-
电催化氧化技术由于具有占地面积小、氧化能力强、无二次污染等特点而广泛应用于难生物降解废水的预处理或未达标废水的深度处理中[1-2]。电催化氧化反应发生在电极和溶液相接触的区域,将污染物传递到电极表面成为电催化氧化反应的基础与前提。有研究[3]表明:传质问题成为了限制电催化氧化技术应用的原因之一。因此,改善系统内的传质对提高电催化氧化的效率及降低能耗有明显作用,增加体系流体速度,会加速污染物由溶液向电极表面的扩散及电极表面生成强氧化物质向溶液中的传递速度,最终提高电催化降解速率。此外,有机物电催化氧化过程受传质控制,为了提高电催化氧化反应器的传质性能,研究者[3-6]从不同角度开展研究,如改变操作参数、开发新型反应器、湍流增强组件、折流板、改变电极的几何结构来优化反应器内流体流动和流体分布等。
由于电催化氧化反应是在电极表面发生的,溶液的对流和扩散会造成反应区域内的流体流动及物质浓度分布不均,不同电极位置的极化程度和反应速度也不相同,因此,了解电极表面的局部传质性能是有益的。尽管实验方法可以获得反应器内的平均传质系数[7-8],然而电催化氧化反应在阴阳极之间进行,加上反应过程伴随着气体析出带来的扰动,目前已有的实验方法难以精确测定反应区域内的某一点的流速。计算机技术的发展为预测反应器内流体的流动提供了可能。
计算流体力学(computional fluid dynamics, CFD)是基于能量、质量及动量定律发展的数值模拟工具,已广泛应用于废水处理领域的研究中[9-11]。与实验方法相比,CFD可以显著地降低实验测试的次数,节约资金和时间。CFD能够精确地模拟水处理单元流体的流动行为,并在有限的时间内模拟反应器的性能,通过改变参数条件寻求最佳设计方案,对反应器设计、优化起到了重要的作用。有研究[11]利用CFD对管式电化学反应器去除废水中的Cr(VI)进行模拟实验,结果表明,较低流速下反应器的进水口位置对系统内的流体扩散有重要的影响,增加流速能够显著促进反应器内的流体扩散和污染物的去除速度。DURAN等[12]利用CFD不同模型对环形管反应器进行模拟,并确定层流模型能够预测Re<1 500的流体流动情况,使用AKN和Reynolds应力模型对反应器模拟的可信度较高。SANTOS等[13]利用CFD对电催化反应器内的流体流动及传质情况进行了模拟,利用传质准数方程对模拟结果进行验证,结果表明,CFD预测与实验所得舍伍德数(Sh)吻合度较高,证明了CFD结果的准确性。张艺龙[14]将CFD用于电催化氧化反应器电极组件与隔板的相对位置进行模拟研究,对反应器进行改进,其能耗较改进前平均降低30.80%。SU等[15]研究结果表明,将网状电极代替传统平板电极后,其可以作为湍流组件增加流体的扰动,且传质无因次方程与实验结果吻合度较高。
本研究从数值仿真角度,采用CFD比较相同有效面积的平板结构和网状结构电极对反应器内部流体分布及速度的影响,并模拟不同进水位置对反应器及电极表面速度分布情况,进而优化电催化反应器的设计,通过确定传质准数方程对模型进行验证。此外,利用极限电流法测定不同进水方式时反应器内的平均传质系数,从而为开发高效传质电催化氧化反应器提供参考。
全文HTML
-
铁氰化钾(99.5%)购于天津市致远化学试剂有限公司,亚铁氰化钾(99.5%)购于天津市天力化学试剂有限公司,碳酸钠(99.8%)购于天津市巴斯夫化工有限公司,超纯水电导率为18.4 Ω·cm−1。
电催化反应器长50 mm,宽40 mm,高80 mm,进出口管的管径为6 mm,电催化反应器内阳极和阴极尺寸为60 mm × 40 mm,其中网状电极内的菱形孔尺寸为4 mm × 8 mm。
-
利用Ansys Design Modeler 建模,用Gambit对模型划分网格并定义边界条件,采用六面体网格对模型进行网格化,采用标准k-ε湍流模型,利用一阶迎风离散格式及SIMPLE算法对模型进行计算。在本研究中,作如下假设:1)流体是稳态的,有跟定的温度和动力学黏度;2)流体为不可压缩的且没有能量变化,由于强制对流的存在,忽略自然对流。进水口采用流量入口边界条件,设定反应器的进口流速为0.045 m·s−1,反应器出口设为出流比为1的出口边界条件,且出水口采用自由出流,对反应器的壁面采用无滑移、无渗透壁面条件。监测进出口质量守恒、各断面平均流速及残差曲线,当其值均小于10−3并最终趋于平稳时,则认定模拟结果收敛。
利用极限电流法测定电催化反应器内流体的传质系数;溶液中的离子通过扩散作用向电极表面移动,当溶液传质过程为扩散控制时,则出现极限电流。极限电流法测试体系采用铁氰根离子的还原过程,电解液由1 mmol·L−1 K3Fe(CN)6和10 mmol·L−1 K4Fe(CN)6组成,并添加1 mol·L−1 Na2CO3作为支持电解质。在不同进水流速下(0、0.015、0.032、0.045和0.062 m·s−1)获得各个反应器的极化曲线,当电流不再随电压增加而变化时为极限电流,传质系数与极限电流成正比[4,16],计算方法见式(1)。
式中:km为传质系数,m·s−1;Ilim为极限电流密度,mA;n为电荷转移数;A为电极面积,cm2;F为法拉第常数,取值96 487 C·mol−1;c0为溶液初始浓度,mol·L−1。
电化学反应器的传质性能[17-18]的计算方法见式(2)和式(3)。
式中:Sh为舍伍德数,m·s−1;de为电化学反应器的特征长度;u为电解液流速,m·s−1;ρ为溶液密度,kg·m−3;μ为介质的运动黏度,m2·s−1;D为溶液的扩散系数,m2·s−1。
1.1. 实验原料、装置
1.2. 实验方法及分析方法
-
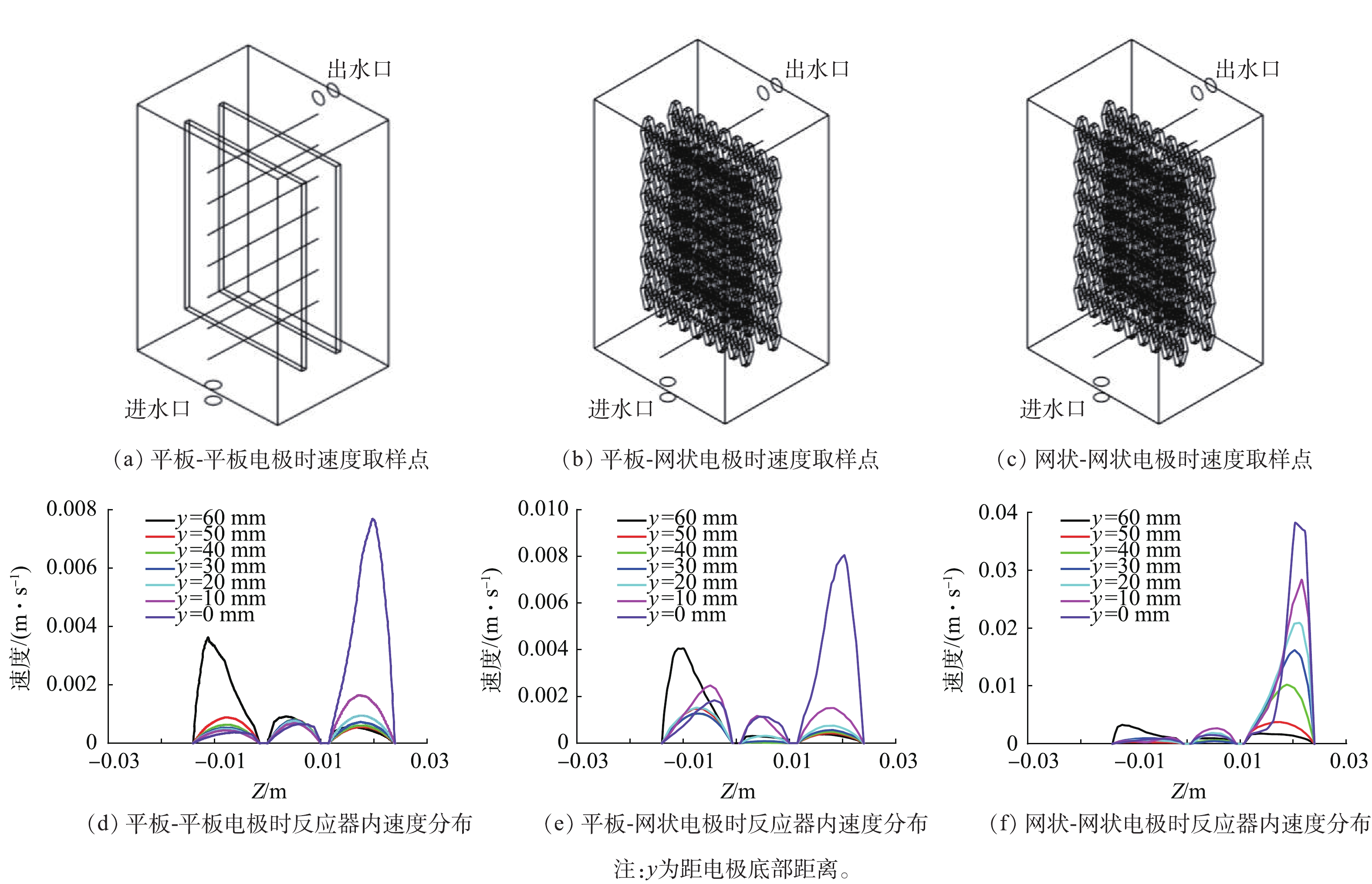
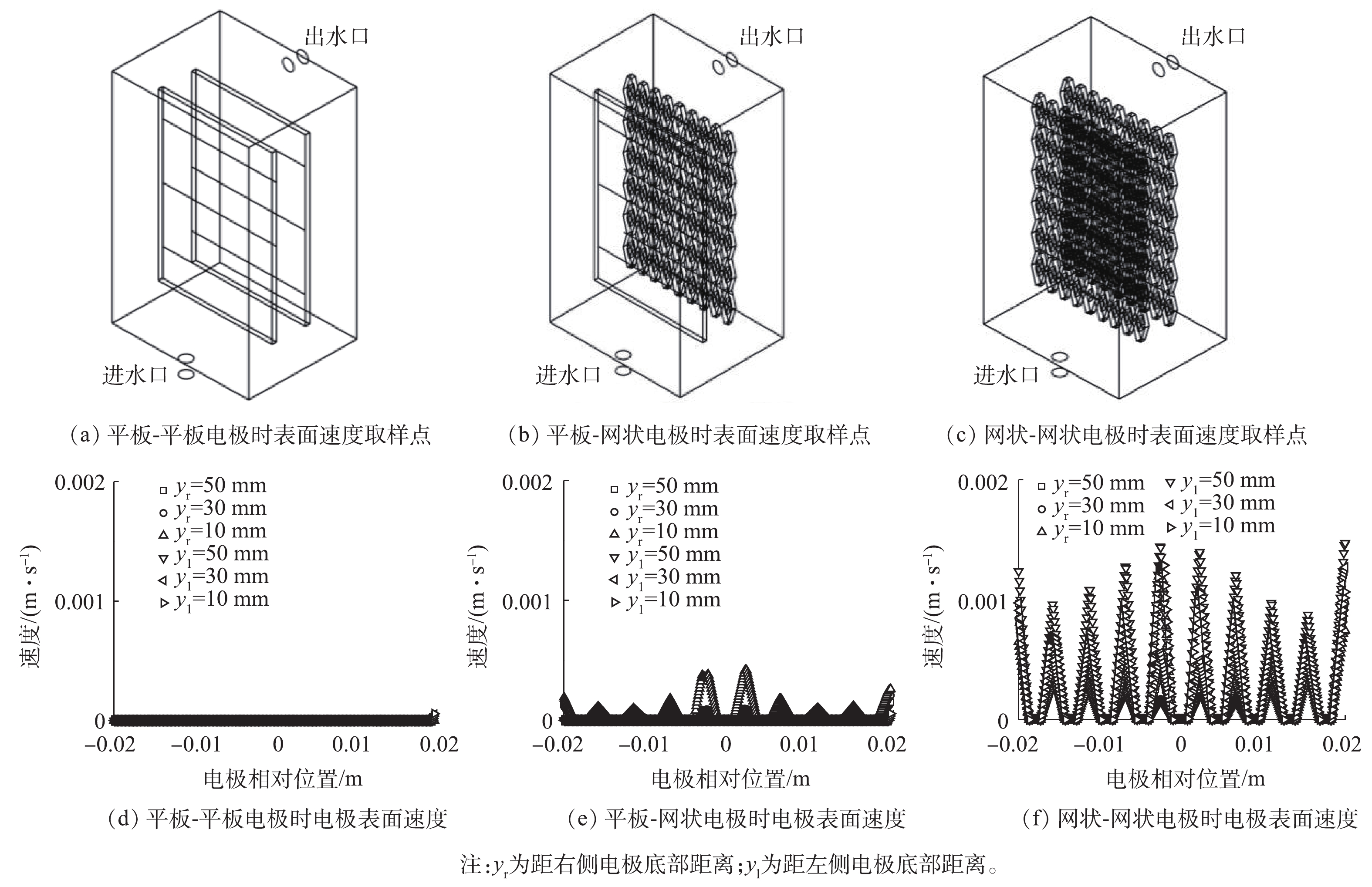
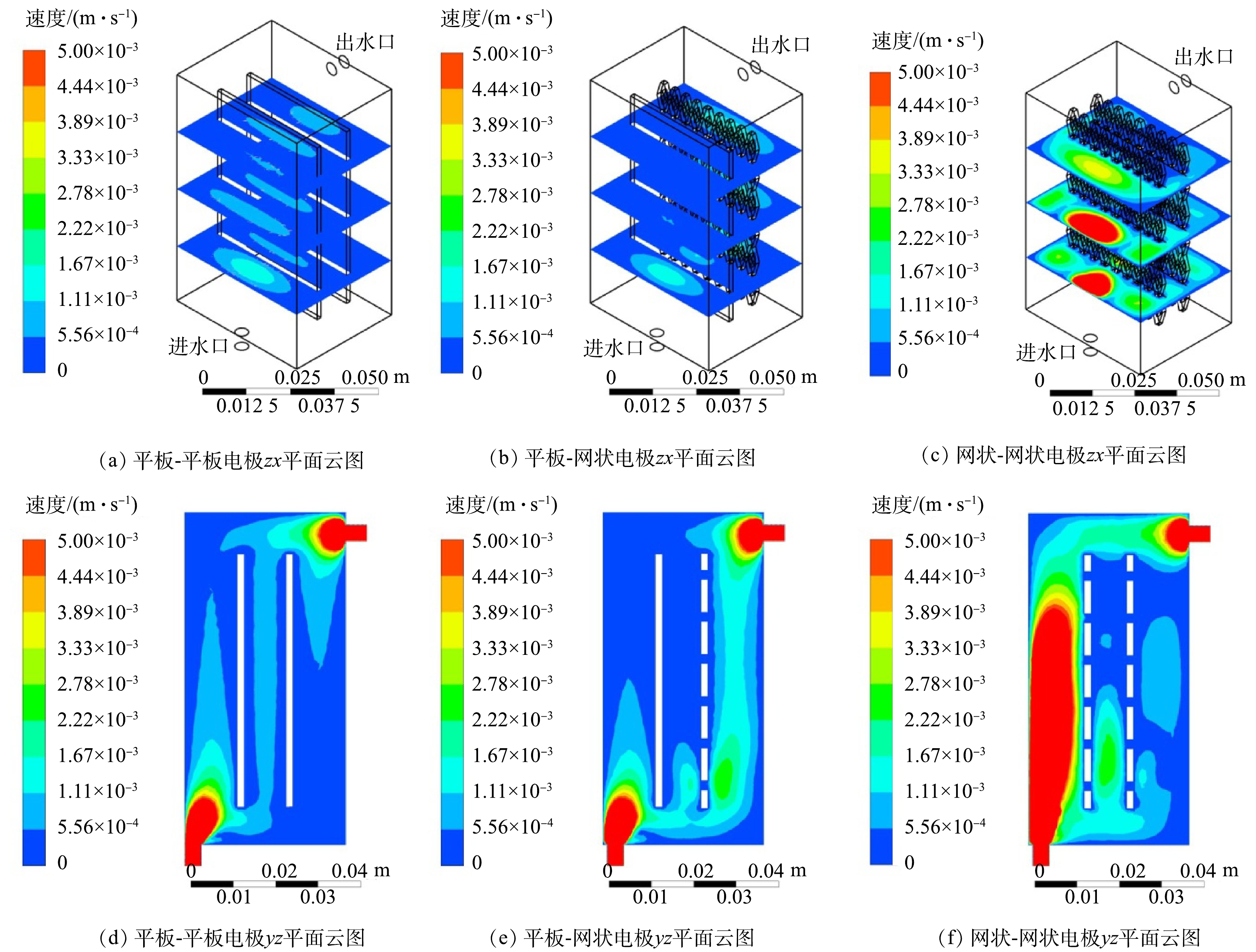
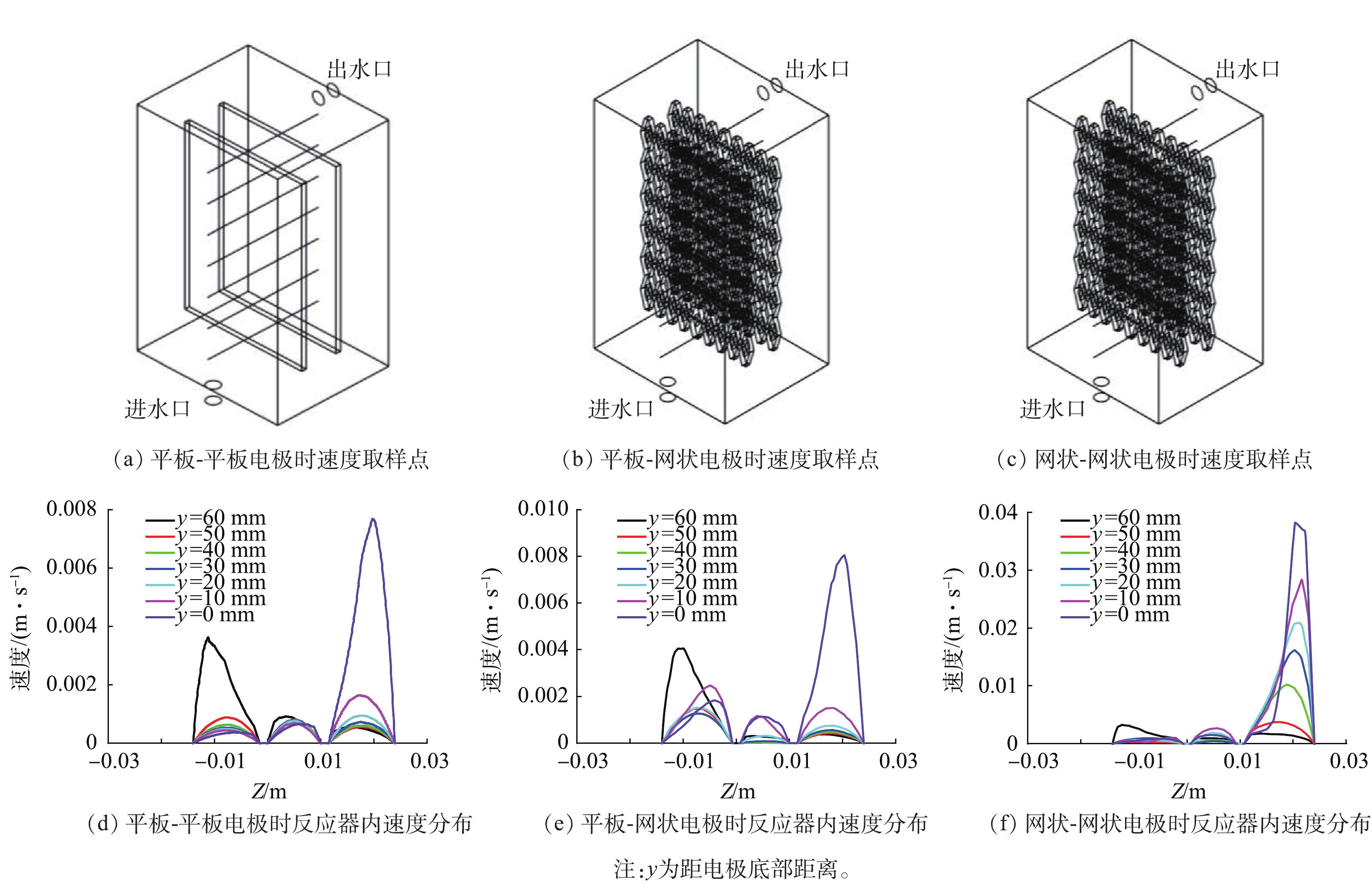
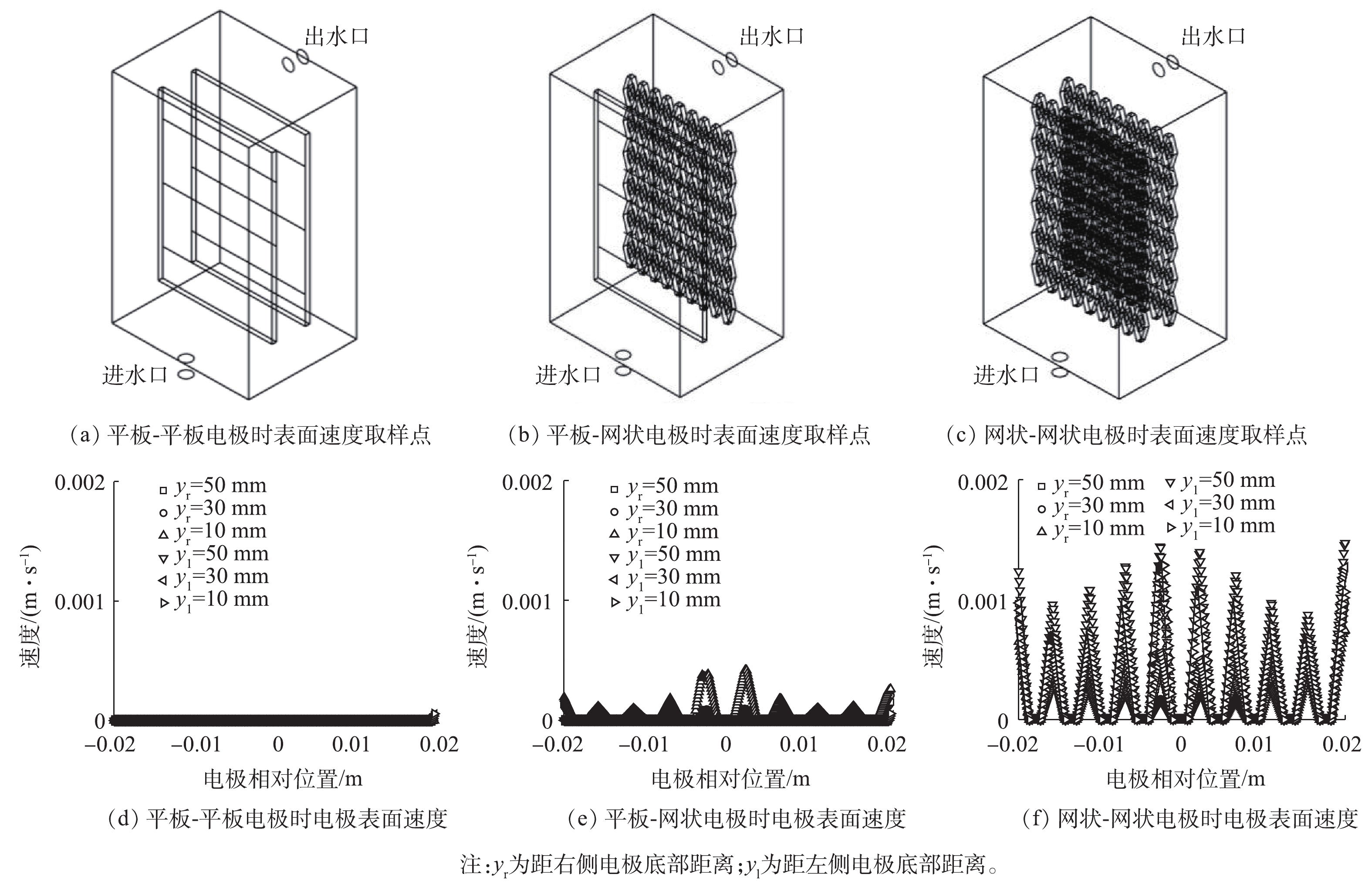
考察了平板-平板、平板-网状、网状-网状电极(图1)结构对反应器内流体分布的影响。当进水流速为0.045 m·s−1时,yz平面及距电极底面10、30和50 mm处的zx平面速度云图如图1所示。可以看出,网状-网状电极的存在使反应器内各个截面的速度云图均大于平板-平板电极及平板-网状电极。当流体进入反应器时速度最大,随着流体持续进入反应器,由于压力降及截面面积的增加使速度逐渐下降。距电极底部不同高度截面反应器内流速如图2所示,网状-网状电极结构y=0 mm处最大流速为0.039 m·s−1,为平板-平板电极和平板-网状电极的4.87倍和5.20倍。不同电极结构的电极表面速度如图3所示,流速在电极表面水平方向较垂直方向均匀,且在相同高度处,网状-网状电极表面的速度最大,在y=10 mm处,网状-网状结构、平板-网状结构、平板-平板结构距电极底部10 mm处右侧电极(yr=10 mm)的最大流速分别为0.001 3、0.000 5 和0 m·s−1。这是由于网状电极的菱形孔结构利于溶液在其中通过,此结构改变了溶液的界面流动方向,对流体的流动起到了导流和阻挡的作用,增强了反应器中的强制对流,进而增加了流体速度。有研究[19]表明,电极表面较高的流速利于污染物与电极之间的接触及产物的及时逸出,降低液层厚度,进而提高传质性能。因此,电催化反应器中的网状电极具有增加溶液扰动、使流体分布更加均匀、促进传质的作用,确定最优条件为网状-网状结构。
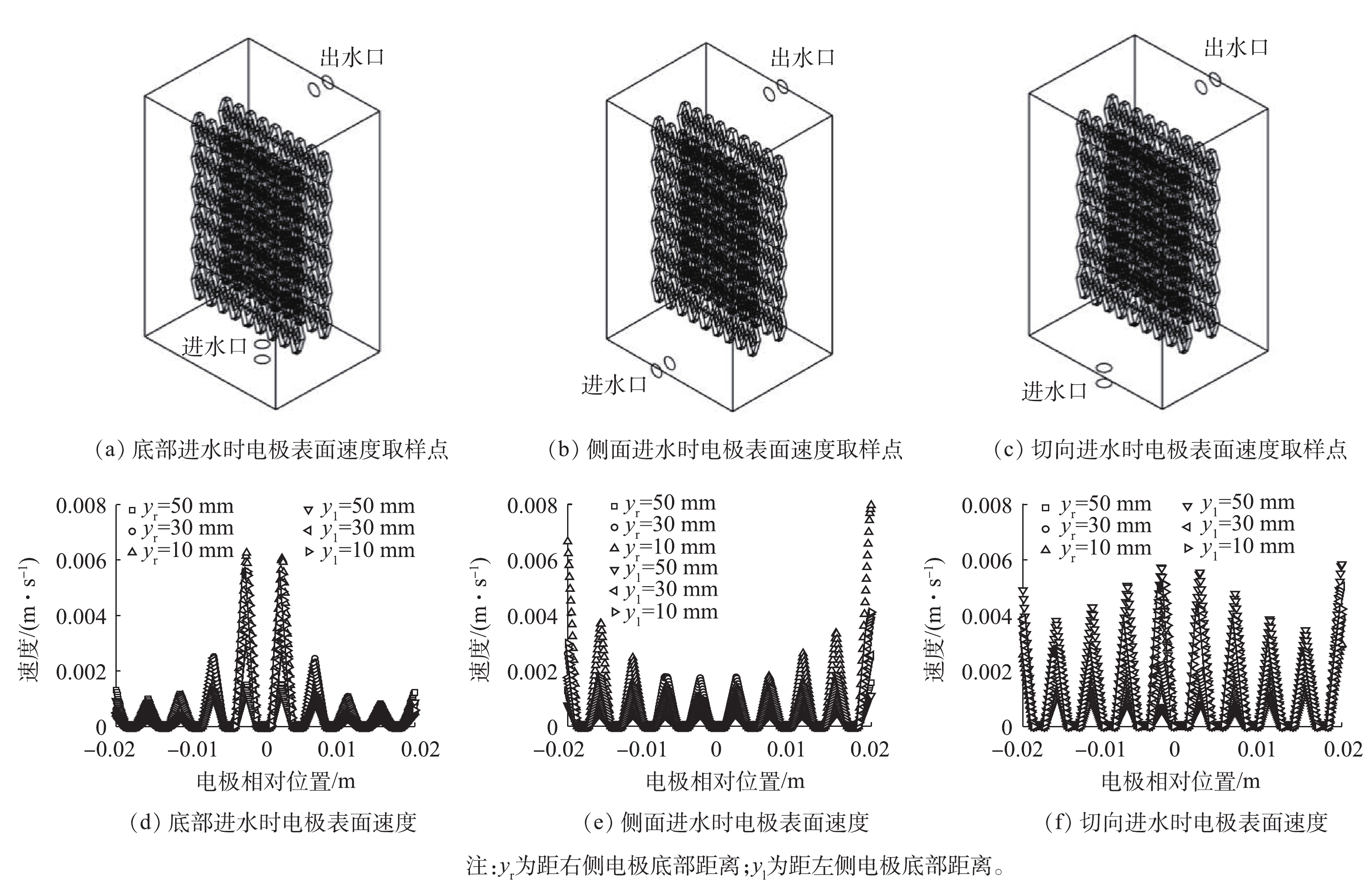
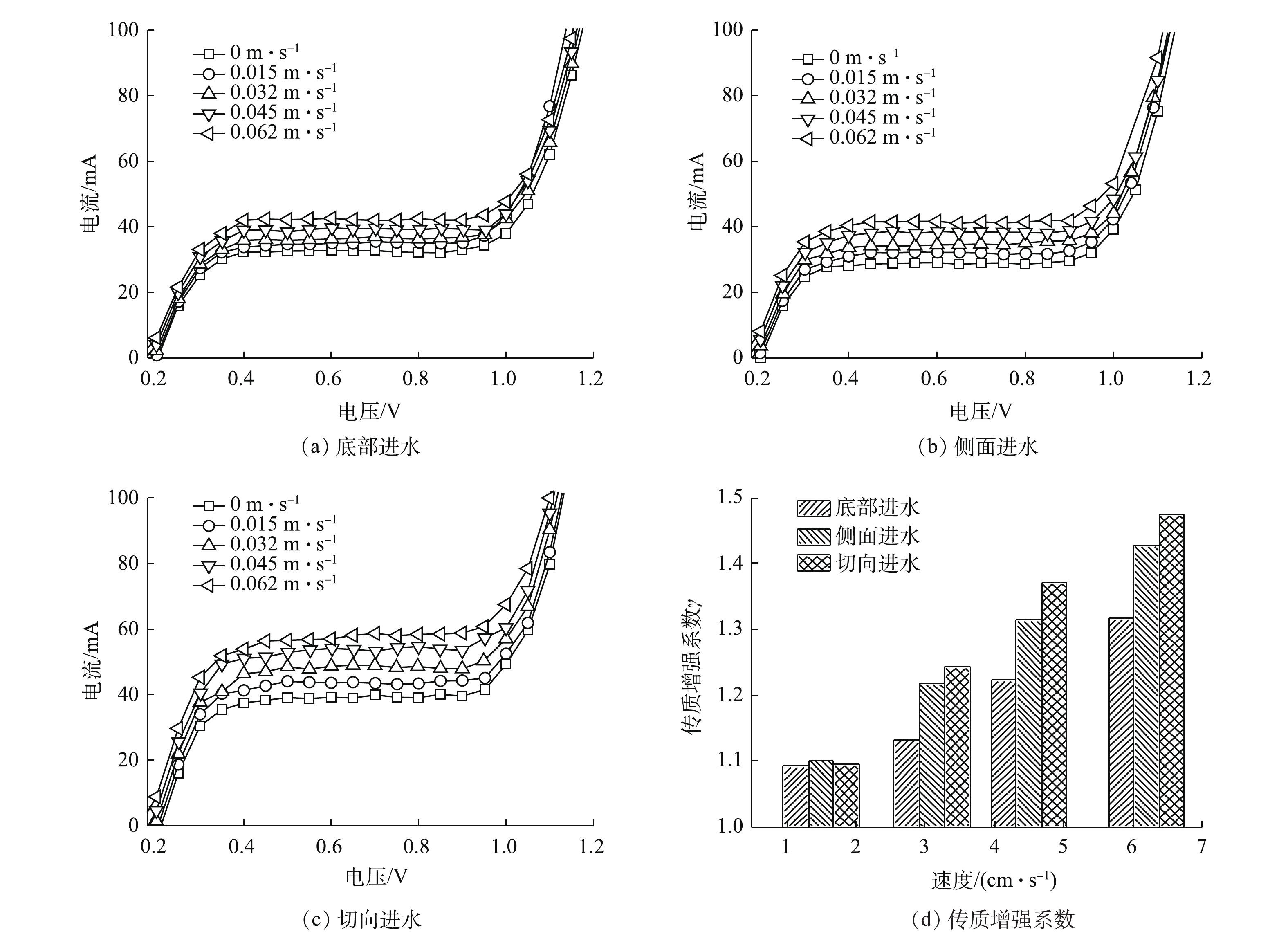
对侧向进水、底部进水及切向进水等不同进水方式进行模拟分析,各进水方式下电极表面速度分布如图4所示。虽然切向进水时电极表面的最大速度(0.001 4 m·s−1)低于底部进水时电极表面最大速度(0.006 m·s−1),但是切向进水时电极表面速度较其他2种情况更加均匀。同时切向进水可以减少出水涡流的发生概率,增加流体向前流动时在反应器内的反应时间,进而提高反应能力;均匀的速度分布可以促进反应过程中溶液分布的一致性及空间利用率。因此,综合考虑后选用切向进水为最优进水方式。
-
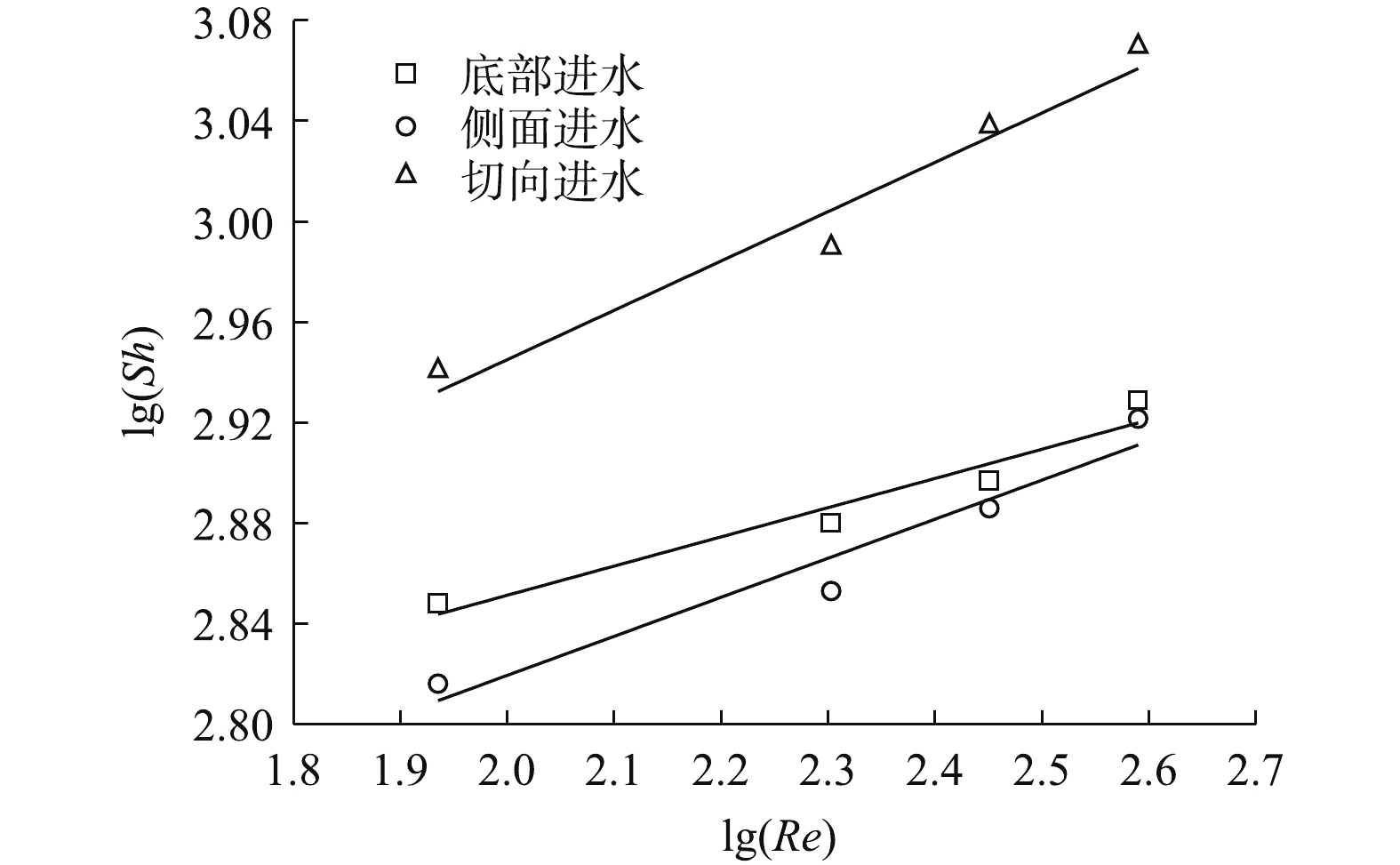
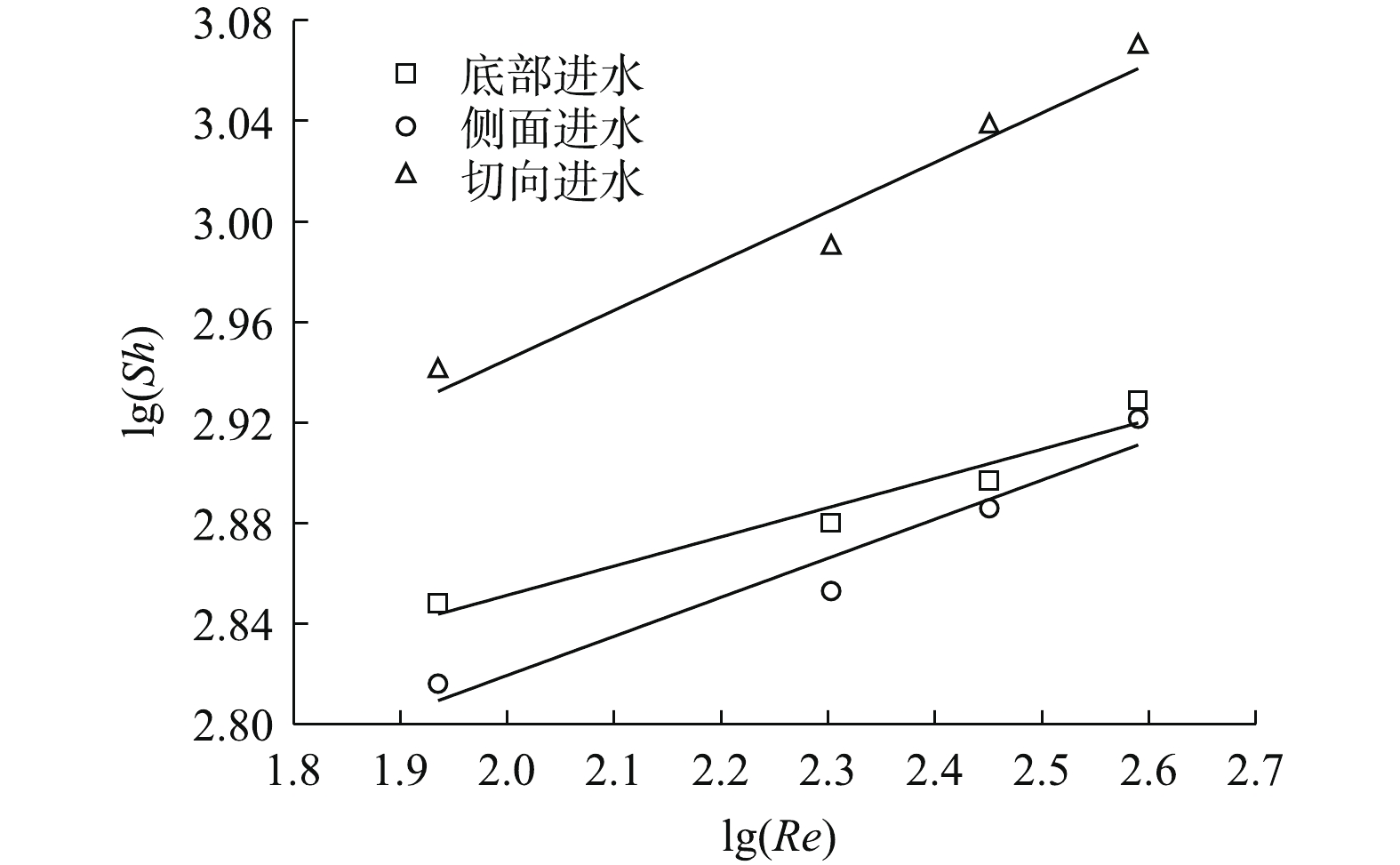
根据式(2)及式(3)对传质方程进行拟合分析,当进水流速由0增至0.062 m·s−1时,切向进水、底部进水及侧面进水的传质关系式分别表示为式(4)~式(6)。
式中:Re指数与动力学有关;其值越大,代表在电极表面产生更多的微湍流,进而有利于污染物在电极表面的传质过程。由图5可知,模拟结果与实验结果吻合度较高,说明此模型可以用来描述电催化反应器内的传质性能。利用极限电流法计算反应器内的平均传质系数,如图6(a)~图6(c)所示。不同进水流速时的极限电流均出现在电压为0.9 V左右处,且随着进水流速的增加,极限电流也随之提高。进水流速为0时,切向进水的电流为39.2 mA,为侧面进水(29.2 mA)和底部进水(32.3 mA)的1.34倍及1.21倍;当进水流速提高至0.062 m·s−1时,切向进水的电流达58.5 mA,为静止时的1.49倍。结果表明,增加进水流速可以显著提高反应器内的传质系数,利用传质增强系数来表示各进水条件下的平均传质性能提高情况,各进水方式的传质增强系数与进水流速呈现正相关(图6(d)),进水流速为0.062 m·s−1时,切向进水γ为1.475,高于底部进水(1.428)及侧面进水(1.317)。这说明在相同进水口分布条件下,与其他2种进水方式相比,增大进水流速更利于提高切向进水时体系的传质系数。尽管没有公式直接说明反应器的传质性能与流体流动状态之间的关系,但有研究[18]表明,尤其当溶液的传质仅受对流控制时,反应器内流体的流速可以间接反映传质系数的大小。切向进水使流体运动的轨迹发生了变化,进而改变了流体在电极表面的速度和电极表面液层的厚度,最终影响反应器内流体的传质过程。传质增强系数γ的计算方法见式(7)。
式中:γ为传质增强系数;Ilim,v为某一流速下的极限电流密度,mA;Ilim,v=0为v=0时的极限电流密度,mA;km,v为某一流速下的反应器内传质系数,m·s−1;km,v=0为v=0时的反应器内传质系数,m·s−1。
2.1. 不同电极结构对反应器流体分布的影响及进水方式的优化
2.2. 模型验证
-
1)利用CFD对电催化反应器内电极结构及进水方式进行模拟优化,结果表明:网状-状网电极的y = 0处最大流速为0.039 m·s−1,为平板-平板和平板-网状电极的4.87倍和5.20倍,网状-网状电极的存在使电极表面能够获得均匀的速度分布,其多孔的结构改变了溶液的流动方向,增强了反应器中的强制对流。
2)切向进水时,电极表面速度较其他2种情况更加均匀,均匀的速度分布促进反应过程中溶液分布的一致性,提高空间利用率。
3)静止条件下,切向进水的极限电流为侧面进水(29.2 mA)和底部进水(32.3 mA)的1.34倍及1.21倍;增大进水流速至0.062 m·s−1,切向进水的极限电流为静止时的1.49倍,增加进水流速可以有效提高反应器的极限电流和传质性能,切向进水时反应器具有最佳的传质性能。
4) 传质拟合结果表明,CFD模拟与实验的吻合度较高,证明CFD模拟结果可以较为真实地反映流体在反应器内的流动情况。




 下载:
下载: