-
大气污染的日益严重和全球变暖的加速到来[1],原因之一是在生活和生产中产生大量尾气、废气和烟气,这些气体含有含碳、氮、硫[2]等元素的化合物不断地污染环境. 目前已经有多种方法吸收这些污染物,但或多或少的存在着一些不足. 例如工业上被广泛应用于吸收二氧化硫的石灰石技术[3]会耗费大量的水资源并排放出二氧化碳,且这种工艺不可逆;通过离子液体吸收废气[4-5]的能力很强且没有副产物生成,但由于离子液体价格昂贵、黏度高、有毒性等因素制约了其发展[6].
低共熔溶剂(deep eutectic solvent, 简称DESs)是一类新型离子液体(ionic liquid,简称IL)类似物[7],其具有离子液体的许多特征和优良性质,同时相较于离子液体,DES合成过程简单、成本低并且无毒性、可生物降解[8],所以DES被视为离子液体的绿色替代溶剂并在溶解[9-10]、材料制备[11]、催化[12-13]以及电化学[14-16]等众多领域都有广泛的应用,目前在污染物气体吸收领域也展现出广阔的前景. DES的最大优点是可以通过简单地改变氢键供体与受体的种类而设计出成千上万种不同的DESs,且不同DESs吸收某种气体的能力各有高低,因此,寻找适用于能高效吸收不同气体的DES是一个重点也是一个难点. 如果通过在实验室开展有限次实验制备DESs再进行吸收不同气体的实验,会耗费大量的人力物力,而使用计算机进行分子模拟实验可以方便地建立各种DESs的模型,对DES的结构、性质以及和气体的相互作用进行模拟计算[17]. 目前国内外已经陆续有学者使用分子模拟技术来模拟DES对气体的吸收,这种方法有利于高效地选择DESs并预测实验结果,同时通过各种数据分析可以更加清晰地展示其中的作用机理. 本文对低共熔溶剂和分子模拟技术进行简要介绍,并对DES吸收有害气体的分子模拟研究进展进行综述,为人们设计不同的DES合成出更加高效的气体捕捉剂提供基本的理论指导.
-
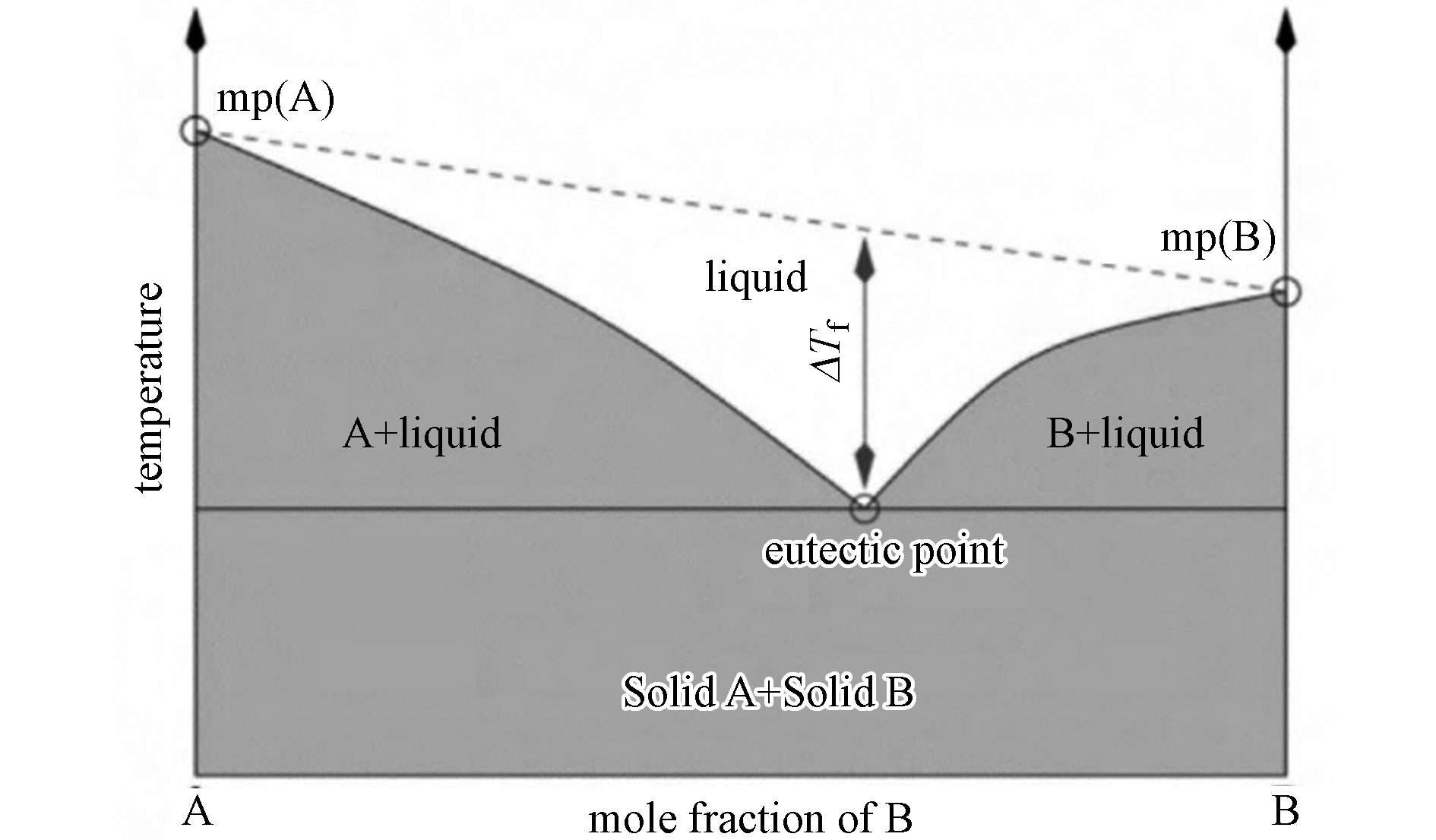
在2003年Abbott等[18]将氯化胆碱和尿素经过混合搅拌制得一种低共熔混合物,这种混合物的熔点比其任意组分的熔点显著降低(图1),通常表现出液态溶剂的性质,故将其命名为低共熔溶剂,也称为低共熔离子液.
DES的制备方法非常简单,只需要将两种或多种组分混合加热便可以获得相应的低共熔混合物[19] 这些不同组分之间一般通过氢键作用力进行结合,由此可将其定义为氢键供体(hydrogen-bonding donor,HBD)和氢键受体(hydrogen-bonding acceptor, HBA)[20].
随着研究的不断深入,近年来人们用不同种类、摩尔比的氢键供体和氢键受体组合制备出多种多样的低共熔溶剂. Smith等[22]提出低共熔溶剂可以由一个通式Cat+X−zY来描述,在这个公式中Cat+代表所有的铵、磷、锍阳离子,X表示碱,一般为卤化物阴离子,Y是一类酸分子,而z则表示其数量. 根据DESs组分的不同可以大致分为4类,如表1所示,类型Ⅰ是季铵盐和金属卤化物体系,但可以形成DESs的非水合金属卤化物较少;而水和金属卤化物与季铵盐组合而成的是第Ⅱ类DESs;类型Ⅲ是季铵盐和HBD形成的一类可以溶解过渡金属的DESs,通过调整HBD就可以改变DESs的物理性质;类型Ⅳ是金属卤化物与HBD的混合物.
低共熔溶剂有着一系列优良的物理化学性质,这使其在溶解[23]、萃取分离、材料制备、催化以及电化学等众多领域都有广泛的应用[24-29],如使用氯化胆碱类DESs对生活中常见的丝瓜络进行处理,可成功分离提取出较高含量的丝瓜络纤维素[30],进一步研究发现氯化胆碱/二水合草酸体系的DESs具有分离三大素和促进纤维素纳米化的双重作用,结合超声辅助处理,可制备含木质素的纳米纤维素[31]. 也有学者以氯化胆碱/三乙醇胺DES为反应介质,用不同原料合成的具有介孔结构的新型钠离子电池的负极材料,且有着很好的电化学性能[32]. 王淑波等[33]利用乙二醇与氯化胆碱(物质的量比为2∶1)DES可以有效破坏乙醇/水混合溶液共沸点的特性,对乙醇/水溶液进行蒸馏脱水,制备出高浓度的燃料级乙醇,由于这种DES的沸点高,萃取过程中能量消耗少,相比于有毒的乙二醇做萃取剂的方法有着显著的优势. 还有研究者将尿素、金属氯化物、三聚氰胺合成的DESs进行热解,使金属氢氧化物均匀的负载到C3N4上得到了一种高效的光催化固氮复合材料[34]. 由此可见,可以通过简单地改变氢键供体与受体的种类而设计出成千上万种具有不同功能作用的DESs,利用DESs的这种可设计性,可以根据实际需要,选取不同的原料,实现其特定结构与功能的构建.
-
分子模拟技术(molecular simulation,MS)是近年来飞速发展的一种理论计算技术,是采用合适的计算机软件,模拟化学体系的微观结构,利用数值运算、分析统计等方法对系统的平衡热力学、统计动力学等性质进行理论预测,建立多维场景以模拟分子的静态、动态结构变化,实现实验手段难以考察的化学分子作用过程与作用位点[35-36],通过计算机对物质的微观结构和运动进行模拟计算可以得到有关物质的一系列性质参数以及宏观特性,并帮助预测实验结果以及阐述作用机理[37]. 研究者只需对简单分子结构进行模拟并拟合其热力学性质就能对DESs进行快速筛选,从大量配体中选择满足需求的组分,同时还能预测各种气体在DESs中的溶解度,并分析其作用机理. 相较于传统实验,分子模拟实验所需成本较低、安全性高、结果准确度高、可以大量节省时间和资源,并且计算机模拟可以得到一些无法从实验室获取的信息[38]. 因此,运用分子模拟技术进行模拟实验正在成为目前的研究热点之一.
-
根据分子模拟方法所运用的理论基础可将其分为量子力学方法和经典力学方法两大类[39],在经典力学方法中又可以划分出多种模拟方法,如蒙特卡罗方法(Monte Carlo method, MC)、分子动力学模拟(molecular dynamics simulation, MD)、分子力学方法(molecular mechanics, MM)等,这些分子模拟方法基于不同的原理通过计算机对原子或分子模型进行计算,进而得出现代实验方法难以测算的数据参数.
-
量子力学计算方法是基于量子力学(quantum mechanics)[40],几乎可以计算出和分子有关的一切性质且与实际实验结果非常相近. 量子力学方法使用最多的计算方法是从头算方法(abinitio method)[41],这种方法通过一定的原理将电子的波函数转化为其他函数形式进行计算以研究分子中电子的行为. 从头算方法计算结果极为精确,但其可以计算的体系非常小并且计算速度极慢. 随后人们通过研究不同的波函数转化方式开发出半经验方法(semi-empirical method),这种方法相比于从头算方法提升了计算速度且扩大了计算体系. 量子力学方法一般被用于计算精确的DESs构型,保证模拟结果的准确性,同时对DESs与气体的相互作用形式进行定性、定量计算. 量子力学方法尽管计算结果精确,但其对电子的计算过程困难,故不太适用于较大体系,人们逐渐开始研究非量子计算方法并取得一系列成果.
-
蒙特卡罗方法[42]是最早用于研究大分子体系的非量子计算方法. 蒙特卡罗方法通过建立体系中分子或原子的随机运动的模型,选取多个随机数,应用统计学中的方法计算概率,从而得到体系的一系列计算结果,随机数选取越多则结果误差越小. 但这种方法只能计算出结果的平均值,并不能对体系的动态过程进行研究,故逐渐被后续发展起来的分子动力学模拟所取代.
-
分子力学方法又被叫做力场方法(force field method),是目前应用较多的研究手段,主要是用来计算分子的能量并找出其平衡构型[43]. 分子力学的基本思想是由Andrews在1930年提出[44]:每个分子的化学键都有其特殊的键长、键角值,分子会减小内部的非键作用(van der Waals力)并使键长、键角值接近原本的数值,从而变为平衡构型. 根据玻恩-奥本海默近似(Born-Oppenheimer approximation,简称BO近似,又称绝热近似)原理,在分子力学中不会去计算电子相互作用,所以计算量远远小于从头算量子力学方法,且在某些情况下分子力学的计算结果与量子力学计算结果相差不大. 因此研究者一般将分子力学方法用于DESs团簇构型的初筛选,随后再进行量子力学计算可以大大缩短模拟时间成本. 分子力学方法很适合研究计算量庞大的大分子体系,如药物、生物大分子等.
-
分子动力学模拟是目前使用最广泛的研究复杂微观系统的技术[45]. 在分子动力学模拟中,一个体系内每个原子核都会受到其他所有原子核以及电子形成的势场作用而做牛顿运动,计算机求解牛顿运动方程就可以得到粒子的运动规律,从而得到体系的种种性质[46]. 分子动力学模拟的动态特性可以快速分析出DESs配体之间的相互作用位点,并提供大量可能的作用构型并结合分子力学模拟以及量子力学模拟进行筛选. 同时分子动力学模拟考虑了更多影响体系变化的因素如温度、压强等,从而使DESs吸收气体模拟结果更加符合实际. 这种模拟方法的结果虽然不如从头算动力学方法计算精确,但其优点就在于可以计算的体系非常大并且计算速度较快. 目前已经有多款分子动力学模拟计算软件被开发并应用于物理、化学、医学等多个领域.
这些模拟方法各有所长,所应用的领域也各不相同,量子力学方法与分子动力学方法是研究分子间相互作用以及其反应性最常用的方法. 量子力学方法研究的是真空条件下分子之间由电子变化而导致的一系列性质和作用,分子动力学模拟则是考虑分子在给定的力场条件下进行牛顿运动,对其运动规律求解得到体系的动力学性质. 蒙特卡洛模拟和分子力学方法由于其计算速度快,相对精度较低,一般只会用于模拟前的大量构型搜索和初步结构优化,减轻后续计算负担.
-
随着分子模拟技术的广泛应用,各大公司不断开发更新不同的模拟软件以满足人们的不同研究需求. 以下将介绍几款目前使用人数较多的分子模拟软件.
-
Gaussian[47]软件是目前较为流行的一款计算功能强大、应用范围广的量子化学软件. 这款软件基于量子力学理论,通过一些简单的命令就可以计算并预测体系几乎所有的性质,如过渡态能量和结构、分子轨道、原子电荷和电势、红外光谱等. Gaussian软件可以研究气、液、固相多种物质形态,并可以很快计算出体系的最稳定结构使结果更加精确.
-
ORCA[48]是一款完全免费的量子化学程序,无需对其代码进行编译可以直接从其官网下载安装,随着ORCA不断对其程序进行更新发展,其功能更加完善且在计算速度、结果精确度上都有着很大的提高,越来越受到研究人员的欢迎. 这款软件最大的优势在于其充分利用了密度拟合技术结合独家的COSX方法,并在计算时默认开启RI加速,使得其在处理较大体系的计算速度以及精度上都有着惊人的提升. 但是,ORCA的短板在于其没有开发出相应的图形界面且输入文件相对较难书写,计算精度相较于Gaussian还有一定不足.
-
GROMACS[49]是一个可以进行分子动力学模拟并对产生的轨迹数据进行分析的程序包. 除了分子动力学方法,GROMACS还可以应用随机动力学和路径积分方法来模拟体系中的分子. 由于程序对键合作用与非键合作用的强大计算能力,使其可以对晶体、液晶、聚合物、生物大分子溶液等多种物质进行模拟研究,并且在GROMACS中不需要再编写代码来分析轨迹数据,程序会直接将其整合输出为图表的形式.
-
Materials Studio[50](简称MS)是一款由美国Accelrys公司开发研制的专门应用于材料科学领域的模拟计算软件,该软件可以很方便地建立模型并对其进行模拟计算得到系列相关性质. 软件涵盖了分子力学、量子力学的密度泛函理论(DFT)[51]、介观模型、分子动力学和统计相关等多种模拟方法,根据计算所使用的不同模拟方法软件细分出多个不同的模块以方便使用,可以帮助解决目前在催化剂、聚合物、固体与表面、晶体与衍射、结晶学和材料特性等多方面的问题.
以上各种软件各有特色,且都有其适用领域,随着分子模拟技术的不断发展进步,这些软件逐渐成为研究各种材料、溶剂和生物医药等领域难以或缺的工具.
-
目前已经有很多研究报道了使用低共熔溶剂吸收各类有害气体如:酸性气体SO2[52-56]和CO2[57-62]、氮氧化物[63]、NH3[64]等. 由于所研究的气体大多对人身体有害,通过计算机分子模拟实验可以避免直接接触,并且相对于实验室实验更加方便,还能加深人们对其中作用机理的理解.
-
气态硫化物是大气污染和损害人身体的主要物质之一,例如在工业上冶炼金属和化石燃料的燃烧等所产生大量的SO2,在一些化工过程还会产生副产品H2S等[65],如果不加以控制,这些气体会慢慢毒害人们的身体,甚至形成酸雨造成极大的破坏[66]. 传统脱硫技术主要是通过碱性脱硫剂与烟气中的硫化物反应而将其固定,但传统方法效率低、成本高还容易产生二次污染[67],发展新技术进行脱硫处理并改善环境问题是社会发展的必然趋势. 使用DESs进行硫化物的捕集很好的弥补了传统技术的不足,是一种极具发展前途的方法. 已报导的可以吸收二氧化硫的DESs已经有很多种,不同种类甚至不同配比的DESs对SO2的吸收能力都不同,通过分子模拟实验找到成本低、稳定并且吸收效果好的DESs对此类研究的后续发展有着重要意义. 其中以氯化胆碱类DESs居多,如Li[68]应用基于量子力学的多种方法对DESs-SO2的相互作用进行研究,希望能帮助人们设计出可以高效吸收SO2的低共熔溶剂. 通过对体系的电荷分析表明,氯化胆碱-甘油在和SO2分子相互作用时会发生电荷的转移,从而使DESs分子带正电,SO2分子带负电,这是DESs吸收SO2的重要因素之一.
Korotkevich等[69]使用LAMMPS软件对氯化胆碱-甘油DES以及和SO2的混合物(图2)进行了从头算分子动力学(AIMD)研究,通过径向分布函数(RDF)分析体系中不同组分间的相互作用力,发现在二氧化硫加入后,由于二氧化硫分子与氯离子形成了强络合物,影响了DESs中原有的氢键网络,原本DESs中氯离子与羟基间的相互作用都变弱,这也解释了加入二氧化硫后体系流动性增强的原因.
郭连晓[70]研究了6种氯化胆碱型DESs(配体分别是乙二醇、丙三醇、硫脲、尿素、丙二酸和乳酸)对SO2的吸收能力和机理,绝大部分DESs吸收二氧化硫的过程都是由二氧化硫分子中的硫与氯离子之间的强相互作用主导的,而二氧化硫分子中的氧与配体和阳离子上的氢形成的相互作用较弱. 在有少量水存在时,DESs吸收二氧化硫的位点并没有改变,并且吸收二氧化硫的结合能要大于无水体系,即水分子提高了对二氧化硫的吸收能力. 水分子的存在还会降低DESs的黏度并提升二氧化硫的吸收速率.
García[71]和其研究团队建立了3种氯化胆碱基DESs与SO2 、CO2和模拟的烟气成分的界面接触模型,并进行分子动力学模拟,以进一步研究DESs在吸收酸性气体时的界面作用机理. 结果表明SO2和CO2的吸收过程相似,都是先在DESs表面大量聚集,经过一段时间后DESs的吸附层进行重组,气体分子之间的相互作用减弱而与DESs阳离子的相互作用增强,气体分子便逐渐开始进入DESs内部,由于这几种DESs和SO2之间更强的相互作用使SO2的吸收过程更加迅速.
刘妍[72]对氯化胆碱和配体(乙二醇、丙三醇、丙二酸、硫脲、尿素)的电荷分布以及结合能进行分析发现,氯化胆碱和配体的物质的量比为1∶2时所形成的DES团簇最稳定. 将5种DESs吸收SO2的过程分别进行分子模拟,发现DESs与二氧化硫的结合方式可以分为两种情况:第一种适用于配体为醇类和酸类的DESs,二氧化硫会进入DESs内部并破坏内部氢键与氯离子形成S—Cl−相互作用. 另一种情况则是DESs的配体是硫脲和尿素时,二氧化硫会附着在DESs表面,不破坏DESs的构型与HBD中电负性较大的原子结合形成O—S、S—S相互作用. 在这两种不同的结合机理下合理进行预测,若DESs中HBD与氯离子的作用力越弱或者二氧化硫与HBD的作用力越强,则DESs吸收二氧化硫的能力越强.
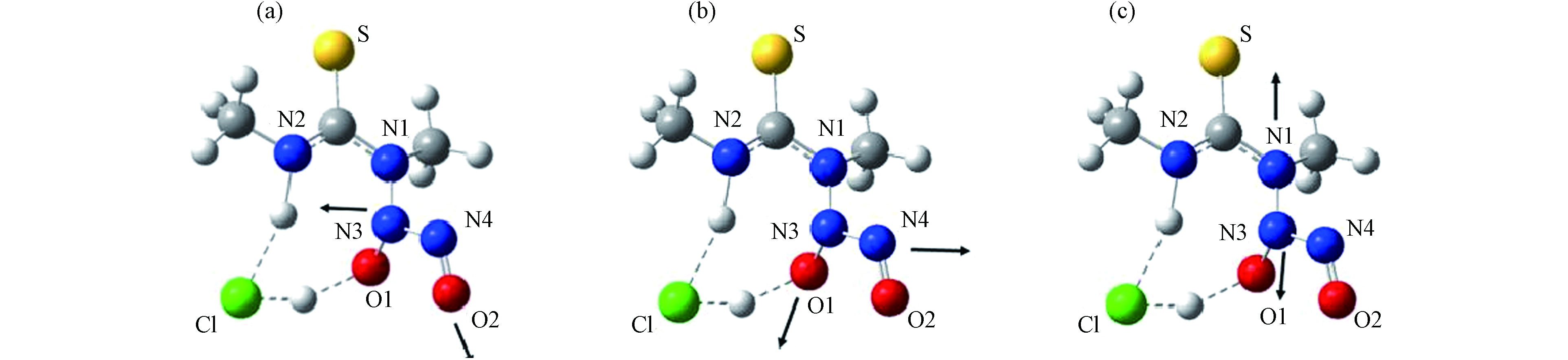
在实际应用中,不仅要求DESs对二氧化硫的吸收能力要好,对SO2的解吸能力一样重要,很多DESs由于和SO2之间强烈的化学相互作用导致后续难以对SO2进行解吸. Hou等[73]以1-丁基-3-甲基咪唑鎓盐(BmimCl)、乙烯脲(EU)和4-甲基咪唑(4-CH3-Im)为原料制备出一种三元DES,这种DES可以在烟气中高效的捕获低浓度的SO2,并具有出色的解吸能力如图3. 使用Gaussian 09软件进行量子化学计算,发现在吸收过程中4-甲基咪唑中的N原子会和SO2中的S原子逐渐靠近,作用力增强. DES中的氯离子会转移N原子上的H+使SO2形成S—OH结构,而乙烯脲可能正是通过影响这一过程来调控DES对SO2的吸收和解吸.
Atilhan等[74]应用密度泛函理论和分子中原子的量子理论(QTAIM)分析了氯化胆碱、1-丁基-3-甲基咪唑氯化物(Bmim)和1-乙基-3-甲基咪唑氯化物(EMIM)作为HBA,乙酰胺、乙酰丙酸、柠檬酸、果糖、乙二醇、甘油和乳酸作为HBD的共11种不同DESs与SO2的相互作用. 首先使用Avogadro软件对所有DESs-SO2构型进行结构和能量优化,并选择其中能量最低的构型进行研究. 对DESs与SO2的相互作用过程模拟发现DESs在吸收SO2过程中自身结构并不会被破坏,理论上可以重复使用. QTAIM分析可以计算出体系中各种相互作用的类型与大小,Reduced Density Gradient(RDG)分析进一步证明了DESs和SO2间主要为范德华型相互作用.
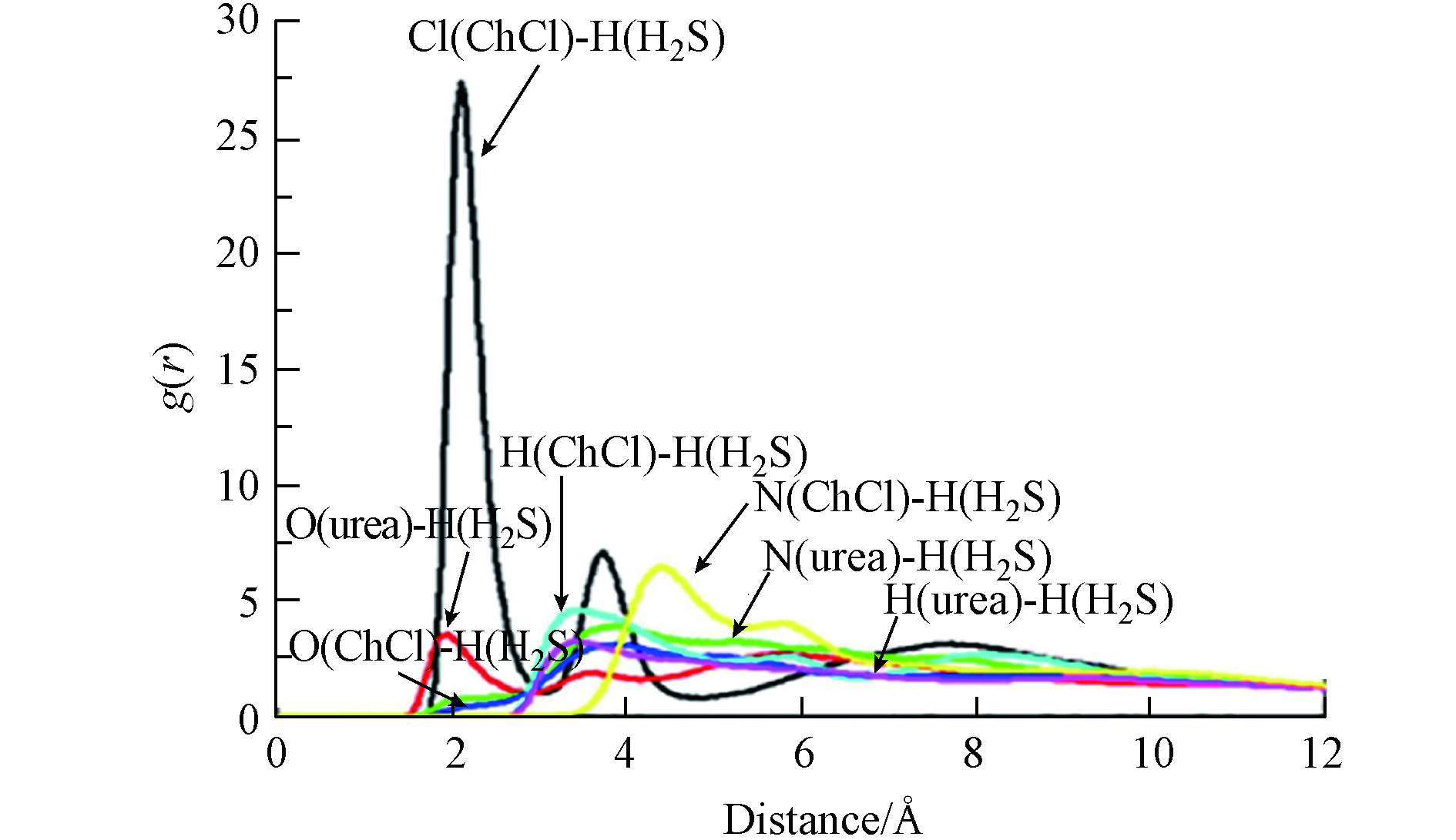
Liu等[75]使用蒙特卡洛(Monte Carlo method)模拟和量子力学方法研究了氯化胆碱-尿素吸收H2S气体的机理,这是目前为数不多的关于DESs吸收H2S气体的分子模拟研究. 体系中的相互作用被RDF分析(图4)清晰的展现出来,吸收过程主要是通过氯离子和H2S中氢原子之间较强的相互作用主导,因此可以通过调整DESs中组分的摩尔比来改变其吸收H2S的能力.
综上所述,通过分子模拟研究DESs对硫化气体的吸收主要体现在对SO2气体的吸收,而各种不同的DESs吸收SO2的机理有所差异,总结来说可以分为以下两种:一是认为二氧化硫中的硫原子一般会与DESs中的氯离子形成强相互作用,而氧原子与DESs中某些原子形成弱相互作用. 二是认为DESs在吸收SO2过程中可能会发生电荷转移导致两者之间相互吸引. 而DESs吸收H2S气体的分子模拟研究不多,主要观点也是认为H2S是通过氢原子和DESs氯原子之间的氢键作用被吸收.
-
随着科技的不断发展进步,各种新型绿色可再生能源也正在不断被开发利用,但化石燃料在未来几年仍然会在能源生产中占很大比重,这不可避免地导致大量CO2排放而加剧全球变暖和海洋酸化,因而CO2气体的捕获自然也成为近年来的研究热点. 目前工业上主要使用烷醇胺[76]吸收二氧化碳气体,但这种方法成本高,且吸收剂容易蒸发或降解,开发合适的二氧化碳捕获技术同样显得尤为关键.
García等[77]首次使用密度泛函理论(DFT)在分子层面上研究了二氧化碳吸收的短程相互作用. 所选用的DESs为氯化胆碱-甘油和氯化胆碱-丙二酸,并且考虑了氯化胆碱与氢键供体在3种不同物质的量比情况下捕获二氧化碳的能力. 对DESs几何构型与能量的计算表明DESs中的主要相互作用在氯离子和羧基或者羟基中的氢原子之间产生. 而不同的DESs构型在吸收CO2时所产生的分子间相互作用不同,CO2分子主要和氯离子、HBD分子作用紧密,同时还会产生部分羟基与CO2的相互作用. 由于氯化胆碱的吸湿性,这类DESs在使用过程中不可避免地会吸收水分,通过研究有水体系下DESs吸收CO2的作用位点与相互作用能表明,H2O分子与DESs的亲和力更强,会抢占CO2的最佳作用位点,但同时会通过电荷的转移增强CO2与DESs的结合强度,相互作用能的计算结果显示水的存在并不会影响DESs对CO2的捕获能力. 为了较为真实地模拟烟气,后续计算模型中加入了一定比例的O2、CO2、N2、H2O分子,自扩散系数的计算结果表明体系中的其他分子不会对CO2吸收造成影响,这也验证了他们之前实验中水与DESs捕获二氧化碳能力关系的结论[71].
Ullah等[78]通过实验与分子模拟相结合的方法,研究了氯化胆碱-乙酰丙酸(LEV)DES的密度和黏度,得出了分子模拟的结果与实验数据较为吻合的结论. 使用密度泛函理论研究了体系中的短程属性,对纯DESs系统和DES-CO2系统进行了径向分布函数和空间分布函数计算,发现DES中的氢键相互作用主要是乙酰丙酸与氯化胆碱中的氯原子产生,在吸收CO2时这种相互作用不会改变,同时CO2分子与乙酰丙酸分子间形成比与离子更强的相互作用.
Salehi等[79]使用蒙特卡罗(MC)模拟方法研究了CO2、CO、H2S等气体在氯化胆碱-尿素、氯化胆碱-乙二醇中的溶解度,溶解度通过亨利常数来表征,研究所采用的力场为GAFF (Generalized AMBER Force Field) . 在分析数据时发现DESs的密度以及径向分布函数(RDF)的计算结果与其他学者的结果有些许出入,这主要是由于选择了不同的模拟方法和不同力场参数所导致. 作者通过降低离子的电荷标度因子(一种力场参数),发现离子间的相互作用也随之降低,体系的密度随之降低,气体在DESs中的溶解度会升高,所以为体系选择合适的力场参数可以使模拟更加接近真实水平. 计算出的亨利常数表明H2S、CO2在这两种DESs中的溶解性较好,CO则相对难溶.
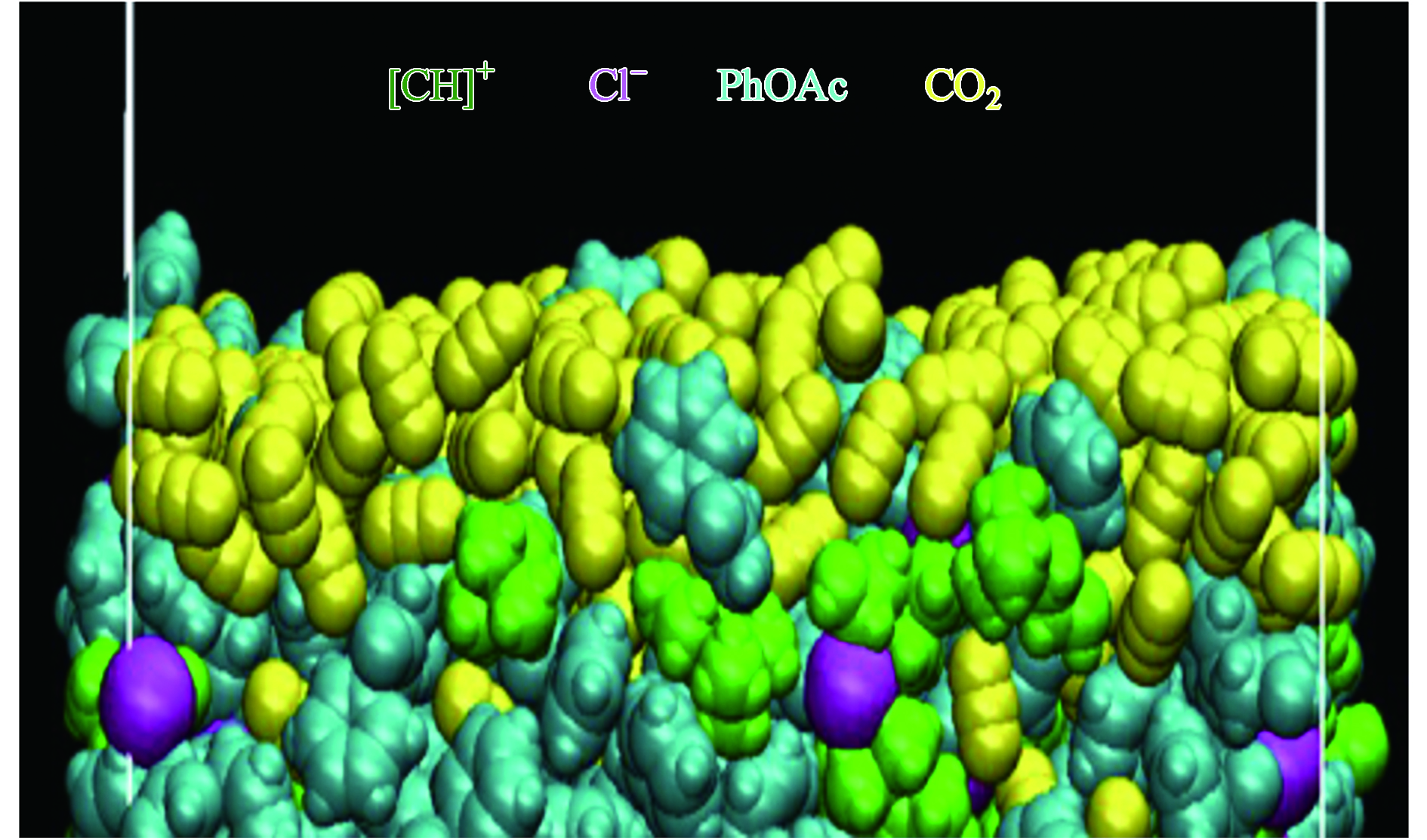
Altamash等[80]使用MDynaMix v.5.2软件对氯化胆碱-苯乙酸的结构、性质进行动力学模拟并研究了其界面行为来推断对CO2捕集的可行性. 采用密度泛函理论计算体系的相互作用能表明,氯化胆碱-苯乙酸相比于之前报道的氯化胆碱-乙酰丙酸对CO2有更好的捕获性能. 在吸收CO2分子过程DES的体积膨胀非常小,即DES可以在不削弱其分子间相互作用的情况下重新排列自身结构. 这个现象在Wang等[81]的实验中也被发现并佐证,在吸收CO2前后,DES的径向分布函数峰的位置与大小几乎相差不大,且DES体系中组分的相互作用能前后变化不超过2%. 对DES吸收烟气中二氧化碳的界面行为模拟如图5表明[80],在吸收CO2时界面处会出现3个不同的吸附区域:最外层的N2吸附层、靠近cl−的水分子吸附层和靠近苯乙酸的CO2分子层. 水分子层的存在虽然不会影响CO2的吸收但会降低被吸附的CO2分子向溶剂内部扩散的速度.
Shen等[82]利用DESs对不同气体的溶解度不同这一特点对甲烷/二氧化碳气体进行分离,希望找到较为有效的气体分离系统. 所使用的DESs体系为氯化胆碱/乙二醇和氯化胆碱/乙酰丙酸,物质的量比均为1∶2,随后建立了带有孔隙的石墨与二氧化钛模型,让混合气体通过填充有DESs的孔隙,主要是通过比较不同条件下气体的溶解性、扩散性和渗透性来表征DESs分离二氧化碳的能力. 然而气体在DESs膜内的渗透性与吸附和扩散过程都紧密相关,不同的模型中哪种行为占据了主导地位就会导致不同的渗透性.
以上研究表明,不同的氯化胆碱基DESs对CO2均有较好的吸收效果,一般是通过氯化胆碱中的氯离子或HBD与CO2产生相互作用. CO2与DESs所产生的氢键相互作用以及范德华相互作用是吸收的主导因素,因此通过选择与CO2具有强相互作用的配体制备DESs可以达到更好的吸收效果. 而在实际应用中还要考虑温度、压强、湿度等因素影响以及吸收后的解吸能力等,这些是分子模拟研究难以完成的.
-
有毒有害的含氮化合物一般包括氮氧化物(NO)和NH3等,其中氮氧化物会破坏臭氧层、形成酸雨造成大气污染,还会影响身体健康导致一系列疾病[83],但目前关于DESs对这类物质吸收的相关研究相对还较少. Hizaddin等[84]通过COSMO-RS模拟对94种DESs脱氮能力进行了实验预测. 发现季铵盐和磷组成的DESs有较好的脱氮潜能,而这种能力的强弱与DESs中阴、阳离子的种类和HBD及其摩尔比都相关.
魏光森[85]使用1,3-二甲基硫脲/四丁基氯化膦DESs吸收NO气体,通过核磁共振氢谱图发现在吸收NO以后DES的氢谱图出现了新的峰,分析发现是1,3-二甲基硫脲中的一个N—H键脱氢后吸收NO分子形成新的结构. 随后通过Gaussian09软件对红外吸收峰的计算也验证了氢谱图的结果,DESs中的一个N原子作为吸收位点吸收了两摩尔的NO分子形成了—N—(NO—)—N=O的结构如图6.
张吕鸿等[86]通过Gaussian软件对以苯甲酸、尿素、咪唑和硫脲为氢键供体,离子液为氢键受体的4种DESs进行电荷密度计算,并将结果与NO吸收能力对比发现,DESs中核心原子电荷密度越低,则其去质子化能力越强,吸收NO的能力越强. DESs吸收NO是通过化学吸附作用,故提高DESs中可与NO反应的组分的比例,就可以提高吸收NO的能力. 在这项实验中以苯甲酸为HBD的DESs吸收NO的效率最高.
除了氮氧化物,氨气(NH3)也是一种有害气体,同时它又是一种重要化工原料,无论是出于大气污染的治理还是原料的回收,研究对氨气的吸收都有重要意义. Deng等[87]用NH4SCN作为氢键受体,甘油、乙二醇、尿素、乙酰胺作为氢键供体制备的DESs吸收NH3,发现每克NH4SCN-甘油(物质的量比为2:3)DESs在303 K和0.10 MPa条件下可以吸收0.223 g的NH3,通过与其他文献的数据比较发现,这个吸收能力高于所有已经报道的DESs. 通过分子动力学模拟和密度泛函理论计算发现NH4SCN-甘油DESs与NH3之间存在的是一种较弱的相互作用. 为了更好地实施绿色可持续发展概念,研究者们[88]选择氯化胆碱和天然糖(木糖、核糖和果糖)等均无毒且可生物降解的原料,混合制备成真正意义上“绿色溶剂”的DESs用于吸收NH3,通过核磁共振氢谱表明在吸收了氨气以后,DESs中羟基的H+峰消失了,说明这类DESs中的羟基是NH3的吸收位点,随后进行的量子化学计算也证明了羟基与NH3之间形成了氢键相互作用.
由于普通二元DESs在吸收氨气时,HBD的酸性强会导致DESs结构被破坏,吸收时产生固体,影响吸收能力,而HBD酸性低或使用中性HBD时氨气的溶解度很低. 因而,李煜惠[89]利用COSMO-RS模拟研究了三元杂化DES(氯化胆碱/间苯二酚/甘油)内部氢键网络的结构与相互作用,探讨了该三元DES吸收NH3的影响因素. 径向分布函数分析表明这种杂化DES组分之间的相互作用在吸收氨气过程中没有较大变化,故其稳定性较高. 且DESs与NH3分子间存在多重氢键作用,这种杂化DES对氨气的溶解能力很强.
从上述研究可以发现,分子模拟作为目前常用的理论分析手段被广泛应用于有害气体治理领域,尤其是结合目前新兴的低共熔溶剂,利用分子结构模型,通过对电荷、能量、分子轨道和相互作用等进行分析,可以帮助研究者有选择性的调控低共熔溶剂组成,并根据应用需要选择合适的溶剂,这种方法已被众多学者证明其准确性(见表2). 这种理论预测方法可以节约大量溶剂筛选的时间和经济成本,机理分析结合实验验证也更加具有说服力,通过低共熔溶剂吸收有害气体的分子模拟研究是非常有潜力和意义的一种方法.
-
从目前众多学者所研究的结果来看,低共熔溶剂这类溶解性强、可自由调控且制备简单的绿色溶剂的确是未来用于吸收各种有害气体的理想溶剂. 而随着计算机技术的不断发展,软件模拟的准确性越来越高,计算机模拟技术成为预测反应进程、分析反应机理的有效手段,不断有学者将其用于DESs吸收有害气体的研究.
对这些研究进行汇总比较发现,大部分气体的吸收过程是通过氯化胆碱类DESs与气体之间的分子间作用力而产生的物理相互作用,但也存在一些DESs与气体分子之间发生化学作用生成化学键. 分子模拟的结果表明在DESs内部组分之间存在较强的氢键网络,吸收有害气体时DESs组分中的某些原子会与气体分子之间产生相互作用并互相吸引以达到吸收气体的目的,这种相互作用的种类以及强弱与DESs和所吸收有害气体的种类有关,同时DESs组分的配比也会对吸收能力产生一定影响. 大部分DESs吸收前后的相互作用对比表明在吸收气体前后内部结构并没有发生变化,即这些DESs在对气体进行解吸后可以循环使用. 但目前缺少对不同体系DESs在相同条件下吸收有害气体的分子模拟对比研究,难以从理论的角度确定适合某种有害气体的最佳吸收剂.
另外在进行分子模拟时也存在一定问题,分子模拟时各种模拟参数的选择尤为重要,合适的参数会使模拟结果更加符合真实情况,例如分子动力学模拟中的分子力场和量子力学中的基组方法都是模拟结果准确与否的关键性因素. 而目前没有明确的使用关系,只能依靠研究者们的不断探索积累经验而确定更加合适的参数,这在一定程度上制约了计算机分子模拟的快速发展. 另一方面,建模以及模拟过程中的操作不同也会对结果造成影响,目前这方面的计算机模拟方法和软件较多,不同研究者使用的软件和操作等的差异会造成无法避免的误差并使实验结果之间的比较分析产生争议. 但在实际应用中,DESs的黏度、pH、极性以及所含基团等因素都会影响到气体吸收效果,同时在吸收过程中,温度、压力、时间、气体流速和水含量等也会对吸收容量及效率产生影响,这些因素很难通过分子模拟体现出来. 希望在不久的将来更多操作简单、适应性强、模拟实验数据准确的分子模拟软件可以被开发出来.
低共熔溶剂吸收有害气体的分子模拟研究进展
Research progress in molecular simulation of deep eutectic solvents absorption of harmful gases
-
摘要: 工业生产中的有害废气如二氧化碳、二氧化硫、硫化氢、氨气、二氧化氮和一氧化氮的排放量日益增加,导致了全球变暖、空气污染和酸雨等一系列问题,对生态环境造成了严重危害. 因此对这些有害气体进行低成本、高效和绿色可持续的捕获一直是人们关注的重点. 低共熔溶剂(deep eutectic solvent,简称DES)作为一种合成简单、成本低、安全无毒、溶解性强且可生物降解的绿色溶剂是这些有害气体的理想吸收剂. 但DESs种类繁多,为不同气体选择合适的吸收剂是一个难点,对有害气体的吸收过程进行计算机分子模拟可以更加深入地了解其中的作用机理并为人们合成更加高效的吸收剂提供理论指导. 本文对低共熔溶剂、分子模拟技术、常用模拟方法以及软件进行了简要介绍和总结,以不同低共熔溶剂吸收各种有害气体的研究出发,从几何构型与能量、分子间相互作用力以及吸收气体时的相互作用能等角度分析讨论了低共熔溶剂与气体之间的作用机理,为未来制备DESs吸收有害气体提供了一定参考依据.Abstract: The emissions of harmful waste gases such as carbon dioxide, sulfur dioxide, hydrogen sulfide, ammonia, nitrogen dioxide and nitric oxide in industrial production are increasing day by day, which leads to a series of problems such as global warming, air pollution and acid rain. It has caused serious harm to the ecological environment. Therefore, the low-cost, efficient, green and sustainable capture of these harmful gases has always been the focus of attention. Deep Eutectic Solvent (DES), as a green solvent with simple synthesis, low cost, safety, non-toxicity, strong solubility and biodegradability, is an ideal absorbent for harmful gases. However, there are many kinds of DESs, so it is difficult to choose suitable absorbents for different gases. Through the computer molecular simulation, we can understand the mechanism of harmful gases absorption and provide theoretical guidance for the synthesis of more efficient absorbent. In this paper, the properties of DESs, computer molecular simulation technology, common simulation methods and software are briefly introduced and summarized. Based on the study of absorbing all kinds of harmful gases by different DESs, the interaction mechanism between DESs and gases is analyzed and discussed from the perspectives of geometry configuration and energy, intermolecular interaction force and the interaction energy of gas absorption, which provides a certain reference for the preparation of DESs to absorb harmful gases in the future.
-
Key words:
- deep eutectic solvents /
- harmful gases /
- molecular simulation.
-
类型 Type 通式 General formula 实例 Terms 类型Ⅰ Cat+X−zMClx M = Zn,Sn,Fe, Al,Ga,In 类型Ⅱ Cat+X−zMClx·yH2O M = Cr,Co,Cu,Ni,Fe 类型Ⅲ Cat+X−zRZ Z = CONH2, COOH, OH 类型Ⅳ MClx+ RZ = MClx−1+·RZ +MClx+1− M = Al, Zn Z=CONH2, COOH, OH 表 2 已报导的低共熔溶剂吸收有害气体的分子模拟研究
Table 2. Reported Molecular Simulation Study of Harmful Gas Absorption by Deep Eutectic Solvents
有害气体 Harmful gases 低共熔溶剂 DESs 软件 Software 相互作用机制 Interaction mechanism 参考文献 Reference SO2 氯化胆碱/丙三醇 LAMMPS SO2···氯离子 [69] SO2 氯化胆碱/乙二醇 Gaussian S(SO2)···氯离子 [72] SO2 氯化胆碱/丙三醇 Gaussian S(SO2)···氯离子 [72] SO2 氯化胆碱/丙二酸 Gaussian S(SO2)···氯离子 [72] SO2 氯化胆碱/尿素 Gaussian S(SO2)···O(尿素) [72] SO2 氯化胆碱/硫脲 Gaussian S(SO2)···S(硫脲) [72] SO2 氯化胆碱/丙三醇 GaussianGAMESS S(SO2)与氯离子、电荷转移相互作用 [68] CO2 氯化胆碱/丙二酸 Gaussian CO2···氯离子、CO2··· -COOH [77] CO2 氯化胆碱/丙三醇 Gaussian CO2···氯离子、CO2··· -OH [77] CO2 氯化胆碱/乙酰丙酸 GaussianMDynaMix C(CO2)··· -COOH(乙酰丙酸) [78] CO2 氯化胆碱/苯乙酸 GaussianMDynaMix C(CO2)···苯环(苯乙酸) 、(CO2)··· -OH(氯化胆碱) [80] H2S 氯化胆碱/尿素 RASPA Gaussian H(H2S)···氯离子、H(H2S)···O(尿素) [75] NO 1,3-二甲基硫脲/四丁基氯化膦 Gaussian NO···1,3-二甲基硫脲化学反应 [85] NO 苯甲酸/氯化四丁基膦 Gaussian NO···苯甲酸化学反应 [86] NH3 硫氰酸铵/丙三醇 Materials studio NH3··· -OH(丙三醇) [87] NH3 氯化胆碱/木糖 Gaussian NH3···氯离子、NH3··· -OH(木糖) [88] CH4 氯化胆碱/乳酸 ORCA MDynaMix H(CH4) ···O(氯化胆碱) [90] CH4 甜菜碱/乳酸 ORCA MDynaMix C(CH4)··· -COOH(乳酸) [90] CH4 丙氨酸/乳酸 ORCA MDynaMix C(CH4)··· -OH(乳酸) [90] -
[1] 吴姗. 生态文明视角下的大气污染现状及防治对策 [J]. 化工设计通讯, 2020, 46(7): 241,248. doi: 10.3969/j.issn.1003-6490.2020.07.156 WU S. Status quo of air pollution from the perspective of ecological civilization and countermeasures [J]. Chemical Engineering Design Communications, 2020, 46(7): 241,248(in Chinese). doi: 10.3969/j.issn.1003-6490.2020.07.156
[2] 徐明. 烟气二氧化硫污染控制技术发展及现状 [J]. 安徽师范大学学报(自然科学版), 2001, 24(2): 187-189. doi: 10.14182/j.cnki.1001-2443.2001.02.026 XU M. The pollution control technology and its development of sulfur dioxide from stack gases [J]. Journal of Anhui Normal University (Natural Science), 2001, 24(2): 187-189(in Chinese). doi: 10.14182/j.cnki.1001-2443.2001.02.026
[3] WANG C, LIU H, LI X Z, et al. A new concept of desulfurization: The electrochemically driven and green conversion of SO2 to NaHSO4 in aqueous solution [J]. Environmental Science & Technology, 2008, 42(22): 8585-8590. [4] 陈秀梅. 功能化离子液体吸收SO2及其机理的研究[D]. 北京: 北京化工大学, 2015. CHEN X M. The research about functional ionic liquid absorbing SO2 and its mechanism[D]. Beijing: Beijing University of Chemical Technology, 2015(in Chinese).
[5] 汤子仟, 吕玲红, 戴中洋, 等. 负载离子液体吸收二氧化碳的分子动力学模拟研究[C]. 中国化学会全国计算化学学术会议暨分子模拟国际论坛, 2017. TANG Z Q, LV L H, DAI Z Y, et al. Molecular dynamics simulation of carbon dioxide absorption by supported ionic liquids[C]. Chinese Chemical Society National Conference on Computational Chemistry and International Forum on Molecular Simulation, 2017.
[6] 石家华, 孙逊, 杨春和, 等. 离子液体研究进展 [J]. 化学通报, 2002, 65(4): 243-250. doi: 10.14159/j.cnki.0441-3776.2002.04.004 SHI J H, SUN X, YANG C H, et al. Progress in the studies on ionic liquids [J]. Chemistry, 2002, 65(4): 243-250(in Chinese). doi: 10.14159/j.cnki.0441-3776.2002.04.004
[7] PAIVA A, CRAVEIRO R, AROSO I, et al. Natural deep eutectic solvents - solvents for the 21st century [J]. ACS Sustainable Chemistry & Engineering, 2014, 2(5): 1063-1071. [8] 韦露, 樊友军. 低共熔溶剂及其应用研究进展 [J]. 化学通报, 2011, 74(4): 333-339. doi: 10.14159/j.cnki.0441-3776.2011.04.011 WEI L, FAN Y J. Progress of deep eutectic solvents and their applications [J]. Chemistry, 2011, 74(4): 333-339(in Chinese). doi: 10.14159/j.cnki.0441-3776.2011.04.011
[9] 杨靖雪, 郎金燕, 王娜, 等. 纤维素在深度共熔溶剂中的溶解性能 [J]. 化工科技, 2020, 28(5): 16-21. doi: 10.3969/j.issn.1008-0511.2020.05.004 YANG J X, LANG J Y, WANG N, et al. Solubility of cellulose in deep eutectic solvents [J]. Science & Technology in Chemical Industry, 2020, 28(5): 16-21(in Chinese). doi: 10.3969/j.issn.1008-0511.2020.05.004
[10] 张依. 低碳烷烃在离子液体和低共熔溶剂中的溶解性能研究[D]. 杭州: 浙江大学, 2017. ZHANG Y. Absorption of light hydrocarbons in ionic liquids and deep eutectic solvents[D]. Hangzhou: Zhejiang University, 2017(in Chinese).
[11] 洪枢. 低共熔溶剂中生物质纳米材料的制备及功能化构建[D]. 南京: 南京林业大学, 2020. HONG S. Preparation and functionalization of bio-nanoparticles with deep eutectic solvents[D]. Nanjing: Nanjing Forestry University, 2020(in Chinese)
[12] 王睿. 乙酰胺类低共熔溶剂@ZIF-8复合催化剂催化降解PET的反应研究[D]. 北京: 北京化工大学, 2020. WANG R. Degradation of PET by acetamide eutectic Solvent@ZIF-8 composite catalyst[D]. Beijing: Beijing University of Chemical Technology, 2020(in Chinese).
[13] 吴晓彬, 田梦媛, 冯柏成, 等. 二乙烯三胺/氯化胆碱低共熔溶剂催化CO2环加成反应 [J]. 化工科技, 2020, 28(4): 1-7. doi: 10.3969/j.issn.1008-0511.2020.04.001 WU X B, TIAN M Y, FENG B C, et al. Cycloaddition of CO2 and epoxide catalyzed by deep eutectic solvent of diethylenetriamine and choline chloride [J]. Science & Technology in Chemical Industry, 2020, 28(4): 1-7(in Chinese). doi: 10.3969/j.issn.1008-0511.2020.04.001
[14] 陆一. 高镍锍在低共熔溶剂中电沉积制备Ni-Mo-Cu的研究[D]. 上海: 上海大学, 2019.LU Y. Electrodeposition of Ni-Mo-Cu coatings from high nickel matte in deep eutectic solvent[D]. Shanghai: Shanghai University, 2019(in Chinese). [15] 杨闯. 低共熔溶剂中多功能纳米多孔铜膜的电化学制备及其催化性能的研究[D]. 昆明: 昆明理工大学, 2017. YANG C. Electrochemical preparation and catalytic performance of multifunctional nanoporous copper films in deep eutectic solvent [D]. Kunming: Kunming University of Science and Technology, 2017(in Chinese).
[16] 张久凌. ChCl-EG低共熔溶剂中电沉积镍、银研究[D]. 沈阳: 沈阳理工大学, 2019. ZHANG J L. Study on electrodeposition of nickel and silver in ChCl-EG eutectic solvent[D]. Shenyang: Shenyang Ligong University, 2019(in Chinese).
[17] PERILLA J R, GOH B C, CASSIDY C K, et al. Molecular dynamics simulations of large macromolecular complexes [J]. Current Opinion in Structural Biology, 2015, 31: 64-74. doi: 10.1016/j.sbi.2015.03.007 [18] ABBOTT A P, CAPPER G, DAVIES D L, et al. Novel solvent properties of choline chloride/urea mixtures [J]. Chemical Communications , 2003(1): 70-71. doi: 10.1039/b210714g [19] 徐环斐, 彭建军, 孔毅, 等. DES在木质纤维素类生物质预处理领域的研究进展 [J]. 中华纸业, 2020, 41(8): 22-29. doi: 10.3969/j.issn.1007-9211.2020.08.003 XU H F, PENG J J, KONG Y, et al. A review on deep eutectic solvent(DES) pretreatment of lignocellulosic biomass [J]. China Pulp & Paper Industry, 2020, 41(8): 22-29(in Chinese). doi: 10.3969/j.issn.1007-9211.2020.08.003
[20] ZHANG Q H, de OLIVEIRA VIGIER K, ROYER S, et al. Deep eutectic solvents: Syntheses, properties and applications [J]. Chemical Society Reviews, 2012, 41(21): 7108-7146. doi: 10.1039/c2cs35178a [21] 徐环斐, 彭建军, 宋晓明, 等. 低共熔溶剂的分子模拟研究进展 [J]. 齐鲁工业大学学报, 2019, 33(5): 1-9. doi: 10.16442/j.cnki.qlgydxxb.2019.05.001 XU H F, PENG J J, SONG X M, et al. Review of molecular sifmulation of deep eutectic solvents [J]. Journal of Qilu University of Technology, 2019, 33(5): 1-9(in Chinese). doi: 10.16442/j.cnki.qlgydxxb.2019.05.001
[22] SMITH E L, ABBOTT A P, RYDER K S. Deep eutectic solvents (DESs) and their applications [J]. Chemical Reviews, 2014, 114(21): 11060-11082. doi: 10.1021/cr300162p [23] ABBOTT A P, CAPPER G, DAVIES D L, et al. Solubility of metal oxides in deep eutectic solvents based on choline chloride [J]. Journal of Chemical & Engineering Data, 2006, 51(4): 1280-1282. [24] 张盈盈, 陆小华, 冯新, 等. 胆碱类低共熔溶剂的物性及应用 [J]. 化学进展, 2013, 25(6): 881-892. ZHANG Y Y, LU X H, FENG X, et al. Properties and applications of choline-based deep eutectic solvents [J]. Progress in Chemistry, 2013, 25(6): 881-892(in Chinese).
[25] HONG S, YUAN Y, ZHANG K T, et al. Efficient hydrolysis of chitin in a deep eutectic solvent synergism for production of chitin nanocrystals [J]. Nanomaterials, 2020, 10(5): 869. doi: 10.3390/nano10050869 [26] YUAN Y, HONG S, LIAN H L, et al. Comparison of acidic deep eutectic solvents in production of chitin nanocrystals [J]. Carbohydrate Polymers, 2020, 236: 116095. doi: 10.1016/j.carbpol.2020.116095 [27] CHEN L, DENG J Q, SONG Y D, et al. Highly stable dispersion of carbon nanotubes in deep eutectic solvent for the preparation of CNT-embedded carbon xerogels for supercapacitors [J]. ChemElectroChem, 2019, 6(22): 5750-5758. doi: 10.1002/celc.201901611 [28] JAUMAUX P, LIU Q, ZHOU D, et al. Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries [J]. Angewandte Chemie International Edition, 2020, 59(23): 9134-9142. doi: 10.1002/anie.202001793 [29] HONG S, YUAN Y, YANG Q R, et al. Choline chloride-zinc chloride deep eutectic solvent mediated preparation of partial O-acetylation of chitin nanocrystal in one step reaction [J]. Carbohydrate Polymers, 2019, 220: 211-218. doi: 10.1016/j.carbpol.2019.05.075 [30] 宋艳丹, 季新伟, 陈玲, 等. 胆碱类低共熔溶剂提取分离丝瓜络纤维素的研究 [J]. 中国造纸学报, 2018, 33(2): 6-12. SONG Y D, JI X W, CHEN L, et al. Extraction and separation of cellulose from loofa sponge with two types of choline-based deep eutectic solvents [J]. Transactions of China Pulp and Paper, 2018, 33(2): 6-12(in Chinese).
[31] HONG S, SONG Y D, YUAN Y, et al. Production and characterization of lignin containing nanocellulose from Luffa through an acidic deep eutectic solvent treatment and systematic fractionation [J]. Industrial Crops and Products, 2020, 143: 111913. doi: 10.1016/j.indcrop.2019.111913 [32] 蒋芳. 低共熔溶剂合成钠离子电池负极材料NaTi2(PO4)3@C的工艺及性能研究[D]. 南宁: 广西大学, 2019. JIANG F. Deep eutectic solvent synthesis of NaTi2(PO4)3@C and its use as anode material for sodium-ion batteries[D]. Nanning: Guangxi University, 2019(in Chinese).
[33] 王淑波, 马尚文, 冯树波, 等. 乙二醇-氯化胆碱低共熔溶剂萃取精馏制取燃料乙醇的研究 [J]. 煤炭与化工, 2019, 42(10): 129-132. doi: 10.19286/j.cnki.cci.2019.10.034 WANG S B, MA S W, FENG S B, et al. Investigation of fuel ethanol preparation by extractive distillation using a deep eutectic solvent as an extractor [J]. Coal and Chemical Industry, 2019, 42(10): 129-132(in Chinese). doi: 10.19286/j.cnki.cci.2019.10.034
[34] MOU H Y, WANG J F, ZHANG D L, et al. A one-step deep eutectic solvent assisted synthesis of carbon nitride/metal oxide composites for photocatalytic nitrogen fixation [J]. Journal of Materials Chemistry A, 2019, 7(10): 5719-5725. doi: 10.1039/C8TA11681D [35] 常旭, 张悦, 蔺海晓, 等. 数值模拟技术在建筑结构加固教学中的应用 [J]. 现代教育技术, 2010, 20(5): 118-120. doi: 10.3969/j.issn.1009-8097.2010.05.030 CHANG X, ZHANG Y, LIN H X, et al. The application of numerical approach in ReinforceMent of building curriculum [J]. Modern Educational Technology, 2010, 20(5): 118-120(in Chinese). doi: 10.3969/j.issn.1009-8097.2010.05.030
[36] 杨莹, 王一, 杨大伟, 等. 分子模拟技术辅助有机化学教学的研究 [J]. 轻工科技, 2020, 36(4): 21-22,25. YANG Y, WANG Y, YANG D W, et al. Research on molecular simulation technology to assist organic chemistry teaching [J]. Light Industry Science and Technology, 2020, 36(4): 21-22,25(in Chinese).
[37] 杨萍, 孙益民. 分子动力学模拟方法及其应用 [J]. 安徽师范大学学报(自然科学版), 2009, 32(1): 51-54. doi: 10.14182/j.cnki.1001-2443.2009.01.007 YANG P, SUN Y M. Method of molecular dynamics simulation and its application [J]. Journal of Anhui Normal University (Natural Science), 2009, 32(1): 51-54(in Chinese). doi: 10.14182/j.cnki.1001-2443.2009.01.007
[38] FRENKEL D, SMIT B, RATNER M A. Understanding molecular simulation: From algorithms to applications [J]. Physics Today, 1997, 50(7): 66. [39] 陈正隆, 徐为人, 汤立达. 分子模拟的理论与实践[M]. 北京: 化学工业出版社, 2007. CHEN Z L, XU W R, TANG L D. Theory and practice of molecular simulation [M]. Beijing: Chemical Industry Press, 2007(in Chinese).
[40] TOMONAGA S I, PRATHER J L. Quantum mechanics [J]. American Journal of Physics, 1964, 32(10): 806-807. [41] FRIESNER R A. Ab initio quantum chemistry: Methodology and applications [J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(19): 6648-6653. doi: 10.1073/pnas.0408036102 [42] FOULKES W M C, MITAS L, NEEDS R J, et al. Quantum Monte Carlo simulations of solids [J]. Reviews of Modern Physics, 2001, 73(1): 33-83. doi: 10.1103/RevModPhys.73.33 [43] 任译, 杨捷, 吴德印, 等. 分子力场进展 [J]. 化学研究与应用, 1998, 10(1): 1-14. REN Y, YANG J, WU D Y, et al. Progress in molecular force field [J]. Chemical Research and Application, 1998, 10(1): 1-14(in Chinese).
[44] ANDREWS D H. The relation between the Raman spectra and the structure of organic molecules [J]. Physical Review, 1930, 36(3): 544-554. doi: 10.1103/PhysRev.36.544 [45] 文玉华, 朱如曾, 周富信, 等. 分子动力学模拟的主要技术 [J]. 力学进展, 2003, 33(1): 65-73. doi: 10.3321/j.issn:1000-0992.2003.01.008 WEN Y H, ZHU R Z, ZHOU F X, et al. An overview on molecular dynamics simulation [J]. Advances in Mechanics, 2003, 33(1): 65-73(in Chinese). doi: 10.3321/j.issn:1000-0992.2003.01.008
[46] 樊康旗, 贾建援. 经典分子动力学模拟的主要技术 [J]. 微纳电子技术, 2005, 42(3): 133-138. doi: 10.13250/j.cnki.wndz.2005.03.009 FAN K Q, JIA J Y. An overview on classical molecular dynamics simulation [J]. Micronanoelectronic Technology, 2005, 42(3): 133-138(in Chinese). doi: 10.13250/j.cnki.wndz.2005.03.009
[47] FRISCH M, TRUCKS G, SCHLEGEL H, et al. Gaussian 98 (Revision A. 11.4) [J]. Journal of the American Chemical Society, 2002, 24(2): 235-247. [48] NEESE F. The ORCA program system [J]. Wiley Interdisciplinary Reviews:Computational Molecular Science, 2012, 2(1): 73-78. doi: 10.1002/wcms.81 [49] LINDAHL E, HESS B, van der SPOEL D. GROMACS 3.0: A package for molecular simulation and trajectory analysis [J]. Molecular Modeling Annual, 2001, 7(8): 306-317. doi: 10.1007/s008940100045 [50] 庄昌清, 岳红, 张慧军. 分子模拟方法及模拟软件Materials Studio在高分子材料中的应用 [J]. 塑料, 2010, 39(4): 81-84. ZHUANG C Q, YUE H, ZHANG H J. Molecular simulation methods and materials studio applications to macromolecular material [J]. Plastics, 2010, 39(4): 81-84(in Chinese).
[51] BÜHL M, KAUPP M, MALKINA O L, et al. The DFT route to NMR chemical shifts [J]. Journal of Computational Chemistry, 1999, 20(1): 91-105. doi: 10.1002/(SICI)1096-987X(19990115)20:1<91::AID-JCC10>3.0.CO;2-C [52] YANG D Z, HOU M Q, NING H, et al. Efficient SO2 absorption by renewable choline chloride–glycerol deep eutectic solvents [J]. Green Chemistry, 2013, 15(8): 2261. doi: 10.1039/c3gc40815a [53] ZHANG K, REN S H, YANG X, et al. Efficient absorption of low-concentration SO2 in simulated flue gas by functional deep eutectic solvents based on imidazole and its derivatives [J]. Chemical Engineering Journal, 2017, 327: 128-134. doi: 10.1016/j.cej.2017.06.081 [54] YANG X Q, ZHANG Y T, LIU F, et al. Deep eutectic solvents consisting of EmimCl and amides: Highly efficient SO2 absorption and conversion [J]. Separation and Purification Technology, 2020, 250: 117273. doi: 10.1016/j.seppur.2020.117273 [55] ZHENG C Y, LI K, ZHANG C, et al. Investigation of guanidinium acetylacetonate and polyethylene glycol mixture as a new reversible and efficient SO2 absorbent [J]. Separation Science and Technology, 2021, 56(15): 2499-2506. doi: 10.1080/01496395.2020.1833218 [56] SUN S Y, NIU Y X, XU Q, et al. Efficient SO2 absorptions by four kinds of deep eutectic solvents based on choline chloride [J]. Industrial & Engineering Chemistry Research, 2015, 54(33): 8019-8024. [57] TRIVEDI T J, LEE J H, LEE H J, et al. Deep eutectic solvents as attractive media for CO2 capture [J]. Green Chemistry, 2016, 18(9): 2834-2842. doi: 10.1039/C5GC02319J [58] GUTIÉRREZ M C, CARRIAZO D, ANIA C O, et al. Deep eutectic solvents as both precursors and structure directing agents in the synthesis of nitrogen doped hierarchical carbons highly suitable for CO2 capture [J]. Energy & Environmental Science, 2011, 4(9): 3535. [59] CUI G, LV M, YANG D Z. Efficient CO2 absorption by azolide-based deep eutectic solvents [J]. Chemical Communications (Cambridge, England), 2019, 55(10): 1426-1429. doi: 10.1039/C8CC10085C [60] REN H W, LIAN S H, WANG X, et al. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture [J]. Journal of Cleaner Production, 2018, 193: 802-810. doi: 10.1016/j.jclepro.2018.05.051 [61] LIN H Q, GONG K, HYKYS P, et al. Nanoconfined deep eutectic solvent in laminated MXene for efficient CO2 separation [J]. Chemical Engineering Journal, 2021, 405: 126961. doi: 10.1016/j.cej.2020.126961 [62] SZE L L, PANDEY S, RAVULA S, et al. Ternary deep eutectic solvents tasked for carbon dioxide capture [J]. ACS Sustainable Chemistry & Engineering, 2014, 2(9): 2117-2123. [63] 田士东, 张生军, 杜秉霖, 等. 新型溶剂离子液体用于NOx排放控制的研究进展 [J]. 当代化工, 2019, 48(12): 2939-2943. TIAN S D, ZHANG S J, DU B L, et al. Research progress in control of NOx emission by novel solvent ionic liquids [J]. Contemporary Chemical Industry, 2019, 48(12): 2939-2943(in Chinese).
[64] 高豹. 低共熔溶剂用于NH3捕集的实验研究[D]. 杭州: 浙江工业大学, 2019.GAO B. Experimental research of deep eutectic solvents for NH3 uptake[D]. Hangzhou: Zhejiang University of Technology, 2019(in Chinese). [65] WU H Y, SHEN M Y, CHEN X C, et al. New absorbents for hydrogen sulfide: Deep eutectic solvents of tetrabutylammonium bromide/carboxylic acids and choline chloride/carboxylic acids [J]. Separation and Purification Technology, 2019, 224: 281-289. doi: 10.1016/j.seppur.2019.04.082 [66] 吴庆魁. 烟气硫氧化物和氮氧化物控制技术小结 [J]. 广东化工, 2011, 38(3): 32-33. doi: 10.3969/j.issn.1007-1865.2011.03.015 WU Q K. Contral technology summary for sulfur oxides and nitrogen oxides in the flue gas [J]. Guangdong Chemical Industry, 2011, 38(3): 32-33(in Chinese). doi: 10.3969/j.issn.1007-1865.2011.03.015
[67] 梁东东, 李大江, 郭持皓, 等. 我国烟气脱硫工艺技术发展现状和趋势 [J]. 有色金属(冶炼部分), 2015(4): 69-73. LIANG D D, LI D J, GUO C H, et al. Development status and trend of flue gas desulfuration in China [J]. Nonferrous Metals (Extractive Metallurgy), 2015(4): 69-73(in Chinese).
[68] LI H P, CHANG Y H, ZHU W S, et al. Theoretical evidence of charge transfer interaction between SO2 and deep eutectic solvents formed by choline chloride and glycerol [J]. Physical Chemistry Chemical Physics:PCCP, 2015, 17(43): 28729-28742. doi: 10.1039/C5CP04172D [69] KOROTKEVICH A, FIRAHA D S, PADUA A A H, et al. Ab initio molecular dynamics simulations of SO2 solvation in choline chloride/glycerol deep eutectic solvent [J]. Fluid Phase Equilibria, 2017, 448: 59-68. doi: 10.1016/j.fluid.2017.03.024 [70] 郭连晓. 氯化胆碱型低共熔溶剂的量化计算及其吸收SO2机理的研究[D]. 北京: 北京化工大学, 2017. GUO L X. A theoretical study on choline chloride-based deep eutectic solvents and their mechanism of SO2 capture[D]. Beijing: Beijing University of Chemical Technology, 2017(in Chinese).
[71] GARCÍA G, ATILHAN M, APARICIO S. Interfacial properties of deep eutectic solvents regarding to CO2 capture [J]. The Journal of Physical Chemistry C, 2015, 119(37): 21413-21425. doi: 10.1021/acs.jpcc.5b04585 [72] 刘妍. 含DES体系中弱相互作用的表征和关联[D]. 北京: 北京化工大学, 2018. LIU Y. Chracterization and correlation of intermolecular weak interactions in DES-containing systems[D]. Beijing: Beijing University of Chemical Technology, 2018(in Chinese).
[73] HOU S, ZHANG C C, JIANG B, et al. Investigation of highly efficient and reversible absorption of SO2 using ternary functional deep eutectic solvents [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(43): 16241-16251. [74] ATILHAN M, ALTAMASH T, APARICIO S. Quantum chemistry insight into the interactions between deep eutectic solvents and SO 2 [J]. Molecules , 2019, 24(16): 2963. doi: 10.3390/molecules24162963 [75] LIU F J, CHEN W, MI J X, et al. Thermodynamic and molecular insights into the absorption of H2S, CO2, and CH4 in choline chloride plus urea mixtures [J]. AIChE Journal, 2019, 65(5): e16574. doi: 10.1002/aic.16574 [76] DUTCHER B, FAN M H, RUSSELL A G. Amine-based CO2 capture technology development from the beginning of 2013-a review [J]. ACS Applied Materials & Interfaces, 2015, 7(4): 2137-2148. [77] GARCÍA G, ATILHAN M, APARICIO S. A theoretical study on mitigation of CO2 through advanced deep eutectic solvents [J]. International Journal of Greenhouse Gas Control, 2015, 39: 62-73. doi: 10.1016/j.ijggc.2015.05.004 [78] ULLAH R, ATILHAN M, ANAYA B, et al. A detailed study of cholinium chloride and levulinic acid deep eutectic solvent system for CO2 capture via experimental and molecular simulation approaches [J]. Physical Chemistry Chemical Physics:PCCP, 2015, 17(32): 20941-20960. doi: 10.1039/C5CP03364K [79] SALEHI H S, HENS R, MOULTOS O A, et al. Computation of gas solubilities in choline chloride urea and choline chloride ethylene glycol deep eutectic solvents using Monte Carlo simulations [J]. Journal of Molecular Liquids, 2020, 316: 113729. doi: 10.1016/j.molliq.2020.113729 [80] ALTAMASH T, ATILHAN M, ALIYAN A, et al. Insights into choline chloride–phenylacetic acid deep eutectic solvent for CO2 absorption [J]. RSC Advances, 2016, 6(110): 109201-109210. doi: 10.1039/C6RA22312E [81] WANG J, CHENG H, SONG Z, et al. Carbon dioxide solubility in phosphonium-based deep eutectic solvents: An experimental and molecular dynamics study [J]. Industrial & Engineering Chemistry Research, 2019, 58(37): 17514-17523. [82] SHEN Y, ABEDIN R, HUNG F R. On the performance of confined deep eutectic solvents and ionic liquids for separations of carbon dioxide from methane: Molecular dynamics simulations [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2019, 35(10): 3658-3671. doi: 10.1021/acs.langmuir.8b03990 [83] 张楚莹, 王书肖, 邢佳, 等. 中国能源相关的氮氧化物排放现状与发展趋势分析 [J]. 环境科学学报, 2008, 28(12): 2470-2479. doi: 10.3321/j.issn:0253-2468.2008.12.011 ZHANG C Y, WANG S X, XING J, et al. Current status and future projections of NOx emissions from energy related industries in China [J]. Acta Scientiae Circumstantiae, 2008, 28(12): 2470-2479(in Chinese). doi: 10.3321/j.issn:0253-2468.2008.12.011
[84] HIZADDIN H F, HADJ-KALI M K, RAMALINGAM A, et al. Extractive denitrogenation of diesel fuel using ammonium- and phosphonium-based deep eutectic solvents [J]. The Journal of Chemical Thermodynamics, 2016, 95: 164-173. doi: 10.1016/j.jct.2015.12.009 [85] 魏光森. 低共熔溶剂制备及其用于吸收一氧化氮的性能研究[D]. 天津: 天津大学, 2018. WEI G S. Preparation of deep eutectic solvents and study of their properties for nitric oxide capture[D]. Tianjin: Tianjin University, 2018(in Chinese).
[86] 张吕鸿, 马号朋, 澹台晓伟, 等. 苯甲酸型低共熔溶剂吸收一氧化氮的性能研究 [J]. 化工学报, 2020, 71(8): 3644-3651. ZHANG L H, MA H P, TANTAI X W, et al. Study on the absorption of nitric oxide by benzoic acid-based deep eutectic solvents [J]. CIESC Journal, 2020, 71(8): 3644-3651(in Chinese).
[87] DENG D S, GAO B, ZHANG C, et al. Investigation of protic NH4SCN-based deep eutectic solvents as highly efficient and reversible NH3 absorbents [J]. Chemical Engineering Journal, 2019, 358: 936-943. doi: 10.1016/j.cej.2018.10.077 [88] LI Z L, ZHONG F Y, HUANG J Y, et al. Sugar-based natural deep eutectic solvents as potential absorbents for NH3 capture at elevated temperatures and reduced pressures [J]. Journal of Molecular Liquids, 2020, 317: 113992. doi: 10.1016/j.molliq.2020.113992 [89] 李煜惠. 低共熔溶剂分离含氮化合物的研究[D]. 杭州: 浙江大学, 2018. LI Y H. Separation of nitrogen compounds by deep eutectic solvents[D]. Hangzhou: Zhejiang University, 2018(in Chinese).
[90] ALTAMASH T, AMHAMED A I, APARICIO S, et al. Combined experimental and theoretical study on high pressure methane solubility in natural deep eutectic solvents [J]. Industrial & Engineering Chemistry Research, 2019, 58(19): 8097-8111. -




 下载:
下载: