-
自然界中过量的磷极易引发赤潮、水华等水体富营养化现象,给整个水生态系统带来安全隐患,如何高效降低水体磷浓度已经成为水污染防治的主要问题之一。吸附法除磷工艺由于其高效清洁、可回收磷、可重复利用、工艺简单等优点,具有广泛的应用前景[1]。黏土矿物(clay minerals)作为常见吸附剂,其独特的层间结构及离子附着现象使其通过离子交换、静电引力和配位作用拥有良好的吸附潜力[2-3],且具有无臭、无毒、比表面积大、化学稳定性高等优点[4-5]。中性条件下天然黏土矿物对离子的吸附能力由大到小分别为蒙脱石>伊利石>高岭石[6]。天然黏土矿物对磷的吸附容量不高,通常采用离子饱和、焙烧、铝铁等高价态阳离子负载方式进行预处理或改性,提高吸附剂的配位结合能力或扩大矿物层间距,更有利于离子吸附[6-7]。
目前,国内外大部分吸附除磷研究主要集中于正态磷酸盐(
H2PO−4,HPO2−4,PO3−4 )这一形态展开。但是,现实水体中水溶性磷酸盐包括正态磷酸盐和非正态磷酸盐(P2O4−7,P3O5−10,(PO3)n−n 等)。非正态磷酸盐由于较难水解或难以被传统方法测定等原因,虽广泛应用于肥料、水处理、食品、采矿、印染等行业,但在磷循环中的重要性往往被人们所忽略[8]。SUNDARESHWAR等[9]发现以焦磷酸盐为主的非正态磷酸盐在海岸湿地沉积物中含量已超过正态磷酸盐沉积物。因此,有效处理水体中的非正态磷酸盐同样是吸附除磷研究的重要内容之一。本研究以蒙脱石型黏土矿物为研究对象,利用焙烧、铝基改性、铁基改性等方式进行定向改性并开展了吸附除磷实验研究,采用吸附模型对批次动力学吸附实验、热力学吸附磷实验结果进行了拟合,分析了不同改性蒙脱石的除磷效果,探究了改性蒙脱石对正态磷酸盐和非正态磷酸盐单独存在及其混合体系下的吸附特性,以期为含磷废水吸附处理技术应用提供参考。
全文HTML
-
为提高蒙脱石吸附容量,强化吸附除磷性能,对蒙脱石样品(SWy,购自美国黏土矿物协会)进行预处理及改性。
在进行预处理(SWy-离子饱和)时,蒙脱石经研钵研磨,200目筛网过筛后,加入0.01 mol·L−1 CaCl2溶液中,搅拌悬浮24 h。将蒙脱石悬液离心(10 000 r·min−1, 20 min),用去离子水洗涤3次以上,直至用2.0 mol·L−1 AgNO3溶液检测,确定上清液中无残余Cl−。将处理后的蒙脱石进行冷冻干燥、研磨过筛后于室温下储存备用[10]。
在制备改性1#蒙脱石(SWy-焙烧)时,称取经预处理的SWy-离子饱和按一定的配合比加入6.0 mg·L−1的NaOH溶液,在85 °C恒温下搅拌12 h,用去离子水洗涤至中性。将处理后的蒙脱石冷冻干燥,在450 °C马弗炉中焙烧4 h,冷却备用,制得改性SWy-焙烧。
在制备改性2#蒙脱石(SWy-Al)时,称取SWy-离子饱和适量,向其中加入0.1 mg·L−1 AlCl3溶液,并在搅拌过程中逐滴加入NaOH溶液,调节至一定pH,置于40 °C恒温水浴磁力搅拌器中搅拌60 h,后用去离子水洗涤3次以上。将以上材料进行冷冻干燥,制得SWy-Al。
在制备改性3#蒙脱石(SWy-Fe)时,称取SWy-离子饱和适量,加入1.0 mg·L−1 Fe(NO3)3·9H2O溶液及5.0 mg·L−1 KOH溶液,后续操作同上段,制得SWy-Fe。
-
分别进行吸附动力学和吸附热力学实验,以考察不同的改性蒙脱石对正态磷酸盐、非正态磷酸盐及其混合体系的吸附特性。
在进行吸附动力学实验时,向250 mL血清瓶中分别加入5.0 mg·L−1的K2HPO4(正态磷酸盐)或HPO3(非正态磷酸盐)、0.3~1.0 g·L−1改性蒙脱石,并以0.1 mol·L−1 KCl作为反应体系电解质。将血清瓶置于27 °C、150 r·min−1摇床中振荡。反应进行至10、30、60、120、180、240 min,用无菌注射器取样,使用紫外分光光度计(SHIMADZU UV-1780)测定上清液总磷(TP)、正态磷酸盐(P)、非正态磷酸盐(PP, PP=TP-P)浓度。
在进行吸附热力学实验时,分别加入0~10.0 mg·L−1的K2HPO4(正态磷酸盐)或HPO3(非正态磷酸盐)、1.0 g·L−1改性蒙脱石,其他实验条件同上。待吸附反应平衡后,测定总磷(TP)、正态磷酸盐(P)、非正态磷酸盐(PP, PP=TP-P)的浓度。
-
Lagergren拟一级动力学拟合方程如式(1)所示。
Ho拟二级动力学拟合方程如式(2)所示。
式中:k1为拟一级方程速率常数,min−1; k2为拟二级方程速率常数,g·(mg·min)−1; qe和qt分别为吸附容量和t时刻的吸附量,mg·g−1。
Langmuir吸附等温线模型方程如式(3)和式(4)所示。
Freundlich吸附等温线模型方程如式(5)所示。
式中:C0为含磷溶液的初始浓度,mg·L−1;Ce为达到吸附平衡时总磷浓度,mg·L−1;qe为蒙脱石平衡吸附量,mg·g−1;qmax为蒙脱石最大吸附量,mg·g−1;KL为Langmuir等温线系数,L·mg−1;KF为Freundlich等温线系数;1/n为吸附常数。
-
透射电镜采用美国FEI公司Tecnai G2 Spirit,并应用仪器配置的一体化X射线能谱器EDAX Analyzer(DPP-II)观察样品微观形貌,同时进行成分及元素像分析(element mapping)。
1.1. 材料制备
1.2. 吸附实验
1.3. 数据处理方法
1.4. 材料性能表征
-
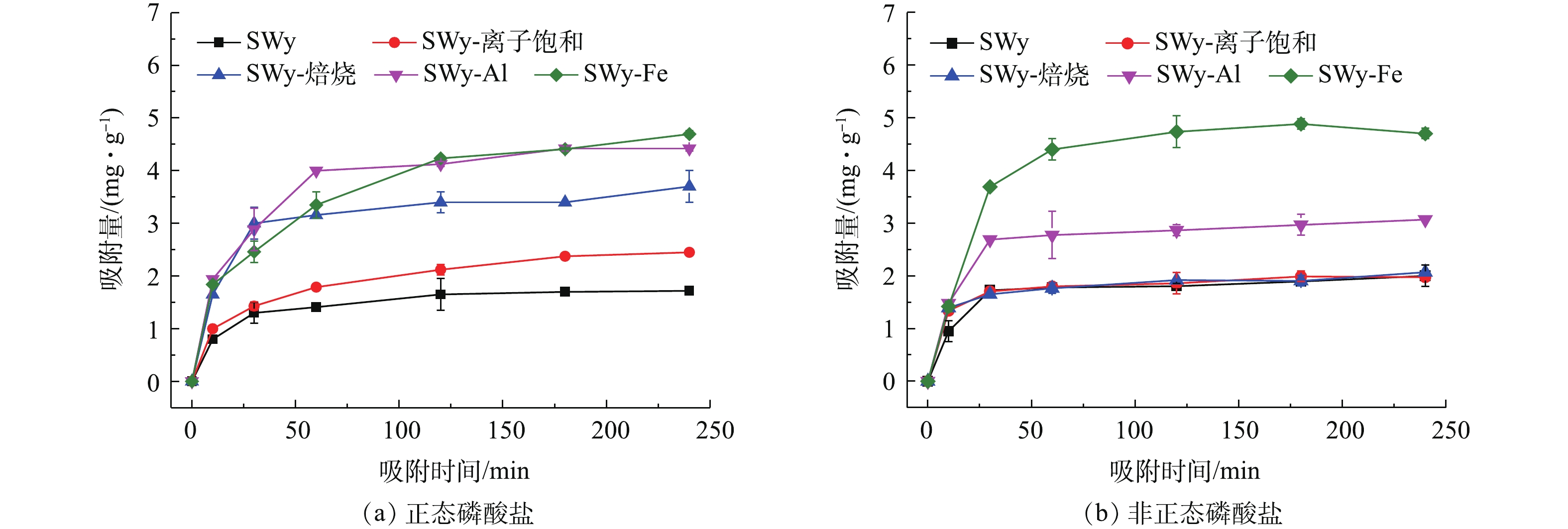
实验考察了不同种类改性蒙脱石对正态磷酸盐/非正态磷酸盐的吸附效果。图1(a)和图1(b)为pH=7.0、反应温度27 °C的条件下,蒙脱石经预处理和不同方式改性后对正态磷酸盐、非正态磷酸盐的吸附结果。如图1所示,3种改性蒙脱石均可提高正态磷酸盐和非正态磷酸盐的吸附效果。吸附4 h后,反应过程达到吸附平衡,天然蒙脱石SWy对正态磷酸盐去除率仅为32.5%;预处理后,SWy-离子饱和的正态磷酸盐去除率升高至46.2%。可交换阳离子Ca2+的引入不仅平衡了蒙脱石样品层间不饱和电荷,而且在吸附反应过程中易于被磷酸盐离子取代,形成磷酸钙复合体,提高蒙脱石对磷酸盐的吸附能力[7]。3种改性蒙脱石SWy-焙烧、SWy-Al、SWy-Fe的投加,对正态磷酸盐的吸附去除率相比SWy,分别提高了37.3%,50.9%和56.1%,3种改性蒙脱石中SWy-Fe对于正态磷酸盐拥有最佳吸附效果,去除率达到88.5%。
类似地,3种改性蒙脱石的加入也均提高了非正态磷酸盐吸附效果。未经预处理的蒙脱石SWy对非正态磷酸盐去除率为40.1%。3种改性蒙脱石SWy-焙烧、SWy-Al、SWy-Fe的投加,使非正态磷酸盐的吸附去除率分别提高了1.3%、20.0%和55.3%。在3种改性材料中,SWy-Fe对于非正态磷酸盐依然拥有最佳除磷效果。
对不同改性蒙脱石吸附结果进行吸附动力学回归分析,拟合结果见表1。磷酸盐在改性蒙脱石上的吸附动力学符合Ho拟二级动力学方程(R2均大于0.98),包含吸附外部液膜扩散、表面吸附、颗粒内扩散等过程[11]。这表明改性蒙脱石对正态磷酸盐、非正态磷酸盐的吸附过程均为多种吸附作用的综合作用结果,物理、化学吸附和扩散作用对吸附过程具有重要影响。
蒙脱石表面具有巨大的比表面积和特殊的层状结构,能进行无选择性多层物理吸附。图1磷吸附反应初期(前30 min),正态和非正态磷酸盐浓度均出现快速下降,这表明磷酸根离子在物理吸附作用下被快速吸附到蒙脱石表面,并随后颗粒物表面发生慢速的选择性化学吸附。
选择性化学吸附阶段,磷酸根作为配体与颗粒物表面的阳离子进行配位结合,形成共价键化合物[12]。Al-OH和Fe-OH官能团与磷酸盐通过形成络合物发生稳定的化学吸附。许海娟等[13]发现吸附过程中形成的磷酸络合物转化成磷酸铁或磷酸铝表面沉淀,降低磷的移动性和生物有效性,进一步促进磷的去除。WANG等[14]的配位电荷分布(LCD)模型验算结果也证明,多价阳离子(Fe3+、Al3+、Ca2+)在增加磷酸根的吸附效果方面展现出协同效应,这与本研究SWy-Al、SWy-Fe总磷去除率优于SWy-离子饱和的实验结果一致。证明了本研究蒙脱石SWy改性过程中Ca2+、Fe3+、Al2+阳离子的负载均有利于增加磷的吸附点位,提高磷吸附效果。实验结果表明SWy-Fe拥有最强的磷吸附能力。
实验考察了不同含量SWy-Fe对正态磷酸盐/非正态磷酸盐的吸附效果。SWy-Fe对于正态磷酸盐和非正态磷酸盐均具有最佳吸附效果,故以此为基础,继续探究改性蒙脱石对正态磷酸盐和非正态磷酸盐的吸附特性,进一步针对性分析改性蒙脱石对正态磷酸盐、非正态磷酸盐单独及混合体系的磷吸附行为。
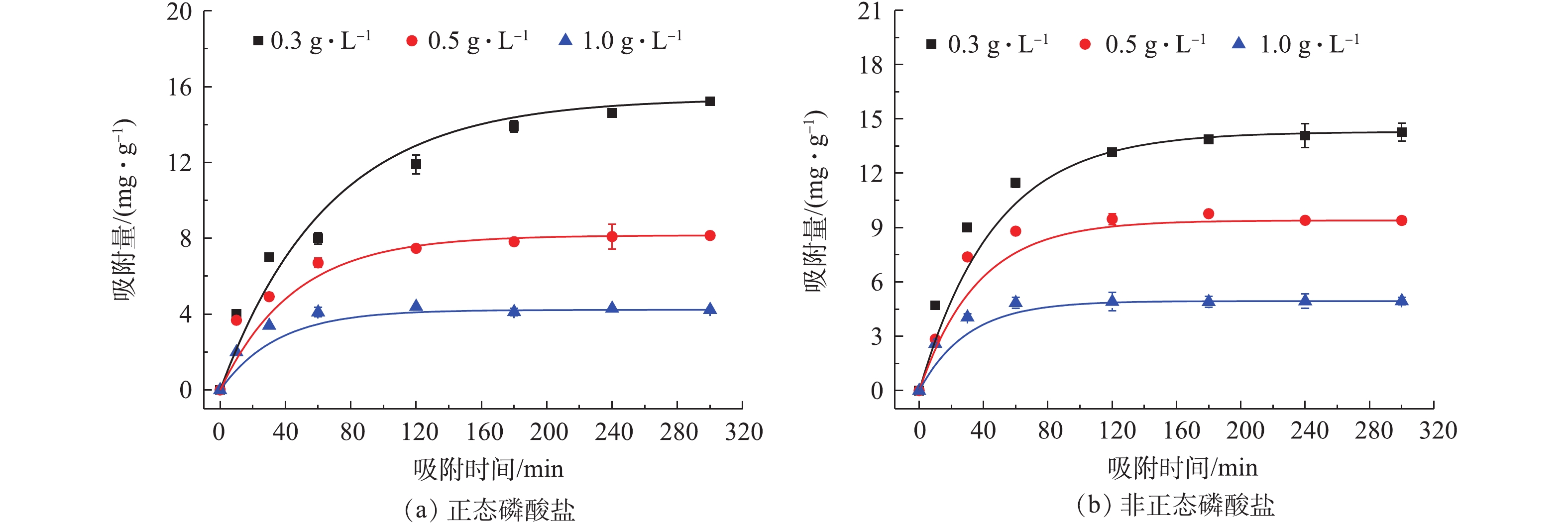
图2为pH=7.0、反应温度27 °C的条件下,不同含量的SWy-Fe分别对初始浓度为5.0 mg·L−1的正态磷酸盐和非正态磷酸盐吸附效果的影响结果。随着吸附剂用量的增大,吸附量显著减小,达到吸附平衡所需时间明显缩短。在吸附初期,吸附剂的活性位点较多[15],以物理吸附为主的磷吸附速率较大。随着吸附时间的延长,体系中总磷浓度降低,且SWy-Fe表面活性位点减少,吸附速率逐渐减小,直至吸附平衡。结果表明,针对正态磷酸盐和非正态磷酸盐,SWy-Fe质量浓度越高,表面活性位点及可利用的表面积越多,磷吸附速率随之增加,吸附平衡时间明显缩短。
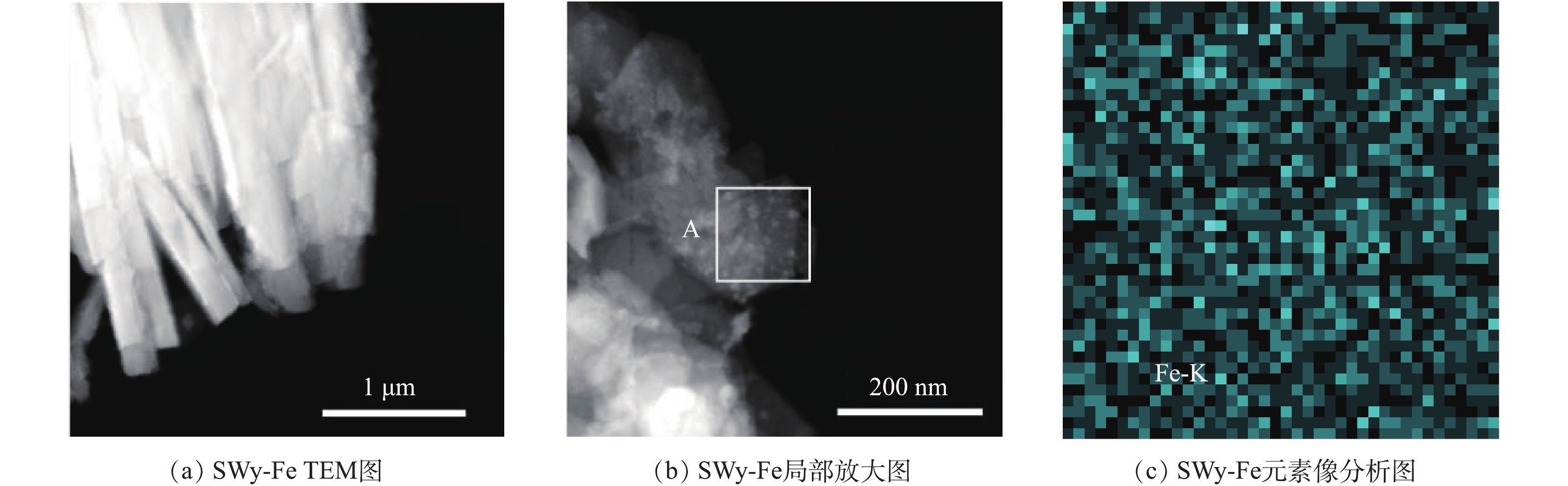
本研究对改性蒙脱石SWy-Fe矿物元素分布进行了表征。改性蒙脱石中SWy-Fe具有最佳磷吸附效果,为研究特异性元素在改性蒙脱石SWy-Fe表面的负载效果,对SWy-Fe样品进行透射电镜分析。图3为紫外-可见光吸收边带值最大的SWy-Fe样品的透射电镜(TEM)及局部放大后的元素面分布图。如图3(a)所示,蒙脱石颗粒呈不规则长条状或薄片状。选取图3(b)中A区域,进行TEM-EDS的成分及元素像分析,表征蒙脱石表面Fe元素分布。图3(c)为所选区域所有点的EDX图累加结果。可以看出,改性样品层面分布较均匀的Fe-K线峰,表明成功在蒙脱石层面负载Fe元素。
-
在pH=7.0,反应温度27 °C条件下,考察了0.5 g·L−1 SWy-Fe分别对初始浓度为0~10 mg·L−1的正态磷酸盐和非正态磷酸盐的吸附效果,并且通过Langmuir和Freundlich模型[16]对所得到的实验数据进行吸附热力学分析。如图4所示,经吸附等温线拟合,SWy-Fe对正态磷酸盐和非正态磷酸盐的吸附均符合Langmuir吸附等温模型,在pH=7.0、27 °C时,其对二者的最大吸附量分为21.9 mg·g−1和18.8 mg·g−1。
-
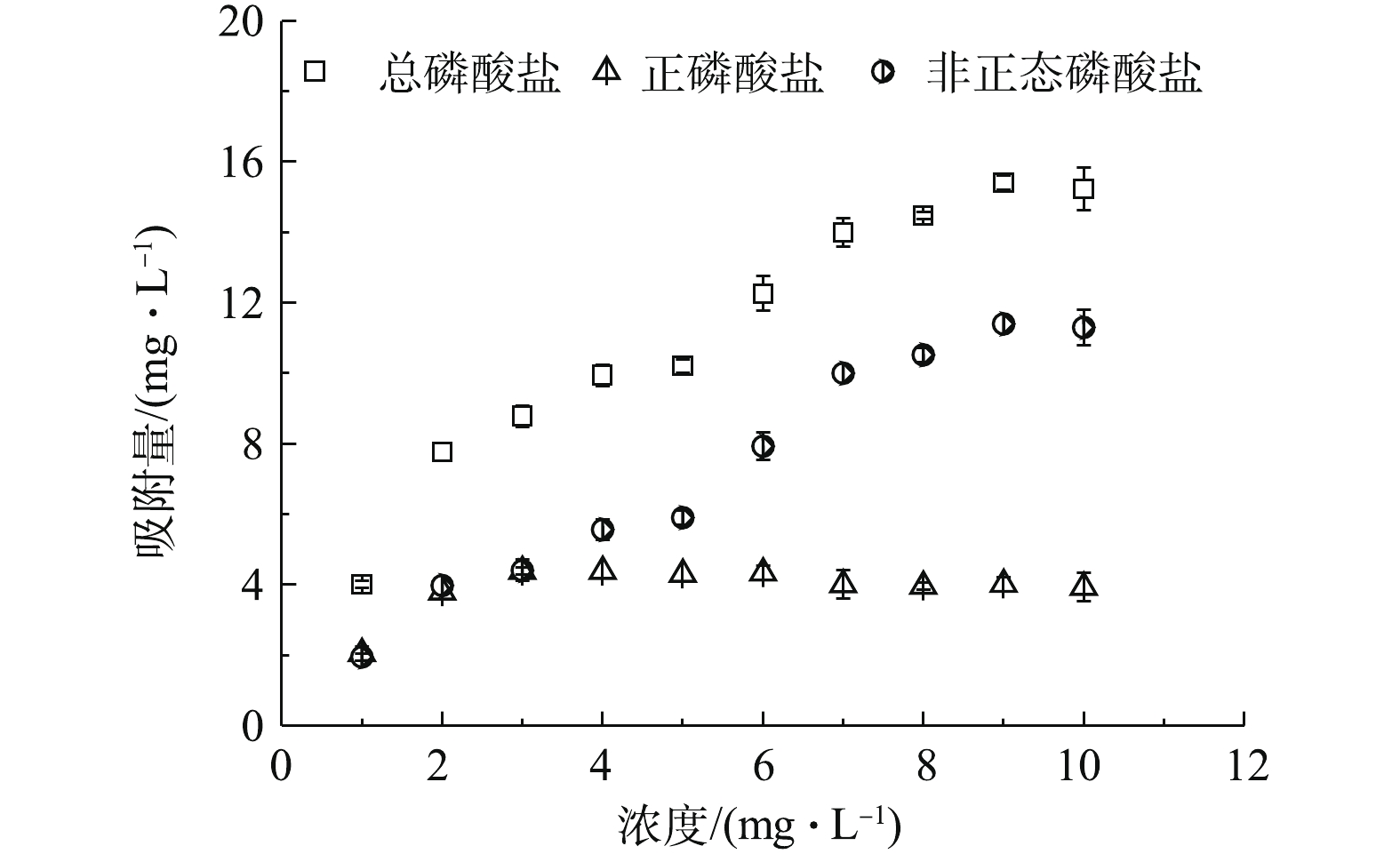
将0.5 g·L−1 SWy-Fe和总磷酸盐溶液混合均匀,其中,正态磷酸盐与非正态磷酸盐质量浓度比(P∶PP)为1∶1~5∶5,分别检测体系中磷酸盐浓度的变化(图5)。如图5所示,在pH=7.0、27 °C下,吸附平衡后,当初始总磷浓度为3.0 mg·L−1以下,正态磷酸盐和非正态磷酸盐吸附量变化趋势几乎完全重合。这表明低浓度总磷混合液(TP=1.0~3.0 mg·L−1)、正态磷酸盐和非正态磷酸盐吸附反应不存在明显的竞争行为,在饱和吸附量内,SWy-Fe对水体中的正态磷酸盐和非正态磷酸盐二者混合体系均具有较好的吸附效果。
2种形态的磷酸盐之间的竞争作用主要发生在初始磷浓度较高的情况。随着总磷浓度的不断上升,两者吸附效果出现了明显差别。在吸附平衡状态下,SWy-Fe对正态磷酸盐的平衡吸附量为4.3 mg·g−1,非正磷酸盐的平衡吸附量为11.4 mg·g−1。这表明当初始总磷浓度高于3 mg·L−1,SWy-Fe对非正态磷酸盐的竞争吸附效果显著优于正态磷酸盐。非正态磷酸盐与Ca2+形成低Ca/P比的钙磷产物[17](Ca∶PP=0.5∶1;Ca∶P=1.1∶1),对于等量的Ca2+,非正态磷酸盐对Ca的利用率高于正态磷酸盐。同理,磷酸盐与Fe3+发生表面络合吸附反应,形成Fe-P络合物,由于偏磷酸根的空间构型,非正态磷酸盐与Fe3+形成低Fe/P比的钙磷产物(Fe∶PP=0.67∶1;Fe∶P=1.1∶1),即SWy-Fe对正态磷酸盐的吸附需要更多的表面活性位点。
2.1. 改性蒙脱石对正态磷酸盐/非正态磷酸盐的吸附动力学特征
2.2. 改性蒙脱石SWy-Fe对正态磷酸盐/非正态磷酸盐的吸附热力学特征
2.3. 改性蒙脱石SWy-Fe对正态磷酸盐和非正态磷酸盐混合体系的吸附热力学特征
-
1)蒙脱石3种改性方式(SWy-焙烧、SWy-Al、SWy-Fe)均可提高蒙脱石对正态磷酸盐和非正态磷酸盐的吸附效果,正态磷酸盐的吸附去除率分别提高37.3%、50.9%和56.1%,非正态磷酸盐吸附去除率分别提高1.3%、20.0%和55.3%,其中SWy-Fe拥有最佳吸附效果。吸附过程符合Ho拟二级动力学方程(R2均大于0.98),为吸附外部液膜扩散、表面吸附、颗粒内扩散等多种吸附过程综合作用的结果。
2) SWy-Fe对正态磷酸盐和非正态磷酸盐的吸附均符合Langmuir吸附热力学模型(R2均大于0.97),饱和吸附量分别为21.9 mg·g−1和18.8 mg·g−1。可交换阳离子Ca2+/Fe3+的引入平衡了蒙脱石样品内类质同象置换引起的不饱和电荷,通过吸附络合作用提高了蒙脱石对磷酸盐的吸附能力。
3) SWy-Fe对正态磷酸盐和非正态磷酸盐混合体系的吸附结果表明,正态磷酸盐和非正态磷酸盐的吸附竞争作用发生在初始总磷浓度高于3.0 mg·L−1的体系中,非正态磷酸盐吸附效果显著优于正态磷酸盐,单位平衡吸附量之比达到2.9∶1.0。这为实际含磷废水吸附处理技术应用提供理论依据。





 下载:
下载: