-
2021年,我国新能源汽车年销售量达352.1×104辆,同比增长1.6倍[1]。锂电池作为新能源汽车的重要组成部分,具有高电压、高比容量、循环寿命长、对环境无污染等诸多优势,成为电源装置中的主导产品[2]。而具有更好的电化学性能、温度稳定性、安全性和循环寿命的磷酸铁锂正极材料的出现[3],更是促进了锂电池和新能源汽车的发展。磷酸铁锂电池在电动船舶、商用车、通讯基站等领域亦具有广阔的应用前景[4-8]。
磷酸铁锂电池经过一定的循环次数,在达到8~10年的使用期限后将会报废,预计到2025年,报废磷酸铁锂动力电池将近100×104 t[9-10],而电池的随意丢弃会污染土壤,导致环境酸碱度增高;同时会造成锂资源的浪费,不符合低碳循环的绿色发展理念。为保障“无废城市”建设的稳步推进,降低固体废物产生的强度,提升固体废物的综合利用水平,打好污染防治攻坚战,对废旧磷酸铁锂电池正极材料的资源化回收利用是势在必行的。
目前,对于废旧磷酸铁锂电池的回收主要是直接物理再生和湿法冶金2种途径[11]。SUN等[12]设计了一条绿色全固态路线,将经过纯化和均质化预处理后的废料与不同量的Li2CO3和20% (质量浓度) 蔗糖混合,在惰性氛围下,350 ℃加热5 h,然后在650 ℃加热10 h重新合成LiFePO4/C材料,在半电池和全电池测试中表现出优异的物理、化学和电化学性能。LIANG等[13]通过添加相应的Li、Fe、P盐后进行球磨和喷雾干燥,在氮气气氛下,650 ℃热处理10 h得到灰白色修复的LiFePO4粉末,修复后的LiFePO4材料的容量高达139 mAh·g−1。直接物理再生不涉及酸碱的使用,避免了二次废液的产生,但是高温煅烧带来的高能耗并不符合绿色化工的理念。湿法冶金主要是将经过预处理后的废旧LiFePO4/C粉末以离子形式浸出到溶液中,经过浓缩、提纯后生成金属盐或其他产物[14]。按照浸出方式的不同可分为无机酸浸法、有机酸浸法、氧化性无机盐浸出法和非氧化性无机盐浸出法[15]。KUMAR等[16]利用100%的柠檬酸作为浸出剂进行浸出实验,Li的浸出率最高达94.83%,Fe、P元素以FePO4形式回收再生LiFePO4/C材料,该实验柠檬酸廉价且易降解,对环境友好,但浸出过程中Fe的损失率达4.05%。ZHANG等[17]用理论量的1.05倍Na2S2O8氧化LiFePO4至FePO4,从废旧LiFePO4电池中选择性回收锂,Li的浸出率最高达99.9%,该方法减少了酸碱的使用,且Li的浸出效率高,但会引入大量Na2SO4。HUANG等[18]用HCl+H2O2浸出废旧LiFePO4 / LiMn2O4混合正极电池材料,固液分离得到含有Fe3+、Mn2+和Li+等离子的浸出液,回收效率高,产品纯度高,但HCl容易挥发而产生毒性。因此,本研究主要选取沸点比较高的H2SO4作为浸出溶剂,同时使用对环境友好的H2O2作为氧化剂,整个浸出工艺符合废旧磷酸铁锂电池电极材料绿色回收的发展趋势。
本研究采用湿法工艺,研究了浸出时间、浸出温度、pH、L/S以及H2O2/Li的摩尔比对废旧磷酸铁锂正极材料中3种离子 (Li、Fe、P) 浸出率的影响,通过浸出液的二次浸出实现了对Li的高效率浸出,并以棒状Li2CO3的形式回收;同时通过控制pH来减少Fe、P的浸出,并以FePO4/C形式进行回收。本研究结果可为选择性高效浸出废旧磷酸铁锂电池电极材料中锂的新工艺开发提供参考。
-
实验所采用的废旧磷酸铁锂电池电极材料来自某废旧磷酸铁锂电池回收企业,Li、Fe、P的质量分数分别为4.40%、34.20%和19.00%。
实验中使用的氢氧化钠 (NaOH) 、过氧化氢 (H2O2) 、浓硫酸 (H2SO4) 、无水碳酸钠 (Na2CO3) 试剂皆为分析纯,使用的溶液皆用去离子水配制。
-
实验所用的仪器包括:pH计 (PB-10,赛多利斯科学仪器北京有限公司) ;集热式恒温加热磁力搅拌器 (DF-101S,巩义市予华仪器有限责任公司) ;循环水式真空泵 (SHZ-D(Ⅲ);巩义市予华仪器有限责任公司) ;电子万用炉 (DL-1,北京市永光明医疗仪器有限公司) ;真空干燥箱 (BPZ-6033LC,上海一恒科学仪器有限公司) ;扫描电子显微镜 (MIRA4 LMH,捷克TESCAN公司以及SU3500,日本株式会社日立制作所) ;X-粉末衍射仪 (D8 Advance,德国Bruker公司) ;火焰原子吸收光谱仪 (240FS AA,美国安捷伦科技有限公司) ;比例光束分光光度计 (U-5100,日本株式会社日立制作所) 。
-
1) 第一次浸出。量取一定量的去离子水放入500 mL烧杯并置于恒温加热磁力搅拌器中进行加热,放入pH计调节pH,加入一定量的原料和双氧水,用一定浓度的硫酸对pH进行调控,反应一段时间后进行固液分离,得到低浓度的含Li+溶液和含FePO4的滤饼。
2) 浸出液的二次浸出。按一定固液比量取第一次浸出的低浓度含Li+溶液 (剩余的低浓度含Li+溶液收集起来用于下一次的二次浸出) ,放入500 mL烧杯并置于恒温加热磁力搅拌器中进行加热,重复第一次浸出的步骤,固液分离后得到高浓度的含Li+溶液和含FePO4的滤饼。
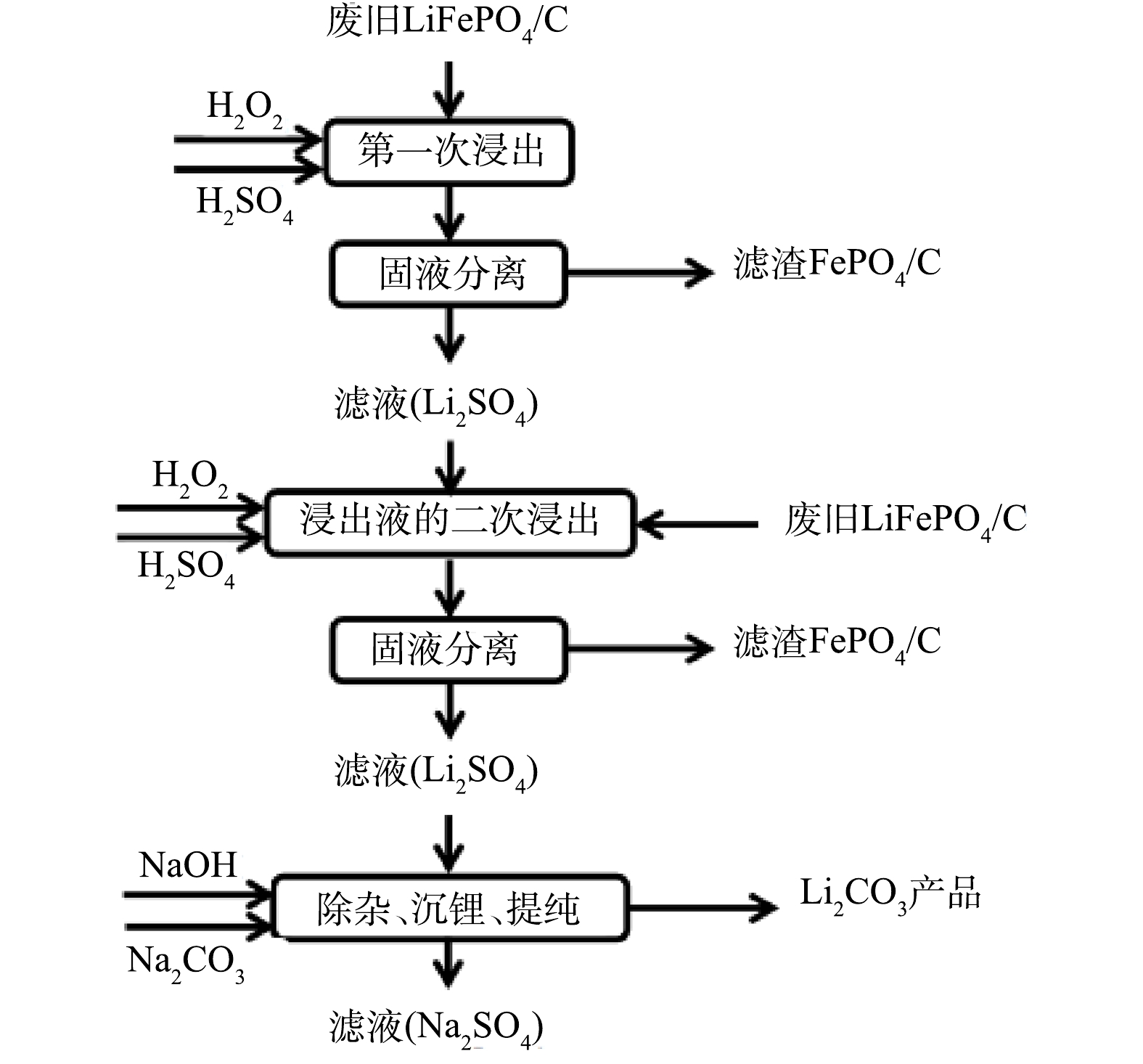
3) 将高浓度的含Li+滤液除杂、沉锂、提纯后得到Li2CO3产品,含FePO4的滤渣收集起来后续处理。具体浸出工艺流程如图1所示。
-
采用扫描电子显微镜观察废旧磷酸铁锂原料、浸出渣和Li2CO3产品的微观形貌;采用X-粉末衍射仪分析浸出渣和Li2CO3产品的物相;采用火焰原子吸收光谱仪定量分析浸出液中的Li、Fe含量;采用比例光束分光光度计定量分析浸出液中的P含量。
根据式 (1) 、式 (2) 计算Li、Fe、P元素的浸出率。
式中:A为从校准曲线查出的稀释试样中的离子质量浓度,mg·L−1;B为试样溶液的稀释倍数;V为试样体积,mL。m1为加入的废旧磷酸铁锂正极材料的质量,g;w为废旧磷酸铁锂正极材料中Li、Fe、P各元素的质量分数;C为第二次浸出时底液的离子质量浓度,mg·L−1;V为第二次浸出时底液的体积,mL。
-
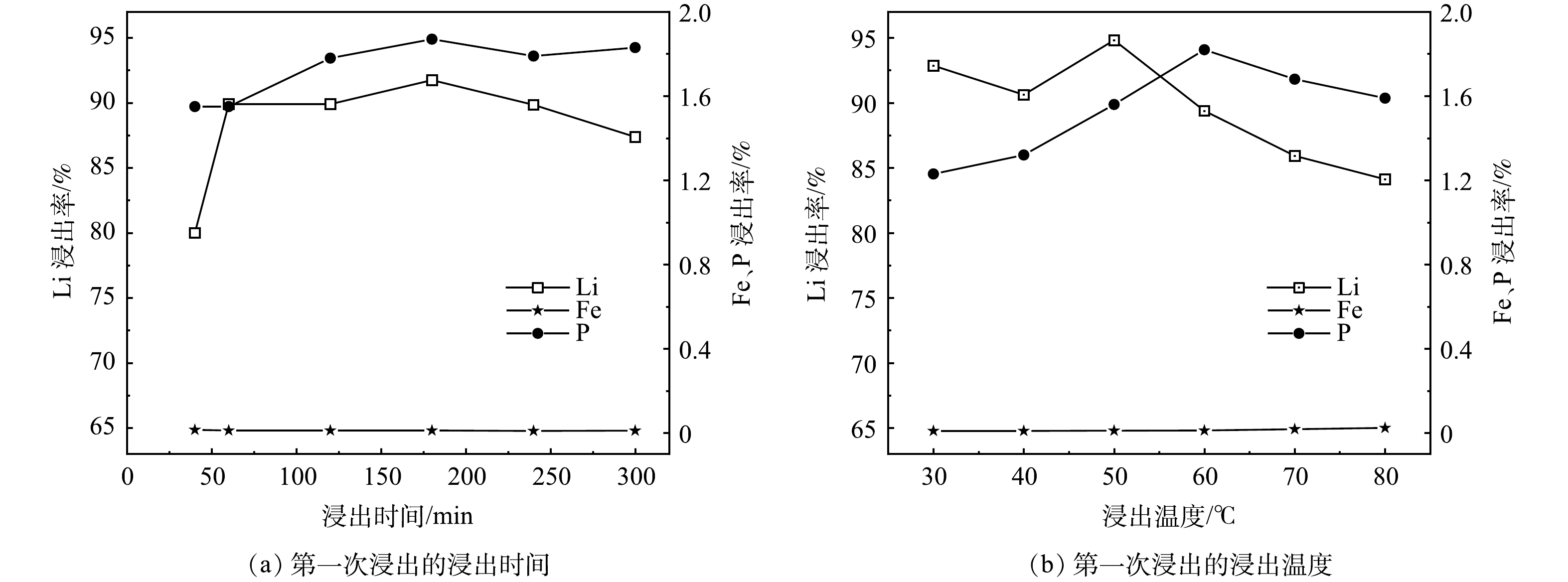
1) 浸出时间对锂、铁、磷浸出率的影响。实验在60 ℃、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.5、pH为3.0的条件下,浸出时间对锂、铁、磷浸出率的影响见图2 (a) 。从图中可以看出,随着反应时间从40 min到60 min,Li的浸出率从80.00%上升到89.90%,60 min后Li的浸出率变化不大,证明60 min后浸出反应基本完成,反应到了180 min时,Li的浸出率达到了最高点91.75%;同时P的浸出率也从1.55%上升到了最高点1.87%,随着反应时间继续增加,Li的浸出率降低,这归因于Li3PO4的形成。实验中Fe和P的浸出率分别在0.02%和1.87%之内,考虑到实验效率和后续Li2CO3的纯度,选取1 h作为最佳反应条件。
2) 浸出温度对锂、铁、磷浸出率的影响。实验在1 h、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.5、pH为3.0的条件下,浸出温度对锂、铁、磷浸出率的影响见图2 (b) 。从图中可以看出,随着浸出温度从30 ℃上升到50 ℃,Li的浸出率达到了最高点94.82%;同时Fe和P的浸出率也存在微量的增长,这是由于温度的上升加快了实验的反应速率,促进了各离子的浸出。随着浸出温度的继续增加,Li的浸出率降低,可归因于高温导致了H2O2分解,影响了Li的浸出,同时Fe和P的浸出率分别在0.03%和1.90%之内,故选取50 ℃作为最佳反应条件。在工业生产应用中考虑到能耗问题,室温下反应也是可行的。
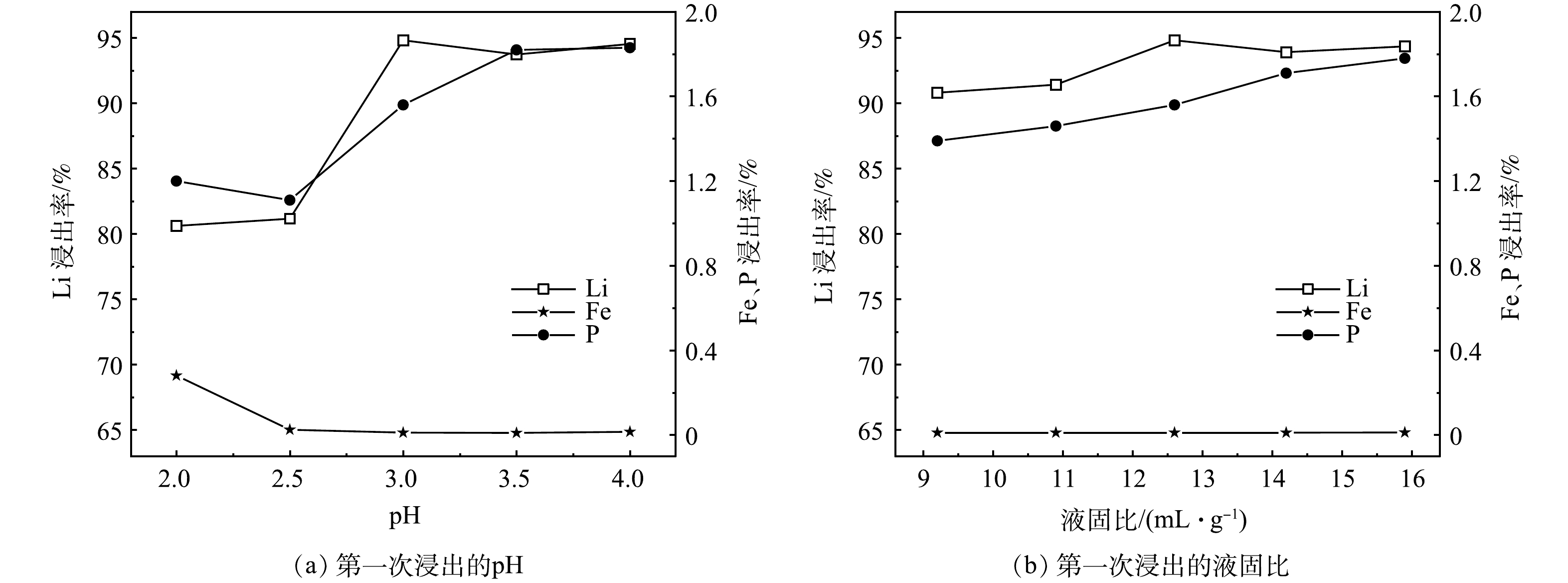
3) pH对锂、铁、磷浸出率的影响。实验在1 h、50 ℃、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.5的条件下,pH对锂、铁、磷浸出率的影响见图3 (a) 。从图中可以看出,随着浸出pH从2.0上升到4.0,Li的浸出率从80.62%上升到94%以上;同时P的浸出率呈现先降后升的趋势,Fe的浸出率呈现先急降后平稳的趋势,这归因于在H+浓度较高的情况下,Fe3+的溶解量较大,少量溶解的Fe3+与PO43-结合重新生成FePO4。为获取较高的Li浸出率和可接受的Fe、P损失,减少PO43-对Li2CO3纯度的影响,选取pH=3.0作为最佳反应条件。在此条件下,根据式 (3) 可得H2SO4/Li的摩尔比为0.50,而实验中H2SO4的摩尔用量为原料中Li摩尔量的0.49~0.50,与理论值基本一致,这表明接近化学计量的H2SO4已经可以达到对Li选择性浸出的预期效果。
4) 液固比对锂、铁、磷浸出率的影响。实验在1 h、50 ℃、H2O2/Li的摩尔比为1.5、pH为3.0的条件下,液固比对锂、铁、磷浸出率的影响见图3 (b) 。从图中可以看出,随着液固比从9.2 mL·g−1上升到12.6 mL·g−1,Li的浸出率最高可达94.82%,继续提高液固比,不仅Li的浸出率并未见大幅度增加,还会增加后期蒸发浓缩时的能耗。因此,选取L/S为12.6 mL·g−1作为最佳反应条件。
5) H2O2/Li的摩尔比对锂、铁、磷浸出率的影响。实验在1 h、50 ℃、L/S为12.6 mL·g−1、pH为3.0的条件下,H2O2/Li的摩尔比对锂、铁、磷浸出率的影响结果见表1。从表中可以看出,随着H2O2/Li的摩尔比从0.5上升到1.8,Li的浸出率不断上升,这是由于H2O2作为强氧化剂,将Fe从+2价氧化为+3价,反应式如式 (4) 所示,这有利于FePO4的形成,促使LiFePO4的溶解和Li的释放,而过量的H2O2会产生大量的气泡。因此,选取H2O2/Li的摩尔比为1.5作为最佳反应条件,在此条件下H2O2的合适添加量约为其理论值的3倍。
因此第一段浸出反应的最佳条件为浸出时间1 h、浸出温度50 ℃、L/S为12.6 mL·g−1、pH为3.0、H2O2/Li的摩尔比为1.5,Li的浸出率最高达94.82%;Fe和P的浸出率分别为0.011%和1.56%,Fe、P损失少。
-
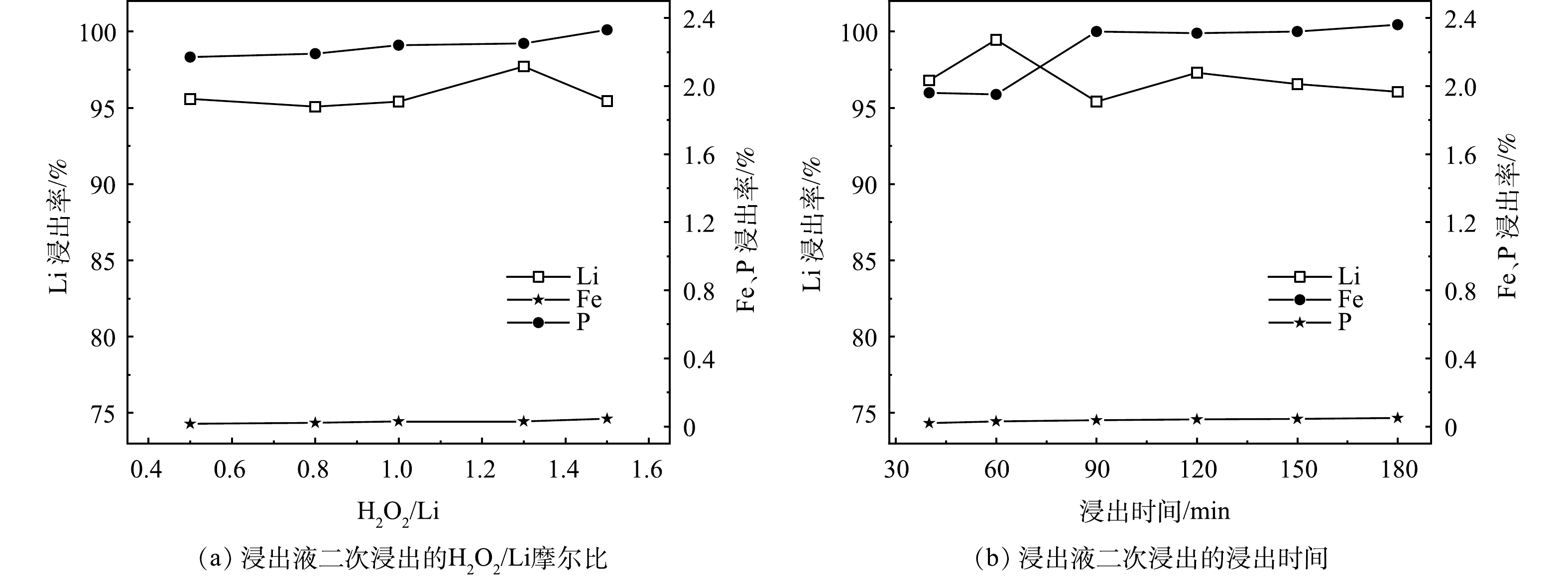
1) H2O2/Li的摩尔比对锂、铁、磷浸出率的影响。实验在50 ℃、L/S为12.6 mL·g−1、1 h、pH为3.0的条件下,H2O2/Li的摩尔比对锂、铁、磷浸出率的影响见图4 (a) 。从图中可以看出,随着H2O2/Li的摩尔比从0.5上升到1.5,Li的浸出率都在95%以上,根据式 (3) 可知,H2O2的理论摩尔量是物料中Li的摩尔量的0.5倍,由实验结果可知,当H2O2实际用量接近理论用量时,Li的浸出率依旧高于第一次浸出实验的最佳浸出率;最高点H2O2/Li的摩尔比为1.3,H2O2的用量约为理论值的2.5倍,比第一次浸出实验用量少了0.5倍。这归因于第一次浸出实验的浸出液中还残留未被分解的H2O2。综合考虑Li的浸出率和生产成本,选取H2O2/Li的摩尔比为1.3作为最佳反应条件,在此条件下,H2O2的体积分数为2.0%。
2) 浸出时间对锂、铁、磷浸出率的影响。实验在50 ℃、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.3、pH为3.0的条件下,浸出时间对锂、铁、磷浸出率的影响见图4 (b) 。从图中可以看出,浸出时间为40 min时,Li基本完成浸出。从40 min到180 min,Li的浸出率都在95%以上;同时Fe和P的浸出率分别在0.05%和2.40%之内,浸出时间为60 min时,Li的浸出率最高可达到99.46%。为获得较高的Li浸出率,选取浸出时间为1 h作为最佳反应条件。
综上,浸出液的二次浸出反应的最佳条件为浸出时间1 h、浸出温度50 ℃、L/S为12.6 mL·g−1、pH为3.0、H2O2/Li的摩尔比为1.3。经过浸出液的二次浸出,Li的浸出率从94.82%上升到最高可达99.46%,而Fe、P的浸出率分别在0.03%、2.3%以内,Fe、P的损失少;H2O2的用量从理论值的3倍降低到2.5倍,减少了H2O2的用量,节约了生产成本;同时,浸出液的二次浸出能提高滤液中锂离子溶度,利于后续沉锂。考虑到Li的浸出率达到了99%以上,而继续将浸出液进行第三次浸出的话,会增加Fe、P损失,降低生产效率,故将浸出液进行二次浸出后,进行下一阶段的除铁、沉锂。如表2所示,对比近几年LiFePO4的回收工艺,本研究相比于LI等[19]和LI等[20]具有更高的浸出率;MAHANDRA等[21]用HCOOH作浸出剂,Li浸出率高,但H2O2的用量同样高;高浸出率需要付出更长的反应时间和更大的能耗[9,22];KUMAR等[16]用100%柠檬酸作浸出剂,绿色环保,但伴随着高的H2O2消耗和较低的Li浸出率。目前研究的双氧水氧化-酸浸-浸出液二次浸出的工艺在高效选择性回收Li上具有一定的优势。
-
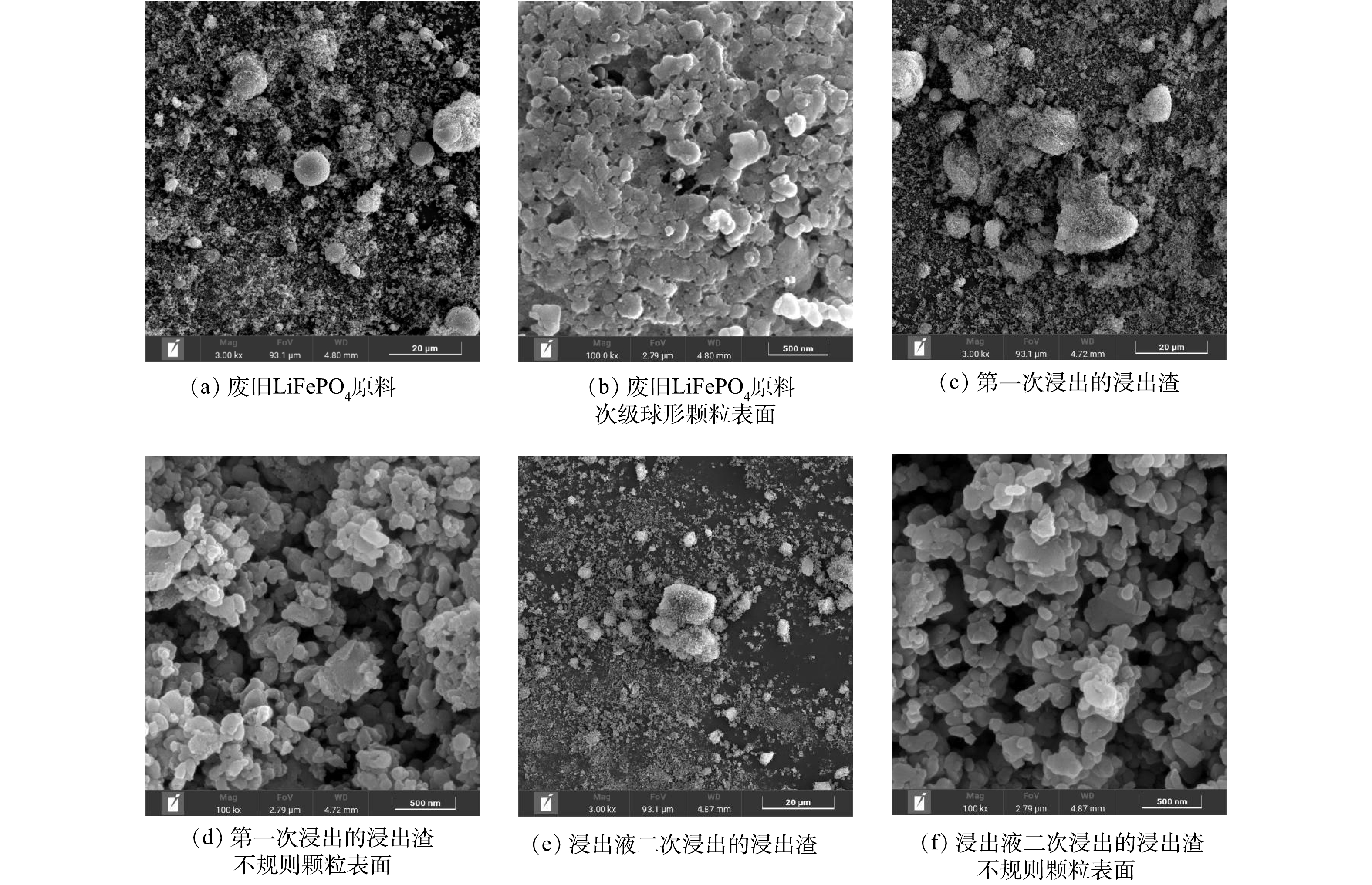
1) 浸出渣的SEM分析。从图5 (a) 可以看出,废旧LiFePO4原料由许多初级球形小颗粒和小部分次级球形大颗粒组成,图5 (b) 是次级球形大颗粒的表面,可以观察到,LiFePO4颗粒之间是紧密团聚在一块。经过第一次浸出后,从图5 (c) 、图5 (d) 可以看出LiFePO4颗粒散开了,看不到球形大颗粒,只看到一些不规则的块状颗粒,表明LiFePO4的溶解从表面渗透到了内部。从图5 (e) 、图5 (f) 可以看出,经过浸出液的循环浸出,浸出渣内部孔隙更加疏松,颗粒之间的团聚情况减弱,这使得反应时溶质的比表面积增大,溶质与溶剂接触得更加充分,Li的浸出反应更加完全。浸出液二次浸出的浸出渣比表面积的提高极有可能是Li浸出率提高将近5%的原因之一;初步判断,在浸出液的二次浸出中,浸出反应促进了LiFePO4从外到内的溶解,打开内部通道,一定的离子浓度减轻了颗粒之间的团聚情况,使得反应中LiFePO4颗粒与酸溶液的接触面积增大,促进了Li的脱嵌,提高了Li的浸出率。
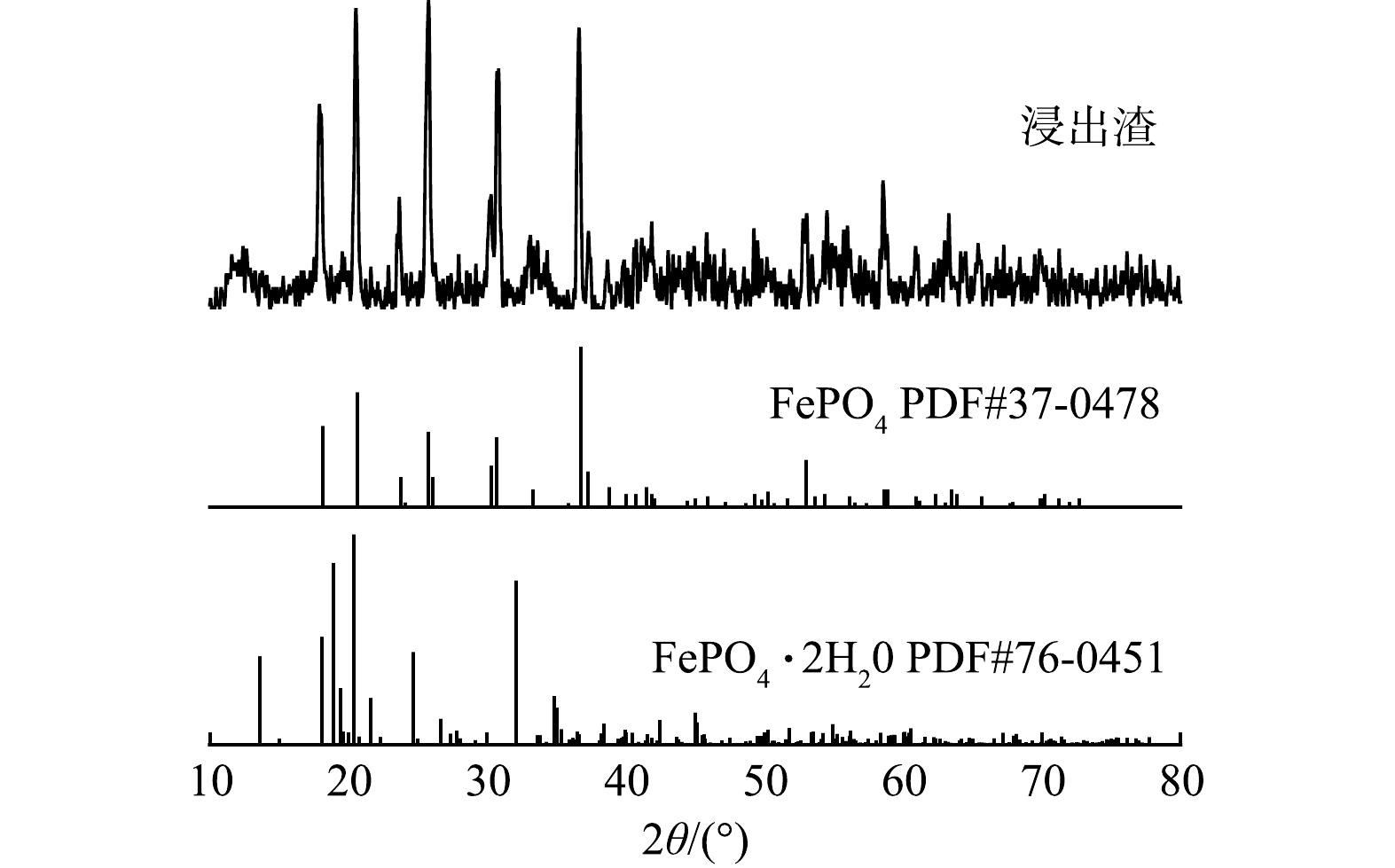
2) 浸出渣的XRD分析。如图6所示,将第一次浸出和浸出液二次浸出的混合浸出渣、标准FePO4 (PDF#37-0478) 和标准FePO4·2H2O (PDF#76-0451) 的XRD分析图进行对比,分析表明,Fe、P主要以橄榄型结构的无水FePO4形式存在于灰色浸出渣中。
-
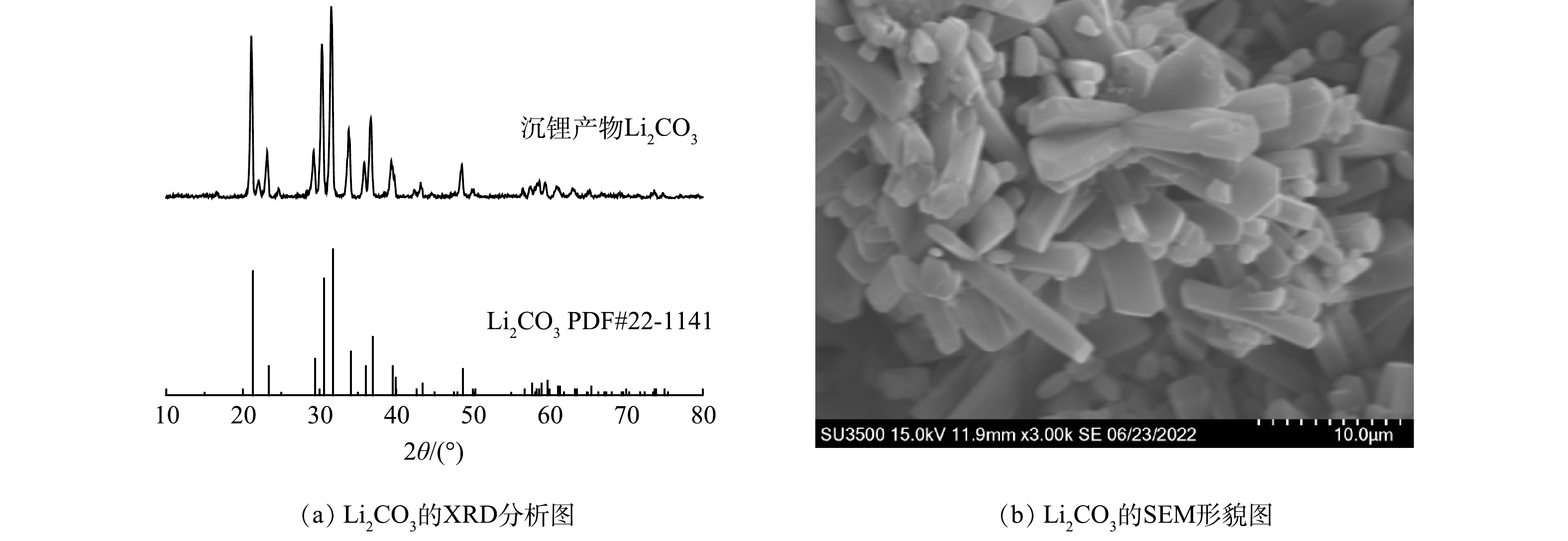
通过加入NaOH溶液除杂、Na2CO3溶液沉锂、沸水洗锂提纯后,得到白色粉末。如图7 (a) 所示,XRD分析表明了该白色粉末为Li2CO3,结晶度高、纯度高。如图7 (b) 所示,SEM形貌图可观察到所得Li2CO3产品棱角清晰,呈棒状结构,长径比大概在1~9之间,结晶效果良好。回收的Li2CO3产品能重新作为电池行业正极材料的重要原料,应用于新能源汽车、移动电子产品、通讯基站等领域,缓解当前锂资源短缺、价格上涨等问题。
-
1) 双氧水氧化-酸浸-浸出液二次浸出工艺的最优条件:1第一次,浸出时间1 h、浸出温度50 ℃、L/S为12.6 mL·g−1、pH为3.0、H2O2/Li的摩尔比为1.5;第二次,浸出时间1 h、H2O2/Li的摩尔比为1.3。
2) 双氧水氧化-酸浸-浸出液二次浸出工艺每段反应时间均仅为1 h;浸出液二次浸出时,H2O2的用量降低到理论值的2.5倍;H2SO4的用量接近于理论值,利于降低后续除杂净化时碱液的使用量。
3) Fe、P的浸出率分别在0.03%、2.3%之内,主要以橄榄型无水FePO4的形式存在于浸出渣中。
4) 浸出液经过一次循环浸出后,Li的浸出率最高可达99.46%,Li以Li2CO3形式进行回收,回收的Li2CO3产品结晶度高、纯度高,可用于锂电池的制备。
废旧磷酸铁锂电池正极材料浸出回收锂工艺
Leaching and recovering technology of lithium from the cathode material of waste lithium iron phosphate batteries
-
摘要: 为实现对废旧磷酸铁锂电池的高效资源化回收,提出了一种双氧水氧化—酸浸—浸出液二次浸出的工艺回收正极材料中的Li。通过浸出液二次浸出,选择性地从LiFePO4/C中浸出Li,既减少了双氧水的用量,又提高了锂离子浸出率。通过扫描电子显微镜 (SEM) 、原子吸收光谱仪 (AAS) 、X-粉末衍射仪 (XRD) 等分析表征手段考察了浸出温度、浸出时间、液固比 (L/S) 、pH以及H2O2/Li的摩尔比对Li、Fe、P元素浸出率的影响。结果表明,通过浸出液的二次浸出,Li的浸出率从94.82%上升到99.46%;同时,Fe和P的浸出率分别控制在0.03%和2.3%之内,为后续Fe、P的回收创造了有利条件。所得的Li2SO4浸出滤液,经过进一步的除杂和沉锂后得到Li2CO3白色粉末;Fe、P以橄榄型无水FePO4的形式存在于灰色浸出渣中。本研究结果可为废旧磷酸铁锂电池正极材料中锂的工业化回收提供参考。Abstract: In order to achieve the efficient resource recovery of waste lithium iron phosphate batteries, this paper proposed a process of oxidation-acid leaching-secondary leaching of the filtrate via hydrogen peroxide to recycle Li from the cathode material. By selectively leaching of Li from LiFePO4/C in secondary leaching of the filtrate, the consumption amount of hydrogen peroxide reduced and the leaching rate of lithium ion was improved. The effects of leaching temperature, leaching time, liquid-solid ratio (L/S), pH and the molar ratio of H2O2/Li on the leaching rates of Li, Fe and P were investigated by scanning electron microscopy (SEM), atomic absorption spectroscopy (AAS), X-ray powder diffraction (XRD) and other analytical detection methods. The results showed that the leaching rate of Li increased from 94.82% to 99.46% through secondary leaching of the filtrate, and the leaching rates of Fe and P were controlled within 0.03% and 2.3%, respectively, further creating favorable conditions for the subsequent recycle of Fe and P. White powder of Li2CO3 was obtained via further impurity removal and lithium deposition of the resulting Li2SO4 filtrate; Fe and P were presented in the gray leaching slag with the form of olive-shaped anhydrous FePO4. This paper provides a reference for the industrial recycling of lithium from the cathode materials of waste lithium iron phosphate batteries.
-
2021年,我国新能源汽车年销售量达352.1×104辆,同比增长1.6倍[1]。锂电池作为新能源汽车的重要组成部分,具有高电压、高比容量、循环寿命长、对环境无污染等诸多优势,成为电源装置中的主导产品[2]。而具有更好的电化学性能、温度稳定性、安全性和循环寿命的磷酸铁锂正极材料的出现[3],更是促进了锂电池和新能源汽车的发展。磷酸铁锂电池在电动船舶、商用车、通讯基站等领域亦具有广阔的应用前景[4-8]。
磷酸铁锂电池经过一定的循环次数,在达到8~10年的使用期限后将会报废,预计到2025年,报废磷酸铁锂动力电池将近100×104 t[9-10],而电池的随意丢弃会污染土壤,导致环境酸碱度增高;同时会造成锂资源的浪费,不符合低碳循环的绿色发展理念。为保障“无废城市”建设的稳步推进,降低固体废物产生的强度,提升固体废物的综合利用水平,打好污染防治攻坚战,对废旧磷酸铁锂电池正极材料的资源化回收利用是势在必行的。
目前,对于废旧磷酸铁锂电池的回收主要是直接物理再生和湿法冶金2种途径[11]。SUN等[12]设计了一条绿色全固态路线,将经过纯化和均质化预处理后的废料与不同量的Li2CO3和20% (质量浓度) 蔗糖混合,在惰性氛围下,350 ℃加热5 h,然后在650 ℃加热10 h重新合成LiFePO4/C材料,在半电池和全电池测试中表现出优异的物理、化学和电化学性能。LIANG等[13]通过添加相应的Li、Fe、P盐后进行球磨和喷雾干燥,在氮气气氛下,650 ℃热处理10 h得到灰白色修复的LiFePO4粉末,修复后的LiFePO4材料的容量高达139 mAh·g−1。直接物理再生不涉及酸碱的使用,避免了二次废液的产生,但是高温煅烧带来的高能耗并不符合绿色化工的理念。湿法冶金主要是将经过预处理后的废旧LiFePO4/C粉末以离子形式浸出到溶液中,经过浓缩、提纯后生成金属盐或其他产物[14]。按照浸出方式的不同可分为无机酸浸法、有机酸浸法、氧化性无机盐浸出法和非氧化性无机盐浸出法[15]。KUMAR等[16]利用100%的柠檬酸作为浸出剂进行浸出实验,Li的浸出率最高达94.83%,Fe、P元素以FePO4形式回收再生LiFePO4/C材料,该实验柠檬酸廉价且易降解,对环境友好,但浸出过程中Fe的损失率达4.05%。ZHANG等[17]用理论量的1.05倍Na2S2O8氧化LiFePO4至FePO4,从废旧LiFePO4电池中选择性回收锂,Li的浸出率最高达99.9%,该方法减少了酸碱的使用,且Li的浸出效率高,但会引入大量Na2SO4。HUANG等[18]用HCl+H2O2浸出废旧LiFePO4 / LiMn2O4混合正极电池材料,固液分离得到含有Fe3+、Mn2+和Li+等离子的浸出液,回收效率高,产品纯度高,但HCl容易挥发而产生毒性。因此,本研究主要选取沸点比较高的H2SO4作为浸出溶剂,同时使用对环境友好的H2O2作为氧化剂,整个浸出工艺符合废旧磷酸铁锂电池电极材料绿色回收的发展趋势。
本研究采用湿法工艺,研究了浸出时间、浸出温度、pH、L/S以及H2O2/Li的摩尔比对废旧磷酸铁锂正极材料中3种离子 (Li、Fe、P) 浸出率的影响,通过浸出液的二次浸出实现了对Li的高效率浸出,并以棒状Li2CO3的形式回收;同时通过控制pH来减少Fe、P的浸出,并以FePO4/C形式进行回收。本研究结果可为选择性高效浸出废旧磷酸铁锂电池电极材料中锂的新工艺开发提供参考。
1. 材料与方法
1.1 实验原料及试剂
实验所采用的废旧磷酸铁锂电池电极材料来自某废旧磷酸铁锂电池回收企业,Li、Fe、P的质量分数分别为4.40%、34.20%和19.00%。
实验中使用的氢氧化钠 (NaOH) 、过氧化氢 (H2O2) 、浓硫酸 (H2SO4) 、无水碳酸钠 (Na2CO3) 试剂皆为分析纯,使用的溶液皆用去离子水配制。
1.2 实验装置
实验所用的仪器包括:pH计 (PB-10,赛多利斯科学仪器北京有限公司) ;集热式恒温加热磁力搅拌器 (DF-101S,巩义市予华仪器有限责任公司) ;循环水式真空泵 (SHZ-D(Ⅲ);巩义市予华仪器有限责任公司) ;电子万用炉 (DL-1,北京市永光明医疗仪器有限公司) ;真空干燥箱 (BPZ-6033LC,上海一恒科学仪器有限公司) ;扫描电子显微镜 (MIRA4 LMH,捷克TESCAN公司以及SU3500,日本株式会社日立制作所) ;X-粉末衍射仪 (D8 Advance,德国Bruker公司) ;火焰原子吸收光谱仪 (240FS AA,美国安捷伦科技有限公司) ;比例光束分光光度计 (U-5100,日本株式会社日立制作所) 。
1.3 实验方法
1) 第一次浸出。量取一定量的去离子水放入500 mL烧杯并置于恒温加热磁力搅拌器中进行加热,放入pH计调节pH,加入一定量的原料和双氧水,用一定浓度的硫酸对pH进行调控,反应一段时间后进行固液分离,得到低浓度的含Li+溶液和含FePO4的滤饼。
2) 浸出液的二次浸出。按一定固液比量取第一次浸出的低浓度含Li+溶液 (剩余的低浓度含Li+溶液收集起来用于下一次的二次浸出) ,放入500 mL烧杯并置于恒温加热磁力搅拌器中进行加热,重复第一次浸出的步骤,固液分离后得到高浓度的含Li+溶液和含FePO4的滤饼。
3) 将高浓度的含Li+滤液除杂、沉锂、提纯后得到Li2CO3产品,含FePO4的滤渣收集起来后续处理。具体浸出工艺流程如图1所示。
1.4 分析方法
采用扫描电子显微镜观察废旧磷酸铁锂原料、浸出渣和Li2CO3产品的微观形貌;采用X-粉末衍射仪分析浸出渣和Li2CO3产品的物相;采用火焰原子吸收光谱仪定量分析浸出液中的Li、Fe含量;采用比例光束分光光度计定量分析浸出液中的P含量。
根据式 (1) 、式 (2) 计算Li、Fe、P元素的浸出率。
第一次浸出液中Li、Fe、P元素浸出率(%)=A×B×V×10−6m1×w×100% (1) 第二次浸出液中Li、Fe、P元素浸出率(%)=A×B×V×10−6m1×w+C×V×10−6×100% (2) 式中:A为从校准曲线查出的稀释试样中的离子质量浓度,mg·L−1;B为试样溶液的稀释倍数;V为试样体积,mL。m1为加入的废旧磷酸铁锂正极材料的质量,g;w为废旧磷酸铁锂正极材料中Li、Fe、P各元素的质量分数;C为第二次浸出时底液的离子质量浓度,mg·L−1;V为第二次浸出时底液的体积,mL。
2. 结果与讨论
2.1 第一次浸出实验结果分析与讨论
1) 浸出时间对锂、铁、磷浸出率的影响。实验在60 ℃、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.5、pH为3.0的条件下,浸出时间对锂、铁、磷浸出率的影响见图2 (a) 。从图中可以看出,随着反应时间从40 min到60 min,Li的浸出率从80.00%上升到89.90%,60 min后Li的浸出率变化不大,证明60 min后浸出反应基本完成,反应到了180 min时,Li的浸出率达到了最高点91.75%;同时P的浸出率也从1.55%上升到了最高点1.87%,随着反应时间继续增加,Li的浸出率降低,这归因于Li3PO4的形成。实验中Fe和P的浸出率分别在0.02%和1.87%之内,考虑到实验效率和后续Li2CO3的纯度,选取1 h作为最佳反应条件。
2) 浸出温度对锂、铁、磷浸出率的影响。实验在1 h、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.5、pH为3.0的条件下,浸出温度对锂、铁、磷浸出率的影响见图2 (b) 。从图中可以看出,随着浸出温度从30 ℃上升到50 ℃,Li的浸出率达到了最高点94.82%;同时Fe和P的浸出率也存在微量的增长,这是由于温度的上升加快了实验的反应速率,促进了各离子的浸出。随着浸出温度的继续增加,Li的浸出率降低,可归因于高温导致了H2O2分解,影响了Li的浸出,同时Fe和P的浸出率分别在0.03%和1.90%之内,故选取50 ℃作为最佳反应条件。在工业生产应用中考虑到能耗问题,室温下反应也是可行的。
3) pH对锂、铁、磷浸出率的影响。实验在1 h、50 ℃、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.5的条件下,pH对锂、铁、磷浸出率的影响见图3 (a) 。从图中可以看出,随着浸出pH从2.0上升到4.0,Li的浸出率从80.62%上升到94%以上;同时P的浸出率呈现先降后升的趋势,Fe的浸出率呈现先急降后平稳的趋势,这归因于在H+浓度较高的情况下,Fe3+的溶解量较大,少量溶解的Fe3+与PO43-结合重新生成FePO4。为获取较高的Li浸出率和可接受的Fe、P损失,减少PO43-对Li2CO3纯度的影响,选取pH=3.0作为最佳反应条件。在此条件下,根据式 (3) 可得H2SO4/Li的摩尔比为0.50,而实验中H2SO4的摩尔用量为原料中Li摩尔量的0.49~0.50,与理论值基本一致,这表明接近化学计量的H2SO4已经可以达到对Li选择性浸出的预期效果。
2LiFePO4+H2SO4+H2O2=2FePO4+Li2SO4+2H2O (3) 4) 液固比对锂、铁、磷浸出率的影响。实验在1 h、50 ℃、H2O2/Li的摩尔比为1.5、pH为3.0的条件下,液固比对锂、铁、磷浸出率的影响见图3 (b) 。从图中可以看出,随着液固比从9.2 mL·g−1上升到12.6 mL·g−1,Li的浸出率最高可达94.82%,继续提高液固比,不仅Li的浸出率并未见大幅度增加,还会增加后期蒸发浓缩时的能耗。因此,选取L/S为12.6 mL·g−1作为最佳反应条件。
5) H2O2/Li的摩尔比对锂、铁、磷浸出率的影响。实验在1 h、50 ℃、L/S为12.6 mL·g−1、pH为3.0的条件下,H2O2/Li的摩尔比对锂、铁、磷浸出率的影响结果见表1。从表中可以看出,随着H2O2/Li的摩尔比从0.5上升到1.8,Li的浸出率不断上升,这是由于H2O2作为强氧化剂,将Fe从+2价氧化为+3价,反应式如式 (4) 所示,这有利于FePO4的形成,促使LiFePO4的溶解和Li的释放,而过量的H2O2会产生大量的气泡。因此,选取H2O2/Li的摩尔比为1.5作为最佳反应条件,在此条件下H2O2的合适添加量约为其理论值的3倍。
表 1 第一次浸出不同H2O2/Li的摩尔比下Li、Fe、P的浸出率Table 1. The leaching rates of Li, Fe, P under the different molar ratios of H2O2/Li in the first leaching %元素 不同H2O2/Li摩尔比 0.5 0.8 1 1.3 1.5 1.8 Li 90.69 93.78 92.86 93.87 94.82 94.46 Fe 0.007 0.008 0.009 0.011 0.011 0.016 P 1.59 1.72 1.76 1.85 1.56 1.83 2Fe2++H2O2+2H+=2Fe3++2H2O (4) 因此第一段浸出反应的最佳条件为浸出时间1 h、浸出温度50 ℃、L/S为12.6 mL·g−1、pH为3.0、H2O2/Li的摩尔比为1.5,Li的浸出率最高达94.82%;Fe和P的浸出率分别为0.011%和1.56%,Fe、P损失少。
2.2 浸出液的二次浸出实验结果分析与讨论
1) H2O2/Li的摩尔比对锂、铁、磷浸出率的影响。实验在50 ℃、L/S为12.6 mL·g−1、1 h、pH为3.0的条件下,H2O2/Li的摩尔比对锂、铁、磷浸出率的影响见图4 (a) 。从图中可以看出,随着H2O2/Li的摩尔比从0.5上升到1.5,Li的浸出率都在95%以上,根据式 (3) 可知,H2O2的理论摩尔量是物料中Li的摩尔量的0.5倍,由实验结果可知,当H2O2实际用量接近理论用量时,Li的浸出率依旧高于第一次浸出实验的最佳浸出率;最高点H2O2/Li的摩尔比为1.3,H2O2的用量约为理论值的2.5倍,比第一次浸出实验用量少了0.5倍。这归因于第一次浸出实验的浸出液中还残留未被分解的H2O2。综合考虑Li的浸出率和生产成本,选取H2O2/Li的摩尔比为1.3作为最佳反应条件,在此条件下,H2O2的体积分数为2.0%。
2) 浸出时间对锂、铁、磷浸出率的影响。实验在50 ℃、L/S为12.6 mL·g−1、H2O2/Li的摩尔比为1.3、pH为3.0的条件下,浸出时间对锂、铁、磷浸出率的影响见图4 (b) 。从图中可以看出,浸出时间为40 min时,Li基本完成浸出。从40 min到180 min,Li的浸出率都在95%以上;同时Fe和P的浸出率分别在0.05%和2.40%之内,浸出时间为60 min时,Li的浸出率最高可达到99.46%。为获得较高的Li浸出率,选取浸出时间为1 h作为最佳反应条件。
综上,浸出液的二次浸出反应的最佳条件为浸出时间1 h、浸出温度50 ℃、L/S为12.6 mL·g−1、pH为3.0、H2O2/Li的摩尔比为1.3。经过浸出液的二次浸出,Li的浸出率从94.82%上升到最高可达99.46%,而Fe、P的浸出率分别在0.03%、2.3%以内,Fe、P的损失少;H2O2的用量从理论值的3倍降低到2.5倍,减少了H2O2的用量,节约了生产成本;同时,浸出液的二次浸出能提高滤液中锂离子溶度,利于后续沉锂。考虑到Li的浸出率达到了99%以上,而继续将浸出液进行第三次浸出的话,会增加Fe、P损失,降低生产效率,故将浸出液进行二次浸出后,进行下一阶段的除铁、沉锂。如表2所示,对比近几年LiFePO4的回收工艺,本研究相比于LI等[19]和LI等[20]具有更高的浸出率;MAHANDRA等[21]用HCOOH作浸出剂,Li浸出率高,但H2O2的用量同样高;高浸出率需要付出更长的反应时间和更大的能耗[9,22];KUMAR等[16]用100%柠檬酸作浸出剂,绿色环保,但伴随着高的H2O2消耗和较低的Li浸出率。目前研究的双氧水氧化-酸浸-浸出液二次浸出的工艺在高效选择性回收Li上具有一定的优势。
表 2 废旧磷酸铁锂电池的不同浸出工艺参数的比较Table 2. Comparison of different leaching process parameters of waste lithium iron phosphate batteries浸出剂 氧化剂 浸出时间/min 浸出温度/ ℃ Li、Fe、P浸出率/% 文献 pH=3.66 H2SO4 2.2 vol% H2O2 120 60 96.85、0.027、1.95 [19] H2SO4/Li=0.56 Na2S2O8/Li=0.45 90 60 97.53、1.39、2.58 [20] HCOOH/Li=3.23 5 vol% H2O2 30 30 99.98、<0.50、<0.50 [21] pH=3.5 H2SO4 600 mL·min-1 空气 300 25 99.30、<0.02、<0.02 [9] H2SO4/Li=0.38 高温活化:600 ℃ 空气气氛 240 85 98.46、0.01、26.59 [22] pH≈3.5 100%柠檬酸 6 vol% H2O2 90 — 94.83、4.05、0.84 [16] pH=3.0 (H2SO4/Li=0.49) 2.2 vol% H2O2 120 50 99.46、0.029、2.25 本研究 2.3 浸出渣分析
1) 浸出渣的SEM分析。从图5 (a) 可以看出,废旧LiFePO4原料由许多初级球形小颗粒和小部分次级球形大颗粒组成,图5 (b) 是次级球形大颗粒的表面,可以观察到,LiFePO4颗粒之间是紧密团聚在一块。经过第一次浸出后,从图5 (c) 、图5 (d) 可以看出LiFePO4颗粒散开了,看不到球形大颗粒,只看到一些不规则的块状颗粒,表明LiFePO4的溶解从表面渗透到了内部。从图5 (e) 、图5 (f) 可以看出,经过浸出液的循环浸出,浸出渣内部孔隙更加疏松,颗粒之间的团聚情况减弱,这使得反应时溶质的比表面积增大,溶质与溶剂接触得更加充分,Li的浸出反应更加完全。浸出液二次浸出的浸出渣比表面积的提高极有可能是Li浸出率提高将近5%的原因之一;初步判断,在浸出液的二次浸出中,浸出反应促进了LiFePO4从外到内的溶解,打开内部通道,一定的离子浓度减轻了颗粒之间的团聚情况,使得反应中LiFePO4颗粒与酸溶液的接触面积增大,促进了Li的脱嵌,提高了Li的浸出率。
2) 浸出渣的XRD分析。如图6所示,将第一次浸出和浸出液二次浸出的混合浸出渣、标准FePO4 (PDF#37-0478) 和标准FePO4·2H2O (PDF#76-0451) 的XRD分析图进行对比,分析表明,Fe、P主要以橄榄型结构的无水FePO4形式存在于灰色浸出渣中。
2.4 回收的碳酸锂分析表征
通过加入NaOH溶液除杂、Na2CO3溶液沉锂、沸水洗锂提纯后,得到白色粉末。如图7 (a) 所示,XRD分析表明了该白色粉末为Li2CO3,结晶度高、纯度高。如图7 (b) 所示,SEM形貌图可观察到所得Li2CO3产品棱角清晰,呈棒状结构,长径比大概在1~9之间,结晶效果良好。回收的Li2CO3产品能重新作为电池行业正极材料的重要原料,应用于新能源汽车、移动电子产品、通讯基站等领域,缓解当前锂资源短缺、价格上涨等问题。
3. 结论
1) 双氧水氧化-酸浸-浸出液二次浸出工艺的最优条件:1第一次,浸出时间1 h、浸出温度50 ℃、L/S为12.6 mL·g−1、pH为3.0、H2O2/Li的摩尔比为1.5;第二次,浸出时间1 h、H2O2/Li的摩尔比为1.3。
2) 双氧水氧化-酸浸-浸出液二次浸出工艺每段反应时间均仅为1 h;浸出液二次浸出时,H2O2的用量降低到理论值的2.5倍;H2SO4的用量接近于理论值,利于降低后续除杂净化时碱液的使用量。
3) Fe、P的浸出率分别在0.03%、2.3%之内,主要以橄榄型无水FePO4的形式存在于浸出渣中。
4) 浸出液经过一次循环浸出后,Li的浸出率最高可达99.46%,Li以Li2CO3形式进行回收,回收的Li2CO3产品结晶度高、纯度高,可用于锂电池的制备。
-
表 1 第一次浸出不同H2O2/Li的摩尔比下Li、Fe、P的浸出率
Table 1. The leaching rates of Li, Fe, P under the different molar ratios of H2O2/Li in the first leaching %
元素 不同H2O2/Li摩尔比 0.5 0.8 1 1.3 1.5 1.8 Li 90.69 93.78 92.86 93.87 94.82 94.46 Fe 0.007 0.008 0.009 0.011 0.011 0.016 P 1.59 1.72 1.76 1.85 1.56 1.83 表 2 废旧磷酸铁锂电池的不同浸出工艺参数的比较
Table 2. Comparison of different leaching process parameters of waste lithium iron phosphate batteries
浸出剂 氧化剂 浸出时间/min 浸出温度/ ℃ Li、Fe、P浸出率/% 文献 pH=3.66 H2SO4 2.2 vol% H2O2 120 60 96.85、0.027、1.95 [19] H2SO4/Li=0.56 Na2S2O8/Li=0.45 90 60 97.53、1.39、2.58 [20] HCOOH/Li=3.23 5 vol% H2O2 30 30 99.98、<0.50、<0.50 [21] pH=3.5 H2SO4 600 mL·min-1 空气 300 25 99.30、<0.02、<0.02 [9] H2SO4/Li=0.38 高温活化:600 ℃ 空气气氛 240 85 98.46、0.01、26.59 [22] pH≈3.5 100%柠檬酸 6 vol% H2O2 90 — 94.83、4.05、0.84 [16] pH=3.0 (H2SO4/Li=0.49) 2.2 vol% H2O2 120 50 99.46、0.029、2.25 本研究 -
[1] 张宏霞, 张衍杰, 马茜, 等. “双碳”目标下新能源产业发展趋势[J]. 储能科学与技术, 2022, 11(5): 1677-1678. doi: 10.12028/j.issn.2095-4239.2022.5.cnkxyjs202205041 [2] 胡华坤, 薛文东, 李勇, 等. 锂离子电池安全性保护措施研究进展[J]. 高分子学报, 2022, 53(5): 457-473. doi: 10.11777/j.issn1000-3304.2021.21392 [3] GANGAJA B, NAIR S, SANTHANAGOPALAN D. Reuse, recycle, and regeneration of LiFePO4 cathode from spent lithium-ion batteries for rechargeable lithium-and sodium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(13): 4711-4721. [4] 刘兰胜. 磷酸铁锂电池应用现状及发展趋势[J]. 电池工业, 2021, 25(5): 263-265. doi: 10.3969/j.issn.1008-7923.2021.05.007 [5] 刘艳良. 浅谈磷酸铁锂电池及其在电动船舶行业应用与发展[J]. 科学技术创新, 2020(15): 43-44. doi: 10.3969/j.issn.1673-1328.2020.15.024 [6] PERCIC M, FRKOVIC L, PUKSEC T, et al. Life-cycle assessment and life-cycle cost assessment of power batteries for all-electric vessels for short-sea navigation[J]. Energy, 2022, 251: 123895. doi: 10.1016/j.energy.2022.123895 [7] YANG J, GU F, GUO J. Environmental feasibility of secondary use of electric vehicle lithium-ion batteries in communication base stations[J]. Resources, Conservation and Recycling, 2020, 156: 104713. doi: 10.1016/j.resconrec.2020.104713 [8] DU J, OUYANG M, WU X, et al. Technological direction prediction for battery electric bus under influence of China's new subsidy scheme[J]. Journal of Cleaner Production, 2019, 222: 267-279. doi: 10.1016/j.jclepro.2019.02.249 [9] JIN H, ZHANG J, WANG D, et al. Facile and efficient recovery of lithium from spent LiFePO4 batteries via air oxidation–water leaching at room temperature[J]. Green Chemistry, 2022, 24(1): 152-162. doi: 10.1039/D1GC03333F [10] 王天雅, 宋端梅, 贺文智, 等. 废弃动力锂电池回收再利用技术及经济效益分析[J]. 上海节能, 2019(10): 814-820. doi: 10.13770/j.cnki.issn2095-705x.2019.10.003 [11] KUMAR J, NEIBER R R, Park J, et al. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: Strategies for highly selective lithium recovery[J]. Chemical Engineering Journal, 2021: 133993. [12] SUN Q, LI X, ZHANG H, et al. Resynthesizing LiFePO4/C materials from the recycled cathode via a green full-solid route[J]. Journal of Alloys and Compounds, 2020, 818: 153292. doi: 10.1016/j.jallcom.2019.153292 [13] LIANG Q, YUE H, WANG S, et al. Recycling and crystal regeneration of commercial used LiFePO4 cathode materials[J]. Electrochimica Acta, 2020, 330: 135323. doi: 10.1016/j.electacta.2019.135323 [14] 周伟, 符冬菊, 刘伟峰, 等. 废旧磷酸铁锂动力电池回收利用研究进展[J]. 储能科学与技术, 2022, 11(6): 1854-1864. doi: 10.19799/j.cnki.2095-4239.2022.0201 [15] 邱宏菊, 郝先东, 桂雨曦, 等. 废旧磷酸铁锂电池正极材料回收技术进展[J]. 现代化工, 2022, 42(7): 60-64+69. doi: 10.16606/j.cnki.issn0253-4320.2022.07.013 [16] KUMAR J, SHEN X, Li B, et al. Selective recovery of Li and FePO4 from spent LiFePO4 cathode scraps by organic acids and the properties of the regenerated LiFePO4[J]. Waste Management, 2020, 113: 32-40. doi: 10.1016/j.wasman.2020.05.046 [17] ZHANG J, HU J, LIU Y, et al. Sustainable and facile method for the selective recovery of lithium from cathode scrap of spent LiFePO4 batteries[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 5626-5631. [18] HUANG Y, HAN G, LIU J, et al. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process[J]. Journal of Power Sources, 2016, 325: 555-564. doi: 10.1016/j.jpowsour.2016.06.072 [19] LI H, XING S, LIU Y, et al. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 8017-8024. [20] LI H, YE H, SUN M, et al. Process for recycle of spent lithium iron phosphate battery via a selective leaching-precipitation method[J]. Journal of Central South University, 2020, 27(11): 3239-3248. doi: 10.1007/s11771-020-4543-3 [21] MAHANDRA H, GHAHREMAN A. A sustainable process for selective recovery of lithium as lithium phosphate from spent LiFePO4 batteries[J]. Resources, Conservation and Recycling, 2021, 175: 105883. doi: 10.1016/j.resconrec.2021.105883 [22] TAO S, LI J, WANG L, et al. A method for recovering Li3PO4 from spent lithium iron phosphate cathode material through high-temperature activation[J]. Ionics, 2019, 25(12): 5643-5653. doi: 10.1007/s11581-019-03070-w -




 下载:
下载: