-
亚硝酸盐氧化菌(nitrite-oxidizing bacteria,NOB)在生物硝化反应中驱动
NO−2 -N氧化为NO−3 -N的过程,对于工业、养殖业水体净化修复至关重要。NOB严格好氧,是一类以CO2为唯一碳源、NO−2 -N为能源的自养细菌,生长代时普遍在10 h以上[1],生长率低,对环境因素极为敏感,这些特点限制了NOB菌株的分离纯化和机理研究[2]。随着工农业废水中重金属和抗生素累积的问题日益突出,过高的重金属、抗生素影响微生物群落结构和代谢功能,进而影响生态系统的净化修复特别是硝化细菌生物脱氮能力,故有必要对其进行进一步探讨。由于NOB分离和纯培养较为困难,目前其菌种资源并不多,国内外研究多集中于以硝化活性污泥为材料的资源开发及其种群生态分布的研究上,且大多为基于氨氧化细菌(ammonia-oxidizing bacteria,AOB)的研究。王勤[3]的研究表明,在生物脱氮系统中,应控制重金属Cu2+、Zn2+、Cr6+、Cd2+的质量浓度分别不高于0.5、30、0.5、5 mg·L−1;TIAN等[4]的研究表明,50 mg·L−1的链霉素可以完全抑制生物膜废水处理系统中的硝化反应;KATIPOGLU等[5]的研究表明,对活性污泥系统连续供给50 mg·L−1的盐酸四环素,硝化细菌活性丧失,NOB在系统中相对丰度降低了10倍。在实际应用上,NOB菌株世代时间长、数量少、对环境敏感,导致硝化效果欠佳,对比探究NOB和硝化菌群在环境胁迫下的活性变化对废水脱氮应用有一定理论意义,而基于不同重金属、抗生素胁迫下比较NOB和硝化菌群脱
NO−2 -N效果的研究尚未见报道。本研究采集珠江水样富集硝化菌群,并分离纯化一株NOB,考察了不同浓度重金属离子和抗生素对菌株亚硝酸盐氧化能力的影响,探究了菌株在环境胁迫下的一般规律,再对比NOB和硝化菌群富集物(初始NOB数量相等情况下)在不同种重金属和抗生素胁迫下的亚硝酸盐氧化活性的变化,以期为NOB的研究及其在工业、养殖业水体净化的实际应用提供参考。
-
所用化学试剂均为分析纯;琼脂糖凝胶DNA回收试剂盒(DP209,北京天根生化科技有限公司);零背景pTOPO-TA克隆载体试剂盒(北京艾德莱生物科技有限公司);TransStart® Tip Green qPCR SuperMix(AQ142-11,北京全式金生物技术公司)。富集硝化菌群的水样取自珠江穗石码头。
紫外-可见光分光光度计(UV-2800A,上海尤尼柯仪器有限公司);立式压力蒸汽灭菌锅(GI80DP,美国致微仪器有限公司);振荡培养箱(ZQLY-180G,上海知楚仪器有限公司);pH测量仪(HQ40d,美国Hach公司);超微量紫外可见光分光光度计(Nano Drop 1000,美国Thermo Fisher公司);实时荧光定量PCR仪(ABI 7500,美国Thermo Fisher公司)。
-
NOB液体培养基:0.5 g·L−1 KCl,0.485 g·L−1 MgSO4·7H2O,1 g·L−1 NaCl,0.3 g·L−1 KH2PO4,0.1 g·L−1 CaCl2·2H2O。以上成分高压蒸汽灭菌冷却后,加入过0.22 μm滤膜除菌的NaHCO3、NaNO2、微量元素母液,使其在培养基中的含量最终分别为2.5 mmol·L−1、10 mmol·L−1、体积分数1‰。在NOB液体培养基中加入质量分数为1.6%的琼脂。硝化菌群富集培养基:NOB液体培养基中NaNO2替换为等浓度NH4Cl。微量元素母液[6]:10 mL 浓HCl,2.1 g·L−1 FeSO4·7H2O,0.062 g·L−1 H3BO3,0.017 g·L−1 CuCl2·2H2O,0.100 g·L−1 MnC12·4H2O,0.036 g·L−1 Na2MoO4·2H2O,0.070 g·L−1 ZnCl2,0.190 g·L−1 CoCl2·6H2O,0.024 g·L−1 NiCl2·6H2O。
-
1)硝化菌群的富集和NOB的分离纯化。将10 mL珠江穗石码头水样接入装有90 mL富集培养基的锥形瓶中,在30 ℃、150 r·min−1条件下振荡培养,每隔2 d取样检测
NH+4 -N、NO−2 -N、NO−3 -N的含量,初始NH+4 -N被AOB氧化为NO−2 -N,接着NO−2 -N被NOB氧化为NO−3 -N,完成硝化过程。当硝化率达90%以上时,以10%体积比接种到新鲜富集培养基继续培养,以此获得稳定硝化菌群富集物,提取富集物DNA送至金唯智生物公司高通量测序。将富集液稀释涂布于NOB固体培养基平板上,在30 ℃恒温培养箱倒置培养28 d以上,挑取NOB单菌落至液体培养基进一步纯化,通过光学显微镜、扫描电镜观察NOB形态。2) NOB的鉴定。以NOB菌液作为模板,用细菌16S rDNA通用引物[7]27F(5′-AGAGTTTGATCCTGGCTCAG-3′)和1492R(5′-TACGGCTACCTTGTTACGACTT-3′)进行PCR。PCR反应体系(25 μL):2 μL NOB菌液,27F和1492R(10 μmol·L−1)各0.5 μL,12.5 μL 2×Master Mix,9.5 μL ddH2O。PCR反应条件:94 ℃ 10 min;95 ℃ 30 s,56 ℃ 30 s,72 ℃ 90 s,35个循环;72 ℃ 10 min。使用胶回收试剂盒回收纯化16S rDNA,采用零背景pTOPO-TA克隆载体试剂盒进行T-A克隆,经菌落PCR检测出阳性转化子并送至金唯智生物科技有限公司测序。将16S rDNA完整序列通过BLAST与Genebank数据库比对后,选择相似度较高的序列,用MEGA 7.0软件采用邻接法构建系统进化树并分析同源性。
3)重金属、抗生素对NOB亚硝酸盐氧化活性的影响。将NOB种子液以10%体积比接种至液体培养基中(初始
NO−2 -N为80 mg·L−1),添加不同重金属、抗生素设置单一变量,在30 ℃、150 r·min−1的条件下振荡培养,每12 h取样检测NO−2 -N含量。通过参考文献[3-5,8]及预实验设定重金属实验组质量浓度梯度为Cu2+(0、1、5、10、20 mg·L−1)、Zn2+(0、1、2、3、5 mg·L−1)、Cd2+(0、1、5、10、20 mg·L−1)、Mn2+(0、5、20、50、70 mg·L−1)、Cr6+(0、0.4、0.6、0.8、1.0 mg·L−1);抗生素实验组质量浓度梯度为卡那霉素(0、1、5、10 mg·L−1)、氨苄青霉素(0、1、2、3 mg·L−1)、链霉素(0、1、3、5 mg·L−1)、盐酸四环素(0、10、20、50 mg·L−1)。4)重金属、抗生素胁迫对NOB及硝化菌群活性的影响。通过绝对定量qPCR检测NOB种子液和硝化菌群富集物中NOB的绝对数量(以nxrA基因拷贝数计):nxrA引物[9]F1370F1(5′-CAGACCGACGTGTGCGAAAG-3′)和F2843F2(5′-TCCACAAGGAACGGAAGGTC-3′)。使用nxrA的胶回收产物作为标准品。采用改良CTAB法提取DNA[10]。绝对定量qPCR体系与程序参考TransStart® Tip Green qPCR SuperMix试剂盒(全式金,北京)说明书。
选择一组合适的重金属离子、抗生素质量浓度(显著影响NOB氧化活性但不完全抑制活性,NOB完全耗完底物时间为无添加组的1.5倍以上),比较NOB初始接种量相等情况下NOB和硝化菌群富集物对重金属、抗生素单一因素胁迫的亚硝酸盐氧化响应。其中重金属组:5 mg·L−1 Cu2+、1 mg·L−1 Zn2+、10 mg·L−1 Cd2+、20 mg·L−1 Mn2+、0.8 mg·L−1 Cr6+;抗生素组:0.5 mg·L−1 卡那霉素、0.5 mg·L−1氨苄青霉素、1 mg·L−1 链霉素、30 mg·L−1 盐酸四环素。NOB和硝化菌群富集物种子液接种量依据qPCR结果设定(见表1),使NOB和硝化菌群实验组中初始NOB数量相等;摇床条件设为30 ℃、150 r·min−1,每12 h取样检测
NO−2 -N含量。 -
NH+4 -N采用靛酚蓝法测定[11];NO−2 -N采用N-(1-萘基)-乙二铵光度法测定[11];NO−3 -N采用离子色谱法测定[12];pH通过pH计(HQ40d,美国Hach公司)测量;NOB nxrA质量浓度通过超微量紫外可见光分光光度计(Nano Drop 1000,美国)测定,标准品拷贝数按照式(1)计算;NOB nxrA基因绝对拷贝数量使用实时荧光定量PCR仪(ABI 7500,美国)和7500 Software v2.3检测分析。式中:c为NOB nxrA质量浓度,ng·μL−1;L为nxrA片段长度,320 bp。
-
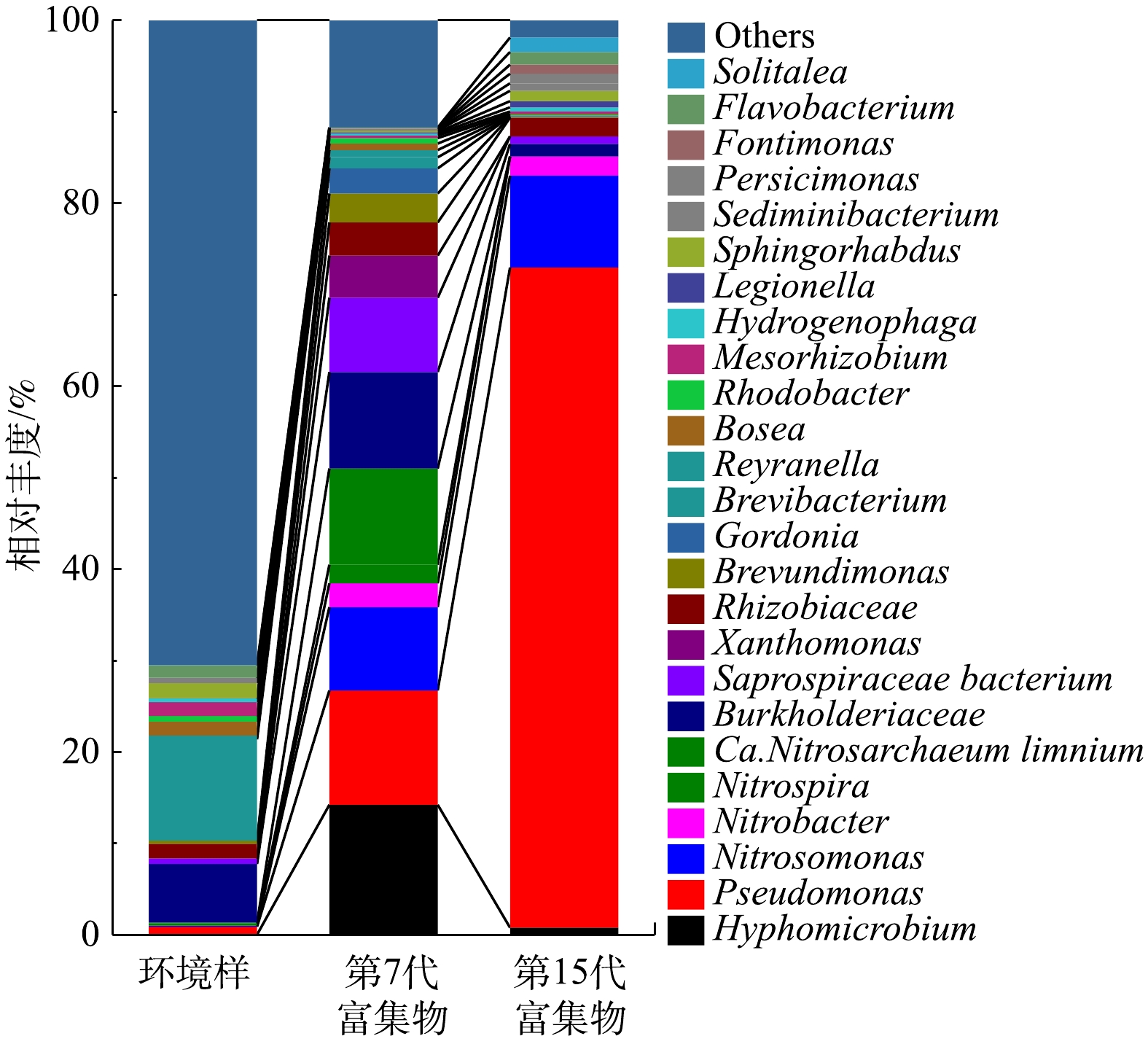
在属水平统计珠江水环境样、富集物群落组成情况如图1所示。在富集培养过程中,AOB、NOB以及Pseudomonas逐渐成为优势菌群。在15代硝化菌群富集培养中,Pseudomonas由环境样0.88%增长到第15代的72.18%,成为富集物的主要优势菌种之一;Nitrosomonas丰度由0.11%升高至10.04%;Nitrobacter的丰度由0.014%上升到2.10%;环境样中Nitrospira、Candidatus Nitrotoga、Nitrospina等属的NOB在第15代富集物中检测不到。有研究表明,Nitrobacter的亚硝酸盐半饱和常数(Km,NO2-)为40~1 380 μmol·L−1,Nitrospira的亚硝酸盐半饱和常数为9~27 μmol·L−1,Nitrospira具有更高
NO−2 -N亲和力,生长速率低,适合在低浓度NO−2 -N下缓慢生长[13-14];Nitrososphaera与Nitrosomonas同样具有这种关系。这解释了10 mmol·L−1NH+4 -N和NO−2 -N的选择压力下Nitrosomonas和Nitrobacter分别成为优势AOB、NOB且丰度升高的现象。 -
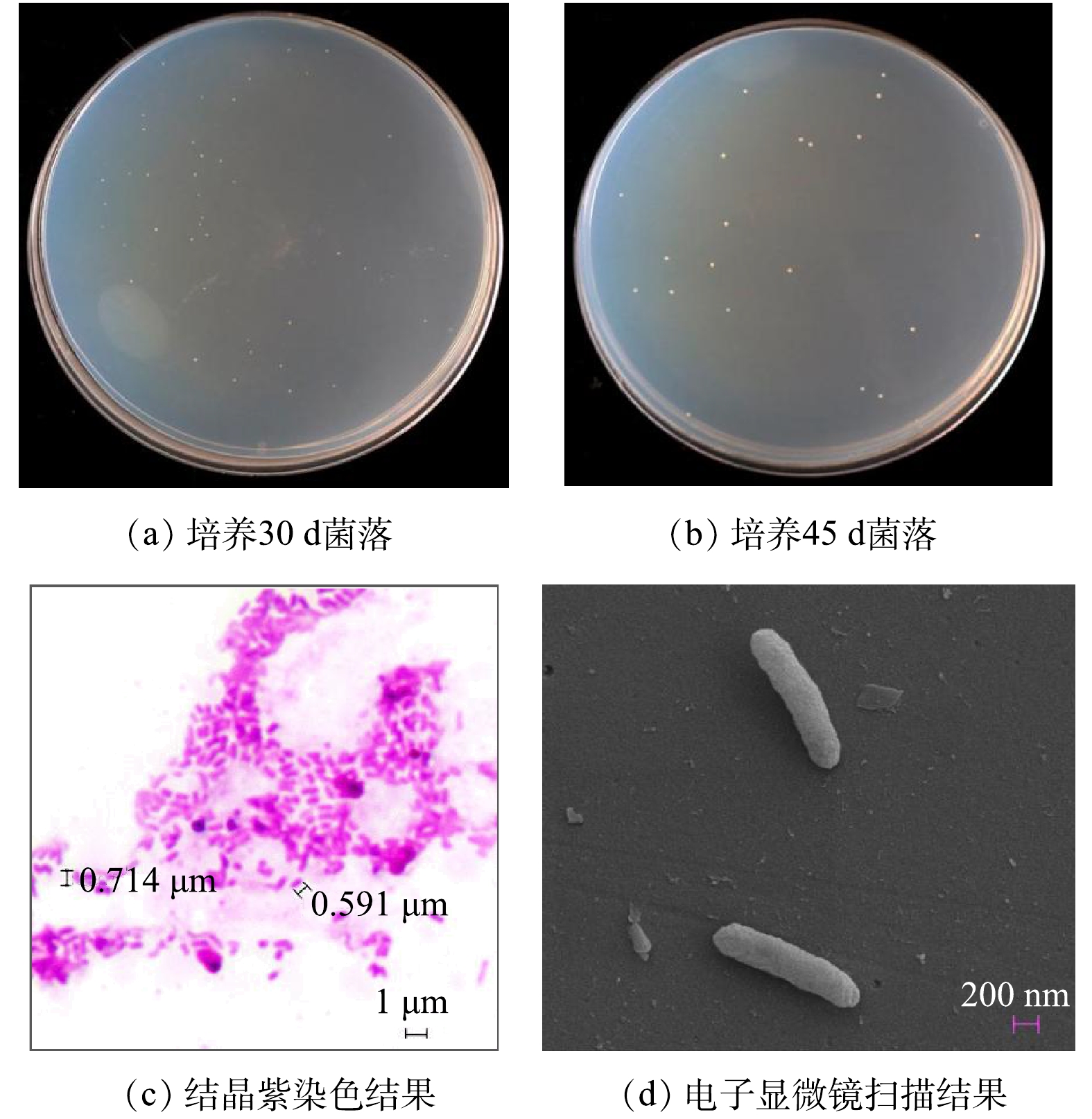
NOB的菌落形态和细胞形态如图2所示。培养30 d,长出来NOB菌落呈针尖大小,色泽淡黄,平实不透明,边缘及表面光滑,直径0.2~0.3 mm,直径随培养时间的延长而增大(图2(a)和图2(b));培养45 d,直径约0.5 mm。结晶紫染色结果显示:NOB为分散排列、无芽孢、两端钝圆的短杆状细菌,细胞大小为0.5~1.0 μm(图2(c))。扫描电镜成像显示:NOB呈杆状,细胞表面完整、光滑(图2(d))。
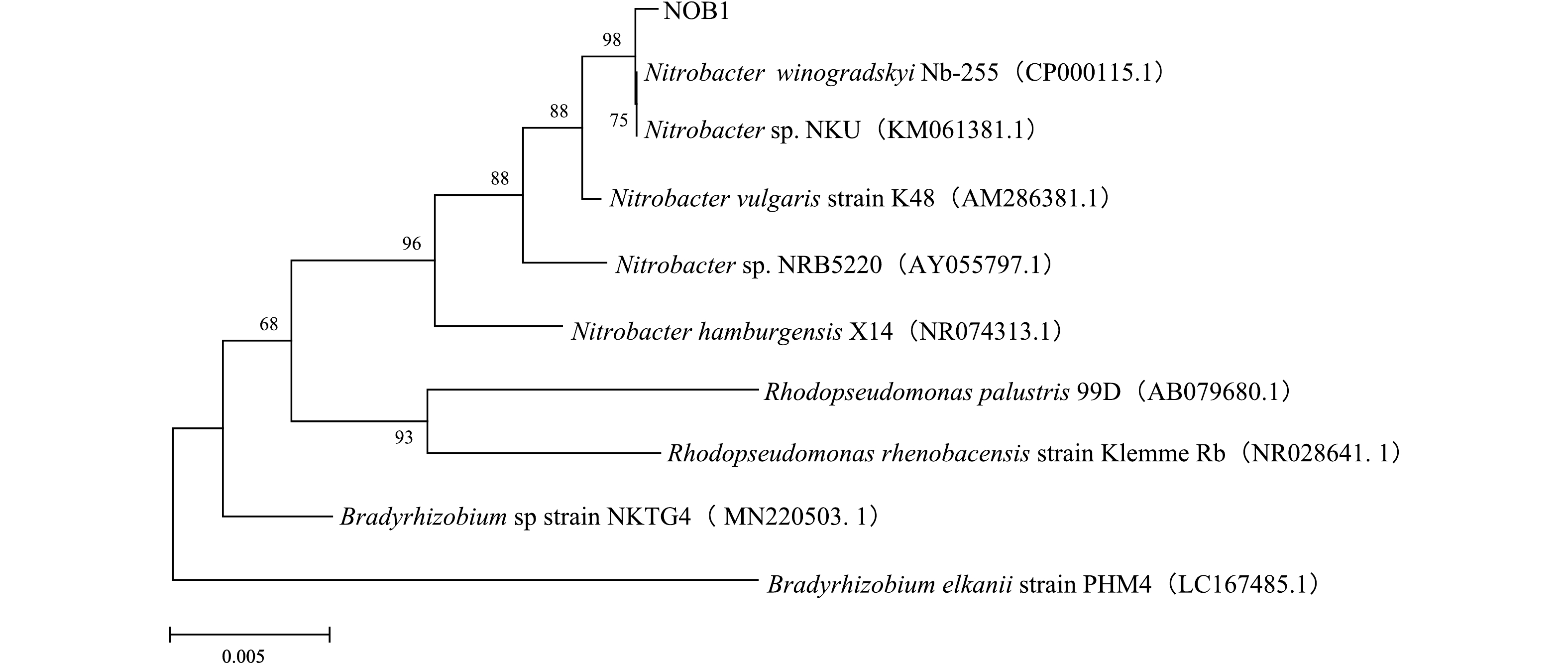
菌株NOB1的16S rDNA序列在GenBank数据库通过BLAST检索对比,结果表明,NOB1与Nitrobacter winogradskyi Nb-255模式菌株相似度最高,达到99.58%。基于16S rDNA序列构建系统发育树,如图3所示,NOB1与Nitrobacter winogradskyi Nb-255同源性最高,且与各Nitrobacter处于同一聚类。
-
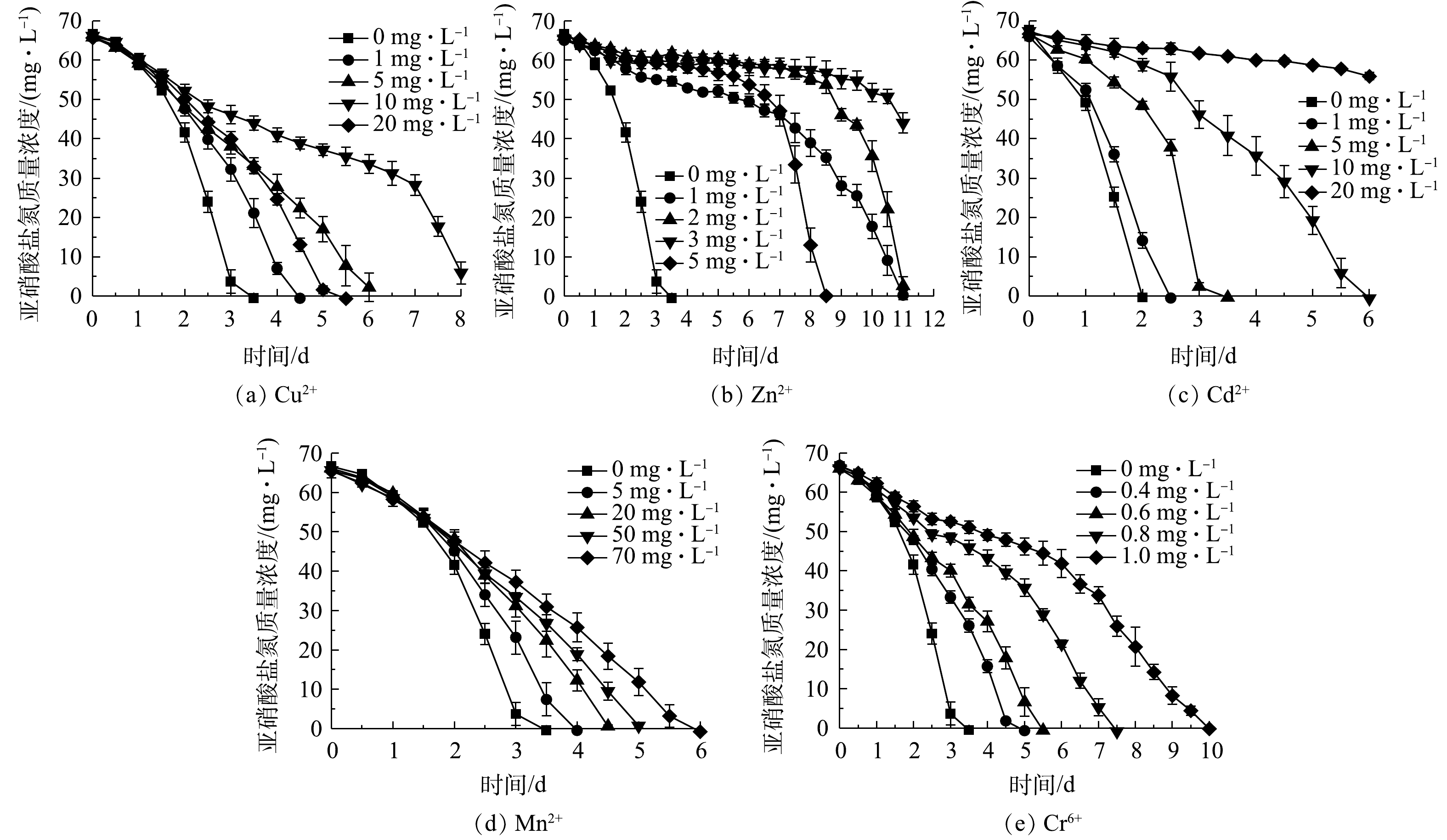
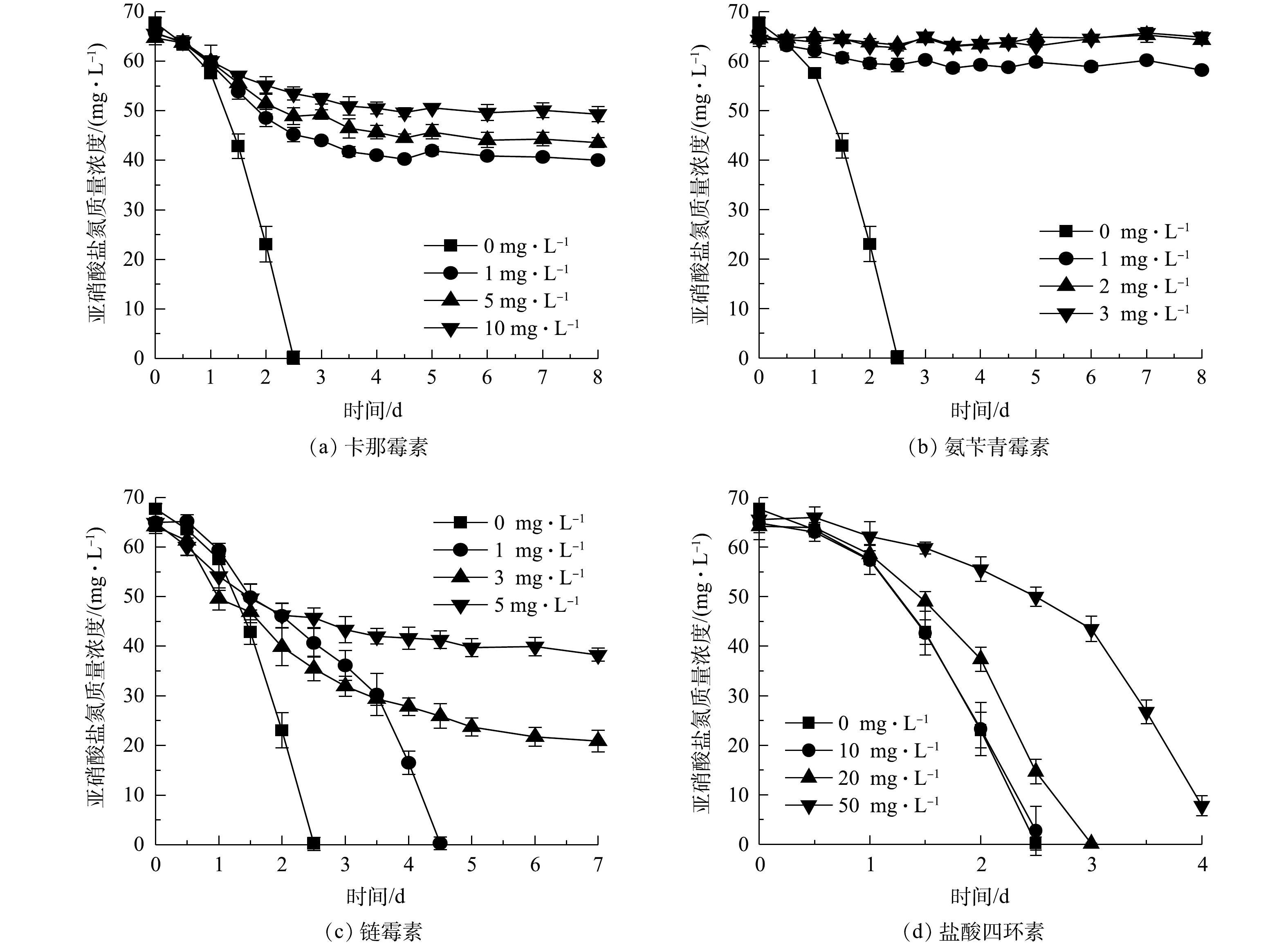
1)重金属对NOB亚硝酸盐氧化活性的影响。不同浓度的Cu2+、Zn2+、Cd2+、Mn2+、Cr6+对NOB亚硝酸盐氧化活性的影响情况如图4所示。可以看出,在5种重金属组中,亚硝酸盐氧化活性随重金属浓度的变化规律基本一致,均随重金属离子浓度升高而其活性抑制作用增强。以Cu2+组为例,Cu2+添加量为0 mg·L−1时亚硝酸盐氧化速率最快,在第3 天基本消耗完底物,脱
NO−2 -N率达到97.58%;在Cu2+添加量为1、5、10 mg·L−1组中,分别在第4.5、6.5、8 天基本消耗完初始添加的NO−2 -N,表明NOB亚硝酸盐氧化速率随Cu2+浓度升高而降低;Cu2+质量浓度为20 mg·L−1时的亚硝酸盐氧化速率介于1 mg·L−1与5 mg·L−1组之间,其原因是Cu2+添加量较高(根据Ksp(Cu(OH)2)=2.2×10−20计算出20 mg·L−1 Cu2+开始沉淀pH为5.92),形成可见的Cu(OH)2沉淀,毒害性随之减小,同样的现象亦存在于5 mg·L−1的Zn2+实验组。从5种重金属离子对NOB亚硝酸盐氧化速率的影响来看,对NOB亚硝酸盐氧化活性抑制作用为Cr6+>Zn2+>Cu2+>Cd2+>Mn2+。重金属对微生物的损害主要体现为破坏细胞膜、抑制生物大分子合成[15]。有学者研究[16-18]表明,当添加Cu2+≥30 mg·L−1、Cd2+≥10 mg·L−1、Cr6+≥5 mg·L−1时对硝化脱氮过程有显著影响,这些研究是以硝化活性污泥作为材料,活性污泥可能通过沉淀、吸附或吸收重金属而降低毒性,相比于单一菌株,活性污泥可能对重金属胁迫有更好的抗性[19],所以这些研究中的结果略高于本研究中的单一NOB菌株。2)抗生素对NOB亚硝酸盐氧化活性的影响。不同浓度卡那霉素、氨苄青霉素、链霉素、盐酸四环素对NOB亚硝酸盐氧化活性的影响如图5所示。可以看出,除盐酸四环素外,其他3种抗生素对NOB亚硝酸盐氧化活性起到显著的抑制作用。空白对照组在第2.5 天氧化完
NO−2 -N;卡那霉素或氨苄青霉素≥1 mg·L−1时,NOB在培养前期表现较弱的亚硝酸盐氧化活性,随着培养时间的延长,NOB受抗生素毒害作用加深,活性逐渐殆尽;NOB对盐酸四环素的耐受性较强,添加盐酸四环素≤10 mg·L−1时NOB亚硝酸盐氧化活性几乎不受影响,盐酸四环素为20 mg·L−1时NOB活性略有降低,在高达50 mg·L−1时,盐酸四环素才对NOB表现出较明显的抑制效果,但脱NO−2 -N率仍能在第4 天达到90.02%。以质量浓度1 mg·L−1抗生素的脱NO−2 -N率为依据判断4种抗生素对NOB亚硝酸盐氧化速率的影响,对NOB亚硝酸盐氧化活性抑制作用强弱顺序为氨苄青霉素>卡那霉素>链霉素>盐酸四环素。 -
1) NOB和硝化菌群绝对定量。NOB和硝化菌群富集物中nxrA绝对定量结果见表1,可以看出NOB纯培养种子液中NOB的绝对拷贝数要高于硝化菌群富集物的,前者数量约为后者的3倍。为使两者初始接种NOB量相等,NOB种子液和硝化菌群种子液的接种量分别为2.000%和6.056%。
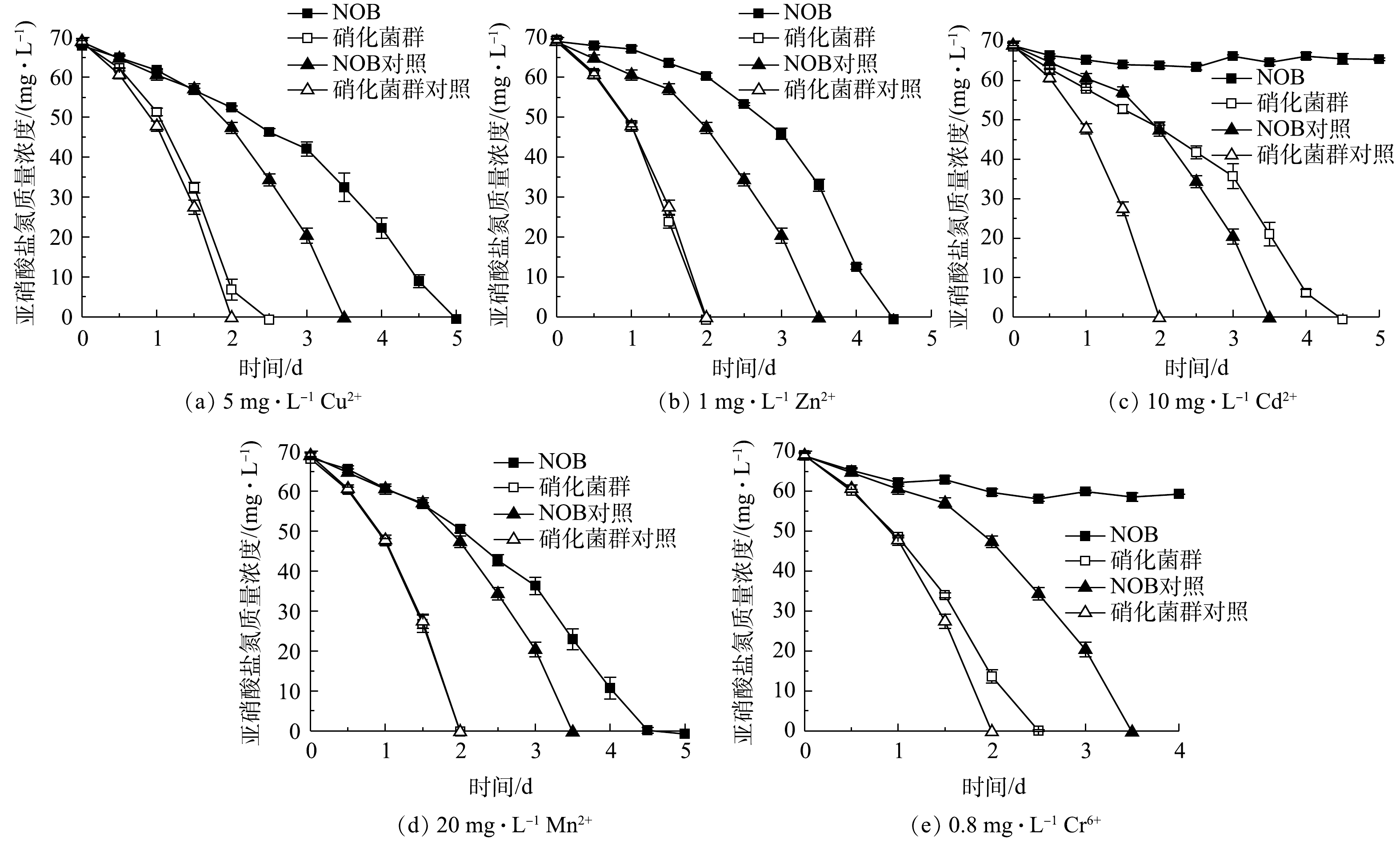
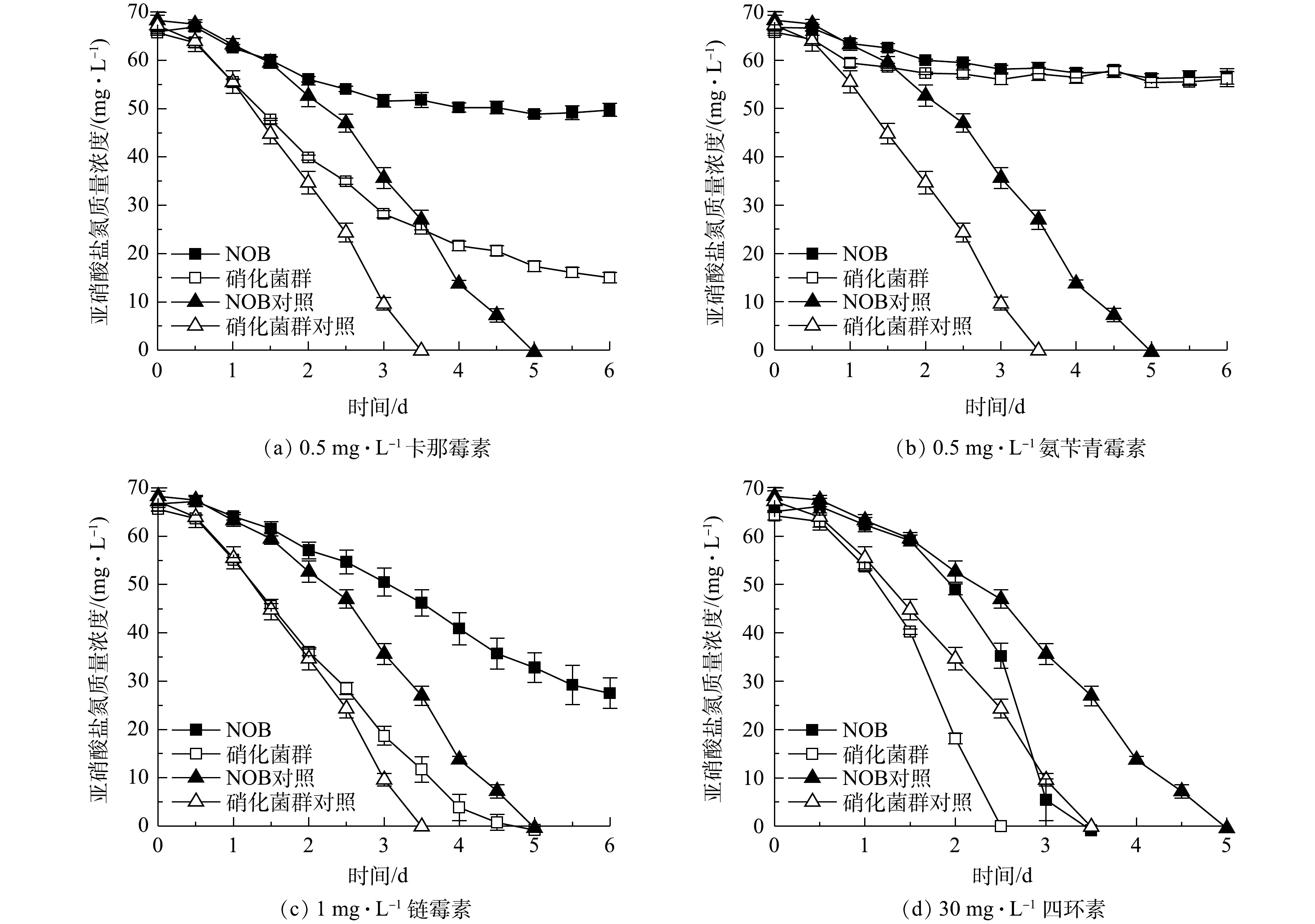
2)重金属胁迫对NOB及硝化菌群活性的影响。重金属胁迫对NOB及硝化菌群活性的影响结果如图6所示。可以看出,在重金属离子胁迫作用下,硝化菌群富集物的亚硝酸盐氧化速率均高于NOB组。以5 mg·L−1 Cu2+组为例,NOB对照和实验组分别于第3.5和5 天氧化完底物,硝化菌群对照和实验组的亚硝酸盐氧化速率更快,在第2 天的脱
NO−2 -N率分别达到100%和90.08%。同时,值得注意的是,硝化菌群对照组和实验组的亚硝酸盐氧化速率几乎一致,而NOB对照组和实验组的亚硝酸盐氧化速率差异显著。这表明NOB组在5 mg·L−1 Cu2+胁迫作用下活性受到显著影响,而硝化菌群组则几乎不受到5 mg·L−1 Cu2+的抑制作用。同样的现象发生也在1 mg·L−1 Zn2+和20 mg·L−1 Mn2+实验组。由此可见,在初始NOB数量相等情况下,NOB纯培养物在铜、锌、镉、锰、铬等重金属胁迫下的抗逆性和稳定性略显不足,而硝化菌群富集物亚硝酸盐氧化活性和稳定性更高,抗重金属胁迫能力更强,实际应用潜力更大。硝化菌群富集物中除了NOB、AOB,还有丰富的微生物群落,其中Pseudomonas、Rhizobiaceae、Burkholderiaceae等占比较大,分别达到72.18%、2.04%、1.39%。有研究[20-22]表明,这些微生物有较强分泌胞外聚合物(extracellular polymeric substances,EPS)能力。EPS上的羧基、羟基、磷酰基等阴离子基团是吸附Cu2+、Zn2+、Cd2+等重金属离子的主要位点[23-24]。此外,有的微生物还有离子通道、重金属外排、氧化还原等复杂的胞内解毒机制,如Cr6+可以竞争SO2−4 转运蛋白结合位点,细菌相关蛋白表达水平升高从而解毒[25]。这些研究结论与本研究中观察到的硝化菌群富集物稳定性更高、亚硝酸盐氧化速率更快等现象一致。3)抗生素胁迫对NOB及硝化菌群活性的影响。4种抗生素胁迫对NOB及硝化菌群活性的影响结果如图7所示。对比NOB与硝化菌群富集物组可以看出,在不同抗生素胁迫下,后者的亚硝酸盐氧化速率均高于前者。以0.5 mg·L−1卡那霉素组为例,在无添加卡那霉素的情况下,NOB和硝化菌群分别在第5、3.5 天消耗完底物。NOB对抗生素敏感,在0.5 mg·L−1卡那霉素胁迫下,NOB和硝化菌群组的亚硝酸盐氧化活性均受到显著抑制作用,培养前期氧化速率较缓慢,随着培养时间的延长受毒害作用加深,亚硝酸盐氧化活性逐步降低,第6 天NOB和硝化菌群组的脱
NO−2 -N率分别为24.60%和77.18%,在抗生素的抑制作用下,硝化菌群的亚硝酸盐氧化能力仍高于NOB。在硝化系统中,微生物主要通过外排泵、核糖体保护机制、以及破坏活性位点等方式抵抗抗生素;ZHANG等[26]的研究表明,Pseudomonas、Flavobacterium和Nocardiopsis的菌群数量与抗生素抗性基因呈正相关。本研究的高通量测序结果(图1)表明,Pseudomonas和Flavobacterium在富集物菌群中占比为73.55%。这可能是硝化菌群富集物在抗生素胁迫下亚硝酸盐氧化活性、稳定性以及抗逆性都比NOB单一菌株更高的原因之一,说明硝化菌群在应对抗生素污染的环境问题具有更大的潜力。同时,值得注意的是,在30 mg·L−1 盐酸四环素条件下,不管是NOB还是硝化菌群富集物,实验组都比对照组先消耗完底物,即30 mg·L−1 盐酸四环素在实验中在一定程度上促进亚硝酸盐氧化而非抑制作用,表明适量浓度的四环素可能对NOB的富集有帮助。宋超[27]在NOB的活性受到四环素抑制的结论基础上进一步研究发现,在硝化污泥中加入较高浓度的四环素,NOB的数量比四环素浓度较低的组更多,硝化性能更好。其可能原因是:四环素刺激EPS的分泌,与EPS的—OH、C—O—C、C=O和C—N等官能团作用,诱导硝化污泥形成结构紧密的聚合体,对部分优势菌种起到保护作用[28-29];四环素被EPS吸附并通过微生物缓慢降解而减弱毒性效应[30];且四环素本身易变质失效。
-
1)以珠江穗石码头水样富集硝化菌群并分离纯化一株NOB菌株,NOB的亚硝酸盐氧化活性随重金属离子(Cu2+、Zn2+、Cd2+、Mn2+、Cr6+)、抗生素(卡那霉素、氨苄青霉素、链霉素、盐酸四环素)浓度升高而受到抑制效应增强。
2)在初始NOB相等情况下,与NOB纯培养物相比,硝化菌群富集物在重金属或抗生素胁迫下的亚硝酸盐氧化速率更快,稳定性更高,对重金属和抗生素的抗逆性更强,对于处理含氮废水有更好的应用潜力。
重金属和抗生素胁迫对亚硝酸盐氧化菌及硝化菌群活性的影响
Effects of heavy metal and antibiotic stresses on the activity of nitrite-oxidizing bacteria and nitrifying bacteria flora
-
摘要: 重金属和抗生素广泛存在工业废水、养殖水体中,对硝化细菌活性有不同程度的影响。为研究重金属和抗生素胁迫对亚硝酸盐氧化菌(nitrite-oxidizing bacteria,NOB)及硝化菌群活性的影响,采集珠江水样富集硝化菌群并分离纯化NOB,研究了不同浓度重金属离子(Cu2+、Zn2+、Cd2+、Mn2+、Cr6+)和抗生素(卡那霉素、氨苄青霉素、链霉素、盐酸四环素)对NOB亚硝酸盐氧化活性的影响,揭示了不同重金属和抗生素胁迫对NOB及硝化菌群富集物活性的影响。结果表明:第15代富集培养的硝化菌群中Nitrosomonas丰度由0.11%升高至10.04%,Nitrobacter丰度由0.014%上升到2.104%;经鉴定,分离纯化的NOB菌株与Nitrobacter winogradskyi相似性为99.58%;NOB的亚硝酸盐氧化活性随重金属离子、抗生素浓度升高而下降;在初始NOB量相等情况下,与NOB组相比,硝化菌群富集物实验组在重金属或抗生素胁迫下,亚硝酸盐氧化速率更快,稳定性更高,对重金属和抗生素的抗逆性更强,对于处理含氮废水的实际应用潜力更大。该研究结果可为NOB的研究和在工业、养殖业水体净化的开发应用提供参考。Abstract: Heavy metals and antibiotics are widely distributed in industrial wastewater and aquaculture water, which have varying degrees of impact on the activity of nitrifying bacteria. In order to study the effects of heavy metals and antibiotics on the nitrite oxidation of nitrite-oxidizing bacteria (NOB) and nitrifying bacteria flora, water sample of the Pearl River was collected to enrich and cultivate nitrifying bacteria flora and isolate NOB, then the effects of different concentrations of heavy metal ions (Cu2+, Zn2+, Cd2+, Mn2+, Cr6+) and antibiotics (kanamycin, ampicillin, streptomycin and tetracycline hydrochloride) on the nitrite oxidation activity of NOB were studied to reveal the effects of heavy metals and antibiotics stresses on the activities of nitrifying bacteria flora and NOB. The results showed that the abundance of Nitrosomonas in the nitrifying bacteria flora after the 15th times enrichment increased from 0.11% to 10.04%, and the abundance of Nitrobacter increased from 0.014% to 2.104%. The isolated and purified NOB was closely related to Nitrobacter winogradskyi, showing a similarity of 99.58%. The nitrite oxidation activity of NOB decreased with the increase of the concentration of heavy metal ions and antibiotics; in comparison with NOB, the nitrifying bacteria flora under the stress of heavy metals and antibiotics had a higher nitrite oxidation rate, more stable and stronger stress resistance to heavy metals and antibiotics, which indicates it has a greater potential on the practical application in nitrogenous wastewater treatment. The results provide a theoretical reference for the research of NOB and its development and application in industry and aquaculture water purification.
-
Key words:
- nitrite-oxidizing bacteria /
- nitrifying bacteria flora /
- heavy metal /
- antibiotic
-
2018年国务院办公厅发布《“无废城市”建设试点工作方案》(国办发〔2018〕128号)[1],2019年4月30日,中华人民共和国生态环境部公布11个“无废城市”建设试点[2],经过为期2年的建设,试点工作已成功验收,试点实践形成了“无废城市”建设指标体系和一批可推广示范模式,其内核不仅更注重生态环境保护,还在于让经济发展过程资源利用率更高、社会效益更好,展现了“无废城市”城市管理理念的先进之处。2022年4月24日,生态环境部公布“十四五”时期“无废城市”建设名单,内蒙古自治区呼和浩特市、包头市、鄂尔多斯市均在“无废城市”建设名单中[3]。“无废城市”旨在通过源头减量、循环利用、绿色生产等方式,实现城市内资源的有效利用和环境的持续改善。当前,“无废校园”是一种建立在“无废城市”概念基础上的先进的校园理念[4-6],“无废校园”已成为“无废城市”建设的重要组成部分,两者的相互促进、协同发展已成为重要趋势[7-8]。它通过资源的高效利用、环境的保护与生态建设、绿色生活理念的倡导等方式,实现校园内废弃物的最小化,推动校园走向可持续发展。高校的固体废物具有种类较多且区域较为集中的特点。因此,针对校园固废产生特性、处置利用及管理方面的顶层设计需求较为迫切。
目前,“无废校园”建设相关研究主要集中在垃圾分类现状讨论、分阶段减量、分类回收等方面。张悦等[9]以中北大学信息商务学院为例探讨高校校园固废分类现状及对策,认为可在垃圾分类措施完善、优化垃圾箱设置的区域针对性和提高学生的环保意识等方面开展。仝伟亮等[10]提出可从源头分类减量、中间资源化回收减量、末端无害化堆肥减量开展,并建立校园固废三级管理体系。朱兴峰等[11]提出可设计出一款智能垃圾分类回收设备推动校园固废分类与资源回收利用。陈成等[12]分析了校园垃圾分类存在的问题,提出可通过建立学校特色的规章制度、加强基础设施建设、应用先进的技术、采取有效地激励与约束措施和加强垃圾分类的宣传教育等对策,提高学生垃圾分类素养。彭希等[13]提出可采用数字化技术提升校园垃圾管理水平。
当前,在高校校园固废处理研究领域,研究者们主要开展校园垃圾分类管理或提升管理水平等方面研究,而在固体废物灰色模型预测方面,主要针对某个区域或某个城市开展研究。如封辰阳等[14]基于浦东地区历史数据,采用灰色预测模型和优化模型,预测了该区未来10年的垃圾产生量及最优回收方案,并得出了政府需要投入的成本;燕飞等[15]基于灰色预测理论,分别用GM(1,1)模型、分数阶GM(1,1)模型和新陈代谢GM(1,1)模型对广州市2015—2019年城镇生活垃圾清运量数据进行建模、检验和比较,结果表明新陈代谢GM(1,1)模型预测精度最高,预测2020—2024年广州市城镇生活垃圾清运量仍呈现长的趋势,在2024年将会突破1 000×104 t。然而,针对“无废校园”建立固废产量等相关预测模型尚未报道,校园固废产量预测是一个重要的环境管理问题,其直接涉及校园环境的全时空质量与学生健康。因此,通过预测固废产生,可以有效规划和调整管理应对方案,改善校园环境质量;同时,还可以通过强化收运与资源化利用体系建设,针对性建立完善回收网络、建立多种回收渠道、推广先进回收技术,有效降低或消除废弃物的对生态环境的影响,在保护自然资源与资源利用最大化的同时,也可推动循环经济发展,促进绿色产业和低碳经济的实现。
在全国“无废城市”持续推进与呼和浩特市已入选“十四五”无废城市建设的背景下,本研究以内蒙古工业大学为例,通过文献调研、实地走访、现场调查等手段,深入研究内蒙古工业大学校园固废产生量、产生规律及校园固废处理方式,并基于GM(1,1)灰色预测模型,结合现有数据特点与规律构建校园固废产量预测模型,以期为“无废校园”建设提供参考。
1. 研究概况
1.1 研究区概况
内蒙古工业大学(新城校区)位于内蒙古自治区呼和浩特市,校区研究区域建筑分布见图1。校园占地面积约215.13 hm2,建筑面积92.37×104 m2,在校师生约1.30×104人,校园固体废物的产出区域包括3区公寓(东区、西区、北区)、各教学楼(第一教学楼、第二教学楼、第三教学楼、第四教学楼、科学楼)、各实验楼(工程技术楼A、B座等)、各餐厅、绿化带和施工场地(施工时)等。
1.2 研究方法与对象
以文献调研、实地走访与调查、问卷调研等多种研究方法及结合灰色预测模型建构,针对住教学区、宿舍区、餐厅等区域等产生的纸张和纸板、塑料制品、厨余垃圾、纺织品等可回收垃圾与电子废物、有害废物、玻璃制品、建筑废弃物等不可回收垃圾。
1)文献调研。确定本研究涉及范围与主题,以CNKI、万方数据知识服务平台、Web of Science等作为文献资料数据库,针对检索结果开展筛选和评估,分析及研究关键文献资料,梳理总结“无废校园”建设现状。
2)实地走访与调查。实地走访与调查内蒙古工业大学(新城校区)垃圾产生、处理及回收过程与方式,以1年为期限,调研频次每周不少于3次,调研不同季节不同时间节点垃圾的产生、收集及处置方式,通过校园代表性的楼宇、区域作为调查重点,开展实地考察并采集数据,基于调研成果分析与整理,摸清垃圾底数与特点。同时,数据的采集与调研时间固定为调研当天垃圾产生过程基本结束、垃圾清运过程之前以及避开可能出现特殊情况导致调研数据受到较大影响的时间段或日期。
3)灰色预测模型。灰色预测法是一种用于预测时间序列数据的方法,可处理具有不确定性和随机性的数据。在生态环境管理领域,灰色模型可以用于预测垃圾产量等具有不确定性的问题。通过建立符合的微分方程模型来预测未来的发展趋势,最终得到预测值[16]。本研究通过采用灰色系统预测模型[17],以灰色系统方法和模型技术,基于人流量、垃圾产量等方面综合评估,选取最具有代表性的某一教学楼连续1周垃圾产量数据,建立灰色预测模型,从而预测该建筑未来几天或更长时间周期的产量,根据结果精确性确定全校预估结果准确性。
灰色预测模型计算过程中,
x(0) x(0) x(1) x(1)1=x(0)1 x(1) x(1)k=∑kj=1x(0)jk=1,2,⋯,N (1) 式(2)称为一阶灰色微分方程,记为GM (1,1)。式(2)中a和u为待辨识参数。
dx(1)dt+ax(1)=u (2) 设参数向量
ˆa yN ˆa=[au]T (3) yN=(x(0)2,x(0)3,…,x(0)N)T (4) B=(−(x(1)2+x(1)1)/21⋮⋮−(x(1)N+x(1)N−1)/21) (5) 求出对应
yN ˆa ˆa ˆa=(BTB)−1BTyN (6) 有响应方程式(7),将春、夏、秋、冬四季的
a u ˆx(1)k+1=(x(1)1−ua)e−ak+ua (7) ˆx(0)k+1=ˆx(1)k+1−ˆx(1)k (8) 4)对比分析法。对比分析法是一种基本的科学探索方法,它是通过将一组具有相似因素的不同性质物体或对象进行对照比较,以确定它们在构造、性质等方面的差异和相似之处。本文通过内蒙古工业大学与安阳工学院各建筑区固废日均产量进行对比,明晰不同区域高校固废产生的特征。
2. 校园固体废物产生规律及模型构建与预测
2.1 校园固体废物产生特性
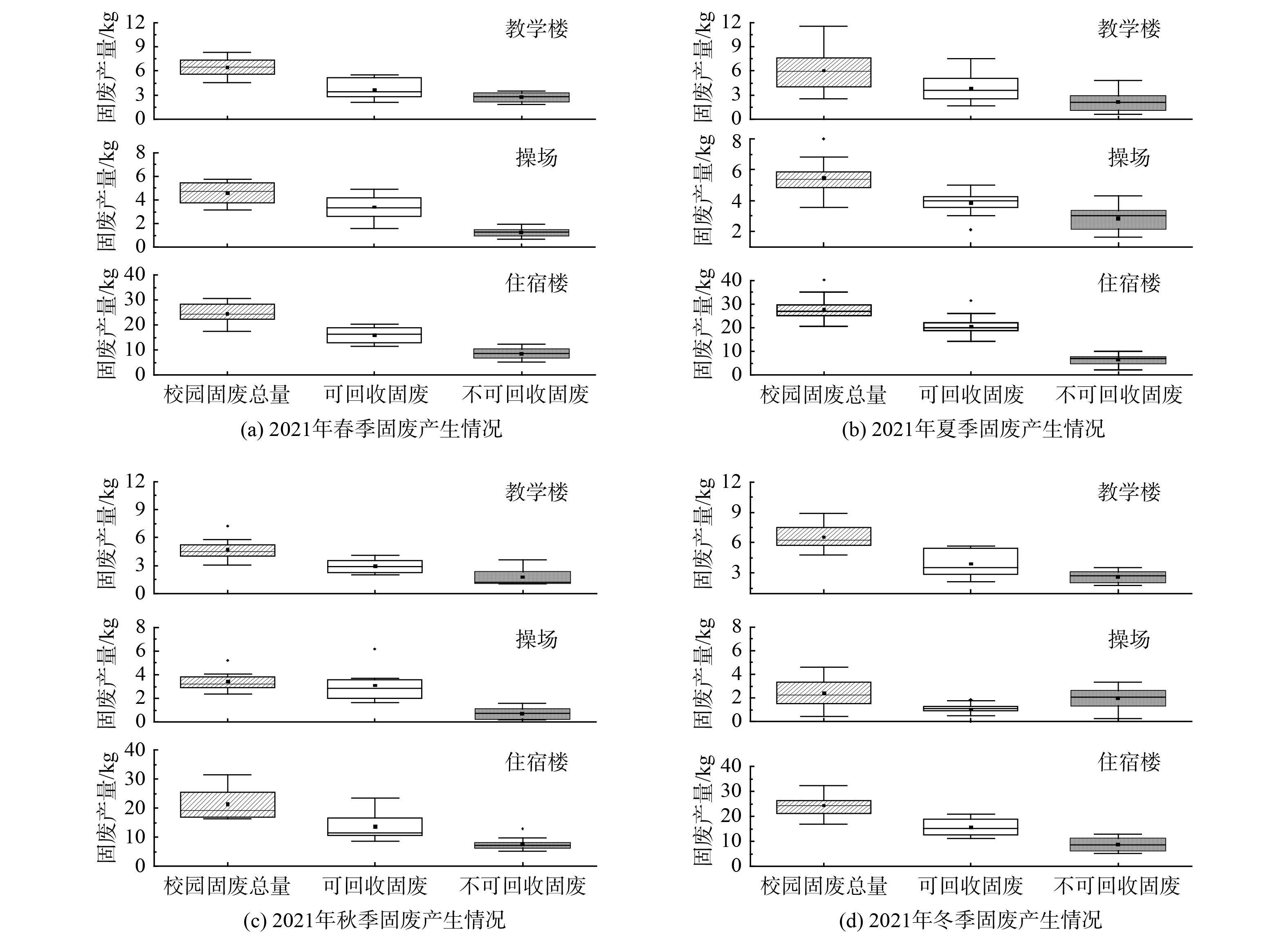
为摸清内蒙古工业大学新城校区校园固体废物产生规律,于2021年开展随机调研该校区某教学楼、操场、某住宿楼的校园固体废物产生情况,分析结果见图2。
通过图2可看出春、夏、秋、冬四季下不同建筑物固废产生量情况,其中教学楼、操场、住宿楼日固废产生均值分别为(6.08±0.77)、(4.50±0.82)、(23.58±5.41)kg,包括可回收固废日产生均值为(3.69±0.96)、(1.3±0.08)、(14.70±3.61)kg,不可回收固废日产生均值为(2.38±0.33)、(3.21±0.83)、(8.88±2.70)kg。综合四季来看,夏季垃圾产量处于全年的高位,在固废总量上体现最明显,其数值为4个季节中最高。
在时间方面分析,春季的产生规律最能体现出住宿区、教学区、运动区的固废量产生特征在时间尺度方面体现为季节性波动较大。图2(a)为春季3区固废产量对比,其中操场的固废总量存在较大波动,且固废总量均值明显偏低,教学楼和宿舍的固废总量较为稳定;可回收固废量方面,教学楼和宿舍的可循环利用固废量远高于操场,且存在较大波动,操场可回收固废量相对稳定但均值较低;不可回收固废方面,相比可回收固废表现出固废产生量较低。图2(b)相较(a)、(c)、(d)图,无论是固废总量还是可回收固废或不可回收固废量均最高;夏季住宿区、运动区的固废量都会呈现出明显的增加趋势,这与夏季昼长夜短,人们活动时间较长有一定关联;同时从全年来看,夏季校园住宿区和教学区生活垃圾产量的高峰期(即5月底)是毕业季,据统计,固废总量均值较其他季节下约高出1.2~2.8倍,该时期学生离校时平均70%~85%的物品被丢弃,日固废产生量也大幅增加。因此,建议可借鉴埃斯库埃拉滨海高级理工学院(ESPOL, Escuela Superior Politécnica del Litoral)建立临时废物收集中心,促进部分有价值的物品二次再利用[18];秋季各类垃圾的产量数据相较其他季节,数据较为稳定,为四季垃圾产量的较低时段;冬季固废量与春、夏两季节比较相对更少,平均每日固废量比夏季固废量减少40%左右。这可能是因为冬季气温偏低,人们户外运动意愿不强烈,因此在运动区活动的人也比夏季时要少得多,表现为固废产量冬季时显著降低,故固废产生总量也自然减少。另外,固废的日产量呈现出非常显著的时间阶段性,具体为周末产量远大于工作日的产量,主要原因是周末学生外出购物频次较高,由此产生大量购物、食品包装垃圾丢弃现象,工作日学生大都在校内上课,购物消费较少,故生活垃圾产量少于休息日。此外,每年学生开学时期校园固废产生量增长明显,寒暑假期间校园内固废产量最少。
在空间方面分析,校园固废产生量较大、产生空间具有集中性、产生时间具有阶段性。调研区域内每日产生固废总量均值为35.26 kg,其中可回收固废均值22.97 kg,可回收固废中纸张和塑料分别占比为33.59%和66.41%。校园固废产生特征主要表现为校园人员较为集中,校园固废会随着人流量的增大而增多,校园废弃物也会急剧增加,体现出校园固废空间集中的特点。例如由于夏季高温炎热,学生相对喜欢饮用矿泉水或冰镇饮料等,因此住宿区、运动区的固废量均会呈现明显的增加趋势,结合毕业季,宿舍区的垃圾产量也会有明显地升高。
综上所述,校园不同区域之间固废产量的差异会随不同季节表现出一定的季节性差异。该数据特征对于进一步深入了解校园固废管理和环境保护方面的差异提供了有价值的信息,有助于制定相应的管理措施和政策[19]。因此,建议在校园固废管理中需根据不同季节的特点采取相应措施,加强垃圾分类、鼓励回收和减少不可回收固废的产生,可大幅降低固废对环境的影响,提高资源利用效率[20]。
2.2 不同建筑物固废产生现状分析
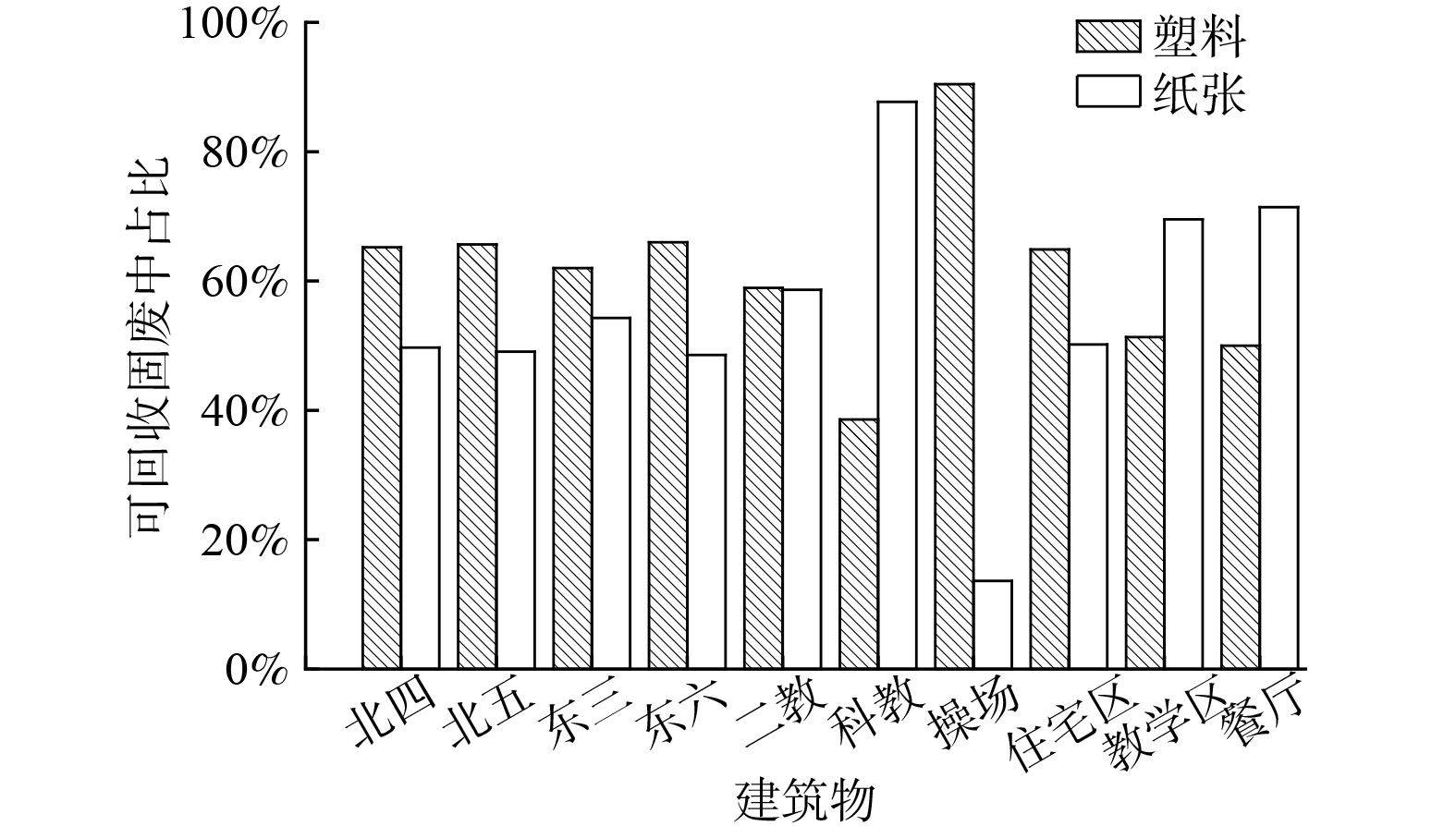
1)校园固体废物产生类别现状。表1为内蒙古工业大学新城校区单建筑物校园固废产量现状,分析表明单个建筑物可回收固废产生总量为32.18 kg,占总体固废产生量的50.59%;不可回收固废产生总量为16.63 kg,占总体固废产生量的26.20%;不可分类固废产生总量为14.67 kg,占总体固废产生量的23.11%;除教学区和宿舍以外其他区域可回收校园固废产量较少,其中餐厅的可回收和不可回收校园固废产量最低。从固废产生总量来看,可回收固废占比较大,因此,校园固废具有易于分类、便于管理的特点。
表 1 内蒙古工业大学新城校区单建筑物校园固废产量数据Table 1. Solid waste production data of single building campus of Xincheng Campus of Inner Mongolia University of Technology建筑物 可回收平均值/kg 不可回收平均值/kg 不可分类平均值/kg 数据属性 北区公寓四号楼 4.87 3.20 2.60 单层 北区公寓五号楼 9.10 4.20 1.00 单层 东区公寓三号楼 4.20 1.67 0.67 单层 东区公寓六号楼 3.60 1.12 0.23 单层 第二教学楼 0.67 0.36 0.00 单层 科学楼 1.42 1.55 5.53 单层 操场 1.74 0.88 0.74 单层 思源餐厅 0.05 0.046 0.00 每人次 馨苑餐厅 0.009 0.048 0.00 每人次 住宿区 5.44 2.55 1.13 每层 教学区 1.05 0.96 2.77 每层 餐厅 0.030 0.047 0.00 每人次 图3为各建筑物可回收固废中塑料和纸张占比情况。发现在校园可回收固废中塑料和纸张的主要分布区域存在明显差异,塑料的产生主要分布在操场和宿舍区域。这可能是因为在操场的学生运动后习惯补充大量水分而产生的矿泉水瓶、饮料瓶等固废,同时由于学生在宿舍停留时间较长,产生固废的时间占比也相对较大;废纸的产生则是主要分布在教学区和宿舍区,从纸张主要类型是快递纸盒和书本便可以看出其产生量大的原因。因此,不同类别校园固废占比分布与其高校人员学习、生活等习惯息息相关。
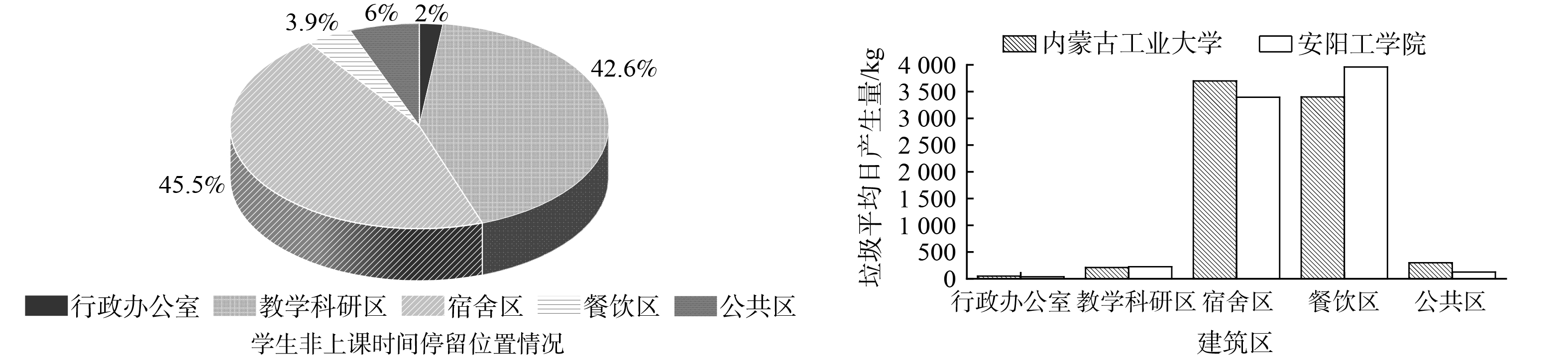
2)与其他院校校园固废产生特征对比分析。通过调研分析学生平时非上课时间的停留时间较长的区域,结果图4(a)分析可得,88.10%的学生非上课时间集中活动在教学科研区和宿舍区,只有11.90%的学生集中活动在公共区、餐饮区以及行政办公区。图4(b)内蒙古工业大学各分区校园固废产生量与安阳工学院2020年的数据对比发现[22],2所学校各分区固废产生量均存在因不同区域功能属性的不同而导致的差异较大,相同区域其固废产生量还是相差不大,其中表现较为明显的是2学校宿舍区和餐饮区固废产量最多,产生了约92.53%以上的固废量,公共区和教学科研区固废产量次之,行政办公区最少。结合图4(a)反映出宿舍区为学生主要居住和活动的主要区域,时间占比长,因此固废产生量较多是毋庸置疑的;对于餐饮区作为人们吃饭和短时间集中人数最多区域之一,固废产生量较高的原因除了一些客观因素比如其产生量占总体固废量近40%,还存在着高校学生就餐浪费率较高等因素,因此高校学生环保意识的应需不断加强,以及“光盘行动”等减少餐后厨余垃圾浪费等一系列环保行动应需多宣传和普及。此外,2所学校日均固废产生量分别为7 655.47和7 041.00 kg,人均分别约0.25和0.30 kg,从数据上来讲,2校区人均日产量仅为文献公布的居民日均固废产生量平均值的24%~30%[21,23],学校生活较社会面生活习惯较单一,学生主要以读书为主,行为方式较简单。因此,校园固废产生垃圾种类偏少,产量相对较低,符合校园固废产生的特点。
2.3 校园固体废物产量模型构建及预测
灰色预测模型一般用在环境影响评价中,确定城市固废与生态环境的正相关关系,设计城市固废生态环境影响评价算法完成影响评价,并为环境影响设计提供理论基础[24-25]。建立灰色模型后,就可以利用该模型对未来的固体废物产生量进行预测,根据模型的输出结果,预测未来的固体废物产生量。本研究以某教学楼一年四季每周固废实际产量数据为依据,建立GM(1,1)灰色预测模型,开展预测校园固废产量,计算结果见表2。
表 2 内蒙古工业大学某教学楼不同季节下固废产量数据GM(1,1)灰色预测模型计算表Table 2. Calculation table of GM(1,1) gray prediction model of solid waste production data in different seasons of a teaching building of Inner Mongolia University of Technology季节 日期 x(0)/kg x(1)/kg 灰色预测 季节 日期 x(0)/kg x(1)/kg 灰色预测 x^(1)/kg x^(0)/kg ε^(0) x^(1)/kg x^(0)/kg ε^(0) 春季 3.05 8.23 8.23 8.23 8.23 0.00 秋季 9.08 5.22 5.22 5.22 5.22 0.00 3.06 6.33 14.56 14.85 6.62 −0.29 9.09 4.69 9.91 11.56 6.34 1.65 3.07 7.72 22.28 21.43 6.58 1.14 9.10 6.49 16.40 17.98 6.42 −0.07 3.08 4.89 27.12 27.96 6.53 −1.64 9.11 7.64 24.04 24.47 6.50 −1.15 3.09 8.28 35.45 34.46 6.49 1.79 9.12 7.88 31.92 31.05 6.57 −1.31 3.10 4.67 40.12 40.90 6.45 −1.78 9.13 8.30 40.22 37.70 6.65 −1.65 3.11 7.19 47.13 47.31 6.41 0.78 9.14 4.21 44.43 44.43 6.73 2.52 3.12 / / 53.68 6.36 / 9.15 / / 51.24 6.81 / 3.13 / / 60.00 6.32 / 9.16 / / 58.13 6.89 / 3.14 / / 66.28 6.28 / 9.17 / / 65.11 6.98 / 3.15 / / 72.52 6.24 / 9.18 / / 72.17 7.06 / 夏季 5.25 5.69 5.69 5.69 5.69 0.00 冬季 12.17 3.07 3.07 3.07 3.07 0.00 5.26 6.26 11.95 11.69 6.00 0.26 12.18 3.77 6.84 6.89 3.82 −0.05 5.27 7.52 19.47 18.50 6.81 0.71 12.19 4.27 11.11 11.15 4.26 0.01 5.28 7.04 26.51 26.24 7.74 −0.70 12.20 4.23 15.34 15.90 4.75 −0.52 5.29 8.02 34.53 35.04 8.80 −0.78 12.21 5.32 20.66 21.20 5.30 0.02 5.30 10.53 45.06 45.03 10.00 0.58 12.22 7.27 27.93 27.11 5.91 1.36 5.31 11.54 56.60 56.38 11.35 0.19 12.23 5.77 33.70 33.71 6.60 −0.83 6.01 / / 69.28 12.90 / 12.24 / / 41.07 7.36 / 6.02 / / 83.93 14.65 / 12.25 / / 49.29 8.21 / 6.03 / / 100.58 16.65 / 12.26 / / 58.46 9.17 / 6.04 / / 119.49 18.91 / 12.27 / / 68.68 10.23 / 通过灰色预测模型计算,以下分别为四季核算过程,
x(0) x(0)值为 x(0)={[8.236.337.724.898.284.677.19][5.696.267.527.048.0210.5311.54][5.224.696.497.647.888.304.21][3.073.774.274.235.327.275.77] (9) 累加次数为1时,则:
x(1)={[8.2314.5622.2827.1235.4540.1247.13][5.6911.9519.4726.5134.5345.0656.6][5.229.9116.4024.0431.9240.2244.43][3.076.8411.1115.3420.6627.9333.70] (10) B={[−11.391;−18.421;−24.701;−31.281;−37.781;−43.621][−8.821;−15.711;−22.991;−30.521;−39.7951;−50.831][−7.561;−13.151;−20.221;−27.981;−36.071;−42.321][−4.951;−8.971;−13.221;−18.001;−24.291;−30.811] (11) 对应,
yN yN={[6.337.724.898.284.677.19]T[6.267.527.048.0210.5311.54]T[4.696.497.647.888.304.21]T[3.774.274.235.327.275.77]T (12) 春、夏、秋、冬四季的
ˆa ˆa={[0.0066;6.6977][−0.1276;4.8982][−0.0119;6.2422][−0.1095;3.2756] (13) ˆx(1)k+1={1014.80−1006.57e−0.0066k−38.387+44.077e0.1276k−524.555+529.775e0.0119k−29.914+32.984e0.1095k (14) 对于春季拟合数据,残差均值
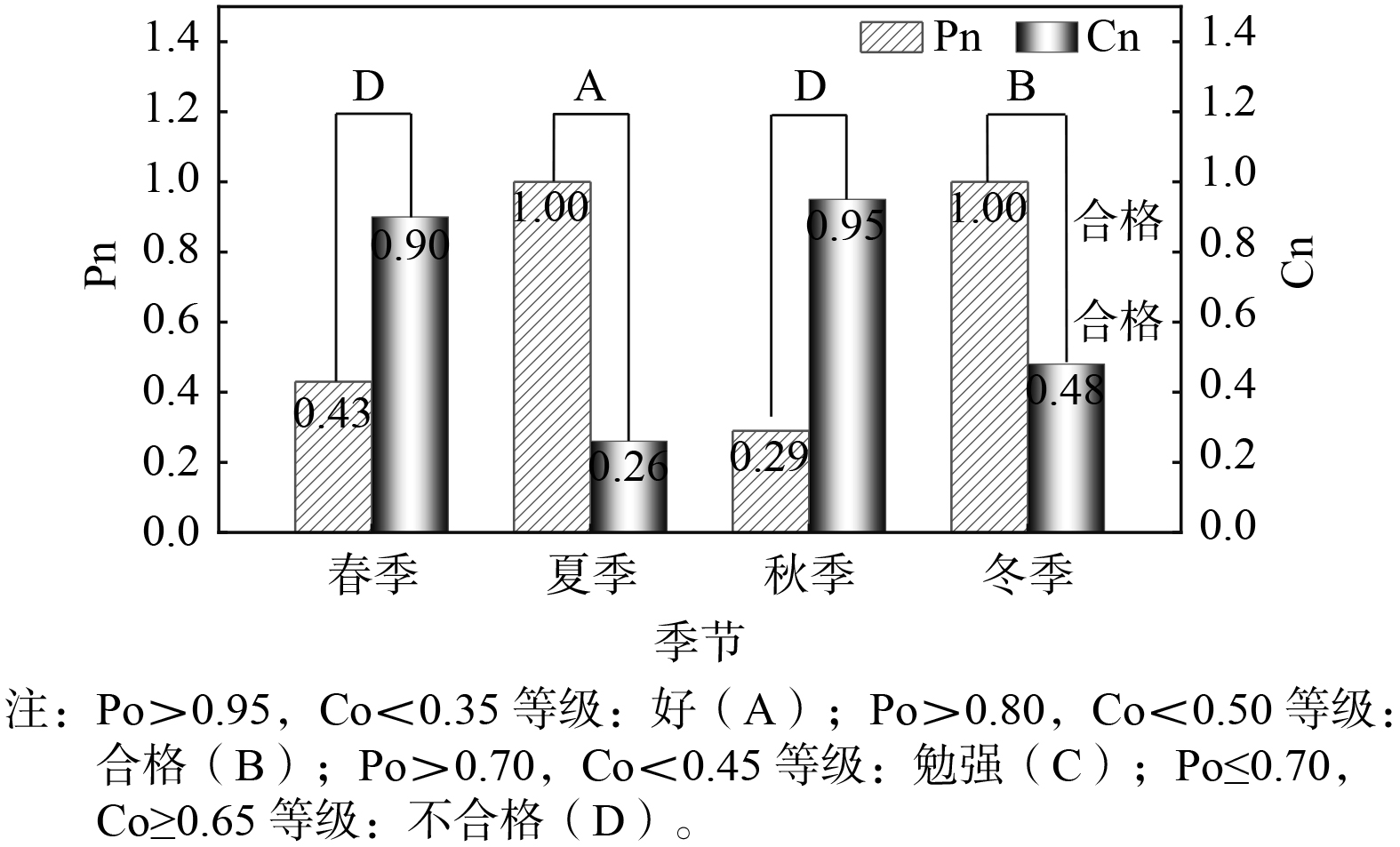
¯ε(0) ¯x(0) c=S1S2=0.90⩾0.65 P=P{|ε0i−¯ε(0)|<0.6745S2}=0.43<0.70 对于夏季拟合数据,残差均值
¯ε(0) ¯x(0) c=S1S2=0.26<0.35 P=P{|ε0i−¯ε(0)|<0.6745S2}=1.00>0.95 对于秋季拟合数据,残差均值
¯ε(0) ¯x(0) c=S1S2= P=P|ε0i−¯ε(0)|<0.6745S2=0.29<0.7 对于冬季拟合数据,残差均值
¯ε(0) ¯x(0) c=S1S2=0.48<0.5 P=P|ε0i−¯ε(0)|<0.6745S2=1.00>0.8 由图5可看出,精度检验等级表明灰色系统预测对夏季、冬季的校园固废拟合精度为好和合格,预测结果正确可靠;春季、秋季的拟合精度效果相对较差。结合实地调研数据可知,因为春季温度变化大等因素,秋季处于新生入学季,人数变化较大,如2023年学生毕业人数为6 836人,其中本科生5 653人,研究生1 183人,学生基本上为秋季入学、夏季毕业,因此该阶段校园固废产生具有量大与种类丰富的特点,因此该阶段校园固废产生规律具有不定性,预测数据与实际固废产量存在一定差异,符合区域季节波动实际情况。因此,通过该模型可实现对夏季和冬季基于某月连续1周的校园固废产量预测未来几天乃至整个季节的固废量做出较为准确预测,精度良好。综上所述,对单一教学楼的分析,可一定程度代表校园产量变化趋势,通过“无废校园”固废产量模型构建,可实现校园固废产量趋势的预判,进而动态调整相应的校园固废收集管理策略,为“无废校园”建设提供参考。
3. 结论与建议
1)内蒙古工业大学校园固废具有产生数量大、产生空间集中、产生时间具有阶段性等特点,且不同区域之间固废产量呈现季节性差异。因此,建议高校根据不同季节垃圾产生的规律及特点采取相应的收集处置措施,可通过建立宣传站、电子回收垃圾装置、建立主动式垃圾回收激励措施等,提升校园固废垃圾收集、处理效率,降低综合成本与效益,助力高校“无废校园”建设。
2)校园固废的产生类别具有易于分类、便于管理的特点,且不同类别校园固废占比与高校人员学习、生活等习惯息息相关。学生非上课时间基本停留在教学科研区和宿舍区,相较于社会面,校园产生的固废种类单一,相对更易进行分类和处理。因此,高校应加强宣传教育、制定相关规定,提高学生固废分类意识和认识度,从而有效促进校园固废源头减量。
3) GM(1,1)灰色预测模型构建可准确反映夏季、冬季校园固废产生规律与特征,实现校园固废产量趋势预判,支撑校园固废收集管理策略动态调整,为“无废校园”建设提供参考。
-
表 1 种子液qPCR结果及接种体积
Table 1. qPCR results and inoculation volume of seed liquid
种子液 nxrA拷贝数/(拷贝·mL−1) 接种量/% NOB 7.54×106±5.82×105 2.000 硝化菌群富集物 2.49×106±4.16×104 6.056 -
[1] BOYABAT N, ÖZER A, BAYRAKÇEKEN S, et al. Comparison of oxidation kinetics of nitrite-oxidizing bacteria: Nitrite availability as a key factor in niche differentiation[J]. Applied and Environmental Microbiology, 2015, 81(2): 745-753. doi: 10.1128/AEM.02734-14 [2] MEIKLEJOHN J. The Isolation of Nitrosomonas europaea in pure culture[J]. Journal of General Microbiology, 1950, 4(2): 185-191. doi: 10.1099/00221287-4-2-185 [3] 王勤. 重金属对生物脱氮的毒性效应研究[D]. 广州: 广州大学, 2009. [4] TIAN Z, PALOMO A, ZHANG H, et al. Minimum influent concentrations of oxytetracycline, streptomycin and spiramycin in selecting antibiotic resistance in biofilm type wastewater treatment systems[J]. Science of the Total Environment, 2020, 720: 137531. [5] KATIPOGLU-YAZAN T, MERLIN C, PONS M N, et al. Chronic impact of tetracycline on nitrification kinetics and the activity of enriched nitrifying microbial culture[J]. Water Research, 2015, 72(1): 227-238. [6] WIDDEL F, BAK F. Gram-negative Mesophilic Sulfate-Reducing Bacteria [M]. New York: Springer, 1992. [7] BAKER G C, SMITH J J, COWAN D A. Review and re-analysis of domain-specific 16S primers[J]. Journal of Microbiological Methods, 2003, 55(3): 541-555. doi: 10.1016/j.mimet.2003.08.009 [8] 王秀蘅, 任南琪, 王爱杰, 等. 铁锰离子对硝化反应的影响效应研究[J]. 哈尔滨工业大学学报, 2003, 35(1): 122-125. doi: 10.3321/j.issn:0367-6234.2003.01.030 [9] SOPHIE W, FRANCK P, XAVIER L R, et al. Development and application of a PCR-denaturing gradient gel electrophoresis tool to study the diversity of Nitrobacter-like nxrA sequences in soil[J]. FEMS Microbiology Ecology, 2008, 63(2): 261-271. doi: 10.1111/j.1574-6941.2007.00416.x [10] GRIFFITHS R I, WHITELEY A S, O'DONNELL A G, et al. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition[J]. Applied and Environmental Microbiology, 2000, 66(12): 5488-5488. doi: 10.1128/AEM.66.12.5488-5491.2000 [11] 中华人民共和国环境保护部, 中国国家标准化管理委员会. 土壤. 氨氮、亚硝酸盐氮、硝酸盐氮的测定. 氯化钾溶液提取-分光光度法: HJ 634-2012[S]. 北京: 中国环境科学出版社, 2012. [12] 中华人民共和国环境保护部, 中国国家标准化管理委员会. 水质 无机阴离子的测定 离子色谱法: HJ/T 84-2001[S]. 北京: 中国环境科学出版社, 2012. [13] LAWSON C, LüCKER S. Complete ammonia oxidation: An important control on nitrification in engineered ecosystems?[J]. Current Opinion in Biotechnology, 2018, 50: 158-165. doi: 10.1016/j.copbio.2018.01.015 [14] BERGMANN D J, HOOPER A B, KLOTZ M G. Structure and sequence conservation of hao cluster genes of autotrophic ammonia-oxidizing bacteria: Evidence for their evolutionary history[J]. Applied and Environmental Microbiology, 2005, 71(9): 5371-5382. doi: 10.1128/AEM.71.9.5371-5382.2005 [15] 王文超, 杨立中, 谭周亮. 重金属对废水生物硝化过程影响研究进展[J]. 环境科学与技术, 2013, 36(S2): 157-161. [16] 张宏扬. Cu(Ⅱ)对生物硝化过程和硝化菌群结构的影响机理研究[D]. 天津: 天津大学, 2013. [17] 张杉. 重金属镉对SBR系统水处理效果及微生物群落影响研究[D]. 北京: 北京化工大学, 2017. [18] 杜振峰. Cr(Ⅵ)对生物硝化过程和硝化菌群结构的影响机理研究[D]. 天津: 天津大学, 2013. [19] LIU Y, LAM M C, FANG H H. Adsorption of heavy metals by EPS of activated sludge[J]. Water Science & Technology, 2001, 43(6): 59-66. [20] CHUG R, MATHUR S, KOTHARI S L, et al. Maximizing EPS production from Pseudomonas aeruginosa and its application in Cr and Ni sequestration[J]. Biochemistry and Biophysics Reports, 2021, 26: 100972. doi: 10.1016/j.bbrep.2021.100972 [21] KUMAR M S, SWARNALAKSHMI K, ANNAPURNA K. Rhizobium Biology and Biotechnology[M]. Cham: Springer, 2017: 257-292. [22] SUN B, HAN P, TAO R, et al. Advances in Applied Biotechnology[M]. Cham: Springer, 2015: 295-303. [23] XING W, FANG L, CAI P, et al. Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria[J]. Environmental Pollution, 2011, 159(5): 1369-1374. doi: 10.1016/j.envpol.2011.01.006 [24] RAJESH A, KUMAR] N. Biosorption of cadmium using a novel bacterium isolated from an electronic industry effluent[J]. Chemical Engineering Journal, 2014, 235: 176-185. doi: 10.1016/j.cej.2013.09.016 [25] HENNE K L, TURSE J E, NICORA C D, et al. Global proteomic analysis of the chromate response in Arthrobacter sp. strain FB24[J]. Journal of Proteome Research, 2009, 8(4): 1704. doi: 10.1021/pr800705f [26] ZHANG J, CHEN M, SUI Q, et al. Impacts of addition of natural zeolite or a nitrification inhibitor on antibiotic resistance genes during sludge composting[J]. Water Research, 2016, 91(15): 339-349. [27] 宋超. 四环素强化生物去除及其在自然水体中迁移转化规律的研究[D]. 济南: 山东大学, 2016. [28] 韩月, 李凯, 王志康, 等. SBR中纳米氧化锌和四环素复合投加系统对污泥胞外聚合物的影响[J]. 环境工程学报, 2019, 13(7): 1623-1633. doi: 10.12030/j.cjee.201810097 [29] 张微. 四环素与胞外聚合物的相互作用及其对污泥耐药性的影响 [D]. 上海: 东华大学, 2014. [30] 石义静. 硝化颗粒污泥的培养及其与四环素相互作用研究 [D]. 济南: 山东大学, 2013. -





 下载:
下载: