-
纳米银(nanosilver,nAg)因其具有强杀菌性而被广泛应用于玩具、衣物、洗手液等生活日用品及医疗用品中。截至2022年2月,在纳米材料数据库(The Nanodatabase)中共登记纳米材料5 224种,其中包含nAg的材料约占总数的1/7[1]。在nAg产品的生命周期中,约60%的nAg在制造、使用、废弃和循环过程中通过污水管网进入市政污水处理厂[2]。由于nAg的抑菌性能,进入污水生物处理系统中的nAg会影响微生物呼吸速率[3],导致污水处理厂净化污水的性能下降[4]。进入污水处理系统中的nAg随污泥排出时也可能带来环境风险,FOSTNER等[5]发现进水中投加10 mg·L−1 nAg,在运行30 d和90 d后的SBRs外排活性污泥进入土壤后,对土壤细菌群落组成有显著影响(P<0.05)。
作为金属纳米材料,nAg进入活性污泥污水处理系统后,必然受到污水组成和系统工艺参数如溶解氧、曝气时间、混合强度等影响,经历团聚[6]、溶解[7]、氧化[8] 、硫化[9]等过程,形态发生变化,从而影响nAg抑菌性能[10]。CHEN等[11]认为,活性污泥系统中的nAg通常与H2S、S2−发生硫化反应转化成其最终环境形态Ag2S,nAg的硫化过程可显著降低其对微生物的毒性[12]。然而,研究者认为污水中可能存在多种金属离子(Mg2+、Fe2+/Fe3+)及生物分子如蛋白质等,均可与Ag+竞争S2-[13],nAg在好氧环境中释放的Ag+远多于厌氧,抑菌能力显著高于厌氧[14]。也有研究表明,nAg在污水处理系统中可能转化为AgCl及AgO等形态[15]。nAg的化学形态显著影响其对活性污泥微生物的毒性效应。1 mg·L−1 AgCl胶体对硝化细菌硝化作用的抑制率为(46±4.0)%,与1 mg·L−1 Ag+对该菌的抑制效果相同[16]。以nAg、Ag+、可溶性银化合物、胶体银等形态存在的Ag,均具有很好的抑菌活性[17]。
研究者对nAg考察了活性污泥污水处理系统中的胁迫效应,明确了污水处理系统中nAg来源和进水浓度[18-19],确定了nAg对污水生物处理系统脱氮除磷功能的干扰[20-21],提出了nAg的生态毒性主不仅来源于nAg自身及还包括其释放的Ag+等[22-24]。但关于nAg在活性污泥污水处理系统中的分布、赋存形态等方面的研究却鲜有报道。基于此,本研究采用序批式反应器模拟活性污泥污水生物处理系统,在进水中分别添加不同浓度的nAg和Ag+,连续运行50 d,以分析污水处理系统中Ag在污泥、出水中的分布及Ag在污泥中的赋存形态,为解析、评估nAg对污水生物处理系统的胁迫效应及外排活性污泥的环境风险提供参考。
-
实验进水为人工模拟中等强度的城市生活污水,主要组成成分[25]为:30 mg·L−1 C6H12O6、400 mg·L−1 CH3COONa、150 mg·L−1 NH4Cl、45 mg·L−1 KH2PO4、20 mg·L−1 MgSO4·7 H2O和1 mL·L−1微量元素溶液。其中,微量元素溶液组成[26]为:150 mg·L−1 H3BO3、150 mg·L−1 CoCl2·6H2O、30 mg·L−1 CuSO4·5H2O、150 mg·L−1 FeCl2·6H2O、30 mg·L−1 KI、120 mg·L−1 MnCl2·2H2O、60 mg·L−1 Na2Mo7O4·2H2O、120 mg·L−1 ZnSO4·7H2O。采用NaHCO3调节污水pH,使其保持在6.5~7.5。
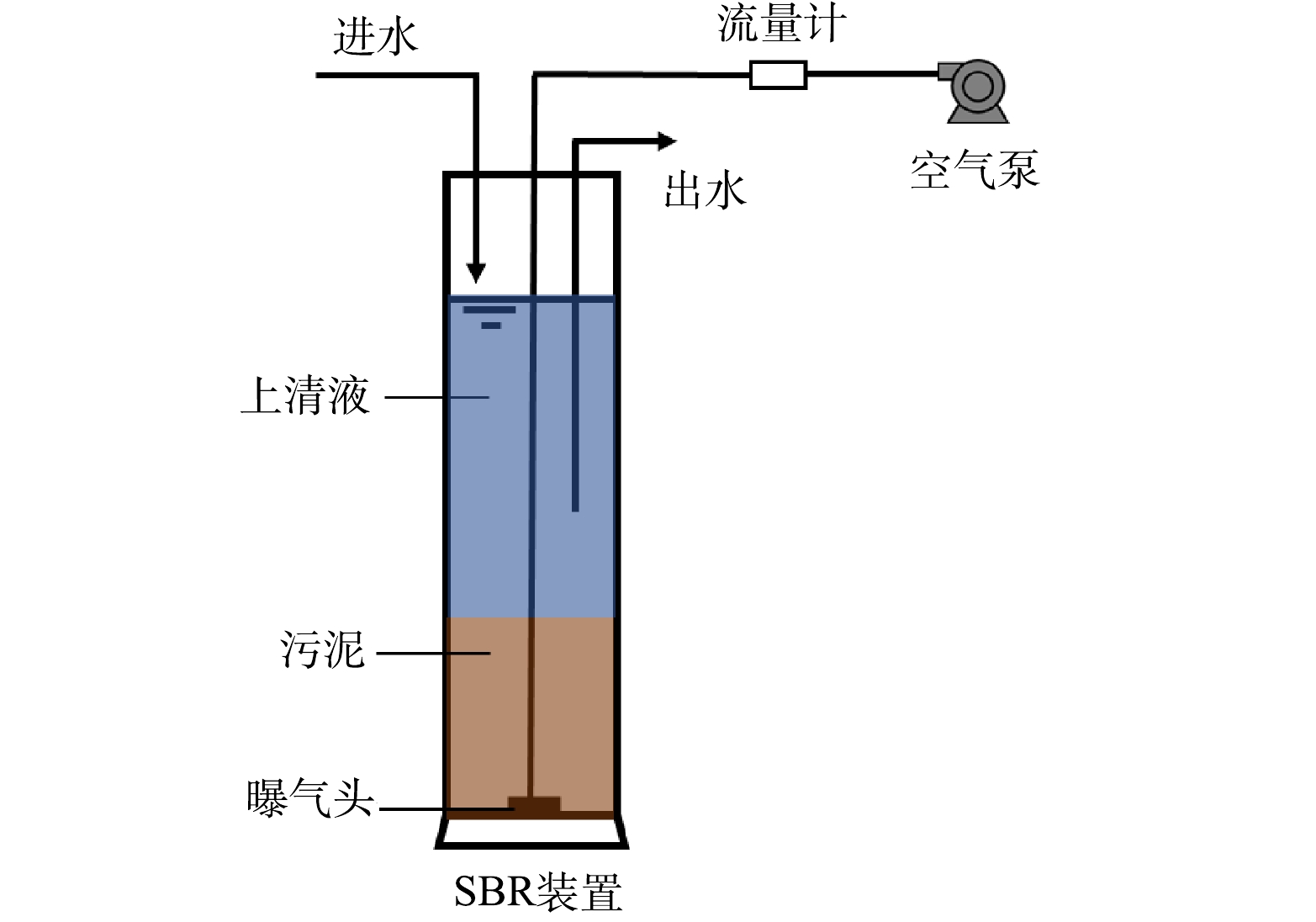
SBR有效体积为1.6 L,采用空气压缩机从底端曝气,空气流速为2.0 L·min−1,实验期间每天运行2个周期,每周期5 h,其中进水15 min,静置90 min,曝气90 min,静置90 min,排水15 min (图1)。运行周期内换水比为50%,其余时间静置,每8 d排泥1次。反应器接种污泥取自南京某市政污水处理厂生化池的回流污泥,反应器内初始污泥混合液悬浮固体(mixed liquor suspended solids,MLSS)质量浓度为4 282~4 628 mg·L−1,污泥容积指数(settling velocity index,SVI)为79~87 mL·g−1。
-
SBRs污水处理系统因具有出水水质好、占地面积小、可以同步脱氮除磷等优点被普遍应用于市政污水和工业污水处理。已有研究表明,市政污水中nAg或Ag质量浓度为16.4~74.7 ng·L−1,活性污泥中nAg或Ag含量为3~14 mg·kg−1[2]。nAg具有广谱抗菌性且不会导致细菌产生抗药性,因而nAg产品在环保、日用品、医疗等领域的使用日趋增多,这可能导致市政污水和活性污泥中Ag浓度不断升高[27]。本研究根据国内外相关研究中所使用的nAg浓度[4,28-30],选取低浓度nAg(1 mg·L−1)和高浓度nAg(10 mg·L−1)作为实验进水中的nAg浓度。采用超滤法[31]测定nAg溶解释放出的Ag+约为nAg质量浓度的30%,因此在进水中分别添加质量浓度为0.3 mg·L−1和3.0 mg·L−1 的Ag+,同步观察nAg溶解释放出的Ag+对污水处理系统的影响。
SBRs运行稳定后(反应器启动后运行约20 d,对污染物的去除效率稳定,污泥沉降性能良好,即达到稳定状态)。在进水中分别加入1 mg·L−1、10 mg·L−1 nAg和0.3 mg·L−1、3 mg·L−1 Ag+,启动反应器。实验所用nAg购自北京德科岛金科技有限公司,表面包被物为聚乙烯吡咯烷酮,平均粒径为10~12 nm;Ag+由AgNO3(国药集团化学试剂有限公司,≥99.8%)与去离子水(电阻率为18 MΩ·cm)配制而成。设置5组反应器:进水中不添加nAg,也不添加Ag+的SBRs为对照(简称CK组),进水中分别添加1 mg·L−1 nAg(简称1-nAg组)、10 mg·L−1 nAg(简称10-nAg组)、0.3 mg·L−1 Ag+(简称0.3-Ag+组)和3.0 mg·L−1 Ag+(简称3-Ag+组),每组SBRs各3个重复,在室温(22~28 ℃)下运行。
实验期间,反应器内MLSS质量浓度为3 800~4 500 mg·L−1,SVI为50~85 mL·g−1,pH为7.73~8.71。一个工作周期(5 h)内活性污泥混合液中溶解氧(dissolved oxygen,DO)为0.2~8.0 mg·L−1,反应器出水DO在3 mg·L−1以上,满足活性污泥微生物脱氮除磷、去除有机物所需要的厌氧、缺氧和好氧生境。
-
1)基本指标。活性污泥混合液MLSS和SVI采用水和水质分析(第四版)[32];DO和pH分别采用便携式溶解氧仪(JPB-607A,上海雷磁仪器厂)和pH测定仪(PB-10,赛多利斯科学仪器(北京)有限公司)测定。
2)活性污泥形态、粒径及Zeta电位。活性污泥形态及元素组成采用扫描电子显微镜(HITACHI,S-3400N Ⅱ,Japan)和X射线能谱仪(HORIBA,EX-250,Japan)测定;污泥絮体粒径及Zeta电位分别由Mastersizer 3000激光粒度分析仪(Malvern Instruments,UK)和Zs90纳米粒度电位仪(Malvern Instruments,UK)测定;活性污泥的胞外聚合物(extracellular polymeric substances,EPS)采用离心法提取[33],其含量以每克总固体悬浮物中含有的EPS量计算。
3) Ag含量测定。取曝气结束前30 min的泥水混合液,低温高速离心(4 ℃,20 000 r·min−1) 30 min、过0.45 µm醋酸纤维滤膜(Whatman,USA),上清液即污水,沉淀部分为污泥。污泥于110 ℃烘箱中烘至恒重,冷却后采用石墨炉-王水消煮法[34]浸出污泥中Ag;污水、污泥及EPS中Ag含量采用电感耦合等离子体质谱仪(ICP-MS,NexION 300,PerkinElmer,USA)测定。
4)活性污泥中Ag的形态。采用X-射线衍射分析仪(Thermo Fisher Scientific,XTRA,USA)和X射线光电子能谱仪(U1VAC-PHI,PHⅠ5000 VersaProbe,Japan)分析Ag在活性污泥中的赋存形态。XRD测定条件为Cu靶,管压40 kV,管流40 mA,扫描范围2Ɵ为30°~90°,步长0.02°。XPS中X射线源为是单色化AlKα,分析活性污泥中C、O、S、N、Ag可能的存在形态。
-
采用Microsoft Excel 2016软件对数据进行统计分析,结果以平均值±标准差(Mean ± SE)表示,数据绘图采用Origin 8.1软件。利用SPSS Statistic 25软件进行数据显著性差异检验,P<0.05 代表数据间存在显著性差异。
-
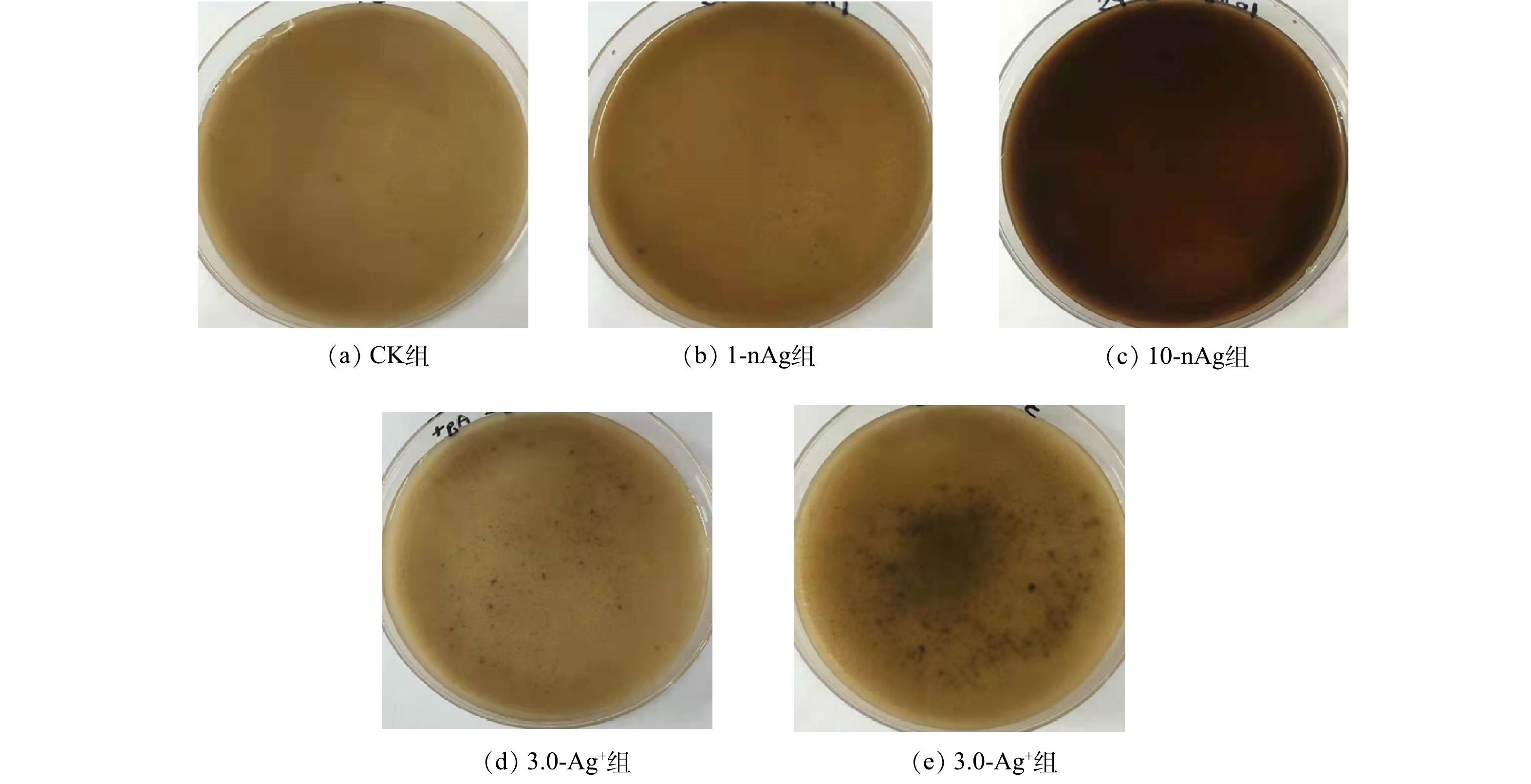
1)活性污泥的形态。进水中投加不同浓度的nAg和Ag+,SBRs运行50 d后,活性污泥混合液形态如图2所示。CK组活性污泥呈黄褐色、毛绒状絮体;1-nAg组中活性污泥颜色略深、有少许黑色颗粒,其他性状与CK组污泥无明显差异;10-nAg组活性污泥呈黑褐色,且该组反应器中MLSS值比CK组低30%;0.3-Ag+组、3.0-Ag+组中活性污泥颜色与CK组及1-nAg组相近,但前者污泥中出现明显的黑色颗粒状物质。SBRs运行50 d后,在进水中分别投加nAg和Ag+会导致活性污泥形态发生改变,高浓度nAg (10 mg·L−1)暴露还可导致活性污泥生物量下降,这与李墨青[35]的研究结果一致。
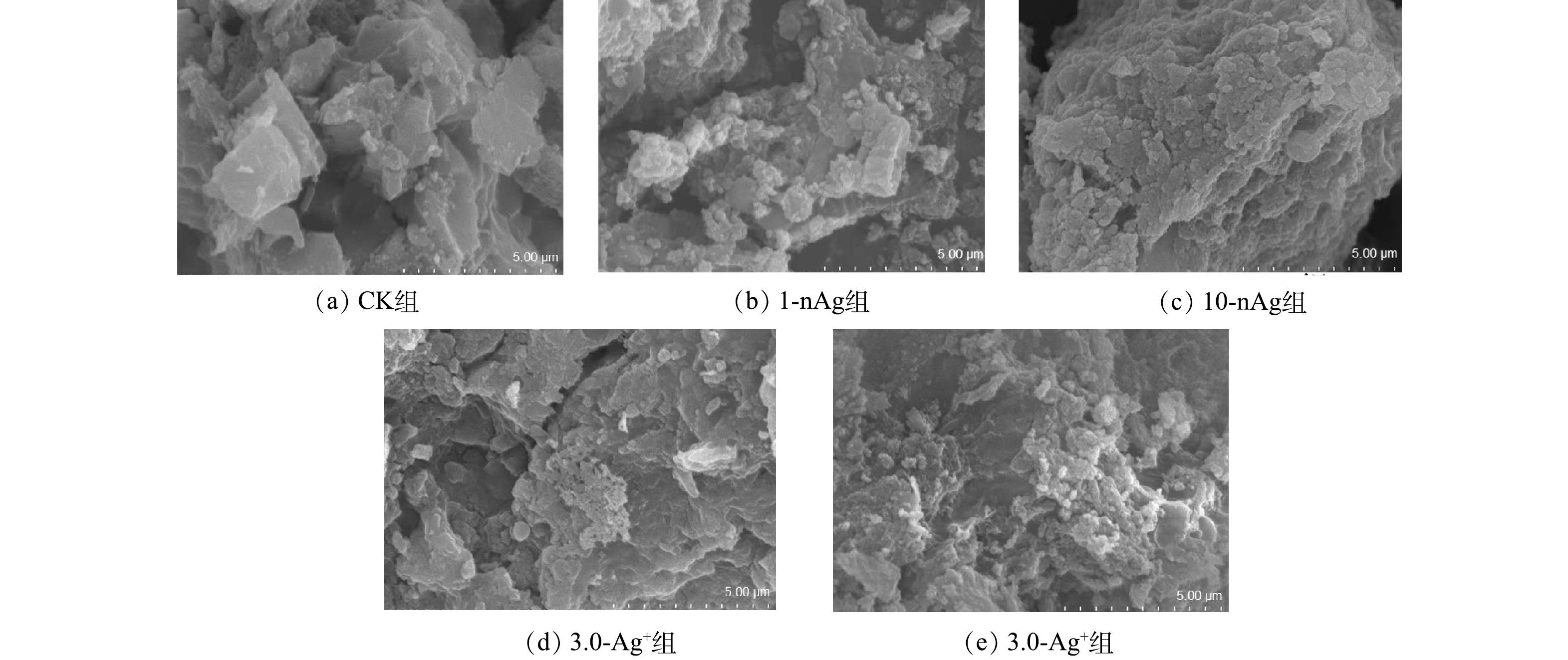
2)活性污泥的微观形貌。采用扫描电镜(scanning electron microscope,SEM)和能谱分析仪(energy dispersive spectroscopy,EDS)观察5组SBRs中活性污泥的微观形貌并分析主要的能谱元素含量。由图3可看出,CK组活性污泥表面粗糙、孔隙明显,进水分别添加nAg和Ag+处理的活性污泥表面结构逐渐致密化、孔隙缩小,其中10-nAg组和3.0-Ag+组中活性污泥结构致密化的现象尤其明显。已有研究表明,活性污泥的生物活性与其孔隙度相关,当受到毒性物质刺激时,活性污泥表面孔隙收缩,降低内部与外界流通性,污泥生物活性减弱[36-37]。这表明进水中的nAg和Ag+可能对活性污泥系统生物活性造成影响,影响后续污水处理效率。
对运行至50 d时的反应器中活性污泥进行能谱元素分析(表1),1-nAg、3.0-Ag+和10-nAg组反应器活性污泥中均检测出Ag元素,分别占所测定元素总质量的0.22%、0.51%和4.38%;0.3-Ag+组的反应器活性污泥中Ag元素低于EDS检测下限(3‰)。由此可见,水中的Ag会被活性污泥所吸附,且进水中Ag含量越高,活性污泥中Ag含量也相应升高[38]。其中,3.0-Ag+组添加的Ag+含量与10-nAg组溶解的Ag+含量相同,但从能谱分析结果可知,3.0-Ag+组活性污泥中Ag含量远低于10-nAg组,这表明活性污泥吸附的不仅仅是nAg溶解释放出的Ag+,还包括nAg或其他形态Ag。
3)活性污泥中EPS的含量。活性污泥微生物分泌的EPS与污泥的沉降及重金属吸附性能密切相关[39]。运行至50 d时,进水中分别添加1 mg·L−1和10 mg·L−1 nAg的活性污泥中EPS含量分别为(33.39±1.59)、(54.10±10.73) mg·g−1,均显著高于CK组(P<0.05),且活性污泥EPS含量随着进水中nAg质量浓度增加而显著增加。进水中分别添加0.3 mg·L−1和3.0 mg·L−1 Ag+的活性污泥EPS含量分别为(22.09±6.89) mg·g−和(27.43±3.17) mg·g−1,与CK组及1-nAg组没有显著性差异(P>0.05),但进水中添加3.0 mg·L−1 Ag+的活性污泥EPS含量显著低于10-nAg组(P<0.05)。在外界毒性物质刺激下,活性污泥的EPS可在微生物细胞外形成保护性缓冲层,减缓细胞与外界基质的接触,从而减轻毒性物质对微生物的影响[40],进水中添加高浓度nAg(10 mg·L−1),其对活性污泥微生物刺激作用大于其释放出的Ag+(3 mg·L−1)。
4)活性污泥絮体粒径及Zeta电位。SBRs运行至第50 天时,各组反应器中活性污泥絮体粒径和Zeta电位如表2所示。与CK组相比,进水中分别添加1 mg·L−1 、10 mg·L−1 nAg和0.3 mg·L−1、3.0 mg·L−1Ag+对活性污泥絮体粒径无显著影响;与CK组及1-nAg组、10-nAg组、0.3-Ag+ 组相比,进水中添加3.0 mg·L−1 Ag+导致污泥絮体的Zeta电位显著上升(P<0.05)。污泥絮体的Zeta电位与絮体的分散稳定性和絮凝效率有关[41],Zeta电位上升代表絮凝体稳定性降低,团聚性增强[35]。进水中添加3.0 mg·L−1 Ag+直接增加了污水中阳离子浓度,可能发挥电中和及压缩双电层作用导致活性污泥絮体的Zeta电位上升。而进水中添加10 mg·L−1 nAg,尽管其在纯水中释放出3.0 mg·L−1 Ag+,但在污水系统中nAg释放Ag+会受到环境中溶解氧、温度及天然有机物等多重因素的影响[42-43],且较易与污水中的阴离子形成化合物,例如CHOI等[44]发现nAg释放的Ag+会与Cl−、SO42−、PO43−等反应生成络合物,从而导致其对污泥絮体Zeta电位的影响与其他各组处理间无显著性差异。
-
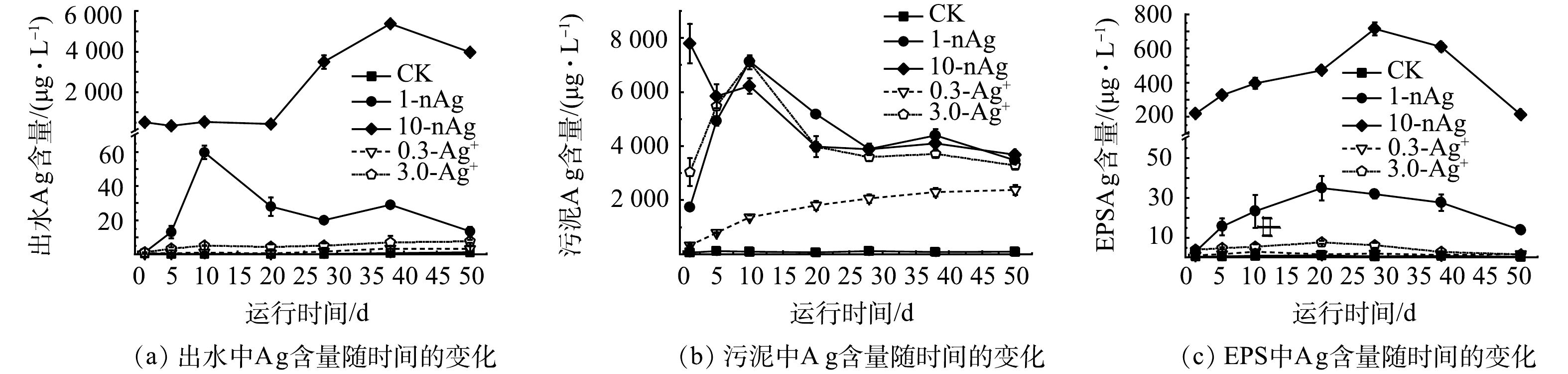
在50 d的运行期内,不同处理组中SBRs出水、活性污泥以及污泥EPS中Ag含量的动态变化如图3所示。由于nAg和Ag+在污水处理系统中易发生形态转变[45],测定时难以区分Ag的真实存在形态,因此,测定反应器各部分的总Ag含量表征进水中nAg和Ag+在SBR中的含量分布。
1)出水中Ag含量。如图4(a)所示,SBRs运行至50 d时,0.3-Ag+和3.0-Ag+组中出水Ag质量浓度分别为(3.18±1.46) μg·L−1和(7.72±0.90) μg·L−1;1-nAg组出水中Ag质量浓度为(13.50±2.55) μg·L−1,10-nAg组出水中Ag质量浓度达(3.96±0.16) mg·L−1。0.3-Ag+、3.0-Ag+组和1-nAg组反应器出水中Ag质量浓度均显著低于10-nAg组。YUAN等[46]连续37 d在SBRs进水中分别添加1 mg·L−1和5 mg·L−1 nAg,出水中总Ag含量分别为(26.0±3.0) μg·L−1和(1.88±0.06) mg·L−1,相差约70倍,与本研究结果相一致。
2)活性污泥中Ag含量。如图4(b)所示,1-nAg、10-nAg和3.0-Ag+组中活性污泥Ag质量浓度在SBRs运行至28 d后趋于稳定,约为3.20~4.30 mg·L−1,运行结束时,这3组反应器中活性污泥Ag质量浓度在3.28~3.67 mg·L−1。以上结果表明反应器运行28 d后活性污泥对Ag的吸附、积累达到稳定值,10-nAg组污泥中Ag的积累达到饱和后,导致出水中Ag含量逐渐升高(图4(a))。进水中添加0.3 mg·L−1 Ag+的SBRs活性污泥中Ag含量随着运行时间延长,持续升高,运行至第 50 天时,污泥中Ag质量浓度为2.38±0.19 mg·L−1。这可能因为进水中Ag含量较低,污泥中Ag含量未达到吸附最大值。SHENG等[38]也发现污泥中Ag积累存在阈值,当污泥中Ag积累达到阈值后,出水中总Ag含量升高。
3)污泥EPS中Ag含量。如图4(c)所示,0.3-Ag+组和3.0-Ag+组的活性污泥EPS中Ag含量与CK组无显著性差异;而1-nAg和10-nAg组的活性污泥EPS中Ag质量浓度明显高于CK和Ag+处理下的活性污泥(P<0.05),分别为(34.79±6.19) μg·L−1和(714.50±37.45) μg·L−1。这表明污泥EPS对污水中nAg具有较强的吸附性。已有研究表明,活性污泥EPS能够捕获污水中nAg并阻止其扩散,有助于消耗纳米微粒诱导产生的活性氧,从而保护微生物细胞膜结构不受到损害[47-48]。
-
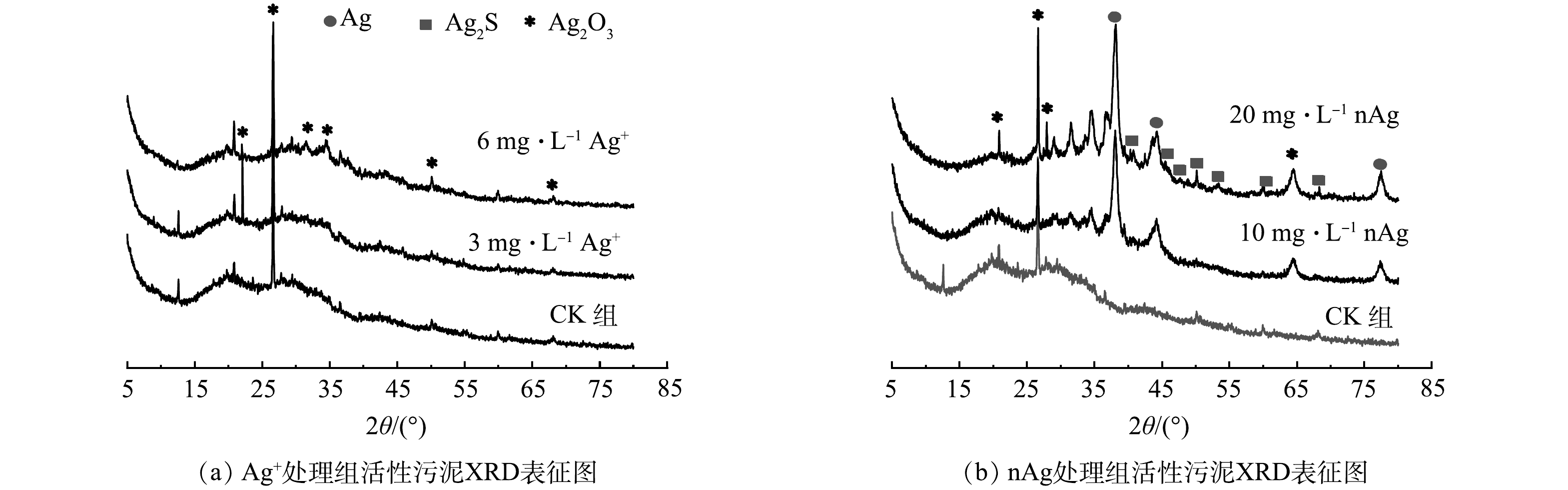
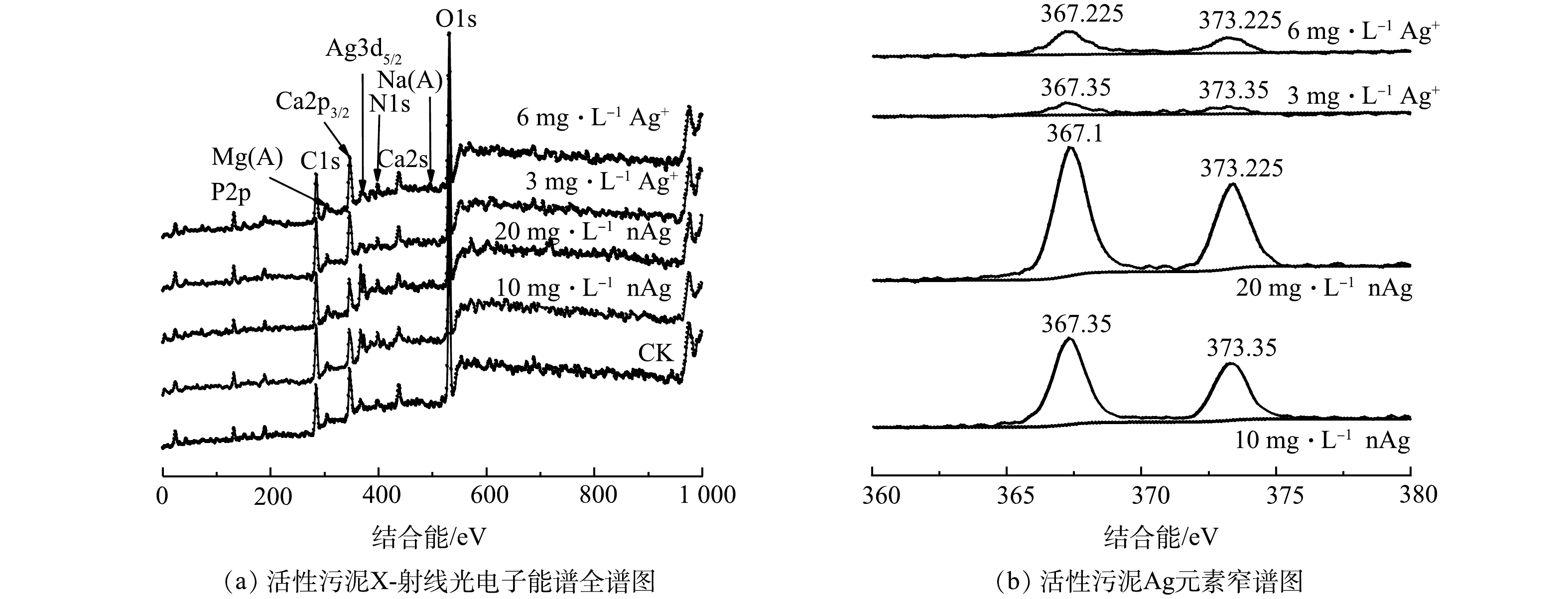
进水中分别添加1 mg·L−1和10 mg·L−1 nAg及0.3 mg·L−1和3 mg·L−1Ag+,SBRs连续运行至第50 天时采用XRD和XPS分析污泥中Ag的形态。XRD表征结果表明,1-nAg组及0.3-Ag+组活性污泥中均未检测到Ag及含Ag化合物,其可能原因是污泥中Ag含量低于所用XRD的检出限(5%)。因此,为了避免仪器的检出下限限制,采用与本文前述相同的反应器运行条件,增设2组反应器,将进水中的nAg和Ag+质量浓度分别增加至20 mg·L−1 nAg和6 mg·L−1 Ag+,在保证采用XRD可以检出污泥中Ag的前提下,进行Ag的形态分析。李金璞等[49-50]的研究表明,进水中分别添加20 mg·L−1 nAg和6 mg·L−1 Ag+对活性污泥理化性状的影响趋势、Ag在SBRs污泥和出水中的分布特征等与进水中分别添加10 mg·L−1 nAg和3 mg·L−1 Ag+的结果较一致,并未影响2.1与2.2中的结论。
1) XRD分析结果。图5为进水中分别添加10 mg·L−1 、20 mg·L−1 nAg和3 mg·L−1 、6 mg·L−1 Ag+以及CK组活性污泥的XRD图谱。进水中添加6 mg·L−1 Ag+处理的活性污泥中存在Ag2O3(PDF40-0909);进水中添加10 mg·L−1 nAg处理的活性污泥中存在Ag0(PDF04-0783)和Ag2O3;进水中添加20 mg·L−1 nAg处理的活性污泥中存在Ag0,Ag2O3和Ag2S(PDF14-0072)。GORHAM等[51]发现在光照下,nAg悬浮液中有氧化银类物质和Ag+存在,而nAg释放的Ag+可被污水中Cl−、S2−等络合沉淀,形成溶度积较小的AgCl和Ag2S沉淀(Ksp[AgCl]=1.8×10−10;Ksp[Ag2S]=6.3×10−50);在活性污泥系统的缺氧池内,nAg也可在2 h内被转化为Ag2S[52]。
本研究SBRs中泥水混合液DO为0.2~8.0 mg·L−1,存在厌氧/缺氧/好氧生境,nAg进入活性污泥系统后可能被氧化为Ag2O3,可能发生硫化形成Ag2S,也有可能未发生形态转化以Ag0的形式存在于污泥中。PVP包被的nAg等电点为3,在pH=6~9的污水中会发生表面羟基化(式(1)),与活性污泥中的R-NH2官能团结合,从而被吸附去除,同时nAg自身形态也会发生变化[53]。CHEN等[54]也发现,进水中添加0.1 mg·L−1 nAg反应4 h后,活性污泥中nAg被转化为Ag2S、Ag0和Ag+等多种形态。
2) XPS分析结果。由图6(a)可知,进水中分别添加10 mg·L−1、20 mg·L−1 nAg和3 mg·L−1、6 mg·L−1 Ag+的活性污泥中富含O、Na、P、Mg、Ca、N等元素,C、O元素原子百分比均高于40%(表3),各活性污泥样品中均检测出Ag,Ag元素原子百分比分别为1.3%、1.9%和0.3%、0.5%,随进水中Ag浓度的上升而上升。图6(b)为进水中添加不同浓度nAg和Ag+处理下活性污泥中Ag元素的XPS能谱图。图6(b)中左峰和右峰数值分别对应Ag原子在3d5/2和3d3/2轨道上的结合能,2峰之间的结合能之差为6.00 eV,各组活性污泥Ag元素在3d5/2轨道上的结合能为367.1~367.4 eV,而XPS能谱分析手册中AgO在3d5/2轨道上的结合能为367.4 eV[55],与本研究Ag元素3d5/2轨道上的结合能最为接近,因此,该结合能下Ag的存在形式可能为氧化物。当污水处理系统中DO充足时,nAg的团聚速率比缺氧状态时快3~8倍[56],活性污泥对nAg的吸附率也更高[54],因此,污水中的nAg可能吸附在污泥中并部分以银的氧化物形式存在。
由以上结果可知,进水中含有nAg和Ag+对污水处理系统中污泥形态、形貌、粒径及Zeta电位均存在影响,且进水中Ag含量越高,其影响越显著,其中nAg对污泥性状的影响大于其溶解释放的Ag+。CHOI等发现在相同条件下,nAg对硝化细菌的抑制率是Ag+的2倍[57];相同浓度的nAg和Ag+对小球藻的毒性作用也并不相同[58]。因此,nAg对污泥生物活性的抑制作用不仅仅来自于溶解的Ag+,与nAg本身的空间结构也有关联,纳米尺度的nAg微粒由于比表面积大,能够吸附在细胞表面,破坏细胞膜导致微生物细胞膜损伤[4],并影响细胞内遗传物质的复制促使细胞凋亡[59]。
进入活性污泥污水处理系统中的nAg,约有2.5%~5.0%的Ag随出水排出,其余部分被活性污泥吸附[60-61],10~30 ℃条件下活性污泥对nAg的最大吸附量为12~30 mg·g−1[54]。在pH、DO及其污水中共存离子等环境因素影响下,污泥中nAg可能被转化为银的氧化物和Ag2S,未转化部分以Ag0的形式存在。有研究表明,银的氧化物和Ag0依然有较强的生物毒性。SHEN等发现金黄色葡萄球菌和大肠杆菌暴露于质量分数为8.5% AgO的复合抗菌材料上20 min后,致死率均达99.99%[62]。暴露1 mg·L−1 Ag0会导致芦苇人工湿地中植物根系活性显著降低[63]。而Ag2S溶解性低,具有很强的环境稳定性,能够有效降低nAg和Ag+ 的毒性。但LI等[64]发现含Ag2S的污水经次氯酸消毒45 min后会溶解出22.3%的Ag+,对后续污水生物处理系统或受纳污水的地表水生态系统产生影响。因此,对于进水中含Ag的活性污泥需要选择恰当的污泥处理方式,如生物法、热处理法和稳定化法等[65],以防止污泥中含银化合物发生形态转化,转化为环境风险更高的银形态。
-
1)进水中分别添加1 mg·L−1、10 mg·L−1 nAg及0.3 mg·L−1 Ag+、3.0 mg·L−1 Ag+ 的SBRs连续运行50 d,与CK相比,进水中投加nAg 和Ag+对活性污泥颜色、形态及污泥EPS数量均有影响,nAg 或Ag+浓度越高,其影响越显著。
2)进水中投加的nAg或Ag+主要吸附、积累在活性污泥中。1-nAg、10-nAg和3.0-Ag+组反应器运行28 d后污泥对Ag的吸附达到稳定值,10-nAg组污泥中Ag积累量达到饱和后,出水中Ag含量逐渐升高,0.3-Ag+组活性污泥Ag含量随着运行时间持续升高,但未达到吸附饱和值。
3) SBRs连续运行50 d,随进水进入反应器的nAg及Ag+受活性污泥系统pH、DO及污水中共存离子等因素影响,部分nAg转化为银的氧化物和Ag2S等,其余以Ag0形态存在;Ag+被转化为银的氧化物和Ag2S等。
纳米银在活性污泥污水处理系统中的分布及形态转化
The distribution and morphology transformation of nanosilver in activated sludge treatment system
-
摘要: 纳米银(nanosilver,nAg)因其优越的抑菌性能成为全球应用最多的纳米材料之一。随着纳米技术的广泛应用,纳米银不可避免进入污水收集和处理系统。在污水生物处理过程中,纳米银可能发生化学形态转变,从而对污水处理微生物产生不同的抑制效应。本研究采用序批式反应器(sequencing batch reactors,SBRs)模拟活性污泥污水处理系统,连续运行50 d,在进水中分别添加1 mg·L−1、10 mg·L−1 nAg和0.3 mg·L−1、3 mg·L−1 Ag+,探究了Ag在活性污泥、出水中的分布以及污泥中Ag的化学形态变化。结果表明,进水中添加的Ag(nAg或Ag+)导致活性污泥颜色、絮体结构和Zeta电位等均发生了变化,进水中Ag含量越高,这些变化越明显;进水中分别添加1 mg·L−1、10 mg·L−1 nAg和3 mg·L−1 Ag+时,运行至第20 天后,活性污泥中Ag质量浓度稳定在3.28~3.67 mg·L−1,而进水中添加0.3 mg·L−1 Ag+时,活性污泥中Ag质量浓度持续升高,运行至第50天,达(2.38±0.19) mg·L−1;进水中分别添加1 mg·L−1 nAg和0.3 mg·L−1、3 mg·L−1 Ag+的反应器在50 d运行期内,出水中Ag质量浓度分别低于60.0、5.0、9.0 μg·L−1,而添加10 mg·L−1 nAg的反应器运行20 d后,出水中Ag含量快速升高,在第50 天时达(3.96±0.16) mg·L−1,这表明进水中添加的Ag主要累积在活性污泥中,活性污泥对进水中的Ag存在吸附饱和现象,超出污泥吸附阈值后,反应器出水中总Ag含量持续升高;对运行至第50 天的各反应器中活性污泥进行了X-射线衍射分析及X射线光电子能谱分析,发现进入活性污泥系统的nAg可以银的氧化物、Ag2S和Ag0等形态存在于污泥中。评价nAg对污水处理微生物的毒性影响应结合nAg的化学形态转化,含有较高浓度Ag的活性污泥在处理处置过程也应评估不同形态Ag的环境影响。Abstract: Nanosilver (nAg) has been used broadly in nanotechnology enhanced consumer products because of its excellent antimicrobial properties. With wide application of nanotechnology, nAg will be inevitably released into sewage collection systems and wastewater treatment plants (WWTPs). During sewage biological treatment, the chemical form transformation of nAg will occur, which will cause different inhibitory effect on the microbial communities in WWTPs. To explore the distribution of Ag in activated sludge and effluent, and chemical form variation of Ag in sludge, the sequencing batch reactors (SBRs) were selected to simulate activated sludge treatment system for 50d running, the test experiments were conducted with addition of 1 mg·L−1 nAg (1-nAg) in influent, 10 mg·L−1 nAg (10-nAg) in influent, 0.3 mg·L−1 Ag+ (0.3-Ag+) in influent or 3 mg·L−1 Ag+ (3-Ag+) in influent, as well as the control (CK) group, respectively. The results showed that the changes in color, floc structure and Zeta potential of activated sludge occurred with the addition of nAg or Ag+ in the influent. The changes became more significant with the increase of nAg or Ag+ concentrations in the influent. The Ag concentrations in activated sludge of 1-nAg, 10- nAg and 3.0-Ag+ groups were ranged from 3.28 to 3.67 mg·L−1 after 20d SBR running. For 0.3-Ag+ group, the Ag concentration in activated sludge continued to increase and reached (2.38±0.19) mg·L−1 at the end of operation. The Ag concentrations in SBR effluent were lower than 5.0 μg·L−1, 9.0 μg·L−1 and 60.0 μg·L−1 with addition of 0.3 mg·L−1, 3 mg·L−1 Ag+ and 1 mg·L−1 nAg during 50 d operation, respectively. However, the Ag concentrations in SBR effluent with 10 mg·L−1 nAg addition increased rapidly after 20d running, and reached (3.96±0.16) mg·L−1 at the operation end. This indicates that Ag mainly accumulated in the activated sludge and the adsorption threshold for Ag occurred on it, when this threshold was exceeded, the total Ag concentration in the effluent continued to increase. X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analysis on SBR activated sludge after 50d running indicated that nAg in activated sludge formed silver oxide, Ag2S or silver complexes. The toxic effects of nAg on microorganisms in WWTP should be evaluated in combination with the chemical transformation of nAg. The environmental impact of Ag should also be assessed in activated sludge treatment and disposal with high concentration Ag.
-
Key words:
- nanosilver /
- silver ion /
- activated sludge /
- sequencing batch reactors /
- morphology transformation
-
突发环境事件是由污染物排放或者生产安全事故、自然灾害等次生的,短时间内可能导致环境质量下降或者造成生态环境破坏的事件[1]。云南省矿产资源极为丰富,尤以有色金属及磷矿著称,被誉为“有色金属王国”,尾矿库泄漏事故次生环境风险突出;地形以高原、山地为主,地势起伏,交通险阻,江河纵横,湖库棋布,道路运输事故引发的突发环境事件高发;位于亚欧板块和印度洋板块交界地带,地质运动活跃导致地震多发和地质灾害频发,易造成企业环保设施受损,导致环境事件发生;地处上游地区,河流和湖泊众多,多数河流具有落差大、水流湍急、流量变化大的特点,且跨国境、跨省界河流多,防范流域突发水污染事件压力大。总体来说,云南省突发环境事件风险特征明显且面临易发多发的高风险态势。
“十四五”期间,云南省突发环境事件的高风险态势加剧,生态环境应急形势更加严峻。在重金属、跨界污染风险突出的形势下,随着原油、成品油输送网络的形成和石化产业链的延伸,应对石化相关产业存储、运输和生产环节的环境风险挑战逐渐增多。加快推进的交通运输建设,加之公路货运仍占主体地位,危险化学品运输次生突发环境事件概率增加;水运业务快速增长,港口、码头环境风险增大。7级地震平静时长突破历史记录,“十四五”时期地震形势更加严峻复杂。基础设施重大工程建设将加剧地质灾害次生突发环境事件的概率。
在把握云南省突发环境事件风险特征的基础上,根据云南省“十四五”经济社会发展规划,深入分析产业结构、运输结构、能源结构布局和重点行业发展变化趋势,提前研判“十四五”云南省生态环境应急形势的新特点新趋势,研究生态环境应急规划的思路和重点,针对性做好风险防控和应急准备,提高应急处置及其保障能力,推进生态环境应急体系和能力现代化,对于妥善应对突发环境事件,维护生态环境安全底线,具有十分重要的现实意义。文章立足于云南省环境应急的现状和问题,结合生态环境应急形势分析,提出了云南省“十四五”生态环境应急规划的思路和建议。
1. “十四五”生态环境应急形势分析
1.1 突发环境事件引发因素短期内难以改变
“十三五”时期,云南省管控违法排污造成突发环境事件的成效显著,因违法排污引起的突发环境事件明显减少,但仍需严厉打击危险废物非法转移和倾倒等违法犯罪活动造成的突发环境事件。生产安全事故、道路运输事故和自然灾害次生的突发环境事件多发频发情况短期内难以改变。
1.1.1 生产安全事故因素
支撑云南省高质量发展的基础仍不牢固,在产业发展方面的短板仍然明显,主要表现在发展方式粗放,制造业产业层次普遍偏低[2]。目前,涉及重大环境风险工艺及物质的石化、化纤、医药、化工、轻工、冶炼、港口/码头、石油天然气及其长输管道等行业在全省均有分布。全省共有尾矿库588座,位居全国第四。2021年,云南省生产事故总量仍然偏大,除道路运输事故外,全省发生各类生产安全事故443起,可能次生突发环境事件的金属非金属矿山事故32起,化工和危险化学品事故5起,工贸行业事故75起[3]。
“十三五”期间,中缅油气管道建成运营,云南省建成投运油气管道总里程达到4 914 km,原油、成品油、天然气三大管网已初成体系,中石油云南石化1 300万吨/年炼油项目建成投产。“十四五”期间,将建设覆盖全省各州、市的天然气支线管道,建成一批原油和成品油储备项目,形成以昆明市为中心的放射状成品油管道输送网络,成品油管道达2 500 km以上,输送能力达3 128万吨/年;将推进石化产业向下游产业链延伸,大力发展功能性化学品、化工新材料等精细化工[2]。
1.1.2 道路运输事故因素
云南省山地面积约占全省总面积的94%左右,地形地貌复杂,道路坡陡弯急,路网安全运行基础薄弱,安全防护设施历史欠账较多,极易发生交通事故并次生突发环境事件。2021年,全省发生道路运输事故1 035起,其中较大事故16起,水上交通事故1起,铁路运输事故9起[3]。
到2025 年,云南省综合交通实体线网总里程将达到36万km,其中高速公路通车里程新增6 000 km、达到1.5万 km,新改建国省道3 000 km,新改建农村公路6万km,铁路营运里程新增1 800 km、达到6 000 km。在建及运营运输机场总数量达到20个。新增及改善航道里程1 000 km、达到5 300 km,新增内河港口泊位60个。预计2021~2035年,公路货运仍占主体地位,货物运输仍然集中在滇中地区,水富港至长江中下游水上运输业务快速增长[4-5]。“十四五”规划的37条国家和地方高速公路项目线路涉及54个集中式饮用水水源保护区,规划的13条铁路项目线路涉及34个集中式饮用水水源地保护区[4-5]。
1.1.3 自然灾害因素
云南省自然灾害种类多、分布地域广、发生频率高,属地质灾害多发频发区和地震多发省份,地质、地震和洪涝等自然灾害诱发突发环境事件风险隐患大,各类灾害风险交织叠加,不确定因素多。2021年与近5年灾害发生频次均值相比,地质灾害增加126.56%、洪涝灾害增加44.23%。2021年因地震灾害共造成10个州(市)的23个县(市、区)不同程度受灾[6]。
云南省地质构造复杂,地层岩性复杂,近地表岩土体破碎,不稳定岩土体广泛分布,稳定性差。复杂脆弱的地质环境背景条件,遭遇高强度降雨(雪)或长时间连续降雨等极端天气以及强烈地震,导致滑坡、泥石流和崩塌等地质灾害多发频发。“十四五”交通、水利和能源等大规模基础设施建设工程将加剧地质灾害的发生。地处印度洋板块与亚欧板块碰撞带附近,地壳运动比较强烈,沿构造线或大的断裂带,常有强烈地震发生,具有频度高、强度大、震源浅、分布广的特征。全省91.2%的国土面积处于7度以上地震高烈度区,1 500万人居住并在活动断层控制的盆地区域内从事生产活动。7级地震平静时长突破历史记录,“十四五”时期震情形势更加严峻复杂,大量长距离、大跨度油气管线等基础设施邻近或直接处于大震危险源地带[7-8]。
1.2 环境风险受体敏感
1.2.1 饮用水水源地
目前,云南省县级及以上城市集中式饮用水水源地共236个,除7个为地下水型饮用水源地外,其他均为湖库型和河流型饮用水水源地;“千吨万人”饮用水水源共330个,湖库型和河流型260个,占比78.8%;乡镇级集中式饮用水源共966个,湖库型和河流型685个,占比70.9%。总体来说,全省湖库型和河流型饮用水水源占比大,环境风险受体敏感性突出。存在交通穿越的县级以上集中式饮用水水源地共69个,因流动源造成突发环境事件的风险较大。“十四五”期间,将新建和续建大、中、小型水库13个[2]。
1.2.2 跨国境和省界河流
云南省是“一带一路”建设、长江经济带两大国家发展战略的重要交汇点,涉及水系包括长江(金沙江)水系、珠江(南盘江)水系、元江(红河)水系、澜沧江(湄公河)水系、怒江(萨尔温江)水系和大盈江(伊洛瓦底江)水系。全省跨国境、跨省界河流众多,与缅甸、越南存在跨国境断面,与西藏、四川、贵州和广西省(自治区)存在跨省界断面。16个州(市)中,8个州市涉及跨国境河流,9个州(市)涉及跨省界河流。跨国境断面共22个,涉及红河水系、澜沧江水系、怒江水系和大盈江水系的河流干流及其一、二级支流共20条。跨省界断面共36个,涉及长江水系、珠江水系、怒江水系和大盈江水系的河流干流及其一、二级支流共26条。
1.3 环境风险源风险突出
云南省产业结构性、布局性环境风险依旧突出。产业结构以资源型产业为主,大部分产业处于全球产业价值链中低端,企业“乱、散、小”问题突出,发展质量亟待提高。各类化工园区、企业依水而建,沿江、沿河10 km范围内风险企业较多。全省“一废一库一品”企业较多。云南省是长江经济带省(市)中尾矿库数量最多的省份,纳入监管尾矿库588座,涉及16个州(市)。截至2021年12月底,全省危险废物经营许可证持证企业共100家。目前,共有重大突发环境事件风险企业90余家,较大突发环境事件风险企业近400家。“十四五”全省按照“大抓产业、主攻工业”思路,将着力扩大工业投资,实现规模以上工业企业数量翻番,大力发展新材料、生物医药、先进装备制造、绿色食品加工、电子信息、化工、卷烟及配套产业[9]。
2. 生态环境应急现状和问题
突发环境事件的妥善应对,需要结合本省环境风险源和风险受体特点,针对风险源可能造成的环境影响范围和程度,提前做好风险管控和应急准备。云南省生态环境应急工作起步较晚,应对突发环境事件的准备基础十分薄弱,存在明显的短板和不足,体制机制还未完全理顺,风险底数尚不清楚,应急保障极不充分,应急能力亟须提升。
2.1 环境应急管理体系需加快完善
当前云南省环境应急管理的体制机制与“十四五”环境安全形势发展的要求不相适应,环境应急管理体制不够完善,联动机制不够健全。云南省生态环境厅突发环境事件应急响应预案以及各州(市)突发环境事件应急预案和响应预案更新滞后,不能满足环境应急预案修订时限和环境应急工作高质量发展要求。缺乏生态环境应急管理制度、管理办法和工作规范,环境应急制度化和规范化工作格局尚未形成。与四川、贵州、广西和西藏省(自治区)签订了跨省(区)流域上下游突发水污染事件联防联控机制合作协议,但省内相邻流域、区域的应急协调联动机制普遍未建立,省政府组成机构间生态环境应急协作联动机制尚未建立。
2.2 突发环境事件风险底数不清
云南省至今未开展过突发环境事件风险专项调查和评估工作,全省突发环境事件风险底数不清,包括环境风险源基本情况、环境风险受体信息、环境风险防控与应急处置能力。因此,不能通过分析建立环境风险源和敏感受体之间的影响关联,不能识别环境风险源及其风险物质特点,不能明确风险源可能造成的环境影响途径、范围和程度。进一步导致政府和部门突发环境事件应急预案编制的支撑基础不牢,开展风险防控和应急准备工作的针对性不足,无法构建与风险水平相适应的环境应急技术和保障能力。
2.3 突发环境事件风险防控针对性不足
由于云南省突发环境事件风险底数不清,尚未建立全省突发环境事件风险源和风险受体分类分级风险管控和隐患排查治理监管机制,未能从源头着手防范化解重特大突发环境事件风险。企业编制的应急预案普遍流于形式、质量不高,针对性、实用性和操作性差,不重视、不执行、不管用的问题突出,事件场景设置不合理,且应急资源种类和数量不足,未与政府预案形成体系。集中式饮用水水源地环境应急预案覆盖不全面,并存在针对性和科学性不足的问题。
2.4 环境应急专业能力亟须提升
云南省于2021年成立了省生态环境应急调查投诉中心,曲靖市和昭通市建立了专职环境应急机构,其余14个州、市均未组建专职的环境应急机构和队伍,开展生态环境应急工作的人员多数为兼职人员,人员流动频繁,难以满足当前敏感严峻的环境应急形势需要。无论专职还是兼职环境应急人员,均缺乏系统性和规范化培训。未针对云南省突发环境事件风险特征和形势开展相关技术开发和应用研究,难以科学支撑复杂、难度较大突发环境事件的应对和处置,全省环境应急队伍专业能力亟须提升。
2.5 环境应急保障不充足
云南省未设立各级环境应急专项资金保障突发环境事件的处置。环境应急装备更新较慢,不能达到应急现场防护和快速监测的要求。应急物资信息库管理有待加强,全省未建设环境应急物资储备库和建立应急物资管理、调运机制。多数企业没有根据自身的环境风险特征储备足量的应急物资。环境应急综合管理和指挥平台开发缓慢,信息化工作有待进一步加强,数据共享机制有待建立,不能有效支撑环境应急管理体系和能力现代化要求。总体来说,一旦遭遇重特大突发环境事件,将面临不能充分保障突发环境事件处置的问题。
3. “十四五”生态环境应急工作思路与建议
李昌林等[10]从国家层面提出突发环境事件应急体系及完善建议,着重强调法律法规的衔接性、应急预案编制技术规范、多元主体参与机制、应急技术研发、应急人才培养和应急物资储备规划。朱文英等[11]也从国家层面提出环境应急管理制度体系发展建议,侧重完善事前防范和管理标准体系、提高事中处置规范化水平、增强事后赔偿和修复规范化水平。云南省生态环境应急工作在发展阶段和发展水平上均同国家环境应急整体发展状况存在较大差距,需立足云南省生态环境应急现状及存在的主要问题,基于突发环境事件风险特征和“十四五”生态环境应急面临的形势,按照“强体系、摸底数、防风险、提能力、促保障”的总体工作思路,基于可推动实现的目标,全面贯彻分类分级的理念和主线,“十四五”期间以突发水环境事件为重心,突出重点行业、重点企业、重点环节、重点风险物质,针对性做好风险防控和应急准备,着力防范和应对重特大突发环境事件发生,推进生态环境应急体系与能力现代化。
3.1 完善应急管理体系,健全联动协作机制
加快修订云南省生态环境厅突发环境事件应急响应预案,理顺厅内应急响应程序和机制,明确厅内各部门分级应对突发环境事件的职能职责。形成以风险评估为基础编制政府及其部门突发环境事件应急预案的导向,推进州(市)政府及生态环境部门按期修订突发环境事件应急预案及应急响应预案。根据环境应急重点工作需要,制定出台一系列管理制度、管理办法和工作规范,提高生态环境应急工作制度化和规范化水平。加强区域、流域和部门间协调协作,推进建立高效顺畅的相邻区域、流域应急协调联动机制,推动建立与应急管理、消防救援、水利、能源、交通运输、自然资源和地震等部门的联动协作机制,尤其要建立与应急管理、消防救援、交通运输和水利部门间的信息共享机制。
3.2 全面开展风险评估,动态掌握风险状况
对16个州(市)开展区域突发环境事件风险评估,全面掌握全省突发环境事件风险底数,为提升政府及其部门应急预案的针对性提供支撑,为实现分类分级、重点精准风险管控奠定基础,针对性做好应急准备,构建与风险水平相适应的环境应急技术和保障能力。同时,将风险评估成果信息化,集成在环境应急管理和指挥系统平台,及时根据环境风险源和风险受体变化情况实时更新,实现突发环境事件风险动态管理目标,提高生态环境应急管理精准化和信息化水平。积极推动流域突发环境风险评估试点工作。
3.3 强化多级风险防控,实现重点精准管控
提升突发环境事件风险管控水平,健全环境风险防范化解机制,突出重点行业和企业,坚持从源头上防范化解重特大突发环境事件风险。在掌握风险底数和实现动态更新的基础上,从企业和流域层面系统构建多层级的突发环境事件风险防控体系,推进风险管控能力现代化。建立企业突发环境事件风险分级管控和隐患排查治理双重防控机制。提升应急预案规范化和精准化管理水平,建立企业应急预案核查管理技术要点和方法体系,开展企业突发环境事件应急预案抽查复核。推动县级及以上集中式饮用水水源地环境应急预案全覆盖。全面推广应用“以空间换时间”的“南阳实践”,以“南阳实践”为抓手防控流域突发水污染事件,实现重点河流“一河一策一图”全覆盖。
3.4 加强专业技术支撑,提高事件应对能力
在目前我国应急管理体系为政府主导的治理模式下,着力推动基层生态环境应急机构和队伍建设,推动建立州(市)、县(区)环境应急专职机构。制定环境应急人员系统性、长期性培训计划,提高应对突发环境事件的专业素质和能力,重点强化基层应急人员信息报告和先期处置能力。重视应急技术深化应用,加强与环境应急技术研究单位的交流合作,结合全省产业结构、运输结构、能源结构布局和重点行业发展水平,根据风险评估成果针对性建立环境应急处置技术库并进一步研究提高应用水平,以支撑现场应急处置。完善环境应急监测和应急处置专家库。推动依托企业和社会组织,组建突发环境事故专业救援队伍。
3.5 建立应急保障体系,提升应急保障能力
推动在省和州(市)层面设立生态环境应急专项资金,确保环境应急资金保障。加强环境应急监测能力建设,探索建立企业、市场、政府多方参与的应急监测保障机制。在继续做好环境应急物资信息库建设和管理的基础上,根据区域行业特点、风险源分布和风险物质类型,研究细化应急物资种类、数量及其储备布局、模式和更新周期,建立实物储备、合同储备和生产储备相结合的应急物资储备模式,推动建设常用应急物资储备库。加快建设环境应急综合管理和指挥平台,初步实现环境应急管理和指挥“一张图”。加强环境应急信息化建设,首要以信息化提高环境风险防控精准化及动态管理水平、促进环境应急物资管理、储备和调运等保障能力。
-
表 1 nAg 和 Ag+处理下SBRs运行至50 d时活性污泥的能谱元素含量
% Table 1. EDS spectra analysis of activated sludgea with addition of different nAg and Ag+ concentrations in influent after 50 days running
% 处理组 Na Mg Al Si P S Cl K Ca Fe Ag CK 1.18 0.45 2.92 1.95 2.80 0.29 0.27 0.39 3.20 0.74 -- 1-nAg 0.87 0.36 1.89 1.21 2.14 0.23 0.18 0.26 2.15 0.45 0.22 10-nAg 0.96 0.48 2.79 1.70 3.62 0.93 0.24 0.45 4.01 0.76 4.38 0.3-Ag+ 1.56 0.48 2.78 1.51 3.08 0.36 0.38 0.47 3.60 0.72 -- 3.0-Ag+ 1.18 0.41 2.36 1.62 2.57 0.39 0.26 0.32 3.16 0.58 0.51 表 2 nAg 和 Ag+处理下SBRs运行至第50 天时活性污泥的粒径及Zeta电位
Table 2. The floc size and Zeta potential of activated sludge in SBRs with addition of different nAg and Ag+ concentrations in influent after 50 days running
处理 污泥粒径(10%)>μm 污泥粒径(90%)>μm Zeta电位/mV CK 80.02±4.84a 12.34±0.69a −13.57±0.83a 1-nAg 105.01±21.28a 15.26±3.15a −11.90±1.15a 10-nAg 92.80±4.47a 13.98±1.19a −11.32±1.93ab 0.3-Ag+ 90.41±8.10a 13.31±0.90a −10.97±0.65ab 3.0-Ag+ 99.70±30.01a 12.06±0.84a −8.35±1.32b 注: 10%和90%表示SBRs中体积分数为10%和90%以上的活性污泥粒径;每列中不同小写字母代表差异显著(P<0.05)。 表 3 nAg 和 Ag+处理下活性污泥中6种元素原子百分比含量
Table 3. Distribution proportion of six elementsin activated sludge in SBRs with addition of different nAg and Ag+ concentrations in influent after 50 days running
处理组 原子百分比/% C1s N1s O1s P2p S2p Ag3d CK 40.98 3.81 48.84 5.82 0.56 10 mg·L−1 nAg 47.42 4.83 41.45 4.25 0.77 1.28 20 mg·L−1 nAg 45.65 5.62 41.74 4.32 0.82 1.85 3 mg·L−1 Ag+ 43.35 4.00 46.37 5.67 0.33 0.3 6 mg·L−1 Ag+ 42.01 3.71 47.28 5.86 0.65 0.49 -
[1] The Danish Ecological Council and Danish Consumer. The Nanodatabase[EB/OL]. [2022-2-1].https://www.nanodb.dk/, 2013. [2] GOTTSCHALK F, T SONDERER, R W SCHOLZ, et al. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions[J]. Environmental Science & Technology, 2009, 43(24): 9216-22. [3] YAZDANBAKHSH A R, RAFIEE M, DARAEI H, et al. Responses of flocculated activated sludge to bimetallic Ag-Fe nanoparticles toxicity: Performance, activity enzymatic, and bacterial community shift[J]. Journal of Hazardous Materials, 2019, 366: 114-123. doi: 10.1016/j.jhazmat.2018.11.098 [4] RASOOL K and LEE D S. Inhibitory effects of silver nanoparticles on removal of organic pollutants and sulfate in an anaerobic biological wastewater treatment process[J]. Journal of Nanoscience and Nanotechnology, 2016, 16(5): 4456-4463. doi: 10.1166/jnn.2016.10984 [5] FORSTNER C, ORTON T G, WANG P, et al. Wastewater treatment processing of silver nanoparticles strongly influences their effects on soil microbial diversity[J]. Environmental Science and Technology, 2020, 54(21): 13538-13547. doi: 10.1021/acs.est.0c01312 [6] FERNANDO I, LU D, and ZHOU Y. Interactive influence of extracellular polymeric substances (EPS) and electrolytes on the colloidal stability of silver nanoparticles[J]. Environmental Science-Nano, 2020, 7(1): 186-197. doi: 10.1039/C9EN00861F [7] VILELA P, LIU H B, LEE S, et al. A systematic approach of removal mechanisms, control and optimization of silver nanoparticle in wastewater treatment plants[J]. Science of the Total Environment, 2018, 633: 989-998. doi: 10.1016/j.scitotenv.2018.03.247 [8] LEVARD C, MITRA S, YANG T, et al. Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli[J]. Environmental Science & Technology, 2013, 47(11): 5738-5745. [9] BRUNETTI G, DONNER E, LAERA G, et al. Fate of zinc and silver engineered nanoparticles in sewerage networks[J]. Water Research, 2015, 77: 72-84. doi: 10.1016/j.watres.2015.03.003 [10] ZHANG C, HU Z, DENG B. Silver nanoparticles in aquatic environments: physiochemical behavior and antimicrobial mechanisms[J]. Water Research, 2016, 88: 403-427. doi: 10.1016/j.watres.2015.10.025 [11] CHEN W P, SONG J H, JIANG S J, et al. Influence of extracellular polymeric substances from activated sludge on the aggregation kinetics of silver and silver sulfide nanoparticles[J]. Frontiers of Environmental Science & Engineering, 2021, 16(2). [12] REINSCH B C, LEVARD C, LI Z, et al. Sulfidation of silver nanoparticles decreases escherichia coli growth inhibition[J]. Environmental Science & Technology, 2012, 46(13): 6992-7000. [13] LEVARD C, REINSCH B C, MICHEL F M, et al. Sulfidation processes of pvp-coated silver nanoparticles in aqueous solution: Impact on dissolution rate[J]. Environmental Science & Technology, 2011, 45(12): 5260-5266. [14] XIU Z, MA J, Alvarez P J J, Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions[J]. Environmental Science & Technology, 2011, 45(20): 9003-9008. [15] KIM B, PARK C S, MURAYAMA M, et al. Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products[J]. Environmental Science & Technology, 2010, 44(19): 7509-7514. [16] CHOI O, DENG K K, KIM N J, et al. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth[J]. Water Research, 2008, 42(12): 3066-3074. doi: 10.1016/j.watres.2008.02.021 [17] TKACHENKO O, KARAS J A, Standardizing an in vitro procedure for the evaluation of the antimicrobial activity of wound dressings and the assessment of three wound dressings[J]. Journal of Antimicrobial Chemotherapy, 2012, 67(7): 1697-1700. [18] MCGILLICUDDY E. , MURRAY I., KAVANAGH S., et al. Silver nanoparticles in the environment: Sources, detection and ecotoxicology[J]. Science of the Total Environment, 2017, 575: 231-246. doi: 10.1016/j.scitotenv.2016.10.041 [19] HAO Z N, F S LI, R LIU, et al. Reduction of ionic silver by sulfur dioxide as a source of silver nanoparticles in the environment[J]. Environmental Science & Technology, 2021, 55(8): 5569-5578. [20] ALIZADEH, S, RAHIM, AA, GUO, B, et al. Impacts of continuous inflow of low concentrations of silver nanoparticles on biological performance and microbial communities of aerobic heterotrophic wastewater biofilm[J]. Environmental Science & Technology, 2019, 53(15): 9148-9159. [21] CHEN Y G, H CHEN, X ZHENG, et al. The impacts of silver nanoparticles and silver ions on wastewater biological phosphorous removal and the mechanisms[J]. Journal of Hazardous Materials, 2012, 239: 88-94. [22] WANG F, Z CHEN, Y WANG, et al. Silver nanoparticles induce apoptosis in HepG2 cells through particle-specific effects on mitochondria[J]. Environmental science & technology, 2022, 56(9): 5706-5713. [23] MO F, H B LI, Y H LI, et al. Exploration of defense and tolerance mechanisms in dominant species of mining area - Trifolium pratense L. upon exposure to silver[J]. Science of the Total Environment, 2022: 811. [24] GAO Y F, W R WU, K X QIAO, et al. Bioavailability and toxicity of silver nanoparticles: Determination based on toxicokinetic-toxicodynamic processes[J]. Water Research, 2021: 204. [25] ALITO C L and GUNSCH C K. Assessing the effects of silver nanoparticles on biological nutrient removal in bench-scale activated sludge sequencing batch reactors[J]. Environmental Science & Technology, 2014, 48(2): 970-976. [26] RAINIER H, LATIFI M A, ROCHEL N. Optimal design and operation of activated sludge processes: State-of-the-art[J]. Chemical Engineering Journal, 2015, 281: 900-920. doi: 10.1016/j.cej.2015.06.125 [27] TANG S H and ZHENG J. Antibacterial activity of silver nanoparticles: Structural effects[J]. Advanced Healthcare Materials, 2018, 7(13). [28] XU Q Y, LI S S, WAN Y P, et al. Impacts of silver nanoparticles on performance and microbial community and enzymatic activity of a sequencing batch reactor[J]. Journal of Environmental Management, 2017, 204: 667-673. doi: 10.1016/j.jenvman.2017.09.050 [29] ZHOU H X and XU G R. Effect of silver nanoparticles on an integrated fixed-film activated sludge-sequencing batch reactor: Performance and community structure[J]. Journal of Environmental Sciences, 2019, 80: 229-239. doi: 10.1016/j.jes.2018.12.016 [30] 段颖, 孙秀玥, 盛涛, 等 纳米银和银离子对活性污泥系统污染物去除效率的影响[J]. 环境工程学报, 2021, 15(7): 2450-2459. [31] JEMEC A, KAHRU A, POTTHOFF A, et al. An interlaboratory comparison of nanosilver characterisation and hazard identification: Harmonising techniques for high quality data[J]. Environment International, 2016, 87: 20-32. doi: 10.1016/j.envint.2015.10.014 [32] 国家环境保护总局. 水和废水监测分析方法(第四版)[M]. 北京: 中国环境科学出版社. 2002. [33] YUAN D Q, WANG Y L, and QIAN X. Variations of internal structure and moisture distribution in activated sludge with stratified extracellular polymeric substances extraction[J]. International Biodeterioration & Biodegradation, 2017, 116: 1-9. [34] MCGRATH S P and CUNLIFFE C H. A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges[J]. Journal of the Science of Food and Agriculture, 1985, 36(9): 794-798. doi: 10.1002/jsfa.2740360906 [35] 李墨青. 纳米银对SBR系统水处理效能及微生物菌群的影响研究[D]. 哈尔滨: 哈尔滨工业大学, 2014. [36] NING Y F, CHEN Y P, SHEN Y, et al. A new approach for estimating aerobic-anaerobic biofilm structure in wastewater treatment via dissolved oxygen microdistribution[J]. Chemical Engineering Journal, 2014, 255: 171-177. doi: 10.1016/j.cej.2014.06.042 [37] ZHANG P, FANG F, CHEN Y P, et al. Composition of EPS fractions from suspended sludge and biofilm and their roles in microbial cell aggregation[J]. Chemosphere, 2014, 117: 59-65. doi: 10.1016/j.chemosphere.2014.05.070 [38] SHENG Z Y, VAN NOSTRAND J D, ZHOU J Z, et al. Contradictory effects of silver nanoparticles on activated sludge wastewater treatment[J]. Journal of Hazardous Materials, 2018, 341: 448-456. doi: 10.1016/j.jhazmat.2017.07.051 [39] SUN J, GUO L, LI Q, et al. Structural and functional properties of organic matters in extracellular polymeric substances (EPS) and dissolved organic matters (DOM) after heat pretreatment with waste sludge[J]. Bioresource Technology, 2016, 219: 614-623. doi: 10.1016/j.biortech.2016.08.042 [40] HONG P-N, HONDA R, NOGUCHI M, et al. Optimum selection of extraction methods of extracellular polymeric substances in activated sludge for effective extraction of the target components[J]. Biochemical Engineering Journal, 2017, 127: 136-146. doi: 10.1016/j.bej.2017.08.002 [41] LI Z, LU P, ZHANG D, et al. Population balance modeling of activated sludge flocculation: Investigating the influence of Extracellular Polymeric Substances (EPS) content and zeta potential on flocculation dynamics[J]. Separation and Purification Technology, 2016, 162: 91-100. doi: 10.1016/j.seppur.2016.02.011 [42] DONG B, LIU G, ZHOU J, et al. Transformation of silver ions to silver nanoparticles mediated by humic acid under dark conditions at ambient temperature[J]. Journal of Hazardous Materials, 2020, 383: 121190. doi: 10.1016/j.jhazmat.2019.121190 [43] LIU J and HURT R H. Ion release kinetics and particle persistence in aqueous nano-silver colloids[J]. Environmental Science & Technology, 2010, 44(6): 2169-2175. [44] CHOI O K and HU Z Q. Nitrification inhibition by silver nanoparticles[J]. Water Science and Technology, 2009, 59(9): 1699-1702. doi: 10.2166/wst.2009.205 [45] HOU L, LI K, DING Y, et al. Removal of silver nanoparticles in simulated wastewater treatment processes and its impact on COD and NH(4) reduction[J]. Chemosphere, 2012, 87(3): 248-52. doi: 10.1016/j.chemosphere.2011.12.042 [46] YUAN Z H, YANG X Y, HU A Y, et al. Assessment of the fate of silver nanoparticles in the A(2)O-MBR system[J]. Science of the Total Environment, 2016, 544: 901-907. doi: 10.1016/j.scitotenv.2015.11.158 [47] SUN X, SHENG Z, and LIU Y. Effects of silver nanoparticles on microbial community structure in activated sludge[J]. Science of the Total Environment, 2013, 443: 828-35. doi: 10.1016/j.scitotenv.2012.11.019 [48] BURKHARDT M, ZULEEG S, KAEGI R, et al. Fate of nanosilver in wastewater treatment plants and their impact on nitrification activity in sewage sludge[J]. Umweltwissenschaften und Schadstoff-Forschung, 2010, 22(5): 529-540. doi: 10.1007/s12302-010-0153-2 [49] 李金璞, 唐珠, 杨新萍. 纳米银对SBRs脱氮效率的影响及外源AHLs的调控作用[J]. 环境工程学报, 2021, 15(9): 2942-2951. doi: 10.12030/j.cjee.202104088 [50] 李金璞. 群体感应AHLs信号分子对活性污泥污水处理系统抵抗纳米银胁迫的作用[D]. 南京: 南京农业大学, 2020. [51] GORHAM J M, ROHLFING A B, LIPPA K A, et al. Storage wars: how citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions[J]. Journal of Nanoparticle Research, 2014, 16(4): 2339. doi: 10.1007/s11051-014-2339-9 [52] KAEGI R, VOEGELIN A, SINNET B, et al. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant[J]. Environmental Science & Technology, 2011, 45(9): 3902-8. [53] LIU J F, CHAO J B, LIU R, et al. Cloud point extraction as an advantageous preconcentration approach for analysis of trace silver nanoparticles in environmental waters[J]. Analytical Chemistry, 2009, 81(15): 6496-6502. doi: 10.1021/ac900918e [54] CHEN L R, FENG W R, FAN J, et al. Removal of silver nanoparticles in aqueous solution by activated sludge: Mechanism and characteristics[J]. Science of the Total Environment, 2020: 711. [55] NIST X-ray Photoelectron Spectroscopy. National Institute of Standards and Technology(Version 4.1 )[EB/OL]. [2021-08-01]. http://srdata.nist.gov/xps/, 2012. [56] ZHANG W, YAO Y, LI K G, et al. Influence of dissolved oxygen on aggregation kinetics of citrate-coated silver nanoparticles[J]. Environmental Pollution, 2011, 159(12): 3757-3762. doi: 10.1016/j.envpol.2011.07.013 [57] CHOI O and HU Z Q. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria[J]. Environmental Science & Technology, 2008, 42(12): 4583-4588. [58] EGOROVA E M. Interaction of silver nanoparticles with biological objects: Antimicrobial properties and toxicity for the other living organisms[J]. Journal of Physics:Conference Series, 2011: 291. [59] BASTOS V, FERREIRA-DE-OLIVEIRA J M P, CARROLA J, et al. Coating independent cytotoxicity of citrate- and PEG-coated silver nanoparticles on a human hepatoma cell line[J]. Journal of Environmental Sciences, 2017, 51: 191-201. doi: 10.1016/j.jes.2016.05.028 [60] JOHNSON A C, JURGENS M D, LAWLOR A J, et al. Particulate and colloidal silver in sewage effluent and sludge discharged from British wastewater treatment plants[J]. Chemosphere, 2014, 112: 49-55. doi: 10.1016/j.chemosphere.2014.03.039 [61] QIU G L, WIRIANTO K, SUN Y L, et al. Effect of silver nanoparticles on system performance and microbial community dynamics in a sequencing batch reactor[J]. Journal of Cleaner Production, 2016, 130: 137-142. doi: 10.1016/j.jclepro.2015.10.051 [62] SHEN W N, FENG L J, FENG H, et al. Ultrafine silver(II) oxide particles decorated porous ceramic composites for water treatment[J]. Chemical Engineering Journal, 2011, 175: 592-599. doi: 10.1016/j.cej.2011.09.121 [63] BAO S, LIANG L, HUANG J, et al. Removal and fate of silver nanoparticles in lab-scale vertical flow constructed wetland[J]. Chemosphere, 2019, 214: 203-209. doi: 10.1016/j.chemosphere.2018.09.110 [64] LI L, XU Z, WIMMER A, et al. New insights into the stability of silver sulfide nanoparticles in surface water: dissolution through hypochlorite oxidation[J]. Environmental Science & Technology, 2017, 51(14): 7920-7927. [65] 陈冠益, 韩克旋, 刘彩霞, 等. 污泥中重金属处理方法[J]. 化学进展, 2021, 33(6): 998-1009. -




 下载:
下载: