Fe3O4的峰位数据相匹配(JCPDS No. 75–1372)[32],说明碳化后MIL-88B-NH2中的铁形成了氧化物. Fe/NC-900的XRD图谱未显示出明显的特征峰,这可能意味着Fe/NC-900中的Fe以高分散状态存在.
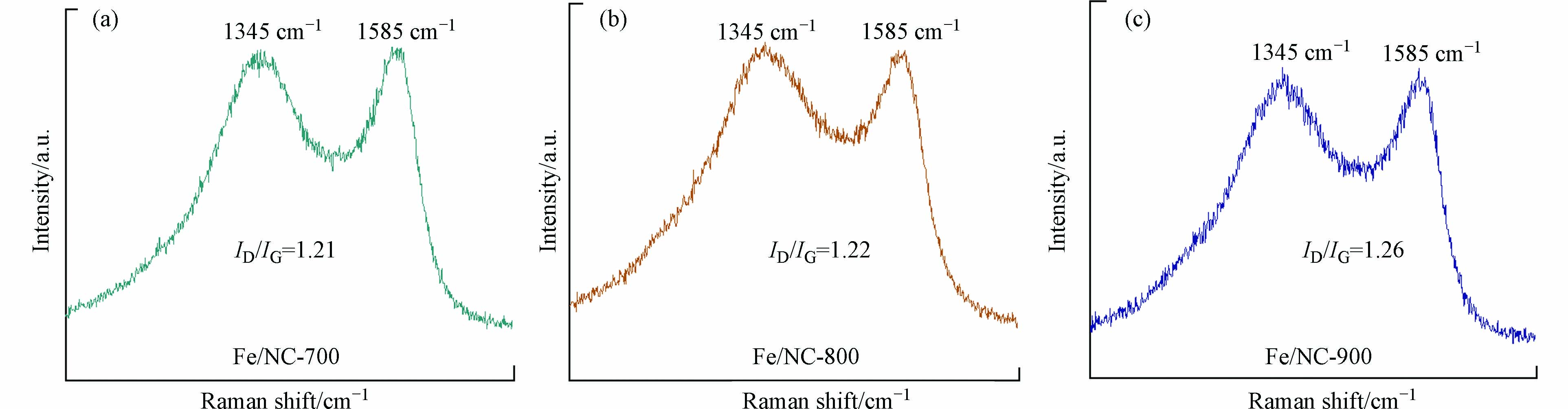
图3是MIL-88B-NH2在不同温度下碳化后的拉曼检测图谱,在1345 cm−1处观察到典型的D带与无序碳相关,代表材料中的缺陷碳,1585 cm−1处显示1个典型的G波段峰值属于sp2杂化石墨碳,这表明存在堆叠和有序的六方碳结构[33]. 经峰拟合后计算两峰面积之比可得ID/IG的值,此值越大代表材料的缺陷程度越大. 图3可以看出,催化剂的缺陷结构随温度的上升而增高,在900 ℃时为1.26.
XPS分析进一步探索了Fe/NC-900的元素组成和价态分布. 如图4(a)全谱所示,Fe/NC-900主要由Fe、C、N和O等元素组成,与扫描电镜EDS结果一致. 图4(b)中,C1s XPS光谱分为两种类型的峰,分别代表C—C键(284.4 eV)和C—O—C键(287.0 eV). Fe/CN-900的高分辨率N1s XPS曲线出现400.7和399.0 eV的两种类型的峰,分别归属于吡咯氮和吡啶氮[34],从峰面积可以看出,吡咯氮含量远大于吡啶氮含量. 在Fe2p图中,Fe/CN-900的Fe2p光谱主要反褶积为分离的2p3/2和2p1/2轨道峰. 在727.16 eV处的峰对应Fe(Ⅱ) Fe 2p1/2的卫星峰,710.3—711.8 eV处对应于Fe(III) Fe 2p3/2,说明前驱体中的Fe3+在制备及煅烧过程中部分转变为Fe2+[35].
-
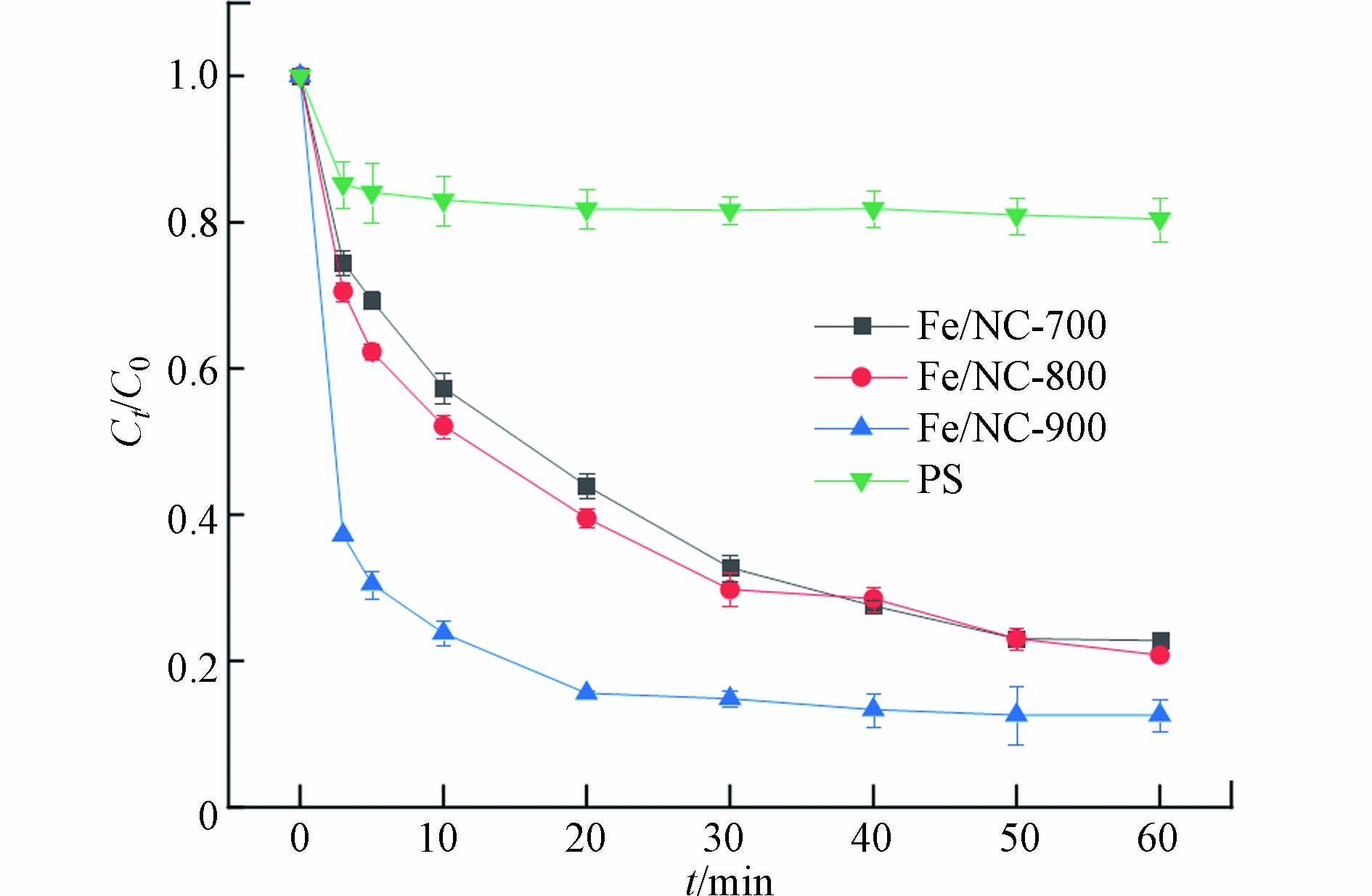
使用不同体系降解OTC的结果如图5所示. 在PS单独作用时只有19.55%的OTC被去除,表明在没有催化剂的情况下PS对OTC的降解能力有限. 当热解温度为700 ℃和800 ℃时,Fe/NC催化剂活化PS去除OTC的效果区别不大,分别为77.19%和79.18%. 当温度提升至900 ℃时,OTC的降解率继续提升到87.39%,说明Fe/NC可以作为PS活化剂有效降解抗生素. 同时,实验结果验证了表征分析结论,当热解温度提高后,Fe/NC-900具有更大的比表面积、孔体积以及较多的缺陷结构,更有利于对PS的活化.
-
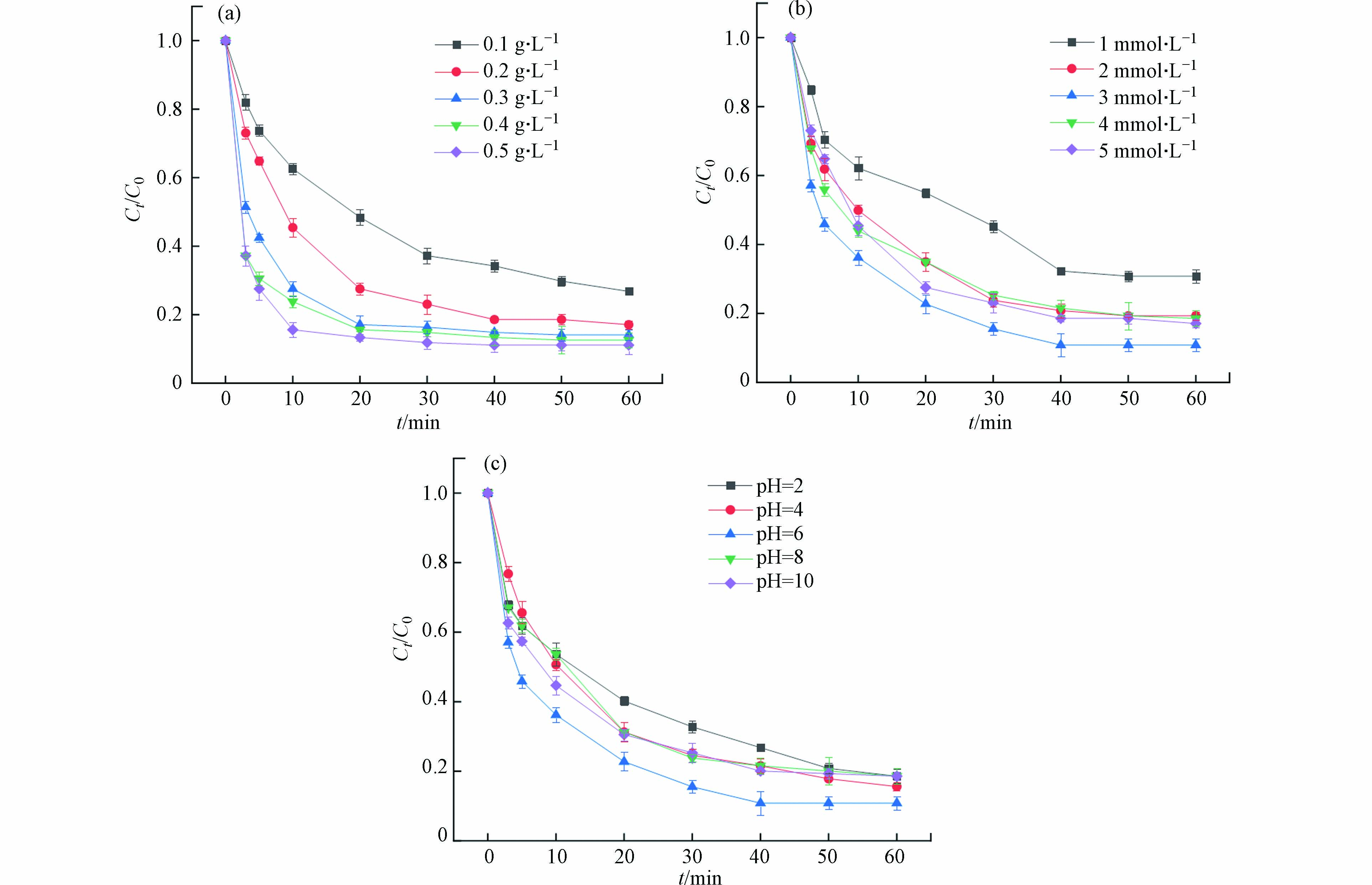
不同Fe/NC-900催化剂投加量对OTC去除的影响如图6(a)所示. 当Fe/NC-900的用量从0.1 g·L−1增加到0.2 g·L−1时,OTC的降解率从73.21%增加到82.91%. 这一结果表明,增加Fe/NC-900催化剂用量可以为PS活化提供更多的活性位点,从而显著提高OTC的去除效率[36]. 但当PS浓度一定时,催化剂活化PS产生的活性氧物质会达到饱和. 因此,随着OTC投加量从0.2 g·L−1增加到0.5 g·L−1,OTC的降解效果仅略有改善(小于6%). 故而在后续反应中Fe/NC-900的用量皆采用0.2 g·L−1.
考察了PS浓度对Fe/NC-900活化PS降解OTC的影响,结果如图6(b)所示. 随着PS浓度从1 mmol·L−1增加到3 mmol·L−1,OTC的降解率从69.18%增加到89.18%. 这一结果可解释为较高的PS浓度有利于生成更多的活性氧物质并提高OTC的降解效率[37]. 然而,随着PS浓度从3 mmol·L−1进一步增加到5 mmol·L−1,OTC的降解率逐渐降低,这归因于过量的PS会消耗活性氧物种. 因此,Fe/NC-900/PS体系中PS的最佳浓度控制在3 mmol·L−1.
反应溶液的初始pH值是影响OTC降解的另一重要因素. 如图6(c)所示,在2.0到6.0的初始pH范围内,OTC的降解效率逐渐升高至89.18%. 这是因为在强酸性条件下,过量的H+会直接淬灭SO4·−以及其他自由基[38]. 随着初始pH值增加到10.0时,OTC的去除率降低到81.42%,这可能是由于碱性条件下自由基的消耗[39]. 总体来说,Fe/NC-900/PS体系在pH值为2—10的范围内,对OTC的去除率都在80%以上,并且波动范围较小,说明Fe/NC-900可作为较稳定的催化剂,对水环境的pH适应范围较广.
-
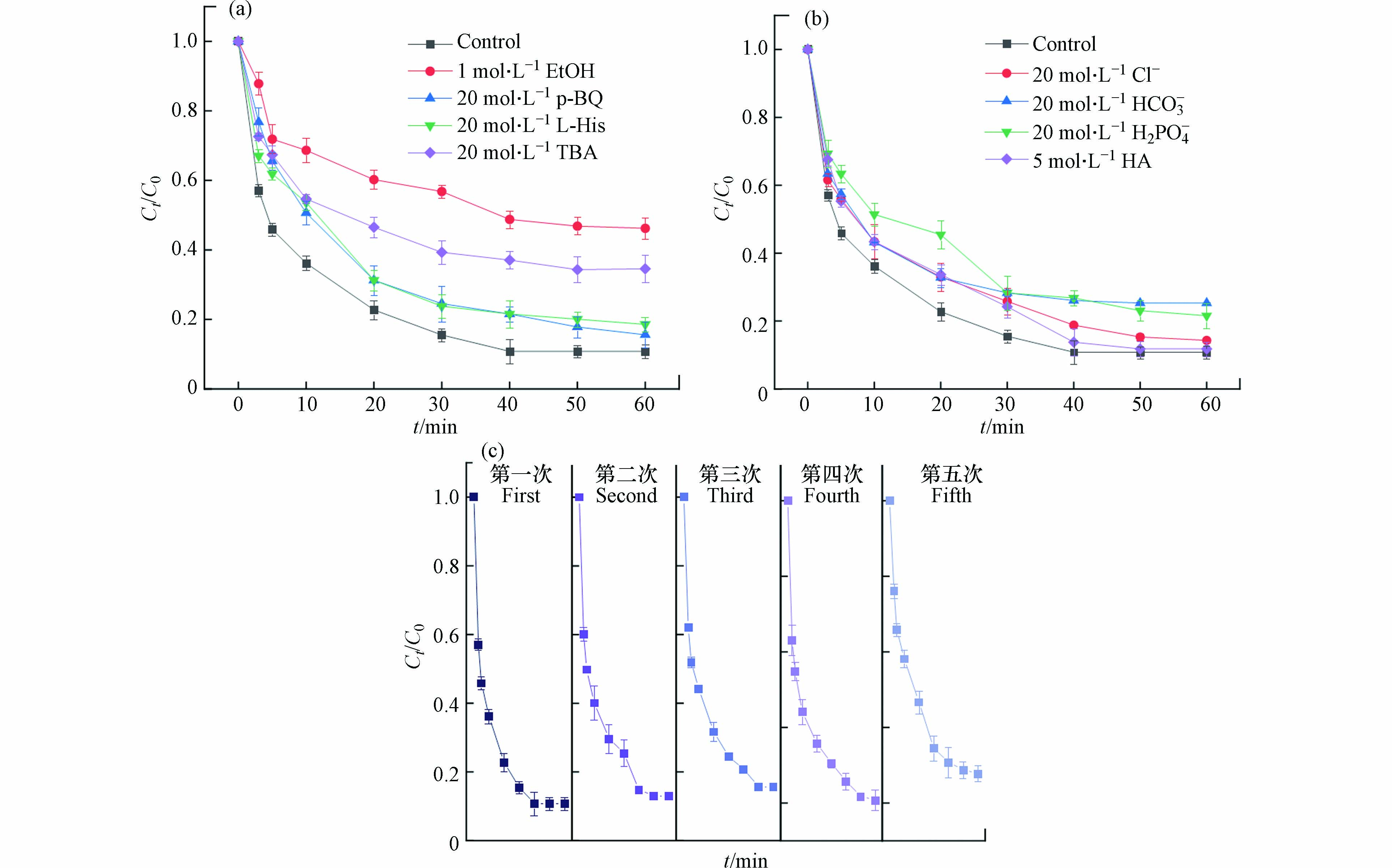
本节进行了活性氧物质淬灭实验,以阐明自由基及非自由基在OTC降解中的作用,如图7(a)所示. 结果表明,4种淬灭剂均对OTC的降解有一定的抑制作用,分别使OTC的降解效率降低至53.79%(EtOH)、65.42%(TBA)、84.4%(p-BQ)和81.42%(L-His). SO4·−被掩蔽后,OTC的去除率仅为53.79%,表明SO4·−在Fe/NC-900/PS体系中对OTC的降解起关键作用.
基于以上分析,提出了Fe/NC-900/PS体系中OTC可能的降解机制. SO4·−可由Fe3+和Fe2+生成,并提供电子以直接与S2O82−相互作用,SO4·−继续与水相互作用产生自由基·OH(公式2和3). 同时,Fe2+也与溶液中的O2反应生成·O2−, 并进一步与·OH反应生成1O2(公式5和6).
-
使用含有不同阴离子和腐殖酸的水溶液模拟真实的水基质(图7b). 从图7可以看出,Cl−和腐殖酸对OTC的去除效果几乎没有影响,说明腐殖酸与OTC之间不存在竞争反应. 在20 mmol·L−1 HCO3−和H2PO4−的存在下,OTC的去除率分别降至74.70%和78.43%,这可能由于这两种阴离子会与体系中产生的自由基反应. 结果表明,在真实的水基质中,材料的活化性能只受到轻微抑制.
图7(c)为Fe/NC-900重复使用5次后对OTC的降解效率图,从OTC的降解趋势可以看出,重复使用后催化剂的活性依然很强,第五次回用后降解率只下降了16.85%. 连续5次OTC的降解率分别为89.18%、86.88%、84.18%、79.29%和72.33%. 说明催化剂活性位点在反应中丧失较少,比较稳定.
-
1)该文成功合成了由MIL-88B-NH2碳化后所得的新型铁基含氮碳材料,并将其用于活化PS降解OTC.
2)研究发现不同热解温度下所得的催化剂活化能力不同. 与Fe/CN-700和 Fe/CN-800相比,Fe/CN-900具有更大的比表面积和孔体积,缺陷结构更多,在活化PS降解OTC的反应体系中催化活性也更强.
3)OTC的降解率受催化剂投加量、PS浓度和初始pH值等因素的影响,Fe/NC-900/PS体系的最佳反应条件为在不调pH的情况下(pH=6),催化剂投加量0.2 g·L−1,PS浓度3 mmol·L−1,此时OTC的去除率达到89.18%.
4)OTC的降解机理为Fe/NC-900表面的Fe与PS反应生成多种自由基(SO4·−、·OH和·O2−)攻击污染物,其中SO4·−对OTC的降解起关键作用.
5)在含有无机阴离子和有机物的水体中Fe/NC-900表现较为稳定,在5次回用后依然保持较高的催化活性. 总而言之,Fe/NC-900是一种PS高效活化剂,为抗生素的去除提供了新的选择.
MOF衍生物(Fe/NC)活化过硫酸盐对土霉素的高效降解
Efficient degradation of oxytetracycline by persulfate activated by MOF derivative (Fe/NC)
-
摘要: 近年来,以有机金属骨架(MOF)为前驱体制备的MOF衍生物得到越来越多的关注. 与MOF相比,热解制得的MOF衍生物具有多变的结构、更高的比表面积和更好的稳定性. 本研究采用高温碳化MOF成功制备了Fe/NC材料,研究了不同因素对Fe/NC活化PS降解土霉素(OTC)的影响. 在OTC初始浓度为20 mg·L−1、PS浓度为3 mmol·L−1、Fe/NC-900投加量为0.2 g·L−1、初始 pH 值为6.0的条件下,反应20 min后,OTC去除率最高达到89.18%. 活性氧物质淬灭实验证实反应体系中存在硫酸根自由基(SO4·−)、羟基自由基(·OH)等多种活性氧物质,其中SO4·−在OTC的降解中起主要作用. 在最优催化工艺参数下探究不同水基质对OTC去除的影响. 其中Cl−和腐殖酸对OTC的降解几乎没有影响,HCO3−和H2PO4−则表现出轻微的抑制作用,说明Fe/NC的适用范围广. 经过五次循环回用后对OTC的降解率仅下降16.85%,表明材料具有较好的重复利用性. 本研究为MOF材料在水污染控制领域中的应用提供了新的理论研究,并为四环素类抗生素的降解提供了新思路.Abstract: In recent years, organometallic skeleton (MOF) derivatives prepared using MOF as precursors have received increasing attention. Compared with MOF, the MOF derivatives produced by pyrolysis have variable structure, higher specific surface area and better stability. In this research, Fe/NC materials were successfully prepared by high temperature carbonization of MOF. The removal efficiency of OTC could reach 89.18% with 20 mg·L−1 initial concentration of OTC, 3 mmol·L−1 PS and 0.2 g·L−1 Fe/NC-900 at 6.0 pH in 20 min. The results of reactive oxygen species quenching experiments confirmed the presence of various reactive oxygen species, among which SO4·− played a primary role in the degradation of OTC. The effects of different water matrix showed that Cl− and humic acid had almost no effect on OTC degradation, while HCO3− and H2PO4− expressed a slight inhibition. After five cycles of recycling, the degradation rate of OTC only declined by 16.85%. This research suggests a new theoretical basis for the application of MOF materials in the field of water contamination control and provides a novel concept for the degradation of tetracycline antibiotics.
-
Key words:
- persulfate /
- MOF derivative /
- oxytetracycline /
- advanced oxidation /
- heterogeneous catalyst
-
随着社会经济的发展,柴油的使用量增加,但是柴油在生产、运输、装卸、加工及使用过程中的泄露会对土壤环境造成一定的污染,直接或间接地危害人类的生命与健康[1-2]。因此,解决柴油污染土壤问题已成为世界各国所共同面临的问题[3]。
目前,针对柴油污染土壤修复的方法主要包括机械、物理、化学和生物修复方法等[4]。其中,机械、物理、化学修复方法具有费用高、容易产生二次污染等不足[5-7]。而生物修复技术是一种高效、环境友好、低成本的技术,能够将柴油等污染物通过微生物代谢转化成无毒的终产物[8-9],因而被广泛应用于修复柴油污染土壤之中[10]。刘沙沙等[11]已成功利用醋酸钙不动杆菌降解柴油以及污染物,经过62 d的生物修复实验,柴油去除率为69.8%。然而,柴油组成的复杂性决定了其降解需要有不同菌株的参与[12],TAO等[13]研究了土著细菌联合体与外源芽孢杆菌(Bacillus subtilis)共同培养降解原油的实验,细菌群落分析结果表明,在确定的共培养条件下,细菌多样性降低,降解效率提高,同时证明芽孢杆菌对长链烷烃有很好的降解效果。
大量的研究证明,微生物在修复有机物污染土壤的过程中具有良好的应用前景,但目前对于构建微生物菌群的研究较少,本研究从柴油污染土壤中筛选、分离出能够降解柴油污染物的微生物,采用组合实验构建优势菌群,探究了其柴油生物降解特性,研究分析了该菌群中各菌种之间的互作机制,为构建降解柴油的菌群提供参考。
1. 材料与方法
1.1 实验仪器与原料
紫外分光光度计(UV-2102C,中国上海),GC-MS(Agilent6890/5975I,安捷伦),台式高速冷冻离心机(H-2050R,中国长沙),恒温振荡培养箱(HZQ-X160,中国太仓)。实验所用试剂均为分析纯。
柴油污染土壤取自上海金山卫金山大道城河路;柴油为市售0#柴油(密度:0.84 kg·L−1);菌种:实验所用菌种均为从柴油污染土壤样品中筛选分离得到。
1.2 实验方法
1)微生物菌种生物量的测定方法。将菌落接种于灭菌的种子培养基中,每2 h取样,采用分光光度计在波长600 nm下测定吸光度值,绘制柴油降解细菌的生长曲线。
2)微生物菌种对柴油降解能力的测定方法。残余柴油浓度采用分光光度法[14]进行测定。菌株对柴油的降解能力采用柴油降解率表示。降解率计算公式如式(1)所示。
R=C0−CtC0×100% (1) 式中:R为柴油降解率;C0为柴油的初始浓度,mg·mL−1;Ct为柴油的降解过程测定浓度,mg·mL−1。
3)微生物高效降解柴油的条件优化实验。以构建好的柴油污染物降解菌群为研究对象,考察了初始pH(5.0、6.0、7.0、8.0、9.0)、初始柴油浓度(1.0、3.0、5.0、7.0、9.0 mL·L−1)、初始接种量(体积比5.0%、10.0%、15.0%)对柴油降解率和细菌生物量的影响结果。在30 ℃,150 r·min−1条件下培养5 d,定时取样并测定其中的柴油降解率以及生物量的变化,每组实验重复3次,取其平均值。
4)微生物多样性测试。将培养24 h的菌群混合液按10%的接种量接种到无机盐培养基中,柴油浓度为7.0 mL·L−1,pH=7.0,在30 ℃,150 r·min−1条件下培养,14 d后,将混合菌进行收集,离心弃上清液,收集菌体,测试微生物多样性,该工作由上海美吉医药科技有限公司完成。
5)微生物降解柴油产物的检测。将筛选获得的高效单菌种分别接种到种子培养基中,培养24 h后,离心收集菌体,稀释使其OD600=1.50,并按照最佳的体积比混合后接种于无机盐培养基中,以不加细菌为对照组,柴油浓度为7.0 mL·L−1,在30 ℃、150 r·min−1的恒温振荡培养箱中培养14 d。然后将培养基取出加入1∶1(体积比)硫酸5.0 mL酸化水样,继续加入0.2 g·L−1的氯化钠破乳[15],然后加入石油醚20.0 mL(60~90 ℃)超声10 min。将上述溶液8 000 r·min−1离心10 min,将上清液转移至另一干净的三角瓶中,下层溶液倒入原三角瓶并用石油醚重新提取1次。合并2次提取液,过膜,利用GC-MS测定培养基内降解产物组成及含量。
6)微生物降解十五烷产物的检测。将本研究所构建的混合菌群接种于添加了十五烷(7.0 mL·L−1)的无机盐培养基中,在30 ℃、150 r·min−1的振荡箱里培养14 d,分别取降解3、6、14 d的培养液,按照上述方法处理并检测。
2. 结果与讨论
2.1 柴油降解菌的富集驯化结果
从柴油污染土壤中筛选获得4株具有较强柴油降解能力的菌株,其菌落形态和柴油降解能力结果见表1。
表 1 菌株的菌落形状及柴油降解能力Table 1. Colony shape and diesel degradability of strains菌株号 菌落形态 菌体形态 菌落颜色 降解率/% 1# 菌落为扁平、边缘不整齐、表面粗糙皱褶 杆状 白色 27.0 2# 菌落透明、光滑、有光泽 球形 白色 29.0 3# 菌落微黄、表面光滑、边缘整齐 杆状 微黄色 32.0 4# 菌落淡红色、湿润、不规则 杆状 淡红色 35.0 2.2 高效降解菌的初步鉴定
微生物初步鉴定结果如表2所示。通过16S rRNA测序结果可知,1#、2#、3#、4#菌株分别与Bacillus sp. VOC18、Enterococcus faecalis、Lysinibacillus、Rhodococcus equi有97%、98%、99%、99%的相似性。本研究分别对1#、2#、3#、4#菌株命名为Bacillus sp. VOC18-L1,Enterococcus faecalis-L2,Lysinibacillus-L3,Rhodococcus equi-L4(简称L1,L2,L3和L4)。
表 2 4种柴油降解菌株的生理生化实验结果Table 2. Physiological and biochemical characteristics of four diesel degrading bacteria实验类型 菌株号 1# 2# 3# 4# 淀粉水解实验 − − − − 明胶实验 + − + + 尿素实验 − + − − 甲基红实验 + − + + V-P实验 + − + − 吲哚实验 − − − − 柠檬酸盐实验 − − − − 硫化氢实验 − − − + 触酶实验 − + − + 葡萄糖发酵实验 + − + + 乳糖发酵实验 − − − − 木糖发酵实验 − − − − 麦芽糖发酵实验 + − + + 蔗糖发酵实验 + − − − 注:“+”表示显阳性,“−”表示显阴性。 2.3 菌株的生长曲线分析结果
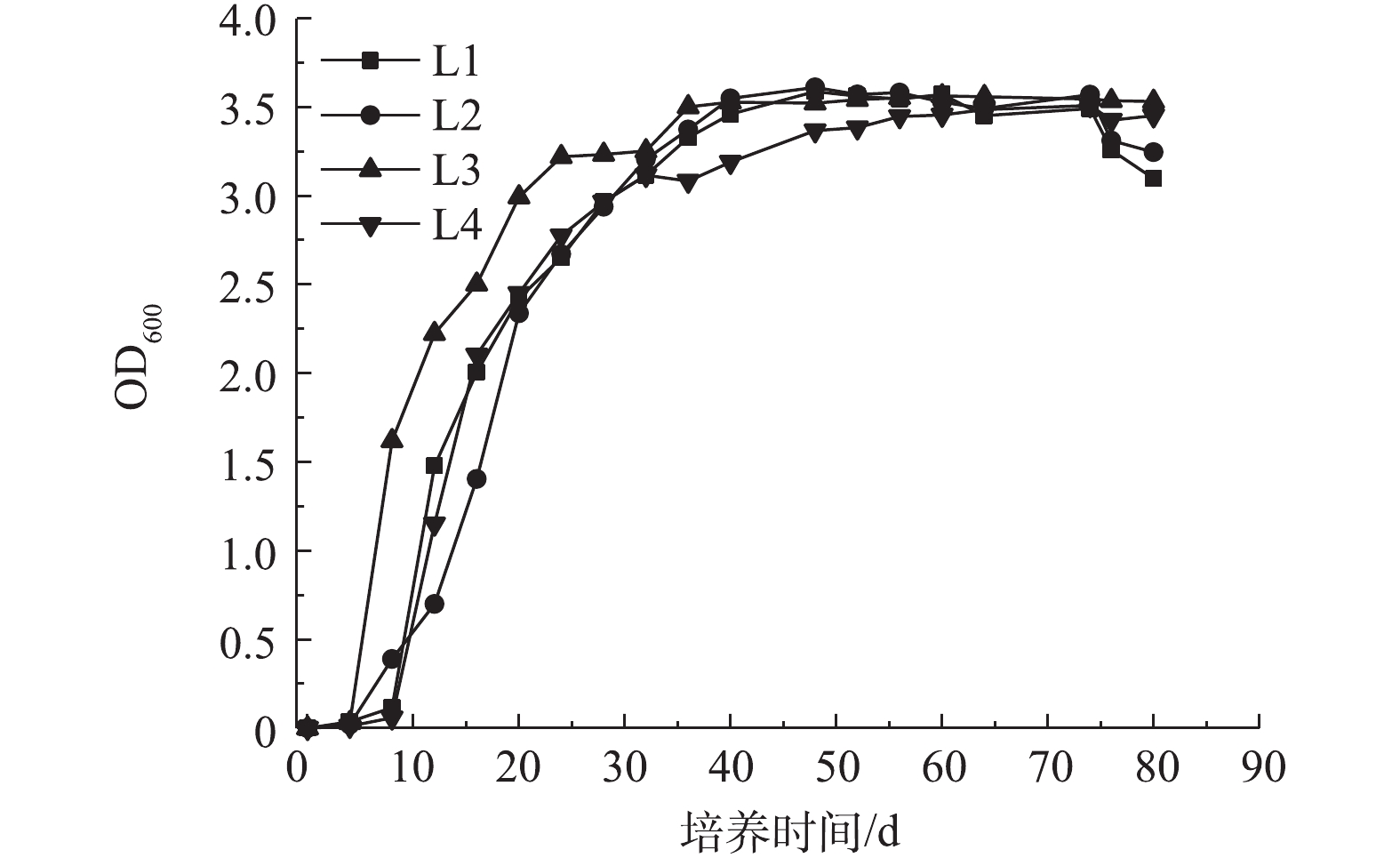
每种菌的生长曲线如图1所示。由图1可知,4种细菌在24 h均进入指数生长阶段,在该阶段,微生物生长速度快,活性较强,因此,后续实验均采用培养24 h的菌液为接种液。
2.4 菌群构建的结果分析
菌群构建的结果如表3所示。由表3可知,编号11的菌群组合在5 d内对柴油的降解率最高,可达到39.6%,这可能是由于不同菌种之间的协同作用,使得混合培养的菌群对于柴油污染物的降解效果要优于单菌,因此,选用该混合菌群作为最佳降解菌群进行后续的研究。
表 3 菌种组合对柴油降解效率的实验结果Table 3. Experimental results of degradation efficiency of diesel oil by species strain combination编号 组合 降解率/% 1 L1+L2 20.9 2 L1+L3 26.2 3 L1+L4 26.1 4 L2+L3 25.8 5 L2+L4 30.9 6 L3+L4 29.3 7 L1+L2+L3 23.5 8 L1+L2+L4 31.5 9 L1+L3+L4 25.0 10 L2+L3+L4 21.1 11 L1+L2+L3+L4 39.6 12 空白 10.9 2.5 柴油降解菌群的混合配比及降解柴油的条件优化
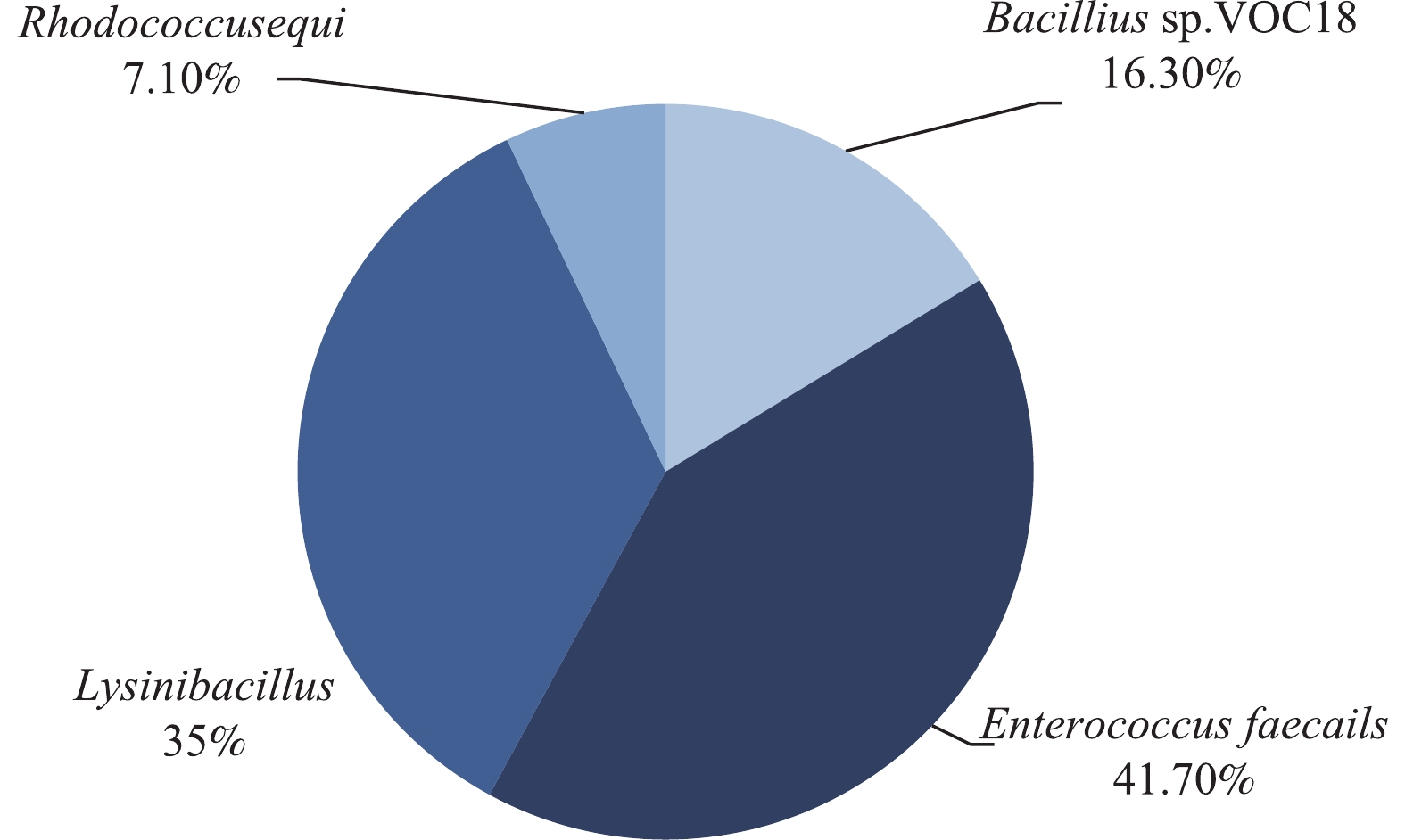
1)采用正交实验法确定柴油降解菌群的最佳菌种混合比例。菌群中各菌种的相对含量对柴油等有机物的降解效率有显著的影响。因此,本研究通过正交实验研究了不同混合比例的菌种对柴油降解效率的影响关系,结果如表4所示。由表4可知,降解效果最好的菌种比例为L1∶L2∶L3∶L4=3∶1∶3∶4,在5 d时,降解柴油效率为52.5%,本研究将此比例的微生物组合命名为OCDL-3134。由于该菌群中的每种细菌都具有特定的作用,因此,柴油降解效率明显得到提高[16-17]。然而,将培养了14 d的微生物进行多样性分析结果如图2所示,由图2可以看出,OCDL-3134经过14 d的培养后,菌种之间的比例变为2∶6∶5∶1,这说明在降解过程中,4种菌根据环境的变化发挥着协同作用,并自行调整他们之间的相对丰度,以实现充分利用柴油污染物的目的。此时L2和L3菌种变为优势菌种,说明在培养后期,L2和L3菌种发挥了重要作用,这与其自身的功能是相一致的。同时也表明确定各种微生物的初始接种比例的菌群构建方案具有一定的合理性。有研究[18]表明,一旦长链烷烃耗尽,就会缺乏碳源和能量用于其生长,而由长链烷烃降解形成的短链烃类化合物则被其他菌种继续代谢利用而进一步降解。
表 4 4种柴油降解菌的接种比例和对应的柴油降解效率表Table 4. Inoculation ratio of four diesel oil degrading bacteria and their diesel oil biodegradation efficiency接种比例(L1∶L2∶L3∶L4) 降解率/% 接种比例(L1∶L2∶L3∶L4) 降解率/% 3∶3∶1∶2 16.5 2∶4∶3∶2 14.8 1∶1∶1∶1 39.3 4∶1∶4∶2 18.5 2∶1∶2∶3 15.3 2∶2∶1∶4 15.7 4∶4∶1∶3 21.4 4∶3∶2∶4 19.1 3∶4∶2∶1 15.6 1∶2∶2∶2 25.5 2∶3∶4∶1 13.7 3∶1∶3∶4 52.5 1∶4∶4∶4 22.3 3∶2∶4∶3 13.2 1∶3∶3∶3 13.1 4∶2∶3∶1 11.7 2)初始pH对OCDL-3134菌体生长和柴油降解效率的影响。环境pH可引起细胞膜电荷的变化,从而影响微生物对营养物质的吸收,影响代谢过程中酶的活性,改变营养物质的可给性和有害物质的毒性。本研究探讨了初始pH对OCDL-3134菌体生长和柴油降解效率的影响。由图3(a)可知,当环境pH过高或过低时,会对微生物产生抑制,这可能是由于此条件严重影响了细菌利用柴油的能量和物质代谢进程,从而影响菌体生长,因此会显著降低柴油的生物降解效率。当pH为7.0时,OCDL-3134对柴油降解的效率达到最佳,此时生物量也达到最高,如图3(b)所示。因此,OCDL-3134对柴油的降解效率和生物量的最佳初始pH均为7.0。
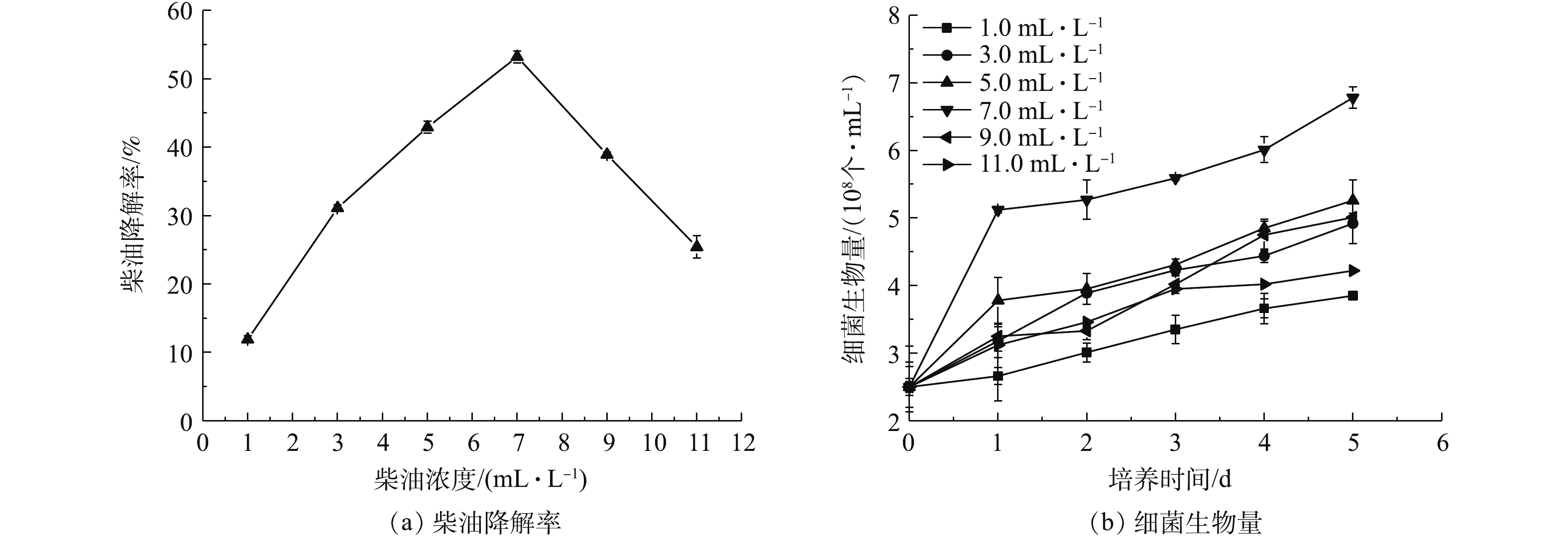
3)柴油浓度对OCDL-3134菌体生长和柴油降解效率的影响。柴油浓度是微生物代谢过程的一个重要因素,对微生物降解性能有一定影响[19]。柴油浓度较低时,碳源不足,细菌生长缓慢,对柴油降解效果不佳。随着柴油浓度的增加,碳源可以满足微生物生长,微生物的降解效果也随之增大。但随着柴油浓度继续增加,柴油降解菌的活性受到抑制,同时培养基表面形成一层油膜,使得溶液内的溶解氧浓度降低,抑制微生物的生长繁殖,从而影响对柴油的降解。如图4(a)所示,随着柴油浓度的增大,微生物的柴油降解率呈先上升后下降的趋势,在柴油浓度为7.0 mL·L−1时,降解效果最好,达到50.0%以上。同时,图4(b)为初始柴油浓度对生物量的影响结果,随着柴油初始浓度的增加,生物量呈先增后降的趋势,结果表明OCDL-3134在初始柴油浓度为7.0 mL·L−1时生物量最佳。
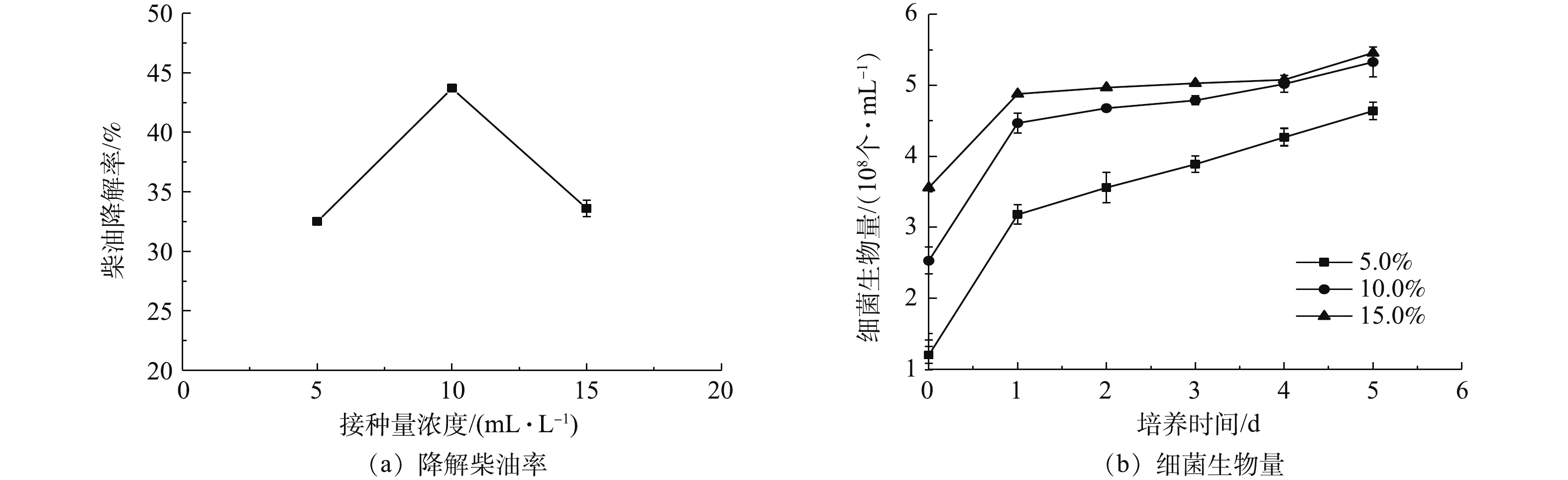
4)接种量对OCDL-3134菌体生长和柴油降解效率的影响。如图5所示,接种量在体积比为10.0%时,微生物对柴油的降解率较高。此后,再增加接种量反而导致新增细胞减少,进而使菌株整体活性下降,降解柴油的后劲不足。15.0%的接种量虽然生物量最多,但由于微生物大量繁殖,造成菌株集中,短时间内消耗了培养基中大量营养成分,不利于新菌株的持续生长,从而影响了菌株对柴油的降解率,因此,根据实验结果最佳接种量为10.0%。
2.6 菌群OCDL-3134降解柴油及十五烷过程中的产物分析
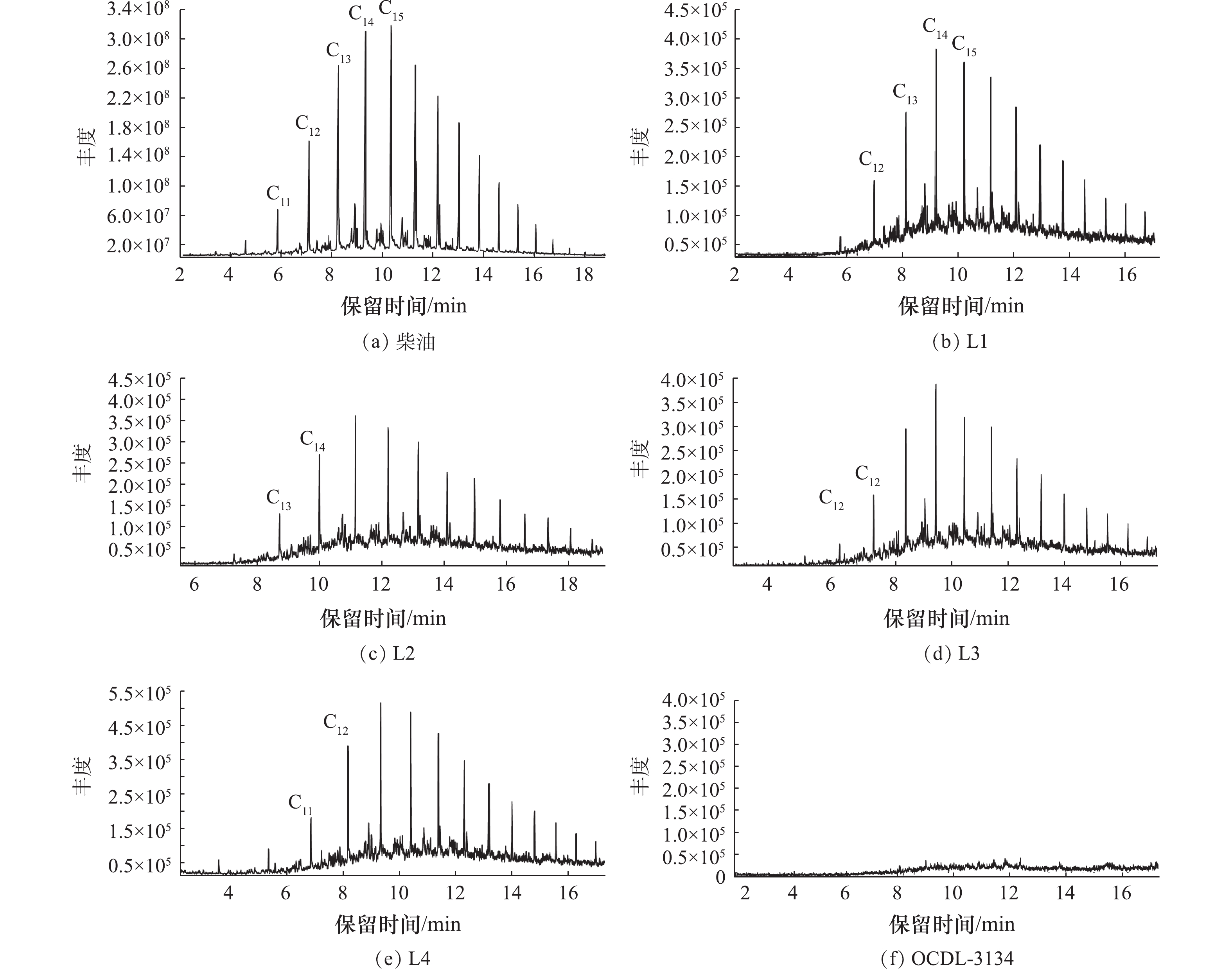
1)菌群OCDL-3134降解柴油过程中的产物分析。各单菌种和OCDL-3134对柴油降解的产物结果如图6所示。图6(a)是原始柴油的组分,由此可知,原始柴油的组分非常复杂,主要包括C13~C24的烷烃。图6(b)是L1培养14 d后的产物,通过与图6(a)对比可发现,L1对短链烷烃降解效果好,推测可能是由于该菌在生长代谢过程中产生了相应的表面活性剂[13],促进了微生物与柴油的接触,从而对短链烷烃具有较好的降解效果;图6(c)是L2培养14 d后的产物,通过与图6(a)对比可发现,整体烃类的含量降低。该菌株具有利用该有机物代谢产酸的能力[20],可以将柴油降解的一些产物分解成小分子的酸,因此,在柴油降解过程中发挥着重要作用;图6(d)是L3培养14 d后的产物。与图6(a)对比可发现,短链以及长链烷烃的含量菌有所减少。该菌能够在好氧条件下代谢简单的碳水化合物,因此,在小分子烃类物质的降解过程具有重要作用,同时在柴油降解的后期可能会具有重要贡献,这一点与图3的结果是一致的;图6(e)是L4培养14 d后产物,与图6(a)对比可发现,整体的烃类含量降低,而环苜蓿烯的含量也有明显的下降。有研究[20]表明,该菌株的主要特性是能够有效降解芳香烃,因此,对于柴油中芳香烃的降解具有重要作用。
综上所述,L1、L2、L3、L4对柴油污染物中的有机烃类物质具有一定的降解效果,并且不同的菌种对不同链长的烃类物质降解效果也有显著差异。然而,L1、L2、L3、L4却不能将柴油完全降解。而L1、L2、L3、L4混合形成的菌群OCDL-3134对柴油降解效果显著,如图6(f)所示,相同时间内,柴油几乎被完全降解,转化成无毒无害的酸类小分子、二氧化碳和水。这表明混合菌对柴油的降解效率显著优于单菌株。
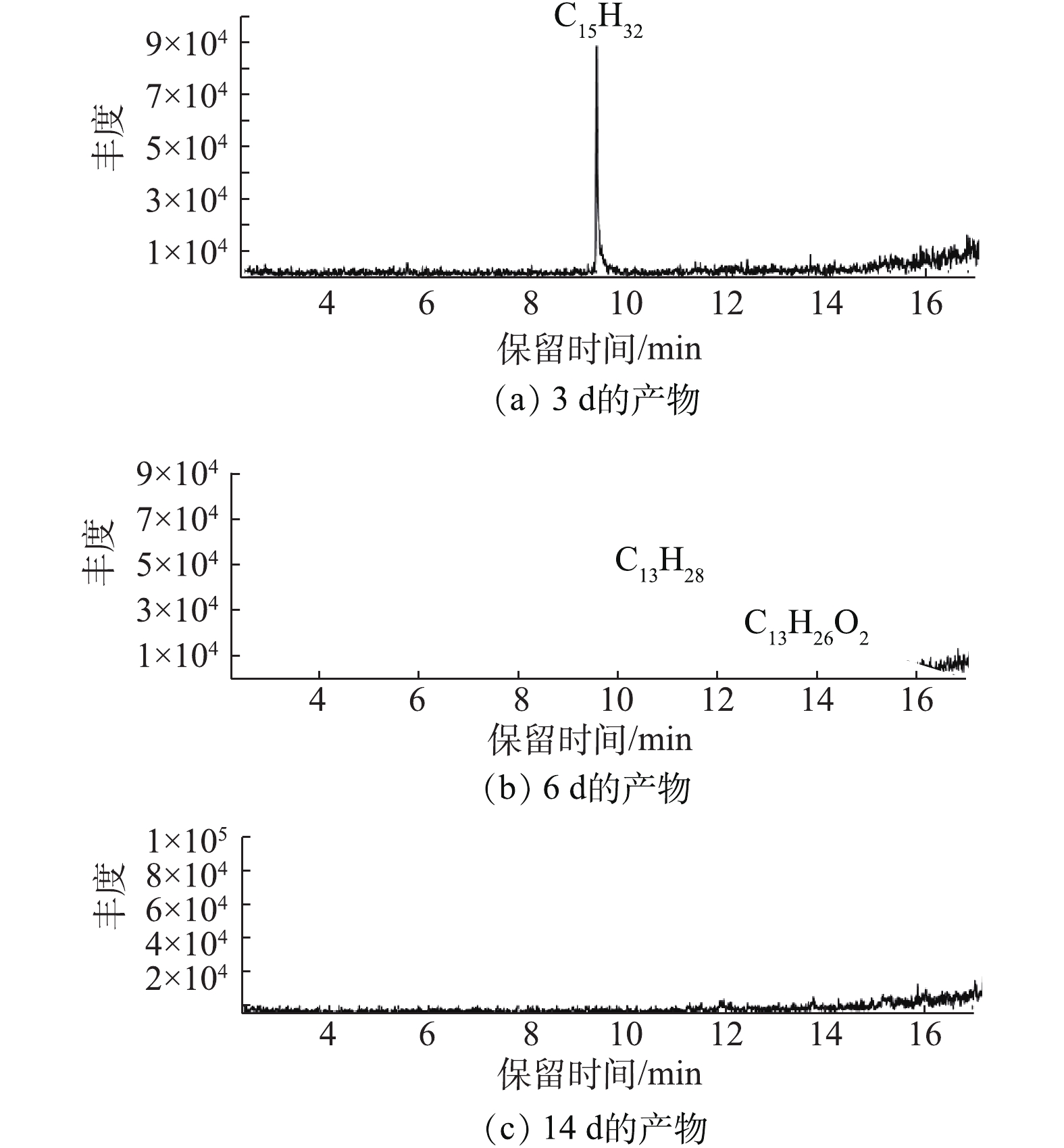
2)菌群OCDL-3134降解十五烷过程中的产物分析。使用GC-MS检测了以柴油作为碳源的微生物降解的产物,实验结果表明,混合菌群对柴油的降解效果显著优于单菌种的效果。对此已有大量研究[21]证明了微生物对柴油等有机污染物的降解作用,但是生物降解长链烷烃的机理研究报道并不多见,而且对其降解途径也缺乏了解。由于柴油属于混合物,且主要的烷烃为十五烷和十六烷,因此,本研究选择十五烷作为研究对象,初步探讨微生物降解十五烷的机理,如图7所示。由图7(a)可知,第3天样品中的主要成分是十五烷,这表明在前3 d菌种要先适应新环境,降解效率低。而到第6天,OCDL-3134中的各菌种在协同作用下将十五烷降解为C13H28、C13H26O2等化合物,如图7(b)所示。据报道[15],微生物对直链烷烃最常见的降解途径为烷烃末端氧化,微生物攻击直链烷烃的末端甲基,由加氧酶、脱氢酶、水化酶等混合功能氧化酶催化,生成伯醇,再进一步氧化为醛和脂肪酸,脂肪酸接着通过氧化进一步代谢,被彻底氧化成二氧化碳和水。如图7(c)所示,在第15天,十五烷以及产物被OCDL-3134彻底降解为水和二氧化碳等小分子物质。
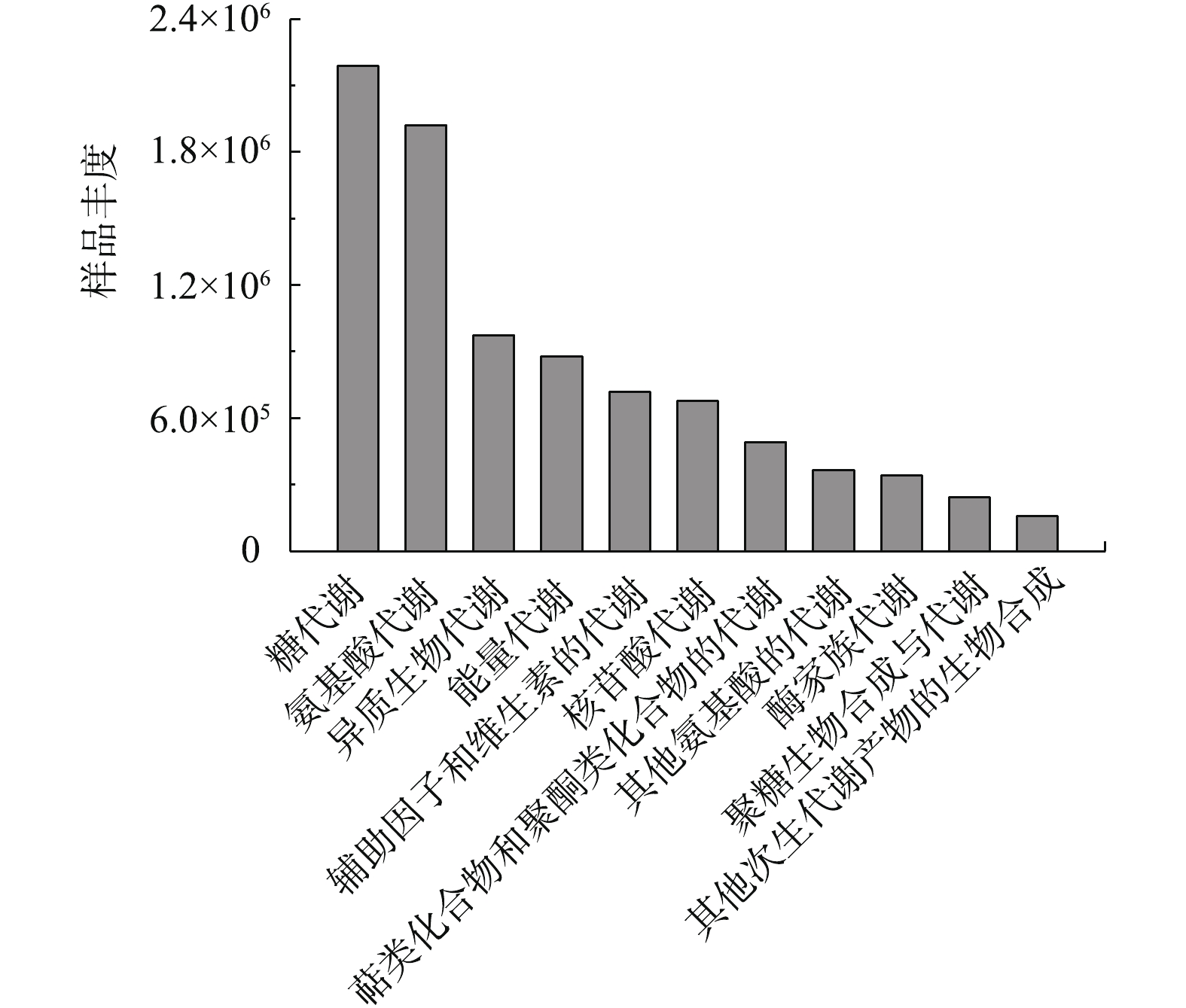
3) OCDL-3134降解柴油过程中细菌代谢功能的预测。图8是从KEGG数据库中获得的代谢丰度图,碳水化合物代谢的丰度最好,表明微生物一开始是对柴油的代谢,而氨基酸的代谢功能丰度是其次的,可能发挥的作用是对中间代谢产物脱氨基,从而进一步代谢成醛、酮、酸等小分子物质,因此,细菌首先利用柴油,并将柴油分解成中间代谢产物,然后经过代谢途径分解成酸类小分子物质。其他的代谢途径如能量代谢、辅因子和维生素的代谢、核酸代谢等也参与进来,最终一起合作完成降解柴油的任务。而且可以发现,4种菌种的异质降解和代谢丰度较好,这一代谢丰度说明微生物具有增强降解柴油的能力,这与TAO等[13]的研究结果相一致,但由于是混合菌,故不能判断是哪一种菌种产生的作用。代谢功能预测进一步证明了混合菌在柴油完全降解方面优于单种菌种。
3. 结论
1)通过排列组合的方式将筛选出的微生物菌种进行组合,得出高效柴油降解菌群OCDL-3134,通过正交实验得出它们之间接种量最优比例为3∶1∶3∶4,同时对混合菌柴油降解性能进行优化,实验测得微生物降解柴油的最优条件为pH=7.0,初始柴油的浓度为7.0 mL·L−1,初始接种量为10.0%,在此优化条件下,测得第14天柴油的最佳降解率为89.0%,这说明所筛选的混合菌种具有较高的应用价值。
2)通过GC-MS检测和微生物多样性功能预测分析证明了微生物对柴油以及十五烷的协同作用高于单菌株的降解效果,4种菌种之间存在协同作用,能够将长链烷烃降解为短链烷烃和小分子物质,并获得自身生长与代谢的能源和碳源。KEGG数据库中获得的代谢丰度图也进一步证明了混合菌在柴油完全降解方面优单种菌种。
-
表 1 Fe/NC-900的元素组成与比重
Table 1. Element composition and specific gravity of Fe/NC-900
元素Element 质量百分比/%Weight percentage 原子百分比/%Atomic percentage C 35.31 56.89 O 14.43 17.45 N 7.97 11.01 Fe 42.29 14.65 *结果来自EDS mapping. 表 2 Fe/NC在不同温度热解产物BET数据
Table 2. BET data of Fe/NC pyrolysis products at different temperatures
催化剂Catalyzer 比表面积/(m2·g−1)Specific surface area 孔体积/(cm2·g−1)Pore volume 孔径尺寸/nm Aperture size Fe/NC-700 195.33 0.27 5.60 Fe/NC-800 256.36 0.18 2.91 Fe/NC-900 429.54 0.41 3.80 -
[1] ZHENG L S, LIN X Q, LIU Y F, et al. Synergistically enhanced oxygen reduction reaction and oxytetracycline mineralization by FeCoO/GO modified cathode in microbial fuel cell[J]. Science of the Total Environment, 2022, 808: 151873. doi: 10.1016/j.scitotenv.2021.151873 [2] ZHANG S, ZHAO S R, HUANG S J, et al. Photocatalytic degradation of oxytetracycline under visible light by nanohybrids of CoFe alloy nanoparticles and nitrogen-/ sulfur-codoped mesoporous carbon[J]. Chemical Engineering Journal, 2021, 420: 130516. doi: 10.1016/j.cej.2021.130516 [3] HE Q L, XIE Z Y, FU Z D, et al. Interaction and removal of oxytetracycline with aerobic granular sludge[J]. Bioresource Technology, 2021, 320: 124358. doi: 10.1016/j.biortech.2020.124358 [4] CHEN J H, YU X L, LI C, et al. Removal of tetracycline via the synergistic effect of biochar adsorption and enhanced activation of persulfate[J]. Chemical Engineering Journal, 2020, 382: 122916. doi: 10.1016/j.cej.2019.122916 [5] LI H Q, HU J T, YAO L F, et al. Ultrahigh adsorbability towards different antibiotic residues on fore-modified self-functionalized biochar: Competitive adsorption and mechanism studies[J]. Journal of Hazardous Materials, 2020, 390: 122127. doi: 10.1016/j.jhazmat.2020.122127 [6] 丛鑫, 王宇, 李瑶, 等. 生物炭及氧化石墨烯/生物炭复合材料对水中抗生素吸附性能研究[J]. 生态环境学报, 2022, 31(2): 326-334. CONG X, WANG Y, LI Y, et al. Adsorption characteristics of biochars and graphene oxide/biochar composites for antibiotics from aqueous solution[J]. Ecology and Environmental Sciences, 2022, 31(2): 326-334 (in Chinese).
[7] QIU G L, CHEN H, SRINIVASA RAGHAVAN D S, et al. Removal behaviors of antibiotics in a hybrid microfiltration-forward osmotic membrane bioreactor for real municipal wastewater treatment[J]. Chemical Engineering Journal, 2021, 417: 129146. doi: 10.1016/j.cej.2021.129146 [8] YIN F B, LIN S Y, ZHOU X Q, et al. Fate of antibiotics during membrane separation followed by physical-chemical treatment processes[J]. Science of the Total Environment, 2021, 759: 143520. doi: 10.1016/j.scitotenv.2020.143520 [9] A D, CHEN C X, ZOU M Y, et al. Removal efficiency, kinetic, and behavior of antibiotics from sewage treatment plant effluent in a hybrid constructed wetland and a layered biological filter[J]. Journal of Environmental Management, 2021, 288: 112435. doi: 10.1016/j.jenvman.2021.112435 [10] 崔迪, 邓红娜, 庞长泷, 等. 生物法去除水环境中磺胺甲恶唑的研究进展[J]. 中国给水排水, 2019, 35(24): 32-38. CUI D, DENG H N, PANG C L, et al. Progress in the study of the removal of sulfamethoxazole by biological methods in water environment[J]. China Water & Wastewater, 2019, 35(24): 32-38 (in Chinese).
[11] TANG L, LIU Y N, WANG J J, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism[J]. Applied Catalysis B: Environmental, 2018, 231: 1-10. doi: 10.1016/j.apcatb.2018.02.059 [12] 齐亚兵. 活化过硫酸盐高级氧化法降解抗生素的研究进展[J]. 化工进展, 2022, 41(12): 6627-6643. QI Y B. Research progress on degradation of antibiotics by activated persulfate oxidation[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6627-6643 (in Chinese).
[13] CHEN N, LEE D, KANG H, et al. Catalytic persulfate activation for oxidation of organic pollutants: A critical review on mechanisms and controversies[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107654. doi: 10.1016/j.jece.2022.107654 [14] OYEKUNLE D T, GENDY E A, IFTHIKAR J, et al. Heterogeneous activation of persulfate by metal and non-metal catalyst for the degradation of sulfamethoxazole: A review[J]. Chemical Engineering Journal, 2022, 437: 135277. doi: 10.1016/j.cej.2022.135277 [15] LEE J, von GUNTEN U, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks[J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. [16] YANG J L, ZHU M S, DIONYSIOU D D. What is the role of light in persulfate-based advanced oxidation for water treatment?[J]. Water Research, 2021, 189: 116627. doi: 10.1016/j.watres.2020.116627 [17] WANG J L, WANG S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. doi: 10.1016/j.cej.2017.11.059 [18] 李小娟, 廖凤珍, 叶兰妹, 等. 金属有机骨架及其衍生材料活化过硫酸盐在水处理中的应用进展[J]. 化工进展, 2019, 38(10): 4712-4721. LI X J, LIAO F Z, YE L M, et al. Progress in the applications of metal-organic frameworks and derivatives activate persulfate in water treatment[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4712-4721 (in Chinese).
[19] 刘路明, 高志敏, 邓兆雄, 等. 过硫酸盐的活化及其在氧化降解水中抗生素的机理和应用[J]. 环境化学, 2022, 41(5): 1702-1717. doi: 10.7524/j.issn.0254-6108.2021010601 LIU L M, GAO Z M, DENG Z X, et al. Activation of persulfate and its mechanism and application in oxidative degradation of antibiotics in water[J]. Environmental Chemistry, 2022, 41(5): 1702-1717 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021010601
[20] CHEN F, WU X L, YANG L, et al. Efficient degradation and mineralization of antibiotics via heterogeneous activation of peroxymonosulfate by using graphene supported single-atom Cu catalyst[J]. Chemical Engineering Journal, 2020, 394: 124904. doi: 10.1016/j.cej.2020.124904 [21] ZHENG X X, NIU X J, ZHANG D Q, et al. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review[J]. Chemical Engineering Journal, 2022, 429: 132323. doi: 10.1016/j.cej.2021.132323 [22] WANG Z B, REN D J, HUANG Y W, et al. Degradation mechanism and pathway of 2, 4-dichlorophenol via heterogeneous activation of persulfate by using[email protected]nanocatalyst[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 654: 130011. doi: 10.1016/j.colsurfa.2022.130011 [23] ZHANG Y F, WEI J, XING L Y, et al. Superoxide radical mediated persulfate activation by nitrogen doped bimetallic MOF (FeCo/N-MOF) for efficient tetracycline degradation[J]. Separation and Purification Technology, 2022, 282: 120124. doi: 10.1016/j.seppur.2021.120124 [24] 何宗文. 钴掺杂铁基骨架材料MIL-101(Fe)@Co活化过硫酸盐降解左氧氟沙星[J]. 邢台职业技术学院学报, 2022, 39((3): ): 101-104. HE Z W. Oxidation of levofloxacin by activated persulfate based on Co-doped iron-based framework material of MIL-101(Fe)@Co[J]. Journal of Xingtai Polytechnic College, 2022, 39((3): ): 101-104 (in Chinese).
[25] WANG J S, YI X H, XU X T, et al. Eliminating tetracycline antibiotics matrix via photoactivated sulfate radical-based advanced oxidation process over the immobilized MIL-88A: Batch and continuous experiments[J]. Chemical Engineering Journal, 2022, 431: 133213. doi: 10.1016/j.cej.2021.133213 [26] WANG D K, WANG M T, LI Z H. Fe-based metal–organic frameworks for highly selective photocatalytic benzene hydroxylation to phenol[J]. ACS Catalysis, 2015, 5(11): 6852-6857. doi: 10.1021/acscatal.5b01949 [27] LI X H, GUO W L, LIU Z H, et al. Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation[J]. Applied Surface Science, 2016, 369: 130-136. doi: 10.1016/j.apsusc.2016.02.037 [28] 雷娟. Co-MOF为前驱体制备的钴基金属氧化物及其甲苯催化氧化性能研究[D]. 太原: 太原理工大学, 2021: 15-18. LEI J. Study on the catalytic oxidation of Co-based metal oxides derived from Co-MOF for toluene[D]. Taiyuan: Taiyuan University of Technology, 2021: 15-18. (in Chinese)
[29] WANG X, YUN Y J, SUN W, et al. A high-performance fluorescence immunoassay based on pyrophosphate-induced MOFs NH2-MIL-88B(Fe) hydrolysis for chloramphenicol detection[J]. Sensors and Actuators B: Chemical, 2022, 353: 131143. doi: 10.1016/j.snb.2021.131143 [30] CHAIKITTISILP W, HU M, WANG H J, et al. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes[J]. Chemical Communications, 2012, 48(58): 7259-7261. doi: 10.1039/c2cc33433j [31] SU Q, LI J, YUAN H Y, et al. Visible-light-driven photocatalytic degradation of ofloxacin by g-C3N4/NH2-MIL-88B(Fe) heterostructure: Mechanisms, DFT calculation, degradation pathway and toxicity evolution[J]. Chemical Engineering Journal, 2022, 427: 131594. doi: 10.1016/j.cej.2021.131594 [32] YANG C, ZHU Y M, CHEN J Q, et al. Graphene/[email protected]carbon hybrid derived from spent MOF adsorbent as efficient persulfate activator for degradation of tetracycline hydrochloride[J]. Chemical Engineering Journal, 2022, 431: 133443. doi: 10.1016/j.cej.2021.133443 [33] YANG L J, LI H, YU Y, et al. Assembled 3D MOF on 2D nanosheets for self-boosting catalytic synthesis of N-doped carbon nanotube encapsulated metallic Co electrocatalysts for overall water splitting[J]. Applied Catalysis B: Environmental, 2020, 271: 118939. doi: 10.1016/j.apcatb.2020.118939 [34] PENG X M, WU J Q, ZHAO Z L, et al. High efficiency degradation of tetracycline by peroxymonosulfate activated with Fe/NC catalysts: Performance, intermediates, stability and mechanism[J]. Environmental Research, 2022, 205: 112538. doi: 10.1016/j.envres.2021.112538 [35] FENG Y, LIAO C Z, KONG L J, et al. Facile synthesis of highly reactive and stable Fe-doped g-C3N4 composites for peroxymonosulfate activation: A novel nonradical oxidation process[J]. Journal of Hazardous Materials, 2018, 354: 63-71. doi: 10.1016/j.jhazmat.2018.04.056 [36] SHEN B, LIU Y G, LIU S B, et al. Catalytic degradation of sulfamethoxazole by persulfate activated with magnetic graphitized biochar: Multiple mechanisms and variables effects[J]. Process Safety and Environmental Protection, 2020, 144: 143-157. doi: 10.1016/j.psep.2020.06.041 [37] WANG L, WANG J Y, HE C, et al. Development of rare earth element doped magnetic biochars with enhanced phosphate adsorption performance[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 561: 236-243. doi: 10.1016/j.colsurfa.2018.10.082 [38] XU H D, ZHANG Y C, LI J J, et al. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols[J]. Environmental Pollution, 2020, 257: 113610. doi: 10.1016/j.envpol.2019.113610 [39] LUO J Y, YI Y Q, YING G G, et al. Activation of persulfate for highly efficient degradation of metronidazole using Fe(II)-rich potassium doped magnetic biochar[J]. The Science of the Total Environment, 2022, 819: 152089. doi: 10.1016/j.scitotenv.2021.152089 -






 DownLoad:
DownLoad: