-
阻燃剂是一类赋予高分子聚合物较难燃烧特性的添加剂,根据其使用方法可以分为两类,添加型阻燃剂和反应型阻燃剂,前者直接添加混合到聚合物中,后者与聚合物化学反应从而产生阻燃成分. 多氯联苯类和多溴联苯醚类阻燃剂因为长距离迁移性以及生物毒性和对环境的污染,已经逐步被世界各国禁止使用[1]. 而有机磷酸酯(OPEs)作为替代品,已经在各行各业广泛用作阻燃剂和增塑剂,例如:塑料、纺织品、电子设备甚至建筑[2,3]. 随着多溴联苯醚(PBDEs)等传统溴化阻燃剂被列入《斯德哥尔摩公约》,OPEs的使用量日益增多,从2001年的18.6万吨增加到2018年的100万吨[4],预计到2025年将增加8%[5]. 但是大部分OPEs是通过物理的方式添加,与材料之间并没有化学键合,因此在使用过程中很容易通过挥发、磨损等方式溶出后进入环境[6]. OPEs是一类以磷酸基团为基础,3个氢被其他基团所取代的化合物,其在环境中常见的种类达十多种,且不同的OPEs的物理化学性质有较大的不同(表1)[7]. OPEs依据侧链基团的不同可以大致分为三大类:带烷基的OPEs、带卤代烷基的OPEs和带苯基的OPEs. 由于不同OPEs取代基不同,理化性质差异也很大. 但也有一定规律可循,如结构相似的OPEs在水中的溶解度就与相对分子质量成反比[2],随着OPEs相对分子量的增加,其疏水性通常逐渐增强,蒸气压随之降低. 从OPEs的核心结构可以表明,水解很有可能是重要的自然衰减机制[8]. 已有研究表明,在水体中带烷基的OPEs可以被光降解,卤代烷基的OPEs不会被光解[9]. 带苯基的OPEs在好氧条件下,能被微生物降解,最近有研究发现了在厌氧条件下能够降解带卤代烷基的OPEs的菌株[10].
目前已经在多种环境介质中检测到OPEs的存在,如大气[11 − 12]、水体[13 − 14]、土壤[15 − 16]、室内灰尘[17 − 18]、降雪[19]、食品包装袋[20]等,甚至出现在人烟稀少的青藏高原[16]和北极[21]. 虽然含量种类差异很大,但是足以表明其在环境中的持久性和长距离传输性. 大量研究表明其具有潜在的致癌、神经毒素、生殖毒性和内分泌干扰等特性[22],并且多种OPEs被多国列入重点关注的污染物[3,10]. 因此,研究OPEs在环境各介质中的分布、毒害效应、迁移转化和绿色高效的修复技术十分重要.
-
目前土壤中OPEs的分布状况研究较少,大部分出现在垃圾填埋场、水处理厂和拆卸场地等[23]. 在不同类型、不同土壤利用状况的土壤中OPEs分布差异很大[24]. 但是,在我国土壤的OPEs中,氯代OPEs占据74%以上,其中又以TCPP为主要OPEs单体[15]. Wang等[15]检测了来自中国各地的土壤样品中19种OPEs,发现OPEs浓度区别较大,最高浓度430 ng∙g−1接近最低浓度4.5 ng∙g−1的一百倍,且发达地区(如华东、华南)的OPEs浓度高于其他地区(如中部、西部),这一结果说明人类活动会带来OPEs污染. 中国生产OPEs的工厂也主要分布于东南沿海地区[25],这是土壤中OPEs的一个重要污染源. 在中国某废弃塑料堆积地中检测到38—
1250 ng∙g−1的OPEs,且在小麦中也检测到了OPEs[23]. 在人烟稀少的青藏高原土壤中也检测到OPEs的存在,且其在垂直方向的分布受到土壤化学性质的影响[16]. 国外也报道了土壤中OPEs检出,如美军的空军基地检测到TMPP和TPHP,这是因为点源排放而引起的污染[26]. 农用地OPEs污染的重要来源之一是农膜. 有研究表明,OPEs可以通过土壤进入食物链从而对人体健康造成潜在风险[27]. -
水体作为流动性很大的介质,在多地都检测出OPEs的存在. 在深海和北极群岛中都监测到OPEs,其中卤代OPEs含量最大,其远距离传输潜力可能更大[13]. 地表水的OPEs大多数都来自污水、垃圾渗滤液以及大气沉降. 黄河流域入海口典型区域的地表水中总OPEs浓度范围为183.81—
1674.52 ng∙L−1,其中TCEP和TCPP是主要组分[14]. 在河南省24个污水处理厂中主要有TCEP、TCPP和TBEP,含量分别为2.50—203、6.70—161、1.60—383 ng∙g−1. 污泥中的OPEs也说明污水处理厂具有一定除去污水中OPEs的能力. 太湖沉积物中的OPEs浓度范围从2012年的3.38—14.25 ng∙g−1(干重)[28]变化到2015年的10.76—335.37 ng∙g−1和2016年的8.06—425.39 ng∙g-1[29],表明太湖沉积物中OPEs水平有所上升,且最高浓度的大幅提升可能意味着这期间产生了新的主要污染源. 张成诺等[14]还研究发现,夏季太湖的水体和沉积物中OPEs的分布多于冬季,并推测这是不同季节的水温和pH存在差异造成的结果. 此外,有研究表明城区雪中OPEs以TCPP为主[19,30],其主要来源于交通运输. 可以看出,TCEP和TCPP是水体中最常见的两种OPEs组分. -
大气可能是OPEs远距离传输的主要载体. 由于OPEs可以通过大气远距离迁移,偏远地区大气中也能检测到一定浓度的OPEs,如南海北部,其来源也被认为是来自大陆的大气传质[6]. Chen等[11]比较了农村与城市的室外灰尘OPEs浓度,发现城市灰尘中OPEs浓度大于农村,但两地都是以TCPP占据主导. 周佳敏等[12]研究了上海市多种室内的灰尘,发现研究生办公室和办公楼灰尘中OPEs浓度比其他环境高1—2个数量级,这可能是因为这些地方的电子产品使用较多. 在多种废弃物回收区开放式和半封闭式工作区的灰尘中OPEs浓度范围分别为
1390 —42700 ng∙g−1和914—7940 ng∙g-1[17],说明半封闭式回收有利于减少OPEs的释放. 韦旭[18]研究发现,电子废弃物拆解厂区室内灰尘中OPEs浓度范围为9547 —311000 ng∙g−1. 对比之下,拆解厂区灰尘中的OPEs浓度一般比附近土壤中高出一个数量级,暴露时有必要做好防护措施. -
已有的研究证实,TCEP、TCIPP和TDCIPP具有神经毒性和致癌作用[2]; TPHP和TBEP能对 DNA产生损伤和对心脏产生毒性效应[31]. 环境中广泛存在有机磷酸酯能通过空气、粉尘吸入或皮肤接触以及饮食摄入等暴露途径对人体健康构成威胁[32]. 有机磷酸酯对水生生态和土壤生态的也存在较大影响.
-
人类长期暴露在含有OPEs的环境会存在健康风险. 由于OPEs作为阻燃剂和增塑剂广泛应用使其容易释放进入环境. 早在2000年已有研究表明办公环境中TPhP的来源可能是电脑的显示器[33]. 周佳敏等[12]发现卤代OPEs是多数室内环境的主要OPEs组分,而烷基OPEs在办公场所、教室等地成为主要OPEs组分,其原因可能是卤代OPEs的使用量大于其余OPEs,且能在环境介质中持久地存在;另一方面,烷基OPEs常用于电子设备的阻燃剂[2],由此可说明室内电子设备是该环境中OPEs的主要来源. 灰尘通常是室内OPEs暴露最主要的途径[34]. 由于在室内灰尘累积时间较长,且附着的OPEs不易光解,因此更容易富集挥发性弱的OPEs. 但是对于某些挥发性强的OPEs(如TCEP)来说,呼吸同样是重要摄入途径. 挪威的一项OPEs暴露研究表明,成年人通过呼吸摄入的OPEs(9.3 ng∙kg−1∙d−1)与通过灰尘摄入的OPEs(8.9 ng∙kg−1∙d−1)相当[35]. 对室外来说,室外环境较开放,可能的来源更为多样,如交通运输[19]、工业排放[11]、住宅扩散[36]等,远距离的大气迁移也是可能的来源.
-
除了直接暴露在含有OPEs的环境中外,摄入含有OPEs的食物和水也会导致人体OPEs的累积. He等[37]调查了澳大利亚昆士兰东南部87个食品样本和5个自来水样本中OPEs的浓度,发现蔬菜中的OPEs浓度最高,平均2.6 ng∙g−1湿重. 他们还发现比起粉尘吸入,膳食摄入是TCEP、TCIPP进入人体更为重要的暴露途径,可以达到总摄入量的75%以上,且食品加工过程是食品内OPEs的主要来源. 比利时的一项研究从市场中食品的OPEs含量推算成年人每日的饮食摄入[38],发现TPhP、TnBP和TEHP的饮食摄入分别为0.3—4.4、3.5—39、23—71 ng∙kg−1∙d−1. 类似地,在食品接触材料中发现了18种OPEs[20],这些OPEs能够迁移到食品中从而危害人体健康. 因此,不仅要关注生活、工作区内的OPEs浓度,食品原产地、食品加工厂区和加工材料的OPEs浓度也不容忽视,食品中的OPEs也应当继续研究.
-
有研究使用高通量毒代动力学模型,可以将一些体内[39]和体外 [40]的毒性数据与人体暴露数据进行对比,进而得到最低风险水平与人类暴露的关系. 其中TPHP和TDCIPP的最低风险水平在人类暴露浓度范围内,因为数据有限,所以其他种类的OPEs的潜在危害也不容忽视[3]. 另一方面,由于儿童更加容易接触到OPEs,儿童的每日摄入量是成年人的2—11倍[41]. OPEs对儿童的潜在危害更严重.
-
已有较多研究通过风险商评价模型(RQ)来评估水生生物的生态风险以及水、沉积物和土壤中残留OPEs的生态毒理学风险[42]. 有报道表明,磷酸三(2,3-二溴丙基)酯(TDBPP)在南京市和太湖流域检测到,且在南京市显示出高风险[43]. 已有多项实验证明OPEs对水生生物的毒性效应. 例如,Hong等[44]发现TPhP能够让鱼类的血脑屏障变得通透并激活其炎症反应,鱼类小脑暴露在TPhP中也会使其学习、记忆能力受损. Yan等[45]将成年河蚬细胞分别暴露于2000 μg∙L−1的TDCIPP和TnBP中,结果表明细胞凋亡受到了显著影响,DNA也受到了损伤,且同浓度下TDCIPP毒性大于TnBP,这说明一些OPEs具有致癌的风险. 另外两项关于TDCIPP对斑马鱼毒性的研究分别表明其能在雌性鱼的大脑、性腺和肝脏中累积,造成雌性鱼的卵子减少、体重减轻、长度变短,也能影响胚胎的发育[46 − 47].
-
目前,许多研究聚焦于植物对OPEs的富集和代谢,而OPEs对土壤生态危害的相关研究较少. 蚯蚓对于土壤生态的重要性不言而喻,有研究表明OPEs能够诱导蚯蚓产生氧化应激[48 − 49],长期暴露实验表明高于500 ng∙g−1的TDCIPP降低了蚯蚓的体重和幼仔出生率,还造成了精囊损伤、皮肤损伤和肌肉萎缩[50],这些影响无疑对土壤生态存在危害. 实验表明TCIPP对植物具有一定的毒性,能够显著抑制黑麦草的生长发育[51]. OPEs在植物体中依赖蒸腾作用来向上转运,因此TPhP这类疏水性强的OPEs容易富集于植物的根部[23],这可能对OPEs土壤危害的相关研究起到一定的启示作用.
-
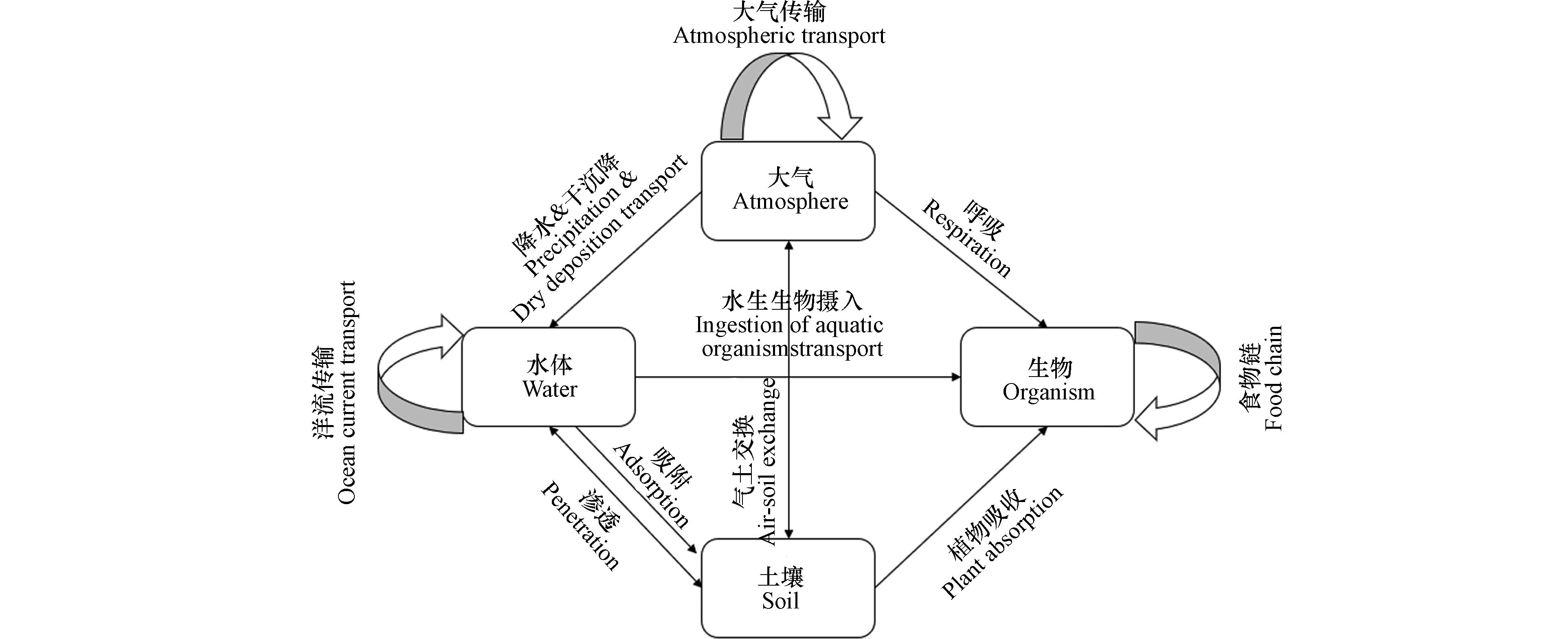
有机磷酸酯在环境中的迁移形式多样,从最基本的在材料中挥发、磨损,到同介质内的大气传输、洋流传输等,再到跨介质的传输,还有生物传输(图1). McDonough等[13]研究了偏远洋区深海和北极地表水里的OPEs,发现浓度意外地高,且氯系OPEs占主导,表明氯系OPEs能够通过洋流长距离运输. 有关南海北部大气颗粒物中OPEs的一项研究也表明洋流传输是OPEs的一个主要传输渠道,同时大气传输和干沉降也是主要渠道,2013年南海北部的OPEs估计干沉降量达到4.98 t[6]. Zhang等[16]分析了青藏高原表层土壤中OPEs的来源,发现主要的源是大气传输. 另一项关于青藏高原冰川中OPEs浓度的研究也表明大气传输占据的重要地位[52]. 在OPEs从亚洲大陆到西北太平洋和北冰洋的长程大气传输中,温度被认为是可能的动力来源[53]. Zhang等[54]研究了华南亚热带水稻田中OPEs在多种基质的分配,认为大气是农田OPEs的主要来源,土壤是主要环境归宿,这意味着农田土壤中OPEs的主要来源是大气沉降、气土交换和灌溉水. 土壤中的OPEs被水稻吸收,但水稻中98%的OPEs通过秸秆重新释放. 鲍美君[24]的研究也证实水稻能够吸收OPEs.
-
水解作用是环境中OPEs很重要的自然转化过程. Wu等[55]通过同位素分析研究了OPEs的水解过程,结果说明OPEs在酸性或中性环境下的水解途径是C—O键断裂,而碱性环境下是P—O键断裂,这个发现区分了不同pH条件下的水解途径. Su等[56]研究了16种磷酸三酯在20℃下中性和不同碱性pH时的水解情况,结果表明大部分磷酸三酯在pH 7—10的水中具有稳定性,但在pH 11—13下更容易水解,水解速率常数范围为
0.0213 —25.7 d−1,且降解速率常数随着磷酸三酯碳链长度的增加而降低,最终产物为磷酸二酯. 这说明环境中的磷酸三酯很可能逐渐转化为磷酸二酯. 金属(氢)氧化物能够催化OPEs的水解. 有研究表明一些金属矿物(如羟基氧化铁、氧化铁等)可以催化OPEs水解过程,使得原本在pH 6下基本不水解的OPEs发生降解,半衰期降低至10 d以下,且催化水解过程受pH影响相对较小,这意味着矿物催化OPEs水解可能是OPEs在自然环境下归趋的决定性因素[57].OPEs在环境介质中也会发生光解. 光解以间接的光解为主,多为照射阳光后产生光敏特性,后经大气中羟基自由基的氧化作用进行的降解[58]. 邢戎光等[59]总结了相关文献,发现虽然OPEs在大气中光解半衰期短,但复杂的大气环境可能会使光解半衰期延长.
OPEs的生物转化值得关注. Zhou等[60]研究了微生物对TCEP的转化,结果表明TCEP的生物转化主要通过磷酸酯键的水解进行,产物为双(2-氯乙基)磷酸酯(BCEP)和单氯乙基磷酸酯(MCEP),也可能通过水解脱氯和氧化途径. 缺氧环境下TCEP和TCPP的微生物转化也有了相关研究[10],微生物Dehalococoides通过单电子转移生成磷酸二酯和乙烯(或丙烯),这与有氧环境下的转化截然不同. 高慧娴[61]对OPEs在植物中的转化进行了总结,认为OPEs在植物体内会通过Ⅰ相代谢和Ⅱ相代谢进行转化. Liu等[62]研究了白羽扇豆和小麦对3种OPEs的生物转化,发现酸性磷酸酶(ACP)是促进OPEs生物转化的重要酶,在缺磷条件下,转化速率会提高,且在转化速率上TPhP>TnBP>TDCPP. OPEs在人体内也会发生转化,Van den Eeed等[63]通过研究人的肝微粒体在体外对OPEs的代谢,得出了TBOEP、TPHP、TCEP、TCIPP和TDCIPP的代谢产物.
-
有机磷酸酯在各种环境介质中被广泛检出[52],其对生态环境和人类健康的潜在危害 [22],使得有机磷酸酯的修复技术也成为环境科学领域的研究重点. 目前主要的修复技术有化学降解法、光催化降解和微生物降解等.
-
化学氧化是有机磷酸酯修复的主要方式之一,其中类芬顿反应克服传统芬顿反应的不足之后,使其得到了更广泛的使用[64]. 目前,已经有了大量关于氧化方法的优化探索以及原理的研究,但是依旧局限于特定的一种或几种OPEs. 刘祖发等[65]研究了TBP和TCEP的芬顿氧化降解,在最佳条件下10 min后降解率分别达到96.8%和74.5%. 并且,降解过程遵循一级反应动力学,pH、
$ {\text{Fe}}^{\text{2+}} $ 、$ {\text{H}}_{\text{2}}{\text{O}}_{\text{2}} $ 和污染物的初始浓度均对反应有所影响. TBP降解过程伴随着酸性中间产物的产生,并存在矿化过程;TCEP 氧化降解过程伴随着脱氯过程. Ambashta等[66]使用超声和磁场作用于TBP,并使用纳米零价铁和纳米铁镍作为催化剂,通过纳米零价铁与氧气作用产生$ {\text{Fe}}^{\text{2+}} $ 和$ {\text{H}}_{\text{2}}{\text{O}}_{\text{2}} $ 来达成类芬顿反应. 在这个反应中,pH、超声的存在、磁场强度都对结果有所影响,在一定条件下能够减少污染物中50%的有机碳. 臭氧具有一定的氧化性,也能用来降解OPEs. Yuan等[67]对比了臭氧和UV/$ {\text{H}}_{\text{2}}{\text{O}}_{\text{2}} $ 两种方法对几类OPEs的降解,结果发现臭氧的降解效率相对较低,这可能是由于臭氧的氧化性不够强. 两种方法对芳香族和脂肪族OPEs的降解效率远高于氯系OPEs;水中的腐殖酸能够提高臭氧对一部分OPEs的降解能力. Li[68]发现水中常见的表面活性剂CTAB能显著加快纳米零价铁对氯系OPEs的吸附降解,这意味着研究环境中常见物质对各种降解方法的影响是一个有意义的研究方向. Chao Li等[69]设计了一种模型用来预测各种OPEs的水·OH反应速率常数($ {k}_{\mathrm{O}\mathrm{H}} $ ),这对于评估OPEs的高级氧化过程具有重大意义. -
光催化降解技术是利用光子激发半导体,从而发生一系列反应,产生羟基和空穴对OPEs中的化学键进行攻击,从而降解OPEs的技术[70]. 光催化降解采用的半导体以
$ {\text{TiO}}_{\text{2}} $ 为多数. 孙敦宇[71]对光催化降解OPEs的研究进展进行了总结,UV/$ {\text{TiO}}_{\text{2}} $ 方法能降解氯系OPEs,如TCPP的10 min降解效率达95.1%[72],这种降解过程受到光源的波长、pH、天然有机物、阴离子和不同的暴露晶面的影响,但控制光催化效率的两个关键因素在于光响应范围和载流子的迁移能力. He等[73]制备了具有001晶面的纳米$ {\text{TiO}}_{\text{2}} $ ,能有效降解TCPP. TCEP在被UV/Fe(Ⅲ)光催化技术降解的同时,还会促进其对六价铬的降解[74]. 王晓寒[75]和Sun[76]等都研究了$ \text{Fe}{\text{TiO}}_{\text{3}} $ 和UV/过硫酸盐降解TCPP的协同效果,发现能够显著提高体系的氧化能力. -
微生物修复的重点在于找到合适的菌种,目前相关研究多是针对某种或某几种OPEs的降解. 在对污泥降解OPEs的研究中发现了五个属的细菌(黄杆菌属、芽孢杆菌属、碱杆菌属、假单胞菌属和巨大芽孢杆菌属)可能具有降解OPEs的能力[77]. 一种PAHs高效降解菌在与鼠李糖脂和植物修复协同作用下,有一定的修复PAHs-OPEs混合污染土壤的潜力[18]. Yang等[78]分离出了能够高效降解TPhP的GYY生物群落,代谢途径是水解、甲氧基化和羟基化,其中的GY-1在最优条件下能够7 d降解98.9%的TPhP. 吴中平[79]富集筛选了土壤中能降解TCPP的菌种,与植物联合降解TCPP的平均降解率可以达到91.14%. 可以看出,在最佳条件下,微生物降解特定的OPEs效率可以非常高,与其他方法结合使用也是有潜力的降解手段.
-
目前关于OPEs的研究已经有很多,包括其分布、暴露、迁移转化和修复技术等,但相关研究依然不够全面,存在一些问题,可以预见今后的研究将更加全面而深入.
(1)OPEs分布相关的研究多集中于水体、沉积物、灰尘等,对在土壤中的分布研究较少,对污染来源的分析也较为有限,多数分析停留于判断多种OPEs的来源异同上. 今后应当研究OPEs在各种环境介质,尤其是土壤中的分布. 同时能对OPEs的来源做出更为详细的判断.
(2)研究的OPEs种类相对有限,一般都集中于最常见的几种氯代、烷基和苯基OPEs上,对于其他种类的OPEs及其代谢产物的研究较为缺乏. 今后的研究范围应覆盖环境中所能见到的所有OPEs及其代谢产物,能对它们在环境中的迁移转化有进一步的研究.
(3)现有OPEs修复技术的研究多集中于物理化学法,虽然微生物修复技术作为一种经济可行的方法,但目前修复效率仍较低. 不同技术组合对OPEs的修复效果及其联合降解机理将成为今后研究的重要方向.
有机磷酸酯的环境行为及其修复技术研究进展
Environmental behavior and remediation technology of organophosphate esters: A review
-
摘要: 有机磷酸酯(OPEs)作为阻燃剂和增塑剂已广泛应用于各行各业. OPEs作为添加型的阻燃剂易于释放进入环境造成污染. 目前,OPEs作为一类新兴的有机污染物,在世界范围内的各种环境介质中能被广泛检出. 本文在对近5年发表的OPEs文献检索和分析的基础上,综述了OPEs在环境分布、危害、迁移转化和修复技术方面的研究进展. 结果表明,OPEs在环境介质中普遍存在,包括土壤、水、沉积物、大气等,可通过环境暴露和饮食进入人体,造成人体健康危害,也对水环境与土壤生态造成影响. 在环境中,OPEs存在多种迁移方式,且具备远距离迁移能力,水解是其重要的环境转化行为. 芬顿氧化法和光降解法是OPEs较有成效的修复技术.Abstract: Organophosphate esters (OPEs) have been extensively utilized in industries as flame retardants and plasticizers. However, the application of OPEs as additive-type flame retardants makes them prone to being easily released into environments, leading to pollution. OPEs, considered as emerging organic pollutants, have been detected in various environmental media worldwide. This paper provides a comprehensive summary of the recent research findings on the environmental distribution, hazards, transportation, transformation, and remediation technology of OPEs based on an extensive search and analysis of published literatures from the past five years. The findings indicate the pervasive presence of OPEs in various environment, such as soil, water, sediment and air. OPEs not only pose risks to human health through environmental exposure and food sources but also exert detrimental effects on water environment and soil ecology. OPEs exhibit diverse migration pathways within the environment, demonstrating their capacity to disperse over long distances. Hydrolysis is the dominant transformation process for OPEs in natural environments, while Fenton oxidation and photo degradation have been identified as effective remediation technologies for addressing OPEs contamination.
-

-
表 1 常见有机磷酸酯的名称、结构图和性质 [7]
Table 1. Name, structure diagram and properties of common organophosphate esters
名称
Name缩写
Abbreviation分子式
Molecular formula结构图
Structure diagram溶解度/(mg∙L−1)
Solubility$ {\mathrm{lg}K}_{\mathrm{O}\mathrm{W}} $ 磷酸三异丙酯 TIPP $ {\mathrm{C}}_{9}{\mathrm{H}}_{21}{\mathrm{O}}_{4}\mathrm{P} $ 
501 2.12 磷酸三乙酯 TEHP $ {\mathrm{C}}_{6}{\mathrm{H}}_{15}{\mathrm{O}}_{4}\mathrm{P} $ 
可溶 0.8 磷酸三丁酯 TnBP $ {\mathrm{C}}_{12}{\mathrm{H}}_{27}{\mathrm{O}}_{4}\mathrm{P} $ 
7.36 4.00 磷酸三(丁氧基乙基)酯 TBEP $ {\mathrm{C}}_{18}{\mathrm{H}}_{39}{\mathrm{O}}_{7}\mathrm{P} $ 
1.96 3.75 磷酸三(1-氯-2-丙基)酯 TCPP $ {\mathrm{C}}_{9}{\mathrm{H}}_{18}{\mathrm{C}\mathrm{l}}_{3}{\mathrm{O}}_{4}\mathrm{P} $ 
1080 2.59 磷酸三(1,3-二氯-2-丙基)酯 TDCPP $ {\mathrm{C}}_{9}{\mathrm{H}}_{15}{\mathrm{C}\mathrm{l}}_{6}{\mathrm{O}}_{4}\mathrm{P} $ 
7 3.65 磷酸三(2-氯乙基)酯 TCEP $ {\mathrm{C}}_{6}{\mathrm{H}}_{12}{\mathrm{C}\mathrm{l}}_{3}{\mathrm{O}}_{4}\mathrm{P} $ 
7820 1.44 磷酸三苯酯 TPhP $ {\mathrm{C}}_{18}{\mathrm{H}}_{15}{\mathrm{O}}_{4}\mathrm{P} $ 
1.03 4.59 磷酸三(4-甲苯基)酯 TPCP $ {\mathrm{C}}_{21}{\mathrm{H}}_{21}{\mathrm{O}}_{4}\mathrm{P} $ 
- 5.11 磷酸三甲苯酯 TMPP $ {\mathrm{C}}_{21}{\mathrm{H}}_{21}{\mathrm{O}}_{4}\mathrm{P} $ 
不溶 - 磷酸三(4-硝基苯基)酯 TnPP $ {\mathrm{C}}_{9}{\mathrm{H}}_{21}{\mathrm{O}}_{4}\mathrm{P} $ 
827 1.87 -
[1] XIA K, LUO M B, LUSK C, et al. Polybrominated diphenyl ethers (PBDEs) in biota representing different trophic levels of the Hudson River, New York: From 1999 to 2005[J]. Environmental Science & Technology, 2008, 42(12): 4331-4337. [2] van der VEEN I, de BOER J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis[J]. Chemosphere, 2012, 88(10): 1119-1153. doi: 10.1016/j.chemosphere.2012.03.067 [3] BLUM A, BEHL M, BIRNBAUM L S, et al. Organophosphate ester flame retardants: Are they a regrettable substitution for polybrominated diphenyl ethers?[J]. Environmental Science & Technology Letters, 2019, 6(11): 638-649. [4] ZHU H K, AL-BAZI M M, KUMOSANI T A, et al. Occurrence and profiles of organophosphate esters in infant clothing and raw textiles collected from the United States[J]. Environmental Science & Technology Letters, 2020, 7(6): 415-420. [5] YE L J, LI J H, GONG S, et al. Established and emerging organophosphate esters (OPEs) and the expansion of an environmental contamination issue: A review and future directions[J]. Journal of Hazardous Materials, 2023, 459: 132095. doi: 10.1016/j.jhazmat.2023.132095 [6] LAI S C, XIE Z Y, SONG T L, et al. Occurrence and dry deposition of organophosphate esters in atmospheric particles over the northern South China Sea[J]. Chemosphere, 2015, 127: 195-200. doi: 10.1016/j.chemosphere.2015.02.015 [7] 廖梓聪, 李会茹, 杨愿愿, 等. 有机磷酸酯(OPEs)的环境污染特征、毒性和分析方法研究进展[J]. 环境化学, 2022, 41(4): 1193-1215. doi: 10.7524/j.issn.0254-6108.2020121601 LIAO Z C, LI H R, YANG Y Y, et al. The pollution characteristics, toxicity and analytical methods of organophosphate esters(OPEs) in environments: A review[J]. Environmental Chemistry, 2022, 41(4): 1193-1215 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020121601
[8] MABEY W, MILL T. Critical review of hydrolysis of organic compounds in water under environmental conditions[J]. Journal of Physical and Chemical Reference Data, 1978, 7(2): 383-415. doi: 10.1063/1.555572 [9] REGNERY J, PÜTTMANN W. Occurrence and fate of organophosphorus flame retardants and plasticizers in urban and remote surface waters in Germany[J]. Water Research, 2010, 44(14): 4097-4104. doi: 10.1016/j.watres.2010.05.024 [10] ZHU X F, DENG S F, FANG Y, et al. Dehalococcoides-containing enrichment cultures transform two chlorinated organophosphate esters[J]. Environmental Science & Technology, 2022, 56(3): 1951-1962. [11] CHEN Y Q, ZHANG Q, LUO T W, et al. Occurrence, distribution and health risk assessment of organophosphate esters in outdoor dust in Nanjing, China: Urban vs. rural areas[J]. Chemosphere, 2019, 231: 41-50. doi: 10.1016/j.chemosphere.2019.05.135 [12] 周佳敏, 赵静, 韦旭, 等. 上海市不同微环境室内灰尘中有机磷酸酯污染特征及健康风险评价[J]. 环境化学, 2023, 42(7): 2317-2327. doi: 10.7524/j.issn.0254-6108.2022102608 ZHOU J M, ZHAO J, WEI X, et al. The pollution characteristics and health risk assessment of organophosphate esters in indoor dust from different microenvironments in Shanghai[J]. Environmental Chemistry, 2023, 42(7): 2317-2327 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022102608
[13] McDONOUGH C A, de SILVA A O, SUN C X, et al. Dissolved organophosphate esters and polybrominated diphenyl ethers in remote marine environments: Arctic surface water distributions and net transport through fram strait[J]. Environmental Science & Technology, 2018, 52(11): 6208-6216. [14] 曹渺, 郭昌胜, 张恒, 等. 黄河流域入海口典型区域有机磷酸酯分布特征和风险评估[J]. 环境科学, 2023, 44(3): 1378-1386. CAO M, GUO C S, ZHANG H, et al. Occurrence distribution and risk assessment of organophosphate esters in A typical area of the estuary in the Yellow River Basin[J]. Environmental Science, 2023, 44(3): 1378-1386 (in Chinese).
[15] WANG Y, YAO Y M, LI W H, et al. A nationwide survey of 19 organophosphate esters in soils from China: Spatial distribution and hazard assessment[J]. Science of the Total Environment, 2019, 671: 528-535. doi: 10.1016/j.scitotenv.2019.03.335 [16] ZHANG Z H, XU Y, WANG Y, et al. Occurrence and distribution of organophosphate flame retardants in the typical soil profiles of the Tibetan Plateau, China[J]. Science of the Total Environment, 2022, 807: 150519. doi: 10.1016/j.scitotenv.2021.150519 [17] WANG Y, SUN H W, ZHU H K, et al. Occurrence and distribution of organophosphate flame retardants (OPFRs) in soil and outdoor settled dust from a multi-waste recycling area in China[J]. Science of the Total Environment, 2018, 625: 1056-1064. doi: 10.1016/j.scitotenv.2018.01.013 [18] 韦旭. 电子废弃物拆解厂区室内环境中有机阻燃剂的污染特征、来源及职业健康风险评价[D]. 上海: 上海第二工业大学, 2022. WEI X. Pollution characteristics, sources and occupational health risk assessment of organic flame retardants in indoor environment of electronic waste dismantling plant[D]. Shanghai: Shanghai Polytechnic University , 2022 (in Chinese).
[19] 李旭, 刘杨, 吕佳佩, 等. 某城区降雪中OPEs污染特征与风险评估[J]. 中国环境监测, 2021, 37(6): 136-146. LI X, LIU Y, LYU J P, et al. Pollution characteristics and risk assessment of organophosphate esters in snow of an urban area[J]. Environmental Monitoring in China, 2021, 37(6): 136-146 (in Chinese).
[20] WANG L, XIAO Q R, YUAN M D, et al. Discovery of 18 organophosphate esters and 3 organophosphite antioxidants in food contact materials using suspect and nontarget screening: Implications for human exposure[J]. Environmental Science & Technology, 2022, 56(24): 17870-17879. [21] FU J E, FU K H, HU B Y, et al. Source identification of organophosphate esters through the profiles in proglacial and ocean sediments from ny-ålesund, the Arctic[J]. Environmental Science & Technology, 2023, 57(5): 1919-1929. [22] GAO F M, ZHANG X H, SHEN X M, et al. Exposure assessment of aryl-organophosphate esters based on specific urinary biomarkers and their associations with reproductive hormone homeostasis disruption in women of childbearing age[J]. Environment International, 2022, 169: 107503. doi: 10.1016/j.envint.2022.107503 [23] WAN W N, ZHANG S Z, HUANG H L, et al. Occurrence and distribution of organophosphorus esters in soils and wheat plants in a plastic waste treatment area in China[J]. Environmental Pollution, 2016, 214: 349-353. doi: 10.1016/j.envpol.2016.04.038 [24] 鲍美君. 典型农田有机磷酸酯和邻苯二甲酸酯的污染特征、植物富集和生态风险[D]. 大连: 大连理工大学, 2021. BAO M J. Pollution characteristics, plant enrichment and ecological risk of organic phosphates and phthalates in typical farmland[D]. Dalian: Dalian University of Technology, 2021 (in Chinese).
[25] HUANG J N, YE L J, FANG M L, et al. Industrial production of organophosphate flame retardants (OPFRs): Big knowledge gaps need to be filled?[J]. Bulletin of Environmental Contamination and Toxicology, 2022, 108(5): 809-818. doi: 10.1007/s00128-021-03454-7 [26] DAVID M D, SEIBER J N. Analysis of organophosphate hydraulic fluids in U. S. air force base soils[J]. Archives of Environmental Contamination and Toxicology, 1999, 36(3): 235-241. doi: 10.1007/s002449900466 [27] EGGEN T, HEIMSTAD E S, STUANES A O, et al. Uptake and translocation of organophosphates and other emerging contaminants in food and forage crops[J]. Environmental Science and Pollution Research, 2013, 20(7): 4520-4531. doi: 10.1007/s11356-012-1363-5 [28] CAO S X, ZENG X Y, SONG H, et al. Levels and distributions of organophosphate flame retardants and plasticizers in sediment from Taihu Lake, China[J]. Environmental Toxicology and Chemistry, 2012, 31(7): 1478-1484. doi: 10.1002/etc.1872 [29] CHEN M H, LIU Y H, GUO R X, et al. Spatiotemporal distribution and risk assessment of organophosphate esters in sediment from Taihu Lake, China[J]. Environmental Science and Pollution Research, 2018, 25(14): 13787-13795. doi: 10.1007/s11356-018-1434-3 [30] MARKLUND A, ANDERSSON B, HAGLUND P. Traffic as a source of organophosphorus flame retardants and plasticizers in snow[J]. Environmental Science & Technology, 2005, 39(10): 3555-3562. [31] CUI D L, BI J, ZHANG Z N, et al. Organophosphorus flame retardant TDCPP-induced cytotoxicity and associated mechanisms in normal human skin keratinocytes[J]. Science of the Total Environment, 2020, 726: 138526. doi: 10.1016/j.scitotenv.2020.138526 [32] HOU M M, SHI Y L, NA G, et al. A review of organophosphate esters in indoor dust, air, hand wipes and silicone wristbands: Implications for human exposure[J]. Environment International, 2021, 146: 106261. doi: 10.1016/j.envint.2020.106261 [33] CARLSSON H, NILSSON U, ÖSTMAN C. Video display units: an emission source of the contact allergenic flame retardant triphenyl phosphate in the indoor environment[J]. Environmental Science & Technology, 2000, 34(18): 3885-3889. [34] LEE H K, KANG H, LEE S, et al. Human exposure to legacy and emerging flame retardants in indoor dust: A multiple-exposure assessment of PBDEs[J]. Science of the Total Environment, 2020, 719: 137386. doi: 10.1016/j.scitotenv.2020.137386 [35] XU F C, GIOVANOULIS G, van WAES S, et al. Comprehensive study of human external exposure to organophosphate flame retardants via air, dust, and hand wipes: The importance of sampling and assessment strategy[J]. Environmental Science & Technology, 2016, 50(14): 7752-7760. [36] VYKOUKALOVÁ M, VENIER M, VOJTA Š, et al. Organophosphate esters flame retardants in the indoor environment[J]. Environment International, 2017, 106: 97-104. doi: 10.1016/j.envint.2017.05.020 [37] HE C, WANG X Y, TANG S Y, et al. Concentrations of organophosphate esters and their specific metabolites in food in southeast Queensland, Australia: Is dietary exposure an important pathway of organophosphate esters and their metabolites?[J]. Environmental Science & Technology, 2018, 52(21): 12765-12773. [38] POMA G, SALES C, BRUYLAND B, et al. Occurrence of organophosphorus flame retardants and plasticizers (PFRs) in Belgian foodstuffs and estimation of the dietary exposure of the adult population[J]. Environmental Science & Technology, 2018, 52(4): 2331-2338. [39] ALZUALDE A, BEHL M, SIPES N S, et al. Toxicity profiling of flame retardants in zebrafish embryos using a battery of assays for developmental toxicity, neurotoxicity, cardiotoxicity and hepatotoxicity toward human relevance[J]. Neurotoxicology and Teratology, 2018, 70: 40-50. doi: 10.1016/j.ntt.2018.10.002 [40] BAJARD L, MELYMUK L, BLAHA L. Prioritization of hazards of novel flame retardants using the mechanistic toxicology information from ToxCast and Adverse Outcome Pathways[J]. Environmental Sciences Europe, 2019, 31(1): 1-19. doi: 10.1186/s12302-018-0176-7 [41] CAO D D, LV K, GAO W, et al. Presence and human exposure assessment of organophosphate flame retardants (OPEs) in indoor dust and air in Beijing, China[J]. Ecotoxicology and Environmental Safety, 2019, 169: 383-391. doi: 10.1016/j.ecoenv.2018.11.038 [42] SUN Y L, ZHU H K. A pilot study of organophosphate esters in surface soils collected from Jinan City, China: Implications for risk assessments[J]. Environmental Science and Pollution Research, 2020, 28: 3344-3353. [43] YAN Z F, FENG C L, JIN X W, et al. Unbalanced pollution and ecological risk of organophosphate esters in Chinese surface water and land use under multiple driving factors[J]. Reviews of Environmental Contamination and Toxicology, 2023, 261(1): 1-15. doi: 10.1007/s44169-023-00025-1 [44] HONG X S, CHEN R, HOU R, et al. Triphenyl phosphate (TPHP)-induced neurotoxicity in adult male Chinese rare minnows (Gobiocypris rarus)[J]. Environmental Science & Technology, 2018: acs. est. 8b04079. [45] YAN S H, WANG Q, YANG L H, et al. A comparison of the toxicity effects of tris(1, 3-dichloro-2-propyl)phosphate (TDCIPP) with tributyl phosphate (TNBP) reveals the mechanism of the apoptosis pathway in Asian freshwater clams (Corbicula fluminea)[J]. Environmental Science & Technology, 2020, 54(11): 6850-6858. [46] ZHU Y, MA X F, SU G Y, et al. Environmentally relevant concentrations of the flame retardant tris(1, 3-dichloro-2-propyl) phosphate inhibit growth of female zebrafish and decrease fecundity[J]. Environmental Science & Technology, 2015, 49(24): 14579-14587. [47] DASGUPTA S, CHENG V, VLIET S M F, et al. Tris(1, 3-dichloro-2-propyl) phosphate exposure during the early-blastula stage alters the normal trajectory of zebrafish embryogenesis[J]. Environmental Science & Technology, 2018, 52(18): 10820-10828. [48] WANG L, HUANG X L, LASERNA A K C, et al. Metabolism of tri-n-butyl phosphate in earthworm Perionyx excavatus[J]. Environmental Pollution, 2018, 234: 389-395. doi: 10.1016/j.envpol.2017.11.098 [49] WANG L, HUANG X L, LASERNA A K C, et al. Untargeted metabolomics reveals transformation pathways and metabolic response of the earthworm Perionyx excavatus after exposure to triphenyl phosphate[J]. Scientific Reports, 2018, 8: 16440. doi: 10.1038/s41598-018-34814-9 [50] ZHU Y, ZHANG J Y, LIU Y X, et al. Environmentally relevant concentrations of the flame retardant tris(1, 3-dichloro-2-propyl) phosphate inhibit the growth and reproduction of earthworms in soil[J]. Environmental Science & Technology Letters, 2019, 6(5): 277-282. [51] 罗庆, 吴中平, 王聪聪, 等. 4种草本植物对氯代有机磷酸酯阻燃剂污染土壤的修复能力研究[J]. 环境工程, 2023, 41(3): 155-162. LUO Q, WU Z P, WANG C C, et al. Remediation capability of four herbs on chlorinated organophosphate flame retardants contaminated soil[J]. Environmental Engineering, 2023, 41(3): 155-162 (in Chinese).
[52] ZOU X, HOU S G, WU S Y, et al. The first detection of organophosphate esters (OPEs) of a high altitude fresh snowfall in the northeastern Tibetan Plateau[J]. Science of the Total Environment, 2022, 838: 155615. doi: 10.1016/j.scitotenv.2022.155615 [53] NA G S, HOU C, LI R J, et al. Occurrence, distribution, air-seawater exchange and atmospheric deposition of organophosphate esters (OPEs) from the Northwestern Pacific to the Arctic Ocean[J]. Marine Pollution Bulletin, 2020, 157: 111243. doi: 10.1016/j.marpolbul.2020.111243 [54] ZHANG Z G, LIN G, LIN T, et al. Occurrence, behavior, and fate of organophosphate esters (OPEs) in subtropical paddy field environment: A case study in Nanning City of South China[J]. Environmental Pollution, 2020, 267: 115675. doi: 10.1016/j.envpol.2020.115675 [55] WU L P, CHLÁDKOVÁ B, LECHTENFELD O J, et al. Characterizing chemical transformation of organophosphorus compounds by 13C and 2H stable isotope analysis[J]. Science of the Total Environment, 2018, 615: 20-28. doi: 10.1016/j.scitotenv.2017.09.233 [56] SU G Y, LETCHER R J, YU H X. Organophosphate flame retardants and plasticizers in aqueous solution: PH-dependent hydrolysis, kinetics, and pathways[J]. Environmental Science & Technology, 2016, 50(15): 8103-8111. [57] FANG Y D, KIM E, STRATHMANN T J. Mineral- and base-catalyzed hydrolysis of organophosphate flame retardants: Potential major fate-controlling sink in soil and aquatic environments[J]. Environmental Science & Technology, 2018, 52(4): 1997-2006. [58] LIU Q F, LI L, ZHANG X M, et al. Uncovering global-scale risks from commercial chemicals in air[J]. Nature, 2021, 600(7889): 456-461. doi: 10.1038/s41586-021-04134-6 [59] 邢戎光, 张蓬, 纪浩, 等. 大气中有机磷酸酯的赋存状况及环境行为[J]. 环境化学, 2024, 43(1): 1-12. doi: 10.1002/etc.5651 XING Rongguang, ZHANG Peng, JI Hao, et al. A review on the occurrence and environmental behavior of organophosphate esters (OPEs) as new pollutants in air[J]. Environmental Chemistry, 2024, 43(1): 1-12(in Chinese). doi: 10.1002/etc.5651
[60] ZHOU X Y, LIANG Y, REN G F, et al. Biotransformation of tris(2-chloroethyl) phosphate (TCEP) in sediment microcosms and the adaptation of microbial communities to TCEP[J]. Environmental Science & Technology, 2020, 54(9): 5489-5497. [61] 高慧娴, 刘宪斌, 田胜艳, 等. 有机磷酸酯在植物体内的吸收、积累、迁移与转化研究进展[J]. 环境化学,2024, 43(1): 186-198. GAO Huixian, LIU Xianbin, TIAN Shengyan, et al. Uptake, accumulation, translocation and transformation of organophosphate esters (OPEs) in plants: A review [J]. Environmental Chemistry, 2024, 43(1): 186-198(in Chinese).
[62] LIU Q, WANG X L, ZHOU J A, et al. Phosphorus deficiency promoted hydrolysis of organophosphate esters in plants: Mechanisms and transformation pathways[J]. Environmental Science & Technology, 2021, 55(14): 9895-9904. [63] van den EEDE N, MAHO W, ERRATICO C, et al. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions[J]. Toxicology Letters, 2013, 223(1): 9-15. doi: 10.1016/j.toxlet.2013.08.012 [64] SUN Y, ZHAO L, TENG Y. Insight into influence mechanisms of pyrite and vernadite on the degradation performance of 2, 2’, 5-trichlorodiphenyl in a pyrophosphate-chelated Fenton-like reaction[J]. Chemical Engineering Journal, 2021, 410: 128345. doi: 10.1016/j.cej.2020.128345 [65] 刘祖发, 丁波, 刘珍珍, 等. Fenton试剂氧化降解水体有机磷酸酯的动力学研究[J]. 亚热带资源与环境学报, 2016, 11(1): 1-8. LIU Z F, DING B, LIU Z Z, et al. A dynamical study on oxidation degradation of OPEs in water by fenton’s reagent[J]. Journal of Subtropical Resources and Environment, 2016, 11(1): 1-8 (in Chinese).
[66] AMBASHTA R D, REPO E, SILLANPÄÄ M. Degradation of tributyl phosphate using nanopowders of iron and iron–nickel under the influence of a static magnetic field[J]. Industrial & Engineering Chemistry Research, 2011, 50(21): 11771-11777. [67] YUAN X J, LACORTE S, CRISTALE J, et al. Removal of organophosphate esters from municipal secondary effluent by ozone and UV/H2O2 treatments[J]. Separation and Purification Technology, 2015, 156: 1028-1034. doi: 10.1016/j.seppur.2015.09.052 [68] LI D, ZHONG Y, ZHU X F, et al. Reductive degradation of chlorinated organophosphate esters by nanoscale zerovalent iron/cetyltrimethylammonium bromide composites: Reactivity, mechanism and new pathways[J]. Water Research, 2021, 188: 116447. doi: 10.1016/j.watres.2020.116447 [69] LI C, WEI G L, CHEN J W, et al. Aqueous OH radical reaction rate constants for organophosphorus flame retardants and plasticizers: Experimental and modeling studies[J]. Environmental Science & Technology, 2018, 52(5): 2790-2799. [70] HOFFMANN M R, MARTIN S T, CHOI W, et al. Environmental applications of semiconductor photocatalysis[J]. Chemical Reviews, 1995, 95(1): 69-96. doi: 10.1021/cr00033a004 [71] 孙敦宇, 杨绍贵, 向伟铭, 等. 有机磷酸酯阻燃剂降解方法的研究进展[J]. 环境化学, 2021, 40(2): 474-486. doi: 10.7524/j.issn.0254-6108.2020051603 SUN D Y, YANG S G, XIANG W M, et al. Research progress on degradation methods of organophosphorus flame retardants[J]. Environmental Chemistry, 2021, 40(2): 474-486 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020051603
[72] YU X L, YIN H, YE J S, et al. Degradation of tris-(2-chloroisopropyl) phosphate via UV/TiO2 photocatalysis: Kinetic, pathway, and security risk assessment of degradation intermediates using proteomic analyses[J]. Chemical Engineering Journal, 2019, 374: 263-273. doi: 10.1016/j.cej.2019.05.193 [73] HE H A, WANG X H, CHENG C, et al. Degradation of organophosphorus flame retardant tri(chloro-propyl)phosphate (TCPP) by (001) crystal plane of TiO2 photocatalysts[J]. Environmental Technology, 2021, 42(10): 1612-1622. doi: 10.1080/09593330.2019.1675771 [74] 李秋月. 三价铁介导紫外光催化降解有机磷酸酯同步去除六价铬的性能与机理研究[D]. 广州: 华南理工大学, 2021. LI Q Y. Study on the performance and mechanism of simultaneous removal of hexavalent chromium from organic phosphate catalyzed by trivalent iron under ultraviolet light[D]. Guangzhou: South China University of Technology, 2021 (in Chinese).
[75] 王晓寒. 基于纳米FeTiO3催化作用的羟基自由基和硫酸根自由基氧化磷酸三氯丙酯(TCPP)研究[D]. 南京: 南京师范大学, 2020. WANG X H. Oxidation of trichloropropyl phosphate (TCPP) by hydroxyl radical and sulfate radical based on nano-FeTiO3 catalysis[D]. Nanjing: Nanjing Normal University, 2020 (in Chinese).
[76] SUN D Y, WANG X H, JI Q Y, et al. Heterogeneous Fenton-like removal of tri(2-chloroisopropyl) phosphate by ilmenite (FeTiO3): Kinetic, degradation mechanism and toxic assessment[J]. Chemosphere, 2022, 307: 135915. doi: 10.1016/j.chemosphere.2022.135915 [77] PANG L, GE L M, YANG P J, et al. Degradation of organophosphate esters in sewage sludge: Effects of aerobic/anaerobic treatments and bacterial community compositions[J]. Bioresource Technology, 2018, 255: 16-21. doi: 10.1016/j.biortech.2018.01.104 [78] YANG Y Y, YIN H, PENG H, et al. Biodegradation of triphenyl phosphate using an efficient bacterial consortium GYY: Degradation characteristics, metabolic pathway and 16S rRNA genes analysis[J]. Science of the Total Environment, 2020, 713: 136598. doi: 10.1016/j.scitotenv.2020.136598 [79] 吴中平. 氯代有机磷酸酯污染土壤的植物-微生物联合修复[D]. 沈阳: 沈阳大学, 2022. WU Z P. Combined Phyto-microbial remediation of chlorinated organophosphate contaminated soils[D]. Shenyang: Shenyang University, 2022 (in Chinese).
-



 下载:
下载:















































