-
我国铅锌工业总规模大,常年位于世界第一,仅2018年,精Zn产量达5.68×106 t,约占全球总量的40%,我国Zn冶炼主流工艺为“焙烧—浸出—电积”,其生产过程中产生大量含Zn、Cd和As等的冶炼废渣,统计表明,平均生产1 t Zn,产生0.96 t废渣[1],废渣历史积存量和年新增量大,难以得到有效的消纳利用,通常采用无害化填埋、堆置储存等方式进行处置。在长期的堆置过程中,受风蚀、淋溶和浸蚀等作用影响,废渣中的重金属释放,对周边人群健康和土壤、地下水等生态环境造成严重威胁[2]。
稳定化是废渣常见的无害化处理方式,通过加入稳定剂降低重金属的迁移性,而稳定剂的选择是关键。目前,常用稳定剂一般包括有机、无机和生物质型3种[3]。其中,无机型药剂因对重金属稳定效果好而广被应用[4-6],其对重金属主要是通过化学键合、物理包容、吸附或形成惰性沉淀物等作用进行稳定[7-8]。目前,研究应用多以含硫、磷、铁、钙、镁等药剂为主[7-11],但多集中在Zn、Pb等个别污染指标,而针对废渣中As、Cd等其他多污染物共存的系统化研究还较为欠缺,特别是个别稳定剂对废渣中As反而存在活化作用则较少关注,受介质类型、污染程度、稳定剂种类、投加量、配伍等因素的影响,不同药剂实际稳定化效果还须进行综合比对和考证。
本研究以湖南某大型冶炼企业渣场堆存的铅锌冶炼废渣为研究对象,采用Na2S·9H2O、(NH4)2HPO4、Na3PO4·12H2O、CaO、MgO为稳定化药剂,并进行了药剂配伍研究,以水浸提法[12]模拟废渣堆存过程的浸蚀淋溶影响,考察了不同药剂对Zn、Cd、Cu、As的综合稳定化效果及其对环境的影响,以期为国内铅锌冶炼废渣的无害化处置提供参考。
全文HTML
-
本研究所用工业废渣取自湖南某大型冶炼企业典型渣堆场。将堆场上性状明显类似的废渣进行现场机械开挖和预混,运至具防渗结构的预存场进行自然风干、人工除杂、混匀、磨碎后,过4 mm筛,取筛下物于小型卧式搅拌机中再次混匀后,用于稳定化实验。
废渣污染特性如表1所示,与《污水综合排放标准》(GB 8978-1996) [13]1级最高允许排放浓度相比,废渣H2O浸出Zn、Cd和Cu超标,分别超标6.60、10.10、1.76倍,废渣pH为2.40,呈强酸性。
稳定化药剂包括Na2S·9H2O、(NH4)2HPO4、Na3PO4·12H2O、CaO、MgO,均为分析纯。
-
称量1 000.00 g(干质量)废渣置于敞口玻璃容器中,将Na2S·9H2O、(NH4)2HPO4和Na3PO4·12H2O 分别配成200 mL的水剂,按废渣Zn、Cd、Cu、As等的理论水浸出量计算药剂添加量,药剂与元素浸出摩尔比设计为2∶1、4∶1、8∶1、16∶1,对应药剂投加质量分数如表2所示,边加药剂边充分混匀搅拌,搅拌时间20 min,CaO和MgO按粉剂添加,根据前期Zn、Cd稳定化实验结果,3种药剂复配配方按质量比Na2S·9H2O∶(NH4)2HPO4∶Na3PO4·12H2O=2∶1∶3进行药剂配伍,设置空白对照(CK),每处理设3次重复,控制水∶渣(质量比)=1:4左右,室温养护7 d后,进行废渣pH、毒性浸出测试。
使用酸度计(pHs-3C型,上海仪电科学仪器股份有限公司)测定供试废渣的pH,测定方法采用《固体废物 腐蚀性测定 玻璃电极法》(GB/T 15555.12-1995)[14];重金属总量测试的前处理采用石墨炉三酸(体积比为HNO3∶HF∶HClO4=3∶2∶2)消解法;毒性浸出采用水浸法[12];消解液和水浸出液中重金属含量采用电感耦合等离子体发射光谱仪(ICP-MS 7500,美国Agilent公司)测定。
修复效果评估根据式(1)~式(3)进行计算。
式中:η(M)为M元素的稳定率;C0、Ct分别为废渣在稳定化前和稳定化后的元素水浸出浓度,mg·L−1;η(Zn, Cd)为Zn和Cd这2种重金属的稳定率均值;η为Zn、Cd、Cu、As 4种元素的综合稳定率均值。
1.1. 供试材料
1.2. 实验方法与分析方法
-
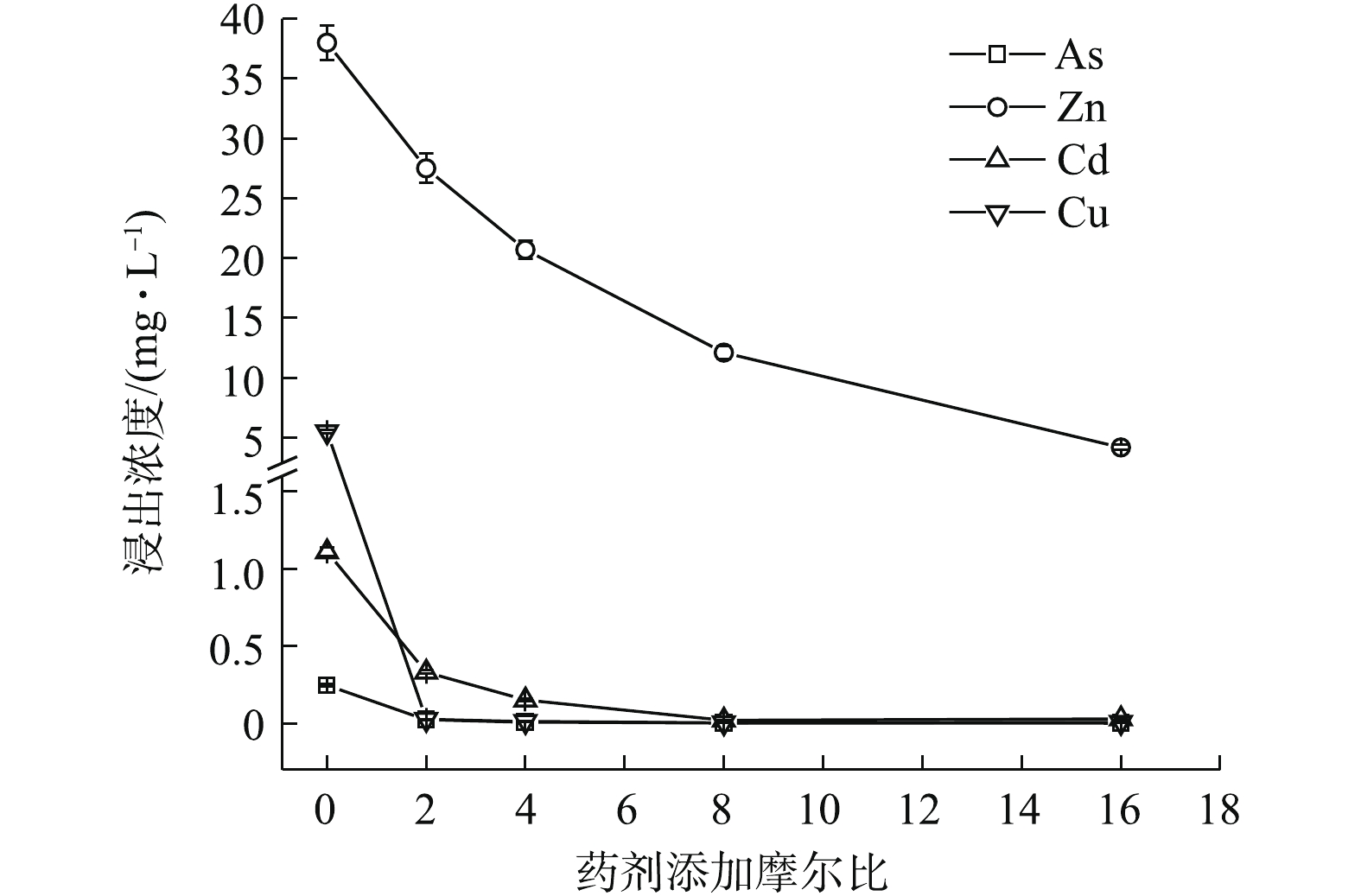
Na2S·9H2O、(NH4)2HPO4、Na3PO4·12H2O均能明显降低4种金属元素的水浸出浓度(图1~图3)。如图1所示,随Na2S·9H2O投加量的增加,各金属元素浸出均呈降低的趋势,Zn、Cd和Cu的稳定率分别为27.63%~88.97%、70.08%~98.05%、99.52%~99.85%。其中,药剂与4种金属元素浸出总量的摩尔比为8 (投加1.21%)时,Cd的浸出浓度由CK的1.11 mg·L−1降至0.02 mg·L−1,达到GB 8978-1996中Cd (0.1 mg·L−1)限标;摩尔比16(投加2.42%)时,Zn浸出才达标,由CK的38 mg·L−1降至4.19 mg·L−1,此时η(Zn)为88.97%、η(Cd)为97.35%,η(Zn, Cd)为93.16%,Na2S·9H2O对3种重金属稳定效果突出,总体稳定效应大小依次为Cu>Cd>Zn,这与其提供的S2-与Zn2+、Cd2+和Cu2+形成金属硫化物沉淀有关[15]。这种难溶物溶解度很低,形成沉淀的先后顺序一般为CuS>CdS>ZnS[16],随投加量的增加,其水解过程中产生的OH−也有利于重金属的稳定。此外,各处理使As浸出降低了90.24%~99.11%,说明其与As形成的惰性沉淀物在应对水浸滤风险能力方面也很显著,但在稳定化过程中有少许H2S逸出。
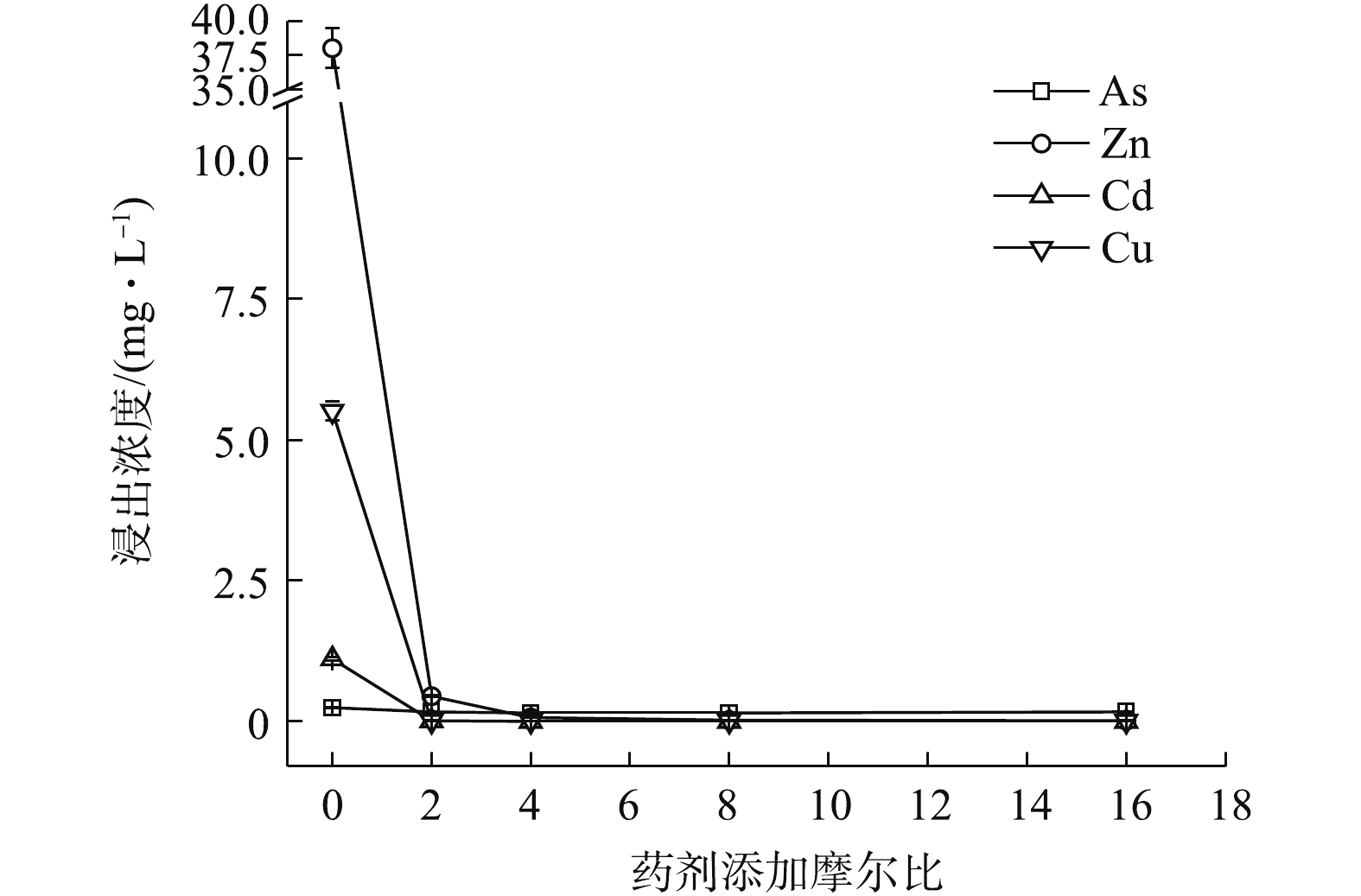
(NH4)2HPO4可明显降低重金属的水浸出毒性。如图2所示,随(NH4)2HPO4投加量的增加,Zn、Cd和Cu浸出浓度均呈不断降低的趋势,稳定率分别为59.52%~99.46%、57.97~99.23%、98.85~99.93%,而As浸出则呈现先降低后回升的趋势,药剂摩尔比≥8后,As浸出浓度开始升高并被活化,药剂摩尔比升高至16时,As浸出浓度被活化1.74倍,浓度为0.68 mg·L−1,超出0.5 mg·L−1限标的36%,药剂摩尔比为4时,As浸出降至最低0.016 mg·L−1,η(As)高达93.52%,但此时Zn和Cd浸出分别降至8.47 mg·L−1和0.23 mg·L−1,并未达标。直到药剂摩尔比为8(投加量为0.64%)时,才使以上4种金属元素均达标,Zn和Cd分别降至0.224 mg·L−1和0.01 mg·L−1,η(Zn)和η(Cd)分别为99.41%和99.08%,η(Zn, Cd)为99.25%,4种元素综合稳定率η达到最高值,为87.42%。因此,0.64%的(NH4)2HPO4投加量综合稳定效果最好,但须控制投加剂量,以免高量添加对As过度活化。
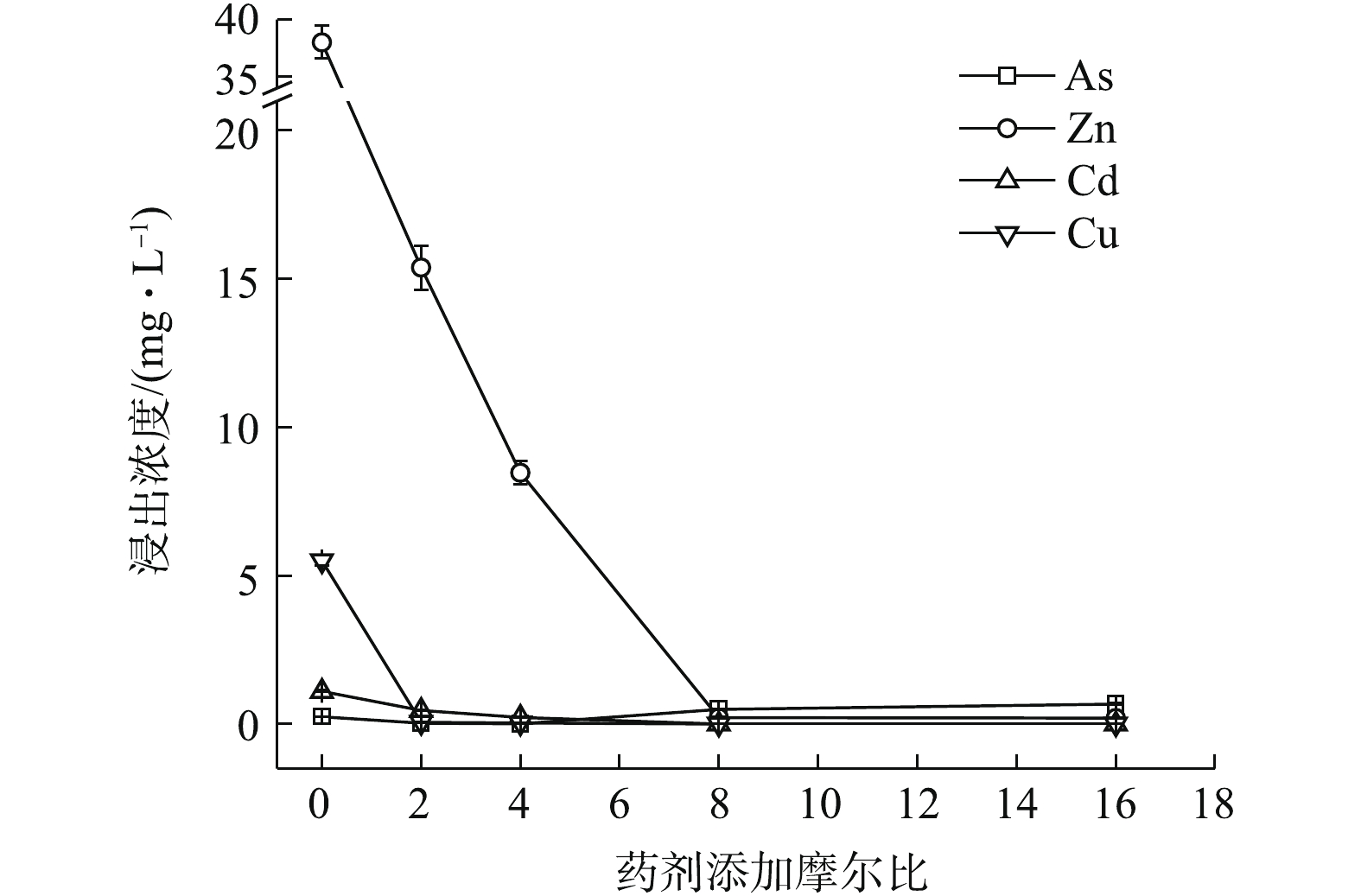
如图3所示,Na3PO4·12H2O稳定化效果突出,最低药剂摩尔比为2 (投加量为0.46%)时,可使4种元素均能达标,Zn、Cd、Cu和As浸出分别降至0.446、0.014、0.004、0.169 mg·L−1,稳定率分别为98.83%、98.70%、99.93%、31.58%,4元素综合稳定率η可达82.26%。其中,η(Zn)和η(Cd)分别为98.83%和98.70%,η(Zn, Cd)为98.76%,稳定率均高于同摩尔比条件下的(NH4)2HPO4(η(Zn) 59.52%,η(Cd) 57.97%,η(Zn, Cd) 58.94%)和Na2S·9H2O(η(Zn)27.63%,η(Cd) 70.08%,η(Zn, Cd) 48.85%),在Na3PO4·12H2O的各处理组中,η(As)均可稳定在34.92%左右。磷酸盐对Cd、Cu等的固定主要是通过与金属发生表面络合吸附和共沉淀等作用所致[8],对Zn主要以诱导或直接吸附[8]以及少量溶解性无定形沉淀反应[17]为主,并可促进Zn的可氧化态向残渣态转变[18]。而P与As化学性质相似,一般认为,
PO3−4 和HPO2−4 会与AsO3−4 形成竞争吸附,使As的流动性增强,但本研究采用单一Na3PO4·12H2O处理强酸性废渣,并未对As产生明显的活化作用,其具体原因尚须进一步的深入研究。由图1~图3可知,对Zn的稳定化效果顺序依次为Na3PO4·12H2O>(NH4)2HPO4>Na2S·9H2O,对Cd和Cu的稳定化效果依次为Na3PO4·12H2O>Na2S·9H2O>(NH4)2HPO4,对As的稳定化效果依次为Na2S·9H2O>(0.16~0.32)% (NH4)2HPO4>Na3PO4·12H2O。其中,(NH4)2HPO4易对As产生活化作用,从药剂投加量角度来看,使4种金属元素同时达标的综合稳定效应依次为0.46% Na3PO4·12H2O(摩尔比为2,η=82.26%)>0.64% (NH4)2HPO4(摩尔比为8,η=87.42%)>2.42% Na2S·9H2O (摩尔比为16,η=96.36%),因此,Na3PO4·12H2O对Zn和Cd稳定效果均最为突出,对As活化效果不明显,且低剂量添加不易导致溶解P过量,因此,在3种药剂中,Na3PO4·12H2O最适合单独处理强酸性废渣。
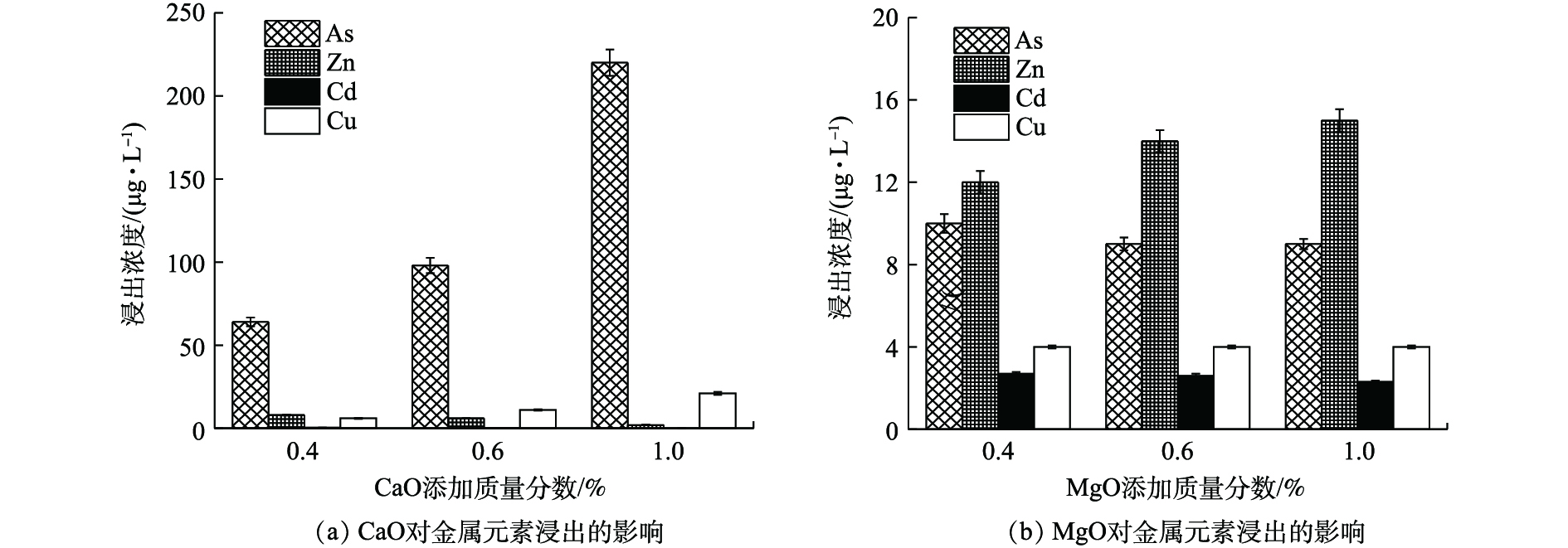
如图4(a)所示,(0.4~1)% CaO处理后,废渣中Zn、Cd、Cu、As的浸出浓度分别降至<10、<0.50、<25、<220 μg·L−1,远低于GB 8978-1996最高限值。其中,Zn、Cd、Cu稳定率均高于96%,这与CaO提高废渣pH、重金属氢氧化物沉淀增多、黏土物质等对重金属吸附性增强[19]、提供的钙离子与金属离子发生同晶替代[20]等作用有关。η(As)则为10.93%~74.09%,低量CaO更有利于As的稳定,CaO对As的稳定作用主要与强氧化性和适当pH条件下易形成CaHAsO4和Ca3(AsO4)2沉淀[21]有关。但本研究表明,当CaO≤1%,随CaO量的增加,As浸出浓度逐渐升高,这可能由于随着OH−浓度增高,负电荷对As的竞争吸附作用逐渐加强,最终导致As的迁移性有所增强[22]。
如图4(b)所示,MgO使废渣中Zn、Cd、Cu、As的浸出浓度分别降至≤15、<3、<5、≤10 μg·L−1,η均高于98%,其中η(As)均高于95%,明显优于CaO,这可能与MgO比表面积大,表面具有镁氧基(Mg―O)活泼反应基团,对重金属的吸附、沉淀等作用更强有关[23]。
由图4可知,CaO、MgO均可作为高效稳定剂,Ca2+、Mg2+虽均会与砷酸根离子形成复杂的络合沉淀物,但CaO的添加会使As浸出反升,4种元素综合稳定效应为0.4% MgO(η=98.90%)>0.4% CaO(η=93.48%)。
-
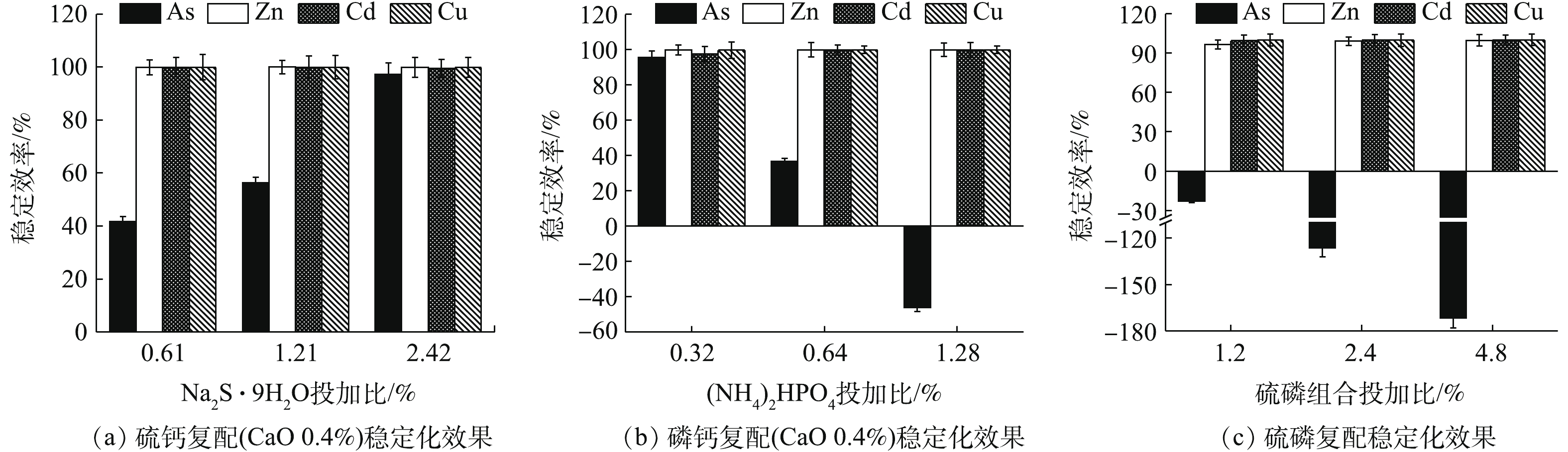
硫钙、磷钙和硫磷组合处理结果如图5所示。各类组合对废渣中的Zn、Cd和Cu的稳定化效果均较为明显,稳定率均高于95%,硫化物的添加有助于As的稳定,磷酸盐过量则对As的稳定化效果产生一定的拮抗作用。由图5(a)可知,0.4% CaO与3种不同投加比的Na2S·9H2O组合处理后,4种元素浸出浓度均达标,Zn、Cd和Cu的稳定率均高于99%,Zn浸出浓度均低于0.07 mg·L−1,Cd和Cu浸出浓度均低于0.01 mg·L−1,随Na2S·9H2O投加量的增加,η(As)明显增强。与单一Na2S·9H2O相比,各处理的硫钙组合均提高了Zn和Cd的稳定效果,其中,η(Zn)从45.53%~88.97%升至99.80%以上,说明CaO的加入有助于增强阳离子金属的稳定效果,协同增效作用明显,但同时一定程度上降低了As的稳定效果,η(As)从96.36%~99.11%降至41.70%~97.17%。在Na2S·9H2O组合比为0.61%和1.21%时,As的稳定化效果反而不如2种药剂单独使用时的效果,不仅未达到协同稳定As的效应,反而弱化了As的稳定效果,这与王浩等[3]的研究结果类似。但CaO与最高投加比(2.42%)Na2S·9H2O组合时,稳定As的能力依次为Na2S·9H2O>硫钙组合>CaO,组合处理对4种元素的综合稳定率η高达99.08%,这也说明硫钙组合稳定As的效果优于CaO,究其原因主要为2个方面:一方面,低量CaO利于降低As的浸出;另一方面,存在碱性条件使As流动性增强的风险[24-25],而硫钙组合对As的稳定化水平则随Na2S·9H2O投加量的增加而增强,在控制低剂量CaO防止因过量而活化As的同时,可使As2S3等更稳定的沉淀作用逐渐占优,As的稳定性增强。综上所述,硫钙组合有利于增强单一Na2S·9H2O对Zn的稳定效果,高剂量时对As的稳定效果优于单一CaO,且对4种元素的综合稳定效果均强于单一处理时的效果,组合处理中先加CaO也大大减少了H2S的逸出,有助于环境友好性,因此,与CaO和Na2S·9H2O单一处理相比,硫钙组合更占优势。
由图5(b)可知,低剂量的0.4% CaO+0.32% (NH4)2HPO4使4种金属元素的稳定率均高于95%,η高达98.13%,优于单一CaO(η 93.48%)和单一(NH4)2HPO4(η 87.42%),与单一(NH4)2HPO4相比,组合处理进一步降低了Zn和As的浸出浓度,但随着组合处理中(NH4)2HPO4投加量的增加,η(As)明显降低,甚至为负值,0.4% CaO+1.28% (NH4)2HPO4处理组的η(As)降为−46.15%。磷钙组合主要弱化了单一(NH4)2HPO4对As的活化作用,CaO与低量(NH4)2HPO4组合更有助于4种金属元素的综合稳定。
考虑到Na2S·9H2O和磷酸盐分别对As和Zn稳定能力强的特点,硫磷复配后的稳定化效果如图5(c)所示。在投加1.2%~4.8%复配剂后,Zn、Cd和Cu的稳定率均高于96%,但As均被活化,η(As)为−171.66%~−23.08%,其中,控制药剂投加量为1.2%,4种金属元素均达标,η仅为68.07%,2.4%和4.8%处理后As浸出超标。硫磷组合剂对As活化性较强,须控制投加量≤1.2%。
2.1. 单一稳定剂
2.2. 无机-无机组合处理
-
1)单一硫化物和磷酸盐对重金属的稳定化结果表明,3种药剂中Na3PO4·12H2O对重金属的稳定效果最好,Na2S·9H2O对As的稳定效果最好,(NH4)2HPO4易对As产生活化作用,从药剂投加量角度考虑,与GB 8978-1996最高允许排放浓度相比,达标稳定4种金属元素综合稳定化效应次序依次为0.46% Na3PO4·12H2O(η 82.26%)>0.64% (NH4)2HPO4(η 87.42%)>2.42% Na2S·9H2O(η 96.36%),Na3PO4·12H2O最优。
2)单一钙基、镁基和无机配伍对重金属的稳定化结果表明,单一MgO(投加比0.4%时,η(Zn) 99.97%,η(Cd) 99.76%)或CaO处理即可使之达标,硫钙组合提高了单一Na2S·9H2O对Zn、Cd的稳定化水平,η(Zn)从45.53%~88.97%升至99.80%以上,0.4% CaO与2.42% Na2S·9H2O的组合对As稳定效果优于单一CaO,对4种金属元素η高达99.08%,优于单一处理;磷钙和磷硫配伍中磷酸盐过量易活化As。各处理满足4种金属元素均达标的综合稳定效应大小顺序依次为0.4% MgO>0.4% CaO>(0.4% CaO+0.61% Na2S·9H2O)>(0.4% CaO+0.32% (NH4)2HPO4)>1.2% (Na2S·9H2O∶(NH4)2HPO4∶Na3PO4·12H2O=2∶1∶3)。
3) MgO、Na3PO4·12H2O、硫钙组合为优选稳定剂,CaO过量和组合剂中磷酸盐过量均不利于As的同时稳定。



 DownLoad:
DownLoad: