-
近年来,随着我国畜禽养殖业的快速发展,养殖废水的不合理排放带来了严重的面源污染[1]。与此同时,养殖废水中磷污染问题也受到了极大关注。一方面,磷是养殖废水中的主要污染物,当排放浓度达到0.02 mg·L−1时,便可引起水体富营养化[2];另一方面,磷矿是一种不可再生资源,对农业生产和磷化学工业具有重要意义[3]。因此,采用高效经济的畜禽养殖废水处理技术使其中的磷元素得以去除的同时实现资源化利用具有重要的现实意义。
目前,常用的除磷技术包括化学沉淀法、生物除磷法、离子交换法以及吸附法[4]。吸附法由于操作简单、成本低廉,尤其是在低磷酸盐浓度条件下吸附效率高,已越来越受到重视[5]。传统的磷吸附剂,如活性炭[6]、海泡石[7]和沸石[8]等,由于处理成本较高,容易产生二次污染或除磷能力有限等问题,在工程化应用中受到限制[9]。在过去的10年中,以果皮、木屑和作物秸秆等天然材料及废弃物材料为基础的生物质吸附剂受到了极大的关注[10]。
生物质材料由于易获取且无二次污染而被认为是一种经济有效且环境友好的吸附剂[11]。其中,海藻能够提供一系列的官能团,包括氨基、羟基、羧基、硫酸盐和咪唑等[12],且作为富营养化的产物,来源广泛,成本低廉。因此,它们可能是一类潜在的生物质吸附材料。但纯海藻类生物质存在导电性差、表面积小、孔隙率低、pH敏感性低等缺点[13],降低了其应用价值。因此,改性是提高其吸附性的必要措施。
镧是一种相对便宜和环境负效应较低的稀土元素[14],能与磷形成稳定沉淀(溶解性产物磷酸镧,pK=26.16)[15]。因此,将镧加载在载体上吸附废水中的磷是一种经济有效的方法。目前,已有研究者采用镧改性膨润土[16]、沸石[17]以及粉煤灰[18]等以增加材料的吸附性能,但采用镧改性海藻等生物质材料用于废水中磷吸附剂的研究还鲜见报道。改性海藻吸附磷酸盐后可直接用作肥料,实现磷元素的资源化再利用,可有效降低吸附工艺产生废物的后续处理费用和对环境的影响。
本研究拟采用镧改性4种海藻作为养殖废水除磷吸附剂,通过SEM和FT-IR等表征手段观察镧改性前、后海藻的表面特征和官能团变化,并探讨在模拟废水中不同吸附剂用量、pH对吸附性能的影响,观察其等温吸附及动力学吸附过程的特点;通过在养猪废水中进行验证,为养殖废水中磷的科学去除和资源化利用提供参考。
-
本研究采用海带(LJ)、石莼(UL)、红藻(RP)和浒苔(EP)4种海藻作吸附废水中磷的基础材料。实验用海藻均购自青岛海兴源生物科技有限公司。海藻经自来水清洗2次后,再用去离子水润洗5次,然后将其置于60 ℃烘箱中干燥24 h,粉碎研磨,过60目筛后,贮存于聚乙烯瓶中备用。
实验用试剂包括氯化镧(LaCl3·7H2O)、氢氧化钠、磷酸二氢钾、钼酸铵、酒石酸锑氧钾、抗坏血酸等,均为分析纯,购自上海国药集团化学药品有限公司。
在制备模拟废水时,称取一定量的KH2PO4溶解于去离子水中,浓度为25 mg·L−1。实验用养猪废水取自四川省某养殖基地,该废水磷酸根浓度为16.83 mg·L−1,pH为8.32,氨氮浓度为498.65 mg·L−1。
-
准确称取粉末状的LJ、UL、RP和EP各10 g,放于150 mL烧杯中,与质量分数为1%的氯化镧溶液以1∶10的固液质量比混合;于磁力加热搅拌器上,30 ℃恒温搅拌9 h,调节pH至9,再继续搅拌3 h,离心分离;用去离子水反复冲洗至上清液为中性,于60 ℃烘箱干燥24 h,粉碎后,过60目筛保存备用。改性后的4种海藻经强酸(HNO3、HClO4、HF)消解至澄清,上清液于0.45 μm微孔滤膜过滤,稀释一定倍数后,用ICP发射光谱仪(ICP,Optima 8000,PerkinElmer,美国)测定其镧元素负载量。经测定,La-LJ、La-RP、La-UL和La-EP中镧的负载量分别为2.02%、1.83%、1.79%和1.46%。
-
4种海藻经镧改性前后的表面形貌用扫描电子显微镜(SEM,Hitachi S4800,日本日立)观察,样品先进行真空喷金,再进行SEM测试。特征官能团采用傅里叶红外光谱仪(FT-IR,Nicolet S10,Thermo Scientific,美国)测定。结构分析采用X射线衍射仪(XRD,D8 ADVANCE,布鲁克公司,德国)测定,用MDI Jade 6.0进行数据分析。
-
称取一定质量的吸附材料于100 mL锥形瓶中,加入50 mL一定浓度的模拟废液,在25 ℃的振荡器中以120 r·min−1的频率振荡。反应一段时间后,以3 500 r·min−1的频率离心5 min,离心后的上清液经0.45 μm微孔滤膜过滤,稀释一定倍数后,用钼锑抗分光光度法测定上清液中磷酸根的浓度。实验中磷酸根的吸附量按照式(1)进行计算。
式中:Qe为平衡吸附量,mg·g−1;C0和Ce为溶液在吸附前后的磷酸根浓度,mg·L−1;V为加入废液的体积,L;m为加入吸附剂的质量,g。
1)吸附剂用量实验。各吸附剂用量分别为0.02、0.05、0.1、0.15、0.2和0.25 g,模拟废液pH为6,浓度为25 mg·L−1,吸附时间为2 h。
2)初始pH实验。各吸附剂用量均为0.1 g,用0.2 mol·L−1的NaOH或HCl溶液将模拟废液pH调为3、4、5、6、7和8,浓度为25 mg·L−1,吸附时间为2 h。
3)动力学实验。各吸附剂用量均为0.1 g,模拟废液pH为6,浓度为25 mg·L−1,吸附时间分别为30、60、90、120、150、180、360和540 min。采用准二级动力学模型(式(2))、粒子内扩散模型(式(3))和Elovich模型(式(4))拟合吸附数据,探究吸附动力学过程。
式中:qt为t时刻镧改性的4种海藻对磷的吸附量,mg·g−1;qe为平衡时的吸附量,mg·g−1;k2为准二级吸附速率常数,g·(mg·min)−1;kip为粒子间扩散速率常数,g·(mg·min1/2)−1;c为边界层厚度,mg·g−1;αs为初始吸附速率,mg·(g·min)−1;βs为任意一次实验的解吸常数,g·mg−1。
4)等温吸附实验。各吸附剂用量均为0.1 g,模拟废液pH为6,浓度分别为10、20、40、60、80和100 mg·L−1,吸附时间为2 h。用Langmuir(式(5))和Freundlich(式(6))模型拟合实验数据。
式中:qe为平衡时的吸附量,mg·g−1;qm为理论最大吸附量,mg·g−1;ka为Langmuir模型常数,L·mg−1;ce为吸附平衡浓度,mg·L−1;kf为Freundlich方程常数;n为常数,1/n表示吸附强度大小。
-
称取4种镧改性的海藻各0.1 g于100 mL锥形瓶,加入经滤膜过滤且pH调为6的养猪废水50 mL(磷酸根浓度16.83 mg·L−1),在25 ℃的振荡器中,以120 r·min−1的转速振荡,反应9 h后,再以3 500 r·min−1的转速离心5 min,离心后的上清液经0.45 μm微孔滤膜过滤,稀释一定倍数后,用钼锑抗分光光度法测定上清液中磷酸根的浓度。
-
用SPSS Version 19.0(SPSS Inc.,芝加哥,伊利诺斯州)统计软件进行数据统计分析。采用单因素方差分析(One-Way ANOVA),每种镧改性海藻在不同吸附剂用量和初始pH条件下,对磷吸附量的差异性以及在同一吸附剂用量或初始pH条件下4种镧改性海藻对磷吸附量的差异性进行检验,采用L-S-D法分析比较平均值,当P<0.05时被认为具有显著差异。
-
1) SEM微观形貌。经镧改性后,4种海藻干化粉末的微观表面均变得粗糙且不规则,表明镧已经成功附着在海藻表面(图1)。EP在未改性前呈表面光滑的不规则体,改性后,其表面粗糙,有大量小颗粒附着,结构出现坍塌,并产生大量孔道,为磷的附着提供了大量的有效吸附位点。UL和RP在未改性前,表面结构均较为致密,整体呈块状结构。经改性后,UL表面变为粗糙的片状结构且形成了很多较小的空隙结构,La-RP呈凹凸状,褶皱区域增加。LJ在改性前,相较于其他3种材料,粗糙多孔的特点最突出,经改性后,La-LJ呈层状表面结构,有较大孔道。
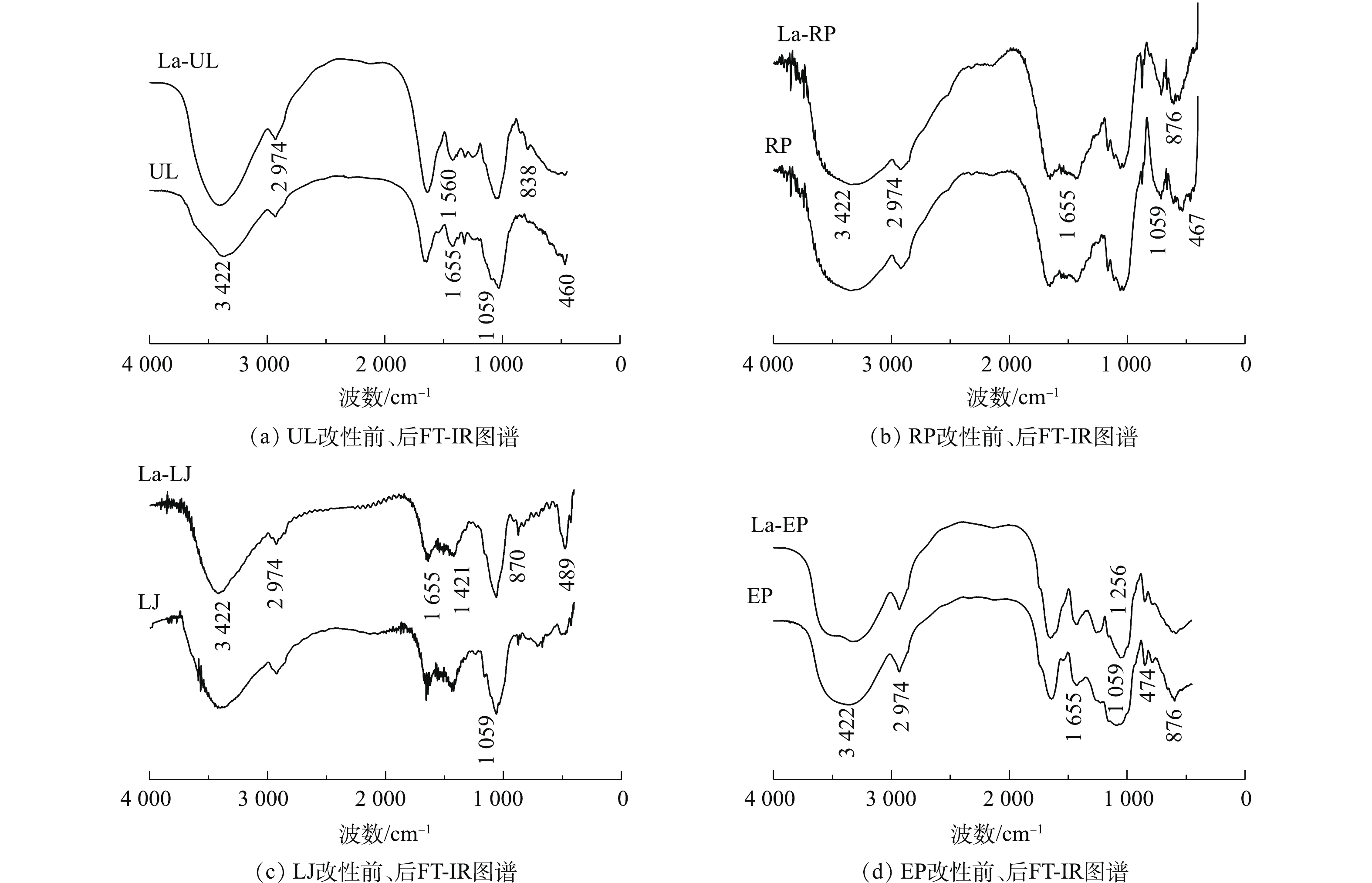
2) FT-IR。4种海藻未改性前,特征吸收峰大致相似(见图2)。在3 422 cm−1处,4种海藻均有较宽谱峰,由—OH伸缩振动产生[19];在2 974 cm−1处的吸收峰是由烷基对碳氢的拉伸振动所产生的[19],1 655 cm−1处的吸收峰为海带中游离水H—O—H键的弯曲振动峰[20],1 059 cm−1处的吸收峰归属于C—OH伸缩振动吸收峰[21]。
经镧改性后,4种海藻在位于3 442 cm−1和1 059 cm−1处的特征吸收峰均一定程度地变弱或变窄。3 422 cm−1处羟基的红外吸收峰减弱的原因可能是由于镧覆盖了部分表面羟基基团,导致海藻表面羟基总量下降,且由于—OH部分被OH−取代,使振动峰变窄[3];1 059 cm−1处C—OH吸收峰的减弱是因为镧的负载形成了C—O—La配位键,从而削弱了C—OH振动峰[21]。此外,La-EP在1 256 cm−1处有新的特征吸收峰,这可能是C—H弯曲振动峰所形成的,而镧改性使其生成C—La键[15]。La-UL、La-RP、La-LJ和La-EP分别在460、467、489和474 cm−1处产生新的特征吸收峰或特征吸收峰有变宽趋势,这是由于La—O键的形成所导致的[22],表明经过改性处理,镧已成功附着在几种海藻的表面。
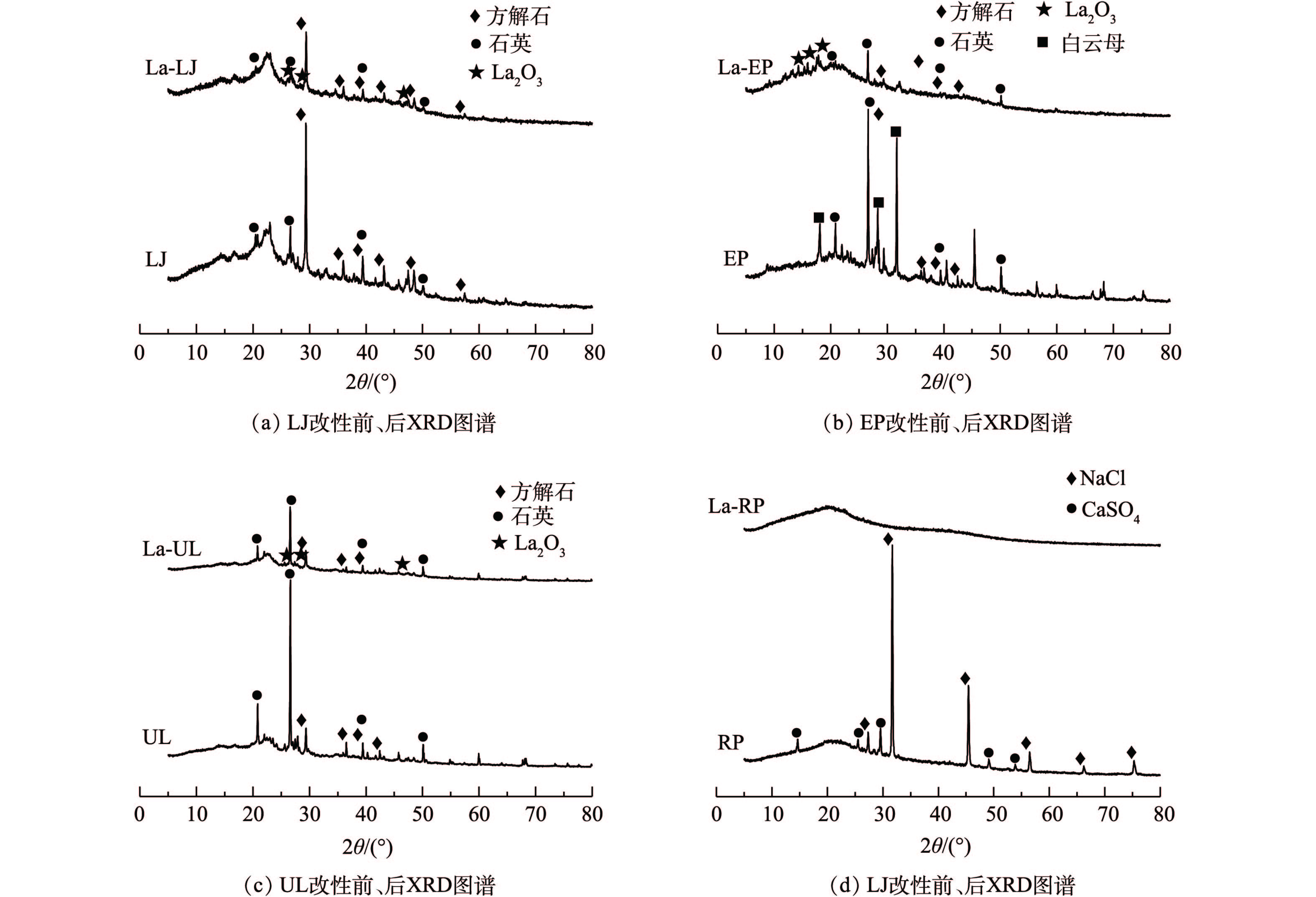
3) XRD。镧改性前后4种海藻的XRD图谱均出现较明显的变化(图3)。未改性前LJ和UL的主要晶相为方解石和石英,EP的主要晶相为方解石、白云母和石英,RP的主要晶相为NaCl和CaSO4。经改性后,镧主要以氧化物的形式存在于海藻表面。LJ、UL和EP主要在13.82°、25.44°、29.07°和47.15°出现La2O3的衍射峰,表明镧已经成功负载于海藻表面。但RP经改性后特征衍射峰消失,呈现非晶态的衍射特征。这可能是由于镧改性使其晶型发生了改变,RP由晶态转变成了非晶态而未显示出特征衍射峰。
-
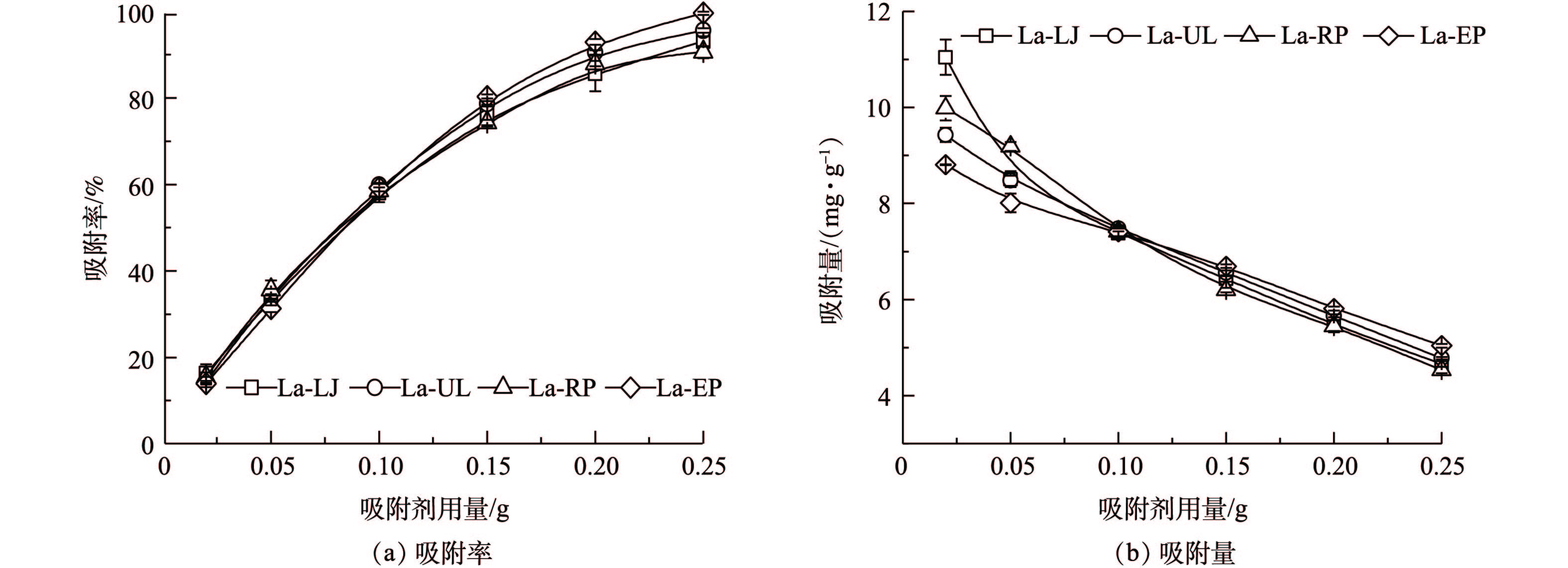
1)吸附剂用量对吸附效果的影响。吸附剂的投加量是一个既影响处理效果又影响处理成本的重要技术参数[20]。在本研究中,当吸附剂用量从0.02 g增加到0.25 g时,4种镧改性海藻对磷的吸附率随吸附剂用量的增加而呈指数趋势(La-LJ、La-UL、La-RP和La-EP的拟合度分别为0.953、0.974、0.977和0.972)升高(图4(a),P<0.05)。这是由于在吸附初期,吸附面积增大,吸附位点增加使吸附率不断增加,但随着吸附的进行,吸附位点逐渐达到饱和,吸附率趋于稳定。而4种镧改性海藻对磷的吸附量则呈指数趋势减少(图4(b))。这可能是由于4种材料的吸附点位未达到吸附饱和状态。当吸附剂用量为0.02 g时,4种镧改性海藻单位质量吸附剂的吸附量均为最高,La-LJ、La-UL、La-RP和La-EP分别为10.85、9.44、9.99和8.81 mg·g−1。在4种材料中,La-LJ的吸附量显著高于其余3种材料(P<0.05),La-EP显著低于其余3种材料(P<0.05)。当吸附剂用量增加至0.25 g时,4种镧改性的海藻对磷的吸附率均达到了90%以上,La-EP对磷的吸附率甚至达到了99%。
在本研究中,磷的吸附量和吸附率随4种镧改性海藻用量的增加所呈现的变化趋势,与镧负载的聚乙烯醇/海藻酸钠水凝胶珠从废水中吸附磷的变化趋势相似[23]。为了兼顾吸附性能与经济效益,本研究在模拟废液初始pH对磷吸附效果、动力学过程和等温吸附过程实验中,均选取0.1 g作为吸附剂投加量。
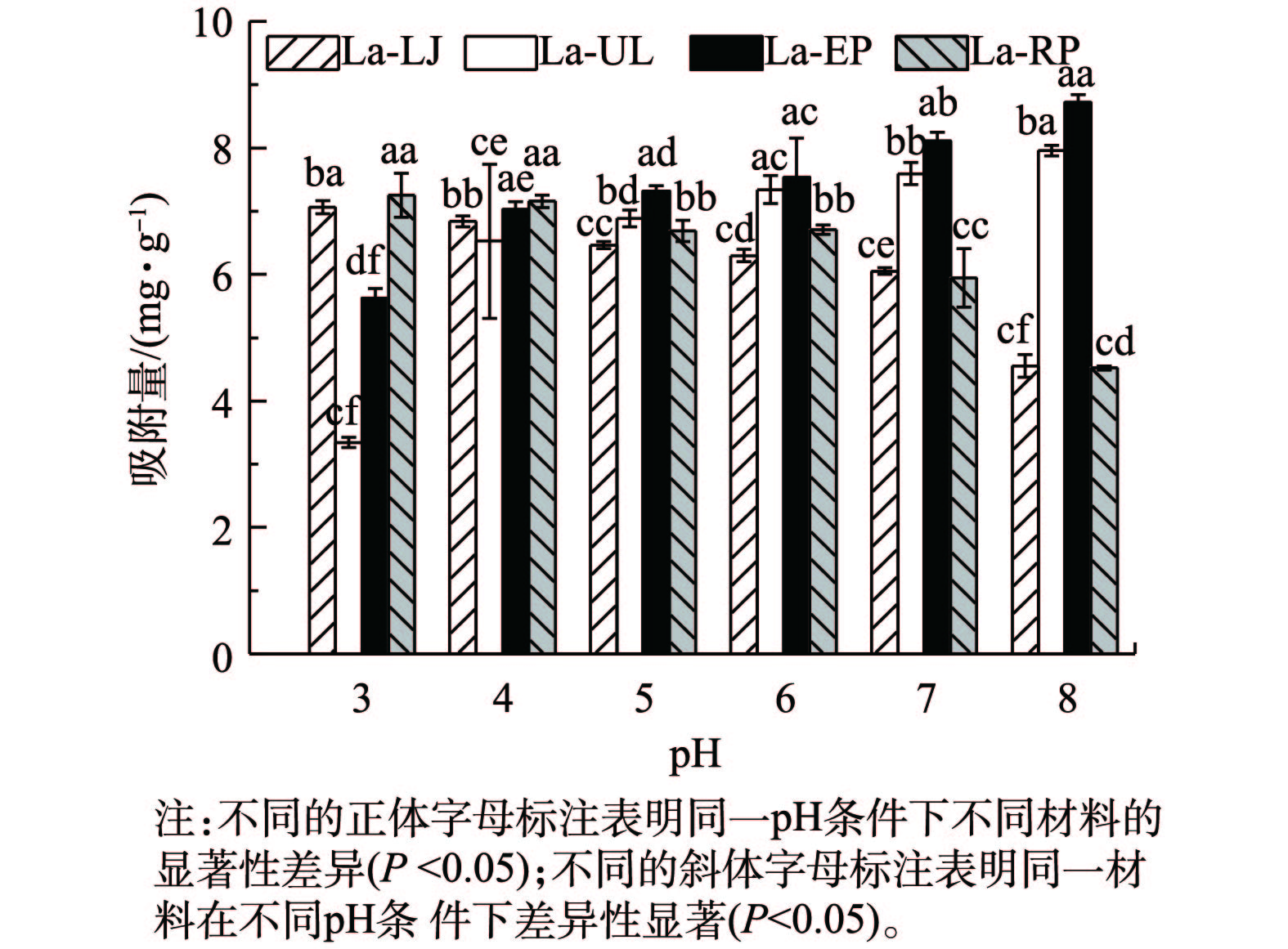
2)模拟废液初始pH对磷吸附效果的影响。溶液pH通过影响溶液中阳离子存在形态及吸附剂表面电荷从而影响吸附量的变化[19]。在本研究中,当初始pH从3上升到8时,4种镧改性海藻对磷的吸附量随pH的升高呈现不同的变化趋势(图5)。其中,La-LJ和La-RP对磷的吸附量随着初始pH的升高而呈现下降的趋势(P<0.05)。这2种材料对磷的吸附量随pH增加的变化趋势与MgO负载的生物炭对废水中磷吸附的变化趋势相似[4]。这可能是由于pH的升高使吸附剂表面的OH−增多,形成带负电荷的反离子层,导致吸附剂与磷酸盐之间的静电排斥力增大,因而吸附阻力也增大造成的[24]。La-UL和La-EP对磷的吸附量则随pH的升高呈现上升的趋势,且2种吸附剂在不同的pH条件下差异显著(P<0.05)。这可能是由于废液pH的升高使负载在海藻表面的镧与水中的羟基结合形成镧羟基化合物,而镧氢氧化物能络合磷酸根离子,从而吸附去除水中的磷。当pH为3时,4种镧改性海藻间吸附量差异显著(P<0.05),La-LJ和La-RP对磷的吸附量达到了最大,分别为7.18 mg·g−1和7.33 mg·g−1。当pH为8时,4种镧改性海藻对磷的吸附量差异显著(P<0.05),La-UL和La-EP对磷的吸附量达到最大,分别为8.06 mg·g−1和8.86 mg·g−1。镧改性的4种海藻在pH升高时,呈现2种不同变化趋势的原因可能是镧的负载形式不同。LJ和RP中镧可能负载在内部,pH的影响主要由表面羟基的静电作用所决定[25];UL和EP中镧可能负载在表面,pH的影响主要由镧氢氧化物的络合作用所决定。因此,La-LJ和La-RP与磷酸盐的反应机制可能由表面的配体交换或离子交换引起,而La-UL和La-EP与磷酸盐的反应机制可能由内部的复合反应引起。
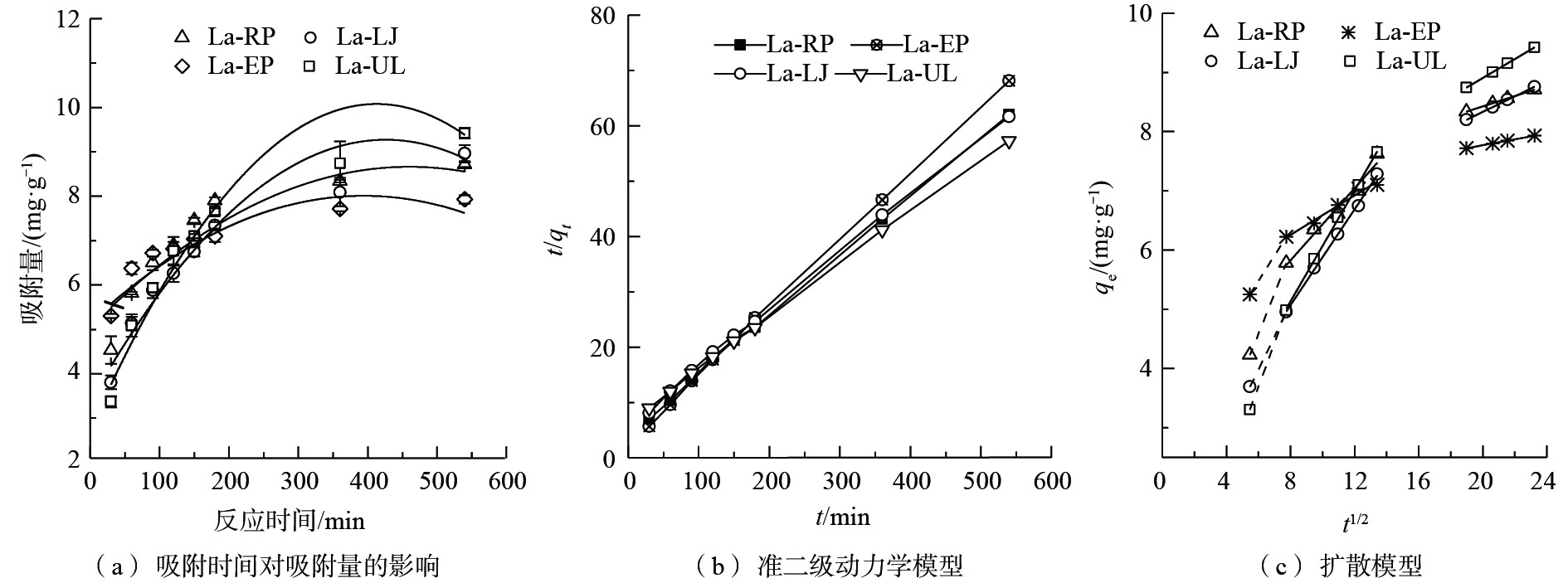
3)动力学吸附过程。吸附时间也是影响废水中磷吸附量的因素之一[26]。当时间从30 min增加至560 min时,4种镧改性海藻对磷的吸附量不断增加并呈现趋于稳定的趋势(图6(a))。这是因为在反应初期,镧改性的海藻提供了大量吸附位点,磷酸根离子快速迁移至吸附剂表面,但随着吸附位点的减少,反应速率逐渐减慢直至达到平衡[4]。其中La-UL相较于其他3种镧改性海藻,随着吸附时间的增加,其吸附量的增加趋势更加明显。这一现象表明,La-UL相较于La-EP、La-RP和La-LJ 3种材料,其对废水中的磷酸根具有更高的亲和能力。
本研究采用准二级动力学模型(图6(b))、粒子内扩散模型(图6(c))和叶诺维奇模型进行拟合。表1和表2给出了准二级动力学模型、叶诺维奇模型和粒子内扩散模型的部分参数。准二级动力学模型假设吸附体系为单层吸附体系,能更好地描述吸附反应的全过程,如液膜扩散、表面吸附和内扩散等[27]。由表1可知,准二级动力学模型的可决系数较高(R2均为0.99以上),表明准二级动力学模型能很好地描述4种镧改性的海藻对废水中磷的吸附过程。这也表明,4种镧改性海藻对磷的吸附速率受化学吸附机理控制,而不是受溶液内的扩散过程所控制[28]。准二级动力学模型表明该吸附过程涉及到吸附剂与吸附物质之间的电子共用和电子转移[29]。通过准二级动力学模型的吸附速率常数,4种镧改性的海藻k2为0.10~0.12 g·(mg·min)−1,远高于镧和铈改性的沸石(k2分别为0.036 9 g·(mg·min)−1和0.004 0 g·(mg·min)−1)[30]。
叶诺维奇模型也能较好地拟合本研究的吸附过程。该模型用来描述化学吸附过程,且假设实际的固体表面能量分布不均匀[11]。比较由叶诺维奇模型获得的吸附速率常数(表1),La-EP的吸附速率常数(1.10)大于其他3种镧改性海藻(La-RP 0.65、La-LJ 0.56、La-UL 0.47)。因此,La-EP比其他3种镧改性海藻能更有效地吸附废水中的磷。
由图6(c)可以看出,4种镧改性的海藻对磷的吸附过程可拟合为3个阶段。每一阶段都可通过拟合直线斜率的变化来确定。曲线的斜率表征了粒子内扩散的速率参数,而截距与边界的厚度成正比。第1阶段在开始前30 min内,直线斜率最大(表2),吸附量随时间的增长快速增加,吸附开始时,反应主要由膜扩散控制,且由于外表面存在大量的有效吸附位点,膜扩散速率很快。在此过程中,磷酸盐从溶液中扩散到镧改性的海藻周围的边界层,吸附剂与磷酸盐之间主要以静电吸引的方式进行吸附[27]。第2阶段为60~180 min,直线斜率减小,此时磷酸盐穿过边界层到达吸附剂的内表面,颗粒内扩散为速率限制步骤。第3阶段为360~540 min,直线斜率最小,在此阶段中,吸附物质与吸附位点相互作用,逐渐达到吸附平衡。4种材料的拟合直线均没有经过原点,表明该吸附过程不仅仅由粒子间的扩散过程控制,还由膜扩散过程控制[26]。此现象与La(OH)3改性蛭石吸附磷的动力学研究结果[28]相似。
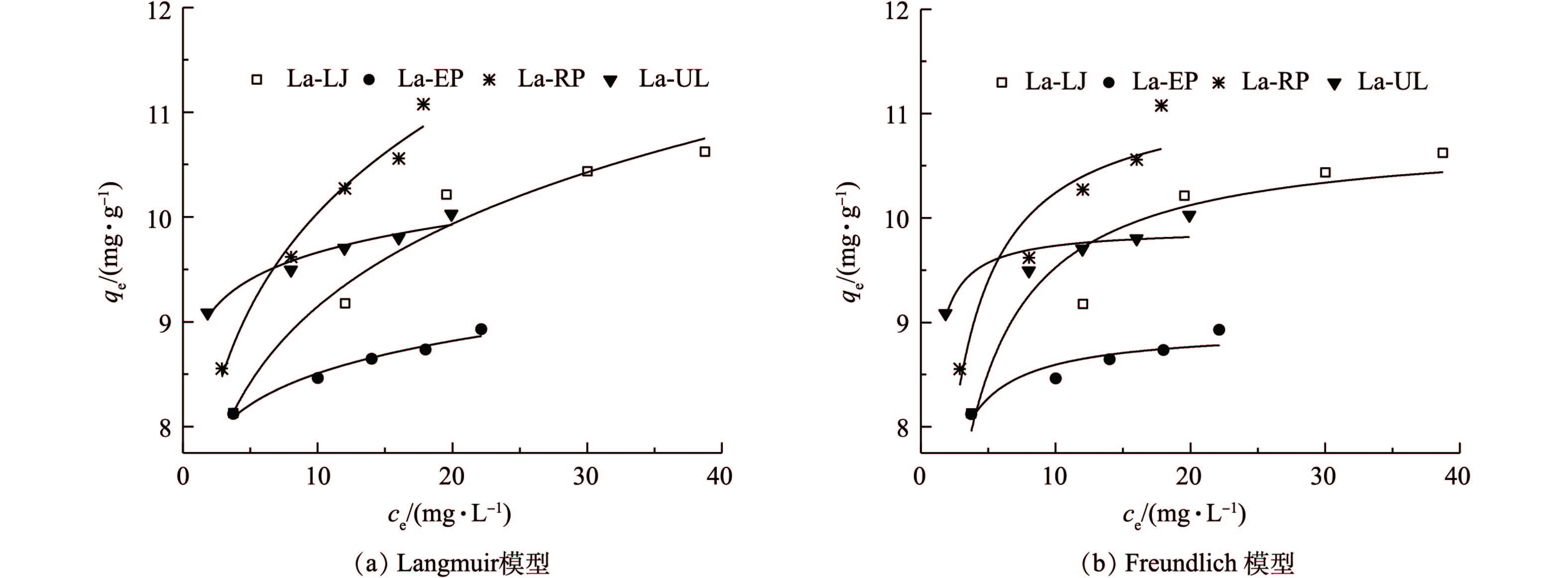
4)等温吸附过程。为探究4种镧改性干化海藻粉末对磷吸附去除的可能性及最大吸附量,采用了Freundlich模型和Langmuir模型模拟实验数据(图7)。由表3可知,通过比较2种模型的R2,4种镧改性的海藻对磷的吸附过程用Freundlich模型拟合比用Langmuir模型拟合效果更好。这表明在实验磷酸根初始浓度为20~100 mg·L−1时,4种镧改性的海藻对磷的吸附过程是一种非均相吸附,并且可以存在多层吸附[28]。由Langmuir模型可知,La-LJ、La-UL、La-RP和La-EP的最大吸附量分别为10.80、8.94、11.25和9.90 mg·g−1,是未改性前(LJ 0.449 mg·g−1、UL 0.237 mg·g−1、RP 0.169 mg·g−1和EP 0.387 mg·g−1)的24~66倍。相比于镧负载的多孔陶粒[29]、镧和铈改性的沸石[30]等,镧改性的海藻具有良好的吸附效果。在Freundlich拟合方程中,参数1/n表示吸附剂吸附性能的强弱,1/n越大,表明吸附性能越强,一般认为1/n为0.1~0.5时吸附容易进行[31]。在本研究中,4种吸附剂的1/n值均小于0.5,表明镧改性的4种海藻对磷的吸附过程均很容易进行(表3)。而且La-RP和La-EP的1/n值均远小于La-LJ和La-UL,表明前两者对磷的吸附性能更好。
-
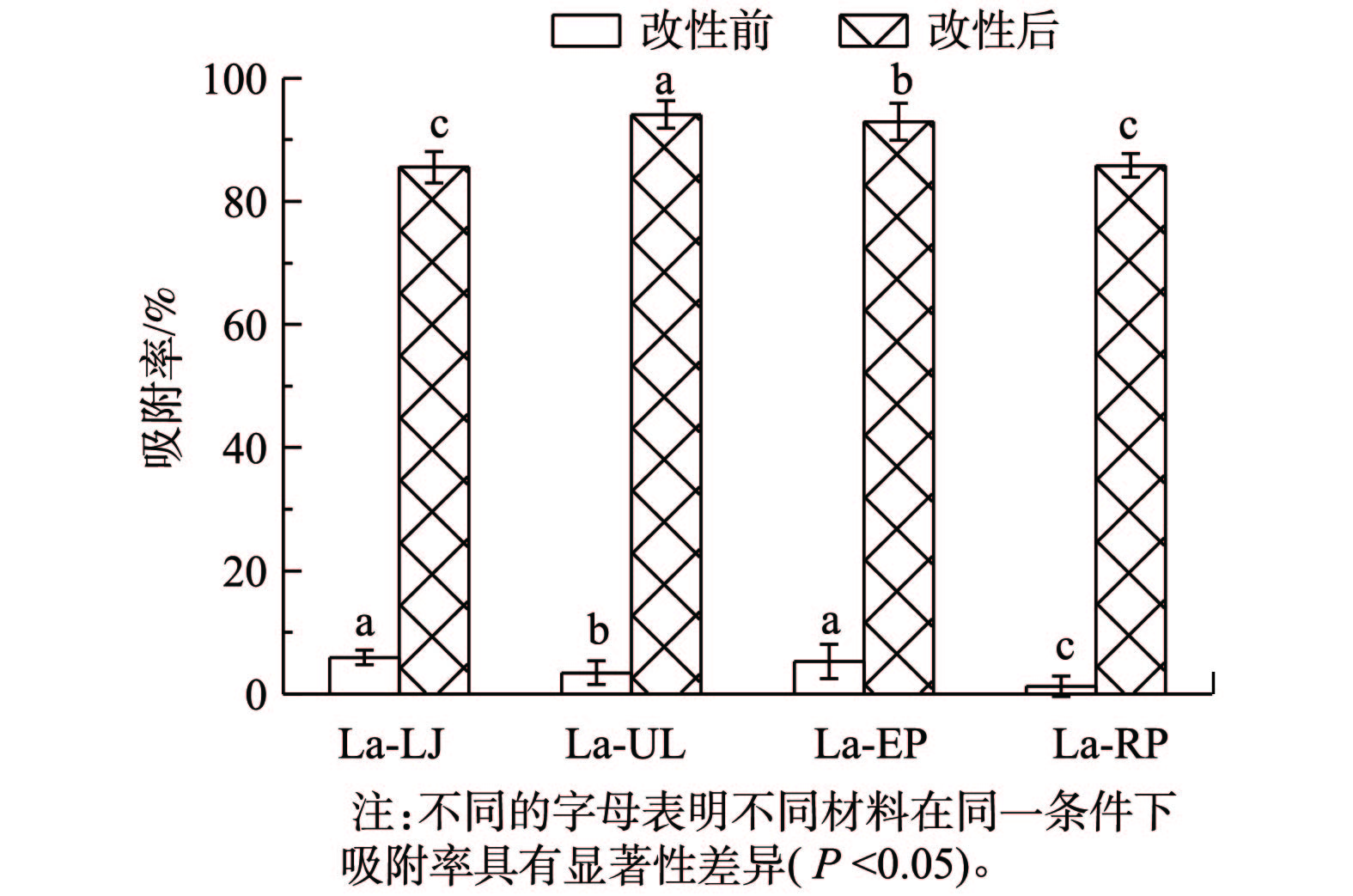
本研究采用养猪废水对比研究4种海藻用镧改性前后对磷吸附的实际效果(图8)。未改性前,LJ、UL、RP和EP对养猪废水中磷的去除率分别为5.34%、2.81%、2.01%和4.60%,而经镧改性后,La-LJ、La-UL、La-EP和La-RP对磷的去除率分别达到85.54%、94.11%、92.88%和85.81%,这表明4种镧改性后的海藻在组成复杂的养猪废水中具有较强的抗干扰能力。其中UL未改性前对磷的去除率较小,但La-UL对养猪废水中磷的吸附率在4种改性海藻中达到了最高。但是,LJ作为未改性前4种海藻中对磷去除率最高的材料,经镧改性后,在养猪废水中对磷的去除率却最低。吸附后废水中剩余磷含量均小于2.5 mg·L−1,可达到《畜禽养殖业污染物排放标准》(GB 18596-2001)。因此,镧改性的4种海藻在废水除磷的应用中具有潜力。此外,本研究采用的镧改性海藻成本相对较低,经吸附后的材料可直接用作肥料,更具有经济价值。
-
1)镧负载改性的海藻干化粉末对磷的吸附去除能力显著提高,La-LJ的吸附效果最好,最大吸附量可达10.85 mg·g−1。
2)当吸附剂用量为0.25 g时,4种镧改性海藻对模拟废水中磷的吸附率均达到90%以上。当pH为3时,La-LJ和La-RP对磷的吸附量最大,分别为7.18 mg·g−1和7.33 mg·g−1;当pH为8时,La-UL和La-EP对磷的吸附量最大,分别为8.06 mg·g−1和8.86 mg·g−1。
3) 用准二级动力学模型和Freundlich吸附等温线模型能更好地拟合4种镧改性海藻粉末对磷的动力学吸附过程和等温吸附过程。
4) 4种海藻经镧改性后对养猪废水中磷的去除率明显提高,能达到85.54%以上,可实现磷达标排放。
4种镧改性海藻粉末对养殖废水中磷的去除
Phosphorus removal from aquaculture wastewater by four types of lanthanum-modified seaweeds powder
-
摘要: 为了解决使用矿物材料除磷产生大量沉积物的问题和实现磷的资源化利用,研究了镧改性的海带(La-LJ)、石莼(La-UL)、红藻(La-RP)和浒苔(La-EP)干化粉末材料对模拟废水和养猪废水中磷的吸附特征。在模拟废水中,随吸附剂用量的增加,4种镧改性的海藻对模拟废水中磷的吸附量均呈指数下降;随初始pH的升高,La-EP和La-UL对磷的吸附量增加,而La-LJ和La-RP对磷的吸附量减少。动力学吸附过程和等温吸附过程分别用准二级动力学模型和Freundlich模型拟合更适合。4种镧改性海藻对磷的最大吸附量为8.94~11.25 mg·g−1,相比于改性前,La-LJ、La-UL、La-RP和La-EP对磷的吸附量分别增加了24、38、66和25倍。在养猪废水中,经4种镧改性海藻吸附处理后,废水含磷浓度降低到2.5 mg·L−1以下,能实现达标排放。因此,镧改性的4种海藻是养猪废水吸附除磷的可行材料。Abstract: In order to solve the problem that a large amount of sediments occurrence when the mineral materials were used to remove phosphorus and to fulfill the phosphorus resource utilization, the adsorption characteristics of phosphorus in simulated wastewater and swine wastewater were studied with lanthanum-modified Laminaria japonica (La-LJ), Ulva lactuca (La-UL), Rhodymenia palmata (La-RP) and Enteromorpha prolifera (La-EP). In simulated water, the adsorption capacities of four lanthanum-modified seaweeds decreased exponentially with the increase of dosage. With the increase of initial pH, the adsorption capacities of La-EP and La-UL increased, while the adsorption capacities of La-LJ and La-RP decreased. The kinetic processes and isothermal adsorption could be better fitted by the pseudo-second order kinetic model and Freundlich adsorption isotherm model, respectively. The maximum adsorption capacities of the four types of lanthanum-modified seaweeds were among 8.94~11.25 mg·g−1. Compared with pre-modification seaweeds, the adsorption capacities of La-LJ, La-UL, La-RP and La-EP increased by 24, 38, 66 and 25 times, respectively. Moreover, the phosphorus concentrations in the swine wastewater after adsorption were reduced to 2.5 mg·L−1, which could meet the corresponding discharge standard in China. Therefore, four types of lanthanum-modified seaweeds could be feasible to remove phosphorus from swine wastewater.
-
Key words:
- water treatment /
- phosphorus removal /
- adsorption /
- seaweeds /
- lanthanum modifcation
-
高氮磷废水的过量排放会导致水体富营养化和生态破坏[1]。微藻是一种光合微生物,能够吸收氮、磷和有机物等,被用处理各种废水[2]。另一方面,微藻细胞脂类含量高是生物柴油生产的主要原料[3-9],因此,将废水处理与微藻生物量生产相结合可以降低二者生产成本。由于微藻对废水中氮/磷的去除是藻细胞生长代谢的结果即平均去除速率和去除率与藻细胞生长速率和生物量呈正相关,而部分细菌和真菌能够促进微藻的生长(如地衣中的细菌和真菌促进其共生绿藻的生长),因此,将微藻与细菌[10-16]或者真菌[17-26]混合培养,利用微藻和细菌或者真菌之间的协同效应促进微藻生长进而提高氮/磷的去除率成为研究热点。
雨生红球藻能够在适宜的条件下快速吸收氮和磷进行自养/混合营养生长,而在不利条件下大量合成脂类和高附加值的虾青素(一种红色类胡萝卜素)[27-28],目前已被用于处理不同的废水,并取得了良好的效果[29-33]。然而,与其他藻类相比,雨生红球藻对有害细菌更敏感,这些细菌严重抑制藻细胞生长,限制了其在废水处理中的应用。实际上,有害细菌对所有微藻的生长均构成严重威胁[34]。为了控制微藻培养过程中的有害细菌,通常采用的方法为添加抗生素、高温处理、强光照射[35-36],以及使用次氯酸钠对废水进行预处理[37]。因此,有效控制有害细菌是利用微藻尤其是雨生红球藻处理废水的关键问题。
在此前的研究[37-38]中我们分离到一种蓝藻共生真菌Simplicillium lanosoniveum(DT06)。DT06能够合成一种新抗生素[39]并且能促进衣藻(Chlamydomonas reinhardtii)生长和脂类合成[40]。因此,本研究将雨生红球藻与真菌DT06在高含氮磷废水中混合培养,以期提高雨生红球藻类生长速率和产量以及废水氮/磷的去除速率和去除率。
1. 材料与方法
1.1 实验材料
1)废水样本。废水来自天津市的某污水处理厂。废水通过0.45 µm滤膜去除不溶性大分子物质,并在4 ℃保存。废水主要性质如下:pH为6.5±0.4;总氮(TN)质量浓度为(553.8±17) mg·L–1;总磷(TP)质量浓度为(90.7±8) mg·L–1,化学需氧量(COD)为(750±22) mg·L–1。
2)微生物菌株。雨生红球藻购自中国武汉水生生物研究所;真菌Simplicillium lanosoniveum DT06由河北工业大学代谢工程与生物合成实验室分离获得,并保藏于中国科学院微生物学研究所菌物标本馆(编号HMAS 242045)。
1.2 微藻接种液以及真菌孢子的制备
1)微藻接种液:5 mL雨生红球藻培养液接种到装有60 mL BBM培养基[27]的100 mL锥形瓶中,置于光照摇床中培养7 d(115 r·min–1、25 °C恒温、60 μmoL·(m2·s)–1持续光照)。雨生红球藻接种液最终的细胞浓度为1.5×105 细胞·mL–1。
2)真菌孢子悬浮液:将真菌DT06划线于PDA培养基平板上,于培养箱(28 ℃)中恒温培养7 d后,从菌落表面轻轻刮取收集DT06孢子,并悬浮于50 mL无菌水中。真菌孢子悬浮液最终细胞浓度为5×106 细胞·mL–1。雨生红球藻细胞和真菌DT06孢子的数量均通显微镜进行计数。
1.3 培养体系的构建
雨生红球藻与真菌DT06混合培养(简称M组):按10%接种量将雨生红球藻接种到含有200 mL废水的500 mL锥形瓶中,并分别接种对应体积的DT06孢子悬浮液,以达到5∶1、10∶1、30∶1、50∶1的细胞数量接种比例(雨生红球藻:DT06)。以雨生红球藻单独培养(1∶0,雨生红球藻:DT06)作为对照(CK)。
雨生红球藻-DT06混合添加NaHCO3培养(简称MC组):在每组含有200 mL废水的500 mL锥形瓶中分别添加不同体积的NaHCO3母液(10 g·L–1),使NaHCO3质量浓度达到0(对照,MCK)、0.2、0.4、0.6和0.8 g·L–1,以最佳细胞接种比例分别接种雨生红球藻和DT06孢子悬浮液。
所有实验均置于光照培养箱中培养12 d(25 °C恒温、60 μmoL·(m2·s)–1持续光照),每天手摇2次,每组实验设置3个重复。
1.4 参数测定方法
1)雨生红球藻生物量。雨生红球藻生物量以细胞干重表示,每隔2 d取培养液并采用显微镜计数法计数,根据式(1)计算雨生红球藻生物量,根据式(2)计算雨生红球藻比生长速率。
X=4.64×10−8N+0.0035 (1) μ=(lnXn−lnX0)/(tn−t0) (2) 式中:X为细胞干质量,g·L–1;N为细胞浓度, 细胞·mL–1;μ为比生长速率,d−1;X0和Xn分别为第t0天和第tn天的雨生红球藻生物量,g·L–1。
2)细菌总数。根据实验室之前的方法[40-42]对废水中细菌总数做了部分修改。灭菌的LB琼脂板接种1 mL稀释105倍的废水样品,并在培养实验相同的条件下培养3 d。总细菌数表示为每毫升菌落形成单位(CFU·mL−1)。
3)废水水质。每隔两天取废水样本进行分析。总氮使用过硫酸钾氧化紫外分光光度法;总磷使用钼锑抗分光光度法;COD 使用重铬酸盐法测定;氮、磷的去除率和去除速率根据式(3)和式(4)进行计算。
N=(N0−Nt)/N0×100% (3) R=(N0−Nt)/(tn−t0) (4) 式中:N为COD和氮、磷的去除率,%;R为COD和氮、磷的去除速率,mg·(L·d)–1;N0和Nt分别为第t0天和第tn天的COD和氮、磷质量浓度,mg·L–1。
4)脂类和虾青素含量。 雨生红球藻脂类和虾青素含量参照我们此前的方法[43]测定。
2. 结果与讨论
2.1 雨生红球藻-真菌DT06混合培养
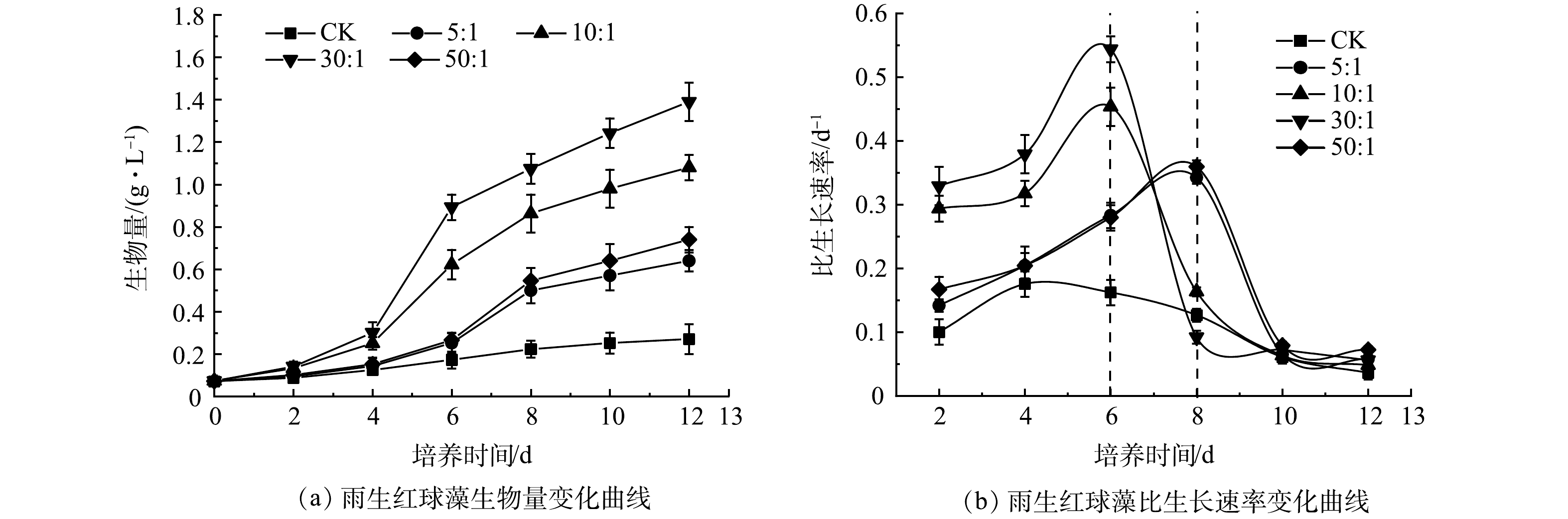
1)混合培养对微藻生长的影响。如图1(a)所示,CK中雨生红球藻的生物量在前8 d内缓慢上升,第10天后趋于平稳,最终达到0.27 g·L–1;而雨生红球藻与DT06混合培养过程中雨生红球藻的生物量在前4 d缓慢上升(适应期),在第6天(10:1、30:1)和第8天(5:1、50:1)快速上升,第8天后趋于平稳。最终,雨生红球藻的生物量在5:1、10:1、30:1和50:1下分别为0.64、1.08、 1.39 和 0.74 g·L–1。
生长动力学分析结果(图1(b))显示, CK中雨生红球藻的比生长速率在第4天达到最大值(0.18 d–1),第6天后逐渐降低至0。雨生红球藻与DT06混合培养过程中雨生红球藻的比生长速率均高于CK。比生长速率在10∶1和30∶1时在第6天达到最大值,分别为0.45 d−1和0.54 d−1;在5∶1和50∶1时在第8天达到最大值,分别为0.34 d−1和0.36 d−1。比生长速率此后逐渐降低至0。雨生红球藻与DT06混合培养过程中30∶1表现出最高的生长速率和最高平均比生长速率(0.25 d−1),因此,后续实验以最佳藻菌细胞比30∶1进行实验。
混合培养中藻类生物量的增加是由于比生长速率的提高,这可归因于2个方面:1)藻类(雨生红球藻)和真菌DT06的共生作用。DT06释放CO2促进雨生红球藻光合作用,并吸收雨生红球藻释放的O2进行有氧代谢,从而解除O2对藻类生长的抑制作用,这与其他菌藻混合培养类似[27,44-47];2)抑制有害细菌的生长。与混合培养相比,对照的生物量异常低,比生长速率过早地下降,表明废水中有害细菌对藻类的生长有显著的抑制作用,混合培养中的生物量持续增加表明DT06释放的抗生素表现出对有害细菌显著的抑制作用。
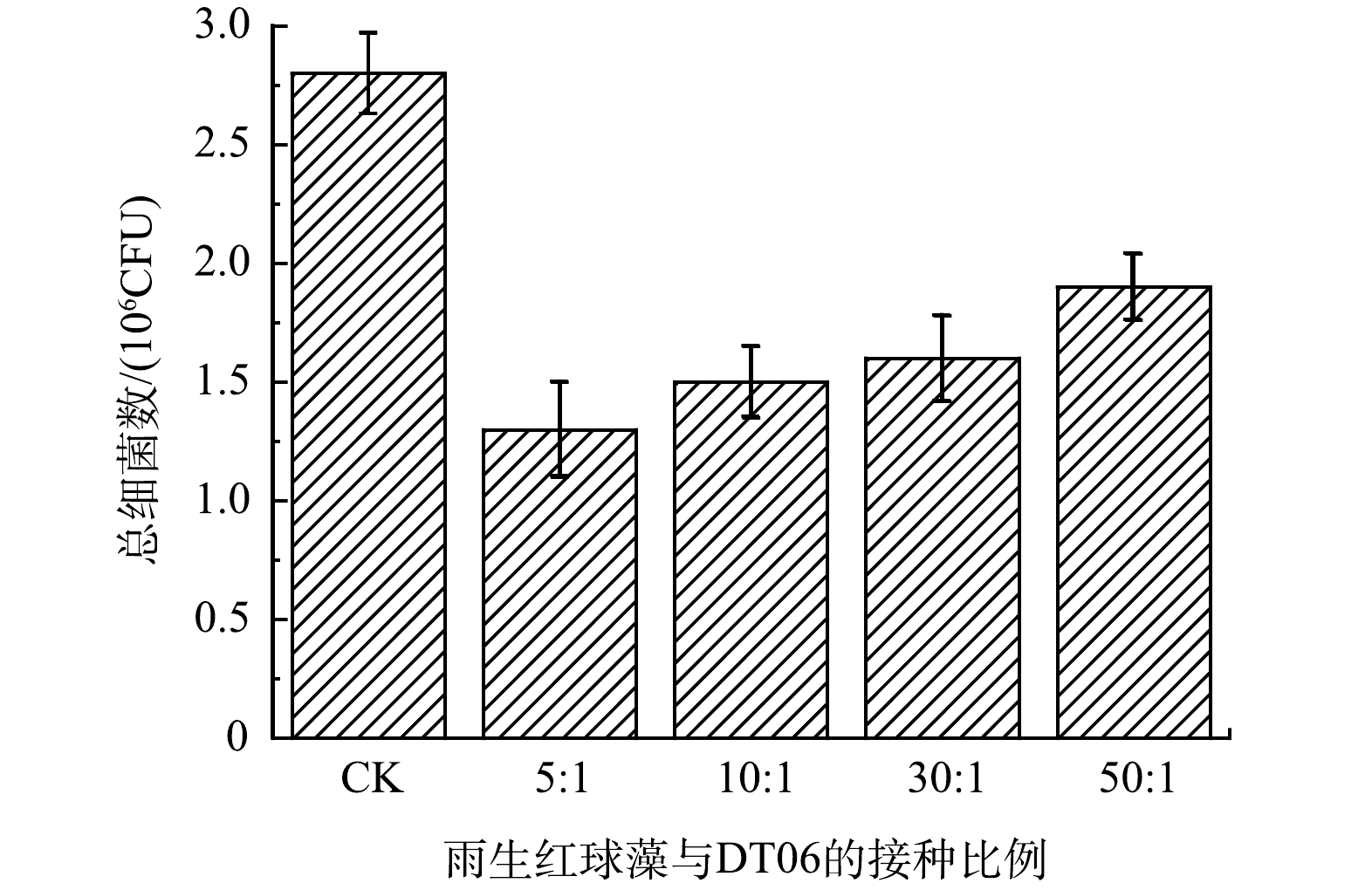
2)总细菌数。如图2所示,实验结束时5∶1、10∶1、30∶1和50∶1中细菌总数分别为1.3、1.5、1.6、1.9×106 CFU。雨生红球藻与DT06混合培养过程中的细菌总数与CK(2.8×106 CFU)相比分别下降了54.8%、46.4%、42.9%和30.4%。这表明DT06能够抑制废水中细菌的增长。
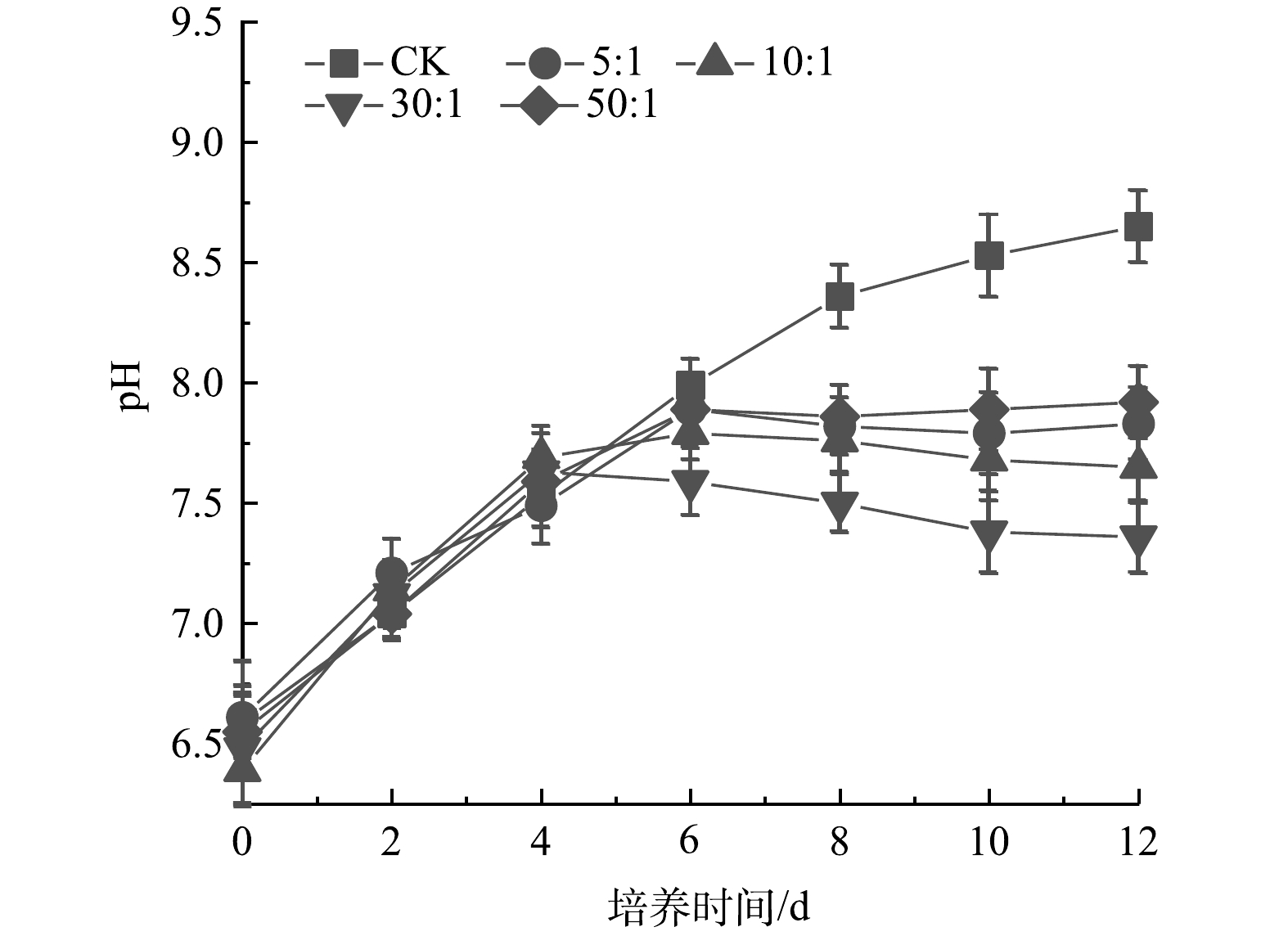
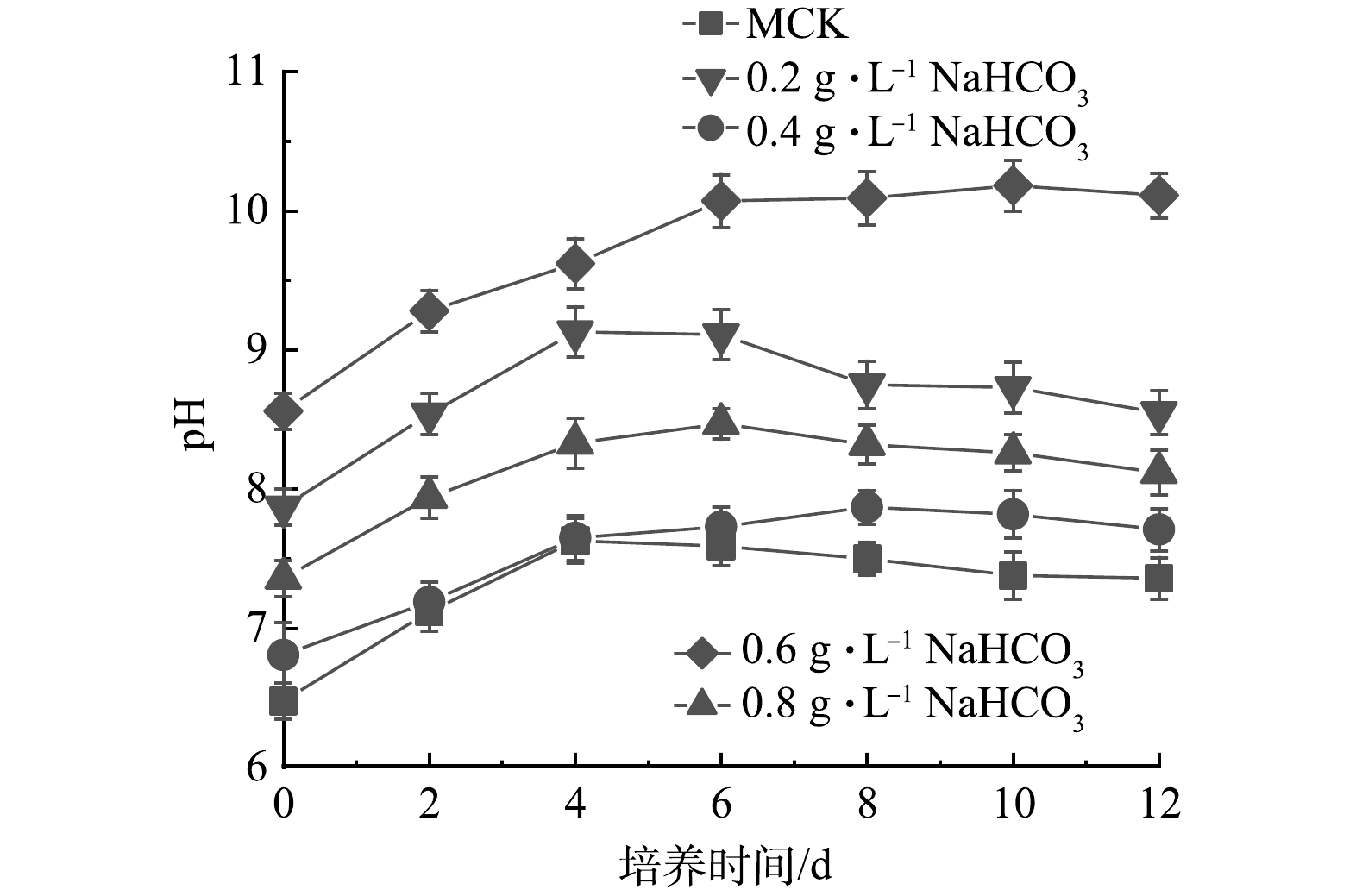
3)如图3所示,CK中pH持续上升,在实验结束时达到8.65。雨生红球藻与DT06混合培养过程中pH在前4 d持续升高,之后保持相对稳定且显著低于CK。实验结束时5: 1、10: 1、30: 1和50: 1的pH分别稳定在7.83、7.65、7.36和7.92。pH快速升高主要原因是雨生红球藻吸收了生理碱性盐(如硝酸盐)。混合培养中pH保持相对稳定,原因是真菌DT06释放的CO2中和培养液的碱性以及雨生红球藻吸收废水中的NH4+降低了培养液的pH。
4)混合培养对COD去除的影响。如图4(a)所示,CK中COD下降缓慢,最终的去除率仅为28.5%,平均去除速率为18.4 mg·(L·d)–1(图4(b))。这表明雨生红球藻和原有的微生物对耗氧有机物(以COD计)的降解能力有限。而在30∶1、10∶1、5∶1和50∶1中,COD分别在第4、6和8天内降至0(去除率100 %)(图4(a)),平均去除率分为183.9、127. 4、96. 8、93.1 mg·(L·d)–1 (图4(b))。结果表明,废水中的难降解耗氧有机化合物(以COD计)可被DT06完全降解为小分子物质和CO2,这些小分子物质被雨生红球藻利用进行混合营养生长。因此,在难降解有机化合物完全降解前后,雨生红球藻的比生长速率快速上升,之后迅速下降(图1(b))。
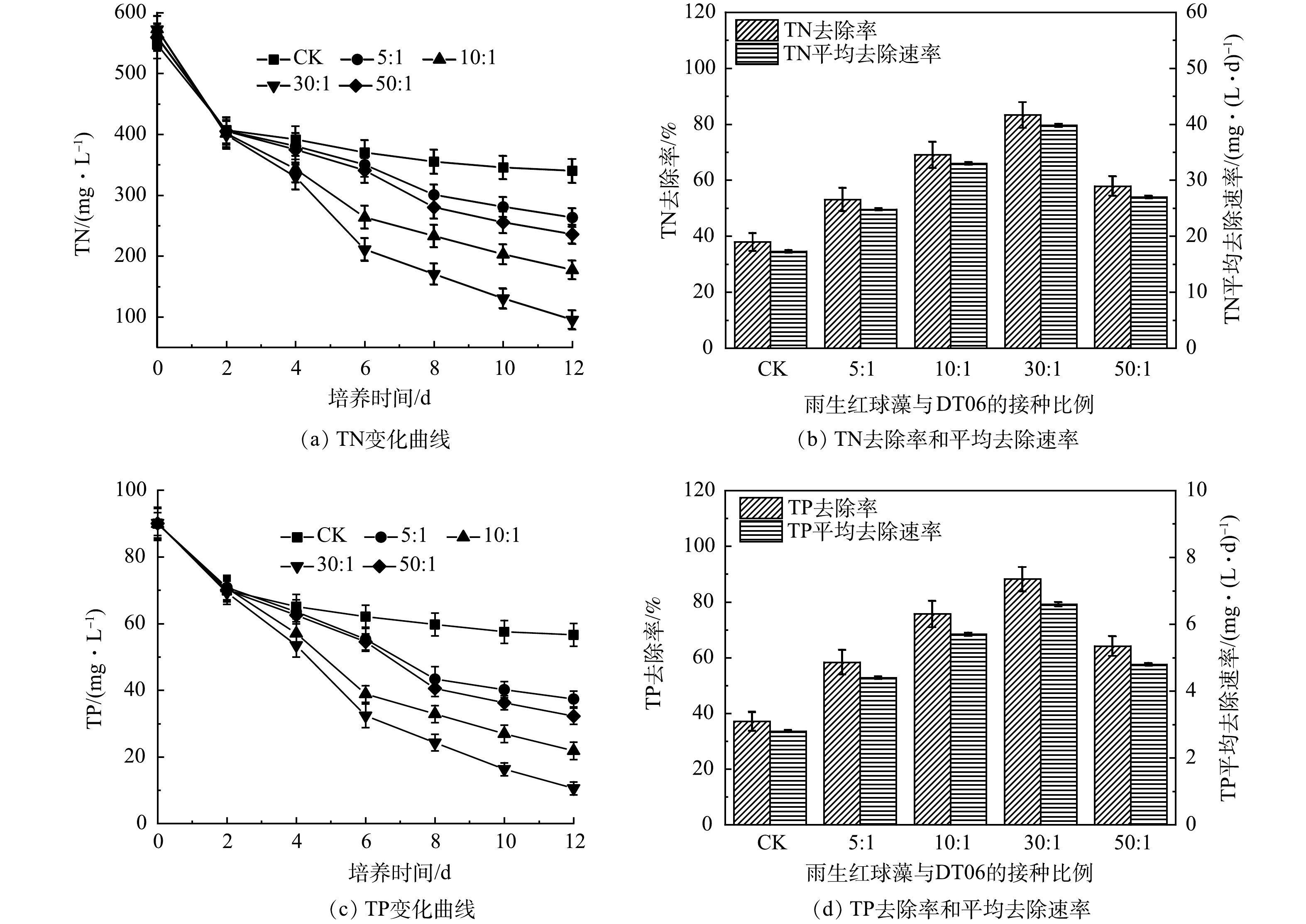
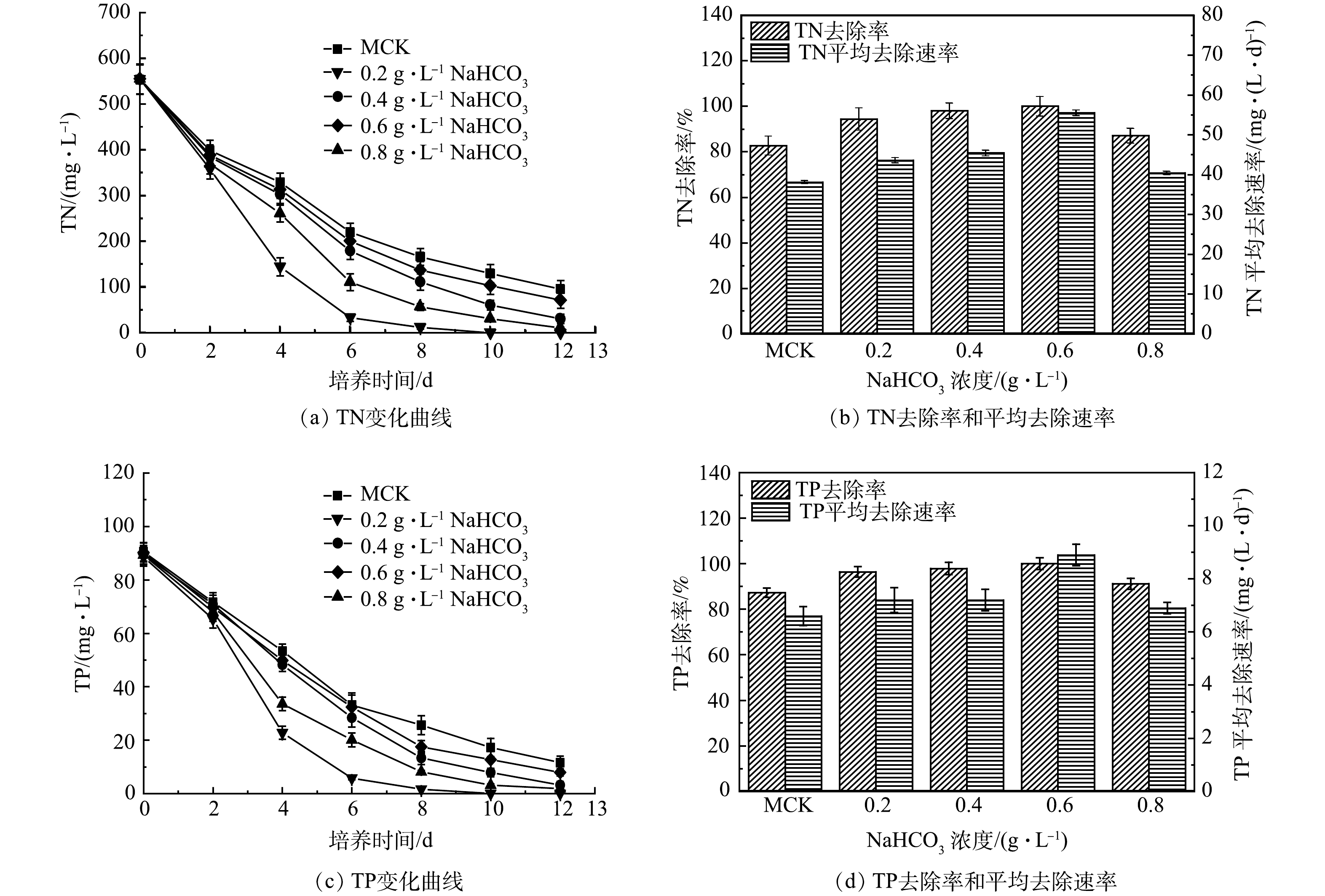
5)混合培养对氮磷去除的影响。如图5(a)所示,CK中TN质量浓度在前2 d迅速下降,之后缓慢下降,最终达到340 mg·L–1,去除率为37.9 %,平均去速除率为17.3 mg·(L·d)–1 (图5(b))。相比之下,雨生红球藻与DT006混合培养过程中TN质量浓度持续下降,下降速度均高于CK(图5(a))。其中, 30∶1中TN去除率最高为83.33%,平均去除速率为39.8 mg·(L·d)–1。而5∶1、10∶1、50∶1中TN的平均去除速率分别为24.8、33.0、27.0 mg·(L·d)–1;去除率分别为53.1%、69.1 %、57.9 % (图5(b))。
TP变化与TN变化规律相似(图5(c)),TP在CK中下降最慢,最终为56.6 mg·L–1;在 30:1中下降最快,最终为10.6 mg·L–1。最低和最高的TP去除率分别为37.1%和88.2%,平均TP去除率分别为2.8 mg·(L·d)–1和6.6 mg·(L·d)–1 (图5(d))。
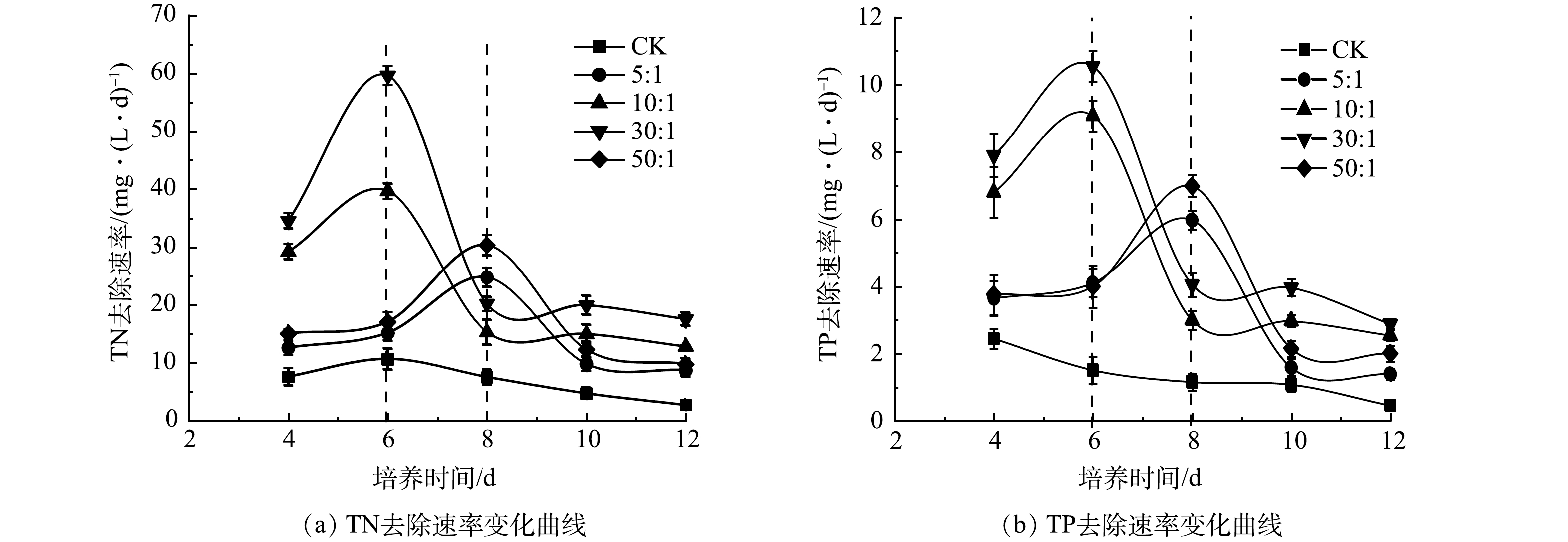
混合培养氮、磷去除率的提高归因于藻类生长速率的提高。如图6所示,在第6天和第8天之前,所有混合培养中的TN和TP去除速率持续增加,随后骤然下降,这与雨生红球藻比生长速率在初始升高和随后下降一致(图1(b))。而如上所述,雨生红球藻比生长速率的骤然下降主要是由于雨生红球藻进行快速异养生长对作为碳源的COD的快速消耗。也就是说,混合培养中有机碳源(如COD)的存在促进了雨生红球藻的生长,进而提高氮、磷的去除率。然而,在实验结束时,雨生红球藻与DT06混合培养组中残余的氮、磷含量仍然很高(图5(a)和5(c))。因此,在混合培养中需要添加额外的碳源来进一步提高氮、磷的去除率。
有研究表明,添加有机碳源会造成不可避免的二次污染[47],并提高废水处理成本。廉价的无机碳源,例如碳酸氢盐(NaHCO3),是产生HCO3−促进雨生红球藻光合营养生长的最佳替代物。因此,为了进一步提高氮磷去除率,本研究在最佳细胞接种比例30∶1的基础上添加NaHCO3进行后续的实验。
2.2 雨生红球藻-真菌DT06混合添加NaHCO3培养
1)添加NaHCO3混合培养对微藻生长的影响。如图7(a)所示,MCK中雨生红球藻生物量在第4天后快速上升,第6天后缓慢上升,最终达到1.36 g·L–1。而添加NaHCO3混合培养过程中雨生红球藻的生物量在第2天后快速上升,第8天后达到稳定期,最终添加0.2、0.4、0.6、0.8 g·L–1 NaHCO3中雨生红球藻的生物量分别为1.58、1.71、1.95、1.44 g·L–1。生长动力学分析结果表明(图7(b)),添加NaHCO3混合培养组中雨生红球藻的比生长速率在第2天上升,并在第4天达到最大值,随后快速下降。添加0.6 g·L–1 NaHCO3中雨生红球藻的比生长速率最高,为0.85 d–1,比MCK(0.51 d–1)高1.66倍。以上结果表明混合培养中添加0.6 g·L–1 的NaHCO3最适合雨生红球藻的生长。
与MCK相比,添加NaHCO3混合培养过程中雨生红球藻的适应期缩短,比生长速率有所升高。这表明NaHCO3产生的HCO3−被雨生红球藻同化为光合底物,从而促进微藻的光合作用。而延长的指数期和比生长速率的下降是由于以下2点:HCO3−的吸收导致pH升高限制了雨生红球藻细胞的生长, 这也是添加0.8 g·L–1 NaHCO3中雨生红球藻的生物量低于添加0.6 g·L–1 NaHCO3的原因(图8);废水中氮、磷质量浓度的下降(图9)导致雨生红球藻细胞生长停止以及孢子的形成(图7)。
2)如图8所示,MCK 中pH在前4 d持续升高,之后稳定在7.3~7.5直到实验结束。由于添加了NaHCO3,添加NaHCO3混合培养过程中初始pH均高于MCK。添加0.2、0.4和0.6 g·L–1 NaHCO3的pH在前4 d逐渐升高,之后保持相对稳定,实验结束时pH分别7.71、8.12和8.55。而添加0.8 g·L–1 NaHCO3的pH持续升高,最终达到10.11。
3)添加NaHCO3混合培养过程中混合培养对氮磷去除的影响。如图9(a)所示,添加NaHCO3混合培养过程中TN质量浓度急剧下降。其中添加0.6 g·L–1 NaHCO3中TN质量浓度下降最快,在第10天达到检出限,达到最高去除率(100%),平均去除速率为55.5 mg·(L·d)–1 (图9(b))。相比之下,添加0.2、0.4、0.8 g·L–1 NaHCO3和MCK中TN质量浓度下降缓慢,最终分别为30.8、10.9、71.5和95.7 mg·L–1。添加0.2、0.4、0.8 g·L–1 NaHCO3和MCK中TN平均去除速率分别为 43.6、45.4、40.4、38.1 mg·(L·d)–1,去除率分别为94.4%、98%、87.1%、82.7%。
TP变化与TN变化规律相似,TP质量浓度在添加0.6 g·L–1 NaHCO3中的第8天便达到检出限,达到最高去除率100%,平均去除速率为8.9 mg·(L·d)–1。而添加0.2、0.4、0.8 g·L–1 NaHCO3和MCK中TP质量浓度在实验结束时分别为3.2、1.9、7.9和11.6 mg·L–1(图9(c))。添加0.2、0.4、0.8 g·L–1 NaHCO3和MCK中TP平均去除速率分别为7.2、7.2、6.9、6.6 mg·(L·d)–1;去除率分别为96.4%、97.9%、91.2%、87.9% (图9(d))。
添加NaHCO3混合培养过程和MCK中TN/TP的变化表明,混合培养中添加NaHCO3促进藻类生长,可提高氮、磷去除率。添加NaHCO3混合培养过程中的TN/TP去除率和平均去除速率(图9(b)和图9(d))与细胞比生长速率和生物量(图7)变化同步,在MC0.6中达到最大值。
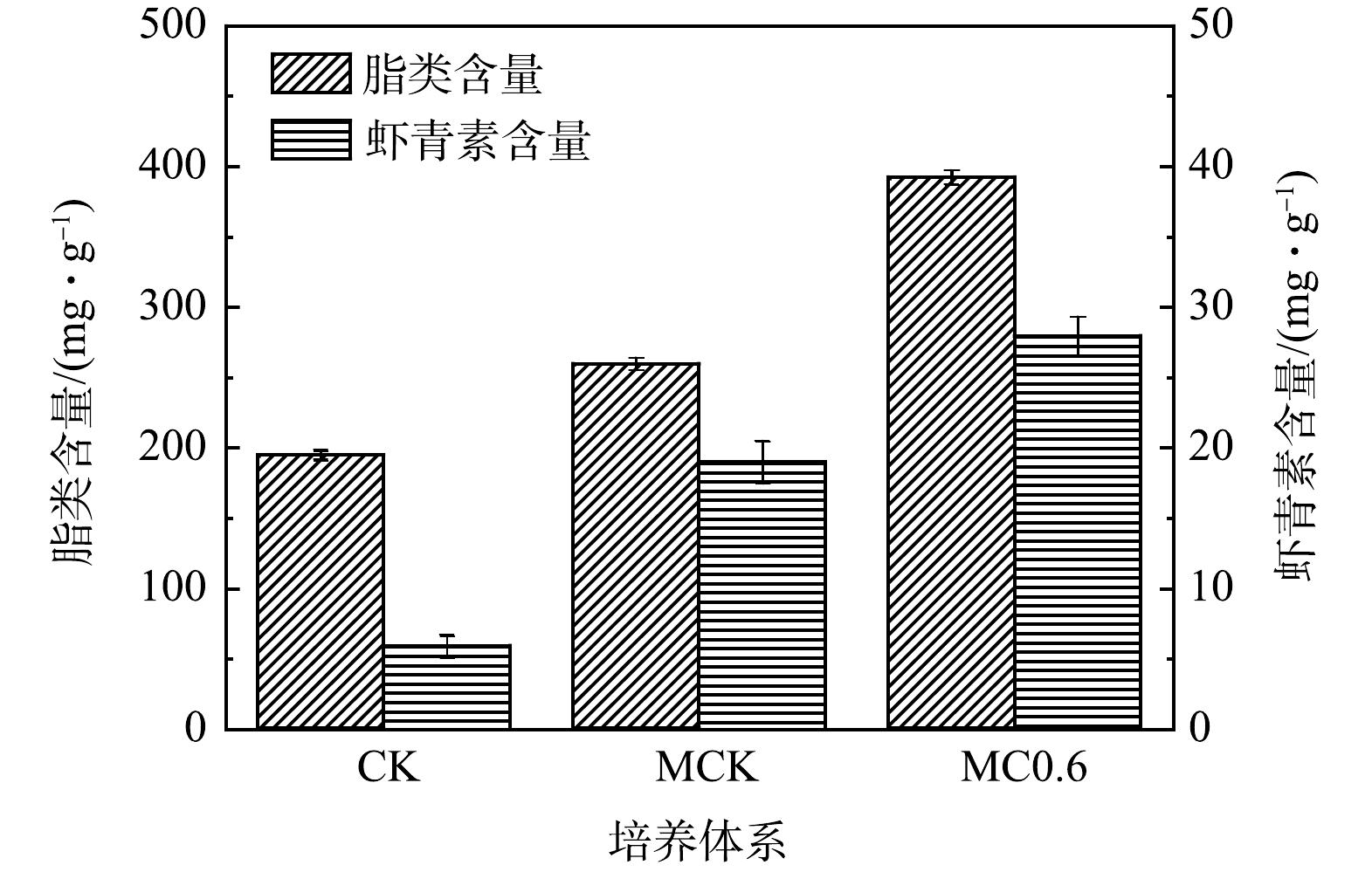
2.3 不同培养体系对脂类和虾青素积累的影响
为了评估不同培养体系对雨生红球藻脂类和虾青素合成的影响,分析比较了雨生红球藻添加0.6 g·L–1 NaHCO3、 30:1(MCK)和CK中的脂类和虾青素含量。如图10所示,添加0.6 g·L–1 NaHCO3中脂类含量最高(392.2 mg·g–1),分别比MCK(259.6 mg·g–1)和CK(194.7 mg·g–1)提高了51.1%和101.4%。添加0.6 g·L–1 NaHCO3中雨生红球藻的虾青素含量达到最高(27.9 mg·g–1),分别是MCK(19.0 mg·g–1)和CK(5.9 mg·g–1)的1.5倍和4.7倍。脂类和虾青素的变化规律相似,主要是由于呈脂溶性虾青素分散在藻类细胞的脂滴中[48],因此,与脂类的合成呈相同的变化趋势(图10)。
与CK和MCK相比,添加0.6 g·L–1 NaHCO3中雨生红球藻的脂类和虾青素含量逐渐增加。主要原因是氮、磷质量浓度的快速下降,尤其是氮(图7(a))。添加0.6 g·L–1 NaHCO3对总氮的快速去除导致早期氮的含量相对不足/缺乏(氮饥饿),使得藻细胞将碳通量引导至脂类合成路径,从而促进脂类和虾青素的合成[48-49]。
3. 结论
1)与雨生红球藻的单独培养(CK)相比,雨生红球藻与DT06混合培养促进了雨生红球藻生长。雨生红球藻与DT06混合培养过程的COD先后均被完全去除,氮、磷的去除效果也得到显著提升。
2)添加NaHCO3的混合培养可进一步促进藻类生长和对氮、磷的去除。在NaHCO3质量浓度为0.6 g·L–1时,雨生红球藻比生长速率达到最高,氮和磷几乎被完全去除,其平均去除速率分别达到55.5 mg·(L·d)–1和8.9 mg·(L·d)–1。
3)在CK、M和MC体系中,MC中雨生红球藻的脂类和虾青素含量最高,分别达到259.6 mg·L–1和27.9 mg·L–1。
-
表 1 4种镧改性海藻对模拟废水中磷吸附的准二级动力学模型和叶诺维奇模型相关参数
Table 1. Parameters of pseudo-second order and Elovich models for phosphorus adsorption in simulationwastewater with the four types of La-seaweeds
吸附剂 准二级动力学模型 叶诺维奇模型 k2 qe R2 αs βs R2 La-LJ 0.10 9.63 0.999 0.56 0.56 0.991 La-UL 0.10 10.48 0.999 0.47 0.47 0.990 La-EP 0.12 8.22 0.999 1.10 1.10 0.977 La-RP 0.11 9.34 0.999 0.65 0.65 0.974 表 2 4种镧改性海藻对模拟废水中磷吸附的粒子内扩散模型相关参数
Table 2. Parameters of intra-particle diffusion model for phosphorous in simulation wastewaterwith the four types of La-seaweeds
吸附剂 第1阶段 第2阶段 第3阶段 kip1 C1 R2 kip2 C2 R2 kip3 C3 R2 La-LJ 0.56 0.65 1 0.40 1.83 0.999 0.13 5.71 0.999 La-UL 0.74 −0.74 1 0.47 1.39 0.999 0.16 5.71 0.999 La-EP 0.43 2.90 1 0.17 4.93 0.973 0.05 6.78 0.999 La-RP 0.68 0.48 1 0.30 3.39 0.959 0.09 6.68 0.999 表 3 4种镧改性海藻对模拟废水中磷的等温吸附模型
Table 3. Isothermal adsorption model parameters for phosphorous in simulation wastewater with the four types of La-seaweeds
材料 Langmuir Freundlich qm/(mg·g-1) ka R2 kf 1/n R2 La-LJ 10.80 0.75 0.89 6.95 0.12 0.96 La-EP 8.94 2.48 0.85 7.56 0.05 0.97 La-RP 11.25 1.02 0.87 7.33 0.14 0.97 La-UL 9.90 5.78 0.77 8.83 0.04 0.94 -
[1] ZHANG X, GUO L, HUANG H, et al. Removal of phosphorus by the core-shell bio-ceramic/Zn-layered double hydroxides (LDHs) composites for municipal wastewater treatment in constructed rapid infiltration system[J]. Water Research, 2016, 96: 280-291. doi: 10.1016/j.watres.2016.03.063 [2] LI J, DAVIS A P. A unified look at phosphorus treatment using bioretention[J]. Water Research, 2016, 90: 141-155. doi: 10.1016/j.watres.2015.12.015 [3] LIU T, CHEN X, WANG X, et al. Highly effective wastewater phosphorus removal by phosphorus accumulating organism combined with magnetic sorbent MFC@La(OH)3[J]. Chemical Engineering Journal, 2018, 335: 443-449. doi: 10.1016/j.cej.2017.10.117 [4] LI R, WANG J J, ZHOU B, et al. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment[J]. Journal of Cleaner Production, 2017, 147: 96-107. doi: 10.1016/j.jclepro.2017.01.069 [5] CHU Y, LI M, LIU J, et al. Molecular insights into the mechanism and the efficiency-structure relationship of phosphorus removal by coagulation[J]. Water Research, 2018, 147: 195-203. doi: 10.1016/j.watres.2018.10.006 [6] HUGGINS T M, HAEGER A, BIFFINGER J C, et al. Granular biochar compared with activated carbon for wastewater treatment and resource recovery[J]. Water Research, 2016, 94: 225-232. doi: 10.1016/j.watres.2016.02.059 [7] YIN H, YUN Y, ZHANG Y, et al. Phosphate removal from wastewaters by a naturally occurring, calcium-rich sepiolite[J]. Journal of Hazardous Materials, 2011, 198: 362-369. doi: 10.1016/j.jhazmat.2011.10.072 [8] WAN C, DING S, ZHANG C, et al. Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application[J]. Separation and Purification Technology, 2017, 180: 1-12. doi: 10.1016/j.seppur.2017.02.031 [9] JIANG Y, LI A, DENG H, et al. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks[J]. Bioresource Technology, 2019, 276: 183-189. doi: 10.1016/j.biortech.2018.12.079 [10] VIEIRA B R C, PINTOR A M A, BOAVENTURA R A R, et al. Arsenic removal from water using iron-coated seaweeds[J]. Journal of Environmental Management, 2017, 192: 224-233. [11] RATHOD M, MODY K, BASHA S. Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii[J]. Journal of Cleaner Production, 2014, 84: 484-493. doi: 10.1016/j.jclepro.2014.03.064 [12] PANDIMURUGAN R, THAMBIDURAI S. Synthesis of seaweed-ZnO-PANI hybrid composite for adsorption of methylene blue dye[J]. Journal of Environmental Chemical Engineering, 2016, 4(1): 1332-1347. doi: 10.1016/j.jece.2016.01.030 [13] HASSELSTRÖM L, VISCH W, GRÖNDAHL F, et al. The impact of seaweed cultivation on ecosystem services: A case study from the west coast of Sweden[J]. Marine Pollution Bulletin, 2018, 133: 53-64. doi: 10.1016/j.marpolbul.2018.05.005 [14] FU H, YANG Y, ZHU R, et al. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum-decorated magnetite[J]. Journal of Colloid and Interface Science, 2018, 530: 704-713. doi: 10.1016/j.jcis.2018.07.025 [15] DONG S, WANG Y, ZHAO Y, et al. La3+/La(OH)3 loaded magnetic cationic hydrogel composites for phosphate removal: Effect of lanthanum species and mechanistic study[J]. Water Research, 2017, 126: 433-441. doi: 10.1016/j.watres.2017.09.050 [16] DING S, SUN Q, CHEN X, et al. Synergistic adsorption of phosphorus by iron in lanthanum modified bentonite (Phoslock®): New insight into sediment phosphorus immobilization[J]. Water Research, 2018, 134: 32-43. doi: 10.1016/j.watres.2018.01.055 [17] XIE J, WANG Z, FANG D, et al. Green synthesis of a novel hybrid sorbent of zeolite/lanthanum hydroxide and its application in the removal and recovery of phosphate from water[J]. Journal of Colloid and Interface Science, 2014, 423: 13-19. doi: 10.1016/j.jcis.2014.02.020 [18] 刘志超, 史晓燕, 李艳根, 等. 镧改性粉煤灰及其脱氮除磷效果研究[J]. 化工新型材料, 2018, 46(2): 205-208. [19] 刘香玉, 孙娟, 赵朝成, 等. CTAB改性膨润土制备及其对海洋溢油的吸附性能[J]. 环境工程学报, 2019, 13(1): 68-78. doi: 10.12030/j.cjee.201808021 [20] 张亚峰, 安路阳, 尚书, 等. 废玻璃/铝渣人工沸石对水中Ca2+的吸附[J]. 环境工程学报, 2019, 13(1): 49-61. doi: 10.12030/j.cjee.201806101 [21] NOVAIS S V, ZENERO M D O, TRONTO J, et al. Poultry manure and sugarcane straw biochars modified with MgCl2 for phosphorus adsorption[J]. Journal of Environmental Management, 2018, 214: 36-44. [22] 胡家朋, 吴代赦, 刘瑞来, 等. 羟基镧改性树脂的制备及其对氟离子的吸附[J]. 安全与环境学报, 2016, 16(5): 237-241. [23] ZHOU A, ZHU C, CHEN W, et al. Phosphorus recovery from water by lanthanum hydroxide embedded interpenetrating network poly (vinyl alcohol)/sodium alginate hydrogel beads[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 554: 237-244. [24] 林丽丹, 王哲, 顾伟, 等. 沸石/水合氧化锆吸附水中的磷[J]. 环境工程学报, 2017, 11(2): 702-708. doi: 10.12030/j.cjee.201509064 [25] 杨金梅, 吕建波, 李莞璐, 等. 壳聚糖载纳米羟基氧化铁对水中磷的吸附[J]. 环境工程学报, 2018, 12(5): 1286-1294. doi: 10.12030/j.cjee.201710002 [26] LI Y, SONG S, XIA L, et al. Enhanced Pb(II) removal by algal-based biosorbent cultivated in high-phosphorus cultures[J]. Chemical Engineering Journal, 2019, 361: 167-179. doi: 10.1016/j.cej.2018.12.070 [27] LUO H, ZENG X, LIAO P, et al. Phosphorus removal and recovery from water with macroporous bead adsorbent constituted of alginate-Zr4+ and PNIPAM-interpenetrated networks[J]. International Journal of Biological Macromolecules, 2019, 126: 1133-1144. doi: 10.1016/j.ijbiomac.2018.12.269 [28] HUANG W, LI D, LIU Z, et al. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents[J]. Chemical Engineering Journal, 2014, 236: 191-201. doi: 10.1016/j.cej.2013.09.077 [29] 石稳民, 付有为, 许智, 等. 镧负载多孔陶粒用于低浓度含磷废水的处理[J]. 环境科学与技术, 2018, 41(11): 110-114. [30] 崔有为, 李杰, 杜兆富. 镧、铈改性沸石对污水除磷性能和机制的比较研究[J]. 中国稀土学报, 2016, 34(4): 460-468. [31] 施川, 张盼月, 郭建斌, 等. 污泥生物炭的磷吸附特性[J]. 环境工程学报, 2016, 10(12): 7202-7208. doi: 10.12030/j.cjee.201508021 -






 下载:
下载: