-
重金属污染一直以来备受关注。砷作为高毒性污染物被广泛认知,矿山开采、冶炼、农业生产、制革、染料、化工等行业生产中往往会产生大量的砷[1-2]。As及其化合物随着径流、入渗等途径进入地下水中,污染水体,并通过不断累积对环境与人体健康造成威胁。在我国,对于砷的排放标准也有着严格的规定[3]。如何有效地处理含As地下水一直以来都是环境工程领域所关注的热点。
As的存在形式及其价态决定了As及其化合物的毒性[4]。As在水体中常以As(Ⅲ)和As(Ⅴ)的形态存在,而在还原条件下的地下水体中,又以As(Ⅲ)为主要存在形态。在目前的地下水As(Ⅲ)污染处理中,中和法[5]、混凝法[6-7]、吸附法[8-9]、离子交换法[10-11]、微生物法[12-13]、电化学法[14-15]均能取得良好的治理效果。但不断寻求提供高效,创新的处理方法,并作为现有地下水As(Ⅲ)污染处理技术的补充或替代仍然是十分必要的。已有研究[16]表明,As(Ⅲ)的毒性比As(Ⅴ)高出数十倍以上,并且迁移性更强,稳定性差,因此,将As(Ⅲ)氧化成更易吸附、稳定的As(Ⅴ)是实现As(Ⅲ)控制的可行途径。SORLINI等[17]研究了二氧化氯、次氯酸盐、高锰酸钾等氧化剂对水体中As的氧化效果,As(Ⅲ)的氧化效率能到达到80%~100%。吕杰蝉等[18]通过感应电芬顿法处理二甲基砷废水,在初始pH为3,电流密度为2 mA·cm−2,初始浓度为5 mg·L−1的条件下,4 h后去除率达到94.4%。LAN等[19]以FeCx/N掺杂的碳纤维复合材料作为催化剂,通过电芬顿反应对含二甲基砷废水进行高效处理,6 h后去除率达到96%。ZHANG等[20]通过非均相芬顿法对畜牧业中的有机砷化合物进行降解,处理3 h后降解率可达到80%以上。尽管对地下水中砷污染处理的研究较少,但这能为我们处理此类地下水提供新的思路,通过强氧化性的羟自由基(·OH)可将As(Ⅲ)氧化为更稳定、毒性低的As(Ⅴ),同时亦可减少使用化学氧化剂带来的风险与成本。
本研究设计了一种新型流通式电芬顿系统,用来处理含As(Ⅲ)地下水。考察了电流强度、pH、流速、曝气速率、电解质浓度等因素对去除效果的影响,并探讨了水体中共存离子的干扰作用及相关的反应机理。此外,对流通式电芬顿系统在连续运行条件下的性能进行了评估,以期为地下水As(Ⅲ)污染的治理提供参考。
全文HTML
-
本研究以湖南某矿区附近城镇的地下水为例,研究中均使用实际地下水作为处理对象。其水质如下:1.87~2.2 mg·L−1 As(Ⅲ)、0.12 mg·L−1 Fe、120 μg·L−1 Mn、75 mg·L−1
SO2−4 、33 mg·L−1 COD、pH为6.25。所用试剂均购自国药试剂集团且为分析纯:水杨酸(salicylic acid)、苯醌(benzoquinone)、氯化钾(KCl)、硫酸(H2SO4)、氢氧化钠(NaOH)。实验中所用水均为超纯水(UPH,优普,18.25 MΩ.cm)。 -
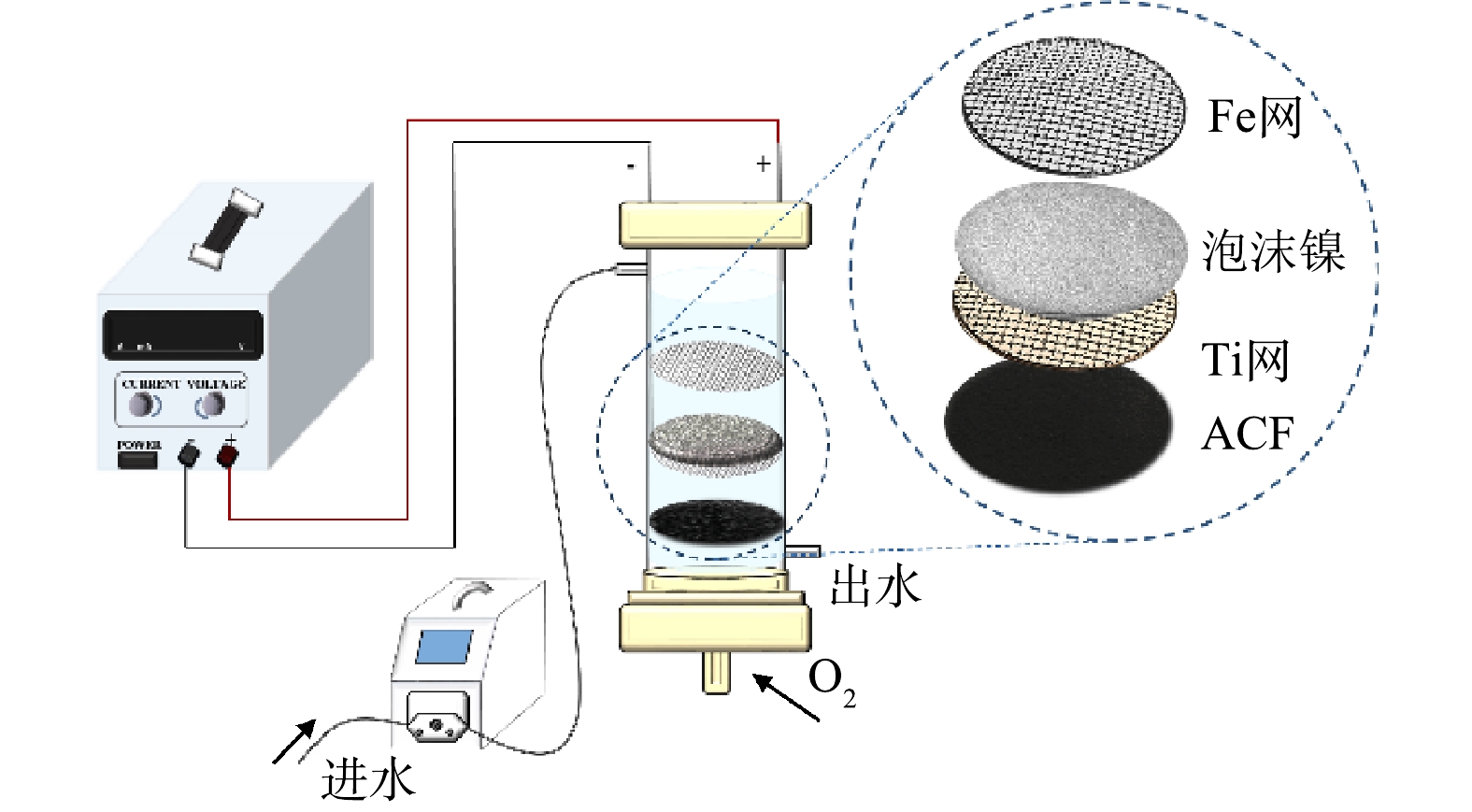
电芬顿反应在亚克力(PMMA)材质的圆柱形反应器(D=60 mm,H=200 mm)中进行,实验装置如图1所示。反应器分为3个部分,阳极为Fe网电极(R=25 mm),阴极为泡沫镍(NF)电极,覆于Ti网上,最下方是活性炭纤维过滤器(ACF),阴阳极通过不锈钢电极夹连接至直流电源(MESTEK DP305)上,并保持恒定距离。将阴极浸泡在地下水中数小时直至饱和后取出再用。通入电流开始反应后,通过蠕动泵(UIP-WIFI-S183, Kamoer)以一定的流速将1 L地下水引入反应器中,通过控制变量对去除率的影响因素进行探究,每组实验设置3组平行。
-
流通式系统中电流的调控通过直流稳压电源(MASTEK DP305)完成,溶液pH的测定通过pH计(METTLER S220)测定。通过原子荧光法测定溶液中As(Ⅲ)和As(Ⅴ),分别通过丁二酮肟分光光度法与邻菲罗啉分光光度法测定总Ni和总Fe的浓度。通过间接分光光度法测定高铁酸盐的浓度[21]。
1.1. 地下水水质与试剂
1.2. 实验装置与步骤
1.3. 分析方法
-
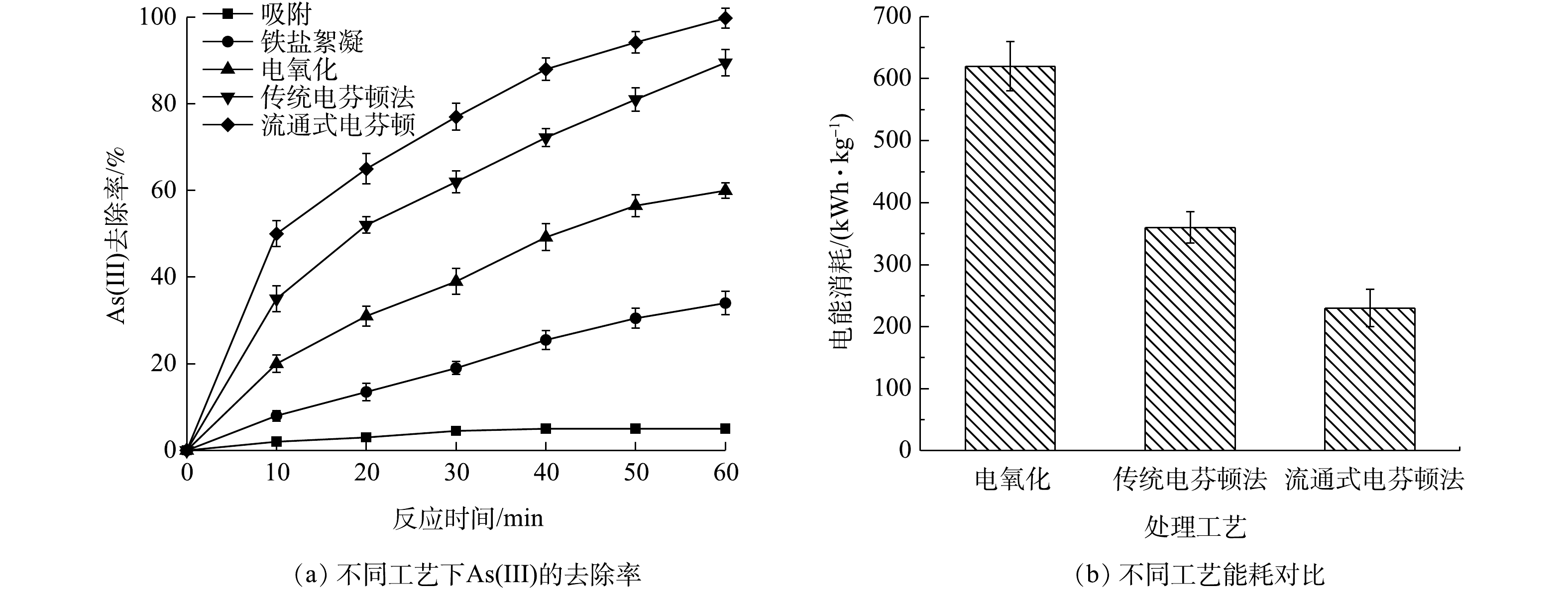
为了高效去除地下水中的As(Ⅲ),设计了一种流通式电芬顿系统。图2(a)为该系统与其他处理工艺去除效率的对比,即吸附(相同装置,不通电)、电氧化、铁盐絮凝、传统电芬顿法(泡沫镍阴极,铁阳极)。可以看出,在吸附条件下,As(Ⅲ)的浓度变化很小。投入一定量的FeCl3·6H2O且不断搅拌后,As(Ⅲ)的去除率为36%。在电氧化处理后,As(Ⅲ)的去除率约为60%。传统电芬顿法处理中,可以对As(Ⅲ)取得92%的去除率,而通过流通式电芬顿系统处理后,As(Ⅲ)的去除率可以达到99.6%左右。这是由于,与集中处理废水相比,垂直流依次通过电极能够提高污染物的传质效率,并且能减少电极间氧化还原作用的干扰,从而可提高对As(Ⅲ)的去除率[22-23]。图2(b)为电化学处理中能耗的对比,可以看出,流通式电芬顿系统的能耗更低,同时,反应中消耗的主要为电能,这避免了化学试剂的消耗,极大地降低了处理成本。
-
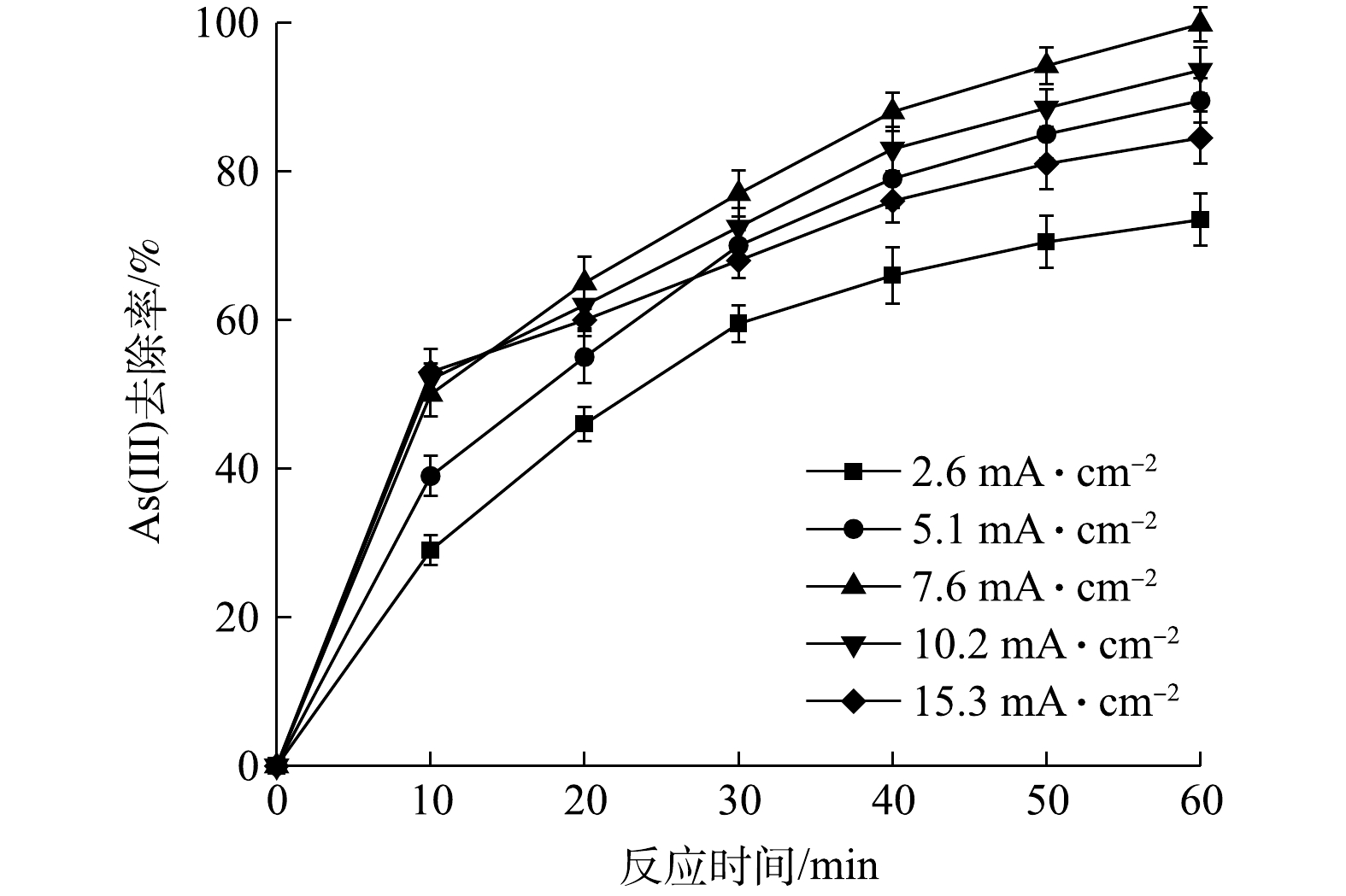
电流密度对电芬顿反应发挥的效用有着至关重要的影响。在电流密度为2.6~15.3 mA·cm−2时,考察了其对去除率的影响。由图3可知,As(Ⅲ)的去除率呈现出先增后减的趋势,在电流密度为7.6 mA·cm−2时(电流强度为150 mA, 电压为3.5 V),As(Ⅲ)的去除率达到最大;当电流密度升至10.2 mA·cm−2 (电流强度为200 mA,电压为4 V)和15.3 mA·cm−2 (电流强度为300 mA,电压为5 V)时,As(Ⅲ)的去除率分别下降至 93.6%和84.5%。这是由于,在一定范围内增大电流密度将提高电极上的电子转移速率,从而促进氧还原反应以及铁的溶解,产生更多的H2O2 ((式(1)~式(3)))。然而,电极上的析氢副反应会在电流密度过高时加剧(式(4)),从而加速电极钝化,同时影响了H2O2的生成[24-25]。因此,在本研究中,当电流密度为7.6 mA·cm−2时能够实现对As(Ⅲ)的高效去除。
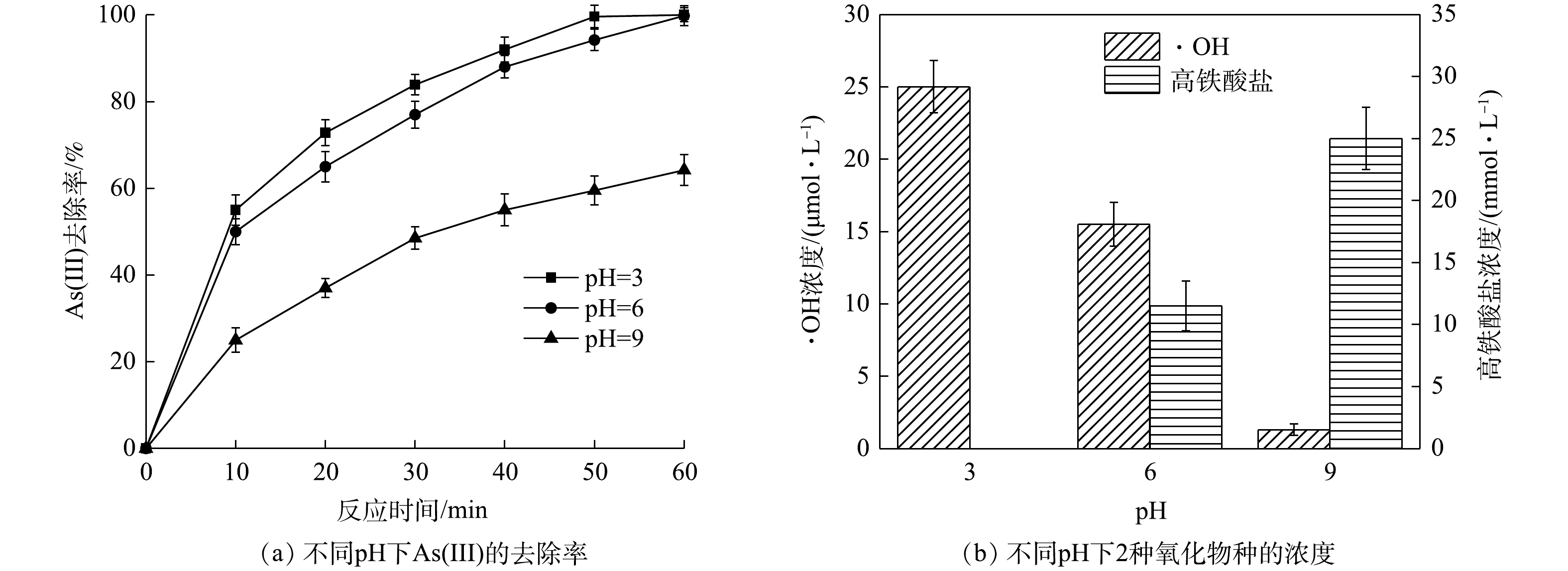
溶液pH对处理效果有着重要影响,故对pH为3~9的条件下溶液中As(Ⅲ)的去除情况进行了探究。如图4(a)所示,当pH为6时,去除率达到99.6%,反应速率略低于pH为3时。由于流通式的处理提升了O2浓度,提高了反应效率,使得电芬顿体系在pH较高的条件下仍能保持较高的H2O2生成效率[26-27]。当pH接近中性时,溶液中的铁离子在电流的作用下会形成具有较强氧化性的高铁酸根[28]。如图4(b)所示,在pH为6时溶液中的羟基自由基与高铁酸盐的浓度均较高。此外,较高的pH增强了阳极溶出Fe的絮凝反应,这些因素在一定程度上减轻了pH对流通式系统处理效率的影响。然而,当pH过高时,H2O2的形成将被抑制(式(5)),从而影响·OH的产生[29-30]。以上结果表明,流通式电芬顿系统能在偏酸性的pH下可保持良好的效果,这极大地节省了调节pH的成本,能够广泛用于As(Ⅲ)污染的地下水体。
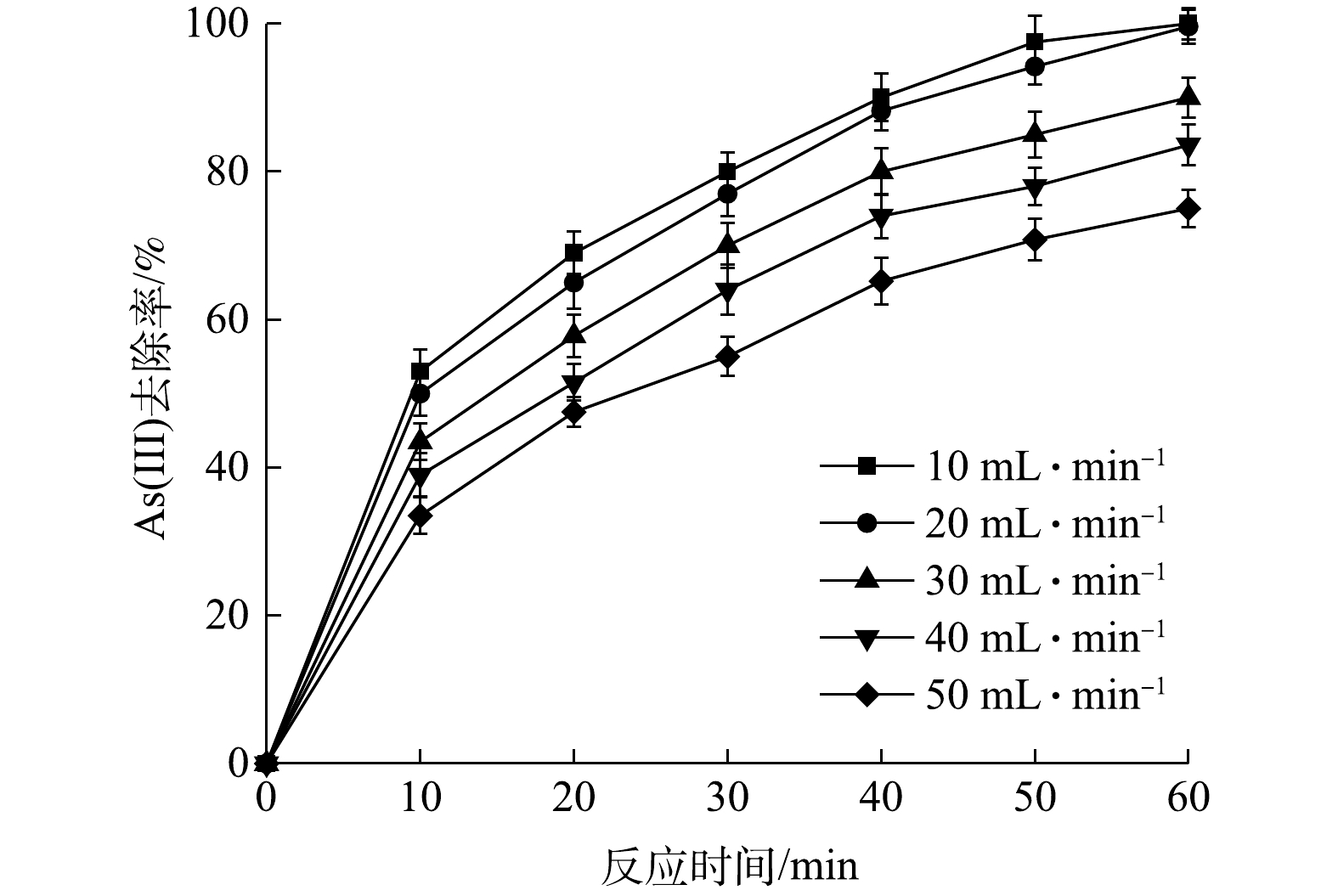
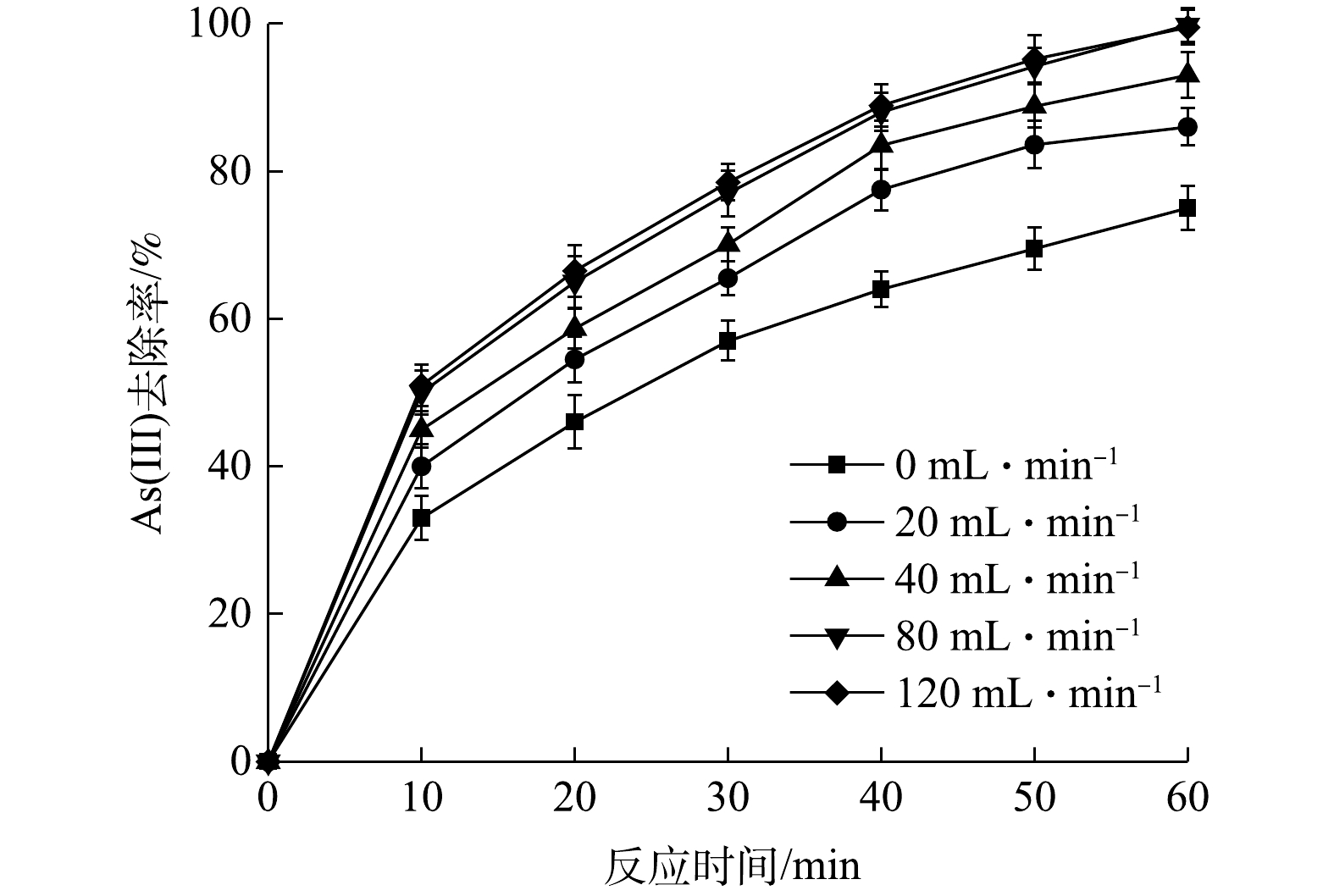
图5反映了流速(10~50 mL·min−1)对As(Ⅲ)去除率的影响。As(Ⅲ)的去除率随着流速的增加而降低(分别为99.9%、99.6%、90%、83.6%、75%)。这是由于流速决定了地下水的停留时间,当流速较低时,As(Ⅲ)能够被更充分的氧化,并且为目标污染物与过滤器提供了更多的接触时间[31]。然而,较低的流速也意味着较高的能耗,控制合适的流速对As(Ⅲ)的去除较为重要。因此,本研究确定最佳流速为20 mL·min−1。
溶解氧含量是限制电芬顿反应的主要因素之一,因此,本实验考察了曝气速率对As(Ⅲ)去除率的影响。如图6所示,As(Ⅲ)的去除率随着曝气速率的提升而增大,曝气速率为80 mL·min−1时去除率达到未曝气条件下的1.35倍。这是由于曝气处理通过鼓动水流提升水体中溶解氧的浓度,增强了H2O2的生成效率(式(6))[32-33]。在继续提高曝气速率后,As(Ⅲ)去除率没有显著的变化,这表示继续增加曝气速率对去除效果的提升有限,溶解氧水平已经达到饱和。因此,当曝气速率确定为80 mL·min−1时,反应效率可维持在较高水平。
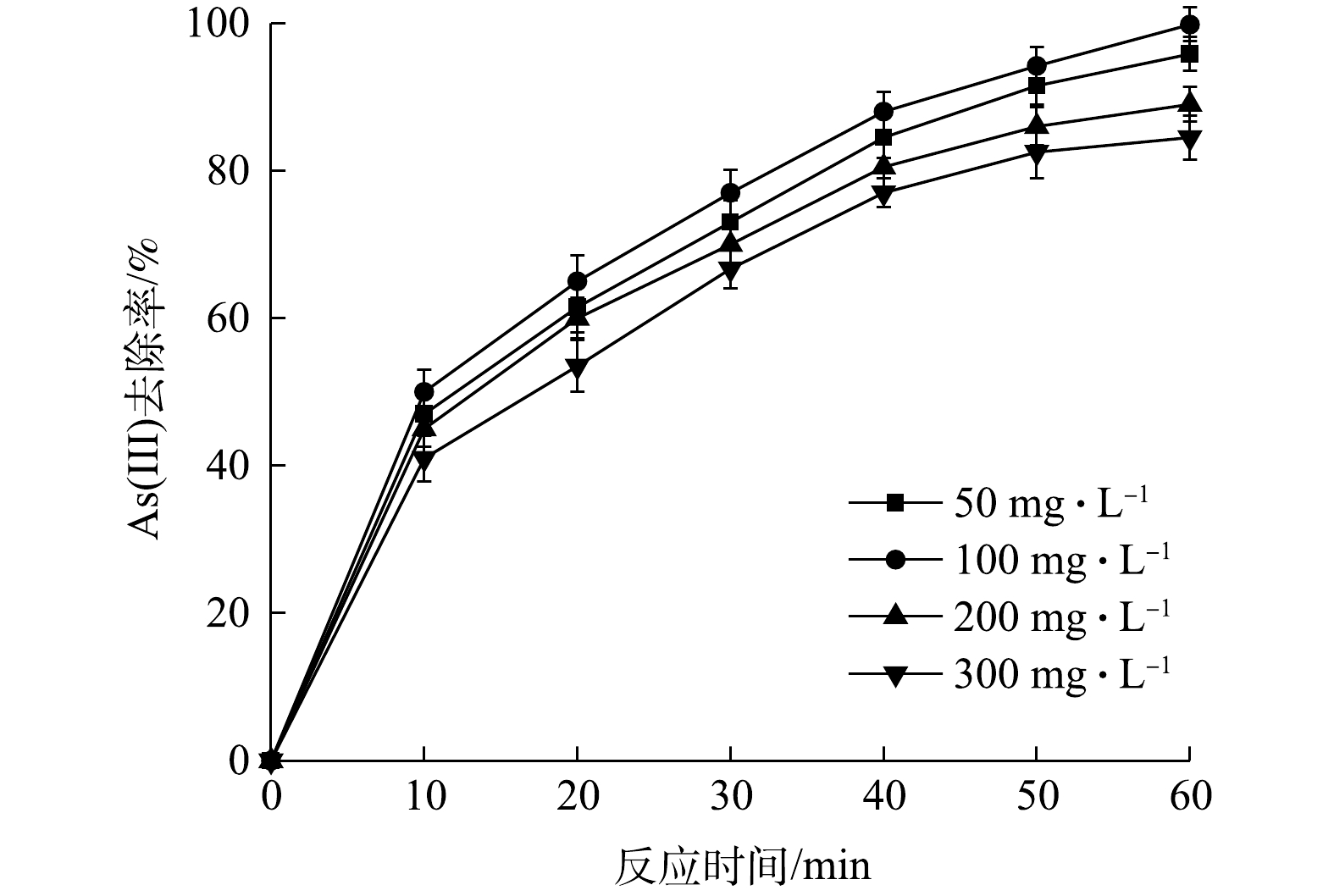
不同浓度电解质对地下水中As(Ⅲ)去除率的影响如图7所示。由图7可知,去除率随着电解质浓度的增加而提升,当电解质质量浓度为100 mg·L−1时去除率最佳。这说明KCl为电流提供了一个合适的通道,增强了溶液的导电性,使电芬顿体系中·OH产生效率得到提升[34-35]。此外,KCl能够避免
CO2−3 、HCO−3 、SO2−4 等作为电解质时与水体中存在的Ca2+反应形成电极表面钝化层,并丰富阳极的溶解,从而提高芬顿反应效率[36-37]。同时,溶液中Cl−可能被电流催化生成次氯酸(HClO)(式(7)~式(8)),从而促进As(Ⅲ)的氧化[38]。然而,过高的电解质浓度并不能进一步提高去除率,这是由于过量的KCl电解质会捕获·OH (式(9)~式(13))。 -
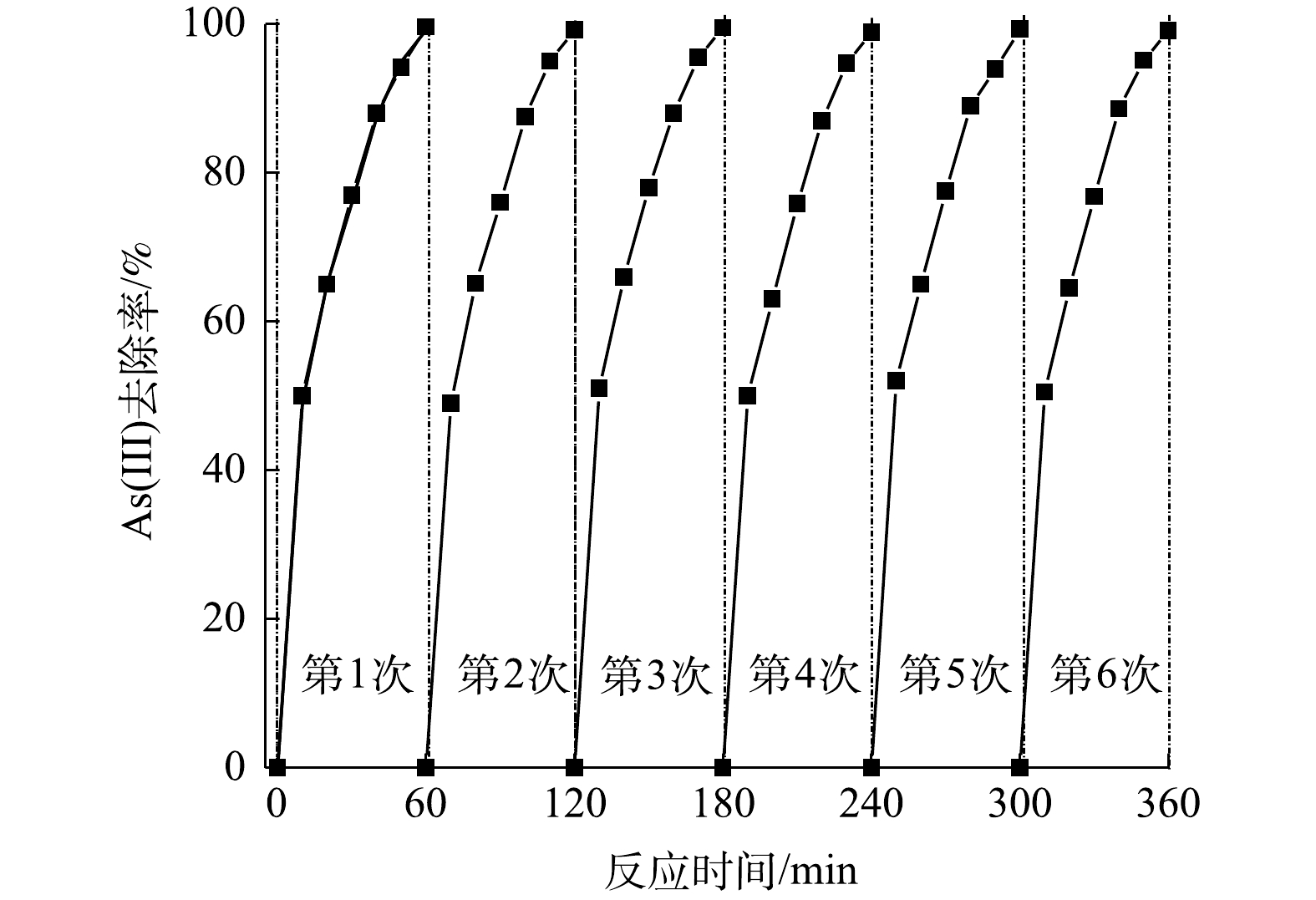
处理系统的使用寿命是评判其实用性的重要指标。因此,在实验中没有对电极材料进行清洗与更换的情况下,考察了连续运行条件下流通式电芬顿系统对地下水中As(Ⅲ)去除效果的变化。由图8可以看出,在最佳反应条件下,经过6次循环实验,As(Ⅲ)的去除率没有发生明显的改变,依次为99.6%、99.2%、99.5%、98.9%、99.3%、99.1%。这表明流通式电芬顿系统在处理含As(Ⅲ)地下水污染时具有良好的稳定性与寿命。
-
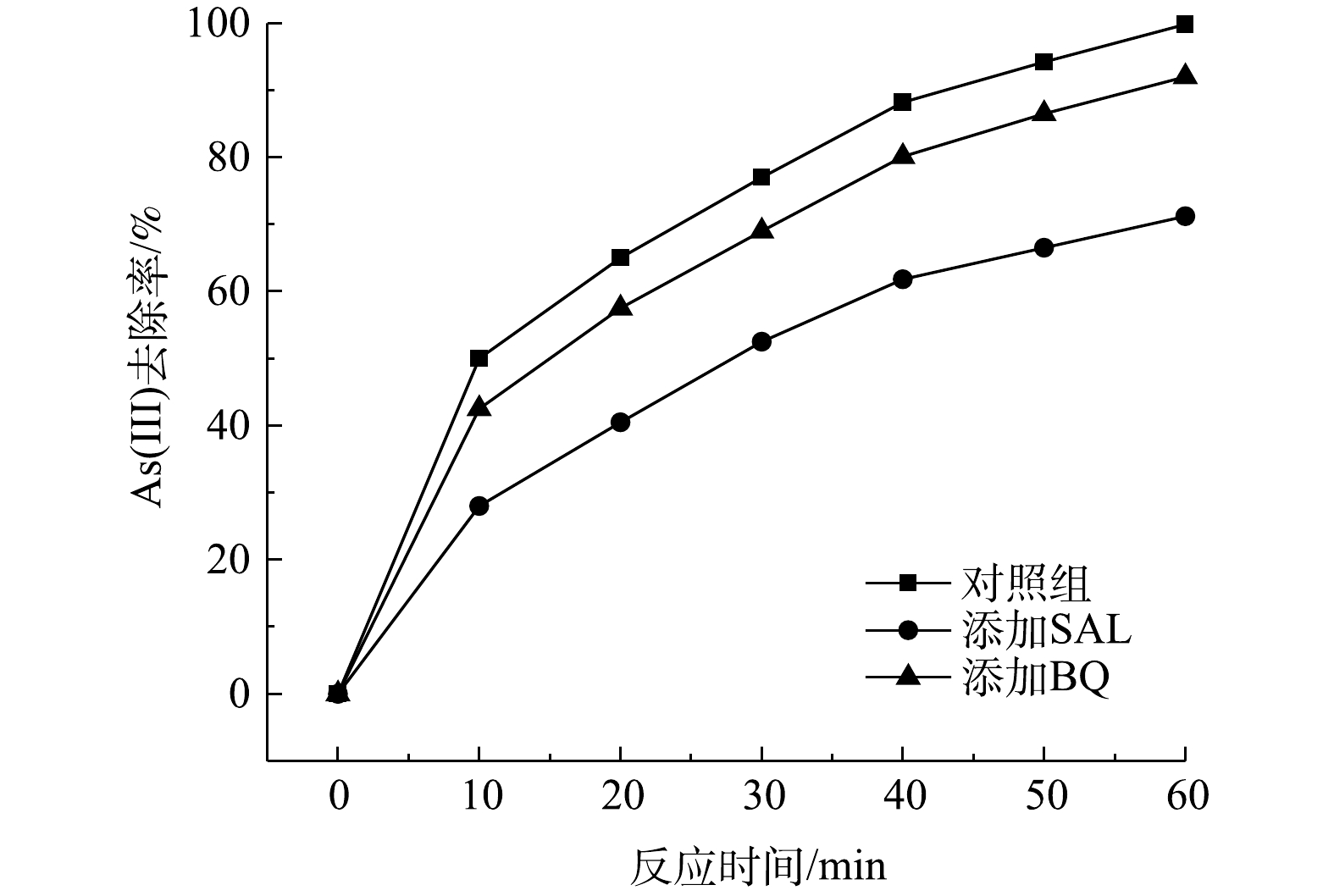
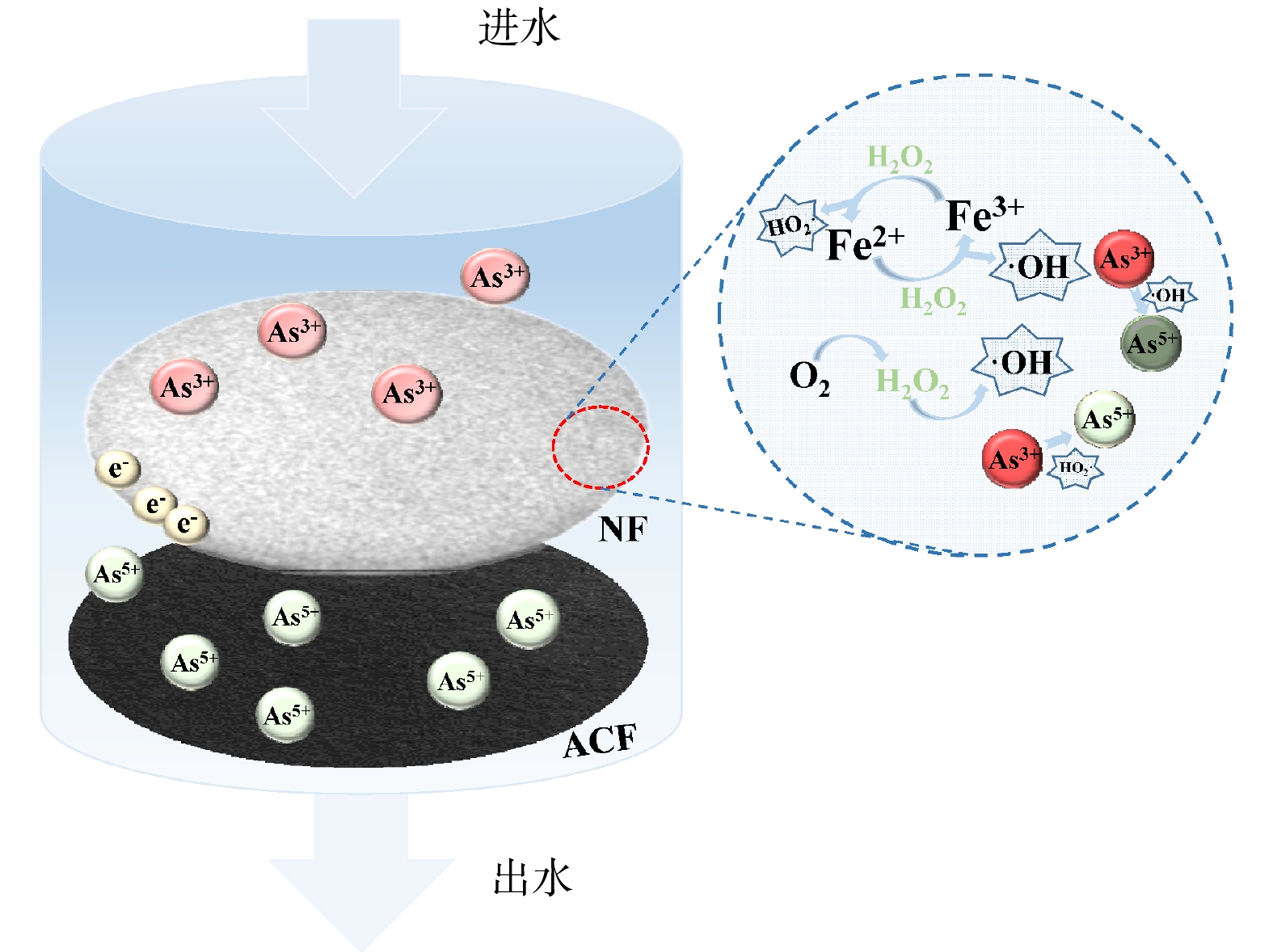
为了探明流通式电芬顿体系中As(Ⅲ)的去除机理,配置一定浓度的水杨酸(SAL)与苯醌(BQ)溶液作为自由基淬灭剂,考察了其对地下水中As(Ⅲ)去除率的影响[39]。由图9可看出,加入水杨酸作为·OH淬灭剂后,As(Ⅲ)的去除率下降至71.2%;而在投加苯醌溶液时,As(Ⅲ)去除率为92%。这说明·OH对As(Ⅲ)/As(Ⅴ)的转化起着主导作用,而过氧化羟基自由基HO2·也能够在一定程度上加速As(Ⅲ)的氧化,二者共同促进了As(Ⅲ)的氧化。如图10所示,去除过程可以合理地总结为:芬顿反应中产生的·OH与HO2·协同对As(Ⅲ)进行高效的氧化(式(14)~式(19))。在此过程中,金属Ni的析出促进了O2的还原(式(20)~式(21)),在一定程度上提高了芬顿反应效率[40-41]。此外,阳极溶出的Fe(III)能对As起到絮凝作用(式(22))[42]。
-
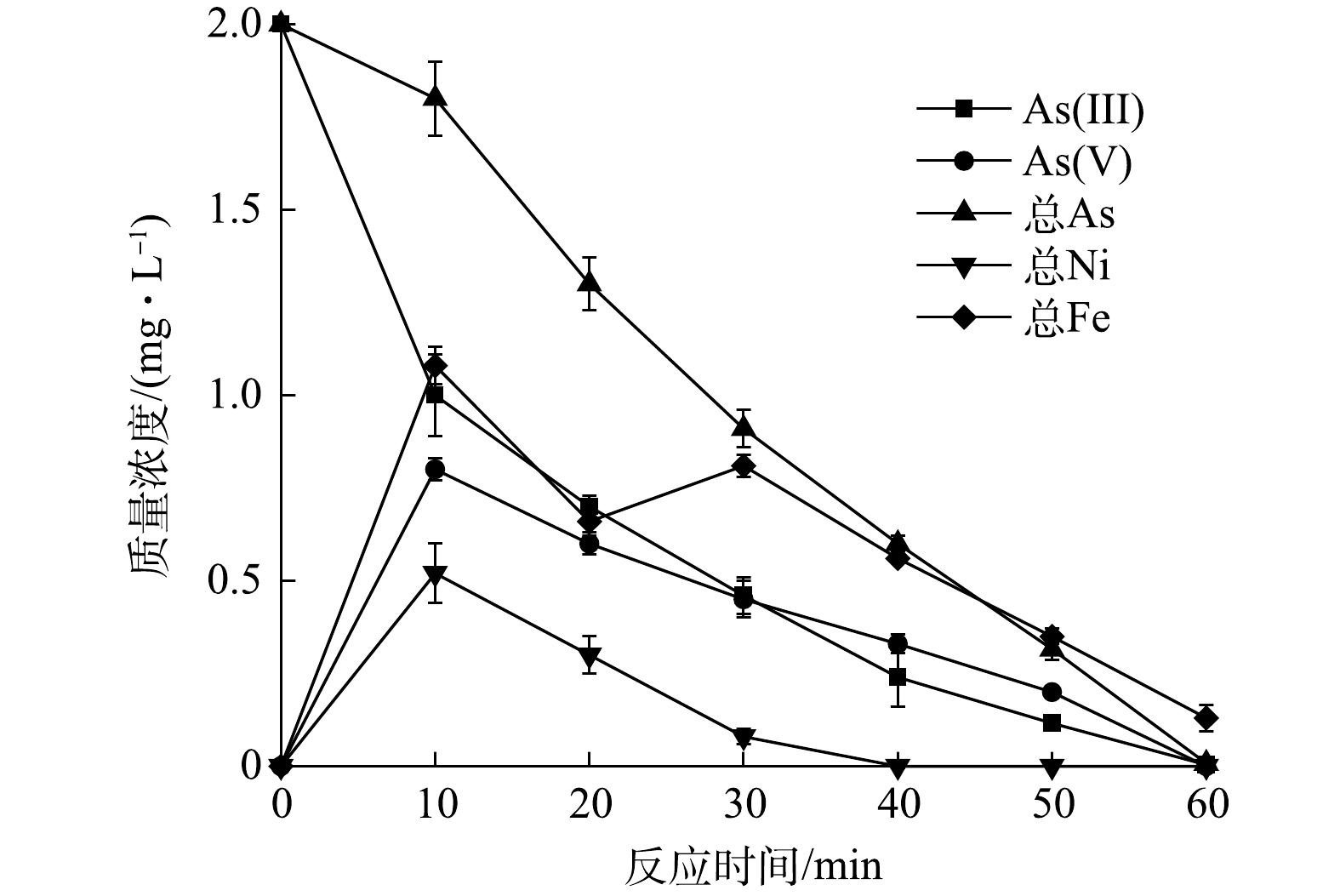
为了去除溶液中的As(Ⅴ)以及可能析出的Ni(II)与Fe(III),反应器中活性炭纤维过滤器被用于对水体进行最后的净化。对出水中As、Ni、Fe类物质的质量浓度变化进行了测定,来进一步证明As(Ⅲ)被高效氧化后去除。如图11所示,反应开始后As(Ⅲ)的质量浓度迅速下降,而As(Ⅴ)质量浓度在初期上升,随后快速地下降。溶液中As(Ⅴ)被活性炭纤维过滤器有效地拦截,以及在阴极表面还原为As。阴极与阳极析出的Ni(II)、Fe(III)同样被过滤器留下。在通电条件下,过滤器还可能存在电吸附反应,加强了对污染离子的截留作用[31],溶液中总As、总Ni、总Fe的浓度变化有力地证实了这一点。此结果表明,流通式电芬顿系统能够有效地对污染水体中As(Ⅲ)进行高效去除,并避免了对水体的二次污染。
2.1. 流通式电芬顿系统的优势
2.2. 操作参数对As(Ⅲ)去除率的影响
2.3. 连续运行实验
2.4. As(Ⅲ)的去除机理
2.5. 污染水体的净化
-
1)设计了一种流通式电芬顿系统且将其用于处理地下水中的高浓度As(Ⅲ)。在电流密度为7.6 mA·cm−2、pH为6、流速为20 mL·min−1、曝气速率为80 mL·min−1、电解质浓度为100 mg·L−1的最佳反应条件下,其可在短时间内对As(Ⅲ)进行高效的处理,As(Ⅲ)去除率可达99.6%以上。
2) ·OH与HO2·能够协同作用,促进As(Ⅲ)的去除。流通式电芬顿系统具有良好的稳定性与寿命,经过连续使用6次后处理效果仍较好。
3) As(Ⅴ)、Ni、Fe可在该系统中与出水进行分离,达成水体的净化,从而可避免二次污染的发生。



 下载:
下载: