-
四溴双酚A(TBBPA)是一种溴代阻燃剂,由于其具有高阻燃性和低廉价格等特点,广泛应用于电子电器产品、家具、油漆、纺织品等日用品中. TBBPA在生产和使用过程中可通过多种途径进入环境. 研究表明,TBBPA具有持久性有机污染物的特征,具有生物累积性、高度亲脂性和难分解性,具有内分泌干扰效应、细胞和神经毒性、免疫毒性等,会对生态系统和人类健康构成严重的威胁[1-4]. TBBPA微溶于水且容易在土壤中聚集,土壤是TBBPA最重要的归宿之一. 近年来,如何有效降解和去除土壤中TBBPA的研究备受关注 .
目前,土壤有机污染物修复技术包括物理修复[5]、生物修复[6-8]和化学修复[9-10],其中化学修复因其低投资、高效率、实施周期短的优点应用广泛. 过硫酸盐(PS)高级氧化技术是最常用的一种化学氧化修复技术,该技术的关键是通过热[11]、紫外光[12]和通过添加过渡金属[13]等方式活化PS. 热活化PS体系中虽然操作简单,但能耗较高,不适用大规模的处理. 在过渡金属活化PS中,亚铁离子(Fe2+)和铁离子(Fe3+)由于含量丰富、环境友好、价格低廉而被广泛用于PS活化,然而Fe2+活化PS技术仍然有一些缺陷,过量的Fe2+会抑制PS的活化,Fe3+还原为Fe2+的速率较慢,难以控制氧化体系中的量[14].
为了提高PS活化性能,本文以典型溴代阻燃剂TBBPA为目标污染物,考虑将Fe2+活化与热活化结合,采用Fe2+耦合热活化PS处理土壤中TBBPA,考察温度、Fe2+初始浓度、PS浓度、初始pH、无机阴离子和其他金属离子对Fe2+/热/PS体系降解TBBPA的影响,以寻求土壤体系中TBBPA降解的最佳反应条件,为土壤中持久性有机污染物的修复提供依据.
-
TBBPA购于上海麦克林生化科技有限公司,甲醇购于美国迈瑞达科技公司,过硫酸钠、五水合硫酸铜、七水合硫酸锌和六水合硫酸镍购于天津津科精细化工研究所,氯化钠、碳酸氢钠、氢氧化钠和七水合硫酸亚铁购于国药集团化学试剂有限公司,浓硫酸购于北京化工厂. 以上药品除甲醇为色谱纯外,其余药品均为分析纯. 实验溶液配制用水为去离子水,高效液相仪器用水为哇哈哈纯净水.
-
LC-20AT 高效液相色谱仪(日本Shimadzu公司);Optima-XPN PN-100 高速离心机(BECKMANCOULTER公司);S400-B多参数pH测试仪(梅特勒-托利多仪器有限公司);FD-A10N-50冷冻干燥机(北京博医康实验仪器有限公司);HH-1-5L恒温水浴锅(江苏科析仪器有限公司);旋转蒸发仪(上海申生科技有限公司);SHA-2恒温振荡器(常州菲普实验仪器厂).
-
供试土壤采自安徽省合肥市瑶海区某农田(117°21'53.424"E, 31°51'23.886"N),采集深度0—20 cm. 去除碎石、树枝杂质,经室内自然干燥,研磨后过60目筛网,储存于棕色玻璃瓶中密封保存. 经检验,土壤中无TBBPA检出. 该土壤基本理化性质见表1.
-
TBBPA配制:精确称取0.250 g TBBPA粉末溶解于500 mL甲醇中,TBBPA浓度为500 mg·L−1.
TBBPA污染土:称取500 g未污染的土壤于2 L烧杯中,加入200 mL甲醇,玻璃棒搅拌均匀后,取30 mL TBBPA(500 mg·L−1)缓缓倒入烧杯中,边搅拌使之充分混匀,接着加甲醇直至液体浸没土壤表面,用玻璃棒搅拌20—30 min,使TBBPA在土壤中均匀混合,置于通风橱中自然风干、老化2周后备用(测定TBBPA约20 mg·kg−1).
-
实验选用过硫酸钠(PS)氧化剂,过渡金属离子选用Fe2+,无机阴离子选用Cl−、
HCO−3 ,其他金属离子选用Cu2+、Zn2+、Ni2+,TBBPA初始浓度约为20 mg·kg−1,水土比3:1,选取因素包括反应温度、Fe2+浓度、PS浓度、pH、无机阴离子和其他金属离子等. 单因素实验设置参数变量下表2.称取2.000 g TBBPA污染土壤于30 mL棕色玻璃瓶中,加入2 mL去离子水和2 mL 25 mmol·L−1的硫酸亚铁溶液,再加入2 mL 50 mmol·L−1的PS储备溶液,使用1 mol·L−1稀硫酸和氢氧化钠溶液调节初始pH为3.0,随即用橡胶塞密封,置于温度55 ℃的恒温振荡器中(转速200 r·min−1)12 h. 预定时间间隔(0.05、0.25、0.5、1、2、4、6、8、12 h)取出放入冰水中(5 min)并加入0.2 mL甲醇,随后将样品放入-20 ℃的冰箱中冷冻4 h后放入冷冻干燥机中冷冻干燥48 h,经预处理,测定土壤中TBBPA浓度.
-
将10 mL甲醇溶剂加入待测棕色玻璃瓶中,置于25 ℃恒温振荡器中振荡8 h后,在剩余土壤中加入10 mL甲醇超声辅助萃取,萃取1 h,转移至离心管分离土壤与上清液. 随后,将离心管中的上清液经滤纸自然过滤转移至茄形烧瓶中,利用旋转蒸发仪将烧瓶中的萃取液旋转蒸发至1 mL,再用甲醇溶液重复多次提取至5 mL容量瓶,定容到刻度线,摇晃均匀,将容量瓶中的液体通过孔隙为0.22 μm的有机滤膜过滤,收集1.5 mL滤液于棕色进样瓶瓶中,用HPLC进行定量分析.
-
(1) TBBPA的测定
本实验采用高效液相色谱仪(HPLC)测定土壤中的TBBPA. HPLC分析条件为:Zorbax Eclipse XDB-C18色谱柱(5 μm,250 mm×4.6 mm),流动相为甲醇:超纯水=85:15,检测波长为230 nm,流速为1.0 mL·min−1,进样体积为10 μL,柱温35 ℃. 对标样进行定性分析,TBBPA在该分析条件下的保留时间为(5.5±0.2) min[10].
(2) 数据分析方法
降解率W计算公式如下所示(公式1),采用准一级反应动力学和二级反应动力学模型(公式2、3)对TBBPA降解进行分析拟合.
式中,t为反应时间(h),C0与Ct分别表示0 h和t h时TBBPA的浓度(mg·kg−1),k为拟合得到的反应动力学常数(h−1).
-
比较了加热、热/Fe2+、热/PS、Fe2+/PS和Fe2+/热/PS体系对土壤中TBBPA降解效果的影响(图1),TBBPA在加热、热/Fe2+、热/PS和Fe2+/PS体系中12 h内的降解率分别为25.0%、32.0%、47.3%和89.9%. 由图可以看出,升高温度对土壤中TBBPA有一定的影响;在热/Fe2+的体系下,TBBPA降解速率小;热/PS和Fe2+/PS体系下TBBPA降解速率有进一步提升,原因主要是热和Fe2+都能活化PS产生较多的
SO·−4 ,降解TBBPA效率较高[15]. 在Fe2+耦合热活化PS体系中,实验条件:[TBBPA]0=20 mg·kg−1,[Fe2+]0=25 mmol·L−1,[PS]0=50 mmol·L−1,初始pH=3.0,温度=55 ℃,反应12 h后,降解效率可达100%,与单一热活化和Fe2+活化方式相比,降解率分别增加了52.7%和10.1%. 因此,Fe2+耦合热活化PS对降解土壤中的TBBPA有促进作用,提高温度更有利于PS产生SO4•−,从而加快降解速率,缩短TBBPA的降解时间. -
(1) 温度
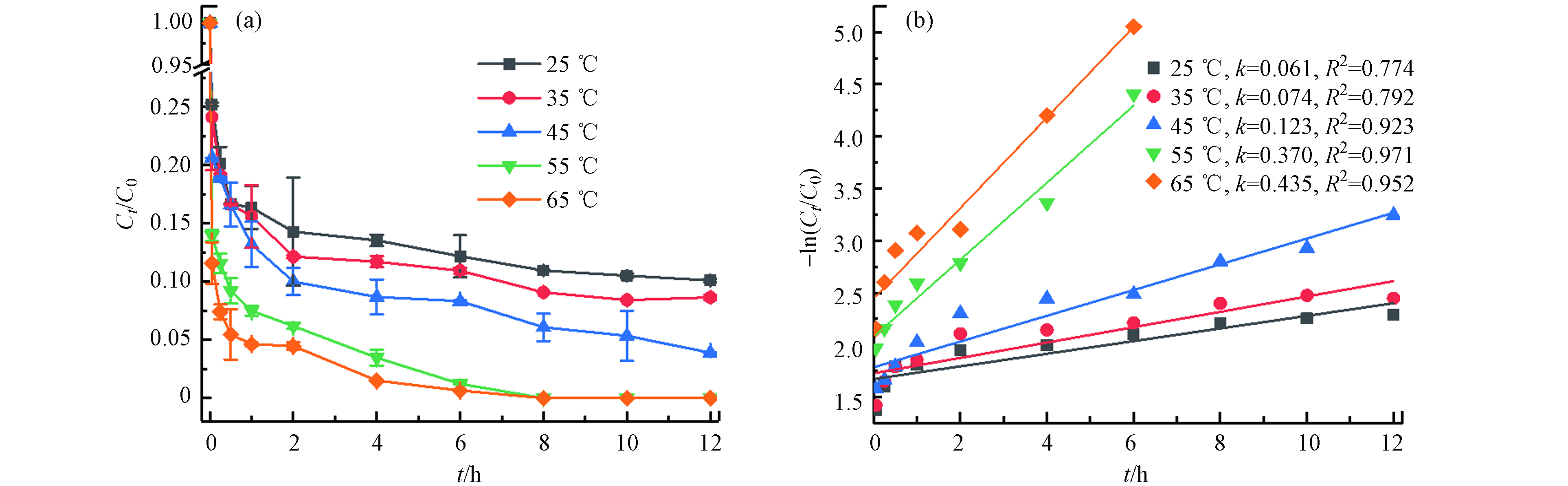
不同温度(25 ℃、35 ℃、45 ℃、55 ℃、65 ℃)对TBBPA在Fe2+/热/PS体系中降解的影响(如图2). 结果表明,TBBPA的降解效率随温度的升高而显著增加,在12 h内25 ℃、35 ℃、45 ℃时TBBPA的降解率分别为89.91%、91.35%、96.10%,55 ℃和65 ℃时降解率则在8 h已经达到100%. 这是由于在Fe2+活化过硫酸盐产生
SO·−4 的基础上(公式4),在体系中升高温度,热量也可以活化过硫酸盐产生SO·−4 (公式5),较多地SO·−4 加快了TBBPA的降解. 同时利用准一级动力学方程拟合表明,TBBPA降解反应均符合准一级反应动力学;在反应温度分别为25 ℃、35 ℃、45 ℃、55 ℃、65 ℃时,其对应的反应速率常数分别为0.061 h−1、0.074 h−1、0.123 h−1、0.370 h−1、0.435 h−1. 在实际的场地修复处理中,较高温度带来高的经济成本,且反应8 h内在55 ℃和65 ℃中降解率均达到100%,因此结合经济因素和降解效能的综合考虑后,选择55 ℃为最适反应温度.(2) Fe2+初始浓度
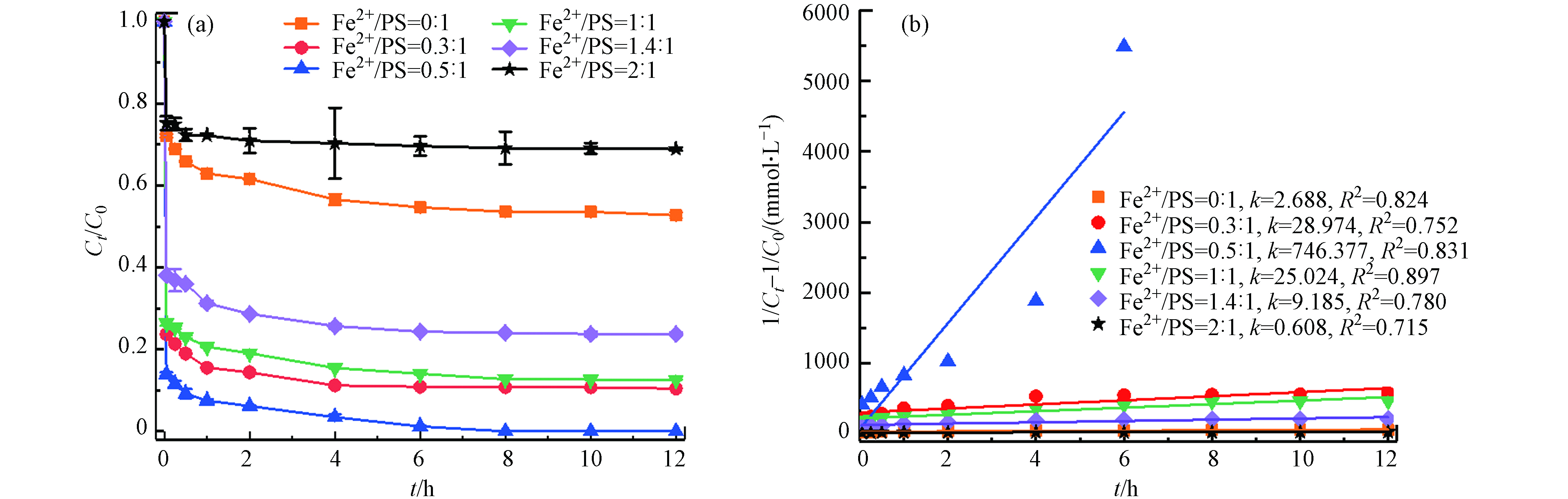
在温度55 ℃、PS浓度为50 mmol·L−1、pH=3的条件下,考察不同初始Fe2+浓度(0、15、25、50、 70、100 mmol·L−1)对土壤中TBBPA降解的影响. 如图3所示,未投加Fe2+时,此时反应仅是热活化反应降解,12 h内TBBPA降解率为47.3%,当Fe2+与PS摩尔比从0.3:1增加到0.5:1,TBBPA降解率从89.6%增加到100%;而继续增大Fe2+与PS摩尔比至2:1,TBBPA的降解效率开始下降,反应结束时仅有31.1%. 由图3(b)可以看出,TBBPA的降解较好拟合二级反应动力学,降解速率常数先增大,然后逐渐减小. 可见,添加Fe2+能够有效活化PS降解土壤中的TBBPA,在Fe2+浓度一定范围内,适当提高Fe2+浓度,能加速TBBPA的降解,在Fe2+与PS摩尔比为0.5:1,降解率最大,随着Fe2+浓度增加,过量的Fe2+也能分解
SO·−4 (公式6),从而抑制TBBPA的降解,去除率开始下降[16-17]. 因此,Fe2+与PS最佳摩尔比为0.5:1.(3) PS浓度
在温度55 ℃、Fe2+浓度为25 mmol·L−1、pH=3的条件下,考察不同PS浓度(15、25、50、70、100 mmol·L−1)对土壤中TBBPA降解的影响. 如图4所示,随着PS浓度的增加,12 h内TBBPA的去除效率从50.1%提高到100%,降解速率常数也从0.014 h−1增至1.135 h−1;TBBPA完全去除所需的时间随PS浓度的增大而缩短,PS浓度50 mmol·L−1、70 mmol·L−1和100 mmol·L−1降解率达到100%的时间分别为8 h、6 h和4 h. 由此可见,增加PS浓度对TBBPA的降解有促进作用. 有研究表明,过量的PS会抑制污染物的降解[18],因为是过量
S2O2−8 会产生高浓度的SO·−4 ,从而SO·−4 作为猝灭剂影响TBBPA降解(公式7、8). 考虑经济问题以及Fe2+与PS最佳摩尔比为0.5:1的情况下,则本研究PS的最佳浓度为50 mmol·L−1.(4) 初始pH值
在温度55 ℃,Fe2+与PS最佳摩尔比0.5:1条件下,进一步研究了不同初始pH值(3.0、5.0、7.0和9.0)对Fe2+耦合热活化PS体系降解TBBPA的影响. 如图5所示,当初始pH分别为3.0、5.0、7.0和9.0时,反应12 h后,TBBP降解率分别为100%、99.62%、99.68%和99.66%,随着pH从3增加到9,反应速率常数从0.370 h−1减小到0.316 h−1,酸性条件下的降解率略高于中性和碱性条件,初始pH为5.0、7.0和9.0时降解效率之间相差不大. 出现这种趋势的原因是,在酸性条件下存在一定的酸催化效应(公式9、10)[19];硫酸亚铁溶液和过硫酸盐溶液自身呈酸性;两者在反应开始前3 min时较为迅速,PS很容易氧化Fe2+成为Fe3+,Fe3+水解也会使pH值降低. 总体来说,Fe2+耦合热活化PS体系具有良好的pH适应性,pH=3.0时最为合适.
-
在场地土壤和地下水的复杂系统中,可能存在无机阴离子对过硫酸盐降解污染物的过程产生一定制约[20]. 因此我们选取氯化钠和碳酸氢钠分别作为Cl−和
HCO−3 的来源进行实验,在Fe2+/热/PS体系中考察不同浓度的Cl−和HCO−3 对污染物降解的影响.(1) Cl−
在最佳反应条件下(温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3),不同浓度的Cl−(0、5、50、100 mmol·L−1)对TBBPA降解的影响(如图6). 反应至4 h时,TBBPA的降解率分别为96.5%、96.2%、98.4%和99.4%;反应至12 h时,降解率分别为100%、99.7%、100%和100%,随着Cl−浓度从0 mmol·L−1增加至100 mmol·L−1,TBBPA完全去除所需时间缩短了2 h. 由图6(b)所示,反应符合准一级动力学模型,其对应的反应动力学常数分别为0.369 h−1、0.367 h−1、0.491 h−1和0.707 h−1. 当Cl−浓度较低时,Cl−与
SO·−4 反应生成了活性较低的Cl•(公式11),低浓度的Cl•对降解体系的影响不显著,而当Cl−浓度较高时,此时反应产生的含氯化合物HClO成为整个体系的主要氧化物质,Cl−浓度越大,促进作用越明显[21-22].(2)
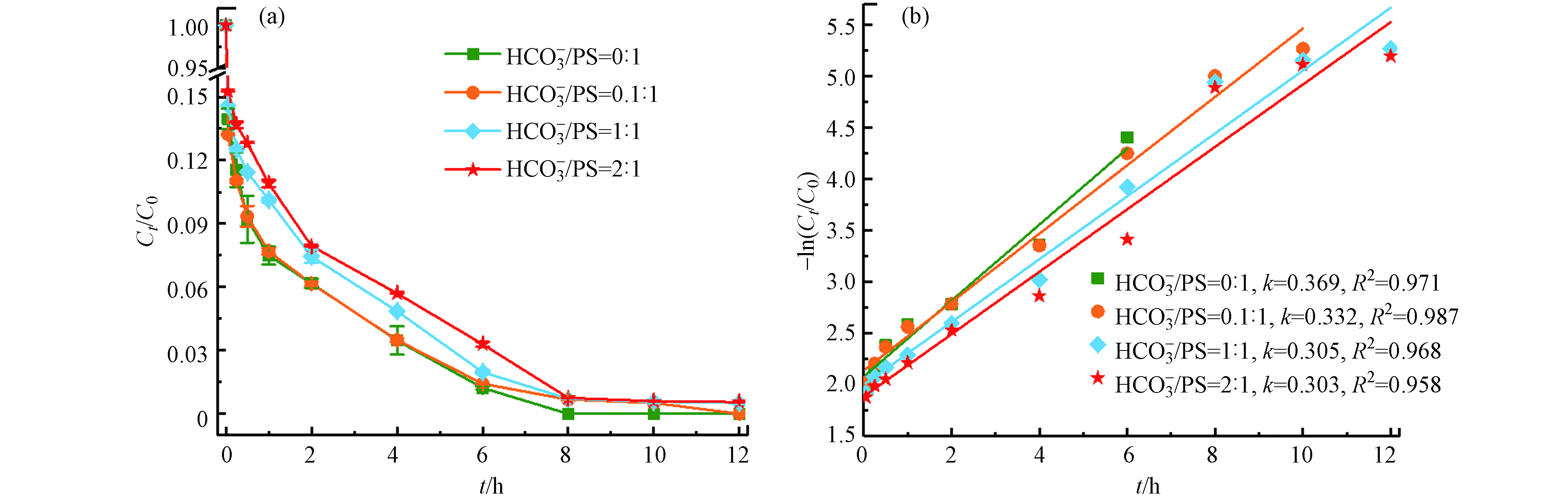
HCO−3 在最佳反应条件下(温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3),不同浓度的
HCO−3 (0、5、50、100 mmol·L−1)对TBBPA降解的影响(如图7).随着
HCO−3 浓度的增大,TBBPA的降解率逐渐降低,反应至6 h时,TBBPA的降解率分别为96.5%、96.5%、95.1%和94.3%;反应至12 h时,降解率分别为100%、100%、99.5%和99.5%. 图7 (b)所示,反应符合准一级动力学模型,其对应的反应动力学常数分别为0.369 h−1、0.332 h−1、0.305 h−1和0.303 h−1. 结果表明,HCO−3 在Fe2+/热/PS体系中抑制了TBBPA的降解,出现这种现象的原因可能是HCO−3 及其转化的CO2−3 与溶液中的SO⋅−4 发生淬灭反应(公式12—14)[19],产生了活性更弱的HCO⋅3 和CO⋅−3 ,减弱了整个反应速率,而且HCO−3 的加入也会致使pH的上升,导致TBBPA降解率降低. -
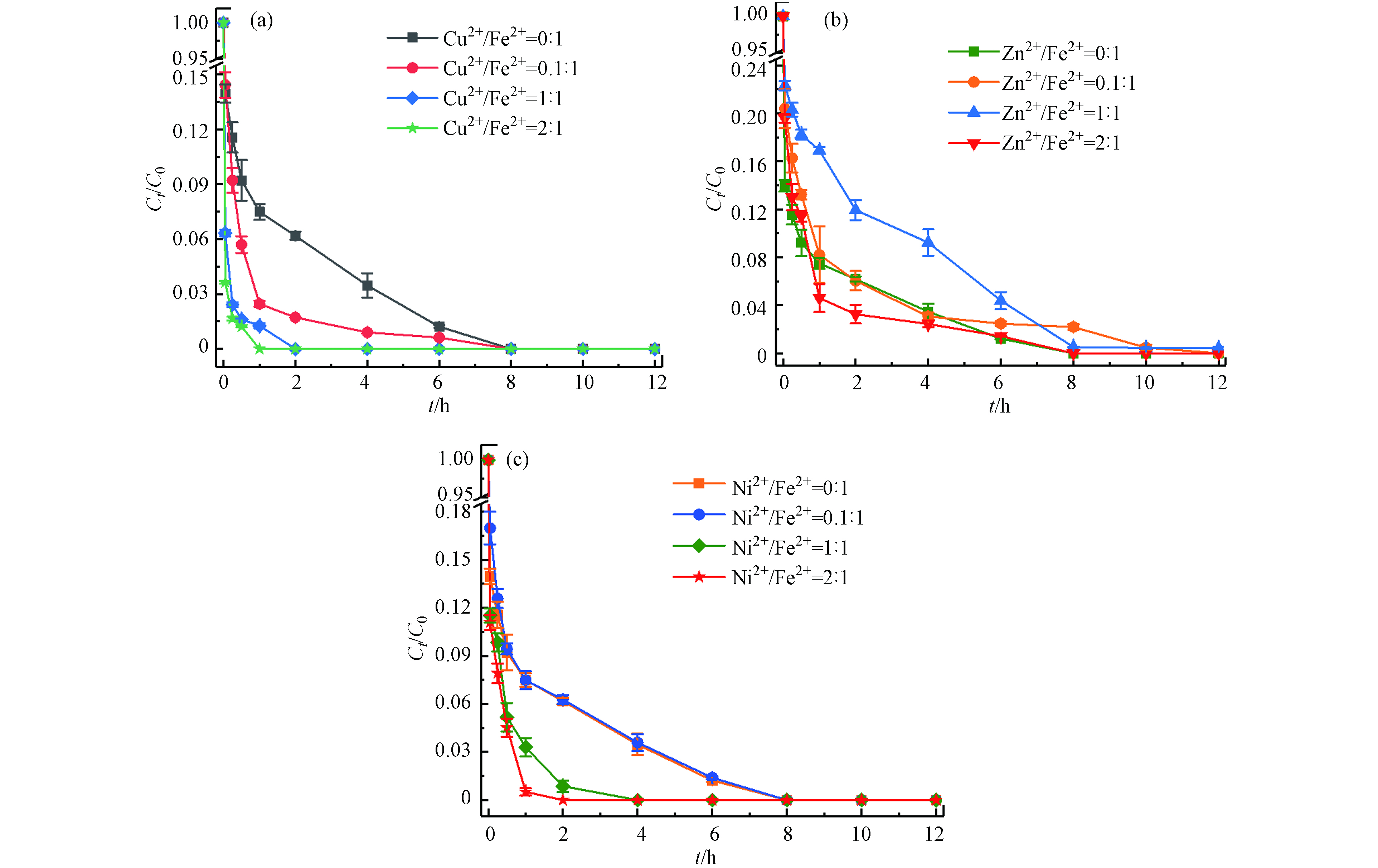
土壤中存在着大量的金属矿物,多种金属的存在也是影响应用的重要因素[23-24]. 本文选择土壤中的几种过渡金属(Cu2+、Zn2+、Ni2+)考察其对Fe2+/热/PS体系降解土壤中TBBPA的影响. 在体系最佳反应条件下(温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3),分别加入M2+/Fe2+摩尔比为0:1、0.1:1、1:1、2:1(M=Cu、Zn、Ni)的过渡金属离子,反应12 h后计算TBBPA降解效率. 结果如图8所示,Cu2+对Fe2+/热/PS体系表现出较好的促进作用,且随着Cu2+浓度的增大,TBBPA完全去除所需的时间从8 h(Cu2+/Fe2+摩尔比0:1)缩短到1 h(Cu2+/Fe2+摩尔比2:1);当增加Ni2+与Fe2+的摩尔比时,Ni2+显现出的促进作用由弱变强;而逐渐增大Zn2+与Fe2+的摩尔比时,对反应速率的影响出现由抑制转变成促进的双重作用. 这可能与M2+/M3+的氧化还原电位有关,△Ep(Cu2+/Cu3+)<△Ep(Ni2+/Ni3+)<△Ep(Zn2+/Zn3+),电位越低意味着催化剂越容易得到电子,有利于PS的活化[25];同时由于金属离子的氧化性Cu2+>Ni2+>Fe2+>Zn2+,Cu2+和Ni2+则更容易促使PS产生更多
SO⋅−4 ,进而生成具有氧化性的Cu3+和Ni3+参与TBBPA降解,而Zn2+增大至一定浓度后才表现出促进作用. -
(1)Fe2+耦合热活化过硫酸盐的方法能高效降解土壤中TBBPA. Fe2+耦合热活化过硫酸盐降解土壤中TBBPA相比于单一热活化和Fe2+活化方式,降解率分别提升52.7%和10.1%.
(2)对Fe2+耦合热活化PS降解TBBPA的影响因素研究表明,TBBPA的降解率随着温度的增加而升高;增大亚铁离子浓度,TBBPA的降解速率呈现出先增加后降低的趋势,Fe2+与PS的最佳摩尔比为0.5:1;增大PS浓度能够显著提高TBBPA的降解率,缩短TBBPA去除时间;Fe2+耦合热活化PS体系具有较宽的pH应用范围且在酸性条件下更有利于降解. 最优条件:温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3.
(3)较高浓度的Cl-对Fe2+/热/PS体系中TBBPA的降解起促进作用,而HCO3-在研究浓度范围内均呈现抑制作用;Cu2+和Ni2+促进Fe2+/热/PS体系对TBBPA的降解,而随着Zn2+浓度的增加,TBBPA的降解速率呈现出先减小后增大的变化.
Fe2+耦合热活化过硫酸盐降解土壤中四溴双酚A
Degradation of tetrabromobisphenol A by ferrous coupling thermally activated persulfate in soil
-
摘要: 有效降解和去除土壤中四溴双酚A(TBBPA)是土壤环境化学研究热点. 本文研究了Fe2+耦合热活化过硫酸盐体系(Fe2+/热/PS)对土壤中TBBPA的降解,并考察温度、Fe2+初始浓度、PS浓度、初始pH值、无机阴离子(Cl−、
HCO−3 )浓度以及金属离子(Cu2+、Zn2+、Ni2+)浓度的影响. 结果表明,Fe2+/热/PS降解TBBPA的效果远高于同等条件下单一热活化和Fe2+活化的情况;提高温度和PS浓度能够促进土壤TBBPA的降解;而过量的亚铁离子会导致TBBPA去除率下降,Fe2+与PS的最佳摩尔比为0.5 : 1;Fe2+耦合热活化PS体系具有较宽的pH应用范围, 在酸性条件下更有利于降解;温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3下,土壤TBBPA初始浓度为20 mg·kg−1,培养12 h,土壤TBBPA的降解效率达到了100%;低浓度Cl−对Fe2+/热/PS体系的影响不显著,高浓度的Cl−会促进TBBPA的降解,而HCO−3 在研究浓度范围内均呈现抑制作用;Cu2+和Ni2+对Fe2+/热/PS体系均表现出较好的促进作用,而随着Zn2+浓度的增加,TBBPA的降解速率呈现出先减小后增大的变化.Abstract: Degradation and removal of tetrabromobisphenol A (TBBPA) from soil is a hot topic in soil environmental chemistry research. In this study, the degradation of tetrabromobisphenol A(TBBPA) by ferrous coupling thermally activated persulfate system (Fe2+/heat/PS) in soil was investigated. The effects of temperature, initial Fe2+ concentration, PS concentration, initial pH, inorganic anions (Cl−, HCO3−) concentration and other metal ions (Cu2+, Zn2+, Ni2+) concentration were also evaluated. The results showed that the degradation rate of TBBPA by Fe2+/heat/PS was much more effective than that the single thermal activation or Fe2+ activation under the same conditions; TBBPA removing effect was found to be enhanced through increasing the temperature and PS concentration; Excess amount of ferrous ion would lead to the declination of TBBPA removal efficiency and the optimal molar ratio of Fe2+/PS was 0.5:1; Fe2+ coupling thermally activated PS system has a wide range of pH applications and the TBBPA was more conducive to degraded under acidic conditions; The degradation efficiency of soil TBBPA reached 100% for 12 hours when the initial concentration of soil TBBPA was 20 mg·kg−1 at 55 ℃, Fe2+ concentration 25 mmol·L−1, PS concentration 50 mmol·L−1 and pH=3; Cl− has dual effect on the removal of TBBPA in the Fe2+/heat/PS system, which inhibited the degradation of TBBPA at low concentrations but promoted it at high concentrations, both HCO3− had an obvious inhibition effect on the degradation of TBBPA. Cu2+ and Ni2+ promoted the degradation of TBBPA by Fe2+/heat/PS system, and the degradation rate of TBBPA decreased first and then increased with the increase of Zn2+ concentration.-
Key words:
- tetrabromobisphenol A /
- homogeneous catalyst /
- ferrous /
- persulfate /
- advanced oxidation
-
四溴双酚A(TBBPA)是一种溴代阻燃剂,由于其具有高阻燃性和低廉价格等特点,广泛应用于电子电器产品、家具、油漆、纺织品等日用品中. TBBPA在生产和使用过程中可通过多种途径进入环境. 研究表明,TBBPA具有持久性有机污染物的特征,具有生物累积性、高度亲脂性和难分解性,具有内分泌干扰效应、细胞和神经毒性、免疫毒性等,会对生态系统和人类健康构成严重的威胁[1-4]. TBBPA微溶于水且容易在土壤中聚集,土壤是TBBPA最重要的归宿之一. 近年来,如何有效降解和去除土壤中TBBPA的研究备受关注 .
目前,土壤有机污染物修复技术包括物理修复[5]、生物修复[6-8]和化学修复[9-10],其中化学修复因其低投资、高效率、实施周期短的优点应用广泛. 过硫酸盐(PS)高级氧化技术是最常用的一种化学氧化修复技术,该技术的关键是通过热[11]、紫外光[12]和通过添加过渡金属[13]等方式活化PS. 热活化PS体系中虽然操作简单,但能耗较高,不适用大规模的处理. 在过渡金属活化PS中,亚铁离子(Fe2+)和铁离子(Fe3+)由于含量丰富、环境友好、价格低廉而被广泛用于PS活化,然而Fe2+活化PS技术仍然有一些缺陷,过量的Fe2+会抑制PS的活化,Fe3+还原为Fe2+的速率较慢,难以控制氧化体系中的量[14].
为了提高PS活化性能,本文以典型溴代阻燃剂TBBPA为目标污染物,考虑将Fe2+活化与热活化结合,采用Fe2+耦合热活化PS处理土壤中TBBPA,考察温度、Fe2+初始浓度、PS浓度、初始pH、无机阴离子和其他金属离子对Fe2+/热/PS体系降解TBBPA的影响,以寻求土壤体系中TBBPA降解的最佳反应条件,为土壤中持久性有机污染物的修复提供依据.
1. 材料与方法(Materials and methods)
1.1 实验试剂
TBBPA购于上海麦克林生化科技有限公司,甲醇购于美国迈瑞达科技公司,过硫酸钠、五水合硫酸铜、七水合硫酸锌和六水合硫酸镍购于天津津科精细化工研究所,氯化钠、碳酸氢钠、氢氧化钠和七水合硫酸亚铁购于国药集团化学试剂有限公司,浓硫酸购于北京化工厂. 以上药品除甲醇为色谱纯外,其余药品均为分析纯. 实验溶液配制用水为去离子水,高效液相仪器用水为哇哈哈纯净水.
1.2 实验仪器
LC-20AT 高效液相色谱仪(日本Shimadzu公司);Optima-XPN PN-100 高速离心机(BECKMANCOULTER公司);S400-B多参数pH测试仪(梅特勒-托利多仪器有限公司);FD-A10N-50冷冻干燥机(北京博医康实验仪器有限公司);HH-1-5L恒温水浴锅(江苏科析仪器有限公司);旋转蒸发仪(上海申生科技有限公司);SHA-2恒温振荡器(常州菲普实验仪器厂).
1.3 土壤的采集
供试土壤采自安徽省合肥市瑶海区某农田(117°21'53.424"E, 31°51'23.886"N),采集深度0—20 cm. 去除碎石、树枝杂质,经室内自然干燥,研磨后过60目筛网,储存于棕色玻璃瓶中密封保存. 经检验,土壤中无TBBPA检出. 该土壤基本理化性质见表1.
表 1 土壤基本理化性质Table 1. Basic physicochemical properties of soilpH 孔隙比 Porosity ratio 饱和度/% Saturation 颗粒成分组成/% Particle composition <0.005 mm 0.005—0.075 mm 0.075—0.25 mm 0.25—0.5 mm 7.10 1.50 95.1 13.3 17.7 47.7 21.3 1.4 污染土制备
TBBPA配制:精确称取0.250 g TBBPA粉末溶解于500 mL甲醇中,TBBPA浓度为500 mg·L−1.
TBBPA污染土:称取500 g未污染的土壤于2 L烧杯中,加入200 mL甲醇,玻璃棒搅拌均匀后,取30 mL TBBPA(500 mg·L−1)缓缓倒入烧杯中,边搅拌使之充分混匀,接着加甲醇直至液体浸没土壤表面,用玻璃棒搅拌20—30 min,使TBBPA在土壤中均匀混合,置于通风橱中自然风干、老化2周后备用(测定TBBPA约20 mg·kg−1).
1.5 实验方法
实验选用过硫酸钠(PS)氧化剂,过渡金属离子选用Fe2+,无机阴离子选用Cl−、
HCO−3 表 2 单因素实验参数变量Table 2. One-way experimental parameter variables单因素名称 Single factor name 参数变量 Parameter variable 温度/℃ 25 35 45 55 65 PS浓度/(mmol·L−1) 15 25 50 70 100 Fe2+浓度/(mmol·L−1) 15 25 50 70 100 初始pH 3.0 5.0 7.0 9.0 — PS与无机阴离子浓度摩尔比 1:0.1 1:1 1:2 — — Fe2+与其他金属离子浓度摩尔比 1:0.1 1:1 1:2 — — 称取2.000 g TBBPA污染土壤于30 mL棕色玻璃瓶中,加入2 mL去离子水和2 mL 25 mmol·L−1的硫酸亚铁溶液,再加入2 mL 50 mmol·L−1的PS储备溶液,使用1 mol·L−1稀硫酸和氢氧化钠溶液调节初始pH为3.0,随即用橡胶塞密封,置于温度55 ℃的恒温振荡器中(转速200 r·min−1)12 h. 预定时间间隔(0.05、0.25、0.5、1、2、4、6、8、12 h)取出放入冰水中(5 min)并加入0.2 mL甲醇,随后将样品放入-20 ℃的冰箱中冷冻4 h后放入冷冻干燥机中冷冻干燥48 h,经预处理,测定土壤中TBBPA浓度.
1.6 土壤样品的预处理
将10 mL甲醇溶剂加入待测棕色玻璃瓶中,置于25 ℃恒温振荡器中振荡8 h后,在剩余土壤中加入10 mL甲醇超声辅助萃取,萃取1 h,转移至离心管分离土壤与上清液. 随后,将离心管中的上清液经滤纸自然过滤转移至茄形烧瓶中,利用旋转蒸发仪将烧瓶中的萃取液旋转蒸发至1 mL,再用甲醇溶液重复多次提取至5 mL容量瓶,定容到刻度线,摇晃均匀,将容量瓶中的液体通过孔隙为0.22 μm的有机滤膜过滤,收集1.5 mL滤液于棕色进样瓶瓶中,用HPLC进行定量分析.
1.7 分析方法
(1) TBBPA的测定
本实验采用高效液相色谱仪(HPLC)测定土壤中的TBBPA. HPLC分析条件为:Zorbax Eclipse XDB-C18色谱柱(5 μm,250 mm×4.6 mm),流动相为甲醇:超纯水=85:15,检测波长为230 nm,流速为1.0 mL·min−1,进样体积为10 μL,柱温35 ℃. 对标样进行定性分析,TBBPA在该分析条件下的保留时间为(5.5±0.2) min[10].
(2) 数据分析方法
降解率W计算公式如下所示(公式1),采用准一级反应动力学和二级反应动力学模型(公式2、3)对TBBPA降解进行分析拟合.
W=(1−CtC0)×100% (1) lnCtC0=−kt (2) 1Ct−1C0=kt (3) 式中,t为反应时间(h),C0与Ct分别表示0 h和t h时TBBPA的浓度(mg·kg−1),k为拟合得到的反应动力学常数(h−1).
2. 结果与讨论(Results and discussion)
2.1 Fe2+耦合热活化PS降解TBBPA
比较了加热、热/Fe2+、热/PS、Fe2+/PS和Fe2+/热/PS体系对土壤中TBBPA降解效果的影响(图1),TBBPA在加热、热/Fe2+、热/PS和Fe2+/PS体系中12 h内的降解率分别为25.0%、32.0%、47.3%和89.9%. 由图可以看出,升高温度对土壤中TBBPA有一定的影响;在热/Fe2+的体系下,TBBPA降解速率小;热/PS和Fe2+/PS体系下TBBPA降解速率有进一步提升,原因主要是热和Fe2+都能活化PS产生较多的
SO·−4 2.2 TBBPA在Fe2+/热/PS体系中降解的影响因素
(1) 温度
不同温度(25 ℃、35 ℃、45 ℃、55 ℃、65 ℃)对TBBPA在Fe2+/热/PS体系中降解的影响(如图2). 结果表明,TBBPA的降解效率随温度的升高而显著增加,在12 h内25 ℃、35 ℃、45 ℃时TBBPA的降解率分别为89.91%、91.35%、96.10%,55 ℃和65 ℃时降解率则在8 h已经达到100%. 这是由于在Fe2+活化过硫酸盐产生
SO·−4 SO·−4 SO·−4 Fe2++S2O2−8→Fe3++SO2−4+SO•−4 (4) S2O2−8+heat→2SO•−4 (5) (2) Fe2+初始浓度
在温度55 ℃、PS浓度为50 mmol·L−1、pH=3的条件下,考察不同初始Fe2+浓度(0、15、25、50、 70、100 mmol·L−1)对土壤中TBBPA降解的影响. 如图3所示,未投加Fe2+时,此时反应仅是热活化反应降解,12 h内TBBPA降解率为47.3%,当Fe2+与PS摩尔比从0.3:1增加到0.5:1,TBBPA降解率从89.6%增加到100%;而继续增大Fe2+与PS摩尔比至2:1,TBBPA的降解效率开始下降,反应结束时仅有31.1%. 由图3(b)可以看出,TBBPA的降解较好拟合二级反应动力学,降解速率常数先增大,然后逐渐减小. 可见,添加Fe2+能够有效活化PS降解土壤中的TBBPA,在Fe2+浓度一定范围内,适当提高Fe2+浓度,能加速TBBPA的降解,在Fe2+与PS摩尔比为0.5:1,降解率最大,随着Fe2+浓度增加,过量的Fe2+也能分解
SO·−4 Fe2++SO•−4→Fe3++SO2−4 (6) (3) PS浓度
在温度55 ℃、Fe2+浓度为25 mmol·L−1、pH=3的条件下,考察不同PS浓度(15、25、50、70、100 mmol·L−1)对土壤中TBBPA降解的影响. 如图4所示,随着PS浓度的增加,12 h内TBBPA的去除效率从50.1%提高到100%,降解速率常数也从0.014 h−1增至1.135 h−1;TBBPA完全去除所需的时间随PS浓度的增大而缩短,PS浓度50 mmol·L−1、70 mmol·L−1和100 mmol·L−1降解率达到100%的时间分别为8 h、6 h和4 h. 由此可见,增加PS浓度对TBBPA的降解有促进作用. 有研究表明,过量的PS会抑制污染物的降解[18],因为是过量
S2O2−8 SO·−4 SO·−4 SO•−4+S2O2−8→•S2O2−8+SO2−4 (7) SO•−4+SO•−4→S2O2−8 (8) (4) 初始pH值
在温度55 ℃,Fe2+与PS最佳摩尔比0.5:1条件下,进一步研究了不同初始pH值(3.0、5.0、7.0和9.0)对Fe2+耦合热活化PS体系降解TBBPA的影响. 如图5所示,当初始pH分别为3.0、5.0、7.0和9.0时,反应12 h后,TBBP降解率分别为100%、99.62%、99.68%和99.66%,随着pH从3增加到9,反应速率常数从0.370 h−1减小到0.316 h−1,酸性条件下的降解率略高于中性和碱性条件,初始pH为5.0、7.0和9.0时降解效率之间相差不大. 出现这种趋势的原因是,在酸性条件下存在一定的酸催化效应(公式9、10)[19];硫酸亚铁溶液和过硫酸盐溶液自身呈酸性;两者在反应开始前3 min时较为迅速,PS很容易氧化Fe2+成为Fe3+,Fe3+水解也会使pH值降低. 总体来说,Fe2+耦合热活化PS体系具有良好的pH适应性,pH=3.0时最为合适.
S2O2−8+H+→HS2O−8 (9) HS2O−8→SO2−4+SO•−4+H+ (10) 2.3 无机阴离子在Fe2+/热/PS体系中的影响
在场地土壤和地下水的复杂系统中,可能存在无机阴离子对过硫酸盐降解污染物的过程产生一定制约[20]. 因此我们选取氯化钠和碳酸氢钠分别作为Cl−和
HCO−3 HCO−3 (1) Cl−
在最佳反应条件下(温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3),不同浓度的Cl−(0、5、50、100 mmol·L−1)对TBBPA降解的影响(如图6). 反应至4 h时,TBBPA的降解率分别为96.5%、96.2%、98.4%和99.4%;反应至12 h时,降解率分别为100%、99.7%、100%和100%,随着Cl−浓度从0 mmol·L−1增加至100 mmol·L−1,TBBPA完全去除所需时间缩短了2 h. 由图6(b)所示,反应符合准一级动力学模型,其对应的反应动力学常数分别为0.369 h−1、0.367 h−1、0.491 h−1和0.707 h−1. 当Cl−浓度较低时,Cl−与
SO·−4 SO•−4+Cl−→Cl•+SO2−4 (11) (2)
HCO−3 在最佳反应条件下(温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3),不同浓度的
HCO−3 随着
HCO−3 HCO−3 HCO−3 CO2−3 SO⋅−4 HCO⋅3 CO⋅−3 HCO−3 HCO−3→CO2−3+H+ (12) SO⋅−4+HCO−3→SO2−4+HCO⋅3 (13) SO⋅−4+CO2−3→SO2−4+CO⋅−3 (14) 2.4 金属离子Cu2+、Zn2+、Ni2+对Fe2+/热/PS体系催化活性的影响
土壤中存在着大量的金属矿物,多种金属的存在也是影响应用的重要因素[23-24]. 本文选择土壤中的几种过渡金属(Cu2+、Zn2+、Ni2+)考察其对Fe2+/热/PS体系降解土壤中TBBPA的影响. 在体系最佳反应条件下(温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3),分别加入M2+/Fe2+摩尔比为0:1、0.1:1、1:1、2:1(M=Cu、Zn、Ni)的过渡金属离子,反应12 h后计算TBBPA降解效率. 结果如图8所示,Cu2+对Fe2+/热/PS体系表现出较好的促进作用,且随着Cu2+浓度的增大,TBBPA完全去除所需的时间从8 h(Cu2+/Fe2+摩尔比0:1)缩短到1 h(Cu2+/Fe2+摩尔比2:1);当增加Ni2+与Fe2+的摩尔比时,Ni2+显现出的促进作用由弱变强;而逐渐增大Zn2+与Fe2+的摩尔比时,对反应速率的影响出现由抑制转变成促进的双重作用. 这可能与M2+/M3+的氧化还原电位有关,△Ep(Cu2+/Cu3+)<△Ep(Ni2+/Ni3+)<△Ep(Zn2+/Zn3+),电位越低意味着催化剂越容易得到电子,有利于PS的活化[25];同时由于金属离子的氧化性Cu2+>Ni2+>Fe2+>Zn2+,Cu2+和Ni2+则更容易促使PS产生更多
SO⋅−4 3. 结论(Conclusion)
(1)Fe2+耦合热活化过硫酸盐的方法能高效降解土壤中TBBPA. Fe2+耦合热活化过硫酸盐降解土壤中TBBPA相比于单一热活化和Fe2+活化方式,降解率分别提升52.7%和10.1%.
(2)对Fe2+耦合热活化PS降解TBBPA的影响因素研究表明,TBBPA的降解率随着温度的增加而升高;增大亚铁离子浓度,TBBPA的降解速率呈现出先增加后降低的趋势,Fe2+与PS的最佳摩尔比为0.5:1;增大PS浓度能够显著提高TBBPA的降解率,缩短TBBPA去除时间;Fe2+耦合热活化PS体系具有较宽的pH应用范围且在酸性条件下更有利于降解. 最优条件:温度55 ℃、Fe2+浓度25 mmol·L−1、PS浓度50 mmol·L−1、pH=3.
(3)较高浓度的Cl-对Fe2+/热/PS体系中TBBPA的降解起促进作用,而HCO3-在研究浓度范围内均呈现抑制作用;Cu2+和Ni2+促进Fe2+/热/PS体系对TBBPA的降解,而随着Zn2+浓度的增加,TBBPA的降解速率呈现出先减小后增大的变化.
-
表 1 土壤基本理化性质
Table 1. Basic physicochemical properties of soil
pH 孔隙比 Porosity ratio 饱和度/% Saturation 颗粒成分组成/% Particle composition <0.005 mm 0.005—0.075 mm 0.075—0.25 mm 0.25—0.5 mm 7.10 1.50 95.1 13.3 17.7 47.7 21.3 表 2 单因素实验参数变量
Table 2. One-way experimental parameter variables
单因素名称 Single factor name 参数变量 Parameter variable 温度/℃ 25 35 45 55 65 PS浓度/(mmol·L−1) 15 25 50 70 100 Fe2+浓度/(mmol·L−1) 15 25 50 70 100 初始pH 3.0 5.0 7.0 9.0 — PS与无机阴离子浓度摩尔比 1:0.1 1:1 1:2 — — Fe2+与其他金属离子浓度摩尔比 1:0.1 1:1 1:2 — — -
[1] GAO C J, XIA L L, WU C C, et al. The effects of prosperity indices and land use indicators of an urban conurbation on the occurrence of hexabromocyclododecanes and tetrabromobisphenol A in surface soil in South China [J]. Environmental Pollution, 2019, 252: 1810-1818. doi: 10.1016/j.envpol.2019.06.128 [2] ALI N, HARRAD S, GOOSEY E, et al. “Novel” brominated flame retardants in Belgian and UK indoor dust: Implications for human exposure [J]. Chemosphere, 2011, 83(10): 1360-1365. doi: 10.1016/j.chemosphere.2011.02.078 [3] BIRNBAUM L S, STASKAL D F. Brominated flame retardants: Cause for concern? [J]. Environmental Health Perspectives, 2004, 112(1): 9-17. doi: 10.1289/ehp.6559 [4] ZHANG K L, HUANG J, ZHANG W, et al. Mechanochemical degradation of tetrabromobisphenol A: Performance, products and pathway [J]. Journal of Hazardous Materials, 2012, 243: 278-285. doi: 10.1016/j.jhazmat.2012.10.034 [5] LABADIE P, TLILI K, ALLIOT F, et al. Development of analytical procedures for trace-level determination of polybrominated diphenyl ethers and tetrabromobisphenol A in river water and sediment [J]. Analytical and Bioanalytical Chemistry, 2010, 396(2): 865-875. doi: 10.1007/s00216-009-3267-x [6] LIU J, WANG Y F, JIANG B Q, et al. Degradation, metabolism, and bound-residue formation and release of Tetrabromobisphenol A in soil during sequential anoxic-oxic incubation [J]. Environmental Science & Technology, 2013, 47(15): 8348-8354. [7] SUN F F, KOLVENBACH B A, NASTOLD P, et al. Degradation and metabolism of tetrabromobisphenol A (TBBPA) in submerged soil and soil-plant systems [J]. Environmental Science & Technology, 2014, 48(24): 14291-14299. [8] 付真真. 污泥菌剂厌氧降解四溴双酚A[D]. 大连: 大连理工大学, 2013. FU Z Z. Anaerobic degradation of tetrabromobisphenol-a by sludge bacterial agent[D]. Dalian: Dalian University of Technology, 2013(in Chinese).
[9] 付欣. 零价纳米铁铜双金属(Cu/nZVI)对土壤中四溴双酚A和四氯双酚A的降解研究[D]. 广州: 华南理工大学, 2016. FU X. The degradation of tetrabromobisphenol A and tetrachlorobisphenol A in soils by copper/nanoscale zero-valent iron(Cu/nZVI) bimetallic particles[D]. Guangzhou: South China University of Technology, 2016(in Chinese).
[10] YUAN X H, LI T L, HE Y Y, et al. Degradation of TBBPA by nZVI activated persulfate in soil systems [J]. Chemosphere, 2021, 284: 131166. doi: 10.1016/j.chemosphere.2021.131166 [11] REZA SAMARGHANDI M, TARI K, SHABANLOO A, et al. Synergistic degradation of acid blue 113 dye in a thermally activated persulfate (TAP)/ZnO-GAC oxidation system: Degradation pathway and application for real textile wastewater [J]. Separation and Purification Technology, 2020, 247: 116931. doi: 10.1016/j.seppur.2020.116931 [12] 李阳, 许玻珲, 邓琳, 等. 紫外活化过硫酸盐降解磷酸氯喹[J]. 环境科学, 2022, 43(9): 4597-4607. LI Y, XU B H, DENG L, et al. Degradation of chloroquine phosphate by UV-activated persulfate[J]. Environmental Science, 2022, 43(9): 4597-4607(in Chinese).
[13] ZHENG X X, NIU X J, ZHANG D Q, et al. Removal of Microcystis aeruginosa by natural pyrite-activated persulfate: Performance and the significance of iron species [J]. Chemical Engineering Journal, 2022, 428: 132565. doi: 10.1016/j.cej.2021.132565 [14] RAO Y F, QU L, YANG H S, et al. Degradation of carbamazepine by Fe(II)-activated persulfate process [J]. Journal of Hazardous Materials, 2014, 268: 23-32. doi: 10.1016/j.jhazmat.2014.01.010 [15] ZHOU Y Y, XIANG Y J, HE Y Z, et al. Applications and factors influencing of the persulfate-based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds [J]. Journal of Hazardous Materials, 2018, 359: 396-407. doi: 10.1016/j.jhazmat.2018.07.083 [16] CHEN K F, KAO C M, WU L C, et al. Methyl tert-butyl ether (MTBE) degradation by ferrous ion-activated persulfate oxidation: Feasibility and kinetics studies [J]. Water Environment Research:a Research Publication of the Water Environment Federation, 2009, 81(7): 687-694. doi: 10.2175/106143008X370539 [17] PENG H J, ZHANG W, LIU L, et al. Degradation performance and mechanism of decabromodiphenyl ether (BDE209) by ferrous-activated persulfate in spiked soil [J]. Chemical Engineering Journal, 2017, 307: 750-755. doi: 10.1016/j.cej.2016.08.129 [18] CHEN J B, QIAN Y J, LIU H M, et al. Oxidative degradation of diclofenac by thermally activated persulfate: Implication for ISCO [J]. Environmental Science and Pollution Research International, 2016, 23(4): 3824-3833. doi: 10.1007/s11356-015-5630-0 [19] AHMAD A, GU X G, LI L, et al. Effects of pH and anions on the generation of reactive oxygen species (ROS) in nZVI-rGo-activated persulfate system [J]. Water, Air, & Soil Pollution, 2015, 226(11): 1-12. [20] LUTZE H V, BIRCHER S, RAPP I, et al. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter [J]. Environmental Science & Technology, 2015, 49(3): 1673-1680. [21] WANG Z H, YUAN R X, GUO Y G, et al. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone reagent: Kinetic analysis [J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 1083-1087. [22] 娄晓祎. 无机阴离子活化过硫酸盐氧化降解典型有机污染物的研究[D]. 上海: 东华大学, 2016. LOU X Y. Inorganic anions mediated persulfate activation for degradation of typical organic pollutants in wastewater[D]. Shanghai: Donghua University, 2016(in Chinese).
[23] NIE W S, MAO Q H, DING Y B, et al. Highly efficient catalysis of chalcopyrite with surface bonded ferrous species for activation of peroxymonosulfate toward degradation of bisphenol A: A mechanism study [J]. Journal of Hazardous Materials, 2019, 364: 59-68. doi: 10.1016/j.jhazmat.2018.09.078 [24] LIU C S, SHIH K, SUN C X, et al. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate [J]. The Science of the Total Environment, 2012, 416: 507-512. doi: 10.1016/j.scitotenv.2011.12.004 [25] XIAN G, KONG S Y, LI Q G, et al. Synthesis of spinel ferrite MFe2O4 (M = Co, Cu, Mn, and Zn) for persulfate activation to remove aqueous organics: Effects of M-site metal and synthetic method [J]. Frontiers in Chemistry, 2020, 8: 177. doi: 10.3389/fchem.2020.00177 -






 DownLoad:
DownLoad: