-
营养盐(氮、磷等)在自然水体中的增加,导致水体生态系统生产力的不断增强,由此引起藻类水华的频繁发生[1]。过量繁殖的藻类可破坏水体生态系统的平衡,降低水域生态景观功能,并对饮用水的供应造成严重威胁[2-3]。传统的藻类水华控制方法主要包括机械打捞、投加絮凝剂或除藻剂以及水生植物控藻等[4]。机械打捞和投加絮凝剂的方法虽然见效快,操作简单,但该方法投资大,打捞/絮凝沉降的藻类不易进行处置,易造成二次污染;除藻剂投加的方法作用时间有限,并可能对水体的生态系统带来不利影响;水生植物控藻的方法对生态系统的影响较小,但处理周期较长,对突发的藻类水华爆发作用有限。
光催化氧化技术作为一种极具潜力的环境净化技术,因其具有较高的氧化能力、环境友好性且成本较低等特点而备受关注[5]。光催化氧化过程中,半导体材料可被光激发产生具有强氧化能力的羟基自由基(·OH),该基团可降解水体中的有机污染物并灭活水中的微生物,因此,许多的研究者将该技术应用于藻类水华的控制[6-7]。PINHO等[8]采用自然光激发的光催化材料对蓝藻水华水体进行处理,研究表明,光催化剂表面产生的活性组分不仅可造成铜绿微囊藻细胞的裂解,还可对藻细胞破裂释放的微囊藻毒素进行降解。
光催化除藻技术相对于传统的除藻方法,具有能耗低、无二次污染等特点,但在实际应用中,目前研究较多的纳米TiO2光催化剂存在禁带较宽、光生电子和空穴易复合等不足。另外,粉体催化剂不易分离,容易流失。为提高光催化剂对可见光的响应,采用较多的方法是对纳米TiO2进行可见光改性;为增加光催化剂的回收性能,通常将催化剂固定到适当的载体上。有研究[9]表明,窄禁带宽度的Ag3PO4与TiO2形成的异质结有助于光生电子-空穴的分离,提高材料的可见光响应,而石墨相氮化碳(g-C3N4)与Ag3PO4复合可提高Ag3PO4的稳定性[10]。
本研究采用光催化剂Ag3PO4和g-C3N4对TiO2进行共修饰,并将催化剂固载到漂浮型载体膨胀珍珠岩上,所制得的漂浮型光催化剂用来对铜绿微囊藻进行灭活,以期获得高效、低耗的藻类水华控制方法。
全文HTML
-
实验用膨胀珍珠岩购自信阳中科矿业有限公司,粒径为2.0~3.0 mm。所用试剂均购于国药集团化学试剂有限公司,为分析纯。铜绿微囊藻(FACHB-913)藻种购于中国科学院水生生物研究所国家淡水藻种库,采用BG11培养基进行培养。培养条件:恒温培养箱中(25±1) ℃下,光照条件2 000 lx,时间设置为12 h昼/12 h夜。
-
将15 g Al(NO3)3·9H2O和12 g CO(NH2)2分别溶于50 mL蒸馏水,形成溶液A及溶液B,将10 g漂洗晾干后的膨胀珍珠岩加入溶液A中并不断搅拌,将溶液B缓慢滴加到混合物,待充分反应2 h后,于105 ℃烘干12 h,随后将颗粒物转移到马弗炉300 ℃下煅烧2 h,得铝盐改性膨胀珍珠岩,命名为mEP。
称取5 g三聚氰胺于马弗炉中,550 ℃下焙烧4 h,冷却后,研磨得到淡黄色g-C3N4粉末。称取1 g的g-C3N4粉末,分散至100 mL无水乙醇中,超声剥离6 h,得到10 mg·mL−1的g-C3N4溶液。
将0.5 mL浓硝酸滴加入9 mL钛酸正四丁酯和20 mL无水乙醇的混合液C中,混合均匀后,滴加6 mL g-C3N4备用溶液,称取4 g mEP,加入混合液C并不断搅拌,待混合均匀后,逐滴加入2.5 mL蒸馏水,使其充分水解。将所制得凝胶陈化24 h后,于105 ℃条件下,烘干12 h,待其水分挥发完全后,将颗粒物于马弗炉中450 ℃下焙烧2 h,筛去粉末,得到成品为g-C3N4-TiO2/mEP。按上述步骤,不加g-C3N4,制得对照样品TiO2/mEP。
准确称量2.55 g AgNO3和0.71 g Na2HPO4,分别溶于30 mL蒸馏水,形成溶液D及溶液E。将上述制得的g-C3N4-TiO2/mEP加入溶液D,在搅拌的条件下,缓慢滴加溶液E,生成的Ag3PO4与TiO2的理论摩尔分数为20%。充分反应后,静置6 h,于60 ℃条件下烘干,再用去离子水漂洗3次后,低温烘干,得最终复合材料,标记为Ag3PO4-g-C3N4-TiO2/mEP-20%。同理制得一系列不同理论摩尔分数复合光催化剂Ag3PO4-g-C3N4-TiO2/mEP-x%(x=5、10、20、30)。
-
光催化剂对藻细胞的吸附和光催化灭活实验均在光催化反应仪(DYYB-F,上海德洋意邦有限公司)中进行,光催化反应仪以500 W的氙灯为光源,添加滤光片滤去紫外光部分。藻细胞的浓度用分光光度计(UV-2700,日本Shimadzu公司)测定培养液在680 nm波长处的吸光度(OD)变化[11]。取对数增长期的藻细胞培养液,于8 000 r·min−1下离心分离5 min,弃去上清液,并用磷酸盐缓冲液(PBS)清洗3次,最后用缓冲液稀释OD680值为0.2,此时细胞浓度约为2.7×106 cells·mL−1,作为实验初始藻细胞溶液。
取50 mL铜绿微囊藻溶液(细胞密度2.7×106 cells·mL−1,pH=7.4)加入到一系列光催化反应管中,分别投入0.1 g可见光催化剂,放入光催化反应仪中,在磁力搅拌器转速为200 r·min−1关灯条件下,进行藻细胞暗吸附,吸附时间为8 h。每隔1 h,取样滴于血球计数板上,使用数码显微镜(BA310,日本Nikon公司)观察计数,测定溶液中铜绿微囊藻的浓度。
与光催化剂吸附藻细胞的操作条件类似,打开光催化反应仪光源,进行光催化除藻实验,间隔1 h,取样滴于血球计数板上,用显微镜观察计数,测定溶液中铜绿微囊藻的浓度变化。
-
光催化剂表征采用的仪器主要有X射线衍射仪(X’pert,德国Bruker公司)、BET自动吸附仪(ASAP2020,美国Micromeritics公司)、傅里叶红外光谱仪(Nicolet 5700,美国热电尼高力仪器公司)、XPS能谱分析仪(PHll600,PerkinElme公司)、紫外可见分光光度计(UV-2550,日本岛津公司)。
1.1. 实验材料与试剂
1.2. 光催化材料制备
1.3. 除藻实验
1.4. 材料表征方法
-
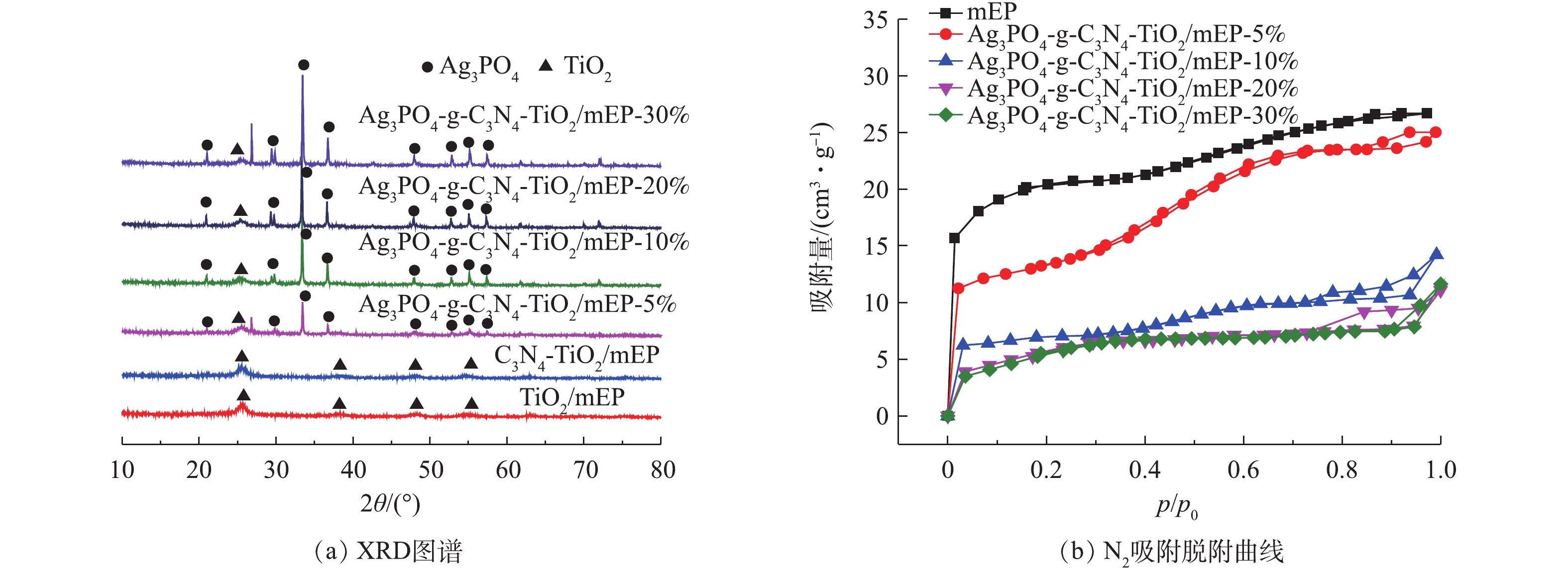
图1(a)为TiO2/mEP、g-C3N4-TiO2/mEP和Ag3PO4-g-C3N4-TiO2/mEP系列材料的XRD图谱,所有复合材料中均检测到锐钛矿型TiO2的晶面特征衍射峰。由于复合的g-C3N4含量较低且g-C3N4结晶度较差,g-C3N4-TiO2/mEP和Ag3PO4-g-C3N4-TiO2/mEP系列材料的XRD衍射谱图中均未检测到明显的g-C3N4衍射峰。Ag3PO4-g-C3N4-TiO2/mEP系列材料中在2θ为20.83º、29.66º、33.27º、36.55º、47.78º、52.68º、54.98º、57.27º处出现的特征峰分别对应于Ag3PO4的(110)、(200)、(210)、(211)、(310)、(222)、(320)、(321)晶面。随着Ag3PO4与TiO2复合摩尔分数的增加,Ag3PO4的各晶面对应的衍射峰的强度也随之增强,但当Ag3PO4/TiO2理论摩尔分数为30%时,Ag3PO4-g-C3N4-TiO2/mEP-30%复合材料的XRD图谱中开始出现杂峰。图1(b)为mEP及不同Ag3PO4/TiO2摩尔比光催化剂材料的N2吸附/脱附等温曲线,其计算所得的比表面积、平均孔径、总孔容数据见表1。由图1(b)可发现,与mEP相比,Ag3PO4-g-C3N4-TiO2/mEP系列催化剂的吸附能力有所下降,这是由于表面复合的催化剂影响到了改性膨胀珍珠岩本身孔隙结构。所有复合材料的N2吸附/脱附等温曲线均符合Ⅳ型等温线,脱附曲线和吸附曲线在相对压力为0.3~0.8发生分离,出现H2型迟滞回线,表明材料中存在介孔结构。由表1可知,mEP表面负载光催化剂后,复合材料的比表面积和孔容出现一定程度的减小,但孔径有一定程度的增加,当Ag3PO4/TiO2理论摩尔分数为20%时,Ag3PO4-g-C3N4-TiO2/mEP-20%光催化材料的孔径明显比其他材料大,达到4.1 nm。
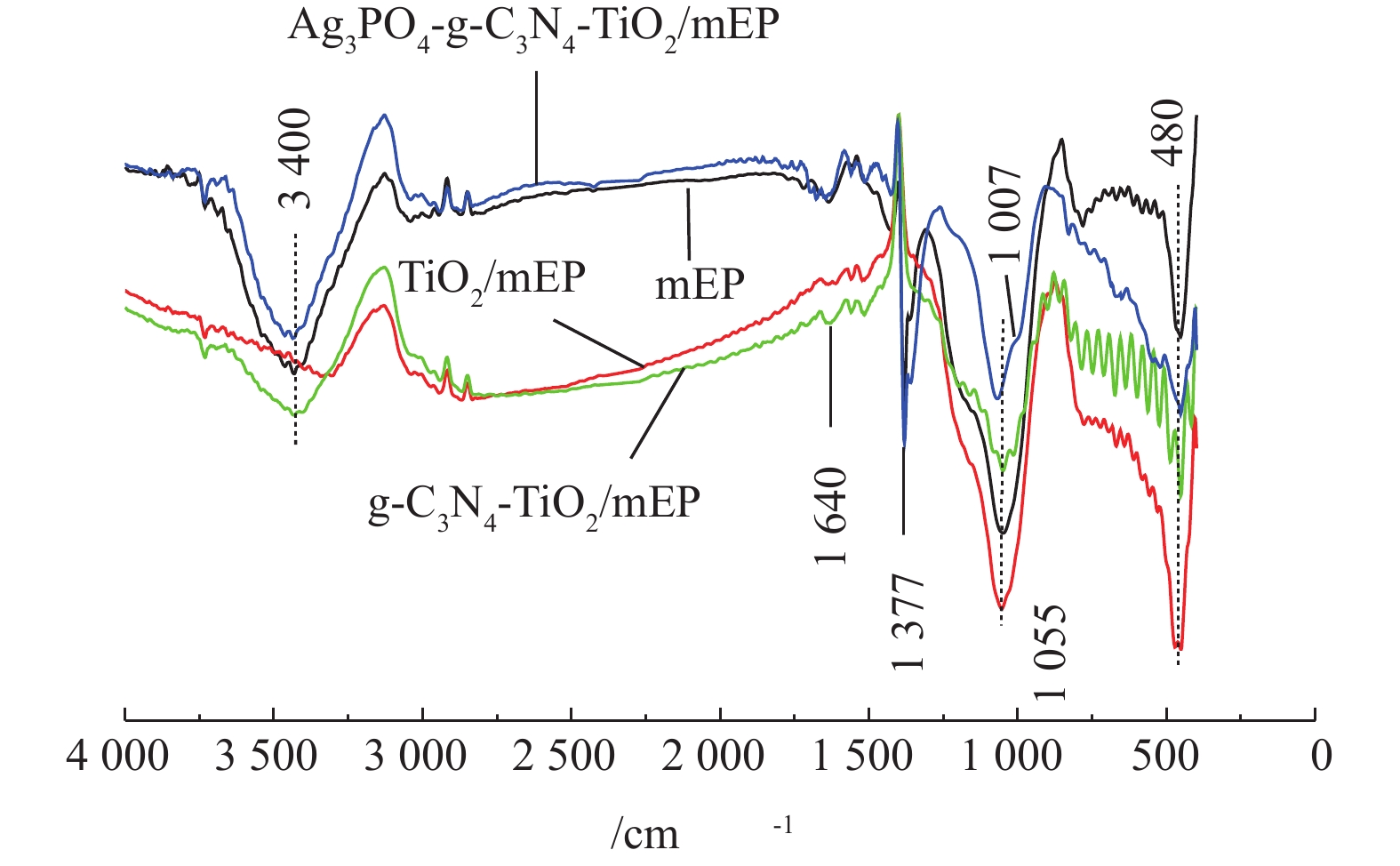
对不同催化剂以及基底mEP采用FTIR进行表面官能团分析,结果如图2所示。这4种材料在480、1 055和3 400 cm−1附近均出现吸收峰,峰位分别为Si− O− Si的弯曲伸缩振动[12]、烷氧基中C− O键的弯曲振动[13]和材料表面的− OH基团[14]。除了mEP外,其他3种复合材料在500~800 cm−1均可观察到一些波峰,主要归因于Ti− O− Ti的伸缩振动[14],表明TiO2成功负载于基底材料上。同时,g-C3N4-TiO2/mEP和Ag3PO4-g-C3N4-TiO2/mEP-20%在1 377 cm−1和1 640 cm−1处可观察到2个峰位,1 377 cm−1处的吸收峰属于芳香CN杂环化合物中C—N伸长振动,而1 640 cm−1处的吸收峰属于C=N的伸缩振动[15],由此推断g-C3N4在催化剂中的存在。另外,Ag3PO4-g-C3N4-TiO2/mEP-20%在1 007 cm−1处出现的吸收峰为磷酸盐(
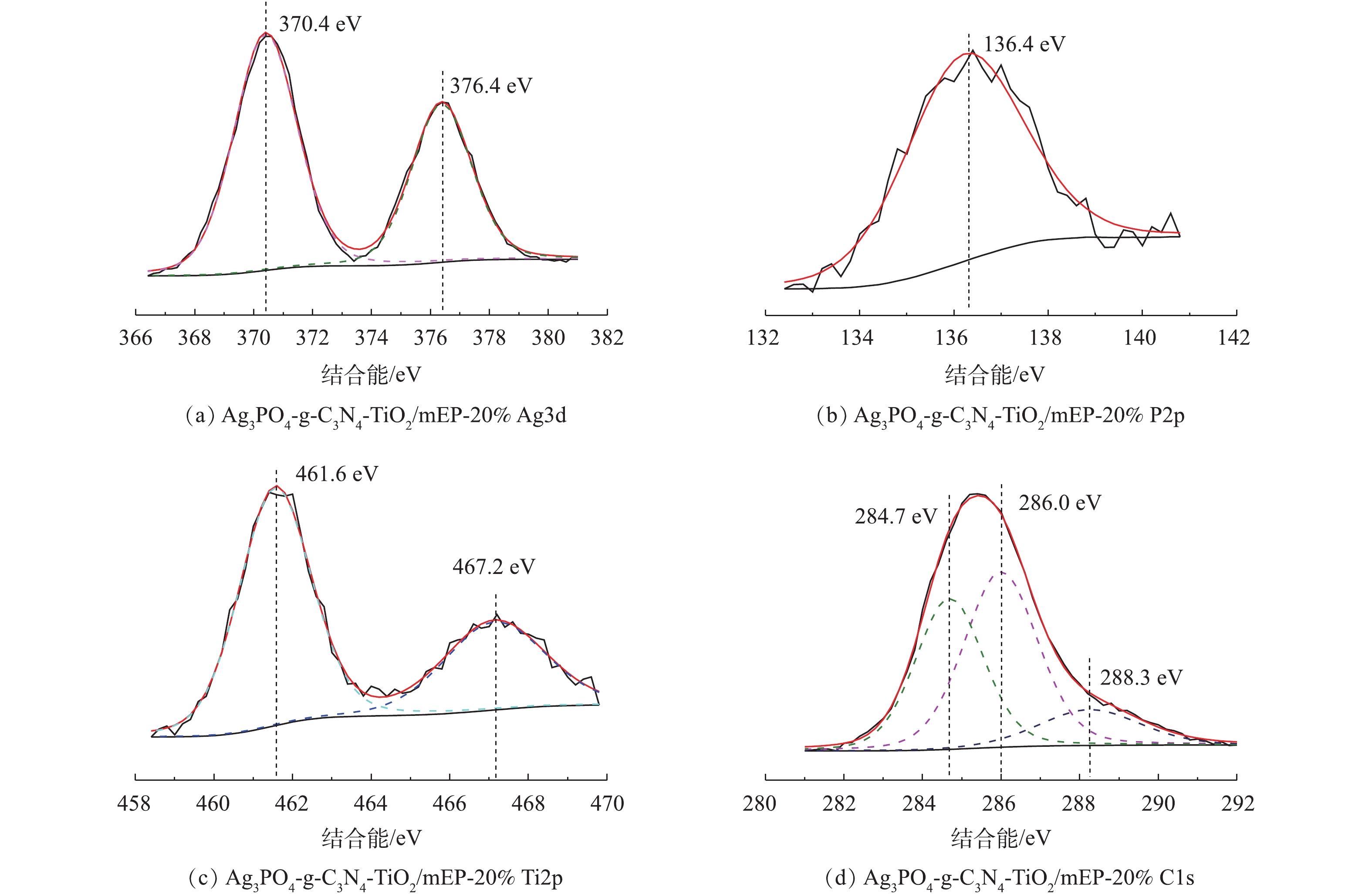
PO3−4 )中P−O的分子振动[16]。通过XPS谱图进一步分析复合光催化材料的元素价态。图3(a)~图3(d)分别为光催化剂Ag3PO4-g-C3N4-TiO2/mEP-20%中Ag3d、P2p、Ti2p、C1s的XPS高分辨谱图。经分峰拟合后,在Ag3d的谱图中(图3(a)),Ag3d5/2和Ag3d3/2的峰位置分别在370.4 eV和376.4 eV且峰型位置对称,表明银主要以Ag+的形式存在[17]。P2p的XPS峰信号(图3(b))出现在136.4 eV处,说明磷在本样品中以P5+的形式存在[18]。Ti2p谱图在461.6 eV和467.2 eV结合能处产生2个峰,分别对应于Ti2p3/2和Ti2p1/2,显示材料中Ti主要以+4价态存在[19]。C1s谱图经分峰拟合后,284.7 eV和288.3 eV处的峰分别对应于C=N[10],而位于286.0 eV处的峰则对应C—O[13]。XPS表征结果表明,Ag3PO4与g-C3N4成功与TiO2复合,催化剂所形成的异质结有助于提高催化剂的可见光响应,提高光生电子与空穴的分离效果。
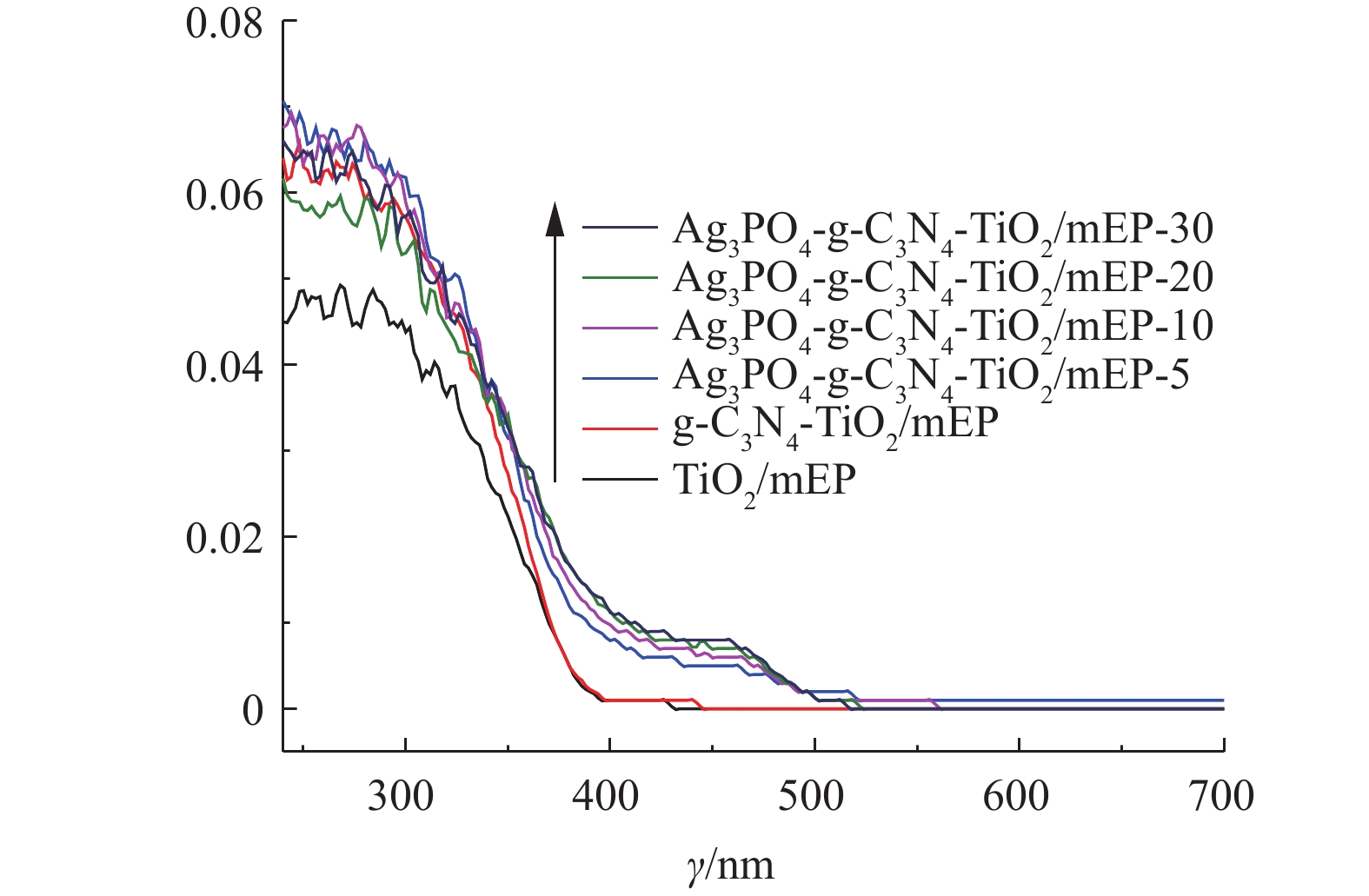
图4为TiO2/mEP、g-C3N4-TiO2/mEP及Ag3PO4-g-C3N4-TiO2/mEP系列复合光催化剂的UV-vis漫反射吸收光谱图。可以看出,TiO2/mEP主要是吸收波长小于400 nm的紫外光,相比于TiO2/mEP复合材料,g-C3N4-TiO2/mEP在可见光区的吸收提升较小。Ag3PO4-g-C3N4-TiO2/mEP复合光催化剂在400 nm和500 nm处出现双吸收带边,表明复合Ag3PO4后,复合催化剂在可见光区域的响应明显增强。随着Ag3PO4负载量的增加,Ag3PO4-g-C3N4-TiO2/mEP复合光催化剂在可见光区的吸收峰强度逐渐增强。
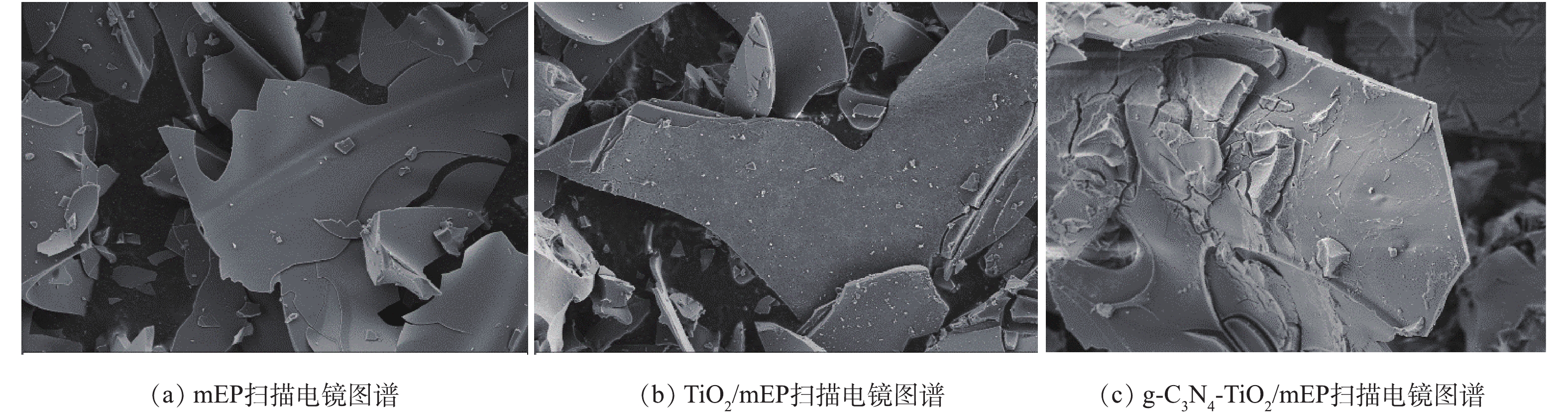
图5为mEP、TiO2/mEP和g-C3N4-TiO2/mEP的SEM扫描电镜图。从图5(a)中可以看出,mEP基底材料外表面有大量凹凸不平的沟壑,其特点是能为复合光催化剂材料提供较大的负载面域。图5(b)中可以清晰地看到纳米TiO2颗粒均匀地分散附着在mEP表面褶皱内。图5(c)中能明显观察到g-C3N4-TiO2复合材料牢固负载于基底材料表面和层间空隙,呈现一定的包裹现象。
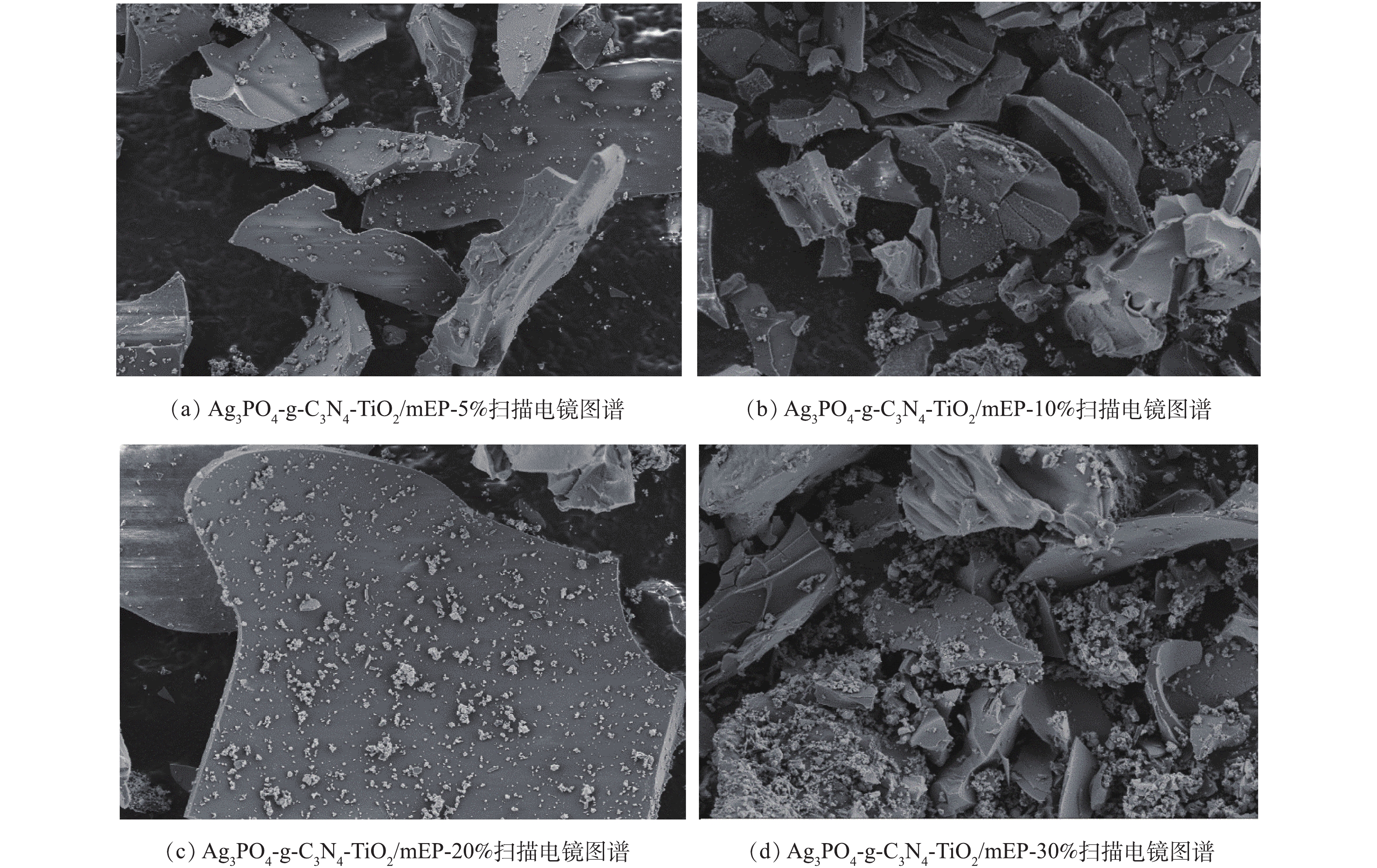
Ag3PO4-g-C3N4-TiO2/mEP系列复合材料的扫描电镜图如图6所示。可以看出,Ag3PO4颗粒分布在凹凸不平的材料表面,随着Ag3PO4/TiO2质量比的增加,材料表面聚集的Ag3PO4颗粒也越多,堆积的颗粒使得材料表面更加粗糙。当Ag3PO4/TiO2摩尔比为0.3时,过多Ag3PO4颗粒在材料表面形成团聚现象,因此,有部分较大颗粒的存在。由图6(c)中可以看出,mEP表面均匀负载了催化剂材料,能为反应过程中藻类的吸附和光催化降解提供有力保障。
-
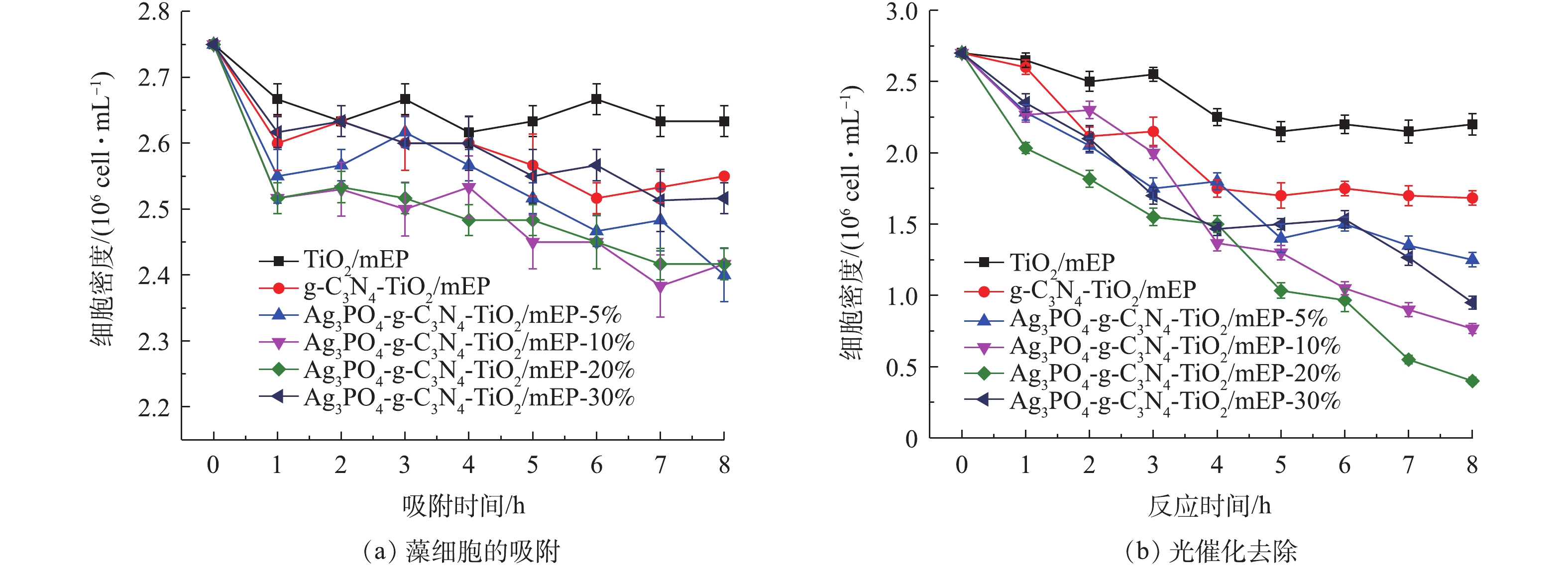
可见光催化除藻是通过催化剂对藻细胞吸附与光催化氧化灭活作用协同完成的。本研究首先考察催化剂对藻细胞的吸附性能,吸附动力学研究结果如图7(a)所示。可以看出,光催化剂对藻细胞均有一定的吸附去除效果。吸附1 h后,藻细胞浓度迅速下降,随着时间的延长,藻细胞浓度变化放缓,对藻细胞的吸附逐渐达到平衡。吸附达到平衡后,TiO2/mEP、g-C3N4-TiO2/mEP、Ag3PO4-g-C3N4-TiO2/mEP-5%、Ag3PO4-g-C3N4-TiO2/mEP-10%、Ag3PO4-g-C3N4-TiO2/mEP-20%和Ag3PO4-g-C3N4-TiO2/mEP-30%对藻细胞的吸附去除率分别为4.24%、7.27%、12.73%、12.12%、12.14%和8.48%,由此可见,Ag3PO4的复合有助于提高催化剂对藻细胞的吸附,但整体对藻细胞的去除率较低。
图7(b)为TiO2/mEP、g-C3N4-TiO2/mEP和Ag3PO4-g-C3N4-TiO2/mEP系列材料光催化灭活藻细胞结果。可以看出,在可见光照射下,TiO2/mEP对藻细胞的去除结果与图7(a)中暗吸附的结果类似,表明TiO2/mEP可见光下无法对藻细胞进行灭活。g-C3N4-TiO2/mEP对藻细胞的去除效果(图7(b))较TiO2/mEP有一定的提升,但提升效果不明显。复合Ag3PO4后,Ag3PO4-g-C3N4-TiO2/mEP系列材料光催化灭活藻细胞的效果均有明显提升,随着Ag3PO4/TiO2摩尔比的增加,藻细胞去除率呈现先增大后减小的趋势。Ag3PO4-g-C3N4-TiO2/mEP-20%催化剂对藻细胞的灭活效果最显著,8 h反应对藻细胞的去除率达到85.19%,该结果远高于催化剂暗吸附对藻细胞的去除效果(12.14%),表明在吸附和光催化灭活协同作用下,该催化剂可实现对藻细胞的高效去除。
-
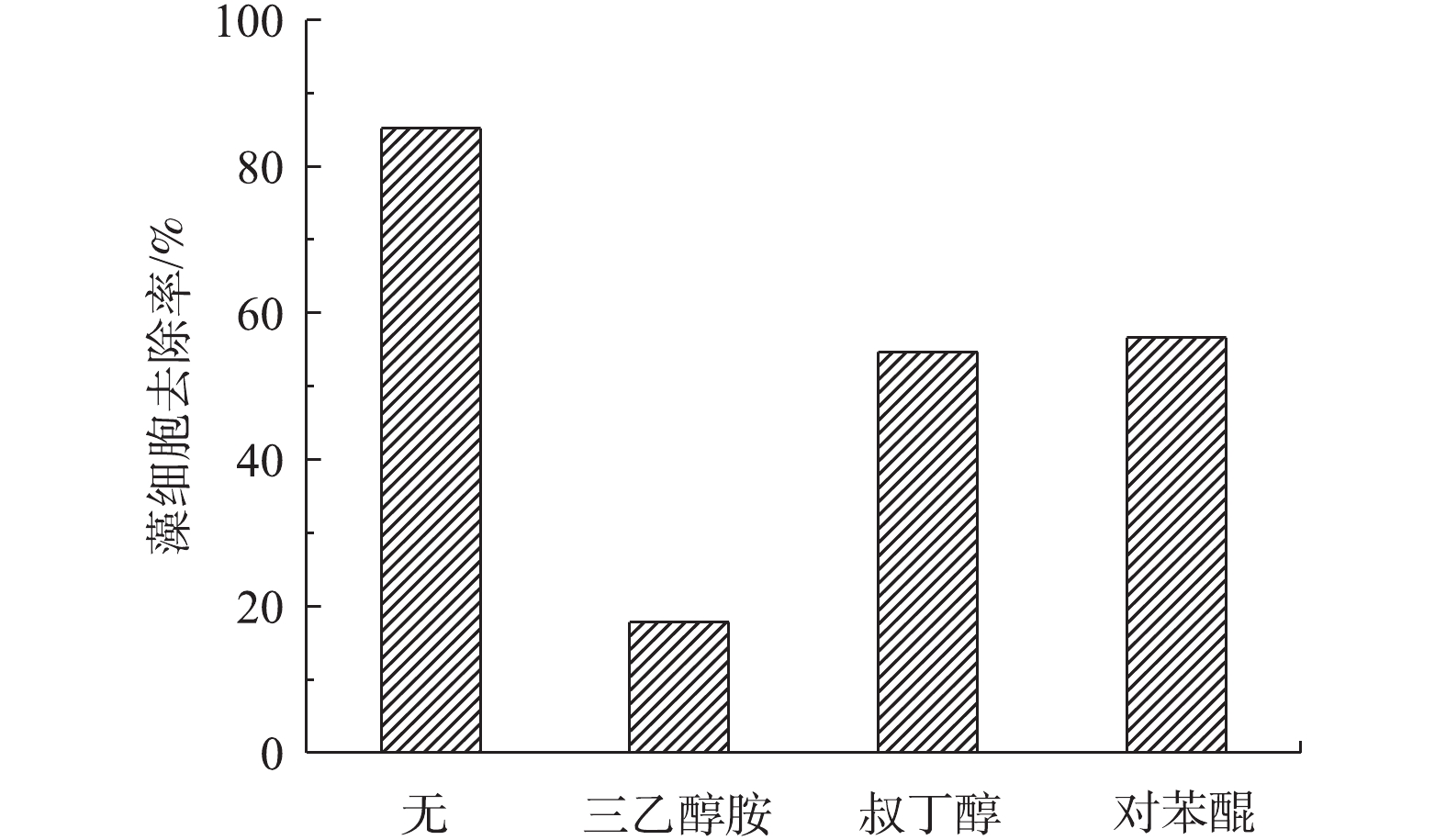
为进一步研究可见光催化除藻过程的作用机理,实验分别选用三乙醇胺(TEOA)、叔丁醇(TBA)、对苯醌(BQ)对光催化反应过程中主要作用的活性基团:价带空穴h+、羟基自由基∙OH和超氧自由基∙
O−2 进行掩蔽[19]。以Ag3PO4-g-C3N4-TiO2/mEP-20%为例,向藻液中分别添加2.0 mmol·L−1的TEOA、20 mmol·L−1的TBA、2.0 mmol·L−1的BQ进行光催化除藻,实验结果如图8所示。添加掩蔽剂后,铜绿微囊藻的去除率均有不同程度的下降;当溶液中存在三乙醇胺时,光催化除藻效率最低,由85.19%下降至17.84%,说明h+在光催化除藻过程中起主要作用,光催化除藻过程中起作用的活性基团贡献率为h+>∙OH>∙O−2 。TiO2在与g-C3N4、Ag3PO4复合后所形成的异质结更有利于抑制光生电子-空穴对的复合。吸附在材料表面的藻细胞可直接被催化剂表面激发产生的光生空穴h+灭活。Ag3PO4-g-C3N4-TiO2/mEP-20%对藻细胞较强的吸附能力(图7(a))更有利于光催化反应过程的进行。实验针对Ag3PO4-g-C3N4-TiO2/mEP-20%,考察其在光催化除藻中的重复利用性能。以8 h为实验周期,每次反应完成后,更换相同浓度的藻液,重复3次。该材料3次重复应用的实验结果如图9所示。可以看出,该催化剂具有良好的化学稳定性。在第1次光降解实验中对藻细胞的去除率为85.19%,随着重复利用次数的增加,催化剂对藻细胞的去除效果有所下降。分析其原因,可能是由于吸附在材料表面的藻细胞以及杀灭的藻细胞所释放的胞内物质影响了材料的进一步吸附,导致去除率下降,但在循环利用3次后,光催化剂对藻细胞的去除率仍可达到74.41%。
-
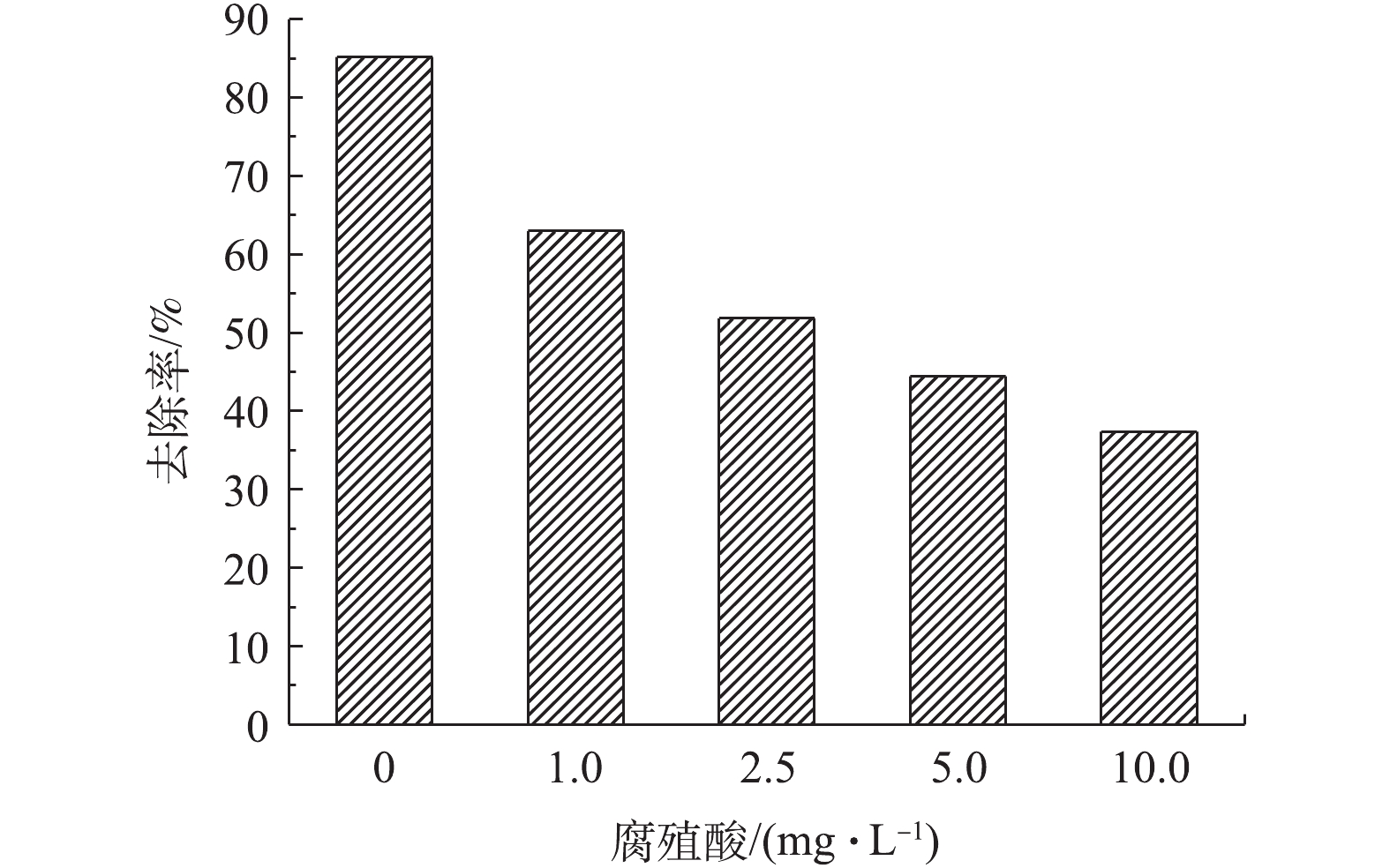
实验考察了自然水体中常见的腐殖酸对Ag3PO4-g-C3N4-TiO2/mEP可见光催化除藻性能的影响(图10)。由图10可以看出,当初始藻细胞浓度为2.7×106 cell·mL−1时,腐殖酸系列浓度由0 mg·L−1上升至10 mg·L−1的过程中,Ag3PO4-g-C3N4-TiO2/mEP-20%对铜绿微囊藻的去除率逐渐降低。未添加腐殖酸时,催化剂对藻细胞的去除率为85.19%,当腐殖酸浓度为10 mg·L−1时,藻细胞的去除率下降至37.40%。溶液中腐殖酸对于光催化过程的影响可能主要存在于2个方面:一方面是腐殖酸与藻细胞的竞争吸附作用,腐殖酸的存在可能在一定程度上占据了一部分材料表面的吸附位点,使得材料对藻细胞的吸附量下降,从而抑制了催化剂对藻细胞的杀灭;另一方面,腐殖酸浓度较高时,其透光性能降低,削减了可见光区光量子入射量,使得材料对光能的利用效率下降,因此,可见光催化活性降低。
实验同时考察了水中叶绿素、
NO−3 和Cr(VI)等对Ag3PO4-g-C3N4-TiO2/mEP可见光催化除藻的影响。结果表明:与腐殖酸类似,叶绿素可以在一定程度上抑制该材料对铜绿微囊藻的光催化去除;低浓度NO−3 具有光敏化效应,会促进材料对铜绿微囊藻的去除,但是随着NO−3 浓度的增加,它会聚集在催化剂表面形成吸附竞争,从而对该材料光催化去除铜绿微囊藻产生抑制作用。Cr(VI)的存在降低了藻类的去除率,但自身也能得到一定的还原。
2.1. 光催化剂表征
2.2. Ag3PO4-g-C3N4-TiO2/mEP的吸附-可见光除藻动力学
2.3. 除藻机理及材料重复利用
2.4. 共存物质对材料光催化除藻的影响
-
1)以铝盐改性膨胀珍珠岩为漂浮型载体,采用溶胶凝胶-浸渍沉积法成功制备了Ag3PO4-g-C3N4-TiO2/mEP光催化剂,该催化剂中Ag3PO4/TiO2的摩尔分数变化可对催化剂的晶型结构、比表面积、表面官能团产生影响。Ag3PO4、g-C3N4与TiO2的复合可提高催化剂的可见光响应。
2) Ag3PO4/TiO2的理论摩尔分数20%时,制得的催化剂对铜绿微囊藻的吸附-光催化灭活效果最佳,单纯暗吸附8 h藻细胞的去除率为12.14%,吸附和光催化协同作用8 h后,藻细胞的去除率达到85.19%。
3)复合Ag3PO4后,光催化剂所形成的异质结有助于促进光生电子和空穴的分离,光催化除藻过程中起作用的活性基团贡献率为h+>∙OH>∙
O−2 ,催化剂在重复利用3次后,藻细胞的去除率仍可达到74.41%,催化剂有较好的稳定。4)有机物(如腐殖酸、叶绿素)、共存离子(如
NO−3 、Cr(VI))都会对材料光催化去除铜绿微囊藻产生影响。腐殖酸和叶绿素可以在一定程度上抑制铜绿微囊藻的光催化去除,低浓度NO−3 具有光敏化效应,Cr(VI)的存在降低了藻类的去除率,但自身也能得到一定的还原。







 下载:
下载: