-
有机磷酸酯(organophosphate esters, OPEs)作为阻燃剂和增塑剂被广泛应用于塑料、泡沫、电子产品和建筑材料中[1-3]. OPEs一般通过物理混合添加到材料中,易通过挥发、浸出、磨损释放到环境中[4],因此广泛存在于各种环境介质中,包括大气[5]、水[6]、土壤[7]、鱼[8],甚至人的头发[9]、尿液[10]和母乳[11]. OPEs可通过皮肤接触、灰尘摄入、呼吸、摄食进入人体,表现出多种毒害效应,包括神经毒性、肾毒性、生殖毒性、致癌性、内分泌干扰性等[12-14]. 邻苯二甲酸酯(phthalates, PAEs)作为增塑剂广泛应用于建材、塑料制品中,改善材料的柔韧性和可塑性[15-16]. PAEs在大气[17]、沉积物[18]、水体[19]、土壤[20]及人体[21]中也被广泛检出. 研究表明,PAEs具有内分泌干扰性,可以干扰内源性激素的合成[22]. 由于两种化合物对生态系统和公众健康的潜在风险,其环境行为已引起普遍关注.
半挥发性有机物(SVOCs)的大气采样可分为主动和被动采样[23],主动采样利用抽气泵采集污染物[24],具有时间短、速度快、准确等优点,但造价贵、体积大、需要电源,不利于多点同时采样. 被动采样器(PAS)利用聚氨酯泡沫(PUF)等对有机物具有较高分配系数的吸附材料,通过空气与材料间的浓度梯度将有机物吸附到材料上[25],具有造价低、无需电源、对空气扰动小、多点同时监测、能反映时间加权平均浓度等优点[26-27],但采样过程中易受到风速等环境因素的干扰,不同采样点间采样速率存在差异. 因此,如何准确获得被动采样器的采样速率是确保其准确定量的关键.
目前PUF-PAS采样速率的评估方法有两种. 一种是基于主动采样校正的采样速率. 通过采样时间内PUF中污染物的日累积量和主动采样测定的大气污染物浓度得到该污染物的采样速率Ra [28],但不能校正不同环境条件下采样速率的差异. 另一种方法是在PUF部署前加入效能参考物(DCs或称PRCs),用以校正风速的环境因素对采样速率造成的影响. 基于PUF中DCs的损失率,计算采样速率RDC,也是目前应用较多的一种方法[29]. 该方法假设PUF对污染物吸收和其替代物释放过程传质方向相反但速率常数相同[30-31]. DCs逸失率在20%—80%之间才能获得相对准确的采样速率[32], 且作DCs的物质通常为同位素标记物,价格昂贵.
当前SVOCs的土-气交换研究主要有两种方法:一种是逸度模型计算法,即通过计算污染物土壤逸度(fs)和大气逸度(fa)的比值关系来判断污染物在两相间的迁移趋势[33-34],但该方法不适用于理化参数缺乏的新型污染物;同时土壤中真实参与土-气交换过程的污染物含量难以准确获取,当污染物浓度较低时,由于环境参数变化带来的不确定性会对最终的结果产生较大影响. 另一种是根据监测的近地面空气中污染物浓度梯度,对土壤-大气交换趋势进行量化,例如近地面空气被动采样器[35-36];相比逸度计算法,该方法能够更直观地反映污染物的垂直浓度分布,表征污染物的土-气交换方向,但也存在被动采样速率不准、受环境因素影响大等问题,需要对采样速率进行校正,以获得较为准确的浓度和趋势.
本研究利用主动采样和添加DCs联合校正采样速率的方法,研究一种可监测土壤垂直方向污染物浓度梯度的新型PUF被动采样器对OPEs和PAEs的采样速率,可以在一定程度上节约了DCs的添加成本,同时解决了主动采样器对空气的扰动和难以同时多点部署以及风速变化影响被动采样速率的不足,弥补了单一校正方法的缺陷,使得浓度结果更加准确,并用于表征近地面大气中OPEs和PAEs的垂直浓度分布,以期获得准确的污染物土-气交换趋势.
全文HTML
-
实验所用正己烷、二氯甲烷、乙酸乙酯、异辛烷均为色谱纯,购自J&K公司;所用无水硫酸钠(分析纯)购自国药公司;所用硅胶(60目)购自Merck公司;标准品购自ACCU公司.
-
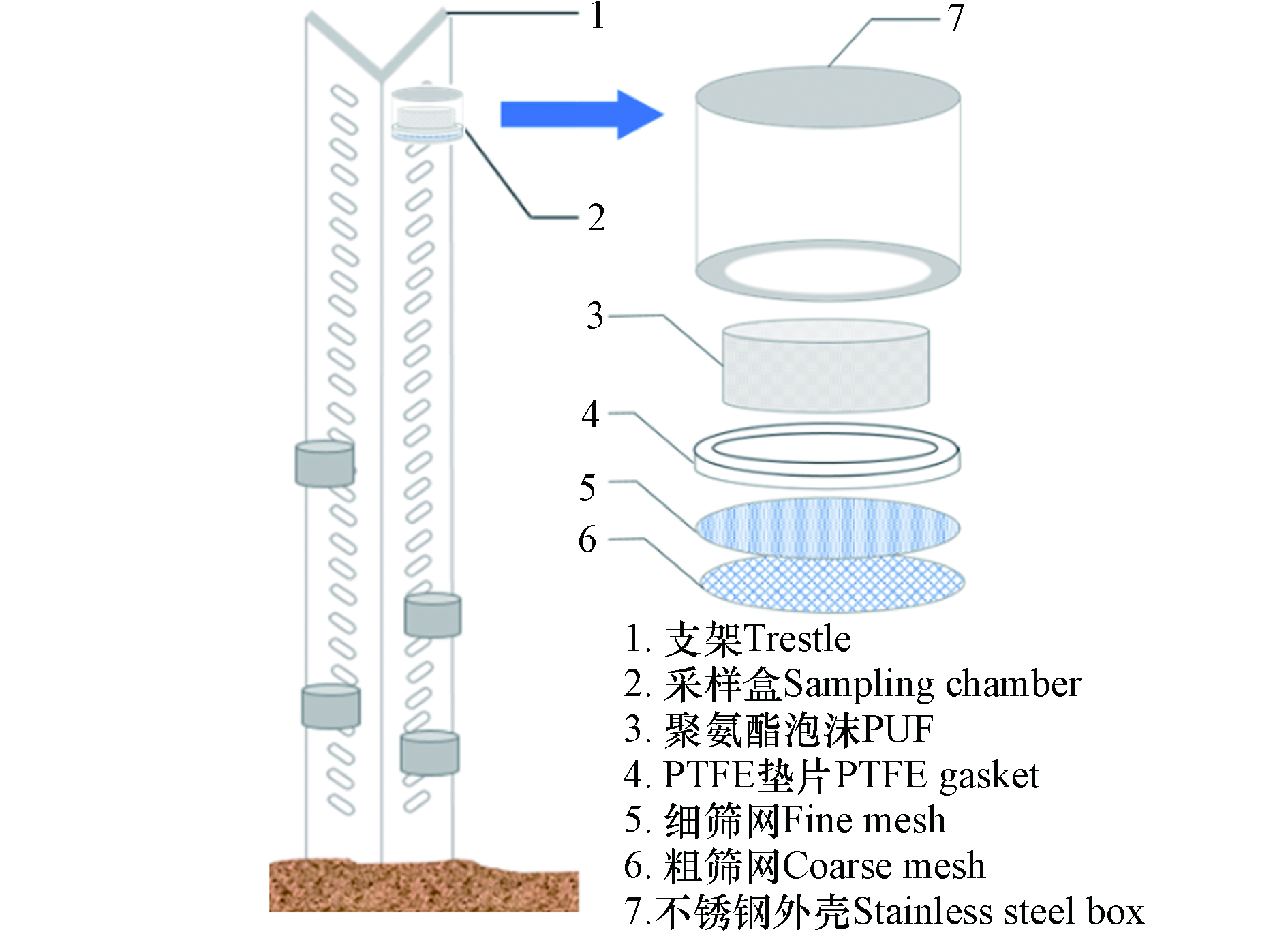
对现有的近地面浓度垂直剖面采样器[36]进行改进,研制出新型垂直浓度梯度被动采样器,如图1所示. 采样盒内径8 cm,高8 cm,为不锈钢材质,顶部封闭,底部中心有直径7 cm的开口,内部由上到下放置PUF、聚四氟乙烯环形垫片、细筛网(200目)、粗筛网(10目). 粗、细筛网可以阻止颗粒物等固体杂质等进入采样盒内,确保尽可能少的采集到颗粒态样品. PUF直径6.5 cm,高2.5 cm,侧面和顶部分别与采样盒内壁间隔0.5 cm和1 cm. 间隔可促进采样盒内部空气流通,提高扩散速率和采样速率.
-
采样时间为2020年9月—11月. 采样前,PUF分别经乙酸乙酯、二氯甲烷索氏提取48 h,并添加DCs (13C12-PCB28, 52, 209). (1)采样速率实验:将新型PAS部署在具地面1.5 m高,分别在采样5、10、20、30、40 d收集3个平行样;同期在距离2 m远处利用主动采样器同步采样,流量为120 L·min−1. (2)垂直浓度梯度实验:新型采样器底部距地面高度为5、10、20、40、130 cm,每个高度3个平行,采样时长40 d.
-
PUF用正己烷/二氯甲烷(1:1,体积比)100 ℃下加速溶剂萃取静态萃取5 min,循环2次. 萃取液浓缩后经硅胶柱净化,加入内标物待测.
11种OPEs和10种PAEs利用岛津GCMS-QP2020配备DB-5MS毛细管柱(30 m×0.25 mm×0.25 μm)测定. 升温程序为:70 ℃,保持2 min,15 ℃·min−1升至300 ℃,保持10 min. 氦气为载气,流速为1.5 mL·min−1.
-
实验中所用的玻璃仪器洗净烘干后,经马弗炉450 ℃烧4 h,使用前使用有机溶剂润洗以去除背景值. 采用内标法定量,分析过程设置了溶剂空白、程序空白、平行样品. 空白样品中目标物含量均<样品含量的10%,OPEs和PAEs的加标回收率分别为73%—95%和71%—102%,最终结果均经过回收率和空白校正.
-
假设被动采样速率R受气体边界层控制;DCs对于气体边界层的传质系数k不依赖于它的PUF-空气分配系数KPUF-A;吸附和逸散的方向是相反的;R等于传质系数k和PUF的表面积A的乘积. 根据公式(1)[37]:
由于空气中DCs浓度为0,即Ci,A=0,因此公式简化为:
两边积分得公式(3)或(4)(稳定DCs校正):
其中,Ci, PUF为PUF中待测物浓度,ng/sample;Ci, A为空气中待测物浓度,ng·m−3,DCs空气中为0;KPUF-A为PUF-空气分配系数,无量纲;
$ K'_{\mathrm{P}\mathrm{U}\mathrm{F}{\text{-}}\mathrm{A}} $ 为PUF-空气分配系数;m3·g−1;k 为传质系数,m·s−1;R为基于DC的采样速率,m3·d−1;APUF为PUF表面积,117.4 cm2;t 为采样时间,d;ρPUF为PUF密度,0.025 g·cm−3;VPUF为PUF体积,82.9 cm3;CDC,0;CDC为DCs初始和结束浓度,ng/sample;当CDC/CDC,0为20%—80%,采样速率相对准确.CDC-stable,0;CDC-stable为稳定DCs初始和结束浓度,ng/sample.稳定DC是指一种难挥发的DC,可以校正采样中PUF的破损、分析损失以及基质效应等造成的误差. 本研究中选取13C12-PCB209作为稳定DC. 其中K’PUF-A可基于辛醇-空气分配系数KOA根据公式(7)[38]计算:由于只有13C12-PCB28和13C12-PCB52回收率在20%—80%范围内,因此获得基于两种DCs的被动采样速率如表1所示. 理论上,基于13C12-PCBs所计算的被动采样速率只适用于PCBs类化合物. 根据双膜理论,SVOCs进入PUF的传质阻力主要由空气边界层厚度控制,同时还与传质系数与PUF-空气分配系数KPUF-A有关[37]. KPUF-A通常与化合物的KOA有关[38]. 同理,DCs的逸散过程也主要受控于空气边界层及化合物性质. 空气边界层的厚度随风速的增加而降低[30],因此采样速率很大程度取决于风速. 本实验选择的DCs的性质(即KOA)与多数目标化合物接近,因此可认为他们具有相近的KPUF-A和相似的逸散特性. 此外,DCs结合主动联合校正可进一步得到更为准确的采样速率. 因此化合物种类不同对采样速率产生的影响可忽略.
-
速率实验中利用主动采样测得大气Σ11OPEs和Σ10PAEs总浓度的平均值分别为(1.54±0.49) ng·m−3和(74.3±10.5) ng·m−3. 基于某线性吸附时间内被动PUF中化合物的日增量(ng·d−1)与该时间段主动采样测得的化合物平均浓度(ng·m−3),可计算出该时间段化合物基于主动的日采样速率RA(m³·d−1),即
式中,t为采样天数(d),ΔMP为t时间PUF中化合物增加量(ng),ΔMA为t时间主动采样测得的化合物含量(ng),VA为该时间段主动采样体积(m³).
-
本实验中,以主动采样校正的采样速率RA作为被动采样40 cm高度上的采样速率,以此为基准,根据不同高度的DCs采样速率RDCx(x为采样高度)与40 cm高度的DCs采样速率RDC40的比值作为校正因子kx,从而得到化合物在不同高度被动样品的主动-DCs联合校正采样速率RS,即
-
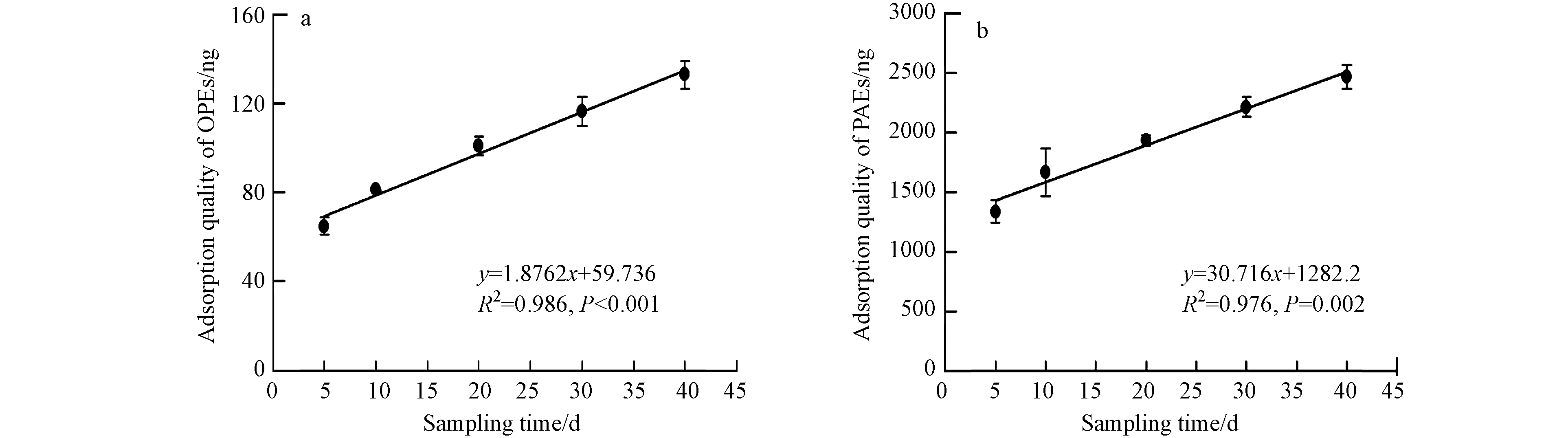
新型被动PUF中Σ11OPEs和Σ10PAEs在同一高度的吸附量随时间变化如图2. 被动吸附一般分为:线性吸附、曲线吸附和吸附平衡3个阶段. 由图2可见,40 d内目标物处于线性吸附阶段.
新型被动采样器OPEs和PAEs单体经主动校正的采样速率RA如表2所示. OPEs和PAEs的采样速率分别为(0.60±0.09)—(2.76±0.29)m3·d−1、(0.50±0.29)—(2.67±0.32)m3·d−1, 与捷克马萨里克大学测得的PAHs((0.3±0.1)—(5.5±0.7)m³·d−1)和PBDEs((0.7±0.3)—(2.4±0.3)m3·d−1)的采样速率接近[39]. 被动采样速率取决于化合物性质、环境浓度、周边风速等气象条件、采样材料及采样器结构等[40]. 经主动校正的采样速率无法表征风速或者环境条件变化引起的被动采样速率的差异.
-
基于主动-DCs联合校正的不同高度PUF中OPEs和PAEs的采样速率范围分别为0.27—3.16 m3·d−1和0.23—3.25 m3·d−1 (表3). 研究表明风速是影响大气气态污染物被动采样的重要因素[48],风速变化改变了空气边界层的厚度,进而影响采样速率. 采样速率随高度逐渐增大,可能是由于距地面越高,空气流速相对越快,因此采样速率越高.
-
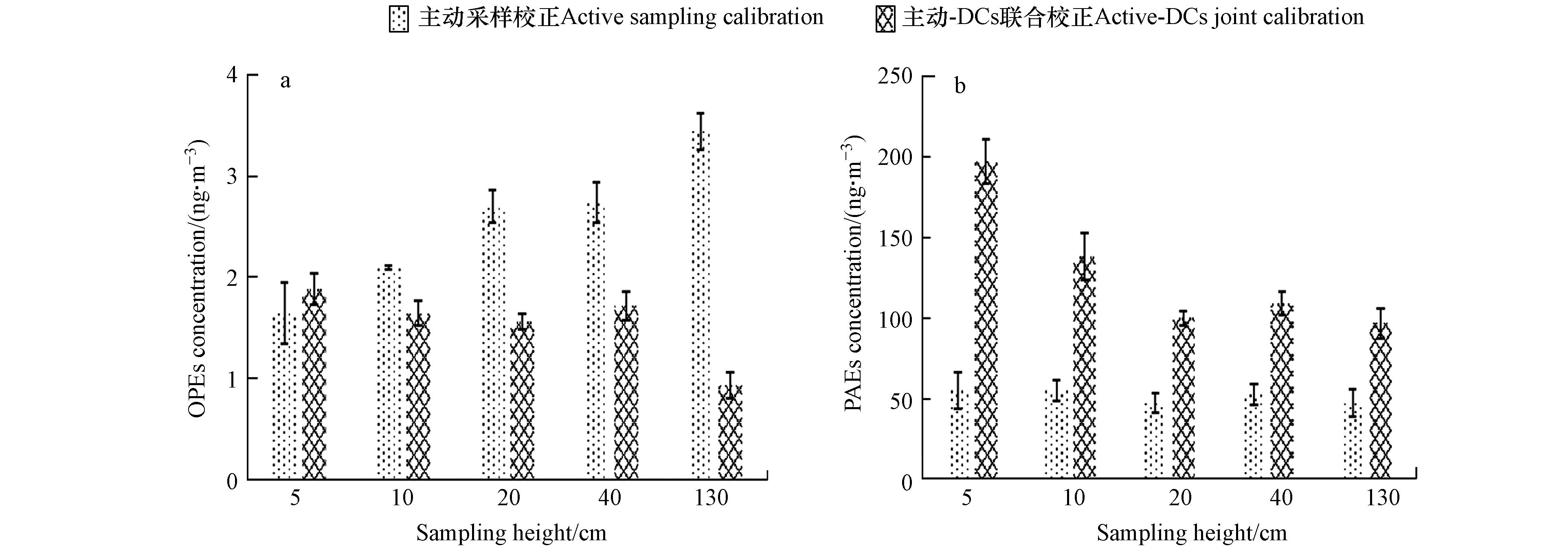
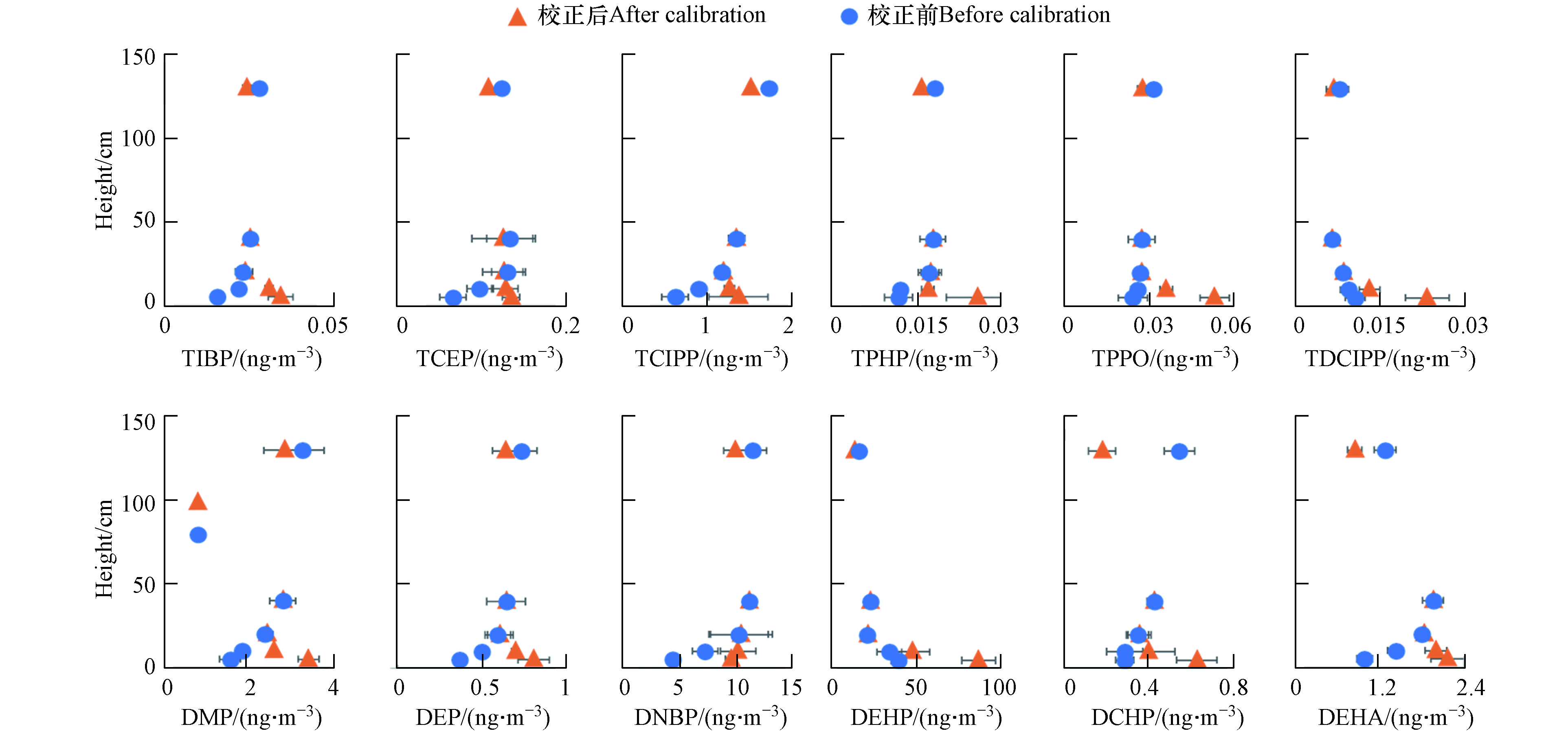
主动校正的不同高度OPEs和PAEs总浓度见图3. 经主动校正的采样速率得到的OPEs和PAEs总浓度分别为(2.52±0.68)ng·m−3和(51.3±3.82)ng·m−3. 组成上,OPEs以TCIPP、TEHP为主,平均占比为45%和41%;PAEs主要单体为DEHP、DIBP和DNBP,平均占比51.16%、20.79%和17.62%. 垂直分布显示, 污染物在地表0—130 cm的高度存在显著浓度梯度变化,表明近地面大气中污染物浓度显著受到土-气交换过程影响. OPEs中大部分单体如TNBP、TCIPP、TIBP、TCEP、TPHP、TPPO等在垂直方向上随高度增加而浓度升高,表现为由大气向土壤沉降; TDCIPP、TMPP为随高度升高浓度降低,表现为由土壤挥发. PAEs中DMP、DEP、DIBP、DNBP、DEHA、DCHP、DEHT、DNOP表现为沉降,而DEHP、BBzP表现为挥发.
-
不同高度PUF中的目标物吸附量受采样速率及污染物浓度共同影响. 基于单一主动校正只能反映不同高度污染物浓度变化导致的差异,不能反应采样速率导致的差异. 基于单一DCs校正能够一定程度反映不同高度采样速率变化导致的吸附量差异,但不能准确反映同一高度不同化合物采样速率差异. 主动-DCs联合校正很好的解决了上述问题,更全面的反映了二者对目标物含量的贡献.
经主动-DCs联合校正采样速率得到的OPEs和PAEs总浓度分别为(1.56±0.36)ng·m−3和(128±41.9)ng·m−3,其中PAEs结果显著高于经主动单独校正的结果(图3). 该区域大气Σ11OPEs浓度略高于南宁市农田大气浓度((0.42±0.33)ng·m−3)[49],略低于北京市室内空气浓度(5.2 ng·m−3)[50],与重庆市农业区附近的室内空气浓度相当(1.79 ng·m−3)[51]. 该区域PAEs大气浓度比重庆农业区大气浓度((3.03±0.32)ng·m−3) [52]高1个数量级,低于北京市住宅空气浓度(498 ng·m−3),与北京市室外大气PM2.5中浓度相当(125 ng·m−3)[53].
组成上,OPEs中TCIPP占比提高至70%. 与重庆农业区大气OPEs组成类似[51],可能由于TCIPP被广泛用作TCEP的替代品[54]. PAEs占比最大的单体由DEHP变为DIBP(58.5%),其次为DEHP(27.2%)和DNBP(9.35%),DEHP、DNBP和DIBP是我国主要生产和使用的增塑剂[52]. 该组成与杭州市大气PAEs的组成略有不同(DEP>DEHP>DIBP)[55].
经主动-DCs联合校正的垂直浓度分布规律显示:TCEP、TCIPP、TIBP、TPHP、TPPO及DMP、DEP、DEHA、DCHP的土-气交换方向趋势发生反转,由之前的沉降变为挥发,其余单体如TNBP、TDCIPP、DNBP、DEHP虽然未发生交换趋势的变化,但环境浓度显著改变,必将导致交换通量发生变化(图4). 单一主动校正的弊端在于假定各高度采样速率相同,而实际上由于垂直方向的风速差异,不同高度的采样速率显著不同. 近地面,由于地表阻力,空气流通速度变慢,因此采样速率降低. 通过添加DCs,根据逸散率的不同,得到不同高度的采样速率,能够更好地校正风速对采样速率造成的影响. DCs校正被动采样速率的方法前期已经被广泛应用[30,32,56],因此将其与主动采样联合校正,能够更准确反映不同高度PUF采样速率的差异,进而获得更准确的污染物土-气交换趋势.
-
(1) 新型垂直浓度梯度被动采样器对OPEs和PAEs的吸附在40 d内均处于线性吸附阶段.
(2) 经主动-DCs联合校正的PUF对OPEs和PAEs采样速率分别为(0.27—3.16)m3·d−1、(0.23—3.25)m3·d−1,采样速率随高度的升高逐渐增大.
(3) 经过主动-DCs联合校正前后, TCEP、TCIPP、TIBP、TPHP、TPPO及的DMP、DEP、DCHP、DEHA的土-气交换趋势发生反转,其余单体如TNBP、TDCIPP、DNBP、DEHP趋势虽然未变,但浓度发生了不同程度的改变,表明基于主动-DCs联合校正被动采样速率能够获得更准确的环境浓度和交换趋势.



 下载:
下载: