-
塑料的广泛使用使得进入环境的塑料越来越多,随着时间的推移逐渐在水体中积累[1]。微塑料因其尺寸小、比表面积大、疏水性高等表面特性,使得微生物更容易黏附在其表面而形成异质性结构的生物膜[2]。在生物膜形成过程中,较高的细菌密度会产生能够与受体蛋白做出有效反应的群体感应(quorum sensing)信号[3]。群体感应是细菌达到一定浓度阈值时通过信号分子分泌、识别,调节基因表达、协调菌群行为的一种细胞交流机制[4]。这种机制赋予细菌一系列社会性能力,包括生物发光、生物膜形成[5]、基因水平转移[6-7]等。群体感应通过多个“信号通路”调节胞外多聚物的产生,从而控制生物膜的形成[8]。生物膜的形成与群体感应有着较强的关联性。微塑料在水环境中大量存在具有时间尺度的持久性、空间尺度的高传输性,还可通过食物链在生态圈中传递的特点,其表面的生物膜上的生态位点富集了很多潜在的致病菌[9]。研究微塑料生物膜群体感应信号分子分泌特征以及信号分子对生物膜形成的调节作用,为探究微塑料生物膜形成过程中群体感应的调控作用机制提供研究基础,同时为治理水环境中微塑料污染提供一种新的思路。
厚壁菌门在微塑料生物膜中常被发现[10]。从属于厚壁菌门的枯草芽孢杆菌能够分泌产生酰基高丝氨酸内酯(AHLs)类群体感应信号分子[11],该类信号分子广泛存在于微生物界的群体中。目前,对生物膜中AHLs类群体感应信号分子鉴定方法通常包括传统的薄层色谱法[12]、气相色谱质谱法[13]。因液相色谱-串联质谱法检测快速、准确性和灵敏度高等优点,近年来被多项研究应用于AHLs的定性与定量[14-17]。由于生物膜产生的信号分子浓度往往低于仪器检出限,需对样品进行浓缩富集。常用的富集方法包括固相萃取(SPE)[18]、液液萃取(LLE)[19]、固相萃取串联液相萃取(SPE-LLE)[20]。SPE和SPE-LLE通常适用于基质影响较大的环境样品[15],由于培养体系中枯草芽孢杆菌生物膜基质干扰相对较小,本研究选用萃取过程相对简单和经济的LLE方法。
本研究针对生物膜上常检出的3-oxo-C6-HSL 、C6-HSL和3-oxo-C8-HSL等 11种AHLs类信号分子 [20-22],采用LLE作为样品的前处理方法,通过对萃取方式和萃取液进行优化来提高微塑料枯草芽孢杆菌生物膜中AHLs回收率,基于超高效液相色谱-串联三重四极杆质谱(UPLC-MS/MS)仪器分析平台,优化色谱质谱分析条件,建立一种快速、准确的微塑料生物膜中AHLs类群体感应信号分子检测方法。使用所建立的方法来测定在生活生产使用最多且水环境中常被检出的聚丙烯(polypropylene,PP)、聚乙烯(polyethylene,PE)、聚苯乙烯(polystyrene,PS)的3种微塑料[23]上生物膜群体感应分泌的11种AHLs类信号分子,并研究其分泌特征。同时,添加外源信号分子来初步探究群体感应信号分子分泌量对生物膜成膜的影响。研究多种AHLs类信号分子化合物的同时定性与定量分析,能为微生物群体感应的进一步研究提供有效且便利的分析方法。
-
本研究采用内标法对目标物进行定量,目前尚无研究报道枯草芽孢杆菌产生C7-HSL,因此选择C7-HSL作为定量目标AHLs的内标物。11种AHLs类信号分子标准品N-(3-氧代己酰)-DL-高丝氨酸内酯(3-oxo-C6-HSL)、N-己酰基-L-高丝氨酸内酯(C6-HSL)、N-(3-氧代辛酰基)-L-高丝氨酸内酯(3-oxo-C8-HSL)、N-辛酰基-DL-高丝氨酸内酯(C8-HSL)、N-(3-氧代癸酰基)-L-高丝氨酸内酯(3-oxo-C10-HSL)、N-癸酰基-L-高丝氨酸内酯(C10-HSL)、N-(3-氧代十二烷酰基)-L-高丝氨酸内酯(3-oxo-C12-HSL)、N-(3-氢氧化十二酰基)-DL-高丝氨酸内酯(3-OH-C12-HSL)、N-十二烷酰-L-高丝氨酸内酯(C12-HSL)、N-(3-氧代十四烷酰)-L-高丝氨酸内酯(3-oxo-C14-HSL)和正十四酰基-DL-高丝氨酸内酯(C14-HSL)和内标物C7-HSL均购于Sigma公司(纯度大于98%);实验所用的聚丙烯、聚乙烯和聚苯乙烯(直径为3.2 mm的球形)均购于阿拉丁公司;K2HPO4、KH2PO4、Na2HPO4·2H2O、NH4Cl、MgSO4·7H2O、CaCl2、FeCl3·6H2O(分析纯)购于上海生工公司;甲醇(色谱纯)购于Tedia公司;甲酸(纯度99%)购于Anaqua公司;乙酸乙酯(分析纯),冰乙酸(分析纯)均购于国药集团;LB培养基、营养琼脂等购于上海生工公司;实验室用水为Milli-Q超纯水。
使用的设备仪器主要包括UPLC-MS/MS(Xevo TQ-S,美国Waters);高速离心机(Centrifuge 5810,德国Eppendorf);超声细胞破碎机(JY92-II,宁波新芝);旋转蒸发仪(RE-100,北京大龙);恒温培养箱(ZWYR-2102,上海智诚);涡旋仪(MX-S,美国SCILOGEX);紫外分光光度计(UV 9100A,北京莱伯泰科)。
-
分别将11种信号分子标准品用甲醇配制成100 mg·L−1的标准储备液,取出1 mL用甲醇和水(V∶V =1∶1)配制成1 mg·L−1的中间混合标准溶液。标准工作溶液按照需要用甲醇和水(V:V= 1∶1)对中间混合标准溶液进行梯度稀释。内标物C7-HSL用甲醇溶解,配制成1 mg·L−1的内标物溶液。所有配制好的溶液放置于−20 ℃避光保存。
用无菌蒸馏水分别配置成含K2HPO4(21.75 g)、KH2PO4(8.5 g)、Na2HPO4·2H2O(33.4 g)和NH4Cl(0.5 g)的溶液A(调至pH = 7.4)、含22.5 g MgSO4·7H2O的溶液B、27.5 g CaCl2的溶液C、0.25 g FeCl3·6H2O的溶液D,并分别定容至1 L。量取10 mL溶液A与800 mL无菌蒸馏水混合,分别加入1 mL溶液 B、C、D后,用无菌蒸馏水定容至1 L,配置成培养体系使用的矿物营养液。
-
枯草芽孢杆菌菌株(Bacillus subtilis)购于广东微生物研究所。枯草芽孢杆菌单菌落接种于含50 mL LB培养基的250 mL三角瓶中,37 ℃振荡培养,12 h后取1 mL菌液于含100 mL LB培养基的500 mL三角瓶中培养,使OD600达到 0.5。取2 mL菌液至含有200 mL矿物营养液的500 mL三角瓶中,将微塑料洗净灭菌后分别加入10 g PP、10 g PE、10 g PS,在37 ℃振荡培养3 d形成生物膜。
-
将生物膜培养体系中的微塑料过滤到滤纸上,用0.9%无菌氯化钠溶液缓慢冲洗微塑料,除去其周围浮游细菌。将清洗过后的微塑料全部转移至50 mL的离心管中,加入足量焦磷酸钠缓冲液(Na4O7P2(50 mmol·L−1)-Tween80(0.05%))[24],涡旋2 min,重复3次。混合涡旋分离得到的生物膜,在10000 r·min−1条件下离心10 min,收集离心后上清液用于提取信号分子,沉积到底部的菌体干燥后测定生物膜重量。
在收集的上清液中加入等体积的乙酸乙酯萃取,振荡15 min,弃水相,混合有机相,重复3次。将上述的萃取液在150 r·min−1,50 ℃条件下旋转蒸干。用无水甲醇再溶解,定容至2 mL。取0.5 mL此溶液,加0.5 mL纯水,过0.22 μm滤膜,待测定。
-
采用UPLC-MS/MS对微塑料生物膜中11种AHLs进行定量分析。色谱柱为ACQUITY BEH C18色谱柱(100 mm × 2.1 mm × 1.7 μm,Waters),流速为0.3 mL·min−1,进样量为5 μL,分析时间为6.5 min,柱温为40 ℃,流动相A为甲醇,B为纯水。质谱选择电离模式为ES+,检测方式为多反应监测(MRM)模式,扫描时间为0.5 s,离子源温度为150 ℃,脱溶剂温度为450 ℃,脱溶剂气和锥孔气为N2,脱溶剂气流速为844 L·h−1,锥孔气流速为154 L·h−1,碰撞气体为氩气,毛细管电压为1.6 kV。
-
将含11种AHLs和1种内标物的标准溶液(浓度均为10 μg·L-1)分别进样,在流动相A甲醇中加入2 mmol·L−1乙酸铵、0.1%甲酸对色谱条件进行优化。在ES+模式下进行全扫描,调整锥孔电压、碰撞电压对质谱条件进行优化。
-
对样品信号分子提取方法进行优化,用等体积的乙酸乙酯,采用振荡15 min和冰浴超声15 min(200 W功率,周期为3 s开、3 s关)两种方式对微塑料生物膜中11种AHLs信号分子进行萃取;采用乙酸乙酯和酸化的乙酸乙酯(0.01%冰乙酸,V/V)两种萃取剂对信号分子萃取剂进行优化。
-
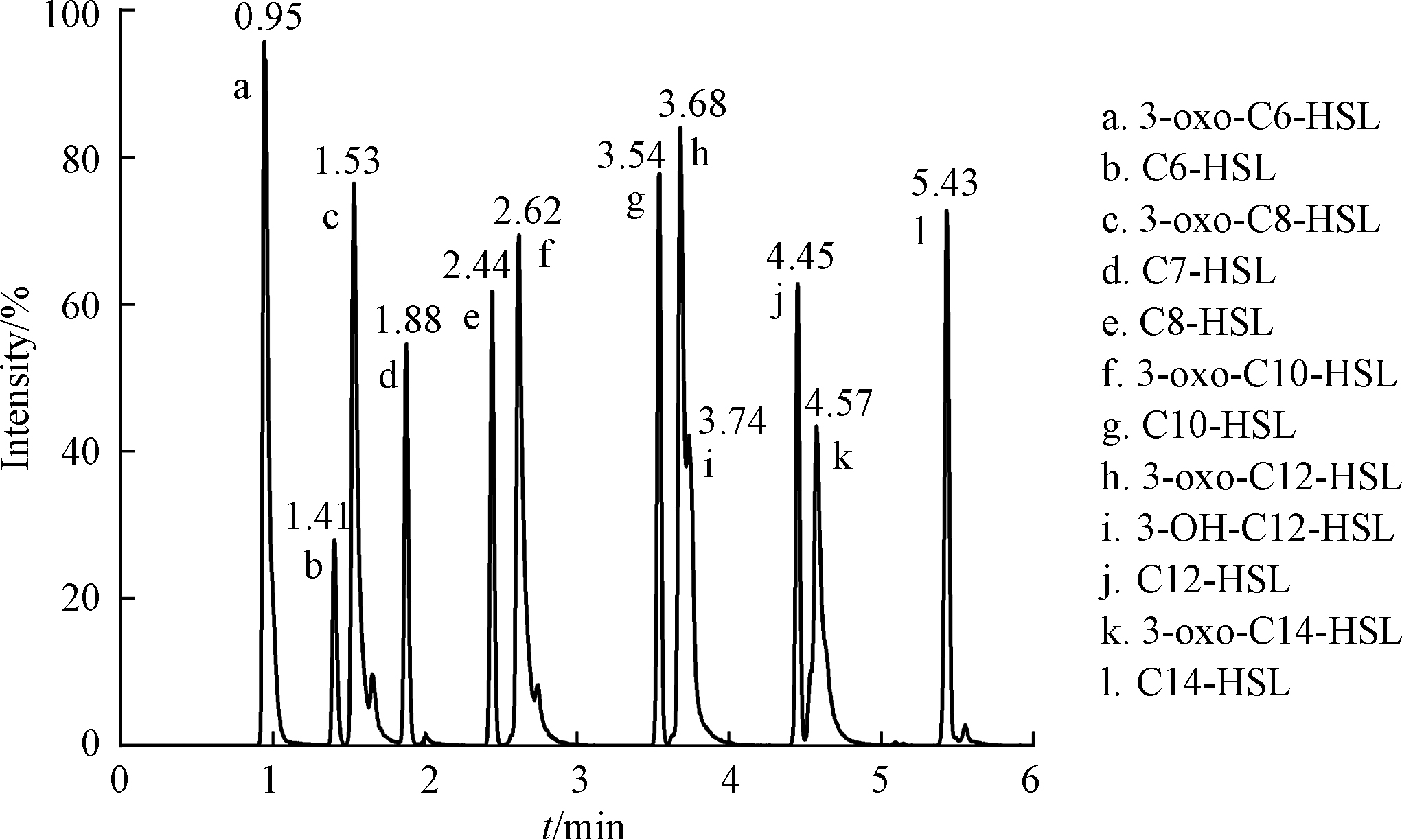
在流动相甲醇加入2 mmol·L−1乙酸铵,提高离子化效率。同时,加入0.1%甲酸,使峰形尖锐,提高灵敏度,降低检出限和定量限。为了更好的分离峰,采用的洗脱梯度为:流动相A的初始比例为50%,在2 min内线性增加到60%,0.5 min线性增加到80%,再用3.5 min增加到100%,最后经0.1 min减少到50%并保持0.4 min。12种AHLs信号分子(包含内标物)的峰被明显分开(图1),3-oxo-C12-HSL和3-OH-C12-HSL异构体在电喷雾离子源正离子模式下,两个峰相交的面积减少,说明流动相优化改善检测效果。虽然内标物C7-HSL与被测信号分子在物理和化学性质上相似,且是被测信号分子的同系物,但是分析结果显示C7-HSL与被测信号分子色谱峰不重叠,适合作为目标群体感应信号分子检测分析方法的内标物。
将11种AHLs和1种内标物分别进样,在ES+模式下进行全扫描,确定其母离子,优化各母离子的锥孔电压使得母离子响应强度最大;并对其子离子进行全扫描,选择其丰度最高的离子作为定量离子,丰度次之的离子作为定性离子,并对锥孔电压、碰撞电压等参数进行优化如表1所示。
-
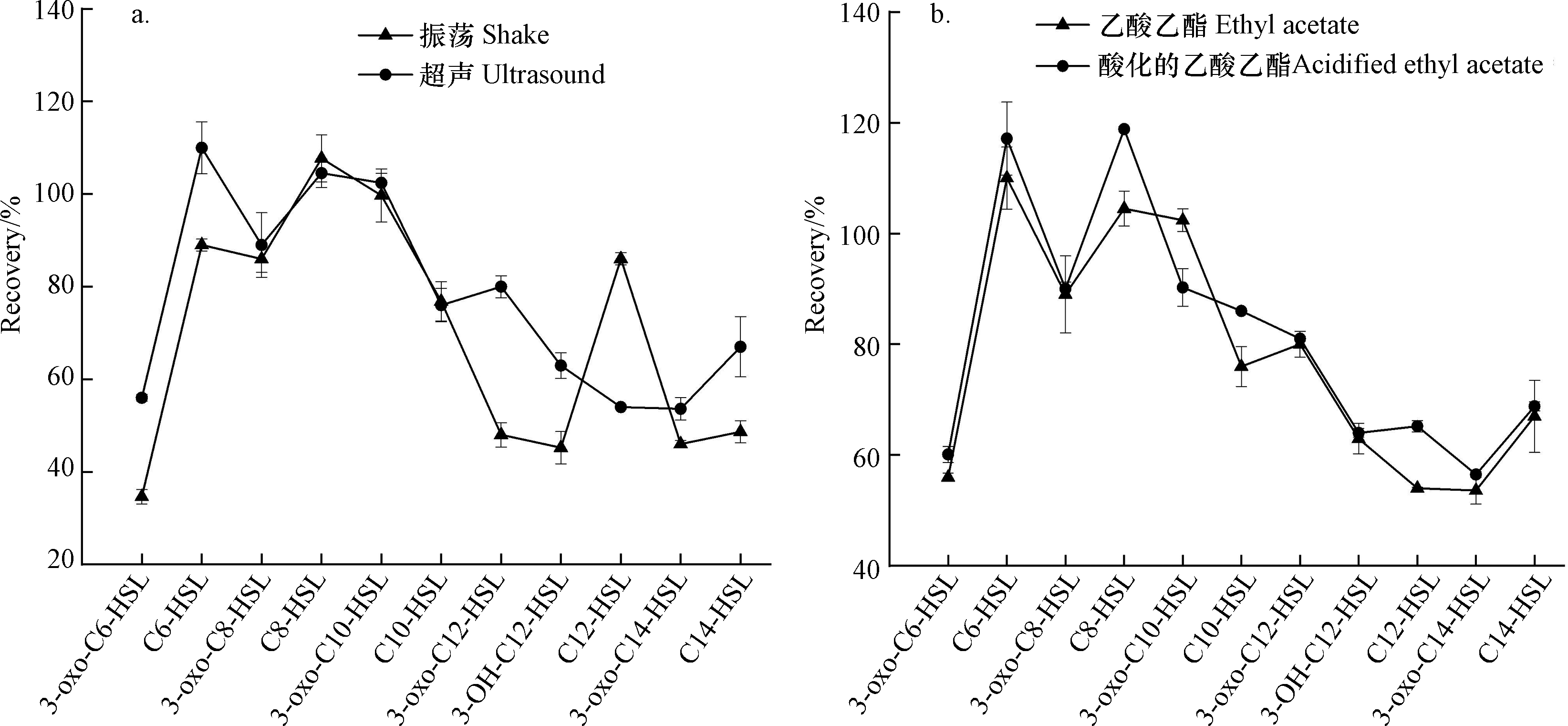
用等体积的乙酸乙酯,采用振荡和冰浴超声两种方式对微塑料生物膜中11种AHLs信号分子进行萃取。经振荡乙酸乙酯萃取的信号分子回收率在34.6%—107.7%,其中对3-oxo-C6-HSL、3-oxo-C12-HSL、3-OH-C12-HSL、3-oxo-C14-HSL和C14-HSL信号分子提取的回收率均低于50%(34.6%—48.7%)(图2)。利用LC-MS/MS测定铜绿假单胞菌产生的群体感应信号分子研究也显示上述物质的回收率较低[16]。然而,超声萃取对11种目标AHLs信号分子回收率在53.6%—110%,绝大多数高于振荡萃取方式下的回收率(图2)。尤其与上述5种低回收率信号分子相比,超声萃取能够提高其回收率达53.7%—80%。
对萃取剂进行优化。采用乙酸乙酯和酸化的乙酸乙酯两种萃取剂对微塑料生物膜中11种AHLs信号分子进行萃取,对应的回收率结果表明,除3-oxo-C10-HSL外,其余10种信号分子在以酸化的乙酸乙酯作为萃取剂得到的回收率相对较高(图2)。主要由于在酸性条件能抑制乙酸乙酯水解,提高萃取效率[25]。因此,采用酸化的乙酸乙酯作为信号分子萃取剂回收率更高。
-
配制0.5、1、2、5、10、20、50 μg·L−1的11种AHLs信号分子混合标准工作溶液,添加内标C7-HSL(10 μg·L−1),以信号分子的峰面积与内标物峰面积比值为纵坐标,以对应的浓度为横坐标做标准工作曲线。11种信号分子的线性回归系数R2均大于0.995(表2),表明目标信号分子在0.5—50 μg·L−1范围内具有良好的线性关系。分别以信噪比(S/N)为3和10来确定检出限和定量限,检出限和定量限分别在0.005—0.01 μg·L−1和0.01—0.02 μg·L−1范围(表2),对目标物分析的灵敏度较高。
-
在样品中加入11种待测AHLs信号分子混合标准溶液,使加标浓度分别为 2、5、10 μg·L−1,同时添加内标物C7-HSL(10 μg·L−1)。利用优化的分析检测方法,在3个加标浓度下11种信号分子回收率分别在54.2%—128.1%、50.6%—109.6%、56.5%—118.8%,相对标准偏差分别为0.2%—10.6%、0.9 %— 5.2%、0.3%—6.6%(表3)。可见,建立的前处理方法对微塑料生物膜中AHLs能实现有效提取、重现性高,可实现对实际生物膜样品AHLs信号分子的准确定量。
-
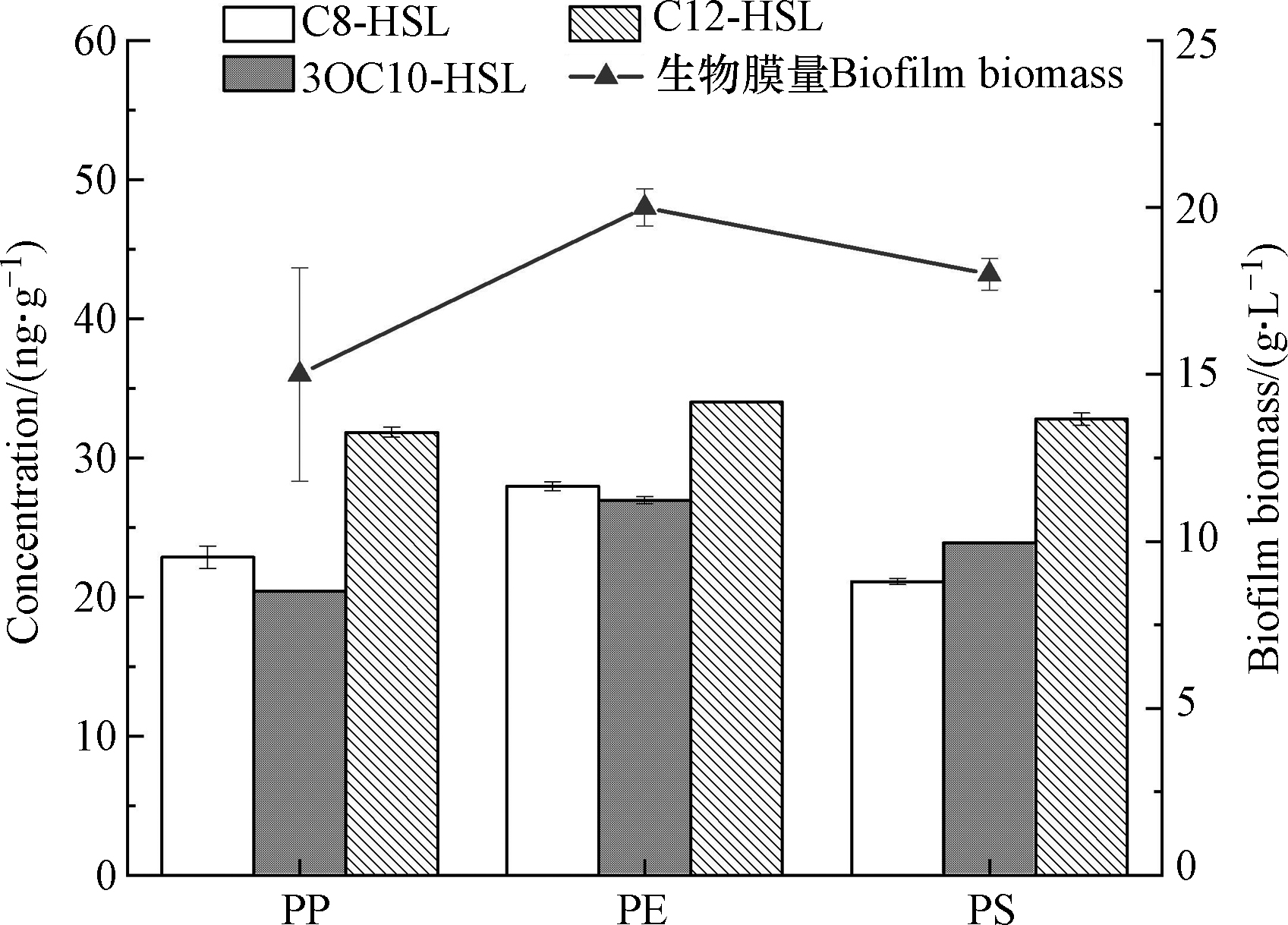
采用构建的LLE-UPLC-MS/MS方法对微塑料上枯草芽孢杆菌生物膜分泌的AHLs信号分子进行定量分析。C8-HSL、C12-HSL和3-oxo-C10-HSL信号分子在3种微塑料枯草芽孢杆菌生物膜中均被检出(图3)。然而,从不同的微塑料PP、PE和PS提取的生物膜中AHLs浓度具有差异,其中PE生物膜中提取的3种AHLs浓度均高于PP和PS。3种微塑料生物膜中C12-HSL产生量最高,其在PE表面生物膜中含量最高达到34 ng·g-1。可见,不同的微塑料生物膜产生的信号分子量存在差异。PE生物膜形成量高(图3),可能是引起其信号分子分泌量高的主要因素。此外,信号分子也会影响生物膜的形成[26]。
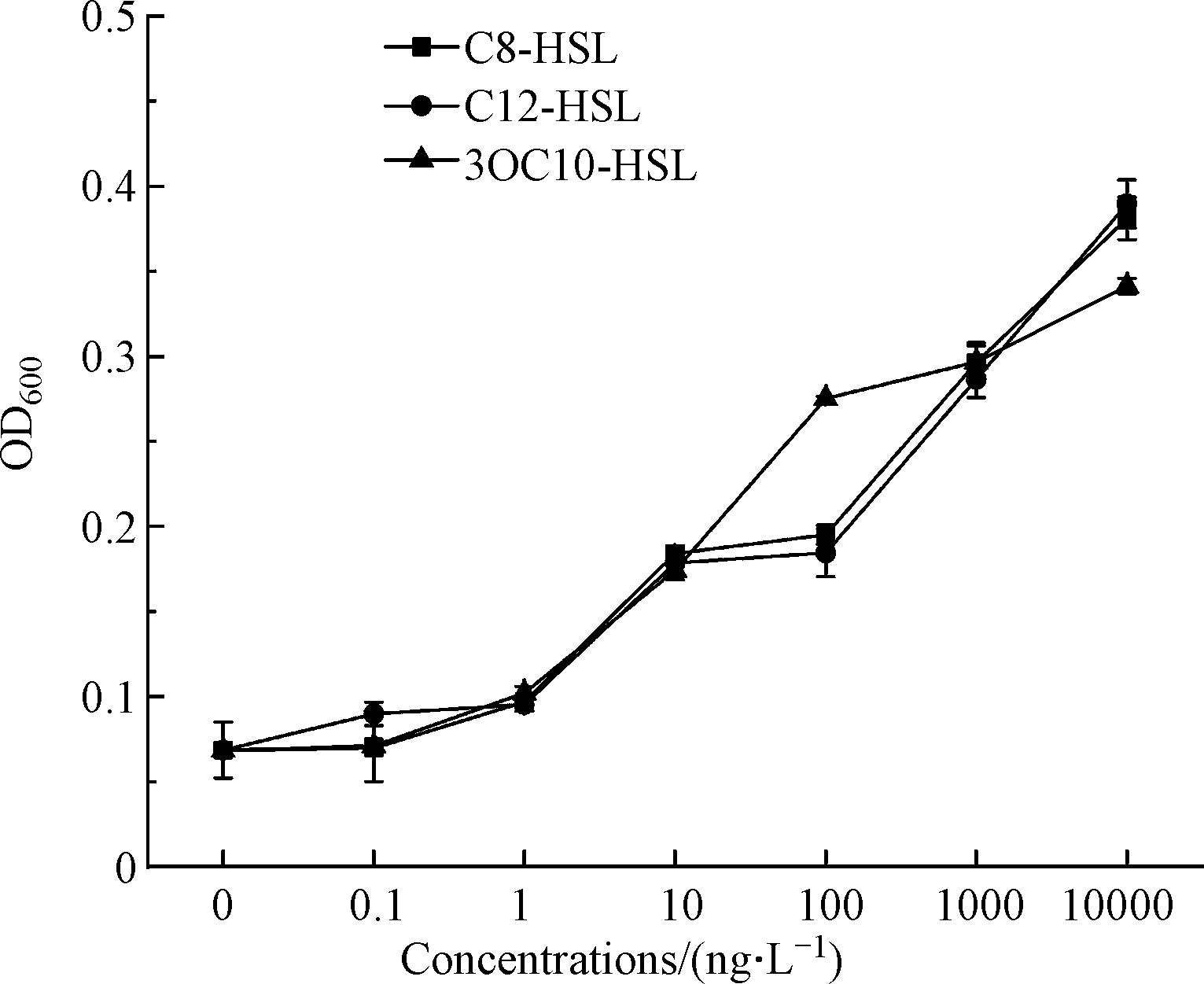
AHLs等群体感应信号分子与生物膜初始附着过程有着极强的相关性[27],信号分子通过调节群体感应从而对微塑料生物膜的形成产生影响。Wu等从饮用水中分离细菌菌株,研究细菌生物膜形成能力与群体感应信号分子生产能力的相关性,添加外源信号分子结果发现1/4的分离株的生物膜得到了显著促进[28]。因此,利用添加外源信号分子研究群体感应信号分子浓度的变化对微塑料上枯草芽孢杆菌生物膜形成的影响。选择检测到的C8-HSL、C12-HSL和3-oxo-C10-HSL作为外源信号分子,分别以0.1、1、10、100、1000、10000 、10000 ng·L-1的7个浓度序列添加到微塑料PE培养体系中,培养3 d后测定细菌成膜量。C8-HSL、C12-HSL和3-oxo-C10-HSL的3种信号分子在低浓度添加时对微塑料生物膜成膜量未产生明显的促进作用;在添加高浓度信号分子时,生物膜成膜量明显增加(图4)。可见,外源添加的群体感应信号分子对枯草芽胞杆菌生物膜的形成会产生促进作用,信号分子浓度越高促进作用越明显。由此证实群体感应作为一种细胞-细胞通信机制[29],可通过分泌信号分子调控胞外聚合物的产生从而影响微塑料生物膜的形成[30]。
-
本研究建立了LLE-UPLC-MS/MS共检测微塑料生物膜群体感应分泌的11种AHLs类信号分子的分析方法,通过对萃取方式、萃取剂、色谱和质谱条件优化,能够实现对信号分子快速、准确的定量分析。利用建立的分析方法对3种不同的微塑料枯草芽孢杆菌生物膜群体感应AHLs类信号分子进行分析测定,3种微塑料生物膜产生的AHLs浓度具有差异性,其中PE生物膜产生的AHLs浓度最高。进一步证实信号分子分泌能够影响微塑料生物膜形成,信号分子浓度越高对生物膜形成促进作用越明显。可见,群体感应作用在微塑料生物膜形成过程中具有重要作用,且不同的微塑料生物膜群体感应对生物膜成膜的调控机制可能存在差异,在分子水平的调控机制仍有待进一步探究。
微塑料生物膜11种AHLs类群体感应信号分子测定及其分泌特征
Determination and secretion characteristics of 11 N-acyl-homoserine lactones signal molecules of quorum sensing in microplastic biofilms
-
摘要: 为研究微塑料生物膜的群体感应效应,利用液液萃取和超高效液相色谱-串联质谱技术,建立了微塑料上枯草芽孢杆菌生物膜分泌的11种酰基高丝氨酸内酯(AHLs)类群体感应信号分子前处理和仪器分析方法。优化后的前处理方法采用酸化的乙酸乙酯(0.01%冰乙酸,V/V)对微塑料生物膜样品中AHLs类信号分子进行萃取,萃取方式采用冰浴超声。微塑料生物膜中AHLs回收率为50.6%—128.1%,相对标准偏差(n = 3)为0.2%—10.6%。对色谱和质谱条件进行优化,11种AHLs在0.5—50 μg·L−1范围内呈良好的线性关系(R2 > 0.995),检出限为0.005—0.01 μg·L−1,定量限为0.01—0.02 μg·L−1。本方法具有快速、准确、灵敏度高等优点。对聚丙烯、聚乙烯、聚苯乙烯等3种微塑料上枯草芽孢杆菌生物膜中11种AHLs分析结果表明,C8-HSL、C12-HSL和3-oxo-C10-HSL在3种微塑料生物膜中均被检出;C12-HSL的产生量最高,且在聚乙烯表面生物膜中含量最高达34 ng·g−1。通过添加外源信号分子研究表明,AHLs分子浓度增加会促进微塑料生物膜形成。
-
关键词:
- 超高效液相色谱-串联质谱 /
- 生物膜 /
- 群体感应 /
- 酰基高丝氨酸内酯 /
- 微塑料
Abstract: In order to study the quorum sensing effect of biofilms on microplastics, a pretreatment and instrumental analysis method for 11 N-acyl-homoserine lactones (AHLs) secreted by Bacillus subtilis biofilms on microplastics was established by liquid-liquid extraction and ultra-performance liquid chromatography-tandem mass spectrometry. The optimized pretreatment method used acidified ethyl acetate (0.01% glacial acetic acid, V/V) to extract AHLs signal molecules from microplastic biofilm samples. The extraction method used ultrasound with ice bath. The recoveries of 11 AHLs in microplastic biofilms ranged from 50.6% to 128.1%, and the relative standard deviations (n = 3) ranged from 0.2% to 10.6%. After the optimization for chromatographic and mass spectrometry condition, the linearities of 11 AHLs were good in the range of 0.5—50 μg·L−1 (R2 > 0.995), the limits of detection were in the range of 0.005—0.01 μg·L−1, and the limits of quantification were in the range of 0.01—0.02 μg·L−1. The method is rapid and accurate, and has high sensitivity. The quantitative analysis for 11 AHLs in Bacillus subtilis biofilms on polypropylene, polyethylene and polystyrene showed that C8-HSL, C12-HSL and 3-oxo-C10-HSL were all detected in the three types of microplastic biofilms; the production amount of C12-HSL was the highest, and was up to 34 ng·g−1 in polyethylene biofilm. The result from the addition of exogenous AHLs indicated the increasing concentrations of AHLs can promote the biofilm formation on microplastics.-
Key words:
- UPLC-MS/MS /
- biofilm /
- quorum sensing /
- AHLs /
- microplastics
-

-
表 1 12种AHLs类信号分子的质谱条件
Table 1. Analysis parameters of 12 AHLs for mass spectrometry
化合物
Compounds母离子(m/z)
Parents ion子离子(m/z)
Daughter ion锥孔电压/ V
Cone碰撞能量/eV
Collision energy3-oxo-C6-HSL 214 102.0*,111 20 10 C6-HSL 200.1 102.0*,99 20 10 3- oxo-C8-HSL 242.2 102.0*,140.9 20 15 C7-HSL 214.3 102.0*,111.3 20 15 C8-HSL 228.2 102.0*,126.6 20 15 3-oxo-C10-HSL 270.2 102.0*,169 20 15 C10-HSL 256 102.0*,155 20 15 3-oxo-C12-HSL 298 102.0*,197 20 15 3-OH-C12-HSL 300.4 102.0*,199.4 20 15 C12-HSL 284.3 102.0*,183.1 20 15 3-oxo-C14-HSL 326.4 102.0*,102 20 15 C14-HSL 312 102.0*,211 20 15 *为定量离子. *Ions are quantitative ions. 表 2 11种AHLs信号分子的线性关系、检出限、定量限
Table 2. The linear regression equation,limit of detection,limit of quantitation of 11 AHLs
化合物
Compounds线性回归方程
Linear regression equations回归系数
Regression coefficient R2检出限/ (μg·L-1)
Limit of detection定量限/ (μg·L-1)
Limit of quantitation3-oxo-C6-HSL y=3.53x+0.44 0.9996 0.005 0.02 C6-HSL y=1.38x+0.09 0.9998 0.01 0.02 3-oxo-C8-HSL y=5.17x+0.31 0.9992 0.005 0.01 C8-HSL y=2.31x+0.57 0.9989 0.005 0.01 3-oxo-C10-HSL y=2.77x+0.10 0.9991 0.005 0.01 C10-HSL y=2.86x+0.80 0.9987 0.005 0.01 3-oxo-C12-HSL y=6.32x+0.44 0.9986 0.005 0.01 3-OH-C12-HSL y=1.38x+0.21 0.9966 0.005 0.01 C12-HSL y=2.44x+0.70 0.9971 0.005 0.01 3-oxo-C14-HSL y=3.70x+0.39 0.9955 0.005 0.01 C14-HSL y=3.27x+0.36 0.9987 0.005 0.02 表 3 11种AHLs的加标回收率和相对标准偏差(n = 3)
Table 3. Recoveries and relative standard deviations of 11 AHLs(n = 3)
化合物
Compounds添加浓度2 μg·L−1 添加浓度5 μg·L−1 添加浓度10 μg·L−1 平均回收率/%
Mean recoveryRSD/% 平均回收率/ %
Mean recoveryRSD/ % 平均回收率/%
Mean recoveryRSD /% 3-oxo-C6-HSL 54.2 1.3 57.2 0.9 60.1 1.5 C6-HSL 128.1 10.6 101.2 2.7 117.1 6.6 3-oxo-C8-HSL 93.0 5.5 52.6 1.6 90.0 1.2 C8-HSL 119.7 5.8 109.6 5.2 118.8 0.5 3-oxo-C10-HSL 99.5 1.6 101.3 5.2 90.2 3.4 C10-HSL 108.9 6.2 60.7 1.3 86.0 0.3 3-oxo-C12-HSL 78.0 4.3 50.6 1.6 79.0 0.4 3-OH-C12-HSL 65.1 2.3 54.2 1.4 64.0 0.8 C12-HSL 65.1 2.5 64.6 0.9 65.2 1.0 3-oxo-C14-HSL 55.3 0.2 58.4 1.6 56.5 0.5 C14-HSL 73.7 2.3 57.2 2.0 66.8 0.8 -
[1] NIU L H, HU J X, LI Y, et al. Effects of long-term exposure to silver nanoparticles on the structure and function of microplastic biofilms in eutrophic water [J]. Environmental Research, 2021: 112182. doi: 10.1016/j.envres.2021.112182 [2] CAI L, WU D, XIA J H, et al. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics [J]. Science of the Total Environment, 2019, 671: 1101-1107. doi: 10.1016/j.scitotenv.2019.03.434 [3] TAHRIOUI A, DUCHESNE R, BOUFFARTIGUES E, et al. Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation [J]. NPJ Biofilms and Microbiomes, 2019, 5: 15. doi: 10.1038/s41522-019-0088-3 [4] 孙锋, 严慧聪, 汪美贞. 细菌群体感应调控多样性及群体感应淬灭 [J]. 微生物学报, 2019, 59(3): 454-467. SUN F, YAN H C, WANG M Z. Advance of the diversity of bacterial quorum sensing and quorum quenching [J]. Acta Microbiologica Sinica, 2019, 59(3): 454-467(in Chinese).
[5] SOLANO C, ECHEVERZ M, LASA I. Biofilm dispersion and quorum sensing [J]. Current Opinion in Microbiology, 2014, 18: 96-104. doi: 10.1016/j.mib.2014.02.008 [6] ZHU L, CHEN T, XU L, et al. Effect and mechanism of quorum sensing on horizontal transfer of multidrug plasmid RP4 in BAC biofilm [J]. Science of the Total Environment, 2020, 698: 134236. doi: 10.1016/j.scitotenv.2019.134236 [7] GUO X P, YANG Y, LU D P, et al. Biofilms as a sink for antibiotic resistance genes (ARGs) in the Yangtze Estuary [J]. Water Research, 2018, 129: 277-286. doi: 10.1016/j.watres.2017.11.029 [8] HAMMER B K, BASSLER B L. Quorum sensing controls biofilm formation in Vibrio cholerae [J]. Molecular Microbiology, 2003, 50(1): 101-104. doi: 10.1046/j.1365-2958.2003.03688.x [9] LIU X W, WANG H X, LI L L, et al. Do microplastic biofilms promote the evolution and co-selection of antibiotic and metal resistance genes and their associations with bacterial communities under antibiotic and metal pressures? [J]. Journal of Hazardous Materials, 2022, 424: 127285. doi: 10.1016/j.jhazmat.2021.127285 [10] 敬双怡, 李岩, 于玲红, 等. SMBBR工艺处理生活污水脱氮效能及其微生物多样性 [J]. 应用与环境生物学报, 2019, 25(1): 206-214. JING S Y, LI Y, YU L H, et al. Denitrification efficiency of the special moving-bed biofilm reactor(SMBBR) process for domestic sewage treatment and its microbial diversity [J]. Chinese Journal of Applied and Environmental Biology, 2019, 25(1): 206-214(in Chinese).
[11] 陈涛. 群感效应对饮用水活性炭深度处理中抗生素抗性基因水平转移的作用及调控研究[D]. 杭州: 浙江大学, 2018. CHEN T. Effects of quorum sensing on antibiotic resistome promotion in drinking water during biological activated carbon treatment[D]. Hangzhou: Zhejiang University, 2018(in Chinese).
[12] 王艳, 张士春, 周进. 一种改良型检测群体感应信号分子的TLC方法 [J]. 生物学杂志, 2020, 37(1): 97-100. doi: 10.3969/j.issn.2095-1736.2020.01.097 WANG Y, ZHANG S C, ZHOU J. A modified TLC method for detecting quorum sensing signal molecules of N-acyl-homoserine lactones in bacteria [J]. Journal of Biology, 2020, 37(1): 97-100(in Chinese). doi: 10.3969/j.issn.2095-1736.2020.01.097
[13] 郭秀春, 郑立, 张魁英, 等. 气相色谱-质谱法检测细菌中N-酰基高丝氨酸内酯类信号分子 [J]. 分析测试学报, 2012, 31(3): 347-350. doi: 10.3969/j.issn.1004-4957.2012.03.020 GUO X C, ZHENG L, ZHANG K Y, et al. Determination of N-acyl-homoserine lactones signal molecules by gas chromatography-mass spectrometry [J]. Journal of Instrumental Analysis, 2012, 31(3): 347-350(in Chinese). doi: 10.3969/j.issn.1004-4957.2012.03.020
[14] 马晨晨, 李柏林, 欧杰, 等. 高效液相色谱-串联质谱法同时测定细菌群体感应效应的11种AHLs类信号分子 [J]. 分析化学, 2010, 38(10): 1428-1432. MA C C, LI B L, OU J, et al. Detection of N-acyl-homoserine lactones class signal molecules of quorum sensing secreted by bacteria using high performance liquid chromatography-mass spectrometry/mass spectrometry [J]. Chinese Journal of Analytical Chemistry, 2010, 38(10): 1428-1432(in Chinese).
[15] HUANG S J, ZHANG H, ALBERT NG T C, et al. Analysis of N-Acy-L-homoserine lactones (AHLs) in wastewater treatment systems using SPE-LLE with LC-MS/MS [J]. Water Research, 2020, 177: 115756. doi: 10.1016/j.watres.2020.115756 [16] LI J L, SUN K, MENG J, et al. Detection of N-acyl-homoserine lactones signal molecules of quorum sensing secreted by denitrification flora in microaerobic nitrogen removal processes by ultra-performance liquid chromatography tandem mass spectrometry [J]. Chinese Journal of Analytical Chemistry, 2016, 44(8): 1165-1170. doi: 10.1016/S1872-2040(16)60948-9 [17] SUN Y P, HE K, YIN Q D, et al. Determination of quorum-sensing signal substances in water and solid phases of activated sludge systems using liquid chromatography-mass spectrometry [J]. Journal of Environmental Sciences, 2018, 69: 85-94. doi: 10.1016/j.jes.2017.04.017 [18] WANG J F, LIU Q J, LI X H, et al. In-situ monitoring AHL-mediated quorum-sensing regulation of the initial phase of wastewater biofilm formation [J]. Environment International, 2020, 135: 105326. doi: 10.1016/j.envint.2019.105326 [19] JIN L, ZHANG X J, SHI H, et al. Identification of a novel N-acyl homoserine lactone synthase, AhyI, in Aeromonas hydrophila and structural basis for its substrate specificity [J]. Journal of Agricultural and Food Chemistry, 2020, 68(8): 2516-2527. doi: 10.1021/acs.jafc.9b07833 [20] WANG J F, DING L L, LI K, et al. Estimation of spatial distribution of quorum sensing signaling in sequencing batch biofilm reactor (SBBR) biofilms [J]. The Science of the Total Environment, 2018, 612: 405-414. doi: 10.1016/j.scitotenv.2017.07.277 [21] 窦懿. 鲍曼不动杆菌群体感应系统信号分子N-酰基高丝氨酸内酯的鉴定以及与耐药基因相关性的研究[D]. 上海: 上海交通大学, 2015. DOU Y. Study on N-acylhomoserine lactones of Acinetobacter baumanii quorum sensing and its relation to drug resistant gene expression[D]. Shanghai: Shanghai Jiaotong University, 2015(in Chinese).
[22] 丁雅娟, 丁蒙丹, 张佳娣, 等. 海洋微生物中的群体感应 [J]. 科技通报, 2019, 35(6): 1-6. DING Y J, DING M D, ZHANG J D, et al. Quorum sensing in marine microorganisms [J]. Bulletin of Science and Technology, 2019, 35(6): 1-6(in Chinese).
[23] OGONOWSKI M, MOTIEI A, ININBERGS K, et al. Evidence for selective bacterial community structuring on microplastics [J]. Environmental Microbiology, 2018, 20(8): 2796-2808. doi: 10.1111/1462-2920.14120 [24] LUO C H, LÜ F, SHAO L M, et al. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes [J]. Water Research, 2015, 68: 710-718. doi: 10.1016/j.watres.2014.10.052 [25] TANG R, ZHU J L, FENG L F, et al. Characterization of LuxI/LuxR and their regulation involved in biofilm formation and stress resistance in fish spoilers Pseudomonas fluorescens [J]. International Journal of Food Microbiology, 2019, 297: 60-71. doi: 10.1016/j.ijfoodmicro.2018.12.011 [26] 朱颖楠, 王旭, 王瑾丰, 等. 外源群体感应-好氧反硝化菌强化生物膜脱氮研究 [J]. 环境科学学报, 2019, 39(10): 3225-3237. ZHU Y N, WANG X, WANG J F, et al. Insight into enhancing nitrogen removal in biofilm by exogenous quorum sensing-aerobic denitrifier (QS-HNAD) [J]. Acta Scientiae Circumstantiae, 2019, 39(10): 3225-3237(in Chinese).
[27] WANG J F, LIU Q J, WU B, et al. Quorum sensing signaling distribution during the development of full-scale municipal wastewater treatment biofilms [J]. Science of the Total Environment, 2019, 685: 28-36. doi: 10.1016/j.scitotenv.2019.05.249 [28] WU Z Y, WANG Q, GUO F, et al. Responses of bacterial strains isolated from drinking water environments to N-acyl-L-homoserine lactones and their analogs during biofilm formation [J]. Frontiers of Environmental Science & Engineering, 2014, 8(2): 205-214. [29] DAVIES D G, PARSEK M R, PEARSON J P, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm [J]. Science, 1998, 280(5361): 295-298. doi: 10.1126/science.280.5361.295 [30] SHIH P C, HUANG C T. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance [J]. Journal of Antimicrobial Chemotherapy, 2002, 49(2): 309-314. doi: 10.1093/jac/49.2.309 -



 下载:
下载: