-
近年来我国水产事业快速发展,养殖品种产量日益增加[1]。在养殖水体中培养微生物絮团的生物絮凝已成为集约化水产养殖的热点[2-3],生物絮凝是利用养殖环境系统中微生物絮团吸收转化水中有害含氮无机物,减少水环境对养殖鱼类的毒害[4-5]。但生物絮凝系统的养殖水磷酸盐高于20 mg·L−1[6],且碳酸氢根(
HCO−3 )、硝氮(NO−3 -N)浓度普遍较高[2-3]。直接排放养殖水易造成湖泊富营养化,环境污染[7]。因此,对养殖水进行除磷是必要的。目前,养殖水除磷技术主要有人工湿地除磷[8]、生物除磷[9]、吸附法[10],吸附法因具有高效快速、易操作、成本廉价、无二次污染等特点而成为研究热点[11]。废弃牡蛎壳因其获取便利,独特的多孔结构[12],被视为一种天然的除磷吸附剂。李林锋等[13]发现天然牡蛎壳吸附除磷的最大吸附量为0.88 mg·g−1。浦晨霞等[14]将牡蛎壳进行高温改性,对总磷的去除率可达90%,吸附量为1.10 mg·g−1。改性有助于提高牡蛎壳吸附效率,通常含铁氧化物改性制备的吸附剂吸附除磷效果更佳[15],目前,含铁化合物改性牡蛎壳吸附除磷鲜有报道。因此,本文选择牡蛎壳粉进行载铁改性,制成载铁牡蛎壳粉(magnetic modified oyster shell powder, magnetic modified OSP),探究了吸附剂添加量、初始总磷(total phosphorus, TP)浓度、pH对载铁牡蛎壳粉在模拟含磷水中吸附除磷的影响,并对其进行了表征,同时选择生物絮凝系统水中含量较多的
HCO−3 、NO−3 -N作为共存离子,考察了共存离子对吸附除磷的影响。通过吸附热动力学探讨了其除磷的吸附机理,以期为实际生物絮凝养殖废水除磷提供参考。
全文HTML
-
载铁牡蛎壳粉制备:取自中国辽宁省某海域海滩上直径7~8 cm废弃天然牡蛎壳,多次清洗,表面洗净后晾干;用中药粉碎机粉碎过100 μm备用。分别取20.00 g牡蛎壳粉,20.00 g FeCl3·6H2O,11.10 g FeSO4·7H2O溶解于800 mL超纯水,充分搅拌均匀,并逐滴加入10.00 mol·L−1 NaOH至混合液pH为10.00,随后在350 r·min−1条件下搅拌1 h,静置1 d后移去上清液,水洗沉淀物至中性,70 ℃干燥24 h,备用[16]。使用分析纯级别的磷酸二氢钾(KH2PO4)、碳酸氢钠(NaHCO3)、硝酸钾(KNO3),分别和超纯水配制溶液。
-
1)吸附剂添加量对除磷效果的影响。于若干250 mL锥形瓶分别加入50 mL,初始TP浓度为20.00 mg·L−1的模拟含磷水,调节初始pH为4.00~6.00,将不同添加量2.00、4.00、6.00、8.00、10.00、12.00、14.00、16.00、18.00、20.00 g·L−1载铁牡蛎壳粉分别装入锥形瓶。混合后在25 ℃,180 r·min−1下振荡24 h,待吸附平衡后,取上清液测定TP,计算TP去除率(η)、吸附量(qe),每个添加量3个平行。
2)初始TP浓度对载铁牡蛎壳粉吸附除磷效果的影响。于若干250 mL锥形瓶分别加入50 mL,初始TP浓度为10.00、20.00、30.00、40.00、50.00、60.00、70.00、80.00、90.00、100.00 mg·L−1的模拟含磷水,调节初始pH为4.00~6.00,准确称取8.00 g·L−1载铁牡蛎壳粉分别装入锥形瓶。混合后在25 ℃,180 r·min−1下振荡24 h,待吸附平衡后,取上清液测定TP,计算TP去除率、吸附量,每个初始TP浓度3个平行。
3) pH对载铁牡蛎壳粉吸附除磷效果的影响。于若干250 mL锥形瓶分别加入50 mL,初始TP浓度为50.00 mg·L−1的模拟含磷水,0.01 mol·L−1 HCl和0.01 mol·L−1 NaOH调节溶液pH初始为2.00、4.00、6.00、8.00、10.00、12.00,准确称取8.00 g·L−1载铁牡蛎壳粉分别装入锥形瓶。混合后在25 ℃,180 r·min−1下振荡24 h,待吸附平衡后,取上清液测定TP,计算TP去除率、吸附量,每个pH 3个平行。
4)共存离子对载铁牡蛎壳粉吸附除磷效果的影响。于若干250 mL锥形瓶分别加入50 mL,初始
HCO−3 、NO−3 -N分别为50.00、100.00、150.00、200.00 mg·L−1,初始TP浓度为50.00 mg·L−1的模拟含磷水,调节初始pH为4.00~6.00,准确称取8.00 g·L−1载铁牡蛎壳粉分别装入锥形瓶。混合后在25 ℃,180 r·min−1下振荡24 h,待吸附平衡后,取上清液测定TP,计算TP去除率、吸附量,每个浓度3个平行。TP去除率和吸附量根据式(1)和式(2)计算。式中:η为TP去除率;C0、Ce分别为TP的初始浓度和吸附平衡浓度,mg·L−1;qe为平衡吸附剂量,mg·g−1;V为水样体积,mL;m为吸附剂质量,g。
5)载铁牡蛎壳粉的解吸。于250 mL锥形瓶中加入50 mL初始TP浓度为50.00 mg·L−1的模拟含磷水,调节初始pH为4.00~6.00,准确称取8.00 g·L−1载铁牡蛎壳粉装入锥形瓶。混合后在25 ℃、180 r·min−1条件下振荡24 h,待吸附平衡后,取上清液测定溶液中TP,计算吸附量;将经吸附的载铁牡蛎壳粉105 ℃烘干数小时,再分别加入0.01、0.10、1.00 mol·L−1 NaOH溶液,混合后于25 ℃,180 r·min−1条件下振荡24 h,待吸附平衡后,取上清液测定溶液中TP浓度Cd、解吸量,解吸率(qd/qe),每个浓度3个平行。
6)吸附热力学实验。于若干250 mL锥形瓶中分别加入50 mL,初始TP浓度为26.40、36.95、47.42、61.17、71.08 mg·L−1的模拟含磷水,调节初始pH为4.00~6.00,分别准确装入8.00 g·L−1载铁牡蛎粉,以180 r·min−1分别在15、25、35 ℃下恒温振荡24 h,待吸附平衡后,取上清液测定TP、吸附量、热力学参数。每个初始TP浓度3个平行。采用Langmuir(式(3))、Freundlich(式(4))方程进行线性拟合,通过式(5)、式(6)、式(7)分别计算出固液分配系数、吉布斯自由能、标准反应焓变、标准反应熵。
式中:K为Langmuir吸附平衡常数;qm为最大吸附量,mg·g−1;Kf为Freundlich吸附平衡常数;KD为固液分配系数,mL·g−1;C0为溶液初始浓度,mg·L−1;V为溶液体积,mL;m为吸附剂质量,m;G0为吉布斯自由能,kJ·mol−1;H0为标准反应焓变,kJ·mol−1;S0为标准反应熵,kJ·(mol·K)−1;R为理想气体常数,kJ·(mol·K)−1;T为热力学温度,K。
7)吸附动力学实验。于若干250 mL锥形瓶中分别加入50 mL初始TP浓度为50.00 mg·L−1的模拟含磷水,调节初始pH为4.00~6.00,分别准确装入8.00 g·L−1载铁牡蛎壳粉。在15、25、35 ℃下以180 r·min−1振荡,于0、10、30、60、120、240、480、720、1 080、1 440 min取样,取上清液测定TP,计算载铁牡蛎壳粉的即时吸附量、吸附活化能,每个温度梯度3个平行。采用准一级动力学方程(式(8))、准二级动力学方程(式(9))以及颗粒内扩散动力学方程(式(10))进行线性模型拟合,并通过式(11)计算吸附活化能。
式中:qt为t时刻吸附量,mg·g−1;k1为准一级动力学速率常数,g·(min·mg)−1;k2为准二级动力学速率常数,g·(min·mg)−1;kp为颗粒内扩散速率常数,g·(min·mg1/2)−1;R为摩尔气体常数,kJ·(mol·K)−1;C为界面层厚度相关系数,mg·g−1;k0为表现频率因子,g·(min·mg)−1;Ea为吸附活化能,kJ·mol−1。
-
pH通过WTW(Multi 3430,德国)测定;
NO−3 -N采用紫外分光光度法;TP采用钼锑抗分光光度法,HCO−3 采用酸碱滴定指示法[17];使用比表面积及孔径分析仪(ASAP,Micromeritics,美国)分析测定壳粉比表面积;使用扫描电子显微镜(SEM,赛默飞FEG-250,美国)分析壳粉形貌;使用X射线衍射(XRD,smartlab ragiku 2019,理学电机公司,日本)分析壳粉晶体结构。 -
实验数据采用Excel进行结果统计,用Origin 9和Adobe Illustrator CC 2019 进行图表绘制。采用SPSS 22.0统计软件对数据进行ANOVA 单因素方差分析,P<0.05为差异性显著,实验数值用平均值±标准差形式表示。
1.1. 材料
1.2. 实验方法
1.3. 指标测定
1.4. 数据分析
-
图1反映了吸附剂添加量、初始TP浓度及pH对载铁牡蛎壳粉吸附除磷的影响。由图1(a)可知,随着载铁牡蛎壳粉添加量的增加,TP去除率上升,吸附量持续下降。当添加量从2.00 g·L−1增至10.00 g·L−1时,TP去除率由(33.05±5.71)%升至(84.61±1.47)%,这是由于壳粉和模拟水的接触面积增加,导致TP去除率迅速升高。但当添加量从10.00 g·L−1增至20.00 g·L−1时,TP去除率变化趋于稳定,为(81.74±1.47)%~(85.61±6.23)%,吸附量由(3.45±0.60) mg·g−1持续降至(0.89±0.01) mg·g−1。虽然高添加量提高了TP去除率,但过多的吸附剂相互掩饰吸附位点,影响吸附位点与磷分子的结合,从而造成吸附量下降,这和梁越敢等[18]的研究结果相似。结果表明,初始TP浓度为 20 mg·L−1时,载铁牡蛎壳粉的最佳添加量为8.00 g·L−1。
-
由图1(b)可知,随着初始TP浓度增大,TP去除率先上升后下降。当初始TP浓度从5.00 mg·L−1增至20.00 mg·L−1时,TP去除率由(44.90±4.16)%迅速升至(84.91±0.94)%;初始TP浓度由20.00 mg·L−1增至50.00 mg·L−1时,TP去除率由(84.91±0.94)%升至(87.35±1.06)%;当初始TP浓度从50.00 mg·L−1增至100.00 mg·L−1时,TP去除率由(87.35±1.06)%降至(74.65±1.38)%。以上结果表明,当溶液中初始TP浓度低于20.00 mg·L−1时,8.00 g·L−1添加量的载铁牡蛎壳粉上吸附位点多,去除率上升迅速,随着吸附的进行,载铁牡蛎壳粉上吸附位点被占据,活性吸附位点减少,吸附接近饱和[19],表现为TP去除率上升逐渐缓慢。随着初始TP浓度增大,吸附位点相对减少,当初始TP浓度为100.00 mg·L−1中,过多的磷分子互相竞争吸附位点,导致TP去除率下降,因此,载铁牡蛎壳粉吸附效率降低。
随着初始TP浓度从5.00 mg·L−1增至100.00 mg·L−1,载铁牡蛎壳粉的吸附量由(0.38±0.04) mg·g−1持续升至(9.87±0.06) mg·g−1。这可能是吸附位点结合了大量的磷分子后,壳粉上活性吸附位点减少,TP去除率下降,但由于溶液中TP浓度过高,吸附去除的TP浓度之差相对较大。因此,吸附量相对上升。结果表明,8.00 g·L−1载铁牡蛎壳粉在初始TP浓度为20.00~50.00 mg·L−1的模拟水中除磷效果最佳。
-
当初始TP浓度为50.00 mg·L−1时,添加量为8.00 g·L−1的载体牡蛎壳粉在酸性条件下载铁牡蛎壳粉的TP去除率、吸附量均高于碱性条件下,由图1(c)可知,当pH在2.00~6.00时,载铁牡蛎壳粉的TP去除率均高于80%。由式(12)~式(14)可知,在酸性条件下,溶液中磷主要以
H2PO−4 和HPO2−4 形式存在[20]。载铁牡蛎壳粉表面具有氧化铁层,氧化铁层在水溶液中容易发生羟基化,这使得吸附位点更具活性,溶液中H2PO−4 、HPO2−4 与吸附位点上的羟基氧化物配位交换[21],表现为TP去除率、吸附量升高。同时,载铁牡蛎壳粉通过静电作用吸附H2PO−4 和HPO2−4 ;当pH为2.00时,TP去除率则高达(95.00±2.02)%,pH为2.00~12.00时,TP去除率下降明显,由(95.00±2.02)%降至(14.67±5.87)%。pH从2.00升至4.00时,吸附量由(2.04±0.02) mg·g−1升至(2.49±0.36) mg·g−1。但随着pH从4.00升至12.00时,吸附量持续降至(0.43±0.24) mg·g−1。随着pH升高,溶液中OH−浓度越来越高,OH−和H2PO−4 、HPO2−4 的相互竞争吸附使得载铁牡蛎壳粉除磷效率降低、吸附量下降。碱性条件使得载铁牡蛎壳粉的等电点为负,静电作用吸附能力下降,TP去除率、吸附量降低[21]。 -
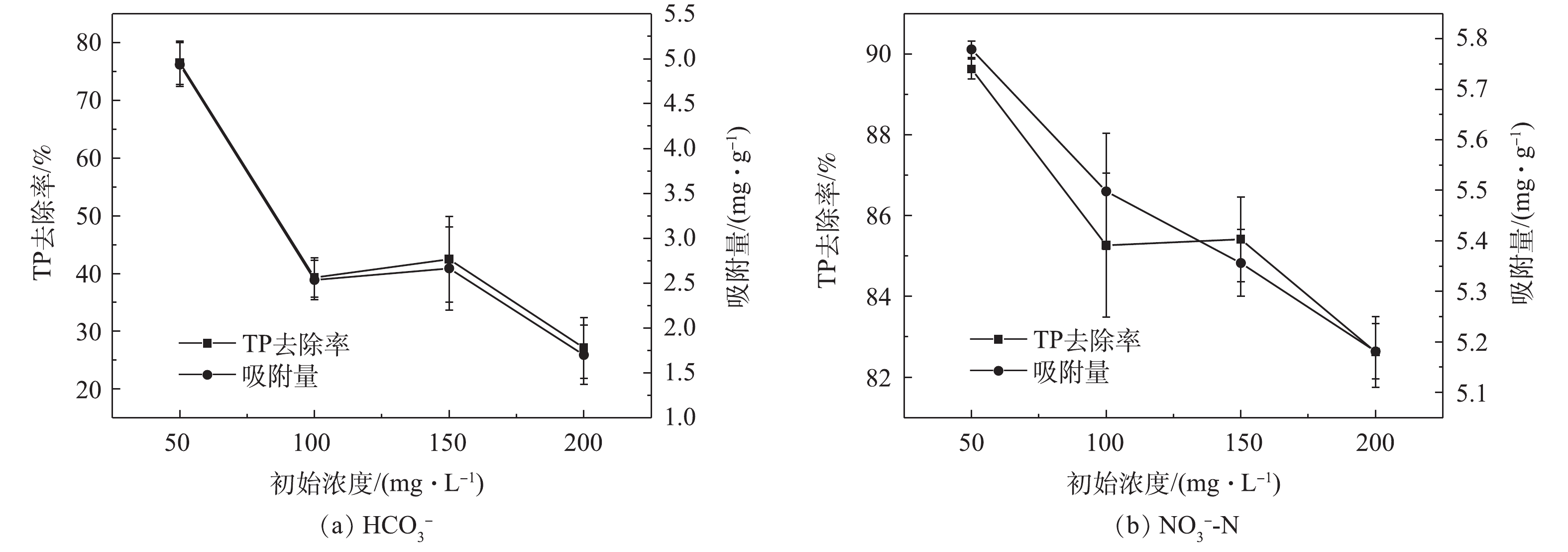
HCO−3 的存在对载铁牡蛎壳粉吸附除磷有明显的抑制作用,由图2(a)可知,当HCO−3 存在时,TP去除率和吸附量均低于(76.54±3.75)%和(4.94±0.24) mg·g−1。当HCO−3 为50.00~200.00 mg·L−1时,TP去除率和吸附量均随着HCO−3 的升高而下降,TP去除率从(76.54±3.75)%下降至(27.11±5.28)%,吸附量从(4.94±0.24) mg·g−1降至(1.70±0.33) mg·g−1。这可能是HCO−3 与壳粉上Fe配位的阴离子发生了交换反应,影响了H2PO−4 、HPO2−4 与壳粉上Fe配位的阴离子发生的交换反应[22],HCO−3 和H2PO−4 、HPO2−4 相互竞争吸附位点。有研究[22]表明,HCO−3 与吸附剂之间的结合力强于H2PO−4 、HPO2−4 与吸附剂之间的结合力,吸附剂上的活性吸附位点优先与HCO−3 结合。因此,当共存离子为HCO−3 时,载铁牡蛎壳粉的TP去除率、吸附量降低,这和梁越敢等[18]和王正芳[21]的研究结果相似。NO−3 -N的存在对载铁牡蛎壳粉吸附除磷的影响较小,由图2(b)可知,当NO−3 -N存在时,TP去除率、吸附量均高于(82.62±0.87)%和(5.18±0.05) mg·g−1。实际生物絮凝系统的养殖废水HCO−3 在100~150 mg·L−1,HCO−3 竞争吸附位点,载铁牡蛎壳粉吸附除磷效率降低。因此未来探究载铁牡蛎壳粉在实际生物絮凝养殖废水中吸附除磷的效果,需先进行养殖废水硝化前处理,降低HCO−3 。 -
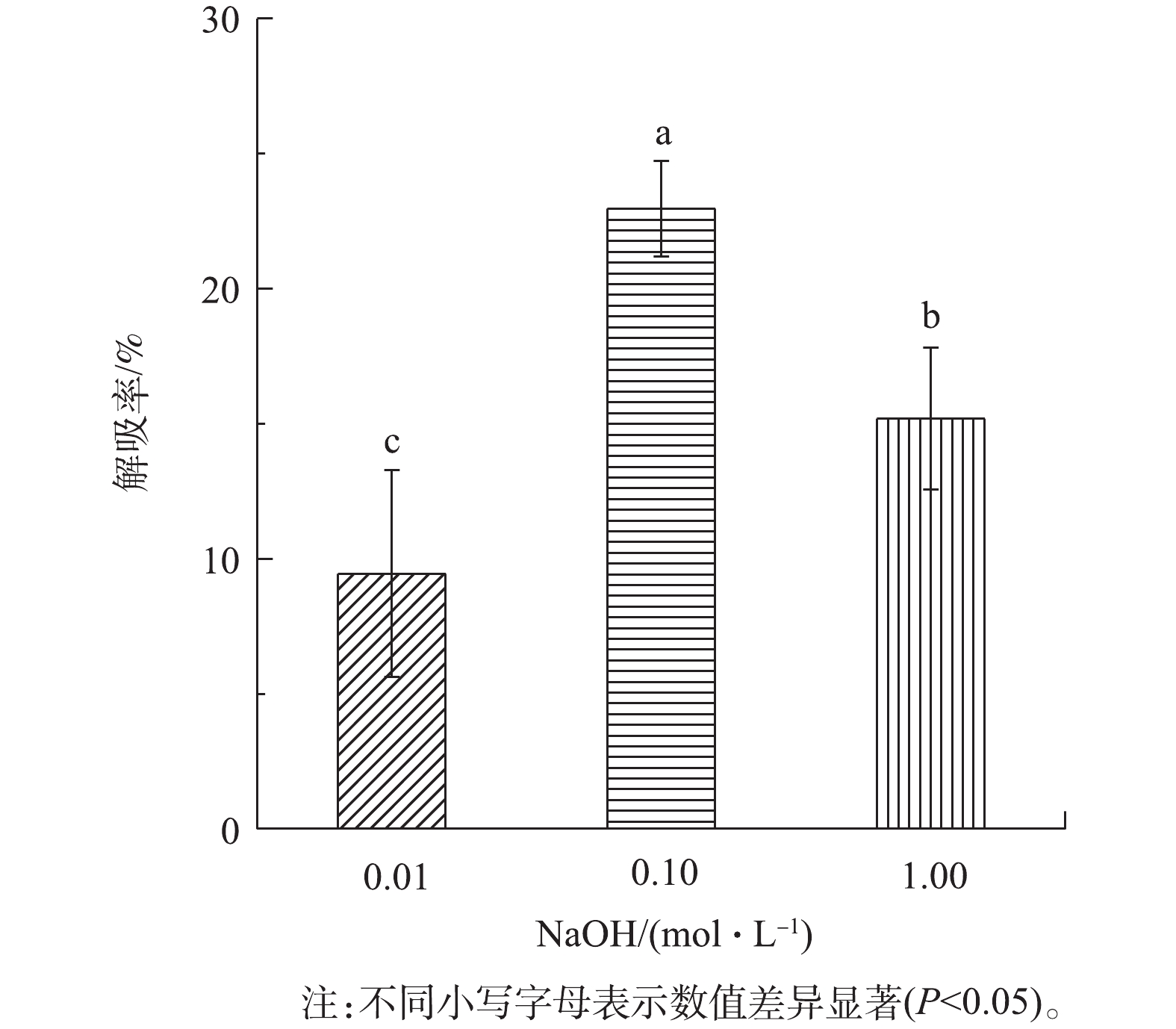
由图3可知,0.10 mol·L−1 NaOH的解吸率为(22.97±1.77)%,显著均高于0.01、1.00 mol·L−1 NaOH的解吸率(P<0.05),表明0.10 mol·L−1 NaOH对载铁牡蛎壳粉的解吸效果较好。但由于牡蛎壳粉的解吸率低于生物炭(42.3%)[21],解吸率过低会影响牡蛎壳粉的广泛应用,因此,对牡蛎壳粉的解吸效果有待进一步研究。
-
天然未处理的牡蛎壳粉和载铁牡蛎壳粉表面结构特征参数由表1可知,天然牡蛎壳粉和载铁牡蛎壳粉比表面积分别为1.18 m2·g−1和0.68 m2·g−1。经处理后,载铁牡蛎壳粉的孔容和平均孔径均增大,分别从0.005 cm3·g−1和16.52 nm增至0.061 cm3·g−1和35.60 nm。这可能是由于物质的比表面积是根据物质内部孔隙计算得出。在经载铁壳粉处理后,表面覆盖上一层含铁物质[23],堵塞了表面部分的孔隙[24]。测量比表面积的N2和壳粉表面的孔隙接触减少,继而导致计算结果降低。壳粉孔径的增大,也表明壳粉表明覆盖一层物质。
将牡蛎壳粉进行SEM分析,样品放大10 000倍,由图4(a)可知,天然牡蛎壳粉表面凹凸不平,存在较多完整、光滑的大片状结构,片状结构之间形成了较多的缺陷位,这和壳粉的比表面积下降结果相一致。由图4(b)可知,吸附后的载铁牡蛎壳粉表面主要为微小颗粒,颗粒细小,分布均匀,原先的大片状结构破碎成小片状结构,片状结构表面粗糙。牡蛎壳粉经载铁处理后,壳粉表面结构发生变化,比表面积下降。有研究[25-26]表明,表面覆盖有氧化铁的吸附剂在电镜照片中呈现亮灰色,形状以团聚或松散的微小颗粒为主,颗粒无规则,分布均匀。载铁牡蛎壳粉的颜色较亮,表面颗粒细小,均匀分散。因此载铁牡蛎壳粉表面含有氧化铁等物质。
将天然牡蛎壳粉和已吸附的载铁牡蛎壳粉进行X射线衍射分析,由图5可知,天然牡蛎壳粉和已吸附的载铁牡蛎壳粉的衍射图谱上均在23°、29.34°、35.92°、39.36°、43.12°、47.44°、48.46°、58°、63.02°、65.50°出现碳酸钙峰值。已吸附的载铁牡蛎壳粉在27.34°、31.06°出现峰形弥散的峰值。这与Fe2(PO)5和Fe4(PO4)2O标准卡片相吻合。由图5可知,牡蛎壳粉表面结合的铁组分主要为Fe2(PO)5和Fe4(PO4)2O。
-
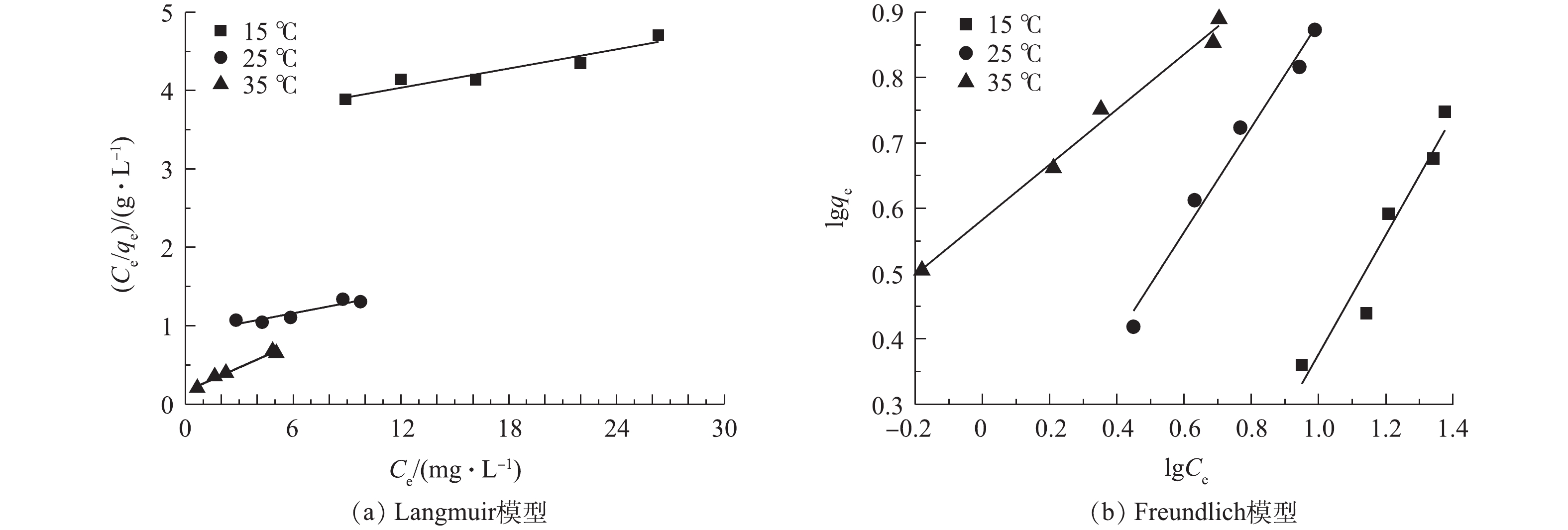
由图6可知,随着温度的升高,2种方程的斜率均上升。由表2可知,在Freundlich方程中,15、25和35 ℃下的R2均大于Langmuir方程中对应的R2,这表明相比Langmuir方程,Freundlich方程更好地描述载铁牡蛎壳粉的吸附除磷行为,载铁牡蛎壳粉表面上的吸附位点的能量分布为指数型。随着温度从15 ℃升至35 ℃,Freundlich方程的吸附常数Kf、拟合相关系数R2也随之升高,1/n随之下降;Langmuir方程的吸附常数K、拟合相关系数R2升高,最大吸附量下降。当1/n为0.1~0.5时,则认为吸附过程容易发生;当1/n>2,吸附过程难发生。Kf越大,表明吸附效果越好。在本研究中,25 ℃和35 ℃下1/n分别为0.80和0.42,这表明载铁牡蛎壳粉在25 ℃和35 ℃条件下更容易吸附除磷。载铁牡蛎壳粉对TP最大吸附量为9.81 mg·g−1,高于天然牡蛎壳除磷(0.88 mg·g−1)[13]和高温改性牡蛎壳除磷(1.10 mg·g−1)[14],但低于镧-多孔沸石(17.2 mg·g−1)和ZrO2(29.71 mg·g−1)。这可能是牡蛎壳粉的比表面积较小,牡蛎壳粉的比表面积(1.18 m2·g−1)低于沸石(64.52 m2·g−1)[27]和ZrO2 (8.83 m2·g−1)[28],由此可见,比表面积小的吸附剂吸附效果较差。
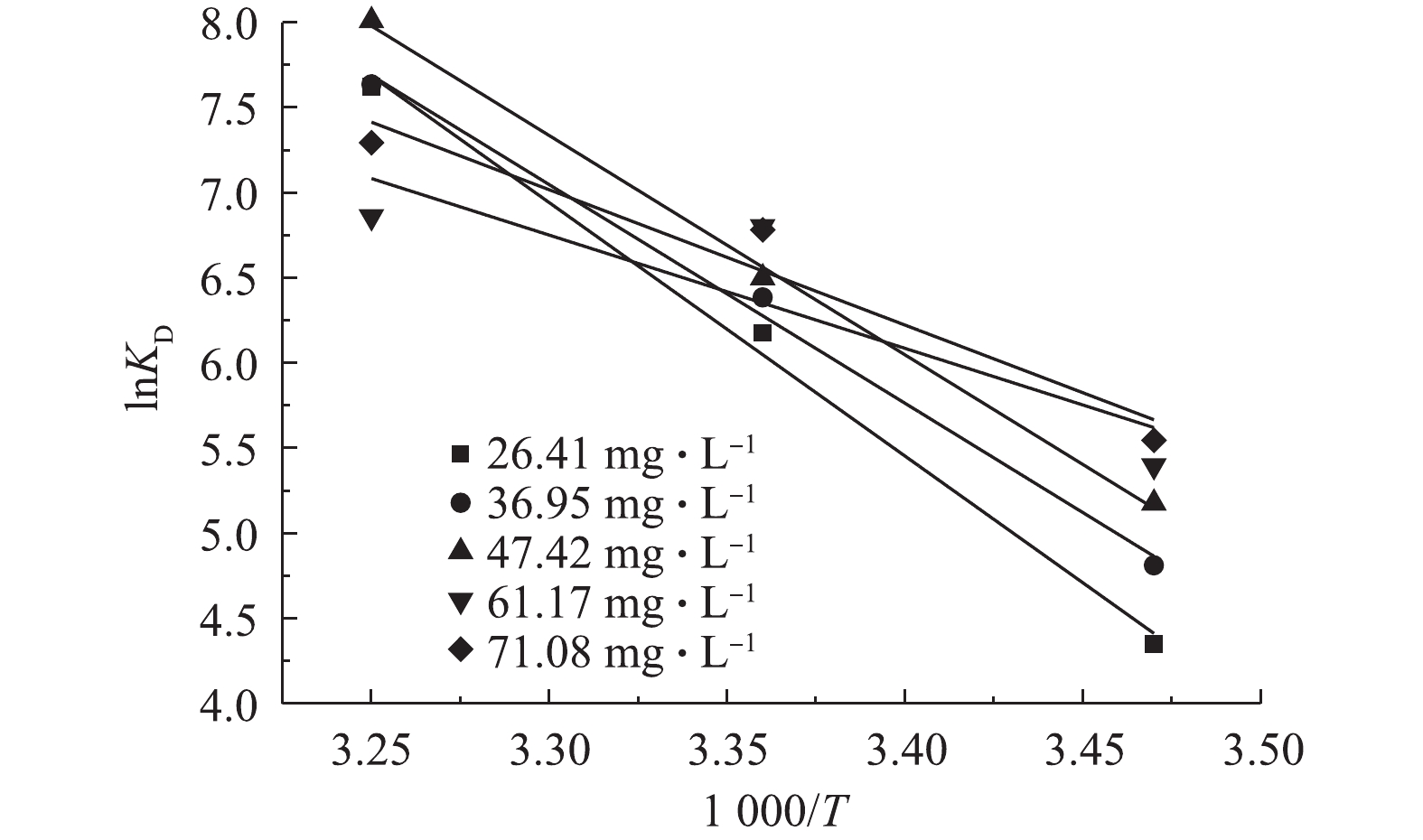
以1 000/T对ln(KD)作图得图7。由式(7)可知,1 000/T对ln(KD)直线斜率和截距可分别求得S0和H0。在不同初始TP浓度、温度条件下,载铁牡蛎壳粉吸附除磷的G0均小于0,说明吸附过程是自发进行,由表3可知。随着温度由15 ℃升至35 ℃,同一TP初始浓度的G0均降低,但其绝对值增大,表明吸附过程的动力较大。在25 ℃和35 ℃下,G0分别先降至−15.93 kJ·mol−1和−18.76 kJ·mol−1,后升至−15.88 kJ·mol−1和−17.08 kJ·mol−1,这表明牡蛎壳粉吸附是一个动态过程。同时,在较大的初始TP浓度条件下,易发生吸附的逆过程[21]。实验中H0、S0均大于0,进一步印证了载铁牡蛎壳粉吸附除磷为吸热反应,高温有助于除磷的效果。同时,牡蛎壳粉表面上磷分子解吸的自由度大于牡蛎壳粉吸附溶液磷分子的自由度[29]。这也可能是由于本实验中振荡强度较大导致的。
-
根据式(8)和式(9),由表4可知,准二级动力学25 ℃和35 ℃的R2均大于准一级动力学对应的R2,且25 ℃和35 ℃下实验检测的吸附量均大于准一级动力学理论计算的吸附量,表明准二级动力学更适合描述载铁牡蛎壳粉吸附除磷的机理。由式(11)可得,载铁牡蛎壳粉吸附除磷的活化能为22.09 kJ·mol−1,说明吸附过程存在物理吸附和化学吸附[30-31],同时由表3可知,H0均大于40 kJ·mol−1,表明载体牡蛎壳粉吸附除磷的过程主要由化学吸附决定[27, 32-34]。为进一步研究载铁牡蛎壳粉对磷吸附的机理和限速因素,采用颗粒内扩散模型拟合得到3段斜率各不相同的直线,且直线均不过原点,结果如图8所示。由表5可知,牡蛎壳粉吸附过程可分为3个阶段:第1阶段在前60 min内,直线斜率随着温度升高而增大,说明高温有助于磷分子穿过水膜面达到牡蛎壳粉表面,该过程为膜扩散主导的吸附过程[27];第2阶段在120~480 min,35 ℃的直线斜率下降,表明牡蛎壳粉表面上的磷分子达到壳粉内部,为颗粒内扩散过程[21];第3阶段为720~1440 min,25 ℃和35 ℃对应的直线斜率最小,这表明磷分子在颗粒内的扩散减弱,吸附接近平衡,吸附过程转变为主导。在15 ℃下,载体牡蛎壳粉的准二级动力学、颗粒内扩散模型拟合参数结果差异较大,这可能是低温抑制了磷分子的运动以及固液相之间扩散,这也说明载铁牡蛎壳粉可适用的温度范围较窄(25~35 ℃)。
2.1. 吸附剂添加量对载铁牡蛎壳粉吸附除磷效果的影响
2.2. 初始TP浓度对载铁牡蛎壳粉吸附除磷效果的影响
2.3. pH对载铁牡蛎壳粉吸附除磷效果的影响
2.4. 共存离子对载铁牡蛎壳粉吸附除磷效果的影响
2.5. 载铁牡蛎壳粉的解吸
2.6. 载铁牡蛎壳粉表征
2.7. 吸附热力学
2.8. 吸附动力学
-
1)当初始TP为20 mg·L−1时,载铁牡蛎壳粉最佳添加量为8.00 g·L−1,在此条件下,载铁牡蛎壳粉对初始TP浓度为20.00~50.00 mg·L−1的模拟水除磷效果最佳,TP去除率由(84.94±0.94)%升至(87.35±1.06)%,吸附量由(2.37±0.03) mg·g−1升至(5.45±0.22) mg·g−1;当pH为2.00~6.00时,载铁牡蛎壳粉TP去除率高于(80.13±3.27)%,吸附量高于(2.04±0.02) mg·g−1;
HCO−3 的存在对载铁牡蛎壳粉吸附除磷有明显的抑制作用;0.10 mol·L−1 NaOH对牡蛎壳粉解吸效果最优,解吸率为(22.97±1.77)%。2)经改性吸附后,牡蛎壳粉比表面积下降、孔径增大。壳粉表面以细小颗粒为主,分布均匀。XRD衍射结果表明,载铁牡蛎壳粉表面覆盖成分主要为Fe2(PO)5和Fe4(PO4)2O。
3)当初始TP浓度为26.40~71.08 mg·L−1和温度为15~35 ℃时,Freundlich吸附模型更好地描述载铁牡蛎壳粉吸附除磷反应,载铁牡蛎壳粉对TP最大吸附量为9.81 mg·g−1。当初始TP浓度为50.00 mg·L−1和温度为15~35 ℃时,准二级动力学方程更好地描述载铁牡蛎壳粉吸附除磷过程,吸附过程存在物理吸附与化学吸附,主要由化学吸附决定。膜扩散和颗粒内扩散为主要限速步骤,配位交换和静电吸附为主要吸附机理。





 下载:
下载: