-
中国是仅次于巴西的世界第二大铁矿石生产国。作为铁矿石生产的副产品,铁尾矿的大量储存对周边环境具有很大的威胁和危害,而环境责任和矿产资源的可持续发展对于采矿业是一个至关重要的问题[1]。据估算,每生产1 t铁精矿就会产生约2.5~3.0 t铁尾矿。截至2013年,我国铁尾矿总量已达到50×108 t,且其数量正不断增加[2]。长期以来,通过堆放和掩埋的方式处理铁尾矿,不仅占用了很大的空间,而且耗费了大量的处理费用[3]。随着我国矿山资源日渐枯竭,矿山尾矿对环境污染的问题日趋严重[4],铁尾矿减量化、无害化和资源化利用迫在眉睫。提取和回收尾矿中的重金属不仅可以实现较高的经济效益,同时也有利于尾矿的无害化处理,具有很好的环境效益,故一直以来受到了广泛关注[5-8]。
近年来,氯化焙烧在城市固体垃圾[9]、污泥飞灰[10]、垃圾焚烧飞灰[11-12]、半导体材料[13]等固体废物中重金属挥发特性方面已有广泛研究。在CaCl2热分解机理和氯化焙烧工艺的研究方面,FRAISSLER等[14]指出,在干燥和潮湿空气条件下,氯的释放发生在CaCl2熔点以上(782 ℃)。在干空气条件下,与O2反应生成Cl2;在湿空气条件下,与H2O反应生成HCl。NOWAK等[9]分别对氮气和空气条件下的氯化挥发进行了热力学平衡计算,发现CaCl2反应释放了Cl2和HCl,Cl2和HCl可进一步与金属再发生氯化反应,是间接氯化反应。YU等[15]发现,相比氧化焙烧,氯化焙烧能在较低温度下挥发重金属。JIAO等[16]发现,CaCl2的添加对飞灰中Cs的挥发效果很好。常耀超等[17]采用氯化焙烧工艺从硫铁矿烧渣中回收有价金属,在焙烧温度为1 250 ℃、焙烧时间为1 h条件下,Cu、Pb、Zn的挥发率分别达到73.1%、93.9%和75.2%。
氯化焙烧受焙烧温度、焙烧时间、焙烧气氛等因素影响。许多研究表明,氯化焙烧可实现重金属的挥发[9-13],可以去除和回收固体废物中的重金属。但目前对氯化焙烧的研究多集中在焙烧温度、氯化剂添加比及焙烧时间的影响上,对于氮气等无氧条件下及空气等有氧条件下CaCl2的热行为的对比实验研究还比较缺乏,对焙烧气氛及焙烧后氯化产物的回收处理方面仍存在不足[18]。本研究对CaCl2和铁尾矿进行氯化焙烧,探究了CaCl2分别在氮气和干空气条件下热处理过程中的热行为和分解机理,并研究了焙烧气氛及焙烧温度对铁尾矿中Cu、Pb、Zn的去除效果的影响及冷凝和吸收液洗涤吸收对挥发烟气中重金属的回收效果。
-
本研究以广东省某山区铁尾矿为研究对象,该铁尾矿主要由硫铁矿、针铁矿、石英组成,含有Fe、Si、Al、S、Cu等元素。以氧化物形式体现各元素含量时,Fe2O3、SiO2、Al2O3和SO3分别占58.45%、15.78%、8.36%和3.35%,Cu、Pb、Zn、Cr、Cd的浓度如表1所示。实验前在通风条件下对铁尾矿样品进行风干,然后在烘箱中105 ℃烘干,直到重量不变,放入样品袋中待用。
-
本研究采用管式炉进行热处理实验,实验装置如图1所示。氯化焙烧实验装置包括供气部分、管式炉和吸收烟气中重金属的尾端部分。实验室管式电炉(SK2-2-130,天津中环试验电炉有限公司)由管径80 mm,长度120 cm的刚玉管和加热器组成。
-
为使铁尾矿样品和无水CaCl2充分混合,本研究采用湿混的方式对样品进行混合。每次实验做2 个平行样,每个样品取CaCl2和铁尾矿样品共20 g,与20 mL纯水混合,并在干燥箱中于105 ℃温度下干燥至恒重[14],时间为12 h。将烘干后的样品在干燥器中冷却至室温,从出气端放入管式炉,并推送到中间加热部分,在800 ℃以下时,按照5 ℃·min−1的速率升温和降温,在800~1 000 ℃时,按照3 ℃·min−1的速率升温和降温。在焙烧温度下,保温60 min。当温度降至100 ℃后,从刚玉管中取出样品,然后在干燥器内冷却至室温。通过分析样品中金属元素的初始量和残留量,按式(1)计算各金属元素的挥发率。
式中:α为挥发率;C为铁尾矿中金属浓度,mg·kg−1;M为铁尾矿添加量,g;c为焙烧后残渣中金属浓度,mg·kg−1;m为焙烧后残渣质量,g。
-
采用热重分析法(NETZSCH TG 209 F1,德国NETZSCH公司)测定无水氯化钙(CaCl2,AR)的热重变化(升温速率:10 ℃·min−1;气氛:干空气和氮气(99.9%));采用X射线荧光光谱(XRF)(EDX-7000,日本岛津公司)鉴定热处理后的铁尾矿残渣的主要成分;采用X射线衍射仪(XRD)(D8 ADVANCE,德国Bruker公司)进行结晶化合物的测定;用6 mL 65%硝酸(AR,HNO3)、2 mL 35%盐酸(AR,HCl)和3 mL 40%氢氟酸(AR,HF)在微波消解仪(MARS6,美国CEM公司)中消解后,用纯水稀释至50 mL,采用火焰原子吸收分光光度计(Z2000,日本日立公司)测定重金属的浓度。
-
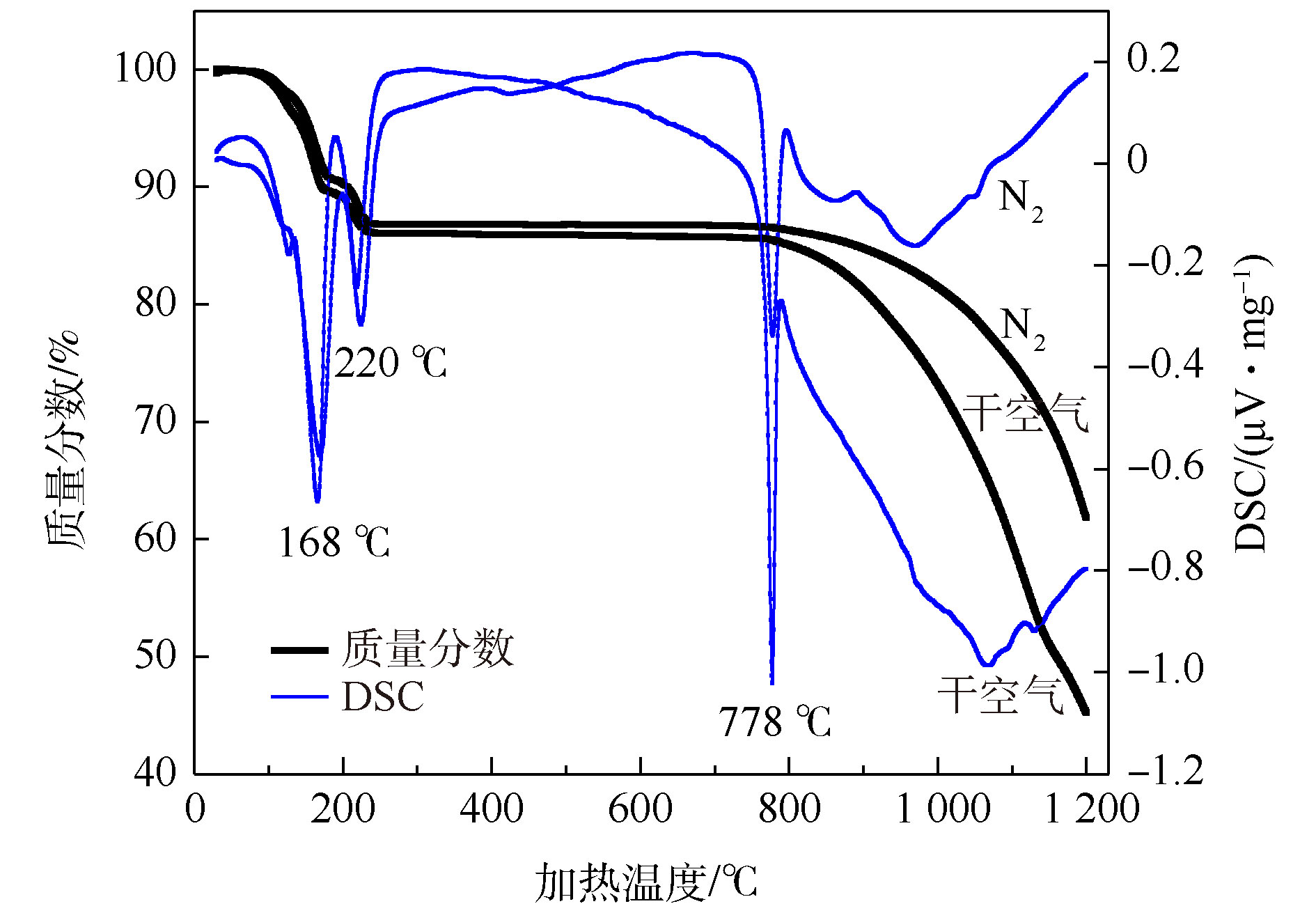
在氮气和干空气条件下,通过热重分析对无水CaCl2加热过程中重量损失和吸放热情况进行分析,结果如图2所示。从图2可以看出,无水CaCl2在168、220、778 ℃时有明显的吸热峰,并伴随着较大幅度的重量损失。在100~168 ℃时,自由水的蒸发导致重量损失并吸收热量;在220 ℃左右时,再次出现重量损失及热量的吸收,应是CaCl2水合物的分解所致;778 ℃是CaCl2的熔点,熔融过程中会吸收大量热量,故出现明显的吸热峰,CaCl2熔融后,开始分解并释放Cl,导致了大幅度的重量损失[14];在温度达到778 ℃后,空气气氛下失重幅度相对较大,吸热峰也相对明显,这可能是因为空气中O2的存在,增加了Cl的释放,且与CaCl2、与H2O和O2均发生反应[14],增加了热量吸收。
为进一步分析CaCl2在热处理过程中的热行为,分别对氮气条件下200、600、1 000 ℃和干空气条件下600、1 000 ℃热处理后的CaCl2残渣进行XRD分析。预处理方法步骤及热处理升、降温程序设置等与铁尾矿样品氯化焙烧实验相同。
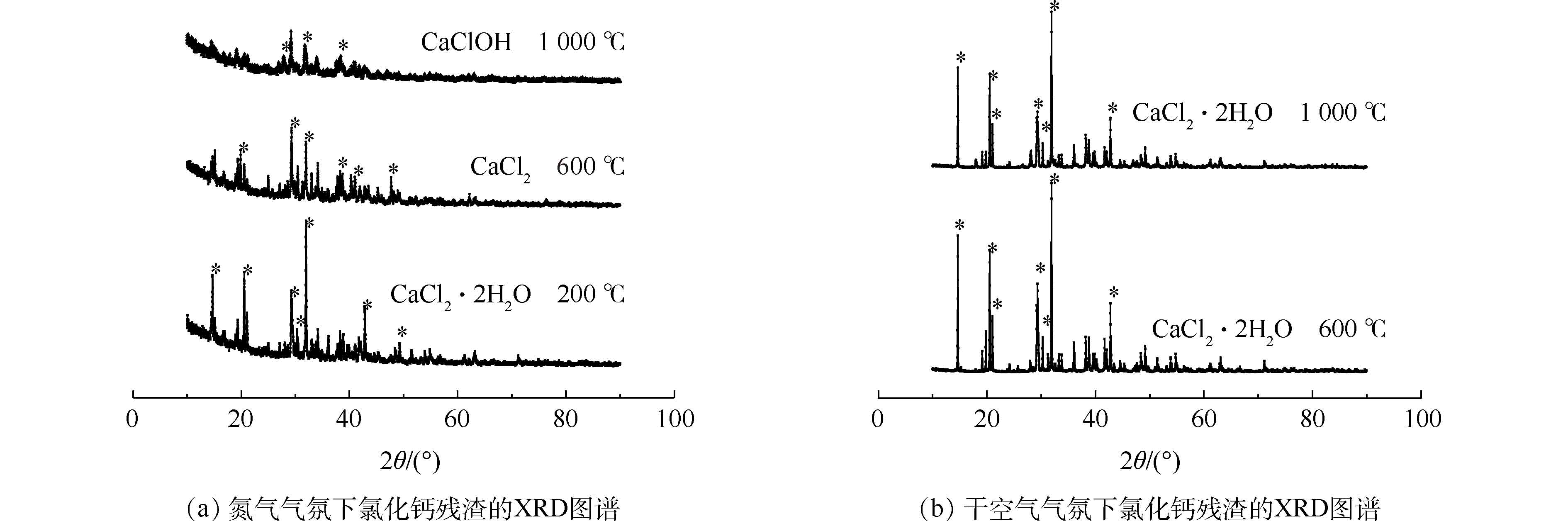
在氮气和空气条件下,热处理后CaCl2残渣的XRD图谱见图3(a)和图3(b)。可以看出,温度升至600 ℃和1 000 ℃后,氮气和空气条件下所得残渣有较大差异。从图3(a)可以看出,在氮气条件下,CaCl2在200 ℃焙烧1 h后的残渣主要成分是CaCl2·2H2O。这是因为预处理时将CaCl2按质量比1∶1溶于水中,所得CaCl2溶液烘干到质量分数超过70%后生成CaCl2·2H2O [19]。在600 ℃焙烧后的残渣中主要成分是CaCl2。可能是温度达到260 ℃后继续升温,CaCl2结晶水逐渐分解形成无水CaCl2[14]。778 ℃是CaCl2的熔点,而CaCl2在1 000 ℃热处理后残渣的主要物质是CaClOH。由此可以推断,778 ℃ CaCl2达到熔点后,与残余H2O反应,生成CaClOH并释放HCl,这与CaCl2·6H2O的分解机理相近[14]。如图3(b)所示,在干空气条件下,进行600 ℃和1 000 ℃热处理后,残渣主要成分均是CaCl2·2H2O,只是1 000 ℃残渣衍射峰强度较低。相比氮气条件下,H2O的损失不明显,这可能是因为CaCl2在H2O和O2存在的情况下,可以分别与H2O和O2反应,放出HCl和Cl2[14],空气中O2的存在减少了H2O的消耗。
-
NOWAK等[9]研究指出,CaCl2和MgCl2作为氯化剂时,效果优于NaCl,在1 000 ℃下焙烧60 min,80%~90% Cu、40% Zn和将近100% Pb挥发出去。以CaCl2为氯化剂,添加量为150 g·kg−1时,各温度下Zn、Cu、Pb的挥发率基本达到最高。在以MgCl2为氯化剂时,Pb在1 000 ℃下焙烧1 h可实现最大程度的挥发[20]。因此,在已有研究基础上,本研究选择在CaCl2添加量为15%、焙烧时间为1 h条件下进行氯化焙烧实验。因需对挥发物进行收集,本研究根据实验需要将气体流量选定为100 mL·min−1。
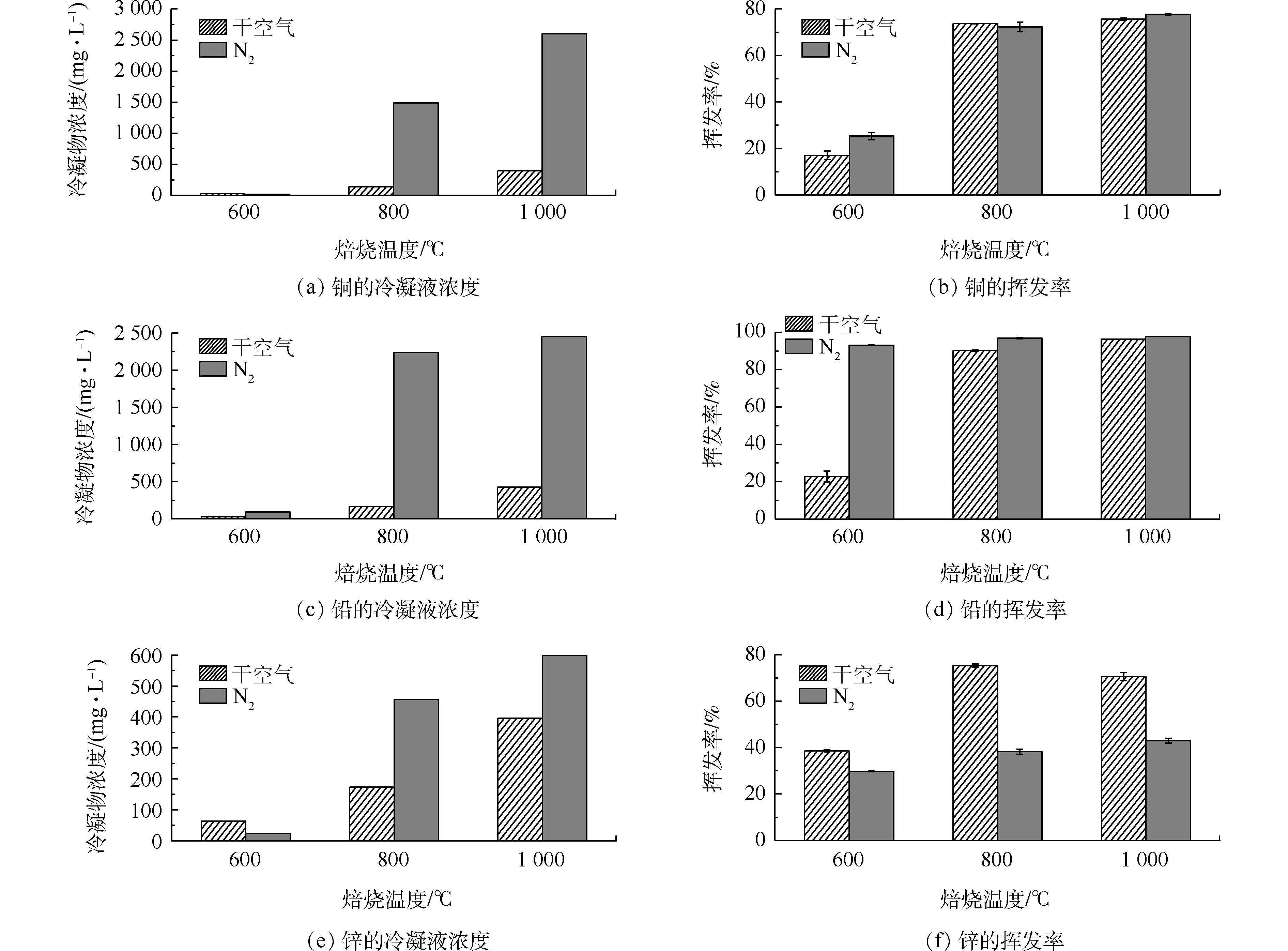
图4(b)、图4(d)、图4(f)为不同气氛、不同温度体系下铁尾矿样品中重金属Cu、Pb、Zn的挥发效果,图4(a)、图4(c)、图4(e)为对应焙烧温度和焙烧气氛条件下的冷凝收集液浓度。从图4(b)和图4(d)中可以看出,对于Cu和Pb,在氮气条件和干空气条件下,挥发率均随着温度的升高而增大,且整体而言,氮气条件下的挥发效果优于干空气条件。Cu的挥发率在600 ℃升至800 ℃时有较为明显的升高,而800 ℃到1 000 ℃挥发率上升程度较小,且各温度下Cu在2种气氛下挥发率相差很小,而氮气条件下Pb在600 ℃时挥发率达到90%,远远超过干空气条件;当温度升至1 000 ℃,氮气和干空气条件下Cu的挥发率分别达到77.7%和75.7%,Pb的挥发率分别达到97.8%和96.4%。在800~1 000 ℃时,2种气氛下Pb的挥发率均超过95%,这与Pb是易挥发性金属元素有关[9]。由此可见,焙烧气氛对Cu的挥发效果影响不大,但对较低温度时Pb的挥发率影响较大;在温度较低时,温度的升高对提高Cu和Pb的挥发率效果较为明显。除800 ℃时Cu在氮气条件下的挥发率略低于空气条件外,整体而言,当固体氯化剂CaCl2用于焙烧铁尾矿的复杂体系中时,氮气+CaCl2条件也优于干空气+CaCl2条件,这与Cu和Pb在氮气-HCl气氛下焙烧的挥发率高于空气-HCl气氛[21]的结论相近。
从图4(f)可以看出,焙烧温度和焙烧气氛对Zn的影响较为明显。在干空气条件下,Zn在焙烧温度为800 ℃时挥发效果最佳,挥发率为75.3%。在氮气条件下,Zn的挥发率随着温度的升高而升高,在1 000 ℃最高达到38.2%,而且在各个温度下的挥发率均远远低于干空气条件下的挥发率,这与氮气-HCl气氛和空气-HCl气氛对比研究的结果[21]不同,其可能的原因是空气-CaCl2气氛下会生成Cl2,而Cl2对于金属的氯化效果好于HCl[14]。与Cu和Pb相比,Zn的挥发率较低,这与Zn的氯化物本身的蒸气压较高有关[21];同时,尾矿中SiO2、Al2O3的大量存在,会使Zn在反应过程中生成硅酸锌、铝酸盐等,降低了挥发率[22]。
-
S元素与Cl元素影响着氯化焙烧过程中重金属的挥发[23],但铁尾矿中S元素和引入的Cl元素的物态变化及迁移转化也可能对环境造成污染。因此,有必要对不同焙烧条件下的焙烧渣进行分析,考察其基本元素组成。
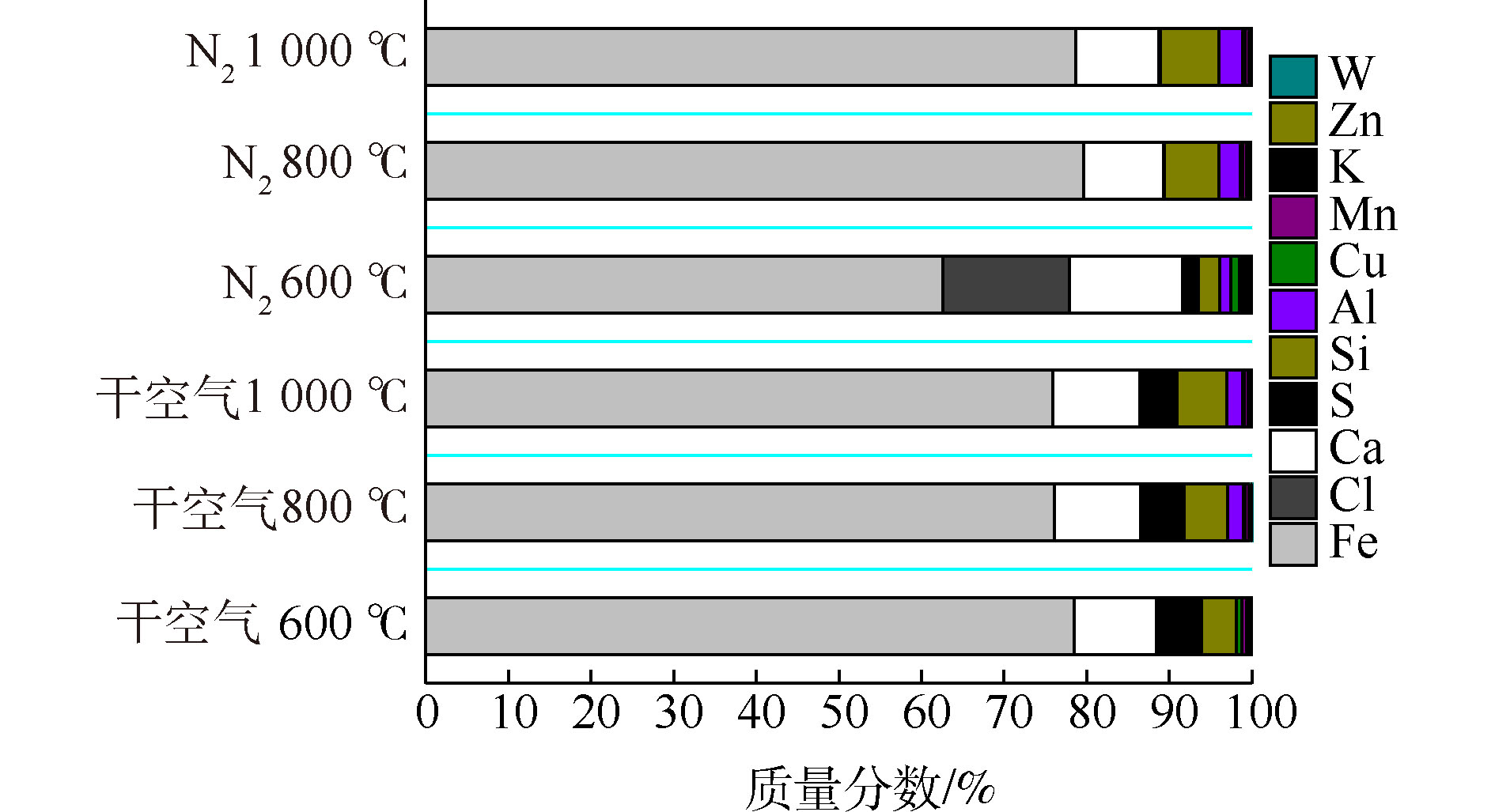
不同焙烧条件下的焙烧渣的成分组成如图5所示。可以看出,氮气条件下焙烧渣中S元素的残留量比干空气条件低。在干空气条件下,S元素质量分数随着温度的增高逐渐降低,但仍是焙烧渣的主要组成元素。这可能是因为,在空气气氛下焙烧铁尾矿的过程中,CaCl2与空气中的O2以及氧化生成的SO2反应生成CaSO4,残留在焙烧渣中[24]。在氮气条件下,600 ℃焙烧后仍残留很多Cl元素和Ca元素,证明了上述氮气条件下CaCl2的分解机理;在800、1 000 ℃下进行氯化焙烧后,残渣中未检测到Cl元素和S元素,主要为Fe、Ca、Si元素及少量的Cu、Zn等元素。
-
采用冷凝管冷凝收集和湿法洗涤回收方式对烟气中挥发成分进行收集和分析,对冷凝收集液进行XRF分析,结果如表2所示。结合图4(a)、图4(c)、图4(e),可以看出,冷凝收集方法对不同气氛下挥发物的收集效果有较大的差异。在同种气氛下,随着温度的升高,冷凝液中各金属浓度随之增高;而同一焙烧温度下,氮气气氛下收集物中各重金属浓度高于干空气条件,尤其是在800 ℃和1 000 ℃条件下,氮气和干空气条件下Pb、Cu的挥发率相近,但冷凝液中相应重金属浓度相差很大,说明用冷凝的方法收集氮气条件下的氯化焙烧挥发物更加有效。
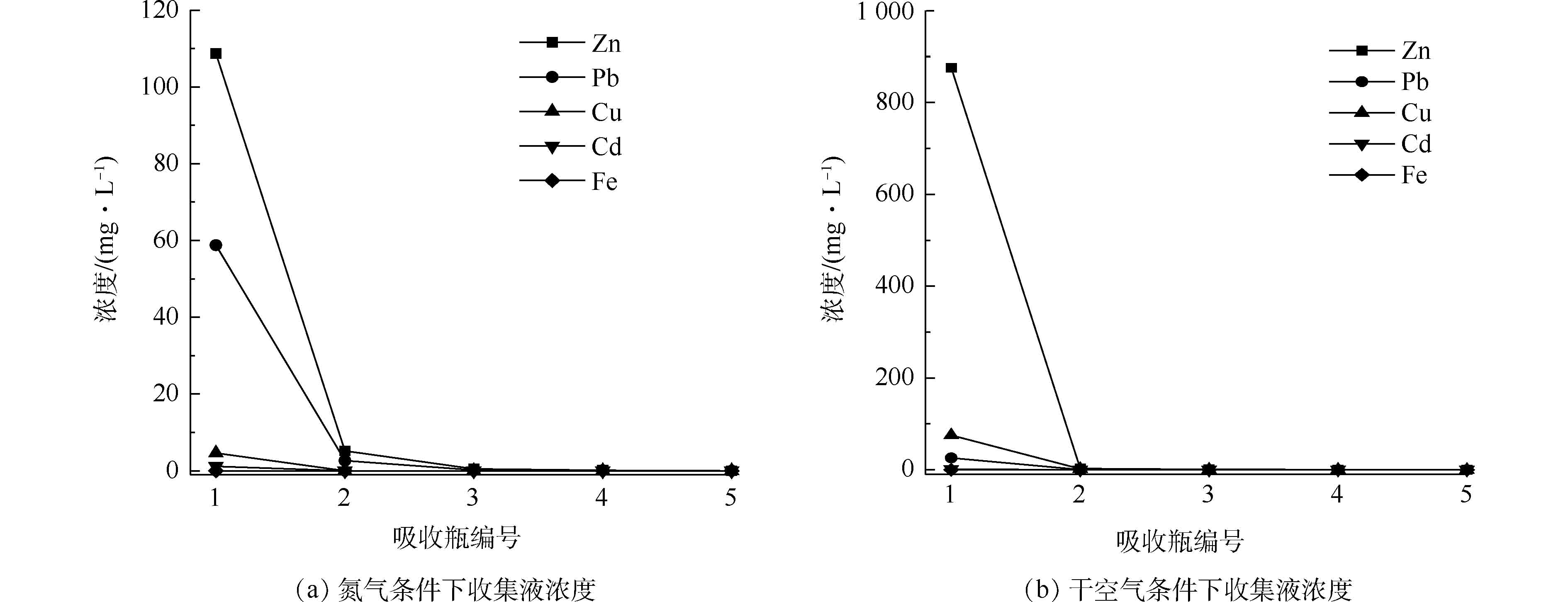
为探究湿法收集方式对氮气和干空气焙烧气氛下的烟气中各元素的回收效果,本研究对烟气端连续5个盛有200 mL 5%稀硝酸的吸收瓶中重金属浓度进行检测,结果如图6所示。可以看出,干空气条件下吸收瓶中金属浓度较高。第3个及以后的吸收瓶基本未检测到各金属元素,由此可见,挥发出的各金属元素在前2个吸收瓶中基本被完全吸收。由第1个吸收瓶中各金属元素的浓度可以看出,吸收瓶中金属Zn、Cu、Pb浓度较高,而Cd、Fe元素浓度很低,这是因为铁尾矿中本身Cd含量较低,而Fe元素属于难氯化挥发金属。整体对比发现,干空气气氛下氯化焙烧的挥发物更易于被湿法洗涤收集。
-
1)在氮气条件下,CaCl2反应生成CaClOH释放Cl;在干空气条件下,CaCl2分别与H2O和O2反应释放Cl。上述反应过程仍需进一步探究。
2)铁尾矿中重金属Cu、Pb、Zn的挥发率Pb>Cu>Zn,且随着温度的升高逐渐增大。
3)选择在1 000 ℃、氮气气氛下对铁尾矿进行氯化焙烧,Cu和Pb可以分别达到80%和90%以上的去除率,选用冷凝方法回收挥发物效果更好;在800℃,选择干空气条件下焙烧,Zn可以实现75%以上的挥发率,并适合采用湿法洗涤吸收方式进行收集。Fe元素在所有实验条件下的收集效果均较差。
4)在氮气条件下,氯化焙烧后的残渣相对于干空气气氛,S元素的残留量低,对环境的潜在威胁较小,更易于处置。
CaCl2氯化焙烧回收铁尾矿中的重金属Cu、Pb、Zn
Recovering Cu, Pb and Zn in iron tailings by chlorination roasting with CaCl2
-
摘要: 针对铁尾矿资源化利用问题,通过添加CaCl2对铁尾矿进行氯化焙烧。使用X射线衍射仪、热重分析法对CaCl2进行了分析,采用原子吸收光谱法、X射线荧光分析对焙烧渣和收集物进行了分析;探究了氮气和干空气条件下CaCl2的作用机理;研究了焙烧气氛和温度对铁尾矿中Cu、Pb、Zn的挥发效果的影响及不同方法对挥发物的收集效果。结果表明:在氮气条件下,CaCl2在反应后生成了CaClOH且释放Cl;在空气条件下,CaCl2分别与H2O和O2反应释放Cl;重金属Cu、Pb、Zn的挥发率次序为Pb>Cu>Zn,且随着温度的升高而增大;在氮气条件下,Pb的挥发效果优于干空气条件,Zn与之相反,而焙烧气氛对Cu的挥发效果影响较小;氮气条件下的烟气中重金属更易于被冷凝收集,干空气条件下的烟气中重金属更易于被湿法洗涤吸收。氯化焙烧处理后,铁尾矿中Cu、Pb、Zn均实现了很大程度的挥发。Abstract: In view of the resource utilization of iron tailings, the iron tailings were chlorination roasted by adding CaCl2. X-ray diffraction analysis and thermogravimetric analysis were used to analyze CaCl2, and atomic absorption spectrometry and X-ray fluorescence were used to analyze the roasted slag and collected materials. The CaCl2 action mechanisms under nitrogen and dry air conditions were investigated, the effects of roasting atmosphere and temperature on the volatilization rates of Cu, Pb and Zn in iron tailings, as well as the effects of different collection methods on the collection rates were studied. The results show that the CaCl2 reaction could generate Ca(OH)Cl and release Cl in nitrogen atmosphere, while CaCl2 reacted with O2 or H2O to release Cl in the dry air. The order of heavy metal volatilization rates was Pb>Cu>Zn, and the rates increased with the increase of temperature. For Pb volatile effect, nitrogen atmosphere was better than air atmosphere, which was contrary for Zn volatile effect, while roasting atmosphere has little influence on Cu volatile effect. In nitrogen atmosphere, the heavy metals in the flue gas were more easy to be collected by condensation, while in the circumstance of dry air, the heavy metals in the flue gas were more easy to be recovered by wet-washing and absorption. After chlorinated roasting, large part of Cu, Pb and Zn in iron tailings volatilized.
-
Key words:
- metal ore /
- iron tailings /
- chlorination roasting /
- volatilization rate /
- heavy metal /
- solid waste
-
中国是仅次于巴西的世界第二大铁矿石生产国。作为铁矿石生产的副产品,铁尾矿的大量储存对周边环境具有很大的威胁和危害,而环境责任和矿产资源的可持续发展对于采矿业是一个至关重要的问题[1]。据估算,每生产1 t铁精矿就会产生约2.5~3.0 t铁尾矿。截至2013年,我国铁尾矿总量已达到50×108 t,且其数量正不断增加[2]。长期以来,通过堆放和掩埋的方式处理铁尾矿,不仅占用了很大的空间,而且耗费了大量的处理费用[3]。随着我国矿山资源日渐枯竭,矿山尾矿对环境污染的问题日趋严重[4],铁尾矿减量化、无害化和资源化利用迫在眉睫。提取和回收尾矿中的重金属不仅可以实现较高的经济效益,同时也有利于尾矿的无害化处理,具有很好的环境效益,故一直以来受到了广泛关注[5-8]。
近年来,氯化焙烧在城市固体垃圾[9]、污泥飞灰[10]、垃圾焚烧飞灰[11-12]、半导体材料[13]等固体废物中重金属挥发特性方面已有广泛研究。在CaCl2热分解机理和氯化焙烧工艺的研究方面,FRAISSLER等[14]指出,在干燥和潮湿空气条件下,氯的释放发生在CaCl2熔点以上(782 ℃)。在干空气条件下,与O2反应生成Cl2;在湿空气条件下,与H2O反应生成HCl。NOWAK等[9]分别对氮气和空气条件下的氯化挥发进行了热力学平衡计算,发现CaCl2反应释放了Cl2和HCl,Cl2和HCl可进一步与金属再发生氯化反应,是间接氯化反应。YU等[15]发现,相比氧化焙烧,氯化焙烧能在较低温度下挥发重金属。JIAO等[16]发现,CaCl2的添加对飞灰中Cs的挥发效果很好。常耀超等[17]采用氯化焙烧工艺从硫铁矿烧渣中回收有价金属,在焙烧温度为1 250 ℃、焙烧时间为1 h条件下,Cu、Pb、Zn的挥发率分别达到73.1%、93.9%和75.2%。
氯化焙烧受焙烧温度、焙烧时间、焙烧气氛等因素影响。许多研究表明,氯化焙烧可实现重金属的挥发[9-13],可以去除和回收固体废物中的重金属。但目前对氯化焙烧的研究多集中在焙烧温度、氯化剂添加比及焙烧时间的影响上,对于氮气等无氧条件下及空气等有氧条件下CaCl2的热行为的对比实验研究还比较缺乏,对焙烧气氛及焙烧后氯化产物的回收处理方面仍存在不足[18]。本研究对CaCl2和铁尾矿进行氯化焙烧,探究了CaCl2分别在氮气和干空气条件下热处理过程中的热行为和分解机理,并研究了焙烧气氛及焙烧温度对铁尾矿中Cu、Pb、Zn的去除效果的影响及冷凝和吸收液洗涤吸收对挥发烟气中重金属的回收效果。
1. 材料与方法
1.1 实验原料
本研究以广东省某山区铁尾矿为研究对象,该铁尾矿主要由硫铁矿、针铁矿、石英组成,含有Fe、Si、Al、S、Cu等元素。以氧化物形式体现各元素含量时,Fe2O3、SiO2、Al2O3和SO3分别占58.45%、15.78%、8.36%和3.35%,Cu、Pb、Zn、Cr、Cd的浓度如表1所示。实验前在通风条件下对铁尾矿样品进行风干,然后在烘箱中105 ℃烘干,直到重量不变,放入样品袋中待用。
表 1 铁尾矿中重金属浓度Table 1. Heavy metal concentration in iron tailingsmg·kg−1 Cu Pb Zn Cr Cd 4 568.00 1 761.68 2 518.70 85.81 2.81 1.2 实验装置
本研究采用管式炉进行热处理实验,实验装置如图1所示。氯化焙烧实验装置包括供气部分、管式炉和吸收烟气中重金属的尾端部分。实验室管式电炉(SK2-2-130,天津中环试验电炉有限公司)由管径80 mm,长度120 cm的刚玉管和加热器组成。
1.3 实验方法
为使铁尾矿样品和无水CaCl2充分混合,本研究采用湿混的方式对样品进行混合。每次实验做2 个平行样,每个样品取CaCl2和铁尾矿样品共20 g,与20 mL纯水混合,并在干燥箱中于105 ℃温度下干燥至恒重[14],时间为12 h。将烘干后的样品在干燥器中冷却至室温,从出气端放入管式炉,并推送到中间加热部分,在800 ℃以下时,按照5 ℃·min−1的速率升温和降温,在800~1 000 ℃时,按照3 ℃·min−1的速率升温和降温。在焙烧温度下,保温60 min。当温度降至100 ℃后,从刚玉管中取出样品,然后在干燥器内冷却至室温。通过分析样品中金属元素的初始量和残留量,按式(1)计算各金属元素的挥发率。
α=(1−cmCM)×100% (1) 式中:α为挥发率;C为铁尾矿中金属浓度,mg·kg−1;M为铁尾矿添加量,g;c为焙烧后残渣中金属浓度,mg·kg−1;m为焙烧后残渣质量,g。
1.4 分析方法
采用热重分析法(NETZSCH TG 209 F1,德国NETZSCH公司)测定无水氯化钙(CaCl2,AR)的热重变化(升温速率:10 ℃·min−1;气氛:干空气和氮气(99.9%));采用X射线荧光光谱(XRF)(EDX-7000,日本岛津公司)鉴定热处理后的铁尾矿残渣的主要成分;采用X射线衍射仪(XRD)(D8 ADVANCE,德国Bruker公司)进行结晶化合物的测定;用6 mL 65%硝酸(AR,HNO3)、2 mL 35%盐酸(AR,HCl)和3 mL 40%氢氟酸(AR,HF)在微波消解仪(MARS6,美国CEM公司)中消解后,用纯水稀释至50 mL,采用火焰原子吸收分光光度计(Z2000,日本日立公司)测定重金属的浓度。
2. 结果与讨论
2.1 CaCl2热行为机理研究
在氮气和干空气条件下,通过热重分析对无水CaCl2加热过程中重量损失和吸放热情况进行分析,结果如图2所示。从图2可以看出,无水CaCl2在168、220、778 ℃时有明显的吸热峰,并伴随着较大幅度的重量损失。在100~168 ℃时,自由水的蒸发导致重量损失并吸收热量;在220 ℃左右时,再次出现重量损失及热量的吸收,应是CaCl2水合物的分解所致;778 ℃是CaCl2的熔点,熔融过程中会吸收大量热量,故出现明显的吸热峰,CaCl2熔融后,开始分解并释放Cl,导致了大幅度的重量损失[14];在温度达到778 ℃后,空气气氛下失重幅度相对较大,吸热峰也相对明显,这可能是因为空气中O2的存在,增加了Cl的释放,且与CaCl2、与H2O和O2均发生反应[14],增加了热量吸收。
为进一步分析CaCl2在热处理过程中的热行为,分别对氮气条件下200、600、1 000 ℃和干空气条件下600、1 000 ℃热处理后的CaCl2残渣进行XRD分析。预处理方法步骤及热处理升、降温程序设置等与铁尾矿样品氯化焙烧实验相同。
在氮气和空气条件下,热处理后CaCl2残渣的XRD图谱见图3(a)和图3(b)。可以看出,温度升至600 ℃和1 000 ℃后,氮气和空气条件下所得残渣有较大差异。从图3(a)可以看出,在氮气条件下,CaCl2在200 ℃焙烧1 h后的残渣主要成分是CaCl2·2H2O。这是因为预处理时将CaCl2按质量比1∶1溶于水中,所得CaCl2溶液烘干到质量分数超过70%后生成CaCl2·2H2O [19]。在600 ℃焙烧后的残渣中主要成分是CaCl2。可能是温度达到260 ℃后继续升温,CaCl2结晶水逐渐分解形成无水CaCl2[14]。778 ℃是CaCl2的熔点,而CaCl2在1 000 ℃热处理后残渣的主要物质是CaClOH。由此可以推断,778 ℃ CaCl2达到熔点后,与残余H2O反应,生成CaClOH并释放HCl,这与CaCl2·6H2O的分解机理相近[14]。如图3(b)所示,在干空气条件下,进行600 ℃和1 000 ℃热处理后,残渣主要成分均是CaCl2·2H2O,只是1 000 ℃残渣衍射峰强度较低。相比氮气条件下,H2O的损失不明显,这可能是因为CaCl2在H2O和O2存在的情况下,可以分别与H2O和O2反应,放出HCl和Cl2[14],空气中O2的存在减少了H2O的消耗。
2.2 不同气氛和不同温度下重金属挥发效果
NOWAK等[9]研究指出,CaCl2和MgCl2作为氯化剂时,效果优于NaCl,在1 000 ℃下焙烧60 min,80%~90% Cu、40% Zn和将近100% Pb挥发出去。以CaCl2为氯化剂,添加量为150 g·kg−1时,各温度下Zn、Cu、Pb的挥发率基本达到最高。在以MgCl2为氯化剂时,Pb在1 000 ℃下焙烧1 h可实现最大程度的挥发[20]。因此,在已有研究基础上,本研究选择在CaCl2添加量为15%、焙烧时间为1 h条件下进行氯化焙烧实验。因需对挥发物进行收集,本研究根据实验需要将气体流量选定为100 mL·min−1。
图4(b)、图4(d)、图4(f)为不同气氛、不同温度体系下铁尾矿样品中重金属Cu、Pb、Zn的挥发效果,图4(a)、图4(c)、图4(e)为对应焙烧温度和焙烧气氛条件下的冷凝收集液浓度。从图4(b)和图4(d)中可以看出,对于Cu和Pb,在氮气条件和干空气条件下,挥发率均随着温度的升高而增大,且整体而言,氮气条件下的挥发效果优于干空气条件。Cu的挥发率在600 ℃升至800 ℃时有较为明显的升高,而800 ℃到1 000 ℃挥发率上升程度较小,且各温度下Cu在2种气氛下挥发率相差很小,而氮气条件下Pb在600 ℃时挥发率达到90%,远远超过干空气条件;当温度升至1 000 ℃,氮气和干空气条件下Cu的挥发率分别达到77.7%和75.7%,Pb的挥发率分别达到97.8%和96.4%。在800~1 000 ℃时,2种气氛下Pb的挥发率均超过95%,这与Pb是易挥发性金属元素有关[9]。由此可见,焙烧气氛对Cu的挥发效果影响不大,但对较低温度时Pb的挥发率影响较大;在温度较低时,温度的升高对提高Cu和Pb的挥发率效果较为明显。除800 ℃时Cu在氮气条件下的挥发率略低于空气条件外,整体而言,当固体氯化剂CaCl2用于焙烧铁尾矿的复杂体系中时,氮气+CaCl2条件也优于干空气+CaCl2条件,这与Cu和Pb在氮气-HCl气氛下焙烧的挥发率高于空气-HCl气氛[21]的结论相近。
从图4(f)可以看出,焙烧温度和焙烧气氛对Zn的影响较为明显。在干空气条件下,Zn在焙烧温度为800 ℃时挥发效果最佳,挥发率为75.3%。在氮气条件下,Zn的挥发率随着温度的升高而升高,在1 000 ℃最高达到38.2%,而且在各个温度下的挥发率均远远低于干空气条件下的挥发率,这与氮气-HCl气氛和空气-HCl气氛对比研究的结果[21]不同,其可能的原因是空气-CaCl2气氛下会生成Cl2,而Cl2对于金属的氯化效果好于HCl[14]。与Cu和Pb相比,Zn的挥发率较低,这与Zn的氯化物本身的蒸气压较高有关[21];同时,尾矿中SiO2、Al2O3的大量存在,会使Zn在反应过程中生成硅酸锌、铝酸盐等,降低了挥发率[22]。
2.3 焙烧渣性质分析
S元素与Cl元素影响着氯化焙烧过程中重金属的挥发[23],但铁尾矿中S元素和引入的Cl元素的物态变化及迁移转化也可能对环境造成污染。因此,有必要对不同焙烧条件下的焙烧渣进行分析,考察其基本元素组成。
不同焙烧条件下的焙烧渣的成分组成如图5所示。可以看出,氮气条件下焙烧渣中S元素的残留量比干空气条件低。在干空气条件下,S元素质量分数随着温度的增高逐渐降低,但仍是焙烧渣的主要组成元素。这可能是因为,在空气气氛下焙烧铁尾矿的过程中,CaCl2与空气中的O2以及氧化生成的SO2反应生成CaSO4,残留在焙烧渣中[24]。在氮气条件下,600 ℃焙烧后仍残留很多Cl元素和Ca元素,证明了上述氮气条件下CaCl2的分解机理;在800、1 000 ℃下进行氯化焙烧后,残渣中未检测到Cl元素和S元素,主要为Fe、Ca、Si元素及少量的Cu、Zn等元素。
2.4 氯化焙烧挥发物定性和定量分析
采用冷凝管冷凝收集和湿法洗涤回收方式对烟气中挥发成分进行收集和分析,对冷凝收集液进行XRF分析,结果如表2所示。结合图4(a)、图4(c)、图4(e),可以看出,冷凝收集方法对不同气氛下挥发物的收集效果有较大的差异。在同种气氛下,随着温度的升高,冷凝液中各金属浓度随之增高;而同一焙烧温度下,氮气气氛下收集物中各重金属浓度高于干空气条件,尤其是在800 ℃和1 000 ℃条件下,氮气和干空气条件下Pb、Cu的挥发率相近,但冷凝液中相应重金属浓度相差很大,说明用冷凝的方法收集氮气条件下的氯化焙烧挥发物更加有效。
表 2 干空气和氮气条件下不同温度热处理后的冷凝液主要成分Table 2. Main compositions of condensate after heat treatment under different temperature in dry air and N2 atmosphere气体和温度条件 H2O Cl Si Zn Cu Pb Fe 干空气-600 ℃ 73.57 26.21 0.20 0.02 0.01 — — 干空气-800 ℃ 73.21 26.54 0.16 0.04 0.02 0.02 — 干空气-1 000 ℃ 71.67 27.93 0.21 0.09 0.04 0.04 — N2-600 ℃ 71.82 27.92 0.17 0.01 0.01 0.03 — N2-800 ℃ 71.09 28.23 0.18 0.11 0.14 0.20 0.01 N2-1 000 ℃ 70.19 28.88 0.19 0.17 0.24 0.22 0.02 注:“—” 未检出。 为探究湿法收集方式对氮气和干空气焙烧气氛下的烟气中各元素的回收效果,本研究对烟气端连续5个盛有200 mL 5%稀硝酸的吸收瓶中重金属浓度进行检测,结果如图6所示。可以看出,干空气条件下吸收瓶中金属浓度较高。第3个及以后的吸收瓶基本未检测到各金属元素,由此可见,挥发出的各金属元素在前2个吸收瓶中基本被完全吸收。由第1个吸收瓶中各金属元素的浓度可以看出,吸收瓶中金属Zn、Cu、Pb浓度较高,而Cd、Fe元素浓度很低,这是因为铁尾矿中本身Cd含量较低,而Fe元素属于难氯化挥发金属。整体对比发现,干空气气氛下氯化焙烧的挥发物更易于被湿法洗涤收集。
3. 结论
1)在氮气条件下,CaCl2反应生成CaClOH释放Cl;在干空气条件下,CaCl2分别与H2O和O2反应释放Cl。上述反应过程仍需进一步探究。
2)铁尾矿中重金属Cu、Pb、Zn的挥发率Pb>Cu>Zn,且随着温度的升高逐渐增大。
3)选择在1 000 ℃、氮气气氛下对铁尾矿进行氯化焙烧,Cu和Pb可以分别达到80%和90%以上的去除率,选用冷凝方法回收挥发物效果更好;在800℃,选择干空气条件下焙烧,Zn可以实现75%以上的挥发率,并适合采用湿法洗涤吸收方式进行收集。Fe元素在所有实验条件下的收集效果均较差。
4)在氮气条件下,氯化焙烧后的残渣相对于干空气气氛,S元素的残留量低,对环境的潜在威胁较小,更易于处置。
-
表 1 铁尾矿中重金属浓度
Table 1. Heavy metal concentration in iron tailings
mg·kg−1 Cu Pb Zn Cr Cd 4 568.00 1 761.68 2 518.70 85.81 2.81 表 2 干空气和氮气条件下不同温度热处理后的冷凝液主要成分
Table 2. Main compositions of condensate after heat treatment under different temperature in dry air and N2 atmosphere
气体和温度条件 H2O Cl Si Zn Cu Pb Fe 干空气-600 ℃ 73.57 26.21 0.20 0.02 0.01 — — 干空气-800 ℃ 73.21 26.54 0.16 0.04 0.02 0.02 — 干空气-1 000 ℃ 71.67 27.93 0.21 0.09 0.04 0.04 — N2-600 ℃ 71.82 27.92 0.17 0.01 0.01 0.03 — N2-800 ℃ 71.09 28.23 0.18 0.11 0.14 0.20 0.01 N2-1 000 ℃ 70.19 28.88 0.19 0.17 0.24 0.22 0.02 注:“—” 未检出。 -
[1] ZHAO S J, FAN J J, WEI S. Utilization of iron ore tailings as fine aggregate in ultra-high performance concrete[J]. Construction & Building Materials, 2014, 50: 540-548. [2] MA B G, CAI L X, LI X G, et al. Utilization of iron tailings as substitute in autoclaved aerated concrete: Physico-mechanical and microstructure of hydration products[J]. Journal of Cleaner Production, 2016, 127: 162-171. doi: 10.1016/j.jclepro.2016.03.172 [3] 李玉凤, 包景岭, 张锦瑞. 铁尾矿资源开发利用现状分析[J]. 中国矿业, 2015(11): 77-81. doi: 10.3969/j.issn.1004-4051.2015.11.017 [4] 邢宁, 吴平霄, 李媛媛, 等. 大宝山尾矿重金属形态及其潜在迁移能力分析[J]. 环境工程学报, 2011, 5(6): 1370-1374. [5] SIRKECI A A, GÜL A, BULUT G, et al. Recovery of Co, Ni, and Cu from the tailings of divrigi iron ore concentrator[J]. Mineral Processing & Extractive Metallurgy Review, 2006, 27(2): 131-141. [6] 李登敏, 杨雪莹, 邓久帅, 等. 从某铁尾矿中回收钛的试验[J]. 现代矿业, 2017(7): 217-218. doi: 10.3969/j.issn.1674-6082.2017.07.067 [7] 李芸邑, 梁嘉良, 刘阳生. 从铁尾矿中回收铁的磁化技术研究[J]. 环境工程, 2014, 32(s1): 634-638. [8] 孙达, 李永聪, 高志明. 从某铁尾矿中回收铜的试验研究[J]. 金属矿山, 2007(9): 119-122. doi: 10.3321/j.issn:1001-1250.2007.09.034 [9] NOWAK B, ROCHA S F, ASCHENBRENNER P, et al. Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: Influence of the chloride type[J]. Chemical Engineering Journal, 2012, 179: 178-185. doi: 10.1016/j.cej.2011.10.077 [10] NOWAK B, ASCHENBRENNER P, WINTER F. Heavy metal removal from sewage sludge ash and municipal solid waste fly ash: A comparison[J]. Fuel Processing Technology, 2013, 105: 195-201. doi: 10.1016/j.fuproc.2011.06.027 [11] 韦琳, 吕晓蕾, 刘阳生, 等. CaCl2高温热处理垃圾焚烧飞灰中重金属的挥发特性[J]. 环境工程学报, 2013, 7(11): 4533-4539. [12] 赵鹏, 景明海, 刘盖, 等. 熔盐法处置高熔点垃圾焚烧飞灰重金属的浸出特性[J]. 环境工程学报, 2018, 12(1): 324-330. [13] NISHINAKA K, TERAKADO O, TANI H, et al. Pyrometallurgical recovery of gallium from GaN semiconductor through chlorination process utilizing ammonium chloride[J]. Materials Transactions, 2017, 58(4): 688-691. doi: 10.2320/matertrans.M2016395 [14] FRAISSLER G, JÖLLER M, BRUNNER T, et al. Influence of dry and humid gaseous atmosphere on the thermal decomposition of calcium chloride and its impact on the remove of heavy metals by chlorination[J]. Chemical Engineering & Processing Process Intensification, 2009, 48(1): 380-388. [15] YU J, SUN L S, MA C, et al. Mechanism on heavy metals vaporization from municipal solid waste fly ash by MgCl2·6H2O[J]. Waste Management, 2016, 49: 124-130. doi: 10.1016/j.wasman.2015.12.015 [16] JIAO F, IWATA N, KINOSHITA N, et al. Vaporization mechanisms of water-insoluble Cs in ash during thermal treatment with calcium chloride addition[J]. Environmental Science and Technology, 2016, 50(24): 13329-13334. [17] 常耀超, 徐晓辉, 王云. 硫铁矿烧渣氯化焙烧扩大试验[J]. 有色金属(冶炼部分), 2013(10): 1-3. doi: 10.3969/j.issn.1007-7545.2013.10.001 [18] 姜平国, 吴朋飞, 汪正兵, 等. 氯化挥发的研究进展[J]. 有色金属科学与工程, 2016, 7(6): 43-49. [19] 李全超, 童张法, 陈志传, 等. 氯化焙烧法制备无水氯化钙的工艺研究[J]. 无机盐工业, 2016, 48(10): 36-39. [20] YU J, QIAO Y, SUN L S, et al. Detoxification of ashes from a fluidized bed waste incinerator[J]. Chemosphere, 2015, 134: 346-354. doi: 10.1016/j.chemosphere.2015.04.045 [21] YU J, SUN L S, XIANG J, et al. Vaporization of heavy metals during thermal treatment of model solid waste in a fluidized bed incinerator[J]. Chemosphere, 2012, 86(11): 1122-1126. doi: 10.1016/j.chemosphere.2011.12.010 [22] LIU J, FALCOZ Q, GAUTHIER D, et al. Volatilization behavior of Cd and Zn based on continuous emission measurement of flue gas from laboratory-scale coal combustion[J]. Chemosphere, 2010, 80(3): 241-247. doi: 10.1016/j.chemosphere.2010.04.028 [23] LIU J Y, LIU C, SUN G, et al. Thermodynamic behaviors of Cu in interaction with chlorine, sulfur, phosphorus and minerals during sewage sludge co-incineration[J]. Chinese Journal of Chemical Engineering, 2018, 26: 1160-1170. doi: 10.1016/j.cjche.2017.10.019 [24] 潘贵, 邵京明, 钱虎, 等. 金精矿焙砂氯化挥发多元素综合提取新技术研究[J]. 世界有色金属, 2017(23): 230-232. -




 DownLoad:
DownLoad: