-
磺胺甲恶唑(sulfamethoxazole,SMX)作为磺胺类典型代表,因其具有抗菌谱广、使用方便、价格低廉等特点,被广泛用于水产养殖、畜牧业以及由各种微生物引起的人类疾病预防和治疗中[1-2]。经现有常规污水处理工艺处理后,尽管COD等指标都能达到排放标准,但仍有许多SMX及其中间代谢产物残留在出水中,经过长年的积累会对地表水以及地下水造成不可修复的损坏[3],继而影响整个生态系统的良性循环,最终危害人类健康[4-7]。因此,寻求高效经济的去除方法,控制SMX在水环境的含量对生态环境与生命安全均具有重要的现实意义[8]。
目前,SMX的降解方法主要有芬顿法、改良芬顿法、吸附法等。苏荣军等[9]利用Fenton氧化体系对SMX制药废水进行了研究,在最佳实验条件下,60 min内,SMX的降解率达到了88.9%;赵天亮等[10]利用光降解方法有效地去除了SMX,但少有研究较为系统地考察水中共存阴离子和腐殖酸对SMX降解的影响。近年来,基于硫酸根自由基(
SO−4⋅ )高级氧化技术被广泛应用于土壤和地下水原位修复和有毒有害难生化降解的有机废水实践中[11-13]。由于SO−4⋅ (E0=2.5~3.1 V)有着比羟基自由基(·OH)(E0=1.8~2.7 V)更高的氧化还原电位,可以氧化水中绝大部分有机物,Fe2+作为被广泛使用的激活剂,其具有消耗速度快和形成Fe3+沉积并阻碍反应的明显缺点[14]。Fe3O4具有较高的催化活性,能够缓慢向溶液中释放Fe2+,且因其磁性也更易实现固液分离,在常温常压下反应就可以进行(式(1)),不产生二次污染的同时还可以重复利用多次,是真正的绿色催化剂。本研究采用共沉淀法制备了具有较高催化活性的磁性纳米Fe3O4,并对其物化性质进行了表征,分别考察了PS浓度、Fe3O4投加量、初始pH、共存阴离子(Cl−、CO2−3 、NO−3 )以及腐殖酸(HA)对SMX降解效果的影响,同时考察了Fe3O4的性能和重复利用效果,进一步对降解SMX反应(式(1))的作用机理进行探讨。
全文HTML
-
试剂:磺胺甲恶唑(>98%)、过硫酸钾(>99.5%)、六水合三氯化铁、硫酸亚铁、盐酸、硫酸、氨水、氢氧化钠、氯化钠、无水碳酸钠、碳酸氢钠、碘化钾、硝酸钠、腐殖酸、分析用甲醇、甲酸、乙醇、叔丁醇。实验用水均采用Milli-Q纯化系统(18.2 mΩ·cm)制备的超纯水。
仪器:THZ-C型恒温振荡器,AUY120型分析天平,梅特勒-托利多pH计,超声波清洗机,DZF-6020真空干燥箱,安捷伦1260高效液相色谱,Tescan Mira 3高分辨率场发射扫描电子显微镜(SEM),Oxford X-MaxN能谱分析仪(EDS),紫外可见分光光度计(UV5500),布鲁克TENSOR-II傅里叶红外光谱仪,DX-2700型X射线衍射仪(XRD),TOC分析仪(岛津TOC-VCPH),ASAP 2460全自动比表面及孔隙度分析仪(BET)。
-
将一定浓度的FeCl3·6H2O溶液和FeSO4·7H2O溶液混合,再将混合液快速加入到装有氨水、置于超声波清洗机中的三口烧瓶中,全程在通入氮气的条件下进行,控制温度为60 ℃。反应30 min后,用磁铁将黑色的磁性Fe3O4纳米材料收集,用去离子水反复冲洗至上清液呈中性后,置于真空干燥箱中60 ℃烘干,研磨后,储存于干燥皿中。样品用扫描电镜-能谱(SEM-EDS)、红外(FT-IR)、X射线衍射(XRD)、比表面(BET)进行表征。
-
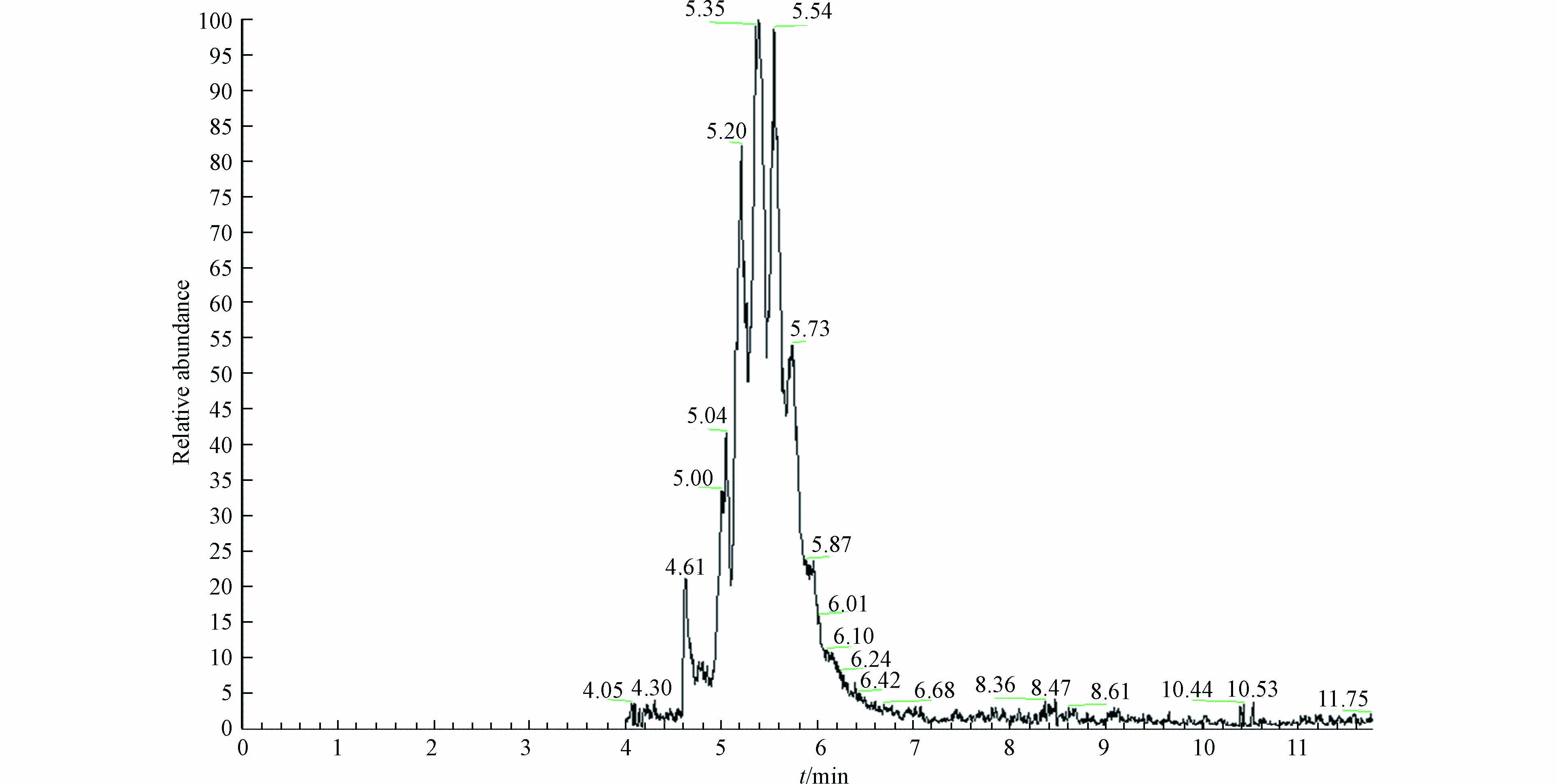
量取50 mL 20 μmol·L−1的SMX溶液于锥型瓶,将其置于摇床中,控制温度为30 ℃,转速为180 r·min−1,实验先投加一定量的Fe3O4反应30 min,待Fe3O4吸附平衡后,加入过硫酸盐溶液,每隔一定时间取水样,立即加入甲醇淬灭反应。反应液经0.22 μm滤膜过滤,利用高效液相色谱(HPLC)进行定量分析。通过单因素实验明确了PS浓度、Fe3O4投加量、初始pH对SMX降解效果的影响,确定PS浓度、Fe3O4投加量、初始pH最佳条件后,在此基础上又考察了共存离子和腐殖酸对SMX降解的影响。通过添加乙醇(EtOH)[15-16]及叔丁醇(TBA)[16]来鉴定体系中
SO−4⋅ 和·OH的存在。实验中,SMX的降解可用拟一级动力学描述,如式(2)所示。式中:Ct为t时刻的SMX浓度,μmol·L−1;C0为SMX初始浓度,μmol·L−1;t为反应时间,min;k为拟一级反应速率常数,min−1。
-
采用分光光度法[17]测定PS浓度。在NaHCO3存在的条件下,PS与KI反应生成黄色络合物,通过紫外可见分光光度计(UV5500,上海元析仪器有限公司)于400 nm处进行测定。SMX浓度采用美国安捷伦1260高效液相色谱仪及C-18的色谱柱进行定量分析。检测条件为:流动相(甲醇/0.1%甲酸)=35∶65,检测波长为270 nm,进样量为10 µL,流量为0.8 mL·min−1,柱温为30 ℃。
1.1. 试剂和仪器
1.2. 催化剂制备和表征
1.3. 实验方法
1.4. 分析方法
-
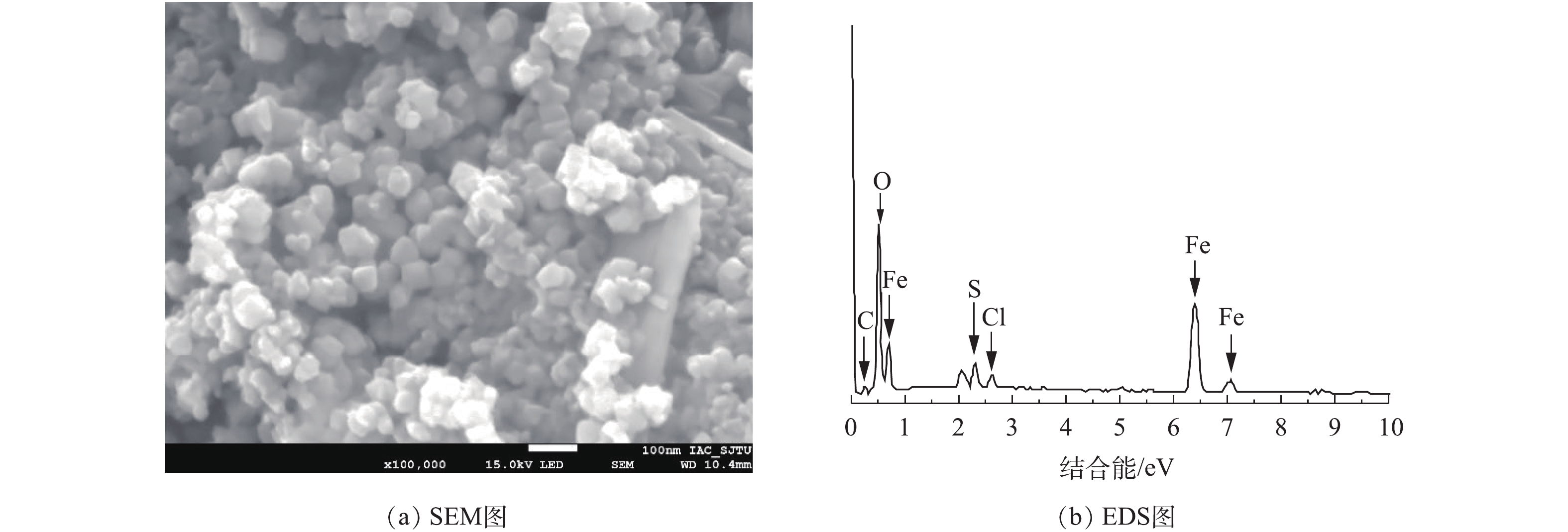
对纳米Fe3O4进行扫描电镜(SEM)、能谱(EDS)、红外光谱(FT-IR)、X射线衍射(XRD)和比表面测试(BET)等分析,具体结果如图1~图4所示。如图1(a)所示,在放大100 000倍的情况下,可清晰地观察到催化剂晶体为立方体颗粒,颗粒均匀,粒径约为40 nm,部分颗粒之间发生了团聚,这说明颗粒之间存在磁性吸引;由图1(b)可知,颗粒中存在C、O、Fe、S、Cl元素,Fe和O含量最高,质量分数分别为39.16%和54.23%。
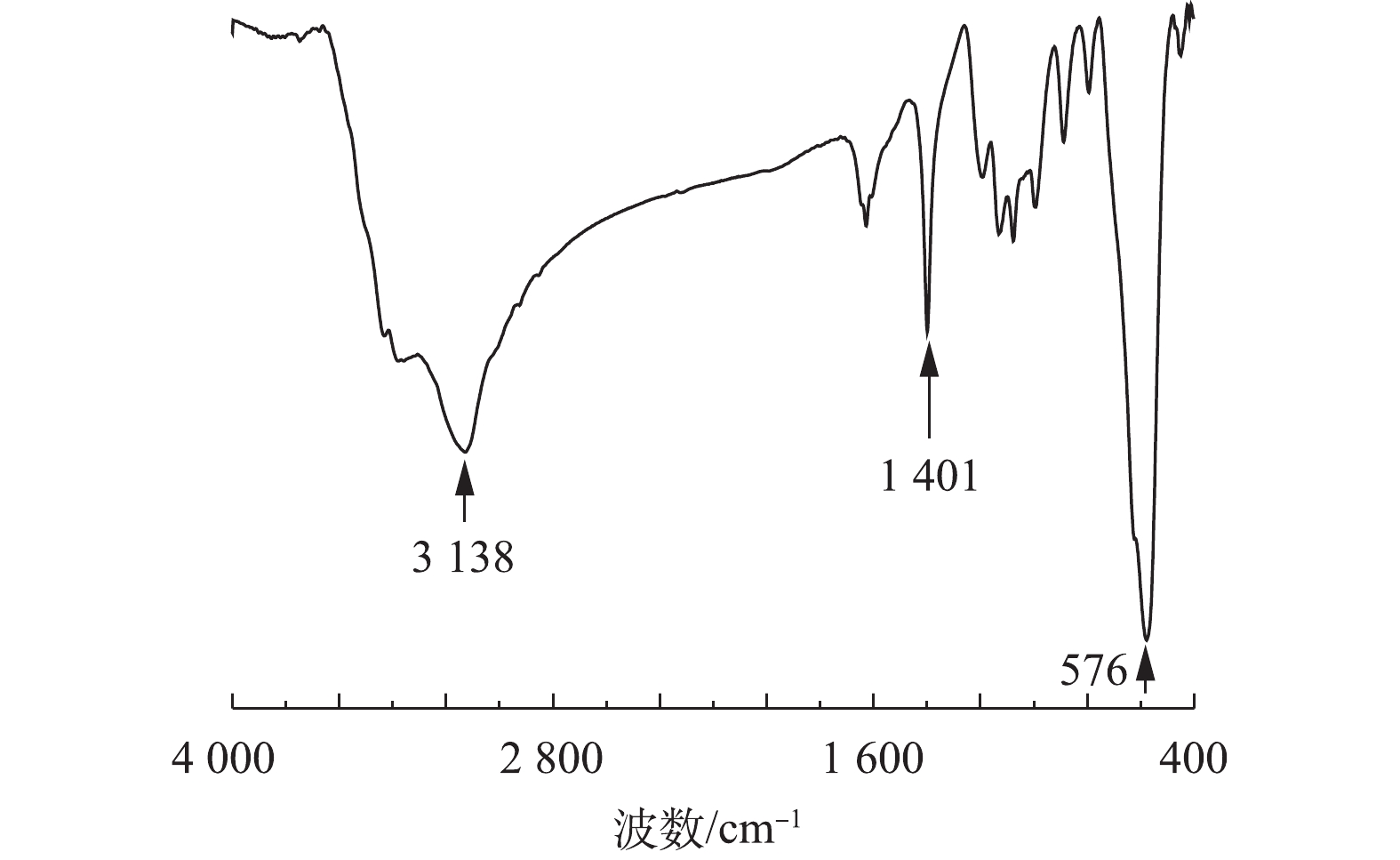
图2为Fe3O4的FT-IR光谱图。由图2可知,在576、1 401、3 138 cm−1处出现3个较强的吸收峰。波数576 cm−1处为Fe—O伸缩振动峰,1 401 cm−1和3 338 cm−1处分别是H—O—H和O—H的伸缩振动峰,这可能是由于少量水分子吸附在Fe3O4表面所导致的。
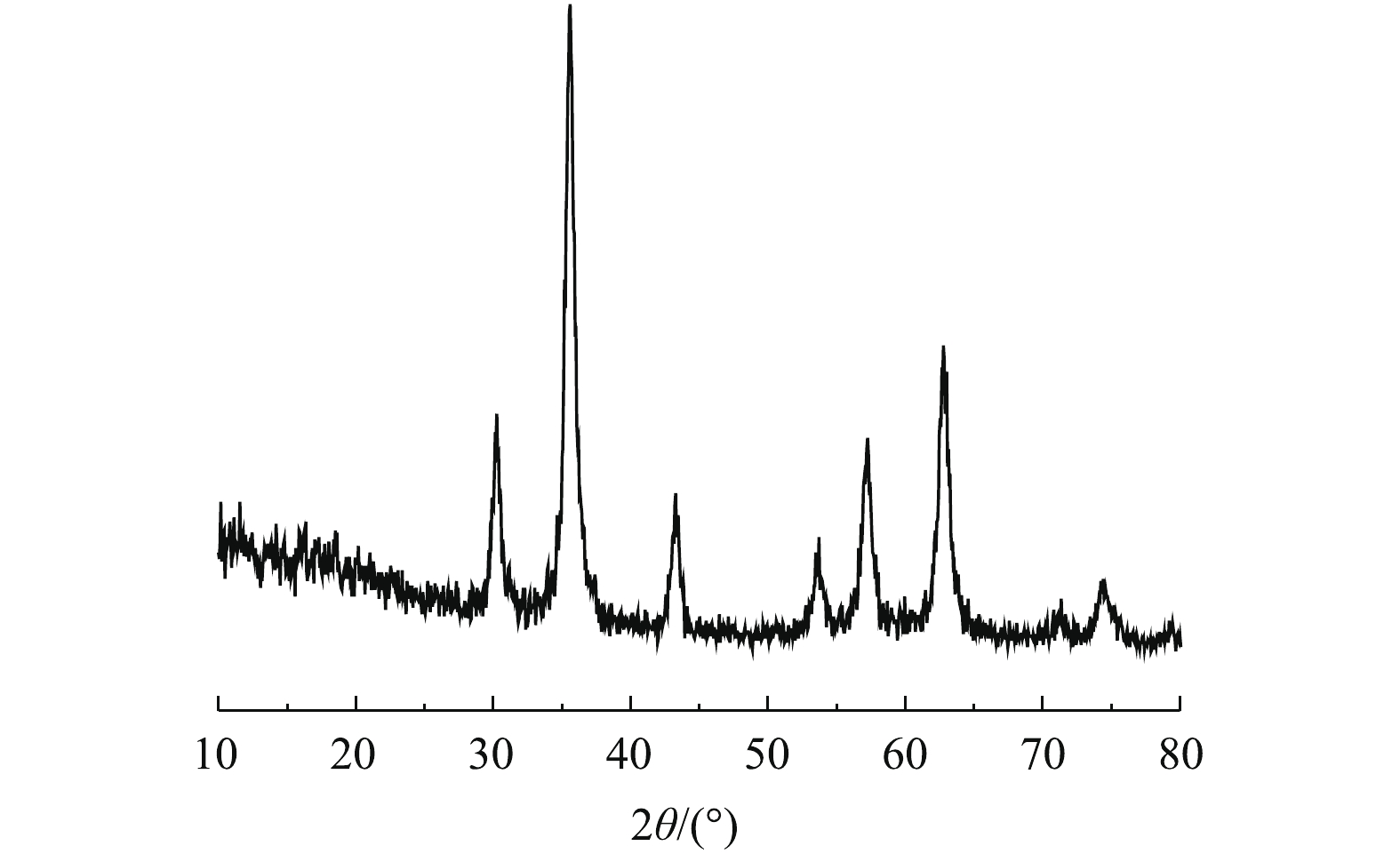
图3为Fe3O4的XRD图谱,将其与Fe3O4标准图谱(JCPDS PDF#65-3107)进行对比,在30.1º、35.4º、43.1º、53.4º、57.0º、62.5º、74.1º处具有较强的衍射峰,与标准Fe3O4所具有的特征衍射峰相吻合,这说明制得的催化剂为纯度较高的Fe3O4。
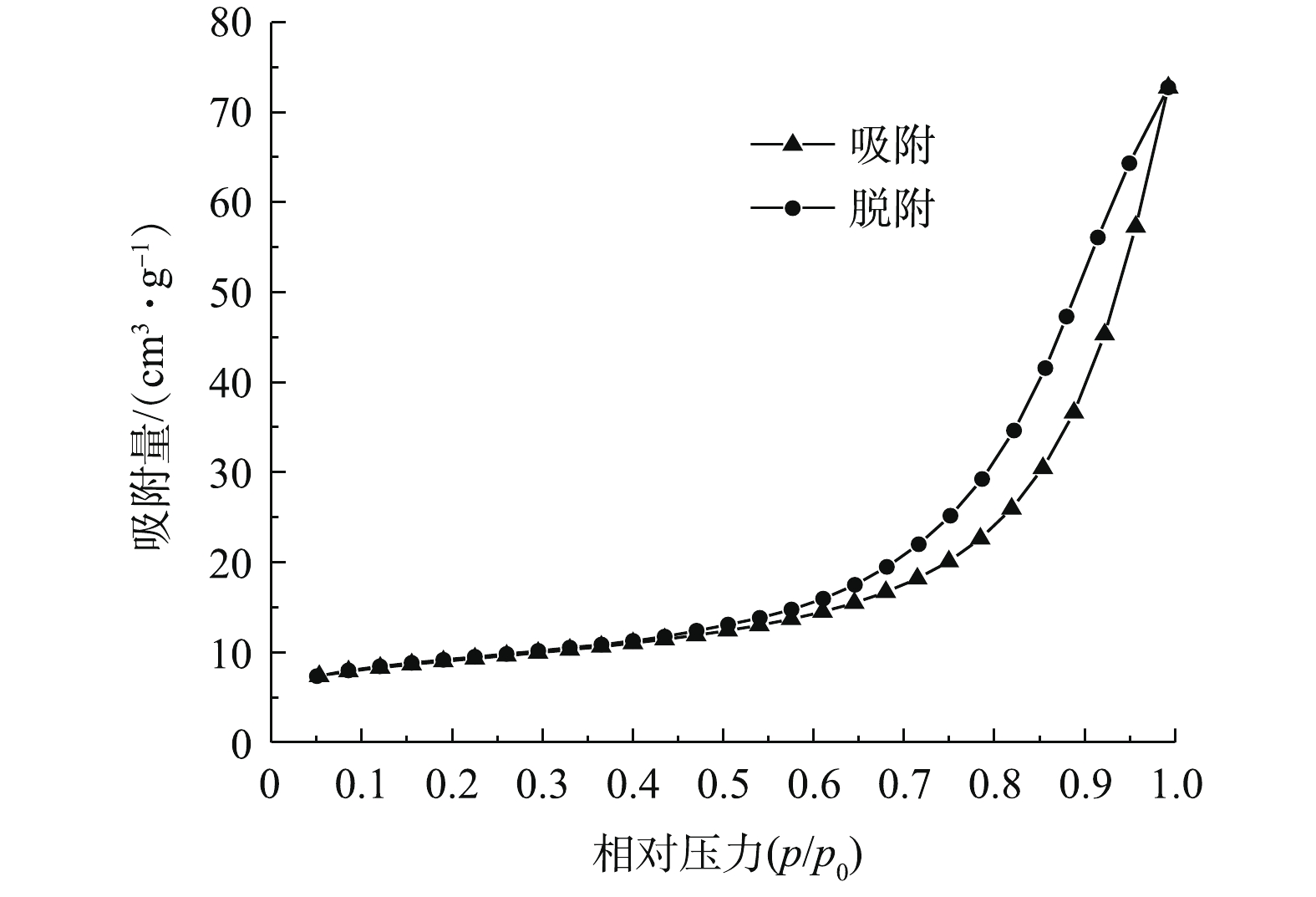
图4为依据Brunauer-Emmett-Teller方程计算得到的Fe3O4吸附氮气的吸附解吸等温曲线。由图4可知,该等温线呈现出明显的IV型,根据IUPAC的分类,说明Fe3O4为典型的中孔吸附材料。其平均孔径为12.936 2 nm,比表面积为30.816 3 m2·g−1,总孔容为0.112 449 cm3·g−1,表明Fe3O4可以提供大量的活性反应位点。
-
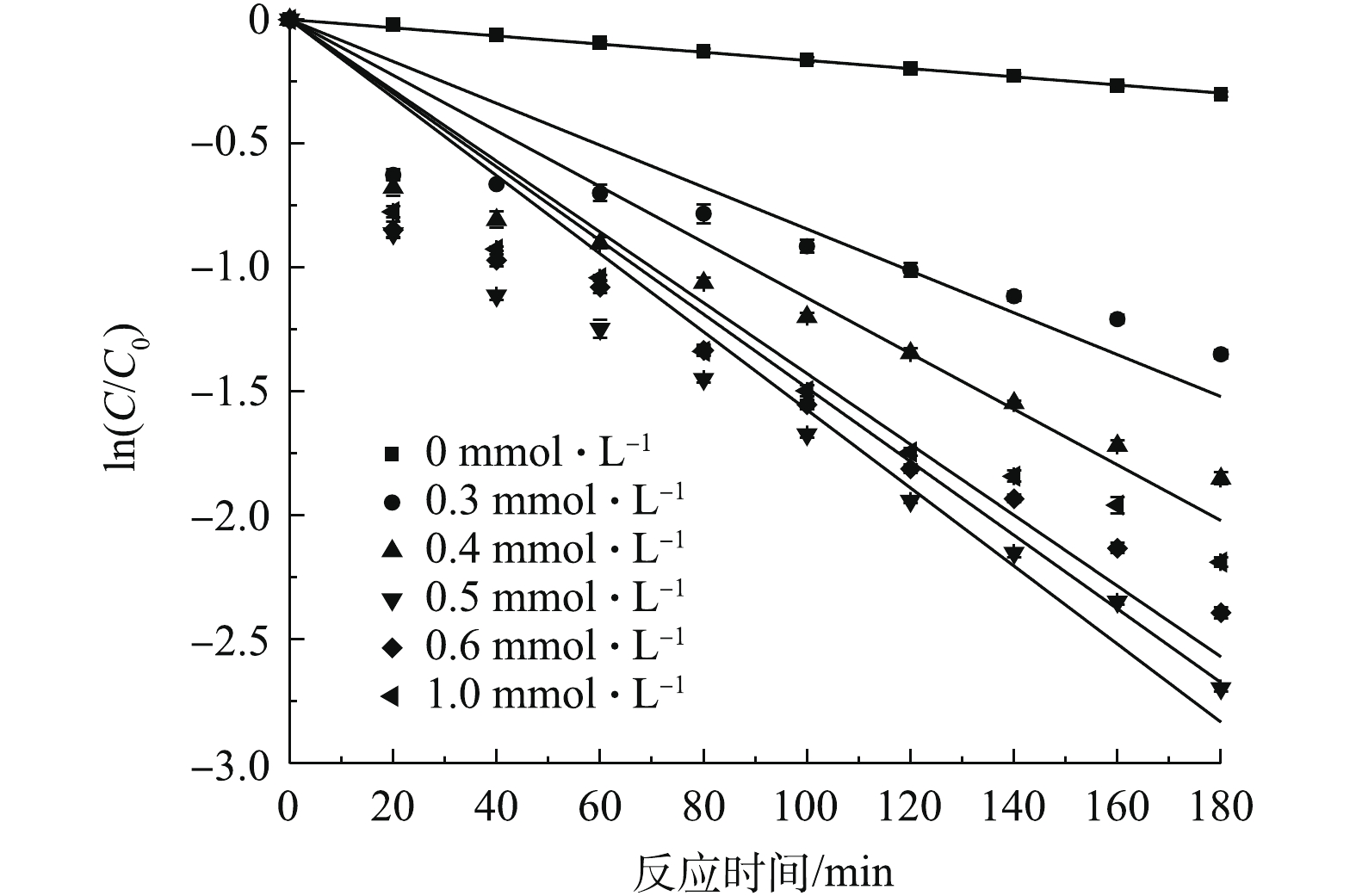
在PS浓度为0~1.0 mmol·L−1的条件下,考察了其对SMX降解效果的影响,结果如图5所示。当PS浓度由0 mmol·L−1提高至0.5 mmol·L−1时,SMX降解速率逐渐提高,反应180 min后,SMX降解速率达到93.3%。分析原因为,随着体系中PS浓度的增加,过硫酸盐可被Fe3O4催化活化,从而产生更多的
SO−4⋅ 参与反应(式(1)),故有利于SMX的降解[12]。当继续提高PS浓度至1.0 mmol·L−1时,SMX的降解率由93.3%降至88.8%。有研究[18]表明,过量的S2O2−8 能够与SO−4⋅ 发生反应(式(3)),过量的SO−4⋅ 也会发生自身的淬灭反应(式(4))。由式(1)可知,催化剂Fe3O4的投加直接影响
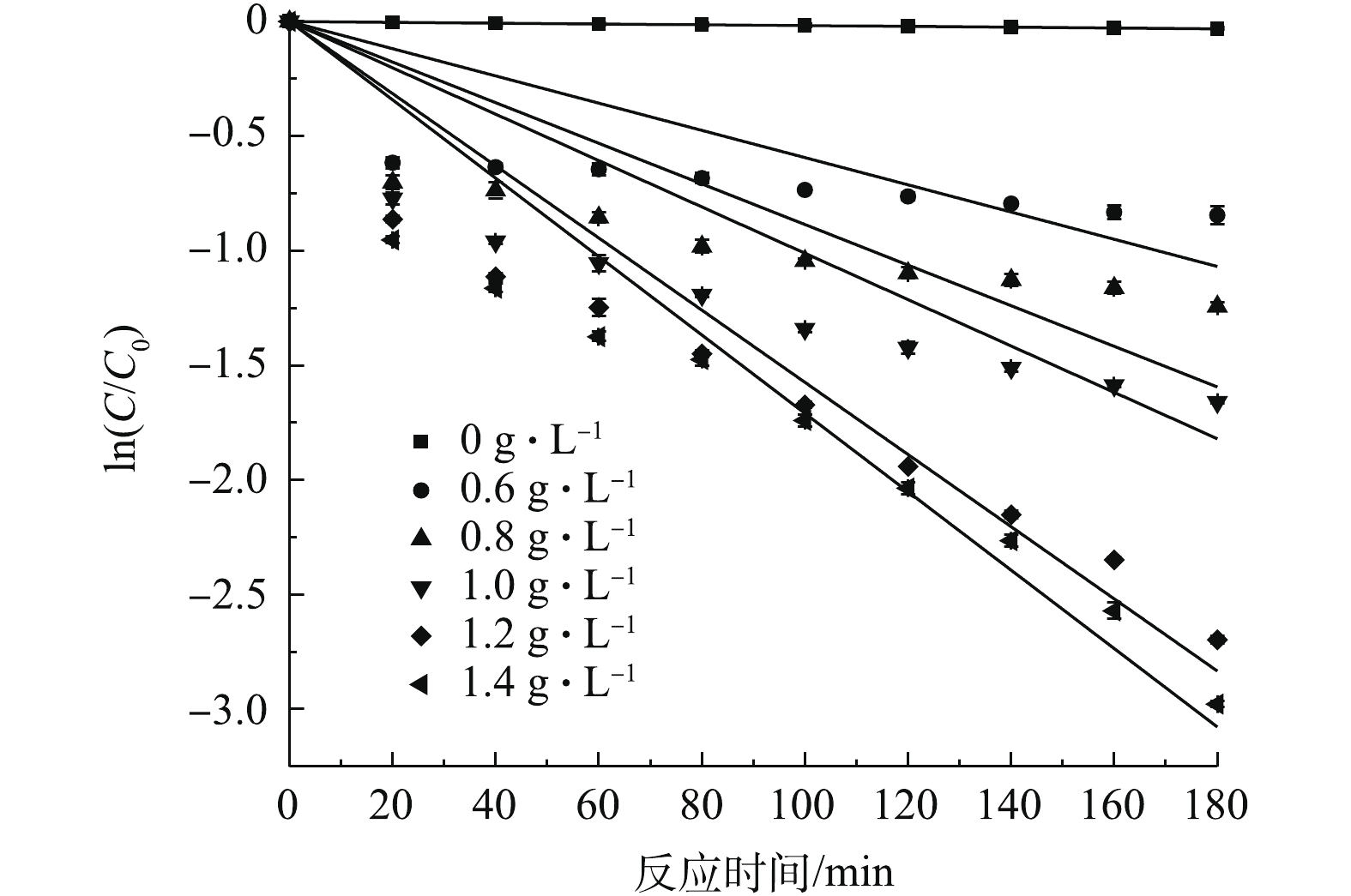
SO−4⋅ 的活化,从而对SMX的降解效果产生影响。在Fe3O4投加量为0~1.4 g·L−1的条件下,考察了SMX的降解效果。由图6可知,在未投加Fe3O4的条件下,SMX基本没有被降解,当Fe3O4投加量增加到1.2 g·L−1时,反应180 min,SMX的降解率可达到93.3%。由式(1)可知,增加Fe3O4的投加量可提供更多的Fe2+,PS被Fe3O4表面的Fe2+激活,从而产生更多的SO−4⋅ 参与反应。此外,磁性Fe3O4表面的Fe2+能够引发一系列类芬顿反应,因此,增加体系中Fe3O4投加量,能提高SMX的降解率。当继续增大Fe3O4至1.4 g·L−1时,SMX降解效果的变化并不明显,这可能是因为在Fe3O4催化活化PS反应中,体系中PS浓度相对不足造成的。图7为不同pH (3、5、7、9、11)对SMX的降解效果的影响。由图7可见,随pH的增大,SMX的降解率逐渐降低。这一现象与
S2O2−8 在不同pH下的不同氧化性有关[19]。在酸性条件下,S2O2−8 不仅可以被Fe3O4激活产生SO−4⋅ ,还可以与H+反应生成SO−4⋅ (式(5)和式(6)),有研究[20]表明,水溶液中SO−4⋅ 也会反应生成·OH(式(7)),从而促进活化PS反应的进行。在碱性条件下,
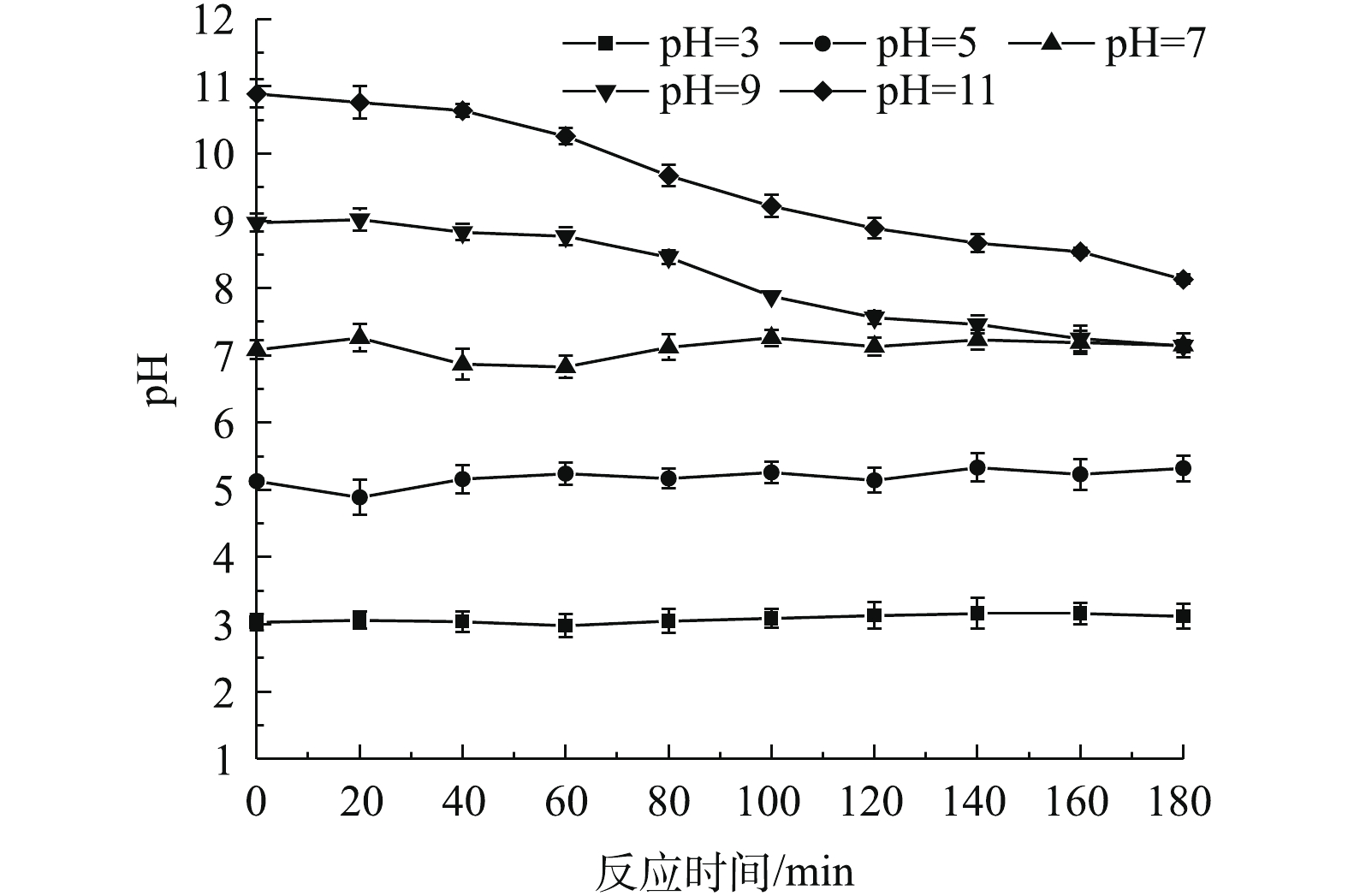
SO−4⋅ 和OH−更易反应生成·OH(式(8)),而·OH的氧化电位低于SO−4⋅ [21];当pH升高时, 体系中Fe2+从Fe3O4表面脱除,反应产生的Fe3+也会生成氢氧化物胶体,从而增加磁性Fe3O4颗粒的团聚,致使其催化活性进一步的降低,因此,碱性条件对降解SMX的抑制作用更加明显。考虑自然状态下实际水体pH多为中性,故选择最适pH为7。反应过程中pH随时间的变化曲线如图8所示,由图8可知,在SMX降解过程中,当初始pH≥9时,反应过程中的pH逐渐下降,而在中性和酸性(3.0~5.0)条件下,反应过程中的pH略有增加,但变化并不显著。初始pH很高时,pH出现明显下降,很可能是由于SMX在降解过程中生成了CO2和较低分子质量的有机酸。鉴于H2CO3是一种弱酸,pKa1和pKa2分别为6.3和10.3,当初始pH处于中性至酸性范围时,释放到溶液中的H+不足以改变溶液的pH,因此,溶液中pH相对稳定,这与GONG等[22]和WANG等[23]的研究结果相似。
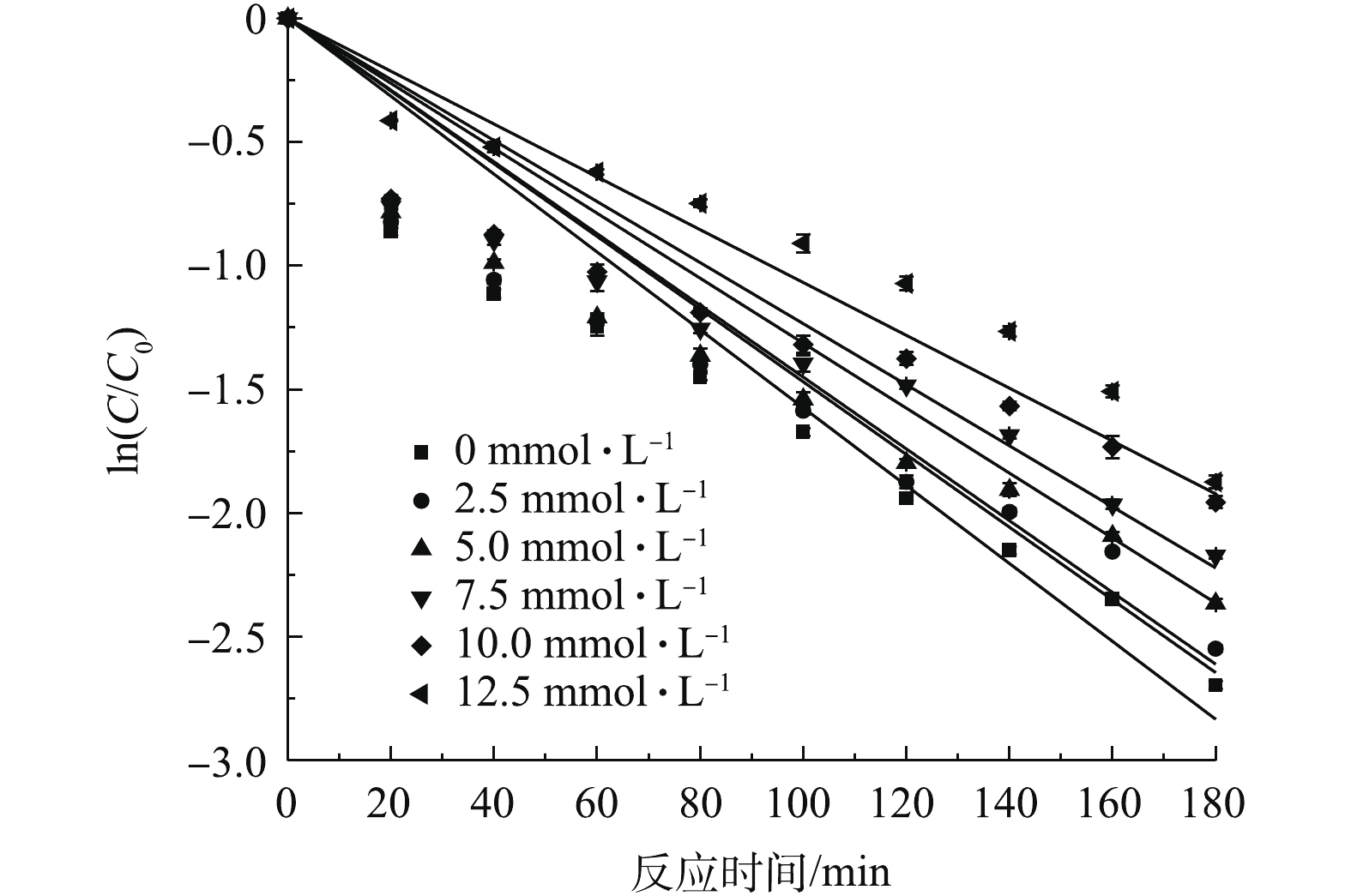
天然水体中存在着大量阴离子,其在维持酸碱平衡等方面发挥着重要的作用。实验考察了Cl−、
CO2−3 、NO−3 这3种阴离子在降解SMX实验中的影响。图9为加入2.5、5.0、7.5、10.0和12.5 mmol·L−1 Cl−时对SMX降解效果的影响。由图9可知,与不投加Cl−相比,在反应180 min后,对应不同的Cl−投加浓度,SMX的降解率分别下降了1.08%、2.65%、4.65%、7.40%、8.61%。结果表明,低浓度的Cl−对于SMX的降解基本无影响,随着Cl−浓度的增加,对SMX降解抑制作用逐渐加强。有研究[24-25]表明,Cl−是一种有效的自由基捕获剂,可与SO−4⋅ 反应生成氯自由基(Cl·) (式(9)),随着Cl−浓度的升高,Cl·又可迅速与Cl−反应,生成活性较低的二氯自由基(Cl−2⋅ )[26](E0=1.36 V)(式(10)~式(12)),减弱了SO−4⋅ 的作用;其次,Cl−也可能会优先吸附到磁性Fe3O4表面的活性部位,使得磁性Fe3O4催化能力降低,导致SO−4⋅ 产生量减少。另外,还有研究[27-28]表明,S2O2−8 和SO−4⋅ 等自由基的活性随溶液中离子强度的增大而降低。不同
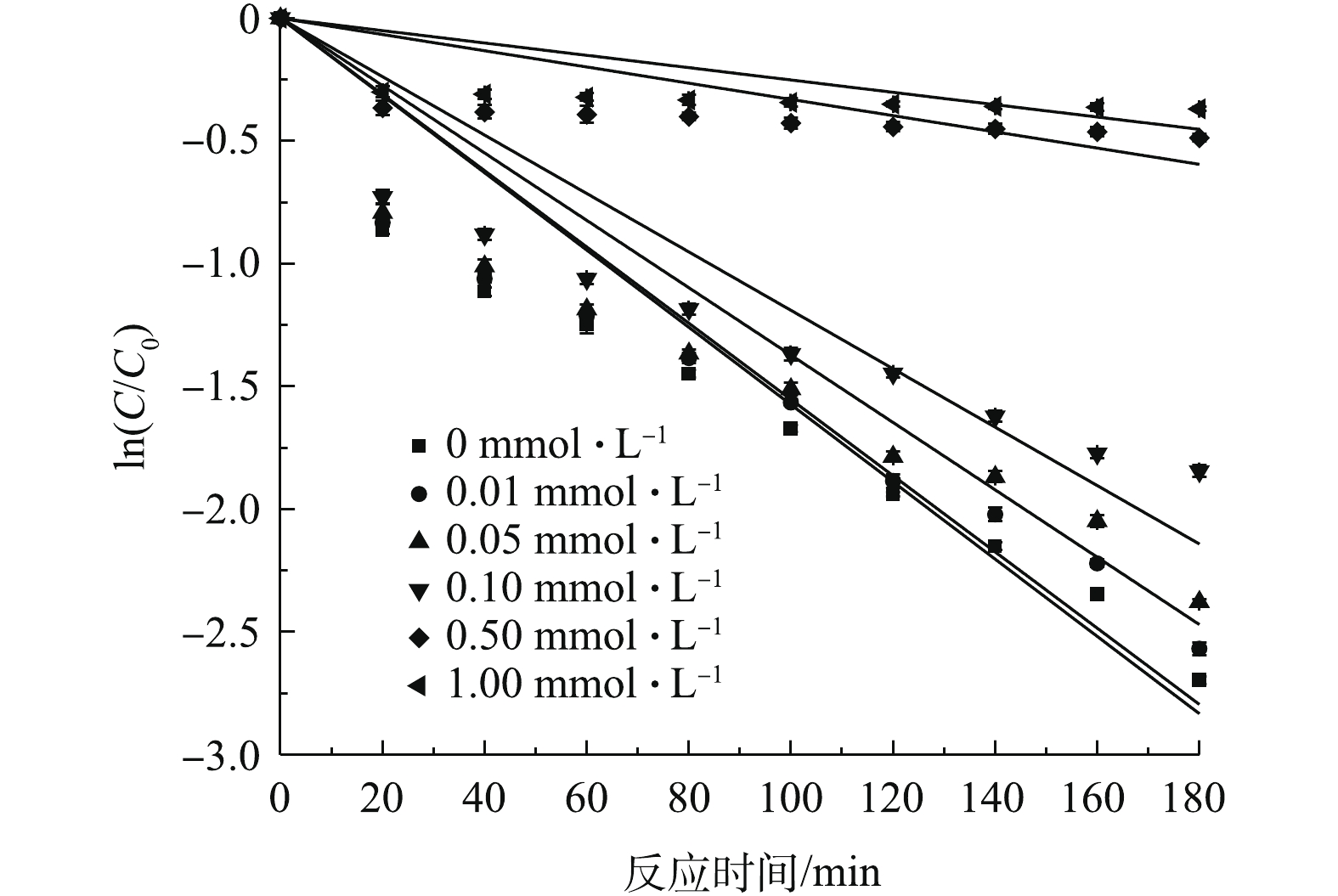
CO2−3 浓度对SMX降解效果的影响如图10所示。结果表明,分别加入0.01、0.05、0.10 mmol·L−1CO2−3 ,反应180 min后,低浓度CO2−3 对SMX的降解效果有轻微抑制作用,SMX的降解率由93.3%降至84%;当CO2−3 浓度提高至1.00 mmol·L−1时,对SMX降解效果的抑制作用明显增强,SMX的降解率下降至31%。其原因可能是:体系中CO2−3 会发生水解,生成HCO−3 ,而2种离子在溶液中的占比是由溶液的pH决定的(式(13)和式(14))。当溶液pH为7.0±0.1时,可以认为溶液中主要以HCO−3 /CO2−3 共同离子存在,能够与SO−4⋅ 反应,产生CO−3⋅ (式(15)和式(16))。CO−3⋅ 也能与有机物反应,但SO−4⋅ 反应速率常数高于CO−3⋅ ,因此,在体系中加入较高浓度的CO2−3 时,SMX降解速率会有所降低。同时,高浓度CO2−3 对SO−4⋅ 也有淬灭作用。GAO等[29]采用热活化过硫酸盐氧化降解三氯生,研究结果表明,碱性物质(如CO2−3 、HCO−3 )的存在对三氯生降解具有抑制效果。不同
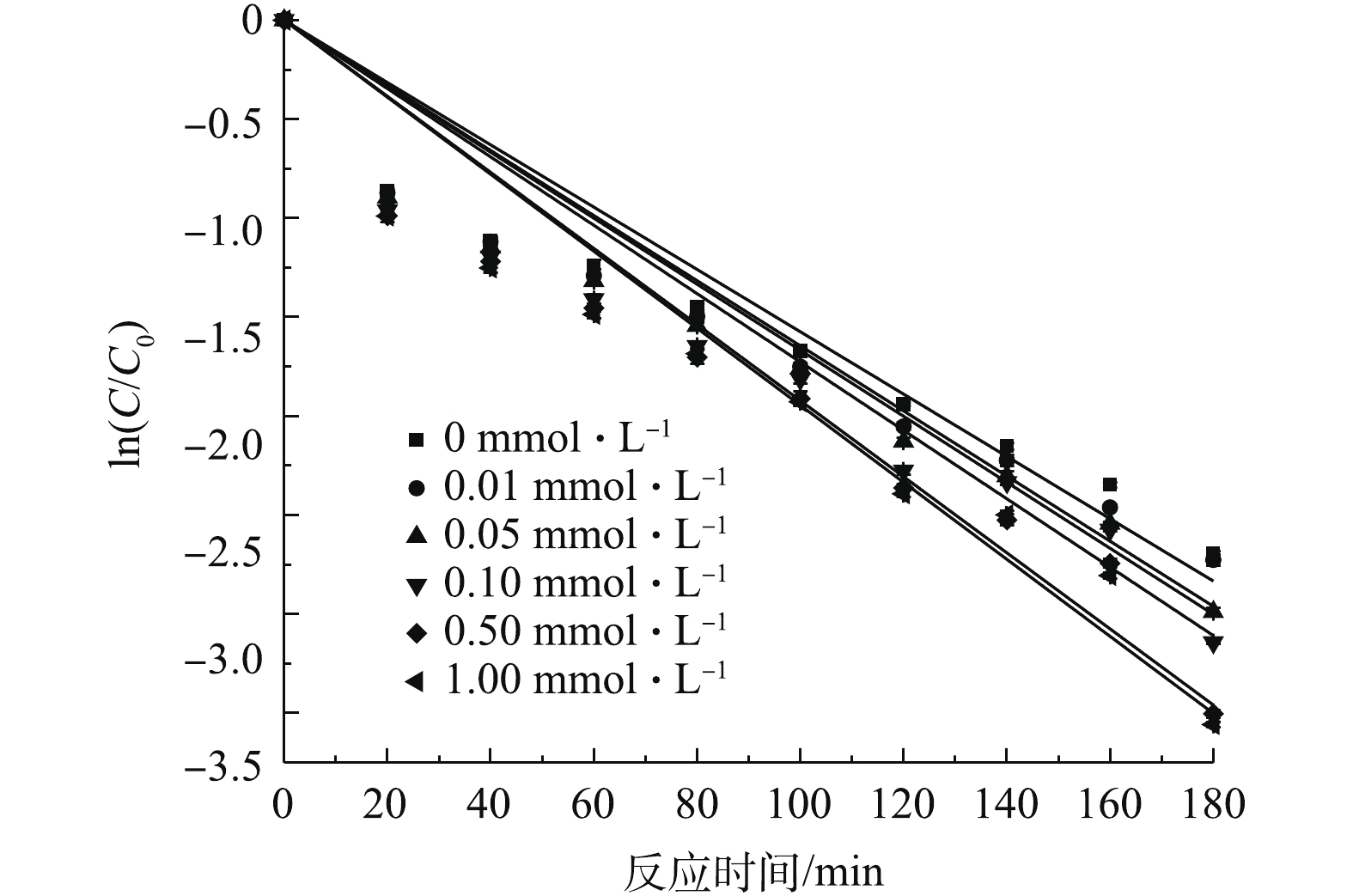
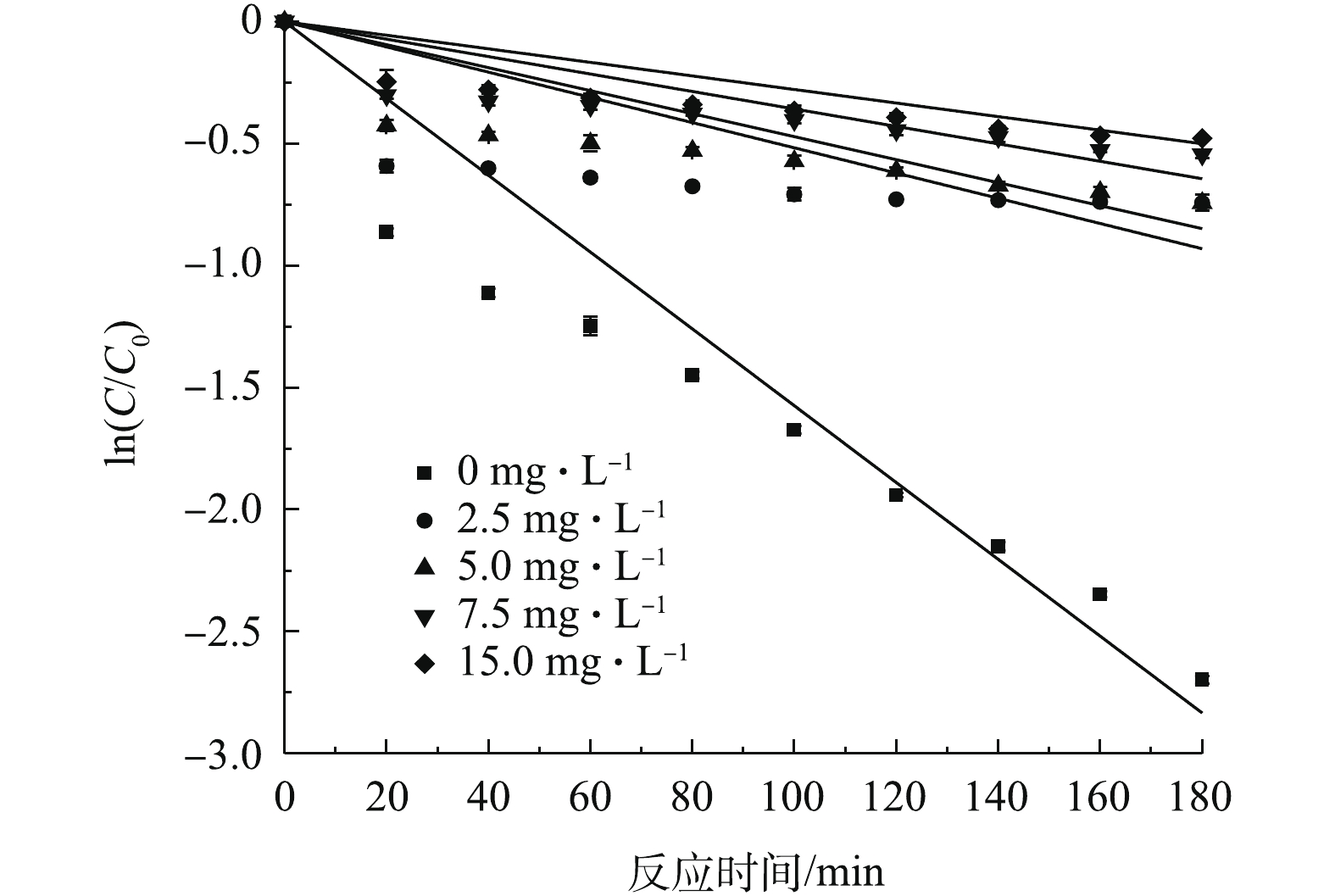
NO−3 浓度对SMX降解效果的影响如图11所示。由图11可知,与不投加NO−3 相比,当反应中分别加入0.01、0.10、0.50、1.00 mmol·L−1的NO−3 ,反应180 min后,SMX的降解率分别增加了0.2%、1.1%、2.2%、2.9%。结果表明,低浓度NO−3 对SMX降解具有轻微的促进作用。有研究[28,30]表明,水中的NO−3 在自然光或者紫外光的照射下发生一系列化学反应(式(17)~式(21)),生成亚硝酸自由基(NO−2⋅ )、硝基自由基(NO−3 ·)、氧自由基(O−·)等活性自由基,其进一步与水反应产生OH·,与SO−4⋅ 协同作用达到强化降解SMX的作用。实验考察了腐殖酸对SMX降解效果的影响。腐殖酸是腐殖质的重要组成部分,是经植物残体腐解后,由碳、氢、氧、氮等元素组成的一类高分子有机弱酸物质。其总量庞大,广泛分布江河湖海,土壤煤矿等各地,对生态平衡中碳循环、矿物迁移积累、土壤肥力等多方面都有影响。实验研究了不同浓度HA对SMX降解效果的影响,如图12所示。由图12可见,随着HA浓度的增加,对SMX降解的抑制作用加强。其原因可能是因为:HA结构复杂且含有大量的苯环、羰基和羧基等多种官能团,和SMX共同竞争
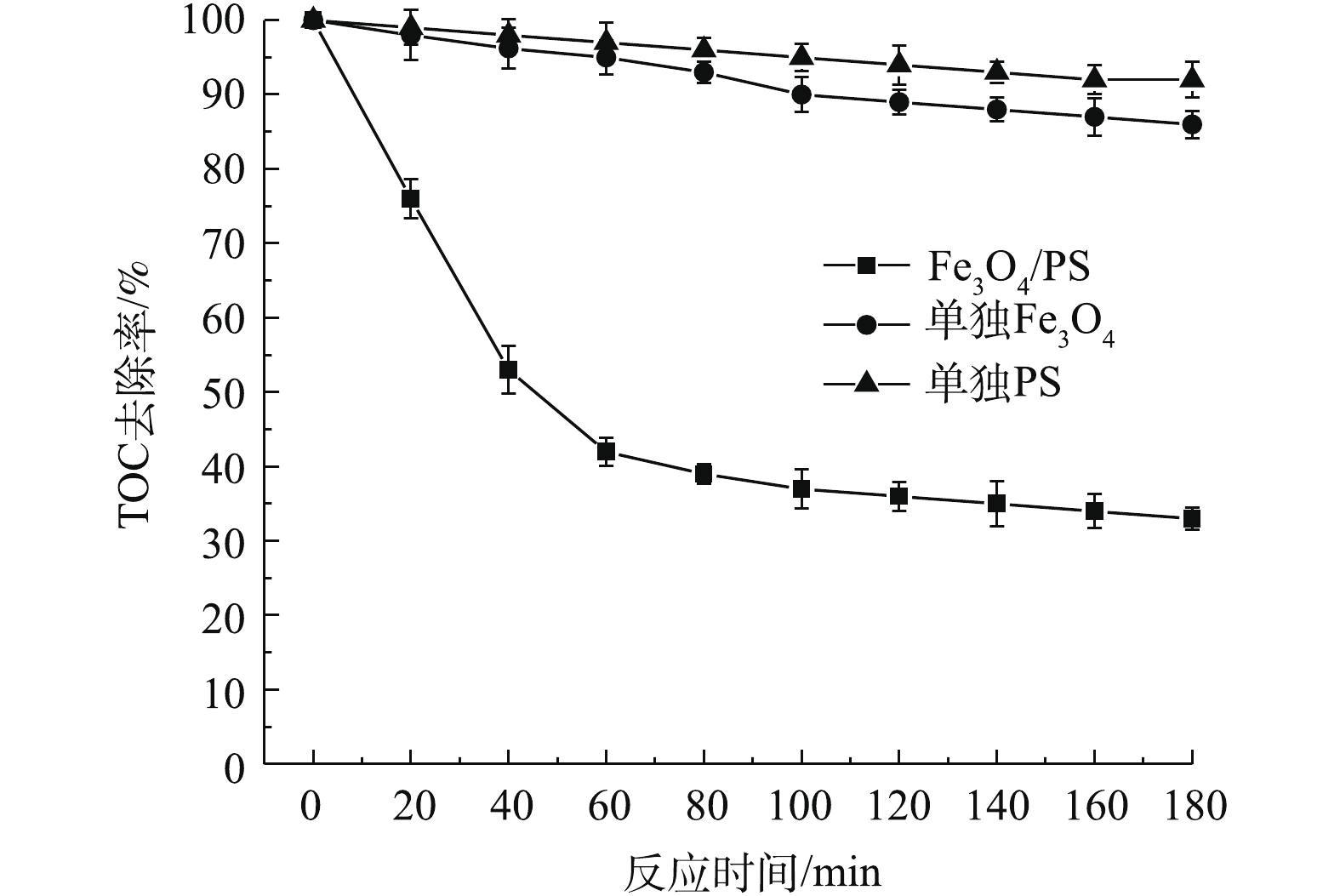
SO−4⋅ ,并可能率先和SO−4⋅ 发生反应,因而对SMX降解效果造成不利影响。实验考察了反应过程中TOC的变化情况。实验将单独PS氧化效果、单独Fe3O4吸附效果与Fe3O4/PS体系降解效果进行了对比,结果如图13所示。由图13可见,单独PS氧化和单独Fe3O4吸附的矿化率很低,难以实现矿化,而Fe3O4活化PS可以实现SMX的有效矿化。
-
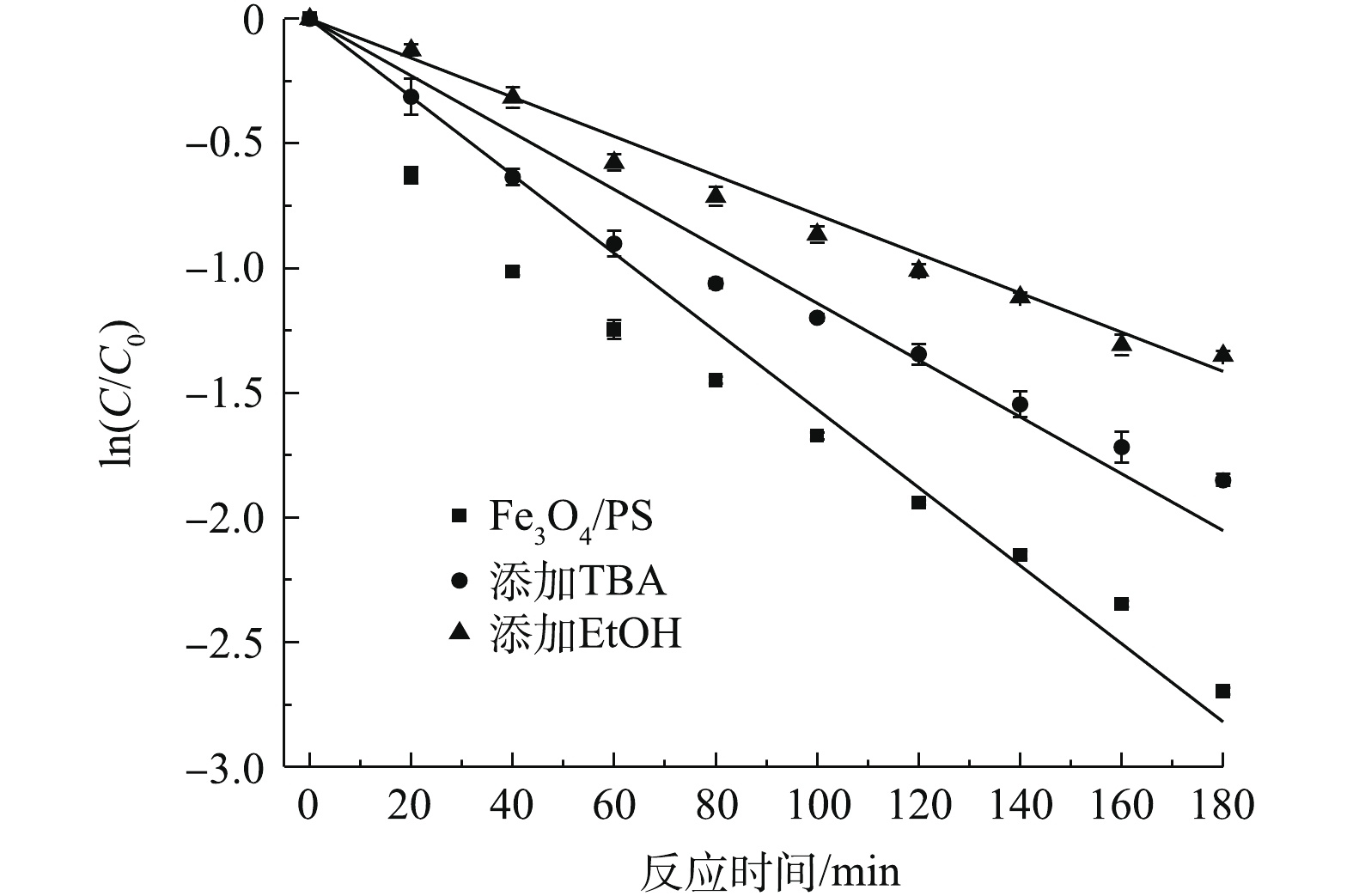
据报道,含有α氢原子的醇与·OH或
SO−4⋅ 具有较高的反应速率,含有α氢的EtOH可以作为辨别过硫酸根与·OH或SO−4⋅ 贡献的淬灭剂,而TBA对SMX降解效果的影响可以用来区分·OH与SO−4⋅ 贡献大小[12]。通过投加EtOH及TBA,对体系中主要自由基进行了鉴定,结果如图14所示。Fe3O4/PS体系中EtOH和TBA的存在均不同程度地抑制了SMX的降解,在添加EtOH后,SMX的降解率下降了19.2%,其速率常数为7.8×10−3 min−1;在添加TBA后,SMX的降解率下降了10.3%,其速率常数为1.14×10−2 min−1。由此可见,EtOH对SMX降解的抑制能力明显强于TBA,体系中SMX降解率的差异表明体系中SO−4⋅ 和·OH是共同存在的。同时,从SMX降解的速率常数可以看出,在添加TBA和EtOH后,SMX降解的速率常数逐级降低。由速率常数递减的差值可以推测,SO−4⋅ 在降解SMX的过程中发挥了主导作用,其贡献率为58.9%,而·OH的贡献率为41.1%。 -
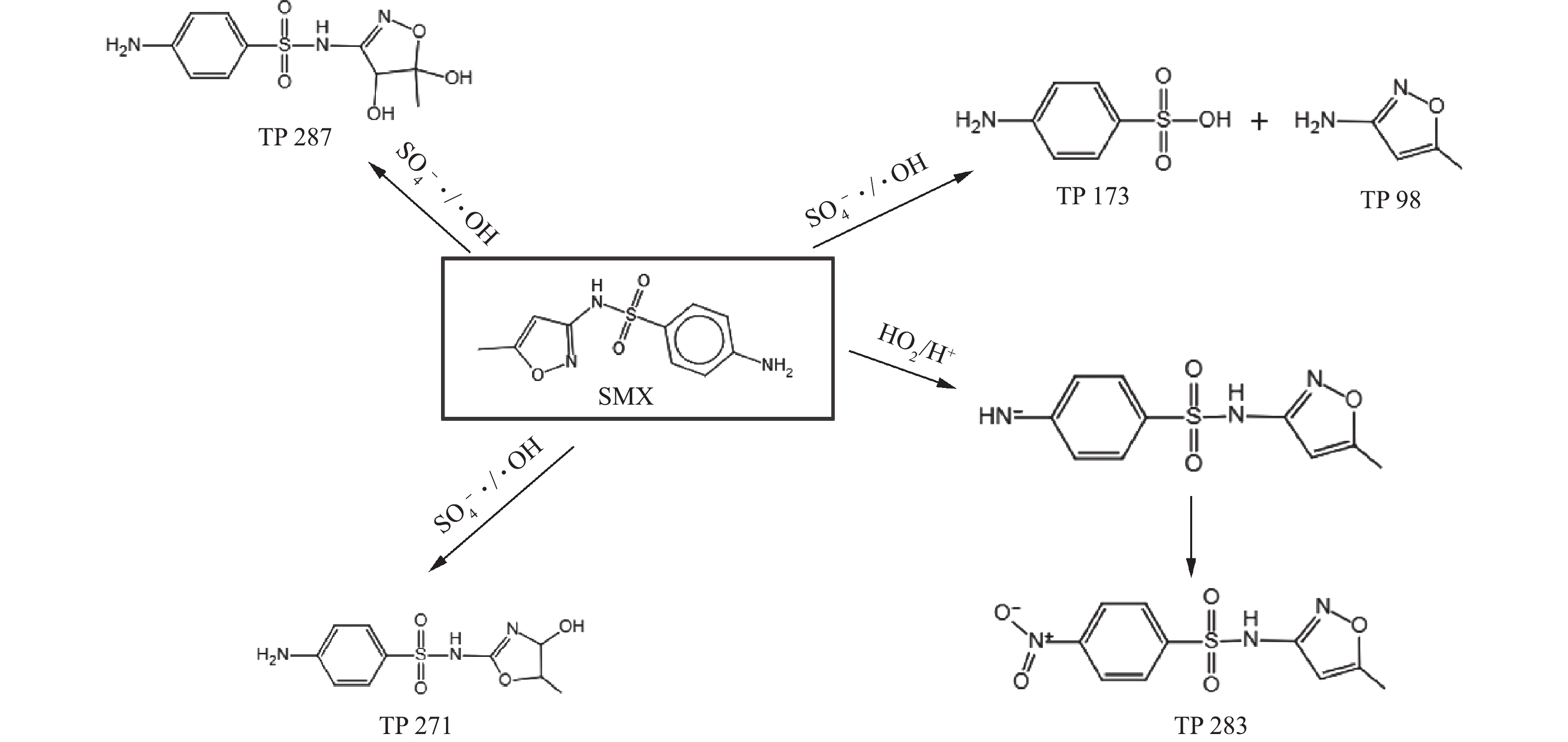
本研究对反应后的降解产物进行了分析,提出了可能的降解路径(如图15所示)。·OH表现出与烯烃双键和苯胺的高反应活性,主要反应途径之一是与位于恶唑环上的双键进行加成反应,这与BUXTON等[15]的研究结果相似。
SO−4⋅ 比·OH更具选择性和亲电性,其主要是通过在烯烃双键的恶唑环上引发亲电攻击,生成醇类化合物(TP 287),恶唑环中的氮原子会增强烯烃双键的电子密度,且比异恶唑环中的烯烃双键表现出更强的供电子能力,这与YANG等[31]的研究结果相似。 -
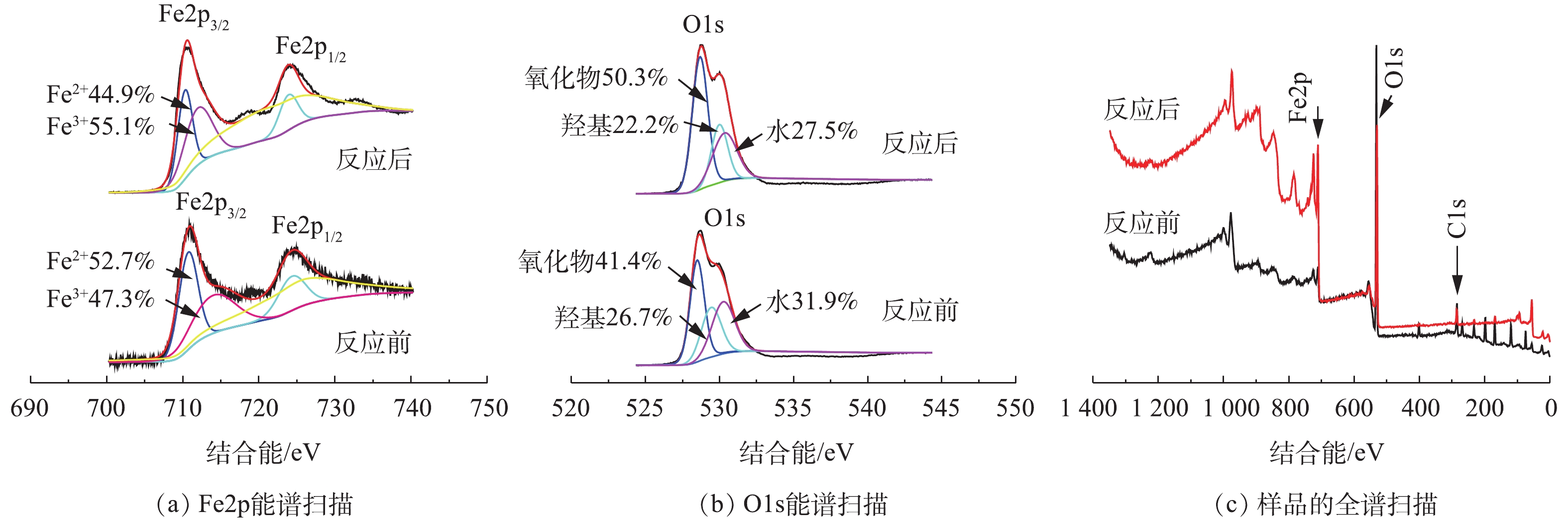
通过比较SMX降解反应前后Fe3O4的X射线光电子能谱 (X-ray photoelectron spectroscopy, XPS),分析了PS活化机理,结果如图16所示。由图16(a)可知:反应前,Fe3O4中的710.6 eV和713.0 eV的铁带为Fe2p,观察到的位置与磁铁矿铁赋值的研究[32]一致;反应后,Fe3O4中的结合能略有增加,这表明Fe3O4中Fe2+和Fe3+组分的变化。Fe3+的比例增加了15.9%,这表明在反应过程中部分Fe2+发生了电子捕获。另外,O1s的XPS光谱如图16(b)所示。反应前,529.4、530.7、531.4 eV处的3个峰证实晶格氧(O2−)、氢氧化物(OH−)和H2O共同存在Fe3O4中,这与先前的研究结果[32-33]一致。活化反应后,Fe3O4中的O2−、OH−和H2O的比例发生变化,O2-的占比有所升高,OH−和H2O的占比降低,这表明催化剂上的OH−和H2O参与反应,并生成了H+和Fe(氧)氢氧化物[34]。Fe3O4表面的Fe2+活化PS可生成
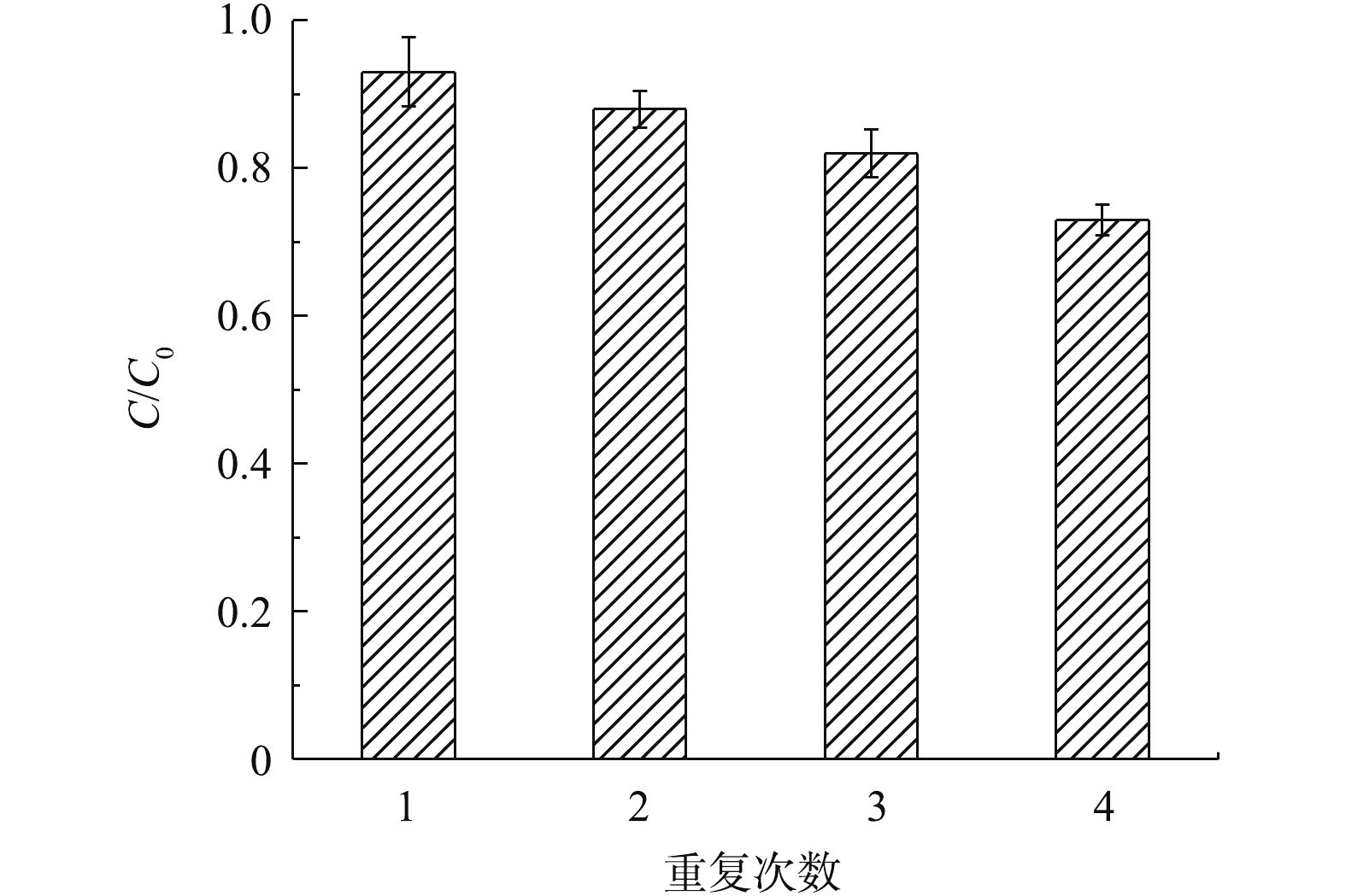
SO−4⋅ (式(1)),通过SO−4⋅ 与H2O的反应生成了∙OH (式(7)),从而参加SMX降解过程。Fe2+可被氧化为Fe3+,并在Fe3O4表面上沉淀下来,从而形成Fe(氧)氢氧化物(式(22)和式(23))。在pH=7.0、SMX浓度为20 μmol·L−1、Fe3O4投加量为1.2 g·L−1、PS浓度为0.5 mmol·L−1、温度为(30±0.5) ℃的最佳实验条件下,对Fe3O4进行重复性实验,结果如图17所示。每次反应进行180 min后,将Fe3O4取出,先用甲醇冲洗3次[35-36],利用相似相溶原理,将沉积包裹在Fe3O4表面的反应代谢产物溶解,再用蒸馏水反复冲洗3次后,加入到下次重复实验中。结果表明,经过4次循环实验后,SMX的降解率分别为93%、88%、82%和73%,Fe3O4表现出了较好的稳定性和催化活性,这说明Fe3O4能够稳定地应用到活化过硫酸盐和处理抗生素废水的高级氧化应用中。
2.1. 纳米Fe3O4表征
2.2. 降解SMX的影响因素及动力学分析
2.3. 自由基鉴别
2.4. 降解机理的分析
2.5. Fe3O4反应特性
-
1)通过共沉淀法制备了纳米Fe3O4,其具有良好的活化PS和降解磺胺甲恶唑的能力。在PS浓度为0.5 mmol·L−1,Fe3O4投加量为1.2 g·L−1,初始pH为中性的条件下,经180 min反应后,SMX的降解率达到了93.3%,且降解过程符合拟一级动力学方程。
2)纳米Fe3O4在酸性或中性条件下对PS均有较好的催化活性,且Fe3O4具有良好的稳定性。XPS光谱分析结果表明,反应过程中Fe2+发生了电子捕获,主要参与了催化活化PS降解SMX的过程。
3)水中低浓度的阴离子Cl−和
CO2−3 对SMX降解反应具有较小的抑制作用,而NO−3 对SMX降解反应有轻微的促进作用。腐殖酸的存在对SMX降解反应产生较强的抑制作用。4)采用EtOH及TBA作为淬灭剂进行自由基识别,结果表明,在Fe3O4/PS体系中,
SO−4⋅ 和·OH同时存在,SO−4⋅ 在SMX降解过程中发挥了主导作用。













 DownLoad:
DownLoad: