-
氯苯(chlorobenzene,CB)是最简单的氯芳烃,自19世纪合成以来,即大量用于生产DDT,至今,氯苯依然是年产量超过100万磅的高产量化学品[1]. 环境中的氯苯大多来源于人类的工业活动,据报道,美国氯苯类化合物的环境排放量可达到每年980吨[2]. 氯苯在自然界中的降解速度较慢,具有很强的生物积累性和生物毒性,有研究显示氯苯除了对中枢神经系统和呼吸系统有影响之外,还可造成肾脏和肝脏的损伤[3].
目前已经有很多研究者关注到氯苯的无害化处理问题,传统的氯苯处理方法主要包括吸附法、生物降解法和化学氧化法. 这些方法大多具有二次污染、效率低、选择性差等特点. 基于单过硫酸盐化合物(PMS)的高级氧化技术因其高氧化效率在降解氯代有机污染物的过程中表现出了优异的性能. 许多研究结果表明,钴氧化物(CoO、CoO2、Co2O3、CoO(OH)、Co3O4)具有活化PMS的良好能力,但单钴氧化物的比表面积非常低,以团聚,导致活性位有限,显著抑制其催化活性[4]. 有研究表明通过将钴氧化物分散在多孔材料的孔道中,可以将活性金属限域在特定孔结构中,从而使活性金属实现高度分散,这种方法可以极大提高钴基材料的催化活性[5]. SBA-15具有较高的比表面积、稳定的结构和有序的孔径,是一种良好的催化剂载体. 由于金属盐与模板剂之间的强相互作用,通过固相研磨法将金属盐与未去除模板的SBA-15充分混合之后,经过焙烧可以得到高金属分散度的催化剂. 因此,在本研究中,采用固相研磨法合成催化剂CoOx@SBA-15,并对其活化PMS降解氯苯的性能进行测试,并进一步探究反应中的各种因素对反应活性的影响机制及反应体系的主要活性物种.
-
试剂:P123(Sigma-Aldrich,99%),正硅酸四乙酯(国药,AR),六水合硝酸钴(阿拉丁,99%),氯苯(麦克林,AR),单过硫酸盐化合物(Sigma-Aldrich,99.9%),2,2,6,6-四甲基哌啶(Sigma-Aldrich,AR),5,5-二甲基-1-吡咯啉-N-氧化物(百灵威,AR),甲醇(TEDIA,HPLC),盐酸(国药,AR),实验中所用水均为去离子水.
-
以SBA-15为载体利用固相研磨法制备限域型CoOx@SBA-15[6]:按文献报道方法,以正硅酸四乙酯(TEOS)为硅源,三嵌段共聚化合物P123为模板剂合成介孔氧化硅SBA-15[7];在室温条件下将一定量的Co(NO3)2·6H2O与1 g未去除模板剂的SBA-15在研钵中混合并研磨1 h得到CoOx@SBA-15;将所得的混合物置于马弗炉中,以2 °C·min−1 的升温速率升温至500 °C,并保持5 h,焙烧所得产物标记为CoOx(X)@SBA-15,其中X是钴的负载量(以质量分数计).
采用传统浸渍法制备CoOx/SBA-15和CoOx/SiO2催化剂. 首先将SBA-15置于马弗炉中焙烧,以1 °C·min−1的升温速率升温到550 °C,并保持6 h,目的是碳化并去除SBA-15中的模板剂;将购得的SiO2置于马弗炉中焙烧,以2 °C·min−1的升温速率升温到300 °C,并保持4 h,目的是去除其中可能存在的杂质;随后将一定量的Co(NO3)2溶液与1 g载体在室温条件下混合搅拌2 h以上,并在90 °C水浴中蒸干,在100 °C烘箱中干燥过夜,干燥后所得材料标记为CoOx(X)/Y,其中X是负载量(以质量分数计),Y是载体.
催化剂透射电镜分析(TEM)在日本JEOL公司,JEM-200CX型透射电子显微镜上检测;X射线衍射分析(XRD):采用日本Rigaku公司D/max-rA型X射线衍射仪,Cu 靶(Kα1,λ=0.154056 nm,扫描速度6(°)· min−1),操作条件:40 kV、30 mA,扫描范围:10°—80°;催化剂中Co含量采用原子吸收光谱(AAS,美国Thermo公司)测定;催化剂比表面积、孔径孔容采用比表面积测定仪(ASAP 2020,美国Micromeritics公司)分析;催化剂的在不同温度的还原状态采用泛泰公司生产的Finesorb-3010程序升温化学吸附仪进行测定.
-
氯苯的降解实验在250 mL三口烧瓶中进行,温度保持在(25±0.5)℃,具体操作方法如下:储备液用去离子水稀释至反应所需浓度,三口烧瓶中溶液总体积为200 mL,随后将一定量的催化剂(5—40 mg)分散在溶液中,搅拌1 h以达到吸附平衡并保证催化剂充分分散. 加入一定量的PMS储备液开始反应,在反应开始后的固定时间(1、3、5、10、20、30、50、70、90、120 min)取出反应溶液,并通过0.22 μm PTFE过滤器(Anpel)进行过滤. 将过滤后的1 mL反应溶液转移到装有0.5 mL甲醇的2 mL棕色液相小瓶中以清除残留的自由基.
使用配备有C-18色谱柱(ZORBAX Eclipse XDB-C18)的高效液相色谱仪(HPLC,1220 Infinity II)检测滤液中的氯苯. 仪器条件:流动相包括纯水和甲醇(30/70,V/V),流速为0.8 mL·min−1. 紫外检测波长为223 nm,柱温30 ℃.
-
催化剂活化PMS产生的自由基采用德国Bruker BioSpin有限公司生产的顺磁共振波谱仪(Electron Paramagnetic Resonance Spectrometer,EMX PLUS(PPMS))检测. 具体方法如下:称取适量催化剂分散在去离子水中,涡旋振荡30 s确保催化剂充分分散,取一定量PMS溶液加入到催化剂的溶液中,并使其充分混匀;在2.5 mL尖头离心管中加入1 mL所得混合溶液和100 µL 1 mol·L−1的自由基捕获剂(5,5-二甲基吡咯啉氧化物(DMPO)或2,2,6,6-四甲基-4-哌啶(TEMP))储备液,自由基捕获剂需溶解在pH = 7.4的磷酸缓冲液中,涡旋振荡30 s;将混合后的溶液转移至EPR样品管中,进行EPR分析. EPR操作参数:中心场为348.0 mT,扫描宽度为20 mT,微波频率为9.77 GHz,调制频率为100 GHz,能量为20 mW.
-
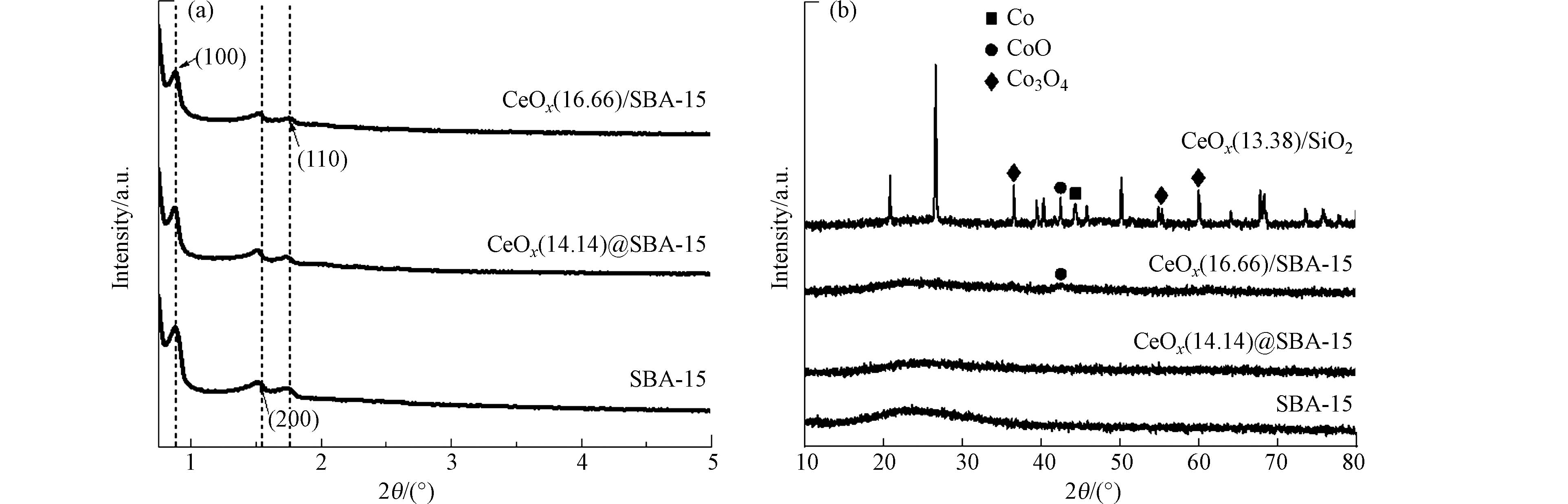
图1(a)是焙烧去除模板后的SBA-15、固相研磨法合成的催化剂CoOx@SBA-15以及浸渍法合成的催化剂CoOx/SBA-15的小角XRD图谱,小角XRD图谱可以用于分析材料的孔结构的有序度. 从图1(a)中可以看出,去除模板后的SBA-15在2θ为0.75°到2°范围内有3个明显的特征衍射峰,分别位于0.88°、1.52°和1.76°,对应于于SBA-15的(100)、(110)和(200)衍射面,该结果表明合成的SBA-15具有高度的二维六方介孔结构和p6mm对称性[7]. 此外,催化剂CoOx@SBA-15和CoOx/SBA-15的图谱中也呈现着3个明显的特征衍射峰,这表明在500 ℃焙烧后载体SBA-15的介孔结构并没有被破坏.
催化剂的广角XRD图谱如图1(b)所示. 从图1可以看出,浸渍法合成的CoOx/SiO2催化剂在2θ 为36.56°、55.3°、59.96°处均有明显的特征衍射峰,此处衍射峰可归属于尖晶石Co3O4的(311)、(422)、(511)晶面,这表明焙烧过程中Co(NO3)2在载体SiO2形成了较大的Co3O4微晶,这是因为金属和载体之间的相互作用较弱[8]. 此外在该材料的XRD图谱上还可以观察到CoO(2θ = 42.9°)和金属Co(2θ = 44.2°)的特征衍射峰,这是因为在焙烧过程中产生的微量C、N会还原部分Co3O4. 从CoOx@SBA-15和CoOx/SBA-15的广角XRD图谱中可以看出,2种材料与载体SBA-15一样均在2θ = 22°附近有一处较宽的衍射峰,可归属于无定型SiO2的特征峰[9]. 其中催化剂CoOx/SBA-15可以观察到一处微弱的CoO(2θ = 42.9°)特征衍射峰,表明位于SBA-15上的Co(NO3)2焙烧时部分形成了CoO分散在SBA-15表面或孔道中,而在CoOx@SBA-15中未观察到明显的衍射峰,这表明金属在CoOx@SBA-15中高度分散.
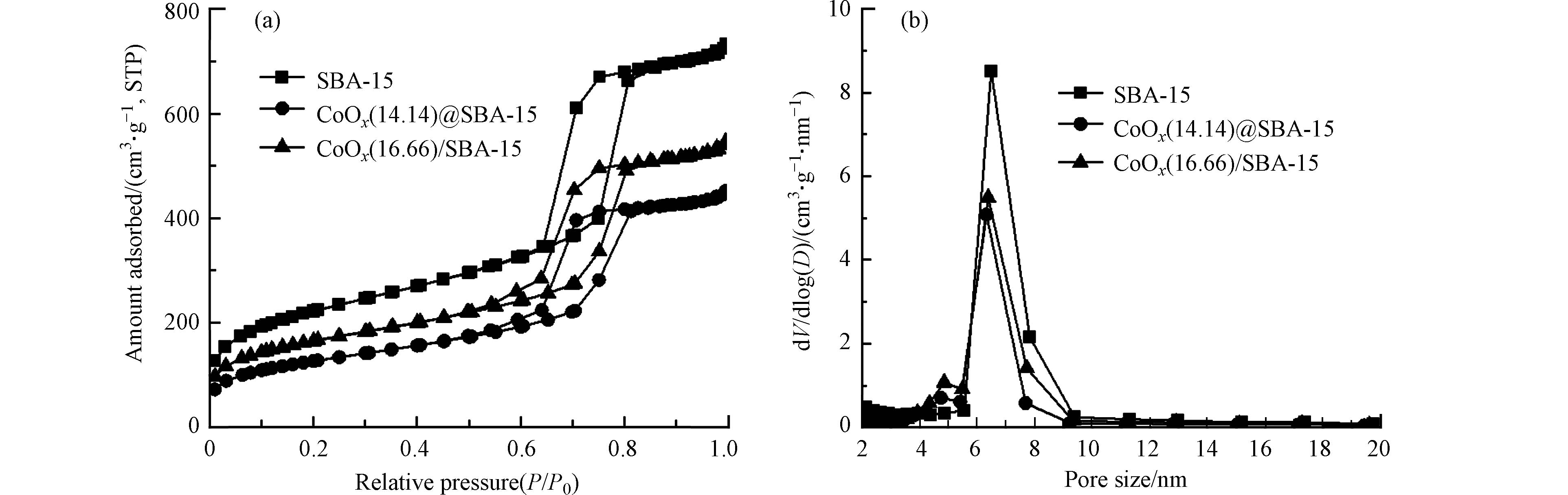
去模板后的载体SBA-15以及催化剂CoOx@SBA-15和CoOx/SBA-15的N2吸附-脱附等温线和孔径分布如图2所示,各材料的结构参数汇总在表1. 从图2(a)中可以看出,SBA-15的N2吸附-脱附等温线在P/P0 0.64—0.85之间出现了明显的H1型回滞环,且曲线为典型的IV型等温线,验证了实验中合成的SBA-15具有有序的介孔结构[10]. CoOx@SBA-15和CoOx/SBA-15的等温线形状与SBA-15类似,分别在P/P0 0.50—0.85和0.49—0.82之间存在H1型回滞环,这说明经过焙烧后的催化剂依旧具有和SBA-15一样的有序介孔结构. 但材料的氮气吸附量呈现出CoOx@SBA-15 < CoOx/SBA-15 < SBA-15的趋势,这是由于固相研磨法合成的催化剂中金属会在焙烧过程中更多地被限域在SBA-15的孔道中,从而影响材料的吸附量. 除此之外,如图2(b)所示,催化剂CoOx@SBA-15和CoOx/SBA-15和载体SBA-15的孔径均集中分布在4—10 nm之间,最可几孔径分别为6.38 nm、6.33 nm和6.49 nm,这表明金属的负载并不会影响SBA-15的中孔结构. 除此之外,从表1中可以看出,CoOx@SBA-15和CoOx/SBA-15的孔容分别为0.68 cm3·g-1和0.82 cm3·g−1,相较于SBA-15的孔容1.10 cm3·g−1有很大降低,孔径和孔容结果也进一步验证了催化剂CoOx@SBA-15上SBA-15对钴氧化物的限域作用.
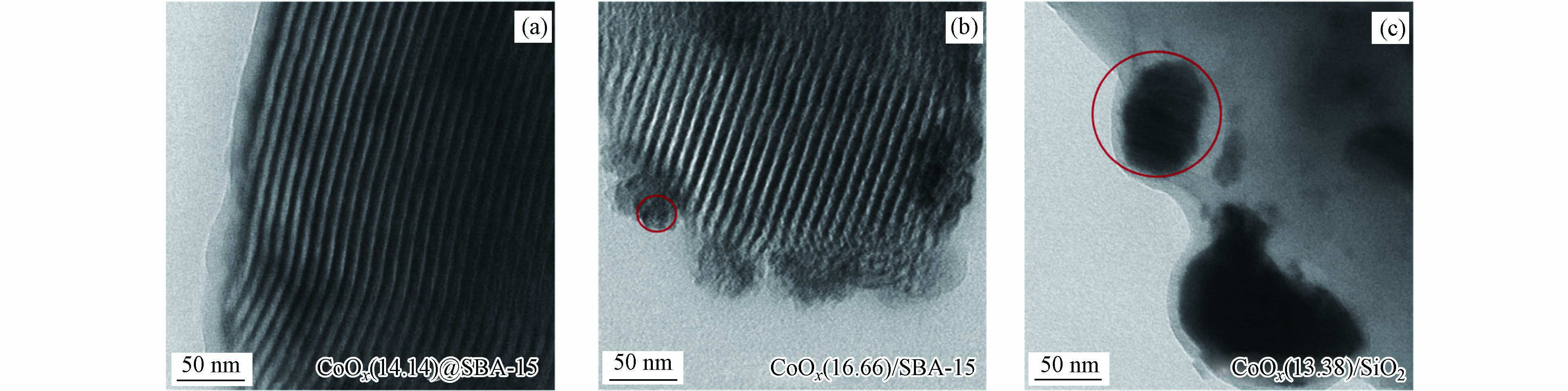
图3为催化剂CoOx@SBA-15、CoOx/SBA-15和CoOx/SiO2的TEM图. 从图3(a)、(b)可以观察到清晰的孔道结构,这与SBA-15的典型孔道结构相一致[7]. 此外CoOx/SBA-15和CoOx/SiO2的TEM图中可以观察到明显的金属颗粒,这是因为传统浸渍法合成的材料易在载体表面形成团聚而呈现出较大的金属颗粒. 对比图3(a)可以发现,利用固相研磨法合成的材料CoOx@SBA-15中金属颗粒借由焙烧过程中和模板剂P123的相互作用高度分散在SBA-15的孔道之中,而未见明显的金属颗粒.
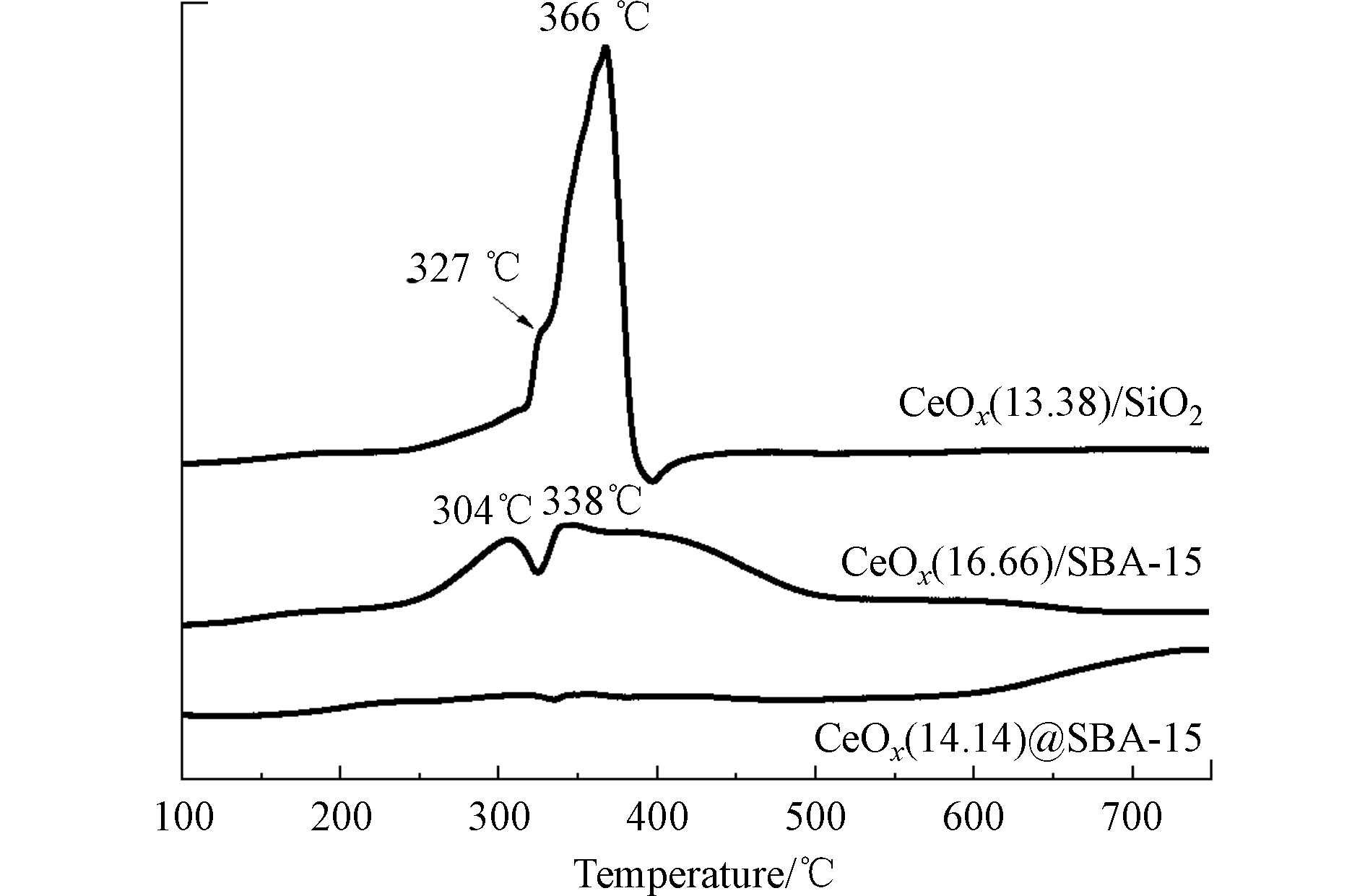
为了进一步探究催化剂中的CoOx和载体之间的相互作用,对3种催化剂进行了氢气程序升温还原实验,所得结果如图4所示. 从图4可以看出,CoOx/SBA-15和CoOx/SiO2的TPR曲线中均可观察到2个明显的连续还原峰,其中CoOx/SiO2的还原峰出现在327°C和366°C附近,分别对应于Co3O4还原为CoO和CoO还原为金属Co两个过程[11],且第二个还原峰的强度较高,这是因为负载在SiO2上的Co3O4的颗粒粒径较大,因而CoO的还原程度较高[12]. CoOx/SBA-15的2个还原峰出现在304°C和338°C附近,且300—500°C之间的还原峰强度较低、范围较广,这归因于SBA-15的介孔结构. 虽然同为浸渍法合成,但相较于CoOx/SiO2,CoOx/SBA-15上的CoOx分散度更高、颗粒更小. 相对地,CoOx@SBA-15的TPR曲线在100—600°C 范围内未观察到明显的还原峰,仅在700°C后出现了强度较弱的还原峰,该还原峰归属于Co2+与SBA-15强相互作用形成的高分散的硅酸钴类物质的还原峰[12],表明CoOx@SBA-15中CoOx为高分散,与TEM和XRD结果一致.
-
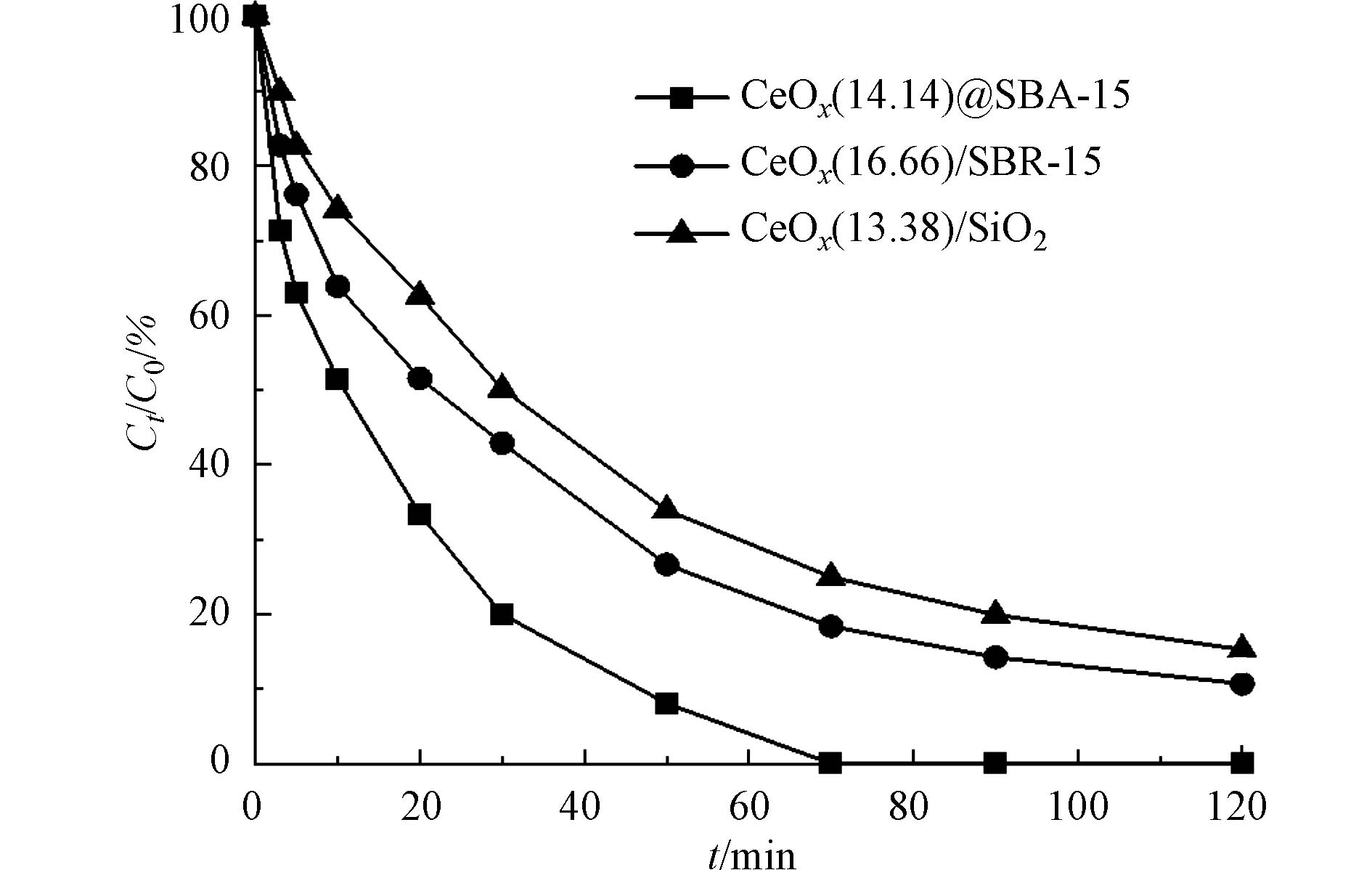
氯苯的初始浓度为0.18 mmol·L−1,催化剂的投加量为50 mg·L−1,PMS浓度为5 mmol·L−1时,分别以CoOx(14.14)@SBA-15、CoOx(16.66)/SBA-15和CoOx(13.38)/SiO2为催化剂在室温条件下进行氯苯的降解实验,所得结果如图5所示. 结果显示,3种催化剂均在活化PMS催化降解氯苯的反应中表现出较强的活性,反应120 min后,氯苯在CoOx(14.14)@SBA-15、CoOx(16.66)/SBA-15和CoOx(13.38)/SiO2 的3种催化剂上去除率分别为100%、89.3%和84.7%. 结合催化剂表征来看,SBA-15的介孔结构使得的催化活性CoOx(16.66)/SBA-15高于CoOx(13.38)/SiO2,具体来说,SBA-15的均匀孔结构有利于活性位点的分散同时还可以促进污染物在催化剂上的扩散,提高氯苯的降解效率. 而CoOx(14.14)@SBA-15的催化活性高于CoOx(16.66)/SBA-15是因为采用固相研磨法合成催化剂的过程中Co2+与模板剂P123互作用,使得CoOx高度分散在SBA-15的孔道中,形成对活性位点的限域作用[13]. 限域在孔道中的活性位点能够更高效地与PMS接触,提高PMS的活化效率,进而提高反应活性.
-
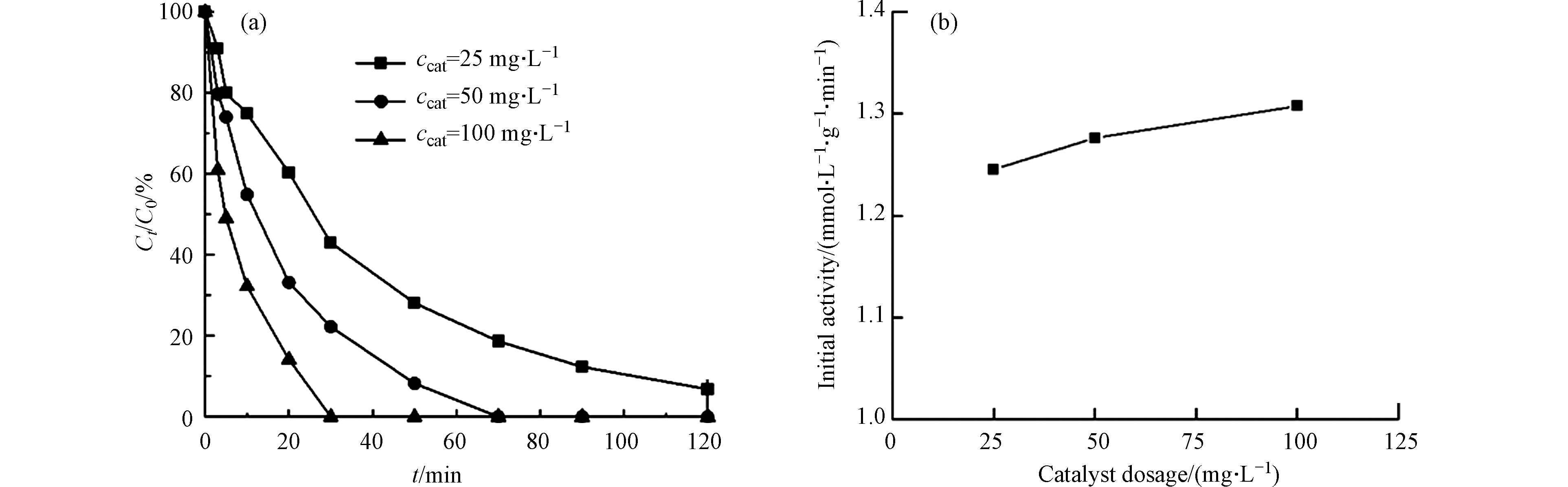
氯苯的初始浓度为0.18 mmol·L−1,PMS浓度为5 mmol·L−1时,以CoOx(14.14)@SBA-15为催化剂探究催化剂投加量对CoOx@SBA-15催化PMS降解氯苯的影响,所得结果如图6所示. 催化剂投加量为50 mg·L−1和100 mg·L−1时,氯苯分别在70 min和30 min时实现完全降解,而当催化剂投加量为25 mg·L−1时,氯苯在120 min时的去除率仅为93.2%,这表明氯苯的降解速率随着催化剂投加量的增加而增大. 通过计算催化剂在最初3 min内的催化活性可以验证反应过程是否受降解中间体的竞争性吸附影响[14],催化活性计算结果如图6(b)所示. 从图6可以看出,不同催化剂投加量时的氯苯降解反应的初活性基本相同,因此该反应不受催化剂传质阻力影响.
-
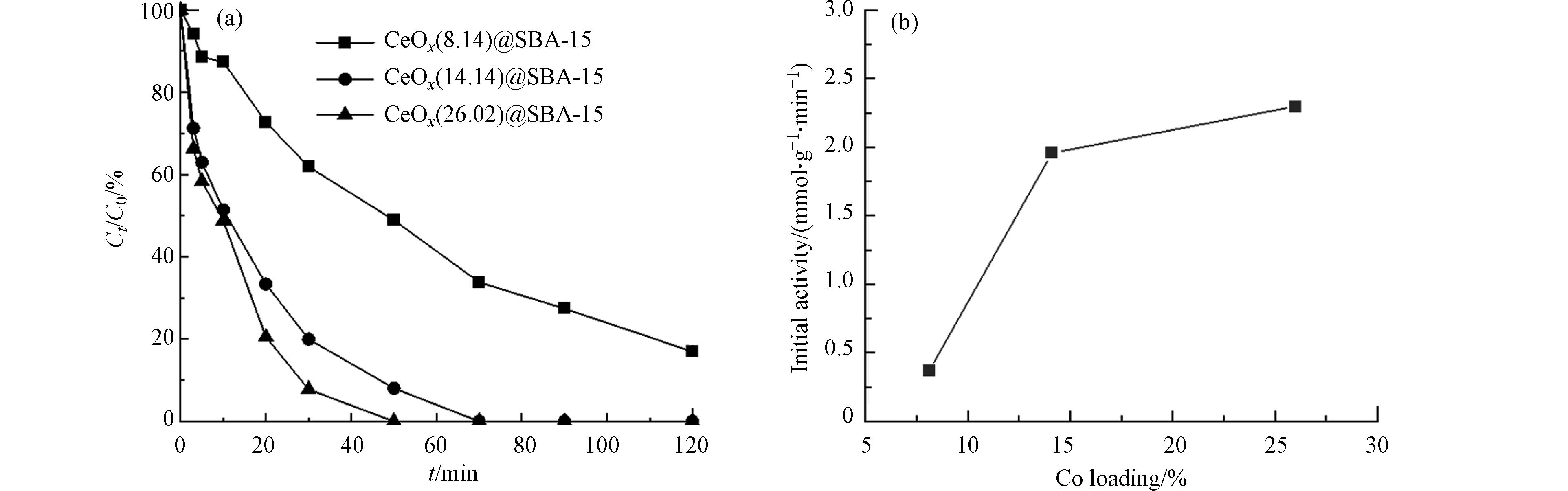
氯苯的初始浓度为0.18 mmol·L−1,催化剂的投加量为50 mmol·L−1,PMS浓度为5 mmol·L−1时,分别以不同负载量的CoOx@SBA-15为催化剂在室温条件下进行氯苯的降解实验,所得结果如图7所示. 从图7可以看出,当负载量为14.14%和26.02%时,CoOx@SBA-15可以在70 min内实现氯苯的完全降解,而当催化剂的负载量为8.14%时,反应120 min后氯苯的去除率仅为83%,氯苯的降解速率随着催化剂负载量的增加而增大. 图7(b)中展示的是不同负载量的CoOx@SBA-15催化剂的反应初活性,随着催化剂负载量的增加,催化反应在3 min内的初活性从0.368增长到2.297 mmol·L−1gCat−1min−1,这是因为负载量的增加使得催化反应的主要活性位点CoOx增多.
-
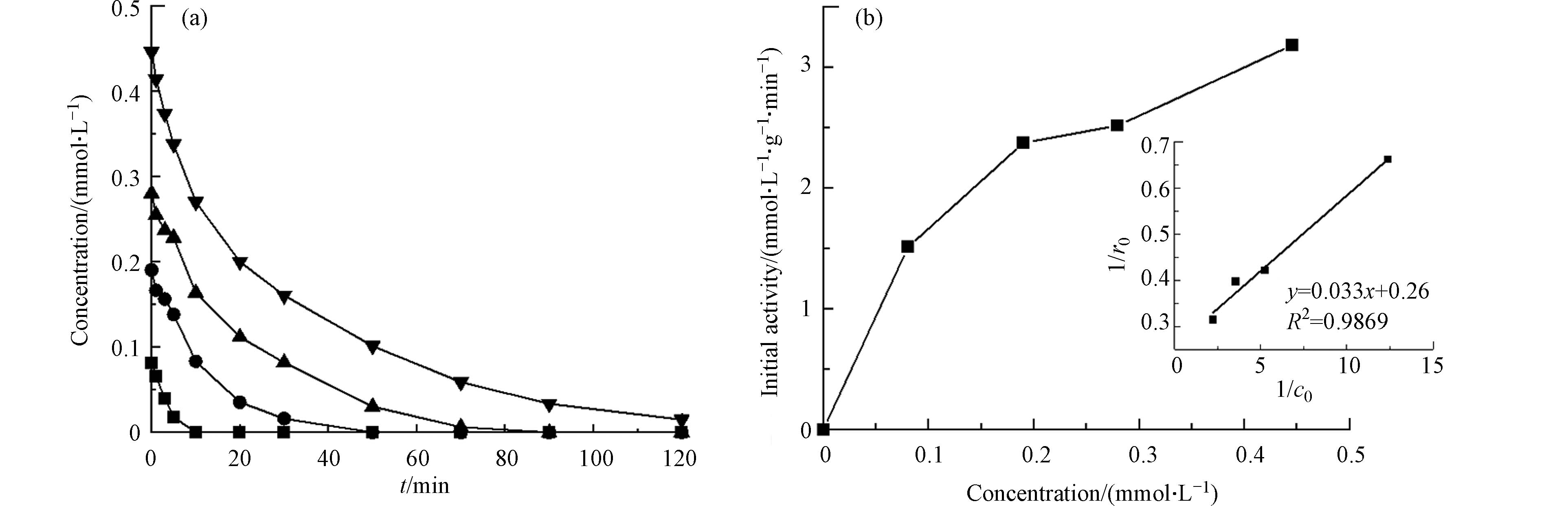
氯苯的初始浓度为0.18 mmol·L−1,催化剂的投加量为50 mg·L−1时,在其他条件不变的情况下,改变每次降解实验中氯苯和PMS的初始浓度,以进一步探究PMS在CoOx(14.14)@SBA-15上的活化机制,所得结果如图8、9所示. 从图8中可以看出,当PMS的浓度固定为5 mmol·L−1,氯苯的初始浓度从0.1 mmol·L−1增加至0.4 mmol·L−1时,氯苯的降解速率随之加快,反应初活性也从1.512 mmol·L−1gCat−1min−1增至3.185 mmol·L−1gCat−1min−1,这是因为随着氯苯初始浓度的增加,氯苯在CoOx(14.1)@SBA-15表面的吸附量不断增加,从而促进了氯苯的降解. 从图9中可以看出,当氯苯的初始浓度固定为0.1 mmol·L−1时,随着PMS浓度从2.5增加到10 mmol·L−1,反应初活性从1.429 mmol·L−1gCat−1min−1增至2.460 mmol·L−1gCat−1min−1,这说明PMS在催化剂表面的吸附量也会影响氯苯的降解反应.
为了进一步探究反应物在催化剂表面的吸附对反应的影响,使用Langmuir-Hinshelwood(L-H)模型对实验数据进行拟合,Langmuir吸附方程为:
其中,θ、b、c分别代表吸附的反应物覆盖在催化剂表面的分数,吸附常数和反应物的浓度. 而在该反应中,可能吸附在催化剂表面的反应物有氯苯和PMS,因此假设氯苯和PMS的初始浓度分别为
c1 和c2 ,当氯苯的初始浓度c1 固定时,θ的计算公式为:式中,
r1 为氯苯的初始降解速率,k为反应速率常数,b1 和b2 分别为氯苯和PMS的吸附常数. 从式4可以看出,当c2 固定时,1/r1 与1/c1 成正比. 同理,当PMS浓度c2 固定时,1/r2与1/c2 也呈正比.L-H模型假设反应速率与吸附在催化剂表面的反应物浓度成正比,进而证明反应物的吸附是催化反应的速率控制步骤. 因此将
1/r1 与1/c1 、1/r2 与1/c2 分别进行拟合,拟合结果如图8(b)和图9(b)的插图所示. 从图中可以看出,当氯苯的初始浓度改变时,1/r1 与1/c1 的线性关系良好(R2 > 0.98),同样的,当PMS的浓度改变时,1/r2 与1/c2 也呈现了良好的线性关系(R2 > 0.98),这表明氯苯和PMS在催化剂表面的吸附均在氯苯的降解过程中起着重要的作用,是反应的速率控制步骤. -
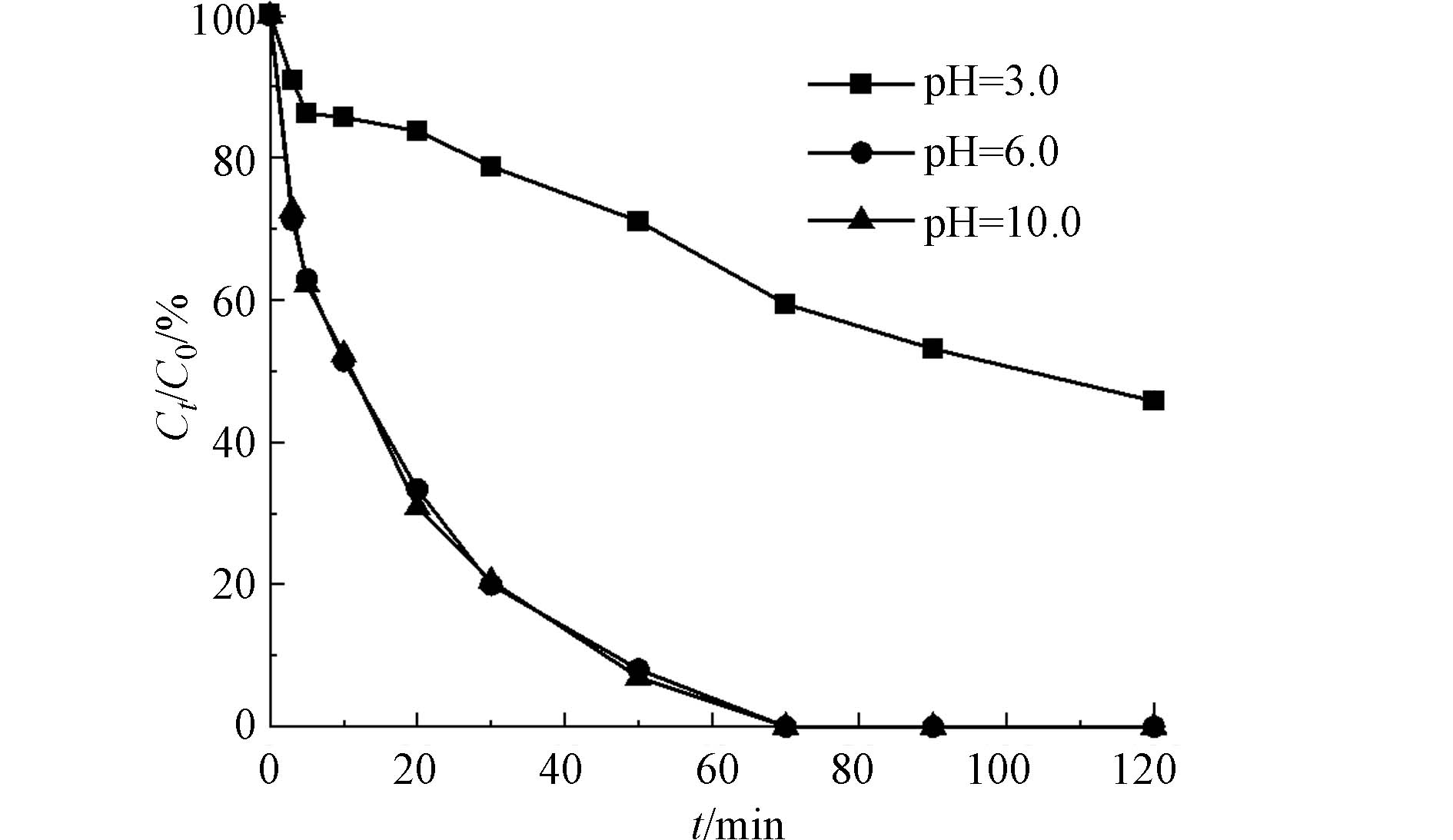
氯苯的初始浓度为0.18 mmol·L−1,PMS浓度为5 mmol·L−1,催化剂的投加量为50 mg·L−1 时,以CoOx(14.14)@SBA-15为催化剂探究反应体系初始pH氯苯降解的影响,所得结果如图10所示. 从图10可看出,当反应体系初始pH为6.0和10.0时,氯苯在70 min时可以实现完全降解,这表明CoOx@SBA-15催化剂在中性和碱性条件下都具有较高的催化活性. 然后当反应体系初始pH为3.0时,120 min时氯苯的去除率仅为54.3%,这表明酸性条件会抑制催化剂活化PMS降解氯苯,这是因为酸性条件下,H+易于HSO5-结合形成氢键,影响PMS的活化[15].
-
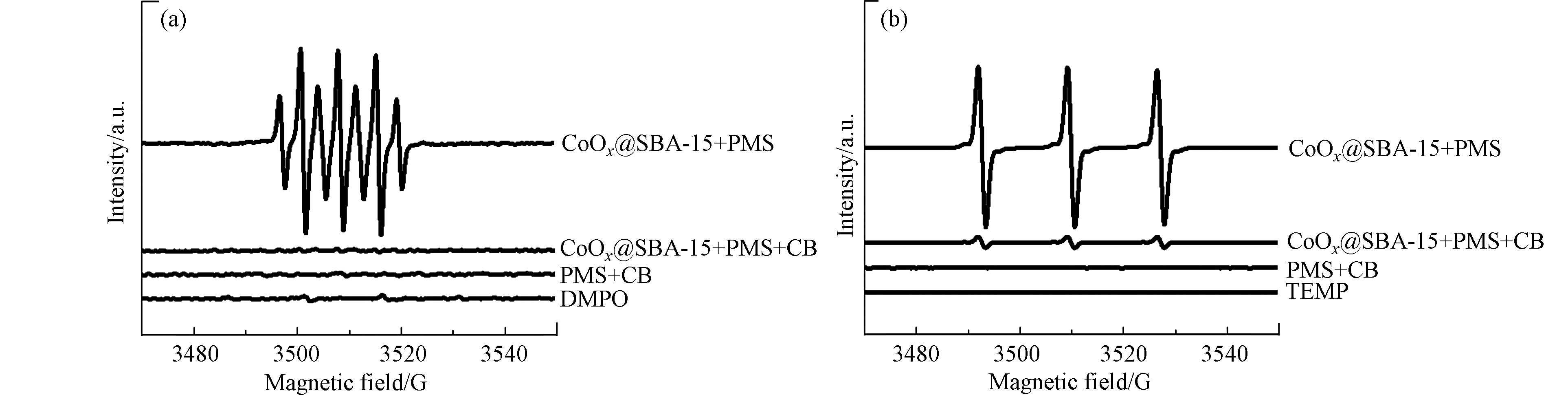
采用EPR技术检测反应体系中的活性自由基,具体方法见1.4,所得结果如图11所示. 从图11(a)可以看出,CoOx@SBA-15+PMS的EPR光谱图中未见明显的DMPO·-OH和DMPO·-
SO−4 特征峰,这表明该体系中没有产生SO·−4 和·OH,但在该体系的光谱中出现了强度为1:2:1:2:1:2:1的7条分裂峰,这类分裂峰可归属于DMPO-X,形成原因为DMPO受单原子直接氧化,类似的EPR信号曾出现在Co2+-PMS体系中[16]. 除此之外,在CoOx@SBA-15+PMS+CB、PMS+CB、DMPO体系的EPR光谱图中还出现了3个等强度的分裂缝,此处可归属于DMPO的分裂峰[17]. CoOx@SBA-15/PMS/CB催化体系的DMPO-EPR结果表明,PMS在催化剂CoOx@SBA-15上的活化存在非自由基过程,PMS会与CoOx@SBA-15表面的活性位点结合形成亚稳态复合物,这种复合物具有强氧化活性,从而促进氯苯的氧化降解. 这也再次验证了,在氯苯的降解过程中,PMS在催化剂表面的吸附是反应的关键步骤. 为了鉴定反应体系中可能存在的其他活性自由基,使用TEMP(2,2,6,6-四甲基-4 哌啶)作为单线态氧(1O2)的捕获剂,所得结果如图11(b)所示,TEMP-EPR光谱图中出现了3个等强度的分裂峰,归属于TEMP-1O2,这表明CoOx@SBA-15/PMS/CB催化体系中存在单线态氧(1O2).为了进一步验证反应体系中存在的自由基种类,在反应体系中加入一定量自由基猝灭剂[18],其中甲醇(Methanol)用于猝灭
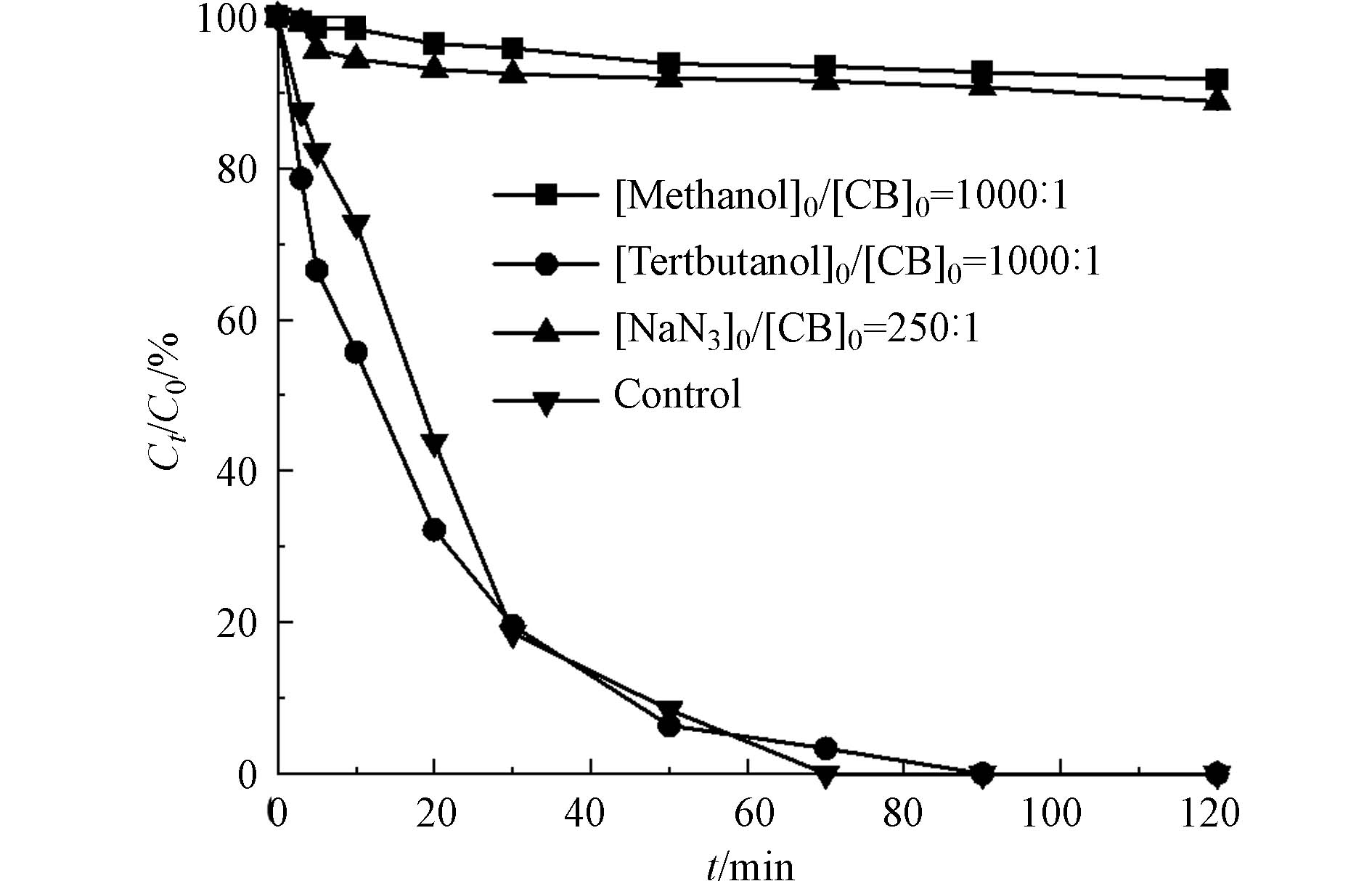
SO·−4 和·OH,叔丁醇(tert-butanol)用于猝灭·OH,叠氮化钠(NaN3)则用于猝灭1O2,实验结果如图12所示. 与Control组相比,当反应体系中存在甲醇和叠氮化钠时,氯苯在120 min时的去除率从100%分别下降到9.3%和10.2%. 而当反应体系中存在叔丁醇时,氯苯的降解反应未被明显抑制,虽然降解速率有所下降但在90 min时可以实现完全降解. 因此反应体系中的主要活性物种是SO·−4 和1O2,·OH的作用较小. -
(1)相较于传统浸渍法合成的催化剂CoOx/SBA-15和CoOx/SiO2,固相研磨法合成的催化剂CoOx@SBA-15的活性位点颗粒更小、分散度更高,因而在氯苯的降解实验中具有更高的催化活性.
(2) CoOx@SBA-15催化活化PMS降解氯苯的反应符合Langmuir-Hinshelwood模型,表明PMS和氯苯在催化剂表面的吸附是该反应的关键步骤.
(3)酸性条件下,H+易于HSO5-结合形成氢键,不利于PMS的活化,进而影响氯苯的降解效率.
(4)自由基抑制试验表明CoOx@SBA-15/PMS/CB催化体系中的主要活性物种为
SO·−4 、·OH和1O2.
SBA-15限域的CoOx催化过硫酸盐降解水中氯苯的机制研究
CoOx confined in SBA-15 as the highly effective catalyst to activate peroxymonosulfate for chlorobenzene degradation
-
摘要: 以中孔SBA-15为载体采用固相研磨法合成限域型催化剂CoOx@SBA-15,采用传统浸渍法合成负载型催化剂CoOx/SBA-15和CoOx/SiO2作为对比. 利用X射线衍射(XRD)、透射电子显微镜测试(TEM)等表征技术对催化剂的结构和组成进行分析,并对催化剂活化PMS降解氯苯的性能进行了研究. 结果表明,CoOx@SBA-15具有更高的催化活性,氯苯的降解反应受氯苯和PMS在催化剂表面吸附控制,CoOx@SBA-15/PMS/CB催化体系中的主要活性物种为
SO·−4 和1O2.-
关键词:
- CoOx@SBA-15 /
- 氯苯降解 /
- PMS活化 /
- 限域催化剂.
Abstract: Supported Co catalysts on SBA-15 were prepared using a solid-state reaction between Co(NO3)2 and organic template-occluded SBA-15. The catalysts were characterized by X-ray diffraction (XRD), N2 adsorption-desorption, H2 temperature-programmed reduction and Transmission Electron Microscope (TEM). Catalytic activities of the catalysts were investigated by activating PMS for chlorobenzene degradation. The results showed that CoOx@SBA-15 had a higher activity than other catalysts. The degradation of chlorobenzene on catalyst followed the Langmuir-Hinshelwood model, reflecting that the activation of adsorbed PMS was the rate controlling step. EPR results showed that the main active species involved in the catalytic system CoOx@SBA-15/PMS/CB wereSO·−4 and 1O2.-
Key words:
- PMS activation /
- chlorobenzene degradation /
- CoOx@SBA-15 /
- confinement atalysts
-
氯苯(chlorobenzene,CB)是最简单的氯芳烃,自19世纪合成以来,即大量用于生产DDT,至今,氯苯依然是年产量超过100万磅的高产量化学品[1]. 环境中的氯苯大多来源于人类的工业活动,据报道,美国氯苯类化合物的环境排放量可达到每年980吨[2]. 氯苯在自然界中的降解速度较慢,具有很强的生物积累性和生物毒性,有研究显示氯苯除了对中枢神经系统和呼吸系统有影响之外,还可造成肾脏和肝脏的损伤[3].
目前已经有很多研究者关注到氯苯的无害化处理问题,传统的氯苯处理方法主要包括吸附法、生物降解法和化学氧化法. 这些方法大多具有二次污染、效率低、选择性差等特点. 基于单过硫酸盐化合物(PMS)的高级氧化技术因其高氧化效率在降解氯代有机污染物的过程中表现出了优异的性能. 许多研究结果表明,钴氧化物(CoO、CoO2、Co2O3、CoO(OH)、Co3O4)具有活化PMS的良好能力,但单钴氧化物的比表面积非常低,以团聚,导致活性位有限,显著抑制其催化活性[4]. 有研究表明通过将钴氧化物分散在多孔材料的孔道中,可以将活性金属限域在特定孔结构中,从而使活性金属实现高度分散,这种方法可以极大提高钴基材料的催化活性[5]. SBA-15具有较高的比表面积、稳定的结构和有序的孔径,是一种良好的催化剂载体. 由于金属盐与模板剂之间的强相互作用,通过固相研磨法将金属盐与未去除模板的SBA-15充分混合之后,经过焙烧可以得到高金属分散度的催化剂. 因此,在本研究中,采用固相研磨法合成催化剂CoOx@SBA-15,并对其活化PMS降解氯苯的性能进行测试,并进一步探究反应中的各种因素对反应活性的影响机制及反应体系的主要活性物种.
1. 材料与方法(Materials and methods)
1.1 试剂与药品
试剂:P123(Sigma-Aldrich,99%),正硅酸四乙酯(国药,AR),六水合硝酸钴(阿拉丁,99%),氯苯(麦克林,AR),单过硫酸盐化合物(Sigma-Aldrich,99.9%),2,2,6,6-四甲基哌啶(Sigma-Aldrich,AR),5,5-二甲基-1-吡咯啉-N-氧化物(百灵威,AR),甲醇(TEDIA,HPLC),盐酸(国药,AR),实验中所用水均为去离子水.
1.2 催化剂的制备与表征
以SBA-15为载体利用固相研磨法制备限域型CoOx@SBA-15[6]:按文献报道方法,以正硅酸四乙酯(TEOS)为硅源,三嵌段共聚化合物P123为模板剂合成介孔氧化硅SBA-15[7];在室温条件下将一定量的Co(NO3)2·6H2O与1 g未去除模板剂的SBA-15在研钵中混合并研磨1 h得到CoOx@SBA-15;将所得的混合物置于马弗炉中,以2 °C·min−1 的升温速率升温至500 °C,并保持5 h,焙烧所得产物标记为CoOx(X)@SBA-15,其中X是钴的负载量(以质量分数计).
采用传统浸渍法制备CoOx/SBA-15和CoOx/SiO2催化剂. 首先将SBA-15置于马弗炉中焙烧,以1 °C·min−1的升温速率升温到550 °C,并保持6 h,目的是碳化并去除SBA-15中的模板剂;将购得的SiO2置于马弗炉中焙烧,以2 °C·min−1的升温速率升温到300 °C,并保持4 h,目的是去除其中可能存在的杂质;随后将一定量的Co(NO3)2溶液与1 g载体在室温条件下混合搅拌2 h以上,并在90 °C水浴中蒸干,在100 °C烘箱中干燥过夜,干燥后所得材料标记为CoOx(X)/Y,其中X是负载量(以质量分数计),Y是载体.
催化剂透射电镜分析(TEM)在日本JEOL公司,JEM-200CX型透射电子显微镜上检测;X射线衍射分析(XRD):采用日本Rigaku公司D/max-rA型X射线衍射仪,Cu 靶(Kα1,λ=0.154056 nm,扫描速度6(°)· min−1),操作条件:40 kV、30 mA,扫描范围:10°—80°;催化剂中Co含量采用原子吸收光谱(AAS,美国Thermo公司)测定;催化剂比表面积、孔径孔容采用比表面积测定仪(ASAP 2020,美国Micromeritics公司)分析;催化剂的在不同温度的还原状态采用泛泰公司生产的Finesorb-3010程序升温化学吸附仪进行测定.
1.3 氯苯的降解实验
氯苯的降解实验在250 mL三口烧瓶中进行,温度保持在(25±0.5)℃,具体操作方法如下:储备液用去离子水稀释至反应所需浓度,三口烧瓶中溶液总体积为200 mL,随后将一定量的催化剂(5—40 mg)分散在溶液中,搅拌1 h以达到吸附平衡并保证催化剂充分分散. 加入一定量的PMS储备液开始反应,在反应开始后的固定时间(1、3、5、10、20、30、50、70、90、120 min)取出反应溶液,并通过0.22 μm PTFE过滤器(Anpel)进行过滤. 将过滤后的1 mL反应溶液转移到装有0.5 mL甲醇的2 mL棕色液相小瓶中以清除残留的自由基.
使用配备有C-18色谱柱(ZORBAX Eclipse XDB-C18)的高效液相色谱仪(HPLC,1220 Infinity II)检测滤液中的氯苯. 仪器条件:流动相包括纯水和甲醇(30/70,V/V),流速为0.8 mL·min−1. 紫外检测波长为223 nm,柱温30 ℃.
1.4 反应活性物种的鉴定
催化剂活化PMS产生的自由基采用德国Bruker BioSpin有限公司生产的顺磁共振波谱仪(Electron Paramagnetic Resonance Spectrometer,EMX PLUS(PPMS))检测. 具体方法如下:称取适量催化剂分散在去离子水中,涡旋振荡30 s确保催化剂充分分散,取一定量PMS溶液加入到催化剂的溶液中,并使其充分混匀;在2.5 mL尖头离心管中加入1 mL所得混合溶液和100 µL 1 mol·L−1的自由基捕获剂(5,5-二甲基吡咯啉氧化物(DMPO)或2,2,6,6-四甲基-4-哌啶(TEMP))储备液,自由基捕获剂需溶解在pH = 7.4的磷酸缓冲液中,涡旋振荡30 s;将混合后的溶液转移至EPR样品管中,进行EPR分析. EPR操作参数:中心场为348.0 mT,扫描宽度为20 mT,微波频率为9.77 GHz,调制频率为100 GHz,能量为20 mW.
2. 结果讨论 (Results and discussion)
2.1 催化剂的表征
图1(a)是焙烧去除模板后的SBA-15、固相研磨法合成的催化剂CoOx@SBA-15以及浸渍法合成的催化剂CoOx/SBA-15的小角XRD图谱,小角XRD图谱可以用于分析材料的孔结构的有序度. 从图1(a)中可以看出,去除模板后的SBA-15在2θ为0.75°到2°范围内有3个明显的特征衍射峰,分别位于0.88°、1.52°和1.76°,对应于于SBA-15的(100)、(110)和(200)衍射面,该结果表明合成的SBA-15具有高度的二维六方介孔结构和p6mm对称性[7]. 此外,催化剂CoOx@SBA-15和CoOx/SBA-15的图谱中也呈现着3个明显的特征衍射峰,这表明在500 ℃焙烧后载体SBA-15的介孔结构并没有被破坏.
催化剂的广角XRD图谱如图1(b)所示. 从图1可以看出,浸渍法合成的CoOx/SiO2催化剂在2θ 为36.56°、55.3°、59.96°处均有明显的特征衍射峰,此处衍射峰可归属于尖晶石Co3O4的(311)、(422)、(511)晶面,这表明焙烧过程中Co(NO3)2在载体SiO2形成了较大的Co3O4微晶,这是因为金属和载体之间的相互作用较弱[8]. 此外在该材料的XRD图谱上还可以观察到CoO(2θ = 42.9°)和金属Co(2θ = 44.2°)的特征衍射峰,这是因为在焙烧过程中产生的微量C、N会还原部分Co3O4. 从CoOx@SBA-15和CoOx/SBA-15的广角XRD图谱中可以看出,2种材料与载体SBA-15一样均在2θ = 22°附近有一处较宽的衍射峰,可归属于无定型SiO2的特征峰[9]. 其中催化剂CoOx/SBA-15可以观察到一处微弱的CoO(2θ = 42.9°)特征衍射峰,表明位于SBA-15上的Co(NO3)2焙烧时部分形成了CoO分散在SBA-15表面或孔道中,而在CoOx@SBA-15中未观察到明显的衍射峰,这表明金属在CoOx@SBA-15中高度分散.
去模板后的载体SBA-15以及催化剂CoOx@SBA-15和CoOx/SBA-15的N2吸附-脱附等温线和孔径分布如图2所示,各材料的结构参数汇总在表1. 从图2(a)中可以看出,SBA-15的N2吸附-脱附等温线在P/P0 0.64—0.85之间出现了明显的H1型回滞环,且曲线为典型的IV型等温线,验证了实验中合成的SBA-15具有有序的介孔结构[10]. CoOx@SBA-15和CoOx/SBA-15的等温线形状与SBA-15类似,分别在P/P0 0.50—0.85和0.49—0.82之间存在H1型回滞环,这说明经过焙烧后的催化剂依旧具有和SBA-15一样的有序介孔结构. 但材料的氮气吸附量呈现出CoOx@SBA-15 < CoOx/SBA-15 < SBA-15的趋势,这是由于固相研磨法合成的催化剂中金属会在焙烧过程中更多地被限域在SBA-15的孔道中,从而影响材料的吸附量. 除此之外,如图2(b)所示,催化剂CoOx@SBA-15和CoOx/SBA-15和载体SBA-15的孔径均集中分布在4—10 nm之间,最可几孔径分别为6.38 nm、6.33 nm和6.49 nm,这表明金属的负载并不会影响SBA-15的中孔结构. 除此之外,从表1中可以看出,CoOx@SBA-15和CoOx/SBA-15的孔容分别为0.68 cm3·g-1和0.82 cm3·g−1,相较于SBA-15的孔容1.10 cm3·g−1有很大降低,孔径和孔容结果也进一步验证了催化剂CoOx@SBA-15上SBA-15对钴氧化物的限域作用.
表 1 SBA-15及钴基催化剂的结构参数Table 1. Structural parameters of SBA-15 and Cobalt-based catalyst样品 Sample SBETa/(m2·g−1) Smicrob/(m2·g−1) Vtc/(cm3·g−1) Vmicrob/(cm3·g−1) DBJHd/nm SBA-15 744 75 1.10 0.051 6.68 CoOx(14.14)@SBA-15 430 38 0.68 0.024 6.70 CoOx(16.66)/SBA-15 552 69 0.82 0.045 6.80 a calculated by Brunauer-Emmett-Teller (BET) method;b calculated by t-plot method;c obtained at P/P0=0.995;d from BJH method. 图3为催化剂CoOx@SBA-15、CoOx/SBA-15和CoOx/SiO2的TEM图. 从图3(a)、(b)可以观察到清晰的孔道结构,这与SBA-15的典型孔道结构相一致[7]. 此外CoOx/SBA-15和CoOx/SiO2的TEM图中可以观察到明显的金属颗粒,这是因为传统浸渍法合成的材料易在载体表面形成团聚而呈现出较大的金属颗粒. 对比图3(a)可以发现,利用固相研磨法合成的材料CoOx@SBA-15中金属颗粒借由焙烧过程中和模板剂P123的相互作用高度分散在SBA-15的孔道之中,而未见明显的金属颗粒.
为了进一步探究催化剂中的CoOx和载体之间的相互作用,对3种催化剂进行了氢气程序升温还原实验,所得结果如图4所示. 从图4可以看出,CoOx/SBA-15和CoOx/SiO2的TPR曲线中均可观察到2个明显的连续还原峰,其中CoOx/SiO2的还原峰出现在327°C和366°C附近,分别对应于Co3O4还原为CoO和CoO还原为金属Co两个过程[11],且第二个还原峰的强度较高,这是因为负载在SiO2上的Co3O4的颗粒粒径较大,因而CoO的还原程度较高[12]. CoOx/SBA-15的2个还原峰出现在304°C和338°C附近,且300—500°C之间的还原峰强度较低、范围较广,这归因于SBA-15的介孔结构. 虽然同为浸渍法合成,但相较于CoOx/SiO2,CoOx/SBA-15上的CoOx分散度更高、颗粒更小. 相对地,CoOx@SBA-15的TPR曲线在100—600°C 范围内未观察到明显的还原峰,仅在700°C后出现了强度较弱的还原峰,该还原峰归属于Co2+与SBA-15强相互作用形成的高分散的硅酸钴类物质的还原峰[12],表明CoOx@SBA-15中CoOx为高分散,与TEM和XRD结果一致.
2.2 CoOx@SBA-15催化活化PMS降解氯苯
2.2.1 不同催化剂催化氯苯降解实验
氯苯的初始浓度为0.18 mmol·L−1,催化剂的投加量为50 mg·L−1,PMS浓度为5 mmol·L−1时,分别以CoOx(14.14)@SBA-15、CoOx(16.66)/SBA-15和CoOx(13.38)/SiO2为催化剂在室温条件下进行氯苯的降解实验,所得结果如图5所示. 结果显示,3种催化剂均在活化PMS催化降解氯苯的反应中表现出较强的活性,反应120 min后,氯苯在CoOx(14.14)@SBA-15、CoOx(16.66)/SBA-15和CoOx(13.38)/SiO2 的3种催化剂上去除率分别为100%、89.3%和84.7%. 结合催化剂表征来看,SBA-15的介孔结构使得的催化活性CoOx(16.66)/SBA-15高于CoOx(13.38)/SiO2,具体来说,SBA-15的均匀孔结构有利于活性位点的分散同时还可以促进污染物在催化剂上的扩散,提高氯苯的降解效率. 而CoOx(14.14)@SBA-15的催化活性高于CoOx(16.66)/SBA-15是因为采用固相研磨法合成催化剂的过程中Co2+与模板剂P123互作用,使得CoOx高度分散在SBA-15的孔道中,形成对活性位点的限域作用[13]. 限域在孔道中的活性位点能够更高效地与PMS接触,提高PMS的活化效率,进而提高反应活性.
2.2.2 催化剂投加量对氯苯降解的影响
氯苯的初始浓度为0.18 mmol·L−1,PMS浓度为5 mmol·L−1时,以CoOx(14.14)@SBA-15为催化剂探究催化剂投加量对CoOx@SBA-15催化PMS降解氯苯的影响,所得结果如图6所示. 催化剂投加量为50 mg·L−1和100 mg·L−1时,氯苯分别在70 min和30 min时实现完全降解,而当催化剂投加量为25 mg·L−1时,氯苯在120 min时的去除率仅为93.2%,这表明氯苯的降解速率随着催化剂投加量的增加而增大. 通过计算催化剂在最初3 min内的催化活性可以验证反应过程是否受降解中间体的竞争性吸附影响[14],催化活性计算结果如图6(b)所示. 从图6可以看出,不同催化剂投加量时的氯苯降解反应的初活性基本相同,因此该反应不受催化剂传质阻力影响.
2.2.3 负载量对氯苯降解的影响
氯苯的初始浓度为0.18 mmol·L−1,催化剂的投加量为50 mmol·L−1,PMS浓度为5 mmol·L−1时,分别以不同负载量的CoOx@SBA-15为催化剂在室温条件下进行氯苯的降解实验,所得结果如图7所示. 从图7可以看出,当负载量为14.14%和26.02%时,CoOx@SBA-15可以在70 min内实现氯苯的完全降解,而当催化剂的负载量为8.14%时,反应120 min后氯苯的去除率仅为83%,氯苯的降解速率随着催化剂负载量的增加而增大. 图7(b)中展示的是不同负载量的CoOx@SBA-15催化剂的反应初活性,随着催化剂负载量的增加,催化反应在3 min内的初活性从0.368增长到2.297 mmol·L−1gCat−1min−1,这是因为负载量的增加使得催化反应的主要活性位点CoOx增多.
2.2.4 表面吸附对氯苯降解的影响
氯苯的初始浓度为0.18 mmol·L−1,催化剂的投加量为50 mg·L−1时,在其他条件不变的情况下,改变每次降解实验中氯苯和PMS的初始浓度,以进一步探究PMS在CoOx(14.14)@SBA-15上的活化机制,所得结果如图8、9所示. 从图8中可以看出,当PMS的浓度固定为5 mmol·L−1,氯苯的初始浓度从0.1 mmol·L−1增加至0.4 mmol·L−1时,氯苯的降解速率随之加快,反应初活性也从1.512 mmol·L−1gCat−1min−1增至3.185 mmol·L−1gCat−1min−1,这是因为随着氯苯初始浓度的增加,氯苯在CoOx(14.1)@SBA-15表面的吸附量不断增加,从而促进了氯苯的降解. 从图9中可以看出,当氯苯的初始浓度固定为0.1 mmol·L−1时,随着PMS浓度从2.5增加到10 mmol·L−1,反应初活性从1.429 mmol·L−1gCat−1min−1增至2.460 mmol·L−1gCat−1min−1,这说明PMS在催化剂表面的吸附量也会影响氯苯的降解反应.
为了进一步探究反应物在催化剂表面的吸附对反应的影响,使用Langmuir-Hinshelwood(L-H)模型对实验数据进行拟合,Langmuir吸附方程为:
θ=bc1+bc (1) 其中,θ、b、c分别代表吸附的反应物覆盖在催化剂表面的分数,吸附常数和反应物的浓度. 而在该反应中,可能吸附在催化剂表面的反应物有氯苯和PMS,因此假设氯苯和PMS的初始浓度分别为
c1 c2 c1 θ=b1c11+b2c2+b1c1 (2) r1=−dcdt=kθ=kb1c11+b1c1+b2c2 (3) 1r1=1k+(1+b2c2)kb11c1 (4) 式中,
r1 b1 b2 c2 1/r1 1/c1 c2 1/r2与1/c2 L-H模型假设反应速率与吸附在催化剂表面的反应物浓度成正比,进而证明反应物的吸附是催化反应的速率控制步骤. 因此将
1/r1 1/c1 1/r2 1/c2 1/r1 1/c1 1/r2 1/c2 2.2.5 反应体系初始pH对氯苯降解的影响
氯苯的初始浓度为0.18 mmol·L−1,PMS浓度为5 mmol·L−1,催化剂的投加量为50 mg·L−1 时,以CoOx(14.14)@SBA-15为催化剂探究反应体系初始pH氯苯降解的影响,所得结果如图10所示. 从图10可看出,当反应体系初始pH为6.0和10.0时,氯苯在70 min时可以实现完全降解,这表明CoOx@SBA-15催化剂在中性和碱性条件下都具有较高的催化活性. 然后当反应体系初始pH为3.0时,120 min时氯苯的去除率仅为54.3%,这表明酸性条件会抑制催化剂活化PMS降解氯苯,这是因为酸性条件下,H+易于HSO5-结合形成氢键,影响PMS的活化[15].
2.3 催化体系中的活性物种
采用EPR技术检测反应体系中的活性自由基,具体方法见1.4,所得结果如图11所示. 从图11(a)可以看出,CoOx@SBA-15+PMS的EPR光谱图中未见明显的DMPO·-OH和DMPO·-
SO−4 SO·−4 为了进一步验证反应体系中存在的自由基种类,在反应体系中加入一定量自由基猝灭剂[18],其中甲醇(Methanol)用于猝灭
SO·−4 SO·−4 3. 结论 (Conclusion)
(1)相较于传统浸渍法合成的催化剂CoOx/SBA-15和CoOx/SiO2,固相研磨法合成的催化剂CoOx@SBA-15的活性位点颗粒更小、分散度更高,因而在氯苯的降解实验中具有更高的催化活性.
(2) CoOx@SBA-15催化活化PMS降解氯苯的反应符合Langmuir-Hinshelwood模型,表明PMS和氯苯在催化剂表面的吸附是该反应的关键步骤.
(3)酸性条件下,H+易于HSO5-结合形成氢键,不利于PMS的活化,进而影响氯苯的降解效率.
(4)自由基抑制试验表明CoOx@SBA-15/PMS/CB催化体系中的主要活性物种为
SO·−4 -
表 1 SBA-15及钴基催化剂的结构参数
Table 1. Structural parameters of SBA-15 and Cobalt-based catalyst
样品 Sample SBETa/(m2·g−1) Smicrob/(m2·g−1) Vtc/(cm3·g−1) Vmicrob/(cm3·g−1) DBJHd/nm SBA-15 744 75 1.10 0.051 6.68 CoOx(14.14)@SBA-15 430 38 0.68 0.024 6.70 CoOx(16.66)/SBA-15 552 69 0.82 0.045 6.80 a calculated by Brunauer-Emmett-Teller (BET) method;b calculated by t-plot method;c obtained at P/P0=0.995;d from BJH method. -
[1] KOWALSKY E S, JENNINGS A A. Worldwide regulatory guidance values for chlorinated benzene surface soil contamination [J]. Journal of Environmental Engineering, 2012, 138(11): 1085-1105. doi: 10.1061/(ASCE)EE.1943-7870.0000574 [2] MALCOLM H. Chlorobenzenes other than hexachlorobenzene: Environmental aspects [Z]. 2004 [3] National Center for Biotechnology Information. Pubchem compound summary for CID 7964, Chlorobenzene [Z]. 2022. [4] LIU X H, YI R, ZHANG N, et al. Cobalt hydroxide nanosheets and their thermal decomposition to cobalt oxide nanorings [J]. Chemistry - an Asian Journal, 2008, 3(4): 732-738. doi: 10.1002/asia.200700264 [5] WANG Y , WU Z , SHI L , et al. Rapid functionalization of mesoporous materials: Directly dispersing metal oxides into as-prepared SBA-15 occluded with template [J]. Advanced Materials, 2005, 17(3): 323-327. doi: 10.1002/adma.200400860 [6] NING X, LU Y Y, FU H Y, et al. Template-mediated Ni(II) dispersion in mesoporous SiO2 for preparation of highly dispersed Ni catalysts: Influence of template type [J]. ACS Applied Materials & Interfaces, 2017, 9(22): 19335-19344. [7] ZHAO D, FENG J, HUO Q, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores [J]. Science, 1998, 279(5350): 548-552. doi: 10.1126/science.279.5350.548 [8] PARK K S, SARAVANAN K, PARK S J, et al. Effects of CO2 on the deactivation behaviors of Co/Al2O3 and Co/SiO2 in CO hydrogenation to hydrocarbons [J]. Catalysis Science & Technology, 2017, 7(18): 4079-4091. [9] ZHAN H J, XIA Q H, LU X H, et al. Selective epoxidation of styrene with air catalyzed by CoOx and CoOx/SiO2 without any reductant [J]. Catalysis Communications, 2007, 8(10): 1472-1478. doi: 10.1016/j.catcom.2006.12.026 [10] LIU H M, LI Y M, WU H, et al. Effects of β-cyclodextrin modification on properties of Ni/SBA-15 and its catalytic performance in carbon dioxide reforming of methane [J]. Catalysis Communications, 2012, 28: 168-173. doi: 10.1016/j.catcom.2012.08.035 [11] VISWANATHAN B, GOPALAKRISHNAN R. Effect of support and promoter in Fischer-Tropsch cobalt catalysts [J]. Journal of Catalysis, 1986, 99(2): 342-348. doi: 10.1016/0021-9517(86)90359-3 [12] ZHANG X Y, GUAN Y Y, XIONG Y, et al. Nitrous oxide decomposition over Co/SBA-15 catalysts: The influence of metal loading, cobalt precursor, and solvent effect [J]. Materials Research Innovations, 2016, 20(7): 487-494. doi: 10.1179/1433075X15Y.0000000062 [13] CHEN W, FAN Z L, PAN X L, et al. Effect of confinement in carbon nanotubes on the activity of Fischer-Tropsch iron catalyst [J]. Journal of the American Chemical Society, 2008, 130(29): 9414-9419. doi: 10.1021/ja8008192 [14] ILINITCH O M, CUPERUS F P, NOSOVA L V, et al. Catalytic membrane in reduction of aqueous nitrates: Operational principles and catalytic performance [J]. Catalysis Today, 2000, 56(1/2/3): 137-145. [15] TAN C Q, GAO N Y, DENG Y, et al. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate [J]. Journal of Hazardous Materials, 2014, 276: 452-460. doi: 10.1016/j.jhazmat.2014.05.068 [16] HUANG Y F, HUANG Y H. Behavioral evidence of the dominant radicals and intermediates involved in Bisphenol A degradation using an efficient Co2+/PMS oxidation process [J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 418-426. [17] CANO M, QUINTANA J, JULIÁ L, et al. Trapping of cyclopropenyl radicals by 5, 5-dimethyl-1-pyrroline-N-oxide [J]. The Journal of Organic Chemistry, 1999, 64(14): 5096-5099. doi: 10.1021/jo9900026 [18] QIN X, FANG S W, ZHAO L, et al. Cobalt super-microparticles anchored on nitrogen-doped graphene for aniline oxidation based on sulfate radicals [J]. Science of the Total Environment, 2017, 601/602: 99-108. doi: 10.1016/j.scitotenv.2017.05.198 -






 下载:
下载: