-
氨氮、硝酸盐氮和亚硝酸盐氮是地下水中无机氮的主要存在形态,近年来,氮污染屡见报道,已成为地下水环境保护的重大问题之一[1-4]。《2018中国生态环境状况公报》[5]显示,全国纳入监测的2 833个浅层地下水监测点中水质超过《地下水质量标准》(GB/T 14848-2017)Ⅲ类标准的点位占76.1%,超标因子除锰、铁、硫酸盐等受水文地质化学背景影响的因子外,氨氮是最为突出的人为污染指标之一。生活垃圾填埋场是地下水氮污染的主要来源之一[6-7],尤其是早期建成的非正规垃圾填埋场,缺乏正规的防渗和渗滤液收集设施,含高浓度氨氮的渗滤液可经土壤层渗入地下水中,造成地下水氮污染。笔者对山地区域某沟谷型非正规生活垃圾填埋场开展的地下水污染调查结果显示,填埋区下游地下水中除氨氮存在一定程度超标外,未见硝酸盐氮和亚硝酸盐氮出现明显累积或超标现象,说明渗滤液中的氨氮在包气带-地下水间的迁移转化以迁移为主。
包气带不仅是地下水的天然屏障,也是地表输入的污染物与同地下水发生联系的重要地带,氨氮经过包气带向地下水迁移转化的过程中,会经过一系列的物理、化学及生物作用,其中,土壤吸附和解吸附是影响铵态氮在包气带中分布的重要环境行为[8-9]。本研究以非正规生活垃圾填埋场所在区域包气带土壤为研究对象,聚焦土壤对铵态氮的吸附解吸附行为,以水、中性盐、酸3类浸提剂将铵态氮区分为水溶态、可交换态和固定态3种赋存形态[10],分别探讨其吸附特征,揭示环境温度、pH、阳离子等要素对其吸附过程的影响,结合解吸附实验量化包气带土壤对铵态氮的固定能力,旨在深化对铵态氮在包气带土壤-地下水迁移规律的认识,为地下水氮污染控制提供参考。
全文HTML
-
供试土样取自重庆市某非正规生活垃圾填埋场下游侧边界,所采土样未受渗滤液污染。该垃圾填埋场利用天然沟谷填埋生活垃圾,未设置防渗层。土壤类型为分布广泛的黄壤,受土壤层厚度影响,采集深度为0~60 cm或0~80 cm,剔除砾石、树枝及根系等杂物后,装袋送回实验室,自然风干后过10目尼龙筛用于吸附和解吸附实验。
供试土壤以砂质黏壤土为主,砂粒平均含量52.06%,粉粒平均含量为27.14%,黏粒平均含量为18.25%,pH=7.1,氧化还原电位(Eh)为213.5 mV,有机质含量为1.48%,阳离子交换量(CEC)为8.09 cmol·kg−1。水溶态铵、可交换态铵、固定态铵含量分别为10.30、2.53、139.73 mg·kg−1。
-
1)吸附动力学实验方法。称取土样(4±0.002 0) g于33根具塞聚乙烯离心管中,分别加入20 mL浓度为200 mg·L−1的氯化铵溶液,调节pH至7.0,在(25±0.5) ℃下、以 120 r·min−1恒温振荡。分别于1、3、5、10、20、30、60、90、120、180、240 min取出3根比色管,快速离心后上清液,经 0.45 µm 膜过滤,测定氨氮浓度,土样经75%乙醇洗去表面附着的
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 后于105 ℃烘干,用于测定水溶态铵、可交换态铵、固定态铵。吸附总量按式(1)计算,各形态铵吸附量按式(2)进行计算。式中:Q为吸附量,mg·kg−1;C1为
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 的初始浓度,mg·L−1;C2为上清液中$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 的剩余浓度,mg·L−1;q1为吸附后土壤中铵态氮的含量,mg·g−1,q0为土壤中铵态氮的初始含量,mg·g−1。2)吸附热力学实验。称取土样(4±0.002 0) g于18根具塞聚乙烯离心管中,分别加入20 mL浓度为50、100、200、400、600、800 mg·L−1的氯化铵溶液,调节pH至7.0,在(25±0.5) ℃下,以 120 r·min−1恒温振荡180 min。达到吸附平衡后,以4 500 r·min−1离心10 min,上清液经 0.45 µm 膜过滤后测定氨氮,土样经75%乙醇洗去表面附着的
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 后于105 ℃烘干,用于测定水溶态铵、可交换态铵、固定态铵。3)影响因素研究。按照2)所述步骤,逐次改变温度、pH、阳离子浓度(CaCl2),考察3个因素对铵态氮吸附热力学的影响。温度分别为15、25、35 ℃,pH分别为3、5、7、9、11,阳离子浓度(CaCl2)为0、0.001、0.005、0.01、0.05、0.1 mol·L−1。
4)解吸附实验方法。污染土样的制备:称取一定量土样按照5∶1的水土比分别加入50、100、200、400、600、800 mg·L−1的氯化铵溶液,混合均匀,按2)中所述步骤达吸附平衡后,用75%乙醇洗去表面附着的
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 后烘干备用。依次称取污染土样(4±0.002 0) g若干份,加入20 mL纯水后进行解吸附,每个样品设置3 个平行样,达到解吸附平衡后取样,按照前述实验步骤测定上清液中的
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 浓度及土样中的水溶态铵、可交换态铵、固定态铵含量。5)测定方法。水溶态铵、可交换态铵、固定态铵的提取按照KOWALENKO等[10]、SILVA等[11]使用的方法,提取试剂及主要参数见表1。水溶态铵、可交换态铵采用纳氏试剂光度法测定,固定态铵采用蒸馏滴定法测定。
pH和Eh分别由pH计和氧化还原电位计测定,有机碳采用重铬酸钾氧化-分光光度法测定,CEC采用醋酸铵交换法测定,土壤机械组成采用简易比重计法测定。
1.1. 实验材料
1.2. 实验方法
-
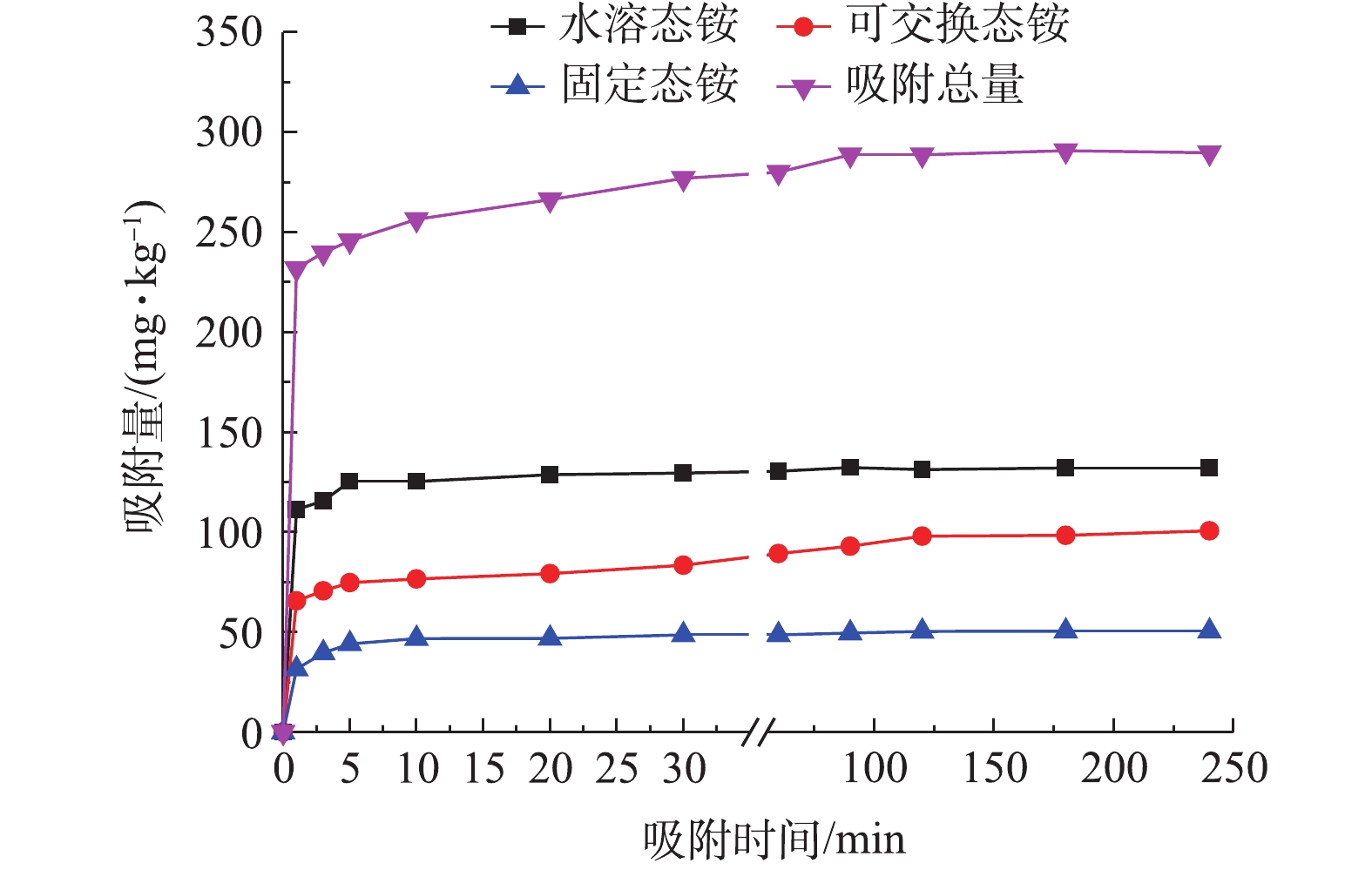
图1显示了黄壤对不同形态铵的吸附动力学实验结果,吸附总量由上清液测试数据计算得出,水溶态铵、可交换态铵、固定态铵吸附量由土样分态提取测定结果计算得出。由图1可知,黄壤与
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 接触的瞬间发生快速吸附,在1 min内吸附总量达到吸附平衡量的80%,随后吸附速率急剧降低,在20 min 内吸附总量达到吸附平衡量的90%,直至90 min后吸附量基本趋于平缓。各形态铵的吸附过程与吸附总量呈现了相似的变化规律,起始吸附速率略有不同,具体表现为水溶态铵>可交换态铵>固定态铵。水溶态铵起始吸附速率略大于吸附总量,1 min内吸附量可达吸附平衡量的84%,5 min内达到吸附平衡量的90%,30 min后基本达到吸附平衡。可交换态铵、固定态铵的吸附进程滞后于铵态氮吸附总量,1 min吸附量分别为其吸附平衡量的65%和62%,达到吸附平衡的时间也相对较长,分别为180 min 和120 min。采用Elovich方程、Lagergren准二级速率方程、抛物线方程3种常用的土壤吸附动力学特征表达模型对实验结果进行了拟合,结果如表2所示。Elovich方程、Lagergren准二级速率方程均能够用于描述铵态氮的吸附过程,相关性显著(R2>0.97),Elovich方程对水溶态铵、可交换态铵和吸附总量吸附过程的拟合效果优于Lagergren准二级速率方程,Lagergren准二级速率方程对固定态铵吸附过程的拟合程度更高,这说明供试黄壤对水溶态铵、可交换态铵以及铵态氮总量吸附过程复杂,伴随吸附能量的变化,而对固定态铵的吸附以化学吸附为主。抛物线方程对铵态氮吸附全过程的拟合结果不收敛,但可用于表达吸附初期阶段(10 min内)的动力学特征(R2=0.895~0.998),说明
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 在土壤颗粒介质内表面的扩散对铵态氮吸附初期阶段具有重要的影响。由Elovich方程、Lagergren准二级速率方程拟合结果可知,供试黄壤对水溶态铵、可交换态铵、固定态铵、铵态氮的吸附量达到各自90%吸附平衡量的时间分别为2.9、63.1、6.1、20.9 min,与实验结果基本吻合。有研究[12-14]表明,铵态氮吸附是快速过程,可在10 min内达到90%吸附平衡量,本实验中水溶态铵、固定态铵吸附结果与已有研究结论一致,但可交换态铵、铵态氮总量的吸附结果与该结论存在较大的差异。可见,分形态研究铵态氮吸附动力学对于深入了解土壤对氨氮的吸附机理及特征十分重要。 -
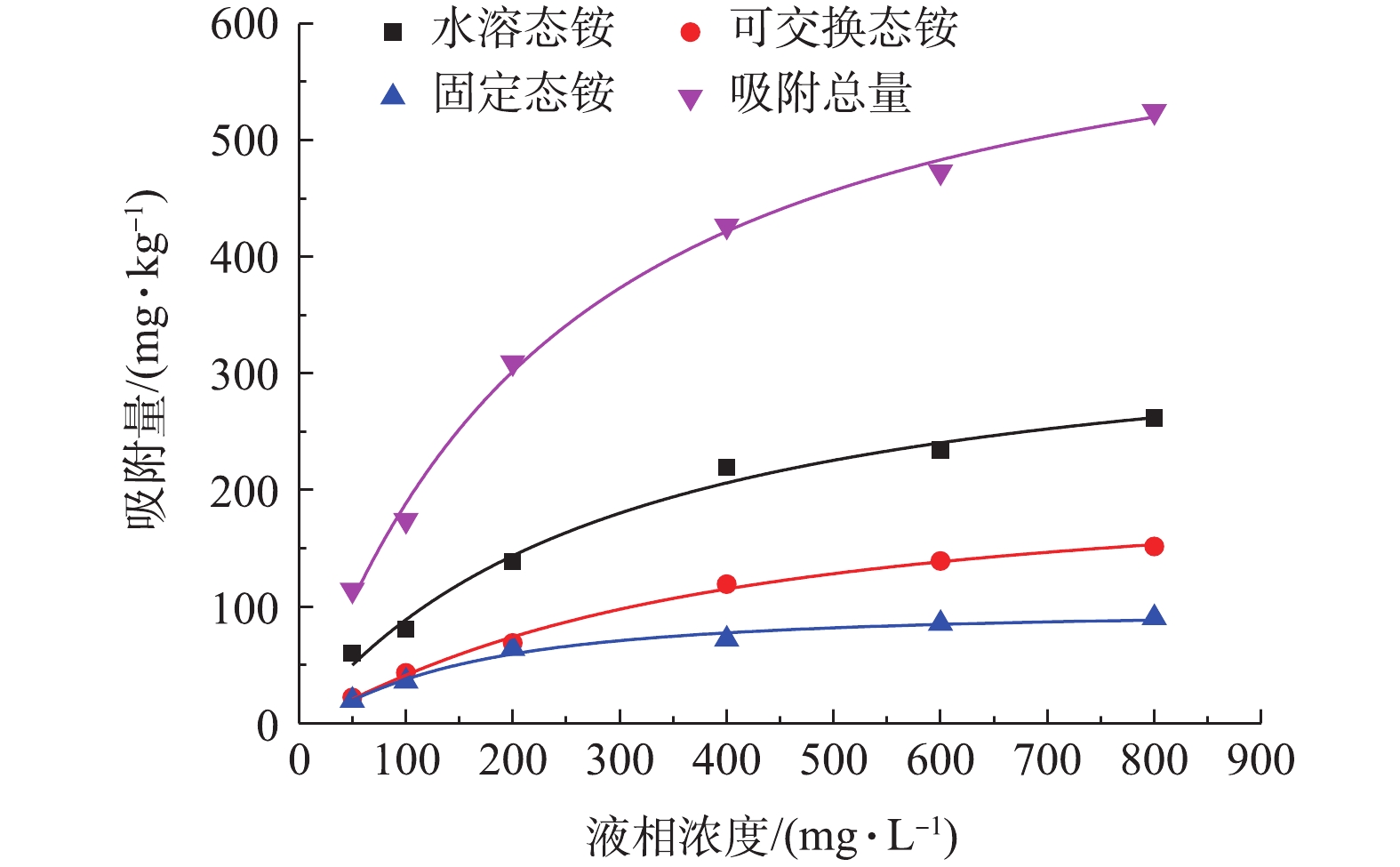
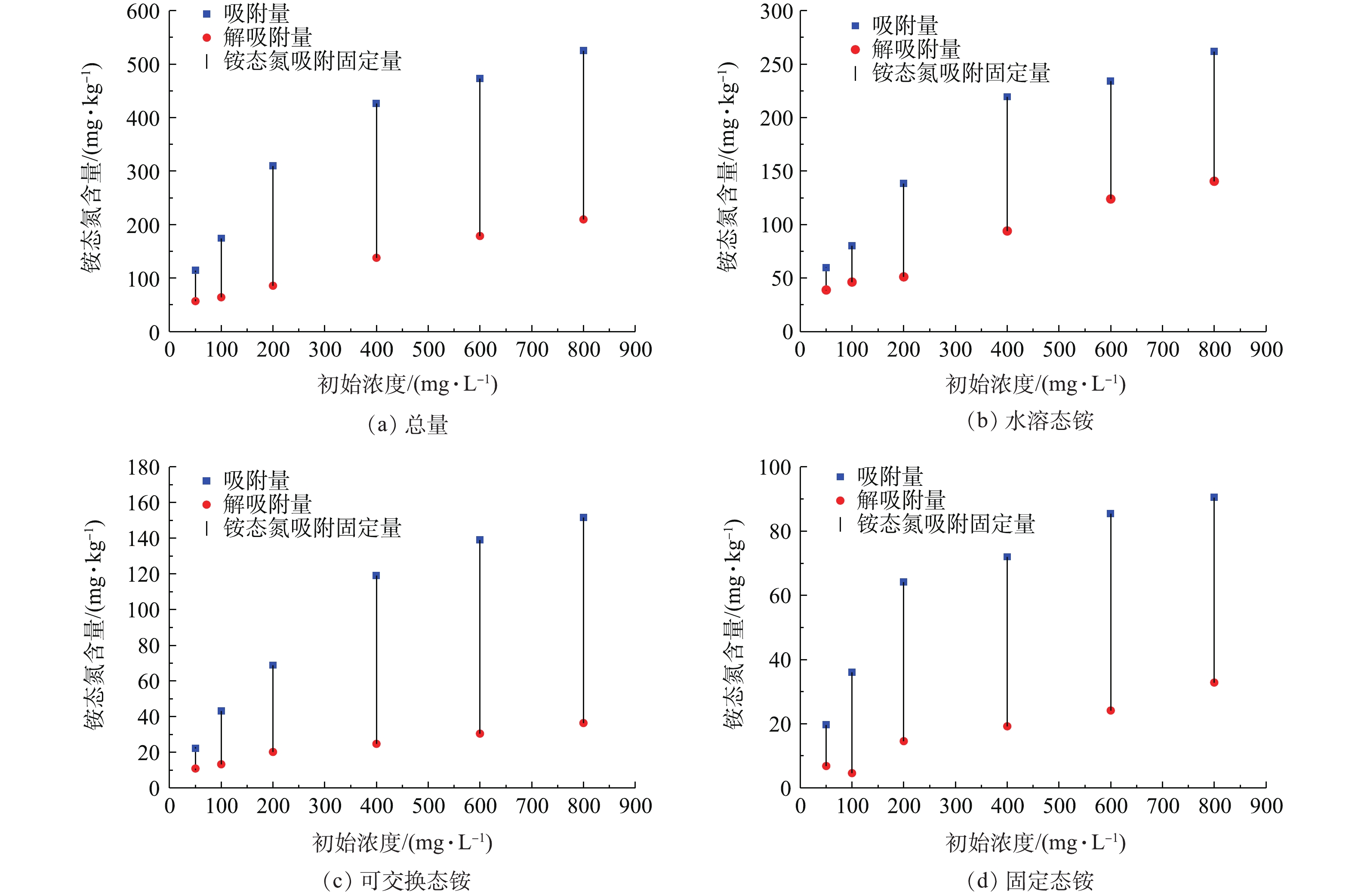
在25 ℃等温条件下,各形态铵在不同初始浓度的吸附结果如图2所示。黄壤对各形态铵吸附量随着氯化铵初始浓度升高而增加。吸附总量、水溶态铵、可交换态铵呈现了相似的变化趋势,初始浓度低于400 mg·L−1时,吸附量随浓度升高呈线性增加,之后增速放缓,逐渐趋于饱和,达到最大吸附量。固定态铵略有不同,初始浓度≤200 mg·L−1时,吸附量随初始浓度增加呈线性增长,之后基本趋于饱和,说明在实验时间内为固定态铵吸附提供的可结合点位有限,饱和吸附量较低。以吸附量与溶液中溶质质量的比值计算吸附率,结果显示吸附率随初始浓度升高而降低,当初始浓度为50 mg·L−1时,黄壤可通过吸附将近一半的
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 吸附在包气带中,吸附率可达45.7%,当浓度升高至800 mg·L−1时,吸附率降为13.1%,说明当外界输入$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 浓度较低时,黄壤可有效阻止$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 向地下水迁移,但对高浓度(高于200 mg·L−1)的$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 输入防护作用相对较小,将有70%以上的$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 穿过包气带进入地下水中,从而造成地下水氮污染。分别采用Langmuir模型、Freundlich模型对吸附结果进行拟合(表3)。结果表明,2种模型均能很好的描述黄壤对铵态氮的等温吸附过程,Langmuir模型的回归拟合效果更好,吸附过程为以单分子吸附为主的物理吸附和化学吸附,拟合结果与已有研究得出的结论[15-16]相一致。由Langmuir方程推算得出铵态氮的最大吸附量为696.49 mg·kg−1,水溶态铵、可交换态铵、固定态铵的最大吸附量分别为363.50、245.64、112.50 mg·kg−1。3种赋存形态铵的最大吸附量之和为721.64 mg·kg−1,略高于吸附总量的拟合结果。这主要归因于以下2点:一方面归因于模型拟合误差,按照3种形态铵吸附量的总和进行拟合计算,得出的最大吸附量为707.91 mg·kg−1,比分形态拟合结果的总和小1.9%;另一方面归因于实验中吸附总量和形态氮所用数据的不同口径,3种形态铵吸附量的总和比吸附总量(由上清液测定)平均低(7.1±4.0)%,拟合中得出的KL也相对较小,导致最大吸附量相对较大。同时,供试黄壤对铵态氮的吸附过程也满足Freundlich方程,这说明吸附过程中存在非均匀性表面的吸附。
-
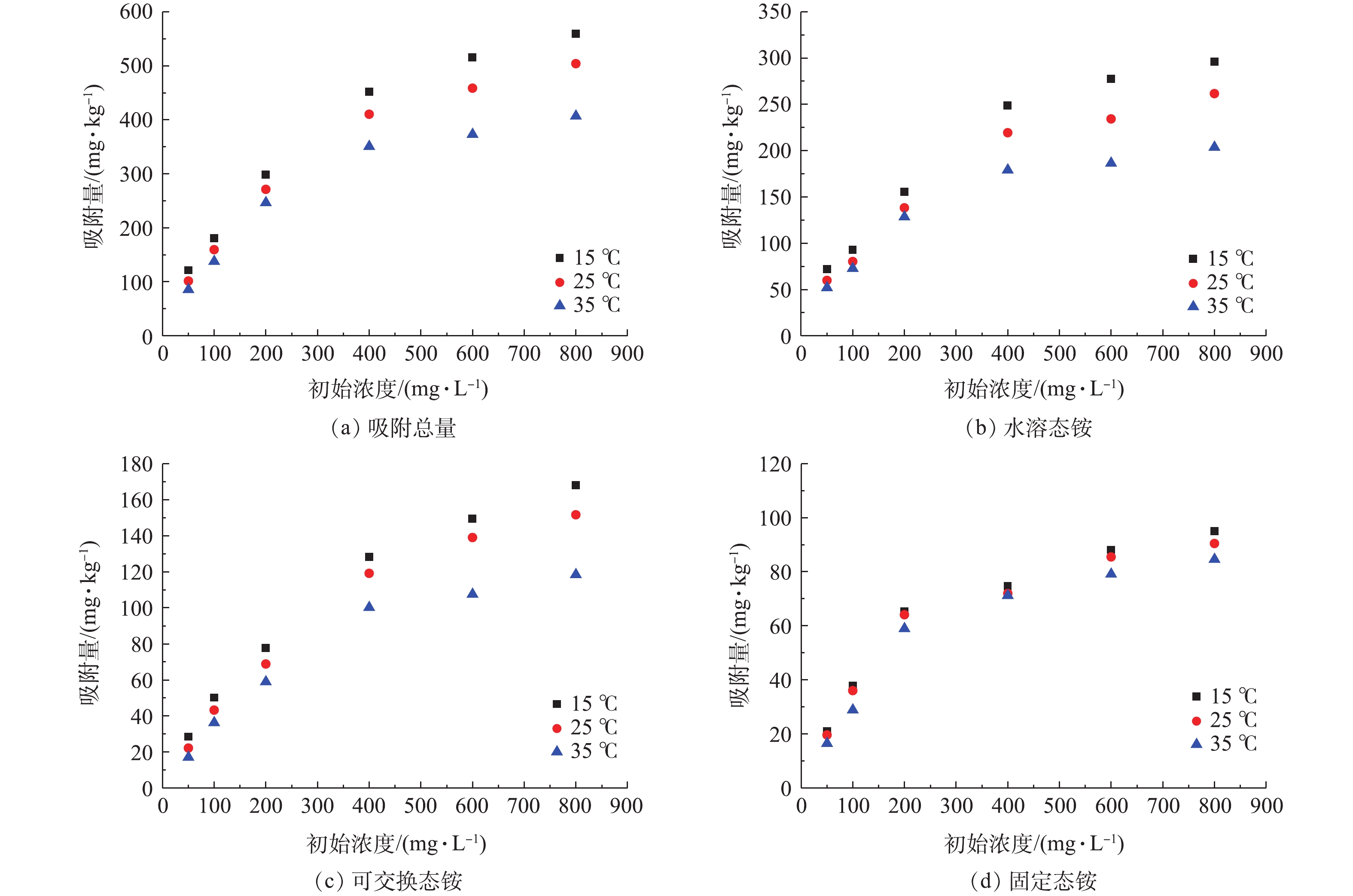
1)温度对铵态氮吸附的影响。有研究[17-20]证明,温度对铵态氮吸附影响显著,为了考察温度对不同形态铵吸附的影响,在pH=7、氯化铵初始浓度分别为50、100、200、400、600、800 mg·L−1情况下,分别考察了在15、25、35 ℃下各形态铵吸附量的变化规律。由图3可知,随着温度的降低,各形态铵吸附量呈现了不同程度的增加,这与LIU等[19]和隋淑梅等[20]的研究结论相同。温度升高对铵态氮的吸附具有抑制作用,氯化铵初始浓度越高,抑制作用越明显,不同形态铵吸附受抑制程度不同。吸附总量、水溶态铵、可交换态铵吸附对温度变化的响应明显,温度由35 ℃降至25 ℃各形态铵吸附量分别增加了(18.0±5.1)% (不同初始浓度吸附量增幅的平均值和方差,下同)、(18.1±8.5)%、(23.5±5.9)%,降至15 ℃时吸附量分别增加了(32.9±7.6)%、(36.4±10.7)%和(40.8±13.5)%;固定态铵吸附受温度的影响较小,25 ℃和15 ℃时的吸附量分别比35 ℃时增加了(11.4±8.8)%和(16.2±10.3)%。另外,在初始浓度为200 mg·L−1时的等温吸附实验结果表明,温度对吸附速率具有促进作用,温度由15 ℃升至35 ℃可使各形态铵达到吸附平衡的时间缩短30~60 min。温度对吸附的影响和吸附能量变化及分子运动活性有关,一方面,铵态氮吸附属于放热反应[14,21],释放的吸附热会导致吸附层温度升高,从而降低吸附介质的活性,促使反应向吸热方向进行(解吸附方向),不利于吸附反应发生;另一方面,温度升高可加剧分子运行,增加分子活性,缩短反应时间。动力学过程研究显示黄壤对水溶态铵和可交换态铵的吸附过程伴随能量变化,因此温度对二者的吸附抑制作用更为显著,而固定态铵主要通过化学吸附被土壤介质矿物或有机组分吸持[22],受温度变化的影响不显著。
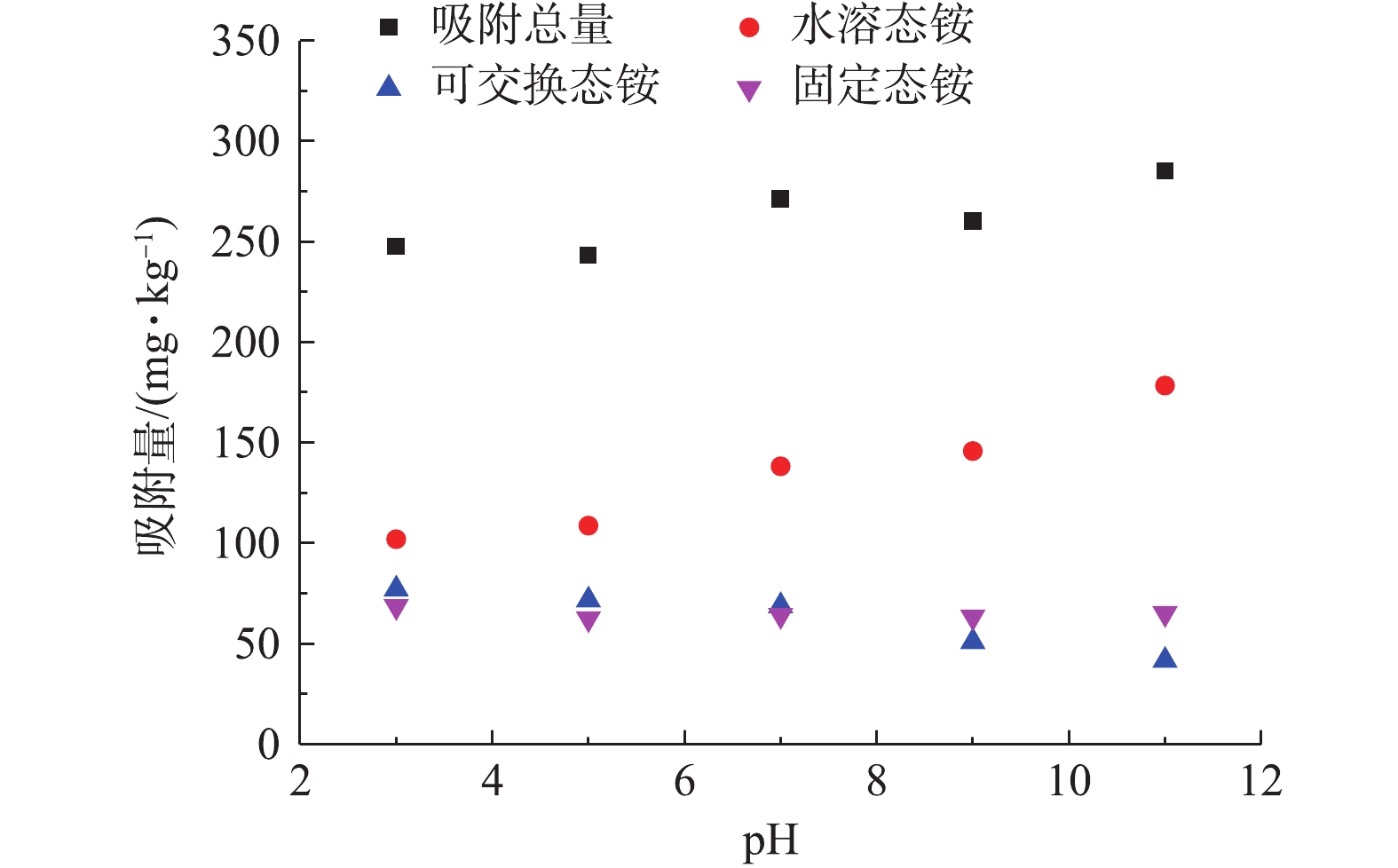
2) pH对铵态氮吸附的影响。土壤环境pH的变化可能会引起含水解官能团土壤胶粒表面电性及电荷量的变化,从而对铵态氮在土壤介质中的吸附产生影响。各形态铵在25 ℃、铵初始浓度为200 mg·L−1、不同pH条件下的吸附情况如图4所示。有研究[23-24]表明,pH升高有利于土壤对铵态氮的吸附,但是本研究结果并未显示出类似的规律。由图4可知,铵态氮吸附总量及固定态铵吸附量随pH变化不显著,水溶态、可交换态铵随pH升高呈现了相反的变化趋势。水溶态铵吸附量随着pH升高逐渐增大,吸附量与pH在P<0.05水平上呈显著正相关 (R2=0.949~0.987),这说明pH升高有利于水溶态铵的吸附。可交换态铵的吸附量与pH在P<0.05水平上呈显著负相关性(R2=0.927~0.989),吸附量随pH的升高而降低,在碱性(pH>7)条件下吸附量降低趋势尤为明显。
水溶态铵、可交换态铵在pH的变化过程中呈现的变化趋势,主要是由铵的水解作用引起的,其水解平衡方程如式(3)所示。
水溶态铵主要以水合态或者分子态与土壤介质表面结合,而可交换态铵主要是以离子形态与土壤介质表面结合[25]。2种形态的铵分别位于上述平衡方程的两侧,当pH增加,溶液的碱性增强,使得水解方程向右进行,水合态铵含量增加,而离子态铵含量减少,从而使得水溶态铵吸附量增加,可交换态铵吸附量减少;反之,当pH降低,方程向左进行,水合态铵减少,离子态铵增加,使得水溶态铵减少,可交换态铵增加。
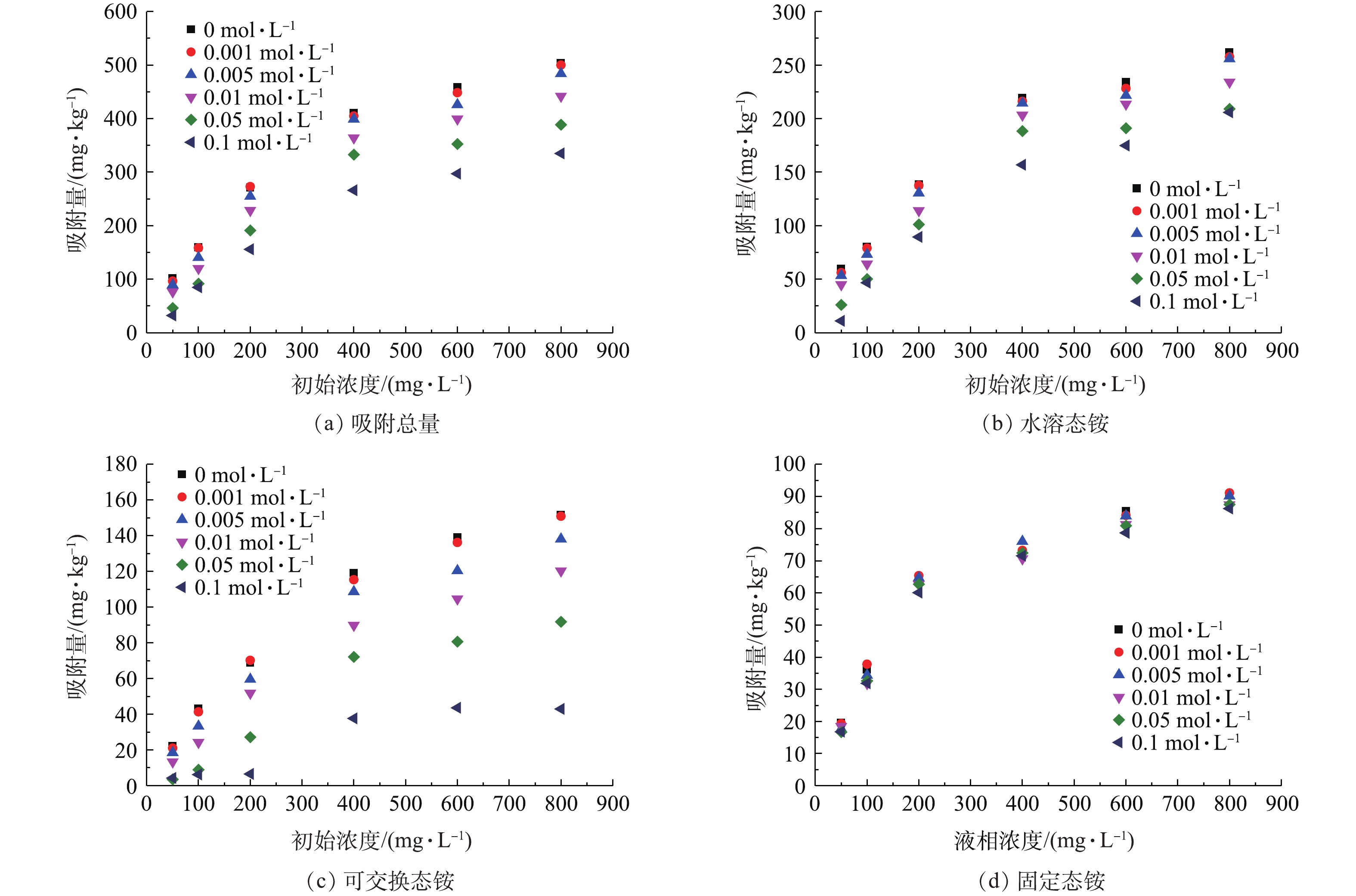
3)阳离子对铵态氮吸附的影响。阳离子对于铵态氮吸附的影响存在两种不同的看法,一部分认为水溶态铵、可交换态铵易被碱金属离子交换[26],阳离子的存在可与铵竞争结合点位[27];有研究[28-29]表明,阳离子可促进铵态氮的吸附。为了探明阳离子对供试土壤铵态氮吸附的影响,选择前期吸附实验过程中浓度变化最显著的Ca2+为典型代表,探讨阳离子对铵态氮吸附过程的影响,实验在pH=7、温度为25 ℃条件下进行,阳离子浓度设置为0、0.001、0.005、0.01、0.05、0.1 mol·L−1,结果如图5所示。
结果表明,随着CaCl2浓度的升高,各形态铵吸附量相对于对照组(C=0 mol·L−1)整体呈现下降趋势,其中,黄壤对水溶态铵、可交换态铵的吸附量受阳离子影响显著,下降趋势明显,吸附量与CaCl2浓度在P<0.05水平上呈显著负相关性,R2分别为0.893~0.989、0.886~0.983,且在吸附过程中Ca2+浓度呈下降趋势,这说明在吸附过程中阳离子与水溶态铵、可交换态铵之间存在竞争吸附点位的情况。固定态铵吸附受阳离子的影响不显著,吸附量降幅较小。
由图5还可以看出,CaCl2对可交换态铵吸附过程的抑制作用最显著,阳离子浓度达到0.05 mol·L−1及以上时,可交换态铵的吸附量相对于对照组出现了锐减。在自然环境中,土壤颗粒一般带负电荷,而
$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 与碱土金属离子均通过离子交换与介质中的吸附点位相结合,因此,加入的Ca2+将会与$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 竞争土壤介质表面、边面及边角的吸附点位,由于Ca2+价态更高,其与土壤胶体间的亲和力更强,因此其交换能力也更强[30],在位点竞争中,$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 处于劣势,故表现出吸附量减少的趋势。虽然Ca2+具有较强的交换能力,由于其水合离子的半径(0.41 nm)比$ {\rm{NH}}_{\rm{4}}^{\rm{ + }}$ 的水合离子半径(0.33 nm)大[31],难以脱掉水化膜进入矿物层间,进而竞争层间吸附点位,因此,Ca2+对固定态铵的吸附影响较小。 -
水溶态铵以分子态或水合态通过H键与土壤介质的极性表面结合,易被水分子重新置换,释放风险较高。可交换态铵以离子形态通过静电力作用与土壤介质表面结合,未被矿物层间的结构固定,具有一定活性,可通过离子交换释放。固定态铵吸附机制与可交换态铵类似,但结合更稳定,被固定在矿物层间,通常无法通过一般的离子交换释放。从不同形态铵与土壤表面的结合强度看,水溶态铵最容易释放,随地表水下渗进入地下水中,其次为可交换态铵,再次为固定态铵。
为了掌握黄壤对各形态铵的固定能力,开展了系列解吸附实验,以吸附量和解吸附量的差值表示固氮量,见图6。结果表明,黄壤对铵态氮具有一定的固定能力,解吸附量远低于吸附量,最高可将超过70%吸附在其表面的铵态氮固定在土壤中。pH、温度、阳离子对于铵态氮的固定存在一定的影响。pH虽然对吸附总量的影响不大,但可显著影响铵态氮的解吸附,解吸附量随着pH的升高呈降低趋势,因此,pH升高可促进铵态氮在黄壤中的固定,pH由3升至11,铵态氮的固定比例由(43.1±7.5)%上升至(67.1±10.3)%。与温度对吸附的影响效果相同,黄壤对铵态氮的固定效果随着温度的升高而降低,固定比例由15 ℃时的(74.4±3.9)%降至35 ℃时的(38.4±15.4)%。Ca2+的存在不利于铵态氮的固定,其浓度越高,对解吸附的促进作用越显著,显著削减土壤对铵态氮的固定效果,当浓度升至0.05 mol·L−1和0.1 mol·L−1时,可导致被土壤吸附的水溶态铵和可交换态铵全部被释放。
由黄壤对各形态铵的固定比例看,固定能力与其赋存形态的活性具有负相关性,活性越高,被固定的比例越低,在25 ℃、pH=7、不添加阳离子条件下,固定态铵、可交换态铵、水溶态铵的被固定比例依次为(73.0±8.6)%、(70.6±10.6)%、(48.5±10.2)%,鉴于各形态铵吸附量的显著差异,因此,由固定量看,仍然是水溶态铵最高、固定态铵最低。
2.1. 铵态氮吸附动力学特征
2.2. 铵态氮吸附热力学特征
2.3. 铵态氮吸附的影响因素
2.4. 黄壤对铵态氮的固定能力
-
1)供试黄壤对铵态氮的吸附动力学特征相似,初始吸附速率及达到吸附平衡的时间不同,初始吸附速率由大到小依次为水溶态铵>吸附总量>可交换态铵>固定态铵,达到吸附平衡的时间先后顺序依次为水溶态铵、吸附总量、固定态铵、可交换态铵。
2)黄壤对水溶态铵、可交换态铵、铵态氮总量的吸附过程符合Elovich方程,吸附过程伴随能量的变化。黄壤对固定态铵的吸附过程符合Lagergren准二级速率方程,吸附以化学吸附为主。吸附初期(前10 min),颗粒内扩散对铵态氮吸附速率具有重要的影响。
3)供试黄壤对铵态氮的吸附量随着初始浓度的增加而增大,但吸附率却随初始浓度升高而降低,由50 mg·L−1时的45.7%降至800 mg·L−1时的13.1%。吸附热力学过程符合Langmuir等温式,25 ℃时,铵态氮最大吸附量(总量)为696.49 mg·kg−1,水溶态铵、可交换态铵、固定态铵最大吸附量分别为363.50、245.64、112.50 mg·kg−1。
4)温度、阳离子浓度对铵态氮吸附总量影响显著,水溶态铵、可交换态铵吸附量随温度及阳离子浓度降低而增加。pH对吸附总量的影响不明显,但可显著影响水溶态铵和可交换态铵的吸附量。固定态铵对温度、阳离子浓度、pH因素的反应不敏感。
5)供试黄壤对铵态氮具有一定固定能力,最高可将超过70%吸附在其表面的铵态氮固定在土壤中。各形态铵在黄壤中固定的难易程度依次为固定态铵>可交换态铵>水溶态铵,固定量大小依次为水溶态铵>可交换态铵>固定态铵。





 下载:
下载: