-
2019年我国废水总氮和氨氮排放量分别为117.6×104 t和46.3×104 t,其中工业源废水的总氮和氨氮排放量分别为13.4×104 t和3.5×104 t [1]。虽然占比不大,但氨氮废水成分复杂、生态毒性大,深度处理和资源化利用难度极高[2],排放至水体中可造成富营养化等多重生态危害。基于微藻培养的废水处理技术是依靠微藻代谢过程将废水中的碳氮磷硫等污染物作为生长必需的营养物,从而转化为微藻生物质和高附加值产物,有望在实现废水处理的同时产生经济效益,具有高效、低耗和绿色、可持续的优势,成为氨氮废水深度处理和资源循环利用技术领域的研究热点[3-7]。
嗜硫原始红藻(Galdieria sulphuraria)作为一种极端环境来源的单细胞微藻,具有极度耐酸、耐高温、耐高氨氮、耐高盐和耐重金属离子等突出生理特性,还可合成高价值的耐热型藻蓝蛋白(Phycocyanin,PC)[8-9]。有研究表明,嗜硫原始红藻可有效去除市政污水、厨余酶解液和工业废水中的氨氮,并可联产高蛋白(主要是藻蓝蛋白),表明该藻在废水处理和资源化利用领域极具应用潜力[10-14]。采用非灭菌的市政废水连续流加培养Galdieria sulphuraria CCMEE 5587. 1,可在去除氨氮的同时抑制粪肠球菌和大肠杆菌等致病菌的繁殖,表明该藻在非灭菌条件下处理废水可大大降低运行成本[12],提升水体安全性。最近,本团队在非灭菌光发酵系统中,利用混养Galdieria sulphuraria UTEX 2919处理高氨氮工业废水(
NH+4 质量浓度为3 360~5 540 mg·L−1),发现在补料分批培养模式下控制葡萄糖的补加,可将NH+4 去除速率显著提高到1.71 g·(L·d)−1[11,15]。在调节葡萄糖添加量以优化培养基C/N后,发现C/N为6时细胞生长、NH+4 去除速率和藻胆蛋白(Phycobiliprotein,PBP)合成都比C/N为9、12和16时显著提高,表明低C/N有利于铵氮同化、生物质和蛋白生产[11]。因此,进一步优化葡萄糖补加模式以维持低C/N,可避免碳源补充过量导致高C/N,有利于氨氮的快速去除并降低碳源成本,这对于非灭菌光发酵体系降本增效尤为重要。目前尚未有此类报道,值得深入研究。为强化铵根去除并联产高蛋白生物质的技术体系、降低碳源成本,本研究针对5 L光发酵罐中的非灭菌光发酵过程,分别采用间歇补糖和连续流加补糖模式,系统比较了其对细胞生长、铵根去除速率、蛋白质合成和藻胆蛋白积累的影响,以期为今后高氨氮废水的高效处理和资源化利用提供参考。
-
嗜硫原始红藻(Galdieria sulphuraria UTEX 2919)购自美国UTEX藻种库(https://utex.org/)。高氨氮工业废水由中国石化催化剂有限公司长岭分公司提供,具体成分见表1。原废水在使用前先经过0.8 μm孔径水相滤膜过滤,再用去离子水稀释至
NH+4 质量浓度为3 300~5 200 mg·L−1后,用于配制废水2MA培养基[11]。废水主要提供了配方中的氮源(硫酸铵)。在废水2MA培养基中加入20 g·L−1葡萄糖为碳源后配置成混养培养基,使用浓硫酸调节培养基初始pH至2.5。将嗜硫原始红藻无菌藻种接种至含已灭菌废水2MA混养培养基的锥形瓶中,置于温度为35 ℃、转速为150 r·min−1和光照强度为200 μmol·(m2·s)−1的恒温光照摇床中,培养5~7 d后细胞生长至对数后期,作为本实验的种子液。
-
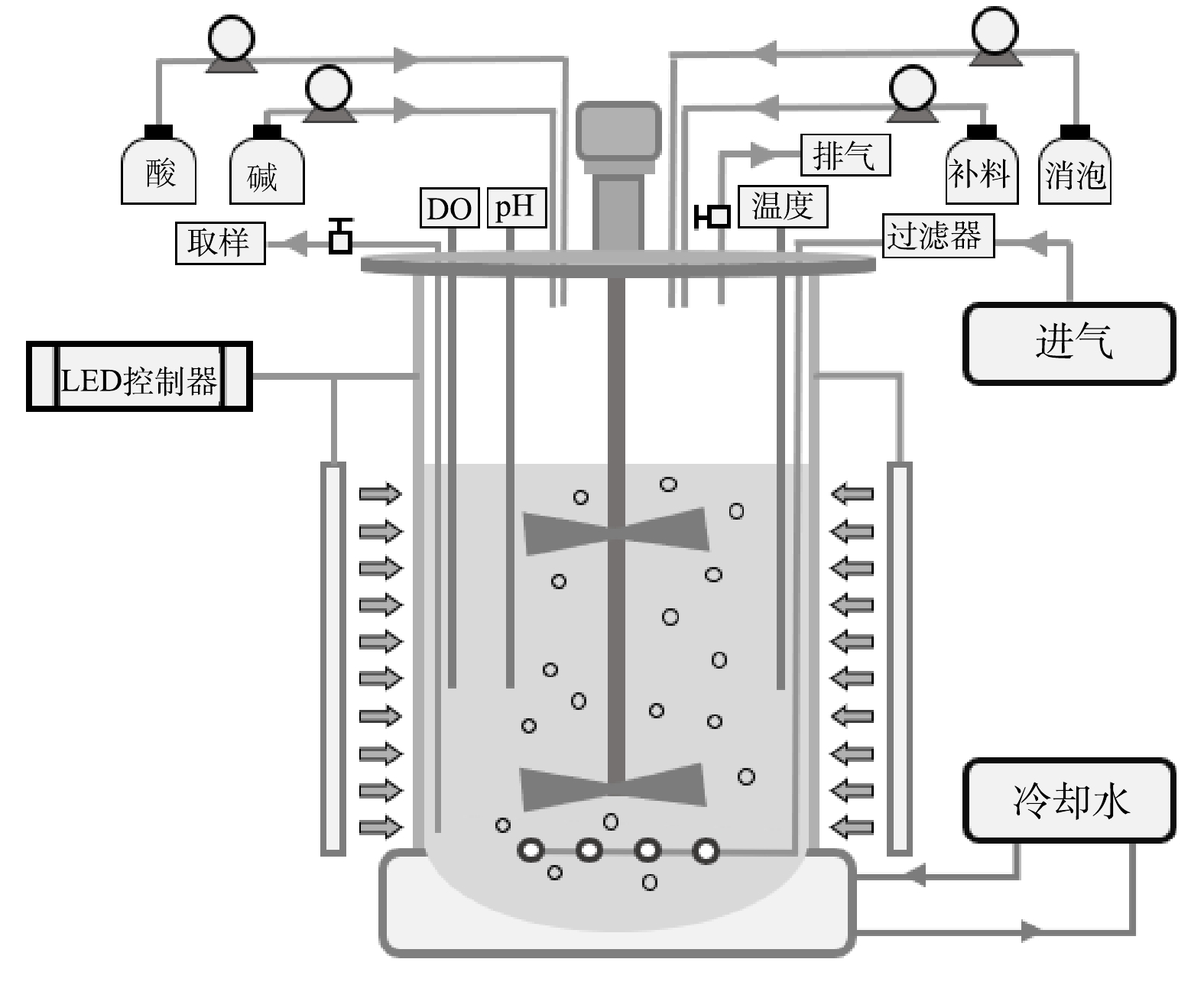
以白光LED灯板四面环绕在5 L玻璃发酵罐外,构建外置光源的光发酵罐(图1),2批次补料分批培养的操作参考郑雅莉[11]的研究方法。配制初始
NH+4 质量浓度约为5 000 mg·L−1的非灭菌废水2MA混养培养基,将离心浓缩后的种子液接种于装有该培养基的5 L光发酵罐中,工作体积为3.5 L。初始培养基中葡萄糖质量浓度为20 g·L−1、接种密度为1.0×108 个·mL−1。光发酵条件为:温度35 ℃、发酵罐外表面光照强度450 μmol·(m2·s)−1、通气量240 L·h−1、搅拌转速350 r·min−1。培养过程中连续自动监测pH和溶解氧变化,每24 h取样测定各水质指标,根据磷酸根消耗情况补加KH2PO4母液至初始水平。当第1批次废水培养基中NH+4 质量浓度下降至≤20 mg·L−1时,放出部分藻液并补加等体积的新鲜非灭菌废水2MA混养培养基至工作体积3.5 L,使NH+4 质量浓度恢复为5 000 mg·L−1,随后进入第2批次培养。当NH+4 质量浓度再次下降至≤150 mg·L−1时结束培养。 -
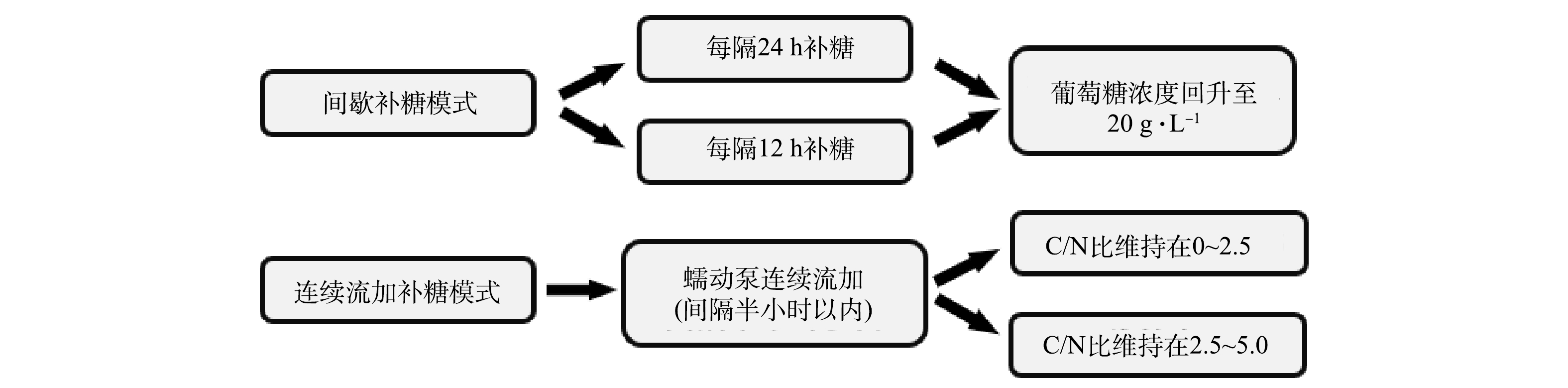
1)间歇补糖模式。在上述分批补料培养过程中,当初始葡萄糖质量浓度首次下降至≤10 g·L−1时,开始补加无菌葡萄糖母液到初始质量浓度20 g·L−1并继续培养。在2台5 L光发酵罐上分别设置补糖操作间隔时间为12 h和24 h的2个实验组(图2)。其中,在12 h实验组中进行了2个批次补料分批培养以验证重现性;在24 h实验组中进行了1个批次培养并至144 h时结束。在此过程中每24 h取样测定细胞密度、生物量质量浓度和水质指标(葡萄糖、铵根、磷酸根质量浓度等),并计算生物量产率、铵根去除速率等。藻液经离心、洗涤、冻干后测定其中蛋白和藻胆蛋白含量及产率等。
2)连续流加补糖模式。在2台5 L光发酵罐上设置2个C/N组,即采用蠕动泵连续流加高浓度葡萄糖母液,使一个发酵罐C/N维持在0~2.5(低C/N组),另一个发酵罐C/N维持在2.5~5.0(高C/N组),2个C/N组中分别进行2个批次光发酵实验(图2)。光发酵过程中每24 h取样分析水质指标(葡萄糖、铵根、磷酸根质量浓度等)并计算该时间点C/N;测定细胞密度和生物量质量浓度,并计算生物量产率、铵根去除速率等。藻液经离心、洗涤、冻干后测定其中蛋白和藻胆蛋白含量及产率等。
-
光发酵液中细胞密度采用美国Beckman Coulter 的CytoFLEX流式细胞仪测定;生物量质量浓度采用干重法测定,即藻液在8 000 r·min−1下离心3 min获得藻泥,用超纯水将藻泥重悬、洗涤、再离心重复2次,置于60 ℃热风干燥箱中烘干至恒重;葡萄糖质量浓度采用深圳市西尔曼科技有限公司的M-100生物传感器测定;
NH+4 和PO3−4 质量浓度均采用意大利HANNA HI83200多参数水质分析仪测定。洗净藻泥在−40 ℃下冻干2~3 d获得冻干藻粉。冻干藻粉中蛋白质含量采用凯氏定氮法测定;藻胆蛋白含量采用美国BioTek Cytation 5多功能微孔板检测仪测定。 -
实验数据采用平均值±标准偏差表示,采用IBM SPSS Statistics 22和Origin 2017进行统计分析。采用单因素方差分析(P<0.05)对数据进行显著性检验,评价实验数据的统计学意义。
-
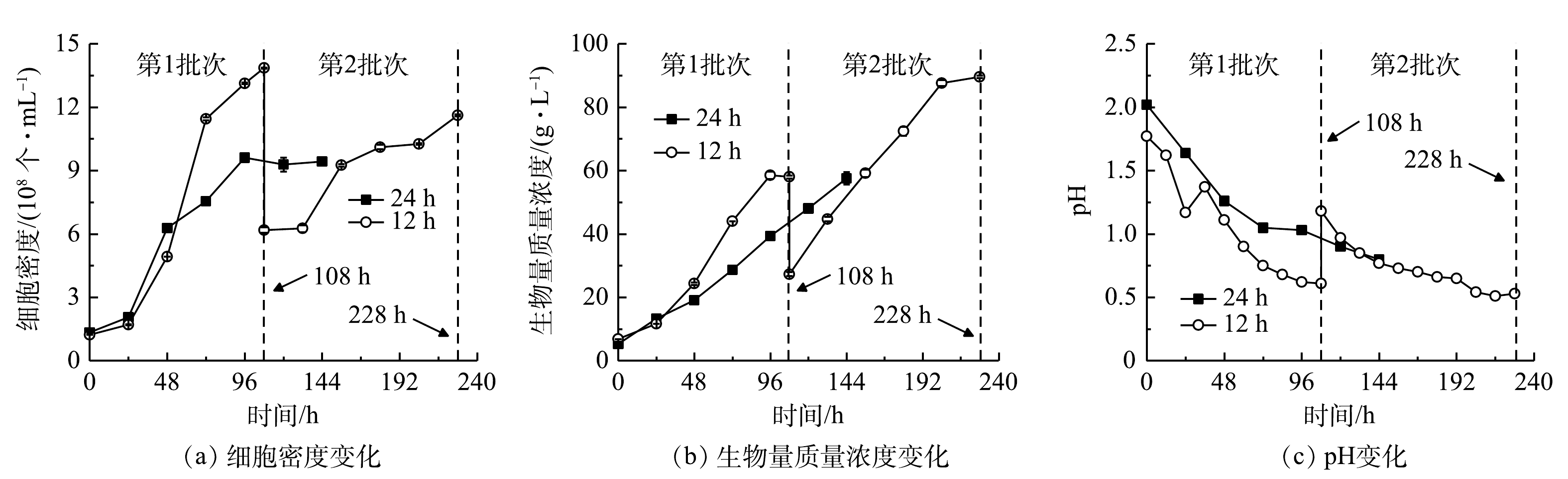
1)细胞生长。细胞密度与生物量质量浓度结果见图3(a)、图3(b),计算得到比生长速率和生物量产率。在24 h组进行了1个批次补料,细胞密度在96 h达到峰值(9.61×108 个·mL−1),最终比生长速率、生物量质量浓度和产率分别为0.33 d−1、57.60 g·L−1和8.73 g·(L·d)−1。在12 h组进行了2个批次分批培养,在108 h时结束第1批次培养,此时培养基中
NH+4 质量浓度降为0 mg·L−1,细胞仍处于对数生长期,最高细胞密度、比生长速率、生物量质量浓度和产率分别为1.39×109 个·mL−1、0.54 d−1、58.00 g·L−1和11.36 g·(L·d)−1;第2批次中最大细胞密度、比生长速率、生物量质量浓度和产率分别为1.16×109 个·mL−1、0.13 d−1、89.55 g·L−1和12.45 g·(L·d)−1。经比较可知,第2批次比生长速率相比第1批次显著降低(P<0.01),而生物量质量浓度和产率则比第1批次提高了54.40%和9.60%。12 h组第1批次培养与24 h组相比,两者生物量质量浓度没有显著差异,而细胞密度、比生长速率和生物量产率则分别提高了46.92%、65.46%和30.21%,差异显著(P<0.01)。这表明缩短补糖间隔时间有利于细胞的快速增长和生物量积累。本研究进行了补加葡萄糖的非灭菌发酵。虽然高氨氮工业废水培养基中含有土著细菌,但该藻仍能快速增殖获得高细胞密度、高生物量质量浓度和产率。这种超强的抗微生物污染、易于形成种群优势的能力,可能与该藻的极度耐酸能力有关。光发酵体系pH快速下降情况见图3(c)。有研究表明,G. sulphuraria CCMEE 5587. 1在非灭菌反应器中处理市政废水时,在去除氨氮、BOD5和磷酸根的同时可有效去除原废水中粪肠球菌和大肠杆菌等致病菌[12],说明培养体系中极低的pH可显著抑制或杀死废水中大部分致病菌[16]。本团队的前期研究中在非无菌体系下培养嗜硫原始红藻UTEX 2919处理高氨氮工业废水,结果表明,原废水中主要细菌的相对丰度显著下降,表明体系中的细菌水平不会对该体系的高效运行产生负面影响[15]。
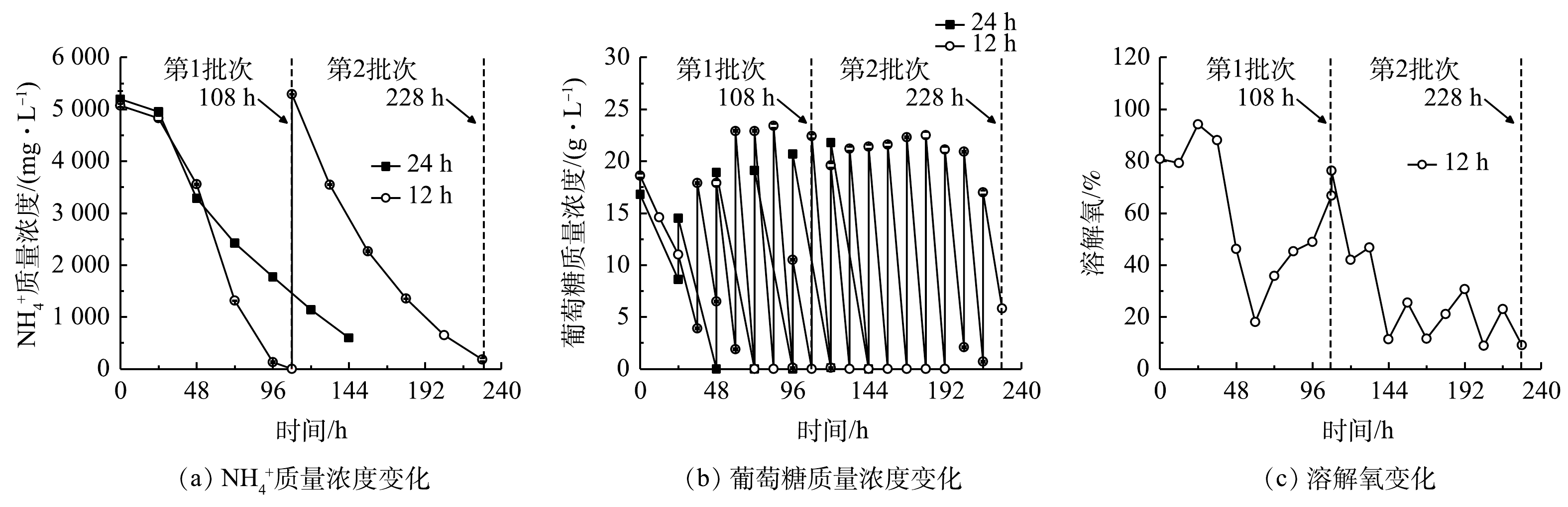
2)葡萄糖消耗和氨氮去除。细胞大量增殖和生物量积累伴随着废水培养基中葡萄糖和
NH+4 的快速消耗。由图4(a)和表2可以看出,当非灭菌废水培养基中初始NH+4 质量浓度为5 070.00~5 290.00 mg·L−1时,12 h组比24 h组的葡萄糖消耗速率和NH+4 去除速率更高,且前者的第1批次培养可达到最高NH+4 去除速率(1 234.38 mg·(L·d)−1),比24 h组提高了61.09%(P<0.01),而葡萄糖消耗速率也提高了57.39%。在12 h组第2批次培养中虽然葡萄糖消耗速率提高了48.87%,但NH+4 去除速率较第1批次下降了17.21%。其原因可能是:虽然微藻生物量有所提高,但葡萄糖补加量、光照强度、搅拌速度、通气量等参数没有提高,无法维持嗜硫原始红藻生长的最佳条件;此外,在第2批次培养中该藻代谢更多的碳源以促进生物量的快速积累并达到最高值89.55 g·L−1(图3(b))。有研究[17]表明,微藻自养时的光合作用效率比混养时更高,产氧速率高于耗氧速率,使得藻液的溶解氧(dissolved oxygen,DO)水平不断上升;而当微藻混养生长时,细胞呼吸作用增强,外部有机碳源(如葡萄糖)的存在可在一定程度上限制光合作用活性,导致微藻耗氧速率高于产氧速率,最终使得藻液的溶解氧水平有所降低。此外,葡萄糖限制或缺乏会抑制微藻的生长[11,18]。本研究结果表明,12 h组的溶解氧水平快速下降至低于10%(图4(c)),这与更高的葡萄糖消耗速率和生物量质量浓度密切相关。这说明低溶解氧水平并未影响细胞高密度发酵和废水培养基中
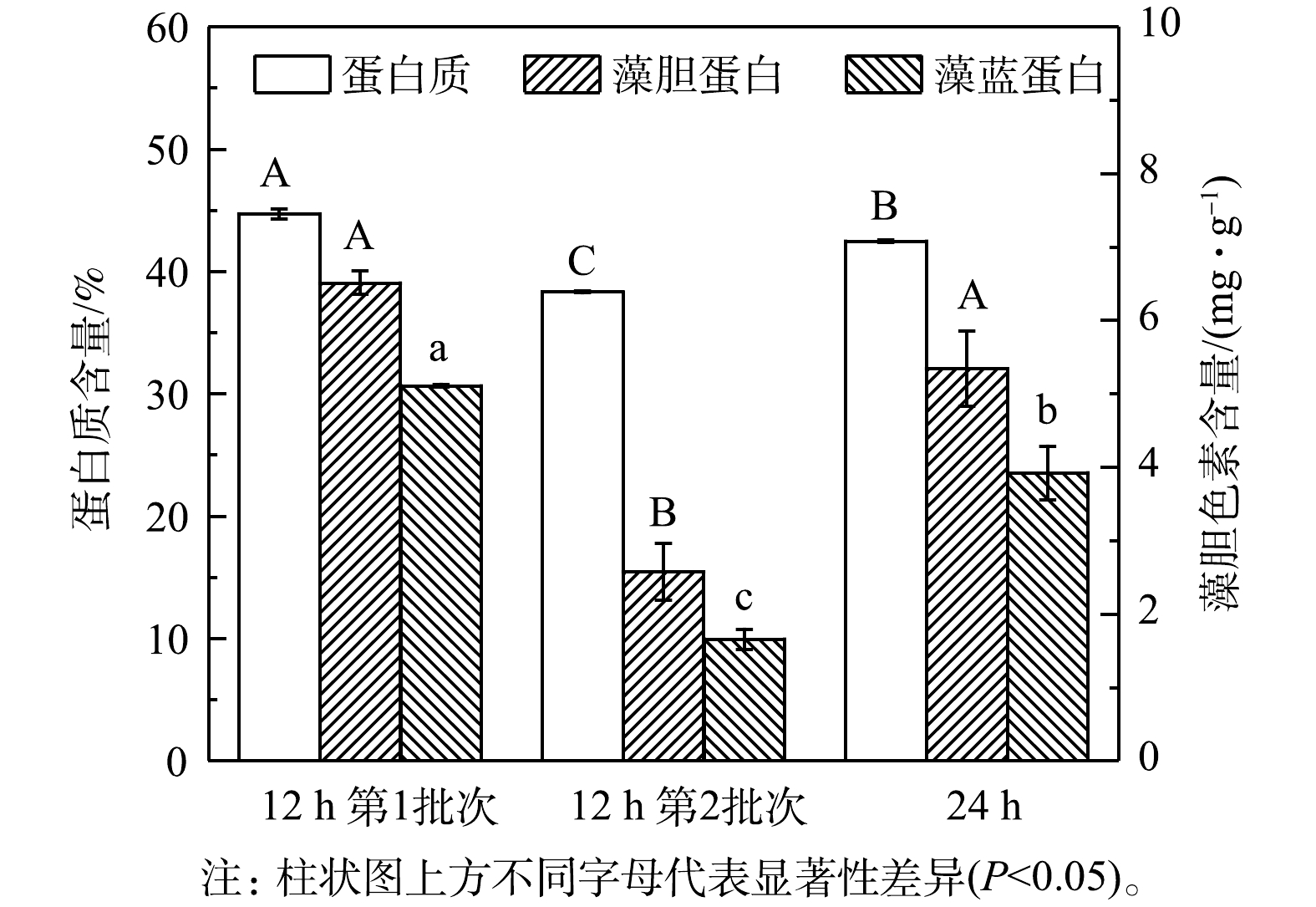
NH+4 的去除速率,最终NH+4 去除率仍能达到96%以上(表2)。3)粗蛋白和藻胆蛋白生产。对光发酵体系中粗蛋白和藻胆蛋白生产进行了分析,结果见图5和表3。在12 h组第1批次培养中可获得最高粗蛋白、藻胆蛋白(PBP)和藻蓝蛋白(PC)含量,分别为44.71%、6.51 mg·g−1和5.11 mg·g−1,比第2批次分别高16.68%、152.71%和208.18%,也比24 h组分别高5.25%、21.90%和30.17%。同时,12 h组中第1批次培养可获得最高葡萄糖对
NH+4 的同化系数YN/G,为45.64 mg·g−1,比第2批次高79.79%(表2)。这表明在第1批次培养中消耗每单位葡萄糖可同化更多的NH+4 ,引起C/N快速下降,从而有利于蛋白质合成和积累。已有研究表明,培养基中C/N可影响微藻代谢流的走向,降低C/N可促进更多碳源流向氮代谢并合成更多含氮化合物(包括蛋白质),相反则会促进更多碳源流向含碳有机物(如碳水化合物、脂质)[19,20]。因此,缩短补糖间隔时间为12 h,不仅有利于细胞快速生长,更有利于氨氮转化为蛋白质。此外,部分藻液采收后补充新鲜培养基以维持培养体积的恒定,能降低藻液中生物量质量浓度从而提高藻液的透光性、优化了罐内的光分布,最终提高了生物量质量浓度[21]。因此,补糖间隔时间为12 h,能显著提高生物量产率并促进废水培养基中NH+4 转化为蛋白质,但葡萄糖消耗量仍较高。后续实验可对补糖模式进行进一步优化,以期达到降本增效的目的。 -
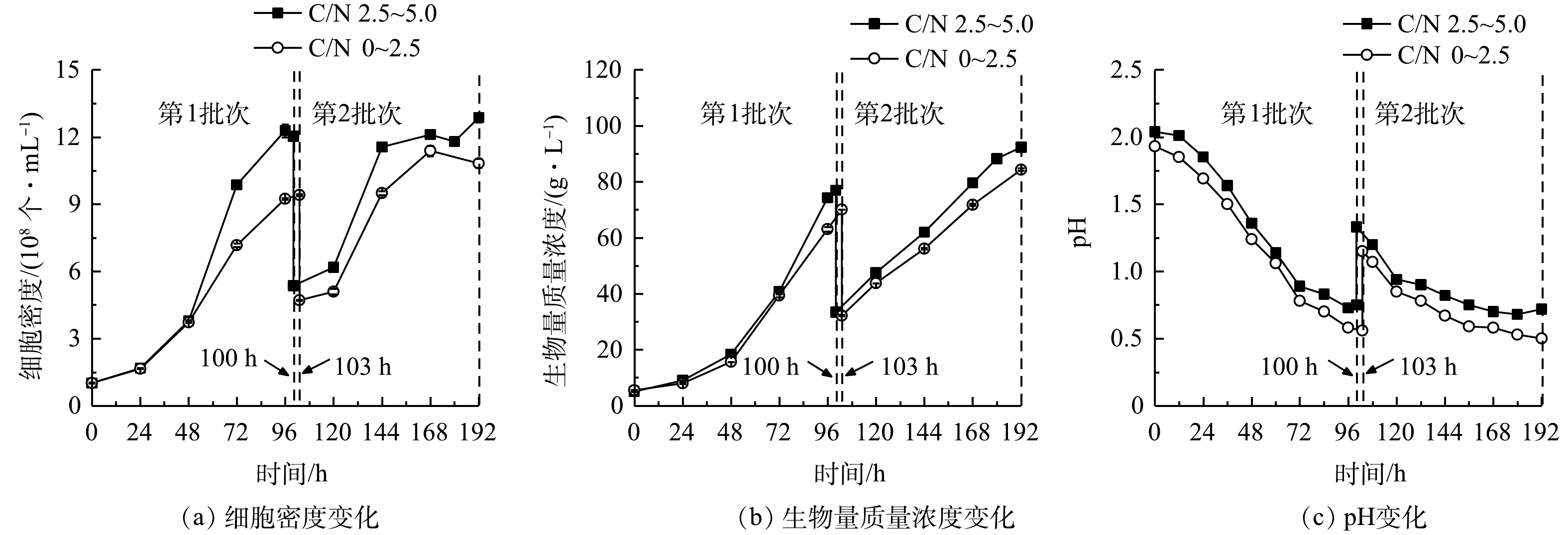
1)细胞生长。连续流加补糖模式下细胞生长、生物量生产和pH变化结果如图6所示,计算后可比较比生长速率和生物量产率(表4)。前述结果表明,高C/N组中第1批次最终细胞密度和生物量质量浓度可达1.20×109 个·mL−1和76.95 g·L−1,比同批次低C/N组分别提高了27.77%和9.77%;在第2批次培养中,高C/N组可达到最大细胞密度(1.29×109 个·mL−1)和生物量质量浓度(92.30 g·L−1),比同批次低C/N组提高了18.85%和9.39%。与低C/N组相比,高C/N组可获得最高的比生长速率(0.59 d−1)和生物量产率(17.24 g·(L·d)−1)(表4)。本研究结果与SLOTH等报道的结果[22]相符,该研究分别利用葡萄糖和硫酸铵为碳氮源,探讨了嗜硫原始红藻在不同C/N下连续流加培养中的细胞生长和PC积累情况,结果表明,在一定范围内随着C/N提高,细胞生长加快、PC合成增加。我们前期研究证实,提高培养基初始C/N能获得混养嗜硫原始红藻更高生物量质量浓度和PBP含量。这说明在一定程度上提高C/N,能促进该藻的细胞生长和生物量积累[11]。
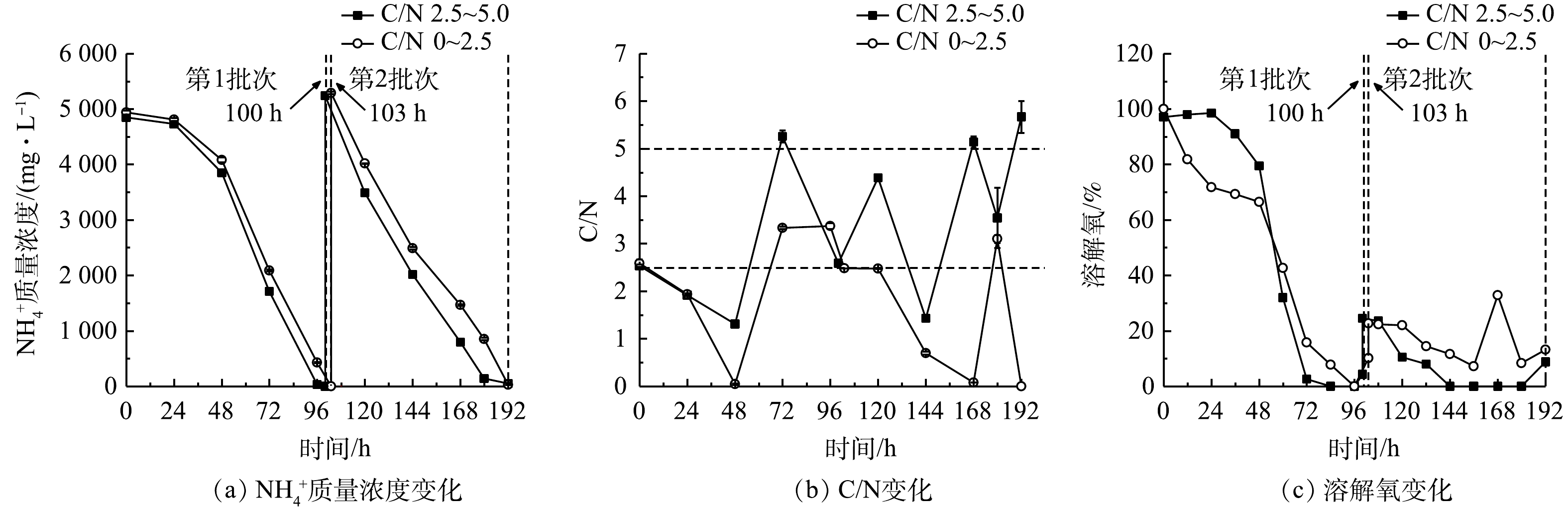
2)葡萄糖消耗和氨氮去除。本研究表明,细胞的大量增殖伴随着废水培养基葡萄糖和
NH+4 的快速消耗,结果如图7(a)、图7(b)和表5所示。高C/N组第1批次的NH+4 去除速率较同批次的低C/N组提高了4.68%,而葡萄糖消耗速率则两者相当;第2批次中NH+4 去除速率达到最高值(1 529.25 mg·(L·d)−1),较同批次的低C/N组提高了7.76%,而葡萄糖消耗速率(42.84 g·(L·d)−1)则降低了9.17%。可见,高C/N组对NH+4 去除作用得到了显著提升。本研究结果与郑雅莉[11]和XU等[23]的报道相符,前者在摇瓶体系中优化兼养培养基中的C/N,发现嗜硫原始红藻UTEX 2919在C/N为3~16范围内,NH+4 去除速率呈先升高后降低趋势,并在C/N为6.0时达到峰值[11];后者比较了普通小球藻(C. vulgaris FACHB-31)在不同C/N下对合成废水总氮的去除情况,结果表明在红光条件下在一定程度上提高C/N至6.0时,该藻对废水总氮的去除效率达74.61%,较C/N为3和9的组别高。这说明,连续流加补糖适当提高培养基C/N,不仅可降低原料(葡萄糖)用量,还能提高废水培养基中NH+4 去除效果[23],达到降本增效(NH+4 去除率接近100%)的目的。高C/N组第2批次中的
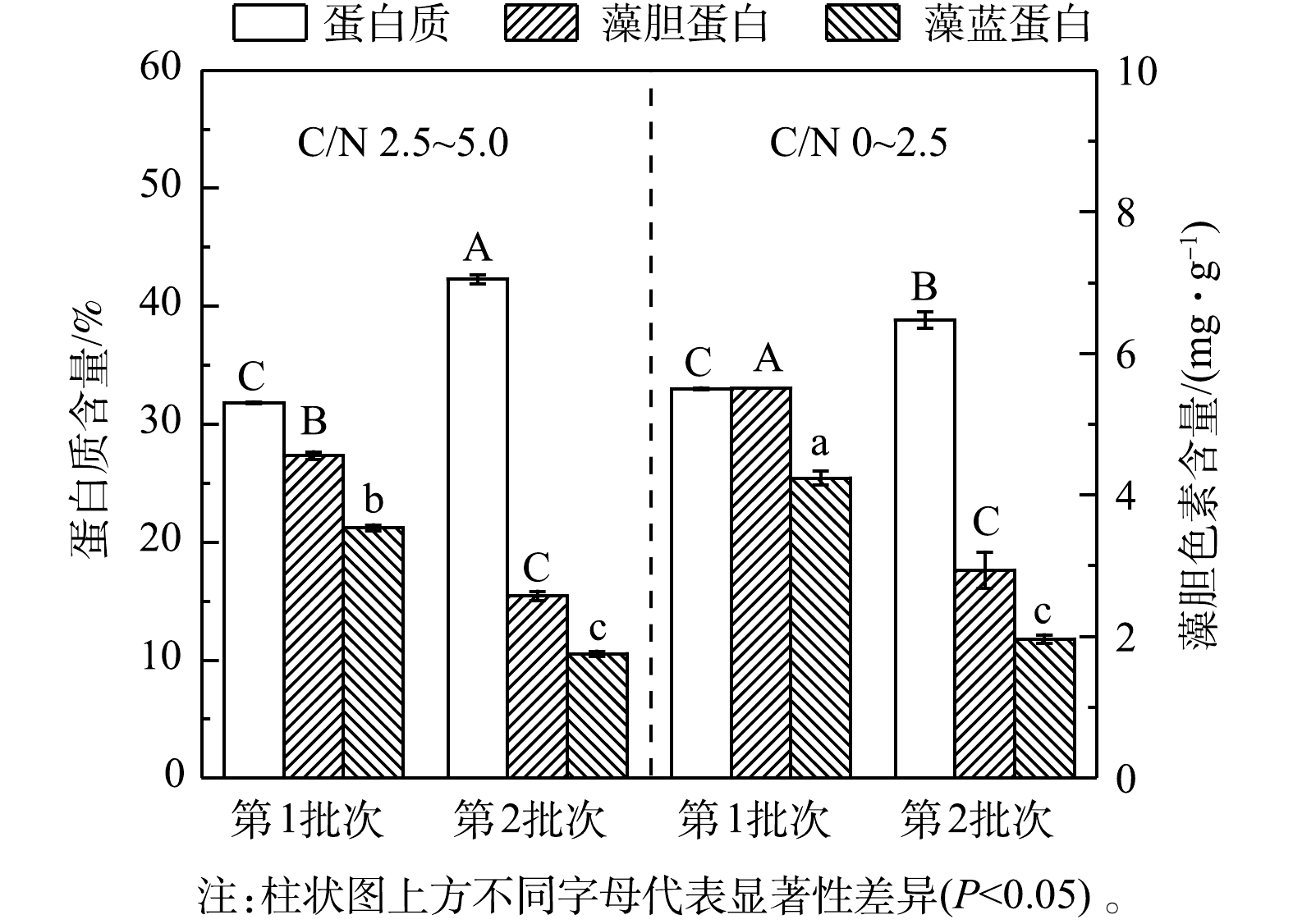
NH+4 去除速率和葡萄糖消耗速率比第1批次分别提高了26.97%和31.66%。这可能是由于经第1批次培养后细胞对高氨氮废水的环境适应性提高,在第2批次中对碳氮的吸收和转化更高效。有研究表明,嗜硫原始红藻可在葡萄糖质量浓度高达200 g·L−1和高浓度无机盐的高渗环境中生长[24],因此,本发酵体系中的废水环境对该藻的生长没有抑制作用,且连续流加补糖为细胞生长提供了充足的有机碳源,有效维持了该藻的混养生长,溶解氧水平亦呈快速下降并维持在极低水平,但未产生生长抑制(图7(c))。3)粗蛋白和藻胆蛋白生产。实验结果表明(图8和表6),在高C/N组的第1批次培养中,最终粗蛋白质含量(31.81%)、PC含量(3.53 mg·g−1)和PC产量(109.21 mg·L−1)较同批次的低C/N组分别降低了3.56%、16.67%和8.11%;但最终蛋白质产量(21.64 g·L−1)却提高了7.07%;在第2批次培养中,最终粗蛋白含量(42.29%)和产量(26.55 g·L−1)达到最高,较同批次的低C/N组别分别提高了8.93%和27.32%; PC含量下降了10.44%,但PC产量却提高了43.68%。这进一步说明:高C/N组有利于提高粗蛋白含量和生物量质量浓度,但PC含量和产量下降可能与混养培养模式有关。一方面,高C/N组提高了细胞生长和蛋白质积累能力;另一方面,在第2批次培养中对糖的代谢加快,呼吸作用增强而光合作用受限制。PC为嗜硫原始红藻的捕光色素复合体组成之一,主要参与该藻的光合作用,当光合作用受抑制时,PC的合成受阻[25],因此,PC的含量与产量均呈下降趋势。
4)间歇补糖和连续流加补糖模式的比较。经比较优化的连续流加补糖(高C/N组)和间歇补糖(补糖间隔时间为12 h)2种模式下废水中
NH+4 的去除效率,发现连续流加补糖模式的高C/N组(表5),最高NH+4 去除速率(1 529.25 mg·(L·d)−1)和平均同化系数YN/G(33.47 mg·g−1)均高于补糖间隔时间为12 h实验组(1 234.38 mg·(L·d)−1和31.52 mg·g−1),且废水处理时间显著缩短了36~50 h。郑雅莉[11]在5L光发酵罐中混养培养嗜硫原始红藻,以补料分批培养模式处理高氨氮工业废水,在前2个批次的培养中平均NH+4 去除速率为1 123.92 mg·(L·d)−1。而本研究采用高C/N的连续流加补糖模式,2个批次培养的平均NH+4 去除速率为1 352.70 mg·(L·d)−1,比前者提高20.36%。这表明高C/N(2.5~5.0)的连续流加补糖模式,可强化非灭菌的光发酵体系对废水中NH+4 的去除效果并降低碳源成本,同时提高粗蛋白的产量和产率(表6)。PC天然无毒,具有良好的荧光特性和抗氧化、抗癌、神经保护以及抗炎等多种生理活性,现已应用于医学检测、药品和功能性食品等领域[26]。来源于嗜硫原始红藻的PC相比于其它来源的PC具有更高热稳定性(在60 ℃下依然保持稳定)[27],因此,更具开发应用潜力。本研究利用嗜硫原始红藻在高效去除废水中铵根的同时高产富含蛋白质、PBP和PC的生物质,获得的生物质有望作为微藻有机肥和畜禽饲料原料,以提高农作物和畜禽的产量和质量,实现废水处理和资源化利用的双赢目标。
-
1)在间歇补糖模式下,缩短补糖间隔时间可促进细胞生长、生物量生产并提高
NH+4 去除速率和去除率。2)采用高C/N(2.5~5.0)的连续流加补糖模式,能进一步强化细胞生长并提高对废水中
NH+4 的去除能力。2个批次的平均NH+4 去除速率为1 352.70 mg·(L·d)−1,第2批次中最高可达到1 529.25 mg·(L·d)−1。3)优化后的高C/N连续流加补糖模式,可降低培养过程中葡萄糖用量,从而降低发酵原料成本,进而可有效提高该技术在高氨氮废水的规模化处理和资源化利用中的应用潜力。
非灭菌光发酵嗜硫原始红藻高效去除工业废水中铵根的补糖模式优化
Optimization of glucose feeding mode for high-efficient ammonium removal from industrial wastewater by non-sterile photo-fermentation of Galdieria sulphuraria
-
摘要: 使用高氨氮工业废水配制混养培养基,在5 L光发酵罐中对嗜硫原始红藻(Galdieria sulphuraria)进行非灭菌条件下的分批和补料分批培养,通过比较间歇补糖和连续流加补糖模式,优化确定了生物量高产率、高
NH+4 去除速率的补糖模式。结果表明,2种模式均能促进生物量生产和NH+4 的去除,其中C/N为2.5~5.0的连续流加补糖是最佳补糖模式。在此模式下,最高NH+4 去除速率达到1 529.25 mg·(L·d)−1,最终生物量质量浓度、蛋白质和藻蓝蛋白(PC)含量分别为92.30 g·L−1、42.29%和1.76 mg·g−1,比12 h间歇补糖模式(最高NH+4 去除速率为1 234.38 mg·(L·d)−1,生物量质量浓度为89.55 g·L−1,蛋白质和PC含量为别为38.32%和1.66 mg·g−1)分别提高了23.89%、3.07%、10.36%和6.02%,葡萄糖消耗量也显著降低。以上构建的基于优化补糖模式的非灭菌光发酵技术能有效促进嗜硫原始红藻生物量生产,可实现废水中NH+4 的高效去除并联产蛋白质,从而达到废水高效脱氮、资源化利用和降本增效等多重目的。Abstract: In this study, the batch and fed-batch cultures of G. sulphuraria were carried out in the mixotrophic medium prepared from non-sterile high-ammonium industrial wastewater in 5 L photo-fermenter. The optimized feeding mode of glucose was achieved by comparative study of intermittent and continuous glucose feeding for high-yield biomass production with highNH+4 removal rate. The results indicated that both feeding modes could enhance biomass production andNH+4 removal, of which the continuous feeding mode with C/N ratio of 2.5~5.0 was selected as the optimal mode. Under this mode, the maximalNH+4 > removal rate was 1 529.25 mg·(L·d)−1, the final biomass concentration, protein and PC content were 92.30 g·L−1, 42.29% and 1.76 mg·g−1, respectively, which were 23.89%, 3.07%, 10.36% and 6.02% higher than those of intermittent glucose feeding mode with operation time of 12 h: maximumNH+4 removal rate of 1 234.38 mg·(L·d)−1, biomass concentration of 89.55 g·L−1, protein content of 38.32%, PC content of 1.66 mg·g−1, respectively. The amount of glucose consumption also decreased significantly. The developed non-sterile photo-fermentation technology based on the optimal glucose feeding mode in this study was effective to enhance the biomass production of G. sulphuraria and achieve the high-effectiveNH+4 removal with coproduction of protein, which could meet the multiple purpose of high-effectiveNH+4 removal from wastewater, resource utilization and lowing cost with enhancing efficiency. -
2019年我国废水总氮和氨氮排放量分别为117.6×104 t和46.3×104 t,其中工业源废水的总氮和氨氮排放量分别为13.4×104 t和3.5×104 t [1]。虽然占比不大,但氨氮废水成分复杂、生态毒性大,深度处理和资源化利用难度极高[2],排放至水体中可造成富营养化等多重生态危害。基于微藻培养的废水处理技术是依靠微藻代谢过程将废水中的碳氮磷硫等污染物作为生长必需的营养物,从而转化为微藻生物质和高附加值产物,有望在实现废水处理的同时产生经济效益,具有高效、低耗和绿色、可持续的优势,成为氨氮废水深度处理和资源循环利用技术领域的研究热点[3-7]。
嗜硫原始红藻(Galdieria sulphuraria)作为一种极端环境来源的单细胞微藻,具有极度耐酸、耐高温、耐高氨氮、耐高盐和耐重金属离子等突出生理特性,还可合成高价值的耐热型藻蓝蛋白(Phycocyanin,PC)[8-9]。有研究表明,嗜硫原始红藻可有效去除市政污水、厨余酶解液和工业废水中的氨氮,并可联产高蛋白(主要是藻蓝蛋白),表明该藻在废水处理和资源化利用领域极具应用潜力[10-14]。采用非灭菌的市政废水连续流加培养Galdieria sulphuraria CCMEE 5587. 1,可在去除氨氮的同时抑制粪肠球菌和大肠杆菌等致病菌的繁殖,表明该藻在非灭菌条件下处理废水可大大降低运行成本[12],提升水体安全性。最近,本团队在非灭菌光发酵系统中,利用混养Galdieria sulphuraria UTEX 2919处理高氨氮工业废水(
NH+4 NH+4 NH+4 为强化铵根去除并联产高蛋白生物质的技术体系、降低碳源成本,本研究针对5 L光发酵罐中的非灭菌光发酵过程,分别采用间歇补糖和连续流加补糖模式,系统比较了其对细胞生长、铵根去除速率、蛋白质合成和藻胆蛋白积累的影响,以期为今后高氨氮废水的高效处理和资源化利用提供参考。
1. 材料与方法
1.1 藻种和种子液培养
嗜硫原始红藻(Galdieria sulphuraria UTEX 2919)购自美国UTEX藻种库(https://utex.org/)。高氨氮工业废水由中国石化催化剂有限公司长岭分公司提供,具体成分见表1。原废水在使用前先经过0.8 μm孔径水相滤膜过滤,再用去离子水稀释至
NH+4 表 1 高氨氮工业废水组成成分[11]Table 1. Composition of high-ammonium industrial wastewater组成成分 质量浓度/(mg·L−1) 组成成分 质量浓度/(mg·L−1) NH+4 9 686.00 Zn2+ 89.98 Na+ 7 623.00 Mn2+ 2.48 Mg2+ 63.49 Co2+ 0.18 K+ 363.00 PO3−4 0.00 Ca2+ 27.66 SO2−4 725.10 Cu2+ 0.21 TOC 32.30 将嗜硫原始红藻无菌藻种接种至含已灭菌废水2MA混养培养基的锥形瓶中,置于温度为35 ℃、转速为150 r·min−1和光照强度为200 μmol·(m2·s)−1的恒温光照摇床中,培养5~7 d后细胞生长至对数后期,作为本实验的种子液。
1.2 补料分批培养
以白光LED灯板四面环绕在5 L玻璃发酵罐外,构建外置光源的光发酵罐(图1),2批次补料分批培养的操作参考郑雅莉[11]的研究方法。配制初始
NH+4 NH+4 NH+4 NH+4 1.3 2种补糖模式的比较研究
1)间歇补糖模式。在上述分批补料培养过程中,当初始葡萄糖质量浓度首次下降至≤10 g·L−1时,开始补加无菌葡萄糖母液到初始质量浓度20 g·L−1并继续培养。在2台5 L光发酵罐上分别设置补糖操作间隔时间为12 h和24 h的2个实验组(图2)。其中,在12 h实验组中进行了2个批次补料分批培养以验证重现性;在24 h实验组中进行了1个批次培养并至144 h时结束。在此过程中每24 h取样测定细胞密度、生物量质量浓度和水质指标(葡萄糖、铵根、磷酸根质量浓度等),并计算生物量产率、铵根去除速率等。藻液经离心、洗涤、冻干后测定其中蛋白和藻胆蛋白含量及产率等。
2)连续流加补糖模式。在2台5 L光发酵罐上设置2个C/N组,即采用蠕动泵连续流加高浓度葡萄糖母液,使一个发酵罐C/N维持在0~2.5(低C/N组),另一个发酵罐C/N维持在2.5~5.0(高C/N组),2个C/N组中分别进行2个批次光发酵实验(图2)。光发酵过程中每24 h取样分析水质指标(葡萄糖、铵根、磷酸根质量浓度等)并计算该时间点C/N;测定细胞密度和生物量质量浓度,并计算生物量产率、铵根去除速率等。藻液经离心、洗涤、冻干后测定其中蛋白和藻胆蛋白含量及产率等。
1.4 分析与检测[11]
光发酵液中细胞密度采用美国Beckman Coulter 的CytoFLEX流式细胞仪测定;生物量质量浓度采用干重法测定,即藻液在8 000 r·min−1下离心3 min获得藻泥,用超纯水将藻泥重悬、洗涤、再离心重复2次,置于60 ℃热风干燥箱中烘干至恒重;葡萄糖质量浓度采用深圳市西尔曼科技有限公司的M-100生物传感器测定;
NH+4 PO3−4 1.5 数据处理与统计分析
实验数据采用平均值±标准偏差表示,采用IBM SPSS Statistics 22和Origin 2017进行统计分析。采用单因素方差分析(P<0.05)对数据进行显著性检验,评价实验数据的统计学意义。
2. 结果与讨论
2.1 间歇补糖模式下细胞生长与废水中铵根的去除
1)细胞生长。细胞密度与生物量质量浓度结果见图3(a)、图3(b),计算得到比生长速率和生物量产率。在24 h组进行了1个批次补料,细胞密度在96 h达到峰值(9.61×108 个·mL−1),最终比生长速率、生物量质量浓度和产率分别为0.33 d−1、57.60 g·L−1和8.73 g·(L·d)−1。在12 h组进行了2个批次分批培养,在108 h时结束第1批次培养,此时培养基中
NH+4 本研究进行了补加葡萄糖的非灭菌发酵。虽然高氨氮工业废水培养基中含有土著细菌,但该藻仍能快速增殖获得高细胞密度、高生物量质量浓度和产率。这种超强的抗微生物污染、易于形成种群优势的能力,可能与该藻的极度耐酸能力有关。光发酵体系pH快速下降情况见图3(c)。有研究表明,G. sulphuraria CCMEE 5587. 1在非灭菌反应器中处理市政废水时,在去除氨氮、BOD5和磷酸根的同时可有效去除原废水中粪肠球菌和大肠杆菌等致病菌[12],说明培养体系中极低的pH可显著抑制或杀死废水中大部分致病菌[16]。本团队的前期研究中在非无菌体系下培养嗜硫原始红藻UTEX 2919处理高氨氮工业废水,结果表明,原废水中主要细菌的相对丰度显著下降,表明体系中的细菌水平不会对该体系的高效运行产生负面影响[15]。
2)葡萄糖消耗和氨氮去除。细胞大量增殖和生物量积累伴随着废水培养基中葡萄糖和
NH+4 NH+4 NH+4 NH+4 NH+4 表 2 间歇补糖模式下葡萄糖和NH+4 Table 2. Parameters of glucose andNH+4 补糖间隔时间/h 葡萄糖消耗速率/( g·(L·d)−1) NH+4 NH+4 NH+4 同化系数YN/G/(mg·g−1) 12(第1批次) 27.04±0.11b 5 070. 00±30.00a 100.00±0.00a 1 234. 38±3.13a 45.64±0.07a 12(第2批次) 40.26±0.38a 5 290. 00±5.00a 96.60±0.57b 1 022. 00±5.00b 25.39±0.12b 24 17.18±0.05c 5 195. 00±155.00a 88.49±0.39c 766.25±25.42c 44.59±1.35a 注:a-c同一列不同字母代表均值之间的显著性差异(P<0.05);YN/G表示每消耗单位葡萄糖对应同化 NH+4 有研究[17]表明,微藻自养时的光合作用效率比混养时更高,产氧速率高于耗氧速率,使得藻液的溶解氧(dissolved oxygen,DO)水平不断上升;而当微藻混养生长时,细胞呼吸作用增强,外部有机碳源(如葡萄糖)的存在可在一定程度上限制光合作用活性,导致微藻耗氧速率高于产氧速率,最终使得藻液的溶解氧水平有所降低。此外,葡萄糖限制或缺乏会抑制微藻的生长[11,18]。本研究结果表明,12 h组的溶解氧水平快速下降至低于10%(图4(c)),这与更高的葡萄糖消耗速率和生物量质量浓度密切相关。这说明低溶解氧水平并未影响细胞高密度发酵和废水培养基中
NH+4 NH+4 3)粗蛋白和藻胆蛋白生产。对光发酵体系中粗蛋白和藻胆蛋白生产进行了分析,结果见图5和表3。在12 h组第1批次培养中可获得最高粗蛋白、藻胆蛋白(PBP)和藻蓝蛋白(PC)含量,分别为44.71%、6.51 mg·g−1和5.11 mg·g−1,比第2批次分别高16.68%、152.71%和208.18%,也比24 h组分别高5.25%、21.90%和30.17%。同时,12 h组中第1批次培养可获得最高葡萄糖对
NH+4 NH+4 NH+4 表 3 间歇补糖模式下粗蛋白和藻胆蛋白生产Table 3. Raw protein and phycobiliprotein production under intermittent feeding mode of glucose补糖间隔时间/h 粗蛋白 藻胆蛋白(PBP) 藻蓝蛋白(PC) 产量/(g·L−1) 产率/( g·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 12(第1批次) 22.39±0.02a 4.98±0.00a 120.17±7.13a 26.70±1.58a 87.29±6.76a 19.40±1.50a 12(第2批次) 21.83±0.03a 4.37±0.03b 67.40±33.03a 13.48±6.61a 25.54±10.22b 4.91±2.04b 24 21.79±0.53a 3.63±0.09c 139.13±1.07a 23.19±0.18a 89.10±2.20a 14.85±0.37a 注:a-c同一列不同字母代表均值之间的差异显著(P<0.05)。 2.2 连续流加补糖模式下细胞生长和废水中铵根的去除
1)细胞生长。连续流加补糖模式下细胞生长、生物量生产和pH变化结果如图6所示,计算后可比较比生长速率和生物量产率(表4)。前述结果表明,高C/N组中第1批次最终细胞密度和生物量质量浓度可达1.20×109 个·mL−1和76.95 g·L−1,比同批次低C/N组分别提高了27.77%和9.77%;在第2批次培养中,高C/N组可达到最大细胞密度(1.29×109 个·mL−1)和生物量质量浓度(92.30 g·L−1),比同批次低C/N组提高了18.85%和9.39%。与低C/N组相比,高C/N组可获得最高的比生长速率(0.59 d−1)和生物量产率(17.24 g·(L·d)−1)(表4)。本研究结果与SLOTH等报道的结果[22]相符,该研究分别利用葡萄糖和硫酸铵为碳氮源,探讨了嗜硫原始红藻在不同C/N下连续流加培养中的细胞生长和PC积累情况,结果表明,在一定范围内随着C/N提高,细胞生长加快、PC合成增加。我们前期研究证实,提高培养基初始C/N能获得混养嗜硫原始红藻更高生物量质量浓度和PBP含量。这说明在一定程度上提高C/N,能促进该藻的细胞生长和生物量积累[11]。
表 4 连续流加补糖模式下细胞生长和生物量生产Table 4. Cell growth and biomass production under continuous feeding mode of glucoseC/N 批次 比生长速率/d−1 生物量产率/( g·(L·d)−1) 2.5~5.0 第1批次 0.59±0.00a 17.24±0.11a 第2批次 0.23±0.00c 15.37±0.15b 0~2.5 第1批次 0.52±0.00b 15.05±0.01b 第2批次 0.22±0.00c 14.07±0.06c 注:a-c同一列不同字母代表均值之间的差异显著(P<0.05)。 2)葡萄糖消耗和氨氮去除。本研究表明,细胞的大量增殖伴随着废水培养基葡萄糖和
NH+4 NH+4 NH+4 NH+4 NH+4 NH+4 NH+4 表 5 连续流加补糖模式下葡萄糖和NH+4 Table 5. Parameters of glucose andNH+4 C/N 批次 葡萄糖消耗速率/( g·(L·d)-1) NH+4 NH+4 NH+4 同化系数YN/G/(mg·g-1) 2.5~5.0 第1批次 32.54±0.00c 4 850. 00±25.00b 99.95±0.05a 1 204. 38±5.63c 37.02±0.17a 第2批次 42.84±0.03b 5 237. 50±7.50a 99.00±0.14c 1 529. 25±5.25a 35.70±0.10b 0~2.5 第1批次 32.20±0.00d 4 937. 50±37.50b 100.00±0.00a 1 150. 49±8.74d 35.73±0.27b 第2批次 47.16±0.00a 5 290. 00±20.00a 99.48±0.14b 1 419. 10±3.37b 30.09±0.07c 注:a-d同一列不同字母代表均值之间的差异显著(P<0.05);YN/G表示每消耗单位葡萄糖对应同化NH4+的量。 高C/N组第2批次中的
NH+4 3)粗蛋白和藻胆蛋白生产。实验结果表明(图8和表6),在高C/N组的第1批次培养中,最终粗蛋白质含量(31.81%)、PC含量(3.53 mg·g−1)和PC产量(109.21 mg·L−1)较同批次的低C/N组分别降低了3.56%、16.67%和8.11%;但最终蛋白质产量(21.64 g·L−1)却提高了7.07%;在第2批次培养中,最终粗蛋白含量(42.29%)和产量(26.55 g·L−1)达到最高,较同批次的低C/N组别分别提高了8.93%和27.32%; PC含量下降了10.44%,但PC产量却提高了43.68%。这进一步说明:高C/N组有利于提高粗蛋白含量和生物量质量浓度,但PC含量和产量下降可能与混养培养模式有关。一方面,高C/N组提高了细胞生长和蛋白质积累能力;另一方面,在第2批次培养中对糖的代谢加快,呼吸作用增强而光合作用受限制。PC为嗜硫原始红藻的捕光色素复合体组成之一,主要参与该藻的光合作用,当光合作用受抑制时,PC的合成受阻[25],因此,PC的含量与产量均呈下降趋势。
表 6 连续流加补糖模式下粗蛋白和藻胆蛋白生产Table 6. Raw protein and phycobiliprotein production under continuous feeding mode of glucoseC/N 批次 粗蛋白 藻胆蛋白(PBP) 藻蓝蛋白(PC) 产量/(g·L−1) 产率/( g·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 2.5~5.0 第1批次 21.64±0.07b 5.19±0.02c 156.61±0.77b 37.59±0.18a 109.21±0.99b 26.21±0.24a 第2批次 26.55±0.06a 6.93±0.02a 80.19±4.89c 20.92±1.27b 38.94±1.28c 10.16±0.34b 0~2.5 第1批次 20.21±0.06c 4.71±0.01d 170.37±3.92a 39.70±0.91a 118.85±1.53a 27.69±0.36a 第2批次 20.85±0.31c 5.62±0.08b 66.79±0.96d 18.01±0.26b 27.11±1.95d 7.31±0.53c 注:a-d同一列不同字母代表均值之间的差异显著(P<0.05)。 4)间歇补糖和连续流加补糖模式的比较。经比较优化的连续流加补糖(高C/N组)和间歇补糖(补糖间隔时间为12 h)2种模式下废水中
NH+4 NH+4 NH+4 NH+4 NH+4 PC天然无毒,具有良好的荧光特性和抗氧化、抗癌、神经保护以及抗炎等多种生理活性,现已应用于医学检测、药品和功能性食品等领域[26]。来源于嗜硫原始红藻的PC相比于其它来源的PC具有更高热稳定性(在60 ℃下依然保持稳定)[27],因此,更具开发应用潜力。本研究利用嗜硫原始红藻在高效去除废水中铵根的同时高产富含蛋白质、PBP和PC的生物质,获得的生物质有望作为微藻有机肥和畜禽饲料原料,以提高农作物和畜禽的产量和质量,实现废水处理和资源化利用的双赢目标。
3. 结论
1)在间歇补糖模式下,缩短补糖间隔时间可促进细胞生长、生物量生产并提高
NH+4 2)采用高C/N(2.5~5.0)的连续流加补糖模式,能进一步强化细胞生长并提高对废水中
NH+4 NH+4 3)优化后的高C/N连续流加补糖模式,可降低培养过程中葡萄糖用量,从而降低发酵原料成本,进而可有效提高该技术在高氨氮废水的规模化处理和资源化利用中的应用潜力。
-
表 1 高氨氮工业废水组成成分[11]
Table 1. Composition of high-ammonium industrial wastewater
组成成分 质量浓度/(mg·L−1) 组成成分 质量浓度/(mg·L−1) NH+4 9 686.00 Zn2+ 89.98 Na+ 7 623.00 Mn2+ 2.48 Mg2+ 63.49 Co2+ 0.18 K+ 363.00 PO3−4 0.00 Ca2+ 27.66 SO2−4 725.10 Cu2+ 0.21 TOC 32.30 表 2 间歇补糖模式下葡萄糖和
NH+4 Table 2. Parameters of glucose and
NH+4 补糖间隔时间/h 葡萄糖消耗速率/( g·(L·d)−1) NH+4 NH+4 NH+4 同化系数YN/G/(mg·g−1) 12(第1批次) 27.04±0.11b 5 070. 00±30.00a 100.00±0.00a 1 234. 38±3.13a 45.64±0.07a 12(第2批次) 40.26±0.38a 5 290. 00±5.00a 96.60±0.57b 1 022. 00±5.00b 25.39±0.12b 24 17.18±0.05c 5 195. 00±155.00a 88.49±0.39c 766.25±25.42c 44.59±1.35a 注:a-c同一列不同字母代表均值之间的显著性差异(P<0.05);YN/G表示每消耗单位葡萄糖对应同化 NH+4 表 3 间歇补糖模式下粗蛋白和藻胆蛋白生产
Table 3. Raw protein and phycobiliprotein production under intermittent feeding mode of glucose
补糖间隔时间/h 粗蛋白 藻胆蛋白(PBP) 藻蓝蛋白(PC) 产量/(g·L−1) 产率/( g·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 12(第1批次) 22.39±0.02a 4.98±0.00a 120.17±7.13a 26.70±1.58a 87.29±6.76a 19.40±1.50a 12(第2批次) 21.83±0.03a 4.37±0.03b 67.40±33.03a 13.48±6.61a 25.54±10.22b 4.91±2.04b 24 21.79±0.53a 3.63±0.09c 139.13±1.07a 23.19±0.18a 89.10±2.20a 14.85±0.37a 注:a-c同一列不同字母代表均值之间的差异显著(P<0.05)。 表 4 连续流加补糖模式下细胞生长和生物量生产
Table 4. Cell growth and biomass production under continuous feeding mode of glucose
C/N 批次 比生长速率/d−1 生物量产率/( g·(L·d)−1) 2.5~5.0 第1批次 0.59±0.00a 17.24±0.11a 第2批次 0.23±0.00c 15.37±0.15b 0~2.5 第1批次 0.52±0.00b 15.05±0.01b 第2批次 0.22±0.00c 14.07±0.06c 注:a-c同一列不同字母代表均值之间的差异显著(P<0.05)。 表 5 连续流加补糖模式下葡萄糖和
NH+4 Table 5. Parameters of glucose and
NH+4 C/N 批次 葡萄糖消耗速率/( g·(L·d)-1) NH+4 NH+4 NH+4 同化系数YN/G/(mg·g-1) 2.5~5.0 第1批次 32.54±0.00c 4 850. 00±25.00b 99.95±0.05a 1 204. 38±5.63c 37.02±0.17a 第2批次 42.84±0.03b 5 237. 50±7.50a 99.00±0.14c 1 529. 25±5.25a 35.70±0.10b 0~2.5 第1批次 32.20±0.00d 4 937. 50±37.50b 100.00±0.00a 1 150. 49±8.74d 35.73±0.27b 第2批次 47.16±0.00a 5 290. 00±20.00a 99.48±0.14b 1 419. 10±3.37b 30.09±0.07c 注:a-d同一列不同字母代表均值之间的差异显著(P<0.05);YN/G表示每消耗单位葡萄糖对应同化NH4+的量。 表 6 连续流加补糖模式下粗蛋白和藻胆蛋白生产
Table 6. Raw protein and phycobiliprotein production under continuous feeding mode of glucose
C/N 批次 粗蛋白 藻胆蛋白(PBP) 藻蓝蛋白(PC) 产量/(g·L−1) 产率/( g·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 产量/(mg·L−1) 产率/( mg·(L·d)−1) 2.5~5.0 第1批次 21.64±0.07b 5.19±0.02c 156.61±0.77b 37.59±0.18a 109.21±0.99b 26.21±0.24a 第2批次 26.55±0.06a 6.93±0.02a 80.19±4.89c 20.92±1.27b 38.94±1.28c 10.16±0.34b 0~2.5 第1批次 20.21±0.06c 4.71±0.01d 170.37±3.92a 39.70±0.91a 118.85±1.53a 27.69±0.36a 第2批次 20.85±0.31c 5.62±0.08b 66.79±0.96d 18.01±0.26b 27.11±1.95d 7.31±0.53c 注:a-d同一列不同字母代表均值之间的差异显著(P<0.05)。 -
[1] 中华人民共和国生态环境部. 2016—2019 年全国生态环境统计公报[R]. 北京: 中华人民共和国生态环境部, 2020. [2] WANG Q, YU Z, WEI D, et al. Mixotrophic Chlorella pyrenoidosa as cell factory for ultrahigh-efficient removal of ammonium from catalyzer wastewater with valuable algal biomass coproduction through short-time acclimation[J]. Bioresource Technology, 2021: 333. [3] NAGARAJAN D, LEE D J, CHEN C Y, et al. Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective[J]. Bioresource Technology, 2020: 302. [4] WOLLMANN F, DIETZE S, ACKERMANN J U, et al. Microalgae wastewater treatment: Biological and technological approaches[J]. Engineering in Life Sciences, 2019, 19(12): 860-871. doi: 10.1002/elsc.201900071 [5] CHEN H, WANG Q. Microalgae-based nitrogen bioremediation[J]. Algal Research-Biomass Biofuels and Bioproducts, 2020: 46. [6] HUSSAIN F, SHAH S Z, AHMAD H, et al. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review[J]. Renewable & Sustainable Energy Reviews, 2021: 137. [7] TORRES-FRANCO A, PASSOS F, FIGUEREDO C, et al. Current advances in microalgae-based treatment of high-strength wastewaters: challenges and opportunities to enhance wastewater treatment performance[J]. Reviews in Environmental Science and Bio-Technology, 2021, 20(1): 209-235. doi: 10.1007/s11157-020-09556-8 [8] SYDNEY E B, SCHAFRANSKI K, VALIO BARRETTI B R, et al. Biomolecules from extremophile microalgae: From genetics to bioprocessing of a new candidate for large-scale production[J]. Process Biochemistry, 2019, 87: 37-44. doi: 10.1016/j.procbio.2019.09.012 [9] ALBERTANO P, CINIGLIA C, PINTO G, et al. The taxonomic position of Cyanidium, Cyanidioschyzon and Galdieria: An update[J]. Hydrobiologia, 2000, 433(1-3): 137-143. [10] SLOTH J K, JENSEN H C, PLEISSNER D, et al. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries[J]. Bioresource Technology, 2017, 238: 296-305. doi: 10.1016/j.biortech.2017.04.043 [11] 郑雅莉. 嗜硫原始红藻混养处理高氨氮工业废水联产藻胆蛋白[D]. 广州: 华南理工大学, 2020. [12] TCHINDA D, HENKANATTE-GEDERA S M, ABEYSIRIWARDANA-ARACHCHIGE I S A, et al. Single-step treatment of primary effluent by Galdieria sulphuraria: Removal of biochemical oxygen demand, nutrients, and pathogens[J]. Algal Research-Biomass Biofuels and Bioproducts, 2019: 42. [13] SELVARATNAM T, PEGALLAPATI A K, MONTELYA F, et al. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters[J]. Bioresource Technology, 2014, 156: 395-399. doi: 10.1016/j.biortech.2014.01.075 [14] HENKANATTE-GEDERA S M, SELVARATNAM T, KARBAKHSHRAVARI M, et al. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration[J]. Algal Research, 2017, 24: 450-456. doi: 10.1016/j.algal.2016.08.001 [15] 魏东, 朱宝君, 郑雅莉. 一种利用极端环境微藻非灭菌发酵法快速脱氨氮的方法及其应用: 中国, CN111960543B[P]. 2021-08-10. [16] DELANKA-PEDIGE H M K, MUNASINGHE-ARACHCHIGE S P, CORNELIUS J, et al. Pathogen reduction in an algal-based wastewater treatment system employing Galdieria sulphuraria[J]. Algal Research-Biomass Biofuels and Bioproducts, 2019: 39. [17] OESTERHELT C, SCHMAELZLIN E, SCHMITT J M, et al. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria[J]. Plant Journal, 2007, 51(3): 500-511. doi: 10.1111/j.1365-313X.2007.03159.x [18] JIN H, ZHANG H, ZHOU Z, et al. Ultrahigh-cell-density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production[J]. Biotechnology and Bioengineering, 2020, 117(1): 96-108. doi: 10.1002/bit.27190 [19] FERNANDES T, FERNANDES I, ANDRADE C A P, et al. Marine microalgae growth and carbon partitioning as a function of nutrient availability[J]. Bioresource Technology, 2016, 214: 541-547. doi: 10.1016/j.biortech.2016.05.001 [20] LU L, WANG J, YANG G, et al. Biomass and nutrient productivities of Tetraselmis chuii under mixotrophic culture conditions with various C: N ratios[J]. Chinese Journal of Oceanology and Limnology, 2017, 35(2): 303-312. doi: 10.1007/s00343-016-5299-3 [21] LIU H, CHEN H, WANG S, et al. Optimizing light distribution and controlling biomass concentration by continuously pre-harvesting Spirulina platensis for improving the microalgae production[J]. Bioresource Technology, 2018, 252: 14-19. doi: 10.1016/j.biortech.2017.12.046 [22] SLOTH J K, WIEBE M G, ERIKSEN N T. Accumulation of phycocyanin in heterotrophic and mixotrophic cultures of the acidophilic red alga Galdieria sulphuraria[J]. Enzyme and Microbial Technology, 2006, 38(1-2): 168-175. doi: 10.1016/j.enzmictec.2005.05.010 [23] XU B, CHENG P, YAN C, et al. The effect of varying LED light sources and influent carbon/nitrogen ratios on treatment of synthetic sanitary sewage using Chlorella vulgaris[J]. World Journal of Microbiology & Biotechnology, 2013, 29(7): 1289-1300. [24] SCHMIDT R A, WIEBE M G, ERIKSEN N T. Heterotrophic high cell-density fed-batch cultures of the phycocyanin-producing red alga Galdieria sulphuraria[J]. Biotechnology and Bioengineering, 2005, 90(1): 77-84. doi: 10.1002/bit.20417 [25] KHAZI M I, DEMIREL Z, DALAY M C. Evaluation of growth and phycobiliprotein composition of cyanobacteria isolates cultivated in different nitrogen sources[J]. Journal of Applied Phycology, 2018, 30(3): 1513-1523. doi: 10.1007/s10811-018-1398-1 [26] SONANI R R, RASTOGI R P, PATEL R, et al. Recent advances in production, purification and applications of phycobiliproteins[J]. World journal of biological chemistry, 2016, 7(1): 100-109. doi: 10.4331/wjbc.v7.i1.100 [27] MOON M, MISHRA S K, KIM C W, et al. Isolation and characterization of thermostable phycocyanin from Galdieria sulphuraria[J]. Korean Journal of Chemical Engineering, 2014, 31(3): 490-495. doi: 10.1007/s11814-013-0239-9 -
























 DownLoad:
DownLoad: