-
全氟和多氟烷基化合物(PFASs)是一类人工合成的有机化合物,具有优异的热稳定性和化学稳定性,疏水疏油、不易生物降解、高表面活性等独特的理化性质[1 − 2]. 自20世纪50年代起,PFASs被广泛应用于商业和工业领域,所涉及产品包括消防泡沫、纸板、杀虫剂、驱虫剂、润滑剂、表面处理剂、个人护理品和食品包装产品等[3 − 4]. 自2001年Giesy和Kannan首次在环境中检测出PFASs起[5] ,PFASs相继在世界各地的水体[6 − 7]、土壤[8 − 9]、空气[10]、沉积物[11]、灰尘[12]、动物[13 − 14]、人体血清[15]、尿液[16]等环境介质及生物样本中广泛检出. 由于PFASs具有环境持久性、生物累积性、多种毒性等特性[17],其进入环境后,会在一定程度上对生态环境及人类健康构成威胁[18]. 已有研究指出PFASs可以通过各种途径进入到人体[19],例如,PFASs可以通过胎盘屏障,进入到胎儿体内,进而对胎儿造成宫内暴露[20]. 近年来,针对于PFASs母婴暴露的相关研究已经成为研究热点. 在以往研究中,研究人员主要通过监测母血、脐带血以及胎盘等样品来探究PFASs的宫内暴露特征[21]. 然而,上述样品采样过程是侵入性的,可能会对采样对象造成损伤,导致难以开展大范围、系统性研究. Vizcaino等[22]指出包括PFASs在内的多种持久性有机污染物可通过胎盘转移进入到胎儿体内,并且在胎便中形成不同程度的累积. 因此,开展胎便中的PFASs的痕量分析研究,可获得PFASs对胎儿的产前暴露相关信息,进而为评估其产前暴露风险提供数据支撑.
胎便指胎儿出生后的几次排便,是一种黑绿色、无味、粘稠的粘性物质. 胎便由水、胃肠道和皮肤脱落的上皮细胞、胎毛、各种胰腺和肠道分泌物,以及吞咽的羊水残留物组成[23],它是一种高度复杂的基质. 与其他研究中使用的胎儿基质(脐带血、尿液和血液)相比,胎便样本数量多,易于收集,非侵入性,更为重要的是胎便可将污染物的累计代谢考虑在内. 除此之外,胎便的样品收集窗口相对较宽,即胎儿出生后3天内的胎便均可用于分析且效果最佳[23].
胎便中积累了妊娠期间暴露于胎儿的多种外源性化合物[24],但其复杂的基质成分导致对其中的污染物进行痕量分析具有一定挑战. 目前,关于胎便中PFASs的分析检测方法仍鲜有报道并亟待研究. 基于此,本研究利用离线固相萃取结合高效液相色谱串联质谱法,建立了胎便中14种PFASs的分析方法. 该方法采用乙腈/水(9∶1,V/V)对胎便进行提取,Envi-Carb与Oasis WAX进行萃取净化,具有操作简便,灵敏度高,准确度和精密度较高的优点. 该方法的建立可为胎便中PFASs的分析检测提供技术基础,为研究PFASs的母婴传递和评估胎儿产前PFASs暴露风险提供技术支撑.
-
PFASs标准品:全氟辛酸(perfluorooctanoic acid, PFOA)、全氟壬酸(perfluorononanoic acid, PFNA)、全氟癸酸(perfluorodecanoic acid, PFDA)、全氟十一烷酸(perfluoroundecanoic acid, PFUdA)、全氟十二烷酸(perfluorododecanoic acid, PFDoA)、全氟十三烷酸(perfluorotridecanoic acid, PFTrDA)、全氟己基磺酸(perfluorohexanesulfonic acid, PFHxS)、全氟庚基磺酸(perfluoroheptanesulfonic acid, PFHpS)、全氟辛基磺酸(Perfluorooctanesulfonic acid,PFOS)、全氟壬基磺酸(perfluorononanesulfonic acid, PFNS)、全氟癸基磺酸(perfluorodecanesulfonic acid, PFDS)、全氟十二烷磺酸(perfluorododecanesulfonic acid, PFDoS)、6:2氯化聚氟烷基醚磺酸(6:2 chlorinated Polyfluoroalkyl ether sulfonic acid, 6:2 Cl-PFESA)、8:2氯化聚氟烷基醚磺酸(8:2 Chlorinated polyfluoroalkyl ether sulfonic acid, 8:2 Cl-PFESA). 13C同位素标记的PFASs内标:13C4-PFOA(13C4-全氟辛酸,MPFOA)、13C5-PFNA(13C5-全氟壬酸,MPFNA),13C2-PFDA(13C2-全氟癸酸,MPFDA),13C2-PFUdA(13C2-全氟十一酸,MPFUdA),18O2-PFHxS(18O2-全氟己基磺酸,MPFHxS),13C4-PFOS(13C4-全氟辛基磺酸,MPFOS). 本研究所使用的PFASs及其同位素标记的化合物标准品均购自加拿大Wellington Laboratories公司,所有标准品纯度均超过98%,详细信息见表1.
甲醇、乙腈(LC-MS级)购自美国Thermo Fisher Scientific公司;醋酸铵(>97%)与氢氧化铵(28%)购自美国Alfa Aesar公司;氢氧化钠、盐酸(优级纯)购自国药集团化学试剂有限公司;Oasis WAX固相萃取柱(150 mg/6 mL)购自美国Waters公司;Envi-carb固相萃取柱(150 mg/6 mL)购自美国Supelco公司;Cleanert PEP(500 mg/6 mL)固相萃取柱购自天津博纳艾杰尔公司. 实验用水为超纯水,由Milli-Q Advantage A10系统制备,电阻率大于18.2 MΩ·cm.
-
色谱柱采用Acquity UPLC BEH C18色谱柱(2.1 mm × 100 mm,1.7 μm,美国Waters公司). 进样量为10 μL,柱温为40 ℃,流速为0.2 mL·min−1. 流动相中A为10 mmol·L−1乙酸铵水溶液,B为乙腈. 梯度洗脱程序为:0—1 min,10% B;1—2 min,线性增加至30% B;2—10 min,线性增加至95% B,保持5 min;15—15.1 min,回到10% B,保持2 min.
-
质谱采用电喷雾负离子模式(ESI−),采用多反应监测(MRM)模式对目标化合物进行定性和定量. 具体仪器参数如下:雾化气流量为3 L·min−1;干燥气流量为5 L·min−1;加热气流量为15 L·min−1;接口温度为300 ℃;DL温度为100 ℃;加热块温度为200 ℃. 14种PFASs的质谱MRM参数见表2.
-
PFASs目标物标准溶液:用移液枪移取目标PFASs标准品,再用甲醇稀释成质量浓度为 500 μg·L−1的目标物混合标准溶液. PFASs内标溶液:用移液枪移取目标PFASs内标,再用甲醇稀释成质量浓度为100 μg·L−1的内标溶液. 所有标准溶液于冰箱中−20 ℃避光保存,使用时用甲醇稀释至所需浓度.
-
0.1%氨甲醇溶液(M/M):取0.8 mL 25%氨水加入到909.33 mL甲醇中搅拌混匀. 25 mmol·L−1 醋酸铵溶液:称取0.3854 g醋酸铵,将其溶解至50 mL超纯水中,再加入1 mL冰醋酸,最后定容至200 mL,搅拌混匀. 0.05 mol·L−1 NaOH甲醇溶液:称取0.2 g NaOH,将其加入到100 mL甲醇中,搅拌混匀即完成配制.
-
首先将胎便样品冷冻干燥5 d,然后用玛瑙研钵和研棒研磨并均质化. 称量0.2 g冻干胎便样品于15 mL聚丙烯离心管中,加入100 μL PFASs内标溶液、4 mL乙腈/水(9:1,V/V),在摇床上混匀10 min(250 r·min−1,4 ℃);完成后进行超声提取20 min;超声完成后离心10 min(4200 r·min−1,4 ℃). 将上清液转移到新的离心管中,并重复上述提取过程一次. 合并两次上清液,进行固相萃取. 用3 mL甲醇活化Envi-Carb柱,上清液过柱并收集过柱液,再用3 mL甲醇洗脱,将洗脱液与过柱液合并后氮吹至1 mL. 再加入12 mL超纯水将其稀释,进行Oasis WAX固相萃取. 首先使用4 mL 0.1%氨甲醇溶液、4 mL甲醇和4 mL水依次对WAX小柱进行活化及平衡,其次将样品溶液以1滴/秒的速度通过WAX柱. 上样完成后,依次用5 mL甲醇和5 mL水进行淋洗,并用4 mL 25 mmol·L−1醋酸铵溶液除杂. 最后,使用3 mL 0.1%氨甲醇进行洗脱. 洗脱液在40 ℃下氮吹浓缩至约200 μL,离心10 min(12000 r·min−1,4 ℃),取上清液待测.
-
称取0.2 g冻干胎便样品,按照“1.4节”中的前处理方法进行提取、过滤和净化,于洗脱后加入3 mL甲醇,即得到基质溶液.
取1 mL 500 μg·L−1 PFASs目标物混合标准溶液,以甲醇为溶剂,逐级稀释配制混合标准工作液,浓度为:1、5、10、25、50、100、250、500 μg·L−1. 然后以基质溶液为溶剂,按照50 μL混合标准工作液,25 μL PFASs内标溶液,425 μL基质溶液的配制方法将混合标准工作液配制成浓度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的基质混合标准工作溶液. 以待测物的质量浓度为横坐标 (
x , μg·L−1),以定量离子色谱峰面积与内标面积之比为纵坐标(y ),绘制基质曲线.按照以上配制步骤,将基质溶液更换为甲醇,分别配制浓度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的溶剂混合标准溶液. 以待测物的质量浓度为横坐标(
x , μg·L−1),以离子色谱峰面积与内标面积之比为纵坐标(y ),绘制溶剂曲线. -
分别以NaOH甲醇(0.05 mol·L−1)、甲醇/MTBE(4:1,V/V)和乙腈/水(9:1,V/V)作为提取溶液,比较3种提取溶液对14种PFASs的提取效果. 结果如图1,对于C-F链大于8的PFCAs(全氟烷基羧酸)和C-F链大于6的PFSAs(全氟烷基磺酸),3种提取溶液的提取效果差异不明显. 但3种提取溶液对PFHpS和PFTrDA呈现出不同的提取效果. 采用NaOH甲醇作为提取溶液时,PFHpS与PFTrDA的回收率分别为142%和67%;而在采用甲醇/MTBE(4:1,V/V)和乙腈/水(9:1,V/V)作为提取溶液时,两种化合物的回收率均超过150%. 上述结果表明NaOH甲醇对于PFHpS和PFTrDA的提取效果优于其它两种提取溶液. 而对于PFDoS、PFDoA的提取,使用0.05 mol·L−1 NaOH甲醇溶液时回收率则较低(19%—50%). 当使用乙腈/水(9:1,V/V)进行提取时,大多数化合物的回收率在73%—91%之间,相较于0.05 mol·L−1 NaOH甲醇溶液(58%—141%)以及甲醇/MTBE(4:1,V/V)(52%—86%)更为理想. 其主要原因可能是因为乙腈/水(9:1,V/V)对PFASs具有较强的亲和力[9],导致其萃取效率高于甲醇/MTBE(4:1,V/V)和0.05 mol·L−1 NaOH甲醇溶液. 因此,本实验采用乙腈/水(9:1,V/V)作为提取溶液.
-
目前,对于PFASs的萃取,使用较多的萃取柱为Oasis WAX萃取柱[25],其主要原因是WAX填料的叔胺官能团在较低的pH下带正电荷,可以有效地吸附阴离子PFASs,具有较好的萃取效果,回收率符合分析方法要求. 而对于复杂基质,一般需采取两步萃取. Envi-Carb萃取柱填料为活性炭,可吸附样品中的色素以及固体颗粒,减轻基质对样品的干扰,提升实验分析的准确性. PEP柱内的填料为官能化聚苯乙烯/二乙烯苯[26-27],由于其同时含有亲水性与疏水性基团,可有效吸附各类极性与非极性化合物,萃取有机物效果也比较好. 而在本研究中,我们比较了Envi-Carb/WAX和PEP/WAX两种萃取方式对于14种PFASs的萃取效果,故设计两组固相萃取实验.
取0.2 g胎便样品于15 mL聚丙烯离心管中,将样品分为等质量的两组:第一组使用Envi-Carb与WAX固相萃取柱进行固相萃取,并按“1.4节”中的实验步骤进行处理;第二组则使用PEP柱与WAX柱进行固相萃取. 具体萃取步骤如下[28 − 29]:
在重复提取两次并合并上清液后,使用PEP固相萃取柱进行萃取操作. 首先,使用10 mL甲醇与10 mL水进行活化;继而将样品以每秒1滴的速度通过PEP柱;上样结束后,用5 mL水淋洗PEP柱并用真空泵进行抽真空处理,持续10 min;最后,使用10 mL甲醇洗脱. 将洗脱液氮吹浓缩至1 mL后,加入12 mL超纯水,之后再使用WAX固相萃取柱进行萃取,步骤与“1.4节”的前处理步骤一致.
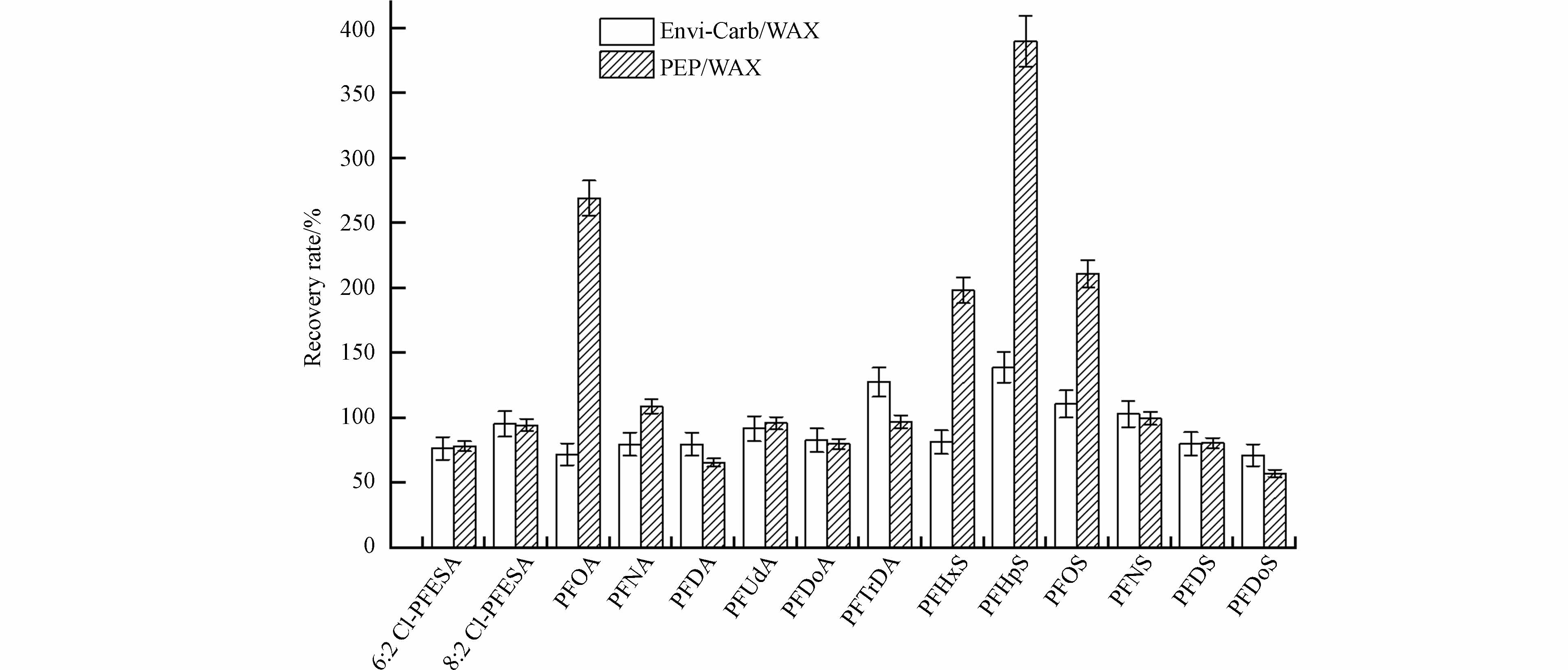
结果如图2所示,在使用Envi-Carb/WAX进行萃取时,14种PFASs的回收率均在可接受范围内(71%—139%);而在使用PEP/WAX萃取时,PFHpS、PFOA、PFHxS、PFOS的回收率则较高(198%—390%). 从结果来看,回收率的差异可能是由于柱内填料的不同. Envi-Carb柱内的填料为活性炭,当在反相条件下使用时对于非极性和极性基质中的有机极性和非极性化合物都具有极强的亲和性,对基质中的干扰物质有较强的吸附能力. 而PEP柱内填料为官能化聚苯乙烯/二乙烯苯,可吸附各类极性与非极性化合物,其更适合对强亲水性化合物的吸附. 因此,Envi-Carb/WAX对于PFASs的萃取效果更好,故选用Envi-Carb/WAX作为本研究中的固相萃取柱.
-
取0.2 g胎便样品于15 mL聚丙烯离心管中,提取剂选用乙腈/水(9:1,V/V). 在WAX柱的萃取过程中,洗脱液分别使用2 mL甲醇与3 mL氨甲醇、3 mL氨甲醇,其余实验步骤与“1.4节”中的步骤相同.
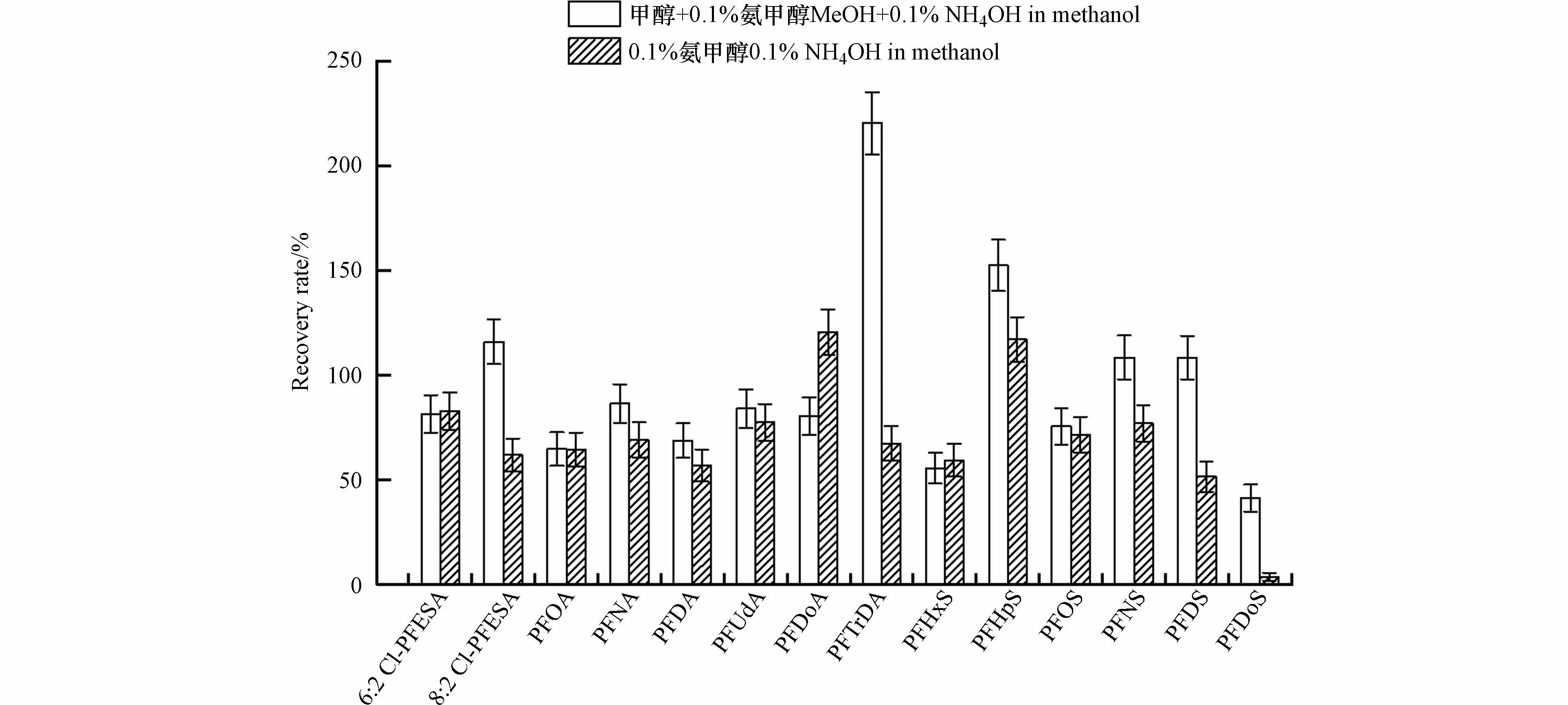
据报道[25],吸附在WAX柱上的阴离子PFASs可以被甲醇洗脱,而氢氧化铵能够促进甲醇对PFASs的洗脱. 因此在本研究中使用了两种洗脱方式,即一组使用甲醇与0.1%氨甲醇进行洗脱,另一组仅使用0.1%氨甲醇洗脱,以此来选择出更好的洗脱方式. 如图3所示,对于PFASs的洗脱,两种洗脱方式的洗脱效果相差不大,而对于PFTrDA的洗脱两种方式有较大差异(220%/67%),对于PFDoS的洗脱则均较低(41%/3%). 但从所有PFASs的回收率结果来看,使用甲醇与氨甲醇洗脱时,回收率结果普遍较高,可能是由于甲醇将吸附在固相萃取柱上的中性干扰物洗脱出来,进而增加了样品的基质效应. 综合考虑各PFASs单体的回收效率、提取时间和提取试剂成本等因素,本研究中选择仅用氨甲醇进行洗脱.
-
用胎便基质溶液配制质量浓度梯度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的PFASs基质混合标准溶液,以各分析物定量离子与内标定量离子的峰面积比值(
y )和分析物对应的质量浓度(x ,μg·L−1)为坐标轴绘制标准曲线,以3倍和10倍信噪比分别计算方法检出限(method detection limits, MDLs)和方法定量限 (method quantification limits, MQLs). 结果表明,14种长链PFASs在0.1—50 μg·L−1范围内线性关系良好,相关系数(r )均大于0.995. MDLs和MQLs分别为0.001—0.149 ng·g−1,0.003—0.495 ng·g−1,结果详见表3. -
在胎便样品中分别添加2 ng·g−1、5 ng·g−1和20 ng·g−1浓度水平的标准物质以进行加标回收实验,每个浓度水平平行测定6次,计算回收率和相对标准偏差. 结果表明,在14种PFASs中,平均回收率为65%—149%,相对标准偏差为3%—22%,能满足定量分析的基本要求. 详细结果见表4.
-
基质效应(Matrix Effect,ME)在定量分析过程中会影响定量的准确度,会对目标化合物的定量结果造成一定的影响,而胎便中的组分较为复杂,因此在使用HPLC-MS/MS进行定量分析时需要考虑其所产生的基质效应. 按照文献方法[30],以胎便基质和纯溶剂基质配制两条浓度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的曲线,并按如下计算基质效应:基质效应(ME) = |(基质匹配校准曲线斜率/纯溶剂标准曲线斜率)–1 |×100%. 当ME< 20%时,为弱基质效应,可忽略,无需采取补偿措施;当20%≤ME≤50%时,为中等程度基质效应,当ME>50%时,为强基质效应,以上两种情况均需采取补偿措施. 结果如表5所示,多数化合物经处理后具有弱基质效应,定量时可忽略基质效应所带来的影响. 但对于经处理后具有中等程度基质效应的化合物(PFHpS、PFDoA、PFOS)来说,则需要考虑基质效应对定量结果的影响,因此需要基质效应曲线来对定量结果进行校准.
-
使用本方法测定了10份胎便样品,同时设置了1个过程空白样品,结果如表6,除8:2 Cl-PFESA、PFDoS未检出外,其余物质均有检出. PFOA和PFHxS的检出率为100%,浓度分别为0.064—0.33 ng·g−1、0.53—2.49 ng·g−1. PFOS的检出率为80%,浓度为<MDLs—1.78 ng·g−1. 其它化合物的检出率均在10%—70%之间,浓度为<MDLs—0.17 ng·g−1之间. 上述结果说明胎便可作为有效的生物样本,评估PFASs的宫内暴露,同时胎便中PFASs的浓度在一定程度上指示了新生儿在PFASs不同单体代谢速率上的差异. 考虑到PFASs经母体暴露于胎儿经历了复杂的传输、转运、代谢等过程,因此以胎便中PFASs的赋存监测作为切入点,开展胎儿PFASs产前暴露水平和经胎盘机制的相关研究,对于评估胎儿的产前暴露风险具有重要意义.
-
本研究通过优化胎便样品前处理过程中的提取溶液、洗脱溶液、固相萃取柱类型等影响因素,结合高效液相色谱串联质谱仪器,建立了同时测定胎便中14种PFASs的分析方法,并系统评估了方法的回收率、精密度、检出限、定量限与基质效应. 结果表明该方法灵敏度高,准确度和精密度较好. 本研究为未来系统性研究胎便中PFASs的赋存特征及暴露风险提供了技术基础.
固相萃取结合高效液相色谱-串联质谱测定胎便中14种全氟和多氟烷基化合物
Determination of 14 kinds of perfluorinated and polyfluoroalkyl compounds in meconium by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry
-
摘要: 全氟和多氟烷基化合物(PFASs)是一类人工合成的物质,由于其热稳定、疏水、疏油等优良性质而被广泛使用于生活和生产中. PFASs具有环境持久性、生物累积性、多种毒性等特性,且可以通过胎盘屏障进入到胎儿体内,进而对胎儿健康产生潜在危害. 胎便中积累了妊娠期间暴露于胎儿的外源性化合物,可用于监测PFASs对胎儿的宫内暴露特征. 本研究基于固相萃取结合高效液相色谱-串联质谱技术,建立了胎便中14种PFASs的分析方法. 采用乙腈/水(9∶1,V/V)对0.2 g冻干胎便样品进行超声提取,提取液经Envi-carb和Oasis WAX小柱固相萃取,0.1%氨甲醇洗脱. 以10 mmol·L−1乙酸铵水溶液和乙腈作为流动相对目标化合物进行梯度洗脱,采用Acquity UPLC BEH C18色谱柱进行分离,基于多反应监测负离子模式采集,内标法定量. 结果表明,在2、5、20 ng·g−1的加标浓度下,14种PFASs的回收率为65%—149%,相对标准偏差为3%—22%,方法检出限(MDLs)为0.001—0.149 ng·g−1,方法定量限(MQLs)为0.003—0.495 ng·g−1. 使用该方法测定了10个胎便样品,ΣPFASs浓度范围为<MDLs—2.49 ng·g−1. 该方法操作简单、便捷、灵敏度高且定量准确,为系统性研究胎便中PFASs的赋存特征及暴露风险提供了技术基础.
-
关键词:
- 胎便 /
- 全氟和多氟烷基化合物 /
- 高效液相色谱-串联质谱 /
- 固相萃取.
Abstract: Perfluorinated and polyfluoroalkyl compounds (PFASs) are a kind of synthetic substances, which have been widely used in daily life and production because of their excellent properties such as heat stability, hydrophobic and oleophobic. PFASs have the characteristics of environmental persistence, bioaccumulation, and multiple toxicity, and can pass across the placental barrier into the fetal, thus posing potential exposure risk to fetal health. Generally, the meconium accumulates exogenous compounds that were exposed to the fetus during pregnancy, as such, the meconium can be used to monitor the exposure characteristics of PFASs to the fetus. A method was established for the determination of 14 kinds of PFASs in meconium by solid phase extraction high performance liquid chromatography-tandem mass spectrometry (SPE HPLC-MS/MS). The freeze-drying meconium sample (0.2 g) was ultrasonically extracted by acetonitrile (ACN)/water (9:1, V/V) and then was extracted by Envi-carb and Oasis WAX cartridges, eluted with 0.1% ammonia in methanol. The sample was separated by using an Acquity UPLC BEH C18 column and eluted gradiently with 10 mmol·L−1 ammonium acetate and acetonitrile as the mobile phase. The samples were detected by tandem mass spectrometry under multiple reaction monitoring (MRM) mode with negative electrospray ionization, and quantified by internal standard method. Results showed that the recoveries of PFASs were 65%—149% at spiked levels of 2, 5, and 20 ng·g−1, and the relative standard deviations (RSD) were 3%—22%. The method detection limits (MDLs) and the method quantitation limits (MQLs) were in the range of 0.001—0.149 ng·g−1 and 0.003—0.495 ng·g−1, respectively. The method was used to detect 10 meconium samples, and the concentrations ranged from <MDLs to 2.49 ng·g−1. This established method is simple, convenient, sensitive and accurate, which will provide a technical basis for the systematic study of the occurrence as well as exposure risk of PFASs in meconium. -
全氟和多氟烷基化合物(PFASs)是一类人工合成的有机化合物,具有优异的热稳定性和化学稳定性,疏水疏油、不易生物降解、高表面活性等独特的理化性质[1 − 2]. 自20世纪50年代起,PFASs被广泛应用于商业和工业领域,所涉及产品包括消防泡沫、纸板、杀虫剂、驱虫剂、润滑剂、表面处理剂、个人护理品和食品包装产品等[3 − 4]. 自2001年Giesy和Kannan首次在环境中检测出PFASs起[5] ,PFASs相继在世界各地的水体[6 − 7]、土壤[8 − 9]、空气[10]、沉积物[11]、灰尘[12]、动物[13 − 14]、人体血清[15]、尿液[16]等环境介质及生物样本中广泛检出. 由于PFASs具有环境持久性、生物累积性、多种毒性等特性[17],其进入环境后,会在一定程度上对生态环境及人类健康构成威胁[18]. 已有研究指出PFASs可以通过各种途径进入到人体[19],例如,PFASs可以通过胎盘屏障,进入到胎儿体内,进而对胎儿造成宫内暴露[20]. 近年来,针对于PFASs母婴暴露的相关研究已经成为研究热点. 在以往研究中,研究人员主要通过监测母血、脐带血以及胎盘等样品来探究PFASs的宫内暴露特征[21]. 然而,上述样品采样过程是侵入性的,可能会对采样对象造成损伤,导致难以开展大范围、系统性研究. Vizcaino等[22]指出包括PFASs在内的多种持久性有机污染物可通过胎盘转移进入到胎儿体内,并且在胎便中形成不同程度的累积. 因此,开展胎便中的PFASs的痕量分析研究,可获得PFASs对胎儿的产前暴露相关信息,进而为评估其产前暴露风险提供数据支撑.
胎便指胎儿出生后的几次排便,是一种黑绿色、无味、粘稠的粘性物质. 胎便由水、胃肠道和皮肤脱落的上皮细胞、胎毛、各种胰腺和肠道分泌物,以及吞咽的羊水残留物组成[23],它是一种高度复杂的基质. 与其他研究中使用的胎儿基质(脐带血、尿液和血液)相比,胎便样本数量多,易于收集,非侵入性,更为重要的是胎便可将污染物的累计代谢考虑在内. 除此之外,胎便的样品收集窗口相对较宽,即胎儿出生后3天内的胎便均可用于分析且效果最佳[23].
胎便中积累了妊娠期间暴露于胎儿的多种外源性化合物[24],但其复杂的基质成分导致对其中的污染物进行痕量分析具有一定挑战. 目前,关于胎便中PFASs的分析检测方法仍鲜有报道并亟待研究. 基于此,本研究利用离线固相萃取结合高效液相色谱串联质谱法,建立了胎便中14种PFASs的分析方法. 该方法采用乙腈/水(9∶1,V/V)对胎便进行提取,Envi-Carb与Oasis WAX进行萃取净化,具有操作简便,灵敏度高,准确度和精密度较高的优点. 该方法的建立可为胎便中PFASs的分析检测提供技术基础,为研究PFASs的母婴传递和评估胎儿产前PFASs暴露风险提供技术支撑.
1. 材料与方法(Materials and methods)
1.1 试剂与材料
PFASs标准品:全氟辛酸(perfluorooctanoic acid, PFOA)、全氟壬酸(perfluorononanoic acid, PFNA)、全氟癸酸(perfluorodecanoic acid, PFDA)、全氟十一烷酸(perfluoroundecanoic acid, PFUdA)、全氟十二烷酸(perfluorododecanoic acid, PFDoA)、全氟十三烷酸(perfluorotridecanoic acid, PFTrDA)、全氟己基磺酸(perfluorohexanesulfonic acid, PFHxS)、全氟庚基磺酸(perfluoroheptanesulfonic acid, PFHpS)、全氟辛基磺酸(Perfluorooctanesulfonic acid,PFOS)、全氟壬基磺酸(perfluorononanesulfonic acid, PFNS)、全氟癸基磺酸(perfluorodecanesulfonic acid, PFDS)、全氟十二烷磺酸(perfluorododecanesulfonic acid, PFDoS)、6:2氯化聚氟烷基醚磺酸(6:2 chlorinated Polyfluoroalkyl ether sulfonic acid, 6:2 Cl-PFESA)、8:2氯化聚氟烷基醚磺酸(8:2 Chlorinated polyfluoroalkyl ether sulfonic acid, 8:2 Cl-PFESA). 13C同位素标记的PFASs内标:13C4-PFOA(13C4-全氟辛酸,MPFOA)、13C5-PFNA(13C5-全氟壬酸,MPFNA),13C2-PFDA(13C2-全氟癸酸,MPFDA),13C2-PFUdA(13C2-全氟十一酸,MPFUdA),18O2-PFHxS(18O2-全氟己基磺酸,MPFHxS),13C4-PFOS(13C4-全氟辛基磺酸,MPFOS). 本研究所使用的PFASs及其同位素标记的化合物标准品均购自加拿大Wellington Laboratories公司,所有标准品纯度均超过98%,详细信息见表1.
表 1 14种PFASs的名称、缩写、化学式及CAS号Table 1. Full names, abbreviations, formulas, CAS number of 14 PFASs化合物名称(中文)Full names in Chinese 化合物名称(英文)Full names in English 缩写Abbreviations 化学式Formulas CAS号CAS number 6:2氯化聚氟烷基醚磺酸 6:2 Chlorinated Polyfluoroalkyl ether sulfonic acid 6:2 Cl-PFESA C8ClF16SO4H 756426-58-1 8:2氯化聚氟烷基醚磺酸 8:2 Chlorinated Polyfluoroalkyl ether sulfonic acid 8:2 Cl-PFESA C10ClF20SO4H 83329-89-9 全氟辛酸 Perfluorooctanoic acid PFOA C8F15O2H 335-67-1 全氟壬酸 Perfluorononanoic acid PFNA C9F17O2H 375-95-1 全氟癸酸 Perfluorodecanoic acid PFDA C10F19O2H 335-76-2 全氟十一烷酸 Perfluoroundecanoic acid PFUdA C11F21O2H 2058-94-8 全氟十二烷酸 Perfluorododecanoic acid PFDoA C12F23O2H 307-55-1 全氟十三烷酸 Perfluorotridecanoic acid PFTrDA C13F25O2H 72629-94-8 全氟己基磺酸 Perfluorohexanesulfonic acid PFHxS C6F13SO3H 355-46-4 全氟庚基磺酸 Perfluoroheptanesulfonic acid PFHpS C7F15SO3H 375-92-8 全氟辛基磺酸 Perfluorooctanesulfonic acid PFOS C8F17SO3H 1763-23-1 全氟壬基磺酸 Perfluorononanesulfonic acid PFNS C9F19SO3H 68259-12-1 全氟癸基磺酸 Perfluorodecanesulfonic acid PFDS C10F21SO3H 335-77-3 全氟十二烷磺酸 Perfluorododecanesulfonic acid PFDoS C12F25SO3H 79780-39-5 甲醇、乙腈(LC-MS级)购自美国Thermo Fisher Scientific公司;醋酸铵(>97%)与氢氧化铵(28%)购自美国Alfa Aesar公司;氢氧化钠、盐酸(优级纯)购自国药集团化学试剂有限公司;Oasis WAX固相萃取柱(150 mg/6 mL)购自美国Waters公司;Envi-carb固相萃取柱(150 mg/6 mL)购自美国Supelco公司;Cleanert PEP(500 mg/6 mL)固相萃取柱购自天津博纳艾杰尔公司. 实验用水为超纯水,由Milli-Q Advantage A10系统制备,电阻率大于18.2 MΩ·cm.
1.2 实验条件
1.2.1 色谱条件
色谱柱采用Acquity UPLC BEH C18色谱柱(2.1 mm × 100 mm,1.7 μm,美国Waters公司). 进样量为10 μL,柱温为40 ℃,流速为0.2 mL·min−1. 流动相中A为10 mmol·L−1乙酸铵水溶液,B为乙腈. 梯度洗脱程序为:0—1 min,10% B;1—2 min,线性增加至30% B;2—10 min,线性增加至95% B,保持5 min;15—15.1 min,回到10% B,保持2 min.
1.2.2 质谱条件
质谱采用电喷雾负离子模式(ESI−),采用多反应监测(MRM)模式对目标化合物进行定性和定量. 具体仪器参数如下:雾化气流量为3 L·min−1;干燥气流量为5 L·min−1;加热气流量为15 L·min−1;接口温度为300 ℃;DL温度为100 ℃;加热块温度为200 ℃. 14种PFASs的质谱MRM参数见表2.
表 2 14种PFASs的质谱多反应监测参数Table 2. MRM parameters of mass spectrometry for 14 PFASs化合物Compound 前体离子(m/z)Precursor ion 子离子(m/z)Product ion Q1 Pre偏差Q1 Pre deviation 碰撞能/eVCollision energy Q3 Pre偏差/VQ3 Pre deviation 对应内标Mass labeled standards 6:2 Cl-PFESA 530.9 351.0* 26 27 15 13C4-PFOS 83.1 38 26 29 8:2 Cl-PFESA 630.9 450.9* 29 20 20 13C4-PFOS 83.1 34 30 30 PFOA 413.1 369.0* 29 11 24 13C4-PFOA 169.1 14 18 16 PFNA 463.1 419.0* 16 11 28 13C5-PFNA 463.0 219.1 13 17 20 PFDA 513.1 469.1* 26 12 22 13C2-PFDA 219.1 24 17 14 PFUdA 562.9 518.9* 20 13 34 13C2-PFUdA 269.1 20 18 24 PFDoA 612.9 568.9* 22 12 26 13C2-PFUdA 169.1 22 27 26 PFTrDA 662.9 618.9* 24 13 20 13C2-PFUdA 169.1 24 26 28 PFHxS 398.9 99.0* 14 38 29 18O2-PFHxS 80.0 14 55 26 PFHpS 449.0 99.0* 16 39 30 18O2-PFHxS 80.0 16 55 28 PFOS 498.9 99.0* 17 40 29 13C4-PFOS 80.0 14 54 26 PFNS 548.9 79.9 20 55 25 13C4-PFOS 99.0* 20 47 30 PFDS 598.9 99.0* 22 49 30 13C4-PFOS 80.0 22 55 29 PFDoS 698.9 99.0* 20 51 14 13C4-PFOS 80.0 24 55 25 13C4-PFOA 417.1 372.1* 14 11 17 — 13C5-PFNA 468.1 423.0* 16 12 27 — 13C2-PFDA 515.1 470.1* 26 12 21 — 13C2-PFUdA 565.1 519.9* 20 12 32 — 18O2-PFHxS 402.9 84.0 19 52 26 — 103.0* 19 36 30 13C4-PFOS 502.9 80.0 24 53 27 — 99.0* 19 46 27 * 定量离子Quantitative ion 1.3 溶液配制
1.3.1 混合标准溶液及内标溶液配制
PFASs目标物标准溶液:用移液枪移取目标PFASs标准品,再用甲醇稀释成质量浓度为 500 μg·L−1的目标物混合标准溶液. PFASs内标溶液:用移液枪移取目标PFASs内标,再用甲醇稀释成质量浓度为100 μg·L−1的内标溶液. 所有标准溶液于冰箱中−20 ℃避光保存,使用时用甲醇稀释至所需浓度.
1.3.2 溶液配制
0.1%氨甲醇溶液(M/M):取0.8 mL 25%氨水加入到909.33 mL甲醇中搅拌混匀. 25 mmol·L−1 醋酸铵溶液:称取0.3854 g醋酸铵,将其溶解至50 mL超纯水中,再加入1 mL冰醋酸,最后定容至200 mL,搅拌混匀. 0.05 mol·L−1 NaOH甲醇溶液:称取0.2 g NaOH,将其加入到100 mL甲醇中,搅拌混匀即完成配制.
1.4 样品前处理
首先将胎便样品冷冻干燥5 d,然后用玛瑙研钵和研棒研磨并均质化. 称量0.2 g冻干胎便样品于15 mL聚丙烯离心管中,加入100 μL PFASs内标溶液、4 mL乙腈/水(9:1,V/V),在摇床上混匀10 min(250 r·min−1,4 ℃);完成后进行超声提取20 min;超声完成后离心10 min(4200 r·min−1,4 ℃). 将上清液转移到新的离心管中,并重复上述提取过程一次. 合并两次上清液,进行固相萃取. 用3 mL甲醇活化Envi-Carb柱,上清液过柱并收集过柱液,再用3 mL甲醇洗脱,将洗脱液与过柱液合并后氮吹至1 mL. 再加入12 mL超纯水将其稀释,进行Oasis WAX固相萃取. 首先使用4 mL 0.1%氨甲醇溶液、4 mL甲醇和4 mL水依次对WAX小柱进行活化及平衡,其次将样品溶液以1滴/秒的速度通过WAX柱. 上样完成后,依次用5 mL甲醇和5 mL水进行淋洗,并用4 mL 25 mmol·L−1醋酸铵溶液除杂. 最后,使用3 mL 0.1%氨甲醇进行洗脱. 洗脱液在40 ℃下氮吹浓缩至约200 μL,离心10 min(12000 r·min−1,4 ℃),取上清液待测.
1.5 基质曲线及溶剂曲线
称取0.2 g冻干胎便样品,按照“1.4节”中的前处理方法进行提取、过滤和净化,于洗脱后加入3 mL甲醇,即得到基质溶液.
取1 mL 500 μg·L−1 PFASs目标物混合标准溶液,以甲醇为溶剂,逐级稀释配制混合标准工作液,浓度为:1、5、10、25、50、100、250、500 μg·L−1. 然后以基质溶液为溶剂,按照50 μL混合标准工作液,25 μL PFASs内标溶液,425 μL基质溶液的配制方法将混合标准工作液配制成浓度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的基质混合标准工作溶液. 以待测物的质量浓度为横坐标 (
x y 按照以上配制步骤,将基质溶液更换为甲醇,分别配制浓度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的溶剂混合标准溶液. 以待测物的质量浓度为横坐标(
x y 2. 结果与讨论(Results and discussion)
2.1 提取溶液优化
分别以NaOH甲醇(0.05 mol·L−1)、甲醇/MTBE(4:1,V/V)和乙腈/水(9:1,V/V)作为提取溶液,比较3种提取溶液对14种PFASs的提取效果. 结果如图1,对于C-F链大于8的PFCAs(全氟烷基羧酸)和C-F链大于6的PFSAs(全氟烷基磺酸),3种提取溶液的提取效果差异不明显. 但3种提取溶液对PFHpS和PFTrDA呈现出不同的提取效果. 采用NaOH甲醇作为提取溶液时,PFHpS与PFTrDA的回收率分别为142%和67%;而在采用甲醇/MTBE(4:1,V/V)和乙腈/水(9:1,V/V)作为提取溶液时,两种化合物的回收率均超过150%. 上述结果表明NaOH甲醇对于PFHpS和PFTrDA的提取效果优于其它两种提取溶液. 而对于PFDoS、PFDoA的提取,使用0.05 mol·L−1 NaOH甲醇溶液时回收率则较低(19%—50%). 当使用乙腈/水(9:1,V/V)进行提取时,大多数化合物的回收率在73%—91%之间,相较于0.05 mol·L−1 NaOH甲醇溶液(58%—141%)以及甲醇/MTBE(4:1,V/V)(52%—86%)更为理想. 其主要原因可能是因为乙腈/水(9:1,V/V)对PFASs具有较强的亲和力[9],导致其萃取效率高于甲醇/MTBE(4:1,V/V)和0.05 mol·L−1 NaOH甲醇溶液. 因此,本实验采用乙腈/水(9:1,V/V)作为提取溶液.
2.2 萃取条件优化
目前,对于PFASs的萃取,使用较多的萃取柱为Oasis WAX萃取柱[25],其主要原因是WAX填料的叔胺官能团在较低的pH下带正电荷,可以有效地吸附阴离子PFASs,具有较好的萃取效果,回收率符合分析方法要求. 而对于复杂基质,一般需采取两步萃取. Envi-Carb萃取柱填料为活性炭,可吸附样品中的色素以及固体颗粒,减轻基质对样品的干扰,提升实验分析的准确性. PEP柱内的填料为官能化聚苯乙烯/二乙烯苯[26-27],由于其同时含有亲水性与疏水性基团,可有效吸附各类极性与非极性化合物,萃取有机物效果也比较好. 而在本研究中,我们比较了Envi-Carb/WAX和PEP/WAX两种萃取方式对于14种PFASs的萃取效果,故设计两组固相萃取实验.
取0.2 g胎便样品于15 mL聚丙烯离心管中,将样品分为等质量的两组:第一组使用Envi-Carb与WAX固相萃取柱进行固相萃取,并按“1.4节”中的实验步骤进行处理;第二组则使用PEP柱与WAX柱进行固相萃取. 具体萃取步骤如下[28 − 29]:
在重复提取两次并合并上清液后,使用PEP固相萃取柱进行萃取操作. 首先,使用10 mL甲醇与10 mL水进行活化;继而将样品以每秒1滴的速度通过PEP柱;上样结束后,用5 mL水淋洗PEP柱并用真空泵进行抽真空处理,持续10 min;最后,使用10 mL甲醇洗脱. 将洗脱液氮吹浓缩至1 mL后,加入12 mL超纯水,之后再使用WAX固相萃取柱进行萃取,步骤与“1.4节”的前处理步骤一致.
结果如图2所示,在使用Envi-Carb/WAX进行萃取时,14种PFASs的回收率均在可接受范围内(71%—139%);而在使用PEP/WAX萃取时,PFHpS、PFOA、PFHxS、PFOS的回收率则较高(198%—390%). 从结果来看,回收率的差异可能是由于柱内填料的不同. Envi-Carb柱内的填料为活性炭,当在反相条件下使用时对于非极性和极性基质中的有机极性和非极性化合物都具有极强的亲和性,对基质中的干扰物质有较强的吸附能力. 而PEP柱内填料为官能化聚苯乙烯/二乙烯苯,可吸附各类极性与非极性化合物,其更适合对强亲水性化合物的吸附. 因此,Envi-Carb/WAX对于PFASs的萃取效果更好,故选用Envi-Carb/WAX作为本研究中的固相萃取柱.
2.3 洗脱液优化
取0.2 g胎便样品于15 mL聚丙烯离心管中,提取剂选用乙腈/水(9:1,V/V). 在WAX柱的萃取过程中,洗脱液分别使用2 mL甲醇与3 mL氨甲醇、3 mL氨甲醇,其余实验步骤与“1.4节”中的步骤相同.
据报道[25],吸附在WAX柱上的阴离子PFASs可以被甲醇洗脱,而氢氧化铵能够促进甲醇对PFASs的洗脱. 因此在本研究中使用了两种洗脱方式,即一组使用甲醇与0.1%氨甲醇进行洗脱,另一组仅使用0.1%氨甲醇洗脱,以此来选择出更好的洗脱方式. 如图3所示,对于PFASs的洗脱,两种洗脱方式的洗脱效果相差不大,而对于PFTrDA的洗脱两种方式有较大差异(220%/67%),对于PFDoS的洗脱则均较低(41%/3%). 但从所有PFASs的回收率结果来看,使用甲醇与氨甲醇洗脱时,回收率结果普遍较高,可能是由于甲醇将吸附在固相萃取柱上的中性干扰物洗脱出来,进而增加了样品的基质效应. 综合考虑各PFASs单体的回收效率、提取时间和提取试剂成本等因素,本研究中选择仅用氨甲醇进行洗脱.
2.4 检出限与定量限
用胎便基质溶液配制质量浓度梯度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的PFASs基质混合标准溶液,以各分析物定量离子与内标定量离子的峰面积比值(
y x r 表 3 14种PFASs的线性范围、回归方程、相关系数、检出限与定量限Table 3. Linear ranges, regression equations, correlation coefficients, MDLs and MQLs of 14 kinds of PFASs化合物Compound 线性范围/(μg·L−1)Linear range 回归方程Regression equation 相关系数Correlation coefficient (r) 检出限/(ng·g−1)MDLs 定量限/(ng·g−1)MQLs 6:2 Cl-PFESA 0.1—50 y=1.3932x+0.0199 0.997 0.002 0.005 8:2 Cl-PFESA 0.1—50 y=1.0309x+0.0261 0.997 0.001 0.003 PFOA 0.1—50 y=0.1993x+0.0161 0.997 0.006 0.020 PFNA 0.1—50 y=0.2244x+0.0042 0.996 0.006 0.018 PFDA 0.1—50 y=0.2412x+0.0073 0.998 0.003 0.011 PFUdA 0.1—50 y=0.2241x+0.0094 0.998 0.007 0.022 PFDoA 0.1—50 y=0.2457x+0.0026 0.995 0.004 0.013 PFTrDA 0.1—50 y=0.2100x+0.0097 0.995 0.008 0.027 PFHxS 0.1—50 y=0.2224x+0.0045 0.997 0.149 0.495 PFHpS 0.1—50 y=0.2858x+0.0079 0.996 0.008 0.025 PFOS 0.1—50 y=0.1127x-0.0028 0.995 0.036 0.120 PFNS 0.1—50 y=0.2018x+0.0014 0.995 0.004 0.012 PFDS 0.1—50 y=0.2018x+0.0063 0.996 0.002 0.007 PFDoS 0.1—50 y=0.1595x+0.0034 0.996 0.002 0.007 2.5 回收率与精密度
在胎便样品中分别添加2 ng·g−1、5 ng·g−1和20 ng·g−1浓度水平的标准物质以进行加标回收实验,每个浓度水平平行测定6次,计算回收率和相对标准偏差. 结果表明,在14种PFASs中,平均回收率为65%—149%,相对标准偏差为3%—22%,能满足定量分析的基本要求. 详细结果见表4.
表 4 样品加标回收率结果Table 4. Recovery results of spiked samples化合物Compound 加标浓度/(ng·g−1)Spiking concentrations 回收率/%Recovery rate 相对标准偏差/%RSD 6:2 Cl-PFESA 2 97 4 5 93 5 20 89 3 8:2 Cl-PFESA 2 99 4 5 97 6 20 94 3 PFOA 2 113 13 5 79 19 20 78 3 PFNA 2 88 8 5 81 4 20 82 4 PFDA 2 93 8 5 87 6 20 87 6 PFUdA 2 88 5 5 84 4 PFUdA 20 84 6 PFDoA 2 79 13 5 72 13 20 75 14 PFTrDA 2 98 20 5 77 19 20 93 16 PFHxS 2 65 12 5 87 11 20 93 5 PFHpS 2 144 10 5 149 8 20 128 8 PFOS 2 136 22 5 100 8 20 93 18 PFNS 2 115 5 5 114 3 20 99 19 PFDS 2 99 6 5 107 4 20 102 4 PFDoS 2 82 16 5 72 12 20 94 7 2.6 基质效应评估
基质效应(Matrix Effect,ME)在定量分析过程中会影响定量的准确度,会对目标化合物的定量结果造成一定的影响,而胎便中的组分较为复杂,因此在使用HPLC-MS/MS进行定量分析时需要考虑其所产生的基质效应. 按照文献方法[30],以胎便基质和纯溶剂基质配制两条浓度为0.1、0.5、1、2.5、5、10、25、50 μg·L−1的曲线,并按如下计算基质效应:基质效应(ME) = |(基质匹配校准曲线斜率/纯溶剂标准曲线斜率)–1 |×100%. 当ME< 20%时,为弱基质效应,可忽略,无需采取补偿措施;当20%≤ME≤50%时,为中等程度基质效应,当ME>50%时,为强基质效应,以上两种情况均需采取补偿措施. 结果如表5所示,多数化合物经处理后具有弱基质效应,定量时可忽略基质效应所带来的影响. 但对于经处理后具有中等程度基质效应的化合物(PFHpS、PFDoA、PFOS)来说,则需要考虑基质效应对定量结果的影响,因此需要基质效应曲线来对定量结果进行校准.
表 5 各分析物基质效应评价Table 5. Matrix effect evaluation for each analyte化合物Compund 溶剂曲线斜率Slope of solvent curve 基质效应曲线斜率Slope of the matrix effect curve 基质效应/%Matrix effect 基质效应评价Matrix effect evaluation 6:2 Cl-PFESA 1.6618 1.3932 16 弱基质效应 8:2 Cl-PFESA 1.1880 1.0309 13 弱基质效应 PFOA 0.2074 0.1993 4 弱基质效应 PFNA 0.2052 0.2244 9 弱基质效应 PFDA 0.2170 0.2412 11 弱基质效应 PFUdA 0.2003 0.2241 12 弱基质效应 PFDoA 0.1862 0.2457 32 中等程度基质效应 PFTrDA 0.1816 0.2100 16 弱基质效应 PFHxS 0.2109 0.2224 5 弱基质效应 PFHpS 0.2317 0.2858 23 中等程度基质效应 PFOS 0.0836 0.1127 35 中等程度基质效应 PFNS 0.1852 0.2018 9 弱基质效应 PFDS 0.2191 0.2018 8 弱基质效应 PFDoS 0.1858 0.1595 14 弱基质效应 2.7 实际样品测定
使用本方法测定了10份胎便样品,同时设置了1个过程空白样品,结果如表6,除8:2 Cl-PFESA、PFDoS未检出外,其余物质均有检出. PFOA和PFHxS的检出率为100%,浓度分别为0.064—0.33 ng·g−1、0.53—2.49 ng·g−1. PFOS的检出率为80%,浓度为<MDLs—1.78 ng·g−1. 其它化合物的检出率均在10%—70%之间,浓度为<MDLs—0.17 ng·g−1之间. 上述结果说明胎便可作为有效的生物样本,评估PFASs的宫内暴露,同时胎便中PFASs的浓度在一定程度上指示了新生儿在PFASs不同单体代谢速率上的差异. 考虑到PFASs经母体暴露于胎儿经历了复杂的传输、转运、代谢等过程,因此以胎便中PFASs的赋存监测作为切入点,开展胎儿PFASs产前暴露水平和经胎盘机制的相关研究,对于评估胎儿的产前暴露风险具有重要意义.
表 6 14种PFASs在胎便中的测定值Table 6. Determination concentration of 14 kinds of PFASs in meconium化合物Compounds 检出率/%Detection rate 浓度/(ng·g−1)Concentrations 最小值Min 第一四分位数P25 中位数Median 第三四分位数P75 最大值Max 6:2 Cl-PFESA 20 <MDLs <MDLs <MDLs <MDLs 0.073 8:2 Cl-PFESA 0 <MDLs <MDLs <MDLs <MDLs <MDLs PFOA 100 0.064 0.083 0.106 0.126 0.326 PFNA 70 <MDLs <MDLs 0.008 0.013 0.029 PFDA 20 <MDLs <MDLs <MDLs <MDLs 0.018 PFUdA 60 <MDLs <MDLs 0.015 0.036 0.089 PFDoA 30 <MDLs <MDLs <MDLs <MDLs 0.007 PFTrDA 70 <MDLs <MDLs 0.015 0.093 0.173 PFHxS 100 0.531 1.244 1.633 1.826 2.486 PFHpS 60 <MDLs <MDLs <MDLs 0.028 0.073 PFOS 80 <MDLs 0.036 0.508 0.669 1.778 PFNS 30 <MDLs <MDLs <MDLs 0.010 0.079 PFDS 10 <MDLs <MDLs <MDLs <MDLs 0.101 PFDoS 0 <MDLs <MDLs <MDLs <MDLs <MDLs 3. 结论(Conclusion)
本研究通过优化胎便样品前处理过程中的提取溶液、洗脱溶液、固相萃取柱类型等影响因素,结合高效液相色谱串联质谱仪器,建立了同时测定胎便中14种PFASs的分析方法,并系统评估了方法的回收率、精密度、检出限、定量限与基质效应. 结果表明该方法灵敏度高,准确度和精密度较好. 本研究为未来系统性研究胎便中PFASs的赋存特征及暴露风险提供了技术基础.
-
表 1 14种PFASs的名称、缩写、化学式及CAS号
Table 1. Full names, abbreviations, formulas, CAS number of 14 PFASs
化合物名称(中文)Full names in Chinese 化合物名称(英文)Full names in English 缩写Abbreviations 化学式Formulas CAS号CAS number 6:2氯化聚氟烷基醚磺酸 6:2 Chlorinated Polyfluoroalkyl ether sulfonic acid 6:2 Cl-PFESA C8ClF16SO4H 756426-58-1 8:2氯化聚氟烷基醚磺酸 8:2 Chlorinated Polyfluoroalkyl ether sulfonic acid 8:2 Cl-PFESA C10ClF20SO4H 83329-89-9 全氟辛酸 Perfluorooctanoic acid PFOA C8F15O2H 335-67-1 全氟壬酸 Perfluorononanoic acid PFNA C9F17O2H 375-95-1 全氟癸酸 Perfluorodecanoic acid PFDA C10F19O2H 335-76-2 全氟十一烷酸 Perfluoroundecanoic acid PFUdA C11F21O2H 2058-94-8 全氟十二烷酸 Perfluorododecanoic acid PFDoA C12F23O2H 307-55-1 全氟十三烷酸 Perfluorotridecanoic acid PFTrDA C13F25O2H 72629-94-8 全氟己基磺酸 Perfluorohexanesulfonic acid PFHxS C6F13SO3H 355-46-4 全氟庚基磺酸 Perfluoroheptanesulfonic acid PFHpS C7F15SO3H 375-92-8 全氟辛基磺酸 Perfluorooctanesulfonic acid PFOS C8F17SO3H 1763-23-1 全氟壬基磺酸 Perfluorononanesulfonic acid PFNS C9F19SO3H 68259-12-1 全氟癸基磺酸 Perfluorodecanesulfonic acid PFDS C10F21SO3H 335-77-3 全氟十二烷磺酸 Perfluorododecanesulfonic acid PFDoS C12F25SO3H 79780-39-5 表 2 14种PFASs的质谱多反应监测参数
Table 2. MRM parameters of mass spectrometry for 14 PFASs
化合物Compound 前体离子(m/z)Precursor ion 子离子(m/z)Product ion Q1 Pre偏差Q1 Pre deviation 碰撞能/eVCollision energy Q3 Pre偏差/VQ3 Pre deviation 对应内标Mass labeled standards 6:2 Cl-PFESA 530.9 351.0* 26 27 15 13C4-PFOS 83.1 38 26 29 8:2 Cl-PFESA 630.9 450.9* 29 20 20 13C4-PFOS 83.1 34 30 30 PFOA 413.1 369.0* 29 11 24 13C4-PFOA 169.1 14 18 16 PFNA 463.1 419.0* 16 11 28 13C5-PFNA 463.0 219.1 13 17 20 PFDA 513.1 469.1* 26 12 22 13C2-PFDA 219.1 24 17 14 PFUdA 562.9 518.9* 20 13 34 13C2-PFUdA 269.1 20 18 24 PFDoA 612.9 568.9* 22 12 26 13C2-PFUdA 169.1 22 27 26 PFTrDA 662.9 618.9* 24 13 20 13C2-PFUdA 169.1 24 26 28 PFHxS 398.9 99.0* 14 38 29 18O2-PFHxS 80.0 14 55 26 PFHpS 449.0 99.0* 16 39 30 18O2-PFHxS 80.0 16 55 28 PFOS 498.9 99.0* 17 40 29 13C4-PFOS 80.0 14 54 26 PFNS 548.9 79.9 20 55 25 13C4-PFOS 99.0* 20 47 30 PFDS 598.9 99.0* 22 49 30 13C4-PFOS 80.0 22 55 29 PFDoS 698.9 99.0* 20 51 14 13C4-PFOS 80.0 24 55 25 13C4-PFOA 417.1 372.1* 14 11 17 — 13C5-PFNA 468.1 423.0* 16 12 27 — 13C2-PFDA 515.1 470.1* 26 12 21 — 13C2-PFUdA 565.1 519.9* 20 12 32 — 18O2-PFHxS 402.9 84.0 19 52 26 — 103.0* 19 36 30 13C4-PFOS 502.9 80.0 24 53 27 — 99.0* 19 46 27 * 定量离子Quantitative ion 表 3 14种PFASs的线性范围、回归方程、相关系数、检出限与定量限
Table 3. Linear ranges, regression equations, correlation coefficients, MDLs and MQLs of 14 kinds of PFASs
化合物Compound 线性范围/(μg·L−1)Linear range 回归方程Regression equation 相关系数Correlation coefficient (r) 检出限/(ng·g−1)MDLs 定量限/(ng·g−1)MQLs 6:2 Cl-PFESA 0.1—50 y=1.3932x+0.0199 0.997 0.002 0.005 8:2 Cl-PFESA 0.1—50 y=1.0309x+0.0261 0.997 0.001 0.003 PFOA 0.1—50 y=0.1993x+0.0161 0.997 0.006 0.020 PFNA 0.1—50 y=0.2244x+0.0042 0.996 0.006 0.018 PFDA 0.1—50 y=0.2412x+0.0073 0.998 0.003 0.011 PFUdA 0.1—50 y=0.2241x+0.0094 0.998 0.007 0.022 PFDoA 0.1—50 y=0.2457x+0.0026 0.995 0.004 0.013 PFTrDA 0.1—50 y=0.2100x+0.0097 0.995 0.008 0.027 PFHxS 0.1—50 y=0.2224x+0.0045 0.997 0.149 0.495 PFHpS 0.1—50 y=0.2858x+0.0079 0.996 0.008 0.025 PFOS 0.1—50 y=0.1127x-0.0028 0.995 0.036 0.120 PFNS 0.1—50 y=0.2018x+0.0014 0.995 0.004 0.012 PFDS 0.1—50 y=0.2018x+0.0063 0.996 0.002 0.007 PFDoS 0.1—50 y=0.1595x+0.0034 0.996 0.002 0.007 表 4 样品加标回收率结果
Table 4. Recovery results of spiked samples
化合物Compound 加标浓度/(ng·g−1)Spiking concentrations 回收率/%Recovery rate 相对标准偏差/%RSD 6:2 Cl-PFESA 2 97 4 5 93 5 20 89 3 8:2 Cl-PFESA 2 99 4 5 97 6 20 94 3 PFOA 2 113 13 5 79 19 20 78 3 PFNA 2 88 8 5 81 4 20 82 4 PFDA 2 93 8 5 87 6 20 87 6 PFUdA 2 88 5 5 84 4 PFUdA 20 84 6 PFDoA 2 79 13 5 72 13 20 75 14 PFTrDA 2 98 20 5 77 19 20 93 16 PFHxS 2 65 12 5 87 11 20 93 5 PFHpS 2 144 10 5 149 8 20 128 8 PFOS 2 136 22 5 100 8 20 93 18 PFNS 2 115 5 5 114 3 20 99 19 PFDS 2 99 6 5 107 4 20 102 4 PFDoS 2 82 16 5 72 12 20 94 7 表 5 各分析物基质效应评价
Table 5. Matrix effect evaluation for each analyte
化合物Compund 溶剂曲线斜率Slope of solvent curve 基质效应曲线斜率Slope of the matrix effect curve 基质效应/%Matrix effect 基质效应评价Matrix effect evaluation 6:2 Cl-PFESA 1.6618 1.3932 16 弱基质效应 8:2 Cl-PFESA 1.1880 1.0309 13 弱基质效应 PFOA 0.2074 0.1993 4 弱基质效应 PFNA 0.2052 0.2244 9 弱基质效应 PFDA 0.2170 0.2412 11 弱基质效应 PFUdA 0.2003 0.2241 12 弱基质效应 PFDoA 0.1862 0.2457 32 中等程度基质效应 PFTrDA 0.1816 0.2100 16 弱基质效应 PFHxS 0.2109 0.2224 5 弱基质效应 PFHpS 0.2317 0.2858 23 中等程度基质效应 PFOS 0.0836 0.1127 35 中等程度基质效应 PFNS 0.1852 0.2018 9 弱基质效应 PFDS 0.2191 0.2018 8 弱基质效应 PFDoS 0.1858 0.1595 14 弱基质效应 表 6 14种PFASs在胎便中的测定值
Table 6. Determination concentration of 14 kinds of PFASs in meconium
化合物Compounds 检出率/%Detection rate 浓度/(ng·g−1)Concentrations 最小值Min 第一四分位数P25 中位数Median 第三四分位数P75 最大值Max 6:2 Cl-PFESA 20 <MDLs <MDLs <MDLs <MDLs 0.073 8:2 Cl-PFESA 0 <MDLs <MDLs <MDLs <MDLs <MDLs PFOA 100 0.064 0.083 0.106 0.126 0.326 PFNA 70 <MDLs <MDLs 0.008 0.013 0.029 PFDA 20 <MDLs <MDLs <MDLs <MDLs 0.018 PFUdA 60 <MDLs <MDLs 0.015 0.036 0.089 PFDoA 30 <MDLs <MDLs <MDLs <MDLs 0.007 PFTrDA 70 <MDLs <MDLs 0.015 0.093 0.173 PFHxS 100 0.531 1.244 1.633 1.826 2.486 PFHpS 60 <MDLs <MDLs <MDLs 0.028 0.073 PFOS 80 <MDLs 0.036 0.508 0.669 1.778 PFNS 30 <MDLs <MDLs <MDLs 0.010 0.079 PFDS 10 <MDLs <MDLs <MDLs <MDLs 0.101 PFDoS 0 <MDLs <MDLs <MDLs <MDLs <MDLs -
[1] BUCK R C, FRANKLIN J, BERGER U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins[J]. Integrated Environmental Assessment and Management, 2011, 7(4): 513-541. doi: 10.1002/ieam.258 [2] LINDSTROM A B, STRYNAR M J, LIBELO E L. Polyfluorinated compounds: Past, present, and future[J]. Environmental Science & Technology, 2011, 45(19): 7954-7961. [3] HERZKE D, OLSSON E, POSNER S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway - A pilot study[J]. Chemosphere, 2012, 88(8): 980-987. doi: 10.1016/j.chemosphere.2012.03.035 [4] ZUSHI Y, HOGARH J N, MASUNAGA S. Progress and perspective of perfluorinated compound risk assessment and management in various countries and institutes[J]. Clean Technologies and Environmental Policy, 2012, 14(1): 9-20. doi: 10.1007/s10098-011-0375-z [5] GIESY J P, KANNAN K. Global distribution of perfluorooctane sulfonate in wildlife[J]. Environmental Science & Technology, 2001, 35(7): 1339-1342. [6] PAN G, ZHOU Q, LUAN X, et al. Distribution of perfluorinated compounds in Lake Taihu (China): Impact to human health and water standards[J]. Science of the Total Environment, 2014, 487: 778-784. doi: 10.1016/j.scitotenv.2013.11.100 [7] WANG P, LU Y L, WANG T Y, et al. Shifts in production of perfluoroalkyl acids affect emissions and concentrations in the environment of the Xiaoqing River Basin, China[J]. Journal of Hazardous Materials, 2016, 307: 55-63. doi: 10.1016/j.jhazmat.2015.12.059 [8] TAN B, WANG T Y, WANG P, et al. Perfluoroalkyl substances in soils around the Nepali Koshi River: Levels, distribution, and mass balance[J]. Environmental Science and Pollution Research, 2014, 21(15): 9201-9211. doi: 10.1007/s11356-014-2835-6 [9] CHEN L, DAI Y Y, ZHOU C, et al. Robust matrix effect-Free method for simultaneous determination of legacy and emerging per- and polyfluoroalkyl substances in crop and soil matrices[J]. Journal of Agricultural and Food Chemistry, 2020, 68(30): 8026-8039. doi: 10.1021/acs.jafc.0c02630 [10] WONG F, SHOEIB M, KATSOYIANNIS A, et al. Assessing temporal trends and source regions of per- and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP)[J]. Atmospheric Environment, 2018, 172: 65-73. doi: 10.1016/j.atmosenv.2017.10.028 [11] ZHAO P J, XIA X H, DONG J W, et al. Short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a turbid river[J]. Science of the Total Environment, 2016, 568: 57-65. doi: 10.1016/j.scitotenv.2016.05.221 [12] YAO Y M, SUN H W, GAN Z W, et al. Nationwide distribution of per- and polyfluoroalkyl substances in outdoor dust in mainland China from eastern to western areas[J]. Environmental Science & Technology, 2016, 50(7): 3676-3685. [13] HOUDE M, de SILVA A O, MUIR D C G, et al. Monitoring of perfluorinated compounds in aquatic biota: An updated review PFCs in aquatic biota[J]. Environmental Science & Technology, 2011, 45(19): 7962-7973. [14] PAN C G, YU K F, WANG Y H, et al. Species-specific profiles and risk assessment of perfluoroalkyl substances in coral reef fishes from the South China Sea[J]. Chemosphere, 2018, 191: 450-457. doi: 10.1016/j.chemosphere.2017.10.071 [15] GAO K, ZHUANG T F, LIU X, et al. Prenatal exposure to per- and polyfluoroalkyl substances (PFASs) and association between the placental transfer efficiencies and dissociation constant of serum proteins-PFAS complexes[J]. Environmental Science & Technology, 2019, 53(11): 6529-6538. [16] PENG L, XU W, ZENG Q H, et al. Distribution characteristics of per- and polyfluoroalkyl substances (PFASs) in human urines of acrylic fiber plant and chemical plant[J]. Environmental Science and Pollution Research, 2021, 28(48): 69181-69189. doi: 10.1007/s11356-021-15355-7 [17] 杨帆, 施致雄. 全氟辛烷磺酸和全氟辛酸的人群暴露水平和毒性研究进展[J]. 环境与健康杂志, 2014, 31(8): 730-734. YANG F, SHI Z X. Human exposure and toxicity of perfluorooctyl sulfonate and perfluorooctanoic acid: A review of recent studies[J]. Journal of Environment and Health, 2014, 31(8): 730-734(in Chinese).
[18] 司圆圆, 张卓婷, 王林钰, 等. 全氟化合物污染特征及生态风险评估[J]. 化工管理, 2020(34): 98-99. SI Y Y, ZHANG Z T, WANG L Y, et al. Pollution characteristics and ecological risk assessment of perfluorinated compounds[J]. Chemical Management, 2020(34): 98-99(in Chinese).
[19] TRUDEL D, HOROWITZ L, WORMUTH M, et al. Estimating consumer exposure to PFOS and PFOA[J]. Risk Analysis, 2008, 28(2): 251-269. doi: 10.1111/j.1539-6924.2008.01017.x [20] FÁBELOVÁ L, BENEITO A, CASAS M, et al. PFAS levels and exposure determinants in sensitive population groups[J]. Chemosphere, 2023, 313: 137530. doi: 10.1016/j.chemosphere.2022.137530 [21] CARIOU R, VEYRAND B, YAMADA A, et al. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns[J]. Environment International, 2015, 84: 71-81. doi: 10.1016/j.envint.2015.07.014 [22] VIZCAINO E, GRIMALT J O, FERNÁNDEZ-SOMOANO A, et al. Transport of persistent organic pollutants across the human placenta[J]. Environment International, 2014, 65: 107-115. doi: 10.1016/j.envint.2014.01.004 [23] GARERI J, KLEIN J, KOREN G. Drugs of abuse testing in meconium[J]. Clinica Chimica Acta, 2006, 366(1/2): 101-111. [24] MEYER-MONATH M, CHATELLIER C, ROUGET F, et al. Development of a multi-residue method in a fetal matrix: Analysis of meconium[J]. Analytical and Bioanalytical Chemistry, 2014, 406(30): 7785-7797. doi: 10.1007/s00216-014-8243-4 [25] PAN Y, WANG J, YEUNG L W Y, et al. Analysis of emerging per- and polyfluoroalkyl substances: Progress and current issues[J]. TrAC Trends in Analytical Chemistry, 2020, 124: 115481. doi: 10.1016/j.trac.2019.04.013 [26] LI M, YANG Z X, YANG M, et al. Determination of furfural in beer by high-performance liquid chromatography with solid-phase extraction[J]. Journal of the Institute of Brewing, 2009, 115(3): 226-231. doi: 10.1002/j.2050-0416.2009.tb00373.x [27] 杨松, 邹楠, 高云, 等. 固相萃取-超高效液相色谱-串联质谱法检测环境水体中18种农药残留[J]. 色谱, 2020, 38(7): 826-832. YANG S, ZOU N, GAO Y, et al. Determination of 18 pesticide residues in environmental water by solid phase extraction-ultra performance liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Chromatography, 2020, 38(7): 826-832(in Chinese).
[28] LI Y, FENG X, ZHOU J, et al. Occurrence and source apportionment of novel and legacy poly/perfluoroalkyl substances in Hai River Basin in China using receptor models and isomeric fingerprints[J]. Water Research, 2020, 168: 115145. doi: 10.1016/j.watres.2019.115145 [29] LIU M L, DONG F F, YI S J, et al. Probing mechanisms for the tissue-specific distribution and biotransformation of perfluoroalkyl phosphinic acids in common carp (Cyprinus carpio)[J]. Environmental Science & Technology, 2020, 54(8): 4932-4941. [30] 黎娟, 乔庆东, 庄景新, 等. 改进的高效液相色谱-串联质谱方法同时测定动物性食品中4种β2-受体激动剂残留[J]. 色谱, 2016, 34(2): 170-175. doi: 10.3724/SP.J.1123.2015.08033 LI J, QIAO Q D, ZHUANG J X, et al. Simultaneous determination of the residues of four β2-agonists in animal foods by modified high performance liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Chromatography, 2016, 34(2): 170-175. doi: 10.3724/SP.J.1123.2015.08033
期刊类型引用(0)
其他类型引用(1)
-






 DownLoad:
DownLoad: