-
稳定同位素在示踪矿物来源和揭示成矿地质环境及成矿机制等方面具有重要意义,其中硫同位素(黄铁矿物)组成在示踪成矿热流体硫的物质来源[1-2]、成矿平衡温度[3]和成矿时代的物理化条件[4]等方面起到了十分重要的作用,是最直接、最有效的指纹元素之一。同时,由于不同硫源和水循环演化时发生的地球化学过程不同,会引起δ34S值差异较大。同理,在不同分馏机制条件下,不同碳源的δ13C值也存在明显差异,其多应用于示踪碳源[5]、碳酸盐岩风化作用速率[6]、地-气界面下有机物氧化分解[7]或微生物活动产生CO2及有机油气地质[8]等方面。诚然,随着同位素分析测试技术日益成熟,同位素研究逐渐拓展到水体环境领域,特别是近年来国内外诸多学者利用34S和13C作为地表水、地下水体中含S、C元素组分来源及形态转化的指示[9-10],随着工农业发展和城市化进程的加快,人类活动(如矿产开采,农用化肥,生活污水等)的输入,也成为含硫组分和含碳组分的来源之一。由于不同来源同位素组成有着特定的“指纹”特征值,加之在形态转化过程中可能发生分馏,使得稳定硫碳同位素在示踪污染源方面的研究成为可能[11-12]。以往34S、13C同位素技术研究在地下热水环境事例众多,技术相对成熟,且主要集中指示地下热水中S、C元素来源及其水-岩作用过程,往往单独或者结合其他稳定同位素在地表水、地下水的研究[13]。结合34S、13C同位素对地表水、岩溶泉的研究也不是很多,邹君宇等[14]也利用了34S、13C同位素对于现代河流中碳-硫耦合循环进行了研究,臧红飞等[15]利用34S、13C同位素对柳林岩溶泉水的补径排区域特征及S、C元素来源进行分析。结合34S、13C同位素对中低温地下热水的研究中,肖琼等[16]运用34S、13C同位素对重庆北温泉的水-岩作用过程进行分析,Marques等[17]利用34S、13C同位素对中低温地下热水中的S、C元素来源进行了示踪。目前34S、13C同位素主要用于示踪地热水中含碳组分和含硫组分来源及其环境特征,如马致远等[18]对关中盆地地下热水赋存环境封闭性和水-岩过程进行了研究,Yang等[19]利用34S、13C同位素技术不仅对中低温地下热水中元素来源进行分析,同时探讨了其水-岩作用过程对环境的意义。
贵州息烽温泉是低温深部承压水的出露点,是著名的偏硅酸-锶、重酸钙镁及氡型地下热水。诚然,自该温泉发现以来,以往诸多学者对该区地下热水的研究主要集中于水化学环境[20]、热储温度和补给来源[21]以及水文地球化学特征及地质成[22]等方面,而对该温泉中硫、碳等元素来源、演化及热储环境和水岩作用等方面鲜有开展研究工作。
本文选取息烽温泉地下热水、地表河流、岩溶泉水为研究对象,基于水文地球化学理论,运用环境硫、碳同位素对各水体中的硫和碳元素来源、水-岩相互作用过程及热储环境进行研究,并对氡气来源进行初步探讨,为该区地热水资源开发利用和科研提供理论参考。
-
息烽温泉地热水位于息烽县温泉镇天台山脚,距贵阳市111 km,离遵义市81 km,距息烽县城41 km,素有“天下第一汤”之称。地理坐标东经106°53′43″,北纬27°19′45″,海拔高度700 m,泉水呈3处天然出露于黑滩河右岸,左岸有1眼地热井,泉口标高695 m,水温恒定常年在53—56 ℃区间,天然涌水量1083 m3·d−1。
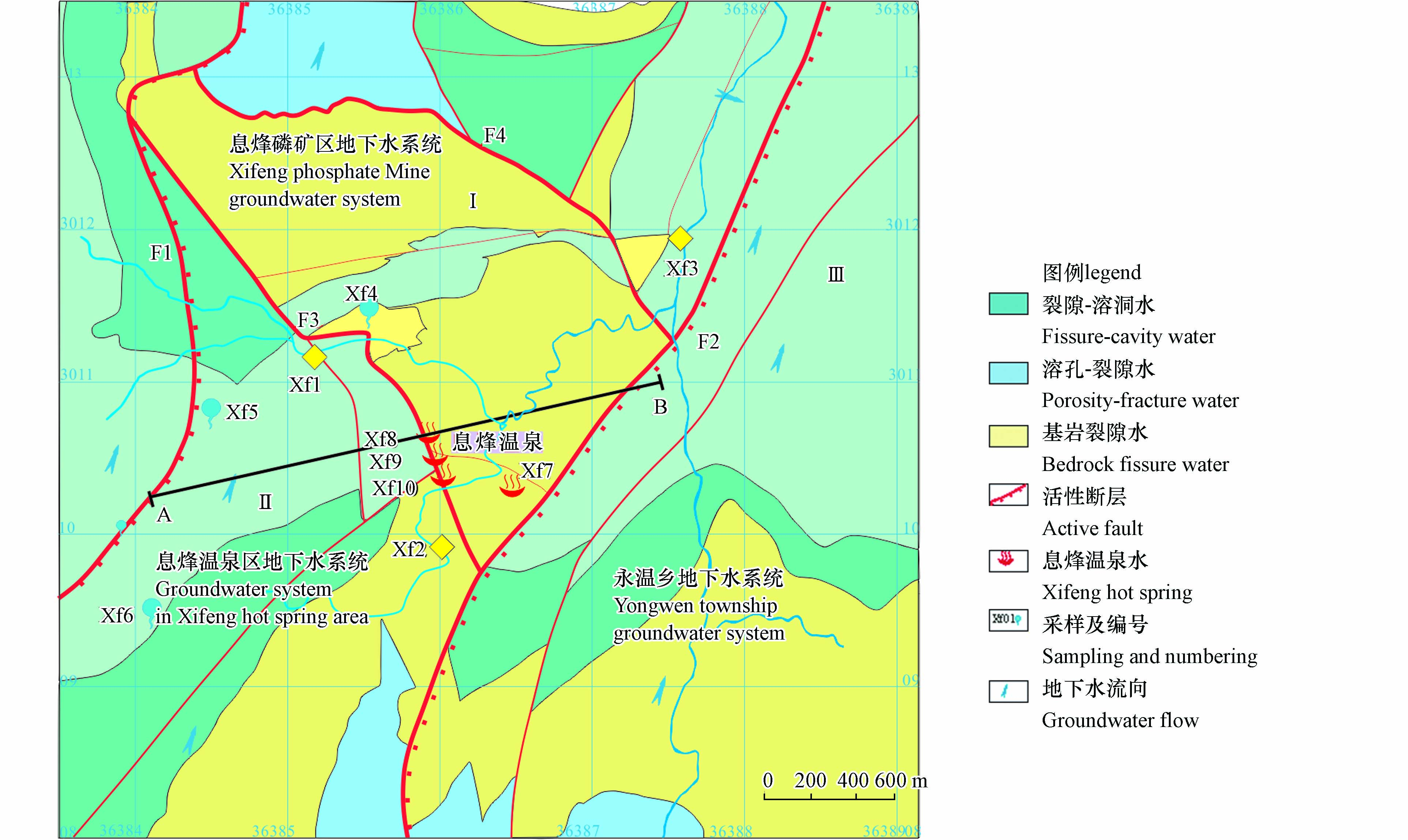
研究区构造上位于凤冈-遵义南北向构造带和黔中东西向构造带的交接复合地区[23],区域构造线方向以北北东向,北东向为主,由西向东依次展布朝阳断层、石头田断层及枫香沟等断层,其中北北东向挽近期活动断裂朝阳断裂(F1)、岩脚断裂(F2)与息烽温泉附近区域石头田-黑滩河断裂(F3)、枫香沟断裂(F4)等阻水断层形成复杂的地垒式地质构造,是区内地热流体运移的主要通道,且以岩脚断裂、石头田-黑滩河断裂、枫香沟断裂及朝阳断裂为分界线[24],划分以北为息烽磷矿区地下水系统Ⅰ,以南为息烽温泉区地下水系统Ⅱ,东侧则为永温乡地下水系统Ⅲ(图1)。息烽温泉区地热水系统Ⅱ与息烽磷矿区地下水系统Ⅰ的系统边界主要以倾向北东,倾角30°—60°,断距大于500 m的压扭型逆冲阻水石头田-黑滩河断为界,息烽温泉沿该断层下盘出露地表。Ⅰ、Ⅱ系统与Ⅲ系统边界则
为北东走向的岩脚断裂为界,该断裂为挽近期活动断裂,发育洋水背斜中段北西翼近轴部,长度大于20 km,走向30°,倾向南东,倾角大于30°,为左旋压扭性断裂。
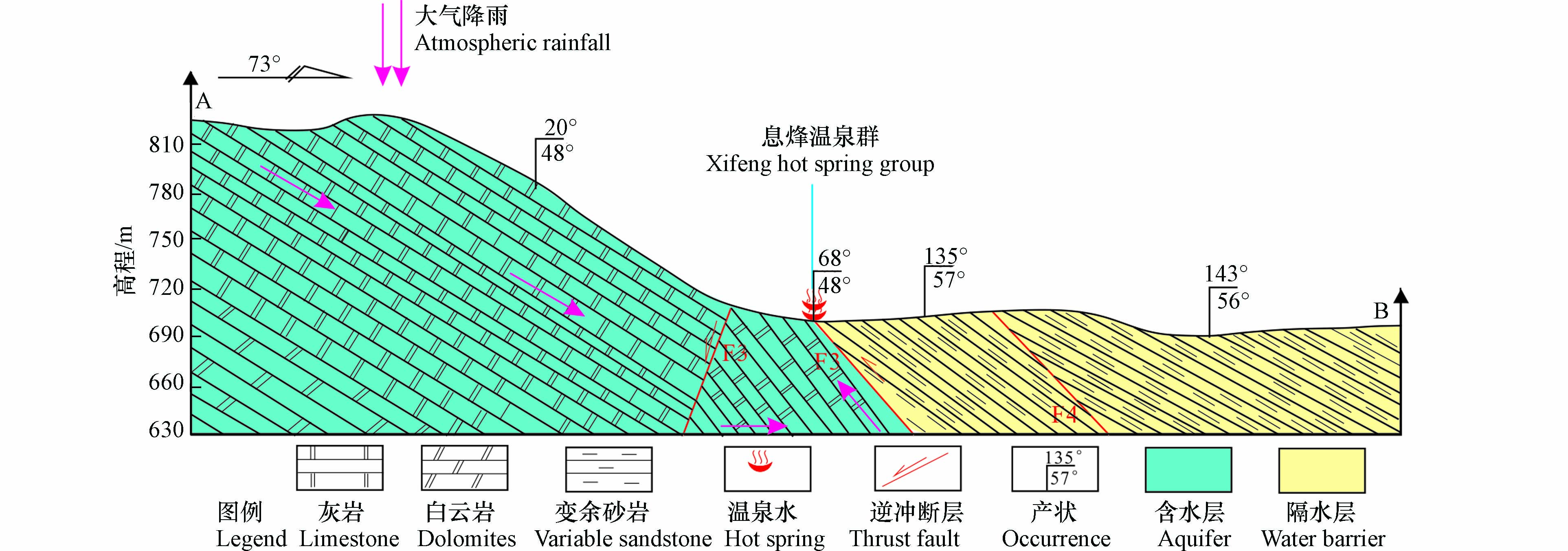
研究区地下水文系统属于热水系统,具有埋藏深、运移时间长、温度高等特征,见图2。区域内地下热水主要接受大气降水补给,其补给区位于盐井冲-白马洞一带。地表出露的上震旦统灯影组、中上寒武统娄山关组地层是区内主要的热储含水层,岩性藻席微晶白云岩、含硅质白云岩夹燧石条带及灰质白云岩,其构造裂隙及古岩溶较发育,含水性丰富且分布均匀,地下水径流模数达到5—7 L·(s·km)−1。上覆地层下寒武统金顶山组、明心寺组及牛蹄塘组碎屑岩是主要隔热盖层,其岩组含风化裂隙水,沿沟谷两侧渗流而出,泉水流量小,地下水径流模数0.1—1 L·(s·km)−1,含水性极弱。接受补给的地下水受岩层走向及断裂构造控制,总体由西向东、南东方向径流,在径流途中与寒武系娄山关组-震旦系灯影组发生水岩相互作用,形成多元素组合体系,后遇阻水黑滩河断裂及前震旦系变质岩、震旦系南坨组碎屑岩的阻隔而排出。
-
2018年4月对息烽地下热水汇水区域共取水样10件,其中息烽温泉3件、地热井1件、地表河流3件、岩溶泉水3件。现场水温、pH、电导率(Ec)用的pHmeterAZ8686、ORPmeter8552便携式水质分析仪(衡欣公司)测定,精度分别为0.1 ℃、±0.02和1 µS·cm−1。阴离子水样采集时,清洗聚乙烯瓶2—3次,现场用直径50 mm、0.45 µm的醋酸纤维脂膜过滤水样后存入596 mL聚乙烯瓶,立即放入便携式冰袋保存,12 h内送至实验室。在实验室用AgNO3滴定法(0.1 mg·L−1)滴定Cl−,NO3−、SO42−用紫外光谱法(0.1 mg·L−1)测定。阳离子测试水样首先进行过滤,然后装于事先用1∶1的 HNO3 溶液清洗过的 50 mL 聚乙烯样瓶中,并立即加1∶1优级纯硝酸溶液 6—8滴,使得 pH< 2。所有阳离子均使用PerkinElmer 公司生产 Optima 2100DV 全谱直读型 ICP -OES (精确度≤0.1%)检测。
水中溶解无机碳(DIC)碳同位素测定,将前处理好的样品加入5滴100%无水磷酸至2 mL反应瓶中密封,使用高纯氦气(99.99%,流速100 mL·min−1)进行600 s的排空处理,并用注射器加入0.2 mL样品。常温(25 ℃)静置10 h,将样品与磷酸反应且平衡后的CO2气体经过70 ℃的熔硅毛细管柱(规格为Poraplot Q,25 m×0.32 mm)与其他杂质气体分离,进入到美国热电公司生产的253plus、Gas Bench稳定气体同位素质谱仪进行测定。设置18个为标准样品(分别为HDIC、KSTD、HNF和HBLS),其标样的δ13C值精度均高于0.1‰。
水样中SO42−的δ34S值测定,仪器设备为美国热电公司的253plus、Flash EA元素分析仪,具体步骤为:将实验前处理好的样品,取500—550 mL水样置于干净烧杯中,加入1:1盐酸,调节pH值至3—4。排除水中碳酸根离子后,加入10 mL的氯化钡溶液(10 mol·L−1)至出现硫酸钡白色沉淀;浸泡、清洗硫酸钡沉淀至中性,转移至蒸发皿中,置于105 ℃烘箱烘干并收集固体硫酸钡。将含有不超过100 μg硫的样品和3—5倍样品的V2O5,包在1个9 mm×5 mm的锡杯里,自动进样器每次投入燃烧反应器中1个样品,通入氧气,使样品在960 ℃下充分燃烧,燃烧产生的所有气体在氦载气流下带入并通过分层充填WO3和Cu丝的氧化还原反应管,使所有气体充分氧化,同时生成的少量SO3通过Cu丝时还原为SO2。气体通过1根色谱柱(Sulphur Separation Column for IRMS/HT;PN 260 070 80)将SO2和其它杂质气体分开后进入质谱仪测试。采用IAEA-SO-5,IAEA-SO-6和NBS 127等3种国际标准物质,标样的分析精度优于0.2‰,实验在北京科荟测试中心完成。
-
结果表明,区内地表水、岩溶泉及地下热水中优势阴离子均以
HCO−3 、SO2−4 为主,其离子浓度大小为HCO−3 >SO2−4 >Cl−,阴离子浓度变化范围为86—191.01 mg·L−1。阳离子以Ca2+、Mg2+为主要特征,其浓度变化为:Ca2+>Mg2+>Na+>K+,阳离子浓度变化范围为20.7—57.4 mg·L−1,其中地下热水中总阳离子浓度(TZ+)值274.3—296.6 mg·L−1均高于地表水和岩溶泉水中TZ+值54.77—118.2 mg·L−1、63.88—184.7 mg·L−1,与大多数地下水有相似的规律:TZ+浓度大于TZ−浓度,其可能是地下热水中存在有机物质。水温为53—56 ℃,pH值7.52—7.72呈弱碱性(表1)。 -
由研究区地表水、岩溶水和地下热水水样的Piper图(图3)可知,阳离子组成均富Ca2+,且分布在Ca-Mg一线上,表现为碳酸盐岩矿物风化作用较强的特征,水中阴离子中都分布在HCO3−-SO42−一端,也体现了碳酸盐的风化,仅有地表洋水河表现为硫酸盐岩的溶解,结合区域地质背景分析,可能受到人类活动等影响。其区域地表水化学类型为HCO3−-Ca·Mg和SO4·HCO3−-Ca·Mg型,岩溶泉水化学类型为HCO3−-Ca·Mg型,息烽地热水化学类型表现为HCO3−·SO4-Ca·Mg型。
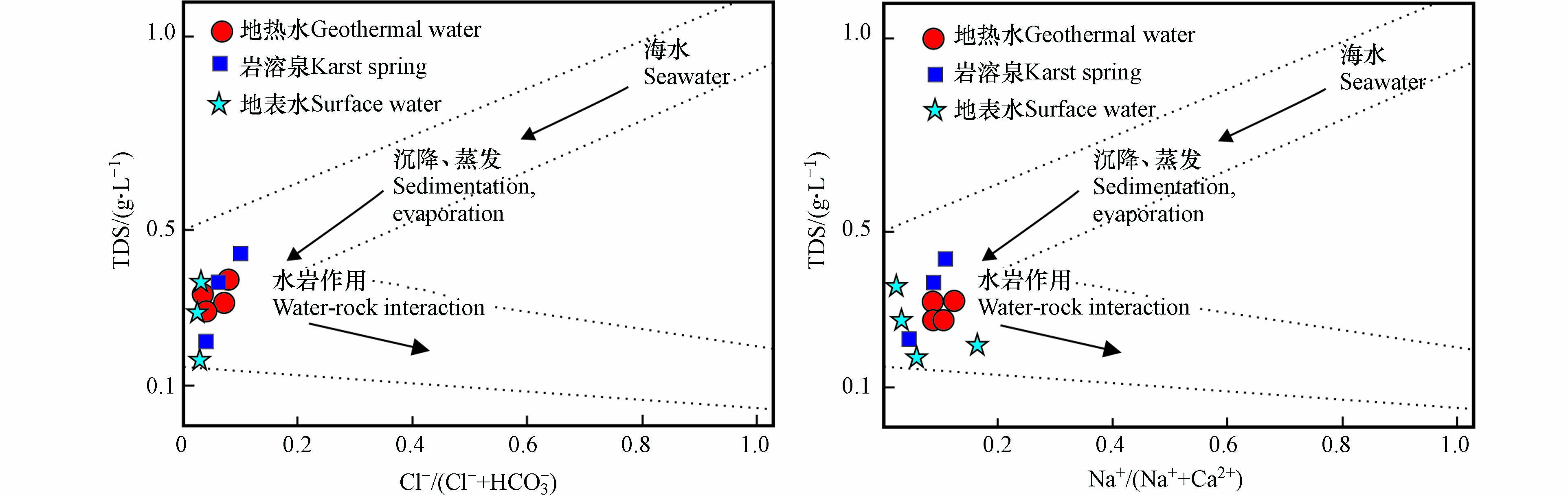
单一元素的比值不能准确地判断出水中元素的来源,利用两组元素及其比值可以去除干扰,获得更准确的结果[25]。吉布斯图能清楚地比较水体中化学组分并评价其形成的原因[26]。图4所示,地热水样点和其他样点都分布Gibbs图的中部,说明各水体中离子组分主要受控于岩石矿物与水之间的相互作用,蒸发浓缩作用和大气降水作用对水样并无影响,然而地热水中TDS略高于岩溶泉,表明浅层较深层水受到一定程度的蒸发浓缩作用的影响,且水在流动的过程中会受到溶滤作用的控制。此外,也受碳酸盐矿物、硫酸盐矿物和硅酸盐矿物的溶解的影响。地热水中Ca2+、Mg2+离子含量。
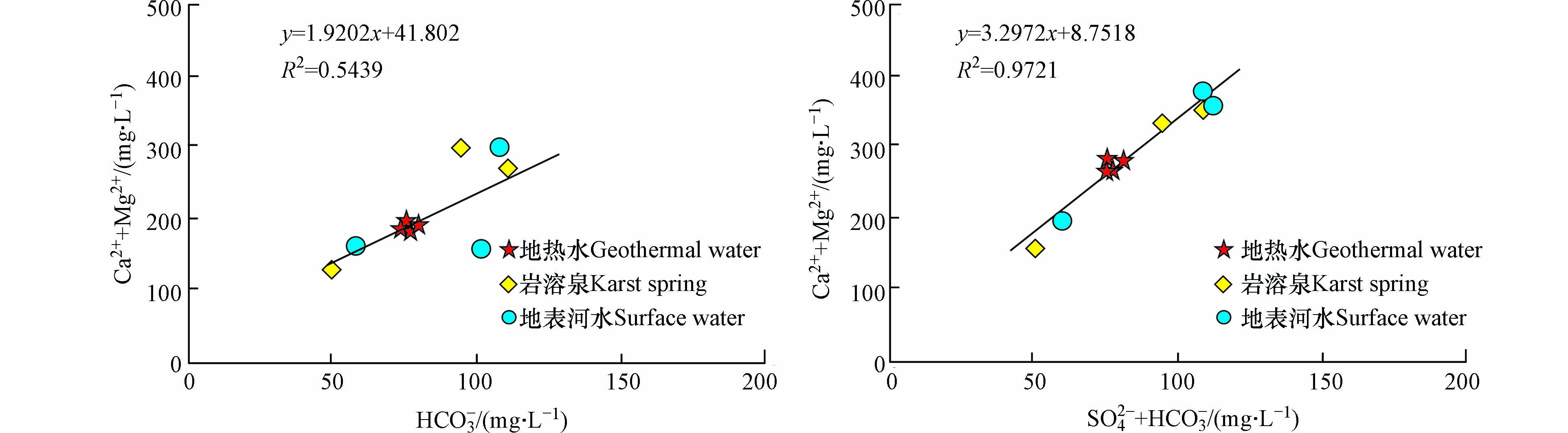
利用阴阳离子当量比确定水中重碳酸离子和硫酸离子来源,若当量比(Ca2++Mg2+):HCO3−= 1:1时,则表示碳酸盐岩风化;若(Ca2++Mg2+):(SO42−+HCO3−)=1:1时,则受硫酸盐的影响。图5表明,地表水、岩溶泉和地下热水中的(Ca2++Mg2+)/ (HCO3−+SO42−)比值都小于1,指示水体中Ca2+、Mg2+、HCO3−、SO42−除主要来源于方解石、白云石等碳酸盐岩矿物的溶解l另外,还存在硫酸盐溶解。
-
地下热水中pH值为7.51—8.36,水中的DIC主要以HCO3−形式存在,因此研究区域水体中的δ13CDIC主要表现为δ13
CHCO−3 [27]。从表1可知,地表水中δ13CDIC值为−12.01‰—−12.96‰,平均值为−12.36‰;岩溶泉水中δ13CDIC值为−12.03‰—−13.50‰,平均为−12.90‰,加之测区内构造裂隙、岩溶管道十分发育,连通性较好,因此认为地表水入渗补给地下水。地下热水中δ13CDIC值为−4.85‰—−9.12‰,平均为−7.70‰,表现出明显的δ13C异常。喀斯特岩溶区地表水和地下水中P2分压分别为45 Pa、 598 Pa,远高于大气P2分压,因此可以忽略大气CO2和大气降水输入的HCO3-[28]。为了更好地探讨研究区温泉水中δ13CDIC的来源。前人研究表明[29],当达到同位素交换反应平衡时,重碳酸盐(HCO3−)与气相CO2的稳定同位素δ13C值之差与绝对温度(T)之间存在以下关系:
由此,已知地下热水中HCO3−的δ13C值和温度值,则可由上式计算出当达到同位素交换平衡时水体中气相CO2的稳定同位素δ13C。根据取样分析地下热水、地表水和岩溶泉水,计算得出的δ13
CHCO−3 和δ13CCO2 值,结果见表1。据研究,纯生物成因CO2气体中的稳定碳同位素值为−25‰[30],资料查明目前国际上公认的深部幔源碳的δ13CCO2 值为−4.7‰—−8.0‰[31],平均值为−6.35‰。根据同位素质量平衡原理,地下热水中的CO2的δ13CCO2 组成可用以下方程表征:计算得出,地表水中δ13
CCO2 值为−19.94‰—−21.22‰;岩溶水中δ13CCO2 值为−20.63‰—−21.23‰,地下热水中的δ13CCO2 值为−10.34‰—−13.99‰,平均值为−12.16‰。郑乐平等对黔中岩溶地区土壤CO2的稳定同位素组成研究认为,在地-气界面,自地表向地下5—15 cm土壤有机物氧化分解和微生物活动产生的CO2的δ13CDIC值−18.0±1.0‰—−20.5±1.0‰[32],因扩散作用而产生4‰左右扩散分馏[30],则土壤中的δ13C值为(−14.0±1.0‰—−16.5±1.0‰),取平均值为−15.25‰,深部幔源δ13CCO2 的平均值为−6.35‰。因此认为,富氡地下热水中参与水-岩反应的CO2为幔源CO2和土壤CO2且土壤中CO2占63%—83%,幔源CO2为17%—37%。表1显示,地表径流水和岩溶泉水中的HCO3−浓度值,其分别203.24 mg·L−1和229.30 mg·L−1,较地下热水体中的HCO3−含量值183.69 mg·L−1,偏高,地下热水中HCO3−含量很多,总体高于地表水、岩溶泉水中HCO3−含量。表明幔源和土壤对CO2的贡献很多,其原因是研究区断层发育,能进入系统的CO2很多,循环时间长,水岩作强烈。 -
自然条件下硫的稳定同位素有:32S、33S、34S和36S的 4种,其相对应丰度值分别为95.02%、0.75%、4.21%、0.02%[33]。由于成岩过程中地质环境差异,引起了硫同位素分馏导致较重的34S同位素在不岩石矿物中的富集程度存在较大差异,因此以硫的重同位素丰度比值相对于国际标准样品同位素丰度比值的千分偏差来表示:
式中,δ34S样品表示样品比值相对国际标准比值的千分偏差;(34S/32S)样品表示样品34S与32S丰度比值;(34S/32S)标准表示国际标准的34S与32S丰度比值。在水体中硫主要以S2−、SO32−、SO42−、HSO4−等离子形式存在,在区内地下热水中S同位素主要以SO42−形式存在,所以δ34S主要表征SO42−中的硫同位素。就地下水而言,由于赋水环境的不同,导致同位素分馏有所差异,其SO42−中δ34S变化范围-13‰—41‰[34]。而测区内地下热水中34S相对富集,δ34S值高于国际标准值;地表水和岩溶泉相对富轻δ34S,δ34S值低于国际标准值,其差异由地层岩性矿物和赋水环境引起。在震旦系灯影组-中上寒武系娄山关组演化期,研究区灯影组-娄山关组含水岩系主要为台地边缘滩相藻白云岩和台地相,特别是在下寒武清虚洞-中上寒武系娄山关组时期沉积了一套海相蒸发岩(石膏)矿物[35-36]。黔东北石阡地区热下热水中硫同位素,其δ34S值为26.35‰—23.1‰与黔北寒武系(清虚洞组、高台组、娄山关组)中石膏矿物中的δ34S值十分相近(表2),并认为地下热水中的硫来源于地层中的海相蒸发硫酸盐。根据区内温泉地下热水系统补、径、排和热储层(灯影组-娄山关组)特征,并结合研究区地层出露情况及地热下水中硫同位素特征值,认为区内地热水中的硫酸盐来源于中上寒武统娄山关组蒸发岩。
上震旦系灯影组白云岩中δ34S值(32.6‰—28.5‰)偏重,这可能是因那时古海洋环境和气候变化导致,寒武系下统清虚洞组-中上统娄山关组时期是区内乃至整个扬子地区处于海相蒸发岩沉积阶段,其中下寒武统清虚洞组、中统高台组及中上统娄山关组地层石膏矿物中δ34S值分别为26.03‰—23.7‰;29.57‰—25.7‰;28.0‰—26.73‰。地下热水中δ34S值为27.52‰—25.41‰,除深部钻井地下热水中的δ34S值为25.41‰外,其他3眼天然出露地下热水中的δ34S值为27.52‰—27.50‰较该点深部井地下热水中δ34S值25.41‰偏重至2.12‰—2.09‰,其可能是天然温泉水受到了地表冷水或大气降水的混合,深部地下热水具有埋深大、较好系统封闭性等特征。因此,深部地下热水中δ34S值可以更好的指示硫元素来源及其热储环境,其δ34S值27.53‰与中上统娄山关组地层石膏矿物中δ34S值更为接近,所以认为区内地热水中硫酸盐主要来源于中上寒武统娄山关组石膏矿物的溶解。
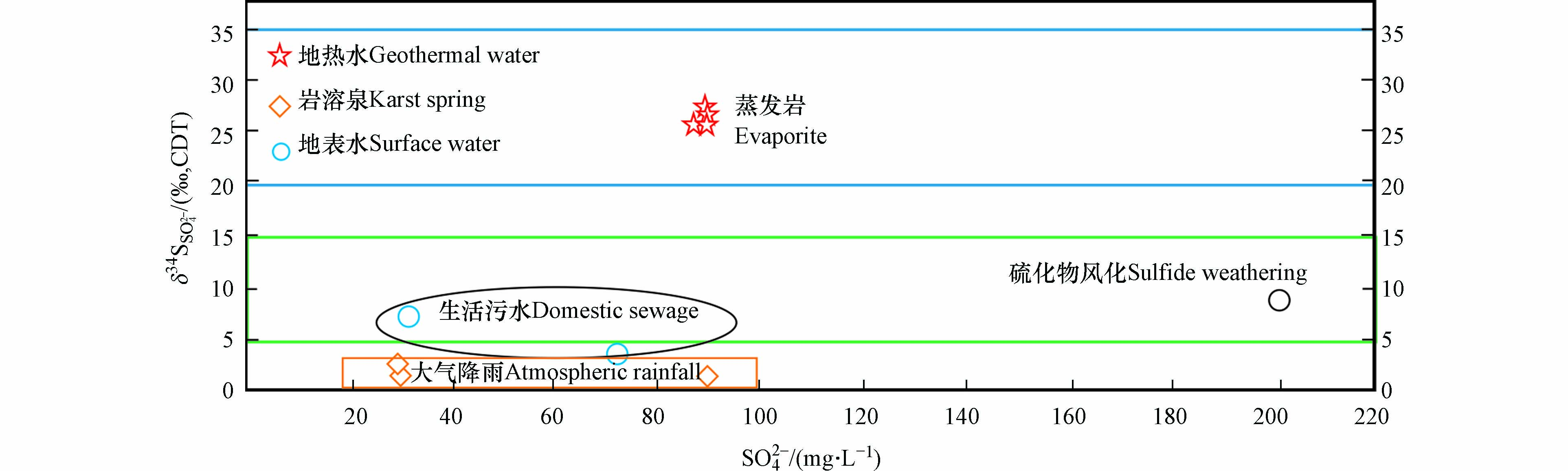
从δ34S-SO42−关系(图6)可知,区域地表水中硫酸盐浓度变化范围为35.0—200.0 mg·L−1差异较大,δ34S同位素值差异(8.67‰—2.04‰)也较大,其主要受硫化物风化和生活污水的影响;在水循环交替活跃潜水带的岩溶泉水中,硫酸盐的浓度值(30.0—90.0 mg·L−1)变化较大,δ34S同位素值为(2.67‰—0.95‰)差异不明显,表现为大气降雨沉降来源。
-
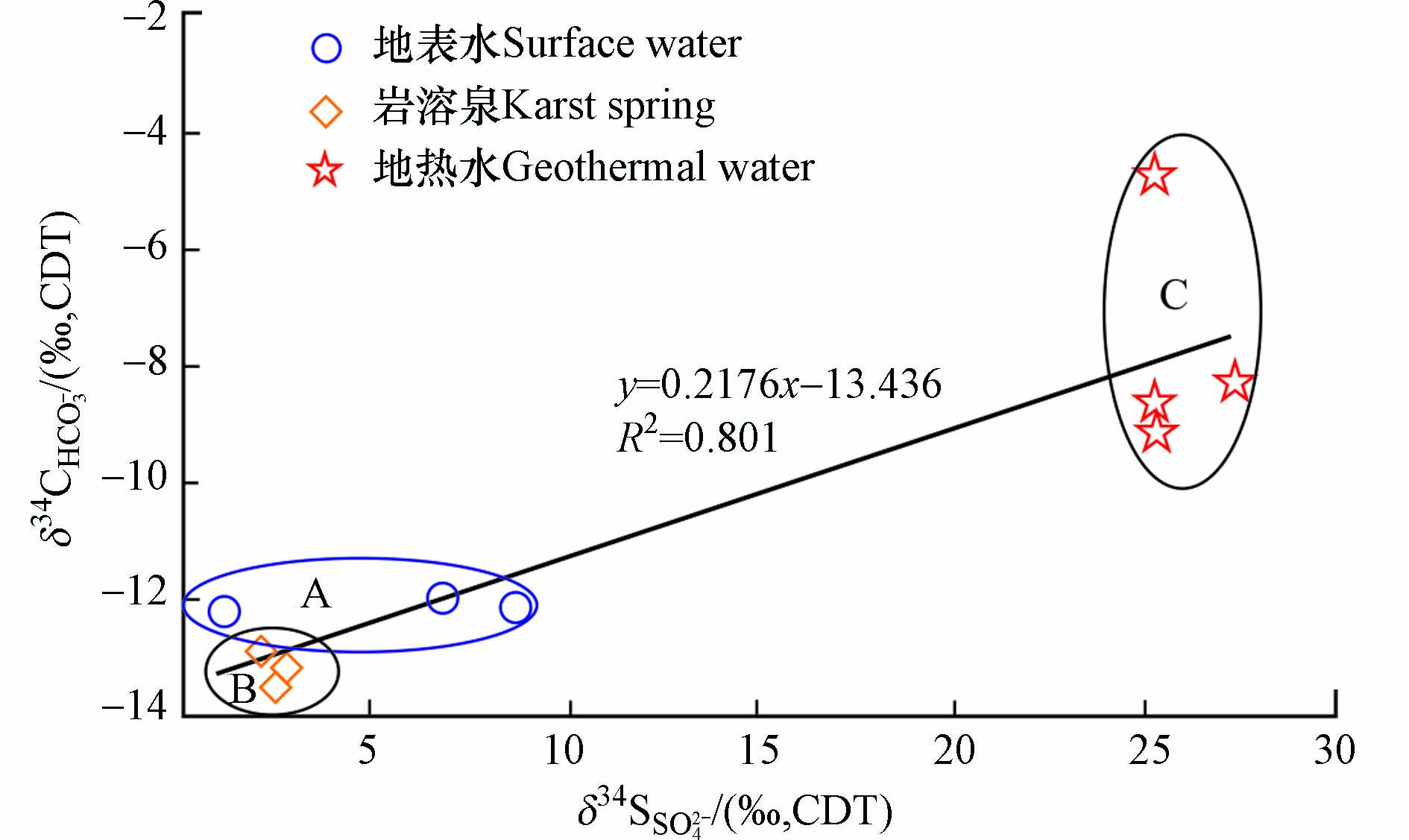
对研究区水体中硫-碳同位素组成特征的研究,有助于分析水体中硫碳元素来源和水岩作用过程。将水体中溶解态DIC和硫酸盐中δ34S值投影于同一张图上进行比较(图7),更能明显的显示地下热水具有较高的δ34S值和δ13C值,异常高的δ34S值(均高于20‰),说明地下热水中SO42−来源于石膏的溶解.
A区为地表径流水中δ13CDIC值(−12.01‰—−12.96‰),B区为岩溶泉水中δ13CDIC值(−12.03‰—−13.50‰),其两者与喀斯特岩溶地区碳酸岩盐风化产物产生的δ13CDIC值(−12.22‰±0.49‰—12.28‰±0.82‰)极为相近,表明研究区地表水、岩溶泉水中HCO3−来源于碳酸盐岩和CO2相互作用的产物。C区为地下热水的δ13CDIC值−4.84‰—−9.12‰,计算其水中的δ13
CCO2 值为−10.62‰—−15.09‰,表现为土壤CO2和深部幔源CO2与碳酸盐岩矿物共同作用的结果。与A区、B区间存在明显差异,说明地下热水系统与地表水系统间的联系较弱,更多的指示着深部CO2在释放过程中与围岩系统间的物质交换。研究表明[37-38],断层氡气Rn通常以CO2、N2和CH4为载体,以对流作用形式从深部运移至浅部,区内地表径流水中Rn浓度为2.2—2.7 Bq·L−1,岩溶泉中Rn浓度为15—28 Bq·L−1,而息烽地下热水中Rn浓度达到144.8 Bq·L−1,已经达到理疗氡泉命名标准。值得注意的是从地表径流水、岩溶泉到地下热水中氡气浓度与碳同位素变化趋势相同。综上,地下热水中DIC来源于碳酸盐岩矿物,在CO2参与下与围岩发生水-岩作用形成的HCO3−。地表水、岩溶泉因出露较浅容易受现代大气中CO2的影响,表现出较轻的δ13CDIC值,地下热水中SO42−来源于石膏的溶解。氡气与研究区域地下热水的温度呈正相关性,至于氡气来源目前还需要做进一步深入研究。 -
(1)地下热水水化学类型为HCO3·SO4-Ca·Mg型,pH呈弱碱性,阴离子主要为
HCO−3 和SO2−4 ,阳离子主要Ca2+和 Mg2+,阴离子浓度变化范围为86—191.01 mg·L−1,阳离子浓度变化范围为20.7—57.4 mg·L−1.(2)地下热水中
HCO−3 >SO2−4 浓度,其δ13CDIC值为−4.85‰—−9.12‰,计算得出地热水中δ13CCO2 值为−10.34‰—13.99‰,初步认为参与水岩反应的CO2为土壤成因和深部幔源成因混合的结果。(3)而地下热水中
SO2−4 的δ34S值为27.52‰—25.41‰,平均值26.47‰,与娄山关组海相蒸发盐岩石膏矿物中δ34S值26.73‰极其相近,结合研究区地下热水热储和补给特征认为地下热水中的硫来源于娄山关组地层中海相蒸发硫酸盐溶解。
富氡地热水中δ34Sso42-、δ 13CDIC同位素特征及其意义—以息烽温泉为例
Characteristics of δ34Sso42- and δ13CDIC isotopes in radon-rich geothermal water and their significance— Xifeng hot spring as an example
-
摘要: 贵州息烽温泉被誉为“亚洲第一氡泉”,富含多种有益元素。选取息烽地热水和与其有关的地表径流水及岩溶泉水为研究对象,利用水文地球化学、δ34
SSO2−4 、δ13CHCO−3 同位素等方法对区内富氡地热水中硫、碳元素来源及水-岩作用过程进行研究。结果显示,地热水水化学类型为HCO3·SO4−Ca·Mg型,pH呈弱碱性,其阴离子主要为HCO−3 和SO2−4 ,阳离子主要Ca2+和 Mg2+,阴离子浓度变化范围为86—191.01 mg·L−1,阳离子浓度变化范围为20.7—57.4 mg·L−1。地热水中δ13CHCO−3 值为−4.85‰—−9.12‰,计算得出CO2的δ13CCO2 值集中在−10.34‰—−13.99‰间,其参与水-岩反应的CO2为幔源和土壤混合成因。δ34SSO2−4 值为25.41‰—27.53‰,与区内寒武系娄山关岩组中石膏中的δ34SSO2−4 值(26.73‰—29.57‰)一致。结合地热水中的阴阳离子含量、δ34SSO2−4 值和δ13CHCO−3 值的分析,可以认为大气降水入渗寒武系娄山关组碳酸盐岩地层发生的水岩反应主要为石膏的溶解,其次CO2进入含水层与围岩发生水岩作用生成HCO−3 。Abstract: Guizhou Xifeng hot spring is known as “the No.1 Radon spring in Asia” and is rich in various beneficial elements. A number of methodologies such as hydrogeochemical analysis and δ34SSO2−4 , δ13CHCO−3 isotope are applied to reveal the source of sulfur and carbon in the radon-rich hot water, related surface water and karst spring in the area. The process of water-rock interaction is also studied. The results show that the hydro-chemical type of the geothermal water isHCO−3 ·SO2−4 −Ca·Mg with a weakly alkaline pH. The anions are mainlyHCO−3 andSO2−4 , the cations are mainly Ca2+ and Mg2+. And the anion concentration frange rom 86—191.01 mg·L−1 while the cation concentration varie from 20.7—57.4 mg·L−1. The δ13CHCO−3 value in geothermal water is −4.85‰—−9.12‰, and the calculated δ13CCO2 value of CO2 is between −10.34‰—−13.99‰. The CO2 involved in the water-rock interaction is caused by the mixing of mantle source and soil. The value of δ34SSO2−4 value is 25.41‰—27.53‰, which is consistent with the δ34SSO2−4 value (26.73‰—29.57‰) in gypsum in the Cambrian Loushan guan Formation. Combined with the analysis of the anion and cation content in the geothermal water, δ34SSO2−4 value and δ13CHCO−3 value, it could be considered that, during the infiltration process of rainwater enters the carbonate formation of the Cambrian Lushanguan Formation, the water-rock reaction is mainly gypsum dissolution. Secondly, CO2 enters the aquifer and interacts with the surrounding rock to produceHCO−3 .-
Key words:
- δ34SSO2−4 /
- δ13CHCO−3 /
- water-rock interaction /
- radon-rich geothermal water /
- Guizhou Xifeng
-
近年来,我国一些发达地区的村落建设了分散式农村污水处理设施,并取得了较好的环境效益,但这些村落污水的治理,仍以COD、氨氮、总磷等污染物的降解为考核目标,而农村居民生活水平和医疗条件在不断提高,村落水环境中EDCs浓度水平也相应增加,其对水环境生态和人类健康危害日益严重[1],尤其是农村地区,EDCs通过灌溉形式,直接被稻、麦、瓜果等农作物吸收,进而进入食物链。EDCs是一种能扰乱生物体新陈代谢平衡的化学物质,主要分为天然产生(E1、E2、E3)及人工合成(EE2)[2]。据报道,各种环境基质中均检测到不同浓度的EDCs,水体中其质量浓度可低至10−6(1 μg·L−1)量级和10−9(1 ng·L−1)量级,而EDCs在极低浓度下就可引起水生生物的生殖发育障碍[3-4]。主要原因在于,EDCs与生物体内的雌激素受体结合而干扰生物内分泌系统正常代谢[5]。Legler等[6]研究发现,当自然水体中E2浓度达到1.0 ng·L−1时,可引起生物体内分泌紊乱。Cappiello等[7]发现不少猝死婴儿体内残留的EDCs含量相对普通新生婴儿较高;Clarke等[8]研究表明,妊娠期女性若接触过量EE2,则将増加母女患乳腺癌的风险。由此可见,当EDCs进入动物食物链,再经过层层传递,最终在人类体内积累,对人体健康损害威胁相应不断增大。
国内外众多学者研究表明,耕作型稻田复合生态系统通过“微生物-稻田湿地”耦合的复合系统对村落污水中的有机污染物进行生物降解,其主要依靠水稻复杂的根系及其附着的生物膜协同净化作用,不但可以达到净化村落污水的效果,还可以产生水稻增肥的效益[9]。但存在类固醇类激素(EDCs)环境污染及生态危害问题。现阶段,人为去除环境中雌激素类污染物主要通过吸附[10]、光催化氧化[11]、生物降解[12]的3种途径使EDCs在环境中迁移、降解。阳春等[10]研究表明,污泥对雌激素的吸附主要来自于污泥中的活性成分,而被生物表面所吸附的雌激素才能被生物降解。光解与氧化作用是EDCs真正的分解过程,因为它不可逆的改变了分子结构,强烈影响其在环境中的归趋,但光降解类固醇雌激素极易受pH的影响,近期有一些研究表明类固醇雌激素光氧化降解后产生的代谢产物仍具有雌激素活性[11]。生物降解通过微生物新陈代谢和自身与周围环境进行物质交换,达到将污染物去除或转化为无害无毒的物质[12],日益受到人们的重视。
本文针对村落污水中的类固醇类激素(EDCs)环境污染及生态危害问题,构建耕作型稻田湿地[13],并以本课题组筛选的农药降解菌HD为EDCs生物强化降解菌[14],考察其对EDCs的降解效能,以期为村落水环境中的EDCs降解机制及环境生态影响评价提供参考。
1. 材料与方法(Materials and methods)
1.1 试验装置与进水水质
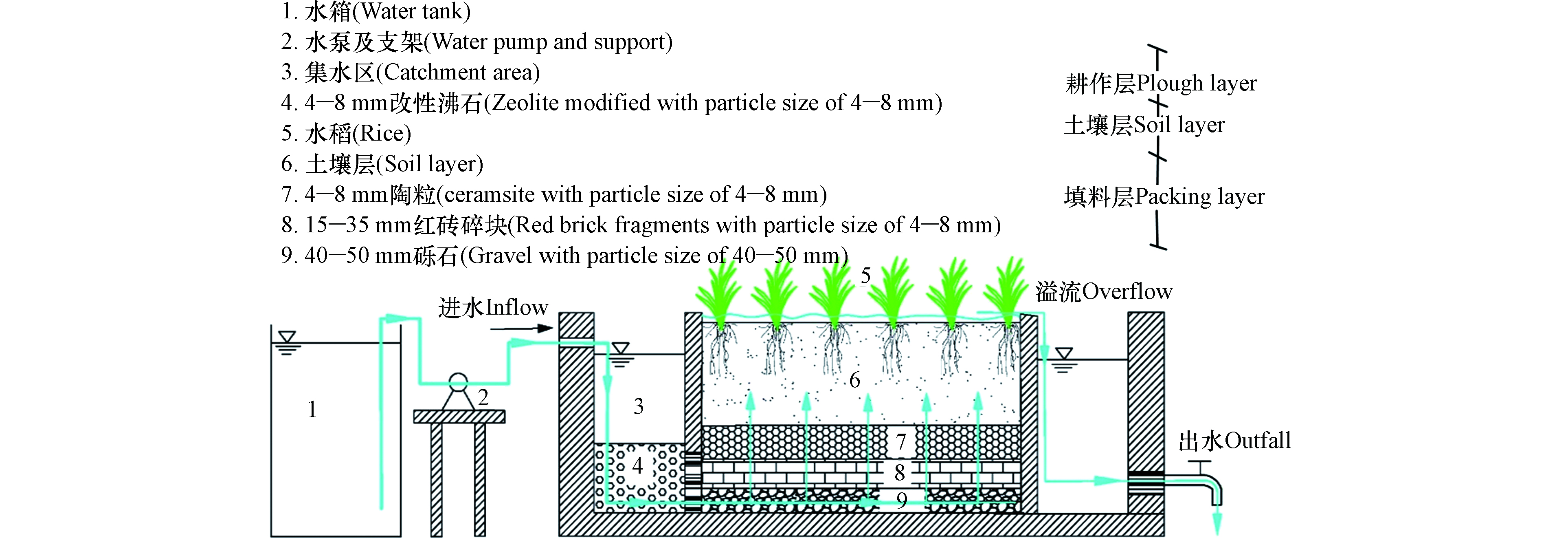
耕作型稻田湿地试验装置分2组,A组为空白对照组,B组为HD菌剂强化组。试验装置如图1所示,整个耕作型稻田湿地装置采用PP板(L×B×H=1 m×0.4 m×0.65 m),由集水区、湿地处理区和出水区3部分所组成,集水区沸石[15]层厚度200 mm,湿地填料层厚度300 mm,自下而上由40—50 mm砾石、15—35 mm红砖碎块、4—8 mm陶粒组成,孔隙率约30.8%;试验进水通过恒流水泵抽入集水区,经集水区的沸石层有效拦截后,再进入稻田湿地。装置内的土壤,取自常州洛阳镇薛家河周边稻田表层15—20 cm处土壤,所取土壤为该地区连年种植水稻土,装置内土壤层厚度为20 cm。耕作层厚度10 cm。水稻秧苗取自常州市洛阳镇薛家河周边水稻田中,种植密度为45株·m−2。
菌剂投加:A、B二组均采用自然进水法生物膜培养,当镜检可见填料上有褐色生物膜和原生动物及COD降解率超过60%时,认为生物挂膜成功。此时在B组中投加HD菌剂,配制100 mL的基础液体培养基(氯化钠:0.5 g,七水合硫酸镁:0.5 g,七水合硫酸亚铁:0.002 g,硫酸铵:1.5 g,氯化钙:0.04 g,磷酸氢二钾:1.5 g,磷酸二氢钾:1.5 g,蒸馏水:1 L,琼脂粉:20 g,pH:7.2—7.4),按照体积分数2.5%的比例接入菌种HD,培养48 h,将菌液混合后,再按照1%的投加比例混入进水中,随进水进入B组耕作型稻田湿地系统,连续投加2周,进行菌剂强化挂膜。A组不投菌,在正常条件下进行生物挂膜,为试验对照组。
农药降解菌HD筛选自南京某废弃农药厂的土壤中,是一株具有降解2,4-二氯苯酚能力的菌,现保藏于中国微生物菌种保藏管理委员会普通微生物中心,保藏编号:CGMCC No. 15123。经理化特性和分子鉴定判断属于摩式假单胞菌属(Pseudomonas mosselii)。
试验进水水质如表1所示。在生活污水的基础上添加少量内分泌干扰物,模拟进水内分泌干扰物浓度波动,如表2所示。
表 1 试验进水水质Table 1. Test water quality指标Index CODcr/(mg·L−1) 总磷/(mg L−1)TP 氨氮/(mg·L−1) NH+4 总氮/(mg·L−1)TN pH 范围 87—156 2.23—5.69 8.75—16.34 10.86—18.33 7.39—7.84 表 2 进水内分泌干扰物浓度(μg·L-1)Table 2. Concentration of endocrine disruptors in water inlet(μg·L-1)类固醇Steroid estrogen E1 Estrone E2 Estradiol EE2 17-α-ethinylestradiol E3 Estriol 原水浓度 10.13—15.25 0.79—1.1 0.88—1.82 6.31—9.58 模拟进水浓度 38.69—60.13 9.86—14.32 10.89—13.21 33.28—54.36 1.2 试验仪器与试剂
表 3 主要试验试剂Table 3. Main experimental reagents药品名称Drug names 分子式Molecular formula 规格Specification 生产单位Production unit E1 C18H22O2 — 阿拉丁 E2 C18H24O2 — 阿拉丁 E3 C18H24O3 — 阿拉丁 EE2 C20H24O3 — 阿拉丁 BSTFA C8H18F3NOSi2 — 阿拉丁 吡啶 C5H5N AR 永华化学科技(江苏) 丙酮 CH3COCH3 AR 国药 正己烷 C6H14 AR 江苏强盛功能化学 二氯甲烷 CH2Cl2 AR 永华化学科技(江苏) 雄烷 C19H23 — 北京谱析科技有限公司 表 4 试验主要仪器Table 4. Experimental main instruments仪器设备Instrument and equipment 型号Model number 生产单位Production unit 多用途高速离心机 SORVALL Thermo electron corporation 行星式球磨机 QM-1SP2 南京大学仪器厂 超声波细胞粉碎机 JY96-Ⅱ 宁波新芝生物科技股份有限公司 气质联用 Trace ISQLT 美国赛默飞科技有限公司 1.3 水样预处理
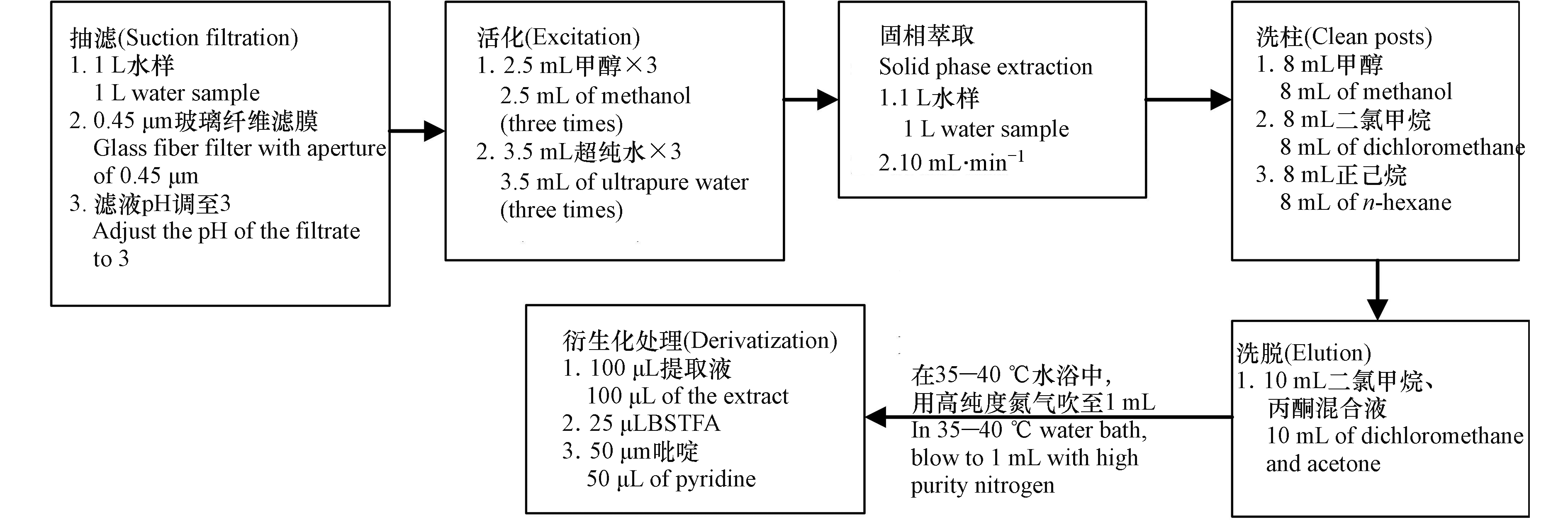
采集1 L水样,GF/F(0.45 μm)滤膜抽滤,滤液用99%的浓硫酸调节至pH3以下。
固相萃取:利用Simon Acti-Carb SPE柱进行固相萃取,首先活化SPE,分别加入2.5 mL甲醇3次,3.5 mL超纯水3次,控制流速在10 mL min−1,进行萃取,待水样萃取完,再分别加入8 mL甲醇、8 mL二氯甲烷、8 mL正己烷进行洗柱,最后用10 mL的二氯甲烷和丙酮的混合溶液淋洗,收集淋洗液,在35—40 ℃的水浴中,用高纯度氮气吹至1 mL,放入冰箱待用,具体步骤见图2。
1.4 土样预处理
稻田土壤采集后,自然风干,去除杂石杂草,用球磨机在400 r·min−1的条件下充分研磨,过20目筛网,混匀后放入冰箱待用。预处理时,称取1 g的样品,放入10 mL的离心管中,加入5 mL溶剂(甲醇∶丙酮=1∶1),超声波萃取10 min,随后以5000 r·min−1转速离心10 min,收集上清液至25 mL离心管中。重复以上步骤2次,合并上清液后,经高纯度氮气吹至1 mL后,定容至20 mL。最后按照图2水样预处理的方法,进行固相萃取。
1.5 衍生化处理
环境中类固醇类EDCs的含量相对较低,且由于这类化学物质都含有—OH官能团,极性较强,若直接采用气质联用测定,在测定过程中容易被色谱柱所吸附,从而造成实测浓度小于实际浓度,因此为降低该类物质的极性,提高其热稳定性,增加了衍生化步骤,即在正常衍生化的步骤中,又增添了吡啶,可以有效降低EE2的衍生产物转变为E1的衍生产物。黄成等[16]对某制药厂污水样品中E1、E2、E3和EE2衍生化后使用气相色谱/质谱法(GC-MS)分析表明,4种目标化合物的加标回收率达到(94.0%±2.9%)—(101.0%±3.8%),说明GC-MS法可应用于污水中雌激素化合物定量检测。
衍生化方法是在1.5 mL色谱进样瓶中加入100 μL的混合标液,通入高纯度氮气将其缓慢吹干,接着在进样瓶中加入25 μL BSTFA和50 μL吡啶,待其反应一定时间后吹干,最后加入V(二氯甲烷)∶V(正己烷)=1∶4的进样溶剂和10 μL 0.01 g·L−1的内标,取1 μL注入GC-MS分析,在空白污水样品中添加100,300、500 ng·L−1 等3个浓度水平的目标物,测定回收率。衍生化的具体反映结构变化如下:

1.6 EDCs的测定条件
试验中E1、E2、EE2、E3选用气质联用进行测定,色谱柱为TG-5MS(30 m×0.25 mm×0.25 μm),气相条件如下:
GC:以氦气为载气,流速1 mL·min−1;不分流方式进样,进样口温度280 ℃,进样体积1 μL;柱初始温度为50 ℃,保持2 min,以12 ℃·min−1程序升温至260 ℃,保持8 min,再以3 ℃·min−1升温至280 ℃,保持5 min;
MS:接口温度280 ℃,传输线温度300 ℃,离子源为EI源,温度250 ℃,电子轰击能量70 eV,溶剂延迟时间12 min,以全扫描模式定性,扫描范围50—600 m/z,以选择离子扫描模式定量;
根据其衍生产物的特征碎片离子分布特征来确定目标产物的实际浓度,衍生产物实际参数如表5所示。
表 5 衍生产物的相应参数Table 5. corresponding parameters of derivative products衍生产物Derivative product 保留时间/min Retention time 特征碎片离子(m/z)Characteristic fragment ion 线性回归方程Equation of linear regression TMS-E1 24.28 342、327、285 Y=(9.43×108)x+(1.02×109);R2=0.91 di-TMS-E2 25.43 416、401、285 Y=(1.08×107))x+(7.55×106);R2=0.91 di-TMS-EE2 27.03 440、425、285 Y=(1.90×107))x+(3.92×106);R2=0.92 Tri-TMS-E3 28.43 504、489、285 Y=(1.14×106)x+(2.38×106);R2=0.90 注:x为目标产物的实际浓度,单位mg·L−1,Y为色谱峰面积. Note:x is the actual concentration of the target product, unit: mg·L−1, Y is the peak area. 2. 结果与讨论(Results and discussion)
2.1 耕作型稻田湿地去除EDCs效能分析
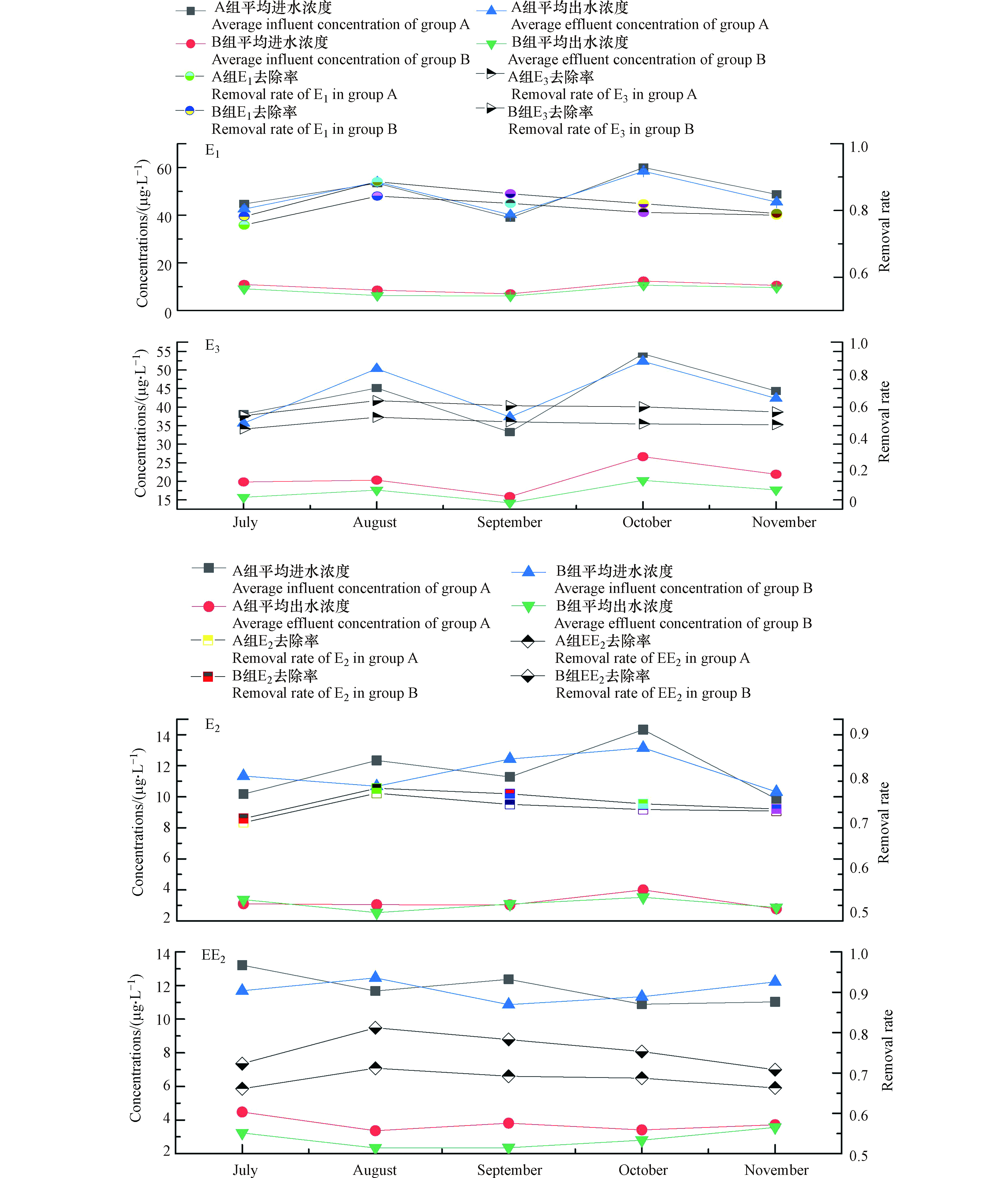
图3是2018年7月至11月,水稻生长过程中,耕作型稻田湿地对EDCs中E1、E2、EE2、E3的去除效果图。从图3可以看出,生活污水中EDCs通过耕作型稻田湿地吸附降解后,A组的E1、E2、EE2、E3的平均去除率为80.1%、72.4%、68.3%、51.4%,B组的平均去除率为82.6%、73.4%、75.6%、60.5%,除了E3外,其余各目标污染物的去除率均在65%以上,Baronti[17]在试验过程中发现E2在生物降解过程中,一部分的E2较易氧化转化成E1,遵循典型的醇氧化为酮原则,而E1通过水和作用又转化为E3,随着中间产物的不断产生,进而导致了出水E3浓度偏高。B组投加HD菌后,可能是假单胞菌HD诱导产生了羟基酶[18],相比A组E1、E2去除率未发生明显变化,EE2和E3去除率增高7%—9%,Huang等[19]发现,在BPA(双酚A)降解的河流底泥中,假单胞菌和鞘单胞菌占据了原有细菌群落的73%,由此可见,向湿地中投加假单胞菌HD有助于提高EDCs的去除率。
湿地经过5个月的连续运行后,投菌组B中的EDCs残留量明显低于未投菌组A。由图3中的实测数据,经计算B组湿地出水中E1、E2、E3和EE2含量相比A组分别降低约26.6%、17.1%、30.3%、13.3%。试验时间跨秋夏两季,夏季EDCs的去除效果要明显优于秋季,这主要是因为夏季正值水稻生长期,水稻根系对EDCs有一定吸收作用,夏季温度较高,微生物活性较强,微生物新陈代谢较为旺盛,运行至秋季时,温度降低,水稻收割,微生物活性逐渐降低,EDCs的降解率并未大幅下降,说明人工湿地中填料层及土壤层对EDCs的去除起到了一定作用。
2.2 HD菌剂强化后耕作型稻田湿地各生物单元对EDCs去除分析
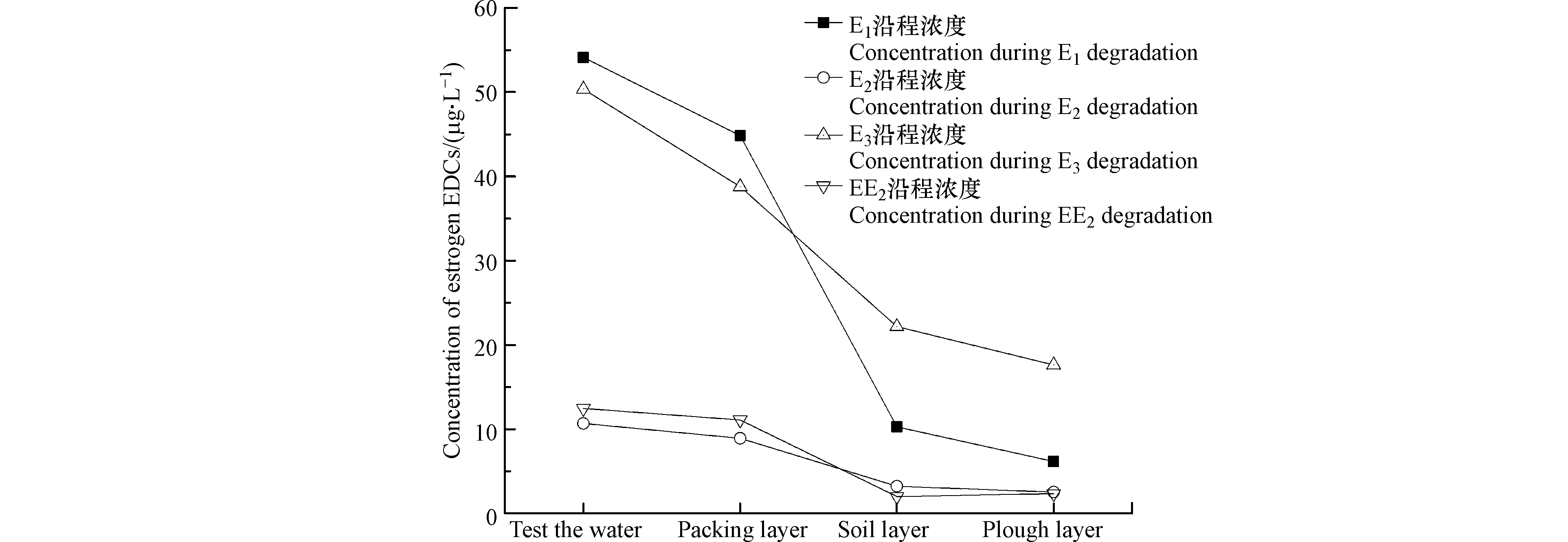
图4为耕作型稻田湿地经HD菌剂强化后EDCs浓度变化趋势图,沿程对E1、E2、E3和EE2的总去除率分别为88.6%、76.3%、64.8%和81.2%。3个生物单元填料层、土壤层、耕作层对E1去除率分别为16.2%、75.1%、28.2%,对E2去除率分别为14.5%、61.8%、18.7%,对E3去除率分别为20.6%、49.8%、10.4%,对EE2去除率分别为8.9%、43.4%、-8.1%,显然土壤层对EDCs的去除贡献占比相对最大,主要是因为土壤中微生物种群丰度要远高于填料层孔隙中生物膜上的微生物种群丰度,同时,土壤中还有分布广泛的水稻根系,通过其根系的泌氧作用,刺激微生物活性,促进微生物新陈代谢,进而加强了微生物降解能力,而填料层的填料作为微生物的代谢场所[20],其主要作用有二:一是直接吸附EDCs,二是作为微生物的载体,在其表面形成微生物集聚(生物膜),为生物膜微生物吸附、吸收、降解EDCs提供平台。其中E1、E2、E3的降解沿程越往后,目标污染物浓度越低,但EE2在经过土壤层的降解后,其浓度却略有提升,一方面,据Ascenzo等[21]研究显示,EE2的生物降解只发生在好氧段,因为EE2拥有乙炔基,从空间结构上来看,这对基团基质和受体的结合有阻碍作用,使得酶活性表达受阻,且在厌氧阶段,会使其由结合态变为游离态,导致EE2不易被降解,另一方面,Li等[22]研究发现,EE2在厌氧条件下更有利用吸附,耕作层分布有植物根系,植物根系的泌氧作用使得填料层吸附作用相比土壤层较弱。同时在降解过程中E3的含量在进水时略低于E1,但随着沿程越往后E3出水浓度高于E1,因为E2在生物降解过程中,一部分的E2较易氧化转化成E1,而E1通过水和作用又转化为E3,随着中间产物的不断产生,进而导致出水E3浓度偏高。
2.3 耕作型稻田湿地水芹种植期土壤中残留EDCs的变化特征
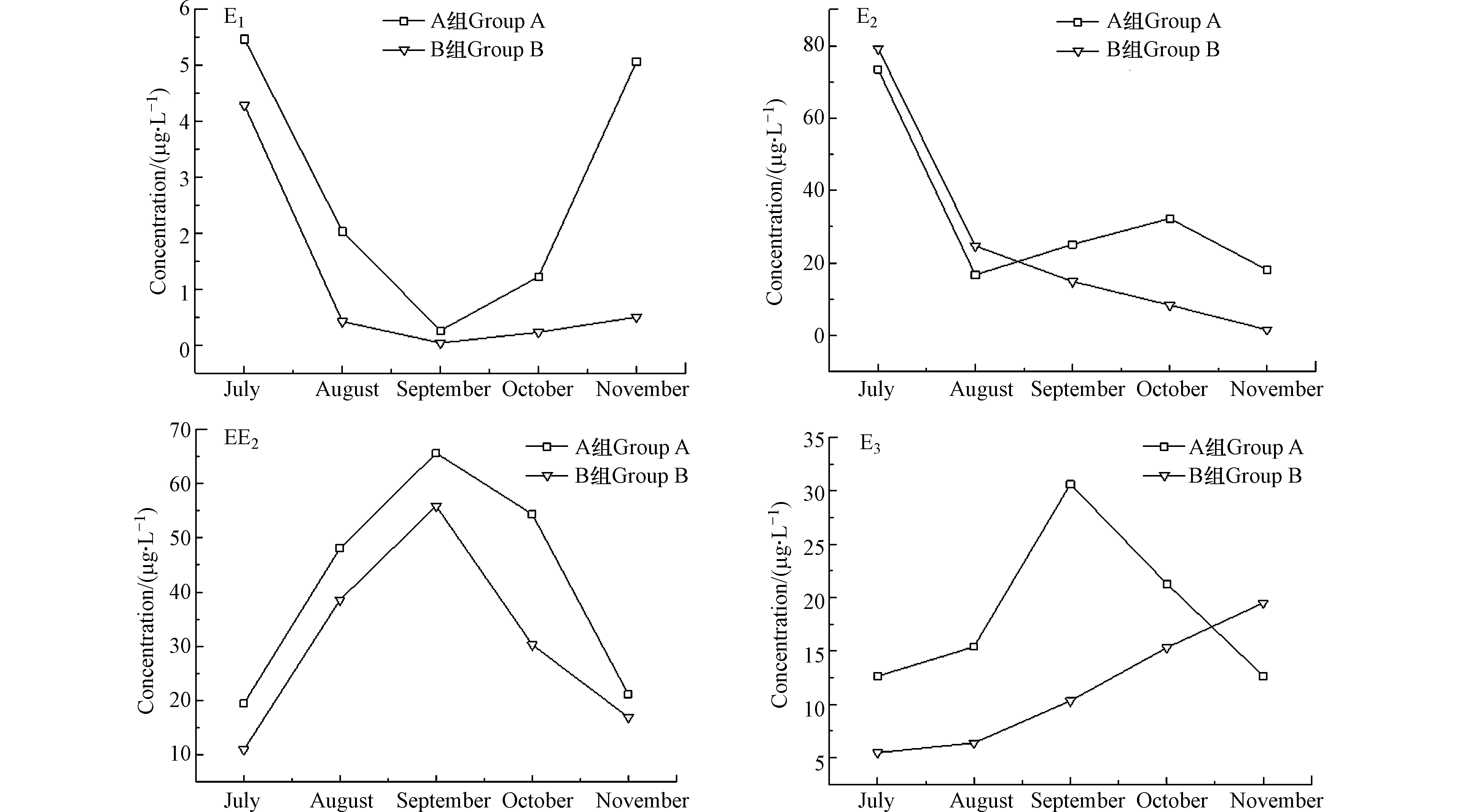
类固醇类EDCs多半有亲脂疏水的特性,易被土壤所吸附,一般类固醇类EDCs在环境中的半衰期为5—25 d不等,土壤中残留的EDCs会在一定时期,通过迁移、浸出到水体中,所以稻田湿地水体中EDCs含量通常偏高[23]。图5反应了耕作型稻田湿地净化过程中,土壤中残留EDCs含量的变化趋势,可看出投菌组B中E1、E2、EE2残留量明显小于A组,可见稻田湿地微生物对EDCs进行生物降解时,反硝化假单胞菌[24]使得反硝化细菌种群丰度提升,有利于E1的生物降解,说明HD菌促进了土壤层中羟基酶的产生,提高了其对EDCs的去除效率。从图5可看出,EE2浓度是先增后减,可能是由于EE2较为稳定不易降解。Ternes等[25]研究EE2的生物降解特性,发现1 mg·L−1的EE2经24 h后几乎无降解。10月水稻收割后,新种植了水芹,据Schroeder等[26]研究发现水芹在24 h内便能通过茎部富集近0.9 ng·L−1的EE2,所以后期EE2浓度又显下降趋势。B组中E3浓度一直呈上升趋势,任海燕等[27]研究发现,对EE2降解中间产物进行质谱分析推测,EE2在降解过程中首先被氧化为E1,后经过一系列生物催化作用生成2-羟基-2,4-二烯-戊酸和2-羟基-2,4-二烯-1,6-己二酸两种中间代谢产物,E1通过水合作转化为E3,又E2在生物降解过程中部分较易被氧化转化为E1,E1通过水合作用又转化为E3,故而导致土壤中E3浓度不断增高。湿地运行初期E1、E2浓度分别增加了85%、25%,EE2、E3浓度下降了60%、30%,这主要是因为生活污水中EDCs的构成主要以天然雌激素中的E1、E2为主,且其主要为结合态形式,极性更强,更易被土壤所吸收[28],且溶解性有机物的共轭物在经细菌酶分解时,亦会产生E1的衍生产物[29],进而导致E1浓度提高。虽然土壤层对于EDCs的降解效果较好,但初期由于土壤中生物种群尚未稳定,所以导致E1、E2的处理效果受限。
3. 结论(Conclusions)
(1)未投菌的A组对雌酮(E1)、雌二醇(E2)、雌三醇(E3)及17α-乙炔基雌二醇(EE2)的平均去除率分别为80.1%、72.4%、51.4%、68.3%,投加HD菌剂强化的B组平均去除率分别为82.6%、73.4%、60.5%、75.6%,A、B两组耕作型稻田湿地对E3去除率相比E1、E2、EE2最低。E2在生物降解过程中较易氧化转化成E1,而E1通过水和作用又转化为E3,随着中间产物的不断产生,出水E3浓度增大。A、B两组耕作型稻田湿地中,除E3外E1、E2、EE2去除率均在65%以上,EE2的生物降解只发生在好氧段,EE2拥有乙炔基,从空间结构上来看,这对基团基质和受体的结合有阻碍作用,使得酶活性表达受阻,且在厌氧阶段,会使其由结合态变为游离态,致使EE2不易被降解。
(2)投加HD菌后,耕作型稻田湿地对E1、E2、E3和EE2的总去除率分别为88.6%、76.3%、64.8%和81.2%。耕作层、土壤层、填料层3个生物单元对EDCs(E1、E2、E3、EE2)去除效果均有提升,其中:对E1去除率分别为16.2%、75.1%、28.2%;对E2去除率分别为14.5%、61.8%、18.7%;对E3去除率分别为20.6%、49.8%、10.4%;对EE2去除率分别为8.9%、43.4%、−8.1%。HD菌对耕作型稻田湿地中微生物群落产生影响,诱导产生的羟基酶有利于降解EDCs,从而提高了EDCs的去除效率。耕作型稻田湿地经HD菌剂强化后,3个生物单元中明显土壤层对EDCs去除贡献相对较大,土壤层微生物种群丰度更大,且土壤层中还分布有广泛的水稻根系,不仅为微生物代谢提供营养物质和氧气,还进一步提高了其生物分解能力。
(3)湿地运行初期,耕作型稻田背景土壤中残留的EDCs含量相对较高,经过5个月的连续运行后,土壤中EDCs含量明显下降,投菌组B中的EDCs残留量要明显低于未投菌组A,B组湿地出水中E1、E2、E3和EE2含量相比A组可分别降低约26.6%、17.1%、30.3%、13.3%。表明HD菌剂能强化耕作型稻田湿地土壤中EDCs的降解。本研究对于村落水环境中的EDCs降解机制及环境生态影响评价有一定的参考价值。
-
表 1 研究区不同类型水化学分析结果
Table 1. Results of different types of water quality analysis in the study area.
地球化学参数Geochemical parameter 常见地球化学组分/(mg·L−1)Common geochemical components 编号 类型Type pH Ec/μS T/℃ TDS K+ Na+ Ca2+ Mg2+ HCO3− SO42− Cl− δ13CCO2/‰ δ13CDIC/‰ XF01 地表水 7.9 730.0 19.9 365 1.90 8.10 71.4 36.23 298.3 74 13.0 −21.22 −12.96 XF02 8.2 289.0 19.8 200.7 2.10 2.80 43.5 15.53 160.62 35 5.02 −20.29 −12.01 XF03 8.3 692.0 25.2 447.8 5.70 18.8 72.2 36.7 150.79 200 16.6 −19.94 −12.13 XF04 岩溶泉 7.3 657.0 16.0 371.1 2.33 4.67 83.9. 27.34 268.32 90 8.02 −20.63 −12.03 XF05 7.5 525.0 18.6 308.4 3.90 3.3 65.2 29.64 295.02 35 6.52 −20.78 −13.17 XF06 7.7 206.0 13.9 171.8 1.90 2.5 41.9 8.47 124.56 30 4.01 −22.29 −13.50 XF07 地热水 7.7 500.0 53.2 487.3 3.50 13.3 54.3 21.65 191.01 86 4.82 −10.62 −4.85 XF08 7.5 503.0 52.6 308.9 3.50 14.0 52.8 21.27 182.58 86 5.02 −14.06 −8.25 XF09 7.6 502.0 53.0 411.2 3.60 13.1 57.4 23.53 182.48 94 6.27 −14.40 −8.61 XF10 7.5 510.0 52.0 382.8 3.5 12 54.3 20.7 178.68 86 4.82 −15.09 −9.12 表 2 黔北地区寒武-上震旦系灯影岩组中石膏矿物中δ34S值统计表
Table 2. Comparison results between geothermal water and simultaneous sedimentary evaporite hot spring water in the study
编号Number 位置Location δ34S/‰ 编号Number 位置Location δ34S/‰ 编号Number 石膏矿物Gypsum mineral [35] δ34S/‰ XF07 息烽地下热水 25.41 SQ1 石阡吴家湾温泉 23.20 YS1 上震旦系灯影组 28.5—32.6 XF08 息烽温泉水 27.52 SQ2 石阡城南温泉 23.10 YS2 下寒武清虚洞组 23.7—26.03 XF09 息烽温泉水 27.53 SQ3 石阡关鱼粮温泉 26.35 YS3 中寒武系高台组 25.7—29.57 XF10 息烽温泉水 27.50 SQ4 石阡甲山镇温泉 26.27 YS4 中上寒武娄山关组 26.73—28.0 平均值 26.99 平均值[35] 24.73 -
[1] SERAFIMOVSKI T, TASEV, GORAN, et al. Sulfur isotope composition in the plesenci native sulfur mineral deposit, republic of macedonia [J]. Procedia Earth & Planetary Science, 2015, 13: 35-38. [2] HIEBERT R S, BEKKER, A, HOULE, M G, et al. Tracing sources of crustal contamination using multiple S and Fe isotopes in the Hart komatiite-associated Ni-Cu-PGE sulfide deposit, Abitibi greenstone belt, Ontario, Canada [J]. Mineralium Deposita, 2016, 51(7): 919-935. doi: 10.1007/s00126-016-0644-1 [3] FEI G C, YU Y F, HUA K Q. Equilibrium temperature calculation of the sulfur isotope in Dongzhongla Pb-Zn deposit in Tibet [J]. Advanced Materials Research, 2015, 1092-1093: 1394-1397. doi: 10.4028/www.scientific.net/AMR.1092-1093.1394 [4] ZHU Q, XIE G, MAO J, et al. Mineralogical and sulfur isotopic evidence for the incursion of evaporites in the Jinshandian skarn Fe deposit, Edong district, Eastern China [J]. Journal of Asian Earth Sciences, 2015, 113: 1253-1267. doi: 10.1016/j.jseaes.2015.05.022 [5] 孙占学, 高柏, 张展适, 等. 赣南地热气体起源的同位素与地球化学证据 [J]. 地质科学, 2014, 49(3): 791-798. doi: 10.3969/j.issn.0563-5020.2014.03.007 SUN Z X, GAO B, ZHANG Z S, et al. Isotopic and geochemical evidences of geothermal gas origins in southern Fujian [J]. Chinese Journal of Geology, 2014, 49(3): 791-798(in Chinese). doi: 10.3969/j.issn.0563-5020.2014.03.007
[6] GONFIANTINI. R, ZUPPI G M. Carbon isotope exchange rate of DIC in karst groundwater [J]. Chemical Geology, 2011, 197(1): 319-336. [7] DATTA S , BISSADA A K , SOCKI R A , et al. The origin of carbon-bearing volatiles in surprise valley hot springs in the Great Basin: Carbon Isotope and water chemistry characterizations[C]// AGU Fall Meeting Abstracts, 2013. [8] 李荣西, 刘建朝, 魏刚峰, 等. 渭河盆地地热水水溶烃类天然气成因与来源研究 [J]. 天然气地球科学, 2009, 20(5): 774-780. LI R X, LIU J C, WEI G F, et al. Study on the genesis and source of geothermal water-soluble hydrocarbon natural gas in the Weihe Basin [J]. Natural Gas Geoscience, 2009, 20(5): 774-780(in Chinese).
[9] 刘颖超, 刘凯, 孙颖, 等. 良乡地热田地热水化学特征及同位素分析 [J]. 南水北调与水利科技, 2015, 5(3): 962-966. LIU Y C, LIU K, SUN Y, et al. Chemical characteristics and isotope analysis of geothermal water in Liangxiang geothermal field [J]. South-to-North Water Transfer and Water Science and Technology, 2015, 5(3): 962-966(in Chinese).
[10] ZHANG D, LIU C. A preliminary study on sulfate reduction bacteria behaviors in groundwater by sulfur and carbon isotopes: A case study in Jiaozuo City, China [J]. Ecotoxicology, 2014, 23(10): 2014-2024. doi: 10.1007/s10646-014-1330-7 [11] LIU M, GUO Q, ZHANG C, et al. Sulfur isotope geochemistry indicating the source of dissolved sulfate in Gonghe geothermal waters, Northwestern China [J]. Procedia Earth and Planetary Science, 2017, 17: 157-160. doi: 10.1016/j.proeps.2016.12.039 [12] STEFÁNSSON A, KELLER N S, ROBIN J G, et al. Multiple sulfur isotope systematics of Icelandic geothermal fluids and the source and reactions of sulfur in volcanic geothermal systems at divergent plate boundaries [J]. Geochimica Et Cosmochimica Acta, 2015, 165: 307-323. doi: 10.1016/j.gca.2015.05.045 [13] ZAMANA L V, ASKAROV S A, BORZENKO S V, et al. Isotopes of sulfide and sulfate sulfur in nitrogen hot springs of the Bauntov Group (Baikal Rift Zone) [J]. Doklady Earth Sciences, 2010, 435(1): 1515-1517. doi: 10.1134/S1028334X10110231 [14] 邹君宇, 韩贵琳. 河流中碳、硫稳定同位素的研究进展 [J]. 地球与环境, 2015, 43(1): 111-121. ZOU J Y, HAN G L. Research progress of carbon and sulfur stable isotopes in rivers [J]. Earth and Environment, 2015, 43(1): 111-121(in Chinese).
[15] 臧红飞, 贾振兴, 郑秀清, 等. 柳林泉域岩溶水水化学及碳硫同位素特征 [J]. 水电能源科学, 2013, 31(12): 28-32. ZANG H F, JIA ZHEN X, ZHENG X Q, et al. Hydrochemical and carbon and sulfur isotope characteristics of karst water in Zhelin Spring Area [J]. Hydropower Energy Science, 2013, 31(12): 28-32(in Chinese).
[16] 肖琼, 沈立成, 杨雷, 等. 重庆北温泉地热水碳硫同位素特征研究 [J]. 水文地质工程地质, 2013, 40(4): 127-133. XIAO Q, SHEN L C, YANG L, et al. Study on carbon and sulfur isotope characteristics of geothermal water in Chongqing North Hot Springs [J]. Hydrogeology and Engineering Geology, 2013, 40(4): 127-133(in Chinese).
[17] MARQUES J M, MATOS C, CARREIRA P M, et al. 2019. Isotopes and geochemistry to assess shallow/thermal groundwater interaction in a karst/fissured-porous environment (Portugal): a review and reinterpretation[J]. Sustainable Water Resources Management, 5(4): 1525-1536. [18] 马致远, 余娟, 李清, 等. 关中盆地地下热水环境同位素分布及其水文地质意义 [J]. 地球科学与环境学报, 2008, 30(4): 396-401. doi: 10.3969/j.issn.1672-6561.2008.04.011 MA Z Y, YU J, LI Q, et al. Environmental isotope distribution of underground hot water in Guanzhong Basin and its hydrogeological significance [J]. Journal of Earth Sciences and Environment, 2008, 30(4): 396-401(in Chinese). doi: 10.3969/j.issn.1672-6561.2008.04.011
[19] YANG P H. , LUO D, HONG A H, et al. Hydrogeochemistry and geothermometry of the carbonate-evaporite aquifers controlled by deep-seated faults using major ions and environmental isotopes [J]. J Hydrol, 2019, 579: 124116. doi: 10.1016/j.jhydrol.2019.124116 [20] 姚在永, 成忠礼, 王俊文. 息烽氡泉环境地球化学的初步研究 [J]. 地球化学, 1982(1): 76-81. YAO Z Y, CHENG Z L, WANG J W. Preliminary study on environmental geochemistry of Xifeng Spring [J]. Geochemistry, 1982(1): 76-81(in Chinese).
[21] 宋小庆, 段启杉, 孟凡涛, 等. 贵州息烽温泉地质成因分析 [J]. 地质科技情报, 2014, 33(5): 216-220. SONG X Q, DUAN Q S, MENG F T, et al. Analysis of geological origin of Guizhou Xifeng Hot Spring [J]. Geological Science And Technology, 2014, 33(5): 216-220(in Chinese).
[22] 吉勤克补子. 贵州息烽温泉水文地球化学特征及地质成因研究[D]. 成都: 成都理工大学, 2015. JIQ K B Z. Hydrogeochemical characteristics and geological genesis of the Xifeng Hot Spring in Guizhou [D]. Chengdu: Chengdu University of Technology, 2015(in Chinese).
[23] 戴传固, 王雪华, 陈建书, 等. 贵州省区域地质志[M]. 北京: 地质出版社, 2012: 556-557. DAI C G, WANG X H, CHEN J S, et al. Regional geology of Guizhou Province [M]. Geological Publishing House, 2012: 556-557(in Chinese).
[24] 贵州息烽磷矿准采标高(600m)以上井下开采与息烽温泉关系勘查论证报告[R]. 贵州省地质矿产勘查开发局114地质大队, 2015. Survey and demonstration report on the relationship between underground mining and Xiyu Hot Spring of Quasi-Phosphorus Mine in Guizhou Province[R]. Geological and Mineral Exploration and Development Bureau of Guizhou Province 114 Geology Brigade, 2015(in Chinese).
[25] 钱会, 马致远, 李培月. 水文地球化学[M]. 北京: 地质出版社, 2012. QIAN H, MA Z Y, LI P Y. Hydrogeochemistry[M]. Beijing: Geological Publishing House, 2012(in Chinese).
[26] 李巧, 周金龙, 高业新, 等. 新疆玛纳斯河流域平原区地下水水文地球化学特征研究 [J]. 现代地质, 2015, 29(2): 238-244. LI Q, ZHOU J L, GAO Y X et al. Hydrogeochemical characteristics of groundwater in the plain area of Manas River Basin in Xinjiang [J]. Modern geology, 2015, 29(2): 238-244(in Chinese).
[27] DAS A, KRISHNASWAMI S, BHATTACHARYA S K. Carbon isotope ratio of dissolved inorganic carbon ( DIC ) in rivers draining the Deccan Traps, India: Sources of DIC and their magnitudes [J]. Earth and Planetary Science Letters, 2005, 236(1-2): 419-429. doi: 10.1016/j.jpgl.2005.05.009 [28] LI S L, LIU C Q, LI J, et al. Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints [J]. Chemical Geology, 2010, 277(3-4): 301-309. doi: 10.1016/j.chemgeo.2010.08.013 [29] DENIES P, LANGMUIR D, HARMON R S. Stable carbon istopic ratios and the extence of a gas phase in the evolution of carbonate waters [J]. Geochimica et Cosmochimica Acta, 1974, 38: 1147-1164. doi: 10.1016/0016-7037(74)90010-6 [30] 王恒纯. 同位素水文地质学概论[M]. 北京: 地质出版社, 1991. WANG H H. Isotope of Hydrogeology [M]. Beijing: Geological Publishing House, 1991 ( in Chinese) .
[31] MOORE J G, BACHELDER J N, CUNNINGHAM C G. CO2 filed vesicles in mid -ocean basalt [J]. Journal Volcano Geothermal Reseach, 1977, 2: 309-327. doi: 10.1016/0377-0273(77)90018-X [32] 郑乐平. 黔中岩溶地区土壤CO2的稳定碳同位素组成研究 [J]. 中国科学(D辑:地球科学), 1999, 29(6): 514-515. ZHENG L P. CO2 stable carbon isotope composition of soil in karst area of central Guizhou [J]. Chinese Science (Series D:Earth Sciences), 1999, 29(6): 514-515(in Chinese).
[33] 高波. 龙子祠泉水化学组分成因及硫同位素分析[J]. 地下水, . 2017, 39(1): 15-17. GAO B. Study on chemical constituents and sulfur isotope analysis of Longzijing spring water[J]. Groundwater, 2017, 39(1): 15-17(in Chinese).
[34] WANG H C. Introduce of Isotopic hydrogeology[M]. Beijing: Geologic Publish House, 1991: 156-158. [35] 韩至钧, 金占省. 贵州省水文地质志. 第四篇热矿水[M]. 北京: 地震出版社, 1996: 252-264. HAN Z W, JIN Z S. Guizhou Province Hydrogeology. The fourth hot mineral water [M]. Beijing: Seismological Press, 1996: 252-264(in Chinese).
[36] 王淑丽, 郑绵平. 我国寒武系膏盐岩分布特征及其对找钾指示 [J]. 矿床地质, 2012(31): 487-488. WANG S L ZHENG M P. Distribution characteristics of cambrian salt rocks in China and their indicators for potassium exploration [J]. Deposit Geology, 2012(31): 487-488(in Chinese).
[37] CIOTOLI G, ETIOPE G, GUERRA M, et al. The detection of concealed faults in the Ofanto Basin using the correlation between soil -gas fracture surveys[J]. Ectonophysics, 1999, T 301: 321 -332. [38] YANG T F, CHOU C Y, CHEN C -H, et al. Exhalation of radon and its carrier gases in SW Taiwan [J]. Radiation Measurements, 2003, 36: 425-429. doi: 10.1016/S1350-4487(03)00164-1 -






























 下载:
下载: