-
水中硬度超标将对日常生活、工业生产造成一定影响,如洗涤剂去污能力降低、锅炉结垢、受热不均,严重时甚至引发爆炸;高硬水还直接威胁人类健康,如导致胃肠功能紊乱,增加患肾结石的风险[1-2]。目前,水的软化方法主要有化学沉淀、离子交换、膜分离和吸附等[3-6]。其中,基于结晶反应的化学沉淀法,其具有操作简单、去除率高、易与净水工艺相结合的特点,特别是软化剂石灰来源广、价格低,已有上百年应用历史,至今仍是最常用的软化技术[7]。
过饱和度S是结晶反应的推动力。高硬水由于Ca2+和/或Mg2+浓度高,相应软化所需
CO2−3 和/或OH−投加量大,导致结晶体系过饱和度S较高。此时若结晶体系为均相结晶体系,则成核速率快,结晶产物数量密度大、颗粒细碎,部分结晶产物沉降性能差,难以实现固液分离,即所谓微晶[8]。微晶的出现不但降低软化效果,而且增加体系出水浊度,需投加大量混凝剂进行混凝促沉才能被去除,由此会产生大量难以脱水的沉淀污泥。以流化床结晶为代表的诱导结晶软化技术,通过外投晶种,将均相结晶调控为以晶种表面结晶为主的非均相结晶,结晶产物粒径可控,加上流化床自身优良的固液分离性能,可有效降低出水微晶含量,应用于高硬水软化优势明显[9-11]。但诱导结晶过程将伴随发生均相结晶,微晶的产生仍不可避免,原水硬度较高时尤为明显,导致软化效果有限[12]。笔者所在的课题组前期开展了CaCO3诱导结晶体系中均相与非均相结晶竞争行为研究,发现体系均相结晶比例随过饱和度的增加而增加,相应微晶产率也会提升[13]。因此,控制诱导结晶体系微晶产率,成为提升高硬水软化效果的关键。
提高反应器内诱导结晶活性位点体积密度可强化体系诱导结晶,达到抑制均相结晶、降低微晶产率效果[1, 14-15]。减小晶种粒径和增加晶种投量均可有效提升诱导结晶活性位点体积密度,但研究者在诱导结晶软化中采用的晶种粒径和投加量差异往往很大。NASON等[16]采用11 μm方解石为晶种,投加量为8~70 mg·L−1;MERCER等[17]采用0.43~3.98 μm方解石为晶种,投加量为100~500 mg·L−1;CHEN等[18]采用200~400 μm石英砂为晶种,投加量为375 g·L−1;顾艳梅等[19]采用200~500 μm砂石为晶种,投加量为225 g·L−1;胡瑞柱等[20]采用100~250 μm石榴石为晶种,投加量为200 g·L−1。上述研究均采用流化床为反应器,但晶种粒径相差上千倍,投加量相差甚至上万倍,这可能对微晶产率和软化效果造成影响。因此,有必要对诱导结晶中微晶的形成与控制进行深入研究,从而为晶种的选择提供理论依据。
考虑到流化床投放晶种粒径过小时容易流失[21-22],为最大程度减小晶种粒径并避免流失,本文选择常用晶种材料中密度最大的Fe3O4为晶种。通过改变Fe3O4投加量,研究高硬水诱导结晶软化中微晶的形成与控制,主要考察了晶种投加量对微晶产率和软化效果的影响,探讨了微晶与结晶体系上清液浊度的关系,分析了聚合氯化铝(PAC)对结晶体系出水的除浊效果。此外,还对诱导结晶体系出水pH的变化进行了研究,对结晶产物的晶型进行了分析。本研究成果可为提升高硬度结晶软化效果和降低沉淀污泥的产量提供参考。
全文HTML
-
高硬度水采用CaCl2配制,沉淀剂采用Na2CO3,均为分析纯,国药集团化学试剂有限公司产品。PAC为分析纯,购自天津光复精细化工。实验用水由Millipore Milli-Q Gradient水净化系统(Billerica,MA)制备,电阻率为18.2 MΩ·cm,pH=6.6~6.8。CaCl2溶液和Na2CO3溶液均现用现配,浓度分别为5 mmol·L−1和0.5 mol·L−1。
Fe3O4购自宝能环保科技公司,平均粒径为45 μm。Fe3O4首先用自来水冲洗至上清液基本清澈,然后8%稀硝酸溶液浸泡除去锈渍和杂质,再用超纯水多次冲洗,烘干至恒重后密封备用。
-
结晶反应采用六联搅拌装置进行。往5个1 L烧杯中分别倒入500 mL的CaCl2溶液后投放Fe3O4,投加量分别为0、0.5、2、5、10 g·L−1,其中0 g·L−1称对照组,其余为实验组。烧杯置于六联搅拌装置,500 r·min−1搅拌5 min形成晶种悬浊液。
调整搅拌转速为300 r·min−1,同时往每个烧杯中加入Na2CO3溶液2.5 mL(对应C/Ca摩尔比为0.5),结晶反应开始启动,反应时间10 min。反应结束后静沉30 min,取20 mL上清液2份,一份测定Ca2+浓度(Ca)、pH和浊度,另一份经0.45 μm滤膜过滤后,测滤液Ca2+浓度(Cb),取沉淀结晶产物进行SEM观测和XRD分析。
在5 L烧杯中投入CaCl2溶液2.5 L和10 g·L−1的Fe3O4进行诱导结晶,其他条件同上。反应结束后立即磁分离,剩余结晶溶液分为4份进行PAC混凝除浊,PAC投加量2、5、7、10 mg·L−1。混凝条件为200 r·min−1 30 s+100 r·min−1 5 min+50 r·min−1 5 min。混凝结束后静沉30 min测上清液浊度。
以上实验均在(25±1) ℃下进行,每个样品进行3次重复。
-
pH和浊度采用pH电极(雷磁PHSJ-3C,上海仪电科学)和浊度仪(WGZ-500B,上海昕瑞仪器)测定。Ca2+测定采用EDTA滴定法。结晶产物形态观测和晶型分析分别采用扫描电子显微镜(SU-8020,日立公司)和X射线衍射仪(D8-Advance,布鲁克公司)进行。
实际流化床结晶工艺中,未结晶到晶种表面且随水流出流化床的微小颗粒为微晶。本文由于在烧杯实验条件下,将反应结束后静置30 min仍未沉降的结晶颗粒视为微晶。结晶体系的总结晶率、微晶产率、Ca2+去除率依次按照式(1)、式(2)和式(3)进行计算。
式中:α为总结晶率;β为微晶产率;γ为钙离子去除率;Ca为反应结束后静沉30 min的上清液Ca2+浓度,mmol·L−1;Cb为反应后出水经0.45 μm滤膜过滤后滤液Ca2+浓度,mmol·L−1。
1.1. 实验材料
1.2. 实验方法
1.3. 分析方法
-
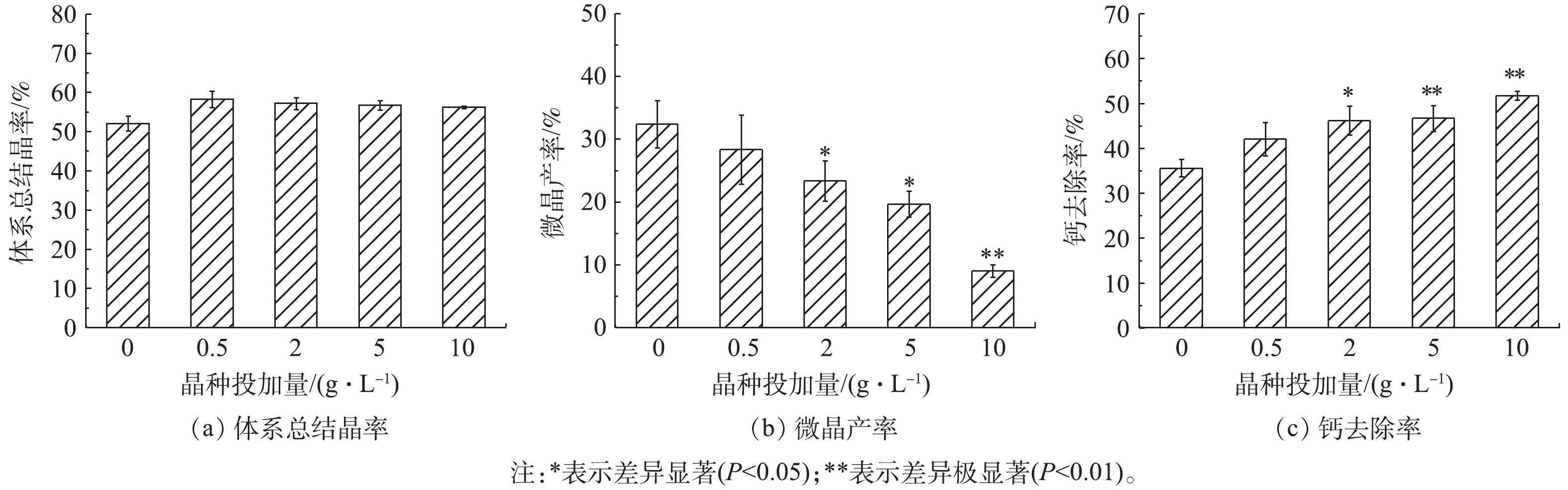
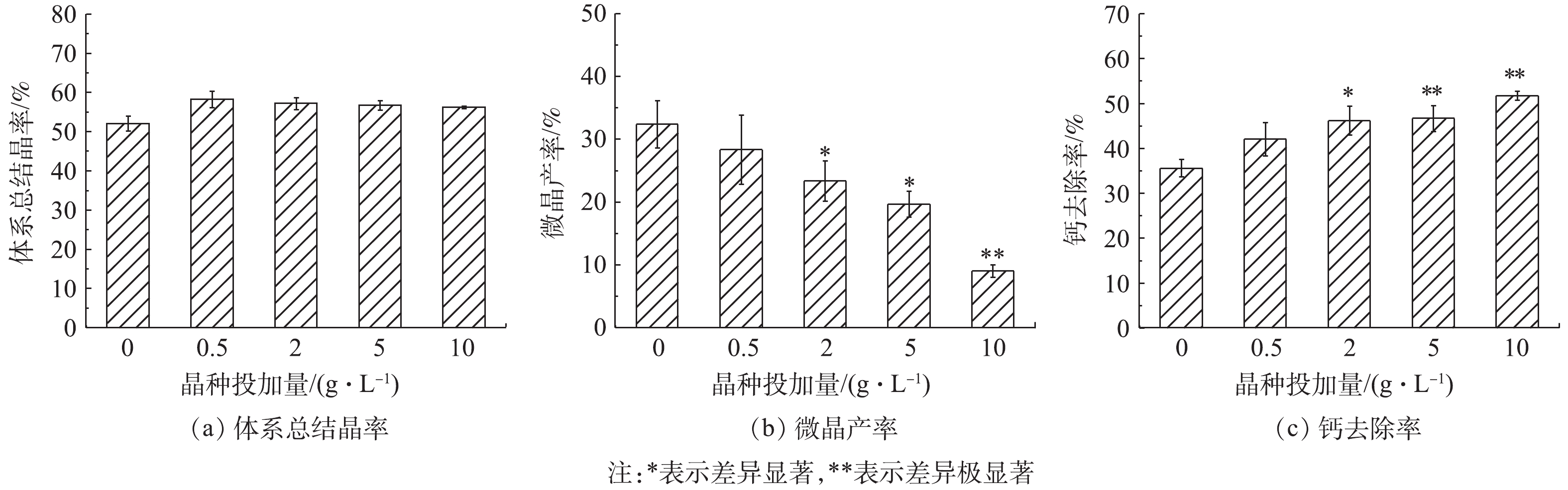
Fe3O4的投加提高了体系总结晶率(图1(a)),但与对照组相比,提高并不显著(P>0.05)。体系微晶产率随Fe3O4投加量增加而降低(图1(b)),与对照组相比,在Fe3O4的投加量2 g·L−1和5 g·L−1时显著降低(P<0.05),在投加量为10 g·L−1时极显著降低(P<0.01)。体系软化效果随Fe3O4投加量增加而提高(图1(c)),与对照组相比,在投加量为2 g·L−1时显著提高(P<0.05),在投加量为5 g·L−1和10 g·L−1时极显著提高(P<0.01)。
总结晶率由结晶体系初始过饱和度S0和临界过饱和度S*之差ΔS决定,ΔS越大,则总结晶率越高。诱导结晶体系中晶种的加入可降低S*[23],但实验中Fe3O4的投加却未引起总结晶率显著提升,说明对照组与实验组结晶体系S*最终处于大致相等水平。根据经典结晶理论,对照组中CaCO3均相结晶过程分成核和晶核成长2个阶段[24]。实验中对照组S0较高(S0=3 775,以方解石Ksp计),可以认为成核过程耗时较短,后续均为晶核成长过程,体系残余构晶离子(Ca2+和
CO2−3 )在晶核表面进行表面结晶,这一过程与诱导结晶的区别仅在于前者晶种为成核阶段生成的晶核,后者晶种为外投Fe3O4。正是因为对照组与实验组ΔS无显著差异,相应总结晶率也无显著差异。对照组钙的总结晶率为(52.0±1.6)%,甚至高于理论值(实验中C/Ca摩尔比=0.5,理论总结晶率50%),这可能是因为来自大气中的CO2经水合、去质子后可提供少量结晶所需构晶离子
CO2−3 [25],所以总结晶率略高于理论值。而实验组的总结晶率均高于理论值,可能是因为Fe3O4的诱导结晶导致体系碱度低于均相结晶体系,相应大气中CO2的补充更明显。在对照组的均相结晶过程中,晶核的形成消耗了大量的构晶离子,导致晶核成长动力不足,最终产物粒度较小,因而固液分离性能不佳,有(32.3±3.1)%的结晶产物即微晶无法顺利沉降。实验组微晶产率随着Fe3O4投加量的增加明显降低,当Fe3O4的投加量为10 g·L−1时,微晶产率仅为(9.0±0.8)%。Fe3O4的投加对微晶产率的削减作用:一方面,因为Fe3O4诱导结晶与均相成核对构晶离子的竞争,降低了均相成核规模,相应微晶数量降低;另一方面,由于Fe3O4诱导结晶的竞争,均相成核期间体系过饱和度小于对照组。GAREA等[8]研究表明,过饱和度越低,均相成核生成的晶核尺寸越大,沉降分离性能越好。
在实际高硬水软化工程中,为了获得预期的硬度去除效果,往往不得不过量投加沉淀剂(
CO2−3 或OH−)或延长结晶反应时间[26],这与图1(c)中显示结果一致:当C/Ca摩尔比为0.5时,由于微晶的形成,对照组钙硬度实际去除率仅为(35.6±1.6)%,远小于理论值50%,此时若要提高软化效果,只有增加CO2−3 投加量或延长反应时间,以增大结晶产物粒径,降低微晶产率。在实验组中投加Fe3O4后,微晶产率得到有效控制,钙去除率显著提升,当Fe3O4投加量为10 g·L−1时,去除率为(51.7±0.8)%,甚至略高于理论值。 -
由图2可知,与对照组相比,在不同Fe3O4投加量的实验组中,结晶体系出水pH的变化均不显著 (P>0.05);但浊度却得到显著降低(P<0.05),Fe3O4投加量越大,浊度降低越明显,当Fe3O4投加量为5 g·L−1和10 g·L−1时,降低为极显著(P<0.01)。
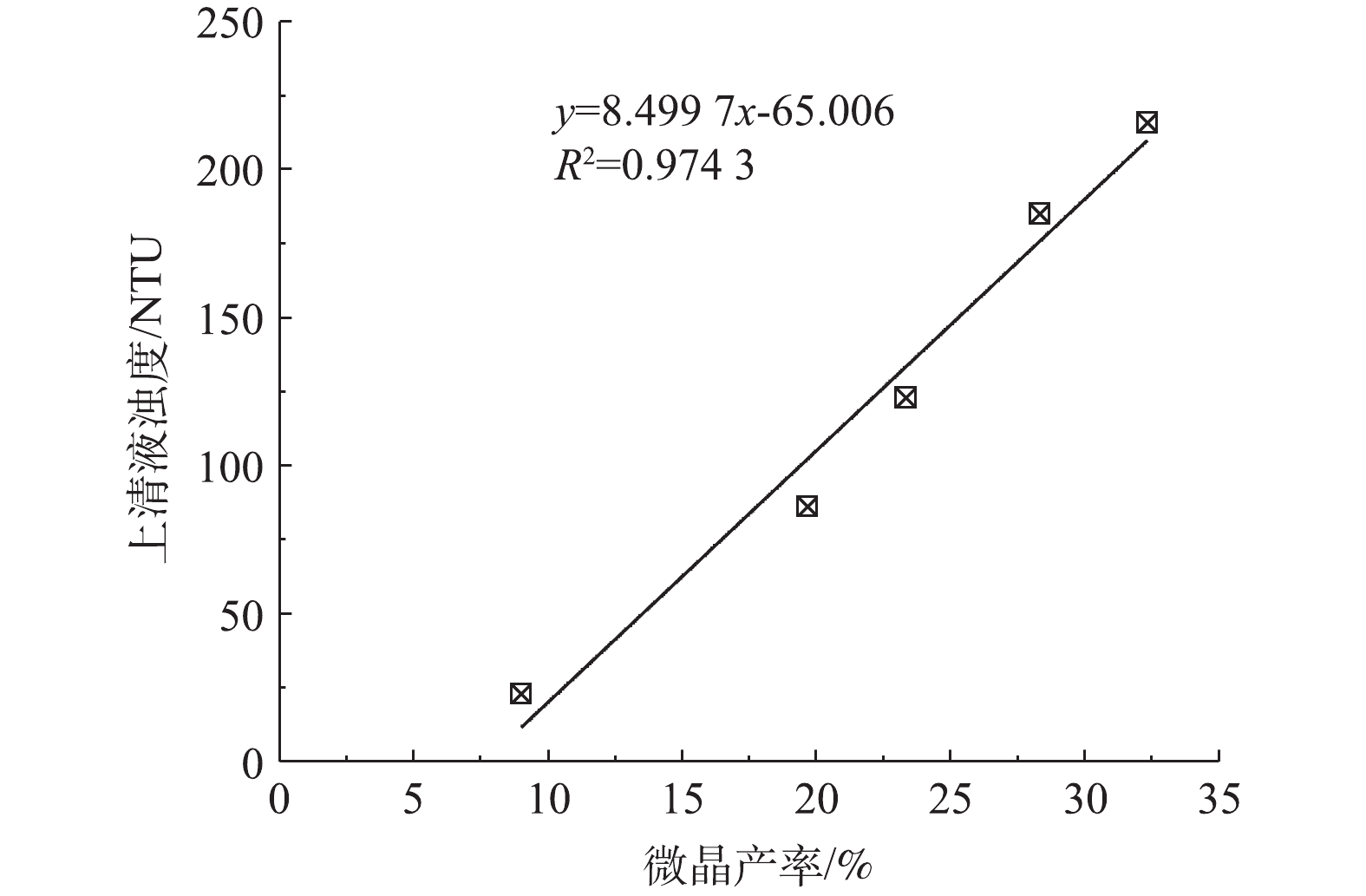
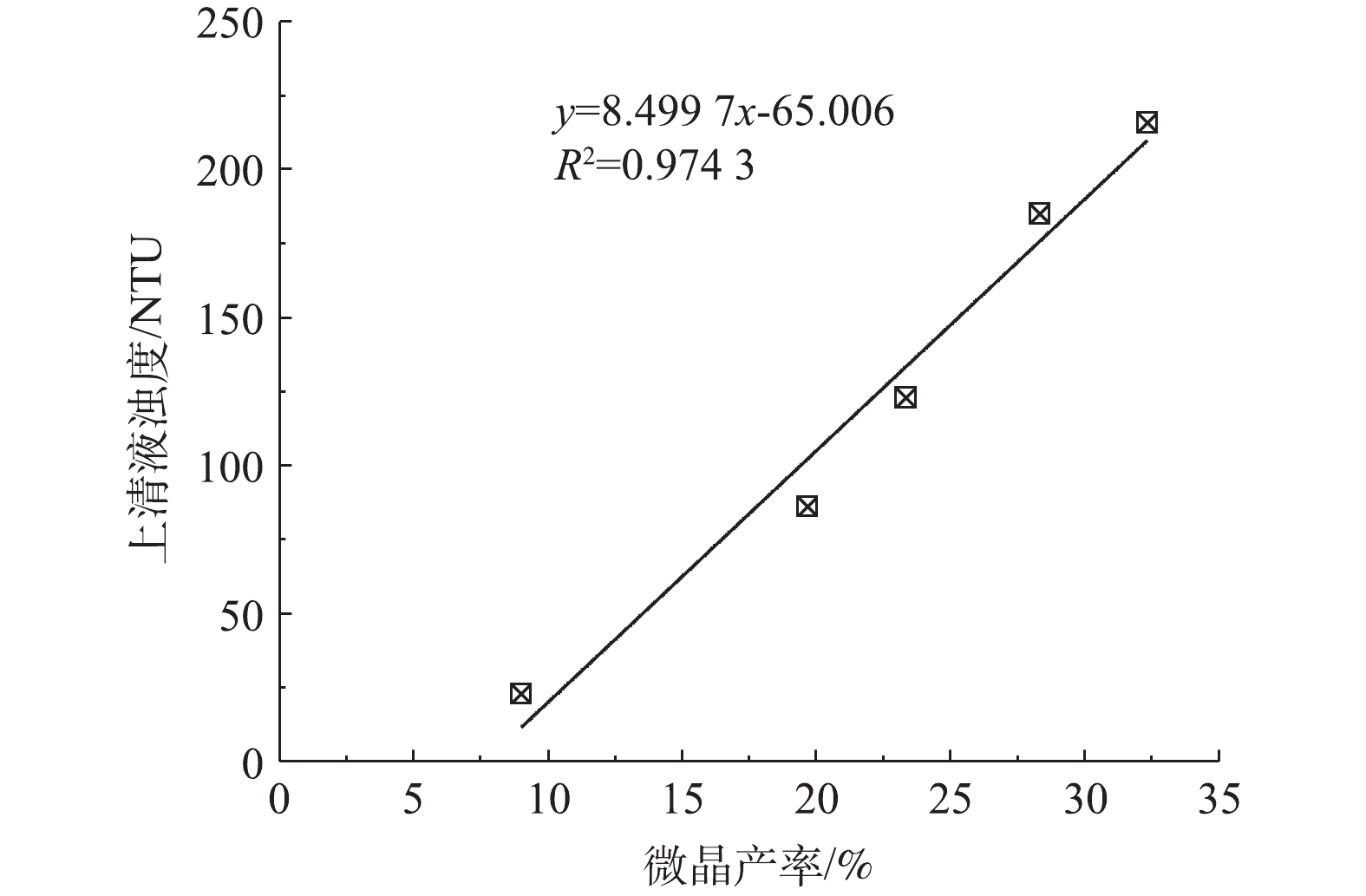
在前面的讨论中已经指出,Fe3O4诱导结晶对体系总结晶率的影响较小,在相同初始Ca2+浓度和C/Ca摩尔比的条件下,这就意味着最终结晶体系
CO2−3 浓度及其与总无机碳浓度摩尔比基本不变,因此,结晶体系出水pH也基本不变。由于Fe3O4诱导结晶优良的微晶控制效果,结晶体系上清液浊度改善明显,这就可以显著降低后续混凝所需混凝剂投量,减小污泥产量。结晶体系上清液浊度与微晶产率的拟合关系表明,两者呈明显正相关关系(图3)。需要指出的是,尽管与对照组相比,Fe3O4诱导结晶体系出水pH无明显降低,仍高于《生活饮用水卫生标准》(GB 5749-2006)规定的8.5,但Fe3O4诱导结晶仍有望用于降低结晶体系出水pH。这是因为相同结晶条件下,Fe3O4诱导结晶提高了钙硬度去除率,若要获得相同软化效果,相应沉淀剂
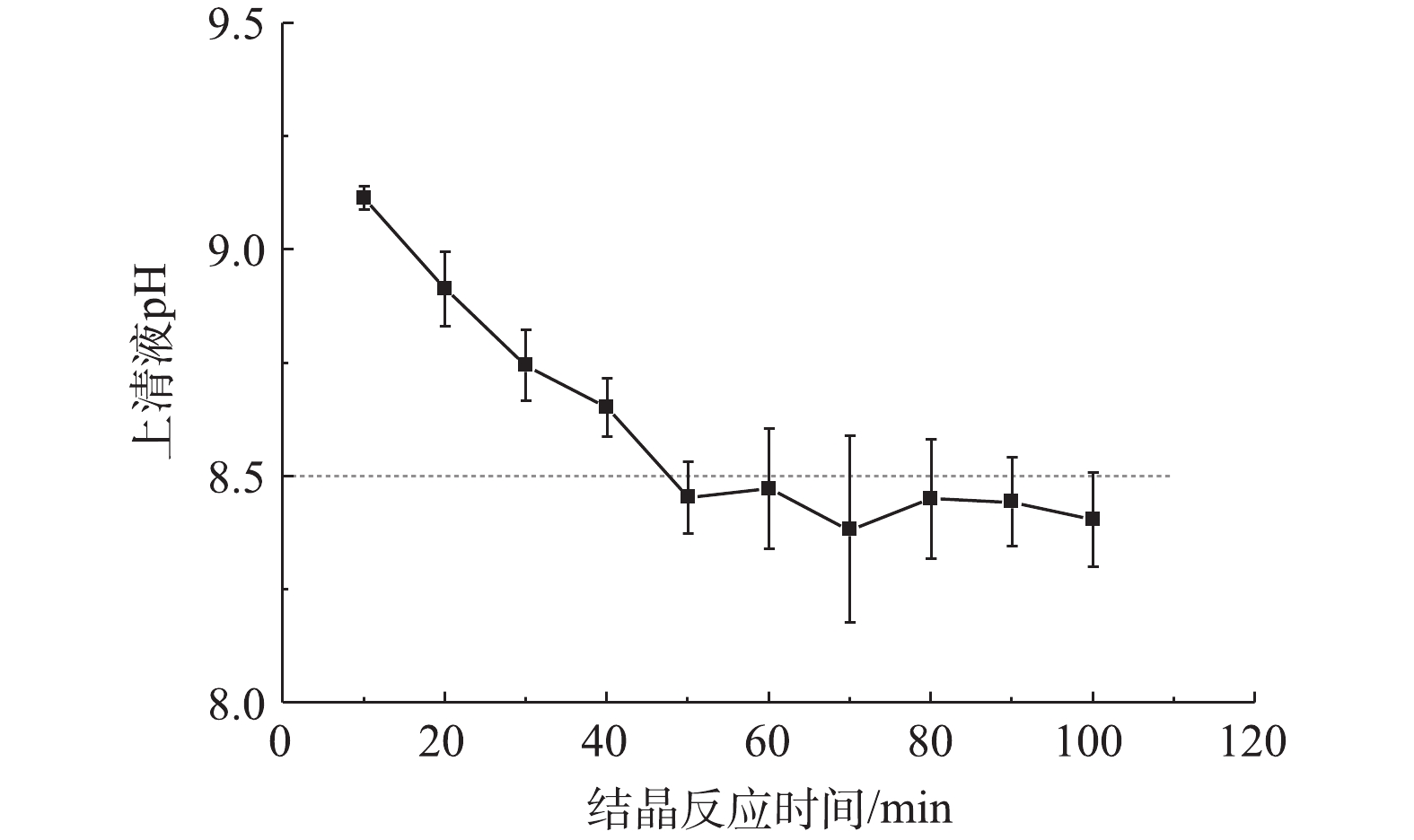
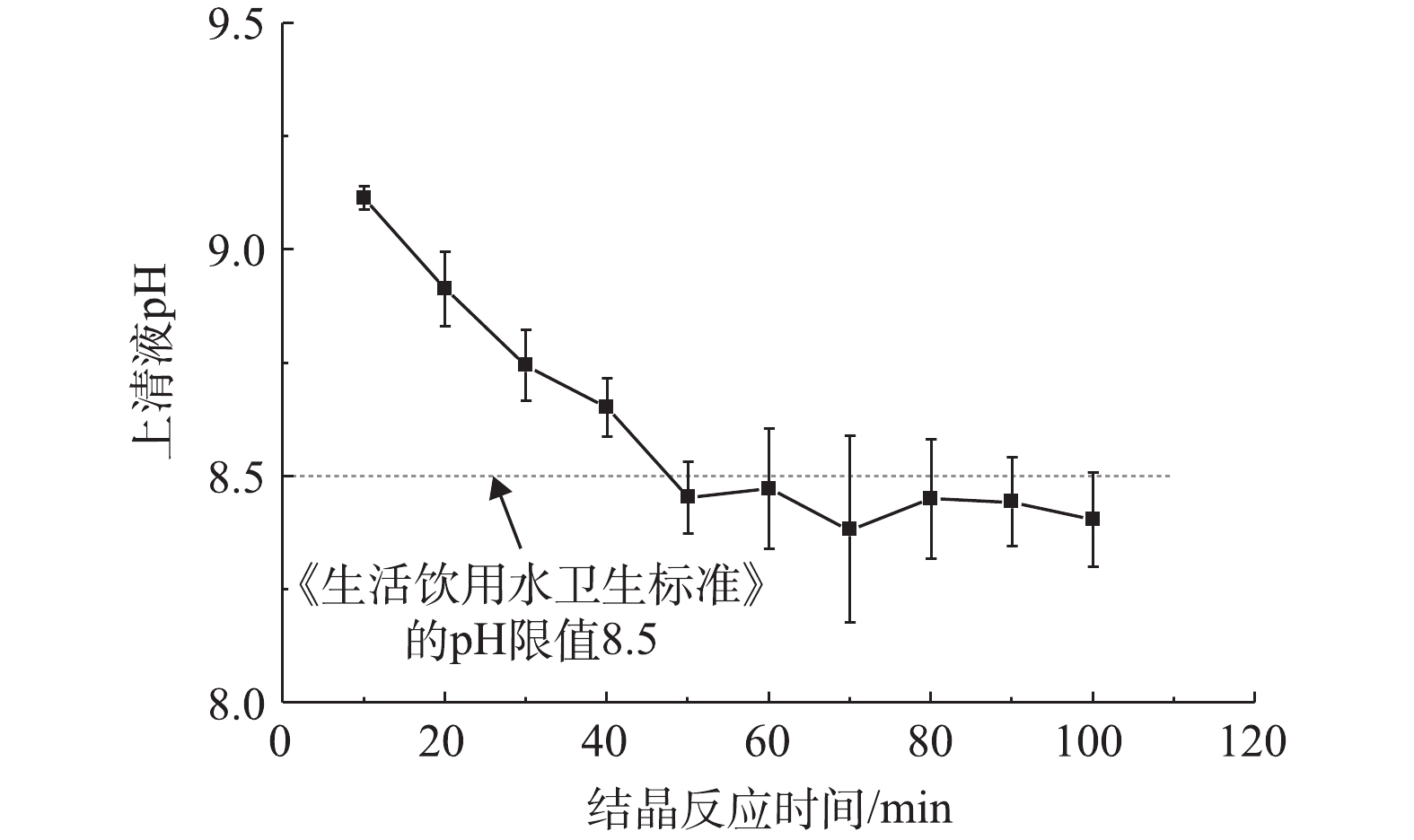
CO2−3 或OH−用量可以减小,这将有效降低出水pH。若要进一步降低诱导结晶体系出水pH,可以适当延长结晶反应时间,如图4所示。当Fe3O4投加量为10 g·L−1时,随着结晶时间的延长,残余构晶离子
CO2−3 浓度继续降低,体系中HCO−3 去质子化过程加强,pH逐渐降低。结晶时间超过50 min后pH稳定在8.38~8.47,达到GB 5749-2006的要求。 -
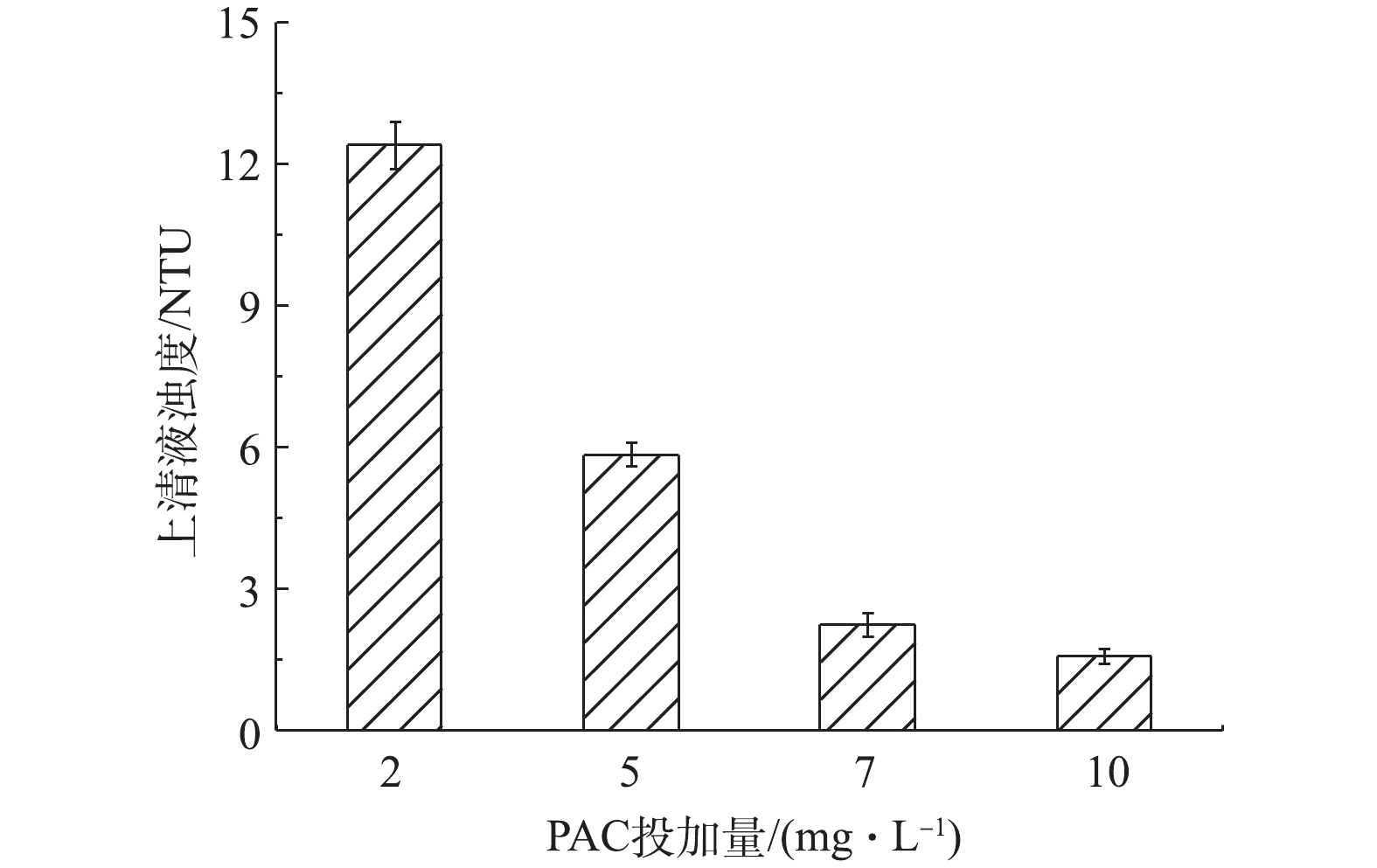
磁分离后结晶体系上清液浊度在40 NTU左右,此时浊度绝大部分由同步发生的均相结晶产物引起。经PAC混凝并静沉30 min后浊度显著降低,且PAC投加量越大,效果越明显(图5)。当PAC投加量为7 mg·L−1,浊度仅为(2.2±0.2) NTU。
混凝沉淀对结晶体系出水除浊效果有限的问题一直困扰高硬度软化。特别是当以石灰为软化剂时,为确保除浊效果,往往不得不加大混凝剂投加量,产生大量沉淀污泥,即便如此,沉淀后出水浊度仍然偏高,容易堵塞滤池。黄明珠等[4]采用“石灰+PAC”软化总硬度为278.5~298.4 mg·L−1的地下水,反应后静沉30 min后,总硬度去除率为50%左右,上清液浊度高达300 NTU以上,经40 mg·L−1的PAC混沉后,浊度仍约为10 NTU。当采用纯碱替代部分石灰时,浊度明显降低。张浩程[27]的研究表明,当石灰、纯碱和混凝剂(PAC)的投加量分别为80~120、30~40和 5~9 mg·L−1时,沉淀后出水浊度可以控制在6.4~12.7 NTU。
本文中实验组由于诱导结晶的竞争,均相结晶得以在较低过饱和度下进行,因而均相结晶产物与对照组相比,数量密度有所降低、粒径增加、微晶产率降低,上清液浊度得到改善(图2(b))。当Fe3O4投加量为10 g·L−1时,不经静沉仅磁分离浊度就可降至40 NTU左右(静沉30 min后进一步降至(22.7±3.9) NTU,见图2(b))。磁分离后悬浊液再经7 mg·L−1的PAC混凝并静沉30 min后,上清液浊度降至(2.2±0.2) NTU,除浊效果优良。
-
图6给出了当Fe3O4投加量为0、0.5和10 g·L−1时结晶产物的扫描电镜图。当Fe3O4投加量为0 g·L−1时,为均相结晶,产物绝大多数是生长完全的斜方六面体或正方体颗粒,晶型完整,表面光滑,粒径为3~7 μm (图6(a))。当Fe3O4投加量0.5 g·L−1时,均相结晶产物数量明显减小(图6(b)),表明Fe3O4诱导结晶一定程度抑制了均相结晶。同时,均相结晶产物仍呈规则斜方六面体或正方体,粒径也不超过10 μm。当继续增大Fe3O4投加量至10 g·L−1时,只有少量均相结晶产物出现,且规则性状逐渐消失(图6(c)),说明均相结晶过程明显被Fe3O4诱导结晶抑制。
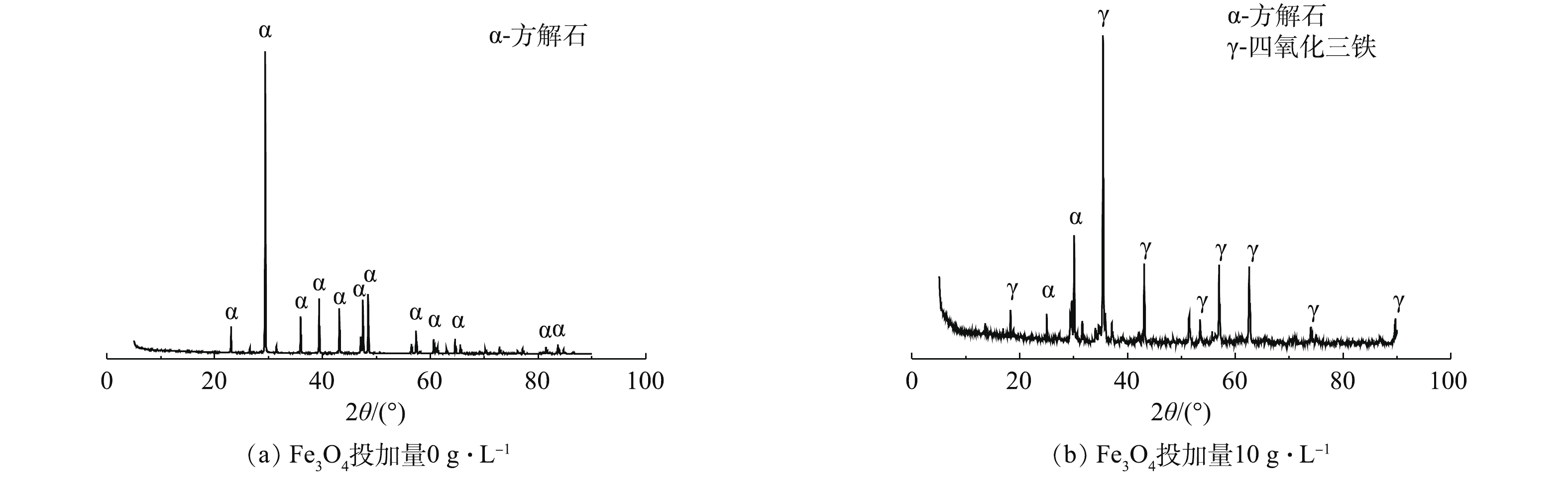
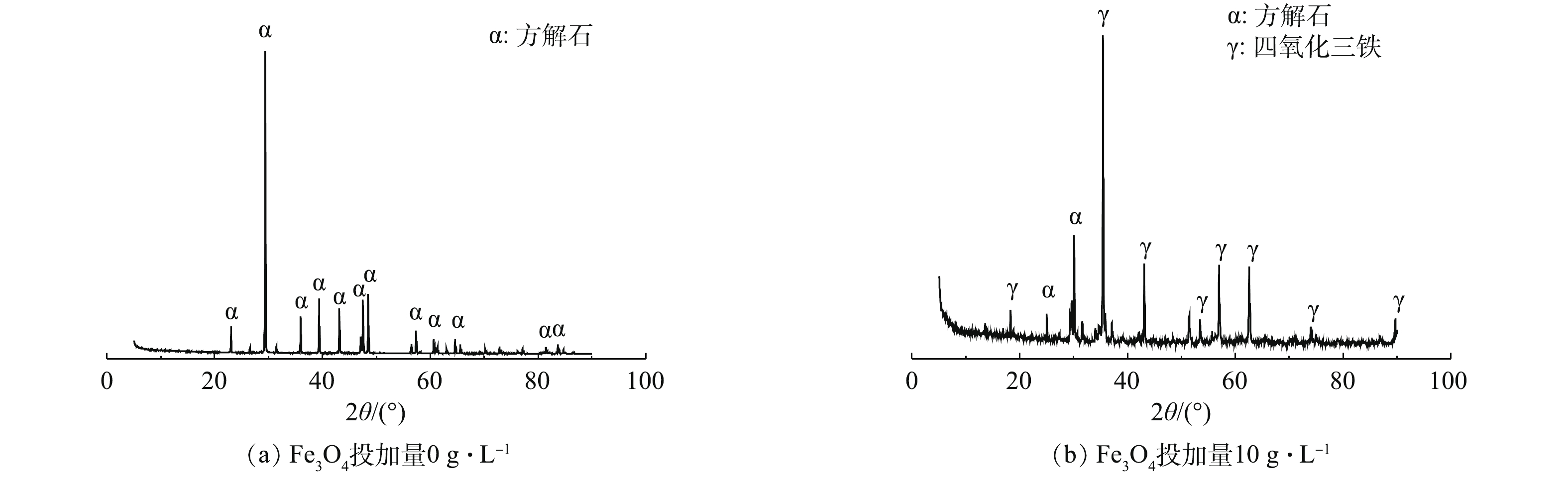
图7给出了均相结晶产物(Fe3O4投加量为0 g·L−1)和Fe3O4诱导结晶产物(Fe3O4投加量为10 g·L−1)的XRD谱图。均相结晶产物的晶型均为方解石(图7(a))。由图7(b)可知,Fe3O4表面的诱导结晶产物晶型同样为方解石。
2.1. Fe3O4投加量对结晶与软化效果的影响
2.2. Fe3O4投加量对结晶体系出水pH和浊度的影响
2.3. Fe3O4诱导结晶体系出水混凝除浊效果
2.4. Fe3O4诱导结晶产物电镜和XRD分析
-
1)与均相结晶软化相比,采用Fe3O4诱导结晶进行高硬水的软化,不会引起体系总结晶率和出水pH的明显变化;但Fe3O4诱导结晶可明显抑制均相结晶,从而降低结晶体系的微晶产率,进而提高软化效果。当Fe3O4投加量为10 g·L−1、C/Ca摩尔比为0.5时,微晶产率仅为(9.0±0.8)%,钙硬度去除率为(51.7±0.8)%,略高于理论值。
2) Fe3O4诱导结晶可以显著降低结晶体系悬浊液浊度,当Fe3O4投加量由0 g·L−1增加至10 g·L−1时,结晶体系经30 min静沉后,浊度可由215.9 NTU降至22.7 NTU;同时Fe3O4诱导结晶悬浊液的混凝除浊性能良好,当Fe3O4投加量为10 g·L−1时,结晶体系经磁分离后,悬浊液采用7 mg·L−1的PAC混凝,浊度就可由40 NTU左右降至2.2 NTU。
3)均相结晶和Fe3O4诱导结晶产物晶型均为方解石。均相结晶结晶产物数量密度随着Fe3O4投加量的增加而减小。





 下载:
下载: