-
我国是世界13个人均水资源最贫乏的国家之一,水资源紧缺严重制约着我国的社会经济发展。将城镇污水再生回用、实现“污水资源化”是解决水资源紧缺的有效途径。补给水环境是再生水的主要用途之一,但其中的磷是造成水体富营养化的关键因素[1-2]。《城市污水再生利用景观环境用水水质》(GB/T 18921-2002)[3]规定观赏性或娱乐性景观环境用水的总磷(total phosphorus,TP)浓度限值为0.5 mg·L−1(湖泊类)或1.0 mg·L−1(河道类)。水体中TP浓度高于0.02 mg·L−1即处于富营养化状态[4],有研究[5-6]表明,污水处理厂出水TP浓度必须控制在0.05 mg·L−1以下。
针对污水处理厂二级处理出水(简称二级出水)低浓度磷的特点,化学沉淀除磷和吸附法除磷不失为经济高效的深度除磷方法。目前,对于化学沉淀除磷,研究者主要关注混凝剂的种类、影响因素和除磷效果[7-9]。对于吸附法除磷,主要集中于吸附材料、吸附机理和吸附性能优劣的研究[10-11]。化学沉淀除磷和吸附法除磷在二级出水中的应用以及对水中不同形态磷的去除效果研究较少。并且前人的研究多数是针对难以达标的水进行化学强化除磷,使出水TP达到规定的排放标准0.5 mg·L−1[12-13]。但是,按照目前的水质标准,再生水若回用于封闭的或流动缓慢的景观环境水体,仍存在爆发水华的风险[14]。因此,对二级出水进行深度除磷,使TP浓度降至更低的水平势在必行。目前,国内对出水TP浓度低于0.05 mg·L−1深度除磷的研究尚未见报道。
针对国内的研究现状,本研究以北京市某城市污水处理厂二级出水为研究对象,将出水TP控制在0.05 mg·L−1以下为研究目的,对磷的形态及浓度分布进行了分析,分别采用化学沉淀法和吸附法对其展开了除磷研究,分析了不同形态磷的去除效果,并对其进行技术经济分析,以期为再生水深度除磷技术提供参考。
-
实验用水取自北京市某城市污水处理厂的二级出水(SS=10.8~12.0 mg·L−1,pH=6.9±0.1),实验所用的化学药品有磷酸二氢钾、钼酸铵、酒石酸锑氧钾、抗坏血酸、浓硫酸、氢氧化钠、氯化铁(FeCl3·6H2O)、聚合氯化铝(PAC,Al2O3含量不少于28%)、硫酸铝(Al2(SO4)3·18H2O)均为分析纯,羟基铁吸附剂为自行制备的羟基铁颗粒,粒径为2 mm。
实验所用仪器有MY3000-6系列混凝实验搅拌仪(武汉市梅宇仪器有限公司)、UVmini-1240紫外可见分光光度计(岛津仪器有限公司)、S-3400N扫描电子显微镜(日立公司)、ASAP 2020M比表面积及孔隙率分析仪(美国麦克公司)、EHN-B11VC1R蠕动泵(保定兰格)。
-
混凝实验使用六联搅拌器,先以200 r·min−1快速混合2 min,再以100 r·min−1慢速搅拌10 min,静置30 min后于液面以下1~2 cm处取样,测定反应体系中各种形态磷的含量。
-
将羟基铁粉末与10%的改性聚乙烯醇(PVA)黏结剂按照一定比例混合,根据湿物料的黏湿程度,确定液固质量比为0.48,再使用摇摆式挤压造粒机通过2 mm的孔径模具挤出,于105 ℃条件下烘干制得羟基铁颗粒。表面形貌的测定采用型号为S-3400N的扫描电子显微镜,将少量粉末或颗粒涂在导电胶上,固定在样品台上进行观察。比表面积及孔隙结构的测定采用型号为ASAP 2020M的比表面积及孔隙率分析仪。
-
吸附实验采用小型固定床吸附柱装置,柱身内径为4 cm,吸附剂装填高度为14.2 cm,采用上向流,设定不同的空床接触时间(EBCT),取样测定出水中各种形态磷的含量。
-
磷的测定采用钼锑抗分光光度法。根据不同的预处理方法,将磷的形态分为TP、可溶性总磷(soluble total phosphorus,STP)、可溶性活性磷酸盐(soluble reactive phosphorus,SRP)、颗粒态磷(particulate phosphorus,PP)及其他溶解磷[15]。原水样直接消解测得TP含量;经0.45 μm滤膜过滤,消解测得STP含量;经0.45 μm滤膜过滤但不消解测得SRP含量;TP减去STP得PP;STP减去SRP得出其他溶解磷含量。pH采用便携式pH计测定。
-
对北京市某城市污水处理厂的二级出水中磷的形态分布及浓度水平进行分析,以期为后续采用合适的除磷方法提供依据。采用瞬时取样的方法[15],于2018年10月份对该厂二级出水进行采集,采样时间为08:00—09:00,对不同形态磷浓度进行了测定分析,结果如表1所示。
由表1可知,该厂二级出水中TP浓度的平均水平为0.332 mg·L−1;各种形态磷含量的分布规律为可溶性总磷>可溶性活性磷酸盐>颗粒态磷>其他溶解性磷,他们占TP的比例依次为75.90%、64.16%、24.10%、11.74%。二级出水中磷的形态分布规律与二级生化处理工艺有关。该厂的二级生化处理系统采用化学强化生物脱氮除磷工艺,即在A2/O工艺曝气池出水渠两端投加化学除磷药剂Al2(SO4)3,通过沉淀污泥回流有效地利用混凝药剂,既促进了反硝化效果、改善了污泥沉降性能,又将出水TP控制在0.5 mg·L−1以内。
-
化学沉淀除磷方法中,混凝剂种类、投加量、水温、pH都会影响混凝效果。由于改变水温比较困难、调节pH会增加处理费用,故本研究重点考察在不改变实验用水的温度和pH条件下,混凝剂种类和投加量对不同形态磷的去除效果的影响,以确定合适的化学除磷药剂及剂量,并与吸附法除磷效果作对比。
-
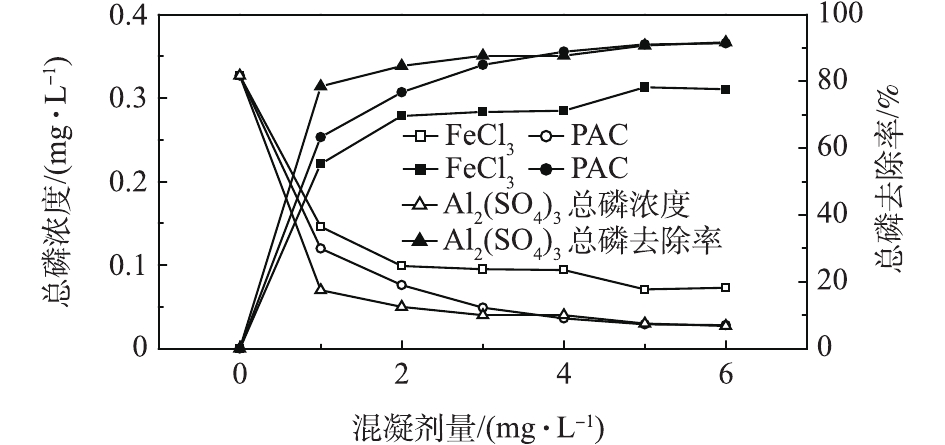
目前,应用较多的混凝剂有铝盐、铁盐和钙盐。由于混凝剂的形态和反应生成的金属磷酸盐沉淀的溶解度均受水体的pH影响,故不同混凝剂有各自最佳的除磷pH范围。一般对于化学沉淀除磷,铝盐的适宜pH为5.0~8.0,铁盐的适宜pH为8.0左右,石灰的适宜pH在10.0以上[16-17]。考虑到该厂二级出水的pH在6.8~7.0,在不调节水体pH的前提下,本研究采用FeCl3、PAC、Al2(SO4)3等3种混凝剂对二级出水进行混凝沉淀实验,考察了不同混凝剂投加量对出水TP的去除效果,以确定适宜的混凝剂种类和投加量。实验水温为(25.0±1.0) ℃,混凝剂投加量范围为1~6 mg·L−1,若无特殊说明,混凝剂的量均以金属离子计。结果如图1所示。由图1可以看出,3种混凝剂中,Al2(SO4)3的除磷效果最好,PAC次之,FeCl3的除磷效果最差。当投加量为1~3 mg·L−1时,TP去除率显著增大,随后趋于平缓。当Al2(SO4)3和PAC的投加量均为3 mg·L−1时,TP去除率分别为87.78%和85.02%,出水TP浓度均低于0.05 mg·L−1。继续增大混凝剂投加量,去除率变化不明显。而FeCl3的投加量为3 mg·L−1时,TP去除率仅为70.95%,即使投加量增大至6 mg·L−1,出水TP浓度仍未能降至0.05 mg·L−1以下,且FeCl3作为混凝剂,反应结束后水体颜色发黄,故其不宜用于该厂二级出水深度除磷。由此可知,Al2(SO4)3和PAC比较适合本研究原水水质,综合考虑除磷效果及投药量的经济因素,确定最佳投药量为3 mg·L−1。
-
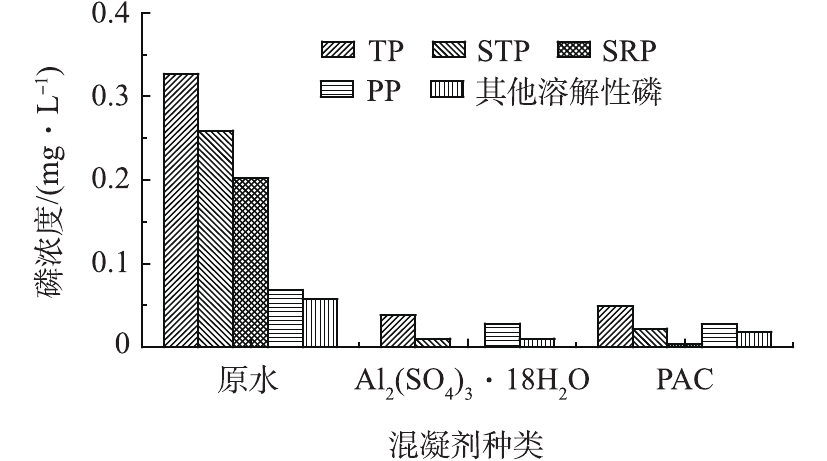
在上述确定的Al2(SO4)3和PAC混凝剂的最佳投加量条件下,对二级出水进行混凝沉淀实验,讨论混凝对不同形态磷的去除效果,结果如图2所示。由图2可知,在混凝沉淀的作用下,各种形态的磷均得到不同程度的去除。其中,SRP的去除效果最好,几乎全部得以去除;而PP和其他溶解性磷的去除效果较差,Al2(SO4)3对SRP、PP及其他溶解性磷的去除率分别为100%、58.8%和82.5%;PAC对SRP、PP及其他溶解性磷的去除率分别为98.0%、60.3%和68.4%。这是由于混凝剂对不同形态磷的作用机理不同造成的。对于同时作为沉淀剂和混凝剂的铝盐来说,一方面,铝盐的水解产物铝离子与SRP发生化学反应生成难溶于水的金属盐沉淀,氢氧化铝絮体可以吸附一部分溶解态磷[18-19];另一方面,作为混凝剂与PP接触使其脱稳、絮凝成较大颗粒,连同金属盐沉淀从水体沉降下来,从而实现磷的去除[20-21]。该水厂二级出水中的磷主要以SRP的形态存在,因此,适宜采用硫酸铝和聚合氯化铝作为混凝剂进行化学除磷。
-
运行维护费用是化学处理系统的重要组成部分,包括药剂费、人工费、电费、维护及污泥处置费用,其中药剂费所占比例较大。根据市售Al2(SO4)3和PAC的价格,结合除磷效果,估算2种药剂的费用。按市售Al2(SO4)3·18H2O价格为800元·t−1,PAC价格为1 900元·t−1计算,对于TP含量为0.327 mg·L−1的原水,深度除磷后出水TP低于0.05 mg·L−1,则每处理1 t废水,Al2(SO4)3·18H2O的药剂费用为0.059 2元,PAC的药剂费用为0.077 0元。综上所述,Al2(SO4)3的除磷效果和药剂费用均比PAC占优势。因此,优先选用Al2(SO4)3作为该厂二级生化出水深度除磷的化学药剂。
-
吸附法除磷作为一种高效低耗的除磷方法近年来逐渐成为国内外的研究热点,该方法利用多孔、大比表面积的吸附材料对磷酸根的吸附亲和力,来实现对水体中磷的去除。本实验所用吸附剂为课题组制备的羟基铁颗粒,对二级出水中磷的吸附效果进行探讨。
-
图3和表2分别为羟基铁粉末与颗粒的表面形貌和比表面积及孔隙结构。由图3(b)可以看出,由摇摆式挤压造粒机制备的羟基铁颗粒呈不规则地颗粒状,有利于增大颗粒的比表面积。对比图3(c)和图3(d)可以看出,黏结剂的加入使松散的粉末聚集在一起,并且颗粒的孔隙结构比较发达。由表2可以看出,颗粒与粉末相比,比表面积和孔容分别减少了39.79%和23.56%,这可能与粘结剂的加入使粉体团聚有关。羟基铁颗粒在25 ℃、pH为7.0时的吸附容量为13.61 mg·g−1。
-
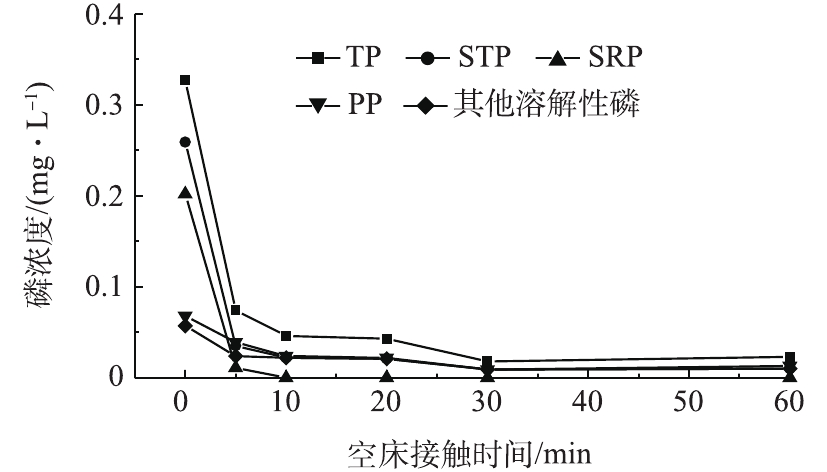
EBCT是固定床操作过程中的一个关键参数,在应用中通常采用整个填料层的容积计算[22]。实验设定EBCT为5~60 min,实验水温为(25.0±1.0) ℃。图4为不同EBCT对应的出水磷浓度。由图4可以看出,吸附剂对TP的去除效果较好,且TP含量的降低大部分归功于SRP含量的降低。PP和其他溶解态磷含量虽然也有一定程度的降低,但幅度很小。当EBCT为5 min时,即可去除二级出水中的大部分TP,出水TP浓度为0.074 mg·L−1。增加EBCT时,各种形态磷的去除率亦随之增大,但其变化幅度减小。当EBCT为10 min时,出水TP浓度由0.327 mg·L−1降至0.046 mg·L−1,SRP全部得以去除,PP和其他溶解态磷分别由0.068 mg·L−1和0.057 mg·L−1降至0.024 mg·L−1和0.022 mg·L−1。继续增加EBCT至30~60 min,PP和其他溶解态磷含量略微减小,但仍未能全部去除。羟基铁对SRP的去除主要是利用磷酸盐与材料表面羟基进行离子交换,并与金属离子形成双配位基内圈配合物[23]。而PP主要是通过滤柱的截留作用得以去除。尽管羟基铁吸附剂对其他形态磷的吸附效果较差,但是二级出水中其他形态磷含量很低,通过吸附仍可将出水TP降至0.05 mg·L−1以下,对控制受纳水体的富营养化具有重要的实际意义。
-
利用羟基铁颗粒吸附除磷,按市售羟基铁、PVA及NaOH的价格分别为9 000、11 000和3 200元·t−1计算,对于TP浓度为0.327 mg·L−1的原水,按深度除磷后出水TP浓度低于0.05 mg·L−1计算,理论上处理1 t二级出水,需消耗羟基铁颗粒20.35 g(其中羟基铁13.75 g,PVA 0.66 g,H2O 5.94 g),所需药剂费用为0.131 01元,高于化学沉淀法除磷的药剂费用。但是,吸附饱和的羟基铁颗粒还可以回收利用,利用100 mL、2 mol·L−1的NaOH溶液对吸附饱和的羟基铁颗粒进行解吸,6 h解吸率达87.27%,解吸后的羟基铁颗粒用蒸馏水清洗几遍至pH不再变化,烘干后可再次用于吸附除磷,NaOH可重复使用。如此重复“吸附-解吸-再吸附”的过程,连续再生3次后,羟基铁颗粒吸附量为初始吸附量的82.3%。从循环使用的角度考虑,羟基铁颗粒除磷的药剂费用将大大降低,羟基铁颗粒循环使用4次后,平均吨水药剂费用可降至0.058 4元(含NaOH费用),略低于Al2(SO4)3的药剂费用。考虑到磷回收的效益,处理1 t二级出水,可回收的磷约0.277 g,如果按磷矿石(30%左右的磷含量)价格为600元·t−1计算,磷回收的收益为0.000 6元·t−1。由此可见,吸附法是一种快速、经济、高效的除磷方法,不仅可以避免产生二次污泥,吸附剂再生后还可以重复利用,还实现了磷资源的回收。
总之,利用化学沉淀法和吸附法处理低浓度含磷废水均能取得良好的处理效果,但从长期的技术经济效益考虑,吸附法优于混凝剂。与化学沉淀法相比,吸附法更符合绿色、节能、可持续发展的污水处理理念。
-
1)所采集的污水处理厂二级出水中TP浓度的平均水平为0.332 mg·L−1,SRP为主要存在形态,占TP浓度的64.16%。与FeCl3相比,Al2(SO4)3和PAC比较适合本实验原水水质,当其投加量为3 mg·L−1时,出水TP可降至0.05 mg·L−1以下。
2)以Al2(SO4)3和PAC作为混凝剂,各种形态的磷均得到不同程度的去除,对SRP的去除效果最好,几乎全部得以去除,而对PP和其他溶解性磷的去除效果较差。通过技术经济比较,Al2(SO4)3的除磷效果和药剂费用均比PAC更占优势。
4)羟基铁颗粒吸附剂对SRP吸附效果显著,对其他溶解性磷吸附效果较差,当空床接触时间大于10 min时,出水TP可降至0.05 mg·L−1以下。从长期的技术经济效益综合考虑,吸附剂优于混凝剂。
城市污水处理厂二级处理出水中磷深度去除技术
Advanced phosphorus removal from the second effluent of municipal sewage treatment plant
-
摘要: 以某城市污水处理厂二级出水为研究对象,分析了其中磷的形态及浓度分布,分别采用FeCl3、PAC、Al2(SO4)3混凝剂和羟基铁颗粒吸附剂开展了基于化学法和吸附法的深度除磷技术的研究。在相同的水质和环境条件下,对不同形态磷的去除效果进行了分析,并对两者的技术经济性进行了对比。结果表明,该厂二级出水中TP浓度的平均水平为0.332 mg·L−1,其中可溶性活性磷酸盐为主要存在形态,占TP浓度的64.16%。与FeCl3相比,Al2(SO4)3和PAC比较适合本实验原水水质,当其投加量为3 mg·L−1时,出水TP可降至0.05 mg·L−1以下。以Al2(SO4)3和PAC作为混凝剂,各种形态的磷均得到不同程度的去除,可溶性活性磷酸盐的去除效果最好,几乎全部得以去除,而颗粒态磷和其他溶解性磷的去除效果较差。通过技术经济比较,Al2(SO4)3在除磷效果和药剂费用上均比PAC占有优势。羟基铁颗粒吸附剂对可溶性活性磷酸盐吸附效果显著,对其他溶解性磷吸附效果较差,当空床接触时间(EBCT)大于10 min时,出水TP可降至0.05 mg·L−1以下。从长期的技术和经济效益综合考虑,吸附剂优于混凝剂。Abstract: In this research, three commonly used coagulants (FeCl3, PAC and Al2(SO4)3) and hydroxyl iron particle adsorbent were used to remove phosphorus from the secondary effluent of a municipal treatment plant. Based on the analysis of the speciation and concentration of phosphorus, the removal effects of different phosphorus forms and technical economy of the coagulation and adsorption methods were discussed under the same water quality and environmental conditions. The results showed that the average TP concentration in the secondary effluent of this plant was 0.332 mg·L−1. The soluble active phosphate was the main form, accounting for 64.16% of TP. Compared with FeCl3, Al2(SO4)3 and PAC were two suitable coagulants for TP removal, and TP concentration in effluent lowered to below 0.05 mg·L−1 at Al2(SO4)3 or PAC dosage of 3 mg·L−1. Additionally, the removal efficiency of particulate phosphorus and other soluble phosphorus was relatively inferior to that of soluble reactive phosphate, the later could be completely removed by Al2(SO4)3 or PAC coagulation. Through the analysis of technical economy of TP removal system, Al2(SO4)3 was selected as the best coagulant. Besides, the adsorption effect of hydroxyl iron adsorbent on soluble reactive phosphate was significant, while on other soluble phosphorus was relatively poor. The TP concentration in effluent was below 0.05 mg·L−1 when the empty bed contact time (EBCT) was longer than 10 min. In view of long-term economic benefits, the performance of adsorbent was better than that of coagulant.
-
我国是世界13个人均水资源最贫乏的国家之一,水资源紧缺严重制约着我国的社会经济发展。将城镇污水再生回用、实现“污水资源化”是解决水资源紧缺的有效途径。补给水环境是再生水的主要用途之一,但其中的磷是造成水体富营养化的关键因素[1-2]。《城市污水再生利用景观环境用水水质》(GB/T 18921-2002)[3]规定观赏性或娱乐性景观环境用水的总磷(total phosphorus,TP)浓度限值为0.5 mg·L−1(湖泊类)或1.0 mg·L−1(河道类)。水体中TP浓度高于0.02 mg·L−1即处于富营养化状态[4],有研究[5-6]表明,污水处理厂出水TP浓度必须控制在0.05 mg·L−1以下。
针对污水处理厂二级处理出水(简称二级出水)低浓度磷的特点,化学沉淀除磷和吸附法除磷不失为经济高效的深度除磷方法。目前,对于化学沉淀除磷,研究者主要关注混凝剂的种类、影响因素和除磷效果[7-9]。对于吸附法除磷,主要集中于吸附材料、吸附机理和吸附性能优劣的研究[10-11]。化学沉淀除磷和吸附法除磷在二级出水中的应用以及对水中不同形态磷的去除效果研究较少。并且前人的研究多数是针对难以达标的水进行化学强化除磷,使出水TP达到规定的排放标准0.5 mg·L−1[12-13]。但是,按照目前的水质标准,再生水若回用于封闭的或流动缓慢的景观环境水体,仍存在爆发水华的风险[14]。因此,对二级出水进行深度除磷,使TP浓度降至更低的水平势在必行。目前,国内对出水TP浓度低于0.05 mg·L−1深度除磷的研究尚未见报道。
针对国内的研究现状,本研究以北京市某城市污水处理厂二级出水为研究对象,将出水TP控制在0.05 mg·L−1以下为研究目的,对磷的形态及浓度分布进行了分析,分别采用化学沉淀法和吸附法对其展开了除磷研究,分析了不同形态磷的去除效果,并对其进行技术经济分析,以期为再生水深度除磷技术提供参考。
1. 材料与方法
1.1 实验材料与仪器
实验用水取自北京市某城市污水处理厂的二级出水(SS=10.8~12.0 mg·L−1,pH=6.9±0.1),实验所用的化学药品有磷酸二氢钾、钼酸铵、酒石酸锑氧钾、抗坏血酸、浓硫酸、氢氧化钠、氯化铁(FeCl3·6H2O)、聚合氯化铝(PAC,Al2O3含量不少于28%)、硫酸铝(Al2(SO4)3·18H2O)均为分析纯,羟基铁吸附剂为自行制备的羟基铁颗粒,粒径为2 mm。
实验所用仪器有MY3000-6系列混凝实验搅拌仪(武汉市梅宇仪器有限公司)、UVmini-1240紫外可见分光光度计(岛津仪器有限公司)、S-3400N扫描电子显微镜(日立公司)、ASAP 2020M比表面积及孔隙率分析仪(美国麦克公司)、EHN-B11VC1R蠕动泵(保定兰格)。
1.2 混凝实验
混凝实验使用六联搅拌器,先以200 r·min−1快速混合2 min,再以100 r·min−1慢速搅拌10 min,静置30 min后于液面以下1~2 cm处取样,测定反应体系中各种形态磷的含量。
1.3 羟基铁颗粒的制备与性能表征
将羟基铁粉末与10%的改性聚乙烯醇(PVA)黏结剂按照一定比例混合,根据湿物料的黏湿程度,确定液固质量比为0.48,再使用摇摆式挤压造粒机通过2 mm的孔径模具挤出,于105 ℃条件下烘干制得羟基铁颗粒。表面形貌的测定采用型号为S-3400N的扫描电子显微镜,将少量粉末或颗粒涂在导电胶上,固定在样品台上进行观察。比表面积及孔隙结构的测定采用型号为ASAP 2020M的比表面积及孔隙率分析仪。
1.4 吸附实验
吸附实验采用小型固定床吸附柱装置,柱身内径为4 cm,吸附剂装填高度为14.2 cm,采用上向流,设定不同的空床接触时间(EBCT),取样测定出水中各种形态磷的含量。
1.5 分析方法
磷的测定采用钼锑抗分光光度法。根据不同的预处理方法,将磷的形态分为TP、可溶性总磷(soluble total phosphorus,STP)、可溶性活性磷酸盐(soluble reactive phosphorus,SRP)、颗粒态磷(particulate phosphorus,PP)及其他溶解磷[15]。原水样直接消解测得TP含量;经0.45 μm滤膜过滤,消解测得STP含量;经0.45 μm滤膜过滤但不消解测得SRP含量;TP减去STP得PP;STP减去SRP得出其他溶解磷含量。pH采用便携式pH计测定。
2. 结果与讨论
2.1 二级出水中磷浓度及形态表征
对北京市某城市污水处理厂的二级出水中磷的形态分布及浓度水平进行分析,以期为后续采用合适的除磷方法提供依据。采用瞬时取样的方法[15],于2018年10月份对该厂二级出水进行采集,采样时间为08:00—09:00,对不同形态磷浓度进行了测定分析,结果如表1所示。
表 1 某污水处理厂二级出水中磷形态及浓度分布Table 1. Phosphorus speciation and concentration in the secondary effluent of a sewage treatment plant采样日期 总磷 可溶性总磷 可溶性活性磷酸盐 颗粒态磷 其他溶解性磷 2018-10-08 0.260 0.201 0.170 0.059 0.031 2018-10-11 0.387 0.288 0.254 0.099 0.034 2018-10-14 0.334 0.237 0.201 0.097 0.036 2018-10-17 0.351 0.273 0.236 0.078 0.037 2018-10-20 0.327 0.259 0.202 0.068 0.057 由表1可知,该厂二级出水中TP浓度的平均水平为0.332 mg·L−1;各种形态磷含量的分布规律为可溶性总磷>可溶性活性磷酸盐>颗粒态磷>其他溶解性磷,他们占TP的比例依次为75.90%、64.16%、24.10%、11.74%。二级出水中磷的形态分布规律与二级生化处理工艺有关。该厂的二级生化处理系统采用化学强化生物脱氮除磷工艺,即在A2/O工艺曝气池出水渠两端投加化学除磷药剂Al2(SO4)3,通过沉淀污泥回流有效地利用混凝药剂,既促进了反硝化效果、改善了污泥沉降性能,又将出水TP控制在0.5 mg·L−1以内。
2.2 化学沉淀对磷的去除规律
化学沉淀除磷方法中,混凝剂种类、投加量、水温、pH都会影响混凝效果。由于改变水温比较困难、调节pH会增加处理费用,故本研究重点考察在不改变实验用水的温度和pH条件下,混凝剂种类和投加量对不同形态磷的去除效果的影响,以确定合适的化学除磷药剂及剂量,并与吸附法除磷效果作对比。
2.2.1 混凝剂种类对出水总磷浓度的影响
目前,应用较多的混凝剂有铝盐、铁盐和钙盐。由于混凝剂的形态和反应生成的金属磷酸盐沉淀的溶解度均受水体的pH影响,故不同混凝剂有各自最佳的除磷pH范围。一般对于化学沉淀除磷,铝盐的适宜pH为5.0~8.0,铁盐的适宜pH为8.0左右,石灰的适宜pH在10.0以上[16-17]。考虑到该厂二级出水的pH在6.8~7.0,在不调节水体pH的前提下,本研究采用FeCl3、PAC、Al2(SO4)3等3种混凝剂对二级出水进行混凝沉淀实验,考察了不同混凝剂投加量对出水TP的去除效果,以确定适宜的混凝剂种类和投加量。实验水温为(25.0±1.0) ℃,混凝剂投加量范围为1~6 mg·L−1,若无特殊说明,混凝剂的量均以金属离子计。结果如图1所示。由图1可以看出,3种混凝剂中,Al2(SO4)3的除磷效果最好,PAC次之,FeCl3的除磷效果最差。当投加量为1~3 mg·L−1时,TP去除率显著增大,随后趋于平缓。当Al2(SO4)3和PAC的投加量均为3 mg·L−1时,TP去除率分别为87.78%和85.02%,出水TP浓度均低于0.05 mg·L−1。继续增大混凝剂投加量,去除率变化不明显。而FeCl3的投加量为3 mg·L−1时,TP去除率仅为70.95%,即使投加量增大至6 mg·L−1,出水TP浓度仍未能降至0.05 mg·L−1以下,且FeCl3作为混凝剂,反应结束后水体颜色发黄,故其不宜用于该厂二级出水深度除磷。由此可知,Al2(SO4)3和PAC比较适合本研究原水水质,综合考虑除磷效果及投药量的经济因素,确定最佳投药量为3 mg·L−1。
2.2.2 混凝对出水不同形态磷浓度的影响
在上述确定的Al2(SO4)3和PAC混凝剂的最佳投加量条件下,对二级出水进行混凝沉淀实验,讨论混凝对不同形态磷的去除效果,结果如图2所示。由图2可知,在混凝沉淀的作用下,各种形态的磷均得到不同程度的去除。其中,SRP的去除效果最好,几乎全部得以去除;而PP和其他溶解性磷的去除效果较差,Al2(SO4)3对SRP、PP及其他溶解性磷的去除率分别为100%、58.8%和82.5%;PAC对SRP、PP及其他溶解性磷的去除率分别为98.0%、60.3%和68.4%。这是由于混凝剂对不同形态磷的作用机理不同造成的。对于同时作为沉淀剂和混凝剂的铝盐来说,一方面,铝盐的水解产物铝离子与SRP发生化学反应生成难溶于水的金属盐沉淀,氢氧化铝絮体可以吸附一部分溶解态磷[18-19];另一方面,作为混凝剂与PP接触使其脱稳、絮凝成较大颗粒,连同金属盐沉淀从水体沉降下来,从而实现磷的去除[20-21]。该水厂二级出水中的磷主要以SRP的形态存在,因此,适宜采用硫酸铝和聚合氯化铝作为混凝剂进行化学除磷。
2.2.3 技术经济比较
运行维护费用是化学处理系统的重要组成部分,包括药剂费、人工费、电费、维护及污泥处置费用,其中药剂费所占比例较大。根据市售Al2(SO4)3和PAC的价格,结合除磷效果,估算2种药剂的费用。按市售Al2(SO4)3·18H2O价格为800元·t−1,PAC价格为1 900元·t−1计算,对于TP含量为0.327 mg·L−1的原水,深度除磷后出水TP低于0.05 mg·L−1,则每处理1 t废水,Al2(SO4)3·18H2O的药剂费用为0.059 2元,PAC的药剂费用为0.077 0元。综上所述,Al2(SO4)3的除磷效果和药剂费用均比PAC占优势。因此,优先选用Al2(SO4)3作为该厂二级生化出水深度除磷的化学药剂。
2.3 吸附剂对磷的吸附去除规律
吸附法除磷作为一种高效低耗的除磷方法近年来逐渐成为国内外的研究热点,该方法利用多孔、大比表面积的吸附材料对磷酸根的吸附亲和力,来实现对水体中磷的去除。本实验所用吸附剂为课题组制备的羟基铁颗粒,对二级出水中磷的吸附效果进行探讨。
2.3.1 羟基铁颗粒的基本特性
图3和表2分别为羟基铁粉末与颗粒的表面形貌和比表面积及孔隙结构。由图3(b)可以看出,由摇摆式挤压造粒机制备的羟基铁颗粒呈不规则地颗粒状,有利于增大颗粒的比表面积。对比图3(c)和图3(d)可以看出,黏结剂的加入使松散的粉末聚集在一起,并且颗粒的孔隙结构比较发达。由表2可以看出,颗粒与粉末相比,比表面积和孔容分别减少了39.79%和23.56%,这可能与粘结剂的加入使粉体团聚有关。羟基铁颗粒在25 ℃、pH为7.0时的吸附容量为13.61 mg·g−1。
表 2 羟基铁粉末与颗粒的主要性能指标Table 2. Properties of hydroxyl iron powder and granular adsorbents吸附剂 粒径/mm BET比表面积/(m2·g−1) 孔容/(m3·g−1) 平均孔径/nm 吸附容量/(mg·g−1) 羟基铁粉末 — 139.731 2 0.147 7 4.229 3 37.45 羟基铁颗粒 2.0 84.132 1 0.112 9 5.369 7 13.61 注:上述吸附容量均指温度为25 ℃、pH为7.0时的吸附容量。 2.3.2 EBCT对出水磷浓度的影响
EBCT是固定床操作过程中的一个关键参数,在应用中通常采用整个填料层的容积计算[22]。实验设定EBCT为5~60 min,实验水温为(25.0±1.0) ℃。图4为不同EBCT对应的出水磷浓度。由图4可以看出,吸附剂对TP的去除效果较好,且TP含量的降低大部分归功于SRP含量的降低。PP和其他溶解态磷含量虽然也有一定程度的降低,但幅度很小。当EBCT为5 min时,即可去除二级出水中的大部分TP,出水TP浓度为0.074 mg·L−1。增加EBCT时,各种形态磷的去除率亦随之增大,但其变化幅度减小。当EBCT为10 min时,出水TP浓度由0.327 mg·L−1降至0.046 mg·L−1,SRP全部得以去除,PP和其他溶解态磷分别由0.068 mg·L−1和0.057 mg·L−1降至0.024 mg·L−1和0.022 mg·L−1。继续增加EBCT至30~60 min,PP和其他溶解态磷含量略微减小,但仍未能全部去除。羟基铁对SRP的去除主要是利用磷酸盐与材料表面羟基进行离子交换,并与金属离子形成双配位基内圈配合物[23]。而PP主要是通过滤柱的截留作用得以去除。尽管羟基铁吸附剂对其他形态磷的吸附效果较差,但是二级出水中其他形态磷含量很低,通过吸附仍可将出水TP降至0.05 mg·L−1以下,对控制受纳水体的富营养化具有重要的实际意义。
2.3.3 技术经济分析
利用羟基铁颗粒吸附除磷,按市售羟基铁、PVA及NaOH的价格分别为9 000、11 000和3 200元·t−1计算,对于TP浓度为0.327 mg·L−1的原水,按深度除磷后出水TP浓度低于0.05 mg·L−1计算,理论上处理1 t二级出水,需消耗羟基铁颗粒20.35 g(其中羟基铁13.75 g,PVA 0.66 g,H2O 5.94 g),所需药剂费用为0.131 01元,高于化学沉淀法除磷的药剂费用。但是,吸附饱和的羟基铁颗粒还可以回收利用,利用100 mL、2 mol·L−1的NaOH溶液对吸附饱和的羟基铁颗粒进行解吸,6 h解吸率达87.27%,解吸后的羟基铁颗粒用蒸馏水清洗几遍至pH不再变化,烘干后可再次用于吸附除磷,NaOH可重复使用。如此重复“吸附-解吸-再吸附”的过程,连续再生3次后,羟基铁颗粒吸附量为初始吸附量的82.3%。从循环使用的角度考虑,羟基铁颗粒除磷的药剂费用将大大降低,羟基铁颗粒循环使用4次后,平均吨水药剂费用可降至0.058 4元(含NaOH费用),略低于Al2(SO4)3的药剂费用。考虑到磷回收的效益,处理1 t二级出水,可回收的磷约0.277 g,如果按磷矿石(30%左右的磷含量)价格为600元·t−1计算,磷回收的收益为0.000 6元·t−1。由此可见,吸附法是一种快速、经济、高效的除磷方法,不仅可以避免产生二次污泥,吸附剂再生后还可以重复利用,还实现了磷资源的回收。
总之,利用化学沉淀法和吸附法处理低浓度含磷废水均能取得良好的处理效果,但从长期的技术经济效益考虑,吸附法优于混凝剂。与化学沉淀法相比,吸附法更符合绿色、节能、可持续发展的污水处理理念。
3. 结论
1)所采集的污水处理厂二级出水中TP浓度的平均水平为0.332 mg·L−1,SRP为主要存在形态,占TP浓度的64.16%。与FeCl3相比,Al2(SO4)3和PAC比较适合本实验原水水质,当其投加量为3 mg·L−1时,出水TP可降至0.05 mg·L−1以下。
2)以Al2(SO4)3和PAC作为混凝剂,各种形态的磷均得到不同程度的去除,对SRP的去除效果最好,几乎全部得以去除,而对PP和其他溶解性磷的去除效果较差。通过技术经济比较,Al2(SO4)3的除磷效果和药剂费用均比PAC更占优势。
4)羟基铁颗粒吸附剂对SRP吸附效果显著,对其他溶解性磷吸附效果较差,当空床接触时间大于10 min时,出水TP可降至0.05 mg·L−1以下。从长期的技术经济效益综合考虑,吸附剂优于混凝剂。
-
表 1 某污水处理厂二级出水中磷形态及浓度分布
Table 1. Phosphorus speciation and concentration in the secondary effluent of a sewage treatment plant
采样日期 总磷 可溶性总磷 可溶性活性磷酸盐 颗粒态磷 其他溶解性磷 2018-10-08 0.260 0.201 0.170 0.059 0.031 2018-10-11 0.387 0.288 0.254 0.099 0.034 2018-10-14 0.334 0.237 0.201 0.097 0.036 2018-10-17 0.351 0.273 0.236 0.078 0.037 2018-10-20 0.327 0.259 0.202 0.068 0.057 表 2 羟基铁粉末与颗粒的主要性能指标
Table 2. Properties of hydroxyl iron powder and granular adsorbents
吸附剂 粒径/mm BET比表面积/(m2·g−1) 孔容/(m3·g−1) 平均孔径/nm 吸附容量/(mg·g−1) 羟基铁粉末 — 139.731 2 0.147 7 4.229 3 37.45 羟基铁颗粒 2.0 84.132 1 0.112 9 5.369 7 13.61 注:上述吸附容量均指温度为25 ℃、pH为7.0时的吸附容量。 -
[1] SCHINDLER D W, HECKY R E, FINDLAY D L, et al. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment[J]. Proceedings of the National Academy of Sciences, 2008, 105(32): 11254-11258. doi: 10.1073/pnas.0805108105 [2] CORRELL D L. The role of phosphorus in the eutrophication of receiving waters: A review[J]. Journal of Environmental Quality, 1998, 27(2): 261-266. [3] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 城市污水再生利用景观环境用水水质: GB 18921-2002[S]. 北京: 中国标准出版社, 2003. [4] 陈旭华. 用尼梅罗(Nemerow)污染指数评价地表水营养状况的探讨[J]. 安全与环境学报, 2003, 3(2): 24-26. doi: 10.3969/j.issn.1009-6094.2003.02.006 [5] RAGSDALE D. Advanced treatment to achieve low concentration of phosphorus[R]. Washington, D C: United States Environment Protection Agency, 2007. [6] WALKER J L, YOUNOS T, ZIPPER C E. Nutrients in lakes and reservoirs: A literature review for use in nutrient criteria development[D]. Blacksburg: Virginia Polytechnic Institute and State University, 2007. [7] OMOIKE A I, VANLOON G W. Removal of phosphorus and organic matter removal by alum during wastewater treatment[J]. Water Research, 1999, 33(17): 3617-3627. doi: 10.1016/S0043-1354(99)00075-5 [8] 李子富, 云玉攀, 曾灏, 等. 城市污水处理厂化学强化生物除磷的实验研究[J]. 中国环境科学, 2014, 34(12): 3070-3077. [9] AGUILAR M I, SAEZ J, LORENS M A, et al. Nutrient removal and sludge production in the coagulation-flocculation process[J]. Water Research, 2002, 36(11): 2910-2919. doi: 10.1016/S0043-1354(01)00508-5 [10] ZENG L, LI X M, LIU J D. Removal of phosphate from aqueous solutions using iron oxide tailings[J]. Water Research, 2004, 38(5): 1318-1326. doi: 10.1016/j.watres.2003.12.009 [11] YOSHIMI S, YOSHIO N. Removal of phosphate by layered double hydroxides containing iron[J]. Water Research, 2002, 36(5): 1306-1312. doi: 10.1016/S0043-1354(01)00340-2 [12] 方华, 万震, 江玒, 等. 二级生化出水化学法深度除磷实验[J]. 南京信息工程大学学报(自然科学版), 2013, 5(5): 462-465. [13] 冯锦梅, 陆俊晨. 二级生化出水除磷及磷形态研究[J]. 污染防治技术, 2012, 25(6): 1-4. [14] 胡洪营, 吴乾元, 黄晶晶, 等. 再生水水质安全评价与保障原理[M]. 北京: 科学出版社, 2011. [15] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. [16] CARAVELLI A H, CONTRERAS E M, ZARITZKY N E. Phosphorus removal in batch systems using ferric chloride in the presence of activated sludge[J]. Hazardous Materials, 2010, 177(1/2/3): 199-208. [17] 孟顺龙, 裘丽萍, 陈家长. 污水化学沉淀法除磷研究进展[J]. 中国农学通报, 2012, 28(35): 264-268. doi: 10.3969/j.issn.1000-6850.2012.35.049 [18] TANADA S, KABAYAMA M, KAWASAKI N, et al. Removal of phosphate by aluminum oxide hydroxide[J]. Journal of Colloid and Interface Science, 2003, 257(1): 135-140. doi: 10.1016/S0021-9797(02)00008-5 [19] MA B W, CHEN G X, HU C Z, et al. Speciation matching mechanisms between orthophosphate and aluminum species during advanced P removal process[J]. Science of the Total Environment, 2018, 642: 1311-1319. doi: 10.1016/j.scitotenv.2018.06.171 [20] 边兴玉, 王文超, 张志斌, 等. 化学辅助除磷工艺药剂投加量的优化研究[J]. 山东建筑大学学报, 2007, 22(2): 513-516. [21] 侯红娟, 王洪洋, 周琪. 低碳、高氮磷城市污水的化学辅助除磷研究[J]. 中国给水排水, 2007, 23(11): 24-27. doi: 10.3321/j.issn:1000-4602.2007.11.007 [22] CRITTENDEN J, BERRIGAN J, HAND D. Design of rapid small scale adsorption tests for a constant diffusivity[J]. Water Pollution Control Federation, 1986, 58(4): 312-319. [23] ZENG H, FISHER B, GIAMMAR D E. Individual and competitive adsorption of arsenate and phosphate to a high-surface-area iron oxide-based sorbent[J]. Environmental Science and Technology, 2008, 42(1): 147-152. doi: 10.1021/es071553d -




 DownLoad:
DownLoad: