-
目前,水中溴酸盐(
${\rm{BrO}}_3^ - $ )和高氯酸盐(${\rm{ClO}}_4^- $ )的危害及毒理学效应被普遍关注[1]。${\rm{BrO}}_3^ - $ 是一种强氧化剂,在天然水体中通常由溴离子(Br−)氧化后形成,而在饮用水消毒过程中是被臭氧氧化形成的一类副产物,在高剂量时,其具有一定的DNA和染色体水平的遗传毒性。因此,${\rm{BrO}}_3^ - $ 已被国际癌症研究机构认定为2B级潜在致癌物,长期饮用含溴酸盐的水可导致致癌率明显升高[1]。我国饮水标准规定,${\rm{BrO}}_3^ - $ 须低于10 μg·L−1,然而近年来有不少有关饮水中${\rm{BrO}}_3^ - $ 含量超标的报道。${\rm{ClO}}_4^ - $ 是一种新兴持久性有毒污染物,广泛应用于制革、火箭固体燃料、烟火、电镀等行业,可通过水的流动或渗滤迁移到地下水、地表水,甚至饮用水中,能引起成人代谢、婴儿发育不良,甚至诱发甲状腺癌等问题[1-2]。美国环保署已将高氯酸盐列入第一批环境污染物候选名单,并建议饮水中的浓度低于20 μg·L−1。由此可见,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 已成为影响人们饮水安全的一大隐患,急须开展${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的治理研究与实践。有研究[3-4]表明,在厌氧和酶催化的环境下,厌氧微生物可以利用有机或无机电子供体(如甲醇、乙醇、单质硫、氢气等),将
${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ 作为电子受体还原成单质离子(Br−、Cl−)。氢基质生物膜反应器(MBfR)是一种将氢自养生物还原技术与中空纤维膜微孔曝气相结合的反应器,已被广泛应用于氧化性物质的去除[5-10]。CHEN等[11]和DEMIREL等[12]利用MBfR去除水中单独${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ ,取得了良好的处理效果,同时他们也发现氢气压力和进水流速是影响去除效能的关键因素[13-17]。夏四清等[9]利用MBfR对多种氧化性污染物的同步去除开展了研究,并对如何实现最优去除效能进行了探索。另外,水中共存硝酸盐(${\rm{NO}}_3^{-} $ -N)产生的影响也引起了广泛的关注,启动驯化时进水中无机氮源采用${\rm{NO}}_3^{-} $ -N,生物膜内存在反硝化菌;${\rm{NO}}_3^{-} $ -N是水中常规的氧化性物质(电子受体),其在水环境中的浓度远高于${\rm{BrO}}_3^ - $ 或${\rm{ClO}}_4^ - $ ;反硝化菌或反硝化过程普遍存在于水环境中,其对${\rm{BrO}}_3^ - $ 或${\rm{ClO}}_4^ - $ 还原降解有一定的影响。因此,研究${\rm{NO}}_3^{-} $ -N共存条件下氢气压力与进水流速对${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 同步去除效能的影响具有重要意义。本研究深入考察了氢气压力与进水流速对
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 去除率、去除通量、当量电子转移通量以及还原动力学的影响,以确定MBfR同步去除${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的适宜氢气压力与进水流速,为MBfR稳定、高效运行提供参考和技术支持。 -
本研究采用的MBfR主要由有机玻璃筒体、膜组件、进出水装置、回流装置、供氢系统等5部分组成(图1)。氢气在膜组件中以微泡或无泡的形式从膜内扩散至膜外,为附着在膜表面的微生物(生物膜)提供氢气(电子供体)。反应器从底部进水,从顶端出水;回流泵不断将顶部的水回流到底部,从而使整个反应器内部水体混合均匀。反应器的主要特征参数如下:高度65 cm,内径6 cm,有效容积1.8 L;中空纤维膜65根,膜长度100 cm,膜外径1.5 mm,膜内径1 mm,膜孔径0.02 μm,膜表面积0.28 m2。
-
进水采用人工模拟配水,以80 mg·L−1的NaHCO3为无机碳源,30 mg·L−1的NaNO3作为氮源(
${\rm{NO}}_3^{-} $ -N为5 mg·L−1)。有研究[7]表明,低浓度${\rm{NO}}_3^{-} $ -N能促进微生物对${\rm{BrO}}_3^ - $ 或${\rm{ClO}}_4^ - $ 的降解,因此,以进水所含5 mg·L−1${\rm{NO}}_3^{-} $ -N作为背景浓度(不再添加${\rm{NO}}_3^{-} $ -N)。利用216 mg·L−1的Na2HPO4·12H2O和236 mg·L−1的KH2PO4作为缓冲溶液调节进水pH,以缓解还原反应过程中pH剧烈升高。此外,配水中含有的营养元素可以满足微生物的代谢生长,具体组分[18-19]如下:ZnSO4·7H2O 0.013 mg·L−1、H3BO3 0.038 mg·L−1、CuCl2·2H2O 0.001 mg·L−1、Na2MoO4·2H2O 0.004 mg·L−1、MnCl2·4H2O 0.004 mg·L−1、CoCl2·6H2O 0.025 mg·L−1、NiCl2.6·H2O 0.001 mg·L−1。向进水中添加目标污染物(${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ )以模拟实际污染水体,并储存在体积为25 L的塑料水桶中,在水桶外套上一层黑色塑料袋进行遮光处理,防止桶内产生藻类。实验开始前,向模拟水体中通入足量氮气来排除水中的溶解氧。MBfR启动驯化阶段与
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 降解系列实验的工况如表1所示。分别从实验室摇瓶装置中提取能够还原${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的混合细菌作为接种微生物,开始挂膜。挂膜期间,流速从0.5 mL·min−1提升至2.0 mL·min−1,内回流速度从0 mL·min−1提升至20 mL·min−1,进水${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 浓度由0.1 mg·L−1提升至1.0 mg·L−1和1.1 mg·L−1。通过观察膜外表面生物固体附着状态以及出水${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 浓度,确定挂膜过程进展。对于每个影响因素系列实验,每次改变条件,首先连续运行反应器2 d后再取样,因为2 d(多于1.0 mL·min−1时的HRT=30 h)足以使系统达到拟稳定状态,该状态定义为溶液混合均匀、浓度达到恒定,但生物膜累积和生物量并没有明显的变化。每个不同系列的工况均每隔2 d取1次样,共取3次做平均处理。 -
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 进水表面负荷分别代表单位时间单位膜面积上承载的进水${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 质量。${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 去除通量分别指单位时间单位膜面积上去除的${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 质量,与进水浓度和出水浓度有关,用于衡量反应体系处理目标污染物的能力。${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 标准化通量则用于比较${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 降解速率的快慢程度。当量电子转移通量用于定量化地评价各污染物对电子供体的竞争关系。参数的计算方法[20-21]分别见式(1)~式(4)。式中:F为
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 进水表面负荷,g·(m2·d)−1;J为${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 去除通量,g·(m2·d)−1;N为${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 标准化通量,m·d−1;Si、Se分别为目标污染物进、出水浓度,g·m−3;Q为进水流量,m3·d−1;A为膜表面积,m2;Eeq为电子受体还原当量电子(e−)转移通量,个·(m2·d)−1;FEWs为还原过程中的当量电子供体换算因子。根据${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ 和${\rm{NO}}_3^{-} $ -N进行完全还原生成Br−、Cl−和N2的化学计量关系,${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ 、N的电子受体的FEWs分别为21.3、12.4和2.8 g·个−1。如1 mol${\rm{BrO}}_3^ - $ (分子质量127.9 g)需要6 mol H+(e−)才能完全还原成Br−(${\rm{BrO}}_3^ - $ +3H2→Br−+3H2O),因此,${\rm{BrO}}_3^ - $ 的当量电子供体换算因子FEWs=127.9/6=21.3 g·个−1。 -
本实验每天从反应器中收集水样,并采用0.22 μm滤膜过滤,然后存入4 ℃冰箱保存,待分析。水样中的
${\rm{BrO}}_3^ - $ 、Br−、${\rm{ClO}}_4^ - $ 、Cl−、${\rm{NO}}_3^{-} $ 等离子浓度均采用离子色谱法测定。所用仪器为离子色谱仪(ICS-1000,美国戴安公司),色谱柱型号为IonPacAS19,保护柱型号为IonPacAG19,淋洗液为60 mmol·L−1 KOH溶液。 -
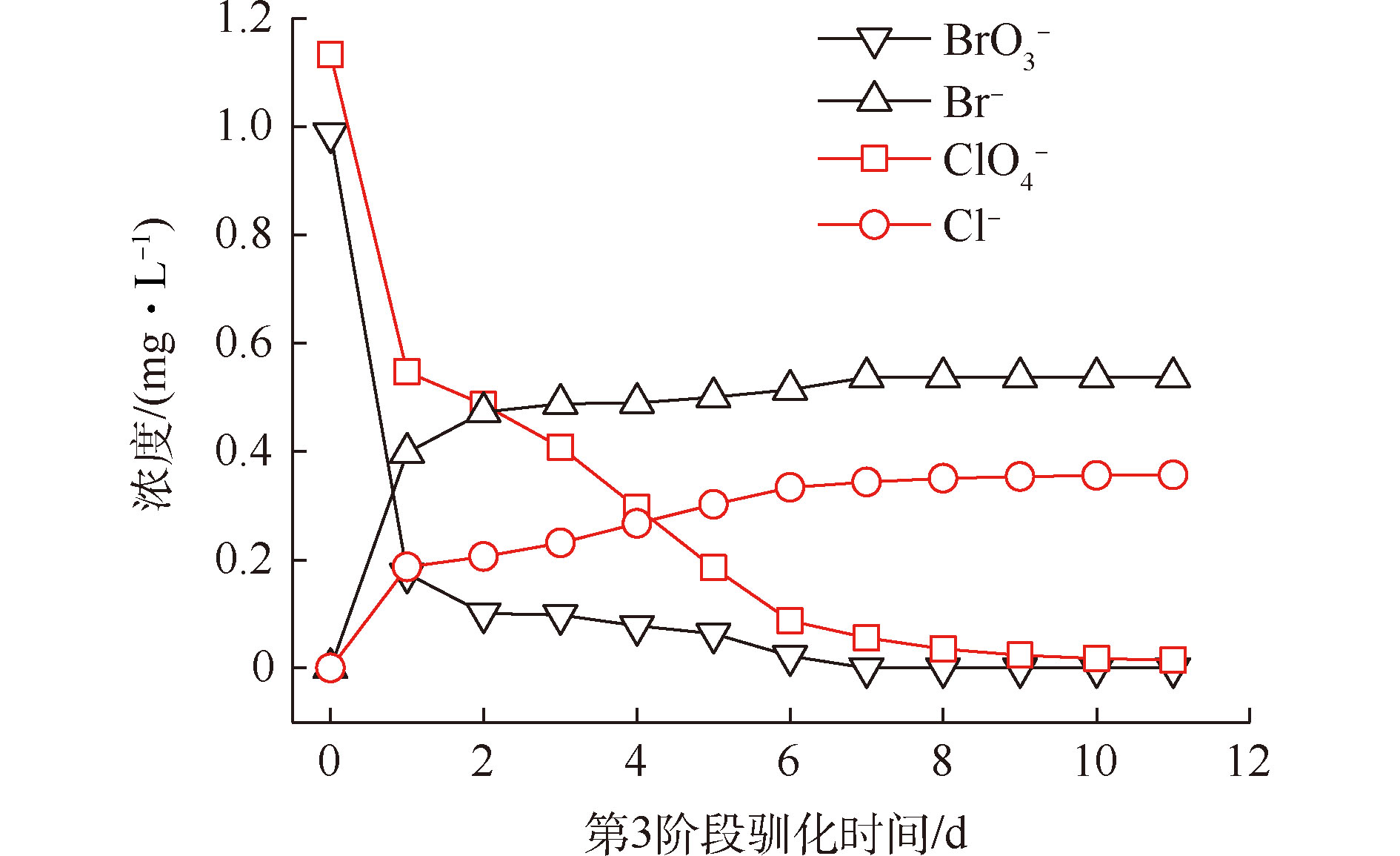
MBfR启动驯化的目的是使中空纤维膜外表面富集能够还原降解
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的微生物(生物膜)。在第1阶段和第2阶段,持续运行39 d后,中空纤维膜外表面附着生长出黄褐色生物絮体,生物膜初始形成。随后进入驯化第3阶段(进水流速2.0 mL·min−1,内回流速度20 mL·min−1,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 浓度分别为1.0 mg·L−1和1.1 mg·L−1),反应器出水${\rm{BrO}}_3^ - $ 、Br−、${\rm{ClO}}_4^ - $ 和Cl−的浓度变化如图2所示。可以看出,随着反应器的连续运行,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的出水浓度均持续降低至0.015 mg·L−1以下(去除率接近100%);同时,出水中Br−和Cl−的浓度持续增加,且生成的Br−和Cl−浓度与水中${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 降低的浓度之间的比值接近于完全反应化学计量关系。因此,可以认为该阶段的最终产物为Br−和Cl−,没有中间产物的产生与累积。 -
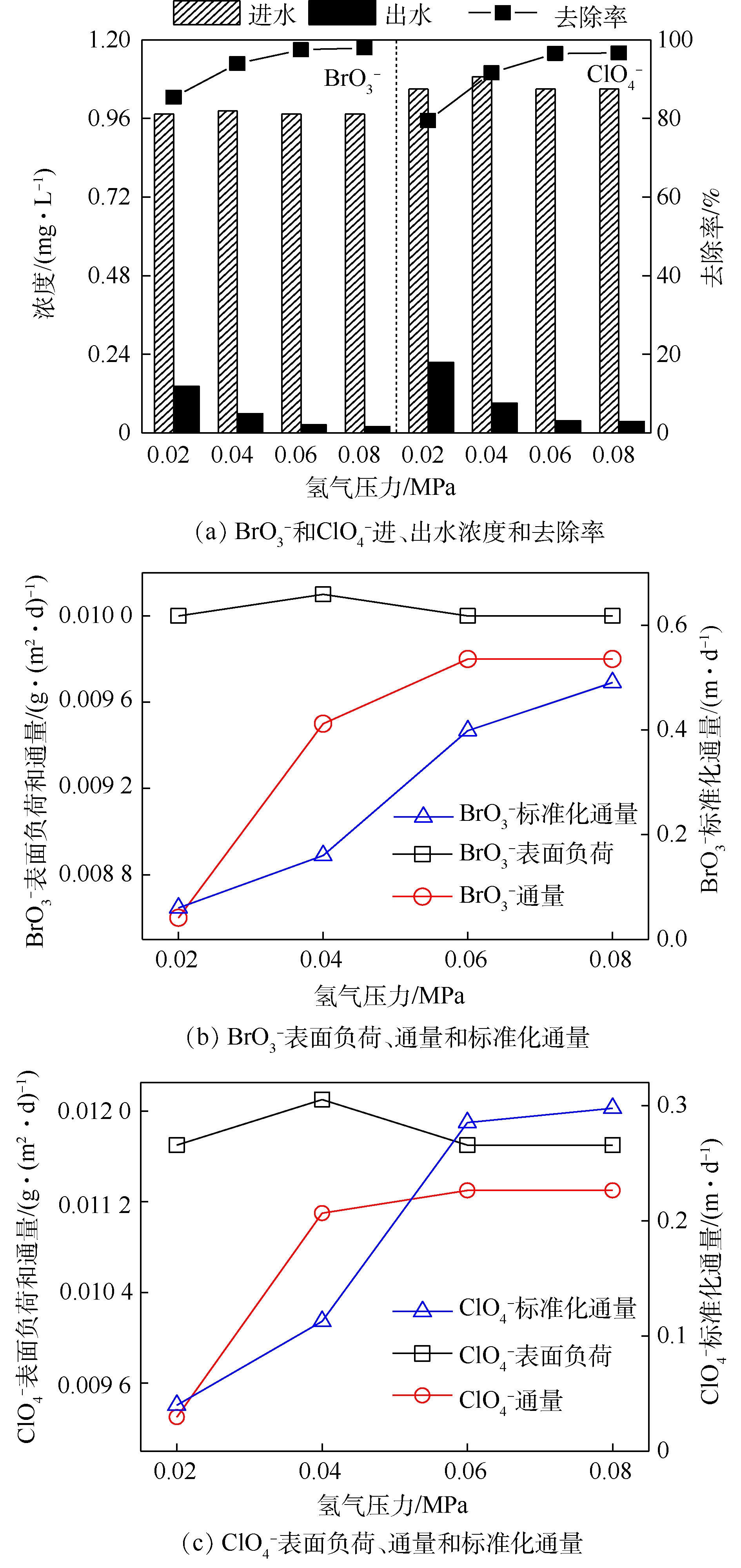
图3显示了氢气压力为0.02、0.04、0.06和0.08 MPa时MBfR对
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除效果。可以看出,BrO3−和${\rm{ClO}}_4^ - $ 的去除率均随氢气压力升高而逐渐增大,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除率分别从85.4%和79.5%升高至97.9%和96.7%;同时,在出水中检测到${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的浓度分别由0.14 mg·L−1和0.23 mg·L−1降至0.02 mg·L−1和0.04 mg·L−1。这说明增大氢气压力提高了系统中氢气的可利用水平,促进了${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的还原降解。由图3(b)和图3(c)可知,随着氢气压力的增大,${\rm{BrO}}_3^ - $ 的去除通量由0.008 6 g·(m2·d)−1升高至0.009 8 g·(m2·d)−1(增大0.1倍),而${\rm{BrO}}_3^ - $ 的标准化通量则出现大幅升高,由0.060 3 m·d−1升高至0.490 4 m·d−1(增大7倍);同时${\rm{ClO}}_4^ - $ 的去除通量由0.009 3 g·(m2·d)−1升高至0.011 3 g·(m2·d)−1(增大0.2倍),标准化通量由0.039 9 m·d−1升高至0.297 7 m·d−1(增大6倍)。由此可见,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除通量和标准化通量虽皆出现了不同程度的上升,但两者标准化通量上升的幅度显著高于去除通量上升的幅度。以上结果表明,增大氢气压力导致${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 出水浓度降低的幅度高于去除通量增加的幅度,这也进一步揭示了两者去除率未随氢气压力升高(大于0.06 MPa)呈线性升高的趋势。因此,从经济和安全角度考虑,最适宜氢气压力为0.04~0.06 MPa。 -
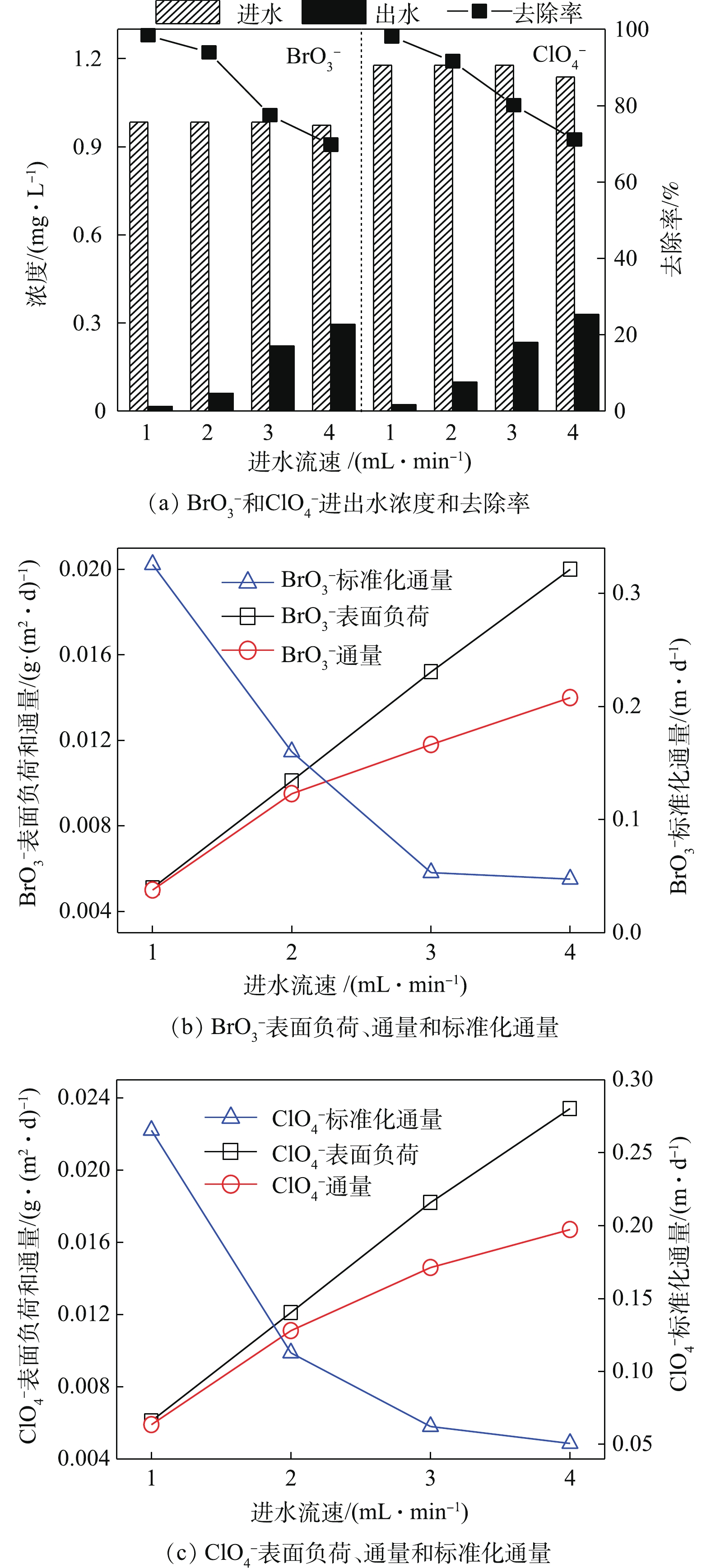
调节进水流速为1.0、2.0、3.0和4.0 mL·min−1,反应器去除
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的效能如图4所示。随进水流速逐渐加快,${\rm{BrO}}_3^ - $ 去除率从98.4%降低至69.7%,${\rm{ClO}}_4^ - $ 去除率由98.1%降低至71.1%,出水${\rm{BrO}}_3^ - $ 的浓度由0.02 mg·L−1升高至0.29 mg·L−1,出水${\rm{ClO}}_4^ - $ 的浓度由0.02 mg·L−1升高至0.33 mg·L−1。结果表明,在上述实验范围内,升高进水流速会明显降低了${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的还原降解效率。由图4(b)和图4(c)看出,随${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的进水表面负荷线性上升,${\rm{BrO}}_3^ - $ 的去除通量由0.005 g·(m2·d)−1升高至0.014 g·(m2·d)−1,但其标准化通量却由0.33 m·d−1降至0.05 m·d−1;同时,${\rm{ClO}}_4^ - $ 的去除通量由0.006 g·(m2·d)−1升高至0.017 g·(m2·d)−1,而其标准化通量由0.27 m·d−1降至0.05 m·d−1。结果表明,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的进水表面负荷增加,即增加单位时间反应器内电子受体总量,随之所需电子供体量也同步增加,但由于氢气压力保持恒定,电子供体量不足于电子受体还原,因此,导致${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除率明显降低。而当进水流速过低时,反应器水力停留时间延长,营养物质供应不足,会对微生物的生长产生不良影响,导致降低处理效率。因此,控制进水流速为2 mL·min−1较适宜于MBfR同步还原降解${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 。夏四清等[9]在利用MBfR处理复合污染水体时发现,当进水流速超过2.0 mL·min−1后,污染物的去除率上升趋势平缓,这与本研究的结果类似。 -
表2和表3列出了调控氢气压力和进水流速时当量电子转移通量及其分配情况。
${\rm{NO}}_3^{-} $ -N、${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的当量电子(e−)通量分别为9.2~36.7、0.23~0.66和0.48~1.34(10−3 个·(m2·d)−1),${\rm{NO}}_3^{-} $ -N的当量电子通量分配最大(92.8%~94.8%)。这说明反硝化过程消耗的电子供体数量最多;其次是${\rm{ClO}}_4^ - $ (3.5%~4.8%),这意味着${\rm{ClO}}_4^ - $ 还原是反应体系中第二大电子供体消耗者;而${\rm{BrO}}_3^ - $ 还原最低(1.7%~2.3%),远低于反硝化。上述结果说明,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 还原对电子供体的竞争性抢夺处于劣势,而反硝化对电子供体的争夺占主导地位。当氢气可利用水平受到限制时,反硝化对电子供体的大量争夺势必使得${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的还原受到较大影响。然而,在本研究条件下,氢气压力的升高对三者当量电子通量的影响幅度并不明显,说明氢气压力满足了生物膜中物质同步还原所需的电子供体,不存在氢气利用受限制的情形。从经济和安全的角度考虑,不宜持续提高氢气压力(达到0.04~0.06 MPa时最佳)。调节进水流速时,三者的当量电子通量均出现不同程度的增长。但是,
${\rm{NO}}_3^{-} $ -N的当量电子通量占比持续增加,而${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的却持续降低(尤其是进水流速达到4.0 mL·min−1时,降低幅度最大)。这同样也证明,${\rm{NO}}_3^{-} $ -N作为电子供体的最大消耗者,对${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 还原起到限制作用。为了提高${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 竞争电子供体的能力,获得更多的电子供体,必须提高${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 当量电子通量和所占比例,这需要调控适宜的进水负荷。当进水流速为2.0 mL·min−1时,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 当量电子通量和所占比例皆处于较高水平,此结果也与前面章节所得结论相吻合。 -
为进一步探讨进水流速和氢气压力影响下
${\rm{BrO}}_3^ - $ 及${\rm{ClO}}_4^ - $ 去除过程的相互竞争性及对各种物质去除性能的影响程度,本研究引入基于稳态生物膜的还原动力学。动力学级数(k')可由稳态生物膜内污染物的去除通量和出水污染物的质量浓度的对数图拟合后得出,计算公式[13, 22]见式(5)。式中:k'为还原动力学级数;J为污染物去除通量,g·(m2·d)−1;Se为反应器出水中污染物的质量浓度,g·m−3。
${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ 和${\rm{NO}}_3^{-} $ -N的还原动力学级数分别为−0.065、−0.099和−0.130 (见表4)。这表明,氢气压力对三者去除效能的影响总体不显著;仅当氢气压力由0.02 MPa升高到0.04 MPa时,三者通量呈现较大幅度上升;继续提高氢气压力,三者的去除通量皆趋于稳定。李海翔等[22]和CHUNG等[23]发现,对氯硝基苯(p-CNB)和砷酸盐(${\rm{AsO}}_4^ {3-} $ )还原对氢气压力变化的敏感性不强烈,基本不受反应过程中氢气浓度水平的影响。随着进水流速的不断提高,${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ 和${\rm{NO}}_3^{-} $ -N的还原动力学级数分别为0.323、0.385和0.755,接近于生物膜内存在的类似于半级反应动力学(厚生物膜中的一种特殊情况)。有研究[20-23]发现,在深层生物膜内,当反应级数小于1时,提高进水负荷并不能使污染物的去除通量呈现线性增加,从而导致反应器中${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除率降低。深层生物膜的扩散限制是影响生物膜内物质还原的重要因素之一。因此,为了进一步提高污染物的去除率,可以适当降低进水流速来解除反应器中的扩散限制。在进水流速影响下,
${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ 和${\rm{NO}}_3^{-} $ -N的还原动力学级数的影响远大于氢气压力对其的影响,这意味着进水流速对三者的去除通量及去除率的影响比氢气压力对其的影响更大。此外,无论调节氢气压力或进水流速,${\rm{NO}}_3^{-} $ -N的还原动力学级数均为最大,其次是${\rm{ClO}}_4^ - $ 和${\rm{BrO}}_3^ - $ 。由此可知,在反应过程中,反硝化对氢气利用的敏感性最强,势必对${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的还原造成潜在的影响。 -
1)氢气压力升高可导致
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除率逐渐升高,但并未随氢气压力增强呈线性升高趋势,而${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除通量也呈先升高后趋于稳定的趋势。反应器去除${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的最适氢气压力为0.04~0.06 MPa。2)随着进水流速的升高,
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除通量也相应增加,但${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 还原所接收的电子相应减少,导致其去除率降低。控制进水流速为2 mL·min−1,更适宜于${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^- $ 的还原降解。3)
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 还原对电子供体的争夺处于劣势,反硝化过程对电子供体产生强烈竞争。相比进水流速而言,调整氢气压力对${\rm{BrO}}_3^ - $ 、${\rm{ClO}}_4^ - $ 和NO3−-N还原当量电子通量及其分配的影响均较小。4)氢气压力升高对
${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 去除效能的影响程度不显著,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ -$ 还原对进水流速的敏感性高于氢气压力。调节氢气压力和进水流速对反硝化的影响程度比对${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 还原的影响更强,因而对${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 去除形成潜在的抑制。
氢气压力和进水流速对氢基质生物膜反应器同步去除溴酸盐和高氯酸盐的影响
Effects of hydrogen pressure and influent flow rate on simultaneous removal of bromate and perchlorate in a hydrogen-based membrane biofilm reactor
-
摘要: 为了考察氢基质生物膜反应器(MBfR)中氢气压力和进水流速对溴酸盐(
${\rm{BrO}}_3^ - $ )和高氯酸盐(${\rm{ClO}}_4^ - $ )同步去除的影响,基于短期系列实验,研究了不同氢气压力和进水流速下${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除效率、去除通量、当量电子转移通量及还原反应动力学。结果表明:氢气压力从0.02 MPa提高至0.08 MPa时,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除率分别升高了12.5%和17.2%,去除通量分别升高了0.001 2 g·(m2·d)−1和0.002 g·(m2·d)−1,但${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 去除率并未随氢气压力持续升高而呈线性升高趋势;当进水流速从1.0 mL·min−1提高至4.0 mL·min−1时,${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除通量由0.005 g·(m2·d)−1和0.006 g·(m2·d)−1分别升高至0.014 g·(m2·d)−1和0.017 g·(m2·d)−1,但${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除率分别从98.4%和98.1%降低至69.7%和71.1%,这说明加快进水流速可导致${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 的去除率明显降低;结合还原反应动力学研究,MBfR运行效能最佳的氢气压力和进水流速分别为0.04~0.06 MPa和2.0 mL·min−1。生物膜当量电子转移通量分析表明,反硝化对电子供体(氢气)的竞争性抢夺比${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 还原更加激烈;还原反应动力学级数揭示了${\rm{BrO}}_3^ - $ 和${\rm{ClO}}_4^ - $ 还原对进水流速加快的敏感性比氢气压力变化更加强烈。为了获得更高的污染物去除效能,可以适当控制进水流速和水中共存${\rm{NO}}_3^{-} $ -N的竞争性抑制。Abstract: In order to investigate the effects of hydrogen pressure and influent flow rate on simultaneous removal of bromate (${\rm{BrO}}_3^ - $ ) and perchlorate (${\rm{ClO}}_4^ - $ ) in a hydrogen-based membrane biofilm reactor (MBfR), the variations of removal efficiency, removal flux, equivalent electron transfer flux and reduction kinetics of${\rm{BrO}}_3^ - $ and${\rm{ClO}}_4^ - $ were studied based on the sequential batch short-term experiments. The results show that, when the hydrogen pressure increased from 0.02 MPa to 0.08 MPa, the removal rates of${\rm{BrO}}_3^ - $ and${\rm{ClO}}_4^ - $ increased by 12.5% and 17.2%, and the removal fluxes increase by 0.0012 g·(m2·d)−1 and 0.002 g·(m2·d)−1, respectively. However, the removal efficiencies of${\rm{BrO}}_3^ - $ and${\rm{ClO}}_4^ - $ did not increase linearly with the continuous increase of hydrogen pressure. When the influent flow rate increased from 1.0 mL·min−1 to 4.0 mL·min−1, the removal fluxes of${\rm{BrO}}_3^ - $ and${\rm{ClO}}_4^ - $ increased from 0.005 g·(m2·d)−1 and 0.006 g·(m2·d)−1 to 0.014 g·(m2·d)−1 and 0.017 g·(m2·d)−1, but the removal efficiencies of${\rm{BrO}}_3^ - $ and${\rm{ClO}}_4^ - $ decreased from 98.4% and 98.1% to 69.7% and 71.1%, respectively, and these indicated that accelerating the flow rate of the reactor significantly decreased${\rm{BrO}}_3^ - $ and${\rm{ClO}}_4^ - $ removal. Based on the study of reduction kinetics, the optimal hydrogen pressure and influent flow rate of MBfR were 0.04~0.06 MPa and 2.0 mL·min−1, respectively. Analysis of the biofilm equivalent electron transfer flux showed that denitrification could more strongly capture electron donor (H2) than${\rm{BrO}}_3^ - $ or${\rm{ClO}}_4^ - $ reduction. The results of analysis of reduction orders revealed that the sensitivity of${\rm{BrO}}_3^ - $ and${\rm{ClO}}_4^ - $ reduction to influent flow rate was stronger than that to hydrogen pressure. In order to achieve a higher removal of pollutants, the influent flow rate and competitive inhibition of nitrate in water needs to be properly controlled.-
Key words:
- hydrogen-based membrane biofilm reactor /
- bromate /
- perchlorate /
- hydrogen pressure /
- influent flow rate
-

-
表 1 MBfR启动驯化与
${\bf{BrO}}_3^ - $ 和${\bf{ClO}}_4^ - $ 降解系列实验工况Table 1. Conditions of acclimation stage and series test of
${\bf{BrO}}_3^ - $ and${\bf{ClO}}_4^ - $ degradation in the MBfR实验阶段 H2压力/
MPa进水流速/
(mL·min−1)进水 ${\rm{NO}}_3^{-} $ -N/
(mg·L−1)内回流速度/
(mL·min−1)进水 ${\rm{BrO}}_3^ - $ /
(mg·L−1)进水 ${\rm{ClO}}_4^ - $ /
(mg·L−1)启动驯化 0.04 0.5 5 0 0.1 0.1 启动驯化 0.04 1.0 5 8 0.1 0.1 启动驯化 0.04 2.0 5 20 1.0 1.1 降解实验 0.02 2.0 5 20 10 1.1 降解实验 0.04 2.0 5 20 10 1.1 降解实验 0.06 2.0 5 20 10 1.1 降解实验 0.08 2.0 5 20 10 1.1 降解实验 0.04 1.0 5 20 10 1.1 降解实验 0.04 2.0 5 20 10 1.1 降解实验 0.04 3.0 5 20 10 1.1 降解实验 0.04 4.0 5 20 10 1.1 表 2 不同氢气压力下电子受体的当量电子通量及其分配
Table 2. Electron-equivalent fluxes of electron acceptors and their distributions at different hydrogen pressure
H2压力/MPa 当量电子通量/(10−3 个·(m2·d)−1) 通量分配/% ${\rm{BrO}}_3^ - $ 
${\rm{ClO}}_4^ - $ 
${\rm{NO}}_3^{-} $ -N
总和 ${\rm{BrO}}_3^ - $ 
${\rm{ClO}}_4^ - $ 
${\rm{NO}}_3^{-} $ -N
0.02 0.40 0.75 18.4 19.55 2.0 3.8 94.1 0.04 0.45 0.90 18.4 19.75 2.3 4.6 93.2 0.06 0.46 0.91 18.4 19.77 2.3 4.6 93.1 0.08 0.46 0.91 18.4 19.77 2.3 4.6 93.1 表 3 不同进水流速下电子受体的当量电子通量及其分配
Table 3. Electron-equivalent fluxes of electron acceptors and their distributions at different influent flow rate
进水流速/(mL·min−1) 当量电子通量/(10−3 个·(m2·d)−1) 通量分配/% ${\rm{BrO}}_3^ - $ 
${\rm{ClO}}_4^ - $ 
${\rm{NO}}_3^{-} $ -N
总和 ${\rm{BrO}}_3^ - $ 
${\rm{ClO}}_4^ - $ 
${\rm{NO}}_3^{-} $ -N
1 0.23 0.48 9.2 9.91 2.3 4.8 92.8 2 0.45 0.90 18.4 19.75 2.3 4.6 93.2 3 0.55 1.17 27.6 29.32 1.9 4.0 94.1 4 0.66 1.34 36.7 38.70 1.7 3.5 94.8 表 4 生物膜内
${\bf{BrO}}_3^ - $ 、${\bf{ClO}}_4^ - $ 和${\bf{NO}}_3^{-} $ -N的还原动力学级数Table 4. Reaction orders of
${\bf{BrO}}_3^ - $ ,${\bf{ClO}}_4^ - $ and${\bf{NO}}_3^{-} $ -N in biofilm影响因素 ${\rm{BrO}}_3^ -$ 
${\rm{ClO}}_4^ - $ 
${\rm{NO}}_3^{-} $ -N
氢气压力 −0.065 −0.099 −0.130 进水流速 0.323 0.385 0.755 -
[1] CHENG H. Improving China’s water resources management for better adaptation to climate change[J]. Climatic Change, 2012, 112(2): 253-282. doi: 10.1007/s10584-011-0042-8 [2] HU Y, CHENG H. Water pollution during China’ s industrial transition[J]. Environmental Development, 2013, 8(1): 57-73. [3] HIJNEN W, VOOGT R, VEENENDAAL H R, et al. Bromate reduction by denitrifying bacteria[J]. Applied & Environmental Microbiology, 1995, 61(1): 239-244. [4] MATOS C T, SVETLOZAR V, CRESPO J O G, et al. Simultaneous removal of perchlorate and nitrate from drinking water using the ion exchange membrane bioreactor concept[J]. Water Research, 2006, 40(2): 231-240. doi: 10.1016/j.watres.2005.10.022 [5] NERENBERG R, RITTMANN B E. Hydrogen-based, hollow-fiber membrane biofilm reactor for reduction of perchlorate and other oxidized contaminants[J]. Water Science & Technology, 2004, 49(11/12): 223-230. [6] XIA S, ZHANG Z, ZHONG F, et al. High efficiency removal of 2-chlorophenol from drinking water by a hydrogen-based polyvinyl chloride membrane biofilm reactor[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1367-1373. [7] LUO J H, WU M, YUAN Z, et al. Biological bromate reduction driven by methane in a membrane biofilm reactor[J]. Environmental Science & Technology Letters, 2017, 4(12): 562-566. [8] 蒋敏敏, 张学洪, 李海翔. 氢基质生物膜反应器在饮用水处理中的研究进展[J]. 净水技术, 2015, 34(s1): 23-26. [9] 夏四清, 梁郡, 李海翔, 等. 利用氢基质生物膜反应器同步去除多种污染物[J]. 同济大学学报(自然科学版), 2012, 40(6): 876-881. doi: 10.3969/j.issn.0253-374x.2012.06.013 [10] ZHANG Y, ZHONG F, XIA S, et al. Autohydrogenotrophic denitrification of drinking water using a polyvinyl chloride hollow fiber membrane biofilm reactor[J]. Journal of Hazardous Materials, 2009, 170(1): 203-209. doi: 10.1016/j.jhazmat.2009.04.114 [11] CHEN X, LIU Y, PENG L, et al. Perchlorate, nitrate, and sulfate reduction in hydrogen-based membrane biofilm reactor: Model-based evaluation[J]. Chemical Engineering Journal, 2017, 316: 82-90. doi: 10.1016/j.cej.2017.01.084 [12] DEMIREL S, BAYHAN I. Nitrate and bromate removal by autotrophic and heterotrophic denitrification processes: Batch experiments[J]. Journal of Environmental Health Science & Engineering, 2013, 11(1): 27. [13] RITTMANN B E, MCCARTY P L. Environmental Biotechnology: Principles and Applications[M]. New York: McGraw-Hill, 2001. [14] 高廷耀, 顾国维, 周琪. 水污染控制工程: 下[M]. 4版. 北京: 高等教育出版社, 2015. [15] ZHAO H P, ONTIVEROS-VALENCIA A, TANG Y, et al. Using a two-stage hydrogen-based membrane biofilm reactor (MBfR) to achieve complete perchlorate reduction in the presence of nitrate and sulfate[J]. Environmental Science & Technology, 2013, 47(3): 1565-1572. [16] LI H, LIN H, XU X, et al. Simultaneous bioreduction of multiple oxidized contaminants using a membrane biofilm reactor[J]. Water Environment Research, 2017, 89(2): 178-185. doi: 10.2175/106143016X14609975746686 [17] DOWNING L S, NERENBERG R. Kinetics of microbial bromate reduction in a hydrogen-oxidizing, denitrifying biofilm reactor[J]. Biotechnology & Bioengineering, 2010, 98(3): 543-550. [18] CHUNG J, RITTMANN B E, WRIGHT W F, et al. Simultaneous bio-reduction of nitrate, perchlorate, selenate, chromate, arsenate, and dibromochloropropane using a hydrogen-based membrane biofilm reactor[J]. Biodegradation, 2007, 18(2): 199-209. doi: 10.1007/s10532-006-9055-9 [19] XIA S, LI H, ZHANG Z, et al. Bioreduction of para-chloronitrobenzene in drinking water using a continuous stirred hydrogen-based hollow fiber membrane biofilm reactor[J]. Journal of Hazardous Materials, 2011, 192(2): 593-598. doi: 10.1016/j.jhazmat.2011.05.060 [20] CHUNG J, NERENBERG R, RITTMANN B E. Bioreduction of selenate using a hydrogen-based membrane biofilm reactor[J]. Environmental Science & Technology, 2006, 40(5): 1664-1671. [21] CHUNG J, NERENBERG R, RITTMANN B E. Bio-reduction of soluble chromate using a hydrogen-based membrane biofilm reactor[J]. Water Research, 2006, 40(8): 1634-1642. doi: 10.1016/j.watres.2006.01.049 [22] 李海翔, 林华, 游少鸿, 等. 氢基质生物膜反应器去除对氯硝基苯的影响因素分析[J]. 环境科学研究, 2015, 28(2): 304-309. [23] CHUNG J, LI X, RITTMANN B E. Bio-reduction of arsenate using a hydrogen-based membrane biofilm reactor[J]. Chemosphere, 2006, 65(1): 24-34. doi: 10.1016/j.chemosphere.2006.03.018 -









































 下载:
下载: