-
煤炭作为我国的大型经济产业,在经济发展中有着举足轻重的作用[1]。2019年我国原煤产量达38.5亿t,同比增长4%,煤炭在未来一段时间内,依旧是我国的主要能源[2]。煤炭生产中,建成使用或已废弃的煤矿会产生大量的废水、岩石废料和尾矿,随着地表径流和地下水的浸出和侵蚀,这些废物中发生了一系列复杂的物理化学反应,如风化、溶解和氧化,造成硫酸和重金属含量高的酸性矿井排水[3-5],一方面影响地表水与地下水的相互关系,通过地下水间接影响地表水水质;另一方面,大量矿井水会直接排入地表水,造成二次污染[6]。对于煤矿开采区的水资源,掌握其水质状况尤为重要,水质的演化特征关系着区域水资源的可持续开发与利用,深入认识水质的变化过程和水环境质量的变化趋势,可为水环境保护、水资源合理开发利用提供依据。
水化学特征可作为河流水质评价以及河流生态系统的重要指标,对流域内人类生活用水、工农业用水有重要的影响。由于水体中的化学成分受区域地质、气候以及人类生产生活等影响,因此,水化学特征在一定程度上可反映流域内的基本特征[7-8],同时也是研究水环境质量的重要方法。对于上述的研究,国内外已经取得了很多成果,研究方法也趋于多样化[9-10]。从20世纪中期开始,运用大量的理论知识和技术手段来研究地表水、地下水水化学特征及演化规律。其中处理水质数据的数学方法有很多,一般包括聚类分析法、主成分分析法、相关分析法、因子分析法等[11-12],基于水质数据的分析,进一步运用piper三线图[13]、Gibbs图[14]、水质模拟法、同位素分析法[15-16]等探究河流离子化学特征及流域主要风化过程的影响[17],继而通过GIS可视化功能将水质结果清晰地展示出来[18]。近年来,人们进行了许多研究来调查水资源和煤矿开采的相互关系与影响。河流水质受到多种因素共同作用,流域水体中离子之间的相互作用、相互影响,构成了一个复杂的水环境系统。针对这种典型区域水体研究,明晰其水化学特征尤为关键,可以进一步分析水质演化规律,以期在煤矿开采过程中保护水资源及煤矿开采区域的水生态[19-20]。
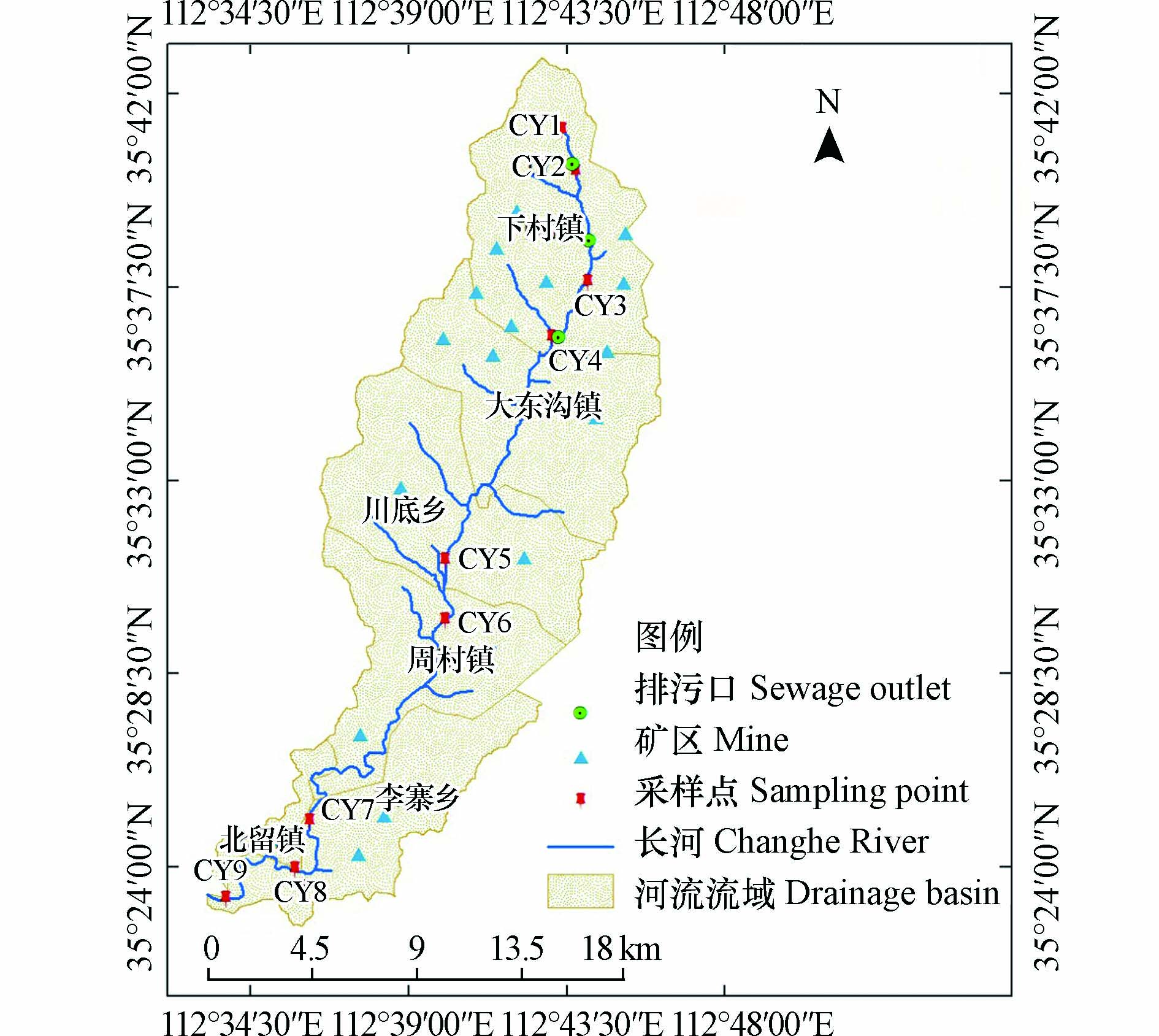
长河流域采煤区地处晋城市泽州县,是晋城煤炭经济带中的重要一环。流域内分布着大量煤炭、煤化工企业,据统计,2019年长河排放污水总量为504万t,由于煤炭工业的快速发展,已经使当地生态环境发生了改变,造成水质型缺水。近年来,晋城市已经开展了多项水环境治理工程,对长河流域地表水和地下水进行水资源保护与治理,但并未从水化学角度深入探究水质成因及变化规律。因此,本研究聚焦采煤区地表水长河,根据当地实际情况,沿程选取9个采样点进行测试分析,明晰地表水水化学特征及影响因素,为当地水资源管理与保护提供科学的建议。
-
研究区位于晋城市泽州县,是晋城重要的煤炭工业带区域,人口总计13.69万人,沁河一大支流长河贯穿整个研究区,起源于武神山,流经泽州县的下村镇、大东沟镇、川底乡、周村镇、李寨乡和北留镇,流域面积317 km2,干流河长54.7 km,当地工农业废水和生活污水,经处理后都排入长河。研究区属于干旱与半干旱地区,降水量小,多年平均降水量为591.8 mm,蒸发强烈,蒸发量1812 mm,导致研究区地表水与地下水水资源相对短缺。区内地势西高东低,北高南低,峰峦叠嶂,丘陵起伏,属典型的土石山地丘陵区。研究区有大量煤炭、煤化工企业,主要集中在中游以上区域,共有18个煤矿,井田面积共计343.43 km2。
-
通过对长河流域实地调研,依据长河多年地表水资料及当地煤炭资源开发利用现状,于2020年8月对长河进行了水体采样,采样点布设考虑当地污废水排放位置及人口分布特征,总计9组表水水样(图1),采集后根据GB3838-2002《地表水环境质量标准》[21]中相关规定进行水样的检测,主要项目为Ca2+、Mg2+、Na+、K+、
HCO−3 、SO2−4 、Cl−、pH和TDS。其中Ca2+、Mg2+、Na+和K+使用火焰原子吸收分光光度法测定;Cl−用硝酸银容量法测定,SO2−4 用离子色谱仪测定,HCO−3 用盐酸滴定法测定,溶解性固体(TDS)采用烘干法测定,pH在现场由多参水质分析仪测定。K+检测限为 0.02 mg·L−1,Na+检测限为0.01 mg·L−1,Ca2+、Mg2+检测限为0.05 mg·L−1,Cl−检测限为1.0 mg·L−1,SO2−4 检测限为0.05 mg·L−1。 -
本文通过Excel进行水化学数据统计,结合研究区水文地质条件综合运用数理统计、Pearson相关性分析、Piper三线图、Gibbs图以及离子比值端元图等方法分析水化学特征和控制因素。其中,Piper三线图和Gibbs图采用Origin2020制图,利用SPSS22分析离子间相关性和系统聚类分析水化学特征。
-
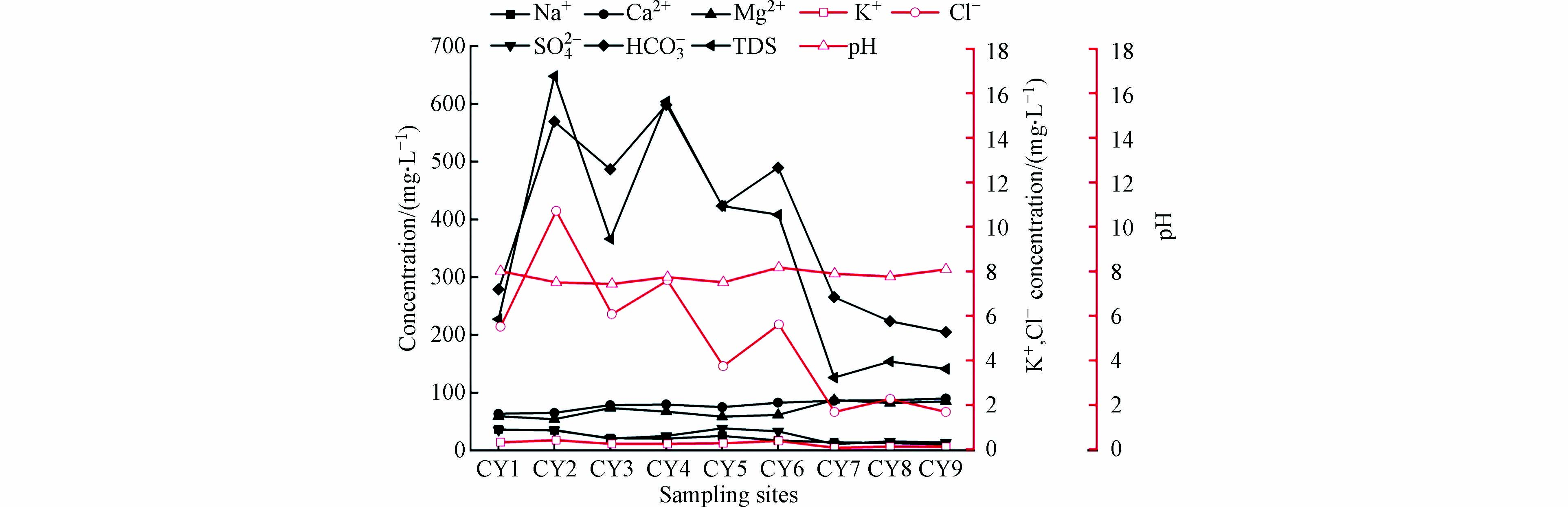
本文共采集地表水样9组,各水化学参数见表1。研究区地表水阳离子质量浓度关系Ca2+> Mg2+> Na+> K+,均值分别为190.3、88.96、48.83、0.56 mg·L−1;阴离子质量浓度关系为
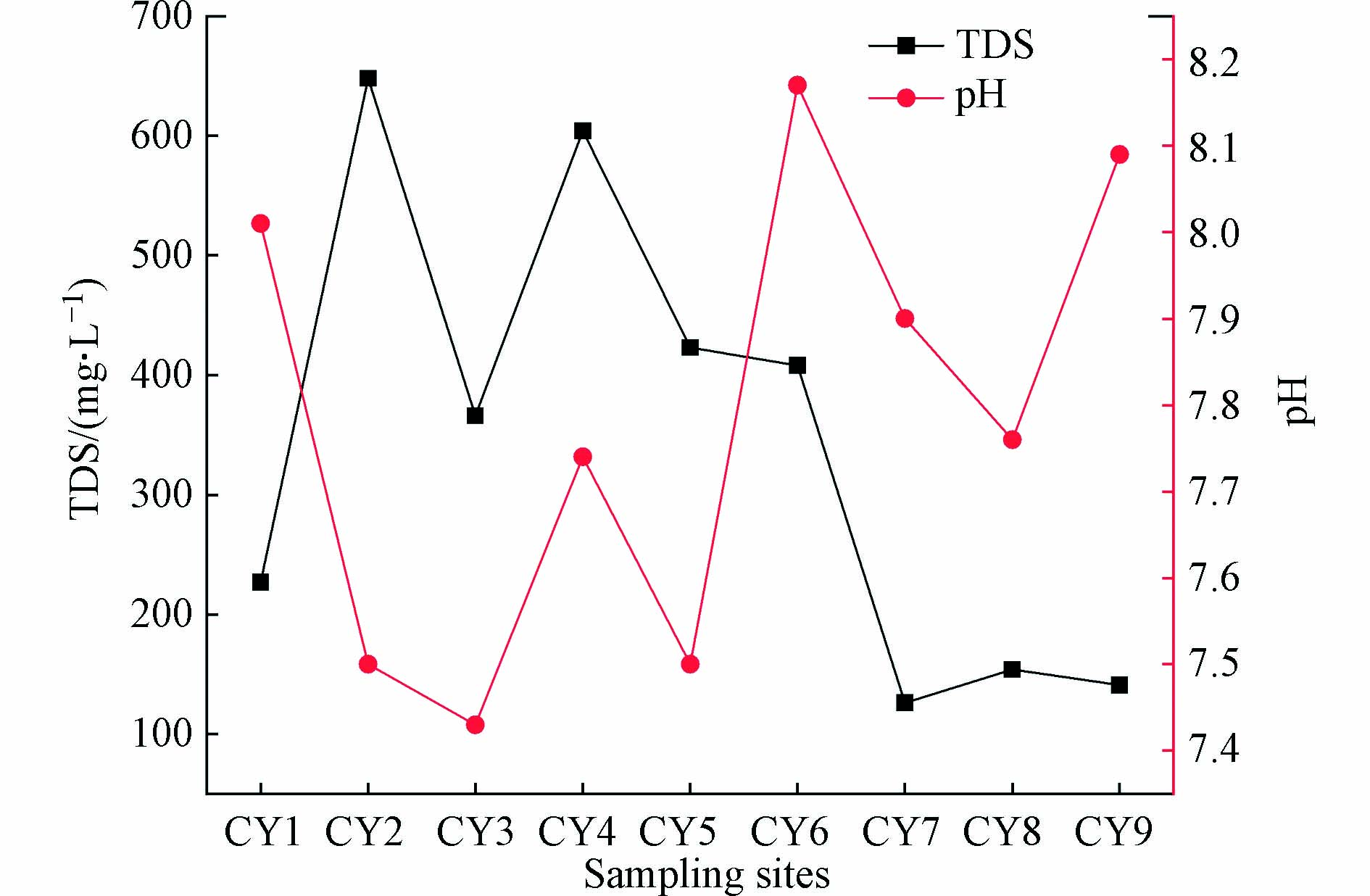
HCO−3 >SO2−4 >Cl−,均值分别为393.29、31.31、6.36 mg·L−1。研究区地表水TDS为126—604 mg·L−1,平均值为344.11 mg·L−1;pH值在7.43—8.17之间,平均值为7.79,属于碱性水;研究区阳离子以Ca2+和Mg2+为主,阴离子主要是HCO−3 。由表1可知,Cl−与TDS的变异系数最大,分别为66.4%与57.0%。水化学Cl−离子受水岩作用影响较小,是一种相对稳定的保守离子,可以较好的示踪生活污水、家禽用水NaCl的输入对水化学的影响[5、22]。本研究水体采样检测结果Cl−离子浓度较低,但其与TDS的变异系数较高,说明演化较为复杂,结合图2沿程变化可以看出,在CY2与CY4两点出现较高值,人类活动对Cl−的输入有限。由图2可知,Na+、Cl−和TDS含量沿程变化规律相似,CY2、CY4两点质量浓度最高,CY3、CY5和CY6相较于前两点浓度略低,其余几个点位浓度明显低于前五点,CY2、CY3、CY4、CY5和CY6位于人口密集的村庄之内,周边有大量的生产与在建煤炭工厂,均需要外排大量的矿井水,对地表水的水质产生了一定的影响。TDS又称溶解性固体总量,是指水中全部溶质的质量,其值越高,说明水中含有的溶解物越多。根据研究区采样点TDS数据绘制出TDS沿长河流域的含量变化图(图3)。由图3可知,长河流域TDS变化趋势为上游至下游TDS逐渐减小,高值主要集中在CY2与CY4,CY3点较低,通过现场实地调研收集资料,采样点CY2与CY4位于研究区上游,人口密集且有大型煤矿工厂,根据泽州县水务局提供的入河排污口基本情况调查表可知,长河流域中上游区域共有大型煤矿4座,污废水排入量共每年360万t,水质检测数据见表2。污废水的排放使得这个区域TDS量高于其他区域。经过中游到下游河道,河流进入山谷,工厂较少且人口稀疏,因此,TDS量明显下降。
由此可以看出,研究区水质好坏受到人为活动的影响较为突出。由图3可以看出,研究区内pH沿程分布规律,在CY2、CY3、CY4和CY5点,水体pH值较低,CY6点pH值最高,与TDS量沿程分布规律呈相反关系,由此可得出,在煤炭开采富集区域,水体的pH值相对较低,呈现弱碱性,下游煤炭、化工工厂较少,水体pH值则略高。
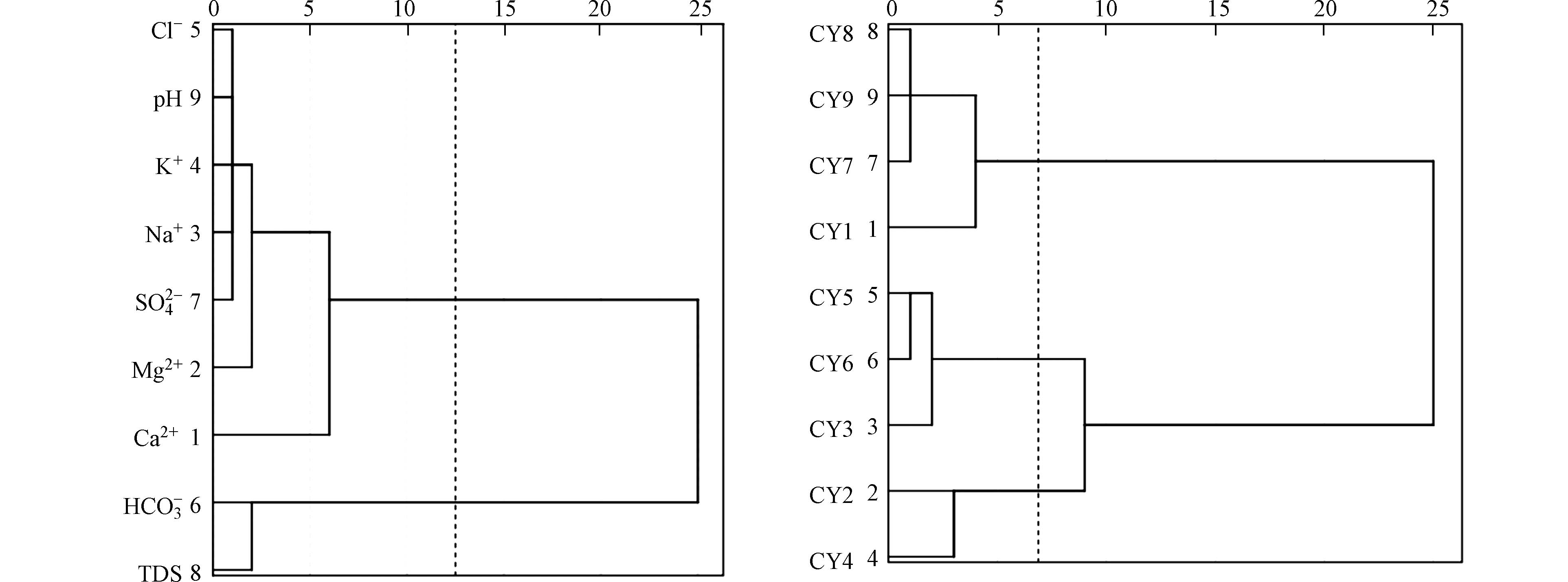
聚类分析是一种多元统计分类的方法,根据不同对象的相似度进行聚合分类。聚类分析分为R型聚类与Q型聚类[23],本文分别采用R与Q型分类对研究区水样进行分析。由图4(a)可知,整体水化学指标可以聚合为3类:第一类为Mg2+、 Na+、K+、Cl−、
SO2−4 和pH,第二类为Ca2+,第三类为HCO−3 与TDS。由图4(b)可知,采样点被聚合为3类:第一类为CY1、CY7、CY8和CY9;第二类为CY3、CY5和CY6;第三类为CY2和CY4。结合研究区采样点布设与相对应离子质量浓度对Q型聚类分析可知,CY1、CY7、CY8和CY9作为研究区的源头与下游入沁口处,水化学离子质量浓度相对较低,被划分为一类;CY3、CY5和CY6作为研究区的中上游,因为煤炭工厂分布较少、人口相对稀疏,离子质量浓度处于中间水平,被划分为一类;而CY2周边有研究区大型矿场成庄煤矿,CY4处于人口最为密集的下村镇,两点质量浓度最高(前文已做分析),因此被划为一类。整个地表水系统大致分为:源头与下游离子浓度最低区域,中、上游离子浓度较高区域和矿区排水离子浓度最高区域。 -
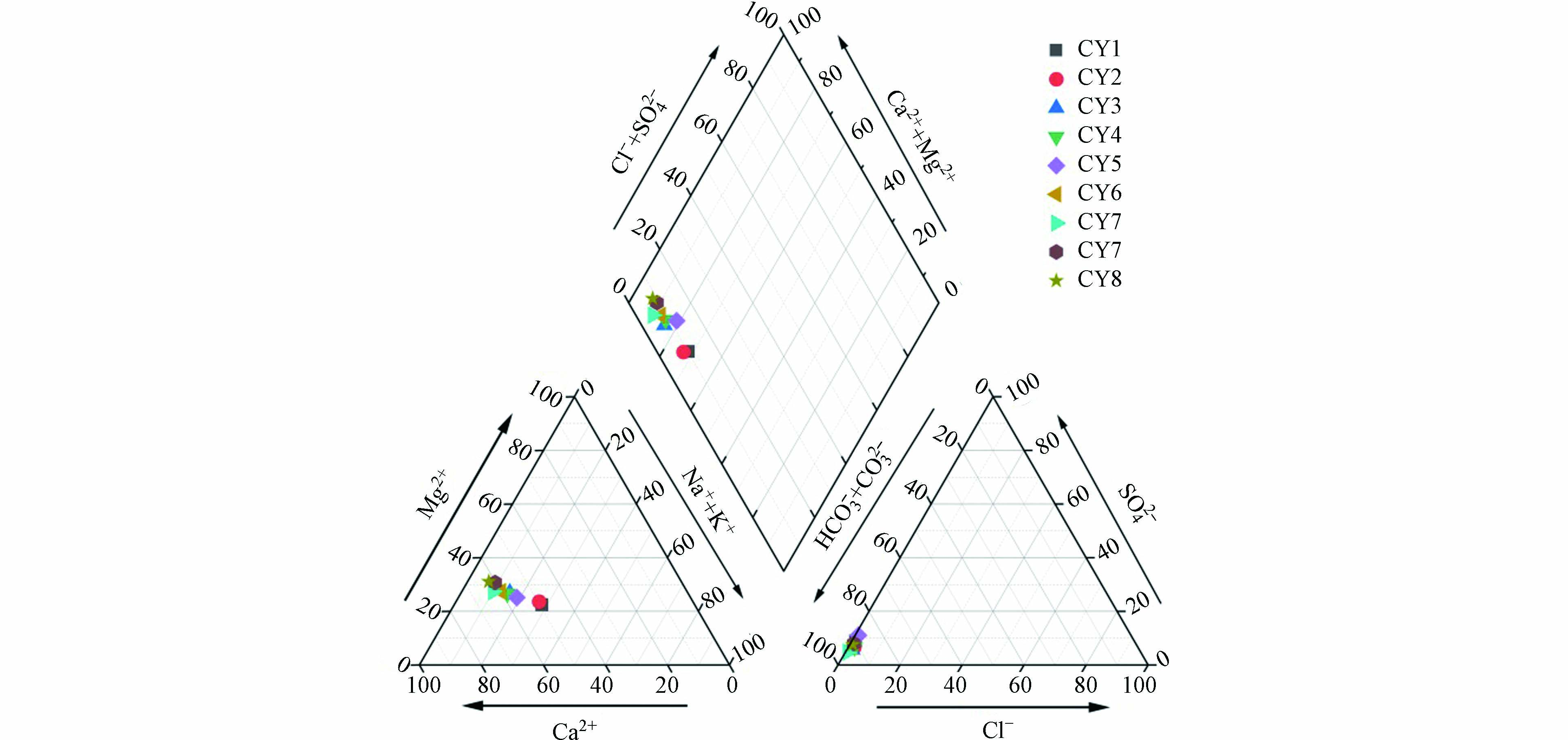
通过piper三线图可以直观地看出地表水的主要离子组成与水化学类型。根据长河流域地表水主要的离子含量绘出piper三线图。由图5可以看出,长河流域地表水样品均靠近
CO2−3 和HCO−3 端元,远离SO2−4 和Cl−端元,阴离子主要以HCO−3 为主;在阳离子三角图中,地表水样品分布靠近Mg2+,Ca2+和Na+含量次之。这些离子可能来源于硅酸盐,碳酸盐以及蒸发岩的风化溶解。所有地表水水样均位于上部菱形的左侧,说明碱土金属超过了碱金属、弱酸超过强酸、次生碱度超过50%。由此分析出,长河流域水化学类型主要是HCO−3 Mg。 -
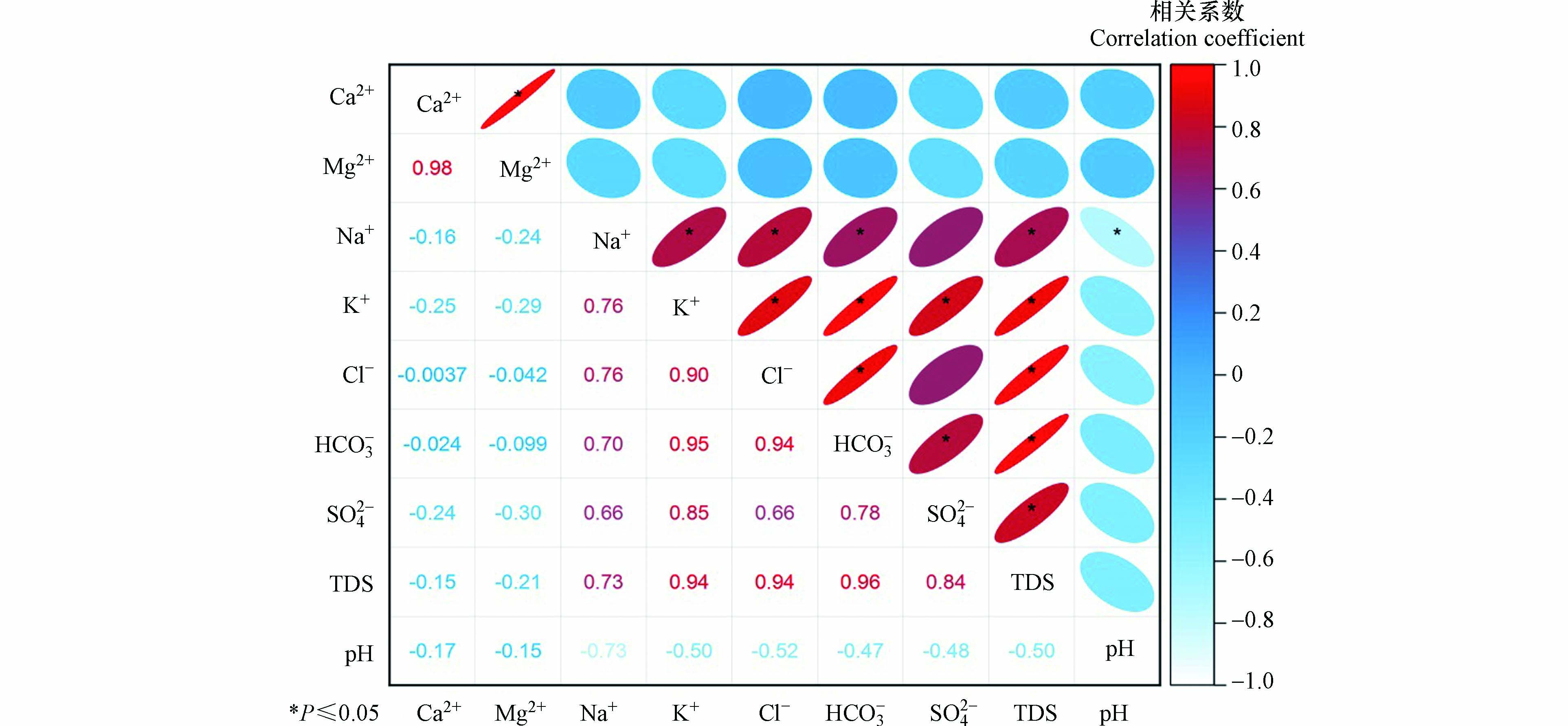
地表水中的各离子指标并不是单独存在的,在一定程度上是存在关系的,通过Pearson相关性分析,初步分析离子的来源。由图6可以看出,TDS与Na+、 K+、
HCO−3 、SO2−4 和Cl−都存在不同程度的相关性,通过临界相关系数可以进一步判断相关程度的大小[24]。临界相关系数见表3。HCO−3 与TDS相关系数为0.96,两者存在极强相关性,可以说明HCO−3 是TDS的主要来源;TDS与Ca2+、Mg2+相关系数分别为-0.15与-0.21,具有负相关性,说明Ca2+、Mg2+两种离子可能发生了化学反应,影响其含量,导致相关性不高;HCO−3 与Na+、Mg2+离子的相关系数为0.70和0.95,存在强相关性和极强相关性,说明这些离子极可能来源于同一物质(碳酸盐);SO2−4 与Ca2+相关系数-0.24,具有负相关性,说明石膏不是SO2−4 的来源,可以反映出研究区采煤外排的矿井水对地表水的影响有限。Na+与Cl−相关系数为0.76,存在强相关性,质量浓度较低,说明Cl−可能来源于工农业污染或者大气降水。 -
研究地表水的影响机制对掌握地表水的水化学成因有着非常重要的影响。研究区地表水水化学影响因素大致分为3种:岩石风化、溶滤作用及离子交换。
-
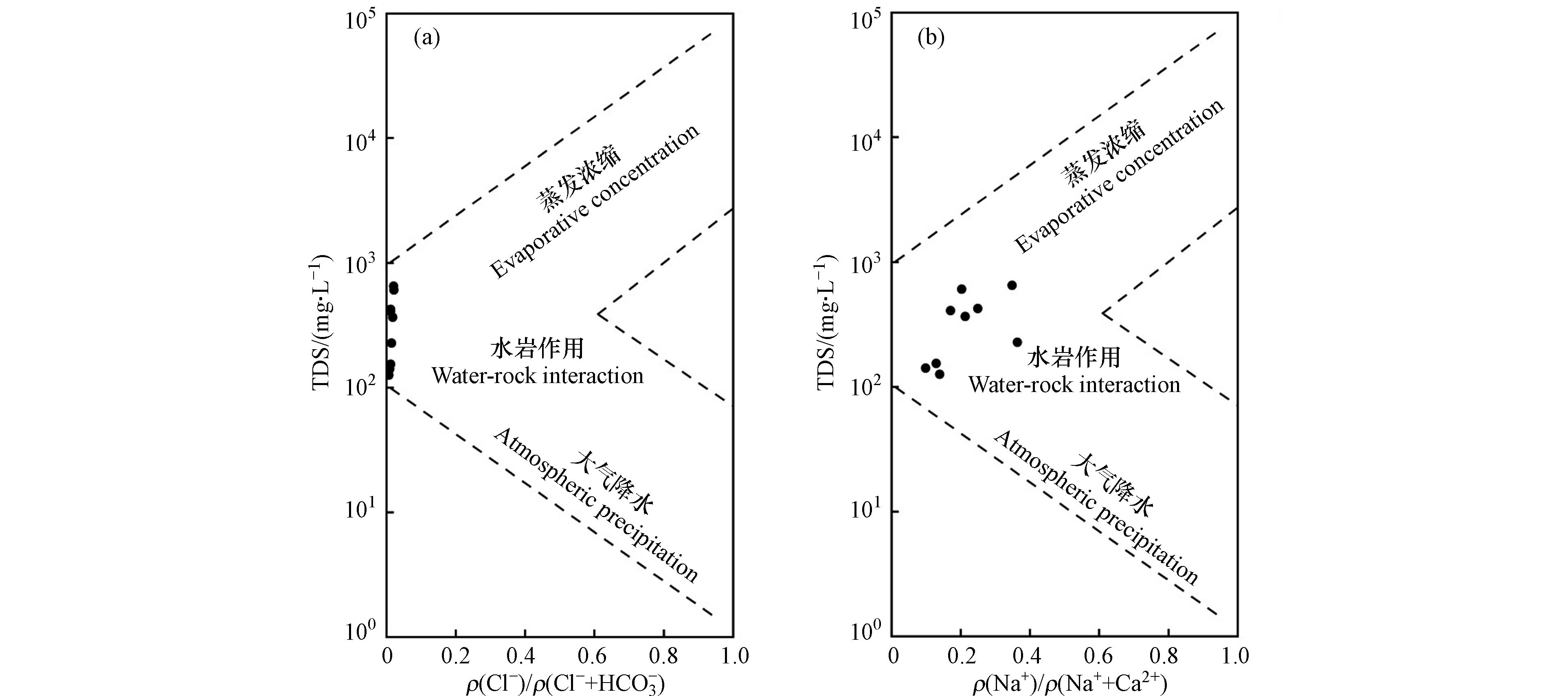
地表水在常年流动的过程中,与河床的矿物质发生反应,同时也有降雨和蒸发作用的影响。Gibbs图通过半对数坐标图直观地表示了地表水的化学组分特征、控制因素和相互关系[14]。通过Gibbs图对长河流域地表水样品进行分析(图7),研究区地表水水样的TDS均低于1000 mg·L−1,ρ(Na+)/ ρ(Na++ Ca2+)比值范围在0.1—0.5之间,ρ(Cl−)/ ρ(Cl−+
HCO−3 )比值均小于0.1,沿着地表水流动的方向,ρ(Na+)/ρ(Na++Ca2+)的比值逐渐降低,所有地表水水样均处于岩石风化控制区域,可以说明研究区地表水的形成机制主要是岩石风化,而蒸发结晶与大气降水的贡献较小。 -
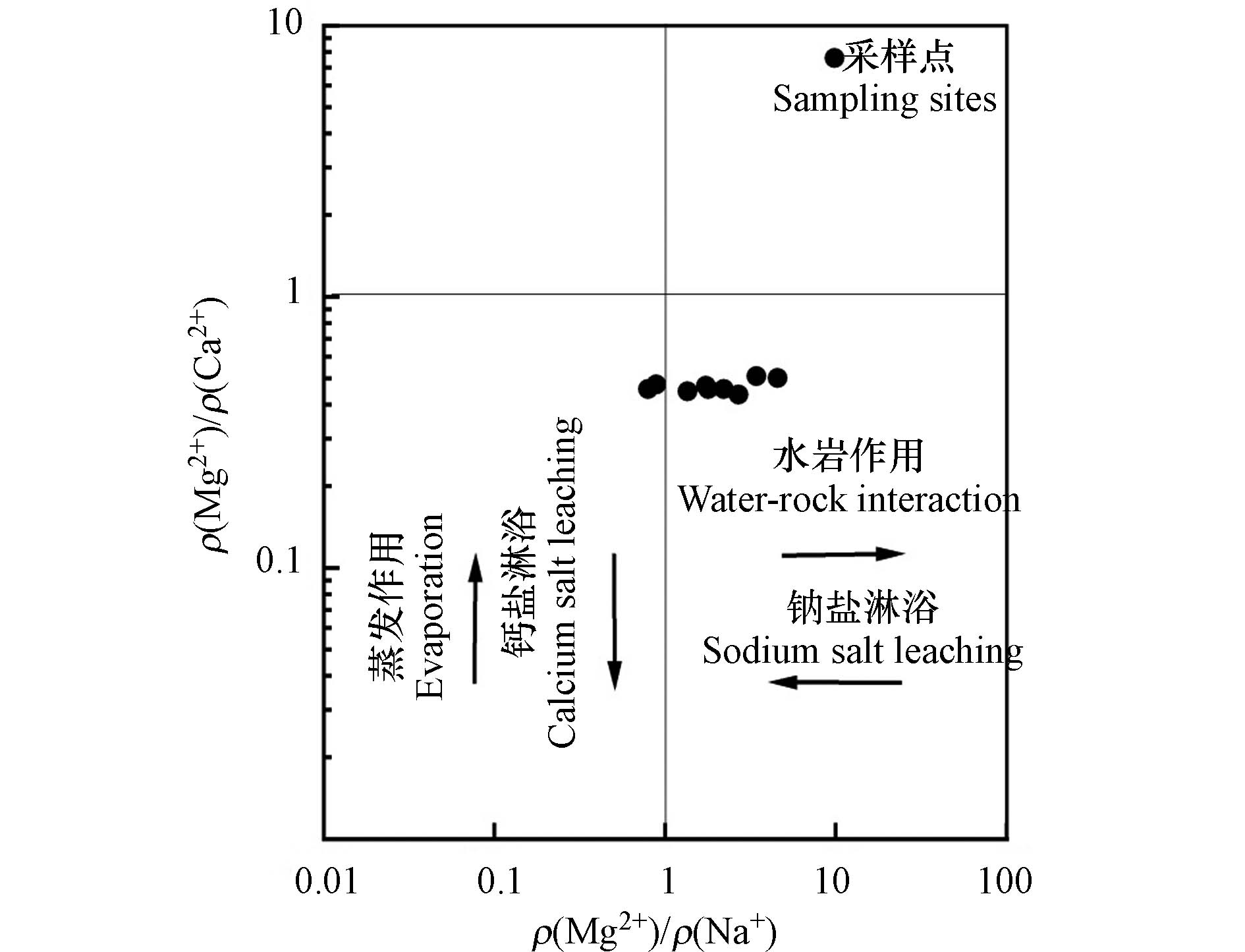
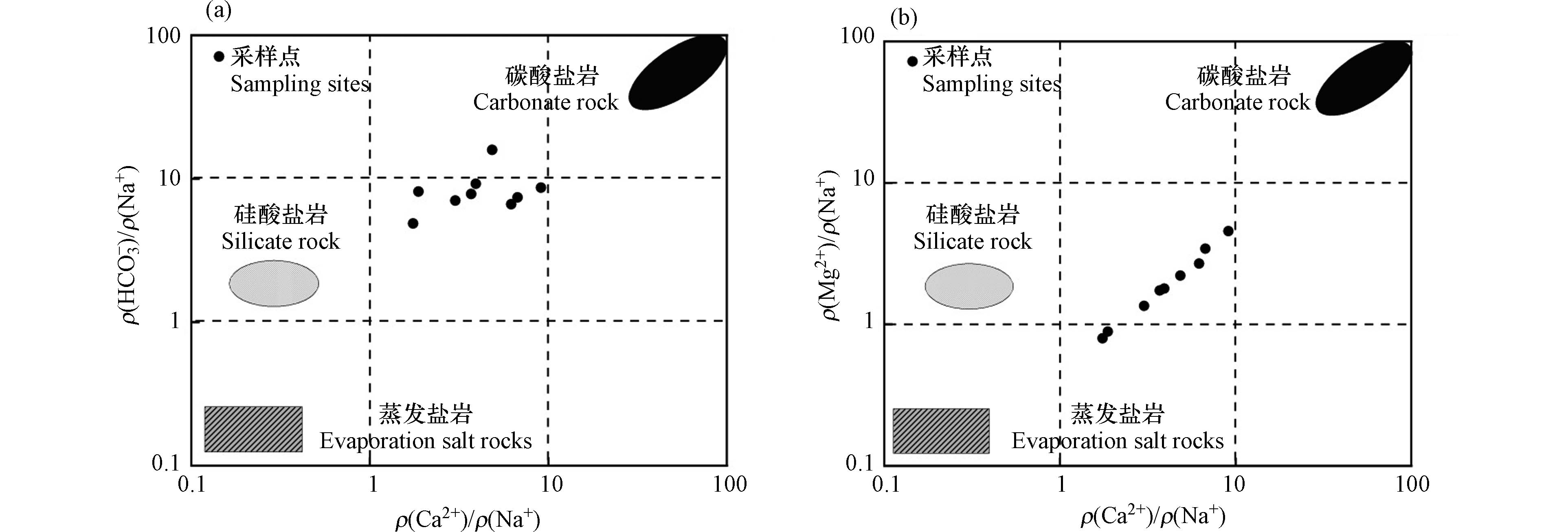
通过分析Mg2+与Ca2+、Na+等离子之间的比例关系,可以反映出地表水的形成作用[25]。长河流域地表水ρ(Mg2+)/ ρ(Na+)比值多数大于1,ρ(Mg2+)/ ρ(Ca2+)比值都小于1(图8),比值说明长河流域地表水受到水岩与钙盐的淋滤共同作用。
-
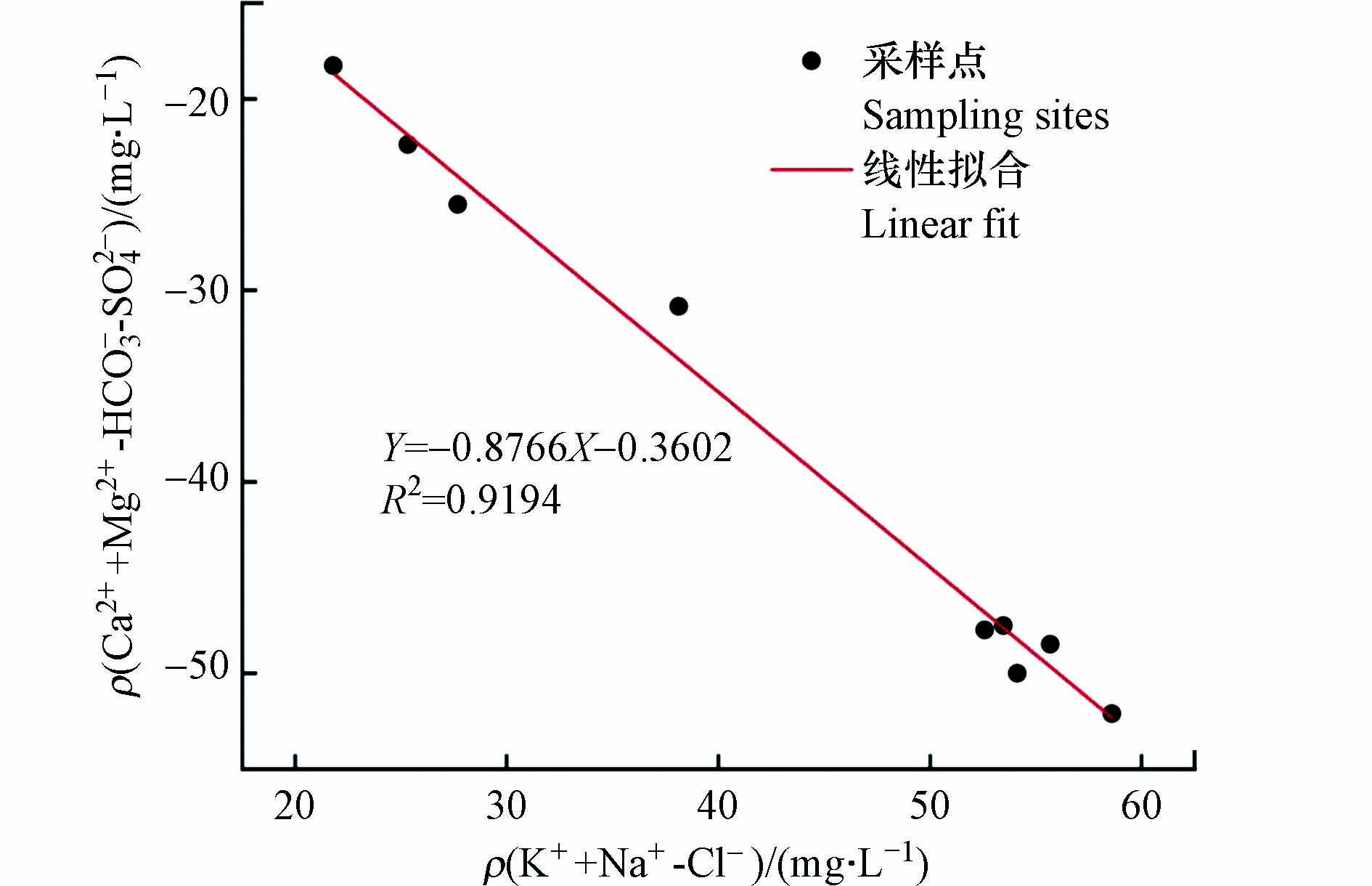
通常情况下,阳离子交换作用会使地表水中一些主要的离子发生变化,也是地表水水化学形成的重要作用[26]。ρ(K++Na++Cl−)/ ρ(Ca2++Mg2+-
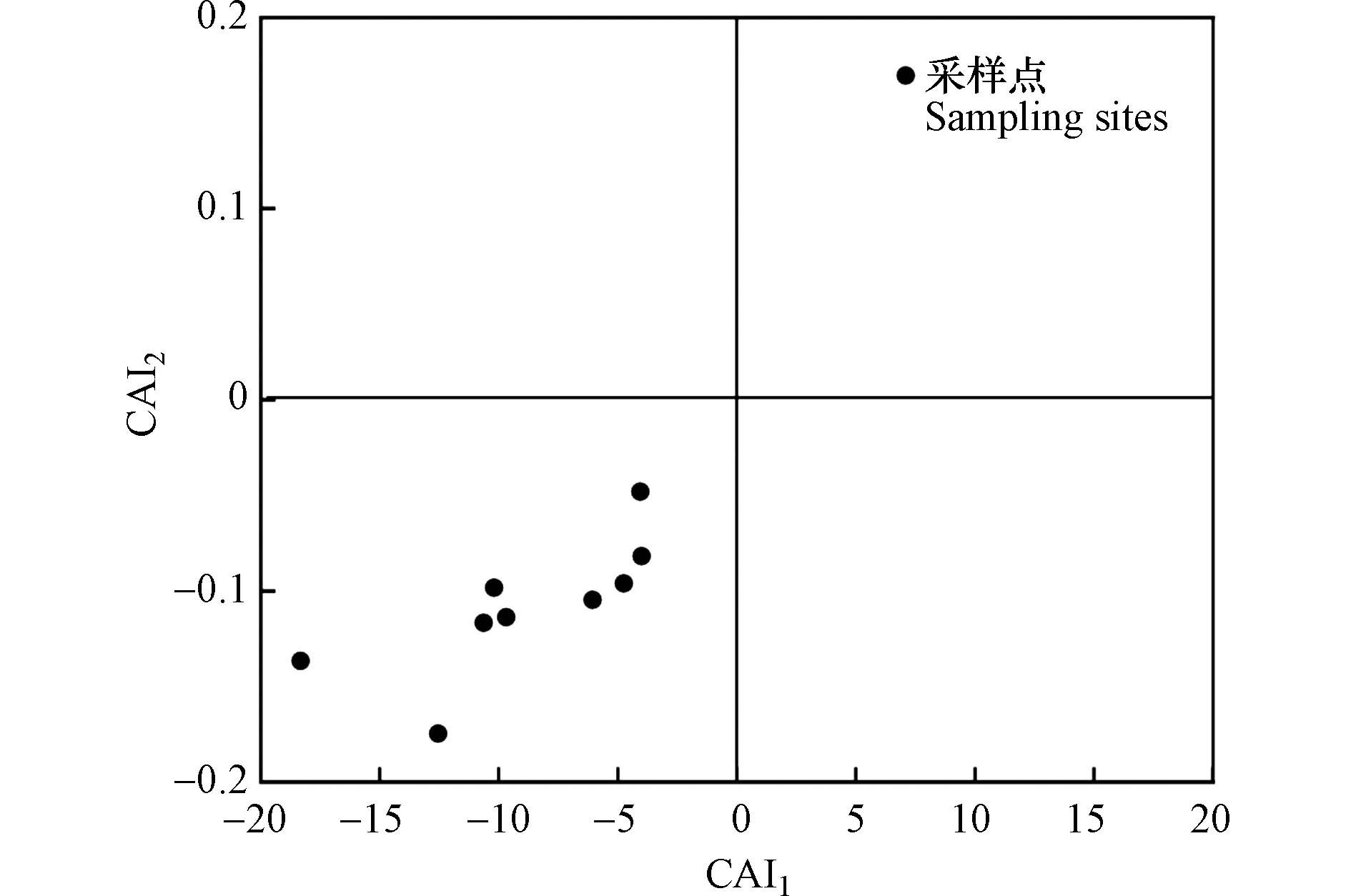
HCO−3 -SO2−4 )的比值可以确定地表水中是否发生了阳离子交换,如若发生了离子交换,则两者拟合后斜率应当为−1。由图9可知,长河流域地表水样品间相关关系为Y=−0.8786X-0.3602,R2=0.9194,更加进一步说明了地表水发生了阳离子交换,而多余的Na+是通过河水中Ca2+与Mg2+交换而得来。Schoeller[27]提出“氯碱指数”CAI1和CAI2来表示阳离子的交换作用及强度。当河水中的Na+、K+交换河床矿物中吸附态的Ca2+、Mg2+时,CAI1与CAI2值为正;反之,当河水中的Ca2+、Mg2+交换河床矿物中吸附态的Na+、K+时,CAI1与CAI2值为负;且阳离子交换作用越强,CAI1与CAI2的绝对值越大。由图10可以看出,CAI1与CAI2均为负值,研究区地表水发生了反阳离子作用,河水中的Ca2+、Mg2+交换河床矿物中吸附态的Na+、K+,也印证了地表水中Na+值远大于Cl−值的原因,与之前的分析一致。
-
从上述讨论可知,长河流域地表水主要受岩石风化作用控制,且主要以水岩与钙盐的淋滤为主,本文引用Gaillardet模型来判断控制水体水化学组成的岩石风化源类型。
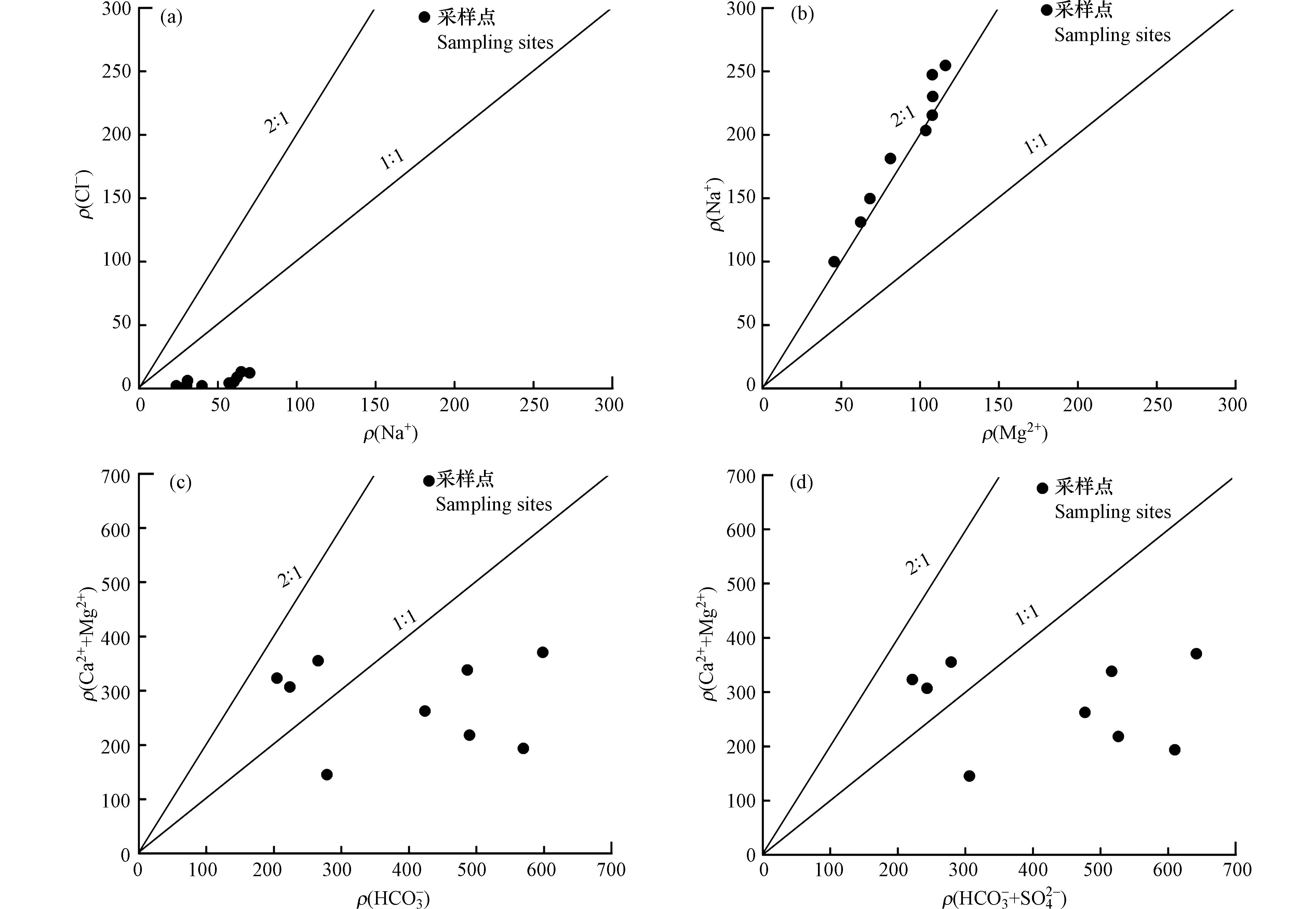
从研究区蒸发岩、硅酸盐和碳酸盐的Ca2+/ Na+与
HCO−3 / Na+、Mg2+/ Na+浓度关系可以看出(图11),地表水样主要集中在硅酸盐岩与碳酸盐岩之间,更靠近硅酸盐岩端元,说明地表水受到硅酸盐岩与碳酸盐岩共同风化溶解的影响,且硅酸盐岩的风化溶解贡献更大。一般情况下,河水中的Na+与Cl−离子主要来源于大气降水、硅酸盐矿物和蒸发岩矿物的溶解,大气降水中的Na+/ Cl−值约为0.86[28-32]。研究区地处内陆,远离海洋,Na+/ Cl−的比值大小会受到大气降水的影响。由图12(a)可知,研究区地表水水样ρ(Na+)/ ρ(Cl−)均大于1,高于大气降水中ρ(Na+)/ ρ(Cl−)的比值,说明大气降水并非研究区地表水中Na+离子的全部来源,Cl−不足以平衡Na+,而多出来的Na+主要来自含钠硅酸盐的风化溶解。水体中ρ(Ca2+)/ ρ(Mg2+)的比值可以判别离子是否来源于白云石、方解石和硅酸盐,若比值等于1,则离子主要来源于白云石的风化溶解;若比值在1—2之间,则来源于方解石的风化溶解;若比值大于2,则离子来源于硅酸盐的风化溶解[33]。由(图12b)可知,研究区ρ(Ca2+)/ ρ(Mg2+)90%的比值基本大于2,则可以说明离子主要来源于硅酸盐的风化溶解。在碳酸盐的溶解中(图12c),研究区ρ(Ca2++Mg2+)/ρ(
HCO−3 )70%的比值大于1,说明多出来的Ca2+和Mg2+来源于碳酸盐的风化,也有一部分来源于阳离子的交换作用。当ρ(Ca2++Mg2+)/ρ(
HCO−3 +SO2−4 )比值等于1时,说明水体中的HCO−3 、SO2−4 、Ca2+和Mg2+都来源于白云石、方解石和石膏的风化溶解,研究区离子比值70%大于1(图12d),可以说明石膏并不是这些离子的主要来源。 -
(1)研究区地表水pH值为7.43—8.17,呈碱性水,水体中阳离子以Ca2+和Mg2+为主,阴离子主要是
HCO−3 ,水化学类型为HCO−3 - Mg2+;TDS随水体流动由上游至下游含量逐渐减小;通过R型聚类分析将水化学指标分为三大类,Q型聚类分析则将采样点分为四大类。(2)TDS与Na+、 K+、
HCO−3 、SO2−4 和Cl−都存在显著的正相关性,HCO3−与TDS相关系数达到0.96,是TDS的主要来源;HCO−3 与Na+、 K+离子相关,离子来源于碳酸盐;SO2−4 与Ca2+不相关,石膏不是SO2−4 的主要来源,SO2−4 来源于外界。(3)通过Gibbs图分析,不同水体中水化学组成主要是受岩石风化作用的控制,引用Gaillardet模型进一步得出多数离子是由硅酸盐岩与碳酸盐岩共同风化溶解作用,少数阳离子是由于离子交换得来,地表水局部河段受到了人类活动影响。
长河流域矿区地表水水化学特征及驱动因子分析
Hydrochemical characteristics and driving factors of surface water in the mining area of Changhe River Basin
-
摘要: 为了研究晋城市长河流域采煤区地表水水质情况、查清其水化学特征,明晰其影响因素及主要离子来源。现场采集了地表水水样9组,采用数理统计方法分析水化学特征,运用Piper三线图分析水化学类型,通过Gibbs图和离子相关分析等方法探讨了地表水主要离子的来源及其影响因素。结果表明,研究区地表水TDS为126—604 mg·L−1,平均值为344.11 mg·L−1;pH值为7.43—8.17,平均值为7.79,属于弱碱性水。地表水阳离子以Ca2+和Mg2+为主,阴离子主要是
HCO−3 ,水化学类型为HCO−3 Mg。通过主要离子的相关性分析可知,TDS与Na+、 K+、HCO−3 、SO2−4 和Cl−都存在显著的正相关性,这些离子对TDS都有贡献。HCO−3 与Na+相关,与K+存在显著相关性,可能来源于含钠或钾硅酸盐。通过水岩模型分析可知,研究区地表水水化学成因主要受岩石风化作用控制,多数离子是由硅酸盐岩与碳酸盐岩风化溶解作用,少数阳离子受到水体离子交换作用影响。相关的研究成果可为研究区水资源规划配置提供科学的参考意见。Abstract: In order to study the water quality of the surface water in the Changhe coal mining area of Jincheng City, find out its hydrochemical characteristics, and clarify its influencing factors and main ion sources. Nine groups of surface water samples were collected on site, and the hydrochemical characteristics were analyzed by mathematical statistics, the hydrochemical types were analyzed by Piper trigraph, and the sources and influencing factors of main ions in surface water were discussed by Gibbs graph and ion correlation analysis. The results showed that : TDS of surface water in the study area was 126—604 mg·L−1, with an average value of 344.11 mg·L−1. The pH value is 7.43—8.17, and the average value is 7.79, which belongs to weakly alkaline water. The main cations in surface water are Ca2+ and Mg2+, the anions are mainlyHCO−3 , and the hydrochemical type isHCO−3 Mg.The correlation analysis of major ions shows that TDS is significantly positively correlated with Na+, K+,HCO−3 ,SO2−4 and Cl-, and these ions all contribute to TDS.HCO−3 is associated with Na+ and significantly associated with K+, and may be derived from sodium or potassium silicates. Through the analysis of the water-rock model, it can be seen that the hydrochemical genesis of the surface water in the study area is mainly controlled by rock weathering, most of the ions are dissolved by the weathering of silicate rock and carbonate rock, and a few cations are affected by water ion exchange. Relevant research results can provide scientific references for water resources planning and allocation in the research area. -
2018年,我国铁尾矿产生量为4.76×108 t,约占全国尾矿产生量的40%。相比较其他种类尾矿,铁尾矿占比最大[1]。而目前铁尾矿的综合利用率却不足30%,远低于发达国家[2]。在我国,铁尾矿主要储存方式为露天堆放,这样不仅有溃坝的风险[3],而且还可能会对周边水体造成污染[4-5]。因此,铁尾矿堆放处理对环境造成的污染问题不容小觑,亟需解决。
铁尾矿对环境造成危害的同时也是一种“放错地方的资源”。铁尾矿中含有大量的SiO2、Al2O3、Fe2O3,这与黏土的成分十分相似,可以代替黏土用作烧结砖的原料。已有大量研究[6-10]证实了铁尾矿制备烧结砖的可行性。严捍东等[8]分别利用铁尾矿、粉煤灰和海泥制备出多孔烧结砖;其结果表明,与粉煤灰相比,铁尾矿更利于减缓烧结砖的泛霜程度。LUO等[9]利用铁尾矿、煤矸石等作为主要原料,并用污泥和页岩作为黏结剂制备烧结砖;结果表明,在烧结温度1 100 ℃、烧结时间3 h的最佳条件下,烧结砖的抗压强度为14.24 MPa、吸水率为17.47%。有研究结果[11-12]表明,将废玻璃加入到烧结砖中会极大地提高黏土烧结砖的力学强度。VORRADA等[11]发现,向黏土砖中添加废弃玻璃,在一定范围内会提高烧结砖的力学强度;在温度为1 100 ℃、玻璃添加量为15%的条件下,会提高2~3倍的力学强度,吸水率可降低至2%~3%。这些研究多数关注在烧结砖力学性能的研究上,而忽略了烧结砖中重金属释放到环境中的潜在风险,特别是对于含有较高重金属的铁尾矿。重金属在烧结砖中的形态分布和浸出特性的问题值得关注。
本研究中,利用铁尾矿、废玻璃和粉煤灰制备烧结砖,以探究其可行性。研究不同温度和配比条件对烧结砖性能的影响和重金属的浸出风险;并研究了在烧结过程中的重金属形态分布、孔径分析、XRD和SEM,以揭示烧结砖的固化机理。本研究结果可为铁尾矿的资源化利用提供数据参考。
1. 材料和方法
1.1 实验原料
供试铁尾矿来自广东省大宝山槽对坑尾矿库,铁尾矿样品置于烘箱中105 ℃烘干,利用行星式球磨机(QM-3SP2,南京南大仪器有限公司)进行球磨,过200目标准筛后储存于密封袋中,置于干燥器中储存。供试废玻璃为高硼硅玻璃,利用球磨机进行球磨,过200目标准筛后储存于密封袋中,置于干燥器中储存。供试粉煤灰来自广东省某燃煤发电厂,粉煤灰样品在烘箱中105 ℃烘干至恒重后,置于干燥器中储存。
1.2 烧结砖的制备
将铁尾矿、粉煤灰和废玻璃按照表1的比例进行充分混合,加入去离子水使得胚料含水率为10%。将胚料放在钢模具内,利用压片机使胚料成型,成型压力为15 MPa。成型后的砖胚置于105 ℃烘箱中,干燥24 h;然后将砖胚置于马弗炉中,设定升温速率为5 ℃·min−1,升温至特定温度(900、1 000、1 100、1 200 ℃),保温时间为2 h。
表 1 烧结砖原料质量分数Table 1. Mass fraction of raw materials to sintered bricks% 样品编号 铁尾矿 粉煤灰 废玻璃 G0 60 40 0 G10 60 30 10 G20 60 20 20 G30 60 10 30 1.3 吸水率实验
将烧结砖样品浸泡在超纯水中,浸泡24 h后取出,用滤纸擦干样品表面,称量样品吸水后的质量,烧结砖样品的吸水率按照式(1)计算。
w=(m2−m1m1)×100% (1) 式中:w是烧结砖的吸水率;m1是烧结砖未浸泡前的质量,g;m2是烧结砖浸泡后取出并擦干的质量,g。
1.4 测定方法
1)采用X射线荧光光谱(XRF)(EDX-7000,日本岛津公司)测定原料中的主要化学成分。
2)原料重金属含量的测定。取0.1 g样品于消解罐中,加入40%氢氟酸2 mL、35%浓盐酸2 mL和65%硝酸6 mL,于微波消解仪中进行消解;消解完成后赶酸,用超纯水稀释至25 mL,利用火焰原子吸收分光光度计(TAS-990F,北京普析通用仪器有限公司)测定总的重金属含量。
3)物相结构分析。采用X射线衍射仪(XRD)(Bruker D8,德国布鲁克公司)进行物相结构分析;扫描角度为10°~80°、步长为0.02°、计数时间为0.03 s。
4)抗压强度。采用万能材料试验机(Instron 5697,美国Instron)测试样品的抗压强度,以式(2)计算烧结砖的抗压强度。
p=FA (2) 式中:p是烧结砖的抗压强度,MPa;F是测量受力面所承受最大的压力,N;A是受力面的面积,mm2。
5)重金属形态分析。烧结砖中的重金属形态分析采用欧共体标准物质局的BCR顺序提取法[13],分为4个形态:弱酸提取态、可还原态、可氧化态、残渣态。
6)孔径分析。采用全自动比表面及孔隙度分析仪(TriStar II 3flex,美国麦克仪器公司)进行孔径分析;以N2作为吸附气体,脱气温度为150 ℃、脱气时间为2 h,采用BJH分析模型处理数据。
7)扫描电镜分析。采用电子扫描电镜(Sigma 300,德国蔡司仪器公司)观察样品微观形貌;利用X射线能谱仪(XFlash6,德国布鲁克公司)做EDS分析。
8)重金属浸出浓度测定。烧结砖重金属浸出浓度通过《固体废物浸出毒性浸出方法-醋酸缓冲溶液法》(HJ/T 300-2007)[14]进行;在样品管中加入1 g样品和20 mL醋酸缓冲溶液,放入翻转式振荡器中以30 r·min−1的转速翻转18 h;翻转完成后,再通过0.45 μm滤膜过滤,用火焰原子吸收分光光度计测定样品浸出液中重金属(Cu、Pb、Zn)的浓度。
2. 结果与讨论
2.1 供试原料制砖可行性
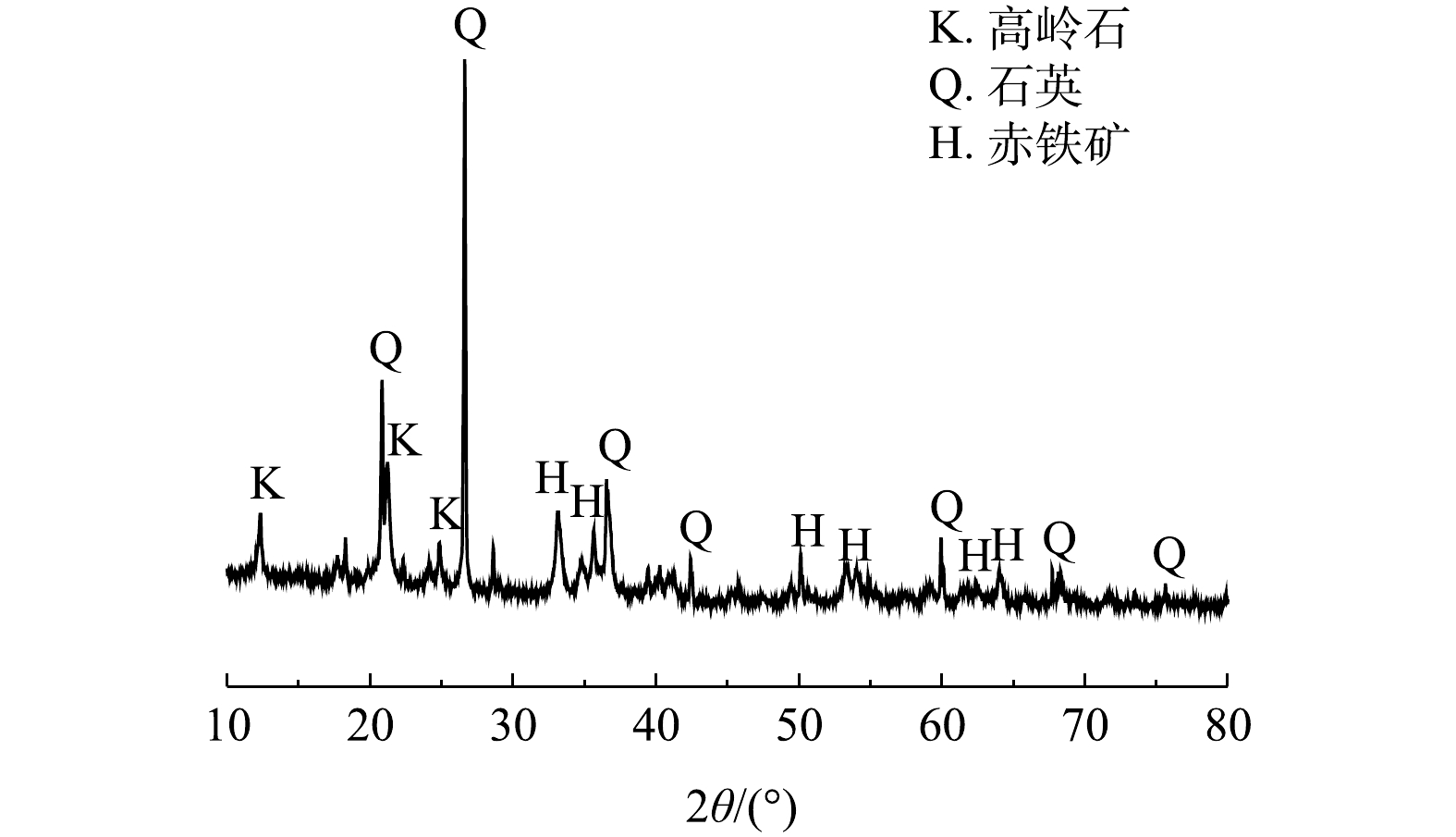
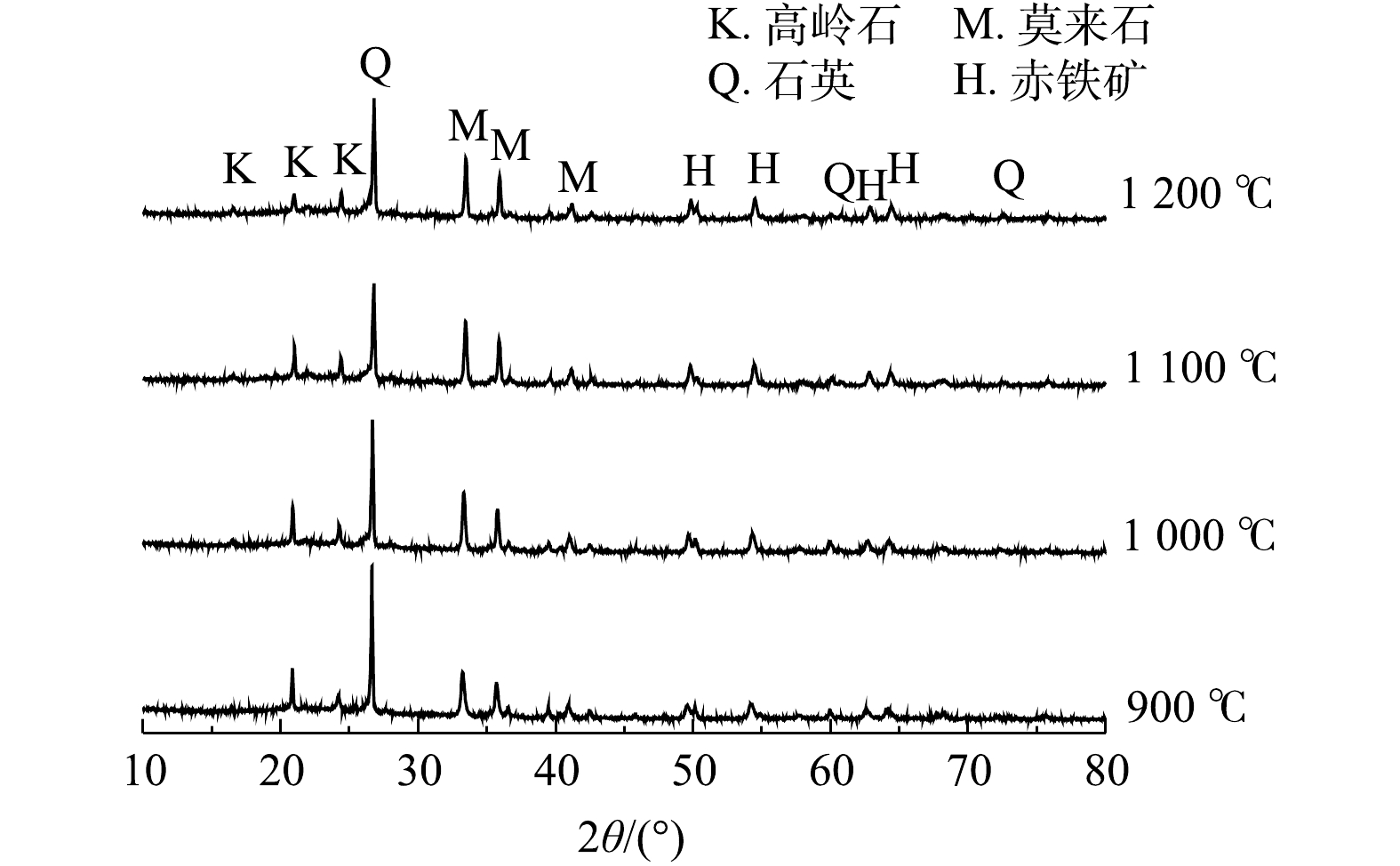
供试铁尾矿外观呈红色的颗粒状,平均粒径为189.24 μm,粒径较小。表2为铁尾矿、粉煤灰、废玻璃的化学组成。通过表2可知,铁尾矿主要化学成分有Fe2O3、SiO2、Al2O3,这些成分的含量与国内外其地区铁尾矿的成分相似[15]。CHEN等[16]指出,铁尾矿中的Fe2O3成分在制备烧结砖的过程中有促进烧结的作用,可以降低烧结砖的烧成温度。粉煤灰中含有大量的SiO2、Al2O3硅铝氧化物,可弥补铁尾矿低硅、低铝的缺点;粉煤灰还含有少部分K2O、Na2O等碱性金属氧化物,因而有助熔的作用。废玻璃的主要成分为SiO2和Na2O,在体系中可以起到黏结作用。另外,从铁尾矿的XRD分析图谱(图1)可以看出,铁尾矿中的主要矿物组成为石英、赤铁矿和高岭石。
表 2 原料化学组成(以质量分数计)Table 2. Chemical composition of raw materials (calculated by mass fraction)% 供试原料 Fe2O3 SiO2 Al2O3 K2O Na2O 其他 铁尾矿 53.78 28.13 13.33 0.86 — 3.90 粉煤灰 6.36 57.01 25.33 2.93 0.88 7.49 废玻璃 0.17 91.70 2.35 0.12 5.12 0.54 注:—为未检出。 从表3可以看出,铁尾矿中重金属种类较多,含量差异较大,是典型的多金属伴生尾矿。其中,Cu、Zn、Pb的含量较高,长期露天堆放会对环境造成隐患。
表 3 铁尾矿中重金属含量Table 3. Heavy metal concentration in iron tailingsmg·kg−1 Cu Pb Zn Cr Cd 2 814.92 1 992.12 1 712.33 63.26 10.84 通过对3种制砖原料理化性质的分析可初步判断,铁尾矿结合粉煤灰、废玻璃制备烧结砖是具有可行性的。
2.2 烧结砖的基本性能
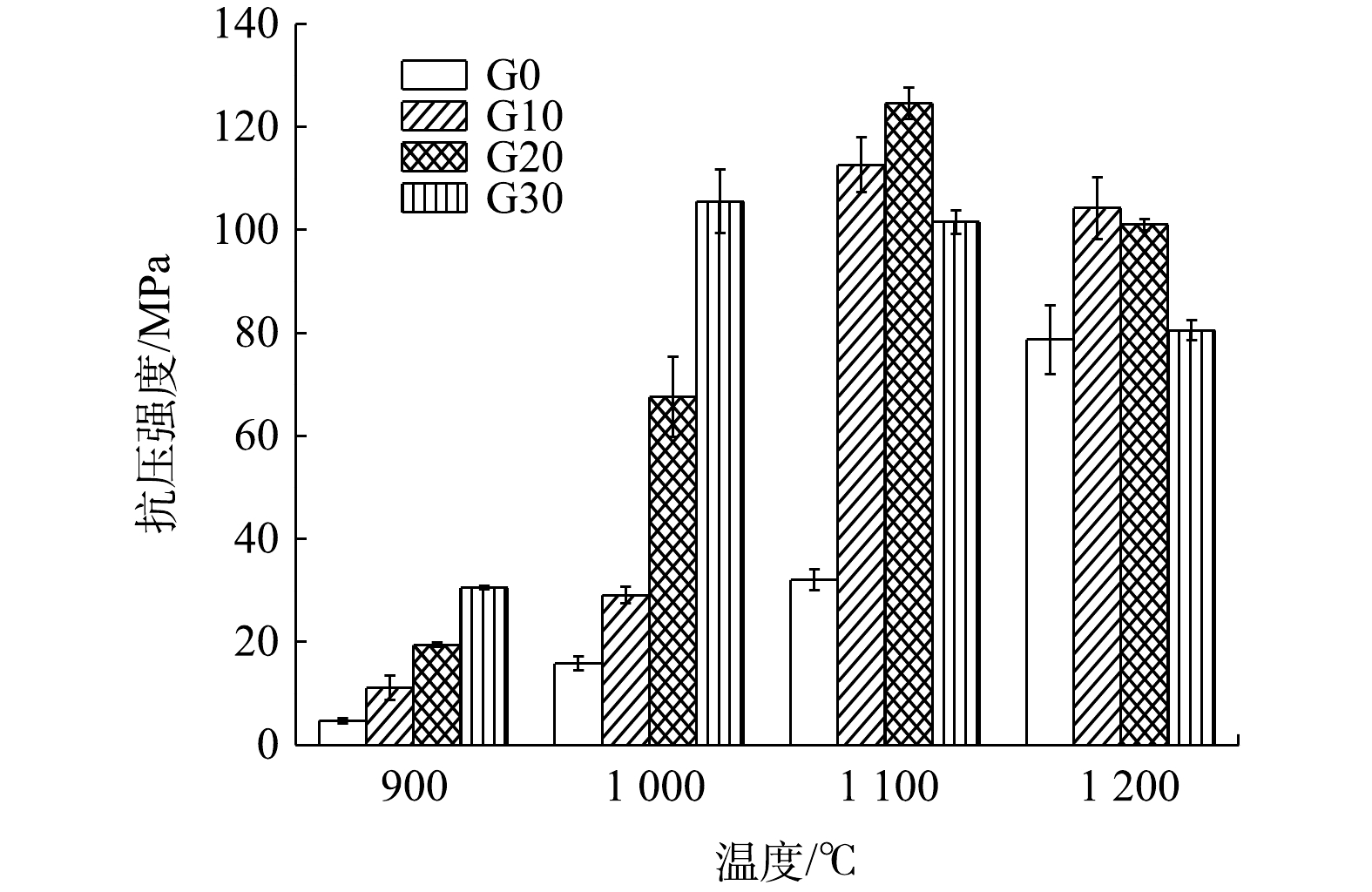
1)烧结砖的抗压强度。抗压强度测试是重要的力学强度指标,可以反映烧结砖承受压缩载荷的能力。图2结果表明,在900~1 100 ℃的条件下,各配比烧结砖的抗压强度均随着烧结温度的上升而增加。在900和1 000 ℃条件下,废玻璃含量对烧结砖抗压强度的影响较为明显。其原因在于,在温度低于1 000 ℃时,铁尾矿与粉煤灰间未发生反应,矿物骨架未形成,导致砖体结构松散[17]。但在此条件下,废玻璃已经达到软化温度,以流动相的形式存在于胚料中,冷却后将铁尾矿和粉煤灰胶结在一起。因此,温度低于1 000 ℃条件下烧结砖主要的力学强度都由玻璃提供[18]。随着烧结温度继续上升至1 000~1 100 ℃,铁尾矿和粉煤灰开始发生反应,烧结砖中的骨架结构形成;废玻璃进一步填充了骨架结构中的孔隙,并进一步提高了烧结砖的力学强度。此条件下所制备烧结砖的力学强度由矿物骨架和废玻璃共同提供。而在1 200 ℃的条件下,烧结砖出现过烧现象[19],呈现较深的红色。此时,砖胚出现膨胀、变形现象,抗压强度出现下降趋势。这是因为,过高的温度破坏了烧结砖的结构。在烧结温度过高或保温时间过长的条件下,烧结砖会形成大量的熔融体,烧结砖中的骨架结构被破坏,导致冷却后出现膨胀、变形的情况,从而降低了砖体的抗压强度。
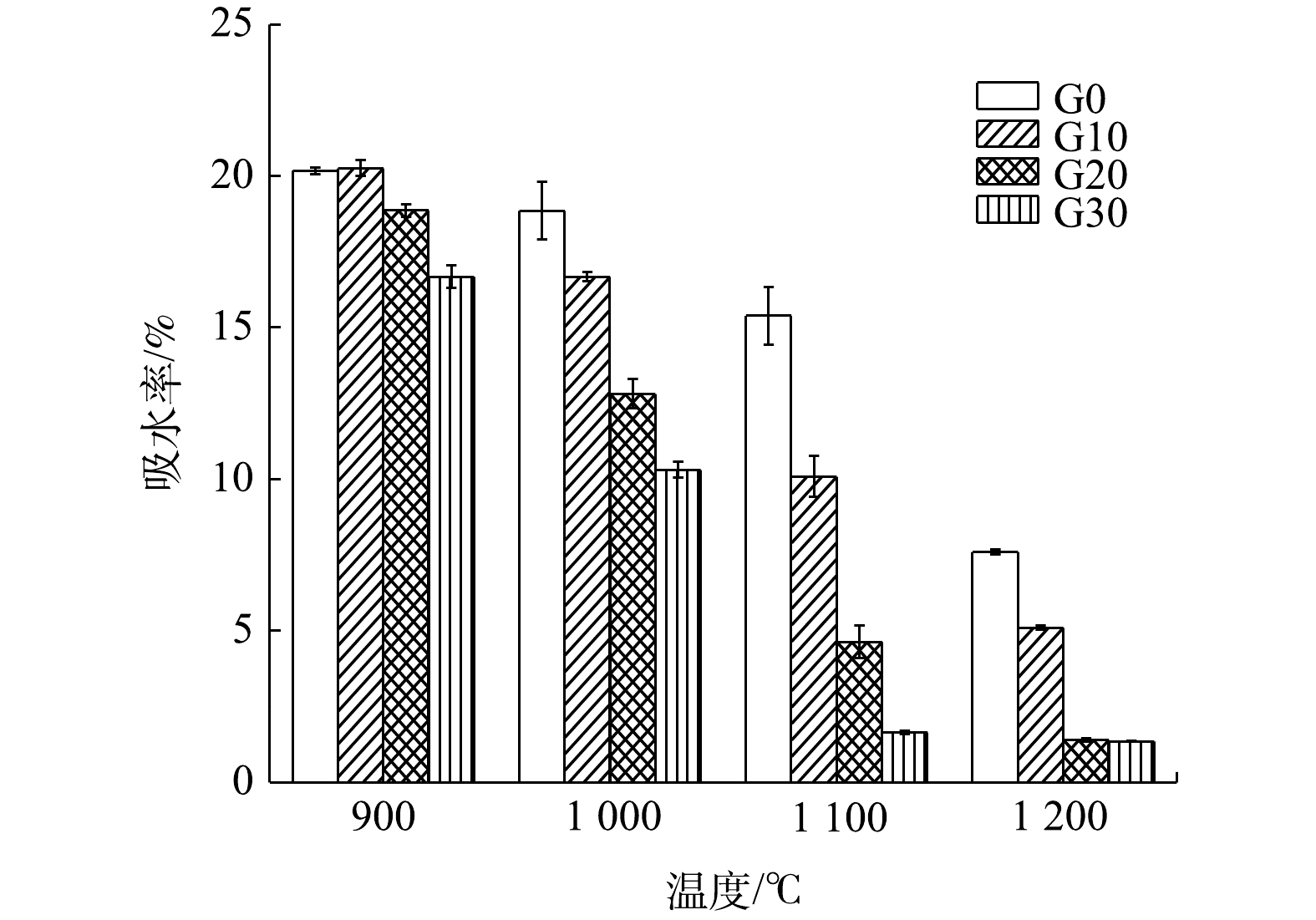
2)烧结砖的吸水率。吸水率可反映烧结砖的内部结构情况。从图3可以看出,在相同配比情况下,烧结砖的吸水率随着烧结温度上升而降低。在900 ℃下,各配比烧结砖的吸水率均在15%以上;而随着烧结温度上升至1 200 ℃,吸水率最低为1.3%。这说明,升高温度有助于烧结砖内部的致密化。在烧结过程中,烧结砖内部的气孔逐渐缩小,内部结构趋于致密化。在相同的烧结温度条件下,吸水率会随着废玻璃含量的增加而降低。
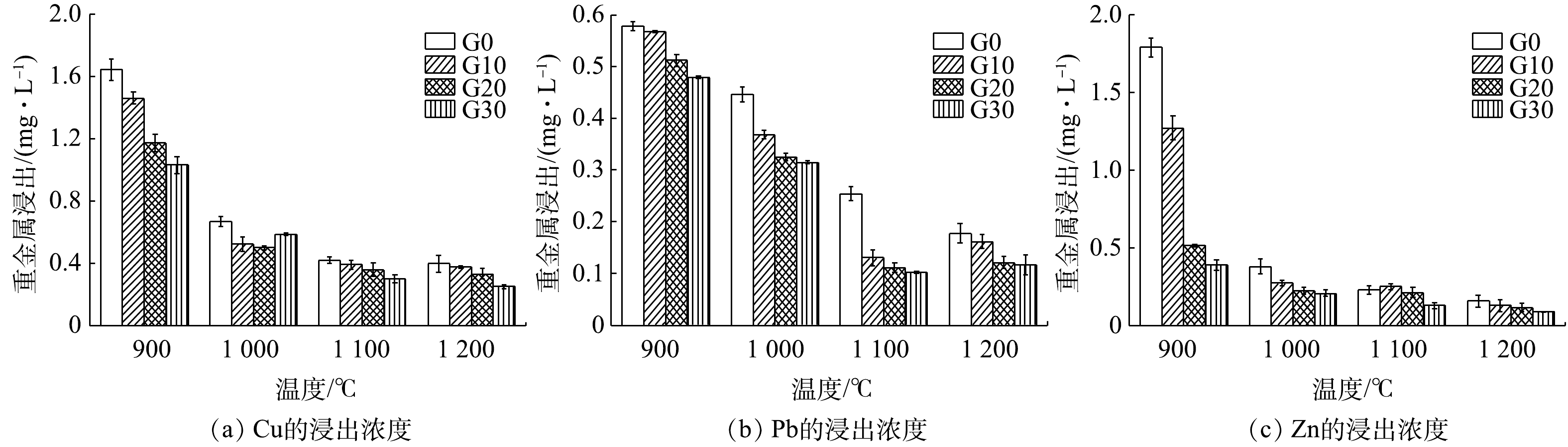
3)烧结砖的重金属浸出毒性。从图4中可以看出,烧结温度在900~1 000 ℃时,烧结砖中Cu、Pb、Zn的浸出浓度会随着废玻璃含量的增加而明显下降。MAO等[20]发现,玻璃在高温条件下的成型过程中会把重金属离子有效地包裹起来,形成玻璃体。当温度到达1 100~1 200 ℃,玻璃含量对重金属浸出的影响极小。这是因为,此时重金属间发生反应主要以稳定的结晶相形式存在,只有较少的重金属以不稳定的形式存在。
Cu、Pb、Zn的浸出浓度均随着烧结温度的上升而减小,最终在1 100 ℃趋于稳定。从图4(a)和(c)中可以看出,Cu和Zn在浸出特征上出现相似的规律;在温度为900 ℃时,重金属浸出浓度都较高,最高可达1.5 mg·L−1。在温度为900~1 000 ℃,3种重金属的浸出浓度下降均超过50%。有研究者[21]发现,Zn和Fe2O3在热反应过程中,可能会形成尖晶石结构ZnFe2O4,从而增加Zn的稳定性,不易被浸出。从图4(b)可以看出,Pb的浸出浓度随着温度的上升而下降。当温度为900时,Pb浸出浓度不足0.6 mg·L−1,远低于Cu和Zn的浸出浓度。这说明,当温度低于900 ℃的条件下,Pb比Cu和Zn更早地发生了固化反应。PbO在700 ℃的条件下,会与Al2O3发生化学反应,生成抗酸蚀能力强的PbAl2O4,从而抵制外界的酸性环境[22]。烧结砖中Cu、Pb、Zn的浸出浓度随烧结温度的升高均呈现降低的趋势。这说明,烧结过程对重金属的固化起到了积极作用,降低了重金属的释放风险。本研究制得的烧结砖中,重金属浸出浓度均低于《危险废物鉴别标准 浸出毒性鉴别》(GB 5085.3-2007)[23]的限值,符合安全标准。
综合分析抗压强度、吸水率、重金属浸出3个方面,采取G20作为最佳配比进行后续实验,以研究烧结过程中烧结砖的重金属形态分布变化、孔隙结构变化、物相结构的转化和微观形貌的变化。
2.3 烧结砖制备的固化机理
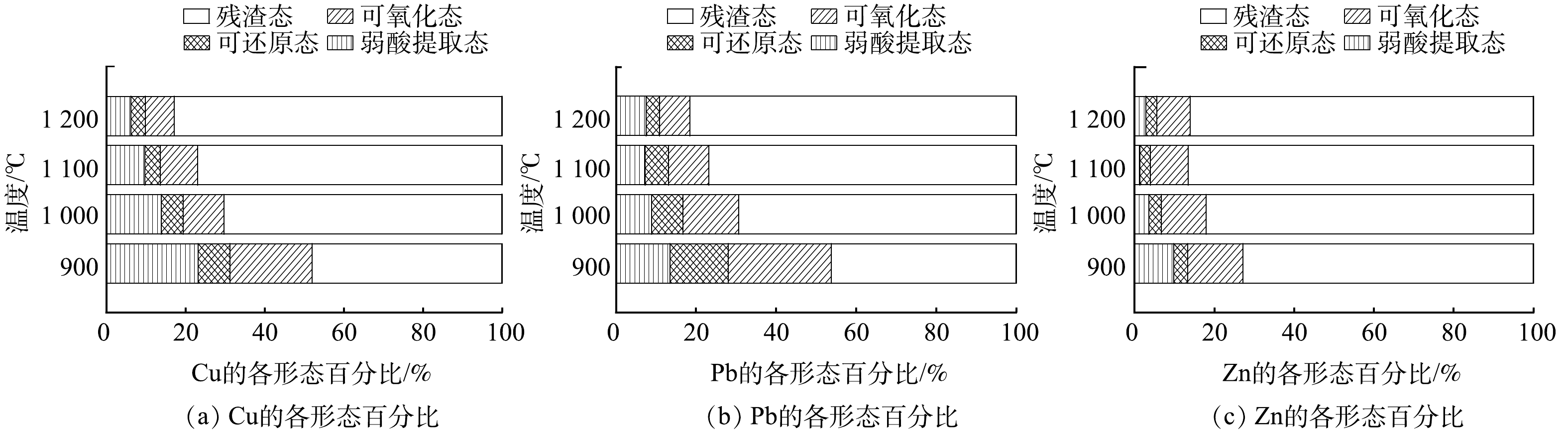
1)烧结砖的重金属形态分布。重金属的形态分布影响着烧结砖的浸出毒性与安全性;同时,也是影响重金属迁移转化能力强弱的重要因素。BCR法把重金属形态分为4种形态:弱酸提取态,是最容易提取的状态,与生物的毒性作用呈正相关;可还原态,是离子键结合的形态;可氧化态,只有在强氧化的条件下才会被释放的状态;残渣态,存在于矿物结晶相中,十分稳定,对生物的毒性作用最小。
图5为G20配比在烧结过程中的重金属各形态分布变化。在温度为900 ℃的条件下,Pb和Cu的稳定性较差,Pb的形态分布为弱酸提取态13.56%、可还原态14.49%、可氧化态25.79%、残渣态46.16%。Cu形态分布为弱酸提取态23.26%、可还原态7.97%、可氧化态20.79%、残渣态47.98%。Pb和Cu的形态特征非常类似,残渣态均不足50%。随着烧结温度的上升,Pb和Cu的残渣态占比上升至81.55%和82.80%。与Pb和Cu相比,Zn的稳定性较好,在900 ℃下,主要形态为残渣态(72.82%)。这说明,Zn主要以结晶相的形式存在,稳定性较好。随着烧结温度上升,Zn的形态转化没有Pb和Cu明显,迁移性较差,因而更稳定。结合图4和图5进行综合分析可知,残渣态占比的上升往往伴随着重金属浸出浓度的下降。这说明重金属在烧结固化过程中,以结晶相的形式而稳定下来。
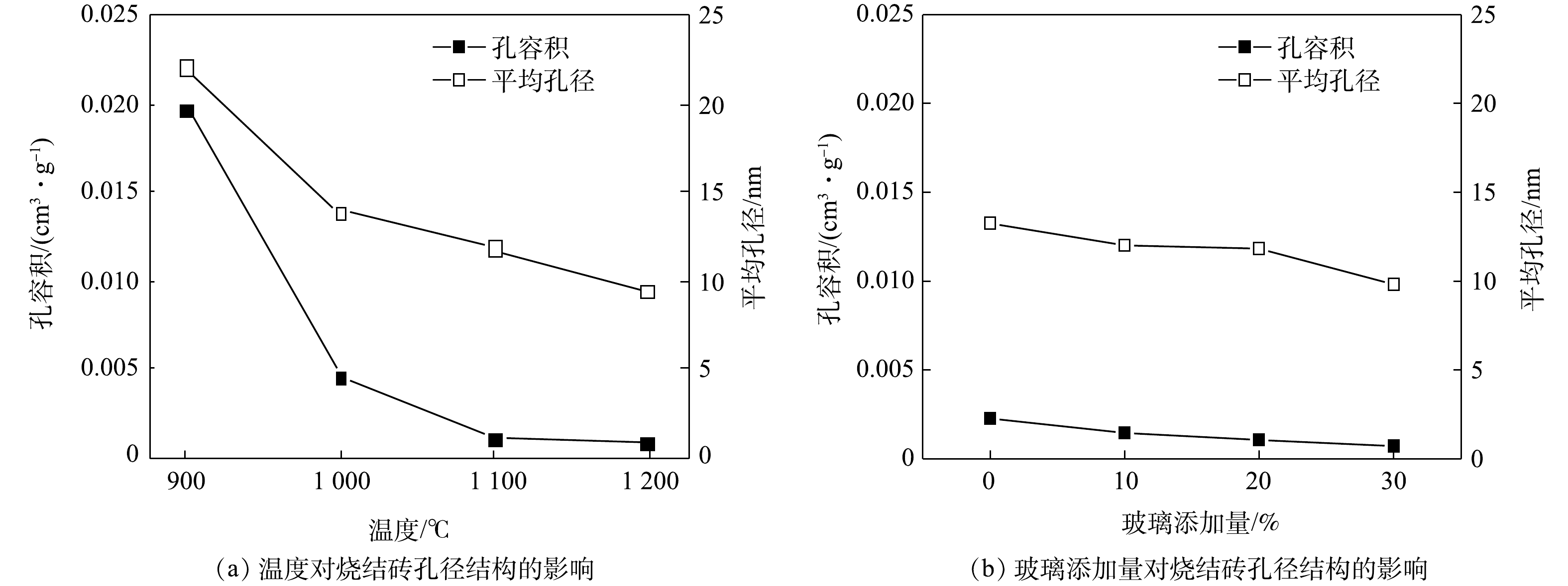
2)烧结砖的孔隙结构变化。孔容积和孔径大小可以反映烧结砖内部的孔隙结构。图6(a)为G20配比烧结砖在烧结过程中孔容积和孔径大小的变化情况。烧结温度对孔容积的影响较为明显。在温度为900 ℃的条件下,孔容积最大,达到了0.019 cm3·g−1;而当温度上升至1 000 ℃时,孔容积迅速下降至0.004 5 cm3·g−1。随着温度继续升高到1 100 ℃,孔容积进一步下降到0.001 1 cm3·g−1;当温度到达1 200 ℃时,孔容积下降到0.001 cm3·g−1。这说明,当烧结温度为900 ℃时,烧结砖内部存在大量的孔隙结构;而随着温度的升高,孔隙结构逐渐减少,趋于致密化。
孔径大小随着温度的升高而降低,当烧结温度为900 ℃时,孔径大小为22.1 nm;而随着温度上升至1 200 ℃,孔径最终降低至9.3 nm。从图6(b)可以看到,废玻璃含量的增加也会降低孔径大小和孔容积。相比较于图6(a),图6(b)中孔容积和孔径大小的变化并不显著。综合分析可知,温度对孔径大小的影响大于废玻璃含量对孔径大小的影响。孔容积和孔径大小的变化趋势与吸水率的变化趋势一致。这说明,孔隙结构可能是影响吸水率大小的内部因素。孔径结构也会影响重金属浸出的反应过程。这是因为,较小的孔径会阻碍浸出液与重金属离子间的接触,而松散的结构则有利于重金属的释放。
3)烧结砖的物相结构变化。图7为G20配比烧结砖在烧结过程中物相结构的变化。从图7中可以看出,烧结砖主要矿物相为高岭石、石英、莫来石和赤铁矿。烧结砖中的赤铁矿相在烧结过程中并未发生明显变化。高岭石和石英的特征峰随着烧结温度上升而降低,莫来石相的特征峰随着烧结温度的上升而升高。有研究者发现[24-25],烧结砖的结构主要是在烧结条件下形成了莫来石晶相结构。因此可推测,在本研究中的烧结过程中,石英相与高岭石相反应生成了莫来石相。莫来石相是一种常见的硅铝酸盐结构,硬度、强度等力学性能较优异,可以有效地增加烧结砖的抗压强度。
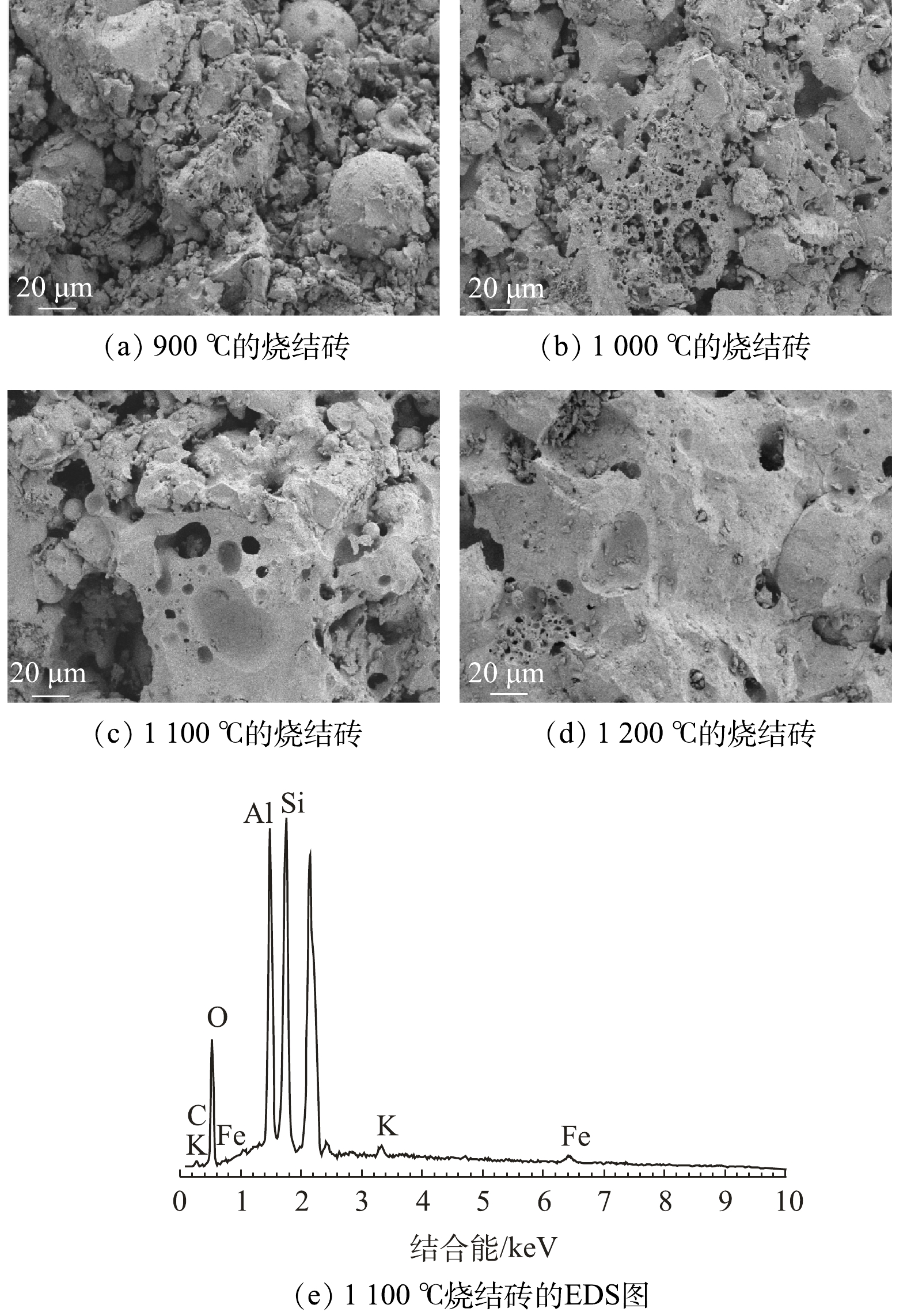
4)烧结砖的微观形貌变化。图8展现了烧结砖在烧结过程中的微观形貌变化。图8(a)显示了900 ℃下烧结砖的微观形态,可以看出,其表面粗糙,有许多大小不一的球形颗粒离散地分布在烧结砖表面。这说明,此时物料间还未开始发生反应,颗粒间的空隙较大。由图8(b)~图8(d)可以看出,随着烧结温度从1 000 ℃升高到1 200 ℃,离散的颗粒不断减少,颗粒间的空隙变小,孔径不断变小。由此可知,烧结过程是一个内部结构致密化的过程,烧结温度的上升会降低孔径大小。经过图8(e)EDS表征,可确定烧结砖结构主要由Si、Al、O元素组成。这说明,烧结砖中矿物骨架结构主要为硅铝酸盐结构。综合XRD结果(图7)可知,烧结砖的物相结构中,只有莫来石相是硅铝酸盐结构。因此推测,图8烧结砖中的硅铝酸盐结构有可能是莫来石结构。
3. 结论
1)在烧结温度为1 100 ℃、玻璃含量为20%的最优条件下,烧结砖的抗压强度可达124 MPa、吸水率为4.63%;其重金属浸出浓度远低于《危险废物鉴别标准 浸出毒性鉴别》(GB 5085.3-2007)的限值。
2)烧结砖的固化机理可能是,在烧结过程中,烧结砖内形成以莫来石相为主的硅铝酸盐结构,以导致烧结砖的孔径和孔容积不断减小,使得烧结砖孔隙结构致密化,提高了烧结砖的强度。
-
表 1 长河流域地表水主要离子质量浓度
Table 1. The mass concentration of main ions in the surface water of the Changhe River Basin
离子Ion 最大值/ (mg·L−1)Max 最小值/ (mg·L−1)Minimum 平均值/ (mg·L−1)Average value 标准差/ (mg·L−1)Standard deviation 变异系数/%Coefficient of variation Ca2+ 254.64 99.82 190.30 53.85 28.3 Mg2+ 116.10 45.50 88.96 25.27 28.4 Na+ 70.10 23.67 48.83 17.58 36.0 K+ 0.84 0.21 0.56 0.24 43.0 Cl− 13.12 2.08 6.36 4.22 66.4 HCO−3 598.23 204.56 393.29 152.42 38.8 SO2−4 53.26 13.54 31.31 13.33 42.6 pH 8.17 7.43 7.79 0.26 3.3 TDS 604.00 126.00 344.10 196.65 57.0 总硬度 500.00 196.00 383.78 103.71 27.0 表 2 长河流域中上游矿区入河排污口水质检测结果(mg·L−1)
Table 2. Test results of water quality of sewage outfalls in the middle and upper reaches of the Changhe River Basin(mg·L−1)
排污口位置Location of sewage outlet TP TN NH−3 NO−3 KMnO4 氯化物Chloride 硫酸盐Sulphate 总硬度Total hardness BOD 下村镇万里村 0.29 0.29 0.68 0.59 8.3 216 176 378 6.41 下村镇中村 0.07 0.74 0.87 0.43 5.4 180 147 412 3.6 下村镇史村 0.09 0.34 0.38 0.61 4.5 173 169 392 2.57 表 3 Pearson相关系数临界值
Table 3. Critical values of Pearson correlation coefficient
皮尔逊相关系数值 Pearson correlation coefficient 相关性 Correlation 0.8—1.0 极强相关 0.6—0.8 强相关 0.4—0.6 中等程度相关 0.2—0.4 弱相关 0.0—0.2 无相关 <0.0 负相关 -
[1] 王金华, 谢和平, 刘见中, 等. 煤炭近零生态环境影响开发利用理论和技术构想 [J]. 煤炭学报, 2018, 43(5): 1198-1209. WANG J H, XIE H P, LIU J Z, et al. Coal development and utilization theory and technical system of near-zero ecological environment impact [J]. Journal of China Coal Society, 2018, 43(5): 1198-1209(in Chinese).
[2] 国家统计局. 中国统计年鉴[J]. 北京: 中国统计出版社, 2019. National Bureau of Statistics. The yearbook of china tourism statistics [J]. Beijing: China Statistics Press, 2019(in Chinese).
[3] AKCIL A, KOLDASS. Acid mine drainage (Amd): Causes, treatment and case studies [J]. Clean Prod, 2006(14): 1139-1145. [4] SUN J, TANG C. Hydrogen and oxygen isotopic composition of karst waters with and without acid mine drainage: impacts at a SW China coalfield [J]. Science of the Total Environment, 2014, 487(4): 123-129. [5] KEFENI, KEBEDE K, MSAGATI T A M. Acid mine drainage: Prevention, treatment options, and resource recovery: A review [J]. Clean Prod, 2017, 151(10): 475-493. [6] 於方, 过孝民, 张强. 中国矿产业的废水污染现状分析与防治对策 [J]. 资源科学, 2004, 26(2): 46-53. doi: 10.3321/j.issn:1007-7588.2004.02.007 YU F, GUO X M, ZHANG Q. Wastewater pollution situation and countermeasures for Chinese mineral industry [J]. Resources Science, 2004, 26(2): 46-53(in Chinese). doi: 10.3321/j.issn:1007-7588.2004.02.007
[7] 赵江涛, 周金龙, 梁川, 等. 新疆焉耆盆地平原区地下水演变的主要水文地球化学过程分析 [J]. 环境化学, 2017, 36(6): 1397-1406. ZHAO J T, ZHOU J L, LIANG C, et al. Hydrogeochemical process of evolution of groundwater in plain area of Yanqi, Xinjiang [J]. Environmental Chemistry, 2017, 36(6): 1397-1406(in Chinese).
[8] 孙英, 周金龙, 魏兴, 等. 巴楚县平原区地下水水化学特征及成因分析 [J]. 环境化学, 2019, 38(11): 2601-2609. SUN Y, ZHOU J L, WEI X, et al. Hydrochemical characteristics and cause analysis of groundwater in the plain area of Bachu County [J]. Environmental Chemistry, 2019, 38(11): 2601-2609(in Chinese).
[9] 沈照理. 水文地球化学基础(一)[J]. 水文地质工程地质, 1983(3): 58-61.SHEN Z L. Basis of Hydrogeochemistry(一)[J]. Hydrogeology & Engineering Geology, 1983(3): 58-61(in Chinese). [10] 王亚平, 王岚, 许春雪, 等. 长江水系水文地球化学特征及主要离子的化学成因 [J]. 地质通报, 2010, 29(Z1): 446-456. WANG Y P, WANG L, XU C X, et al. Hydro-geochemistry and genesis of major ions in the Yangtze River, China [J]. Geological Bulletin of China, 2010, 29(Z1): 446-456(in Chinese).
[11] 周小平, 彭吟雪, 马雷, 等. 氢氧同位素对淮南潘集矿区地下水的指示作用 [J]. 合肥工业大学学报(自然科学版), 2019, 42(4): 536-540. ZHOU X P, PENG Y X, MA L, et al. Indicating function of hydrogen and oxygen isotope to the groundwater of Huainan Panji mine area [J]. Journal of Hefei University of Technology, 2019, 42(4): 536-540(in Chinese).
[12] JIANG L, YAO Z, LIU Z, et al. Hydrochemistry and its controlling factors of rivers in the source region of the Yangtze River on the Tibetan Plateau [J]. Journal of Geochemical Exploration, 2015, 155: 76-83. doi: 10.1016/j.gexplo.2015.04.009 [13] PIPER A M. A graphical interpretation of water analysis [J]. Trans Am Geophys Union, 1944, 25: 914-928. doi: 10.1029/TR025i006p00914 [14] GIBBS R J. Mechanisms controlling world water chemistry [J]. Science, 1970, 170: 1088-1090. doi: 10.1126/science.170.3962.1088 [15] JIA Y F, GUO H M, XI B D, et al. Sources of groundwater salinity and potential impact on arsenic mobility in the western Hetao Basin, Inner Mongolia [J]. Total Environ, 2017, 601/602: 691-702. doi: 10.1016/j.scitotenv.2017.05.196 [16] FEI L, SHOU W, LI S W. Coupling hydrochemistry and stable isotopes to identify the major factors affecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China [J]. Journal of Geochemical Exploration, 2019, 205: 106305. [17] 张未. 格尔木昆仑山前冲洪积扇地下水水文地球化学作用[D]. 西安: 长安大学, 2018. ZHANG W. Hydrogeochemical process of groundwater in the Alluvial Fan of Mt. Kunlun in Golmud[J]. Xi’an: Chang’an University, 2018(in Chinese).
[18] FANG Y H, ZHENG T Y, ZHENG X L, et al. Assessment of the hydrodynamics role for groundwater quality using an integration of GIS, water quality index and multivariate statistical techniques[J]. Journal of Environmental Management, 2020, 273: 111185 [19] GU D. Theory framework and technological system of coal mine underground reservoir [J]. China Coal Soc, 2015, 40(2): 239-246. [20] GUO Q, XIONG X, JIANG J. Analysis of isotopic and hydrochemical characteristics of different waters in Kuye Riber Basin [J]. Soil Water Conserv, 2016, 30(2): 237-242. [21] GB3838—2002, 《地表水环境质量标准》[S]. GB3838-2002, 《Environmental quality standards for surface water》[S]. (in Chinese)
[22] HUANG H, CHEN Z, WANG T, et al. Characteristics and processes of hydrogeochemical evolution induced by long-term mining activities in karst aquifers, southwestern China [J]. Environmental Science and Pollution Research, 2019, 26(29): 30055-30068. doi: 10.1007/s11356-019-05984-4 [23] BELKHIRI L, BOUDOUKHAA, MOUNI L A. Multivariate statistical analysis of groundwater chemistry date [J]. International Journal of Environmental Research, 2010, 5(2): 537-544. [24] 戴维·穆尔. 统计学的世界[M]. 第8版. 北京: 中信出版社, 2007: 345-346. DAVID M. Statistics: Concepts and controversies. [M]. 8th Edition. Beijing: China Citic Press, 2007: 345-346(in Chinese).
[25] LI P, TIAN R, LIU R. Solute geochemistry and multivariate analysis of Water Quality in the Guohua Phosphorite Mine, Guizhou Province, China [J]. Exposure and Health, 2019(11): 81-94. [26] 李平, MAGZUMNUROLLA, 梁志杰, 等. 渠井用水比例对土壤脱盐与地下水化学特征的影响 [J]. 中国农业科学, 2017, 50(3): 526-536. doi: 10.3864/j.issn.0578-1752.2017.03.011 LI P, MAGZUM N, LIANG Z J, et al. Effects of canal well water ratios on root layer soil desalination and groundwater hydrochemical characteristics [J]. Scientia Agricultura Sinica, 2017, 50(3): 526-536(in Chinese). doi: 10.3864/j.issn.0578-1752.2017.03.011
[27] SCHOELLER H. Qualitative evaluation of groundwater resource: Methods and techniques of groundwater investigation and development [J]. Water Research, 1967, 33: 44-52. [28] 吕婕梅, 安艳玲, 吴起鑫, 等. 清水江流域岩石风化特征及其碳汇效应 [J]. 环境科学, 2016, 37(12): 4673-4674. LV J M, AN Y L, WU Q X, et al. Rock weathering characteristics and the atmospheric carbon sink in the chemical weathering processes of Qingshuijiang River Basin [J]. Environmental Science, 2016, 37(12): 4673-4674(in Chinese).
[29] 李甜甜, 季宏兵, 江用彬, 等. 赣江上游河流水化学的影响因素及DIC来源 [J]. 地理学报, 2007, 62(7): 764-775. doi: 10.3321/j.issn:0375-5444.2007.07.009 LI T T, JI H B, JIANG Y B, et al. Hydro-geochemistry and the sources of DIC in the upriver tributaries of the Ganjiang River [J]. Acta Geographica Sinica, 2007, 62(7): 764-775(in Chinese). doi: 10.3321/j.issn:0375-5444.2007.07.009
[30] 张涛, 蔡五田, 李颖智, 等. 尼洋河流域水化学特征及其控制因素 [J]. 环境科学, 2017, 38(11): 4537-4545. ZHANG T, CAI W T, LI Y Z, et al. Major ionic features and their possible controls in the water of the Niyang River Basin [J]. Environmental Science, 2017, 38(11): 4537-4545(in Chinese).
[31] 孙厚云, 王晨昇, 卫晓锋, 等. 大兴安岭南段巴音高勒流域水化学特征及驱动因子 [J]. 环境化学, 2020, 39(9): 2507-2519. doi: 10.7524/j.issn.0254-6108.2020032102 SUN H Y, WANG C S, WEI X F, et al. Hydrochemical characteristics and driving factors in the water of the Bayingaole Basin, Southern Great Xing’an Range [J]. Environmental Chemistry, 2020, 39(9): 2507-2519(in Chinese). doi: 10.7524/j.issn.0254-6108.2020032102
[32] 王剑, 罗朝晖, 陈植华, 等. 滇东北毛坪铅锌矿区水化学特征及成因 [J]. 环境化学, 2018, 37(6): 1421-1431. WANG J, LUO C H, CHEN Z H, et al. Characteristics and controlling factors of water chemistry in Maoping lead-zinc mine area, Northeast Yunnan, China [J]. Environmental Chemistry, 2018, 37(6): 1421-1431(in Chinese).
[33] TIWA R, SINGH A K. Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh [J]. Journal of the Geological Society of India, 2014, 83(3): 329-343. doi: 10.1007/s12594-014-0045-y -
















 DownLoad:
DownLoad: