-
大量研究表明,高级催化氧化法的复合水处理方法经常表现出协同效应,如光/芬顿催化、光催化/臭氧,电催化/紫外光等[1-5]。同样,光电耦合催化体系也存在着明显的协同效应。安太成等[6]发现光电耦合系统中有机物的降解速率远远大于单一的光降解和电降解;XIAO等[7]发现光电耦合系统可以将垃圾渗滤液的COD和氨氮去除率分别提高22.2%和33.4%;HURWITZ等[4]发现在光电同步降解苯酚中,TOC降解率明显大于单一的光催化和电催化;PIRES等[8]发现光电耦合催化对于污水中微生物的破坏也具有一定协同性。这说明光电耦合催化的协同性体现在很多方面,并可以大大提高有机物的降解效率。掌握了协同效应的产生原因,便可通过人为控制其影响因素来直接或者间接控制协同效应的大小。这样,不仅可以节约成本,还可以提高降解效率,故其意义重大。
针对协同效应的产生机理问题,HURWITZ等[4]认为光电耦合体系中紫外光照促进了电催化子系统中的HClO的分解,产生了强氧化性物质Cl·,进而促进了有机物的降解;有研究[9]认为在光助电芬顿体系中,当电芬顿反应将60%的TOC去除后,剩下的小分子物质就极易被紫外灯直接照射分解,很快达到100%的矿化,由此产生协同作用;JAAFARZADEH等[10]认为在光电臭氧体系中,紫外光的存在激活了溶液中的活性物质,产生了更多的 · OH进而产生较强的协同效应。
3,4-二甲基苯胺(3,4-DMA)是一种难降解有机污染物[11-12],并广泛存在于制药和印染废水中。此类废水具有极强的毒性和致癌性[11-14],且此类废水具有高盐、难降解和可生化性差的特点,使得传统的生物法处理效果较差,效率较低[15-16]。因此,本研究以3,4-DMA废水为研究对象,建立一种量化协同效应大小的评判标准,通过不同体系下对其降解速率的研究和羟基自由基的检测,以探讨不同影响因素下的协同效应及协同效应的产生原因。
-
3,4-二甲基苯胺(C8H11N)、乙酰丙酮(C5H8O2)、氢氧化钠(NaOH)、二甲亚砜(C2H6OS)、亚硝酸钠(NaNO2)、氨基磺酸铵(H6N2O3S),均为分析纯;愈创木酚溶液(C7H8O2,95%);P25纳米二氧化钛(TiO2);Ti/IrO2阳极和石墨阴极。
pH计(PB-10型,北京赛多利斯仪器系统有限公司);紫外-可见光分光光度计(UV-1100,上海美谱达仪器有限公司);直流稳压电源(RXN305D,深圳市兆信电子科技有限公司);恒温磁力搅拌器(85-2,常州越新仪器制造有限公司)。
-
反应容器由有机玻璃加工而成,实验装置如图1所示,规格为16.0 cm×12.0 cm×12.0 cm,有效容积为1.0 L。外部用锡箔纸包裹,底部设置有恒温磁力加热搅拌器。中心可根据实验要求设置不同功率(6、12、18 W)的半浸没式石英套管紫外光灯。阳极极板(Ti/IrO2)和石墨阴极极板平行固定在反应器内,间距为50.0 mm。直流电源可调节电压范围为0~30.0 V。
-
配置含6 000 mg·L−1 NaCl、5.0 mg·L−1的3,4-DMA溶液,经过预实验得到最优反应条件,取300 mg TiO2粉末加入反应器中,避光搅拌30.0 min,紫外光灯预热5.0 min调节直流电源,保持电流密度恒为2.5 mA·cm−2。反应开始,间隔2.0 min取样并用0.22 μm聚醚矾膜过滤,然后检测3,4-DMA的残留浓度。在检测 · OH时,反应液换为由蒸馏水配置的适量浓度二甲亚砜和6 000 mg·L−1 NaCl的混合液,间隔4.0 min取样并用0.22 μm聚醚矾膜过滤,然后定量检测 · OH含量。电催化实验中不加入紫外灯和TiO2,光催化实验中不加入电极;电助光催化实验中,在反应容器的外壁设置2块10.0 cm×12.0 cm不锈钢铁板,不锈钢铁板与直流电源连接,形成一个静电场。在所有实验中均保持300 r·min−1的搅拌速度。
-
将3,4-DMA在降解一定时间后的残留浓度实验数据直接用一级动力学公式进行拟合,计算方法见式(1)和式(2)。
式中:C0为3,4-DMA的初始浓度,mg·L−1;Ct为3,4-DMA降解t时间后的残留浓度,mg·L−1;k为一级动力学反应速率常数,min−1。
-
3,4-DMA的检测采用甲氧基苯酚分光光度法[17],在456.0 nm处对水体中3,4-DMA进行测定。 · OH的检测以二甲基亚砜为分子探针,414.0 nm波长处通过测乙酰丙酮法生成的黄色化合物的分光光度值,从而定量表征 · OH生成量[18]。
-
为定量分析协同效应,现提出协同度来表征协同效应的大小,计算公式见式(3)。
式中:f为协同度;k为光电耦合催化氧化系统降解3,4-DMA反应速率常数,min−1;ki为子系统单独反应时的反应速率常数,min−1。
式中:θi为子系统的贡献度。将式(4)代入式(3)可得到式(5)和式(6)。
式(5)的物理意义是协同效应对系统效率的贡献度;式(6)的物理意义是各子系统的贡献度与协同度之和,数值等于1。
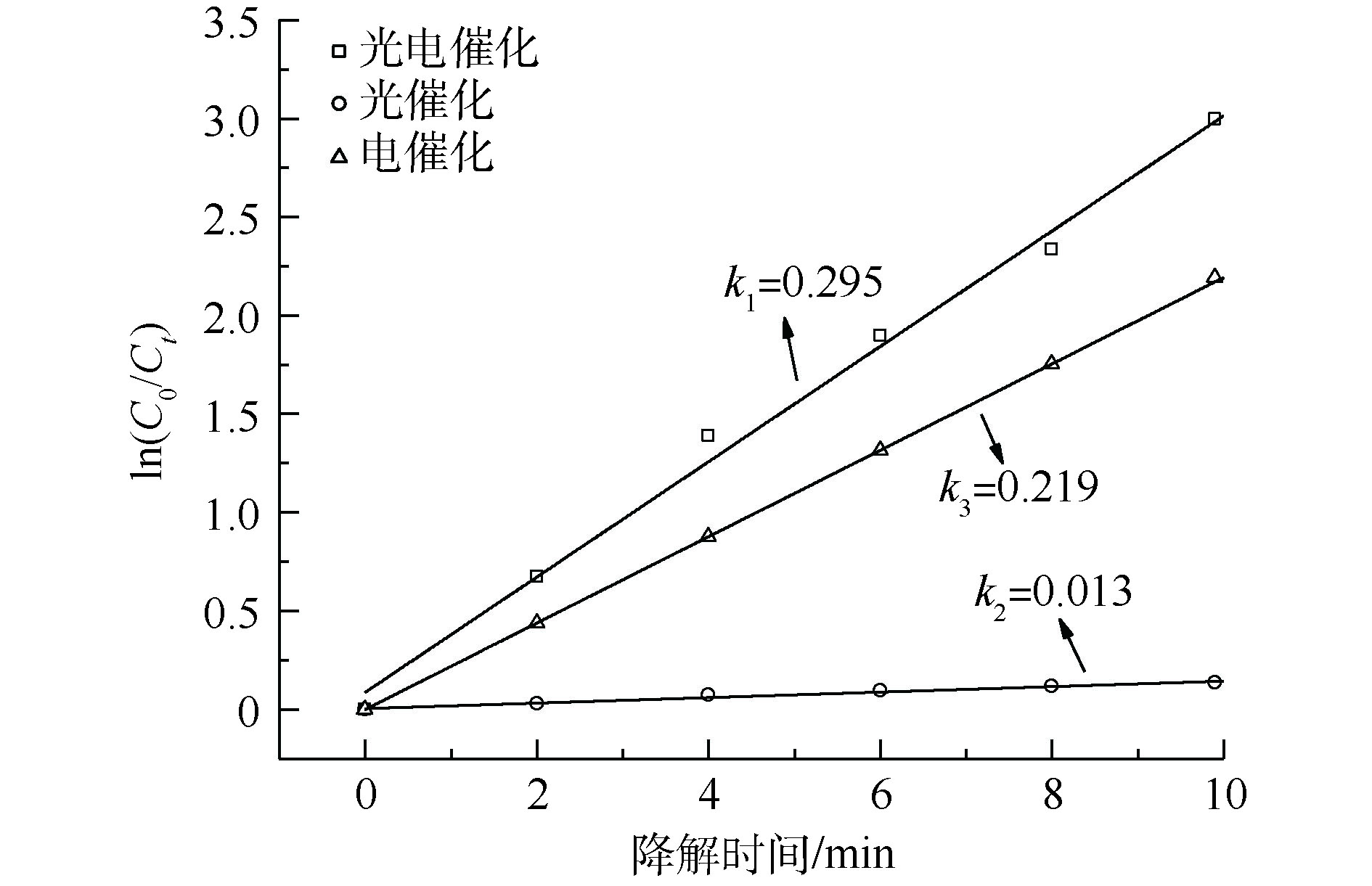
考察了光电催化、光催化和电催化体系中3,4-DMA的动力学结果,如图2所示。发现3种反应体系中3,4-DMA的降解均符合准一级动力学规律,其反应动力学常数k分别为0.295、0.013和0.219 min−1。基于反应速率常数计算,光催化的贡献度为4.41%,电催化贡献度为74.23%,光电耦合催化系统的协同度为21.36%,这说明光电耦合催化氧化系统存在明显的协同效应。
-
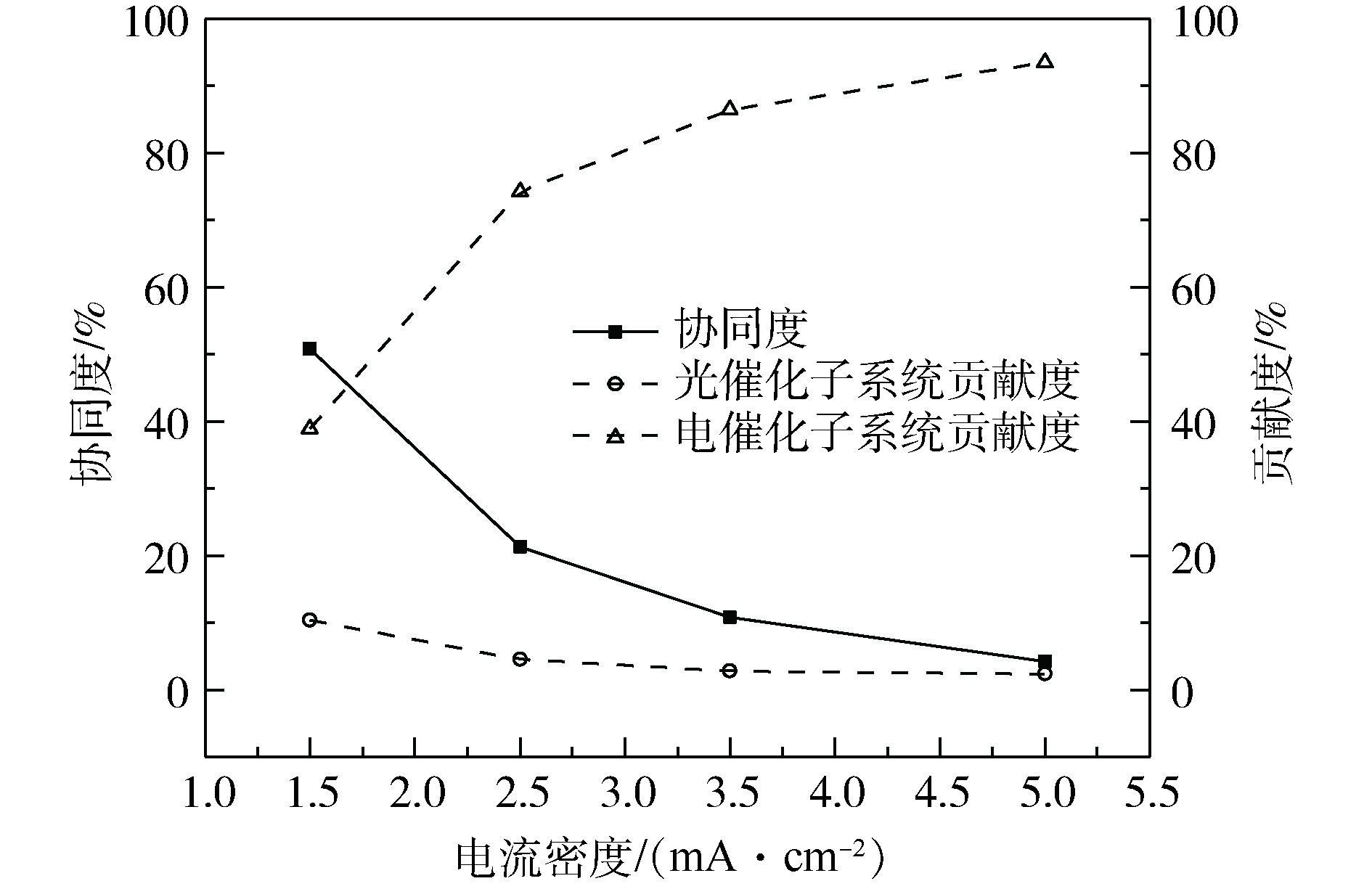
电流密度随着外加电场场强的增加而升高。分别考察了不同电流密度下的光催化、电催化子系统的贡献度和光电耦合体系协同度,如图3所示。当电流密度从1.5 mA·cm−2增加到5.0 mA·cm−2时,体系的协同度从50.78%逐渐下降到4.18%,但始终大于零,表明不同电流密度下光电耦合系统均具有协同效应,且这种协同效应在1.5~5.0 mA·cm−2电流密度范围内,随着电流密度的增加而减小。
外加电场的协同作用可体现在其对于光催化子系统的促进作用上。于是在反应器外壁添加2块金属极板,施加不同的电压进行电助光催化降解实验,结果如图4所示。随着外电场增加,3,4-DMA的降解率先增加后减小;当外加电场强度从0 V·cm−1增加到1.0 V·cm−1,反应50 min后3,4-DMA降解率从49.47%提高到57.41%;当外加静电场的强度提高到1.5 V·cm−1时,降解率最高,达到74.10%。相比较于未加电场的光催化降解率增加了25.60%,尽管在2.0 V·cm−1和2.5 V·cm−1时降解率有所下降,但其相对于未加电场时的降解率仍然有较明显提高。因此,外加静电场后,光催化3,4-DMA的降解率显著提高。这一结果说明,外加静电场存在某种增益作用,能够提高光催化的降解效率。
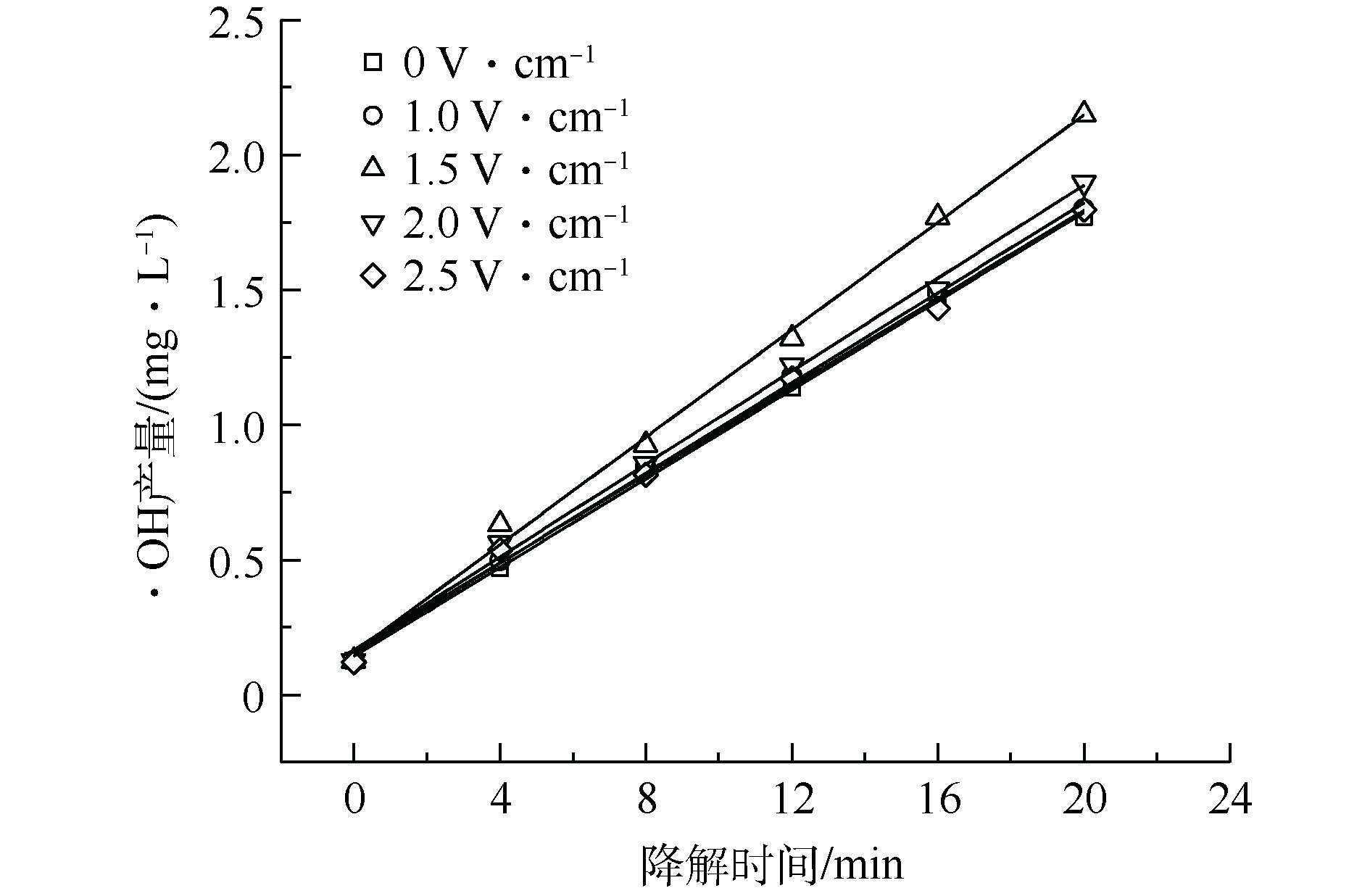
由于光催化降解有机物是基于 · OH氧化的反应机理[1, 5],为验证外电场的施加能够增加 · OH产量而引发协同效应的推测,故用二甲亚砜-乙酰丙酮法定量检测不同外加电场的光催化实验中 · OH的产量[19-20],拟合结果如图5所示。
从图5中可以看出,有外加静电场辅助的光催化系统产生了更多的 · OH。按零级动力学模型计算 · OH产率如表1所示,可以发现其产率先增加后减小,这与3,4-DMA降解率变化规律一致。同时,外加1.5 V·cm−1静电场后 · OH的产率相比较于未加电场时的产率提高了20.73%。这表明,外加静电场提高光催化降解速率的实质是外加电场有效促进了光生空穴和光生电子的分离,从而促进生成更多的 · OH,使得3,4-DMA与 · OH的碰撞概率大大增加,进而增加了反应速率,由此引发了协同效应。随着外电压的增加,协同效应先增加后减少。但协同部分的变化量相对于电催化子系统的变化量较小,故表现为图3所示的协同度的下降。
-
不同光照强度下的协同结果如表2所示。可以看出,光电耦合催化系统的协同度随着光照强度的增加而增加。当光照强度从6 W提升到12 W和18 W时,系统的协同度从21.35%增加到27.32%和33.24%。因此,提高光照强度能提高光电耦合催化系统的协同度。
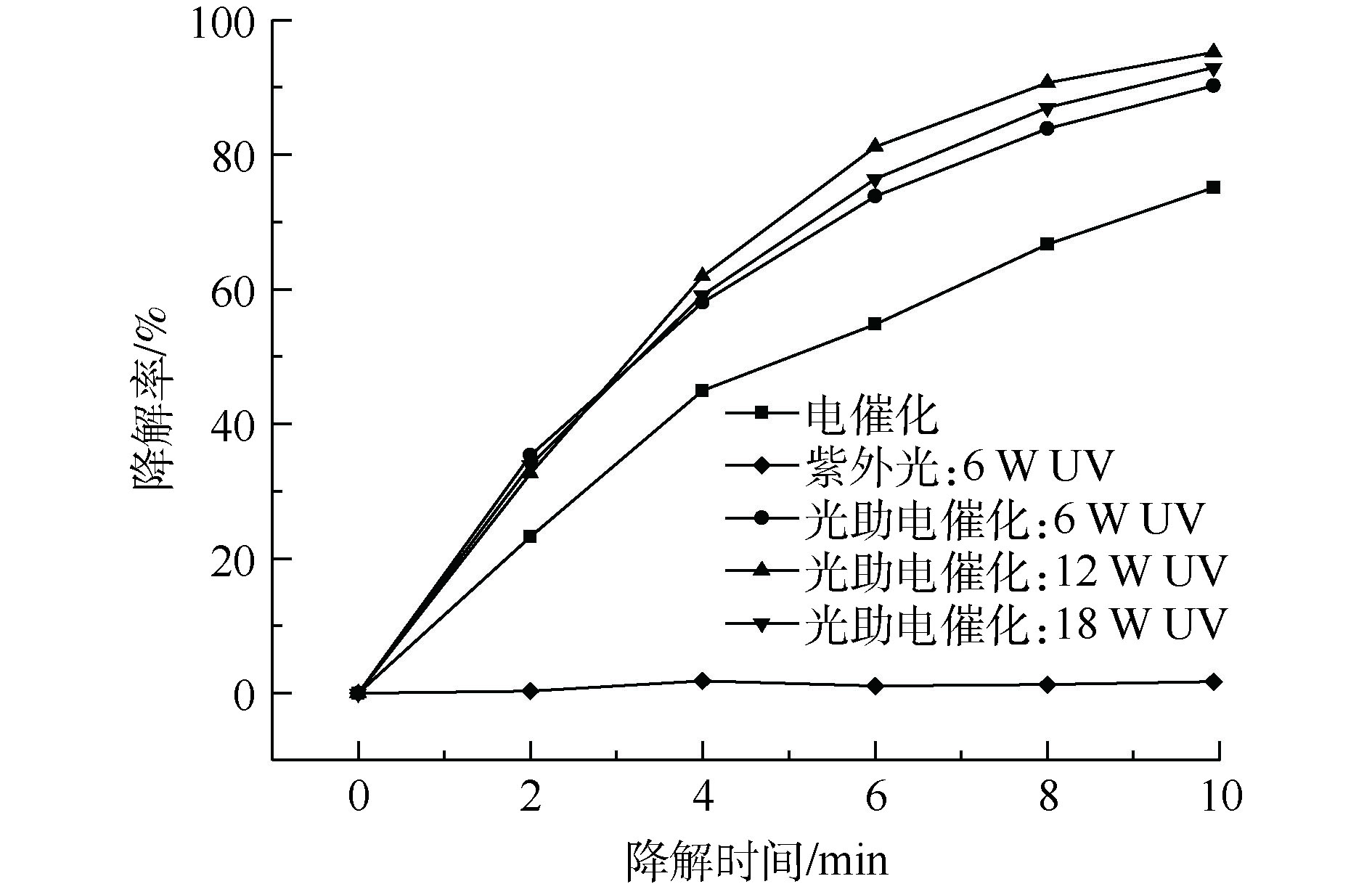
紫外光强的协同性可能体现在其对电催化子系统的促进作用上。将紫外灯由潜入液面移动至液面上方(仍然在反应器内)进行光助电催化实验,结果如图6所示。当只有紫外光照射下,降解10 min时,3,4-DMA浓度仅有少量降低,说明在紫外光条件下3,4-DMA化学性质相对稳定,可降解的概率很小;在紫外光辅助的电催化反应进行到10 min时,6、12、18 W紫外灯照射下3,4-DMA的降解率分别为90.22%、95.21%和93.02%,而3,4-DMA的电催化降解率仅为75.09%,说明紫外光对3,4-DMA的降解率有较大的提升。
另一方面,从表3所示的光助电催化实验的反应速率常数来看,当光照强度为6、12、18 W时,与没有光照的对照组对比,光助电催化的反应速率常数分别提升了5.97%、42.29%和22.14%。由此可见,光照对电催化具有显著的促进作用。这进一步证明了紫外光使得电催化和光电耦合催化体系产生协同效应,且在一定范围内,随着紫外灯光强的提高,协同度不断提高。这一实验结果与HURWITZ等[4]研究结果相一致。他们将光强的协同作用归因于电催化产生了氯气,氯气溶解于水产生次氯酸(式(7)),而光照使次氯酸分解产生氯自由基和 · OH(式(8)),正是这些活性氧物质的生成强化了电催化的催化效果。
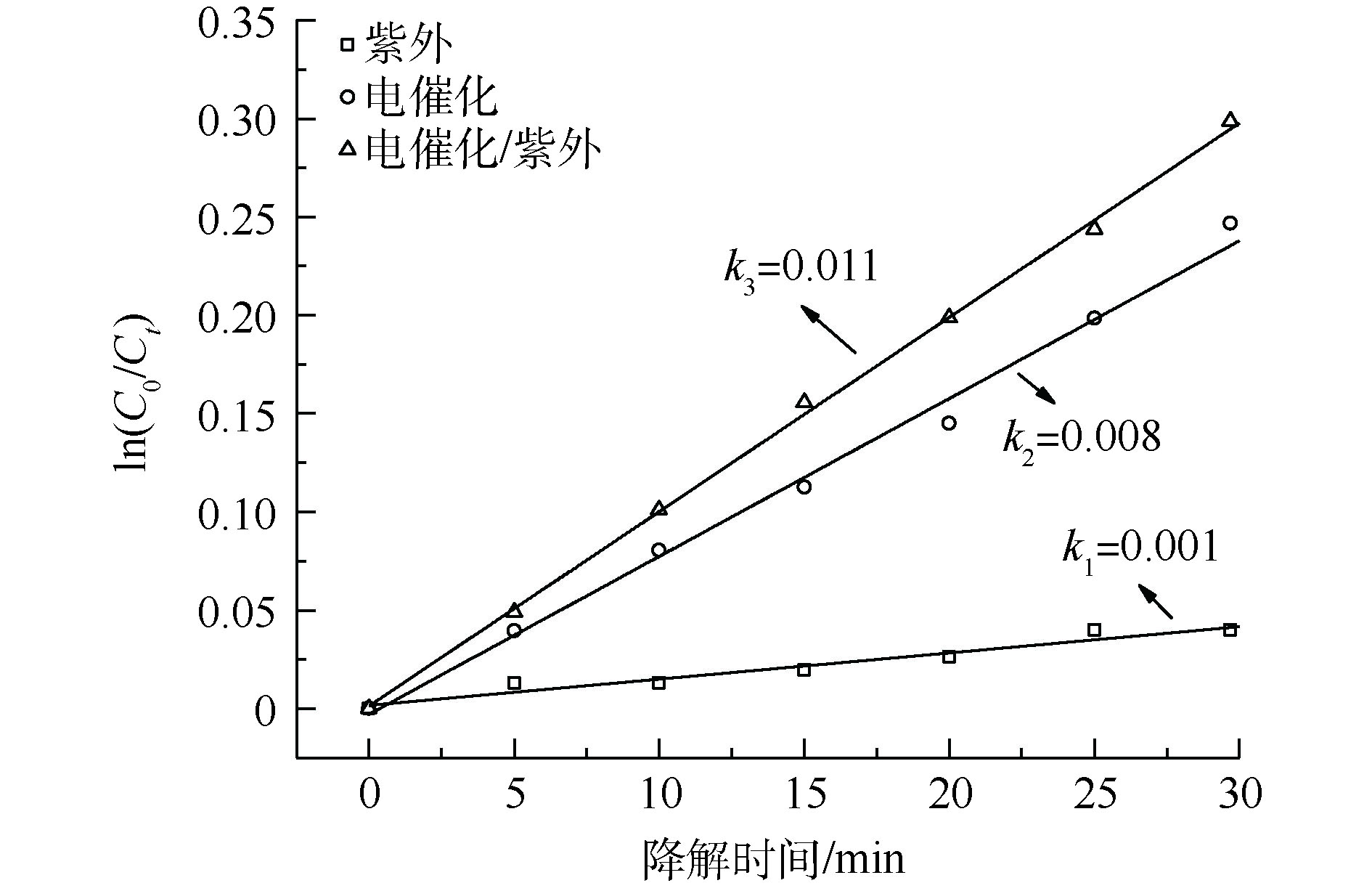
硫酸钠电解质对于Ti/IrO2阳极是惰性电解质,即不会产生活性物质,例如硫酸根自由基[21]。为了证实HURWITZ等[4]的结论,以硫酸钠为电解质进行实验,结果如图7所示。由图7可见,外加6 W紫外光灯时,紫外、电催化和紫外/电催化系统一级反应速率常数分别为0.001、0.008和0.010 min−1,其仍存在协同作用且协同度为18.18%。这说明硫酸钠电解质的电催化体系中紫外光仍可以促进有机物的降解,这与HURWITZ等[4]的结论相悖,说明协同效应与氯类活性物质无关。
从光化学的角度分析协同效应的原因,本研究中采用的是253.7 nm紫外光,依据E= 1 240/λ可得,光量子能量为4.88 eV,超过3,4-DMA中C—C(3.45 eV)、C—H(4.31 eV)、C—O(3.39 eV)、C—N(3.17 eV)等化学键的键能,故紫外光可以激发3,4-DMA,处于激发态的3,4-DMA更容易被电催化系统产生的活性氧化物质等氧化降解,也可以更加有效地利用光催化系统产生的 · OH。因此,可以将紫外光对光电耦合催化系统的协同作用归因于光量子的激发作用,从而提高了 · OH、氯类活性氧化物质的利用率。
-
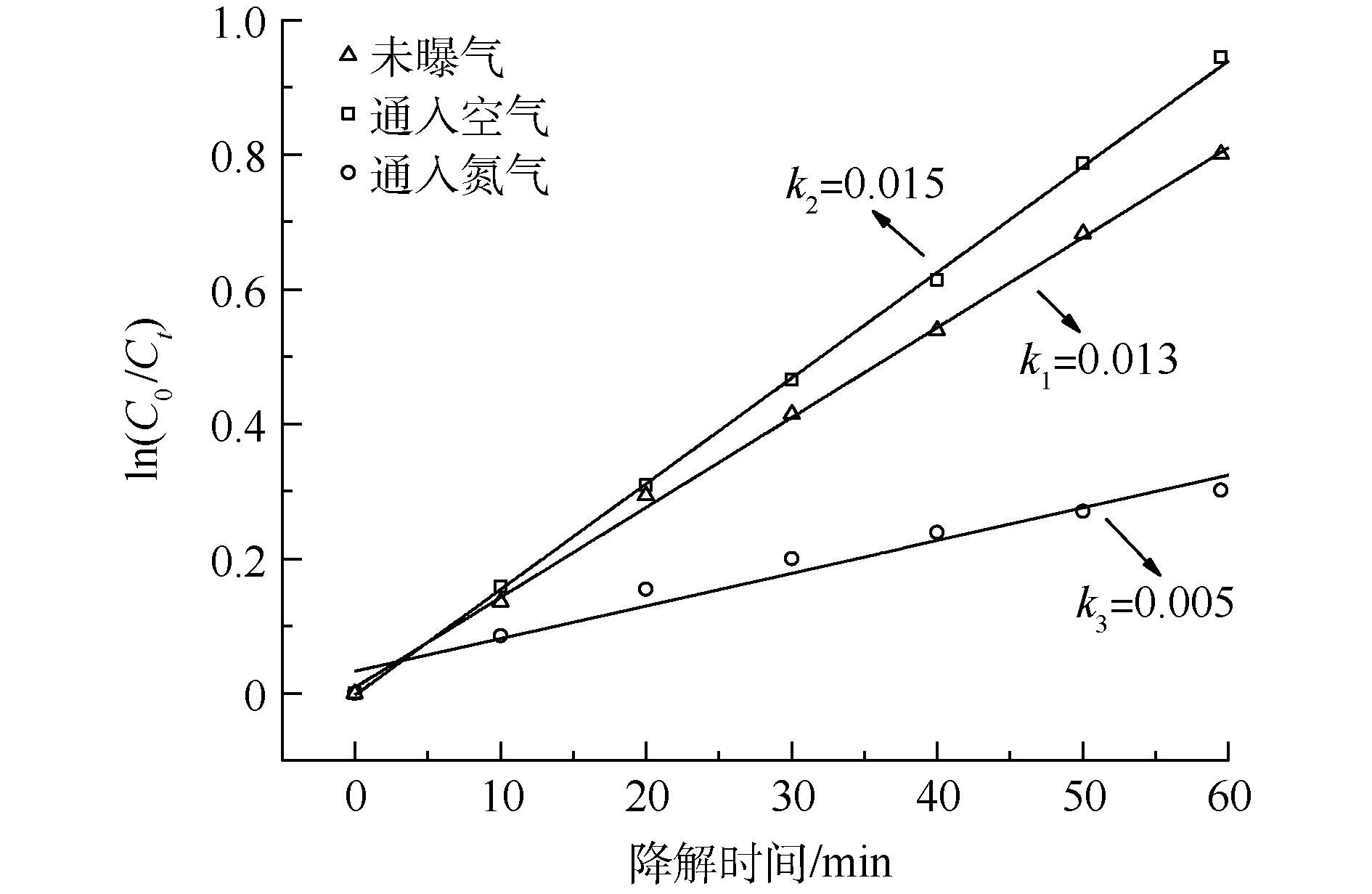
在电催化降解有机物的过程中,析氧反应是一个竞争性副反应,是降低电流效率的重要因素之一[22-23]。但溶解氧一般认为是光催化反应中的电子受体,能够有效地捕获光生电子,对处理难降解有机物非常重要[23]。若能够实现副反应产物氧气的高效利用,则必然出现协同效应。因此,需要重点研究析氧副反应及其与协同度的关系,于是开展了不同气氛条件下的光催化氧化降解3,4-DMA实验,结果如图8所示。
从图8可看出,对比未曝气条件,在氮气氛围时,光催化过程明显得到抑制,一级动力学反应速率常数从0.013 min−1降低到0.004 min−1,降低了69.23%。其原因是,由于在充N2时测得水体中的溶解氧仅为0.96 mg·L−1,而在未曝气条件下水体中溶解氧的浓度为8.36 mg·L−1,溶解氧浓度下降导致光催化降解效率大幅度降低。相反,当曝气为空气时,测定水体中溶解氧浓度提高到8.78 mg·L−1,反应速率常数提高到0.015 min−1,提高了15.38%。可见,溶解氧浓度对光催化反应具有重要影响,充足的溶解氧是光催化快速反应的必要条件。因此,析氧副反应可能利于协同作用。
有研究表明,溶液的pH越大,析氧反应越剧烈[24]。因此,可以通过考察不同pH条件下的光电耦合催化系统的协同度来佐证析氧副反应的协同作用。不同pH条件下的电催化、光催化和光电耦合催化系统降解3,4-DMA等实验的结果如表4所示。可见,在碱性环境下的协同度明显高于酸性环境下的协同度(29.02%与2.15%)。由于在碱性环境下的析氧反应比酸性环境下更为剧烈,电催化副反应析出的高浓度溶解氧为光催化系统提供了电子受体,可以有效捕获光生电子,进而提高羟基自由基等活性物质的产生(式(9)~式(12)),大大提升了光催化的降解速率,从而提高了光电耦合催化系统的协同度。因此,析氧副反应可以提高协同效应。
-
1)在光电耦合催化氧化降解3,4-DMA的体系中,光催化子系统和电催化子系统之间存在协同效应。采用准一级反应动力学常数计算,光催化、电催化子系统的贡献分别为4.41%和74.23%,光电耦合催化系统协同度达到21.36%。
2)影响协同效应的因素为:首先,外加电场促进了体系产生了更多的 · OH;其次,紫外光照使得3,4-DMA及其降解中间产物受到激发而提高了对 · OH等活性氧化物质的利用率;最后,电催化过程中的析氧副反应为光催化提供了电子受体,从而提高了系统的总体降解效率。
光电耦合技术处理3,4-二甲基苯胺废水的协同效应
Synergistic effects of hybrid photo-electrocatalytic degradation of 3,4-dimethylaniline wastewater
-
摘要: 高级催化氧化法的复合水处理工艺经常出现协同效应,但对于协同效应的产生机理仍不清楚。因此,针对光电耦合催化氧化体系,采用协同度为量化指标,研究了不同光照、电流密度、曝气强度、初始pH等条件对光电耦合催化体系降解3, 4-二甲基苯胺(3,4-DMA)的协同效应的影响;并通过光助电催化实验、电助光催化实验和羟基自由基检测实验,探讨了协同效应存在的原因。结果表明,光电耦合催化氧化系统存在协同效应,且协同度受外部条件的影响。协同效应产生的原因主要包括:外加电场促进体系产生了更多的羟基自由基;紫外光照使得3,4-DMA及其降解中间产物受到激发而提高了对羟基自由基等活性氧物种的利用率;电催化过程中的析氧副反应为光催化提供了电子受体,从而提高了系统的总体降解效率。这为深入研究和人为调控协同效应提供了新的方法。Abstract: The synergistic effects often occur in many advanced oxidation processes, while its mechanisms are still unclear. Hence, in this study, the synergetic level was taken as an indicator, and the effects of illumination, current intensity, aeration intensity and initial pH on the synergistic effects of hybrid photo-electrocatalytic degradation of 3,4-dimethylaniline (3,4-DMA) were determined. Furthermore, photo-assisted electrocatalytic, electro-assisted photocatalytic and hydroxyl radicals detection experiments were carried out to explore the degradation mechanism. The results indicated that the synergistic effects occurred in the hybrid photo-electrocatalysis system, and the synergetic level was affected by external conditions. The reasons for the synergetic effects were ascribed to the following items. The applied electric fields promoted the yield of hydroxyl radicals. The ultraviolet radiation improved the utilization of active oxides such as hydroxyl radicals by stimulating 3,4-DMA and its intermediates. The oxygen evolution side reaction in electrocatalysis provided electron acceptors for photocatalysis, which could enhance the degradation efficiency of the whole treatment system. This research provided a new method for further study and regulation of the synergetic effects.
-
表 1 不同外加电场下的光催化的 · OH产率和R2
Table 1. · OH yield and R2 under different applied electric fields in photocatalytic system
场强/(V·cm−1) 产率/(mg·(L·min)−1) R2 0 0.082 0.999 1.0 0.083 0.998 1.5 0.099 0.996 2.0 0.086 0.996 2.5 0.085 0.992 表 2 不同光照强度下的协同作用
Table 2. Synergistic effects under different light intensities
反应类型 k/min−1 R2 光照强度/W f /% 光催化1) 0.013 0.998 6 — 电催化2) 0.219 0.996 6 — 光电耦合催化3) 0.295 0.994 6 21.35 光催化1) 0.023 0.998 12 — 电催化2) 0.219 0.996 12 — 光电耦合催化3) 0.333 0.996 12 27.32 光催化1) 0.030 0.993 18 — 电催化2) 0.219 0.996 18 — 光电耦合催化3) 0.373 0.999 18 33.24 注:1)光照强度为6 W;2) 电流密度为2.5 mA·cm−2;3) 电流密度为2.5 mA·cm−2、光照强度为6 W。所有实验3,4-DMA的浓度5 mg·L−1,室温,氯化钠浓度为6 000 mg·L−1,初始pH均为6.4,搅拌转速300 r·min−1。 表 3 光助电催化时3,4-DMA降解速率常数
Table 3. Degradation rate constant of 3,4-DMA by photo-assisted electrocatalytic systems
光照强度/W k/min−1 R2 增效作用/% 0 0.219 0.996 0 6 0.232 0.998 5.97 12 0.312 0.990 42.29 18 0.267 0.993 22.14 表 4 初始pH对光电耦合催化系统协同度的影响
Table 4. Effect of initial pH on synergetic level of hybrid photo-electrocatalytic oxidation
反应类型 k/min−1 R2 pH f/% 光催化1) 0.008 0.993 3 — 电催化2) 0.447 0.973 3 — 光电耦合催化3) 0.465 0.986 3 2.15 光催化1) 0.013 0.998 6.4 — 电催化2) 0.219 0.996 6.4 — 光电耦合催化3) 0.295 0.994 6.4 17.96 光催化1) 0.021 0.992 9 — 电催化2) 0.160 0.978 9 — 光电耦合催化3) 0.255 0.987 9 29.02 注:1) 光照强度为6 W;2) 电流密度为2.5 mA·cm−2;3) 电流密度为2.5 mA·cm−2、光照强度为6 W。所有实验3,4-DMA的浓度5.0 mg·L−1,室温,氯化钠浓度为6 000 mg·L−1,搅拌速度300 r·min−1,初始pH分别为3、6.4、9。 -
[1] AMMAR H B, BRAHIM M B, ABDELHEDI R, et al. Enhanced degradation of metronidazole by sunlight via photo-Fenton process under gradual addition of hydrogen peroxide[J]. Journal of Molecular Catalysis A: Chemical, 2016, 420: 222-227. doi: 10.1016/j.molcata.2016.04.029 [2] DE TORRES-SOCIAS E, FERNANDEZ-CALDERERO I, OLLER I, et al. Cork boiling wastewater treatment at pilot plant scale: Comparison of solar photo-Fenton and ozone (O3,O3/H2O2). Toxicity and biodegradability assessment[J]. Chemical Engineering Journal, 2013, 234(19): 232-239. [3] SHARMA J, MISHRA I M, KUMAR V. Mechanistic study of photo-oxidation of bisphenol-A (BPA) with hydrogen peroxide (H2O2) and sodium persulfate (SPS)[J]. Journal of Environmental Management, 2016, 166: 12-22. doi: 10.1016/j.jenvman.2015.09.043 [4] HURWITZ G, PORNWONGTHONG P, MAHENDRA S, et al. Degradation of phenol by synergistic chlorine-enhanced photo-assisted electrochemical oxidation[J]. Chemical Engineering Journal, 2014, 240(4): 235-243. [5] ZHANG W, ZHOU S, SUN J, et al. Impact of chloride ions on UV/H2O2 and UV/Persulfate advanced oxidation processes[J]. Environmental Science & Technology, 2018, 52(13): 7380-7389. [6] 安太成, 张文兵, 朱锡海, 等. 一种新型光电催化反应器的研制及甲酸的光电催化深度氧化[J]. 催化学报, 2003, 24(5): 338-342. doi: 10.3321/j.issn:0253-9837.2003.05.007 [7] XIAO S, PENG J, SONG Y, et al. Degradation of biologically treated landfill leachate by using electrochemical process combined with UV irradiation[J]. Separation & Purification Technology, 2013, 117(39): 24-29. [8] PIRES R H, BRUGNERA M F, ZANONI M V B, et al. Effectiveness of photoelectrocatalysis treatment for the inactivation of Candida parapsilosis sensu stricto in planktonic cultures and biofilms[J]. Applied Catalysis A: General, 2016, 511: 149-155. doi: 10.1016/j.apcata.2015.11.036 [9] GARCIA-SEGURA S, EL-GHENYMY A, CENTELLAS F, et al. Comparative degradation of the diazo dye direct yellow 4 by electro-Fenton, photoelectro-Fenton and photo-assisted electro-Fenton[J]. Journal of Electroanalytical Chemistry, 2012, 681: 36-43. doi: 10.1016/j.jelechem.2012.06.002 [10] JAAFARZADEH N, BARZEGAR G, GHANBARI F. Photo assisted electro-peroxone to degrade 2,4-D herbicide: The effects of supporting electrolytes and determining mechanism[J]. Process Safety and Environmental Protection, 2017, 111: 520-528. doi: 10.1016/j.psep.2017.08.012 [11] 杨唯艺, 李孟, 张倩, 等. K2FeO4氧化降解3,4-二甲基苯胺的机理研究[J]. 中国环境科学, 2018, 38(5): 1744-1751. doi: 10.3969/j.issn.1000-6923.2018.05.017 [12] JING D, CAO Y F, YUAN L I. Preparation of 3,4-dimethylaniline by raney nickel catalyst[J]. Journal of Dalian Polytechnic University, 2012, 31(5): 339-341. [13] 李捍东, 王建涛, 周志祥. 苯系化学品理化毒理信息手册[M]. 北京: 中国环境科学出版社, 2015. [14] 周文敏, 傅德黔, 孙宗光. 水中优先控制污染物黑名单[J]. 中国环境监测, 1990, 6(4): 3-5. [15] XU X, CHENG Y, ZHANG T, et al. Treatment of pharmaceutical wastewater using interior micro-electrolysis/Fenton oxidation-coagulation and biological degradation[J]. Chemosphere, 2016, 152: 23-30. doi: 10.1016/j.chemosphere.2016.02.100 [16] WAND J, CHU L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview[J]. Radiation Physics & Chemistry, 2016, 125: 56-64. [17] 杨晓芬, 赵美萍, 李元宗, 等. 水中苯胺类化合物的分光光度法测定[J]. 分析化学, 2002, 30(5): 540-543. doi: 10.3321/j.issn:0253-3820.2002.05.007 [18] 崔崇威, 孙士权, 黄君礼. 羟自由基捕捉剂的比较分析[J]. 哈尔滨商业大学学报(自然科学版), 2004, 20(3): 342-345. doi: 10.3969/j.issn.1672-0946.2004.03.025 [19] 刘婷, 尤宏, 陈其伟, 等. 光助非均相芬顿体系中羟基自由基的荧光光谱法测定与影响因素研究[J]. 环境科学, 2009, 30(9): 2560-2564. [20] 邰超, 韩丹, 阴永光, 等. 二甲亚砜捕获-高效液相色谱测定天然水体中羟基自由基的光化学生成[J]. 环境化学, 2015, 34(2): 212-218. [21] MOREIRA F C, BOAVENTURA R A R, BRILLAS E, et al. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewater[J]. Applied Catalysis B: Environmental, 2017, 202: 217-261. doi: 10.1016/j.apcatb.2016.08.037 [22] REIER T, OEZASLAN M, STRASSER P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials[J]. ACS Catalysis, 2012, 2(8): 1765. doi: 10.1021/cs3003098 [23] 杨世迎. TiO2光催化降解有机污染物的初始步骤机理研究[D]. 杭州: 浙江大学, 2005. [24] REIER T, NONG H N, TESCHNER D, et al. Electrocatalytic oxygen evolution reaction in acidic environments-reaction mechanisms and catalysts[J]. Advanced Energy Materials, 2017, 7(1): 1601275. doi: 10.1002/aenm.v7.1 -





 下载:
下载: