-
大环内酯类抗生素(MLs)是一类分子结构中含有12—16碳内酯环的抗菌药物的总称,广泛用于人类疾病治疗和畜牧养殖[1]. 2009年MLs在兽用抗生素中全球销量最高,2010年MLs位居全球抗生素消费量第三[2]. 2013年中国MLs总消费量为42200 t,占抗生素总消费量的26%[3]. 但由于人体和动物对抗生素的吸收率不高,5%—90%的抗生素以母体或代谢产物形态通过尿液或粪便排出体外,成为一类新的环境污染物. 相对于持久性有机污染物(POPs),MLs具有较短的环境半减期,但因其在环境中不断输入,持续存在,从而表现为“假持久性”[4-8]. MLs特别是红霉素、克拉霉素、阿奇霉素和罗红霉素,经常在全球地表水中被检测到[9]. 抗生素在水环境中不仅会选择性抑杀环境微生物,还会诱导细菌产生抗药性,对生态环境和人类健康构成威胁[10]. 2018年,欧盟将红霉素、克拉霉素、阿奇霉素等MLs作为潜在水污染物纳入地表水污染物监测清单,要求成员国严格监控,评估其对水环境的危害和风险[11].
进入环境中的抗生素会发生一系列的转化行为. 其中,光降解是MLs在环境中的重要消减途径[12-15],而且光解强烈影响此类污染物的生态毒理效应[16-18]. 鉴于MLs是一类普遍存在的新污染物,难以被生物降解,且光降解是决定其环境归趋的重要因素,因此,有必要揭示其环境存在状况、分布特征及光化学转化行为,这对于该类污染物的环境归趋和暴露评价具有重要意义[4,19]. 中国是世界上最大的抗生素生产、消费国,水环境中抗生素的浓度和检出频率高于一些发达国家,环境分布特征可能与其他国家不同[20-21]. 并且,鉴于我国水域的多样性,抗生素类污染物在我国水环境中的分布状况和光化学行为可能呈现复杂、多样的特点. 目前,我国水体中MLs的分布特征及环境光化学行为被广泛关注,相关研究水平持续提升,也出现了一些新的发展趋势. 本文将探讨我国水环境中MLs的存在状况、时空分布差异,总结国内外该类抗生素水环境光化学行为的最新研究进展,其中将重点讨论其光降解动力学、影响因素和反应路径等.
-
大部分抗生素类化合物的蒸汽压较小、亲水性强,因此该类污染物主要存在于水环境中[19]. 目前在中国水环境中已检测到94种抗生素[22]. 通过总结我国与其他国家典型地表水体中MLs的浓度水平(表1),发现在环境水体中常检出的MLs为红霉素、罗红霉素、克拉霉素、螺旋菌素、泰乐菌素等,其在水中和沉积物中的浓度水平分别为ng·L−1级和ng·g−1级. 其中,红霉素和罗红霉素的检出浓度较高,例如在太湖中这两种MLs的最高浓度分别为624.8 ng·L−1和218.3 ng·L−1[23]. 然而,泰乐菌素和克拉霉素检出浓度相对较低,阿奇霉素在湖泊中的残留浓度研究较少.
红霉素已于2002年在中国水产养殖中被禁用,但2012年仍有红霉素使用的相关数据[38]. 在中国的水产品中抗生素的残留水平为0.01—100 ng·g−1湿重(ww),MLs残留平均浓度为7.6 ng·g−1 ww,其中在广东江阳的海陵岛周边养殖场采集的虾类样品中脱水红霉素残留浓度高达15090 ng·g−1[38-39]. 南京固城湖流域蟹塘3个养殖季节,水中脱水红霉素平均浓度为307.27 ng·L−1,夏季高达2450 ng·L−1,这对藻类具有较高的风险,对蟹池中的水生生物也存在潜在危害[40]. 除了水产养殖业,集中畜牧业对抗生素也有很大需求,牲畜粪便和养殖废水是环境中抗生素的重要来源,在我国农场牲畜粪便中检测到MLs的中值浓度为9.6 ng·g−1,动物废水中为13.8 ng·L−1[41]. 抗生素排放后最终进入到水环境,可能污染饮用水水源. 若抗生素不能被净水厂有效去除,进入饮用水管网,将对饮用水安全构成隐患[42]. 李辉等[43]在南京市饮用水源地的7个采样点中共检出5种MLs,其中阿奇霉素的检出浓度最高达65.2 ng·L−1,检出率为64.71%. 另外,厦门莲花水库中MLs浓度也较高,阿奇霉素的浓度最高(232.61 ng·L−1),检出率为75%,其次为罗红霉素,高达72.58 ng·L−1[44]. 同时,由于抗生素的吸附作用,沉积物中也存在残留. 重庆桃溪河表层沉积物中检出的罗红霉素中值浓度为0.4 ng·g−1,脱水红霉素中值为0.1 ng·g−1 [45];在辽河检出的罗红霉素中值浓度为5.51 ng·g−1 (最大值29.6 ng·g−1),红霉素为3.61 ng·g−1 (40.3 ng·g−1)[46];而珠江检出浓度更高,分别为罗红霉素24.7 ng·g−1 (133 ng·g−1)和红霉素24.4 ng·g−1 (385 ng·g−1)[47].
如表1所示,就全球范围看,中国水环境中MLs的残留浓度处于相对较高的水平. MLs在中国安徽宛溪河、长江嘉陵江重庆段、山东莱州湾小清河、北京潮白河等水体中的均值明显高于法国奥杜尔河口、伊比利亚河、美国东南部河流、南非Umgeni河等. 其中,在长江嘉陵江重庆段,水中MLs高达1409 ng·L−1,沉积物中为866.78 ng·g−1;在长江下游检测到沉积物中MLs浓度更高,达到6780 ng·g−1. 中国河流湖泊和沿海水体中的抗生素浓度普遍高于其他国家,且来源复杂[20-21, 48]. 这些研究表明,MLs已成为我国普遍存在的新型有机污染物,其环境存在、分布特征、迁移转化及生态效应引起高度重视.
-
中国地表水环境中MLs的浓度水平存在时空分布差异. 在时间尺度上,不同季节、水期特定水域MLs的分布特征存在差异. Pan等[49]调查了上海浦东新区地表水中的MLs,如脱水红霉素和替米考星,发现MLs在雨季时的浓度相对较低. 廖杰等[44]也发现,厦门莲花水库的MLs在不同水期的浓度变化较大,枯水期的浓度远高于平水期和丰水期. 造成这种差异的原因可能是因为冬季是流感高发季节,MLs的使用量更大,并且冬季枯水期降雨量少,溪流流量较小,水体自净能力较差,导致枯水期的抗生素残留浓度最高;而夏季丰水期强降水导致污染物被稀释[44]. 此外,强烈的阳光和高温导致的快速光解和生物降解也可能是夏季丰水期浓度较低的另一个原因[7]. 除了降雨量、温度、光照强度和微生物活性等因素会影响抗生素的在环境中的季节变化外,农田播种、水产养殖投苗、病虫害防治时抗生素药物的使用方式和时期也与之密切相关[50-51]. 另外,潮汐对抗生素的空间分布和迁移也有很大影响,特别是浓度波动范围较大的抗生素,如红霉素、罗红霉素等[25].
在空间尺度上,MLs在不同的水域和区域的污染分布也存在差异. 通过总结计算我国地表水、近岸海水以及沉积物中MLs平均浓度,得到我国水环境中该类抗生素的空间分布状况,如图1所示. 从图1可以看出,MLs在内陆与沿海地区水体中的污染程度有差异. 位于内陆地区的江汉平原河流、长江嘉陵江重庆段、安徽宛溪河、河北白洋淀、北京潮白河、西安渭河等水体中都存在较高浓度的MLs污染,其中湖北江汉平原地表水中平均浓度达324 ng·L−1. 沿海地区的北部湾、青岛胶州湾、上海浦东河流、上海南陈河等水体中MLs污染较轻. 然而,山东莱州湾污染较高,污染水平为82 ng·L−1,长江下游的沉积物中MLs浓度均值达400 ng·g−1. 对于不同MLs,在不同水域的检出浓度和检出率也存在一定差异,例如河北白洋淀、山东南四湖、湖南洞庭湖等中罗红霉素的平均浓度最高,而在安徽宛溪河、吉林松花江、长江嘉陵江重庆段中平均浓度最高的为红霉素,在厦门莲花水库中阿奇霉素的浓度最高. 以上差异可能与水体稀释能力以及不同地区抗生素的使用种类与用量、环境条件等有关[52]. 从目前可检索到的资料来看,我国西部地区MLs的污染检测数据还比较缺乏(图1).
在同一水域或地区,还发现抗生素在近海海域和河流中的检出频率和浓度均高于在临近湖泊和地下水中的数值[42]. 例如,Zhao等[7]发现,抚仙湖流入河流中MLs的平均浓度是抚仙湖的5.6倍,这可能与抗生素水平迁移期间的稀释和降解有关. Yao等[50]对比了旱、雨季时江汉平原沙湖镇的地表水和附近地下水的抗生素污染状况,发现相较于地表水中阿奇霉素、红霉素等MLs的高浓度(平均浓度324 ng·L−1),地下水中MLs的污染程度较低,平均浓度仅有4.70 ng·L−1,且与地表水的MLs的组成略有不同,推测地下水中积累的抗生素主要来自于河流或小溪,并受土壤和含水层系统的地球化学过程的影响.
-
在表层水体中,普遍存在着抗生素等有机污染物的直接光解、自敏化光解等表观光解,以及间接光降解[72-75]. 总结前人研究,发现MLs也可以发生3种光解反应[76-78].
-
直接光解是指污染物分子吸收光子后,直接发生的光降解行为. 与直接光解不同,自敏化光解是指污染物分子吸收光后,生成的激发三重态并将能力转移给其他物质(如H2O、O2),产生活性氧物种(ROS,如羟基自由基(·OH)、单线态氧(1O2)、超氧阴离子自由基(O2·−)等),生成的ROS再将污染物物氧化降解[79-82]. 由于到达地球表面的太阳光一般是λ > 290 nm,因此只有紫外-可见吸收光谱与光源的发射光谱有重叠,即在290 nm以上有光吸收的MLs才有可能在自然环境中发生直接光解或自敏化光解[83]. 某些MLs可以吸收太阳光,所以在前人研究中, MLs发生了直接光解和自敏化光解,其光解反应遵循准一级反应动力学[15, 84-85]. 通过公式(1)可以得到直接光解的一级速率常数k:
式中,C0和Ct分别为反应0时刻和t时刻的MLs浓度.
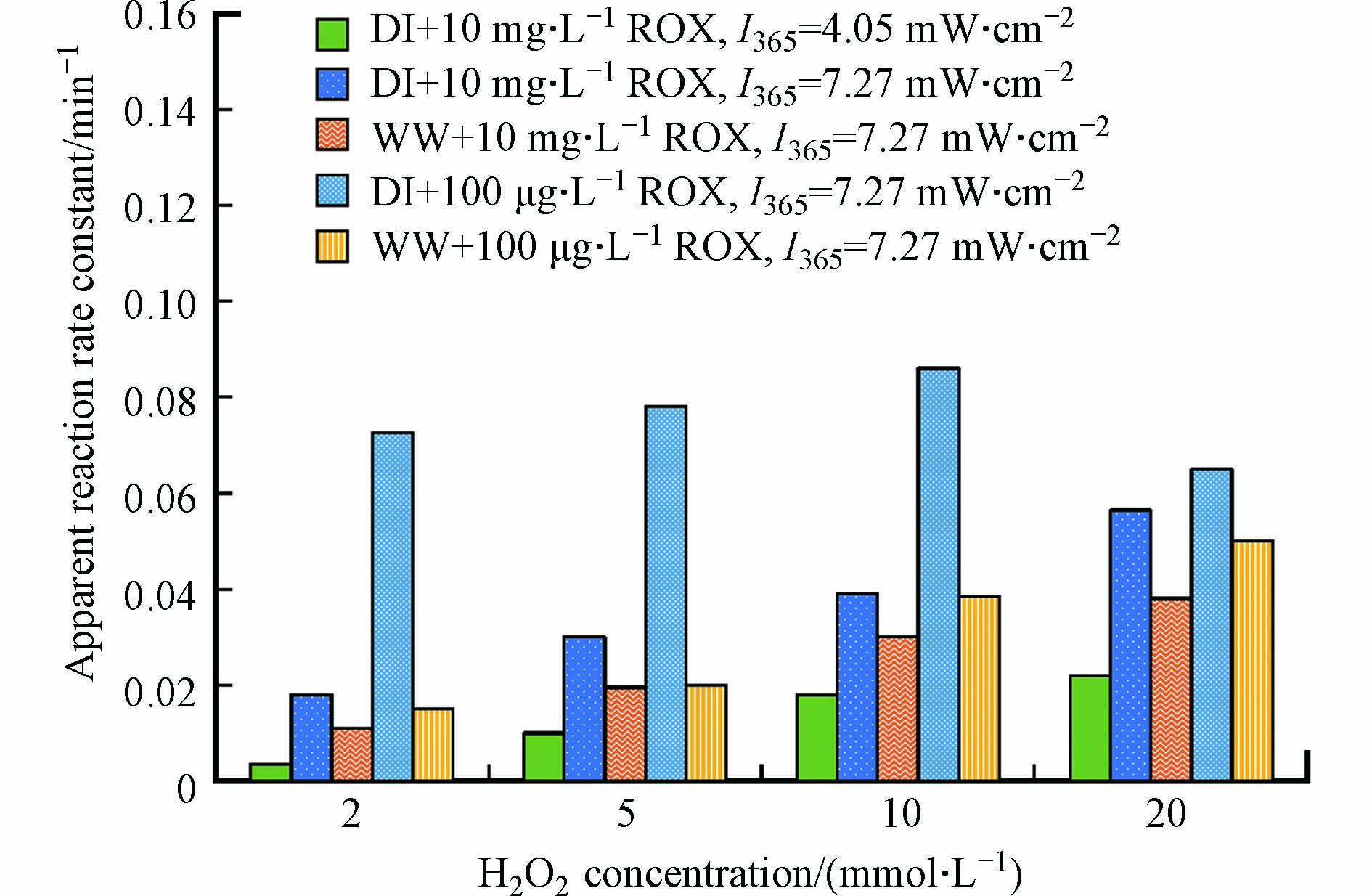
基于光降解k的测定,常海莎[84]发现在紫外光和模拟日光照射下,螺旋霉素、罗红霉素、克拉霉素和泰乐菌素这4种MLs的光降解速率有很大差异,但在纯水中均发生了直接光降解和自敏化光降解,并以直接光降解为主. 添加异丙醇、对苯醌、叠氮化钠和山梨酸可以猝灭ROS从而抑制抗生素的光降解(图2),对比各反应速率常数可知,MLs在水中的光降解过程中存在∙OH、1O2和O2·−参与的自敏化光降解. 以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)和2,2,6,6-四甲基-4-哌啶(TEMP)为·OH和1O2的自旋捕获剂,测定MLs在纯水体系下的电子自旋共振波谱以验证产生的ROS[86]. 实验证实在纯水中罗红霉素本身吸收光可产生·OH和1O2,并且产生的1O2更多,但由于1O2与罗红霉素的反应速率常数较小,导致纯水中罗红霉素光降解速率较低[15].
为了衡量光化学反应对光子的利用效率,需要对MLs的量子产率进行测定. 通常将待测化合物(称为底物s)的溶液和露光计置于相同的光照条件下,进行光化学实验,通过公式(2)计算量子产率(Φs):
式中,a为露光计,其量子产率(已知)为Φa;k为光化学反应速率常数;Lλ为光源在波长λ处的光强;ελ为MLs或露光计在波长λ处的摩尔吸光系数. 常用的化学露光计有草酸铁钾、PNA/pyr(对硝基苯甲醛/吡啶)[87].
前人测定并计算了MLs在不同光源照射下的Φs,总结在表2中. 例如,模拟太阳下纯水中的MLs的量子产率呈现螺旋霉素 > 泰乐菌素 > 克拉霉素 > 罗红霉素[84]. Jia等[9]测得克拉霉素的量子产率5.6 × 10−5比其他研究者[84]测得的量子产率6.3 × 10−3小两个数量级,存在的差异可能与实验测得的反应速率常数k或累计光吸收系数(∑Lλελ)有关.
溶液pH值和水成分会影响抗生素的存在形态,从而影响其光吸收特性. Voigt等[88]测得在溶液pH = 3、7、9时,阿奇霉素、红霉素和泰乐菌素在254 nm光照下的量子产率,发现阿奇霉素在pH 3时的量子产率高于在pH 7和pH 9时的量子产率,而红霉素与泰乐菌素则是在pH 7时的量子产率较大. Batchu等[85]测定了在不同UV光源(254 nm和350 nm)照射下,罗红霉素、红霉素在纯水、淡水和海水(pH = 6—9)中的量子产率,发现罗红霉素在淡水中的量子产率最大,即Φ254 =4.9 × 10−2,Φ350 =7.0 × 10−4;而红霉素在海水中的量子产率最大,分别为Φ254 = 4.5 × 10−3和Φ350 =7.0 × 10−4. 这说明pH和水成分对于MLs光解速率快慢有一定影响,且不同MLs的量子产率在不同溶液中的变化规律并不统一.
-
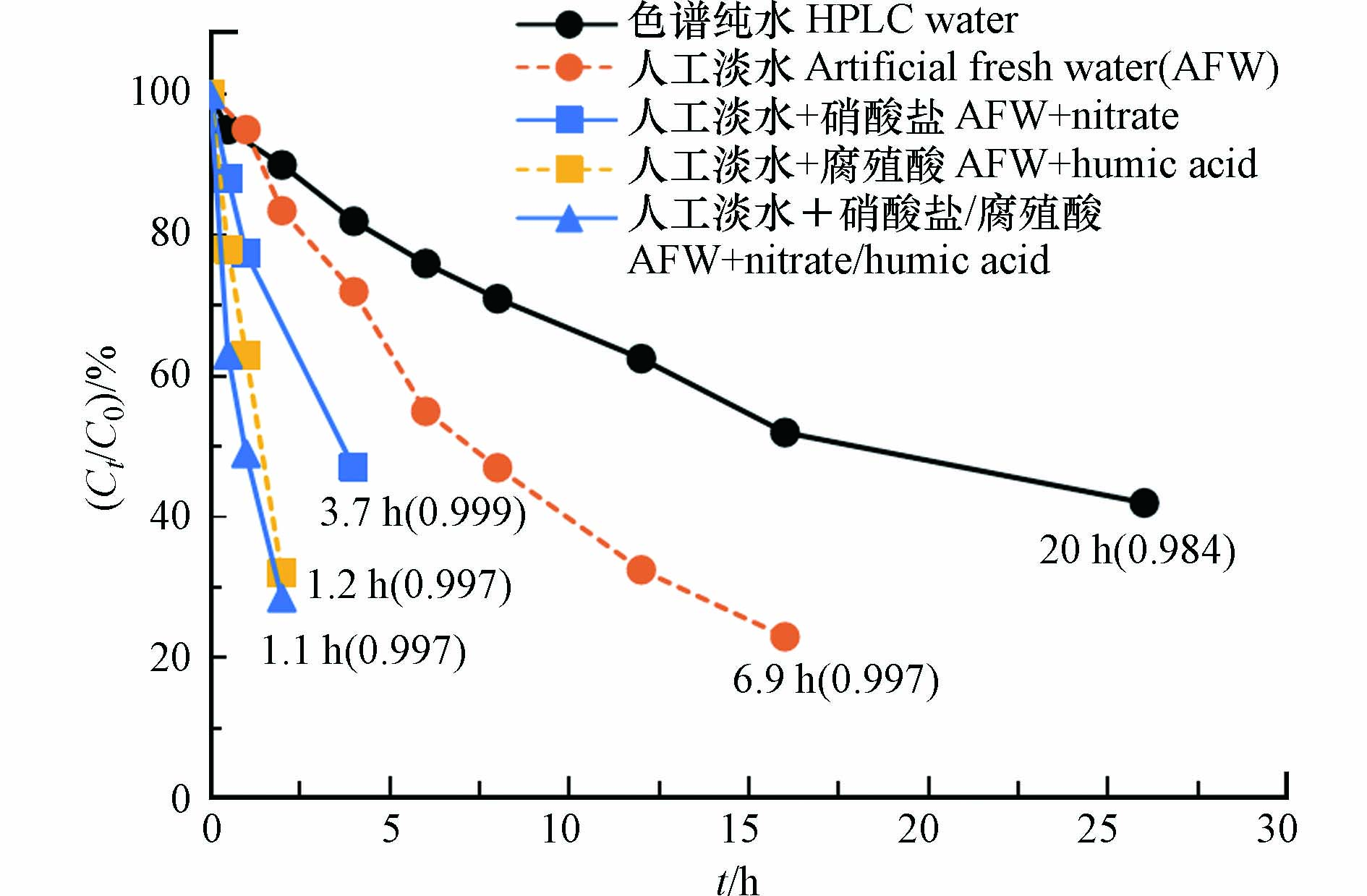
间接光解是指敏化剂吸收光子,然后将能量转移给污染物而引起的分解反应. 前人研究表明,在溶解性有机质(DOM)、硝酸盐等敏化剂存在时,MLs可以发生间接光解,这对于水环境中某些MLs的消减较直接光解更为重要[9, 77, 89-90]. 罗红霉素在淡水和海水的光解速率相对于在纯水中有显著提高,表明天然水中存在重要的间接光解或光敏化过程[85]. 这可能是由于淡水基质中具有高溶解度的有机碳,其作为MLs转化过程中的光敏剂,能吸收较宽范围的波长能量并产生ROS将MLs氧化降解[91]. 海水中存在大量Cl−,Cl−可捕获·OH生成具有强氧化性的Cl·和Cl2·−,从而影响MLs的间接光解[92-93]. Tong等[77]报道了MLs阿奇霉素在模拟太阳光下在色谱纯水、人工淡水、含腐殖酸(HA)/硝酸盐的人工淡水中的光解作用,结果表明阿奇霉素在色谱纯水中降解速率最慢,而在添加了硝酸盐(5 mg·L−1)和HA (0.5 mg·L−1)的人工淡水中降解加速,说明阿奇霉素可被硝酸盐和HA等敏化而发生间接光解(图3).
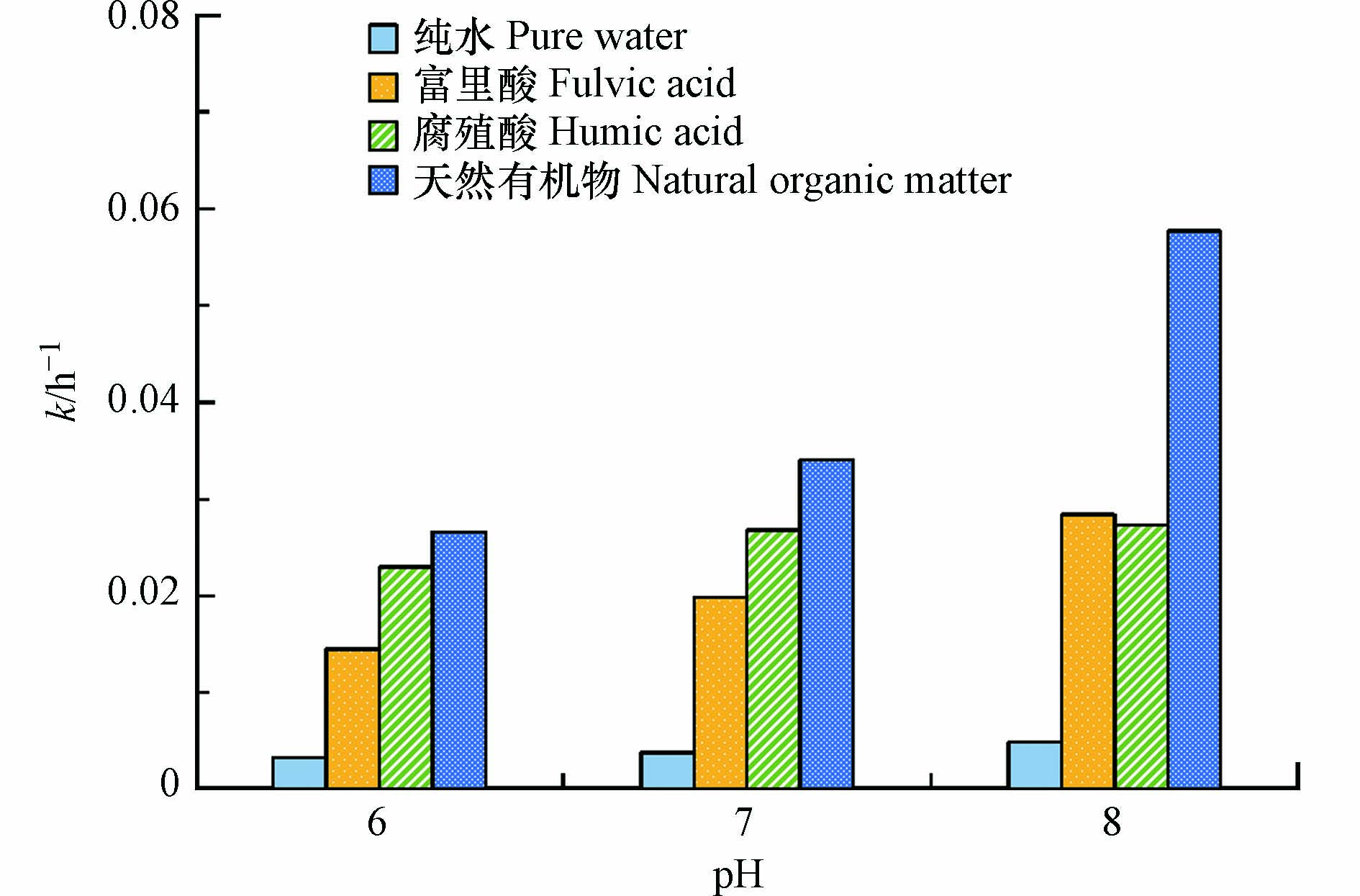
对于MLs的间接光解,一些研究者还对其转化动力学进行了定量研究. Jia等[9]发现MLs在苏瓦尼河天然有机物(SRNOM)溶液中的光降解速率为罗红霉素 ≈ 克拉霉素 > 阿奇霉素 > 红霉素,半减期(t1/2)分别为(7.2 ± 0.2) h、(7.2 ± 0.2) h、(9.4 ± 0.3) h和(11.2 ± 0.4) h. 与纯水中的对照实验相比,SRNOM溶液中的光解速率提高了约2—5倍,表明间接光解在MLs的光转化中起着关键作用,并且MLs与1O2反应的双分子反应速率常数在1.22 × 105 L·mol−1·s−1 (克拉霉素)—2.42 × 105 L·mol−1·s−1 (阿奇霉素)之间,与·OH反应的双分子反应速率常数在 2.18 × 109 L·mol−1·s−1 (阿奇霉素)—4.97 × 109 L·mol−1·s−1 (罗红霉素)之间. 吕宝玲等[15]研究了环境pH条件下(pH为6、7和8),苏瓦尼河腐殖酸(SRHA)、SRNOM和Pony湖富里酸(PLFA)对罗红霉素光降解的影响,在DOM存在下,罗红霉素的光降解反应速率常数均高于纯水中(图4). 并且1O2对罗红霉素光解的贡献率为0.21%—11.33%,·OH对罗红霉素光解的贡献率高达76.76%—98.70%. 根据以上研究可以看出,相较于1O2,·OH对MLs光降解的贡献更大一些.
1O2具有选择性,易于和硫化物、烯、共轭二烯、苯酚类化合物发生反应[94],而·OH没有选择性,能与绝大多数抗生素发生反应[72, 95-96]. 前人针对MLs与·OH反应的动力学进行探究. Voigt等[88]发现在UVC的辐照下,泰乐菌素的降解速度远快于阿奇霉素和红霉素,H2O2的加入使泰乐菌素的降解速度加快了2倍,而阿奇霉素和红霉素的降解速度在相同条件下提高了5倍. 同时,作者还研究了H2O2对螺旋霉素在UVC下的光解[97],发现在没有H2O2的情况下,动力学速率常数为(0.67 ± 0.13) min−1,在10 mg·L−1和30 mg·L−1 H2O2的存在下,速率常数分别增加到(1.41 ± 0.4) min−1和(2.60 ± 3.76) min−1;加入10 mg·L−1 H2O2使反应速率增加了1倍,而加入30 mg·L−1 H2O2并没有增加3倍. Li等[3]也发现仅用UV辐照(120 min)不会使罗红霉素浓度显著降低,而添加H2O2后,不同溶液条件下罗红霉素的降解均遵循准一级动力学(图5). 图5中同一溶液条件下罗红霉素的表观速率常数值随H2O2浓度增加先增大后减少. Li等[3]还利用竞争动力学模型估算了罗红霉素(ROX)与·OH反应的双分子反应速率常数kROX-·OH:
式中,[ROX]0和[ROX]t是反应时间0和t时的ROX浓度;[pCBA]0和[pCBA]t是反应时间0和t时对氯苯甲酸的浓度;kpCBA-·OH是pCBA与·OH反应的双分子反应速率常数(已知). 由公式(3)计算得到kROX-·OH为(5.68 ± 0.34) × 109 L·mol−1·s−1,与其他类抗生素的双分子反应速率常数处于同一数量级[4].
以上研究均表明H2O2的加入量对所引起的MLs降解增量并非线性关系,较高的H2O2初始浓度理论上可以产生更多的·OH,但实际并非如此. 这可能是因为H2O2与MLs相互竞争所导致的H2O2光屏蔽效应或过量H2O2对·OH的猝灭作用,即H2O2消耗·OH产生HO2·+,且当H2O2的浓度足够高时会自动分解为O2和H2O促进了H2O2的消耗[98-100].
-
水中溶解性物质如DOM、Cl−、NO3−、HCO3−、Fe(Ⅲ)、DO(溶解氧)等具有光化学活性的物质,以及pH值等理化性质都是影响抗生素光解动力学的因素[83]. 前人较多研究报道了这些水环境因子对MLs光降解动力学的影响,对此我们进行了总结,如表3所示.
天然水环境中广泛存在着NO2−/NO3−、Fe(Ⅲ)、HCO3−、Cl−等溶解性物质,在不同条件下能促进或抑制MLs的光化学转化(表3). 水中NO2−/NO3−的光化学性质不稳定,其光解能产生多种活性中间体,是天然水体中∙OH的重要来源之一[75, 104]. Fe(Ⅲ)在水中能形成水合物,通过阳光照射产生Fe(Ⅱ)和∙OH[105]. 此外,Fe(Ⅲ)还能与污染物形成配合物进而被降解. Vione等[78]采用分光光度滴定法证明了Fe(Ⅲ)分别与克拉霉素和罗红霉素形成了Fe(Ⅲ)-MLs配合物,在MLs溶液中滴加FeCl3后,其吸收峰(λmax = 361 nm)发生红移(3—5 nm). Fe(Ⅲ)的加入一定程度上促进了MLs的光解,作者认为发生的主要反应为Fe(Ⅲ)-MLs的直接光解,Fe(Ⅲ)光致∙OH对MLs氧化作用几乎可以忽略.
具有光活性的溶解性物质也会对MLs光解起到抑制作用[3, 84]. 常海莎[84]考察了分别添加NO3−、NO2−、Fe3+和Fe2+后4种MLs的光化学行为,发现其光解均符合准一级动力学方程,且水中不同浓度的溶解性物质对MLs存在双重作用,即各自表现出促进/抑制. Li等[3]发现在UV/H2O2体系下,NO3−和NO2−、Fe3+、Cu2+、Mg2+均抑制了罗红霉素的降解,抑制作用为Fe3+ > Cu2+ > Mg2+,NO2− > NO3− > HCO3− > Cl−. 造成该现象的原因是,这些离子在水中既可作为光敏剂或生成配合物促进光降解,亦可作为光掩蔽剂或∙OH捕获剂,从而抑制MLs的光解[81, 106].
淡水中常见的DOM有腐殖酸(HA)、天然有机物(NOM)等. 前人研究中,HA也可能对水中抗生素的光解产生促进或抑制作用[15, 75, 82, 107-108]. Li等[90]证实罗红霉素的表观光降解动力学常数随着HA和NOM浓度的增加而增大,在20 mg·L−1 HA和NOM的存在下,罗红霉素的光降解动力学速率常数分别是不含DOM时的4.0倍和3.6倍. 而HA的加入却抑制了泰乐菌素的光解[102],向溶液中分别添加10 mg·L−1和100 mg·L−1 HA,泰乐菌素在6 h后的光解效率从50%依次减小到30%和16%. 这种抑制现象可能也是由于HA产生光掩蔽效应或捕获ROS所造成的.
分子中具有酸碱解离基团的抗生素化合物,pH能显著影响其存在形态和光化学反应活性[72, 102]. MLs具有多个酸碱解离基团,随溶液pH变化可出现阳离子、中性离子、阴离子等价态[85, 109]. 不同离解形态的抗生素分子吸收光谱不同,量子产率也可能有差异,从而导致其光解速率常数变化[110-111]. 对于间接光解和自敏化光解,pH会影响ROS的生成速率以及抗生素与ROS的反应速率[83, 96]. 在DOM的影响下,罗红霉素的光降解反应速率常数表现出随pH升高而增大的趋势,∙OH对罗红霉素光解贡献率随pH的升高而降低,1O2对罗红霉素光解的贡献率随pH升高而升高[15]. Li等[3]也发现在UV/H2O2体系下,当pH值从4升高到9时,罗红霉素的反应速率常数从0.0162 min−1增加到0.0309 min−1. 在pH 8—9时的罗红霉素降解率几乎是pH 4—5时的两倍,这表明碱性条件在某种程度上更有利于其降解. 这种现象可能归因于罗红霉素发生解离.
综上所述,水环境因子(溶解性物质、pH等)对MLs的光化学行为具有一定的影响,同一因子对不同抗生素的抑制/促进作用不同,同一因子在不同浓度、不同光照条件下对同一抗生素光降解的抑制/促进作用也会有所不同. 虽然这些因素对MLs光解的影响趋势没有统一的规律,但可以看出,MLs表现出的光化学反应活性强弱往往是多重因素共同作用下的结果. MLs存在不同解离形态,且真实水体中多因素共存,光化学转化行为将更为复杂,不仅需深入探究单一因素对MLs不同解离形态光解的影响,而且需要进一步研究多种因素的复合影响、作用机制及相对贡献.
-
在抗生素光化学转化过程中,生成的产物可能保留药物活性[112]或增加菌株的敏感性[85],甚至毒性增强,造成更高的生态风险[101]. 因此鉴定光化学反应的中间产物和最终产物,进而推断光化学反应的路径,对于污染物的生态风险性评价具有重要意义[4]. MLs类抗生素可能发生的光降解路径主要有:光氧化、光致异裂、光致水解等[83].
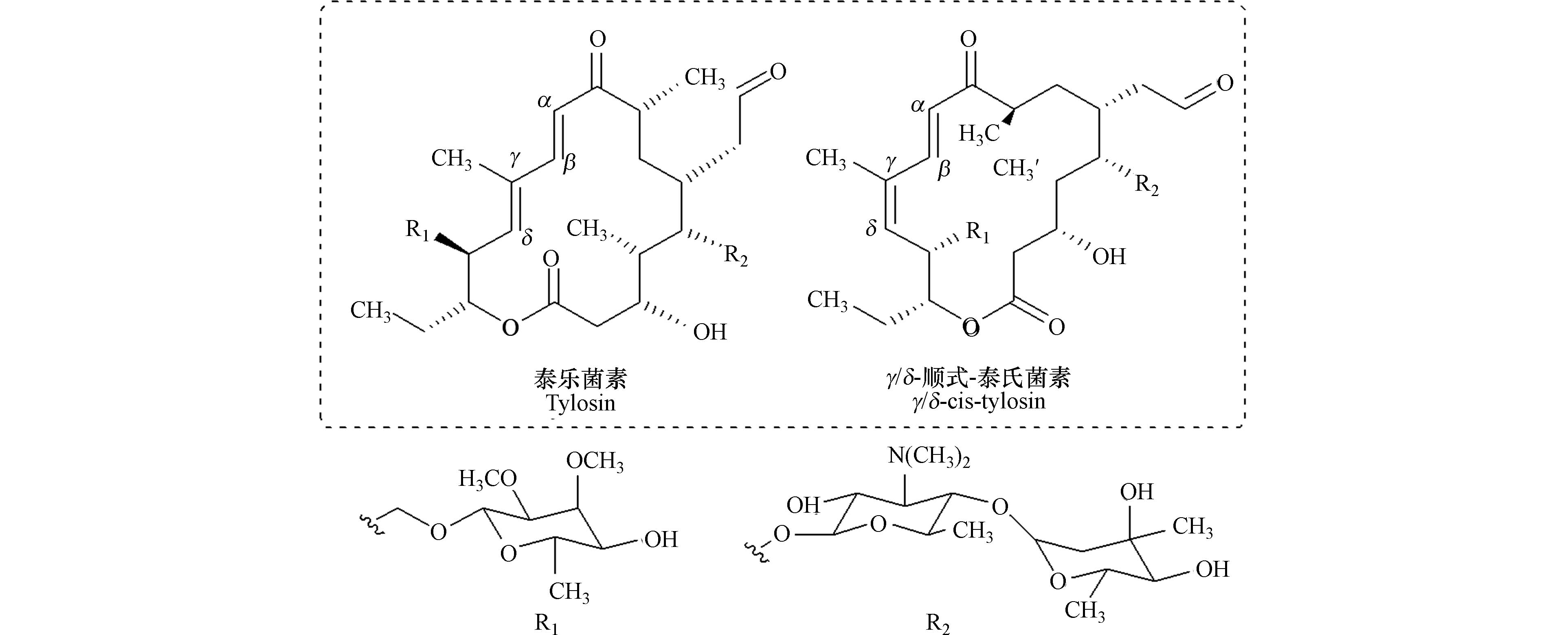
对于直接光解,前人发现对于结构相似的MLs,在一定条件下能够互相转化,如Batchu等[85]对RODW(反渗透去离子水)溶液中罗红霉素的光解产物进行分析,首次发现罗红霉素在254 nm的UV光照下侧链能发生裂解,形成红霉素. 此外,MLs还存在光异构化现象[9, 76]. Werner等[76]通过动力学、质谱和质子核磁共振,证实了在模拟太阳光下,泰乐菌素发生了光异构化. 在其光解产物中没有观察到新的质核比m/z的分子,表明该产物是泰乐菌素的一种异构体,作者认为该异构化是围绕泰乐菌素的内酯环酮二烯发色团末端的γ/δ双键旋转而成(图6). γ/δ-顺式-泰乐菌素异构体对大肠杆菌DH5α生长的抑制活性低于泰乐菌素.
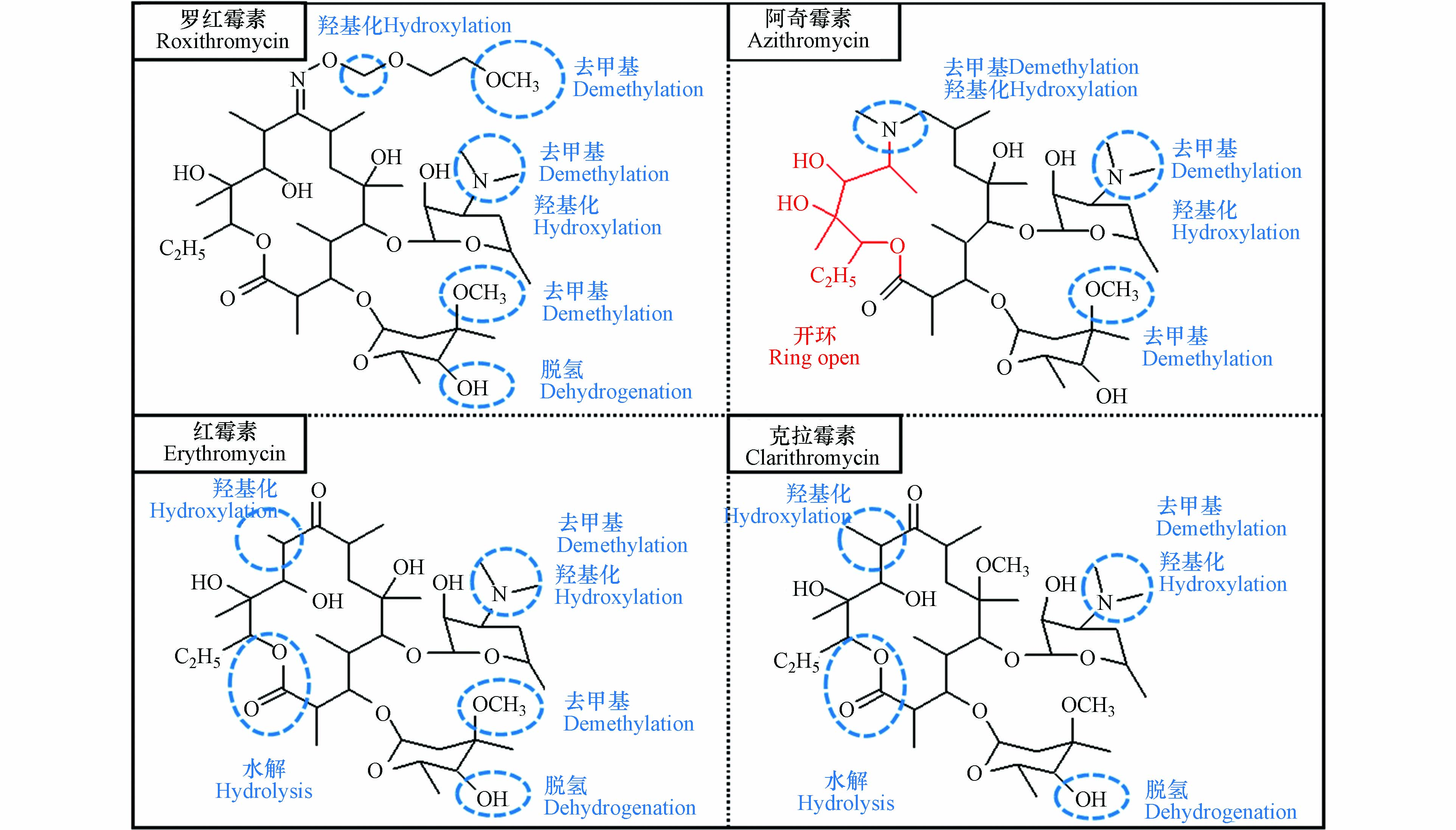
真实的水环境是一个复杂体系,间接光解是MLs在水环境中的一种重要消减途径,前人对MLs光解产物的研究多以DOM存在的体系为主,去甲基是MLs常见的转化路径[3, 9, 77, 90, 101]. Li等[90]采用HPLC-LTQ-Orbitrap XL-MS研究在SRNOM和SRHA存在下罗红霉素的光解产物,该条件下罗红霉素主要被DOM光敏化产生的ROS氧化,其光转化的主要途径为N-去甲基、O-去甲基以及侧链、红霉脱氧糖胺或红霉支糖的裂解(图7). 去甲基位点位于侧链、红霉支糖以及红霉脱氧糖胺的叔胺,这与Li等[3]所观察到的·OH对罗红霉素的攻击位点以及阿奇霉素与·OH反应位点[77]相似.
目前,多数研究并没有根据光解类型(如直接光解、自敏化光解和间接光解)对MLs转化产物加以区分. Gozlan等[101]采用LC-MS鉴定了MLs在不同pH缓冲液、含腐殖酸的pH = 7的缓冲液及自来水和二级废水中的光解产物,发现红霉素、阿奇霉素、克拉霉素和罗红霉素均发生了N-去(二)甲基化和N-氧化,反应位点在红霉脱氧糖胺部分. 此外,即使是同一类抗生素,其结构仍会有一定的差异,因此反应路径存在一些异同. Jia等[9]对典型MLs在DOM水溶液中的光转化路径进行了对比(图8),这4种MLs除了均能发生的去甲基,罗红霉素常见反应还有脱氢和羟基化;阿奇霉素还会发生水合反应;克拉霉素与红霉素能发生羟基化、脱氢、水解;特别的是,阿奇霉素、克拉霉素和红霉素在内酯环上存在反应位点,阿奇霉素的内酯环能开环裂解. 前人的一些研究中也观察到了内酯环的开环裂解:紫外辐射引起双键的光激发导致螺旋霉素内酯环的裂解[97],泰乐菌素的内酯环通过紫外吸收和随后的光反应而被破坏,Fe(Ⅲ)-ROX配合物直接光解导致了内酯环的裂解[78].
由此可见,MLs光转化过程中主要的反应为去甲基、羟基化以及红霉脱氧糖胺和红霉支糖的断裂,且N-去甲基形成的产物最为丰富. 在水环境中,MLs在结构上的变化特别是开环裂解,分别对其原本的抑菌活性和毒性有着怎样的影响,仍需进一步探究.
-
目前我国抗生素滥用问题依旧严峻,尤其是当今全球新冠疫情防控局势紧张,大量抗生素进入环境并持续存在,成为典型的新污染物. 中国重视新污染物治理,MLs是检出率较高的抗生素类新污染物. 本文评述了我国环境水体中MLs的存在状况与时空分布差异,重点讨论了MLs的光解动力学、水环境因子对光解的影响,以及光降解路径与机理等. 我国对该类新污染物的相关研究方兴未艾,一些重点问题值得关注:
(1) MLs的污染数据和分布特征有待丰富并深入探究. 我国关于MLs的环境污染数据不够丰富,特别是在内陆地区,需要开展进一步的监测,以满足优先控制新污染物“筛检”的需求. 进一步,可以借助主成分-多元线性回归(PCA-MLA)分析、Pearson相关性分析等手段了解抗生素来源及其残留的影响因素,深入探究其在不同介质,不同季节、地点的分布差异,以及水文、人类活动及水质指标对其分布的影响.
(2) MLs的光化学转化动力学和机制需要深入研究. MLs分子量大、结构复杂、可解离,天然水环境复杂多变,光化学行为很大程度上受pH、溶解性物质以及光源、温度等环境因子的影响. 需要系统研究MLs不同解离形态在水环境乃至结冰环境中的“复合”光化学转化行为,如表观光解和间接光解、产物和转化路径的规律;深入探究多环境因子对MLs光转化的复合效应,将实验室模拟和野外实验体系建立联系并进行优化,提高实验值推算至实际环境的准确性,以构建实际水环境适用的抗生素光化学转化动力学模型. 激光闪光光解技术(LFP)、电子顺磁共振(EPR)和化学测定法等可检测ROS的种类和丰度,而产物鉴定可借助LC/MS、核磁共振波谱仪(NMR)等设备,并利用维恩图、层次聚类等辅助产物解析.
(3) MLs的风险及光致毒性需要进一步评估. 通过不同的毒性受试生物体(如费氏弧菌、大型蚤、藻类和斑马鱼)以及抑菌实验菌体(如枯草芽孢杆菌、荧光假单胞菌)获得的风险评估可能存在不同的结论,需要多种营养级的生物、更丰富的菌株为对象进行实验,探索毒性作用机制和演变规律. 并且,应关注MLs的光致毒性,以阐明光解过程中母体及其中间产物的毒性效应和抗菌活性演变趋势.
我国水体中大环内酯类抗生素的分布特征及环境光化学行为
Occurrence and photochemical behavior of macrolide antibiotics in the aquatic environment of China
-
摘要: 大环内酯类抗生素(MLs)作为一类新型有机污染物,广泛存在于水环境中,表现为“假持久性”,并且能够导致环境菌群抗药性产生. 本文总结大量文献,分析了我国环境水体中MLs的存在状况与浓度水平,并对该类抗生素浓度水平的时空分布差异进行了讨论,同时总结了水环境中MLs光化学行为的最新研究进展,介绍了其光解动力学以及水环境因子对光解的影响,阐述了光降解路径与机理,最后对该类抗生素的环境存在特征及光化学转化研究进行了展望.Abstract: Macrolide antibiotics (MLs), as a new type of organic pollutants, are existing widely in the environment and show pseudo-persistent, which could induce bacterial resistance in the environment. Based on a large number of peer-reviewed literatures, this review analyzed the occurrence and levels of MLs in the aquatic environment of China, as well as the seasonal and spatial distribution. Furthermore, the current research progress on the aqueous photochemical behavior of MLs was explored. The photodegradation kinetics and effects of aqueous environmental factors were discussed. The corresponding photodegradation pathways and mechanisms for typical MLs were summarized. Finally, the research prospects about the environmental occurrence and photochemical transformation of MLs were proposed.
-
Key words:
- macrolide antibiotics /
- occurrence /
- distribution /
- photodegradation /
- photochemical transformation.
-
铅锌矿在中国矿产资源体系中占据着至关重要的地位,是工业领域的“关键基石”. 我国已勘铅锌资源主要集中于7个省区,储量约占全国66%,其中就包含甘肃省[1]. 在矿业开采与工业加工进程中,废液未经妥善处理随意排放,废渣堆积成山,加上雨水冲刷,致使 Cd、Hg等有害成矿、伴矿元素不断迁移、扩散,对周边土壤造成污染. 同时,由于土壤重金属污染的累积性[2],使其在土壤中的浓度越来越高,最终对生态系统造成危害[3]. 统计数据显示,我国有超过2000万 hm2的农田土壤存在重金属污染情况,当中被工业废渣污染的农田约10万 hm2,因采矿而污染的土壤面积约20万 hm2[4]. 一旦土壤重金属富集至特定程度,便会对农作物质量产生直接影响,有研究表明,国内每年由于土壤重金属污染而导致的粮食安全损失高达1.2×108 t,直接造成的经济损失逾 200 亿元[5]. 其毒性效应可经食物链传递至人体,引发骨骼疼痛、肾脏疾病等健康问题,对人类健康构成了极大的威胁 [6].
目前国内外学者对土壤重金属源解析的方法主要包括判别污染源类别和精准解析污染源两部分[7 − 9],前者包括相关性分析法、主成分分析法和聚类分析法等,后者包括绝对因子得分-多元线性回归法和正定矩阵因子分解法(positive matrix factorization,PMF)等. PMF是对污染源进行精确分析的首选方法,该方法操作简单,可同时满足不确定性和非负性约束[10],能够自动化地处理错误和缺失数据,不需要源组分图就能得到精确、可信的结果,因此被美国环保署优先推荐使用[11]. PMF 源解析虽能识别土壤重金属污染源,但难以精准量化其生态风险和健康风险,从而无法确定首要管控要素[11]. 为此,学者们将PMF模型与生态风险、健康风险评价模型进行整合,成功提出重金属污染特定源的风险评价方法[12]. 马杰等[13]将PMF模型和健康风险模型结合,探讨了不同污染源影响下重庆市农产品主产区的土壤健康风险,确定研究区工业源和As为首要管控要素,重金属源解析成为后期尾矿库及其周围土壤修复或复垦的重要依据.
甘肃省陇南市矿产资源丰富,尾矿库数量多,以铅锌矿和金矿尾矿为主,占比分别为72.54%和15.5%[14]. 当前,铅锌尾矿库及周边农田是土壤重金属污染的重点关注区域. 本文以此为研究对象,测定其土壤重金属含量,分析其污染状况,并在源解析的基础上结合生态风险和健康风险评估模型来量化各污染源相应风险贡献率,从而确定首要控制因素. 研究结果将为尾矿库周边农田土壤污染现状的调查和评价提供科学有效的理论依据.
1. 材料与方法(Materials and methods)
1.1 研究区概况
研究区地处徽县江洛镇赵湾村,隶属于甘肃省陇南市. 县名源自城北徽山之下的徽山驿,在北纬33°32'—34°10'、东经105°34'—106°26'之间,东接两当县,南连陕西省略阳县,西接成县,北通天水市秦州区与麦积区,西北衔西和县,总面积
2699 km2. 江洛镇处于徽县北部山地向中部河谷丘陵过渡区域,地势自北向南渐次降低,地形分为东南部河谷区,西北部山区;境内最高峰海拔2002 m. 江洛镇属暖温带大陆性气候;多年来气温的平均值为11 ℃,无霜期平均每年可达180 d,降水量的平均值为700 mm,降雨每年集中在7—9月. 2022年全县耕地总面积38.98万亩. 江洛镇境内已探明地下矿藏有铅、锌、硫、铁、金、煤等矿产资源,特别是铅、锌矿储量较大[15]. 该尾矿库为山谷型,采用湿式排放方式,尾矿库现状总坝高26 m,初期坝坝高为15 m,堆积坝坝高11 m,库内堆存尾砂量约为23万m3. 该尾矿库未填埋,在工矿生产和矿渣储存过程中,土壤中的粉尘会在大气中沉积,受降雨和径流的影响,对矿区周围环境造成了严重影响,从而可能会引发一系列的生态环境安全问题.1.2 样品的采集与处理
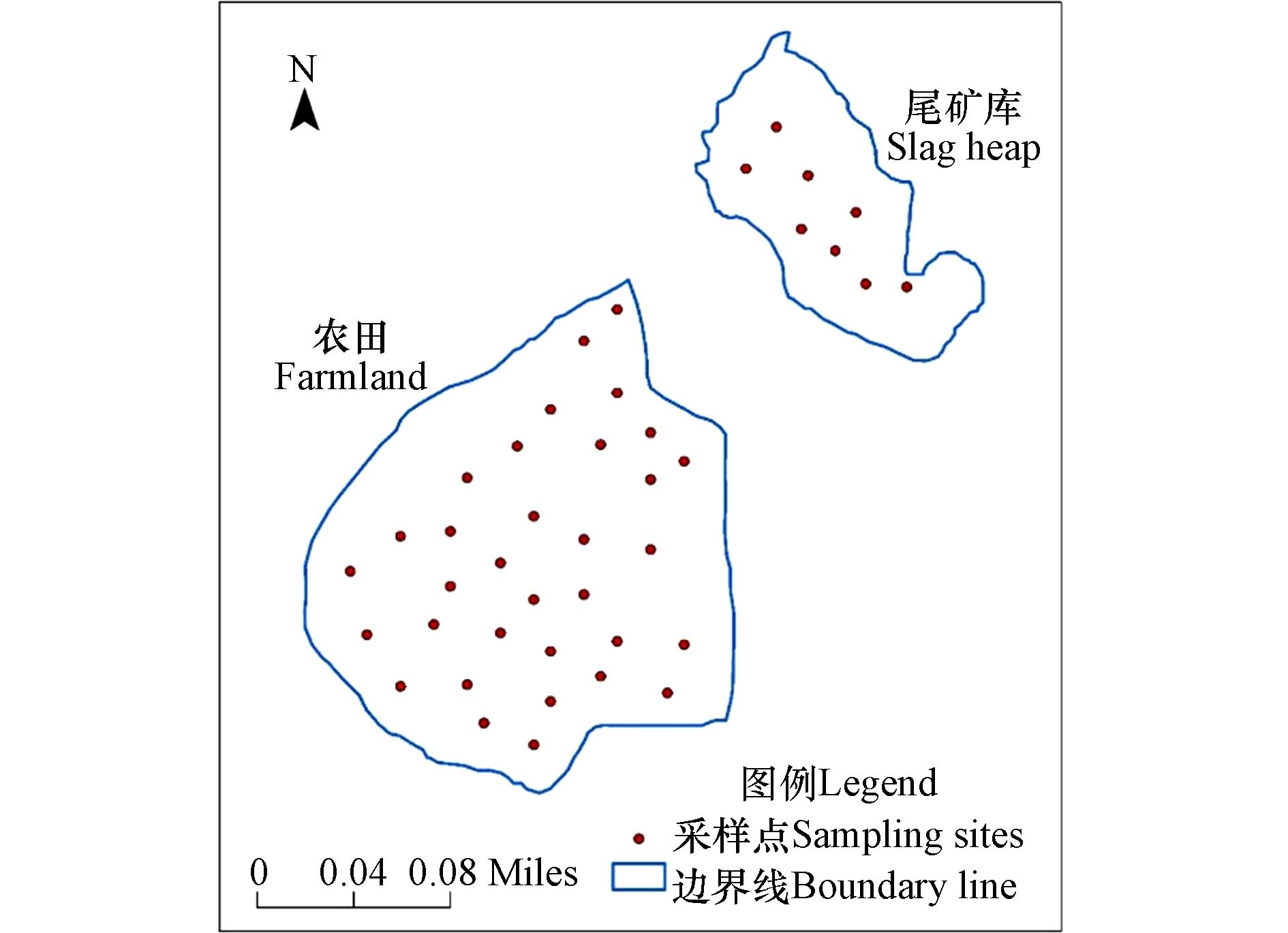
本文于2023年8月在甘肃省陇南市某铅锌尾矿库及其周边农田(北纬33°53'27''—33°53'43''、东经105°48'27''—105°48'41'')进行样品采集. 采用了简单随机布点法并结合专业判断法来布点. 为确保采样点布局的科学性与均匀性,本研究充分借助了地理信息系统的空间分析能力,结合网格化精密的划分手段,对整个研究区域进行布局(采样面积约为3.22 km2)[1]. 采样时用GPS定位采样点经纬度,并根据样点采集顺序对其编号,在现场共布置了41个采样点,其中尾矿库8个,周边农田33个,如图1所示. 在每个采样点采集表层土壤(0—20 cm),样品放在密封袋里保存. 采集的样品经自然风干、去除杂物、研磨和筛分后,一份用于测定土壤pH,一份用于测定土壤重金属含量. 采用电感耦合等离子体质谱仪(ICP-MS,NexION 2000)测定经盐酸-硝酸-氢氟酸-高氯酸微波消解(CEM MARS6)后样品中的镉(Cd)、铬(Cr)、铜(Cu)、镍(Ni)、铅(Pb) 和锌(Zn) 含量,其检出限分别为0.03、2.00、0.70、2.00、1.00 mg·kg−1和5.00 mg·kg−1;采用原子荧光光度计(AFS-930d)测定经硝酸-盐酸消解后样品中的砷(As)和汞( Hg)含量,其检出限分别为0.20 mg·kg−1和0.002 mg·kg−1. 样品测试环节均进行空白实验与平行样测定,结果精密度符合实验允许误差要求,同时用国家标准土样予以质量管控,所测元素的加标回收率为95%—106%,分析误差为±5%.
1.3 评价标准
由于农田研究区土壤pH值范围为6.00—8.18,平均值为7.85,中位数为7.75,其中pH > 7.5占比达93.94%,且变异系数小于0.1. 故选用《土壤环境质量农用地土壤污染风险管控标准》(GB15618-2018)中pH>7.5的风险筛选值(mg·kg−1)和风险管制值(mg·kg−1)分别作为农田土壤的一级和二级评价标准. 《土壤环境质量建设用地土壤污染风险管控标准》(GB36600-2018)中第二类用地的风险筛选值(mg·kg−1)和风险管制值(mg·kg−1)分别作为尾矿库土壤的一级和二级评价标准,如表1所示.
表 1 土壤环境质量评价标准Table 1. Soil environmental quality evaluation criteria指标Index 风险筛选值Risk filter value 风险管制值Risk control value 尾矿库(第二类用地) 农田(pH>7.5) 尾矿库(第二类用地) 农田(pH>7.5) Cu 18000 100 36000 − Cr − 250 − 1300 Ni 900 190 2000 − Zn − 300 − − As 60 25 140 100 Cd 65 0.60 172 4.0 Pb 800 170 2500 1000 Hg 38 3.40 82 6.0 注:“−”表示无此项,下同. Note: "−" means that there is no such item, the same below. 1.4 污染负荷指数法
污染负荷指数法在土壤重金属污染水平评估中应用广泛,既能考量单个样点污染状况,又可评定多重金属污染区域状况[16]. 污染负荷指数法的计算如下式(1–3):
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) 式中,
CFi Ci Bi PLIzone 表 2 污染负荷指数分级标准Table 2. Classification standard of the pollution load indexCF PLI 等级Level 污染程度Contamination degrees CF<1 PLI<1 0 无污染 1≤CF<3 1≤PLI<3 1 轻度污染 3≤CF<6 3≤PLI<6 2 中度污染 6≤CF 6≤PLI 3 重度污染 1.5 PMF源解析
PMF模型是将重金属含量矩阵分解为因子得分矩阵、因子载荷矩阵和残差矩阵[19]. 见式(4–7):
stringUtils.convertMath(!{formula.content}) (4) 式中,
Xij Gjk Fki Eij stringUtils.convertMath(!{formula.content}) (5) 式中,
Uij Uij stringUtils.convertMath(!{formula.content}) (6) 当土壤重金属含量高于MDL时,则由式(6)计算得出:
stringUtils.convertMath(!{formula.content}) (7) 式中,
c δ 1.6 基于源导向的土壤重金属生态风险评估
本研究将PMF模型与综合生态风险评价指数法(Nemerow integrated risk index,NIRI)有机结合,来量化各污染源对生态风险的贡献[20]. 具体计算公式如下(8–12):
stringUtils.convertMath(!{formula.content}) (8) 式中,
Ck∗ij Ci stringUtils.convertMath(!{formula.content}) (9) stringUtils.convertMath(!{formula.content}) (10) 式中,
ERkij Bi Eir Tir stringUtils.convertMath(!{formula.content}) (11) stringUtils.convertMath(!{formula.content}) (12) 式中,
EIRIkij NIRIij ERkijmax Eirmax ERkijave Eirave Eir NIRI 表 3 生态风险指数分级标准Table 3. Ecological risk index classification criteriaEir 生态风险等级Ecological risk level NIRI 生态风险等级Ecological risk level Eir 轻微 NIRI<40 轻微 40≤ Eir 中等 40≤NIRI<80 中等 80≤ Eir 强 80≤NIRI<160 强 160≤ Eir 很强 160≤NIRI<320 很强 Eir 极强 NIRI≥320 极强 1.7 基于源导向的土壤重金属健康风险评估
采用美国环保署(USEPA)推荐的健康风险评估模型,量化土壤重金属对人体健康造成的风险[23]. 土壤重金属健康风险评估模型中暴露参数和不同暴露途径的参考剂量(RfD)和致癌斜率因子(SF)值参照中国生态环境部发布的HJ 25.3-2019中的风险评估模型参数推荐值和美国环保署发布的相关参数[24 − 26]. 对于致癌风险,致癌风险值低于10−6时,表示对人体健康风险不显著,视为风险可以忽略不计;致癌风险值为10−6—10−4时,表示对人体健康有风险;致癌风险值大于10−4时,表示有显著风险,认为风险是不可接受的[27]. 基于源导向的土壤重金属风险评估是先根据PMF模型得到不同源的贡献率,并结合研究区健康风险评估结果,计算不同源对人体健康风险的贡献率[28],具体计算公式如下(13–16):
stringUtils.convertMath(!{formula.content}) (13) stringUtils.convertMath(!{formula.content}) (14) stringUtils.convertMath(!{formula.content}) (15) stringUtils.convertMath(!{formula.content}) (16) 式中,
HQj Fij HQi Dj,HQ CRj CRi Dj,CR 1.8 数据处理与分析
研究数据处理采用SPSS 27和EXCEL,进行描述性统计分析,利用EPA PMF 5.0软件进行溯源解析,使用Origin 2024和ArcGIS软件处理数据并绘制图形.
2. 结果与讨论(Results and discussion)
2.1 土壤重金属污染评价
土壤中As、Cd、Cr、Cu、Ni、Pb、Zn和Hg元素含量描述性统计结果如表4所示,研究区尾矿库土壤中As、Cd、Cr、Cu、Ni、Pb、Zn和Hg含量范围分别为21.64—43.69、0.25—1.04、11.23—16.95、26.07—51.85、7.13—14.61、653.99—925.67、412.96—603.57 mg·kg−1和7.65—9.45 mg·kg−1,其中只有Pb有50.00%的样点超出一级标准. 研究区农田土壤中As、Cd、Cr、Cu、Ni、Pb、Zn和Hg的含量范围分别为10.20—26.85、1.35—3.30、68.76—134.98、12.64—23.60、11.44—29.20、39.12—92.61、66.65—139.35 mg·kg−1和2.48—4.33 mg·kg−1. 根据评价标准(表1),农田土壤中Cd所有样点的含量均超过一级标准,As有3.03%的样点超过了一级标准,Hg有39.39%的样点超过了一级标准,Cr、Cu、Ni、Pb和Zn含量均未超过一级标准. 尾矿库和农田土壤中所有重金属含量均未超过二级标准. 污染负荷指数法结果(图2)表明,农田土壤中8种重金属的
CFi PLI — CFi PLI 1000 万亩[29],农业活动频繁,结合PMF的结果,说明这2种重金属污染与农业活动和成土母质有关. 这与李多杰等[30]对内蒙古兴安盟某铅锌矿的研究结果相似,矿区周围的土壤受到明显的Zn和Pb污染,而铅锌矿周边土壤中的Cd和Cr污染归因于成土母岩风化和人为活动的共同作用.表 4 土壤重金属含量描述性统计分析Table 4. Descriptive statistical analysis of soil heavy metal content项目Item 采样地Sampling site 最小值/( mg·kg−1)Minimum 最大值/( mg·kg−1)Maximum 平均值/( mg·kg−1)Average value 标准差Standard deviation 变异系数Standard deviation 超标率Excess ratio 以一级标准为评价标准 以二级标准为评价标准 As 尾矿库 21.64 43.69 32.04 7.26 22.66% 0% 0% 农田 10.20 26.85 16.04 2.86 17.83% 3.03% 0% Cd 尾矿库 0.25 1.04 0.67 0.25 37.31% 0% 0% 农田 1.35 3.30 2.25 0.41 18.22% 100% 0% Cr 尾矿库 11.23 16.95 14.57 2.38 16.33% − − 农田 68.76 134.98 98.66 18.37 18.62% 0% 0% Cu 尾矿库 26.07 51.85 43.70 10.23 23.41% 0% 0% 农田 12.64 23.60 18.17 1.98 10.89% 0% − Ni 尾矿库 7.13 14.61 10.19 2.48 24.34% 0% 0% 农田 11.44 29.20 20.04 4.61 23.01% 0% − Pb 尾矿库 653.99 925.67 795.70 101.05 12.70% 50% 0% 农田 39.12 92.61 56.68 12.93 22.81% 0% 0% Zn 尾矿库 412.96 603.57 508.20 60.45 11.89% − − 农田 66.65 139.35 90.25 17.36 19.23% 0% − Hg 尾矿库 7.65 9.45 8.28 0.60 7.24% 0% 0% 农田 2.48 4.33 3.37 0.50 14.84% 39.39% 0% pH 尾矿库 8.26 8.58 8.42 0.16 1.90% − − 农田 6.00 8.18 7.85 0.75 9.59% − − 变异系数(coefficient of variance,CV)是一种反映重金属元素含量空间分布离散程度的指标[31],根据CV值的大小,可分为低度变异(CV<0.1)、中度变异(0.1≤CV<0.36)、高度变异(0.36≤CV<1)和极度变异(CV>1)[32]. 由表4可知,尾矿库研究区域土壤中Cd属于高度变异;As、Cu、Ni、Cr、Pb和Zn属于中度变异;Hg属于低度变异. 农田研究区域表层土壤中重金属的变异系数均在0.1—0.36之间,均属于中度变异. 因此,研究区域土壤中重金属含量的空间分布差异显著,地理环境和人类活动对其影响较大.
2.2 农田土壤重金属污染源解析
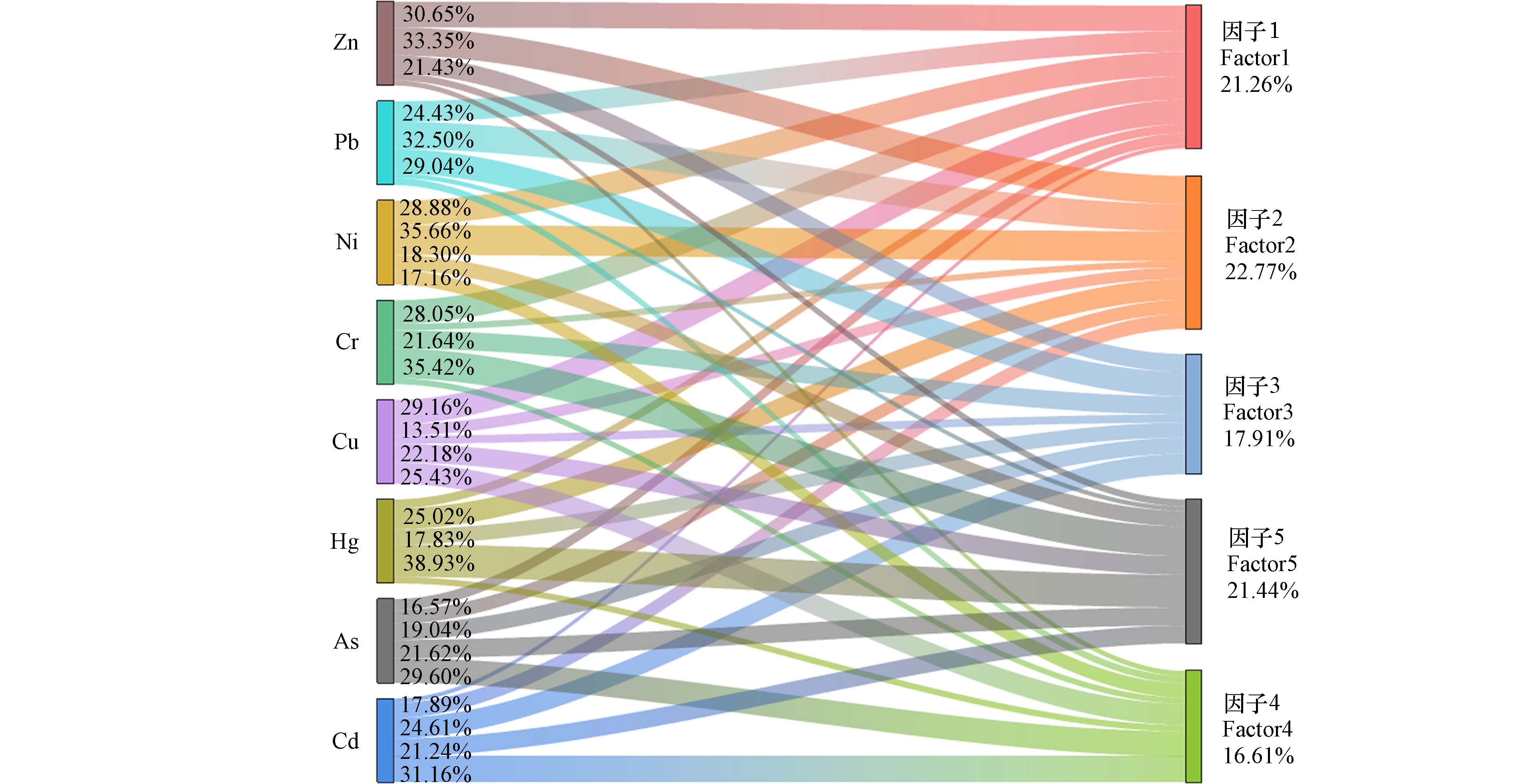
本研究设定4至7个因子数量,经多番运行调试,最终选定5个因子,获取了较低的Q值 42.43,此时实测值与预测值拟合效果最好,且多数残差处于−3—3区间. 8 种元素的R2系数为0.545—0.951,表明PMF的整体拟合效果较好,能够很好地满足研究需求,并解释了原始数据中包含的信息. PMF模型的解析结果如图3所示,5个污染源的占比分别为 21.26%、22.77%、17.91%、16.61%和 21.44%.
由图3可知,因子1主要载荷元素包括Cr、Cu、Ni、Pb和Zn,其贡献率分别为28.05%、29.16%、28.88%、24.43%和30.65%. 据报道,Ni和Cr含量的变化与成土母质、成土过程和地质活动密切相关[33]. 农田土壤中Cr、Cu和Ni均未超过一级评价标准,且含量较低,这表明Cr、Cu和Ni主要是受成土母质的影响,属于自然源. 而农田土壤中Pb和Zn的含量较高,且有研究表明铅锌矿影响区土壤中Pb、 Zn和Cu主要来自于矿山开采活动[34],故污染主要来源于周边的铅锌尾矿库. 故因子1是由自然源和尾矿库源的混合源.
因子2主要载荷元素包括Ni、Pb、Zn和Hg,其贡献率分别为35.66%、32.50%、33.35%和25.02%,变异系数分别为0.23、0.23、0.19和0.15,均属于中度变异,受人类活动影响较大. 有研究表明,铅锌矿区在开采和冶炼活动中所产生的三废中含有大量的Zn、Pb、Cd、As和Hg等元素[7],排放后通过大气降尘进入周围土壤,导致土壤污染. 经调查,农田研究区右侧是废弃工厂,工矿活动及矿渣堆存期间的扬尘会通过大气沉降在土壤中积累,受降雨等影响导致Ni、Pb、Zn和Hg的二次富集. 故因子2是工业降尘源.
因子3 主要载荷元素包括Pb、Cd、Zn和Cr,其贡献率分别为29.04%、24.61%、21.43%和21.64%. 汽车发动机、镀锌构件及轮胎等因机械摩擦、化学侵蚀引发的磨损与腐蚀,以及燃油燃烧或泄露和尾气排放等交通活动,都会将Cd、Pb和Zn元素释放到周边环境中[35,36]. 由因子1可知,Cr含量变化与成土过程显著相关,但研究区Cr的超标率96.97%,表明受到了人类活动的影响. 研究发现Cr还与金属零件和镀铬配件磨损有关[37]. 考虑研究区农田周围有交通运输道路及汽修厂,故因子3是交通源.
因子4主要载荷元素包括As、Cd 和Cu,其贡献率分别为29.60%、31.16%和25.43%,变异系数分别0.18、0.18和0.11,属于中度变异,受人类活动影响较大. 除草剂和杀虫剂的大量使用会造成As的大量积累,磷肥中含有的微量As也是土壤As的重要来源之一[38]. 农家肥中含有较多的Cu、Cd元素,农药、化肥长期投入到农用地中会导致重金属Cu、Cd元素的大量积累[39]. 故因子4是农业源.
因子5主要载荷元素包括As、Cd、Cr、Cu、Ni和Hg,其贡献率分别为21.62%、21.24%、35.42%、22.18%、18.30%和38.93%. Cd污染的主要来源包括汽油燃烧、农药和化肥、垃圾堆积、铅锌矿开采和冶炼等[40]. Cr、Cu、Ni主要受成土母质的影响,同时也有研究表明,农业投入品 (化肥、农药、有机肥等)中含有的Hg、As、Cu、Zn、Cd、Cr等元素易残留在土壤中,如磷肥中Cr含量在几十到几百mg·kg−1[8],As含量在20到50 mg·kg−1,施用不合理时,土壤中Cr、As这类元素含量会升高[39, 41]. 长期的尾矿库堆存、物料转运及加工等人为活动也会向周围环境释放Cd、Hg、As等元素[42],故因子5是由多种类型的活动组成的综合源.
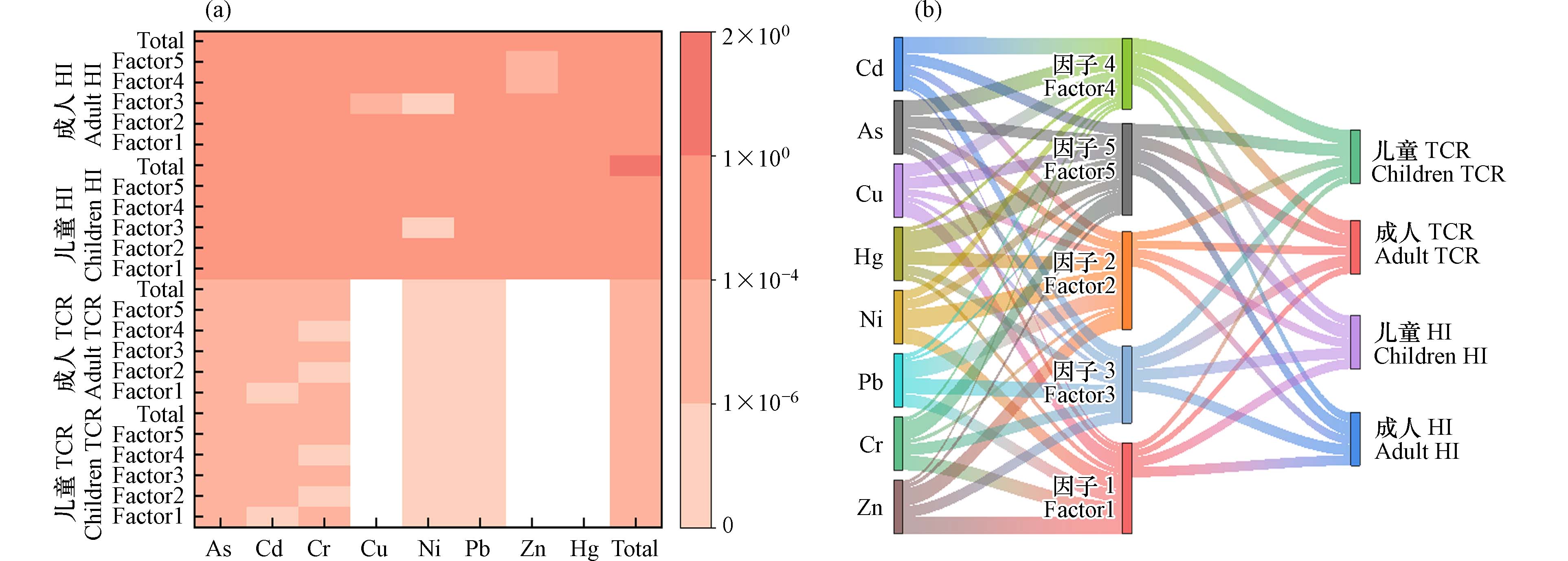
2.3 农田土壤重金属特定源-综合生态风险评估
研究区农田土壤中重金属的
Eir NIRI Eir 2243.04 )>Cd(582.06)>Pb(15.08)>As(12.73)>Cu(3.77)>Ni(2.85)>Cr(2.82)>Zn(1.30). 除了属于极强生态风险的Hg和Cd元素之外,其余元素均属于轻微生态风险,说明造成研究区生态风险的首要污染元素为Hg和Cd. 这与陈希瑶等[43]人研究中国土壤重金属的生态风险结果一致,对土壤造成生态风险的主要元素是Hg和Cd. 此外,研究区农田土壤重金属的综合生态风险指数(NIRI 1673.76 —2921.86 ,平均值为2271.48 ,属于极强生态风险.表 5 农田土壤重金属潜在生态危害系数(Eir Table 5. Evaluation results of potential ecological hazard coefficients (Eir As Cd Cr Cu Ni Pb Zn Hg Eir 8.10 348.44 1.96 2.62 1.62 10.40 0.96 1651.74 Eir 21.31 853.45 3.86 4.90 4.15 24.63 2.01 2883.33 Eir 12.73 582.06 2.82 3.77 2.85 15.08 1.30 2243.04 NIRI 1673.76 — 2921.86 研究区特定源-综合生态风险的评价结果如图4所示,按其贡献率的高低对5 种污染源进行排序:因子5(综合源)(38.37%)> 因子2(工业降尘源)(24.67%)> 因子3(交通源)(17.62%)> 因子1(自然源和尾矿库源的混合源)(10.47%)> 因子4(农业源)(8.86%),表明综合源对农田研究区影响的贡献率最大. 由于Hg元素自身毒性很高,且在研究区土壤中,Hg为首要的生态危害元素,在因子5中贡献率达到了38.93%,导致因子5是对综合生态风险贡献最高的污染源. 本研究中对综合生态风险贡献最高的污染源与以工业降尘源为主要污染源的PMF源解析结果不一致. 这与李军等[21]对敦煌市主城区的土壤重金属的研究结果一致,Hg元素负荷高的工业降尘源虽然不是重金属的最高贡献源,但却是综合生态风险的最高贡献源. 这表明重金属贡献率高的污染源并不一定具有高生态风险.
2.4 农田土壤重金属特定源-人体健康风险评估
图5a 显示了针对特定来源的人体健康风险评估模型的结果. 对于非致癌风险,不同污染源对儿童总
HI HI HI HI HI TCR TCR 对于研究区农田土壤重金属、污染源与健康风险关系如图5b所示,就致癌风险而言,因子4(农业源)是导致成人和儿童致癌风险的首要污染源,贡献率分别为25.87%和26.92%. 其次是因子5(综合源),对成人的贡献率是23.77%,对儿童的贡献率是23.12%,污染因子以As为主,对成人的贡献率是51.79%,对儿童的贡献率是54.70%;对于非致癌风险而言,污染源贡献率的排列顺序:因子5(综合源)> 因子3(交通源)> 因子1(自然源和尾矿库源的混合源)> 因子4(农业源)> 因子2(工业降尘源). 因子5(综合源)是导致儿童非致癌风险的首要污染源,贡献率为25.17%,最主要的污染因子也是As元素,贡献率为43.57%. 这可能与As元素本身毒性有关,其有较低的RfD和较高的SF [46]. 这一结果不仅与杨杰等[47]研究鄂西某铜铅锌尾矿库周边农田土壤重金属风险评估的结果一致,还与张丽瑞等[48]研究甘肃省农田土壤潜在有毒元素污染的典型区域白阴市东大沟的健康风险评价结果一致,As元素是造成当地人群致癌风险影响的主要因子. 考虑到农业源和多种类型活动共同作用的综合源是造成研究区人体健康风险的主要因子,因而工业和农业等人类活动排放的重金属对人体存在健康风险,未来可通过提升工业废物排放标准,建立合适的管控机制以及减少农药和化肥用量等途径减少污染物进入环境的量,从而减少重金属对人体的危害 [45].
3. 结论(Conclusion)
1)研究区尾矿库和农田土壤中重金属含量均低于国家风险管制值,但尾矿库土壤中Pb有50.00%的样点超出国家风险筛选值,农田土壤中Cd、As和Hg分别有100%、3.03%和39.39%的样点超出国家风险筛选值. 总体而言,尾矿库土壤中重金属的污染程度较农田土壤明显偏高,其中尾矿库土壤以Hg、Pb、Zn和Cd污染为主,整体上属于中度污染水平,占比为100%;农田土壤以Hg和Cd污染为主,整体上属于轻度污染水平,占比为93.94%.
2)研究区农田土壤重金属分别受自然源及尾矿库源的混合源(贡献率为21.26%)、工业降尘源(贡献率为22.77%)、交通源(贡献率为17.91%)、农业源(贡献率为16.61%)和由多种类型活动组成的综合源(贡献率为 21.44%)的共同影响.
3)综合源对研究区生态风险贡献率为38.37%,为优先控制污染源,Hg为生态风险优先控制污染元素;农田重金属对成人不构成非致癌健康风险,但综合源是引起当地儿童非致癌风险的优先控制污染源,贡献率为25.17%,农业源是引起当地成人和儿童致癌风险的优先控制污染源,贡献率分别为25.87%和26.92%,健康风险的优先控制污染元素均为As.
-
表 1 我国与其他国家地表水中大环内酯类抗生素的浓度水平对比
Table 1. Concentration difference of macrolide antibiotics in typical surface waters between China and other countries
地表水体Surface waters 采样季节Sampling seasons 水体/(ng·L−1)Water 沉积物/(ng·g−1)Sediment 参考文献References 安徽宛溪河 2018秋 1—147.1(均值35.73) — [8] 长江嘉陵江重庆段 2018春、秋 ND—1409(均值75.003) ND—866.78(均值39.758) [24] 山东莱州湾小清河 2015春、秋、冬,2017春 ND—223(均值81.55) — [25] 长江下游 2018秋 ND—30(均值1.79) ND—6780(均值400) [26] 北京潮白河 2017冬 <782(均值36.017) <8.96(均值1.317) [27] 东海沿海水域 2018春 2.9—77(均值33.6) 0.6—60.3(均值5.2) [28] 北部湾 2015秋 <0.41—40.7(均值2.111) <0.013—1.34(均值0.198) [29] 太湖 2010春 ND—624.8(均值79.9) ND—120.3(均值44.6) [23] 西班牙东部地中海 2018夏、秋,2019冬 <5—1617(均值41.4) — [30] 韩国荣山江 2016春、夏、秋 6.2—475.1(均值42.7) — [31] 法国阿杜尔河口 — <0.06—<8.1 — [32] 黎巴嫩河流 2016春 <23—2806 — [33] 伊比利亚河 2010秋,2011秋 0.09—153.72(均值2.172) 1.13—23.92(均值12.543) [34] 美国东南部河流 2009冬季,2010春、夏、秋、冬 0.2—2.7(中值1.4) — [35] 南非Umgeni河 2013冬、春 0.21—22.57(中值5.65) — [36] 美国迈阿密河 — 14.7—356 — [37] ND:未检出,not detected;—没有数据,data unavailable. 表 2 不同水溶液中大环内酯类抗生素(MLs)在254 nm、350 nm或(模拟)日光下的摩尔吸光系数(ε)、量子产率(Φ)及反应速率常数(k)
Table 2. Molar absorption coefficients(ε), quantum yields(Φ) and reaction rate constants(k) of the typical macrolide antibiotics in different water solutions measured at 254 nm, 350nm or (simulated) sunlight
化合物Compounds 溶液条件Condition Φ/(mol·Einstein−1) k/min−1 参考文献References 254 nm 350 nm 模拟日光Simulated sunlight 254 nm 350 nm 日光Sunlight 罗红霉素 纯水 1.2 × 10−3 2.0 × 10−4 — 1.7 × 10−3 1.2 × 10−4 9.5× 10−5 [85] 淡水 4.9 × 10−2 7.0 × 10−4 — 1.7 × 10−2 3.9× 10−4 2.0× 10−4 海水 1.1 × 10−2 4.0 × 10−4 — 3.0 × 10−2 2.4× 10−4 3.5× 10−4 红霉素 纯水 3.0 × 10−4 2.0 × 10−4 — 3.6 × 10−4 1.1× 10−4 2.1× 10−4 [85] 淡水 3.0 × 10−3 4.0 × 10−4 — 4.5 × 10−3 3.9× 10−4 1.7× 10−4 海水 4.5 × 10−3 7.0 × 10−4 — 2.2 × 10−3 1.5× 10−4 4.8× 10−5 阿奇霉素二水合物A pH 3 1.2 — — 4.7 × 10−1 — — [88] pH 7 1.0 — — 3.9 × 10−1 — — pH 9 4.9 × 10−1 — — 1.9 × 10−1 — — 阿奇霉素二水合物B pH 3 1.8 — — 6.9 × 10−1 — — pH 7 8.1 × 10−1 — — 3.1 × 10−1 — — pH 9 7.3 × 10−1 — — 2.8 × 10−1 — — 游离红霉素A pH 3 9.0 × 10−2 — — 1.0 × 10−1 — — pH 7 5.5 × 10−1 — — 5.9 × 10−1 — — pH 9 1.9 × 10−1 — — 2.1 × 10−1 — — 游离红霉素B pH 3 5.0 × 10−2 — — 5.0 × 10−2 — — pH 7 6.1 × 10−1 — — 6.6 × 10−1 — — pH 9 2.0 × 10−1 — — 2.2 × 10−1 — — 酒石酸泰乐菌素A pH 3 4.0 × 10−2 — — 2.5 — — pH 7 4.0 × 10−2 — — 2.5 — — pH 9 2.0 × 10−2 — — 1.2 — — 酒石酸泰乐菌素B pH 3 2.0 × 10−2 — — 1.4 — — pH 7 3.0 × 10−2 — — 1.7 — — pH 9 1.0 × 10−2 — — 6.7 × 10−1 — — 螺旋霉素 纯水 — — 1.4 × 10−2 3.6 × 10−5 — — [84] 红霉素 纯水 — — 3.5 × 10−3 8.3 × 10−5 — — 克拉霉素 纯水 — — 6.3 × 10−3 1.3 × 10−4 — — 泰乐菌素 纯水 — — 7.6 × 10−3 4.8 × 10−5 — — 红霉素 纯水 — — 5.4 × 10−4 — — — [9] 克拉霉素 纯水 — — 5.6 × 10−5 — — — —没有数据,data unavailable. 表 3 水环境因子对大环内酯类抗生素光降解动力学的影响
Table 3. Effects of aqueous environmental factors on photodegradation kinetics of macrolide antibiotics
水环境因子Factors 化合物Compounds 光源、溶液条件Light, solution condition 对光解影响Effect 参考文献References 天然有机物 罗红霉素、克拉霉素、阿奇霉素、红霉素 1700 W氙灯(λ > 290 nm);pH 7 促进 [9] 罗红霉素 500 W中压汞灯(λ > 290 nm);pH 6、pH 7和pH 8 促进 [15] 罗红霉素 500 W中压汞灯 (λ > 290 nm) 促进 [90] 腐殖酸 罗红霉素 500 W中压汞灯(λ > 290 nm);pH 6、pH 7和pH 8 促进 [15] 罗红霉素 500 W中压汞灯(λ > 290 nm) 促进 [90] 阿奇霉素 500 W氙灯(λ > 290 nm) ;pH 7.3 促进 [77] 罗红霉素、克拉霉素、阿奇霉素、红霉素 太阳光;pH 7 促进 [101] 泰乐菌素 500 W高压汞灯(主波长365 nm) 抑制 [102] 螺旋霉素 250 W高压汞灯 抑制 [103] 罗红霉素、螺旋霉素 250 W高压汞灯(λ > 200 nm) 抑制 [84] 克拉霉素、泰乐菌素 低浓度促进,高浓度抑制 克拉霉素、螺旋霉素 1000 W高压汞灯(λ > 290 nm) 抑制 罗红霉素、泰乐菌素 低浓度促进,高浓度抑制 富里酸 罗红霉素 500 W中压汞灯(λ > 290 nm);pH 6、pH 7和pH 8 促进 [15] NO3− 螺旋霉素 250 W高压汞灯 促进 [103] 阿奇霉素 500 W氙灯(λ > 290 nm) ;pH 6.6 促进 [77] 螺旋霉素、泰乐菌素 250 W高压汞灯(λ > 200 nm) 促进 [84] 罗红霉素 抑制 克拉霉素 低浓度抑制,高浓度促进 罗红霉素、螺旋霉素、泰乐菌素 1000 W高压汞灯(λ > 290 nm) 促进 克拉霉素 低浓度抑制,高浓度促进 罗红霉素 500 W中压汞灯;UV/H2O2;pH 7 抑制 [3] 泰乐菌素 500 W高压汞灯(主波长365 nm) 抑制 [102] NO2− 螺旋霉素、泰乐菌素 250 W高压汞灯(λ > 200 nm) 促进 [84] 罗红霉素 抑制 克拉霉素 低浓度促进,高浓度抑制 罗红霉素、克拉霉素、螺旋霉素、泰乐菌素 1000 W高压汞灯(λ > 290 nm) 抑制 罗红霉素 500 W中压汞灯,UV/H2O2;pH 7 抑制 [3] 螺旋霉素 250 W高压汞灯 抑制 [103] Fe(Ⅲ) 罗红霉素、克拉霉素 中压汞灯(λ > 290 nm) 促进 [78] 螺旋霉素 250 W高压汞灯(λ > 200 nm) 促进 [84] 罗红霉素、克拉霉素、泰乐菌素 抑制 克拉霉素 1000 W高压汞灯(λ > 290 nm) 促进 罗红霉素 抑制 螺旋霉素 低浓度促进,高浓度抑制 泰乐菌素 低浓度抑制,高浓度促进 罗红霉素 500 W中压汞灯,UV/H2O2;pH 7 抑制 [3] Fe(Ⅱ) 克拉霉素 1000 W高压汞灯(λ > 290 nm) 促进 [84] 罗红霉素、螺旋霉素 抑制 泰乐菌素 低浓度抑制,高浓度促进 克拉霉素、泰乐菌素 250 W高压汞灯(λ > 200 nm) 抑制 螺旋霉素 低浓度促进,高浓度抑制 罗红霉素 低浓度抑制,高浓度促进 Cu2+、Mg2+、Cl−、HCO3− 罗红霉素 500 W中压汞灯;UV/H2O2;pH 7 抑制 [3] Ca2+、SO42− 罗红霉素 500 W中压汞灯;UV/H2O2;pH 7 无显著影响 [3] -
[1] SCHLÜSENER M P, BESTER K, SPITELLER M. Determination of antibiotics such as macrolides, ionophores and tiamulin in liquid manure by HPLC-MS/MS [J]. Analytical and Bioanalytical Chemistry, 2003, 375(7): 942-947. doi: 10.1007/s00216-003-1838-9 [2] SENTA I, KRIZMAN-MATASIC I, TERZIC S, et al. Comprehensive determination of macrolide antibiotics, their synthesis intermediates and transformation products in wastewater effluents and ambient waters by liquid chromatography-tandem mass spectrometry [J]. Journal of Chromatography. A, 2017, 1509: 60-68. doi: 10.1016/j.chroma.2017.06.005 [3] LI W, XU X J, LYU B L, et al. Degradation of typical macrolide antibiotic roxithromycin by hydroxyl radical: Kinetics, products, and toxicity assessment [J]. Environmental Science and Pollution Research International, 2019, 26(14): 14570-14582. doi: 10.1007/s11356-019-04713-1 [4] 葛林科, 张思玉, 谢晴, 等. 抗生素在水环境中的光化学行为 [J]. 中国科学:化学, 2010, 40(2): 124-135. doi: 10.1360/zb2010-40-2-124 GE L K, ZHANG S Y, XIE Q, et al. Progress in studies on aqueous environmental photochemical behavior of antibiotics [J]. Scientia Sinica Chimica), 2010, 40(2): 124-135(in Chinese). doi: 10.1360/zb2010-40-2-124
[5] JIANG X S, ZHU Y Q, LIU L Q, et al. Occurrence and variations of pharmaceuticals and personal-care products in rural water bodies: A case study of the Taige Canal (2018-2019) [J]. Science of the Total Environment, 2021, 762: 143138. doi: 10.1016/j.scitotenv.2020.143138 [6] YANG L, WANG T Y, ZHOU Y Q, et al. Contamination, source and potential risks of pharmaceuticals and personal products (PPCPs) in Baiyangdian Basin, an intensive human intervention area, China [J]. Science of the Total Environment, 2021, 760: 144080. doi: 10.1016/j.scitotenv.2020.144080 [7] ZHAO B, XU J M, ZHANG G D, et al. Occurrence of antibiotics and antibiotic resistance genes in the Fuxian Lake and antibiotic source analysis based on principal component analysis-multiple linear regression model [J]. Chemosphere, 2021, 262: 127741. doi: 10.1016/j.chemosphere.2020.127741 [8] DING Y, CUI K P, LV K, et al. Revealing the hydrological transport and attenuation of 14 antibiotics in a low-flow stream [J]. Science of the Total Environment, 2021, 761: 143288. doi: 10.1016/j.scitotenv.2020.143288 [9] JIA X, LIAN L S, YAN S W, et al. Comprehensive understanding of the phototransformation process of macrolide antibiotics in simulated natural waters [J]. ACS ES& T Water, 2021, 1(4): 938-948. [10] SHAH S Q A, COLQUHOUN D J, NIKULI H L, et al. Prevalence of antibiotic resistance genes in the bacterial flora of integrated fish farming environments of Pakistan and Tanzania [J]. Environmental Science & Technology, 2012, 46(16): 8672-8679. [11] SENTA I, KOSTANJEVECKI P, KRIZMAN-MATASIC I, et al. Occurrence and behavior of macrolide antibiotics in municipal wastewater treatment: Possible importance of metabolites, synthesis byproducts, and transformation products [J]. Environmental Science & Technology, 2019, 53(13): 7463-7472. [12] BOREEN A L, ARNOLD W A, MCNEILL K. Photodegradation of pharmaceuticals in the aquatic environment: A review [J]. Aquatic Sciences, 2003, 65(4): 320-341. doi: 10.1007/s00027-003-0672-7 [13] EDHLUND B L, ARNOLD W A, MCNEILL K. Aquatic photochemistry of nitrofuran antibiotics [J]. Environmental Science & Technology, 2006, 40(17): 5422-5427. [14] KNAPP C W, CARDOZA L A, HAWES J N, et al. Fate and effects of enrofloxacin in aquatic systems under different light conditions [J]. Environmental Science & Technology, 2005, 39(23): 9140-9146. [15] 吕宝玲, 李威, 于筱莉, 等. 溶解性有机质对罗红霉素光降解的影响研究 [J]. 环境科学学报, 2019, 39(3): 747-754. LÜ B L, LI W, YU X L, et al. Effect of dissolved organic matter on the photodegradation of roxithromycin [J]. Acta Scientiae Circumstantiae, 2019, 39(3): 747-754(in Chinese).
[16] LATCH D E, PACKER J L, STENDER B L, et al. Aqueous photochemistry of triclosan: Formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products [J]. Environmental Toxicology and Chemistry, 2005, 24(3): 517-525. doi: 10.1897/04-243R.1 [17] JIAO S J, ZHENG S R, YIN D Q, et al. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria [J]. Chemosphere, 2008, 73(3): 377-382. doi: 10.1016/j.chemosphere.2008.05.042 [18] JUNG J, KIM Y, KIM J, et al. Environmental levels of ultraviolet light potentiate the toxicity of sulfonamide antibiotics in Daphnia magna [J]. Ecotoxicology (London, England), 2008, 17(1): 37-45. doi: 10.1007/s10646-007-0174-9 [19] 葛林科, 任红蕾, 鲁建江, 等. 我国环境中新兴污染物抗生素及其抗性基因的分布特征 [J]. 环境化学, 2015, 34(5): 875-883. doi: 10.7524/j.issn.0254-6108.2015.05.2014082501 GE L K, REN H L, LU J J, et al. Occurrence of antibiotics and corresponding resistance genes in the environment of China [J]. Environmental Chemistry, 2015, 34(5): 875-883(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.05.2014082501
[20] LIU X H, LU S Y, GUO W, et al. Antibiotics in the aquatic environments: A review of lakes, China [J]. Science of the Total Environment, 2018, 627: 1195-1208. doi: 10.1016/j.scitotenv.2018.01.271 [21] ZHANG Q Q, YING G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. [22] LI Z, LI M, ZHANG Z Y, et al. Antibiotics in aquatic environments of China: A review and meta-analysis [J]. Ecotoxicology and Environmental Safety, 2020, 199: 110668. doi: 10.1016/j.ecoenv.2020.110668 [23] XU J, ZHANG Y, ZHOU C B, et al. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China [J]. Science of the Total Environment, 2014, 497/498: 267-273. doi: 10.1016/j.scitotenv.2014.07.114 [24] WANG G G, ZHOU S H, HAN X K, et al. Occurrence, distribution, and source track of antibiotics and antibiotic resistance genes in the main rivers of Chongqing City, southwest China [J]. Journal of Hazardous Materials, 2020, 389: 122110. doi: 10.1016/j.jhazmat.2020.122110 [25] LI J, CUI M, ZHANG H. Spatial and temporal variations of antibiotics in a tidal river [J]. Environmental Monitoring and Assessment, 2020, 192(6): 336. doi: 10.1007/s10661-020-08313-2 [26] ZHANG G D, LU S Y, WANG Y Q, et al. Occurrence of antibiotics and antibiotic resistance genes and their correlations in Lower Yangtze River, China [J]. Environmental Pollution, 2020, 257: 113365. doi: 10.1016/j.envpol.2019.113365 [27] ZHANG Y X, CHEN H Y, JING L J, et al. Ecotoxicological risk assessment and source apportionment of antibiotics in the waters and sediments of a peri-urban river [J]. Science of the Total Environment, 2020, 731: 139128. doi: 10.1016/j.scitotenv.2020.139128 [28] LI F F, CHEN L J, CHEN W D, et al. Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening [J]. Marine Pollution Bulletin, 2020, 151: 110810. doi: 10.1016/j.marpolbul.2019.110810 [29] ZHANG R L, PEI J Y, ZHANG R J, et al. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood [J]. Ecotoxicology and Environmental Safety, 2018, 154: 27-35. doi: 10.1016/j.ecoenv.2018.02.006 [30] FONSECA E, HERNÁNDEZ F, IBÁÑEZ M, et al. Occurrence and ecological risks of pharmaceuticals in a Mediterranean River in Eastern Spain [J]. Environment International, 2020, 144: 106004. doi: 10.1016/j.envint.2020.106004 [31] NA T W, KANG T W, LEE K H, et al. Distribution and ecological risk of pharmaceuticals in surface water of the Yeongsan River, Republic of Korea [J]. Ecotoxicology and Environmental Safety, 2019, 181: 180-186. doi: 10.1016/j.ecoenv.2019.06.004 [32] MIOSSEC C, LANCELEUR L, MONPERRUS M. Multi-residue analysis of 44 pharmaceutical compounds in environmental water samples by solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry [J]. Journal of Separation Science, 2019, 42(10): 1853-1866. doi: 10.1002/jssc.201801214 [33] MOKH S, EL KHATIB M, KOUBAR M, et al. Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: A case study natural water sources in Lebanon [J]. Science of the Total Environment, 2017, 609: 830-841. doi: 10.1016/j.scitotenv.2017.07.230 [34] OSORIO V, LARRAÑAGA A, ACEÑA J, et al. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers [J]. Science of the Total Environment, 2016, 540: 267-277. doi: 10.1016/j.scitotenv.2015.06.143 [35] PADHYE L P, YAO H, KUNG'U F T, et al. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant [J]. Water Research, 2014, 51: 266-276. doi: 10.1016/j.watres.2013.10.070 [36] AGUNBIADE F O, MOODLEY B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa [J]. Environmental Monitoring and Assessment, 2014, 186(11): 7273-7291. doi: 10.1007/s10661-014-3926-z [37] PANDITI V R, BATCHU S R, GARDINALI P R. Online solid-phase extraction-liquid chromatography-electrospray-tandem mass spectrometry determination of multiple classes of antibiotics in environmental and treated waters [J]. Analytical and Bioanalytical Chemistry, 2013, 405(18): 5953-5964. doi: 10.1007/s00216-013-6863-8 [38] LIU X, STEELE J C, MENG X Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review [J]. Environmental Pollution, 2017, 223: 161-169. doi: 10.1016/j.envpol.2017.01.003 [39] CHEN H, LIU S, XU X R, et al. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure [J]. Marine Pollution Bulletin, 2015, 90(1/2): 181-187. [40] WANG W X, GU X H, ZHOU L J, et al. Antibiotics in crab ponds of lake Guchenghu Basin, China: Occurrence, temporal variations, and ecological risks [J]. International Journal of Environmental Research and Public Health, 2018, 15(3): 548. doi: 10.3390/ijerph15030548 [41] HUANG F Y, AN Z Y, MORAN M J, et al. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009−2019) [J]. Journal of Hazardous Materials, 2020, 399: 122813. doi: 10.1016/j.jhazmat.2020.122813 [42] 刘鹏霄, 王旭, 冯玲. 自然水环境中抗生素的污染现状、来源及危害研究进展 [J]. 环境工程, 2020, 38(5): 36-42. LIU P X, WANG X, FENG L. Occurrences, resources and risk of antibiotics in aquatic environment: A review [J]. Environmental Engineering, 2020, 38(5): 36-42(in Chinese).
[43] 李辉, 陈瑀, 封梦娟, 等. 南京市饮用水源地抗生素污染特征及风险评估 [J]. 环境科学学报, 2020, 40(4): 1269-1277. LI H, CHEN Y, FENG M J, et al. Pollution characteristics and risk assessment of antibiotics in Nanjing drinking water sources [J]. Acta Scientiae Circumstantiae, 2020, 40(4): 1269-1277(in Chinese).
[44] 廖杰, 魏晓琴, 肖燕琴, 等. 莲花水库水体中抗生素污染特征及生态风险评价 [J]. 环境科学, 2020, 41(9): 4081-4087. LIAO J, WEI X Q, XIAO Y Q, et al. Pollution characteristics and risk assessment of antibiotics in Lianhua reservoir [J]. Environmental Science, 2020, 41(9): 4081-4087(in Chinese).
[45] 陈永山, 章海波, 骆永明, 等. 苕溪流域典型断面底泥14种抗生素污染特征 [J]. 环境科学, 2011, 32(3): 667-672. CHEN Y S, ZHANG H B, LUO Y M, et al. Investigation of 14 selected antibiotics in sediments of the typical cross sections of tiaoxi river [J]. Environmental Science, 2011, 32(3): 667-672(in Chinese).
[46] ZHOU L J, YING G G, ZHAO J L, et al. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in Northern China [J]. Environmental Pollution, 2011, 159(7): 1877-1885. doi: 10.1016/j.envpol.2011.03.034 [47] YANG J F, YING G G, ZHAO J L, et al. Simultaneous determination of four classes of antibiotics in sediments of the Pearl Rivers using RRLC-MS/MS [J]. Science of the Total Environment, 2010, 408(16): 3424-3432. doi: 10.1016/j.scitotenv.2010.03.049 [48] LYU J, YANG L S, ZHANG L, et al. Antibiotics in soil and water in China-a systematic review and source analysis [J]. Environmental Pollution, 2020, 266: 115147. doi: 10.1016/j.envpol.2020.115147 [49] PAN C Y, BAO Y Y, XU B T. Seasonal variation of antibiotics in surface water of Pudong New Area of Shanghai, China and the occurrence in typical wastewater sources [J]. Chemosphere, 2020, 239: 124816. doi: 10.1016/j.chemosphere.2019.124816 [50] YAO L L, WANG Y X, TONG L, et al. Seasonal variation of antibiotics concentration in the aquatic environment: A case study at Jianghan Plain, central China [J]. Science of the Total Environment, 2015, 527/528: 56-64. doi: 10.1016/j.scitotenv.2015.04.091 [51] 刘昔, 王智, 王学雷, 等. 我国典型区域地表水环境中抗生素污染现状及其生态风险评价 [J]. 环境科学, 2019, 40(5): 2094-2100. LIU X, WANG Z, WANG X L, et al. Status of antibiotic contamination and ecological risks assessment of several typical Chinese surface-water environments [J]. Environmental Science, 2019, 40(5): 2094-2100(in Chinese).
[52] SARMAH A K, MEYER M T, BOXALL A B A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment [J]. Chemosphere, 2006, 65(5): 725-759. doi: 10.1016/j.chemosphere.2006.03.026 [53] ZHANG R J, ZHANG R L, YU K F, et al. Occurrence, sources and transport of antibiotics in the surface water of coral reef regions in the South China Sea: Potential risk to coral growth [J]. Environmental Pollution, 2018, 232: 450-457. doi: 10.1016/j.envpol.2017.09.064 [54] CHEN Y H, CUI K P, HUANG Q L, et al. Comprehensive insights into the occurrence, distribution, risk assessment and indicator screening of antibiotics in a large drinking reservoir system [J]. Science of the Total Environment, 2020, 716: 137060. doi: 10.1016/j.scitotenv.2020.137060 [55] 王娅南, 黄合田, 彭洁, 等. 贵州草海喀斯特高原湿地水环境中典型抗生素的分布特征 [J]. 环境化学, 2020, 39(4): 975-986. doi: 10.7524/j.issn.0254-6108.2019090103 WANG Y N, HUANG H T, PENG J, et al. Occurrence and distribution of typical antibiotics in the aquatic environment of the wetland Karst plateau in Guizhou [J]. Environmental Chemistry, 2020, 39(4): 975-986(in Chinese). doi: 10.7524/j.issn.0254-6108.2019090103
[56] LIU S, WANG C, WANG P F, et al. Anthropogenic disturbances on distribution and sources of pharmaceuticals and personal care products throughout the Jinsha River Basin, China [J]. Environmental Research, 2021, 198: 110449. doi: 10.1016/j.envres.2020.110449 [57] CHEN L L, LI H P, LIU Y, et al. Distribution, residue level, sources, and phase partition of antibiotics in surface sediments from the inland river: A case study of the Xiangjiang River, south-central China [J]. Environmental Science and Pollution Research International, 2020, 27(2): 2273-2286. doi: 10.1007/s11356-019-06833-0 [58] WANG Y Q, LIU Y, LU S Y, et al. Occurrence and ecological risk of pharmaceutical and personal care products in surface water of the Dongting Lake, China-during rainstorm period [J]. Environmental Science and Pollution Research International, 2019, 26(28): 28796-28807. doi: 10.1007/s11356-019-06047-4 [59] WANG W X, ZHOU L J, GU X H, et al. Occurrence and distribution of antibiotics in surface water impacted by crab culturing: A case study of Lake Guchenghu, China [J]. Environmental Science and Pollution Research International, 2018, 25(23): 22619-22628. doi: 10.1007/s11356-018-2054-7 [60] 杨俊, 王汉欣, 吴韵斐, 等. 苏州市水环境中典型抗生素污染特征及生态风险评估 [J]. 生态环境学报, 2019, 28(2): 359-368. YANG J, WANG H X, WU Y F, et al. Occurrence, distribution and risk assessment of typical antibiotics in the aquatic environment of Suzhou City [J]. Ecology and Environmental Sciences, 2019, 28(2): 359-368(in Chinese).
[61] 徐晖, 吴明红, 徐刚. 高效液相色谱-串联质谱法对水环境中12种抗生素的检测 [J]. 上海大学学报(自然科学版), 2017, 23(3): 483-490. XU H, WU M H, XU G. Determination of 12 antibiotics in aqueous environment by high performance LC-MS/MS [J]. Journal of Shanghai University (Natural Science Edition), 2017, 23(3): 483-490(in Chinese).
[62] 王大祥, 王倩倩. 淮河流域浉河区水环境中抗生素污染分布特征分析研究 [J]. 环境科学与管理, 2020, 45(3): 63-66. WANG D X, WANG Q Q. Analysis on the distribution of antibiotics pollution in the water environment of Weihe River area in Huaihe River basin [J]. Environmental Science and Management, 2020, 45(3): 63-66(in Chinese).
[63] WANG J W, WEI H, ZHOU X D, et al. Occurrence and risk assessment of antibiotics in the Xi'an section of the Weihe River, northwestern China [J]. Marine Pollution Bulletin, 2019, 146: 794-800. doi: 10.1016/j.marpolbul.2019.07.016 [64] ZHANG G D, LIU X H, LU S Y, et al. Occurrence of typical antibiotics in Nansi Lake's inflowing rivers and antibiotic source contribution to Nansi Lake based on principal component analysis-multiple linear regression model [J]. Chemosphere, 2020, 242: 125269. doi: 10.1016/j.chemosphere.2019.125269 [65] LU S, LIN C Y, LEI K, et al. Occurrence, spatiotemporal variation, and ecological risk of antibiotics in the water of the semi-enclosed urbanized Jiaozhou Bay in Eastern China [J]. Water Research, 2020, 184: 116187. doi: 10.1016/j.watres.2020.116187 [66] LEI K, ZHU Y, CHEN W, et al. Spatial and seasonal variations of antibiotics in river waters in the Haihe River Catchment in China and ecotoxicological risk assessment [J]. Environment International, 2019, 130: 104919. doi: 10.1016/j.envint.2019.104919 [67] LI N, ZHANG X B, WU W, et al. Occurrence, seasonal variation and risk assessment of antibiotics in the reservoirs in North China [J]. Chemosphere, 2014, 111: 327-335. doi: 10.1016/j.chemosphere.2014.03.129 [68] ZHANG L W, DU S Y, ZHANG X, et al. Occurrence, distribution, and ecological risk of pharmaceuticals in a seasonally ice-sealed river: From ice formation to melting [J]. Journal of Hazardous Materials, 2020, 389: 122083. doi: 10.1016/j.jhazmat.2020.122083 [69] JIA J, GUAN Y J, CHENG M Q, et al. Occurrence and distribution of antibiotics and antibiotic resistance genes in Ba River, China [J]. Science of the Total Environment, 2018, 642: 1136-1144. doi: 10.1016/j.scitotenv.2018.06.149 [70] CHEN H Y, JING L J, TENG Y G, et al. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks [J]. Science of the Total Environment, 2018, 618: 409-418. doi: 10.1016/j.scitotenv.2017.11.054 [71] DONG D M, ZHANG L W, LIU S, et al. Antibiotics in water and sediments from Liao River in Jilin Province, China: Occurrence, distribution, and risk assessment [J]. Environmental Earth Sciences, 2016, 75(16): 1-10. [72] GE L K, DONG Q Q, HALSALL C, et al. Aqueous multivariate phototransformation kinetics of dissociated tetracycline: Implications for the photochemical fate in surface waters [J]. Environmental Science and Pollution Research International, 2018, 25(16): 15726-15732. doi: 10.1007/s11356-018-1765-0 [73] GE L K, NA G S, ZHANG S Y, et al. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes [J]. Science of the Total Environment, 2015, 527/528: 12-17. doi: 10.1016/j.scitotenv.2015.04.099 [74] LI K, ZHANG P, GE L K, et al. Concentration-dependent photodegradation kinetics and hydroxyl-radical oxidation of phenicol antibiotics [J]. Chemosphere, 2014, 111: 278-282. doi: 10.1016/j.chemosphere.2014.04.052 [75] 黄宏, 李圆杏, 杨红伟. 水环境中抗生素的光降解研究进展 [J]. 环境化学, 2013, 32(7): 1335-1341. doi: 10.7524/j.issn.0254-6108.2013.07.029 HUANG H, LI Y X, YANG H W. Research progress on photodegradation of antibiotics in aqueous solution [J]. Environmental Chemistry, 2013, 32(7): 1335-1341(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.07.029
[76] WERNER J J, CHINTAPALLI M, LUNDEEN R A, et al. Environmental photochemistry of tylosin: Efficient, reversible photoisomerization to a less-active isomer, followed by photolysis [J]. Journal of Agricultural and Food Chemistry, 2007, 55(17): 7062-7068. doi: 10.1021/jf070101h [77] TONG L, EICHHORN P, PÉREZ S, et al. Photodegradation of azithromycin in various aqueous systems under simulated and natural solar radiation: Kinetics and identification of photoproducts [J]. Chemosphere, 2011, 83(3): 340-348. doi: 10.1016/j.chemosphere.2010.12.025 [78] VIONE D, FEITOSA-FELIZZOLA J, MINERO C, et al. Phototransformation of selected human-used macrolides in surface water: Kinetics, model predictions and degradation pathways [J]. Water Research, 2009, 43(7): 1959-1967. doi: 10.1016/j.watres.2009.01.027 [79] COGAN S, HAAS Y. Self-sensitized photo-oxidation of Para-indenylidene-dihydropyridine derivatives [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2008, 193(1): 25-32. doi: 10.1016/j.jphotochem.2007.06.003 [80] MARTIN N H, JEFFORD C W. Self-sensitized photo-oxygenation of 1-benzyl-3, 4-dihydroisoquinolines [J]. Helvetica Chimica Acta, 1981, 64(7): 2189-2192. doi: 10.1002/hlca.19810640725 [81] GE L K, CHEN J W, QIAO X L, et al. Light-source-dependent effects of main water constituents on photodegradation of phenicol antibiotics: Mechanism and kinetics [J]. Environmental Science & Technology, 2009, 43(9): 3101-3107. [82] GE L K, CHEN J W, WEI X X, et al. Aquatic photochemistry of fluoroquinolone antibiotics: Kinetics, pathways, and multivariate effects of main water constituents [J]. Environmental Science & Technology, 2010, 44(7): 2400-2405. [83] 葛林科. 水中溶解性物质对氯霉素类和氟喹诺酮类抗生素光降解的影响[D]. 大连: 大连理工大学, 2009. GE L K. Effects of aqueous dissolved matter on photodegradation of phenicol and fluoroquinolone antibiotics[D]. Dalian: Dalian University of Technology, 2009(in Chinese).
[84] 常海莎. 大环内酯类抗生素在水体中的光降解及毒性变化研究[D]. 石河子: 石河子大学, 2018. CHANG H S. Study on the photodegradation and change of toxicity of macrolide antibiotics in aqueous environment[D]. Shihezi: Shihezi University, 2018(in Chinese).
[85] BATCHU S R, PANDITI V R, O'SHEA K E, et al. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate [J]. Science of the Total Environment, 2014, 470/471: 299-310. doi: 10.1016/j.scitotenv.2013.09.057 [86] WANG H L, WANG M, WANG H, et al. Aqueous photochemical degradation of BDE-153 in solutions with natural dissolved organic matter [J]. Chemosphere, 2016, 155: 367-374. doi: 10.1016/j.chemosphere.2016.04.071 [87] QU S, KOLODZIEJ E P, CWIERTNY D M. Phototransformation rates and mechanisms for synthetic hormone growth promoters used in animal agriculture [J]. Environmental Science & Technology, 2012, 46(24): 13202-13211. [88] VOIGT M, JAEGER M. On the photodegradation of azithromycin, erythromycin and tylosin and their transformation products - A kinetic study [J]. Sustainable Chemistry and Pharmacy, 2017, 5: 131-140. doi: 10.1016/j.scp.2016.12.001 [89] LATCH D E, STENDER B L, PACKER J L, et al. Photochemical fate of pharmaceuticals in the environment: Cimetidine and ranitidine [J]. Environmental Science & Technology, 2003, 37(15): 3342-3350. [90] LI W, LYU B L, LI J P, et al. Phototransformation of roxithromycin in the presence of dissolved organic matter: Characteriazation of the degradation products and toxicity evaluation [J]. Science of the Total Environment, 2020, 733: 139348. doi: 10.1016/j.scitotenv.2020.139348 [91] DODD M C, KOHLER H P, von GUNTEN U. Oxidation of antibacterial compounds by ozone and hydroxyl radical: Elimination of biological activity during aqueous ozonation processes [J]. Environmental Science & Technology, 2009, 43(7): 2498-2504. [92] CHAMBERLAIN E, ADAMS C. Oxidation of sulfonamides, macrolides, and carbadox with free chlorine and monochloramine [J]. Water Research, 2006, 40(13): 2517-2526. doi: 10.1016/j.watres.2006.04.039 [93] VIONE D, MAURINO V, MINERO C, et al. Phenol chlorination and photochlorination in the presence of chloride ions in homogeneous aqueous solution [J]. Environmental Science & Technology, 2005, 39(13): 5066-5075. [94] MILL T. Predicting photoreaction rates in surface waters [J]. Chemosphere, 1999, 38(6): 1379-1390. doi: 10.1016/S0045-6535(98)00540-2 [95] LUO X, WEI X X, CHEN J W, et al. Rate constants of hydroxyl radicals reaction with different dissociation species of fluoroquinolones and sulfonamides: Combined experimental and QSAR studies [J]. Water Research, 2019, 166: 115083. doi: 10.1016/j.watres.2019.115083 [96] GE L K, ZHANG P, HALSALL C, et al. The importance of reactive oxygen species on the aqueous phototransformation of sulfonamide antibiotics: Kinetics, pathways, and comparisons with direct photolysis [J]. Water Research, 2019, 149: 243-250. doi: 10.1016/j.watres.2018.11.009 [97] VOIGT M, SAVELSBERG C, JAEGER M. Photodegradation of the antibiotic spiramycin studied by high-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight mass spectrometry [J]. Toxicological & Environmental Chemistry, 2017, 99(4): 624-640. [98] LITTER M I, QUICI N. Photochemical advanced oxidation processes for water and wastewater treatment [J]. Recent Patents on Engineering, 2010, 4(3): 217-241. doi: 10.2174/187221210794578574 [99] WANG J L, SONG M R, CHEN B Y, et al. Effects of pH and H2O2 on ammonia, nitrite, and nitrate transformations during UV254nm irradiation: Implications to nitrogen removal and analysis [J]. Chemosphere, 2017, 184: 1003-1011. doi: 10.1016/j.chemosphere.2017.06.078 [100] BENSALAH N, KHODARY A, ABDEL-WAHAB A. Kinetic and mechanistic investigations of mesotrione degradation in aqueous medium by Fenton process [J]. Journal of Hazardous Materials, 2011, 189(1/2): 479-485. [101] GOZLAN I, KOREN I. Identification, mechanisms and kinetics of macrolide degradation product formation under controlled environmental conditions [J]. Journal of Environmental Analytical Chemistry, 2016, 3(1): 100171. [102] 张倩, 杨琛, 莫德清, 等. 水溶液性质对泰乐菌素光降解的影响 [J]. 农业环境科学学报, 2014, 33(12): 2444-2449. ZHANG Q, YANG C, MO D Q, et al. Effects of aqueous solution properties on tylosin photolysis [J]. Journal of Agro-Environment Science, 2014, 33(12): 2444-2449(in Chinese).
[103] 常海莎, 闫豫君, 鲁建江, 等. 螺旋霉素在水溶液中的光降解 [J]. 环境化学, 2018, 37(6): 1343-1350. doi: 10.7524/j.issn.0254-6108.2017091703 CHANG H S, YAN Y J, LU J J, et al. Photodegradation of spiramycin in aqueous solution [J]. Environmental Chemistry, 2018, 37(6): 1343-1350(in Chinese). doi: 10.7524/j.issn.0254-6108.2017091703
[104] TERCERO ESPINOZA L A, NEAMŢU M, FRIMMEL F H. The effect of nitrate, Fe(Ⅲ) and bicarbonate on the degradation of bisphenol A by simulated solar UV-irradiation [J]. Water Research, 2007, 41(19): 4479-4487. doi: 10.1016/j.watres.2007.06.060 [105] NEAMŢU M, POPA D M, FRIMMEL F H. Simulated solar UV-irradiation of endocrine disrupting chemical octylphenol [J]. Journal of Hazardous Materials, 2009, 164(2/3): 1561-1567. [106] HALLADJA S, AMINE-KHODJA A, TER HALLE A, et al. Photolysis of fluometuron in the presence of natural water constituents [J]. Chemosphere, 2007, 69(10): 1647-1654. doi: 10.1016/j.chemosphere.2007.05.035 [107] GE L K, CHEN J W, ZHANG S Y, et al. Photodegradation of fluoroquinolone antibiotic gatifloxacin in aqueous solutions [J]. Chinese Science Bulletin, 2010, 55(15): 1495-1500. doi: 10.1007/s11434-010-3139-y [108] FISHER J M, REESE J G, PELLECHIA P J, et al. Role of Fe(Ⅲ), phosphate, dissolved organic matter, and nitrate during the photodegradation of domoic acid in the marine environment [J]. Environmental Science & Technology, 2006, 40(7): 2200-2205. [109] 齐会勉, 吕亮, 乔显亮. 抗生素在土壤中的吸附行为研究进展 [J]. 土壤, 2009, 41(5): 703-708. QI H M, LV L, QIAO X L. Progress in sorption of antibiotics to soils [J]. Soils, 2009, 41(5): 703-708(in Chinese).
[110] WEI X X, CHEN J W, XIE Q, et al. Distinct photolytic mechanisms and products for different dissociation species of ciprofloxacin [J]. Environmental Science & Technology, 2013, 47(9): 4284-4290. [111] ZHANG Z C, XIE X D, YU Z Q, et al. Influence of chemical speciation on photochemical transformation of three fluoroquinolones (FQs) in water: Kinetics, mechanism, and toxicity of photolysis products [J]. Water Research, 2019, 148: 19-29. doi: 10.1016/j.watres.2018.10.027 [112] BONVIN F, OMLIN J, RUTLER R, et al. Direct photolysis of human metabolites of the antibiotic sulfamethoxazole: Evidence for abiotic back-transformation [J]. Environmental Science & Technology, 2013, 47(13): 6746-6755. 期刊类型引用(5)
1. 邓威,翟健梁,赖淏,陆福洋,宗有杰,熊锐,关博文,常明丰. 微生物-碳化改性钢渣及其对水泥水化特性影响研究进展. 复合材料学报. 2025(01): 119-132 .  百度学术
百度学术
2. 王鹏伟,樊恒辉,任冠洲,谢非含,张星宇,霍江茹. 碳酸酐酶对仿岩溶碳酸氢钙生成速率的影响及其作用机理. 水利与建筑工程学报. 2025(01): 118-124+199 .  百度学术
百度学术
3. 王玉杰,张艳梅,栾金义,赵之平. 酶催化固碳过程及其强化技术研究进展. 化工进展. 2024(01): 232-245 .  百度学术
百度学术
4. 谢昕,王春辉,于荣珍,徐恒,周昊,孙志明,王建兵. CO_2干式生物甲烷化试验研究. 能源环境保护. 2024(03): 109-116 .  百度学术
百度学术
5. 刘鹏,曹源兴,程钰,白云波. 碳酸酐酶增强微生物矿化固土效果的试验研究. 岩土力学. 2024(09): 2554-2564 .  百度学术
百度学术
其他类型引用(0)
-






 下载:
下载: