-
随着工业生产技术发展,重金属对全球水环境的污染日趋严重. 重金属废水无序排放严重污染周边的水体环境,直接或间接地危害人类健康[1],亟待有效处理. 其中酸性重金属废水如酸性矿山废水和电镀废水因其pH低、重金属种类多含量高、毒性大、成分复杂等特点,处理尤为困难[2-3]. 用于酸性重金属废水处理的传统工业水处理技术如加碱沉淀、吸附、离子交换和膜处理等存在成本高、二次污染严重、运行时间长、操作复杂等缺点[4-7]. 而电絮凝技术因其去除效率高、无需外加药剂、设备简单、絮凝产物可资源化等特点逐渐发展成为重金属废水处理领域研究热点[8-11].
对电絮凝所产生絮体产量和种类的调控是优化电絮凝工艺效能的关键[12]. 已有研究表明电絮凝溶液中不同支持电解质种类以及曝气条件会导致不同矿物的生成[12-14]. 针铁矿(goethite)、磁铁矿(magnetite)、赤铁矿(hematite)、水铁矿(ferrihydrite)和绿锈(green rust)等是电絮凝过程中常见的一次或二次生成的铁矿物[14]. 其中绿锈和磁铁矿通常在较低溶解氧(DO)浓度的电絮凝过程中产生,且都具有层状双金属氧化物(LDHs)结构,因而二者具有较高的比表面积和反应活性. 近年来,磷酸亚铁矿物蓝铁矿因其具备对砷、铀、钴和氯化有机化合物的吸附和还原能力而备受关注[15],然而电絮凝形成蓝铁矿(vivianite)的研究甚少,这与磷酸盐在中性条件下对铁阳极的钝化作用有关. 目前针对电絮凝吸附固载重金属的研究主要聚焦于中性或碱性条件[8-9, 12, 16],例如,有研究报道了中性和碱性条件下不同电解质阴离子和溶解氧浓度对于铁基电絮凝成矿种类的影响规律和影响机制[12] . 现阶段,酸性条件下铁电絮凝成矿过程缺乏关注;实际上,探讨酸性铁电絮凝用于实际重金属废水处理具有一定应用价值,这是因为阴极自产碱有利于提高系统pH,进而有效节省碱耗;较低pH能够提高溶液导电率及铁阳极法拉第效率,还使得在中性和碱性条件下易导致阳极钝化的电解质(如磷酸盐),在酸性条件下能够适用于电絮凝处理[17].
本研究着重讨论了酸性条件下铁基电絮凝的成矿规律及其对典型重金属的固载效果. 通过构建铁阳极、不锈钢阴极的电絮凝体系,考察不同支持电解质(NaCl、Na2SO4、NaH2PO4、NaNO3)和有无曝气对成矿种类的影响,以及不同铁矿物对重金属去除效果的影响;其次,通过检测溶液态铁Feaq(铁离子、亚铁离子及其溶液态羟基结合物种等)浓度在不同条件下铁电絮凝的变化规律,帮助深入解了酸性铁电絮凝的反应过程. 研究成果为酸性重金属废水的铁电絮凝处理提供理论参考,为电絮凝产物的资源化回收提供数据支撑.
-
硫酸镉 8/3水合物(CdSO4·8/3 H2O)、硫酸铜五水合物(CuSO4·5 H2O)、硫酸镍六水合物(NiSO4·6 H2O)、氯化铅(PbCl2)、硫酸钠(Na2SO4)、氯化钠(NaCl)、硝酸钠(NaNO3)、磷酸二氢钠 二水合物(NaH2PO4·2 H2O)购自阿拉丁化学试剂有限公司,硫酸、盐酸和硝酸购自广州化学试剂厂,以上试剂均为分析纯. 实验用水均为去离子水. 铁片电极(DT-3型,瑞源钢铁有限公司,江苏),不锈钢片电极(304型,中润宏发不锈钢有限公司,深圳),电极的表面积均为16 cm2(4 cm × 4 cm). 每次实验之前,铁片用粗砂布抛光,用稀释的HCl溶液(按重量5%)除锈,并用去离子水洗涤.
-
电絮凝实验在300 mL玻璃烧杯中进行,均使用铁片作为阳极,不锈钢作为阴极. 阴阳两极平行放置,电极间距为15 mm(有文献报道此间距下铁电絮凝去除重金属效率高[18]). 使用直流电源施加5 mA·cm−2的恒定电流于Fe阳极和不锈钢阴极,电流大小的选取以确保实现高的法拉第效率及重金属去除效率为依据[19]. 反应时间为30 min,磁力搅拌器控制转速为500 r·min−1. 实验分为曝气组和未曝气组,曝气组实验:在反应过程中持续向溶液中通入O2以模拟高DO浓度废水的电絮凝过程,未曝气组:将溶液暴露于空气中以模拟低DO浓度废水的电絮凝过程. 利用CdSO4、CuSO4、NiNO3和Pb(NO3)2储备液配制浓度分别为10、20、10、50 mg·L−1的重金属溶液,初始浓度的选取参考实际酸性矿山废水和工业废水中常见重金属污染数据[18, 20]. 针对每一种重金属溶液分别加入4种支持电解质NaCl、Na2SO4、NaH2PO4和NaNO3(10 mmol·L−1). 利用NaOH和电解质对应的酸溶液(NaH2PO4体系用硫酸溶液)调节初始溶液pH为3,并在反应过程中利用便携式pH计检测溶液pH变化. 开始试验后,以指定的时间间隔用注射器采集样品,通过0.45 μm的聚四氟乙烯膜过滤,收集滤液,分别测定其中的重金属浓度和Feaq浓度. 反应结束后,采集1 mL悬液并立即转移至4 mol·L−1 HCl溶液中溶解,用于后续测定实际产铁量[Fee]以计算法拉第效率. 对剩余悬液抽滤,然后将固体冷冻干燥、称重以便后续进行进一步分析和表征. 所有实验至少进行 3 次,结果以平均值 ± 标准差表示.
-
理论铁的溶解浓度(
[Fet] )根据法拉第电解定律根据公式1计算. 式中,CL是电絮凝体系的电荷负载量,MFe是铁的摩尔质量(55.845 g·mol−1),F是法拉第常数(96 485 C·mol−1),z是电荷转移系数,铁的电荷转移系数z = 2.实验中电絮凝条件为I = 80 mA,L = 0.3 L,t = 30 min,带入公式2即电荷负载量CL为480 C·L−1. 式中,I是实验施加恒定电流大小,t是反应时间,L是反应溶液体积.
最后计算可得实验中电絮凝理论溶解量为138.91 mg·L−1. 将实验组反应后悬液溶解测定的实际产铁量
[Fee] 除以理论溶解量[Fet] ,即可得法拉第效率. -
采用X射线衍射光谱(XRD,马尔文帕纳科公司,荷兰)分析铁矿物的物相组成,扫描范围为10°—80°,扫描速度为2(°)·min−1;用扫描电镜(SEM,FEI公司,美国)观察铁矿物表面形貌,并辅助分析铁矿物的种类;用火焰原子吸收光谱(AAS,岛津公司,日本)测定溶液中Cu(Ⅱ)、Cd(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的含量,以确定产生的铁矿物对重金属的去除效果. 采用邻菲啰啉分光光度法,在510 nm波长处测定溶液态铁(Feaq)的浓度,以分析铁电絮凝的成矿过程.
-
收集上述电絮凝反应后的固体物质,对其进行冷冻干燥后,取0.2 g样品于离心管中,并加入10 mL 0.4 mol·L−1 HCl,然后置于旋转振荡仪以200 r·min−1反应0.5 h,离心收集上清液,测试重金属浸出含量,记为吸附态含量;再将离心后含有残余固体的离心管内加入10 mL 4 mol·L−1 HCl,然后置于旋转振荡仪中反应0.5 h,离心收集上清液,测试重金属浸出含量,记为结构态含量[21-22].
-
为了探讨电解质和曝气条件对酸性铁基电絮凝体系成矿量的影响,对沉淀进行称重. 如图1a所示,NaNO3体系成矿量极少,这是因为NO3−对铁阳极有钝化作用. 据报道,中性铁电絮凝在NO3−和PO43−存在的情况下,铁阳极会形成一层纳米尺度的钝化层,钝化层的产生抑制了铁阳极的Fe(Ⅱ)溶出[17]. 而在本研究中,磷酸盐体系明显浑浊,产生了相对较多铁矿物,这是因为在酸性条件下,磷酸盐的阳极钝化作用被减弱,具体机理由后文详述. 曝气条件下溶液中DO浓度从7.5 mg·L−1逐渐上升,并维持至饱和浓度8.2 mg·L−1左右;而在不曝气条件下,由于阳极产生Fe(Ⅱ)的消耗,溶液中DO浓度将由7.5 mg·L−1在15 min内逐渐降低至0. 图1a结果表明,由于DO浓度影响,曝气相较于不曝气产生的铁矿物量略高.
采用XRD和SEM对固体物质组成和表面形貌进行表征,以明晰不同溶液条件下铁基电絮凝产生铁矿物的种类. 首先,利用XRD对不同条件下电絮凝作用30 min后产生的铁矿物沉淀进行物相区分. 图1b和1c表明,在未曝气条件下,NaCl、Na2SO4和NaH2PO4体系产生沉淀中的主要矿物分别为磁铁矿、绿锈和蓝铁矿;而在曝气条件下,NaCl和Na2SO4体系主要为无定型铁矿物. 未曝气条件下NaCl、Na2SO4和NaH2PO4体系产生铁矿物颜色分别为黑色、绿色和蓝绿色,与XRD结果相符;而在曝气条件下,NaCl和Na2SO4体系产生红棕色固体,NaH2PO4体系产生白色固体,结合XRD表征结果和体系中可能发生的反应[14, 23],认为两者分别为氢氧化铁和磷酸铁. SEM结果如图1d(1)—(5)所示,电絮凝产生的磁铁矿呈现颗粒状结构,绿锈呈现层状结构,蓝铁矿呈现扁平状堆叠结构;而在曝气条件下,所产生的氢氧化铁和磷酸铁分别呈现出层状和絮状结构,这与文献中记载的各类沉淀微观形貌结构一致[12, 14].
-
酸性铁基电絮凝过程中,发现同一体系中溶液颜色在某一时刻后显著变深,且这一时刻后重金属浓度骤降. 据此推测酸性铁基电絮凝的反应过程可分为两个阶段. 为了验证这一观点,并讨论不同的电解质和曝气条件对两个阶段发生时间节点的影响,对电絮凝过程中不同时间节点的溶液态铁Feaq浓度进行取样检测,结果如图2a、2b所示. 在曝气和未曝气条件下,各个支持电解质体系中,Feaq浓度随着反应时间的推移均呈现先上升后下降的趋势. 这可能与Feaq浓度和pH两方面因素有关:一方面,Feaq浓度不断上升,增加了氧化成矿(式7—11)的底物浓度[17];另一方面,随着阴极不断产生OH−,溶液的pH逐渐升高(如图2c、2d所示),Fe(Ⅲ)与OH−的结合增强从而加快了成矿速度[24]. 这导致了该电絮凝体系由Fe(0)氧化反应(式3、式4)为主导向Feaq成矿反应(式7—11)为主导的转变,即表现为铁电絮凝从积累阶段向成矿阶段的转变(图2a、b虚线前后).
不同的电解质组成和曝气条件会导致阳极法拉第效率以及Feaq氧化沉淀速率的不同[25],从而影响成矿. 为了验证上述推断,计算了不同条件下电絮凝反应30 min后的法拉第效率. 如表1所示,NaCl、Na2SO4体系的法拉第效率均高于0.9,表明此时阳极表面主要发生Fe(0)氧化反应(式3、式4)[26-28],而NaH2PO4体系的法拉第效率略低,但是仍高于已有报道中,相应体系在近中性pH条件下的法拉第效率[12]. 这是因为:首先低的pH会导致H2O/O2的E0上升,从而阻碍在给定的阳极电位条件下电解水反应的发生;其次,低pH会导致Fe(Ⅱ)更难被O2氧化,降低了产生的铁氧化物附着在铁阳极表面阻止Fe(Ⅱ)进一步析出的可能性. 而NaNO3体系法拉第效率低于0.2,表明阳极表面主要发生电解水反应(式5)[17]. 这是因为硝酸盐能够氧化Fe(0),生成一层薄的(羟基)铁氧化物钝化膜,抑制离子扩散[29]. 除不同的法拉第效率会影响Fe(Ⅱ)/Fe(Ⅲ)溶出从而影响Feaq浓度外,不同体系Feaq的氧化沉淀速度也会影响Feaq峰值浓度和达峰位置. 例如Cl−存在的电絮凝体系中会产生氯自由基从而提高氧化成矿率[13],因而NaCl体系的Feaq峰值浓度低且达峰时间更早;而相较于蓝铁矿,绿锈的成矿速度更快,故其Feaq峰值浓度更低且达峰更早[12]. 相比于未曝气条件,曝气条件下Feaq的浓度更低,这是因为溶解氧浓度的增加对Fe(Ⅱ)氧化为Fe(III)速度有促进作用,从而提升了成矿率[24]. 该结论与曝气条件下电絮凝体系最终产生沉淀物质量高于不把曝气条件相符.
基于上述结果,在酸性条件下的铁基电絮凝过程中需要经历两个阶段——积累阶段和成矿阶段,铁矿物的形成以及重金属的固载吸附均发生于成矿阶段. 这两个阶段中铁阳极氧化溶出Fe(Ⅱ)和溶液体相中Feaq沉淀分别占据主导地位. 这两个阶段发生的时间节点主要取决于Feaq积累阶段中的法拉第效率,以及在成矿阶段的Feaq成矿效率,两者都与溶液的支持电解质种类及曝气条件有关,并与其共同决定了形成铁矿物的种类和质量. 首先,在积累阶段,阳极Fe(0)的氧化(式3、式4)、和阴极电解水析氢产碱(式6)是主要反应. 这一阶段阳极附近会产生并积累Fe(Ⅱ),并伴随体相pH上升,但是溶液中固体物质的质量不会明显增加. 而随着溶液中Feaq浓度和pH的增加,成矿(式7—11)速率逐渐增加,直至超过Fe(0)的氧化(式3、式4)速率,在这个节点之后,溶液中的固体物质显著增加.
-
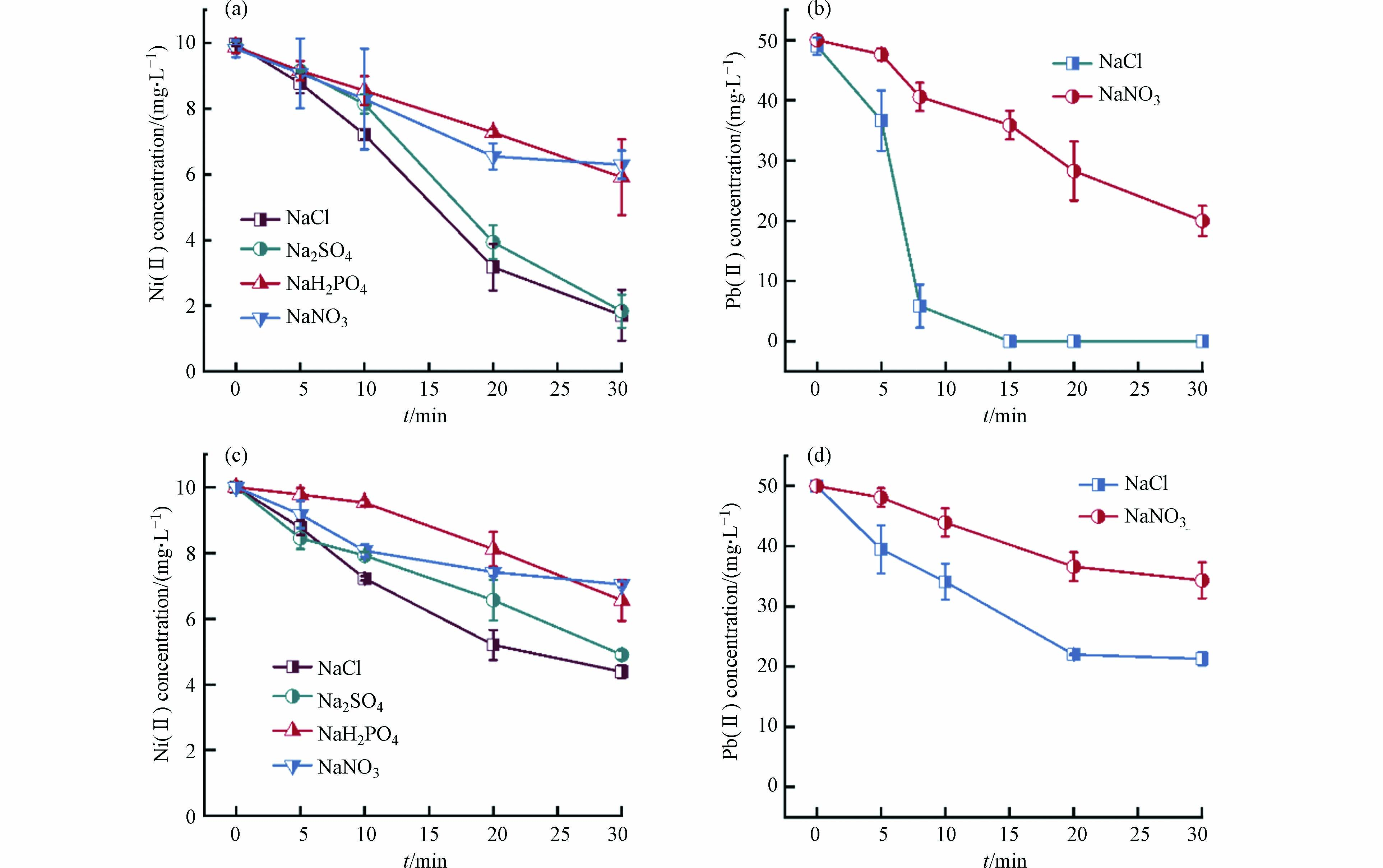
为研究上文中产生不同铁矿物物种对不同重金属的吸附固载效果,向不同支持电解质和有无曝气的电絮凝体系中加入常见重金属阳离子Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)和Pb(Ⅱ),并研究其去除情况. 如前文所述,NaH2PO4作为支持电解质的溶液在酸性未曝气的条件下主要产生蓝绿色的蓝铁矿,而曝气条件下,蓝铁矿则会被充足的氧气氧化为白色的磷酸铁沉淀. 如图3所示,二者对Cd(Ⅱ)、Cu(Ⅱ)均有优良的去除效果,30 min去除率均能达到99%以上. 在未曝气条件下,NaCl和Na2SO4体系分别产生的磁铁矿和绿锈,该体系对Cu(Ⅱ)的去除率也能达到99%以上,但在对Cd(Ⅱ)的去除率上,磁铁矿(85.0%)要高于绿锈(36.5%). 曝气组中NaCl或Na2SO4体系中产生的氢氧化铁对Cd(Ⅱ)、Cu(Ⅱ)的固载效果较差,其去除率分别为35.5%和72.9%. 这是因为除了内球络合吸附,蓝铁矿和绿锈、磁铁矿均因为晶格中具有Fe(Ⅱ),能够被相似离子半径和价态的Pb(Ⅱ)、Cd(Ⅱ)、Cu(Ⅱ)同晶替代[30]. 因此电絮凝产生的不同矿物对Cd(Ⅱ)去除效果强弱顺序可概括为:磷酸铁≈蓝铁矿>磁铁矿>绿锈>氢氧化铁;对Cu(Ⅱ)去除效果强弱顺序为:磁铁矿>蓝铁矿≈磷酸铁>氢氧化铁.
对于Ni(Ⅱ)和Pb(Ⅱ),NaH2PO4电解质体系则无法展现良好的吸附性能. 如图4a、4c所示,未曝气条件下NaH2PO4体系电絮凝对于Ni(Ⅱ)的去除效果十分有限,低于NaCl、Na2SO4体系下81.6%的去除率,与NaNO3体系效果接近,去除率仅约为40.9%. 这与Cu(Ⅱ)和Cd(Ⅱ)的结果差别很大. 结合上文法拉第效率的计算结果可排除阳极钝化的可能性,推测是由于蓝铁矿比表面积小、表面正电荷较强以及官能团种类较单一的特点导致其对Ni(Ⅱ)吸附能力较弱[31],但其对于这三种重金属去除效果的差异的微观机理还有待进一步阐明. 磁铁矿具有非化学计量缺陷同时氧骨架较为灵活,因而具有很大的容量通过Fe(Ⅱ)替代来容纳各种金属离子,故其对Cu(Ⅱ)、Cd(Ⅱ)、Pb(Ⅱ)、Ni(Ⅱ)均有较好的去除能力[32]. 绿锈因为特殊的LDHs而具有内表面,因此也有不错的反应特性,此外在绿锈的形成过程中,Cu(Ⅱ)、Cd(Ⅱ)、Ni(Ⅱ)等也能通过同晶替代Fe(Ⅱ)从而被共沉淀固载. 曝气条件下NaH2PO4体系产生的磷酸铁对Ni(Ⅱ)的去除率仅为34.4%,低于NaCl和Na2SO4体系所产生氢氧化铁的56.1%.
对于酸性溶液中Pb(Ⅱ)的去除,结果如图4b、4c所示,未曝气条件下的NaCl体系效果最好,其次是曝气条件下的NaCl体系,这反映出磁铁矿固载Pb(Ⅱ)的效果强于氢氧化铁. 二者均优于NaNO3电解质体系. 这是由于磁铁矿对于Pb(Ⅱ)的特殊作用导致的,即Pb(Ⅱ)主要通过双齿双核共享配位作用吸附在磁铁矿表面,无吸附容量限制[33]. 因此,电絮凝产生的不同矿物对Ni(Ⅱ)去除效果强弱顺序可概括为:磁铁矿≈绿锈>蓝铁矿≈氢氧化铁>磷酸铁;对Pb(Ⅱ)去除效果强弱顺序为:磁铁矿>氢氧化铁. 未比较Na2SO4和NaH2PO4体系对Pb(Ⅱ)的吸附固载效果是因为PbSO4和Pb(H2PO4)2溶解度较低,Pb(Ⅱ)在这两种支持电解质的废水中无法以稳定的离子形式存在.
需要说明的是,在重金属的电絮凝处理过程中,除了矿物的固载作用,还可能存在阴极还原以及阳极置换的氧化还原过程. 以Cu(Ⅱ)为例,通过惰性电极替代Fe阳极后,发现在不同电解质和曝气条件实验组中Cu(Ⅱ)处理后浓度均为约15 mg·L−1,说明Cu(Ⅱ)的阴极还原确实存在,对溶液中Cu(Ⅱ)去除的贡献占20%. 对于阳极置换反应,由于阳极附近有大量带正电荷的Fe(Ⅱ)/Fe(III)物种和铁氧化物,带相同电荷的Cu(Ⅱ)难以接近,故认为其影响可忽略不计.
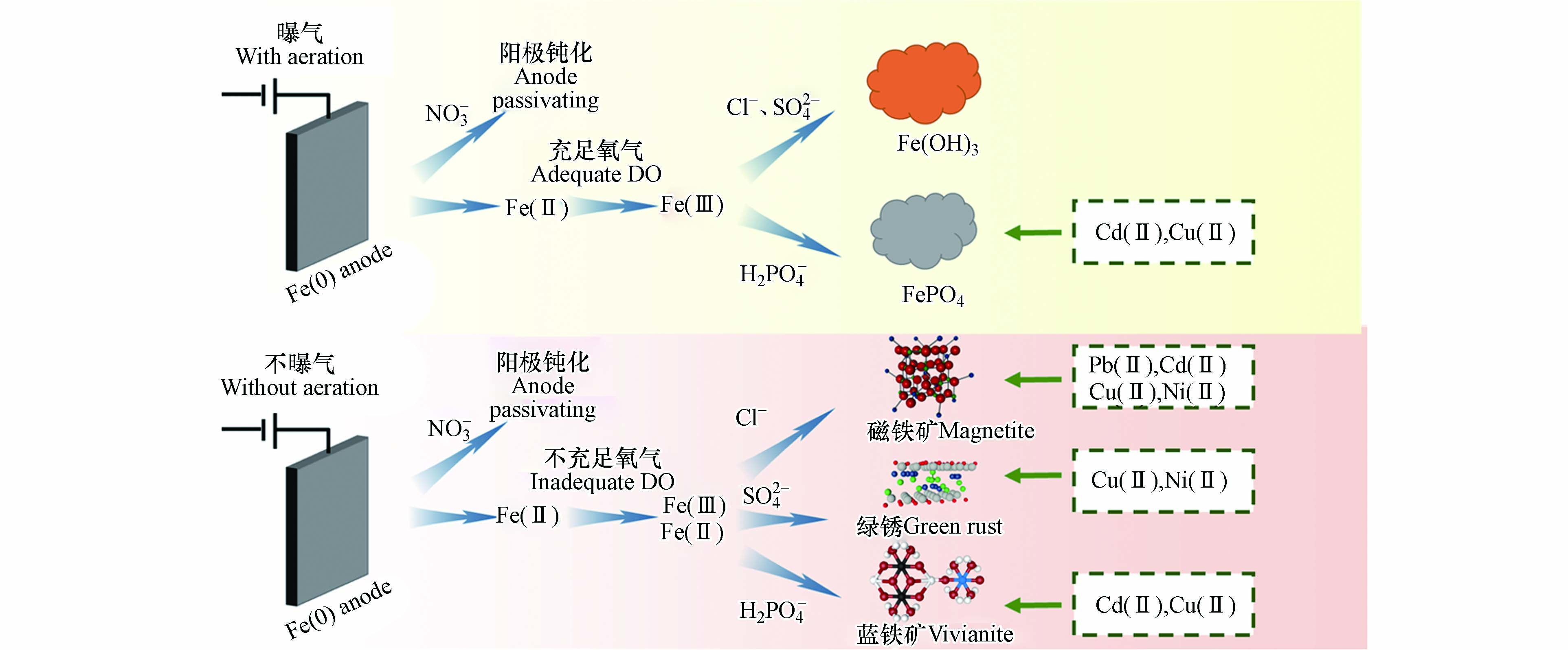
图5为不同电解质和曝气条件下酸性铁基电絮凝的成矿过程、成矿种类及其对四种重金属离子Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的吸附固载效果. 使用NaNO3作为支持电解质会使铁阳极钝化,导致低成矿量和重金属去除效率. 在其他电解质体系中,在无曝气的条件下NaCl、Na2SO4、NaH2PO4体系分别产生磁铁矿、绿锈和蓝铁矿;曝气条件下NaCl、Na2SO4体系产生氢氧化铁、NaH2PO4体系产生磷酸铁. 而产生的不同种类铁矿物对四种重金属的吸附效果各有所长,Cu(Ⅱ)和Ni(Ⅱ)易被吸附在磁铁矿和绿锈上,而磁铁矿对Pb(Ⅱ)、Cd(Ⅱ)的吸附效率高于绿锈;蓝铁矿和磷酸铁对Cu(Ⅱ)和Cd(Ⅱ)固载效果优异,而对Ni(Ⅱ)的固载效果较差;氢氧化铁絮体对四种重金属的固载效果均较差.
-
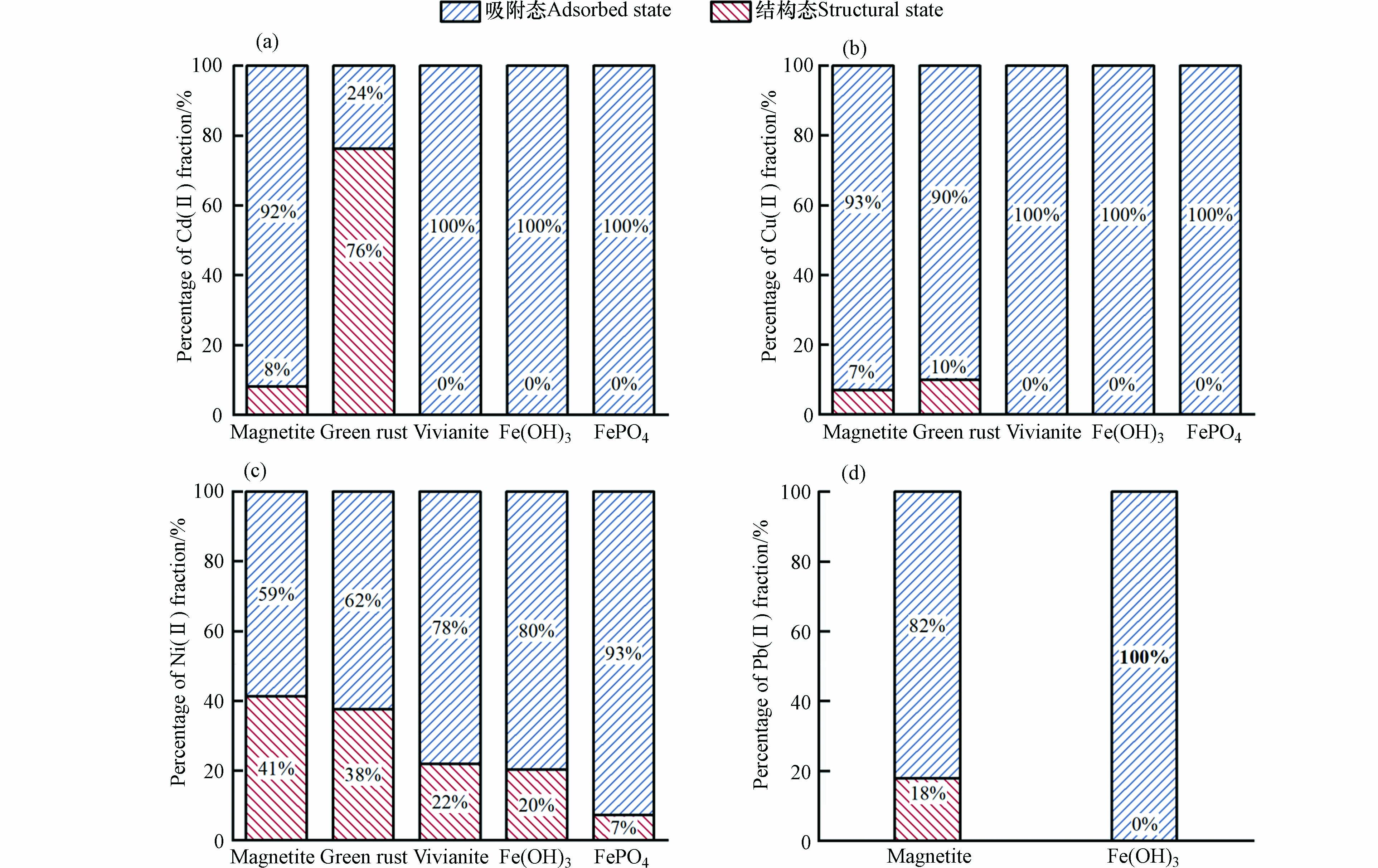
重金属的赋存形态直接影响其在介质中的迁移转化能力、毒性和生物活性[34]. 而酸性条件下产生的铁矿物稳定固载重金属的能力尤为重要,为此对电絮凝结束后产生的固体沉淀进行盐酸提取实验,研究了不同种类铁矿物对Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的的4种重金属离子的固载稳定性. 如图6所示,绿锈对Cd(Ⅱ)的固载后,结构态比例高达76%,对Cu(Ⅱ)、Ni(Ⅱ)的结构态比例分别为10%和38%. 这是因为在绿锈的形成过程中,Cu(Ⅱ)和Ni(Ⅱ)能够在绿锈的形成过程中通过对相似离子半径的Fe(Ⅱ)同晶替代,从而结合进入绿锈的晶格之中被共沉淀固载[16]. 磁铁矿对于4种重金属Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的结构态比例分别为8%、7%、41%和18%. 这是由于磁铁矿的原子组成和原子结构较为灵活,同样促使其能够通过同晶替代Fe(Ⅱ)容纳不同的重金属阳离子[35]. 磷酸铁和氢氧化铁对Cd(Ⅱ)、Cu(Ⅱ)的固载形式为吸附态,对Ni(Ⅱ)的结构态比例也低于22%. 这是因为磷酸铁、氢氧化铁和蓝铁矿对pH较为敏感,易溶于酸. 综上,电絮凝产生的绿锈和磁铁矿对重金属的固载稳定性更高,而磷酸铁、氢氧化铁和蓝铁矿的固载稳定性相对较低,对重金属固载稳定性较低的电絮凝铁泥的进一步处置,更需要注意其可能产生的环境影响.
-
因为在实际的重金属废水中,经常同时存在多种重金属,故为了验证酸性条件铁基电絮凝对多种重金属的同步去除效果,开展了Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)同时存在的电絮凝实验. 结果如图7所示,所有不同支持电解质种类和曝气条件的溶液体系对Cu(Ⅱ)和Ni(Ⅱ)的固载效果均与单一去除效果类似,说明其他共存阳离子的存在对Cu(Ⅱ)和Ni(Ⅱ)的去除没有显著影响. 而对于Cd(Ⅱ)的去除,除了NaNO3体系在单一去除实验中去除效果本身很差之外,NaCl和Na2SO4体系的去除速率也显著低于单一去除实验的去除速率,这说明在同步去除实验中Cd的固载受到了其他共存重金属离子的影响,这是因为Cd(Ⅱ)具有较低的电负性和较大的水化半径[36-37] ,使得Cd(Ⅱ)与铁矿物的亲和力要弱于Cu(Ⅱ)和Ni(Ⅱ),从而在竞争吸附中处于较弱的地位. 而NaH2PO4体系仍然保持较高的去除速率,说明该体系对Cd(Ⅱ)的固载过程除了吸附,还可能存在其他机理. 例如,随着pH升高,H2PO4−会逐渐转化为HPO42−和PO43−,其中,HPO42−和PO43−与Cd(Ⅱ)所形成沉淀的溶解度更低,从而将Cd(Ⅱ)固载下来. 因为这一过程仅与溶液中H2PO4−的浓度和pH有关,而不受其他重金属的影响,故NaH2PO4体系中Cd(Ⅱ)能够保持较高的去除速率.
-
(1)酸性铁电絮凝分为两个阶段. 其中第一阶段Feaq浓度逐渐升高,成矿量少,重金属去除较低;第二阶段Feaq浓度达到峰值并开始下降,成矿量逐步增加,重金属去除速率骤然提升.
(2)不同电解质(NaCl、Na2SO4、NaH2PO4、NaNO3)和曝气与否影响下酸性铁电絮凝会产生不同种类铁矿物. 其中,NaNO3体系会导致铁阳极钝化,抑制成矿;其他电解质在曝气条件下,电絮凝产生固体包括氢氧化铁(NaCl/Na2SO4体系)和磷酸铁(NaH2PO4体系);不曝气条件下,产生铁矿物包括磁铁矿(NaCl体系)、绿锈(Na2SO4体系)和蓝铁矿(NaH2PO4体系).
(3)重金属吸附结果表明,磁铁矿对Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)、Cd(Ⅱ)等都具有优异的固载效果,绿锈对Cu(Ⅱ)和Ni(Ⅱ)的吸附能力高于Cd(Ⅱ);蓝铁矿和磷酸铁对溶液中重金属Cd(Ⅱ)和Cu(Ⅱ)也有良好的去除效率,但对Ni(Ⅱ)去除效果较差,且其重金属固载稳定性低于磁铁矿和绿锈. 在Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)同时存在的重金属废水中,电絮凝对Cu(Ⅱ)和Ni(Ⅱ)的吸附效果与单一重金属废水结果一致,而对Cd(Ⅱ)的吸附效果则显著下降.
电解质及曝气条件对酸性铁电絮凝成矿及其固载重金属的影响
Impacts of supporting electrolyte and aeration conditions on Fe mineral formation and heavy metal immobilization in Fe-electrocoagulation at acidic pH
-
摘要: 本文针对酸性重金属废水开展了一系列铁电絮凝实验,重点考察不同电解质种类以及曝气与否对形成铁矿物种类和絮凝性能的影响. 研究发现,酸性电絮凝过程可分为铁积累阶段和成矿阶段,积累阶段主要是Fe(Ⅱ)/Fe(Ⅲ)的生成与富集,矿物形成和重金属去除主要发生在成矿阶段. 使用NaNO3作为电解质易使铁阳极钝化,导致低成矿量和重金属去除率;在无曝气条件下NaCl、Na2SO4、NaH2PO4电解质体系产生的主要铁矿物分别为磁铁矿、绿锈和蓝铁矿;曝气条件下NaCl、Na2SO4体系产生氢氧化铁,NaH2PO4体系产生磷酸铁. 重金属固载实验结果表明,磁铁矿和绿锈对Cd(Ⅱ)、Cu(Ⅱ)和Ni(Ⅱ)均表现出优异的吸附效果,但磁铁矿对于Cd(Ⅱ)的吸附效率高于绿锈;蓝铁矿和磷酸铁对Cu(Ⅱ)和Cd(Ⅱ)吸附效果优异,对Ni(Ⅱ)较差;氢氧化铁絮体对4种重金属的固载效果均较差. 浸出实验说明磁铁矿和绿锈对重金属固载稳定性优于其他铁矿物. 研究结果为铁电絮凝技术应用于酸性重金属废水处理提供重要证据.Abstract: In this study, a series of experiments on Fe-electrocoagulation were conducted to treat acidic heavy metal wastewaters. The effects of the type of supporting electrolyte and aeration on the performance of electrocoagulation and the species of Fe minerals were investigated. The results of the research showed that the acidic electrocoagulation process can be divided into two stages: Fe accumulation stage and Fe mineralization stage. The accumulation stage mainly involves the generation and enrichment of Fe(Ⅱ)/Fe(Ⅲ), while mineral formation and immobilization of heavy metals mainly occur in the latter stage. The use of NaNO3 as the supporting electrolyte resulted in the passivation of Fe anodes, leading to a low mineralization rate and heavy metal removal efficiency. Without aeration, Fe-electrocoagulation systems with NaCl, Na2SO4, and NaH2PO4 as supporting electrolytes generated magnetite, green rust, and vivianite, respectively. Under aeration conditions, iron hydroxide was formed in NaCl and Na2SO4 electrolyte systems, while iron phosphate was produced in the NaH2PO4 electrolyte system. Magnetite and green rust were found to have high adsorption efficiencies for the heavy metals Cd(Ⅱ), Cu(Ⅱ), and Ni(Ⅱ), with magnetite demonstrating a higher adsorption efficiency for Cd(Ⅱ) than green rust. Meanwhile, vivianite and iron phosphate effectively fixed Cu(Ⅱ) and Cd(Ⅱ), while Ni(Ⅱ) had poor adsorption on both minerals. The adsorption efficiencies of the four types of heavy metals for iron hydroxide were low. The results of leaching experiments showed that the adsorption stability of heavy metals on magnetite and green rust was better than that on vivianite, iron hydroxide, and iron phosphate. Overall, the findings of this study provide important evidence for the potential use of Fe-electrocoagulation as an effective method for treating acidic heavy metal wastewaters.
-
Key words:
- electrocoagulation /
- supporting electrolyte /
- aeration /
- iron minerals /
- heavy metal immobilization
-
随着工业生产技术发展,重金属对全球水环境的污染日趋严重. 重金属废水无序排放严重污染周边的水体环境,直接或间接地危害人类健康[1],亟待有效处理. 其中酸性重金属废水如酸性矿山废水和电镀废水因其pH低、重金属种类多含量高、毒性大、成分复杂等特点,处理尤为困难[2-3]. 用于酸性重金属废水处理的传统工业水处理技术如加碱沉淀、吸附、离子交换和膜处理等存在成本高、二次污染严重、运行时间长、操作复杂等缺点[4-7]. 而电絮凝技术因其去除效率高、无需外加药剂、设备简单、絮凝产物可资源化等特点逐渐发展成为重金属废水处理领域研究热点[8-11].
对电絮凝所产生絮体产量和种类的调控是优化电絮凝工艺效能的关键[12]. 已有研究表明电絮凝溶液中不同支持电解质种类以及曝气条件会导致不同矿物的生成[12-14]. 针铁矿(goethite)、磁铁矿(magnetite)、赤铁矿(hematite)、水铁矿(ferrihydrite)和绿锈(green rust)等是电絮凝过程中常见的一次或二次生成的铁矿物[14]. 其中绿锈和磁铁矿通常在较低溶解氧(DO)浓度的电絮凝过程中产生,且都具有层状双金属氧化物(LDHs)结构,因而二者具有较高的比表面积和反应活性. 近年来,磷酸亚铁矿物蓝铁矿因其具备对砷、铀、钴和氯化有机化合物的吸附和还原能力而备受关注[15],然而电絮凝形成蓝铁矿(vivianite)的研究甚少,这与磷酸盐在中性条件下对铁阳极的钝化作用有关. 目前针对电絮凝吸附固载重金属的研究主要聚焦于中性或碱性条件[8-9, 12, 16],例如,有研究报道了中性和碱性条件下不同电解质阴离子和溶解氧浓度对于铁基电絮凝成矿种类的影响规律和影响机制[12] . 现阶段,酸性条件下铁电絮凝成矿过程缺乏关注;实际上,探讨酸性铁电絮凝用于实际重金属废水处理具有一定应用价值,这是因为阴极自产碱有利于提高系统pH,进而有效节省碱耗;较低pH能够提高溶液导电率及铁阳极法拉第效率,还使得在中性和碱性条件下易导致阳极钝化的电解质(如磷酸盐),在酸性条件下能够适用于电絮凝处理[17].
本研究着重讨论了酸性条件下铁基电絮凝的成矿规律及其对典型重金属的固载效果. 通过构建铁阳极、不锈钢阴极的电絮凝体系,考察不同支持电解质(NaCl、Na2SO4、NaH2PO4、NaNO3)和有无曝气对成矿种类的影响,以及不同铁矿物对重金属去除效果的影响;其次,通过检测溶液态铁Feaq(铁离子、亚铁离子及其溶液态羟基结合物种等)浓度在不同条件下铁电絮凝的变化规律,帮助深入解了酸性铁电絮凝的反应过程. 研究成果为酸性重金属废水的铁电絮凝处理提供理论参考,为电絮凝产物的资源化回收提供数据支撑.
1. 材料与方法(Materials and methods)
1.1 实验材料
硫酸镉 8/3水合物(CdSO4·8/3 H2O)、硫酸铜五水合物(CuSO4·5 H2O)、硫酸镍六水合物(NiSO4·6 H2O)、氯化铅(PbCl2)、硫酸钠(Na2SO4)、氯化钠(NaCl)、硝酸钠(NaNO3)、磷酸二氢钠 二水合物(NaH2PO4·2 H2O)购自阿拉丁化学试剂有限公司,硫酸、盐酸和硝酸购自广州化学试剂厂,以上试剂均为分析纯. 实验用水均为去离子水. 铁片电极(DT-3型,瑞源钢铁有限公司,江苏),不锈钢片电极(304型,中润宏发不锈钢有限公司,深圳),电极的表面积均为16 cm2(4 cm × 4 cm). 每次实验之前,铁片用粗砂布抛光,用稀释的HCl溶液(按重量5%)除锈,并用去离子水洗涤.
1.2 电絮凝实验
电絮凝实验在300 mL玻璃烧杯中进行,均使用铁片作为阳极,不锈钢作为阴极. 阴阳两极平行放置,电极间距为15 mm(有文献报道此间距下铁电絮凝去除重金属效率高[18]). 使用直流电源施加5 mA·cm−2的恒定电流于Fe阳极和不锈钢阴极,电流大小的选取以确保实现高的法拉第效率及重金属去除效率为依据[19]. 反应时间为30 min,磁力搅拌器控制转速为500 r·min−1. 实验分为曝气组和未曝气组,曝气组实验:在反应过程中持续向溶液中通入O2以模拟高DO浓度废水的电絮凝过程,未曝气组:将溶液暴露于空气中以模拟低DO浓度废水的电絮凝过程. 利用CdSO4、CuSO4、NiNO3和Pb(NO3)2储备液配制浓度分别为10、20、10、50 mg·L−1的重金属溶液,初始浓度的选取参考实际酸性矿山废水和工业废水中常见重金属污染数据[18, 20]. 针对每一种重金属溶液分别加入4种支持电解质NaCl、Na2SO4、NaH2PO4和NaNO3(10 mmol·L−1). 利用NaOH和电解质对应的酸溶液(NaH2PO4体系用硫酸溶液)调节初始溶液pH为3,并在反应过程中利用便携式pH计检测溶液pH变化. 开始试验后,以指定的时间间隔用注射器采集样品,通过0.45 μm的聚四氟乙烯膜过滤,收集滤液,分别测定其中的重金属浓度和Feaq浓度. 反应结束后,采集1 mL悬液并立即转移至4 mol·L−1 HCl溶液中溶解,用于后续测定实际产铁量[Fee]以计算法拉第效率. 对剩余悬液抽滤,然后将固体冷冻干燥、称重以便后续进行进一步分析和表征. 所有实验至少进行 3 次,结果以平均值 ± 标准差表示.
1.3 法拉第效率的计算
理论铁的溶解浓度(
[Fet] [Fet]=CL⋅MFe/(F⋅z) (1) 实验中电絮凝条件为I = 80 mA,L = 0.3 L,t = 30 min,带入公式2即电荷负载量CL为480 C·L−1. 式中,I是实验施加恒定电流大小,t是反应时间,L是反应溶液体积.
CL=It/L (2) 最后计算可得实验中电絮凝理论溶解量为138.91 mg·L−1. 将实验组反应后悬液溶解测定的实际产铁量
[Fee] [Fet] 1.4 固相矿物表征与液相金属含量分析
采用X射线衍射光谱(XRD,马尔文帕纳科公司,荷兰)分析铁矿物的物相组成,扫描范围为10°—80°,扫描速度为2(°)·min−1;用扫描电镜(SEM,FEI公司,美国)观察铁矿物表面形貌,并辅助分析铁矿物的种类;用火焰原子吸收光谱(AAS,岛津公司,日本)测定溶液中Cu(Ⅱ)、Cd(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的含量,以确定产生的铁矿物对重金属的去除效果. 采用邻菲啰啉分光光度法,在510 nm波长处测定溶液态铁(Feaq)的浓度,以分析铁电絮凝的成矿过程.
1.5 重金属赋存形态分析
收集上述电絮凝反应后的固体物质,对其进行冷冻干燥后,取0.2 g样品于离心管中,并加入10 mL 0.4 mol·L−1 HCl,然后置于旋转振荡仪以200 r·min−1反应0.5 h,离心收集上清液,测试重金属浸出含量,记为吸附态含量;再将离心后含有残余固体的离心管内加入10 mL 4 mol·L−1 HCl,然后置于旋转振荡仪中反应0.5 h,离心收集上清液,测试重金属浸出含量,记为结构态含量[21-22].
2. 结果与讨论(Results and discussion)
2.1 电解质种类和曝气条件对酸性条件下铁基电絮凝形成矿物种类的影响
为了探讨电解质和曝气条件对酸性铁基电絮凝体系成矿量的影响,对沉淀进行称重. 如图1a所示,NaNO3体系成矿量极少,这是因为NO3−对铁阳极有钝化作用. 据报道,中性铁电絮凝在NO3−和PO43−存在的情况下,铁阳极会形成一层纳米尺度的钝化层,钝化层的产生抑制了铁阳极的Fe(Ⅱ)溶出[17]. 而在本研究中,磷酸盐体系明显浑浊,产生了相对较多铁矿物,这是因为在酸性条件下,磷酸盐的阳极钝化作用被减弱,具体机理由后文详述. 曝气条件下溶液中DO浓度从7.5 mg·L−1逐渐上升,并维持至饱和浓度8.2 mg·L−1左右;而在不曝气条件下,由于阳极产生Fe(Ⅱ)的消耗,溶液中DO浓度将由7.5 mg·L−1在15 min内逐渐降低至0. 图1a结果表明,由于DO浓度影响,曝气相较于不曝气产生的铁矿物量略高.
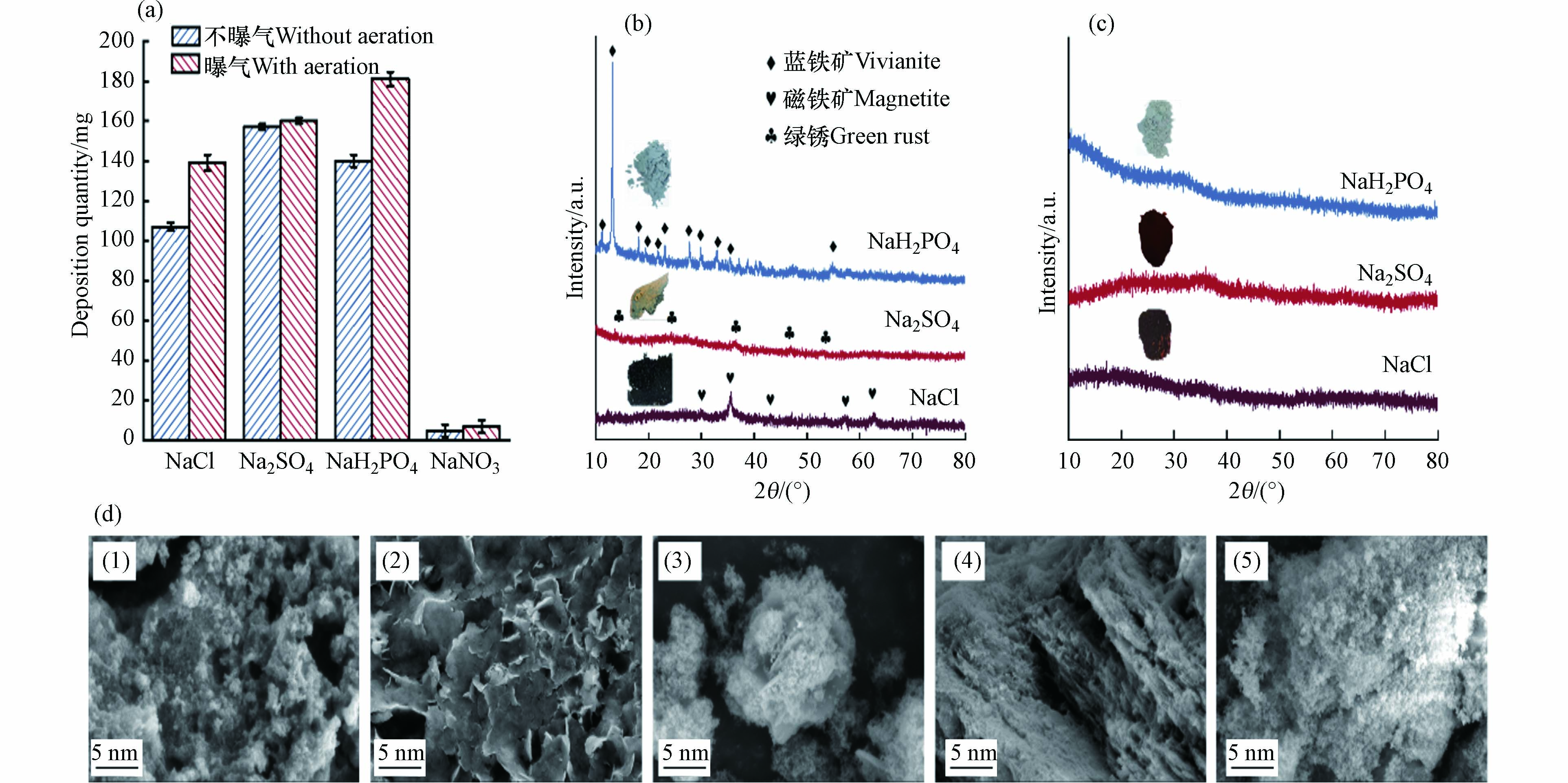 图 1 (a)不同电絮凝体系成矿量,(b)不曝气和(c)曝气情况下于不同支持电解质中产生铁矿物XRD图和(d)不同支持电解质体系(1)NaCl、(2)Na2SO4、(3)NaH2PO4、(4)NaCl(曝气)和(5)NaH2PO4(曝气)成矿的SEM图Figure 1. (a) Quantities of Fe minerals formed under different conditions; XRD patterns of Fe minerals produced in different supporting electrolytes (b) without and (c) with aeration; (d) SEM images of Fe minerals produced in different supporting electrolytes: (1) NaCl (without aeration), (2) Na2SO4 (without aeration), (3) NaH2PO4 (without aeration), (4) NaCl (with aeration), and (5) NaH2PO4 (with aeration)
图 1 (a)不同电絮凝体系成矿量,(b)不曝气和(c)曝气情况下于不同支持电解质中产生铁矿物XRD图和(d)不同支持电解质体系(1)NaCl、(2)Na2SO4、(3)NaH2PO4、(4)NaCl(曝气)和(5)NaH2PO4(曝气)成矿的SEM图Figure 1. (a) Quantities of Fe minerals formed under different conditions; XRD patterns of Fe minerals produced in different supporting electrolytes (b) without and (c) with aeration; (d) SEM images of Fe minerals produced in different supporting electrolytes: (1) NaCl (without aeration), (2) Na2SO4 (without aeration), (3) NaH2PO4 (without aeration), (4) NaCl (with aeration), and (5) NaH2PO4 (with aeration)采用XRD和SEM对固体物质组成和表面形貌进行表征,以明晰不同溶液条件下铁基电絮凝产生铁矿物的种类. 首先,利用XRD对不同条件下电絮凝作用30 min后产生的铁矿物沉淀进行物相区分. 图1b和1c表明,在未曝气条件下,NaCl、Na2SO4和NaH2PO4体系产生沉淀中的主要矿物分别为磁铁矿、绿锈和蓝铁矿;而在曝气条件下,NaCl和Na2SO4体系主要为无定型铁矿物. 未曝气条件下NaCl、Na2SO4和NaH2PO4体系产生铁矿物颜色分别为黑色、绿色和蓝绿色,与XRD结果相符;而在曝气条件下,NaCl和Na2SO4体系产生红棕色固体,NaH2PO4体系产生白色固体,结合XRD表征结果和体系中可能发生的反应[14, 23],认为两者分别为氢氧化铁和磷酸铁. SEM结果如图1d(1)—(5)所示,电絮凝产生的磁铁矿呈现颗粒状结构,绿锈呈现层状结构,蓝铁矿呈现扁平状堆叠结构;而在曝气条件下,所产生的氢氧化铁和磷酸铁分别呈现出层状和絮状结构,这与文献中记载的各类沉淀微观形貌结构一致[12, 14].
2.2 酸性条件下铁基电絮凝过程中Feaq浓度及pH随时间变化
酸性铁基电絮凝过程中,发现同一体系中溶液颜色在某一时刻后显著变深,且这一时刻后重金属浓度骤降. 据此推测酸性铁基电絮凝的反应过程可分为两个阶段. 为了验证这一观点,并讨论不同的电解质和曝气条件对两个阶段发生时间节点的影响,对电絮凝过程中不同时间节点的溶液态铁Feaq浓度进行取样检测,结果如图2a、2b所示. 在曝气和未曝气条件下,各个支持电解质体系中,Feaq浓度随着反应时间的推移均呈现先上升后下降的趋势. 这可能与Feaq浓度和pH两方面因素有关:一方面,Feaq浓度不断上升,增加了氧化成矿(式7—11)的底物浓度[17];另一方面,随着阴极不断产生OH−,溶液的pH逐渐升高(如图2c、2d所示),Fe(Ⅲ)与OH−的结合增强从而加快了成矿速度[24]. 这导致了该电絮凝体系由Fe(0)氧化反应(式3、式4)为主导向Feaq成矿反应(式7—11)为主导的转变,即表现为铁电絮凝从积累阶段向成矿阶段的转变(图2a、b虚线前后).
不同的电解质组成和曝气条件会导致阳极法拉第效率以及Feaq氧化沉淀速率的不同[25],从而影响成矿. 为了验证上述推断,计算了不同条件下电絮凝反应30 min后的法拉第效率. 如表1所示,NaCl、Na2SO4体系的法拉第效率均高于0.9,表明此时阳极表面主要发生Fe(0)氧化反应(式3、式4)[26-28],而NaH2PO4体系的法拉第效率略低,但是仍高于已有报道中,相应体系在近中性pH条件下的法拉第效率[12]. 这是因为:首先低的pH会导致H2O/O2的E0上升,从而阻碍在给定的阳极电位条件下电解水反应的发生;其次,低pH会导致Fe(Ⅱ)更难被O2氧化,降低了产生的铁氧化物附着在铁阳极表面阻止Fe(Ⅱ)进一步析出的可能性. 而NaNO3体系法拉第效率低于0.2,表明阳极表面主要发生电解水反应(式5)[17]. 这是因为硝酸盐能够氧化Fe(0),生成一层薄的(羟基)铁氧化物钝化膜,抑制离子扩散[29]. 除不同的法拉第效率会影响Fe(Ⅱ)/Fe(Ⅲ)溶出从而影响Feaq浓度外,不同体系Feaq的氧化沉淀速度也会影响Feaq峰值浓度和达峰位置. 例如Cl−存在的电絮凝体系中会产生氯自由基从而提高氧化成矿率[13],因而NaCl体系的Feaq峰值浓度低且达峰时间更早;而相较于蓝铁矿,绿锈的成矿速度更快,故其Feaq峰值浓度更低且达峰更早[12]. 相比于未曝气条件,曝气条件下Feaq的浓度更低,这是因为溶解氧浓度的增加对Fe(Ⅱ)氧化为Fe(III)速度有促进作用,从而提升了成矿率[24]. 该结论与曝气条件下电絮凝体系最终产生沉淀物质量高于不把曝气条件相符.
表 1 不同支持电解质和曝气条件对电絮凝处理酸性废水法拉第效率的影响Table 1. Effects of type of supporting electrolyte and aeration on Faradic efficiency of electrocoagulation in treating acidic wastewater支持电解质Supporting electrolyte 曝气条件Aeration condition 法拉第效率Faradic efficiency NaCl without aeration 0.91 with aeration 0.92 Na2SO4 without aeration 0.92 with aeration 0.92 NaH2PO4 without aeration 0.89 with aeration 0.83 NaNO3 without aeration 0.19 with aeration 0.12 Fe(0)−2e−→Fe(Ⅱ) (3) Fe(0)−3e−→Fe(Ⅲ) (4) 2H2O−4e−→4H++O2 (5) 2H2O+2e−→H2↑+2OH− (6) Fe(Ⅱ,Ⅲ)+OH−→Magnetite↓ (7) Fe(Ⅱ,Ⅲ)+OH−+SO2−4→Greenrust↓ (8) Fe(Ⅱ,Ⅲ)+OH−+H2PO2−4→Vivianite↓ (9) Fe(Ⅲ)+3OH−→Fe(OH)3↓ (10) Fe(Ⅲ)+H2PO2−4→FePO4↓+2H+ (11) 基于上述结果,在酸性条件下的铁基电絮凝过程中需要经历两个阶段——积累阶段和成矿阶段,铁矿物的形成以及重金属的固载吸附均发生于成矿阶段. 这两个阶段中铁阳极氧化溶出Fe(Ⅱ)和溶液体相中Feaq沉淀分别占据主导地位. 这两个阶段发生的时间节点主要取决于Feaq积累阶段中的法拉第效率,以及在成矿阶段的Feaq成矿效率,两者都与溶液的支持电解质种类及曝气条件有关,并与其共同决定了形成铁矿物的种类和质量. 首先,在积累阶段,阳极Fe(0)的氧化(式3、式4)、和阴极电解水析氢产碱(式6)是主要反应. 这一阶段阳极附近会产生并积累Fe(Ⅱ),并伴随体相pH上升,但是溶液中固体物质的质量不会明显增加. 而随着溶液中Feaq浓度和pH的增加,成矿(式7—11)速率逐渐增加,直至超过Fe(0)的氧化(式3、式4)速率,在这个节点之后,溶液中的固体物质显著增加.
2.3 酸性条件下铁基电絮凝产生矿物吸附重金属效果
为研究上文中产生不同铁矿物物种对不同重金属的吸附固载效果,向不同支持电解质和有无曝气的电絮凝体系中加入常见重金属阳离子Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)和Pb(Ⅱ),并研究其去除情况. 如前文所述,NaH2PO4作为支持电解质的溶液在酸性未曝气的条件下主要产生蓝绿色的蓝铁矿,而曝气条件下,蓝铁矿则会被充足的氧气氧化为白色的磷酸铁沉淀. 如图3所示,二者对Cd(Ⅱ)、Cu(Ⅱ)均有优良的去除效果,30 min去除率均能达到99%以上. 在未曝气条件下,NaCl和Na2SO4体系分别产生的磁铁矿和绿锈,该体系对Cu(Ⅱ)的去除率也能达到99%以上,但在对Cd(Ⅱ)的去除率上,磁铁矿(85.0%)要高于绿锈(36.5%). 曝气组中NaCl或Na2SO4体系中产生的氢氧化铁对Cd(Ⅱ)、Cu(Ⅱ)的固载效果较差,其去除率分别为35.5%和72.9%. 这是因为除了内球络合吸附,蓝铁矿和绿锈、磁铁矿均因为晶格中具有Fe(Ⅱ),能够被相似离子半径和价态的Pb(Ⅱ)、Cd(Ⅱ)、Cu(Ⅱ)同晶替代[30]. 因此电絮凝产生的不同矿物对Cd(Ⅱ)去除效果强弱顺序可概括为:磷酸铁≈蓝铁矿>磁铁矿>绿锈>氢氧化铁;对Cu(Ⅱ)去除效果强弱顺序为:磁铁矿>蓝铁矿≈磷酸铁>氢氧化铁.
对于Ni(Ⅱ)和Pb(Ⅱ),NaH2PO4电解质体系则无法展现良好的吸附性能. 如图4a、4c所示,未曝气条件下NaH2PO4体系电絮凝对于Ni(Ⅱ)的去除效果十分有限,低于NaCl、Na2SO4体系下81.6%的去除率,与NaNO3体系效果接近,去除率仅约为40.9%. 这与Cu(Ⅱ)和Cd(Ⅱ)的结果差别很大. 结合上文法拉第效率的计算结果可排除阳极钝化的可能性,推测是由于蓝铁矿比表面积小、表面正电荷较强以及官能团种类较单一的特点导致其对Ni(Ⅱ)吸附能力较弱[31],但其对于这三种重金属去除效果的差异的微观机理还有待进一步阐明. 磁铁矿具有非化学计量缺陷同时氧骨架较为灵活,因而具有很大的容量通过Fe(Ⅱ)替代来容纳各种金属离子,故其对Cu(Ⅱ)、Cd(Ⅱ)、Pb(Ⅱ)、Ni(Ⅱ)均有较好的去除能力[32]. 绿锈因为特殊的LDHs而具有内表面,因此也有不错的反应特性,此外在绿锈的形成过程中,Cu(Ⅱ)、Cd(Ⅱ)、Ni(Ⅱ)等也能通过同晶替代Fe(Ⅱ)从而被共沉淀固载. 曝气条件下NaH2PO4体系产生的磷酸铁对Ni(Ⅱ)的去除率仅为34.4%,低于NaCl和Na2SO4体系所产生氢氧化铁的56.1%.
对于酸性溶液中Pb(Ⅱ)的去除,结果如图4b、4c所示,未曝气条件下的NaCl体系效果最好,其次是曝气条件下的NaCl体系,这反映出磁铁矿固载Pb(Ⅱ)的效果强于氢氧化铁. 二者均优于NaNO3电解质体系. 这是由于磁铁矿对于Pb(Ⅱ)的特殊作用导致的,即Pb(Ⅱ)主要通过双齿双核共享配位作用吸附在磁铁矿表面,无吸附容量限制[33]. 因此,电絮凝产生的不同矿物对Ni(Ⅱ)去除效果强弱顺序可概括为:磁铁矿≈绿锈>蓝铁矿≈氢氧化铁>磷酸铁;对Pb(Ⅱ)去除效果强弱顺序为:磁铁矿>氢氧化铁. 未比较Na2SO4和NaH2PO4体系对Pb(Ⅱ)的吸附固载效果是因为PbSO4和Pb(H2PO4)2溶解度较低,Pb(Ⅱ)在这两种支持电解质的废水中无法以稳定的离子形式存在.
需要说明的是,在重金属的电絮凝处理过程中,除了矿物的固载作用,还可能存在阴极还原以及阳极置换的氧化还原过程. 以Cu(Ⅱ)为例,通过惰性电极替代Fe阳极后,发现在不同电解质和曝气条件实验组中Cu(Ⅱ)处理后浓度均为约15 mg·L−1,说明Cu(Ⅱ)的阴极还原确实存在,对溶液中Cu(Ⅱ)去除的贡献占20%. 对于阳极置换反应,由于阳极附近有大量带正电荷的Fe(Ⅱ)/Fe(III)物种和铁氧化物,带相同电荷的Cu(Ⅱ)难以接近,故认为其影响可忽略不计.
图5为不同电解质和曝气条件下酸性铁基电絮凝的成矿过程、成矿种类及其对四种重金属离子Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的吸附固载效果. 使用NaNO3作为支持电解质会使铁阳极钝化,导致低成矿量和重金属去除效率. 在其他电解质体系中,在无曝气的条件下NaCl、Na2SO4、NaH2PO4体系分别产生磁铁矿、绿锈和蓝铁矿;曝气条件下NaCl、Na2SO4体系产生氢氧化铁、NaH2PO4体系产生磷酸铁. 而产生的不同种类铁矿物对四种重金属的吸附效果各有所长,Cu(Ⅱ)和Ni(Ⅱ)易被吸附在磁铁矿和绿锈上,而磁铁矿对Pb(Ⅱ)、Cd(Ⅱ)的吸附效率高于绿锈;蓝铁矿和磷酸铁对Cu(Ⅱ)和Cd(Ⅱ)固载效果优异,而对Ni(Ⅱ)的固载效果较差;氢氧化铁絮体对四种重金属的固载效果均较差.
2.4 电化学产生铁矿物与重金属结合形态
重金属的赋存形态直接影响其在介质中的迁移转化能力、毒性和生物活性[34]. 而酸性条件下产生的铁矿物稳定固载重金属的能力尤为重要,为此对电絮凝结束后产生的固体沉淀进行盐酸提取实验,研究了不同种类铁矿物对Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的的4种重金属离子的固载稳定性. 如图6所示,绿锈对Cd(Ⅱ)的固载后,结构态比例高达76%,对Cu(Ⅱ)、Ni(Ⅱ)的结构态比例分别为10%和38%. 这是因为在绿锈的形成过程中,Cu(Ⅱ)和Ni(Ⅱ)能够在绿锈的形成过程中通过对相似离子半径的Fe(Ⅱ)同晶替代,从而结合进入绿锈的晶格之中被共沉淀固载[16]. 磁铁矿对于4种重金属Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)的结构态比例分别为8%、7%、41%和18%. 这是由于磁铁矿的原子组成和原子结构较为灵活,同样促使其能够通过同晶替代Fe(Ⅱ)容纳不同的重金属阳离子[35]. 磷酸铁和氢氧化铁对Cd(Ⅱ)、Cu(Ⅱ)的固载形式为吸附态,对Ni(Ⅱ)的结构态比例也低于22%. 这是因为磷酸铁、氢氧化铁和蓝铁矿对pH较为敏感,易溶于酸. 综上,电絮凝产生的绿锈和磁铁矿对重金属的固载稳定性更高,而磷酸铁、氢氧化铁和蓝铁矿的固载稳定性相对较低,对重金属固载稳定性较低的电絮凝铁泥的进一步处置,更需要注意其可能产生的环境影响.
2.5 多种重金属同时存在的相互影响
因为在实际的重金属废水中,经常同时存在多种重金属,故为了验证酸性条件铁基电絮凝对多种重金属的同步去除效果,开展了Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)同时存在的电絮凝实验. 结果如图7所示,所有不同支持电解质种类和曝气条件的溶液体系对Cu(Ⅱ)和Ni(Ⅱ)的固载效果均与单一去除效果类似,说明其他共存阳离子的存在对Cu(Ⅱ)和Ni(Ⅱ)的去除没有显著影响. 而对于Cd(Ⅱ)的去除,除了NaNO3体系在单一去除实验中去除效果本身很差之外,NaCl和Na2SO4体系的去除速率也显著低于单一去除实验的去除速率,这说明在同步去除实验中Cd的固载受到了其他共存重金属离子的影响,这是因为Cd(Ⅱ)具有较低的电负性和较大的水化半径[36-37] ,使得Cd(Ⅱ)与铁矿物的亲和力要弱于Cu(Ⅱ)和Ni(Ⅱ),从而在竞争吸附中处于较弱的地位. 而NaH2PO4体系仍然保持较高的去除速率,说明该体系对Cd(Ⅱ)的固载过程除了吸附,还可能存在其他机理. 例如,随着pH升高,H2PO4−会逐渐转化为HPO42−和PO43−,其中,HPO42−和PO43−与Cd(Ⅱ)所形成沉淀的溶解度更低,从而将Cd(Ⅱ)固载下来. 因为这一过程仅与溶液中H2PO4−的浓度和pH有关,而不受其他重金属的影响,故NaH2PO4体系中Cd(Ⅱ)能够保持较高的去除速率.
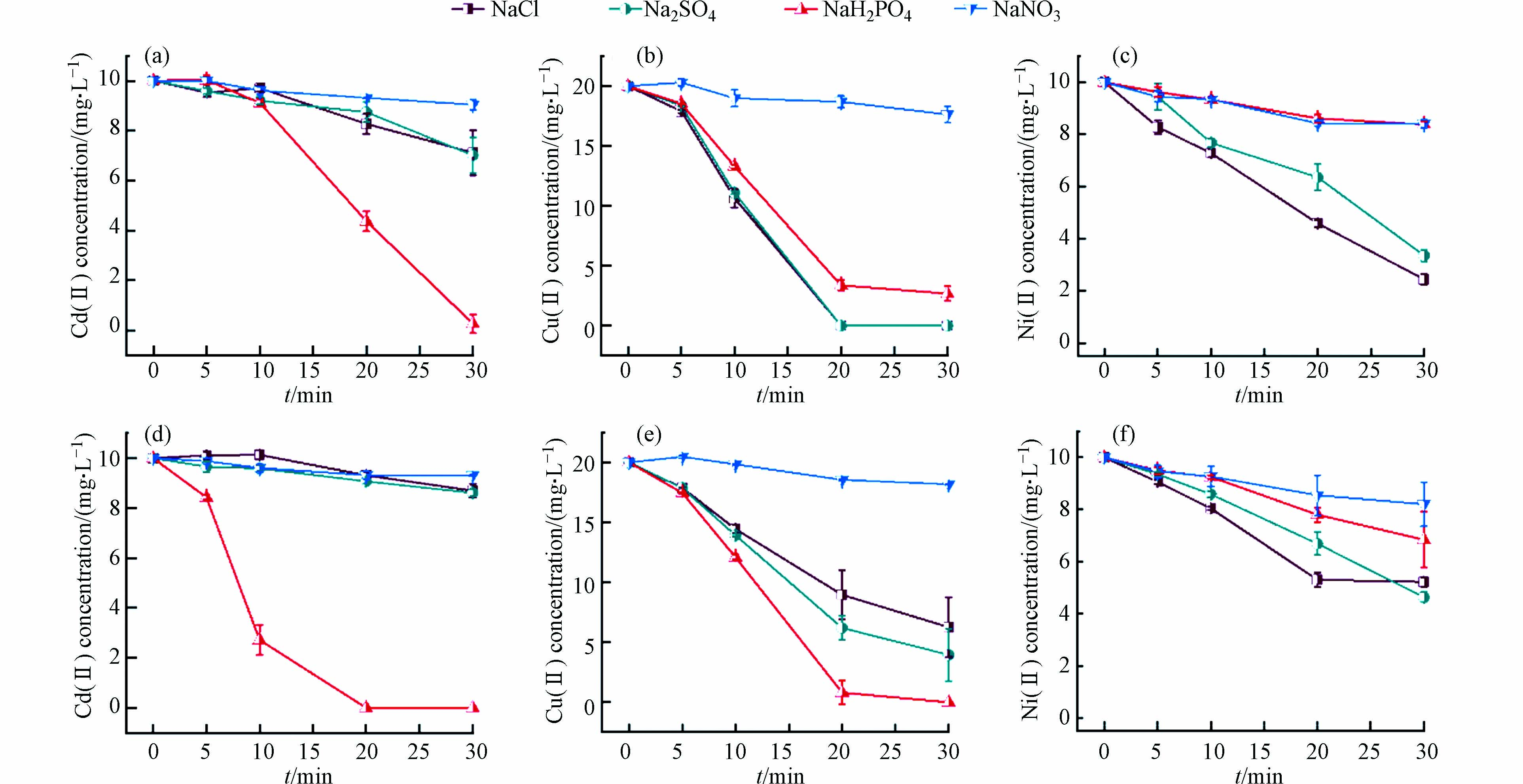 图 7 电解质和曝气条件对铁电絮凝同步去除Cd(Ⅱ)(a. 不曝气、d. 曝气)、Cu(Ⅱ) (b. 不曝气、e. 曝气)、Ni(Ⅱ) (c. 不曝气、f. 曝气)的影响Figure 7. Effects of supporting electrolyte and aeration conditions on the simultaneous removal of Cd(Ⅱ) (a. without and d. with aeration), Cu(Ⅱ) (b. without and e. with aeration), and Ni(Ⅱ) (c. without aeration, f. with aeration) by Fe-electrocoagulation
图 7 电解质和曝气条件对铁电絮凝同步去除Cd(Ⅱ)(a. 不曝气、d. 曝气)、Cu(Ⅱ) (b. 不曝气、e. 曝气)、Ni(Ⅱ) (c. 不曝气、f. 曝气)的影响Figure 7. Effects of supporting electrolyte and aeration conditions on the simultaneous removal of Cd(Ⅱ) (a. without and d. with aeration), Cu(Ⅱ) (b. without and e. with aeration), and Ni(Ⅱ) (c. without aeration, f. with aeration) by Fe-electrocoagulation3. 结论(Conclusions)
(1)酸性铁电絮凝分为两个阶段. 其中第一阶段Feaq浓度逐渐升高,成矿量少,重金属去除较低;第二阶段Feaq浓度达到峰值并开始下降,成矿量逐步增加,重金属去除速率骤然提升.
(2)不同电解质(NaCl、Na2SO4、NaH2PO4、NaNO3)和曝气与否影响下酸性铁电絮凝会产生不同种类铁矿物. 其中,NaNO3体系会导致铁阳极钝化,抑制成矿;其他电解质在曝气条件下,电絮凝产生固体包括氢氧化铁(NaCl/Na2SO4体系)和磷酸铁(NaH2PO4体系);不曝气条件下,产生铁矿物包括磁铁矿(NaCl体系)、绿锈(Na2SO4体系)和蓝铁矿(NaH2PO4体系).
(3)重金属吸附结果表明,磁铁矿对Cu(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)、Cd(Ⅱ)等都具有优异的固载效果,绿锈对Cu(Ⅱ)和Ni(Ⅱ)的吸附能力高于Cd(Ⅱ);蓝铁矿和磷酸铁对溶液中重金属Cd(Ⅱ)和Cu(Ⅱ)也有良好的去除效率,但对Ni(Ⅱ)去除效果较差,且其重金属固载稳定性低于磁铁矿和绿锈. 在Cd(Ⅱ)、Cu(Ⅱ)、Ni(Ⅱ)同时存在的重金属废水中,电絮凝对Cu(Ⅱ)和Ni(Ⅱ)的吸附效果与单一重金属废水结果一致,而对Cd(Ⅱ)的吸附效果则显著下降.
-
图 1 (a)不同电絮凝体系成矿量,(b)不曝气和(c)曝气情况下于不同支持电解质中产生铁矿物XRD图和(d)不同支持电解质体系(1)NaCl、(2)Na2SO4、(3)NaH2PO4、(4)NaCl(曝气)和(5)NaH2PO4(曝气)成矿的SEM图
Figure 1. (a) Quantities of Fe minerals formed under different conditions; XRD patterns of Fe minerals produced in different supporting electrolytes (b) without and (c) with aeration; (d) SEM images of Fe minerals produced in different supporting electrolytes: (1) NaCl (without aeration), (2) Na2SO4 (without aeration), (3) NaH2PO4 (without aeration), (4) NaCl (with aeration), and (5) NaH2PO4 (with aeration)
图 7 电解质和曝气条件对铁电絮凝同步去除Cd(Ⅱ)(a. 不曝气、d. 曝气)、Cu(Ⅱ) (b. 不曝气、e. 曝气)、Ni(Ⅱ) (c. 不曝气、f. 曝气)的影响
Figure 7. Effects of supporting electrolyte and aeration conditions on the simultaneous removal of Cd(Ⅱ) (a. without and d. with aeration), Cu(Ⅱ) (b. without and e. with aeration), and Ni(Ⅱ) (c. without aeration, f. with aeration) by Fe-electrocoagulation
表 1 不同支持电解质和曝气条件对电絮凝处理酸性废水法拉第效率的影响
Table 1. Effects of type of supporting electrolyte and aeration on Faradic efficiency of electrocoagulation in treating acidic wastewater
支持电解质Supporting electrolyte 曝气条件Aeration condition 法拉第效率Faradic efficiency NaCl without aeration 0.91 with aeration 0.92 Na2SO4 without aeration 0.92 with aeration 0.92 NaH2PO4 without aeration 0.89 with aeration 0.83 NaNO3 without aeration 0.19 with aeration 0.12 -
[1] 刘爱荣, 李季, 王伟, 等. 纳米零价铁处理含重金属工业废水研究进展 [J]. 环境化学, 2022, 41(4): 1278-1291. doi: 10.7524/j.issn.0254-6108.2021082203 LIU A R, LI J, WANG W, et al. Advance of heavy metal-loading industrial wastewater treatment with nanoscale zero-valent iron [J]. Environmental Chemistry, 2022, 41(4): 1278-1291(in Chinese). doi: 10.7524/j.issn.0254-6108.2021082203
[2] RODRÍGUEZ-GALÁN M, BAENA-MORENO F M, VÁZQUEZ S, et al. Remediation of acid mine drainage [J]. Environmental Chemistry Letters, 2019, 17(4): 1529-1538. doi: 10.1007/s10311-019-00894-w [3] 欧阳纶, 高念平. 用酸洗废液合成铁氧体净化电镀废水研究 [J]. 环境化学, 1984, 3(6): 59-61. OU Y L, GAO N P. Study on purification of electroplating wastewater by synthesizing ferrite from pickling waste liquid [J]. Environmental Chemistry, 1984, 3(6): 59-61(in Chinese).
[4] KOŁODYŃSKA D, KRUKOWSKA J, THOMAS P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon [J]. Chemical Engineering Journal, 2017, 307: 353-363. doi: 10.1016/j.cej.2016.08.088 [5] CUI L, WANG Y, GAO L, et al. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property [J]. Chemical Engineering Journal, 2015, 281: 1-10. doi: 10.1016/j.cej.2015.06.043 [6] AL-OTHMAN Z A, NAUSHAD M, INAMUDDIN. Organic–inorganic type composite cation exchanger poly-o-toluidine Zr(IV) tungstate: Preparation, physicochemical characterization and its analytical application in separation of heavy metals [J]. Chemical engineering journal (Lausanne, Switzerland:1996), 2011, 172(1): 369-375. [7] KURNIAWAN T A, CHAN G Y S, LO W, et al. Physico–chemical treatment techniques for wastewater laden with heavy metals [J]. Chemical Engineering Journal, 2006, 118(1): 83-98. [8] KOBYA M, GEBOLOGLU U, ULU F, et al. Removal of arsenic from drinking water by the electrocoagulation using Fe and Al electrodes [J]. Electrochimica Acta, 2011, 56(14): 5060-5070. doi: 10.1016/j.electacta.2011.03.086 [9] BALASUBRAMANIAN N, KOJIMA T, SRINIVASAKANNAN C. Arsenic removal through electrocoagulation: Kinetic and statistical modeling [J]. Chemical Engineering Journal, 2009, 155(1): 76-82. [10] BUZZI D C, VIEGAS L S, RODRIGUES M A S, et al. Water recovery from acid mine drainage by electrodialysis [J]. Minerals Engineering, 2013, 40: 82-89. doi: 10.1016/j.mineng.2012.08.005 [11] 信帅帅, 孙彤, 江波. 整流电絮凝技术对缺氧地下水中As(Ⅲ)的原位修复 [J]. 环境化学, 2019, 38(1): 195-201. doi: 10.7524/j.issn.0254-6108.2018020401 XING S S, JIANG T, JIANG B. Rectified-alternating-current electrocoagulation for As(III) remediation in the anoxic groundwater [J]. Environmental Chemistry, 2019, 38(1): 195-201(in Chinese). doi: 10.7524/j.issn.0254-6108.2018020401
[12] DUBRAWSKI K L, van GENUCHTEN C M, DELAIRE C, et al. Production and transformation of mixed-valent nanoparticles generated by Fe(0) electrocoagulation [J]. Environmental Science & Technology, 2015, 49(4): 2171-2179. [13] KEYIKOGLU R, CAN O T, AYGUN A, et al. Comparison of the effects of various supporting electrolytes on the treatment of a dye solution by electrocoagulation process [J]. Colloid and Interface Science Communications, 2019, 33: 100210. doi: 10.1016/j.colcom.2019.100210 [14] van GENUCHTEN C M, BEHRENDS T, KRAAL P, et al. Controls on the formation of Fe(Ⅱ, Ⅲ) (hydr)oxides by Fe(0) electrolysis [J]. Electrochimica Acta, 2018, 286: 324-338. doi: 10.1016/j.electacta.2018.08.031 [15] MUEHE E M, MORIN G, SCHEER L, et al. Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides [J]. Environmental Science & Technology, 2016, 50(5): 2281-2291. [16] XU L, XU X, WU D. Initial dissolved oxygen-adjusted electrochemical generation of sulfate green rust for cadmium removal using a closed-atmosphere Fe–electrocoagulation system [J]. Chemical Engineering Journal, 2019, 359: 1411-1418. doi: 10.1016/j.cej.2018.11.032 [17] van GENUCHTEN C M, DALBY K N, CECCATO M, et al. Factors affecting the Faradaic efficiency of Fe(0) electrocoagulation [J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4958-4968. doi: 10.1016/j.jece.2017.09.008 [18] BHAGAWAN D, POODARI S, POTHURAJU T, et al. Effect of operational parameters on heavy metal removal by electrocoagulation [J]. Environmental Science and Pollution Research, 2014, 21(24): 14166-14173. doi: 10.1007/s11356-014-3331-8 [19] 周好磊, 李少林, 魏宏斌, 等. 低电流电絮凝法去除废水中重金属离子的研究 [J]. 中国给水排水, 2017, 33(5): 85-88. ZHOU H L, LI H L, WEI H B, et al. Removal of heavy metal ions from wastewater by low current electrocoagulation technology [J]. China Water & Wastewater, 2017, 33(5): 85-88(in Chinese).
[20] 蒋克彬, 彭松, 张小海. 铅酸蓄电池厂含铅废水处理工程实例 [J]. 蓄电池, 2008(2): 84-86. JINAG K B, PENG S, ZHANG X H. A project example to treat lead wastewater from lead-acid battery plant [J]. Chinese LABAT Man, 2008(2): 84-86(in Chinese).
[21] MUEHE E M, OBST M, HITCHCOCK A, et al. Fate of Cd during microbial Fe(Ⅲ) mineral reduction by a novel and Cd-tolerant Geobacter species [J]. Environmental Science & Technology, 2013, 47(24): 14099-14109. [22] 刘承帅, 李芳柏, 陈曼佳, 等. Fe(Ⅱ)催化水铁矿晶相转变过程中Pb的吸附与固定 [J]. 化学学报, 2017, 75(6): 621-628. doi: 10.6023/A17030093 LIU C S, LI F B, CHEN M J, et al. Adsorption and stabilization of lead during Fe(Ⅱ)-catalyzed phase transformation of ferrihydrite [J]. Acta Chimica Sinica, 2017, 75(6): 621-628(in Chinese). doi: 10.6023/A17030093
[23] 李立平, 李煜乾. 不同晶体类型磷酸铁的制备及电化学性能的研究进展 [J]. 化工技术与开发, 2022, 51(8): 27-32. LI L P, LI Y Q. Research process on preparation and electrochemical properties of several crystal forms of iron phosphate [J]. Technology & Development of Chemical Industry, 2022, 51(8): 27-32(in Chinese).
[24] BAE Y, CROMPTON N M, SHARMA N, et al. Impact of dissolved oxygen and pH on the removal of selenium from water by iron electrocoagulation [J]. Water Research, 2022, 213: 118159. doi: 10.1016/j.watres.2022.118159 [25] LAKSHMANAN D, CLIFFORD D A, SAMANTA G. Ferrous and ferric ion generation during iron electrocoagulation [J]. Environmental Science & Technology, 2009, 43(10): 3853-3859. [26] HAN M, SONG J, KWON A. Preliminary investigation of electrocoagulation as a substitute for chemical coagulation [J]. Water Supply, 2002, 2(5-6): 73-76. doi: 10.2166/ws.2002.0152 [27] MATTESON M J, DOBSON R L, GLENN R W, et al. Electrocoagulation and separation of aqueous suspensions of ultrafine particles [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 1995, 104(1): 101-109. [28] MILLS D. A new process for electrocoagulation [J]. Journal Awwa, 2000, 92(6): 34-43. doi: 10.1002/j.1551-8833.2000.tb08957.x [29] REINSCH B C, FORSBERG B, PENN R L, et al. Chemical transformations during aging of zerovalent iron nanoparticles in the presence of common groundwater dissolved constituents [J]. Environmental Science & Technology, 2010, 44(9): 3455-3461. [30] SHI M, MIN X, KE Y, et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr)oxides [J]. Science of the Total Environment, 2021, 752: 141930. doi: 10.1016/j.scitotenv.2020.141930 [31] 陈琼姗, 周睿, 许璋奕, 等. 异化还原铁泥合成蓝铁矿/微生物复合材料固载铅研究 [J]. 环境科学学报, 2022, 42(11): 221-231. CHEN Q S, ZHOU R, XU Z Y, et al. Synthesis of vivianite/bacteria composites by dissimilatory reduction of iron sludge for lead immobilization [J]. Acta Scientiae Circumstantiae, 2022, 42(11): 221-231(in Chinese).
[32] ZHANG J, ZHANG C, WEI G, et al. Reduction removal of hexavalent chromium by zinc-substituted magnetite coupled with aqueous Fe(II) at neutral pH value [J]. Journal of Colloid and Interface Science, 2017, 500: 20-29. doi: 10.1016/j.jcis.2017.03.103 [33] LIANG X, WEI G, XIONG J, et al. Adsorption isotherm, mechanism, and geometry of Pb(Ⅱ) on magnetites substituted with transition metals [J]. Chemical Geology, 2017, 470: 132-140. doi: 10.1016/j.chemgeo.2017.09.003 [34] 李宝, 张智慧, 王志奇, 等. 山东南四湖底泥典型重金属的形态分布、稳定度与风险评价 [J]. 环境化学, 2022, 41(3): 940-948. doi: 10.7524/j.issn.0254-6108.2020112501 LI B, ZHANG Z H, WANG Z Q. Fraction distribution, stability and risk assessment of typical heavy metals in sediment of Nansi Lake, Shandong Province, China [J]. Environmental Chemistry, 2022, 41(3): 940-948(in Chinese). doi: 10.7524/j.issn.0254-6108.2020112501
[35] LI Y, WEI G, LIANG X, et al. Metal substitution-induced reducing capacity of magnetite coupled with aqueous Fe(Ⅱ) [J]. Acs Earth and Space Chemistry, 2020, 4(6): 905-911. doi: 10.1021/acsearthspacechem.0c00089 [36] KINNIBURGH D G, JACKSON M L, SYERS J K. Adsorption of alkaline earth, transition, and heavy metal cations by hydrous oxide gels of iron and aluminum [J]. Soil Science Society of America Journal, 1976, 40(5): 796-799. doi: 10.2136/sssaj1976.03615995004000050047x [37] LIANG Y, TIAN L, LU Y, et al. Kinetics of Cd(Ⅱ) adsorption and desorption on ferrihydrite: experiments and modeling [J]. Environmental Science:Processes & Impacts, 2018, 20(6): 934-942. 期刊类型引用(1)
1. 黄晓惠,刘云飞,宋微娜,赵艳红,邵景景,宋春来. 矿物加工工程专业“物理化学”课程教学探索与实践——以电化学章节的教学设计与思考为例. 化工时刊. 2024(06): 106-109 .  百度学术
百度学术
其他类型引用(0)
-









 下载:
下载: