-
颗粒物是环境污染与控制相关研究的重要对象[1]。尽管给水处理工艺通常能够保证出厂水的水质达标,但出厂水经过管网到达用户端的输配过程中难以避免发生水质变化[2]。其中,管网疏松沉积物再悬浮引发自来水变色(如“黄水”)是全世界自来水投诉中最常见的问题[3]。然而,管网疏松沉积物的结构特征及其潜在健康危害目前尚不明确。
管网疏松沉积物来源复杂,包括出厂水中的残余物质和管道的腐蚀产物等。由于给水管网中广泛使用铁质管材和管件,管网铁腐蚀产物释放是导致用户龙头“黄水”的主要原因[4-7]。ZHUANG等[8]利用合成的铁颗粒物实验首次提出,铁颗粒物导致的“黄水”不仅是感官性状问题,且铁颗粒物可通过2种机制产生潜在毒性:1)铁颗粒物(主要是针铁矿)与微量有机污染物通过特定的共价键结合方式强化电子转移,产生活性氧物种(reactive oxygen species,ROS)而增强细胞损伤[8-9];2)微量有机污染物能够显著提升铁颗粒物的比表面积,从而增强其对水中污染物的富集能力[10]。实际管网条件下生成的疏松沉积物组成往往非常复杂,以往对于“黄水”带来的风险都是基于管网的水质和水力条件进行评价和预测[11],然而“黄水”可能引发的毒性风险至今尚无评价标准[12-13]。水质的评价不能仅以饮用水标准限值为判据, 更为重要的是以水质安全风险的综合控制为依据[14]。因此,“黄水”中沉积物颗粒的风险亟需受到重视。
本研究在北方某城市频繁发生“黄水”的区域采集了管网疏松沉积物,对样品的表面形貌、元素组成、表面电位和粒径等结构特征进行了表征,利用人体健康肝脏细胞对样品的细胞毒性进行了测试,并开展了疏松沉积物的细胞毒性与其结构特征之间的相关性分析,初步明确了给水管网疏松沉积物的风险因素。
-
对中国北方某城市频繁发生“黄水”的小区进行了实地调研,截取了7个小区的入楼管管道,管材为镀锌钢管,管径均为DN40,管龄约为(15±5) a。利用软毛刷刷取管道表层疏松沉积物,将获得的疏松沉积物冷冻干燥,再通过90目(160 μm)筛网进行筛分,样品编号1#~7#。
-
管网疏松沉积物样品的表面形貌和元素组成利用场发射扫描电子显微镜(SEM,H-7500)获得,微观结构照片利用显微镜(Leica MGD41)拍摄;表面电位和粒径使用zeta电位分析仪(Malvern,Zetasizer2000)测定;样品晶体结构利用X射线衍射光谱仪(XRD, Rigaku,D/Max-2200)测试;样品粗糙度(Ra)利用原子力显微镜(AFM,Nanosurf C3000, Switzerland)测定。以DMPO作为捕获剂添加浓度为10 mmol·L−1的H2O2,利用电子顺磁共振谱仪(ESR,Bruker A300-10/12)在无光照条件下对样品产生自由基的能力进行测定。
-
以体外细胞毒性实验(MTT法)进行了疏松沉积物样品的毒性检测。实验所用的LO2人体健康肝脏细胞购买自赛柏慷生物有限公司。主要步骤为:在5% CO2、37 ℃条件下,加入疏松沉积物分散液(100 mg·L−1)和细胞共培养24 h;然后吸走原有培养基,加入PBS轻微地清洗2次,再加入200 μL含0.5 mg·mL−1 MTT的培养基,继续培养4 h后,吸走原有培养基,最后加入150 μL DMSO,振荡5 min,利用全波长酶标仪(EPOCH2, Biotek)在570 nm进行检测。实验结果以细胞存活率表示(见式(1))。
-
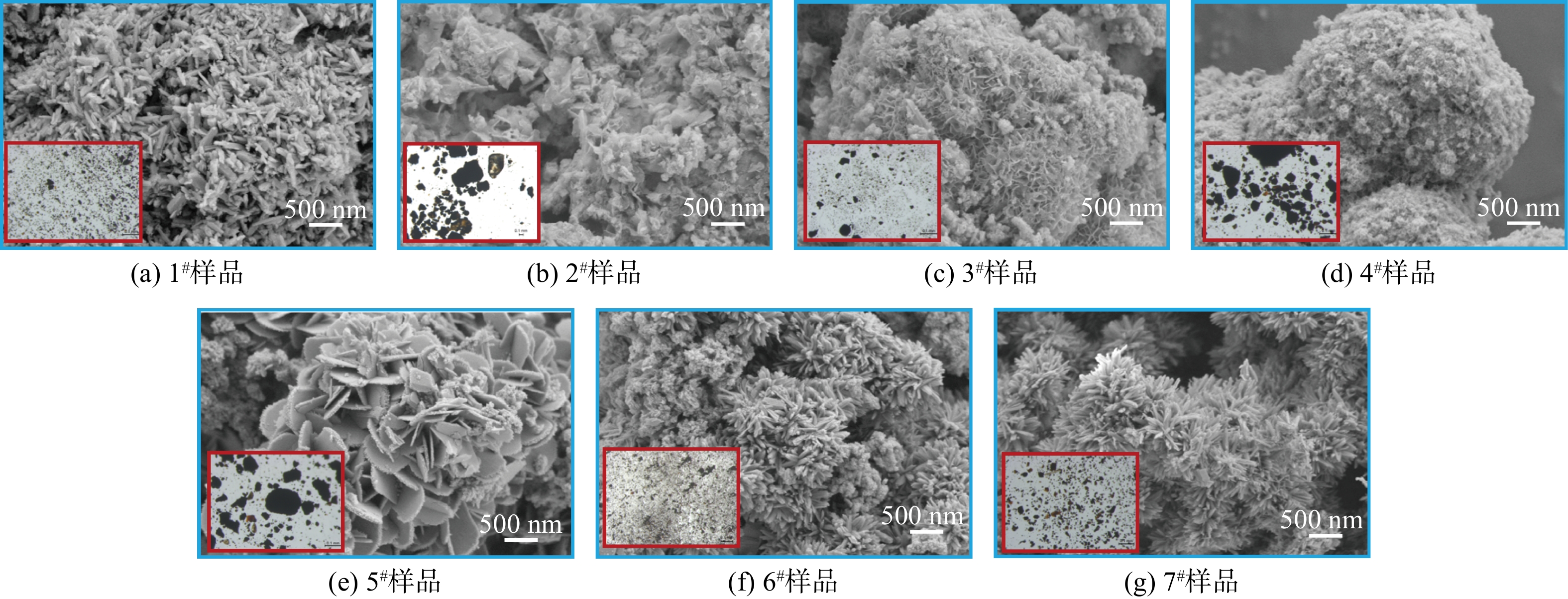
管网疏松沉积物样品1#~7#的SEM照片如图1所示。样品微观形貌中大多存在锋利的针刺状结构,这种结构可能来自于亚单元纳米颗粒取向聚集生长[15]。在所有样品SEM照片中,样品6#的表面针刺最密集且最为锋利,其在显微镜照片(图1内嵌图)中的结构也更加分散和细密,这可能是由于细密锋利的微观结构有助于抑制颗粒之间的团聚作用[16],而具有松散结构的沉积物更易于再悬浮到水中,导致“黄水”的风险更高。
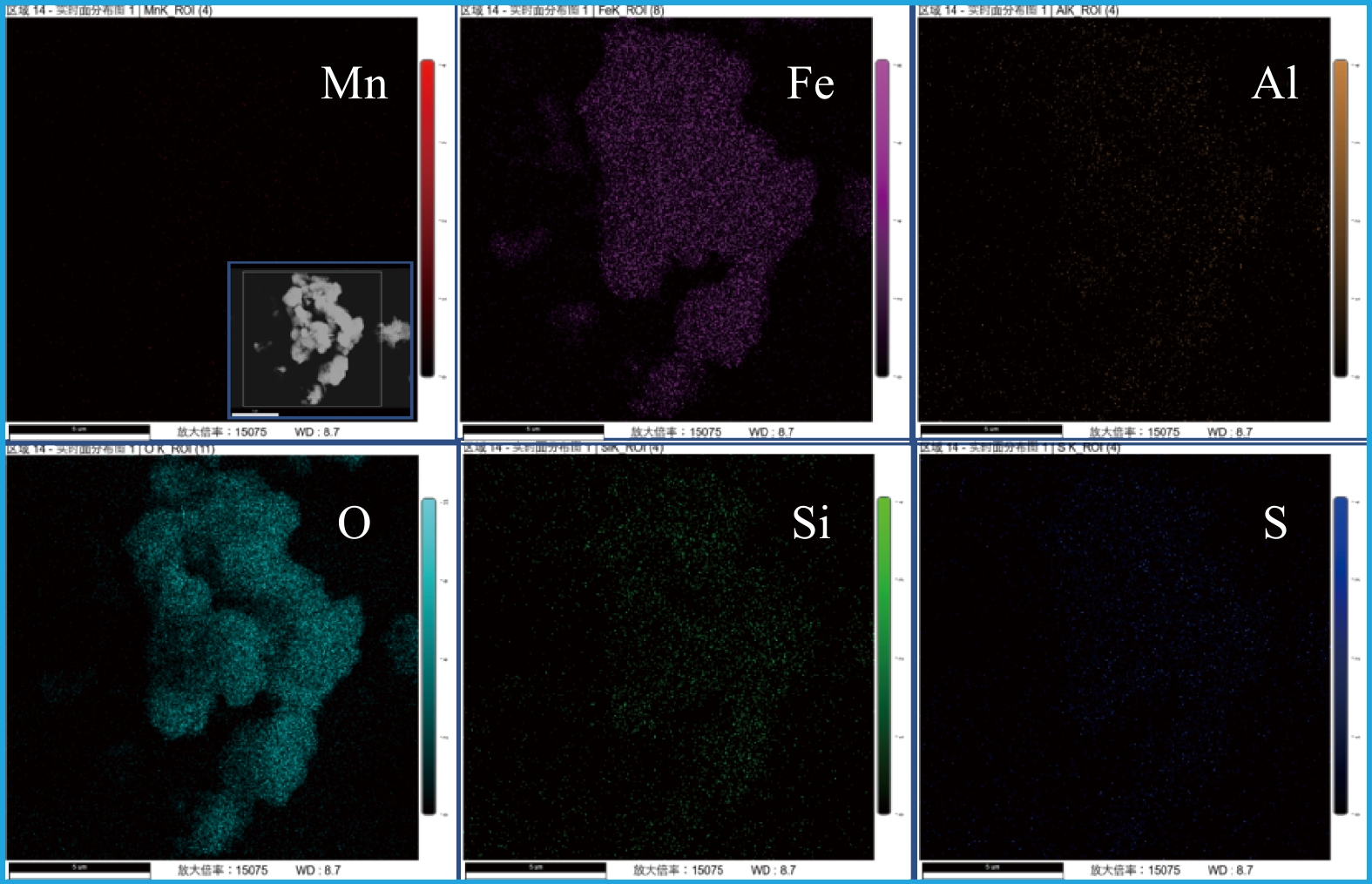
在所有样品的元素组成中(见表1),占比最大的均为铁元素,质量占比为55%~80%,这与以往研究中镀锌钢管管垢中含量最高为铁元素一致[17]。除铁元素以外,样品中还检测到一定量的锰、铜、铝、铅、砷等金属元素。对样品6#进行进一步的元素面扫(见图2)发现,铁元素和氧元素分布均匀,故可推测,样品的主要成分为铁氧化物。此外,样品中的针刺状结构可能源于铁氧化物的取向聚集生长。
-
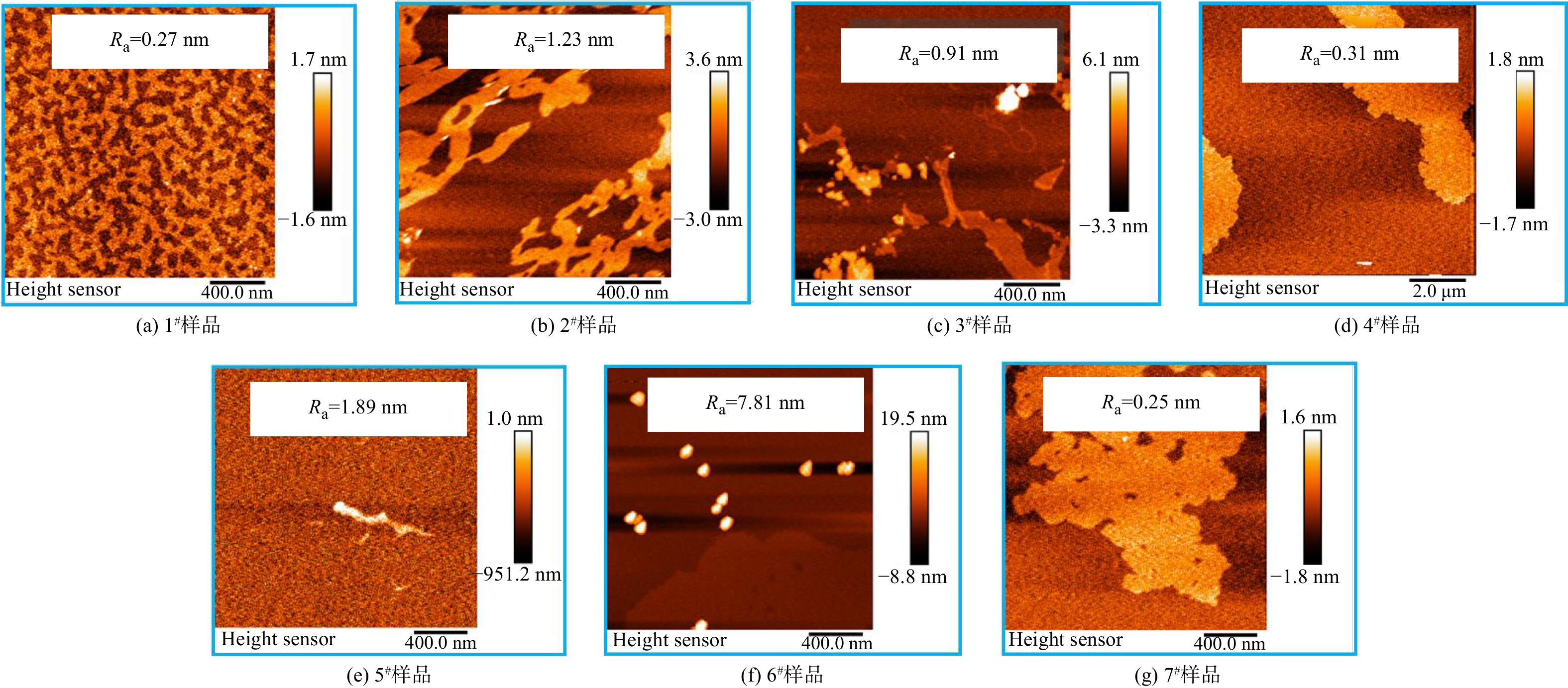
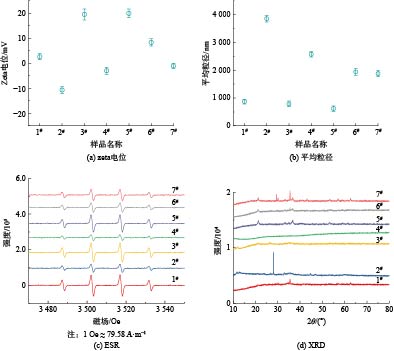
AFM测试结果(见图3)表明,样品粗糙度(Ra)为0.25~7.81 nm,具有最为细密锋利微观形貌的样品6#粗糙度最大,其表面粗糙度明显高于其他样品可能是由于细密锋利的针刺结构导致。样品的zeta电位分布在−15~20 mV,可见,管网疏松沉积物的表面电位既有可能呈正电性也有可能呈负电性。样品平均粒径范围为500~4 000 nm,表明其中包含具有较高毒性风险的纳米尺寸细颗粒。有研究表明,水中颗粒物粒径越大时水体色度变化越明显[18],可见,2#样品的致色风险最高。由于氧化应激是纳米颗粒损伤细胞的重要机制[19],故对样品产生羟基自由基的能力进行了测试(见图4),发现所有样品均能够与双氧水反应产生羟基自由基,其反应位点可能是沉积物中丰富的铁基组分[20]。通过XRD对管网疏松沉积物的晶体成分进行分析,结果如图4和表2所示,表明样品中主要晶体成分是多种铁氧化物,包括Fe2O3(α-Fe2O3、γ-Fe2O3)、FeOOH(α-FeOOH、β-FeOOH、γ-FeOOH、δ-FeOOH)、Fe3O4等,也含有少量SiO2和CaCO3晶体。大多样品中的铁氧化物晶体以γ-Fe2O3为主,但样品6#中晶体占比最高的是γ-FeOOH,故推测样品6#细密锋利的针刺状结构可能与γ-FeOOH晶体生长有关。
-
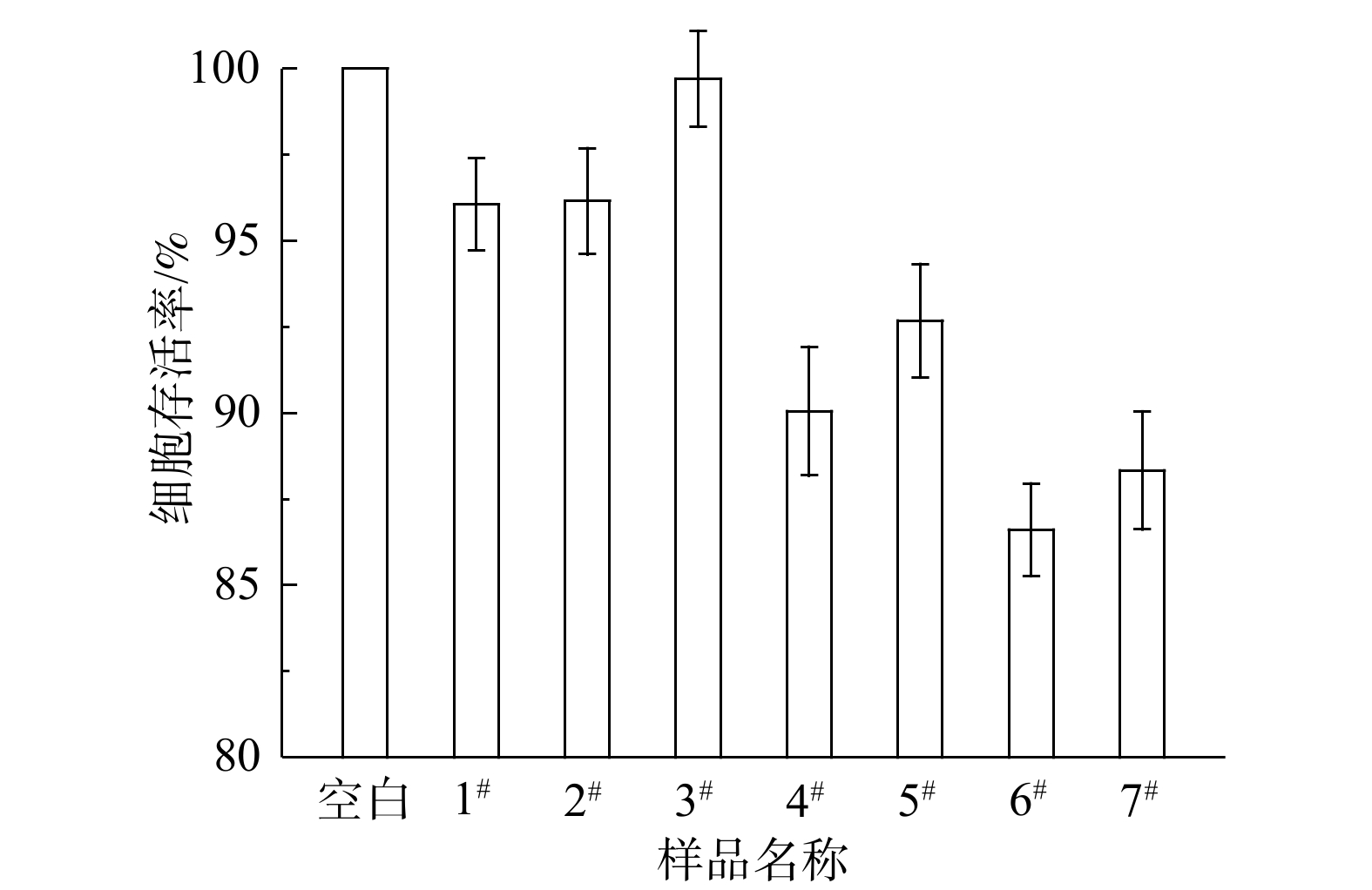
现有对饮用水毒性风险的研究大多聚焦于饮用水中的消毒副产物[21-22]和持久性有机污染物(POPs)[23-24]等有机污染物,以及重金属[25]等无机污染物。饮用水中颗粒物的毒性风险尚未引起重视。通常情况下,当滞留水放出后“黄水”会逐渐消失[26],但由于管网疏松沉积物含有细颗粒,即使是在龙头水放水放至无色时,疏松沉积物颗粒仍会不可避免地存在于水中,用户在使用龙头水时很有可能发生颗粒物的暴露。利用人体健康肝脏细胞测试了管网疏松沉积物样品分散液的MTT细胞毒性。利用样品配置分散液时,在100 mg·L−1下肉眼可见明显颜色,故在此浓度下研究了样品的细胞毒性。当样品分散液的质量浓度为100 mg·L−1时,人体健康肝脏细胞的存活率为86.61%~99.71%(如图5所示),可见疏松沉积物的毒性风险不可忽视。具有最为细密锋利结构的样品6#的细胞存活率最低,故推测疏松沉积物样品的形貌对于毒性具有重要影响。
-
主成分分析法旨在保证原始数据信息丢失最小的情况下对原始的高维变量进行降维处理,以少数的综合变量代替原有的多维变量,简化评价工作[27]。虽然粒径是影响颗粒物毒性的最常见因素[28],但在管网疏松沉积物样品的铁含量、zeta电位、粗糙度和平均粒径中(见表3),与毒性相关性最显著的是粗糙度,样品的粗糙度与细胞存活率呈现负相关,Pearson相关系数为−0.507。即粗糙度越大的疏松沉积物,毒性越高,表明形貌对样品毒性的影响比粒径更大。这是由于样品中普遍存在着针刺状结构,所以粗糙度越高的样品具有更丰富更锋利的表面,导致其对细胞的划伤能力更强;此外,样品的平均粒径与zeta电位具有负相关性(Pearson相关系数为−0.851),即粒径越大的样品zeta电位较低,可见表面电荷相同时,带负电的沉积物颗粒比带正电的更难以悬浮进入水中,健康风险较低。
如表4所示,在管网疏松沉积物的晶体组成与毒性的相关性中,与样品毒性相关性最显著的是γ-FeOOH,与细胞存活率具有负相关性(Pearson相关系数为=−0.762),即γ-FeOOH含量越高的管网疏松沉积物,毒性越大。结合粗糙度与毒性具有相关性的结果推测,γ-FeOOH可能是通过增加颗粒表面锋利程度进而增大样品毒性。李玉仙等[29]发现给水管网余氯较低时不稳定成分γ-FeOOH含量明显增加;牛璋彬等[30]发现给水管网余氯较低时铁释放现象严重。因此,在余氯不足时,“黄水”具有更高的毒性风险,一方面是由于γ-FeOOH晶体成分增多导致疏松沉积物形成毒性更高的颗粒物结构,另一方面是因为铁释放加剧形成更多的疏松沉积物导致颗粒物浓度增大。
-
1) “黄水”小区的管网疏松沉积物中含量最高的金属元素是铁元素,主要晶体成分是铁氧化物。样品微观形貌中大多存在锋利的针刺状结构;疏松沉积物的zeta电位为−15~20 mV、平均粒径为500~4 000 nm,粗糙度为0.25~7.81 nm。
2) 管网疏松沉积物在100 mg·L−1下对应人体健康肝脏细胞的细胞存活率为86.61%~99.71%。粗糙度与疏松沉积物毒性的相关性最显著,表明形貌对疏松沉积物毒性具有显著影响;γ-FeOOH是与管网疏松沉积物毒性相关性最显著的晶体组分,其对毒性的贡献是通过增加样品形貌锋利程度造成的。余氯不足时可能会因铁释放加剧和γ-FeOOH含量增大,造成更高的毒性风险。
给水管网疏松沉积物的结构特征及其风险识别
Structure characteristics and health risk of loose deposits in drinking water distribution system
-
摘要: 自来水变色问题通常被视为感官问题,而导致自来水变色的管网疏松沉积物的结构特征及其潜在危害尚不明确。采集了我国北方某城市频繁发生自来水“黄水”的7个小区管网中的疏松沉积物,并进行了分析。结果表明:所有疏松沉积物样品中含量最高的金属元素都是铁元素、主要晶体成分是铁氧化物,样品颗粒微观形貌大多具有锋利的针刺状结构;样品zeta电位范围为−15~20 mV、平均粒径范围为500~4 000 nm、粗糙度范围为0.25~7.81 nm。体外细胞毒性实验结果显示,沉积物可产生一定的细胞毒性(样品浓度100 mg·L−1时人体健康肝脏细胞存活率为86.61%~99.71%)。通过主成分分析发现,疏松沉积物毒性与粗糙度的相关性比与粒度的高,表明形貌对样品毒性的影响比粒径更大;γ-FeOOH是与疏松沉积物毒性相关性最显著的晶体组分,其对毒性的贡献可能是通过增加颗粒表面锋利程度造成的,余氯不足时可能会因铁释放加剧和γ-FeOOH含量增大造成更高的毒性风险。本研究结果可为全面认识给水管网疏松沉积物的风险提供参考。Abstract: Discoloration of tap water caused by loose deposits is usually regarded as a sensory problem, but structural characteristics and health risk of the loose deposits are not clear. In this study, we collected loose deposits from the residential areas from the section where frequently occurs “yellow water” in a northern city in China. Characterization results showed that in loose deposit samples, the highest metal element was iron and the main crystal component was iron oxide. The morphology of the samples contained sharp fiber-like structures. The zeta potentials of the samples were −15~20 mV, the average sizes were 500~4 000 nm, and the roughness values were 0.25~7.81 nm. The samples had certain toxicity to health human liver cells (the cell viability was 86.61%~99.71% under loose deposits 100 mg·L−1). Principal component analysis showed that roughness had the highest correlation with toxicity, which indicated that morphology had greater influence on sample toxicity than size. Among crystal components, γ-FeOOH had the highest correlation with toxicity, and its contribution to toxicity may due to sharpness increase. When the residual chlorine is insufficient, it will lead to higher toxicity risk due to the increase of iron release and γ-FeOOH content. Therefore, iron particles have great effects on the toxicity of loose deposits. This study provides an important basis for the comprehensive understanding on the risk of loose deposits in drinking water distribution system.
-
Key words:
- drinking water distribution system /
- loose deposits /
- yellow water /
- toxicity
-
我国在过去的几十年里,由于金属的大量开采和冶炼[1]、污水灌溉[2]、化肥的不合理施用[3],镉砷在农田土壤中不断地积累。镉砷是毒性较大的重金属,农田土壤中重金属总量超标现象会通过食物链对生态系统和人类的健康构成威胁。根据原环境保护部和国土资源部2014年4月公布的《全国土壤污染状况调查公报》[4],全国镉污染物点位超标率为7.0%,位居重金属污染物榜首;砷污染物点位超标率为2.7%,砷超标的问题也不容忽视。因此,土壤镉、砷超标的环境问题亟待解决。
修复重金属污染农田有多种修复技术,其中使用修复材料将农田土壤的重金属钝化的技术因其具有成本低廉、修复时间短、效果显著的优点[5],具有广泛的应用前景。然而,GONG等[6]认为,这些钝化技术的研究大多停留在条件容易控制的实验室研究阶段,实际应用条件的复杂性可能会影响修复效果及可行性。近年来,针对镉污染土壤现场修复有效的钝化材料包括生物炭材料[7]、磷酸盐材料[8]、黏土矿物[9]等,黏土矿物因其来源丰富、成本低廉、效果显著等特点已被广泛研究[6]。TAHERVAND等[10]发现,黏土矿物具有很强的吸附重金属的能力,但黏土矿物对重金属的吸附存在选择性。目前,研究用黏土矿物进行砷的固定修复的研究比较少,砷污染土壤的修复材料以铁基材料为主[11]。
镉砷是2种性质极不相同的元素,农田重金属污染大多是复合污染,适合镉单一污染修复的材料和适合砷单一污染修复的材料能否用于农田镉、砷复合污染修复尚有待研究。国内外的学者们普遍研究单一修复材料对农田重金属的钝化效果,而不同修复材料之间的修复效果缺乏比较。因此,本研究比较分析了不同修复材料(硅藻土、膨润土、海泡石、人造沸石)和农田调理剂对农田土壤镉、砷的钝化效果,探究了修复材料处理后土壤中的镉、砷的形态以及土壤理化性质的变化,通过种植水稻并检测水稻内的镉、砷含量来检验修复效果,从而为镉砷污染农田土壤现场修复提供参考。
1. 材料与方法
1.1 供试土壤与供试材料
供试土壤场地位于中国广东省佛山市三水区,农田大小约为6 660 m2,黄棕色黏质土,选取其中的42个点对农田的基本情况进行分析,土壤的基本理化性质如下:总镉0.95~1.50 mg·kg−1,总砷11.41~17.06 mg·kg−1,总铅30.92~42.82 mg·kg−1,总锌39.35~69.70 mg·kg−1,速效磷16.9~86.1 mg·kg−1,碱解氮73.2~117 mg·kg−1,有机质25.7~95.4 g·kg−1,阳离子交换量4.33~7.12 cmol·kg−1,pH为5.22~5.70。供试的修复材料有硅藻土、膨润土、海泡石、人造沸石和农田调理剂,均为化学纯,粒径均在微米级;农田调理剂是由硅、镁、磷等元素构成的复合材料。供试的水稻品种为油牙占。
1.2 实验设计与样品采集
修复材料处理方法如图1所示。育苗工作完成后,投加修复材料7 d后插秧,修复材料处理10 d后(插秧3 d后)施加尿素,修复材料处理17 d和24 d后施加氮磷复合肥。从插秧前到水稻分蘖期期间都是淹水处理,保证田间持水量达到60%~80%,到水稻分蘖数足够的时候开始晒田。
在修复材料处理30 d(水稻秧苗期)、60 d(分蘖期)、100 d(成熟期)时按蛇形取样法随机采集每块实验田的土壤样品,然后进行风干处理,在水稻成熟后按蛇形取样法随机采集水稻样品,每块实验田采集20株水稻。
1.3 分析方法
土壤重金属总量的测定参考电感耦合等离子体质谱法[12];土壤重金属有效形态含量的测定参考电感耦合等离子体质谱法[13];Cd和As形态分布的测定参考Tessier提出的顺序提取程序(SEP)[14];参考钼锑抗分光光度法[15]检测土壤速效磷含量;参考《森林土壤氮的测定》[16]检测土壤碱解氮含量;参考分光光度法[17]检测土壤有机质含量;参考分光光度法[18]检测土壤阳离子交换量;参考《土壤pH的测定》[19]检测土壤的pH;参考《食品中镉的测定》[20]和《食品中总砷及无机砷的测定》[21]测定水稻中Cd和As的含量。
2. 结果与讨论
2.1 实验田各时期重金属总量
表1为实验田各时期Cd和As总量的平均值,总Cd含量高于风险筛选值,低于风险管控值,可能存在食用农产品不符合质量安全标准等土壤污染风险[22]。理论上,钝化材料的投加不会影响重金属在土壤中的总量,经检验,各组别的总Cd和总As的含量之间无显著差异(P<0.05)。土壤重金属总量在秧苗期、分蘖期、成熟期之间也无显著差异(P<0.05)。
表 1 实验田各时期Cd和As总量Table 1. Total cadmium and arsenic content in paddy field during different periodsmg·kg−1 样品 总Cd浓度 总As浓度 秧苗期 分蘖期 成熟期 秧苗期 分蘖期 成熟期 CK 1.22 1.13 1.16 14.10 14.05 14.28 D 1.16 1.13 1.13 13.20 13.62 14.01 F1 1.06 1.08 1.15 17.06 14.06 14.15 AZ1 1.06 1.09 1.07 16.84 13.49 13.83 S1 1.12 1.11 1.01 15.66 13.25 13.37 B1 1.10 1.09 0.99 15.19 13.24 13.32 F2 1.09 1.12 1.02 15.76 13.07 13.94 AZ2 1.07 1.12 1.00 13.96 12.81 12.85 S2 1.05 1.11 0.97 14.02 12.77 11.44 B2 0.99 1.10 0.95 16.76 11.41 11.17 2.2 钝化材料对DTPA提取重金属有效性的影响
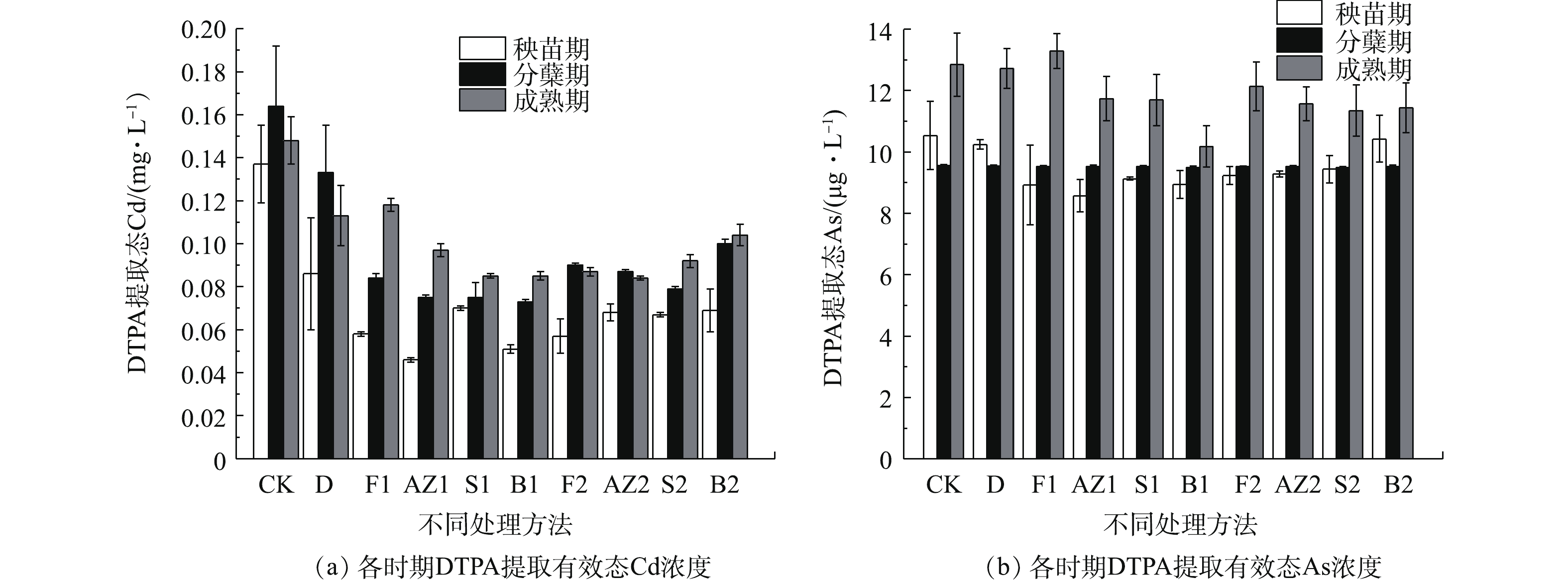
1)钝化材料对DTPA提取Cd有效性的影响。水稻的秧苗期、分蘖期、成熟期的DTPA浸出的平均Cd浓度如图2(a)所示,从秧苗期到分蘖期浸出液的平均Cd浓度有所提升,其原因可能是秧苗期水稻淹水程度较高,分蘖期水稻淹水程度较低,淹水程度高可以降低水稻吸收重金属Cd的效率[23-24]。跟CK对比,各种材料的浸出Cd浓度下降了20.3%~43.2%,S1、S2在处理初期就有较好的钝化效果,而F1和F2在成熟期的钝化效果最好,其原因可能是海泡石处理后迅速提高了土壤的pH和土壤中酶的活性[24],从而提高土壤中Cd的稳定性,而调理剂可能是通过与Cd形成磷酸盐沉淀,所以反应时间更长。
2)钝化材料对DTPA提取As有效性的影响。水稻的秧苗期、分蘖期、成熟期的DTPA浸出的平均As浓度如图2(b)所示,空白对照组浸出液的平均As浓度没有随时间发生显著变化,其他修复材料处理后浸出液的平均As浓度变化趋势与CK保持一致。跟CK相比,各组修复材料对砷都没有明显的修复效果,但维持着土壤中有效形态的As含量,没有造成有效态As显著提升的副作用。修复材料的种类和投加量对As的有效形态含量的改变无显著影响,因此,可以认为本研究中的硅藻土、调理剂、海泡石、人造沸石、膨润土等修复材料能显著降低土壤中DTPA提取Cd有效性的效果。
2.3 钝化材料对重金属SEP形态分布的影响
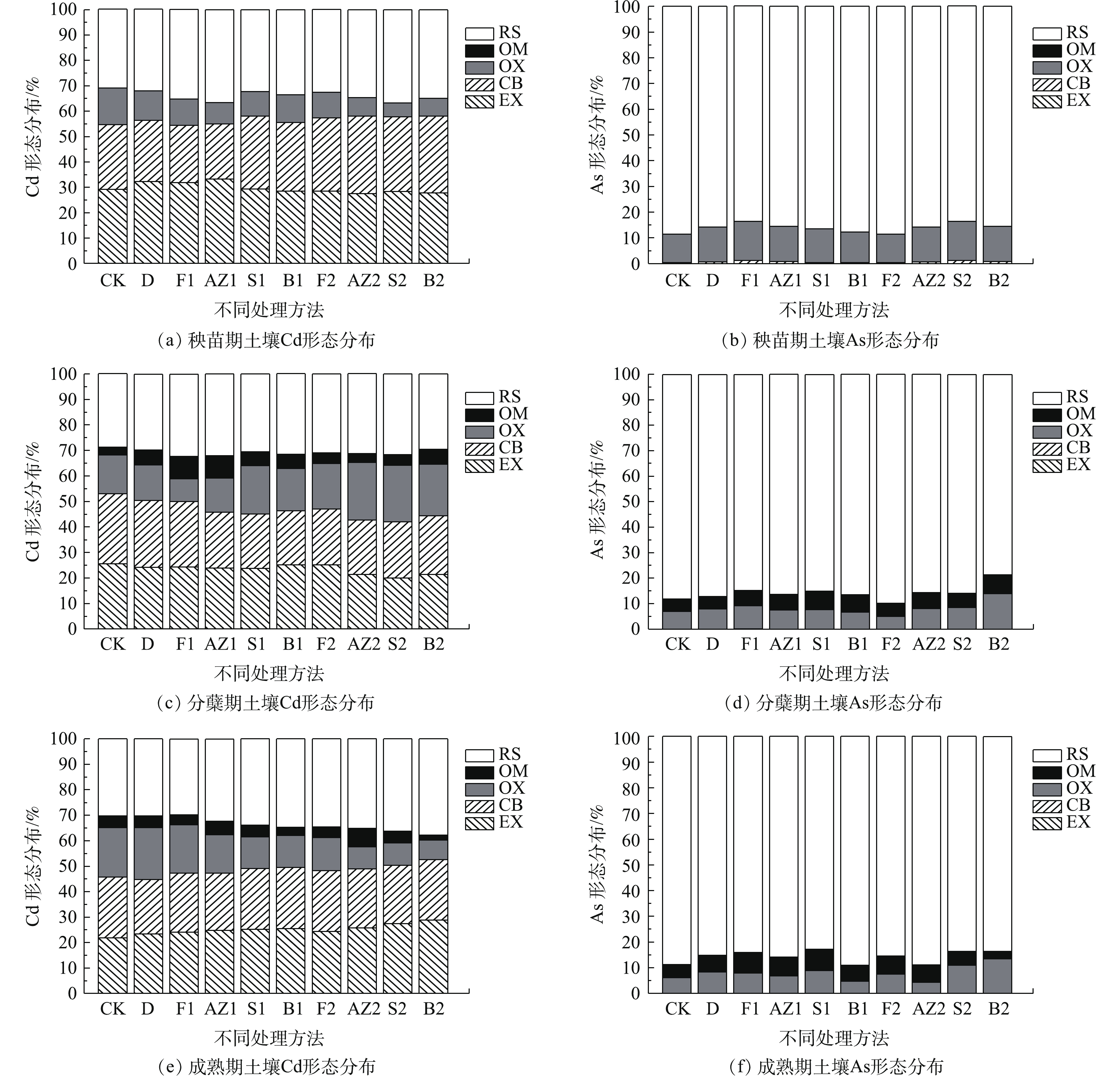
通过SEP连续提取法估算土壤中真实的重金属形态分布和机理[25-26]。在SEP连续提取法中,土壤中的重金属可以根据其在土壤中的稳定性划分为可交换态(EX)、碳酸盐结合态(CB)、铁锰氧化态(OX)、有机结合态(OM)和残余态(RS),各种形态的稳定性顺序为EX<CB<OX<OM<RS。结合图3(a)、图3(c)、图3(e)来看,CK从秧苗期到成熟期的OM形态上升可能是秧苗期后给农田中的水稻追肥所致,修复材料处理后RS形态与CK相比有所提高,其中AZ2、S2、B2的提高幅度比较明显,这说明人造沸石、海泡石、膨润土可能主要以沉淀的方式固定Cd。结合图3(b)、图3(d)、图3(f)来看,空白对照组的OM形态的As含量有所上升,这说明可能是秧苗期前后投加的肥料使OX形态的砷转化为OM形态,或者是由其他农艺因素引起的。其余修复材料处理后,As的各种形态变化趋势与CK相似,这说明修复材料对土壤中As的形态变化无显著影响。
2.4 土壤理化性质的变化
不同修复材料处理后土壤的pH如表2所示。在秧苗期,pH提高的幅度依次为人造沸石粉>海泡石粉>调理剂>膨润土,投加硅藻土后农田土壤的pH反而略有下降。在分蘖期和成熟期,经修复材料处理后农田土壤的pH的变化趋势基本与秧苗期的一致,硅藻土的pH与CK基本一致,其余修复材料处理后的土壤pH均有不同程度地提高,提高的幅度依次为人造沸石粉>海泡石粉>膨润土>调理剂。
表 2 土壤理化性质的变化Table 2. Changes of basic physical and chemical properties of soil阶段 样品 pH 速效磷/(mg·kg−1) 碱解氮/(mg·kg−1) 有机质/(g·kg−1) 阳离子交换量/(cmol·kg−1) 秧苗期 CK 6.02±0.01 33.9±10.2 148±6 32.1±0.8 7.53±0.01 D 5.91±0.07 29.3±2.2 165±0 30.5±0.3 7.74±0.07 F1 6.93±0.04 42.0±4.2 142±25 28.3±5.6 9.16±0.03 AZ1 7.61±0.01 25.1±9.1 137±10 27.6±4.5 7.66±0.30 S1 6.99±0.05 27.4±6.0 204±4 22.8±2.7 8.41±0.64 B1 6.19±0.13 28.7±6.0 164±15 24.0±0.1 8.75±0.01 F2 6.95±0.01 119±27.5 170±5 27.1±4.2 9.38±0.13 AZ2 7.30±0.25 31.1±4.7 292±4 23.7±1.1 8.42±0.55 S2 7.36±0.13 38.3±0.5 320±4 20.0±1.1 9.71±0.02 B2 6.23±0.08 28.4±6.4 330±3 26.1±0.1 8.92±0.13 分蘖期 CK 5.60±0.03 15.9±1.0 88.6±0.2 36.9±0.2 6.10±0.30 D 5.40±0.12 17.3±3.3 81.8±4.8 27.6±5.2 7.70±1.30 F1 5.93±0.04 26.9±1.8 84.7±3.9 30.1±5.3 7.04±1.16 AZ1 6.86±0.02 14.8±0.3 85.5±1.1 38.2±2.3 7.60±0.20 S1 6.49±0.11 14.7±1.8 86.6±9.6 29.3±0.5 9.80±0.20 B1 6.55±0.00 17.2±0.1 77.1±0.1 34.3±0.6 7.14±0.74 F2 6.35±0.01 35.0±4.7 85.6±8.4 22.8±6.9 5.00±0.40 AZ2 6.47±0.03 33.0±2.3 74.0±0.2 14.8±7.4 5.30±0.10 S2 6.38±0.02 22.1±0.1 96.5±7.4 33.9±1.0 5.50±0.30 B2 5.92±0.01 34.1±0.4 73.7±3.0 34.1±8.9 6.64±0.04 成熟期 CK 5.89±0.01 21.3±5.9 95.8±14.9 21.9±3.8 5.88±0.04 D 5.62±0.02 20.7±0.3 106±4.2 33.1±1.8 6.24±0.04 F1 6.18±0.02 45.5±3.4 86.0±3.5 34.4±2.0 7.74±1.30 AZ1 6.64±0.01 21.5±0.9 89.0±0.1 35.7±4.8 6.14±0.30 S1 6.33±0.21 19.5±0.4 95.4±3.3 35.8±0.9 6.27±0.19 B1 6.29±0.02 9.90±0.6 94.4±11.8 30.2±4.5 6.27±0.19 F2 6.27±0.04 41.5±2.7 87.8±1.7 31.3±0.2 5.11±0.19 AZ2 7.07±0.02 26.1±0.7 98.2±8.7 23.4±6.4 5.11±0.19 S2 6.93±0.08 23.6±1.0 101±5.4 22.2±1.5 6.76±0.10 B2 6.59±0.06 29.6±0.8 76.9±0.2 15.0±3.6 8.32±0.49 土壤溶液中的氮、磷元素是植物的主要氮源和磷源,也是衡量土壤肥力的重要指标[24];同时,还有研究表明,速效磷有利于土壤中的Cd形成沉淀[27]。不同修复材料处理后土壤的速效磷含量如表2所示,在秧苗期,经F2处理后,速效磷含量有所提高,与CK存在显著差异,其余修复材料处理后与CK没有较大差异;在成熟期,F1和F2处理后土壤的速效磷含量与CK相比,提升幅度最大,这说明使用调理剂能在较短时间内大幅度提高土壤速效磷含量,其余修复材料处理后,速效磷与CK一致。
不同修复材料处理后,土壤的碱解氮含量如表2所示,在秧苗期,土壤碱解氮的含量高于分蘖期和成熟期,其原因可能是秧苗期前后施加了尿素和复合肥。在秧苗期,经修复材料S1、AZ2、S2、B2处理后,农田土壤的碱解氮含量与CK相比明显提高。在分蘖期和成熟期,各种修复材料处理后,农田土壤的碱解氮含量与空白对照组相比没有显著性差异,这说明修复材料投加后的短时间内,土壤的碱解氮含量会有所提升,经过一段时间后,会恢复到空白对照组的水平。
土壤有机质是土壤肥力的物质基础,其对土壤肥力有多方面的作用[28]。不同修复材料处理后土壤的有机质含量如表2所示,在秧苗期,经修复材料S1和S2处理后,农田的有机质含量与CK相比有所下降,这说明海泡石粉使用初期容易使土壤的有机质减少。在分蘖期,各组实验田的有机质含量与CK相比基本上没有显著差异;在成熟期,除B2处理后农田的有机质含量与CK相比有所下降,其余修复材料处理后农田的有机质含量与CK相比没有显著差异。
阳离子交换量可以用来估算土壤吸收、保留和交换阳离子的能力。不同修复材料处理后土壤的阳离子交换量如表2所示。在秧苗期和分蘖期,部分修复材料处理后的阳离子交换量有所提高,与CK存在显著差异;在成熟期,只有B2处理后的阳离子交换量与CK相比存在较大差异,其原因可能是膨润土富含大量的阳离子,阳离子与土壤中的重金属离子发生交换[29]。这说明修复材料投加后的短时间内土壤的阳离子交换量会有所提升,经过一段时间后,阳离子交换量基本上会恢复到没有修复材料处理过的空白对照组的水平。
2.5 水稻产量
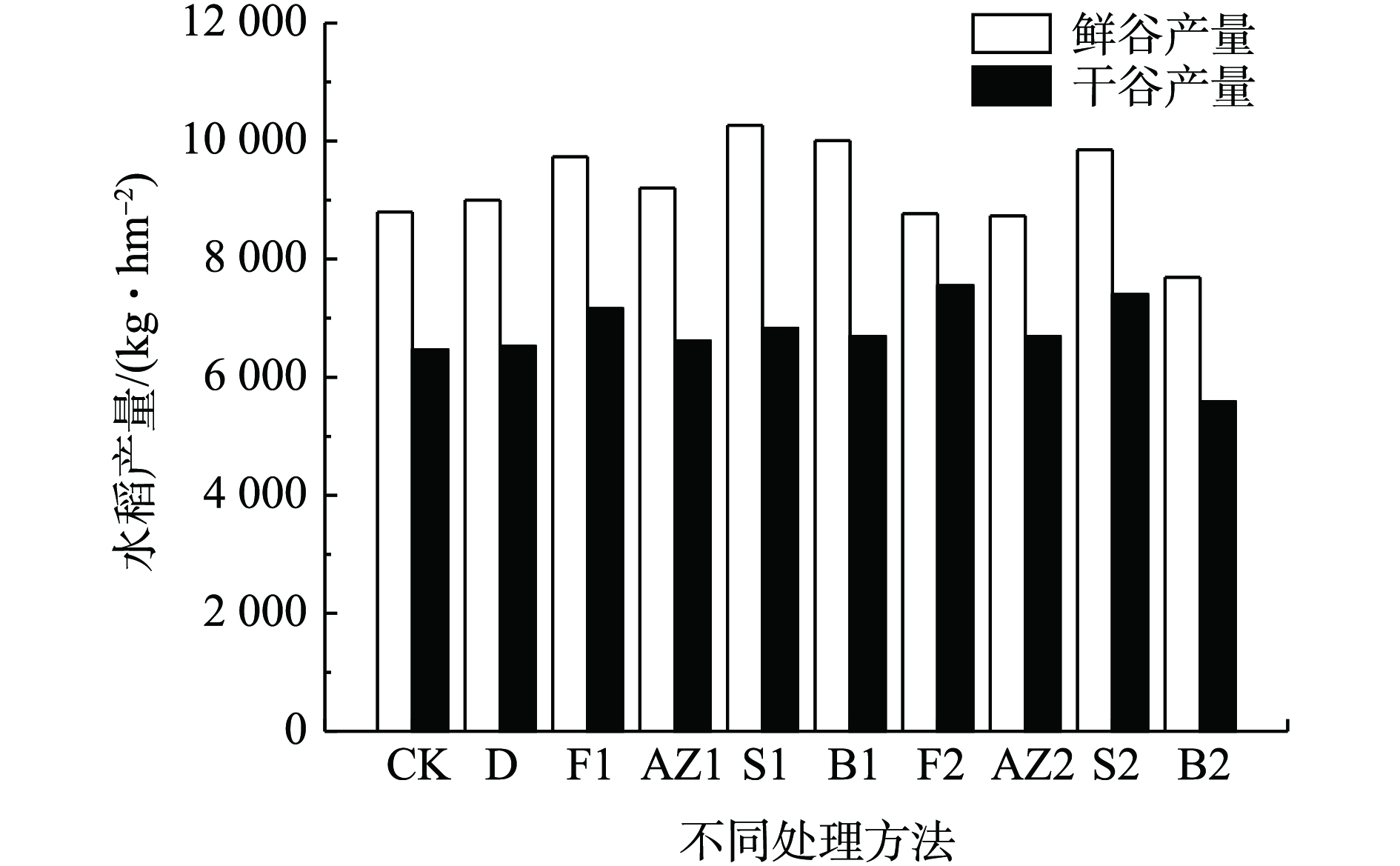
水稻的产量是评价修复材料是否适合农田修复的重要指标,水稻的产量因品种不同、气候不同等因素存在差异。不同修复材料处理后水稻的收成情况如图4所示,各组的水稻产量存在差异,空白对照组的鲜谷产量为8 800 kg·hm−2。经修复材料处理后,水稻的鲜谷产量均有不同程度地增加,其中S1、B1、S2和F1处理后的鲜谷产量最高。空白对照组的干谷产量为6 466 kg·hm−2,除B2外,经修复材料处理后,水稻的干谷产量均有不同程度地增加,其中F2、S2和F1处理后的干谷产量最高,分别为7 549、7 400、7 160 kg·hm−2,分别增产16.74%、14.43%、10.72%。水稻增产的原因可能是因为修复材料缓解了Cd抑制水稻生长的不良影响[30],提高土壤pH从而提高植物的酶活性;同时,磷是作物生长的必需元素,结合土壤速效磷含量的数据可知,调理剂提高了土壤速效磷浓度,有利于水稻的光合作用、碳水化合物运输、氮元素的代谢和脂肪生成,从而提高水稻的产量[31]。
2.6 水稻重金属含量
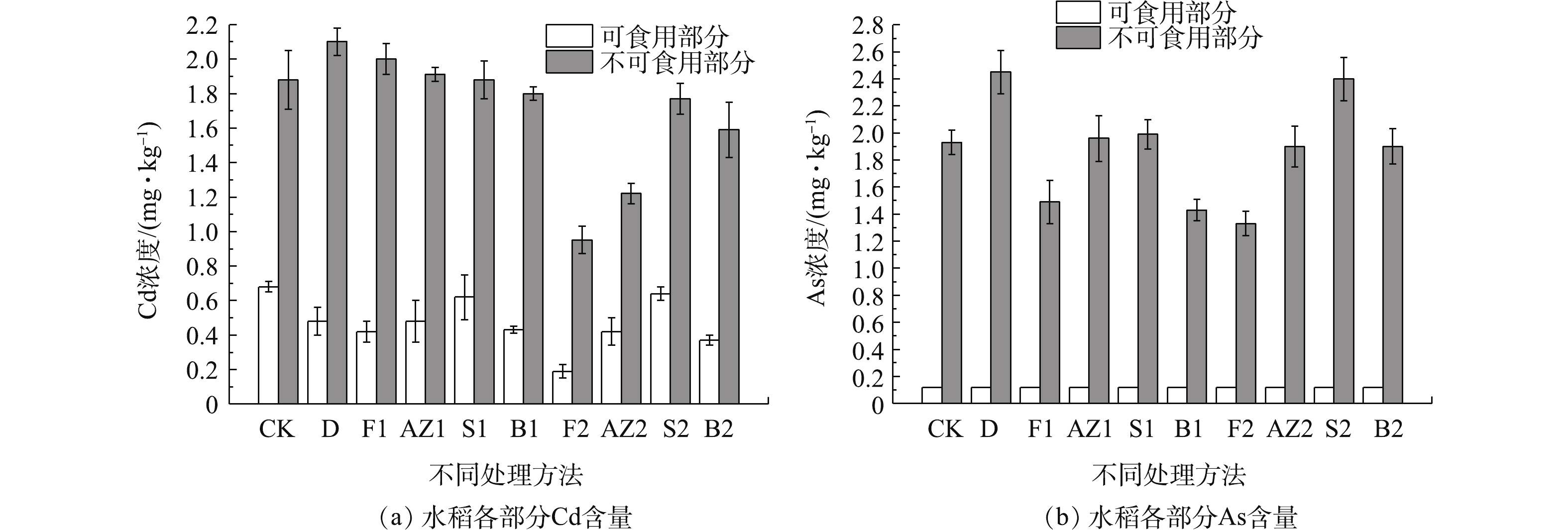
农田修复最关键的是让水稻可食用部分的重金属含量可以满足人体健康食用。水稻可食用部分的Cd含量如图5(a)所示,空白对照组水稻可食用部分的平均Cd含量为《食品中污染物限量》[32]中标准值(0.20 mg·kg−1)的3.4倍。F2处理后水稻可食用部分的Cd含量最低,平均含量为0.19 mg·kg−1,符合《食品中污染物限量》[32]的食用标准值,其他修复材料处理后的平均Cd含量均超出相关标准[32],F2处理后水稻可食用部分的Cd含量与CK相比下降了72%,与MENG等[33]使用的碱性修复材料的修复效果相似,F2处理后速效磷含量和Cd残余态含量均有所提高,因此,调理剂的修复机理可能是调理剂以磷酸盐沉淀固定Cd[27]。B2和AZ2处理后水稻可食用部分与CK相比分别下降了46%和38%,效果没有调理剂明显,膨润土可能是通过离子交换抑制水稻吸收Cd,人造沸石可能是通过表面吸附和沉淀来钝化重金属[34]。F1处理后,水稻可食用部分的Cd含量为0.42 mg·kg−1,与F2存在显著差异,其余修复材料也有相似的规律。这说明在一定范围内,投加量越高,修复材料的修复效果越好,修复材料的投加量和修复材料的种类相比,修复材料的投加量对修复材料修复效果的影响更大。
水稻可食用部分的As含量如图5(b)所示,空白对照组水稻可食用部分的As含量为0.12 mg·kg−1,未超过《食品中污染物限量》的标准值(0.20 mg·kg−1)[32]。修复材料处理后,水稻可食用部分的As含量均是0.12 mg·kg−1,As含量也未超过标准值。
水稻不可食用部分的Cd含量如图5(a)所示,空白对照组的不可食用部分Cd的平均浓度为1.88 mg·kg−1,F2、AZ2和B2处理后水稻的不可食用部分Cd的平均浓度与CK存在显著差异,其中F2与CK相比下降了49.5%,下降幅度最大。其余的修复材料处理后,水稻的不可食用部分Cd与CK没有太大的差异。
水稻不可食用部分的As含量如图5(b)所示,F2与CK相比下降了31.1%,B1和F1与CK相比下降了25.9%和22.8%。其余的修复材料处理后,与CK没有显著差异。海泡石粉、膨润土的投加量对阻止As进入水稻不可食用部分有显著的影响,海泡石粉、膨润土的投加量越高,则会促进As进入水稻的不可食用部分,原因可能是海泡石粉、膨润土的投加量越高,土壤的pH提升越显著,导致含砷沉淀物中的As得到释放,As的迁移能力得到提高[35]。
3. 结论
1)跟其他修复材料相比,调理剂的修复效果最好,既能有效地阻止Cd进入水稻的可食用部分和不可食用部分,也能提升水稻干谷产量,且提升幅度比其他修复材料更高。因此,调理剂具有修复Cd和As污染农田的应用前景。1 kg·m−2投加量的调理剂修复效果比0.5 kg·m−2的调理剂更好。
2)膨润土具备修复Cd污染农田的能力。0.5 kg·m−2投加量的膨润土能有效地阻止Cd进入水稻的可食用部分和不可食用部分,提升水稻干谷产量。但1 kg·m−2投加量的膨润土会减少水稻干谷产量,故膨润土的投加量要根据实际情况考虑。
3)人造沸石具备修复Cd污染农田的能力,能有效地阻止Cd进入水稻的可食用部分。投加人造沸石粉对水稻产量没有较大的影响。1 kg·m−2投加量的人造沸石修复效果比0.5 kg·m−2的人造沸石更好。
4)海泡石粉可以降低农田土壤中Cd的迁移能力,但无法有效地阻止Cd和As进入水稻的可食用部分和不可食用部分。海泡石粉处理后能提高水稻干谷产量。
5)硅藻土能抑制Cd进入水稻的可食用部分,促进Cd和As进入水稻的不可食用部分。因此,这种情况下建议不要将水稻的不可食用部分还田,以减少农田中重金属的总量。投加硅藻土对水稻产量没有较大的影响。
-
表 1 管网疏松沉积物样品的元素组成
Table 1. Elemental compositionof the loose deposits in pipe network
% 序号 C N O As Al Si S Pb Mn Fe Cu 1# 2.40 0.29 30.90 0.46 0.55 0.55 0.55 0.33 1.66 61.65 0.32 2# 4.47 0.36 31.96 0.10 1.20 1.71 0.13 0.54 0.59 57.99 0.95 3# 2.59 0.23 18.37 0.33 1.12 1.23 0.13 0.70 0.99 73.23 1.08 4# 15.17 0.30 2.50 0.03 0.28 0.10 0.00 0.21 0.66 78.82 1.94 5# 8.53 0.38 30.70 0.27 0.41 0.60 0.32 1.02 0.43 56.20 1.14 6# 5.54 0.32 33.62 0.37 0.57 0.87 0.22 0.74 0.32 56.39 1.02 7# 3.30 0.34 32.53 0.61 1.04 1.02 0.12 0.58 0.31 59.21 0.93 表 2 管网疏松沉积物样品的晶体组成
Table 2. Crystal composition of the loose deposits in pipe network
% 样品编号 Fe3O4 CaCO3 α-Fe2O3 γ-Fe2O3 δ-FeOOH α-FeOOH Fe γ-FeOOH β-FeOOH FeCO3 SiO2 1# 15 0 37 28 0 13 0 0 7 0 0 2# 3 14 3 57 0 2 1 9 3 4 3 3# 0 5 7 19 17 16 1 4 5 4 25 4# 6 34 11 13 4 5 0 11 1 1 14 5# 13 0 5 0 0 8 0 14 35 0 26 6# 9 0 9 11 0 14 0 27 8 0 21 7# 8 5 6 7 0 7 0 10 9 0 48 表 3 管网疏松沉积物性质与细胞存活率的Pearson相关系数
Table 3. Pearson correlation coefficient of structure property and cell viability
zeta电位 粗糙度 平均粒径 PV zeta电位 1 0.225 −0.851* 0.203 粗糙度 1 0.032 −0.507 平均粒径 1 −0.191 PV 1 注:*代表显著性水平为0.05。 表 4 管网疏松沉积物晶体组成与细胞存活率相关系数
Table 4. Correlation coefficient of crystal composition and cell viability
Fe3O4 CaCO3 α-Fe2O3 γ-Fe2O3 δ-FeOOH α-FeOOH Fe γ-FeOOH β-FeOOH FeCO3 SiO2 PV Fe3O4 1 −0.411 0.600 −0.364 −0.686 0.095 −0.804* 0.072 0.533 −0.859* −0.103 −0.335 CaCO3 1 −0.190 0.179 0.100 −0.589 0.067 −0.081 −0.488 0.252 −0.210 −0.097 α-Fe2O3 1 0.091 −0.159 0.381 −0.359 −0.477 −0.177 −0.372 −0.512 0.197 γ-Fe2O3 1 −0.042 −0.349 0.679 −0.359 −0.527 0.671 −0.682 0.519 δ-FeOOH 1 0.494 0.592 −0.347 −0.262 0.625 0.114 0.588 α-FeOOH 1 −0.038 0.002 0.016 −0.112 0.084 0.184 Fe 1 −0.336 −0.339 0.981** −0.235 0.738 γ-FeOOH 1 0.212 −0.344 0.270 −0.762* β-FeOOH 1 −0.417 0.293 −0.089 FeCO3 1 −0.273 0.711 SiO2 1 −0.441 RV 1 注:*代表显著性水平为0.05。 -
[1] 汤鸿霄. 环境科学中的化学问题环境水质学中的几个化学前沿问题[J]. 化学进展, 2000, 12(4): 415-422. doi: 10.3321/j.issn:1005-281X.2000.04.008 [2] 刘书明. 饮用水管网输配过程中颗粒物与微量污染物的复合污染效应及其水质风险[J]. 环境工程学报, 2021, 15(1): 1-2. doi: 10.12030/j.cjee.202008046 [3] VREEBURG J H G, BOXALL J B. Discolouration in potable water distribution systems: A review[J]. Water Research, 2007, 41(3): 519-529. doi: 10.1016/j.watres.2006.09.028 [4] 张晓健, 牛璋彬. 给水管网中铁稳定性问题及其研究进展[J]. 中国给水排水, 2006, 22(2): 13-16. doi: 10.3321/j.issn:1000-4602.2006.02.004 [5] 顾军农. 多水源供水模式下管网腐蚀产物释放与应对措施初探[J]. 城镇供水, 2010(1): 66-70. [6] SARIN P, SNOEYINK V L, BEBEE J, et al. Physico-chemical characteristics of corrosion scales in old iron pipes[J]. Water Research, 2001, 35(12): 2961-2969. doi: 10.1016/S0043-1354(00)00591-1 [7] 邱微, 王立友, 樊庆锌, 等. 给水球墨铸铁管腐蚀特性及腐蚀对水质的影响[J]. 哈尔滨工业大学学报, 2016, 48(8): 61-66. doi: 10.11918/j.issn.0367-6234.2016.08.010 [8] ZHUANG Y, HAN B, CHEN R, et al. Structural transformation and potential toxicity of iron-based deposits in drinking water distribution systems[J]. Water Research, 2019, 165: 114999. doi: 10.1016/j.watres.2019.114999 [9] ZHUANG Y, SHEN C, GU Y, et al. Effect of trichloroacetic acid on iron oxidation: Implications on the control of DBPs and deposits in drinking water[J]. Water Research, 2021, 189: 116632. doi: 10.1016/j.watres.2020.116632 [10] ZHUANG Y, HAN B, CHEN R, et al. Mechanism study on organic pollutant accumulation by iron-based particles in drinking water conditions[J]. Chemical Engineering Journal, 2020, 396: 125157. doi: 10.1016/j.cej.2020.125157 [11] 周全, 陈汝硕, 高红涛, 等. 基于模糊综合评价法的城市供水管网黄水风险区域预测及应对措施[J]. 给水排水, 2015, 51(8): 101-104. doi: 10.3969/j.issn.1002-8471.2015.08.030 [12] LIU G, ZHANG Y, KNIBBE W J, et al. Potential impacts of changing supply-water quality on drinking water distribution: A review[J]. Water Research, 2017, 116: 135-148. doi: 10.1016/j.watres.2017.03.031 [13] 李欣, 马建薇, 袁一星. 城市给水管网水质研究与进展[J]. 哈尔滨工业大学学报, 2004, 36(10): 1392-1396. doi: 10.3321/j.issn:0367-6234.2004.10.033 [14] 刘锐平, 曲久辉. 饮用水质健康风险的末端控制[J]. 科学通报, 2009, 54(3): 273-277. [15] CHAN C S, STASIO C D, WELCH S A, et al. Microbial polysaccharides template assembly of nanocrystal fibers[J]. Science, 2004, 303(5664): 1656-1658. doi: 10.1126/science.1092098 [16] ZHUANG Y, KONG Y, LIU Q, et al. Alcohol-assisted self-assembled 3D hierarchical iron (hydr)oxide nanostructures for water treatment[J]. Crystengcomm, 2017, 19(39): 5926-5933. doi: 10.1039/C7CE01320E [17] 牛璋彬, 王洋, 张晓健, 等. 给水管网中管内壁腐蚀管垢特征分析[J]. 环境科学, 2006, 27(6): 1150-1154. doi: 10.3321/j.issn:0250-3301.2006.06.022 [18] 刘东坡, 张金松, 靳军涛. 城市供水管网通水初期水质变化规律研究[J]. 中国给水排水. 2019, 35(17): 43-49. [19] OBERDÖRSTER G. Pulmonary effects of inhaled ultrafine particles[J]. International Archives of Occupational and Environmental Health, 2000, 74(1): 1-8. [20] ZHUANG Y, LIU Q, KONG Y, et al. Enhanced antibiotic removal through a dual-reaction-center Fenton-like process in 3D graphene based hydrogels[J]. Environmental Science: Nano, 2019, 6(2): 388-398. [21] 庞维海, 杨帆, 楚文海, 等. 饮用水中氯代乙酰胺的细胞毒性和遗传毒性[J]. 同济大学学报(自然科学版), 2014, 42(12): 1873-1878. doi: 10.11908/j.issn.0253-374x.2014.12.014 [22] 楚文海, 沈杰, 栾鑫淼, 等. 疫情防控期间污水处理厂强化消毒下的水环境次生风险实证研究[J]. 给水排水, 2020, 56(6): 1-5. [23] 徐明, 阚海东, 桑楠, 等. 持久性有毒污染物环境健康研究的现状与思考[J]. 中国科学院院刊, 2020, 35(11): 1337-1343. [24] HARRAD S, WEMKEN N, DRAGE D S, et al. Perfluoroalkyl substances in drinking water, indoor air and dust from Ireland: Implications for human exposure[J]. Environmental Science & Technology, 2019, 53(22): 13449-13457. [25] PRAT O, VERCOUTER T, ANSOBORLO E, et al. Uranium speciation in drinking water from drilled wells in southern finland and its potential links to health effects[J]. Environmental Science & Technology, 2009, 43(10): 3941-3946. [26] 石宝友, 李涛, 顾军农, 等. 北方某市水源更换过程中管网黄水产生机制的探讨[J]. 供水技术, 2010, 4(4): 12-15. doi: 10.3969/j.issn.1673-9353.2010.04.004 [27] 冯利华. 环境质量的主成分分析[J]. 数学的实践与认识, 2003(8): 32-35. [28] PARK J, LIM D H, LIM H J, et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles[J]. Chemical Communication, 2011, 47(15): 4382-4384. doi: 10.1039/c1cc10357a [29] 李玉仙, 王敏, 李礼, 等. 水源切换条件下管网管垢稳定性和水质腐蚀性判定指标探讨[J]. 给水排水, 2015, 51(2): 110-114. doi: 10.3969/j.issn.1002-8471.2015.02.027 [30] 牛璋彬, 王洋, 张晓健, 等. 给水管网中管内壁腐蚀管垢特征分析[J]. 环境科学, 2006, 27(2): 310-314. doi: 10.3321/j.issn:1001-0742.2006.02.019 -






 下载:
下载: