-
大环内酯类抗生素(MLs)是一类分子结构中含有12—16碳内酯环的抗菌药物的总称,广泛用于人类疾病治疗和畜牧养殖[1]. 2009年MLs在兽用抗生素中全球销量最高,2010年MLs位居全球抗生素消费量第三[2]. 2013年中国MLs总消费量为42200 t,占抗生素总消费量的26%[3]. 但由于人体和动物对抗生素的吸收率不高,5%—90%的抗生素以母体或代谢产物形态通过尿液或粪便排出体外,成为一类新的环境污染物. 相对于持久性有机污染物(POPs),MLs具有较短的环境半减期,但因其在环境中不断输入,持续存在,从而表现为“假持久性”[4-8]. MLs特别是红霉素、克拉霉素、阿奇霉素和罗红霉素,经常在全球地表水中被检测到[9]. 抗生素在水环境中不仅会选择性抑杀环境微生物,还会诱导细菌产生抗药性,对生态环境和人类健康构成威胁[10]. 2018年,欧盟将红霉素、克拉霉素、阿奇霉素等MLs作为潜在水污染物纳入地表水污染物监测清单,要求成员国严格监控,评估其对水环境的危害和风险[11].
进入环境中的抗生素会发生一系列的转化行为. 其中,光降解是MLs在环境中的重要消减途径[12-15],而且光解强烈影响此类污染物的生态毒理效应[16-18]. 鉴于MLs是一类普遍存在的新污染物,难以被生物降解,且光降解是决定其环境归趋的重要因素,因此,有必要揭示其环境存在状况、分布特征及光化学转化行为,这对于该类污染物的环境归趋和暴露评价具有重要意义[4,19]. 中国是世界上最大的抗生素生产、消费国,水环境中抗生素的浓度和检出频率高于一些发达国家,环境分布特征可能与其他国家不同[20-21]. 并且,鉴于我国水域的多样性,抗生素类污染物在我国水环境中的分布状况和光化学行为可能呈现复杂、多样的特点. 目前,我国水体中MLs的分布特征及环境光化学行为被广泛关注,相关研究水平持续提升,也出现了一些新的发展趋势. 本文将探讨我国水环境中MLs的存在状况、时空分布差异,总结国内外该类抗生素水环境光化学行为的最新研究进展,其中将重点讨论其光降解动力学、影响因素和反应路径等.
-
大部分抗生素类化合物的蒸汽压较小、亲水性强,因此该类污染物主要存在于水环境中[19]. 目前在中国水环境中已检测到94种抗生素[22]. 通过总结我国与其他国家典型地表水体中MLs的浓度水平(表1),发现在环境水体中常检出的MLs为红霉素、罗红霉素、克拉霉素、螺旋菌素、泰乐菌素等,其在水中和沉积物中的浓度水平分别为ng·L−1级和ng·g−1级. 其中,红霉素和罗红霉素的检出浓度较高,例如在太湖中这两种MLs的最高浓度分别为624.8 ng·L−1和218.3 ng·L−1[23]. 然而,泰乐菌素和克拉霉素检出浓度相对较低,阿奇霉素在湖泊中的残留浓度研究较少.
红霉素已于2002年在中国水产养殖中被禁用,但2012年仍有红霉素使用的相关数据[38]. 在中国的水产品中抗生素的残留水平为0.01—100 ng·g−1湿重(ww),MLs残留平均浓度为7.6 ng·g−1 ww,其中在广东江阳的海陵岛周边养殖场采集的虾类样品中脱水红霉素残留浓度高达15090 ng·g−1[38-39]. 南京固城湖流域蟹塘3个养殖季节,水中脱水红霉素平均浓度为307.27 ng·L−1,夏季高达2450 ng·L−1,这对藻类具有较高的风险,对蟹池中的水生生物也存在潜在危害[40]. 除了水产养殖业,集中畜牧业对抗生素也有很大需求,牲畜粪便和养殖废水是环境中抗生素的重要来源,在我国农场牲畜粪便中检测到MLs的中值浓度为9.6 ng·g−1,动物废水中为13.8 ng·L−1[41]. 抗生素排放后最终进入到水环境,可能污染饮用水水源. 若抗生素不能被净水厂有效去除,进入饮用水管网,将对饮用水安全构成隐患[42]. 李辉等[43]在南京市饮用水源地的7个采样点中共检出5种MLs,其中阿奇霉素的检出浓度最高达65.2 ng·L−1,检出率为64.71%. 另外,厦门莲花水库中MLs浓度也较高,阿奇霉素的浓度最高(232.61 ng·L−1),检出率为75%,其次为罗红霉素,高达72.58 ng·L−1[44]. 同时,由于抗生素的吸附作用,沉积物中也存在残留. 重庆桃溪河表层沉积物中检出的罗红霉素中值浓度为0.4 ng·g−1,脱水红霉素中值为0.1 ng·g−1 [45];在辽河检出的罗红霉素中值浓度为5.51 ng·g−1 (最大值29.6 ng·g−1),红霉素为3.61 ng·g−1 (40.3 ng·g−1)[46];而珠江检出浓度更高,分别为罗红霉素24.7 ng·g−1 (133 ng·g−1)和红霉素24.4 ng·g−1 (385 ng·g−1)[47].
如表1所示,就全球范围看,中国水环境中MLs的残留浓度处于相对较高的水平. MLs在中国安徽宛溪河、长江嘉陵江重庆段、山东莱州湾小清河、北京潮白河等水体中的均值明显高于法国奥杜尔河口、伊比利亚河、美国东南部河流、南非Umgeni河等. 其中,在长江嘉陵江重庆段,水中MLs高达1409 ng·L−1,沉积物中为866.78 ng·g−1;在长江下游检测到沉积物中MLs浓度更高,达到6780 ng·g−1. 中国河流湖泊和沿海水体中的抗生素浓度普遍高于其他国家,且来源复杂[20-21, 48]. 这些研究表明,MLs已成为我国普遍存在的新型有机污染物,其环境存在、分布特征、迁移转化及生态效应引起高度重视.
-
中国地表水环境中MLs的浓度水平存在时空分布差异. 在时间尺度上,不同季节、水期特定水域MLs的分布特征存在差异. Pan等[49]调查了上海浦东新区地表水中的MLs,如脱水红霉素和替米考星,发现MLs在雨季时的浓度相对较低. 廖杰等[44]也发现,厦门莲花水库的MLs在不同水期的浓度变化较大,枯水期的浓度远高于平水期和丰水期. 造成这种差异的原因可能是因为冬季是流感高发季节,MLs的使用量更大,并且冬季枯水期降雨量少,溪流流量较小,水体自净能力较差,导致枯水期的抗生素残留浓度最高;而夏季丰水期强降水导致污染物被稀释[44]. 此外,强烈的阳光和高温导致的快速光解和生物降解也可能是夏季丰水期浓度较低的另一个原因[7]. 除了降雨量、温度、光照强度和微生物活性等因素会影响抗生素的在环境中的季节变化外,农田播种、水产养殖投苗、病虫害防治时抗生素药物的使用方式和时期也与之密切相关[50-51]. 另外,潮汐对抗生素的空间分布和迁移也有很大影响,特别是浓度波动范围较大的抗生素,如红霉素、罗红霉素等[25].
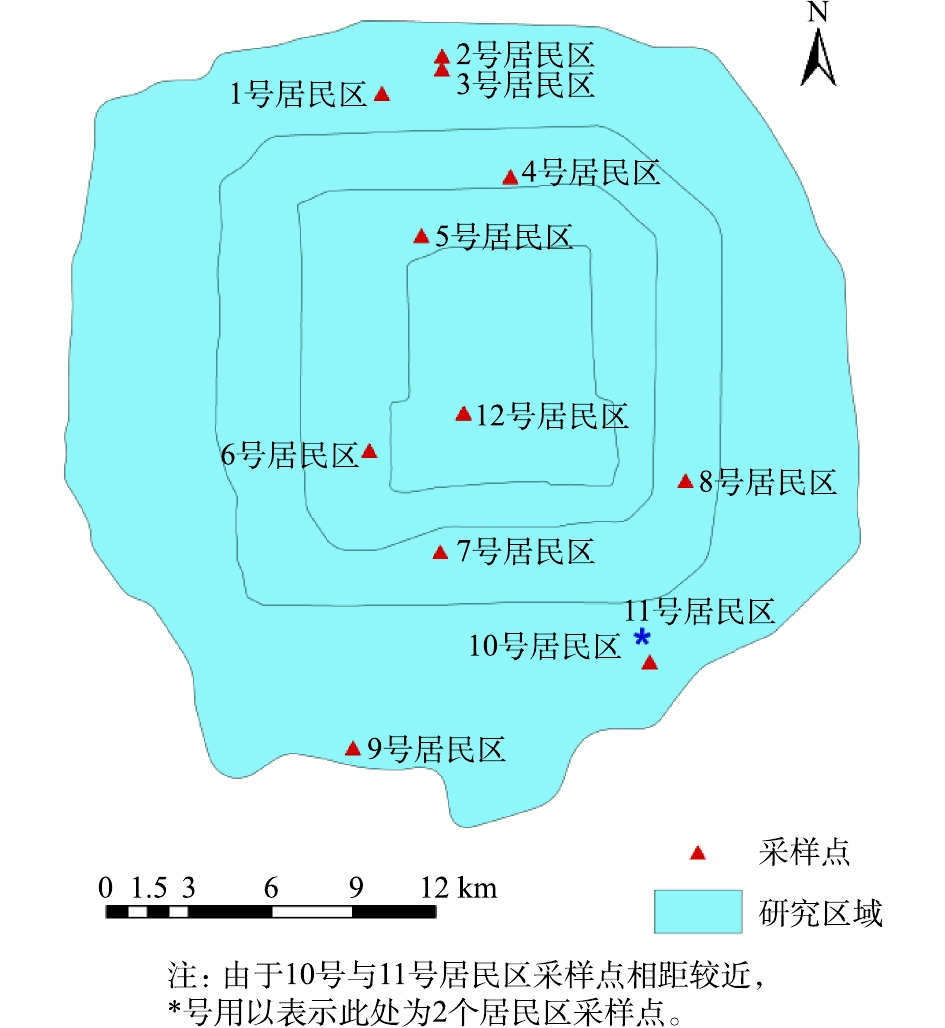
在空间尺度上,MLs在不同的水域和区域的污染分布也存在差异. 通过总结计算我国地表水、近岸海水以及沉积物中MLs平均浓度,得到我国水环境中该类抗生素的空间分布状况,如图1所示. 从图1可以看出,MLs在内陆与沿海地区水体中的污染程度有差异. 位于内陆地区的江汉平原河流、长江嘉陵江重庆段、安徽宛溪河、河北白洋淀、北京潮白河、西安渭河等水体中都存在较高浓度的MLs污染,其中湖北江汉平原地表水中平均浓度达324 ng·L−1. 沿海地区的北部湾、青岛胶州湾、上海浦东河流、上海南陈河等水体中MLs污染较轻. 然而,山东莱州湾污染较高,污染水平为82 ng·L−1,长江下游的沉积物中MLs浓度均值达400 ng·g−1. 对于不同MLs,在不同水域的检出浓度和检出率也存在一定差异,例如河北白洋淀、山东南四湖、湖南洞庭湖等中罗红霉素的平均浓度最高,而在安徽宛溪河、吉林松花江、长江嘉陵江重庆段中平均浓度最高的为红霉素,在厦门莲花水库中阿奇霉素的浓度最高. 以上差异可能与水体稀释能力以及不同地区抗生素的使用种类与用量、环境条件等有关[52]. 从目前可检索到的资料来看,我国西部地区MLs的污染检测数据还比较缺乏(图1).
在同一水域或地区,还发现抗生素在近海海域和河流中的检出频率和浓度均高于在临近湖泊和地下水中的数值[42]. 例如,Zhao等[7]发现,抚仙湖流入河流中MLs的平均浓度是抚仙湖的5.6倍,这可能与抗生素水平迁移期间的稀释和降解有关. Yao等[50]对比了旱、雨季时江汉平原沙湖镇的地表水和附近地下水的抗生素污染状况,发现相较于地表水中阿奇霉素、红霉素等MLs的高浓度(平均浓度324 ng·L−1),地下水中MLs的污染程度较低,平均浓度仅有4.70 ng·L−1,且与地表水的MLs的组成略有不同,推测地下水中积累的抗生素主要来自于河流或小溪,并受土壤和含水层系统的地球化学过程的影响.
-
在表层水体中,普遍存在着抗生素等有机污染物的直接光解、自敏化光解等表观光解,以及间接光降解[72-75]. 总结前人研究,发现MLs也可以发生3种光解反应[76-78].
-
直接光解是指污染物分子吸收光子后,直接发生的光降解行为. 与直接光解不同,自敏化光解是指污染物分子吸收光后,生成的激发三重态并将能力转移给其他物质(如H2O、O2),产生活性氧物种(ROS,如羟基自由基(·OH)、单线态氧(1O2)、超氧阴离子自由基(O2·−)等),生成的ROS再将污染物物氧化降解[79-82]. 由于到达地球表面的太阳光一般是λ > 290 nm,因此只有紫外-可见吸收光谱与光源的发射光谱有重叠,即在290 nm以上有光吸收的MLs才有可能在自然环境中发生直接光解或自敏化光解[83]. 某些MLs可以吸收太阳光,所以在前人研究中, MLs发生了直接光解和自敏化光解,其光解反应遵循准一级反应动力学[15, 84-85]. 通过公式(1)可以得到直接光解的一级速率常数k:
式中,C0和Ct分别为反应0时刻和t时刻的MLs浓度.
基于光降解k的测定,常海莎[84]发现在紫外光和模拟日光照射下,螺旋霉素、罗红霉素、克拉霉素和泰乐菌素这4种MLs的光降解速率有很大差异,但在纯水中均发生了直接光降解和自敏化光降解,并以直接光降解为主. 添加异丙醇、对苯醌、叠氮化钠和山梨酸可以猝灭ROS从而抑制抗生素的光降解(图2),对比各反应速率常数可知,MLs在水中的光降解过程中存在∙OH、1O2和O2·−参与的自敏化光降解. 以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)和2,2,6,6-四甲基-4-哌啶(TEMP)为·OH和1O2的自旋捕获剂,测定MLs在纯水体系下的电子自旋共振波谱以验证产生的ROS[86]. 实验证实在纯水中罗红霉素本身吸收光可产生·OH和1O2,并且产生的1O2更多,但由于1O2与罗红霉素的反应速率常数较小,导致纯水中罗红霉素光降解速率较低[15].
为了衡量光化学反应对光子的利用效率,需要对MLs的量子产率进行测定. 通常将待测化合物(称为底物s)的溶液和露光计置于相同的光照条件下,进行光化学实验,通过公式(2)计算量子产率(Φs):
式中,a为露光计,其量子产率(已知)为Φa;k为光化学反应速率常数;Lλ为光源在波长λ处的光强;ελ为MLs或露光计在波长λ处的摩尔吸光系数. 常用的化学露光计有草酸铁钾、PNA/pyr(对硝基苯甲醛/吡啶)[87].
前人测定并计算了MLs在不同光源照射下的Φs,总结在表2中. 例如,模拟太阳下纯水中的MLs的量子产率呈现螺旋霉素 > 泰乐菌素 > 克拉霉素 > 罗红霉素[84]. Jia等[9]测得克拉霉素的量子产率5.6 × 10−5比其他研究者[84]测得的量子产率6.3 × 10−3小两个数量级,存在的差异可能与实验测得的反应速率常数k或累计光吸收系数(∑Lλελ)有关.
溶液pH值和水成分会影响抗生素的存在形态,从而影响其光吸收特性. Voigt等[88]测得在溶液pH = 3、7、9时,阿奇霉素、红霉素和泰乐菌素在254 nm光照下的量子产率,发现阿奇霉素在pH 3时的量子产率高于在pH 7和pH 9时的量子产率,而红霉素与泰乐菌素则是在pH 7时的量子产率较大. Batchu等[85]测定了在不同UV光源(254 nm和350 nm)照射下,罗红霉素、红霉素在纯水、淡水和海水(pH = 6—9)中的量子产率,发现罗红霉素在淡水中的量子产率最大,即Φ254 =4.9 × 10−2,Φ350 =7.0 × 10−4;而红霉素在海水中的量子产率最大,分别为Φ254 = 4.5 × 10−3和Φ350 =7.0 × 10−4. 这说明pH和水成分对于MLs光解速率快慢有一定影响,且不同MLs的量子产率在不同溶液中的变化规律并不统一.
-
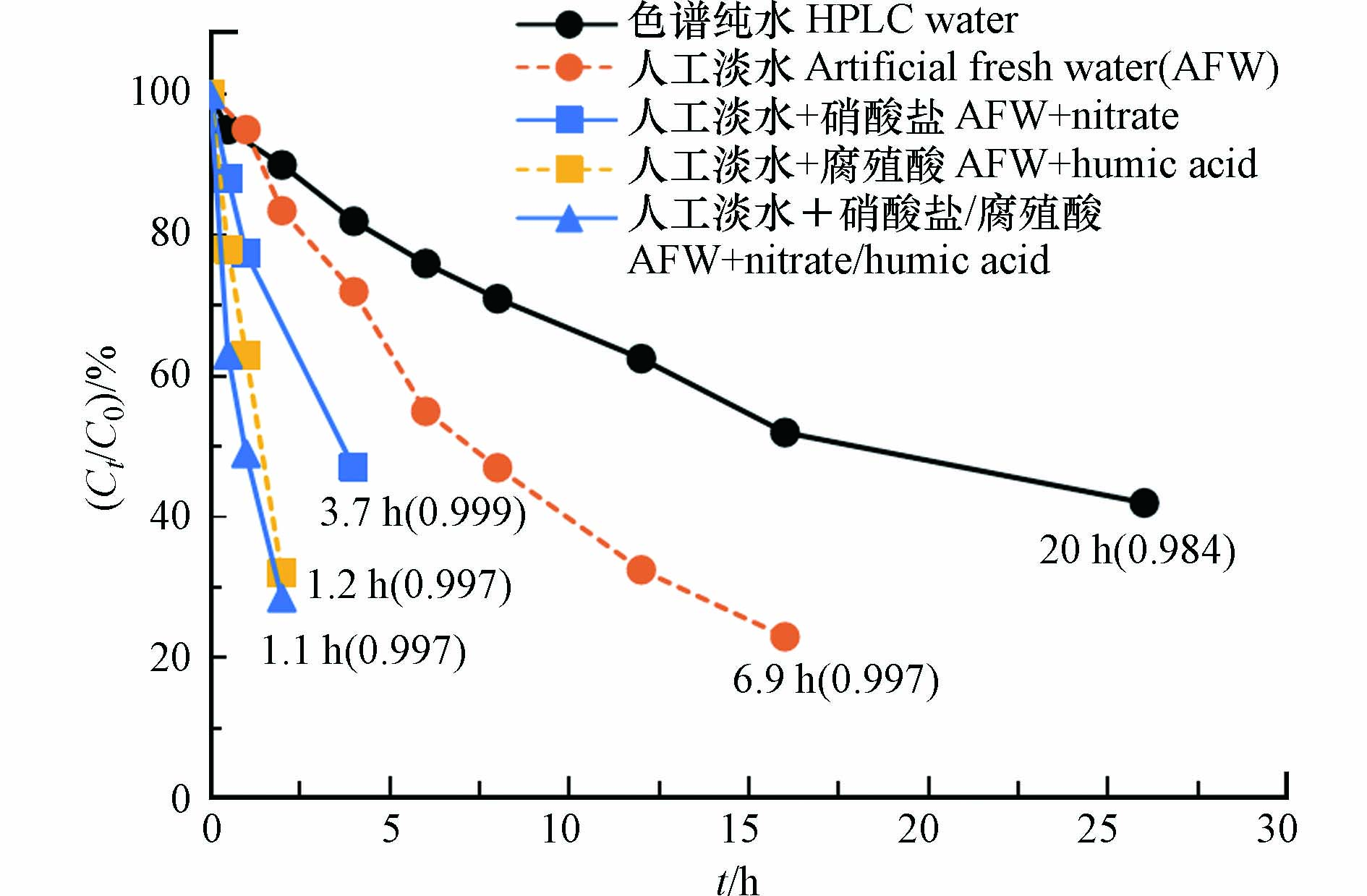
间接光解是指敏化剂吸收光子,然后将能量转移给污染物而引起的分解反应. 前人研究表明,在溶解性有机质(DOM)、硝酸盐等敏化剂存在时,MLs可以发生间接光解,这对于水环境中某些MLs的消减较直接光解更为重要[9, 77, 89-90]. 罗红霉素在淡水和海水的光解速率相对于在纯水中有显著提高,表明天然水中存在重要的间接光解或光敏化过程[85]. 这可能是由于淡水基质中具有高溶解度的有机碳,其作为MLs转化过程中的光敏剂,能吸收较宽范围的波长能量并产生ROS将MLs氧化降解[91]. 海水中存在大量Cl−,Cl−可捕获·OH生成具有强氧化性的Cl·和Cl2·−,从而影响MLs的间接光解[92-93]. Tong等[77]报道了MLs阿奇霉素在模拟太阳光下在色谱纯水、人工淡水、含腐殖酸(HA)/硝酸盐的人工淡水中的光解作用,结果表明阿奇霉素在色谱纯水中降解速率最慢,而在添加了硝酸盐(5 mg·L−1)和HA (0.5 mg·L−1)的人工淡水中降解加速,说明阿奇霉素可被硝酸盐和HA等敏化而发生间接光解(图3).
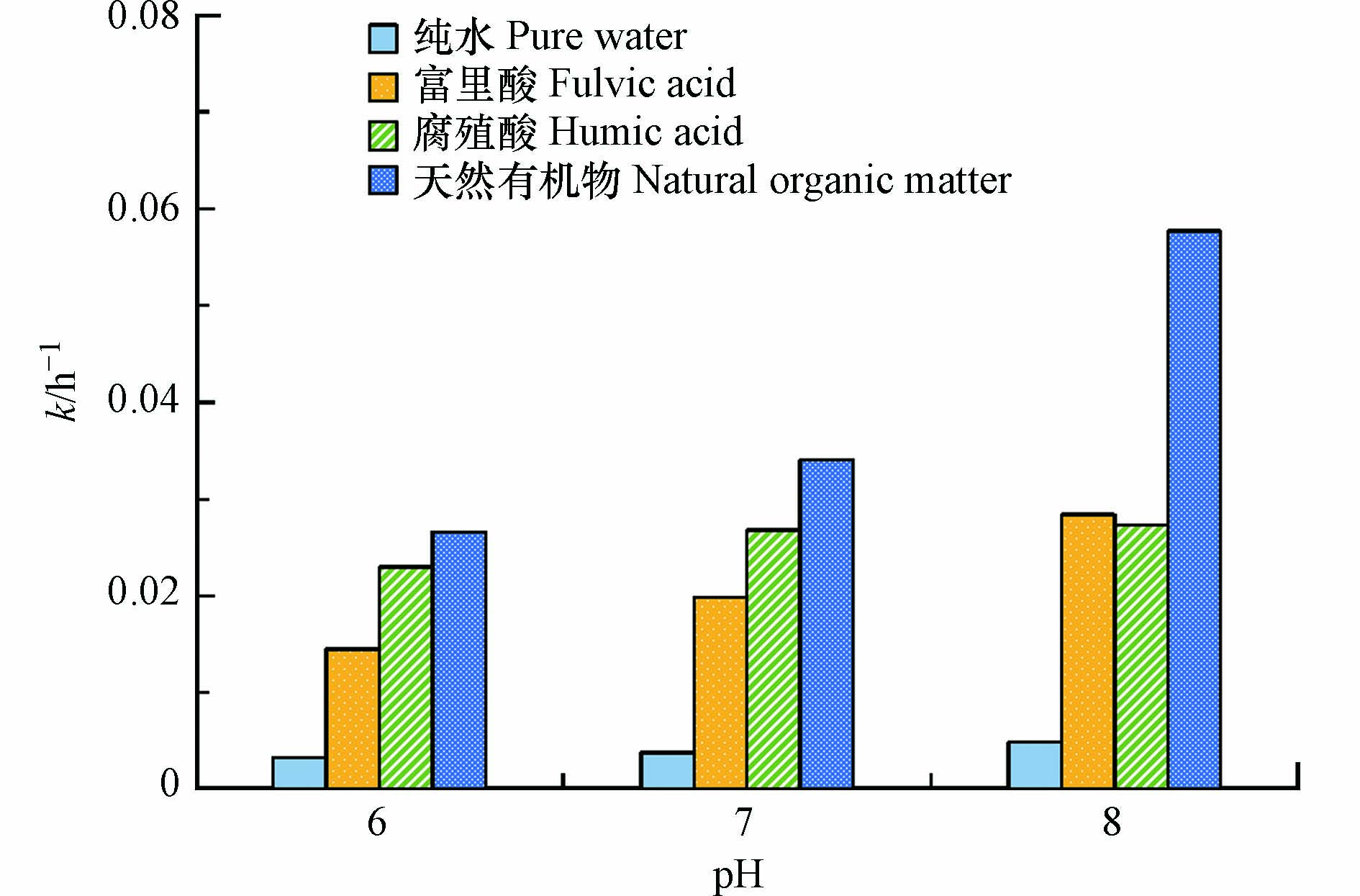
对于MLs的间接光解,一些研究者还对其转化动力学进行了定量研究. Jia等[9]发现MLs在苏瓦尼河天然有机物(SRNOM)溶液中的光降解速率为罗红霉素 ≈ 克拉霉素 > 阿奇霉素 > 红霉素,半减期(t1/2)分别为(7.2 ± 0.2) h、(7.2 ± 0.2) h、(9.4 ± 0.3) h和(11.2 ± 0.4) h. 与纯水中的对照实验相比,SRNOM溶液中的光解速率提高了约2—5倍,表明间接光解在MLs的光转化中起着关键作用,并且MLs与1O2反应的双分子反应速率常数在1.22 × 105 L·mol−1·s−1 (克拉霉素)—2.42 × 105 L·mol−1·s−1 (阿奇霉素)之间,与·OH反应的双分子反应速率常数在 2.18 × 109 L·mol−1·s−1 (阿奇霉素)—4.97 × 109 L·mol−1·s−1 (罗红霉素)之间. 吕宝玲等[15]研究了环境pH条件下(pH为6、7和8),苏瓦尼河腐殖酸(SRHA)、SRNOM和Pony湖富里酸(PLFA)对罗红霉素光降解的影响,在DOM存在下,罗红霉素的光降解反应速率常数均高于纯水中(图4). 并且1O2对罗红霉素光解的贡献率为0.21%—11.33%,·OH对罗红霉素光解的贡献率高达76.76%—98.70%. 根据以上研究可以看出,相较于1O2,·OH对MLs光降解的贡献更大一些.
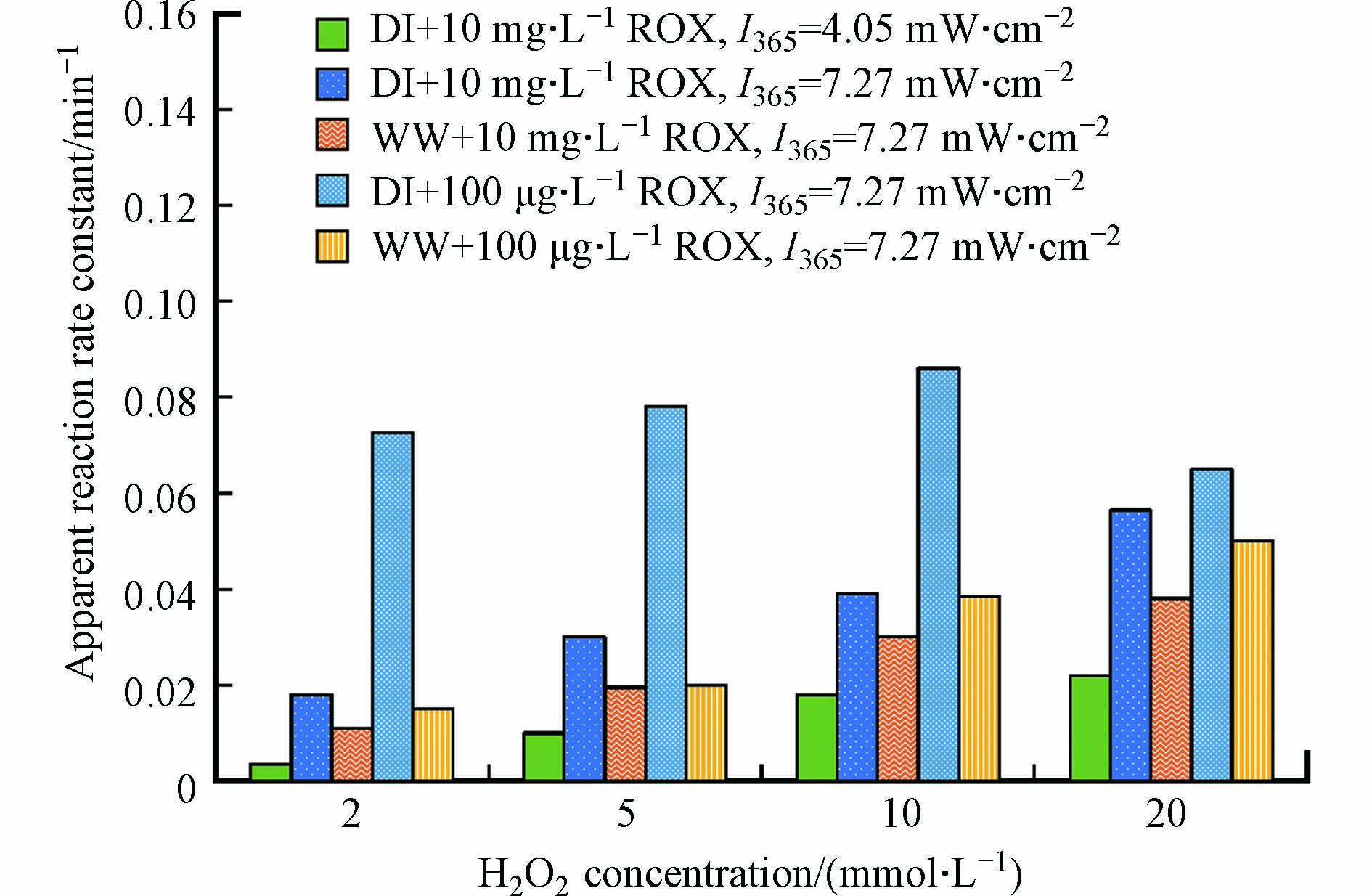
1O2具有选择性,易于和硫化物、烯、共轭二烯、苯酚类化合物发生反应[94],而·OH没有选择性,能与绝大多数抗生素发生反应[72, 95-96]. 前人针对MLs与·OH反应的动力学进行探究. Voigt等[88]发现在UVC的辐照下,泰乐菌素的降解速度远快于阿奇霉素和红霉素,H2O2的加入使泰乐菌素的降解速度加快了2倍,而阿奇霉素和红霉素的降解速度在相同条件下提高了5倍. 同时,作者还研究了H2O2对螺旋霉素在UVC下的光解[97],发现在没有H2O2的情况下,动力学速率常数为(0.67 ± 0.13) min−1,在10 mg·L−1和30 mg·L−1 H2O2的存在下,速率常数分别增加到(1.41 ± 0.4) min−1和(2.60 ± 3.76) min−1;加入10 mg·L−1 H2O2使反应速率增加了1倍,而加入30 mg·L−1 H2O2并没有增加3倍. Li等[3]也发现仅用UV辐照(120 min)不会使罗红霉素浓度显著降低,而添加H2O2后,不同溶液条件下罗红霉素的降解均遵循准一级动力学(图5). 图5中同一溶液条件下罗红霉素的表观速率常数值随H2O2浓度增加先增大后减少. Li等[3]还利用竞争动力学模型估算了罗红霉素(ROX)与·OH反应的双分子反应速率常数kROX-·OH:
式中,[ROX]0和[ROX]t是反应时间0和t时的ROX浓度;[pCBA]0和[pCBA]t是反应时间0和t时对氯苯甲酸的浓度;kpCBA-·OH是pCBA与·OH反应的双分子反应速率常数(已知). 由公式(3)计算得到kROX-·OH为(5.68 ± 0.34) × 109 L·mol−1·s−1,与其他类抗生素的双分子反应速率常数处于同一数量级[4].
以上研究均表明H2O2的加入量对所引起的MLs降解增量并非线性关系,较高的H2O2初始浓度理论上可以产生更多的·OH,但实际并非如此. 这可能是因为H2O2与MLs相互竞争所导致的H2O2光屏蔽效应或过量H2O2对·OH的猝灭作用,即H2O2消耗·OH产生HO2·+,且当H2O2的浓度足够高时会自动分解为O2和H2O促进了H2O2的消耗[98-100].
-
水中溶解性物质如DOM、Cl−、NO3−、HCO3−、Fe(Ⅲ)、DO(溶解氧)等具有光化学活性的物质,以及pH值等理化性质都是影响抗生素光解动力学的因素[83]. 前人较多研究报道了这些水环境因子对MLs光降解动力学的影响,对此我们进行了总结,如表3所示.
天然水环境中广泛存在着NO2−/NO3−、Fe(Ⅲ)、HCO3−、Cl−等溶解性物质,在不同条件下能促进或抑制MLs的光化学转化(表3). 水中NO2−/NO3−的光化学性质不稳定,其光解能产生多种活性中间体,是天然水体中∙OH的重要来源之一[75, 104]. Fe(Ⅲ)在水中能形成水合物,通过阳光照射产生Fe(Ⅱ)和∙OH[105]. 此外,Fe(Ⅲ)还能与污染物形成配合物进而被降解. Vione等[78]采用分光光度滴定法证明了Fe(Ⅲ)分别与克拉霉素和罗红霉素形成了Fe(Ⅲ)-MLs配合物,在MLs溶液中滴加FeCl3后,其吸收峰(λmax = 361 nm)发生红移(3—5 nm). Fe(Ⅲ)的加入一定程度上促进了MLs的光解,作者认为发生的主要反应为Fe(Ⅲ)-MLs的直接光解,Fe(Ⅲ)光致∙OH对MLs氧化作用几乎可以忽略.
具有光活性的溶解性物质也会对MLs光解起到抑制作用[3, 84]. 常海莎[84]考察了分别添加NO3−、NO2−、Fe3+和Fe2+后4种MLs的光化学行为,发现其光解均符合准一级动力学方程,且水中不同浓度的溶解性物质对MLs存在双重作用,即各自表现出促进/抑制. Li等[3]发现在UV/H2O2体系下,NO3−和NO2−、Fe3+、Cu2+、Mg2+均抑制了罗红霉素的降解,抑制作用为Fe3+ > Cu2+ > Mg2+,NO2− > NO3− > HCO3− > Cl−. 造成该现象的原因是,这些离子在水中既可作为光敏剂或生成配合物促进光降解,亦可作为光掩蔽剂或∙OH捕获剂,从而抑制MLs的光解[81, 106].
淡水中常见的DOM有腐殖酸(HA)、天然有机物(NOM)等. 前人研究中,HA也可能对水中抗生素的光解产生促进或抑制作用[15, 75, 82, 107-108]. Li等[90]证实罗红霉素的表观光降解动力学常数随着HA和NOM浓度的增加而增大,在20 mg·L−1 HA和NOM的存在下,罗红霉素的光降解动力学速率常数分别是不含DOM时的4.0倍和3.6倍. 而HA的加入却抑制了泰乐菌素的光解[102],向溶液中分别添加10 mg·L−1和100 mg·L−1 HA,泰乐菌素在6 h后的光解效率从50%依次减小到30%和16%. 这种抑制现象可能也是由于HA产生光掩蔽效应或捕获ROS所造成的.
分子中具有酸碱解离基团的抗生素化合物,pH能显著影响其存在形态和光化学反应活性[72, 102]. MLs具有多个酸碱解离基团,随溶液pH变化可出现阳离子、中性离子、阴离子等价态[85, 109]. 不同离解形态的抗生素分子吸收光谱不同,量子产率也可能有差异,从而导致其光解速率常数变化[110-111]. 对于间接光解和自敏化光解,pH会影响ROS的生成速率以及抗生素与ROS的反应速率[83, 96]. 在DOM的影响下,罗红霉素的光降解反应速率常数表现出随pH升高而增大的趋势,∙OH对罗红霉素光解贡献率随pH的升高而降低,1O2对罗红霉素光解的贡献率随pH升高而升高[15]. Li等[3]也发现在UV/H2O2体系下,当pH值从4升高到9时,罗红霉素的反应速率常数从0.0162 min−1增加到0.0309 min−1. 在pH 8—9时的罗红霉素降解率几乎是pH 4—5时的两倍,这表明碱性条件在某种程度上更有利于其降解. 这种现象可能归因于罗红霉素发生解离.
综上所述,水环境因子(溶解性物质、pH等)对MLs的光化学行为具有一定的影响,同一因子对不同抗生素的抑制/促进作用不同,同一因子在不同浓度、不同光照条件下对同一抗生素光降解的抑制/促进作用也会有所不同. 虽然这些因素对MLs光解的影响趋势没有统一的规律,但可以看出,MLs表现出的光化学反应活性强弱往往是多重因素共同作用下的结果. MLs存在不同解离形态,且真实水体中多因素共存,光化学转化行为将更为复杂,不仅需深入探究单一因素对MLs不同解离形态光解的影响,而且需要进一步研究多种因素的复合影响、作用机制及相对贡献.
-
在抗生素光化学转化过程中,生成的产物可能保留药物活性[112]或增加菌株的敏感性[85],甚至毒性增强,造成更高的生态风险[101]. 因此鉴定光化学反应的中间产物和最终产物,进而推断光化学反应的路径,对于污染物的生态风险性评价具有重要意义[4]. MLs类抗生素可能发生的光降解路径主要有:光氧化、光致异裂、光致水解等[83].
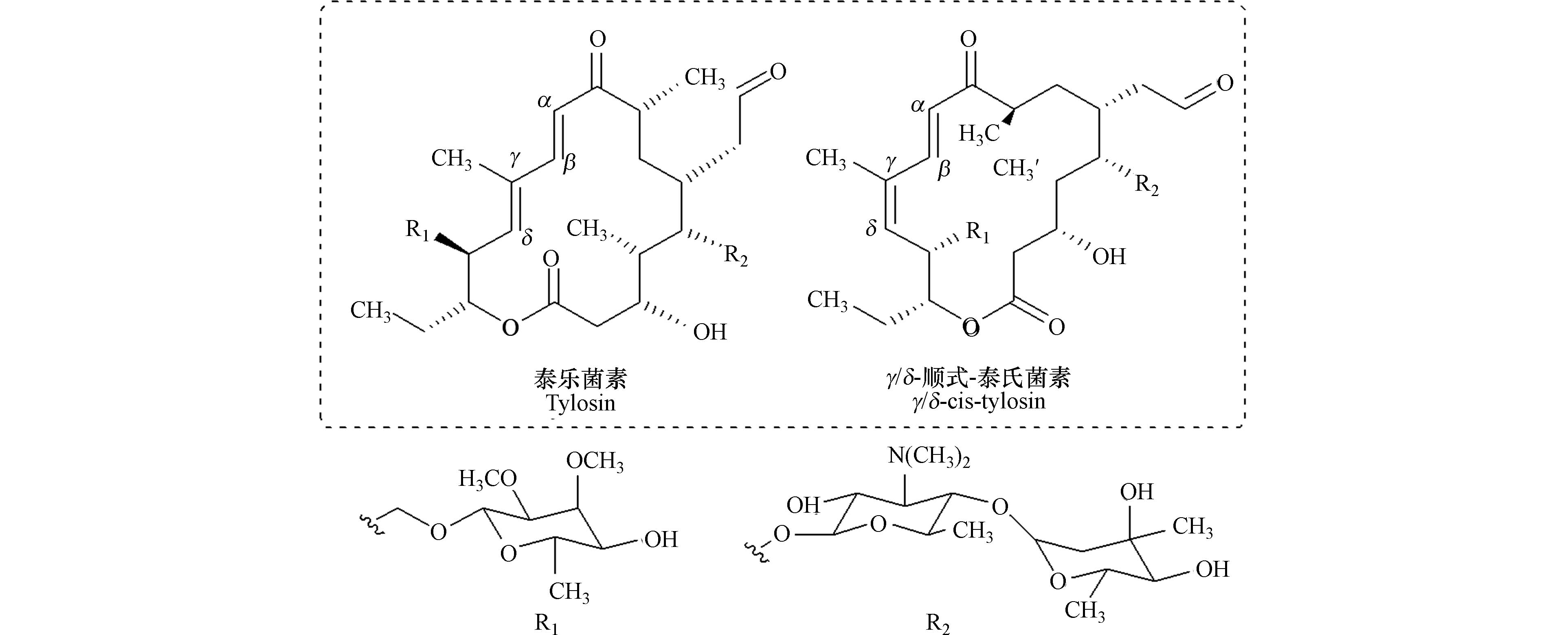
对于直接光解,前人发现对于结构相似的MLs,在一定条件下能够互相转化,如Batchu等[85]对RODW(反渗透去离子水)溶液中罗红霉素的光解产物进行分析,首次发现罗红霉素在254 nm的UV光照下侧链能发生裂解,形成红霉素. 此外,MLs还存在光异构化现象[9, 76]. Werner等[76]通过动力学、质谱和质子核磁共振,证实了在模拟太阳光下,泰乐菌素发生了光异构化. 在其光解产物中没有观察到新的质核比m/z的分子,表明该产物是泰乐菌素的一种异构体,作者认为该异构化是围绕泰乐菌素的内酯环酮二烯发色团末端的γ/δ双键旋转而成(图6). γ/δ-顺式-泰乐菌素异构体对大肠杆菌DH5α生长的抑制活性低于泰乐菌素.
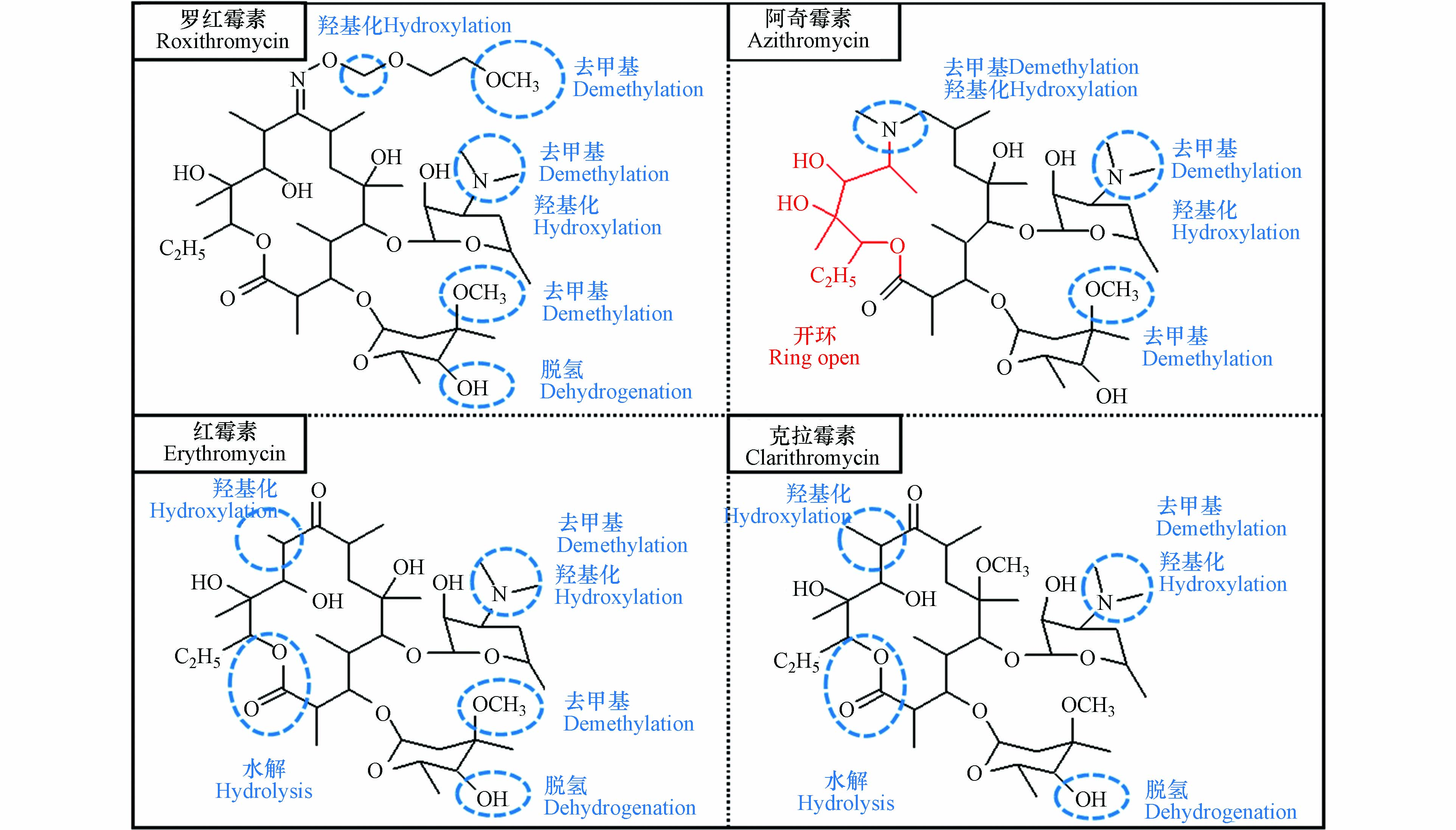
真实的水环境是一个复杂体系,间接光解是MLs在水环境中的一种重要消减途径,前人对MLs光解产物的研究多以DOM存在的体系为主,去甲基是MLs常见的转化路径[3, 9, 77, 90, 101]. Li等[90]采用HPLC-LTQ-Orbitrap XL-MS研究在SRNOM和SRHA存在下罗红霉素的光解产物,该条件下罗红霉素主要被DOM光敏化产生的ROS氧化,其光转化的主要途径为N-去甲基、O-去甲基以及侧链、红霉脱氧糖胺或红霉支糖的裂解(图7). 去甲基位点位于侧链、红霉支糖以及红霉脱氧糖胺的叔胺,这与Li等[3]所观察到的·OH对罗红霉素的攻击位点以及阿奇霉素与·OH反应位点[77]相似.
目前,多数研究并没有根据光解类型(如直接光解、自敏化光解和间接光解)对MLs转化产物加以区分. Gozlan等[101]采用LC-MS鉴定了MLs在不同pH缓冲液、含腐殖酸的pH = 7的缓冲液及自来水和二级废水中的光解产物,发现红霉素、阿奇霉素、克拉霉素和罗红霉素均发生了N-去(二)甲基化和N-氧化,反应位点在红霉脱氧糖胺部分. 此外,即使是同一类抗生素,其结构仍会有一定的差异,因此反应路径存在一些异同. Jia等[9]对典型MLs在DOM水溶液中的光转化路径进行了对比(图8),这4种MLs除了均能发生的去甲基,罗红霉素常见反应还有脱氢和羟基化;阿奇霉素还会发生水合反应;克拉霉素与红霉素能发生羟基化、脱氢、水解;特别的是,阿奇霉素、克拉霉素和红霉素在内酯环上存在反应位点,阿奇霉素的内酯环能开环裂解. 前人的一些研究中也观察到了内酯环的开环裂解:紫外辐射引起双键的光激发导致螺旋霉素内酯环的裂解[97],泰乐菌素的内酯环通过紫外吸收和随后的光反应而被破坏,Fe(Ⅲ)-ROX配合物直接光解导致了内酯环的裂解[78].
由此可见,MLs光转化过程中主要的反应为去甲基、羟基化以及红霉脱氧糖胺和红霉支糖的断裂,且N-去甲基形成的产物最为丰富. 在水环境中,MLs在结构上的变化特别是开环裂解,分别对其原本的抑菌活性和毒性有着怎样的影响,仍需进一步探究.
-
目前我国抗生素滥用问题依旧严峻,尤其是当今全球新冠疫情防控局势紧张,大量抗生素进入环境并持续存在,成为典型的新污染物. 中国重视新污染物治理,MLs是检出率较高的抗生素类新污染物. 本文评述了我国环境水体中MLs的存在状况与时空分布差异,重点讨论了MLs的光解动力学、水环境因子对光解的影响,以及光降解路径与机理等. 我国对该类新污染物的相关研究方兴未艾,一些重点问题值得关注:
(1) MLs的污染数据和分布特征有待丰富并深入探究. 我国关于MLs的环境污染数据不够丰富,特别是在内陆地区,需要开展进一步的监测,以满足优先控制新污染物“筛检”的需求. 进一步,可以借助主成分-多元线性回归(PCA-MLA)分析、Pearson相关性分析等手段了解抗生素来源及其残留的影响因素,深入探究其在不同介质,不同季节、地点的分布差异,以及水文、人类活动及水质指标对其分布的影响.
(2) MLs的光化学转化动力学和机制需要深入研究. MLs分子量大、结构复杂、可解离,天然水环境复杂多变,光化学行为很大程度上受pH、溶解性物质以及光源、温度等环境因子的影响. 需要系统研究MLs不同解离形态在水环境乃至结冰环境中的“复合”光化学转化行为,如表观光解和间接光解、产物和转化路径的规律;深入探究多环境因子对MLs光转化的复合效应,将实验室模拟和野外实验体系建立联系并进行优化,提高实验值推算至实际环境的准确性,以构建实际水环境适用的抗生素光化学转化动力学模型. 激光闪光光解技术(LFP)、电子顺磁共振(EPR)和化学测定法等可检测ROS的种类和丰度,而产物鉴定可借助LC/MS、核磁共振波谱仪(NMR)等设备,并利用维恩图、层次聚类等辅助产物解析.
(3) MLs的风险及光致毒性需要进一步评估. 通过不同的毒性受试生物体(如费氏弧菌、大型蚤、藻类和斑马鱼)以及抑菌实验菌体(如枯草芽孢杆菌、荧光假单胞菌)获得的风险评估可能存在不同的结论,需要多种营养级的生物、更丰富的菌株为对象进行实验,探索毒性作用机制和演变规律. 并且,应关注MLs的光致毒性,以阐明光解过程中母体及其中间产物的毒性效应和抗菌活性演变趋势.
我国水体中大环内酯类抗生素的分布特征及环境光化学行为
Occurrence and photochemical behavior of macrolide antibiotics in the aquatic environment of China
-
摘要: 大环内酯类抗生素(MLs)作为一类新型有机污染物,广泛存在于水环境中,表现为“假持久性”,并且能够导致环境菌群抗药性产生. 本文总结大量文献,分析了我国环境水体中MLs的存在状况与浓度水平,并对该类抗生素浓度水平的时空分布差异进行了讨论,同时总结了水环境中MLs光化学行为的最新研究进展,介绍了其光解动力学以及水环境因子对光解的影响,阐述了光降解路径与机理,最后对该类抗生素的环境存在特征及光化学转化研究进行了展望.Abstract: Macrolide antibiotics (MLs), as a new type of organic pollutants, are existing widely in the environment and show pseudo-persistent, which could induce bacterial resistance in the environment. Based on a large number of peer-reviewed literatures, this review analyzed the occurrence and levels of MLs in the aquatic environment of China, as well as the seasonal and spatial distribution. Furthermore, the current research progress on the aqueous photochemical behavior of MLs was explored. The photodegradation kinetics and effects of aqueous environmental factors were discussed. The corresponding photodegradation pathways and mechanisms for typical MLs were summarized. Finally, the research prospects about the environmental occurrence and photochemical transformation of MLs were proposed.
-
Key words:
- macrolide antibiotics /
- occurrence /
- distribution /
- photodegradation /
- photochemical transformation.
-
城市绿地为居民提供了良好的环境、景观和游憩场所,城市绿地功能的发挥离不开土壤,城市的居住适宜程度和环境质量以及人类的生活品质等都与城市绿地土壤密切相关[1-2]。然而,随着城市化的发展,城市土壤环境问题日渐严峻,大量污染物进入土壤当中,尤为突出的是土壤重金属和多环芳烃(PAHs)污染日益加剧[3-4]。因此,土壤重金属和多环芳烃污染问题是阻碍城市发展、影响城市居民健康的重要因素之一。
城市土壤中的重金属和PAHs主要来自大气中吸附有环境污染物的细颗粒物的干湿沉降[5-6],而城市中的植被格局在很大程度上,影响着大气颗粒物的传输和沉降过程[7]。目前,城市中观尺度上土地利用类型、城市化程度、城区植被类型及覆盖度等因素对土壤重金属和多环芳烃的影响均有较多研究[8-10]。由于不同的土地利用类型(商业区、居民区、工业区等)对土壤污染物的累积影响较大[11-12],因此,选择相同的土地利用类型来研究某种因素的影响能够较为明确地揭示目标因素的影响作用。居民区是城市区域中人口最为集中的地方之一,且占地面积较大,与人居环境质量息息相关[13]。有研究[14]表明,杭州市居民区土壤重金属元素均有不同程度的积累,以Cd最为明显。居民区土壤重金属可能是人体重金属的重要来源之一[15]。陈秀端等[16]发现,在西安市居民区土壤中,重金属污染对成人与儿童的健康都具有风险。其中,儿童所接受的重金属元素As、Ba、Co、Cr、Pb和Zn污染的健康风险远高于成人。吴新民等[17]应用MIELKE等[18]提出的儿童健康标准的土壤Pb全量指标进行研究,发现南京市老居民区土壤Pb浓度超标75%以上。此外,有研究[19-21]表明,不同城市化强度、城市土地利用类型以及植被类型对土壤PAHs累积均具有显著影响。然而,以上的研究均在城市区域尺度上进行,对于小尺度(如居民区尺度)水平上植被格局对土壤污染物累积的影响研究较少。
小尺度水平上的植被格局是城市绿地园林设计和规划的重要内容,研究小尺度水平上植被格局对土壤重金属和PAHs等污染物累积的影响能够为城市绿地园林设计和绿地管理提供依据,能够使其同时兼顾环境美观和健康。因此,本研究选择居民区土地利用类型作为研究对象,对不同植被格局下,居民区土壤重金属和PAHs累积特征进行调查分析,旨在探明小尺度水平上,植被格局对土壤污染物累积的影响及其程度。
1. 材料与方法
1.1 调查区域介绍
本次研究区为北京市五环内区域(北纬39°45′17″~40°01′13″,东经116°12′18″~116°33′13″),五环内区域覆盖了包括东城区、西城区、海淀区、朝阳区、石景山区以及丰台区在内的6个行政管辖区,截至2017年末,该区域人口数量约为1.2×107[22]。
1.2 居民区植被格局概况
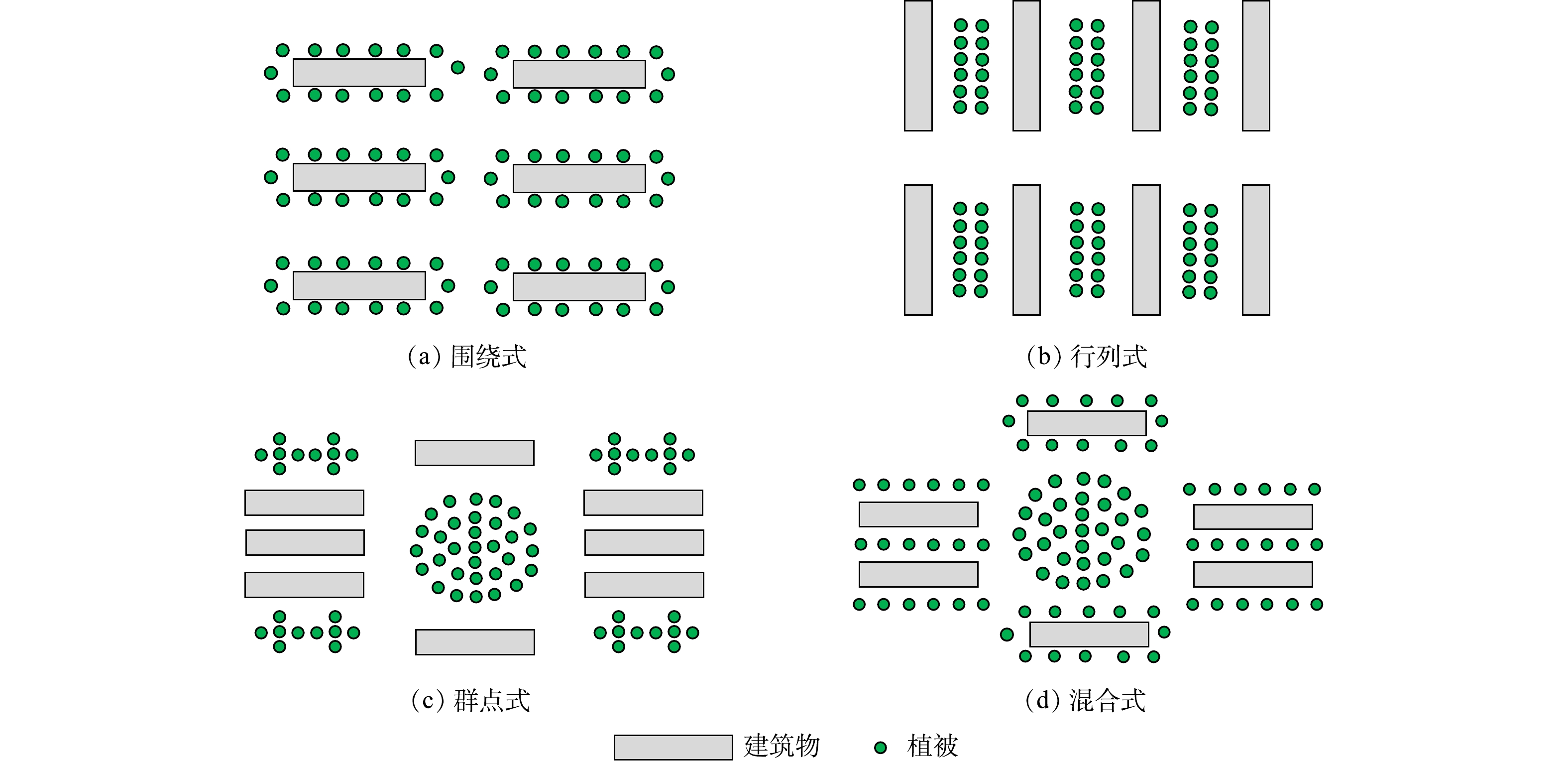
居民区是城市极具代表性的土地利用类型,其内部绿地依托建筑物的布局形式呈现不同的植被格局。我国居民区常见的植被格局类型包括围绕式、行列式、混合式和群点式等,不同的居民区植被格局如图1所示。围绕式绿地格局(图1(a))一般集中围绕在建筑物周边分布,面积较大,并且可分为单周边及双周边;行列式绿地格局(图1(b))通常依托建筑物的布局总体呈现线性排列布局形式,为了便于居民使用,多为楼间绿地;群点式绿地格局(图1(c))通常依托建筑物呈点状或组群布局,以中心公共绿地作为核心,延伸到周边绿地,且该类型居民区的建筑多为散点分布,建筑物密度较高;混合式绿地格局(图1(d))综合了以上3种形式,该类型可能包含以上多种布置形式,但基本仍以其中一种布局为主导[23]。
1.3 采样点选择及采样方法
本研究采样主要针对不同植被格局类型居民区内公共绿地草本植物覆盖的表层土壤。在北京市五环区域内,沿南北方向上选取了12个居民区(图2),每种植被格局(围绕式、行列式、混合式、群点式)分别选取3个具有代表性的居民区。为了消除区域尺度和/或中观尺度污染物分布格局对结果的影响,本次研究的调查点设置充分考虑到主要重金属污染物和多环芳烃在北京市五环内的分布特征,即2类污染物的浓度随着离市中心距离的增加而呈现降低的趋势[24-25]。因此,每种植被格局的3个代表性居民区分别分布在五环到市中心的范围内。根据居民区公共绿地的面积和分布,在每个居民区的公共绿地分别设置2~3个采样点。围绕式和行列式植被格局由于整个小区绿地斑块分布较为均匀,而且斑块面积大小类似,因此在小区不同方位分别布置3个采样点;群点式和混合式在大面积主要公共绿地斑块布置2个采样点,在超过100 m2的小面积绿地斑块增加1个采样点。于每个采样点在10 m×10 m面积内,采用5点混合法采集表层(0~10 cm)土壤,采集1 kg土壤样品于自封袋中,共采集31个土壤样品。土壤样品运送至实验室后,一部分样品冷冻干燥并过100目筛后,装入棕色玻璃瓶中,储存于−25 ℃冰箱中,供PAHs分析。剩余的样品自然风干后去除植物叶片、根系等杂质,磨碎后,使样品通过100目筛,以供测试土壤金属元素指标。
1.4 实验材料
金属元素检测试剂:盐酸(HCl)、硝酸(HNO3)、氢氟酸(HF)、高氯酸(HClO4)均为优级纯;超纯水(Mili-Q)。
金属元素检测仪器:赶酸电热板(GS-Ⅲ,南京瑞尼克科技开发有限公司)、电感耦合等离子体光谱仪(ICP-OES,Prodigy7,美国Leeman labs公司)、电感耦合等离子体质谱仪(ICP-MS,NexION300x,美国PerkinElmer公司)。
多环芳烃检测试剂:丙酮(CH3COCH3)、正己烷(C6H14)、二氯甲烷(CH2Cl2)均为农残级;无水硫酸钠(Na2SO4)为分析纯。
标准样品:EPA16种优控多环芳烃混标;替代物选用氘代三联苯和2-氟联苯;内标选用5种半挥发物氘代多环芳烃(氘代菲、氘代屈、氘代萘、氘代苊、氘代苝)。
多环芳烃检测仪器:快速溶剂萃取仪(ASE 350型,美国戴安公司)、旋转蒸发仪(RE-201D型,郑州科泰实验设备有限公司)、固相萃取仪(Visiprep DL SPE 12孔,美国Supelco公司)、氮吹仪(DC-12型,上海安谱实验科技股份有限公司)、串联四极杆气相色谱-质谱联用仪(7890B-7000D)。
1.5 实验方法
土壤金属元素检测采用四酸法 (HCl-HNO3-HF-HClO4,10∶3∶3∶1)[26];土壤PAHs检测采用加速溶剂萃取-旋蒸浓缩-GC/MS分析方法[27-28]。
使用电感耦合等离子体光谱仪测定重金属Mn及其他金属元素Na、K、Fe、Ca、Mg和Al的浓度[29];采用电感耦合等离子体质谱仪测定重金属Cr、Ni、Cu、Zn、Cd和Pb的浓度[26]。在实验过程中,使用国家土壤标准物质GBW07401进行质量控制,测得标准回收率为72.1%~103.2%。土壤PAHs分析过程采用空白、样品平行样、内标和替代物控制等进行质量控制,内标回收率为50.8%~98.4%,所有多环芳烃浓度的数据均高于方法检测限。
2. 结果与讨论
2.1 研究区土壤重金属污染物识别及统计学分析
研究区表层土壤中的7种重金属浓度统计学特征如表1所示。Cu的平均值、最大值以及变异系数均高于其相应的背景值,其中,变异系数高达88.1%,约是其背景值的4倍;土壤Cd的平均值、最小值和中位值均远高于其对应背景值;在土壤Pb的所有统计值中,除中位值外,均大于其对应的背景值,变异系数约为背景值的3倍;土壤Zn的最大值和变异系数略高于背景值,其他值低于背景值。除此之外,其他3种重金属元素的各项统计值均低于相应的背景值。因此,可以表明,居民区土壤中Cu、Zn、Cd和Pb存在一定程度的累积。
表 1 研究区表层土壤中7种重金属浓度及其变异系数Table 1. Concentrations and variation coefficients of seven heavy metals in surface soil of study area重金属元素 浓度最小值/(mg·kg−1) 浓度最大值/(mg·kg−1) 浓度平均值/(mg·kg−1) 浓度中位值/(mg·kg−1) 变异系数/% 实测 背景1) 实测 背景1) 实测 背景1) 实测 背景1) 实测 背景1) Cr 32.7 50.6 63.4 163 45.9 68.1 46.5 64.4 15.0 23.4 Ni 14.5 17.0 28.2 48.9 21.9 29 22.2 27.4 14.4 25.7 Cu 13.0 15.0 159 101 29.1 23.6 22.3 23.7 88.1 19.8 Zn 33.2 48.2 245 226 71.8 102.6 65.1 97.5 37.6 34.5 Cd 0.104 0.005 0.235 0.339 0.156 0.074 0.150 0.073 19.1 78.9 Pb 13.2 10.0 132 46.0 31.2 25.4 23.2 24.1 85.6 24.8 Mn 335 419 648 1039 400 705 384 685 16.0 22.7 注:1)背景值参考已有研究中的数值[30]。 在环境领域的研究中,主成分分析法用于对一组数据进行降维,通过提取少数综合指标来反映多个因素之间的关系[31]。元素之间的相关关系往往能够反映土壤重金属来源,同时也能够判断出哪些土壤元素浓度受到人为因素的明显影响。所以,为了进一步分析居民区土壤中重金属的来源,识别出受到人为扰动较强的首要污染物,在调查重金属元素的基础上,本研究还同时调查了6种金属元素(Na、K、Fe、Ca、Mg、Al)的浓度,并对以上 13 种金属元素进行了主成分分析,结果如表2所示。可以看出,本研究共提取4个主成分,累积解释总变量方差为84.7%。主成分1占总变量方差的30.4%,具有较高载荷的元素为Cu、Zn和Pb;主成分2占24.3%,占较高载荷的元素为 Fe、Mg和Al;主成分3占17.5%,载荷较高的元素有Cr和Ni;主成分4占12.5%,较高载荷的是Na和K元素。元素Cd、Mn和Ca这3种元素分别在2个甚至3个主成分上的载荷值都类似。说明这些元素的累积受到多个因素的贡献相似。Cu、Zn、Cd和Pb通常是城市土壤中普遍存在的受人为活动影响较为严重的重金属污染物,而Fe、Mg和Al元素尽管受到人为活动干扰较大(如建筑材料等垃圾输入),但是由于其在成土母质中的浓度也非常高,因此,人类活动对这些元素在土壤中的浓度的影响相对较小[32]。除了点源污染,Cr和Ni在土壤中的浓度与成土母质有关。Na和K元素在土壤中来源广泛,并且浓度很高,其在土壤中的浓度变化一般也较大。由此可见,北京市居民区土壤中Cu、Zn、Cd和Pb的主要来源为人为源,并且重金属浓度变化受人类活动影响较大。
表 2 研究区土壤各元素浓度旋转后的因子载荷分析Table 2. Principal component loadings of elements concentrations in studied soils after rotation元素 主成分1 主成分2 主成分3 主成分4 Cr 0.281 0.078 0.886 −0.003 Ni 0.065 0.001 0.914 0.117 Cu 0.960 0.135 0.037 −0.064 Zn 0.951 0.064 0.200 −0.050 Cd1) 0.664 0.153 0.476 −0.170 Pb 0.904 0.118 0.140 −0.206 Mn1) 0.737 0.606 0.114 −0.008 Na −0.209 −0.135 −0.096 0.808 K −0.032 0.178 0.168 0.879 Fe 0.227 0.857 −0.045 −0.031 Ca1) 0.361 0.589 0.499 −0.327 Mg −0.023 0.905 0.244 0.039 Al 0.131 0.880 −0.068 0.068 注:1)表示在2组以上主成分中占有相似比例的载荷。 元素浓度是否服从正态分布是评价土壤重金属是否存在人为活动干扰的依据之一。当浓度数据为非正态分布时,说明存在人为扰动[33]。如表3所示,在原始数据中,只有Cr、Ni、Cd、Ca、Mg和Al等6种元素的K-S检验为不显著(P>0.05),呈现为正态分布,这说明人为干扰因素较少;而其他7种元素的浓度都呈现明显的人为干扰趋势。
表 3 土壤样品中各元素浓度的偏度、峰度、正态性检验(K-S检验)Table 3. Skewness, kurtosis and normal test (K-S testing) of heavy metal concentrations in studied soils元素 峰度 偏度 K-S p Cr 0.053 0.100 0.200 Ni −0.342 −0.321 0.200 Cu 22.0 4.22 0* Zn 14.3 3.17 0* Cd 0.316 0.529 0.200 Pb 7.21 2.60 0* Mn 6.31 2.02 0.008* Na 2.83 1.49 0* K −0.245 −.0725 0.004* Fe 0.637 0.922 0.017* Ca −0.257 −0.164 0.200 Mg 2.29 1.23 0.068 Al 1.61 0.678 0.200 注:*表示差异显著(P<0.05)。 综合土壤中重金属元素浓度与背景值的统计比较、主成分分析以及正态分布检验结果发现,这些小区土壤中的重金属Cu、Zn、Pb这3种元素与人为活动因素相关性较大;Cd元素有一定的相关性,但是影响相对较小。本研究结果与前期在北京市建成区及建成区居民区的调查研究结果一致,即,主要重金属污染物为Cu、Zn、Cd和Pb[24, 29];同时,有相关研究[29]表明,北京市建成区这4种典型重金属主要来源于交通排放,并且随着城市化发展在土壤中逐年累积。
2.2 研究区土壤16种多环芳烃浓度分布及来源分析
在研究区内,居民区表层土壤PAHs浓度统计结果如表4所示。研究区土壤16种PAHs总量(Σ16PAHs)的浓度范围为88.1~2 844 μg·kg−1,中位值为327 μg·kg−1,远超过土壤中内源性 PAHs 的浓度(1~10 μg·kg−1)[34]。因此,研究区土壤多环芳烃主要受外源输入影响较大(表4)。并且,在16种PAHs中,除Nap和Flu外,其余14种单体的变异系数均大于80%。
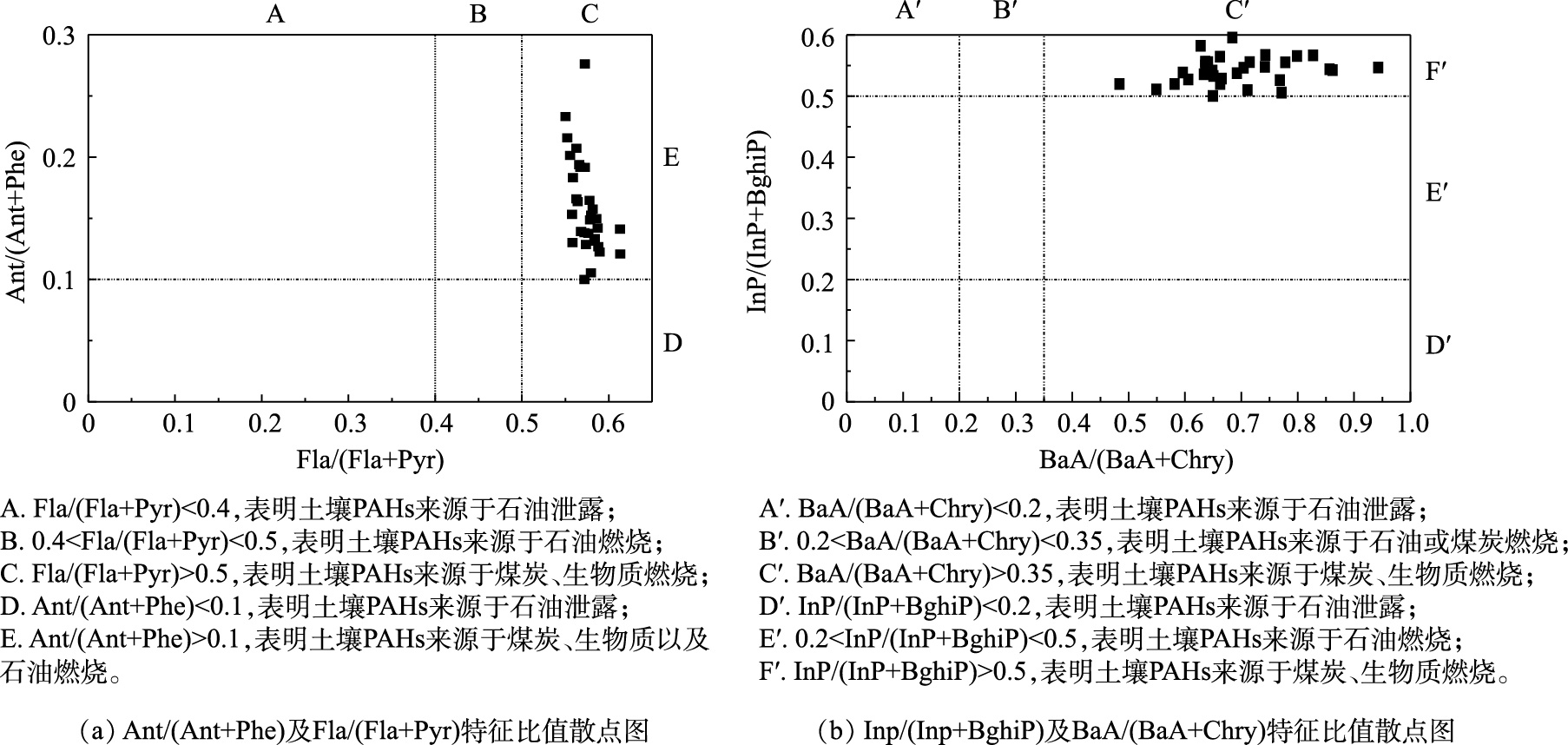
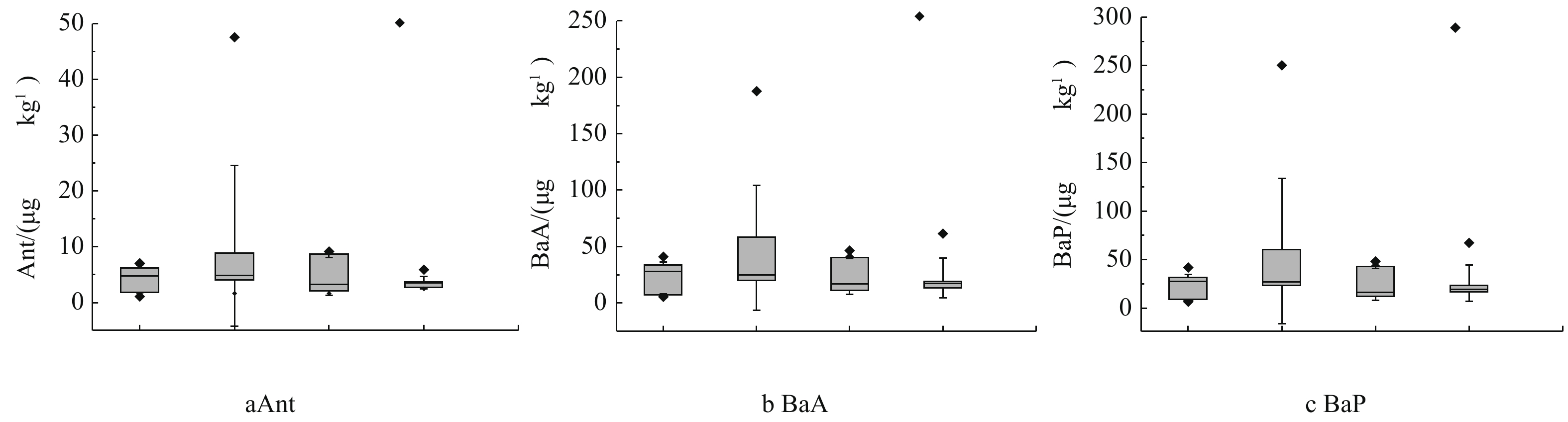
表 4 研究区表层土壤中PAHs浓度Table 4. PAHs concentrations in surface soil of study area化合物 缩写 环数 极小值/(μg·kg−1) 中位值/(μg·kg−1) 极大值/(μg·kg−1) 变异系数/% 萘 Nap 2 3.79 15.4 33.9 51.6 苊 Ace 3 1.23 3.49 28.6 103 苊烯 Acy 3 0.140 1.05 8.25 114 芴 Flu 3 0.710 2.60 13.1 72.9 菲 Phe 3 5.60 26.6 204 105 蒽 Ant 3 1.07 3.72 47.5 140 荧蒽 Fla 4 9.29 30.5 346 124 芘 Pyr 4 7.09 22.7 283 132 苯并(a)蒽 BaA 4 5.68 19.8 188 111 屈 Chry 4 7.99 29.2 289 115 苯并(b)荧蒽 BbF 5 11.3 41.3 269 85.8 苯并(k)荧蒽 BkF 5 6.39 22.4 226 118 苯并(a)芘 BaP 5 6.60 23.3 250 130 二苯并(a,h)蒽 DahA 5 2.60 10.5 91.4 113 苯并(g,h,i)苝 BghiP 6 9.09 34.3 288 108 茚并(1,2,3-cd)芘 InP 6 9.44 44.1 288 94.4 低环PAHs LMW PAHs 2~3 12.6 49.9 326 85.6 高环PAHs HMW PAHs 4~6 75.5 278 2 518 111 总PAHs Σ16PAHs 2~6 88.1 327 2 844 107 PAHs同分异构体比值法是用于来源分析的主要手段[35-36]。YUNKER等[37]认为:当Ant/(Ant+Phe)<0.1时,表明PAHs主要来源于石油源;当Ant/(Ant+Phe)>0.1时,表明PAHs主要来源于燃烧源;当Fla/(Fla+Pyr)<0.4时,表明PAHs主要来自石油源;当0.4<Fla/(Fla+Pyr)<0.5时,表明PAHs来源于来自燃油排放尾气;当Fla/(Fla+Pyr)>0.5时,表明PAHs主要来源于煤炭和生物质燃烧。根据Ant/(Ant+Phe)(图3(a)),除了个别样点以外,绝大部分样点的燃烧来源明显;所有样点的Fla/(Fla+Pyr)比值都大于0.5,即PAHs主要由生物质、煤炭高温燃烧产生。同时,BaA/(BaA+Chry)和InP/(InP+BghiP)也可以用来判定PAHs来源[38](图3(b))。当BaA/(BaA+Chry)<0.2或InP/(InP+BghiP)<0.2时,表明PAHs为石油源;当0.2<BaA/(BaA+Chry)<0.35时,一般认为PAHs来源于石油或煤炭燃烧混合源;当0.2<InP/(InP+BghiP)<0.5时,PAHs的来源主要为石油燃烧,包括汽车排放和原油燃烧;当InP/(InP+BghiP)>0.5或者BaA/(BaA+Chry)>0.35时,表明PAHs主要来源为生物质或者煤炭燃烧。样点的PAHs几乎都来源于生物质和煤炭燃烧(图3(b)),汽车燃料燃烧来源相关性较小。产生这一结果的原因可能是,燃烧过程产生的细颗粒物,在城市区域中均匀背景沉降所导致。PENG等[25]的研究也表明,北方城市集中供暖期所引起的长期煤炭燃烧是北方城市土壤中多环芳烃的主要来源。
2.3 不同植被格局土壤重金属污染物累积特征比较
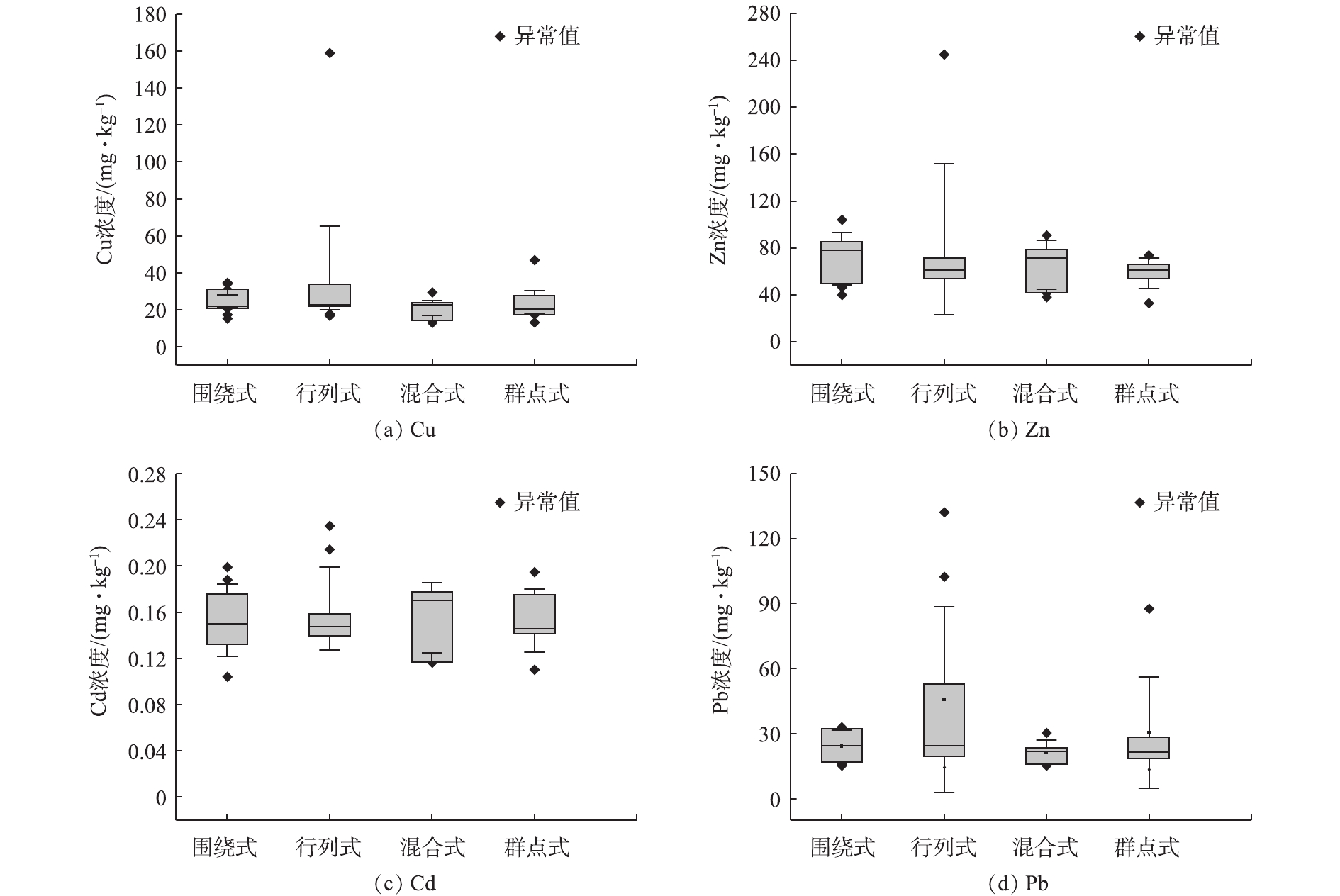
不同植被格局的样点土壤重金属污染物Cu、Zn、Cd和Pb的浓度数值分布如图4所示。可以看出,重金属Cu(图4(a))和Pb(图4(d))的中位值在4种植被格局中的变化不明显,而Cd(图4(c))和Zn(图4(b))元素的中位值稍有变化,即围绕式和混合式稍高,行列式和群点式稍低。从数据分布的情况看:首先,在4种植被格局中,行列式植被格局土壤中的重金属污染物浓度数据分散性相对较高;其次,Cd元素浓度分散性比其他3种元素高(图4(c)),Cu(图4(a))和Pb元素(图4(d))在围绕式和混合式植被格局土壤中的浓度分布较为集中,Zn元素在除了行列式以外的3种植被格局土壤中的分布都较为稳定。从采样调查研究的角度来说,对于行列式植被格局的小区绿地,需要适当增加样点数量才能获得较为客观的重金属浓度;而从环境管理的角度来说,行列式植被格局更有可能吸纳重金属污染物而起到净化环境的作用。土壤Cd元素浓度的分散性较大主要原因是由于城市土壤Cd浓度一般较低。本研究中Cd的浓度大多为0.1~0.2 mg·kg−1,导致系统误差的影响较大。因此,在城市土壤调查中对于Cd元素最需要关注的是异常高值。
2.4 不同植被格局土壤16种PAHs的累积特征比较
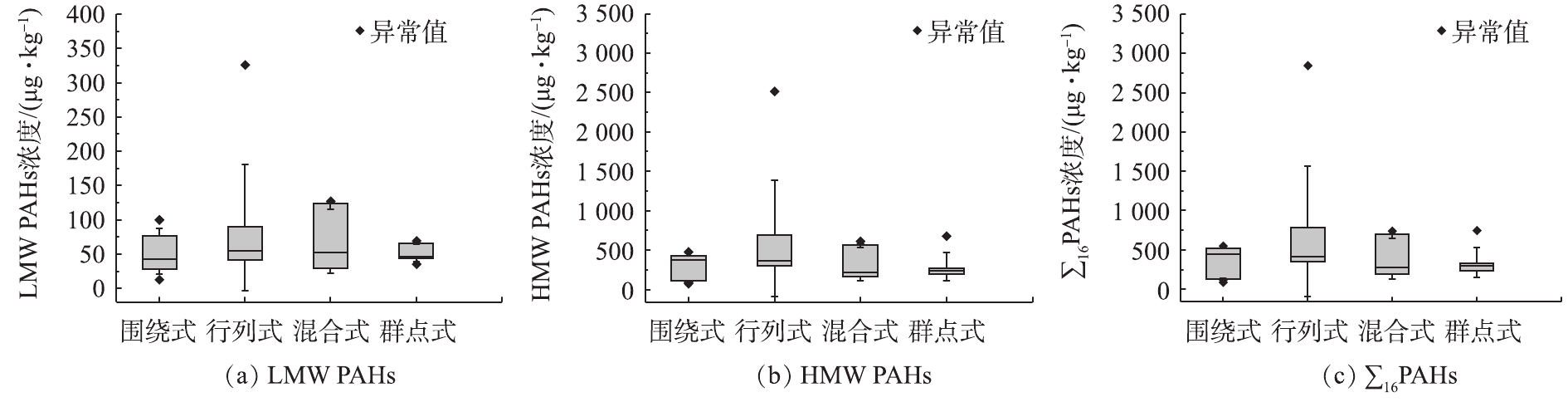
图5分别展示了LMW PAHs(图5(a))、HMW PAHs(图5(b))和∑16PAHs(图5(c))在4种植被格局下的浓度数值分布。不同植被格局下,居民区土壤∑16PAHs浓度中位值顺序为:围绕式(444 μg·kg−1)>行列式(408 μg·kg−1)>群点式(292 μg·kg−1)>混合式(271 μg·kg−1)。行列式植被格局小区,土壤∑16PAHs浓度中位值在4种格局小区内处于较高水平;并且,该格局类型下,土壤∑16PAHs最大值高达2 844 μg·kg−1,约为其他3种植被格局的4~5倍。此外,与重金属的浓度分布类似,行列式植被格局的土壤中LMW PAHs、HMW PAHs和∑16PAHs的浓度分布都较为分散;而且,HMW PAHs和∑16PAHs浓度的中位值在混合式和群点式植被格局土壤中较低。有研究[37]指出,Ant、BaA和BaP这3种PAHs的光敏性最强,尤其在夏季消失很快。因此,土壤中,这3种PAHs通常不会远距离迁移,因而被认为是最好的本地排放指标。由图6可知,相较于其他3种植被格局,Ant(图6(a))、BaA(图6(b))和BaP(图6(c))这3种PAHs在行列式植被格局土壤中的浓度水平均较为分散,说明在这种植被格局下,当地即时产生的PAHs变异也较大。同时,群点式植被格局的土壤中这3种PAHs浓度的中位值都最低,说明在这种植被格局下当地产生的PAHs在土壤中累积较少。植被覆盖类型能够影响下垫面地表土壤中重金属和多环芳烃的浓度,有研究[25]发现,城市中16种优控多环芳烃主要经由大气沉降过程进入土壤,而这一过程受到植被垂直结构的影响较大。对北京市的调查[27]发现,乔木覆盖下的土壤污染物的浓度明显比草地或灌木-草本覆盖类型高。其中,林灌草和林地土壤中多环芳烃浓度是草地土壤的2~3倍(P<0.05)。由于行列式植被格局大多由乔木或乔木-灌木的植被覆盖类型组成,而群点式植被格局中的大面积绿地由于会考虑到居民户外活动的方便,多为草地,这可能是导致行列式植被格局土壤中污染物含量相对较高,而群点式植被格局相对较低的原因。
3. 结论
1)本研究结果表明,所选的31个典型居民区绿地土壤样点主要重金属污染物为Cu、Zn、Cd、Pb;土壤PAHs来源主要与煤炭及生物质燃烧有关,石油及其产物燃烧不是土壤PAHs的主要来源。
2)行列式植被格局的土壤中重金属污染物Cu、Zn、Cd、Pb及高环和低环PAHs的浓度分布都较为分散,污染物高浓度的样点也往往出现在这种植被格局的绿地土壤样品中。这是因为行列式植被格局的绿地植被覆盖类型往往为乔木或乔木-灌木类型。
3)群点式植被格局的土壤中重金属污染物和Ant、BaA和BaP这3种光敏性较强的本地指示性PAHs浓度都较其他3种植被格局土壤低。这与群点式植被格局的绿地植物覆盖类型多为草地有关。
4)在对行列式植被格局小区进行采样调查时,需要布置多个采样点才能获得代表性污染物浓度;同时,行列式植被格局有利于土壤截获污染物,发挥绿地土壤吸纳污染物的生态功能,而群点式植被格局不利于土壤截获和吸纳污染物。但是,该植被格局下绿地环境质量相对较好,适合居民户外活动。
-
表 1 我国与其他国家地表水中大环内酯类抗生素的浓度水平对比
Table 1. Concentration difference of macrolide antibiotics in typical surface waters between China and other countries
地表水体Surface waters 采样季节Sampling seasons 水体/(ng·L−1)Water 沉积物/(ng·g−1)Sediment 参考文献References 安徽宛溪河 2018秋 1—147.1(均值35.73) — [8] 长江嘉陵江重庆段 2018春、秋 ND—1409(均值75.003) ND—866.78(均值39.758) [24] 山东莱州湾小清河 2015春、秋、冬,2017春 ND—223(均值81.55) — [25] 长江下游 2018秋 ND—30(均值1.79) ND—6780(均值400) [26] 北京潮白河 2017冬 <782(均值36.017) <8.96(均值1.317) [27] 东海沿海水域 2018春 2.9—77(均值33.6) 0.6—60.3(均值5.2) [28] 北部湾 2015秋 <0.41—40.7(均值2.111) <0.013—1.34(均值0.198) [29] 太湖 2010春 ND—624.8(均值79.9) ND—120.3(均值44.6) [23] 西班牙东部地中海 2018夏、秋,2019冬 <5—1617(均值41.4) — [30] 韩国荣山江 2016春、夏、秋 6.2—475.1(均值42.7) — [31] 法国阿杜尔河口 — <0.06—<8.1 — [32] 黎巴嫩河流 2016春 <23—2806 — [33] 伊比利亚河 2010秋,2011秋 0.09—153.72(均值2.172) 1.13—23.92(均值12.543) [34] 美国东南部河流 2009冬季,2010春、夏、秋、冬 0.2—2.7(中值1.4) — [35] 南非Umgeni河 2013冬、春 0.21—22.57(中值5.65) — [36] 美国迈阿密河 — 14.7—356 — [37] ND:未检出,not detected;—没有数据,data unavailable. 表 2 不同水溶液中大环内酯类抗生素(MLs)在254 nm、350 nm或(模拟)日光下的摩尔吸光系数(ε)、量子产率(Φ)及反应速率常数(k)
Table 2. Molar absorption coefficients(ε), quantum yields(Φ) and reaction rate constants(k) of the typical macrolide antibiotics in different water solutions measured at 254 nm, 350nm or (simulated) sunlight
化合物Compounds 溶液条件Condition Φ/(mol·Einstein−1) k/min−1 参考文献References 254 nm 350 nm 模拟日光Simulated sunlight 254 nm 350 nm 日光Sunlight 罗红霉素 纯水 1.2 × 10−3 2.0 × 10−4 — 1.7 × 10−3 1.2 × 10−4 9.5× 10−5 [85] 淡水 4.9 × 10−2 7.0 × 10−4 — 1.7 × 10−2 3.9× 10−4 2.0× 10−4 海水 1.1 × 10−2 4.0 × 10−4 — 3.0 × 10−2 2.4× 10−4 3.5× 10−4 红霉素 纯水 3.0 × 10−4 2.0 × 10−4 — 3.6 × 10−4 1.1× 10−4 2.1× 10−4 [85] 淡水 3.0 × 10−3 4.0 × 10−4 — 4.5 × 10−3 3.9× 10−4 1.7× 10−4 海水 4.5 × 10−3 7.0 × 10−4 — 2.2 × 10−3 1.5× 10−4 4.8× 10−5 阿奇霉素二水合物A pH 3 1.2 — — 4.7 × 10−1 — — [88] pH 7 1.0 — — 3.9 × 10−1 — — pH 9 4.9 × 10−1 — — 1.9 × 10−1 — — 阿奇霉素二水合物B pH 3 1.8 — — 6.9 × 10−1 — — pH 7 8.1 × 10−1 — — 3.1 × 10−1 — — pH 9 7.3 × 10−1 — — 2.8 × 10−1 — — 游离红霉素A pH 3 9.0 × 10−2 — — 1.0 × 10−1 — — pH 7 5.5 × 10−1 — — 5.9 × 10−1 — — pH 9 1.9 × 10−1 — — 2.1 × 10−1 — — 游离红霉素B pH 3 5.0 × 10−2 — — 5.0 × 10−2 — — pH 7 6.1 × 10−1 — — 6.6 × 10−1 — — pH 9 2.0 × 10−1 — — 2.2 × 10−1 — — 酒石酸泰乐菌素A pH 3 4.0 × 10−2 — — 2.5 — — pH 7 4.0 × 10−2 — — 2.5 — — pH 9 2.0 × 10−2 — — 1.2 — — 酒石酸泰乐菌素B pH 3 2.0 × 10−2 — — 1.4 — — pH 7 3.0 × 10−2 — — 1.7 — — pH 9 1.0 × 10−2 — — 6.7 × 10−1 — — 螺旋霉素 纯水 — — 1.4 × 10−2 3.6 × 10−5 — — [84] 红霉素 纯水 — — 3.5 × 10−3 8.3 × 10−5 — — 克拉霉素 纯水 — — 6.3 × 10−3 1.3 × 10−4 — — 泰乐菌素 纯水 — — 7.6 × 10−3 4.8 × 10−5 — — 红霉素 纯水 — — 5.4 × 10−4 — — — [9] 克拉霉素 纯水 — — 5.6 × 10−5 — — — —没有数据,data unavailable. 表 3 水环境因子对大环内酯类抗生素光降解动力学的影响
Table 3. Effects of aqueous environmental factors on photodegradation kinetics of macrolide antibiotics
水环境因子Factors 化合物Compounds 光源、溶液条件Light, solution condition 对光解影响Effect 参考文献References 天然有机物 罗红霉素、克拉霉素、阿奇霉素、红霉素 1700 W氙灯(λ > 290 nm);pH 7 促进 [9] 罗红霉素 500 W中压汞灯(λ > 290 nm);pH 6、pH 7和pH 8 促进 [15] 罗红霉素 500 W中压汞灯 (λ > 290 nm) 促进 [90] 腐殖酸 罗红霉素 500 W中压汞灯(λ > 290 nm);pH 6、pH 7和pH 8 促进 [15] 罗红霉素 500 W中压汞灯(λ > 290 nm) 促进 [90] 阿奇霉素 500 W氙灯(λ > 290 nm) ;pH 7.3 促进 [77] 罗红霉素、克拉霉素、阿奇霉素、红霉素 太阳光;pH 7 促进 [101] 泰乐菌素 500 W高压汞灯(主波长365 nm) 抑制 [102] 螺旋霉素 250 W高压汞灯 抑制 [103] 罗红霉素、螺旋霉素 250 W高压汞灯(λ > 200 nm) 抑制 [84] 克拉霉素、泰乐菌素 低浓度促进,高浓度抑制 克拉霉素、螺旋霉素 1000 W高压汞灯(λ > 290 nm) 抑制 罗红霉素、泰乐菌素 低浓度促进,高浓度抑制 富里酸 罗红霉素 500 W中压汞灯(λ > 290 nm);pH 6、pH 7和pH 8 促进 [15] NO3− 螺旋霉素 250 W高压汞灯 促进 [103] 阿奇霉素 500 W氙灯(λ > 290 nm) ;pH 6.6 促进 [77] 螺旋霉素、泰乐菌素 250 W高压汞灯(λ > 200 nm) 促进 [84] 罗红霉素 抑制 克拉霉素 低浓度抑制,高浓度促进 罗红霉素、螺旋霉素、泰乐菌素 1000 W高压汞灯(λ > 290 nm) 促进 克拉霉素 低浓度抑制,高浓度促进 罗红霉素 500 W中压汞灯;UV/H2O2;pH 7 抑制 [3] 泰乐菌素 500 W高压汞灯(主波长365 nm) 抑制 [102] NO2− 螺旋霉素、泰乐菌素 250 W高压汞灯(λ > 200 nm) 促进 [84] 罗红霉素 抑制 克拉霉素 低浓度促进,高浓度抑制 罗红霉素、克拉霉素、螺旋霉素、泰乐菌素 1000 W高压汞灯(λ > 290 nm) 抑制 罗红霉素 500 W中压汞灯,UV/H2O2;pH 7 抑制 [3] 螺旋霉素 250 W高压汞灯 抑制 [103] Fe(Ⅲ) 罗红霉素、克拉霉素 中压汞灯(λ > 290 nm) 促进 [78] 螺旋霉素 250 W高压汞灯(λ > 200 nm) 促进 [84] 罗红霉素、克拉霉素、泰乐菌素 抑制 克拉霉素 1000 W高压汞灯(λ > 290 nm) 促进 罗红霉素 抑制 螺旋霉素 低浓度促进,高浓度抑制 泰乐菌素 低浓度抑制,高浓度促进 罗红霉素 500 W中压汞灯,UV/H2O2;pH 7 抑制 [3] Fe(Ⅱ) 克拉霉素 1000 W高压汞灯(λ > 290 nm) 促进 [84] 罗红霉素、螺旋霉素 抑制 泰乐菌素 低浓度抑制,高浓度促进 克拉霉素、泰乐菌素 250 W高压汞灯(λ > 200 nm) 抑制 螺旋霉素 低浓度促进,高浓度抑制 罗红霉素 低浓度抑制,高浓度促进 Cu2+、Mg2+、Cl−、HCO3− 罗红霉素 500 W中压汞灯;UV/H2O2;pH 7 抑制 [3] Ca2+、SO42− 罗红霉素 500 W中压汞灯;UV/H2O2;pH 7 无显著影响 [3] -
[1] SCHLÜSENER M P, BESTER K, SPITELLER M. Determination of antibiotics such as macrolides, ionophores and tiamulin in liquid manure by HPLC-MS/MS [J]. Analytical and Bioanalytical Chemistry, 2003, 375(7): 942-947. doi: 10.1007/s00216-003-1838-9 [2] SENTA I, KRIZMAN-MATASIC I, TERZIC S, et al. Comprehensive determination of macrolide antibiotics, their synthesis intermediates and transformation products in wastewater effluents and ambient waters by liquid chromatography-tandem mass spectrometry [J]. Journal of Chromatography. A, 2017, 1509: 60-68. doi: 10.1016/j.chroma.2017.06.005 [3] LI W, XU X J, LYU B L, et al. Degradation of typical macrolide antibiotic roxithromycin by hydroxyl radical: Kinetics, products, and toxicity assessment [J]. Environmental Science and Pollution Research International, 2019, 26(14): 14570-14582. doi: 10.1007/s11356-019-04713-1 [4] 葛林科, 张思玉, 谢晴, 等. 抗生素在水环境中的光化学行为 [J]. 中国科学:化学, 2010, 40(2): 124-135. doi: 10.1360/zb2010-40-2-124 GE L K, ZHANG S Y, XIE Q, et al. Progress in studies on aqueous environmental photochemical behavior of antibiotics [J]. Scientia Sinica Chimica), 2010, 40(2): 124-135(in Chinese). doi: 10.1360/zb2010-40-2-124
[5] JIANG X S, ZHU Y Q, LIU L Q, et al. Occurrence and variations of pharmaceuticals and personal-care products in rural water bodies: A case study of the Taige Canal (2018-2019) [J]. Science of the Total Environment, 2021, 762: 143138. doi: 10.1016/j.scitotenv.2020.143138 [6] YANG L, WANG T Y, ZHOU Y Q, et al. Contamination, source and potential risks of pharmaceuticals and personal products (PPCPs) in Baiyangdian Basin, an intensive human intervention area, China [J]. Science of the Total Environment, 2021, 760: 144080. doi: 10.1016/j.scitotenv.2020.144080 [7] ZHAO B, XU J M, ZHANG G D, et al. Occurrence of antibiotics and antibiotic resistance genes in the Fuxian Lake and antibiotic source analysis based on principal component analysis-multiple linear regression model [J]. Chemosphere, 2021, 262: 127741. doi: 10.1016/j.chemosphere.2020.127741 [8] DING Y, CUI K P, LV K, et al. Revealing the hydrological transport and attenuation of 14 antibiotics in a low-flow stream [J]. Science of the Total Environment, 2021, 761: 143288. doi: 10.1016/j.scitotenv.2020.143288 [9] JIA X, LIAN L S, YAN S W, et al. Comprehensive understanding of the phototransformation process of macrolide antibiotics in simulated natural waters [J]. ACS ES& T Water, 2021, 1(4): 938-948. [10] SHAH S Q A, COLQUHOUN D J, NIKULI H L, et al. Prevalence of antibiotic resistance genes in the bacterial flora of integrated fish farming environments of Pakistan and Tanzania [J]. Environmental Science & Technology, 2012, 46(16): 8672-8679. [11] SENTA I, KOSTANJEVECKI P, KRIZMAN-MATASIC I, et al. Occurrence and behavior of macrolide antibiotics in municipal wastewater treatment: Possible importance of metabolites, synthesis byproducts, and transformation products [J]. Environmental Science & Technology, 2019, 53(13): 7463-7472. [12] BOREEN A L, ARNOLD W A, MCNEILL K. Photodegradation of pharmaceuticals in the aquatic environment: A review [J]. Aquatic Sciences, 2003, 65(4): 320-341. doi: 10.1007/s00027-003-0672-7 [13] EDHLUND B L, ARNOLD W A, MCNEILL K. Aquatic photochemistry of nitrofuran antibiotics [J]. Environmental Science & Technology, 2006, 40(17): 5422-5427. [14] KNAPP C W, CARDOZA L A, HAWES J N, et al. Fate and effects of enrofloxacin in aquatic systems under different light conditions [J]. Environmental Science & Technology, 2005, 39(23): 9140-9146. [15] 吕宝玲, 李威, 于筱莉, 等. 溶解性有机质对罗红霉素光降解的影响研究 [J]. 环境科学学报, 2019, 39(3): 747-754. LÜ B L, LI W, YU X L, et al. Effect of dissolved organic matter on the photodegradation of roxithromycin [J]. Acta Scientiae Circumstantiae, 2019, 39(3): 747-754(in Chinese).
[16] LATCH D E, PACKER J L, STENDER B L, et al. Aqueous photochemistry of triclosan: Formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products [J]. Environmental Toxicology and Chemistry, 2005, 24(3): 517-525. doi: 10.1897/04-243R.1 [17] JIAO S J, ZHENG S R, YIN D Q, et al. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria [J]. Chemosphere, 2008, 73(3): 377-382. doi: 10.1016/j.chemosphere.2008.05.042 [18] JUNG J, KIM Y, KIM J, et al. Environmental levels of ultraviolet light potentiate the toxicity of sulfonamide antibiotics in Daphnia magna [J]. Ecotoxicology (London, England), 2008, 17(1): 37-45. doi: 10.1007/s10646-007-0174-9 [19] 葛林科, 任红蕾, 鲁建江, 等. 我国环境中新兴污染物抗生素及其抗性基因的分布特征 [J]. 环境化学, 2015, 34(5): 875-883. doi: 10.7524/j.issn.0254-6108.2015.05.2014082501 GE L K, REN H L, LU J J, et al. Occurrence of antibiotics and corresponding resistance genes in the environment of China [J]. Environmental Chemistry, 2015, 34(5): 875-883(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.05.2014082501
[20] LIU X H, LU S Y, GUO W, et al. Antibiotics in the aquatic environments: A review of lakes, China [J]. Science of the Total Environment, 2018, 627: 1195-1208. doi: 10.1016/j.scitotenv.2018.01.271 [21] ZHANG Q Q, YING G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. [22] LI Z, LI M, ZHANG Z Y, et al. Antibiotics in aquatic environments of China: A review and meta-analysis [J]. Ecotoxicology and Environmental Safety, 2020, 199: 110668. doi: 10.1016/j.ecoenv.2020.110668 [23] XU J, ZHANG Y, ZHOU C B, et al. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China [J]. Science of the Total Environment, 2014, 497/498: 267-273. doi: 10.1016/j.scitotenv.2014.07.114 [24] WANG G G, ZHOU S H, HAN X K, et al. Occurrence, distribution, and source track of antibiotics and antibiotic resistance genes in the main rivers of Chongqing City, southwest China [J]. Journal of Hazardous Materials, 2020, 389: 122110. doi: 10.1016/j.jhazmat.2020.122110 [25] LI J, CUI M, ZHANG H. Spatial and temporal variations of antibiotics in a tidal river [J]. Environmental Monitoring and Assessment, 2020, 192(6): 336. doi: 10.1007/s10661-020-08313-2 [26] ZHANG G D, LU S Y, WANG Y Q, et al. Occurrence of antibiotics and antibiotic resistance genes and their correlations in Lower Yangtze River, China [J]. Environmental Pollution, 2020, 257: 113365. doi: 10.1016/j.envpol.2019.113365 [27] ZHANG Y X, CHEN H Y, JING L J, et al. Ecotoxicological risk assessment and source apportionment of antibiotics in the waters and sediments of a peri-urban river [J]. Science of the Total Environment, 2020, 731: 139128. doi: 10.1016/j.scitotenv.2020.139128 [28] LI F F, CHEN L J, CHEN W D, et al. Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening [J]. Marine Pollution Bulletin, 2020, 151: 110810. doi: 10.1016/j.marpolbul.2019.110810 [29] ZHANG R L, PEI J Y, ZHANG R J, et al. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood [J]. Ecotoxicology and Environmental Safety, 2018, 154: 27-35. doi: 10.1016/j.ecoenv.2018.02.006 [30] FONSECA E, HERNÁNDEZ F, IBÁÑEZ M, et al. Occurrence and ecological risks of pharmaceuticals in a Mediterranean River in Eastern Spain [J]. Environment International, 2020, 144: 106004. doi: 10.1016/j.envint.2020.106004 [31] NA T W, KANG T W, LEE K H, et al. Distribution and ecological risk of pharmaceuticals in surface water of the Yeongsan River, Republic of Korea [J]. Ecotoxicology and Environmental Safety, 2019, 181: 180-186. doi: 10.1016/j.ecoenv.2019.06.004 [32] MIOSSEC C, LANCELEUR L, MONPERRUS M. Multi-residue analysis of 44 pharmaceutical compounds in environmental water samples by solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry [J]. Journal of Separation Science, 2019, 42(10): 1853-1866. doi: 10.1002/jssc.201801214 [33] MOKH S, EL KHATIB M, KOUBAR M, et al. Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: A case study natural water sources in Lebanon [J]. Science of the Total Environment, 2017, 609: 830-841. doi: 10.1016/j.scitotenv.2017.07.230 [34] OSORIO V, LARRAÑAGA A, ACEÑA J, et al. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers [J]. Science of the Total Environment, 2016, 540: 267-277. doi: 10.1016/j.scitotenv.2015.06.143 [35] PADHYE L P, YAO H, KUNG'U F T, et al. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant [J]. Water Research, 2014, 51: 266-276. doi: 10.1016/j.watres.2013.10.070 [36] AGUNBIADE F O, MOODLEY B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa [J]. Environmental Monitoring and Assessment, 2014, 186(11): 7273-7291. doi: 10.1007/s10661-014-3926-z [37] PANDITI V R, BATCHU S R, GARDINALI P R. Online solid-phase extraction-liquid chromatography-electrospray-tandem mass spectrometry determination of multiple classes of antibiotics in environmental and treated waters [J]. Analytical and Bioanalytical Chemistry, 2013, 405(18): 5953-5964. doi: 10.1007/s00216-013-6863-8 [38] LIU X, STEELE J C, MENG X Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review [J]. Environmental Pollution, 2017, 223: 161-169. doi: 10.1016/j.envpol.2017.01.003 [39] CHEN H, LIU S, XU X R, et al. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure [J]. Marine Pollution Bulletin, 2015, 90(1/2): 181-187. [40] WANG W X, GU X H, ZHOU L J, et al. Antibiotics in crab ponds of lake Guchenghu Basin, China: Occurrence, temporal variations, and ecological risks [J]. International Journal of Environmental Research and Public Health, 2018, 15(3): 548. doi: 10.3390/ijerph15030548 [41] HUANG F Y, AN Z Y, MORAN M J, et al. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009−2019) [J]. Journal of Hazardous Materials, 2020, 399: 122813. doi: 10.1016/j.jhazmat.2020.122813 [42] 刘鹏霄, 王旭, 冯玲. 自然水环境中抗生素的污染现状、来源及危害研究进展 [J]. 环境工程, 2020, 38(5): 36-42. LIU P X, WANG X, FENG L. Occurrences, resources and risk of antibiotics in aquatic environment: A review [J]. Environmental Engineering, 2020, 38(5): 36-42(in Chinese).
[43] 李辉, 陈瑀, 封梦娟, 等. 南京市饮用水源地抗生素污染特征及风险评估 [J]. 环境科学学报, 2020, 40(4): 1269-1277. LI H, CHEN Y, FENG M J, et al. Pollution characteristics and risk assessment of antibiotics in Nanjing drinking water sources [J]. Acta Scientiae Circumstantiae, 2020, 40(4): 1269-1277(in Chinese).
[44] 廖杰, 魏晓琴, 肖燕琴, 等. 莲花水库水体中抗生素污染特征及生态风险评价 [J]. 环境科学, 2020, 41(9): 4081-4087. LIAO J, WEI X Q, XIAO Y Q, et al. Pollution characteristics and risk assessment of antibiotics in Lianhua reservoir [J]. Environmental Science, 2020, 41(9): 4081-4087(in Chinese).
[45] 陈永山, 章海波, 骆永明, 等. 苕溪流域典型断面底泥14种抗生素污染特征 [J]. 环境科学, 2011, 32(3): 667-672. CHEN Y S, ZHANG H B, LUO Y M, et al. Investigation of 14 selected antibiotics in sediments of the typical cross sections of tiaoxi river [J]. Environmental Science, 2011, 32(3): 667-672(in Chinese).
[46] ZHOU L J, YING G G, ZHAO J L, et al. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in Northern China [J]. Environmental Pollution, 2011, 159(7): 1877-1885. doi: 10.1016/j.envpol.2011.03.034 [47] YANG J F, YING G G, ZHAO J L, et al. Simultaneous determination of four classes of antibiotics in sediments of the Pearl Rivers using RRLC-MS/MS [J]. Science of the Total Environment, 2010, 408(16): 3424-3432. doi: 10.1016/j.scitotenv.2010.03.049 [48] LYU J, YANG L S, ZHANG L, et al. Antibiotics in soil and water in China-a systematic review and source analysis [J]. Environmental Pollution, 2020, 266: 115147. doi: 10.1016/j.envpol.2020.115147 [49] PAN C Y, BAO Y Y, XU B T. Seasonal variation of antibiotics in surface water of Pudong New Area of Shanghai, China and the occurrence in typical wastewater sources [J]. Chemosphere, 2020, 239: 124816. doi: 10.1016/j.chemosphere.2019.124816 [50] YAO L L, WANG Y X, TONG L, et al. Seasonal variation of antibiotics concentration in the aquatic environment: A case study at Jianghan Plain, central China [J]. Science of the Total Environment, 2015, 527/528: 56-64. doi: 10.1016/j.scitotenv.2015.04.091 [51] 刘昔, 王智, 王学雷, 等. 我国典型区域地表水环境中抗生素污染现状及其生态风险评价 [J]. 环境科学, 2019, 40(5): 2094-2100. LIU X, WANG Z, WANG X L, et al. Status of antibiotic contamination and ecological risks assessment of several typical Chinese surface-water environments [J]. Environmental Science, 2019, 40(5): 2094-2100(in Chinese).
[52] SARMAH A K, MEYER M T, BOXALL A B A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment [J]. Chemosphere, 2006, 65(5): 725-759. doi: 10.1016/j.chemosphere.2006.03.026 [53] ZHANG R J, ZHANG R L, YU K F, et al. Occurrence, sources and transport of antibiotics in the surface water of coral reef regions in the South China Sea: Potential risk to coral growth [J]. Environmental Pollution, 2018, 232: 450-457. doi: 10.1016/j.envpol.2017.09.064 [54] CHEN Y H, CUI K P, HUANG Q L, et al. Comprehensive insights into the occurrence, distribution, risk assessment and indicator screening of antibiotics in a large drinking reservoir system [J]. Science of the Total Environment, 2020, 716: 137060. doi: 10.1016/j.scitotenv.2020.137060 [55] 王娅南, 黄合田, 彭洁, 等. 贵州草海喀斯特高原湿地水环境中典型抗生素的分布特征 [J]. 环境化学, 2020, 39(4): 975-986. doi: 10.7524/j.issn.0254-6108.2019090103 WANG Y N, HUANG H T, PENG J, et al. Occurrence and distribution of typical antibiotics in the aquatic environment of the wetland Karst plateau in Guizhou [J]. Environmental Chemistry, 2020, 39(4): 975-986(in Chinese). doi: 10.7524/j.issn.0254-6108.2019090103
[56] LIU S, WANG C, WANG P F, et al. Anthropogenic disturbances on distribution and sources of pharmaceuticals and personal care products throughout the Jinsha River Basin, China [J]. Environmental Research, 2021, 198: 110449. doi: 10.1016/j.envres.2020.110449 [57] CHEN L L, LI H P, LIU Y, et al. Distribution, residue level, sources, and phase partition of antibiotics in surface sediments from the inland river: A case study of the Xiangjiang River, south-central China [J]. Environmental Science and Pollution Research International, 2020, 27(2): 2273-2286. doi: 10.1007/s11356-019-06833-0 [58] WANG Y Q, LIU Y, LU S Y, et al. Occurrence and ecological risk of pharmaceutical and personal care products in surface water of the Dongting Lake, China-during rainstorm period [J]. Environmental Science and Pollution Research International, 2019, 26(28): 28796-28807. doi: 10.1007/s11356-019-06047-4 [59] WANG W X, ZHOU L J, GU X H, et al. Occurrence and distribution of antibiotics in surface water impacted by crab culturing: A case study of Lake Guchenghu, China [J]. Environmental Science and Pollution Research International, 2018, 25(23): 22619-22628. doi: 10.1007/s11356-018-2054-7 [60] 杨俊, 王汉欣, 吴韵斐, 等. 苏州市水环境中典型抗生素污染特征及生态风险评估 [J]. 生态环境学报, 2019, 28(2): 359-368. YANG J, WANG H X, WU Y F, et al. Occurrence, distribution and risk assessment of typical antibiotics in the aquatic environment of Suzhou City [J]. Ecology and Environmental Sciences, 2019, 28(2): 359-368(in Chinese).
[61] 徐晖, 吴明红, 徐刚. 高效液相色谱-串联质谱法对水环境中12种抗生素的检测 [J]. 上海大学学报(自然科学版), 2017, 23(3): 483-490. XU H, WU M H, XU G. Determination of 12 antibiotics in aqueous environment by high performance LC-MS/MS [J]. Journal of Shanghai University (Natural Science Edition), 2017, 23(3): 483-490(in Chinese).
[62] 王大祥, 王倩倩. 淮河流域浉河区水环境中抗生素污染分布特征分析研究 [J]. 环境科学与管理, 2020, 45(3): 63-66. WANG D X, WANG Q Q. Analysis on the distribution of antibiotics pollution in the water environment of Weihe River area in Huaihe River basin [J]. Environmental Science and Management, 2020, 45(3): 63-66(in Chinese).
[63] WANG J W, WEI H, ZHOU X D, et al. Occurrence and risk assessment of antibiotics in the Xi'an section of the Weihe River, northwestern China [J]. Marine Pollution Bulletin, 2019, 146: 794-800. doi: 10.1016/j.marpolbul.2019.07.016 [64] ZHANG G D, LIU X H, LU S Y, et al. Occurrence of typical antibiotics in Nansi Lake's inflowing rivers and antibiotic source contribution to Nansi Lake based on principal component analysis-multiple linear regression model [J]. Chemosphere, 2020, 242: 125269. doi: 10.1016/j.chemosphere.2019.125269 [65] LU S, LIN C Y, LEI K, et al. Occurrence, spatiotemporal variation, and ecological risk of antibiotics in the water of the semi-enclosed urbanized Jiaozhou Bay in Eastern China [J]. Water Research, 2020, 184: 116187. doi: 10.1016/j.watres.2020.116187 [66] LEI K, ZHU Y, CHEN W, et al. Spatial and seasonal variations of antibiotics in river waters in the Haihe River Catchment in China and ecotoxicological risk assessment [J]. Environment International, 2019, 130: 104919. doi: 10.1016/j.envint.2019.104919 [67] LI N, ZHANG X B, WU W, et al. Occurrence, seasonal variation and risk assessment of antibiotics in the reservoirs in North China [J]. Chemosphere, 2014, 111: 327-335. doi: 10.1016/j.chemosphere.2014.03.129 [68] ZHANG L W, DU S Y, ZHANG X, et al. Occurrence, distribution, and ecological risk of pharmaceuticals in a seasonally ice-sealed river: From ice formation to melting [J]. Journal of Hazardous Materials, 2020, 389: 122083. doi: 10.1016/j.jhazmat.2020.122083 [69] JIA J, GUAN Y J, CHENG M Q, et al. Occurrence and distribution of antibiotics and antibiotic resistance genes in Ba River, China [J]. Science of the Total Environment, 2018, 642: 1136-1144. doi: 10.1016/j.scitotenv.2018.06.149 [70] CHEN H Y, JING L J, TENG Y G, et al. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks [J]. Science of the Total Environment, 2018, 618: 409-418. doi: 10.1016/j.scitotenv.2017.11.054 [71] DONG D M, ZHANG L W, LIU S, et al. Antibiotics in water and sediments from Liao River in Jilin Province, China: Occurrence, distribution, and risk assessment [J]. Environmental Earth Sciences, 2016, 75(16): 1-10. [72] GE L K, DONG Q Q, HALSALL C, et al. Aqueous multivariate phototransformation kinetics of dissociated tetracycline: Implications for the photochemical fate in surface waters [J]. Environmental Science and Pollution Research International, 2018, 25(16): 15726-15732. doi: 10.1007/s11356-018-1765-0 [73] GE L K, NA G S, ZHANG S Y, et al. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes [J]. Science of the Total Environment, 2015, 527/528: 12-17. doi: 10.1016/j.scitotenv.2015.04.099 [74] LI K, ZHANG P, GE L K, et al. Concentration-dependent photodegradation kinetics and hydroxyl-radical oxidation of phenicol antibiotics [J]. Chemosphere, 2014, 111: 278-282. doi: 10.1016/j.chemosphere.2014.04.052 [75] 黄宏, 李圆杏, 杨红伟. 水环境中抗生素的光降解研究进展 [J]. 环境化学, 2013, 32(7): 1335-1341. doi: 10.7524/j.issn.0254-6108.2013.07.029 HUANG H, LI Y X, YANG H W. Research progress on photodegradation of antibiotics in aqueous solution [J]. Environmental Chemistry, 2013, 32(7): 1335-1341(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.07.029
[76] WERNER J J, CHINTAPALLI M, LUNDEEN R A, et al. Environmental photochemistry of tylosin: Efficient, reversible photoisomerization to a less-active isomer, followed by photolysis [J]. Journal of Agricultural and Food Chemistry, 2007, 55(17): 7062-7068. doi: 10.1021/jf070101h [77] TONG L, EICHHORN P, PÉREZ S, et al. Photodegradation of azithromycin in various aqueous systems under simulated and natural solar radiation: Kinetics and identification of photoproducts [J]. Chemosphere, 2011, 83(3): 340-348. doi: 10.1016/j.chemosphere.2010.12.025 [78] VIONE D, FEITOSA-FELIZZOLA J, MINERO C, et al. Phototransformation of selected human-used macrolides in surface water: Kinetics, model predictions and degradation pathways [J]. Water Research, 2009, 43(7): 1959-1967. doi: 10.1016/j.watres.2009.01.027 [79] COGAN S, HAAS Y. Self-sensitized photo-oxidation of Para-indenylidene-dihydropyridine derivatives [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2008, 193(1): 25-32. doi: 10.1016/j.jphotochem.2007.06.003 [80] MARTIN N H, JEFFORD C W. Self-sensitized photo-oxygenation of 1-benzyl-3, 4-dihydroisoquinolines [J]. Helvetica Chimica Acta, 1981, 64(7): 2189-2192. doi: 10.1002/hlca.19810640725 [81] GE L K, CHEN J W, QIAO X L, et al. Light-source-dependent effects of main water constituents on photodegradation of phenicol antibiotics: Mechanism and kinetics [J]. Environmental Science & Technology, 2009, 43(9): 3101-3107. [82] GE L K, CHEN J W, WEI X X, et al. Aquatic photochemistry of fluoroquinolone antibiotics: Kinetics, pathways, and multivariate effects of main water constituents [J]. Environmental Science & Technology, 2010, 44(7): 2400-2405. [83] 葛林科. 水中溶解性物质对氯霉素类和氟喹诺酮类抗生素光降解的影响[D]. 大连: 大连理工大学, 2009. GE L K. Effects of aqueous dissolved matter on photodegradation of phenicol and fluoroquinolone antibiotics[D]. Dalian: Dalian University of Technology, 2009(in Chinese).
[84] 常海莎. 大环内酯类抗生素在水体中的光降解及毒性变化研究[D]. 石河子: 石河子大学, 2018. CHANG H S. Study on the photodegradation and change of toxicity of macrolide antibiotics in aqueous environment[D]. Shihezi: Shihezi University, 2018(in Chinese).
[85] BATCHU S R, PANDITI V R, O'SHEA K E, et al. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate [J]. Science of the Total Environment, 2014, 470/471: 299-310. doi: 10.1016/j.scitotenv.2013.09.057 [86] WANG H L, WANG M, WANG H, et al. Aqueous photochemical degradation of BDE-153 in solutions with natural dissolved organic matter [J]. Chemosphere, 2016, 155: 367-374. doi: 10.1016/j.chemosphere.2016.04.071 [87] QU S, KOLODZIEJ E P, CWIERTNY D M. Phototransformation rates and mechanisms for synthetic hormone growth promoters used in animal agriculture [J]. Environmental Science & Technology, 2012, 46(24): 13202-13211. [88] VOIGT M, JAEGER M. On the photodegradation of azithromycin, erythromycin and tylosin and their transformation products - A kinetic study [J]. Sustainable Chemistry and Pharmacy, 2017, 5: 131-140. doi: 10.1016/j.scp.2016.12.001 [89] LATCH D E, STENDER B L, PACKER J L, et al. Photochemical fate of pharmaceuticals in the environment: Cimetidine and ranitidine [J]. Environmental Science & Technology, 2003, 37(15): 3342-3350. [90] LI W, LYU B L, LI J P, et al. Phototransformation of roxithromycin in the presence of dissolved organic matter: Characteriazation of the degradation products and toxicity evaluation [J]. Science of the Total Environment, 2020, 733: 139348. doi: 10.1016/j.scitotenv.2020.139348 [91] DODD M C, KOHLER H P, von GUNTEN U. Oxidation of antibacterial compounds by ozone and hydroxyl radical: Elimination of biological activity during aqueous ozonation processes [J]. Environmental Science & Technology, 2009, 43(7): 2498-2504. [92] CHAMBERLAIN E, ADAMS C. Oxidation of sulfonamides, macrolides, and carbadox with free chlorine and monochloramine [J]. Water Research, 2006, 40(13): 2517-2526. doi: 10.1016/j.watres.2006.04.039 [93] VIONE D, MAURINO V, MINERO C, et al. Phenol chlorination and photochlorination in the presence of chloride ions in homogeneous aqueous solution [J]. Environmental Science & Technology, 2005, 39(13): 5066-5075. [94] MILL T. Predicting photoreaction rates in surface waters [J]. Chemosphere, 1999, 38(6): 1379-1390. doi: 10.1016/S0045-6535(98)00540-2 [95] LUO X, WEI X X, CHEN J W, et al. Rate constants of hydroxyl radicals reaction with different dissociation species of fluoroquinolones and sulfonamides: Combined experimental and QSAR studies [J]. Water Research, 2019, 166: 115083. doi: 10.1016/j.watres.2019.115083 [96] GE L K, ZHANG P, HALSALL C, et al. The importance of reactive oxygen species on the aqueous phototransformation of sulfonamide antibiotics: Kinetics, pathways, and comparisons with direct photolysis [J]. Water Research, 2019, 149: 243-250. doi: 10.1016/j.watres.2018.11.009 [97] VOIGT M, SAVELSBERG C, JAEGER M. Photodegradation of the antibiotic spiramycin studied by high-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight mass spectrometry [J]. Toxicological & Environmental Chemistry, 2017, 99(4): 624-640. [98] LITTER M I, QUICI N. Photochemical advanced oxidation processes for water and wastewater treatment [J]. Recent Patents on Engineering, 2010, 4(3): 217-241. doi: 10.2174/187221210794578574 [99] WANG J L, SONG M R, CHEN B Y, et al. Effects of pH and H2O2 on ammonia, nitrite, and nitrate transformations during UV254nm irradiation: Implications to nitrogen removal and analysis [J]. Chemosphere, 2017, 184: 1003-1011. doi: 10.1016/j.chemosphere.2017.06.078 [100] BENSALAH N, KHODARY A, ABDEL-WAHAB A. Kinetic and mechanistic investigations of mesotrione degradation in aqueous medium by Fenton process [J]. Journal of Hazardous Materials, 2011, 189(1/2): 479-485. [101] GOZLAN I, KOREN I. Identification, mechanisms and kinetics of macrolide degradation product formation under controlled environmental conditions [J]. Journal of Environmental Analytical Chemistry, 2016, 3(1): 100171. [102] 张倩, 杨琛, 莫德清, 等. 水溶液性质对泰乐菌素光降解的影响 [J]. 农业环境科学学报, 2014, 33(12): 2444-2449. ZHANG Q, YANG C, MO D Q, et al. Effects of aqueous solution properties on tylosin photolysis [J]. Journal of Agro-Environment Science, 2014, 33(12): 2444-2449(in Chinese).
[103] 常海莎, 闫豫君, 鲁建江, 等. 螺旋霉素在水溶液中的光降解 [J]. 环境化学, 2018, 37(6): 1343-1350. doi: 10.7524/j.issn.0254-6108.2017091703 CHANG H S, YAN Y J, LU J J, et al. Photodegradation of spiramycin in aqueous solution [J]. Environmental Chemistry, 2018, 37(6): 1343-1350(in Chinese). doi: 10.7524/j.issn.0254-6108.2017091703
[104] TERCERO ESPINOZA L A, NEAMŢU M, FRIMMEL F H. The effect of nitrate, Fe(Ⅲ) and bicarbonate on the degradation of bisphenol A by simulated solar UV-irradiation [J]. Water Research, 2007, 41(19): 4479-4487. doi: 10.1016/j.watres.2007.06.060 [105] NEAMŢU M, POPA D M, FRIMMEL F H. Simulated solar UV-irradiation of endocrine disrupting chemical octylphenol [J]. Journal of Hazardous Materials, 2009, 164(2/3): 1561-1567. [106] HALLADJA S, AMINE-KHODJA A, TER HALLE A, et al. Photolysis of fluometuron in the presence of natural water constituents [J]. Chemosphere, 2007, 69(10): 1647-1654. doi: 10.1016/j.chemosphere.2007.05.035 [107] GE L K, CHEN J W, ZHANG S Y, et al. Photodegradation of fluoroquinolone antibiotic gatifloxacin in aqueous solutions [J]. Chinese Science Bulletin, 2010, 55(15): 1495-1500. doi: 10.1007/s11434-010-3139-y [108] FISHER J M, REESE J G, PELLECHIA P J, et al. Role of Fe(Ⅲ), phosphate, dissolved organic matter, and nitrate during the photodegradation of domoic acid in the marine environment [J]. Environmental Science & Technology, 2006, 40(7): 2200-2205. [109] 齐会勉, 吕亮, 乔显亮. 抗生素在土壤中的吸附行为研究进展 [J]. 土壤, 2009, 41(5): 703-708. QI H M, LV L, QIAO X L. Progress in sorption of antibiotics to soils [J]. Soils, 2009, 41(5): 703-708(in Chinese).
[110] WEI X X, CHEN J W, XIE Q, et al. Distinct photolytic mechanisms and products for different dissociation species of ciprofloxacin [J]. Environmental Science & Technology, 2013, 47(9): 4284-4290. [111] ZHANG Z C, XIE X D, YU Z Q, et al. Influence of chemical speciation on photochemical transformation of three fluoroquinolones (FQs) in water: Kinetics, mechanism, and toxicity of photolysis products [J]. Water Research, 2019, 148: 19-29. doi: 10.1016/j.watres.2018.10.027 [112] BONVIN F, OMLIN J, RUTLER R, et al. Direct photolysis of human metabolites of the antibiotic sulfamethoxazole: Evidence for abiotic back-transformation [J]. Environmental Science & Technology, 2013, 47(13): 6746-6755. -




 下载:
下载: