-
铬是地下水、地表水和工业场所中最常检测到的重金属之一[1],在电镀、冶金、制革、颜料等行业得到了非常广泛的应用,形态主要有Cr(Ⅵ)和Cr(Ⅲ)。因Cr(Ⅵ)易溶于水,且在地下水中迁移速率快而被重点关注[2-3]。2018年,在全国10 168个国家级地下水水质监测点中,发现个别监测点中的六价铬、铅、锌、砷、汞和镉等重(类)金属超标。因此,防止Cr(Ⅵ)进入地下水中成为刻不容缓的研究课题。近年来,国内外对含土壤、地表和地下水系统中Cr(Ⅵ)的处理进行了大量的研究。目前,黄铁矿、零价铁等含铁矿物是还原处理Cr(Ⅵ)的高效、低成本的还原材料[4-6]。黄铁矿(FeS2)是地球表面较丰富的天然铁硫矿物之一,一般是矿物分离后的尾矿废弃物[7]。作为土壤和地下水中活性铁还原剂之一,能有效去除地下水中的有机和无机污染物[3]。本研究将黄铁矿通过机械球磨的方法进行活化,使天然黄铁矿的粒径降至纳米级,从而提高黄铁矿反应活性[8],将其用于处理含铬土壤和地下水。可渗透反应屏障(permeable reactive barrier,PRB)是一种现代新型的地下水污染修复技术,这种被动修复技术因其在处理各种污染物方面的良好性能,尤其比其他现场技术成本更低,从而受到高度的关注[9]。PRB对于上游迁移而来的污染物羽流,可形成原位处理区,将污染物固定于该区域,阻止其到达下游[10]。在20世纪90年代初,多种材料已被作为PRBs的介质用来去除重金属、氯化溶剂、芳香烃和农药等[11]。
纳米天然黄铁矿晶体具有稳定性好且反应活性大的优点,适用于长效PRB工艺。目前,国内外关于用天然纳米黄铁矿去除重金属的研究较少。本研究将自然环境中大量存在的天然黄铁矿进行活化,制备具有小尺寸效应和活化表面的纳米黄铁矿,将其作为PRB介质,探索开发修复土壤和地下水中重金属原位固定技术,为纳米天然黄铁矿处理土壤和地下水中Cr(Ⅵ)及原位固定其他重金属提供参考。
全文HTML
-
实验过程中用到的药品及试剂:黄铁矿(FeS2)标本来自北京远山长矿物标本公司;重铬酸钾(K2Cr2O7)的规格为分析纯,购于北京化工厂;无水乙醇(C2H5OH)为分析纯,购于天津市福晨化学试剂厂;实验所用的过20目筛的细石英砂和过18目筛的粗石英砂均购于市场;混合气体(体积分数为90%的N2、5%的CO2、5%的H2),购于太原宜虹气体工业有限公司;高纯氩气(体积分数为99.999%),购于太原北方气体有限公司;高纯氮气(体积分数为99.999%),购于太原宜虹气体工业有限公司。
-
实验所用的主要仪器:电感耦合等离子体发射光谱仪(ICP),型号为Optima7300V,购自美国PE公司;COY低氧手套箱,购自天美(中国)科学仪器有限公司;PM系列行星式球磨机,型号QM-3SP04,产商为卓的仪器设备(上海)有限公司;X射线衍射仪(XRD),型号为XRD-6000,生产产商为日本岛津公司;TEM透射电镜,型号JEM-2010,购自日本JEOL公司;蠕动泵,型号为BS100-1A+DG4B,购自保定思诺流体科技有限公司,自动部分收集器,型号为BSZ-100,购自上海沪西分析仪器有限公司。
-
使用行星式球磨机制备纳米级黄铁矿粉末,球磨机筒体有效体积为50 mL,磨筒和磨球为不锈钢材质。在机械活化前,使用1 mol·L−1稀盐酸浸泡黄铁矿1 h,再用去离子水冲洗,静置干燥后放入低氧手套箱内平衡。磨球分别用直径为8、3和2 mm的大、中、小球,其个数比为1∶1∶4,磨球与黄铁矿质量比为10∶1[12];加入20 mL水溶液和一定量的乙醇(可有效地提高黄铁矿粉体在液相中的分散稳定性)作为助磨剂和分散剂,机械球磨(活化)一定时间后,设定转速为450 r·min−1,球磨后的黄铁矿真空冷冻干燥24 h后,在氮气和氩气保护下的低氧手套箱中用研钵研碎,称取实验所需质量的微粒,放入乙醇介质中,用超声清洗仪进行超声,分散均匀后,置于低氧手套箱内备用。
-
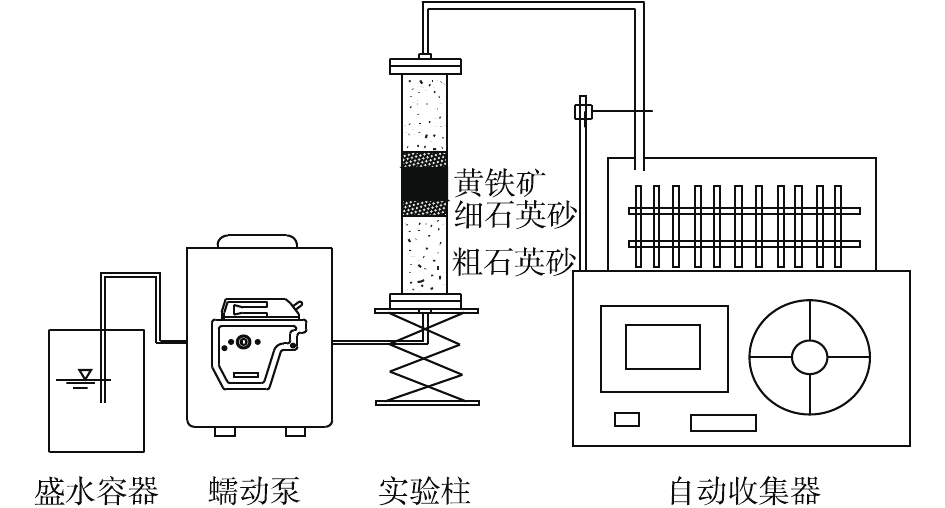
地下水模拟PRB反应装置为自行设计的有机玻璃圆柱,柱长为10 cm,内径为1.5 cm。称取2.829 16 g重铬酸钾溶于1 L去离子水,配制2 000 mg·L−1的标准储备液,避光保存于4 ℃的冰箱。实验所用的溶液浓度均稀释配制,通氮气1 h后备用;将圆柱内壁打磨后,开始装填,实验柱体组成分3部分,由下至上依次为溶液进水区(粗石英砂和细石英砂部分)、反应主体区(装填黄铁矿部分)和溶液出水区(细石英砂和粗石英砂部分)。进水口、出水口与石英砂之间用尼龙纱布和医用棉花进行分隔,防止羽流将装置冲透。按照柱体布置顺序由下至上均匀装入,每装填约2 cm后夯实一次,夯实后的表面进行划毛,防止分层,随后重复操作直至装填完成。柱内装填石英砂总质量为28.54 g,黄铁矿用量为2 g,高度约为1 cm,用螺栓将柱体和法兰盘拧紧固定,确保柱内水在蠕动泵的恒流压力下不向外溢出。将有机玻璃柱连接到饱和装置进行饱和,饱和结束后,用软胶管将蠕动泵和PRB装置连接起来,按照稳定的进液流速0.1 mL·min−1,从土柱下方通入待处理溶液,出水口的溶液连接到自动收集器,实验装置如图1所示。
本实验主要研究了纳米FeS2作为透水反应性屏障(PRB)介质对Cr(Ⅵ)的去除效果,当污染物受到自然水力梯度的影响并流经介质时,可与其发生反应,导致污染物转化为危害更小的化合物,或将其固定在反应介质中[13]。通过对纳米FeS2的形态表征和Cr(Ⅵ)浓度的监测,探索了天然纳米黄铁矿去除土壤中Cr(Ⅵ)的反应机理。本次动态吸附实验在(25±2) ℃下进行,柱体孔隙体积为11.59 mL,通过记录出水水样的体积,作为Cr(Ⅵ)穿透曲线的横坐标,出水Cr(Ⅵ)浓度、pH、Fe3+浓度作纵坐标。吸附过程起始阶段收集样品时间为30 min,吸附后期,逐渐延长取样时间,调整为60、90、120 min,直至Cr(Ⅵ)浓度趋于稳定后,结束吸附实验。
吸附实验结束后,对PRB反应装置进行解吸附实验,主要探索在解吸附后反应介质FeS2对Cr(Ⅵ)的还原固定效果。当测得出水Cr(Ⅵ)的浓度达到峰值并趋于平稳后,用脱氧去离子水代替重铬酸钾溶液,以相同的流速继续供液,直至渗出液中Cr(Ⅵ)浓度降至检出下限后,结束解吸附实验。
1.1. 实验材料
1.2. 主要仪器
1.3. 天然纳米级黄铁矿的制备
1.4. 地下水模拟PRB柱实验
-
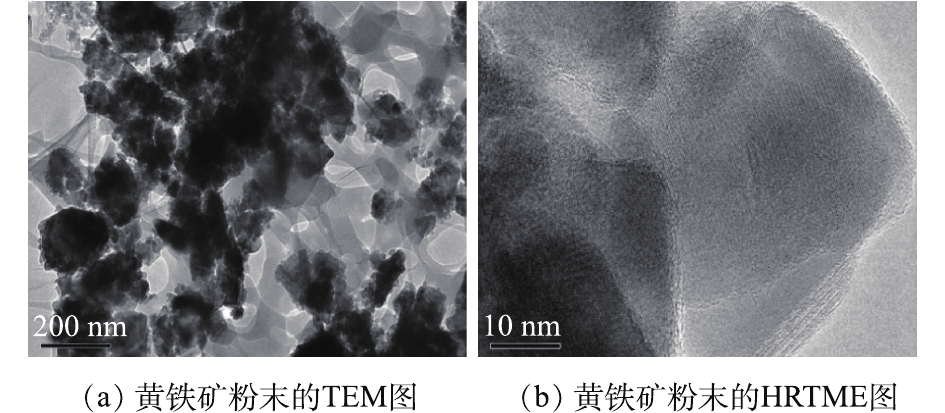
通过粒度分析仪测得黄铁矿的比表面积为11.988 m2·g−1,平均粒径在100 nm左右。图2(a)为黄铁矿球磨24 h超声分散30 min后的TEM表征结果,图2(b)为黄铁矿粉末超声30 min后的HRTME图像[8]。可以看出,黄铁矿粒径可以达到纳米级别,界面距离较大,形状主要为不规则的片状[14],由于乙醇的加入使得分散性改善,减少了纳米黄铁矿的团聚现象。
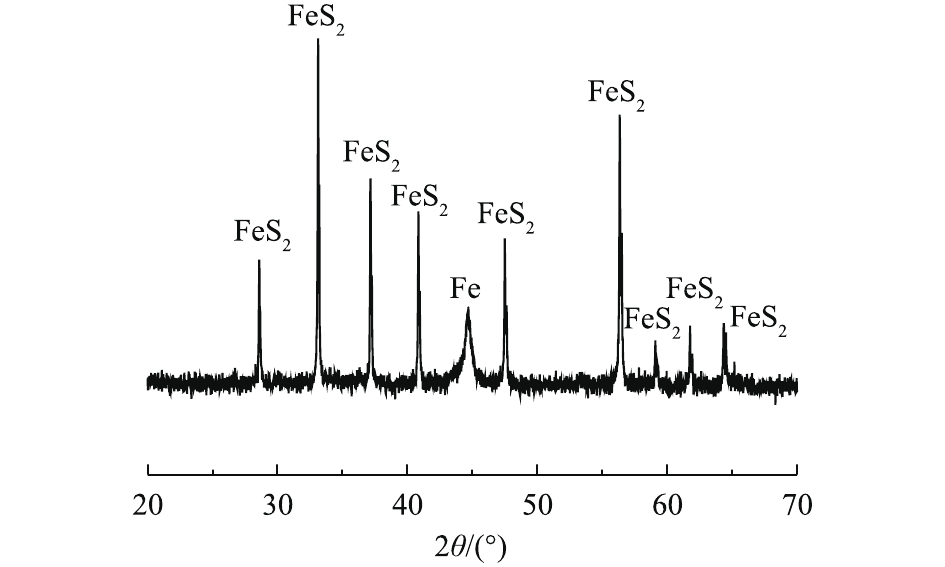
天然纳米黄铁矿的XRD表征结果如图3所示。结果表明,制备的纳米微粒中除含有少量机械活化过程中产生的零价铁之外,无其他物相存在。
-
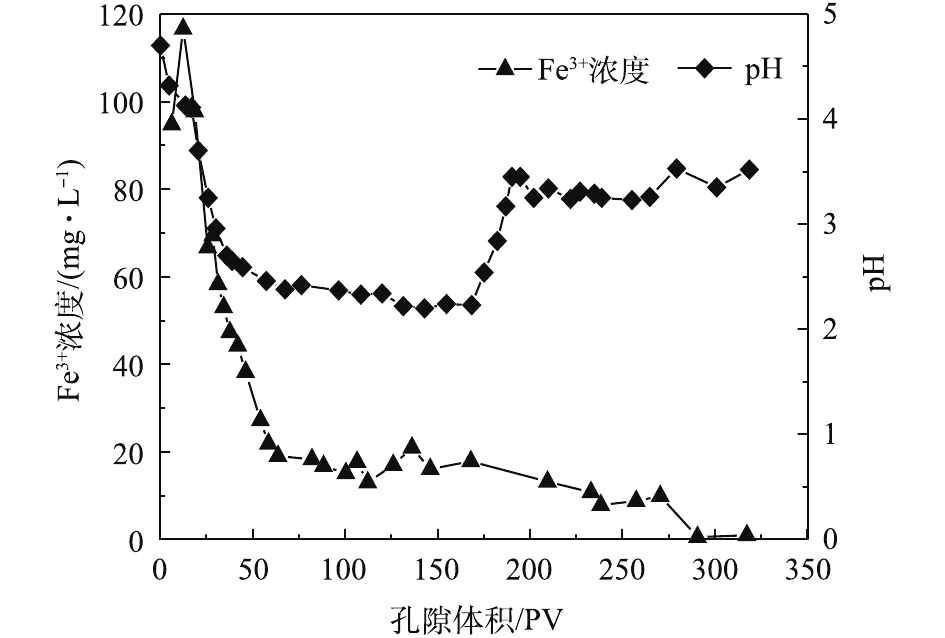
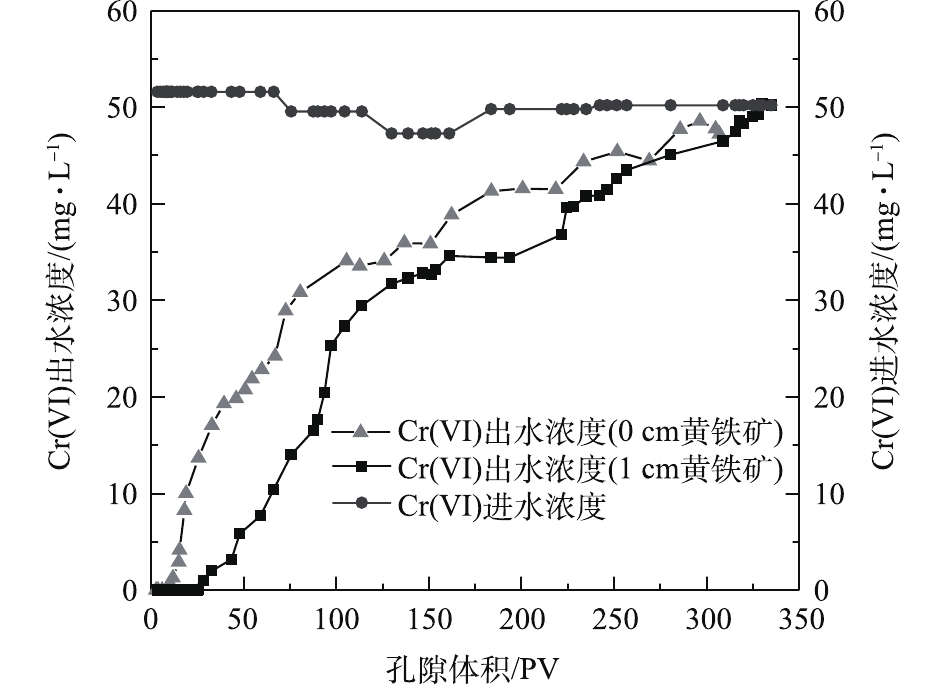
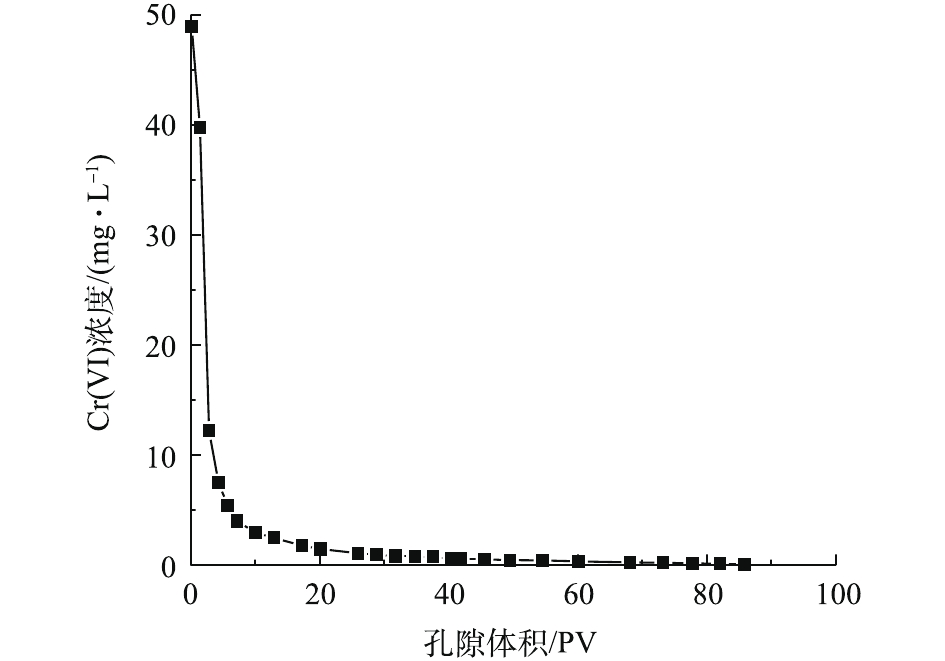
本次PRB实验装置中的进水Cr(Ⅵ)浓度基本控制在50 mg·L−1左右。调节进水流速稳定在0.1 mL·min−1,记录柱体出水体积,测定出水Cr(Ⅵ)的浓度,以出水Cr(Ⅵ)浓度为纵坐标,出水孔隙体积(pore volume, PV)数为横坐标,绘制Cr(Ⅵ)的穿透曲线,结果如图4所示。根据穿透曲线的形状特点,可大致将Cr(Ⅵ)的吸附过程分为吸附传质阶段和吸附饱和阶段2个阶段[15]。由图4可知,当装填0 cm黄铁矿的石英砂柱为8个孔隙体积时,溶液开始穿透,在第280个PV时,达到峰值并趋于稳定,对Cr(Ⅵ)几乎没有阻滞效果。但在石英砂柱内装填1 cm黄铁矿PRB反应层后,溶液穿透点和浓度峰值分别滞后了17个孔隙体积和55个孔隙体积。这说明纳米级天然黄铁矿可以降低Cr(Ⅵ)在土壤中的迁移速率,因为前期纳米黄铁矿吸附位点较多,产生的正电荷对
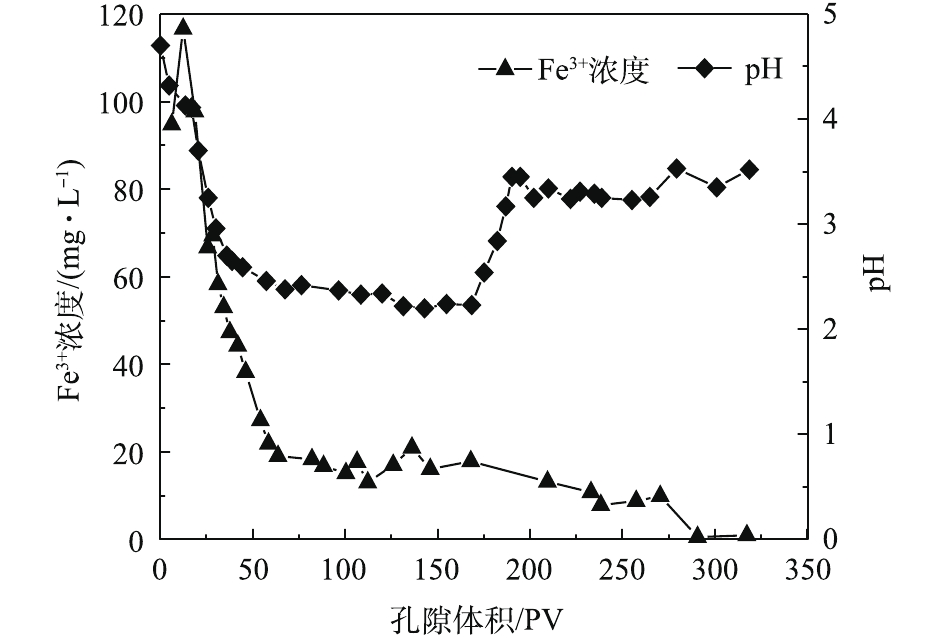
HCrO−4 有较强的静电吸引力,Cr(Ⅵ)被纳米黄铁矿还原成Cr(III),同时生成对应的氢氧化物被固定在土壤中[16],同时,1 g纳米黄铁矿约吸附了50 mg·L−1的Cr(Ⅵ)溶液7.379 9 mg。在25~153个孔隙体积期间内,穿透曲线斜率较大,吸附速率较大,处于吸附传质阶段。随着孔隙体积的增加,黄铁矿的吸附位点减少,吸附过程逐渐趋于平稳直至吸附饱和[15]。实验过程中定出水pH和Fe3+浓度的变化结果如图5所示。pH从4.7开始缓慢降低,当孔隙体积为150个PV时,整个过程处于强酸性条件;这一区间内的Fe3+浓度急剧下降。随着孔隙体积的增加,在吸附饱和阶段,pH增大至3.5左右并趋于平稳时,酸性条件减弱;Fe3+浓度降低,并接近于0。
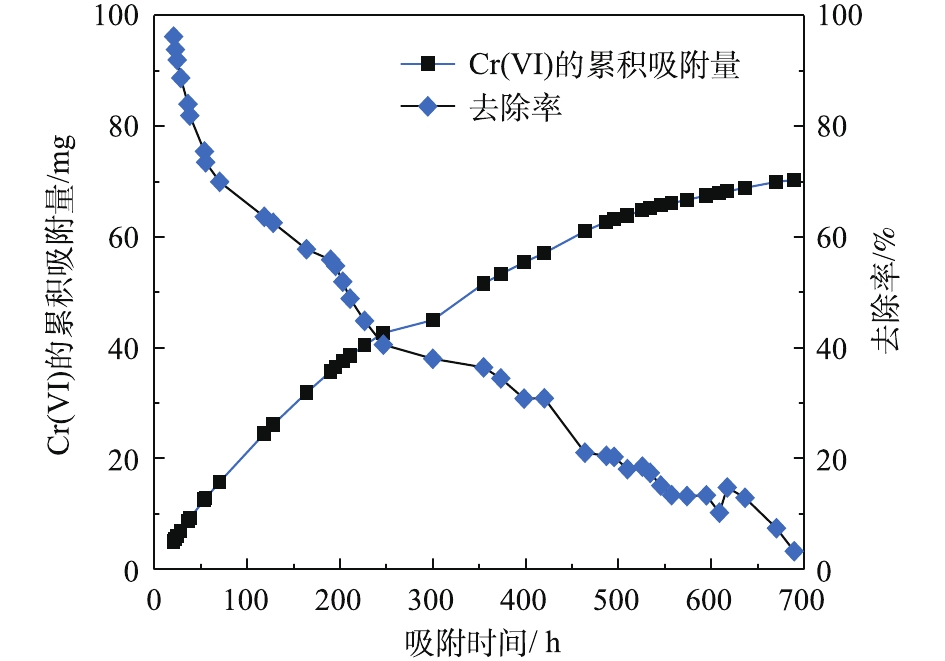
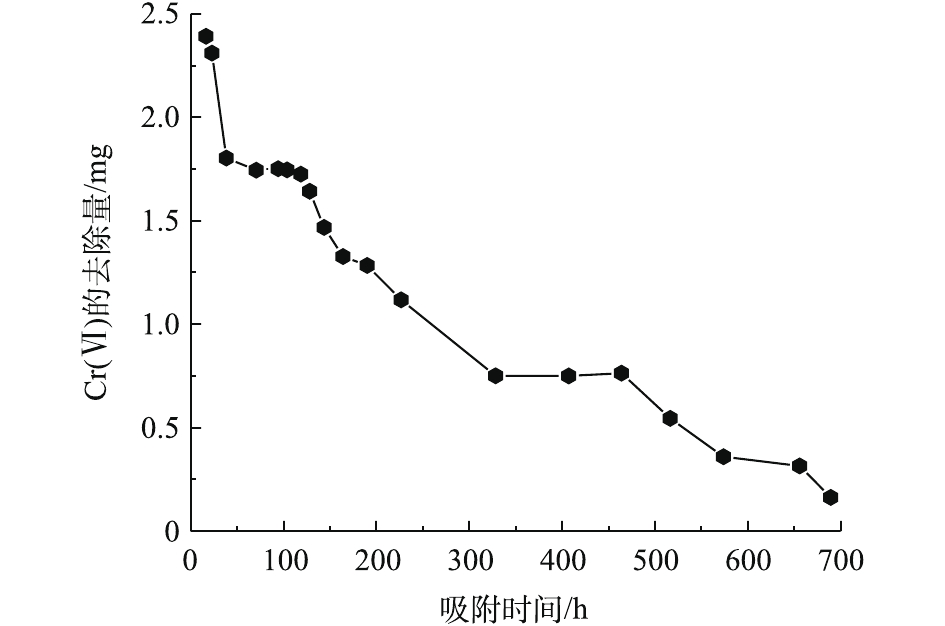
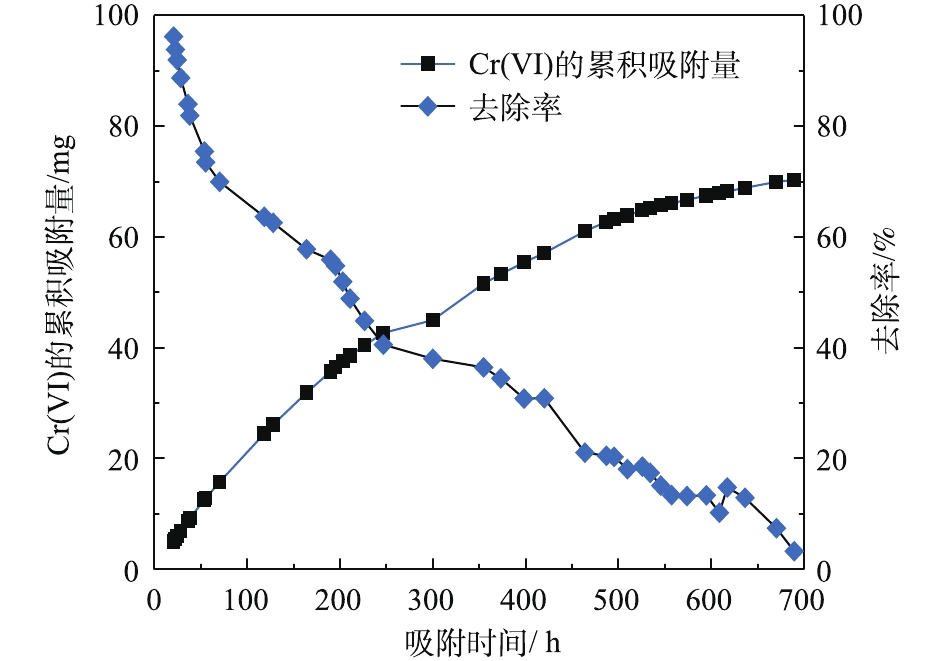
由图6可知,PRB实验装置内的黄铁矿在处理前期对Cr(Ⅵ)的处理量与后期的处理量差距较大(前期处理量>后期处理量),且不稳定。这说明前期吸附过程中,黄铁矿对Cr(Ⅵ)的去除率高,表面反应不均衡。而后期对Cr(Ⅵ)去除率较低,整体波动不大。KANTAR等[17]发现,当pH<6时,黄铁矿表面带正电荷,随着时间的延长,表面氧化产物逐渐堆积。在本研究所使用的非缓冲体系中,H+的消耗导致了Cr3+氢氧化物和混合的Fe3+/Cr3+氢氧化物沉积在黄铁矿表面上,占据了与Cr(Ⅵ)反应的表面位置,并阻止了黄铁矿释放Fe2+离子[18]。因此,黄铁矿还原Cr(Ⅵ)的速率由99.92%迅速降低到3.25%,Cr(Ⅵ)的累积吸附量随时间的延长逐渐增大,总吸附量为70.29 mg,结果如图7所示。
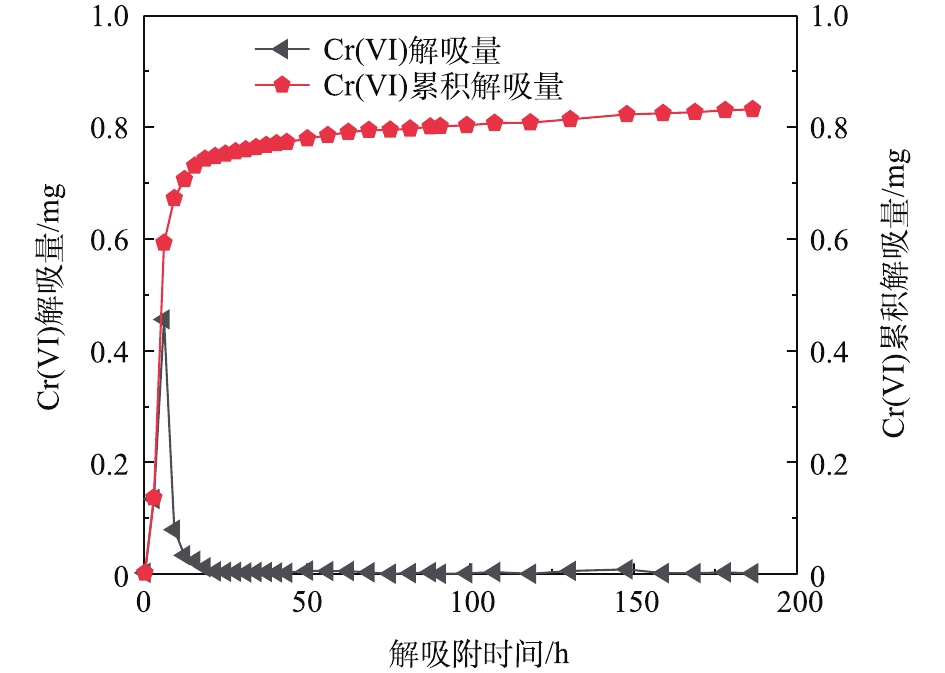
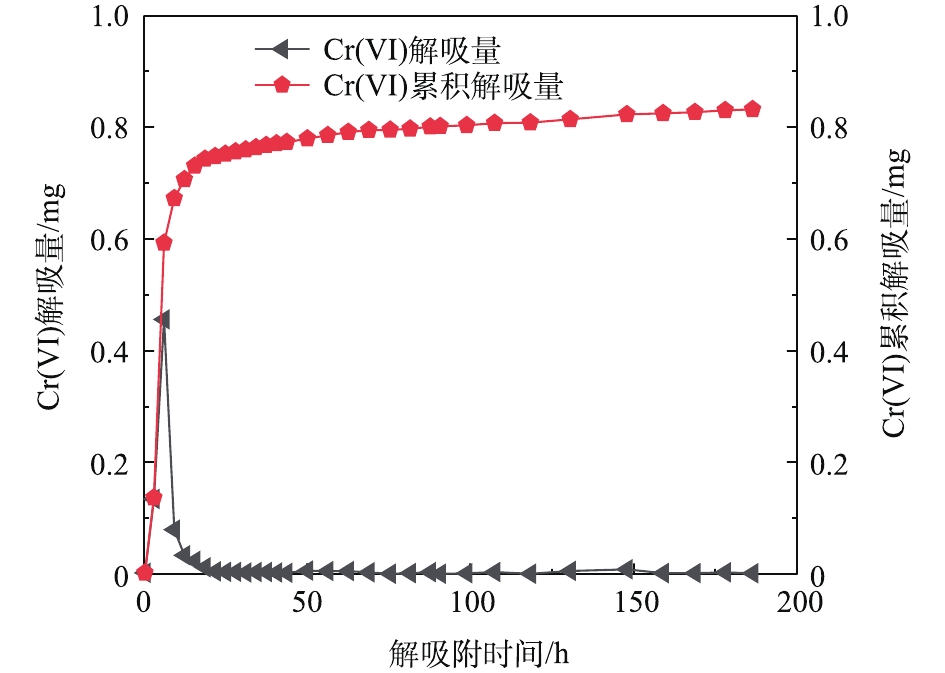
当测得出水溶液的浓度低于初始浓度并达到平衡时,进入解吸附实验阶段,以同样的流速继续泵入脱氧去离子水,收集样品时间为40 min。图8为Cr(Ⅵ)的解吸附曲线,当孔隙体积为1~10个PV时,Cr(Ⅵ)浓度从平衡时的48.5 mg·L−1迅速降到2.9 mg·L−1,之后呈缓慢降低的趋势。对应图9的Cr(Ⅵ)的解吸附曲线,分别表现出较高的解吸量(0.05~0.46 mg)和累积解吸量(0.748 mg)。
结合整个动态吸附过程和解吸附过程,得出黄铁矿PRB反应器对Cr(Ⅵ)的动态运行结果,其中溶液穿透时间为16.3 h,耗竭时间为670.3 h。在运行结束后,2 g纳米黄铁矿还原固定Cr(Ⅵ)共计69.458 mg。
-
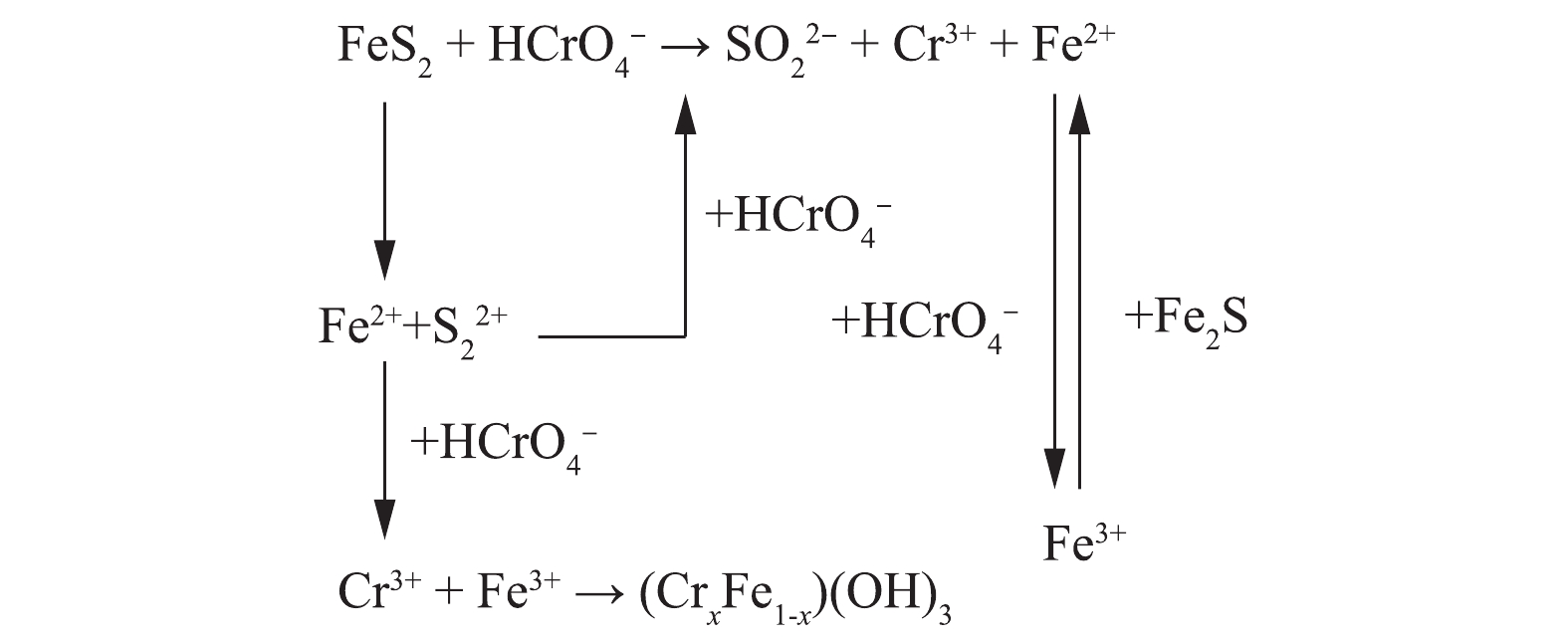
在纳米黄铁矿填充的PRB反应器中,研究了黄铁矿对模拟地下水中的铬污染废水的处理效果。在整个运行过程中,黄铁矿表面发生一系列氧化还原反应,吸附过程分为2个不同的阶段:第1阶段为黄铁矿在酸性条件下的溶解过程,释放出Fe2+和
S2−2 ,随着Fe3+和SO2−4 的生成,这些离子很容易被Cr(Ⅵ)氧化,使得产生的Cr(Ⅵ)的减少;在第2阶段,由于黄铁矿表面化合物的积累,从而其表面钝化。在解吸附过程中,受到物理迁移的作用,溶质从高浓度向低浓度迅速扩散,同时也受到Fe2+的还原作用,Fe2+又将Cr(Ⅵ)还原成为Cr(Ⅲ),从而降低了Cr(Ⅵ)的浓度。卢龙等[19-20]提出,黄铁矿在酸性条件下,其表面容易溶解,并证实在低温弱酸性下溶解反应速率慢,但在酸性强度高时,不需要外界条件就可以发生溶解(式(1)和式(2))。假设溶解的过程产生于晶格畸变,式(3)中Fe2+通过扩散,从晶格内部到表面,最后生成FeOH+。根据Ksp(Fe(OH)2)=8×10−16,得到当pH=6.45时反应便可发生,此时黄铁矿表面硫含量大于铁的含量。当硫开始在黄铁矿表面生成S2−和S时,导致溶液pH增大,S2− 和S被氧化为
SO2−4 的反应复杂且从晶相中参与反应较为缓慢,是影响和决定整个溶解过程中反应速度的关键步骤,SO2−4 的形成会引发FeS2的继续溶解。表面溶解必先经历对硫的还原过程,反应如式(4)~式(6)所示。
式(6)中的关键是要有供电子体存在,Fe2+的电子逸出及其脱离晶格能的束缚为这个过程提供了可能。图6为黄铁矿对Cr(Ⅵ)的去除量随时间的变化。结果表明,随着反应时间的延长,对Cr(Ⅵ)的去除量逐渐降低至较低水平,在320 h后,去除量下降明显。结合图7可知,体系初始pH的下降为PRB反应器内营造了酸性条件,但在320 h之后,pH突然增大,Fe3+浓度相较于之后的降低幅度也大很多。这可能是由于氧化铁在黄铁矿表面沉积,从而导致表面钝化[21]。这一阶段的反应,除了式(7)中Cr(Ⅵ)与黄铁矿的直接反应外,还必须包括黄铁矿的溶解和Fe2+及
S2−2 的后续氧化,即式(8)~式(10)。在大多数条件下,Fe2+在水相中还原Cr(Ⅵ)的速度非常快,因此,只要存在Cr(Ⅵ),Fe2+的浓度就很低,且吸附后的Cr(Ⅵ)与FeS2之间存在电子转移。Cr(Ⅵ)完全还原为Cr(Ⅲ)后,Fe2+与黄铁矿反应成为第2阶段的主要反应,使Fe2+增加,Fe3+随之减少,Fe3+继续被黄铁矿还原为Fe2+(式(11))。
显然,黄铁矿表面仍有部分Fe2+吸附。Cr(Ⅵ)与黄铁矿表面的直接反应还包括Cr(Ⅵ)物质对黄铁矿的吸附, Cr(Ⅵ)还原为Cr2S3,因为Cr2S3很不稳定,容易被水解,与此同时,氧化铁物种的形成和沉积黄铁矿表面,黄铁矿表面行为不活跃,因此,硫酸浓度降低,导致废水pH上升,以固态CrxFe1−x(OH)3的形式存在于黄铁矿表面[22](式(12))。图10总结了黄铁矿还原固定Cr(Ⅵ)的主要反应途径。
在解吸附阶段,由图8~图10可知,在用去离子水连续对反应装置进行解吸附的过程中,较短时间内,Cr(Ⅵ)的浓度就降到最低值。这说明此过程中的物理扩散,使溶质从高浓度区域向低浓度区域迅速下降,同时也受到Fe2+的还原作用,因为反应装置是一个相对厌氧的环境,Fe2+又将Cr(Ⅵ)还原成为Cr(Ⅲ),从而降低了Cr(Ⅵ)的浓度。
2.1. 纳米级黄铁矿的结构表征
2.2. 纳米黄铁矿PRB的运行效果
2.3. PRB反应机理的探讨
-
1)通过柱实验研究了黄铁矿对Cr(Ⅵ)的动态反应(吸附)和解吸附性能,动态吸附阶段FeS2对Cr(Ⅵ)的还原固定分为2个阶段:在酸性条件下,FeS2可以快速的将Cr(Ⅵ)还原为Cr(Ⅲ);随着黄铁矿表面被Fe3+和Cr3+的化合物占据反应位点,反应(吸附)达到饱和。解吸附时,去离子水受到物理迁移的作用,使溶质浓度从高向低迅速扩散,同时也受到Fe2+的还原作用,Fe2+又将Cr(Ⅵ)还原成为Cr(Ⅲ),从而降低了Cr(Ⅵ)的浓度。
2)采用机械球磨活化方法制备纳米级黄铁矿,在PRB反应装置中能够有效地处理含铬废水并将其原位固定在土壤中,2 g的微纳米天然黄铁矿介质固定了约69.458 mg的Cr(Ⅵ),当铬溶液到达穿透点时,去除率达到99.9%。
3)在模拟地下水实验中,用天然纳米黄铁矿原位固定铬污染废水的效果显著。平均1 g黄铁矿可吸附50 mg·L−1的含铬废水约1 854.4 mL,并且能将Cr(Ⅵ)原位固定于PRB中,有效地控制了含铬废水在土壤和地下水中的迁移。






 下载:
下载: