-
2,4-二氯苯氧乙酸(2,4-D)是一种含有苯氧乙酸基团的选择性除草剂,由于其具有廉价易得、高选择性等优点广泛用于去除农业和林业中的阔叶杂草[1]. 但是由于其具有急性神经毒性和慢性毒性,对皮肤和眼睛有刺激作用;慢性毒性表现为对血液、肝、肾的毒性及抑制某些酶的活力,抑制某些蛋白质的合成[2]. 并且其在环境中难以降解、具有生物累积性,会对人体和自然环境造成潜在危害.
因此2,4-D及其衍生物被世界卫生组织(WHO)定义为中度毒物,并且规定饮用水当中的2,4-D浓度不得超过100 mg·L−1 [3],因此探究高效安全的技术去除环境中残留的2,4-D是十分必要的.
高级氧化技术是一种使用强氧化物质与水中溶解性污染物反应的方法,目前高级氧化技术主要包括芬顿法和类芬顿法、臭氧类高级氧化法、光催化氧化技术、电化学氧化技术和基于硫酸根自由基(
${\rm{SO}}_4^{\cdot- } $ )的新型高级氧化技术等[4].由于传统的芬顿技术存在Fe2+利用率低、反应pH要求严格、易产生二次污染等缺点,基于硫酸根自由基的高级氧化技术能够解决传统芬顿技术的诸多问题[5]. 氧化剂过一硫酸盐(PMS)具有不对称结构,更容易被活化产生更多的活性物质,同时其标准氧化电位E0=2.5—3.1V,高于常用氧化剂的氧化性,在反应中pH适用范围更广并且硫酸根自由基比羟基自由基稳定时间更长[6],因此基于硫酸根自由基的高级氧化技术成为近年来处理有机污染物的研究热点.
本文采用一步水热法合成了NPF-FeCo2O4催化剂,该催化剂能够高效活化PMS产生硫酸根自由基降解2,4-D. 此外,分别采用了TEM、XPS等对材料进行表征分析,同时研究了不同pH、不同阴离子等条件下对2,4-D的去除效率,最后通过淬灭试验和EPR测试对反应机理进行探究.
-
2,4-二氯苯氧乙酸(2,4-D)、2,4-二氯苯酚(2,4-DCP)、活性红、磺胺甲恶唑(SMX)、过硫酸氢钾(PMS)、六水合硝酸钴(Co(NO3)2·6H2O)、九水合硝酸铁(Fe(NO3)3·9H2O)、柠檬酸钾、尿素、聚丙烯酰胺(PAM)、1-丁基-3-甲基咪唑磷酸二丁酯盐、1-丁基-3-甲基咪唑六氟磷酸盐、L-组氨酸、对苯醌、无水乙醇、甲醇、叔丁醇,试验用水为去离子水.
-
NPF三掺杂的FeCo2O4由一步水热法合成[7]. 首先,将0.45 g 1-丁基-3-甲基咪唑六氟磷酸盐溶解在含有0.6549 g CO(NO3)2·6H2O,0.4539 g Fe(NO3)3·9H2O,2.064 g柠檬酸钾,0.6 g尿素,0.525 g PAM的60 mL去离子水中,充分搅拌后将得到的混合物转移至100 mL高压釜,在200 ℃下保持24 h,冷却至室温后,用蒸馏水和乙醇洗涤产物,最终在真空条件下80 ℃干燥12 h,得到N、P、F三掺杂的催化剂NPF-FeCo2O4,简称NPF-FCO,用同样的方法制作了不加离子液体和加1-丁基-3-甲基咪唑磷酸二丁酯盐作为杂原子掺杂剂的催化剂FeCo2O4和NP-FeCo2O4,分别简称FCO和NP-FCO.
-
通过透射电子显微镜(TEM,JEM-2100,日本JEOL公司)对 NPF-FeCo2O4催化剂进行形貌表征,通过X射线光电子能谱仪(美国ThermoFischer,ESCALAB Xi+)对催化剂的元素组成以及价态进行分析,通过X射线衍射仪(日本理学rigaku Ultima IV)对催化剂反应前后晶型变化进行测试,采用傅立叶红外光谱(赛默飞IN10)确定样品中的官能团,通过电子自旋共振波谱仪(Bruker EMXPLUS)测定体系中产生的自由基.
-
在室温条件下,取100 mL的浓度为0.1 mmol·L−1的2,4-D溶液于150 mL的烧杯中,加入一定量的催化剂,磁力搅拌30 min,加入一定量的PMS启动反应. 在一定时间间隔取样,取样时通过0.45 μm滤膜过滤,并提前加入100 μL甲醇淬灭样品中剩余的自由基.
采用配有285 nm紫外检测器的高效液相色谱仪(Thermofisher,Ultimate 3000)测定2,4-D的浓度,流动相种类及比例为水(含有0.5%的乙酸):甲醇 = 40:60,检测波长为 285 nm,流速为 1 mL·min−1.
-
图1(a)为不掺杂离子液体的FeCo2O4 TEM图像,可以看出原始的FeCo2O4 催化剂呈现出均匀的球形形态. 图1(b)−(c)表示随着离子液体的掺杂,催化剂的平均尺寸逐渐减小,并且NP-FeCo2O4和NPF-FeCo2O4催化剂开始形成表面粗糙的不规则球形. 表明在水热过程中离子液体的掺杂影响了FeCo2O4的形态和尺寸. 图1(d)为循环5次使用后的NPF-FeCo2O4催化剂,可以看出经过5次循环后催化剂仍然呈现较规则的圆球状.
-
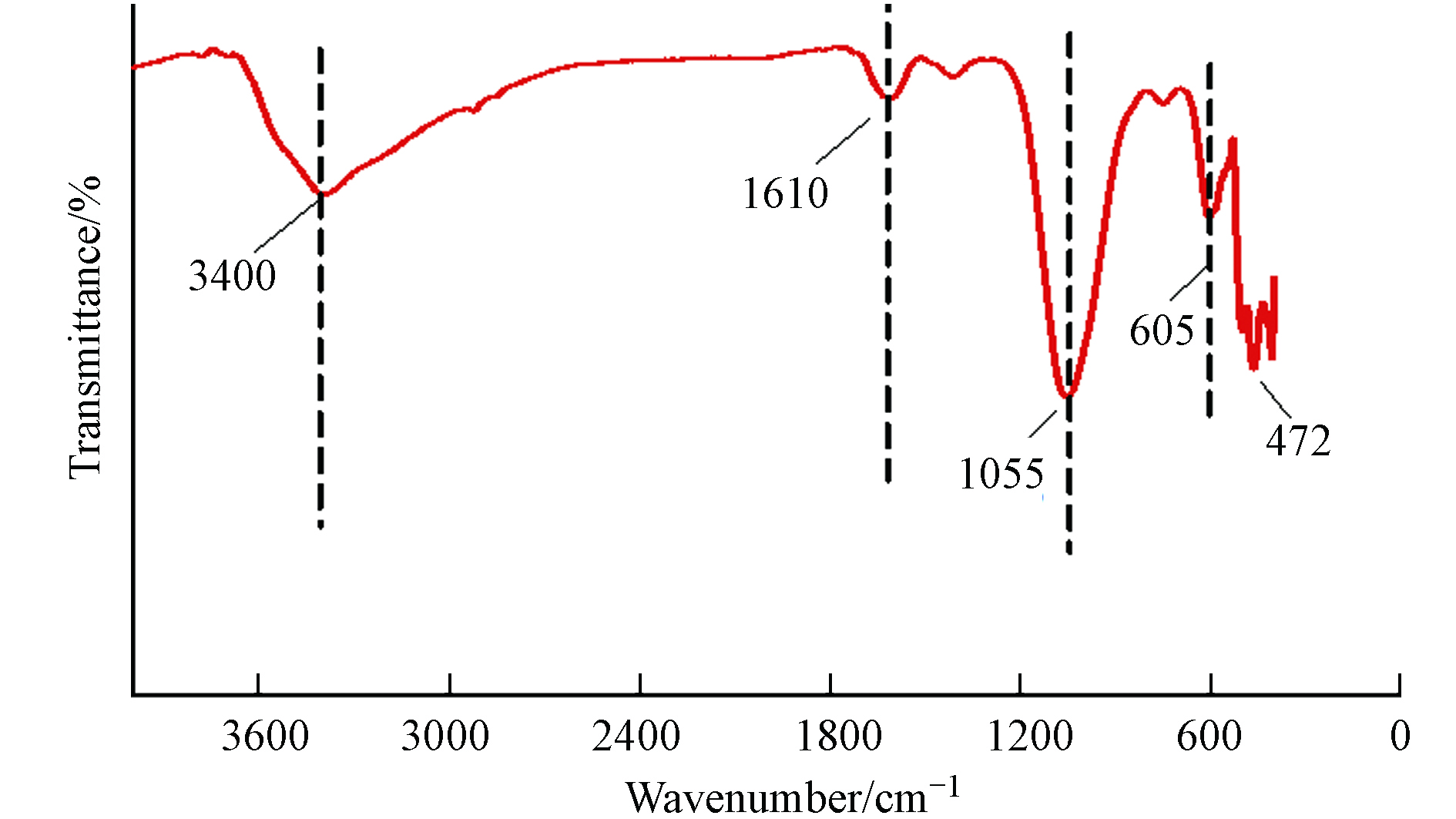
为了确定金属和氧之间的键合状态以及样品中的官能团,对NPF-FeCo2O4催化剂进行了傅里叶红外光谱表征. 如图2所示,傅里叶红外光谱证明材料中存在尖晶石结构. 红外光谱在400—700 cm−1区域的两个吸收带对应于尖晶石FeCo2O4结构. 500 cm−1附近的吸收峰对应尖晶石结构四面体体系中金属-氧键的伸缩振动,即Fe—O. 600—700 cm−1间的吸收峰对应于八面体系统中金属键的拉伸[8],1610 cm−1和3400 cm−1处的拉伸和弯曲模式是羟基(OH)引起的[9].
-
如图3所示,从拟合的XPS光谱图可以看出,合成的催化剂含有Co、Fe、O、C、N、P和F元素. 位于781.3 eV和783.5 eV的峰属于Co 2p3/2,797.4 eV处的峰属于Co 2p1/2,同时也产生了787.7 eV和803.0 eV的卫星峰. 结合能为781.3 eV和783.5 eV的峰说明Co2+氧化物物种的存在,而797.4 eV的峰属于Co3+氧化物种[10]. 715.7 eV和724.2 eV的峰分别属于Fe3+的2p3/2和2p1/2,表明存在Fe3+阳离子,712.1 eV的峰属于Fe2+的2p3/2[11].
由NPF-FeCo2O4的O1s谱图可以看出有3个峰,530.7 eV的峰值属于晶格氧(O1),结合能为 532.3 eV的峰属于表面羟基(O2),531.3 eV处的峰对应于氧空位(O3)[7]. 可以看出,相较于FeCo2O4和NP-FeCo2O4,NPF-FeCo2O4催化剂的氧空位含量最高,而表面羟基含量最低. 相较于NP-FeCo2O4,NPF-FeCo2O4在P 2p谱图中出现了在133.1 eV处的P—O3(氧空位缺陷)新峰[12],表明N/P/F的共掺杂能够通过表面羟基的损失将氧空位结合到NPF-FeCo2O4中. 同时,在N 1s谱图中,相较于NP-FeCo2O4催化剂,NPF-FeCo2O4出现了峰值为398.4 eV的吡啶N,Co—N的含量高于NP-FeCo2O4,在Fe的2p谱图中,Fe3+/Fe2+的相对面积比随着掺杂元素的增加在逐渐增加,表明Co—N和Fe—N物种的形成能够促进Co/Fe向N原子的电荷转移[11].
因此,由XPS图可以得出,加入含有N、P、F元素的离子液体,能够增加Fe3+/Fe2+氧化还原对、催化剂的氧空位和Co—N、Fe—N、P—O、吡啶N等表面活性位点,他们之间的相互作用有利于提高催化剂的催化性能[7].
-
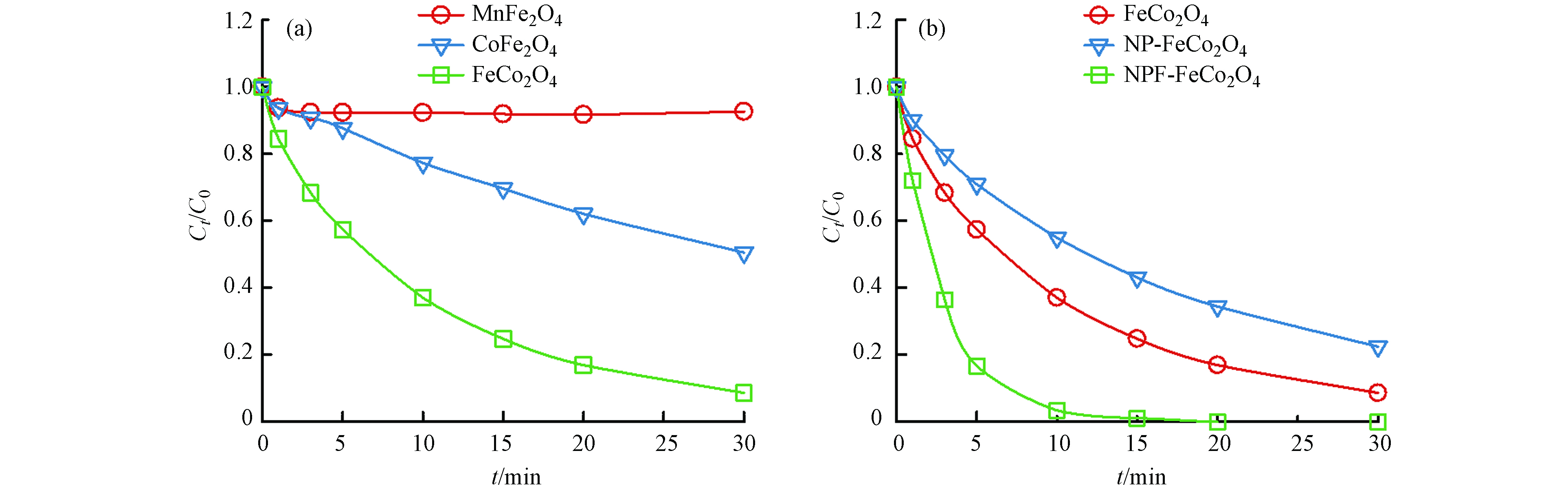
分别对Mn、Fe、Co的3种过渡金属采用同样的方法合成了不同的催化剂,通过催化PMS降解2,4-D的效率来判断催化活性.
如图4所示,在相同条件下,FeCo2O4作为催化剂活化PMS降解2,4-D的效果最好,30 min内去除率能达到90%,但是不能完全降解2,4-D. 因此选择FeCo2O4进行后续的改性研究,如图4(b)中,改性后的NPF-FeCo2O4催化剂在20 min内活化PMS对2,4-D的去除率可达到100%,表明离子液体的加入能够提高FeCo2O4催化性能.
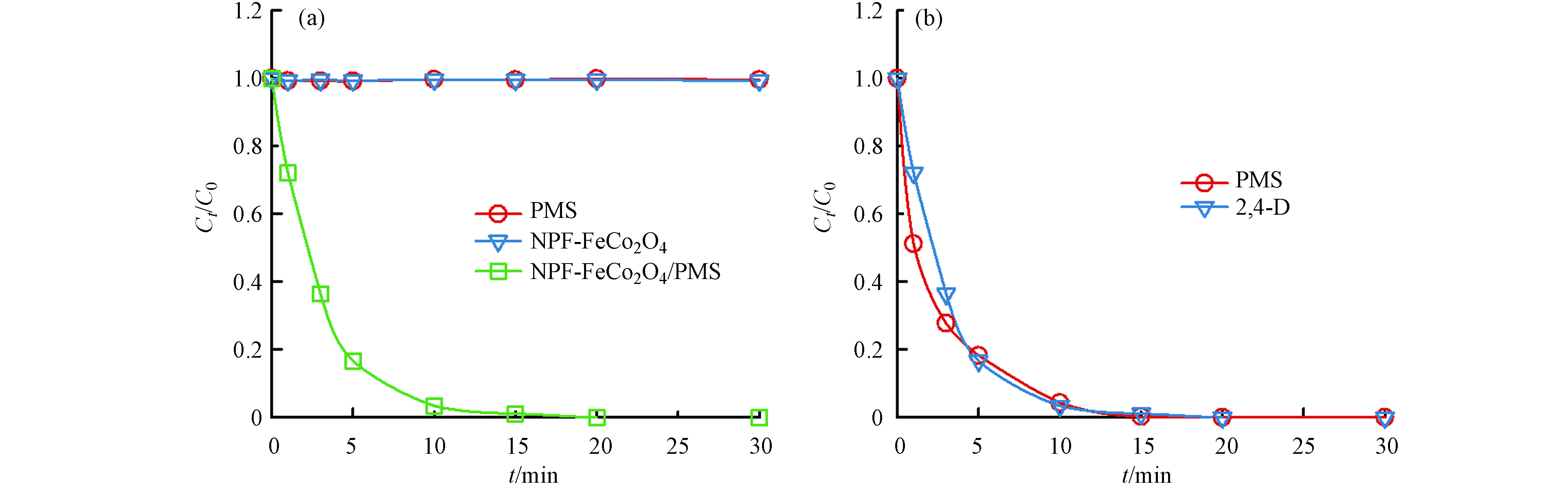
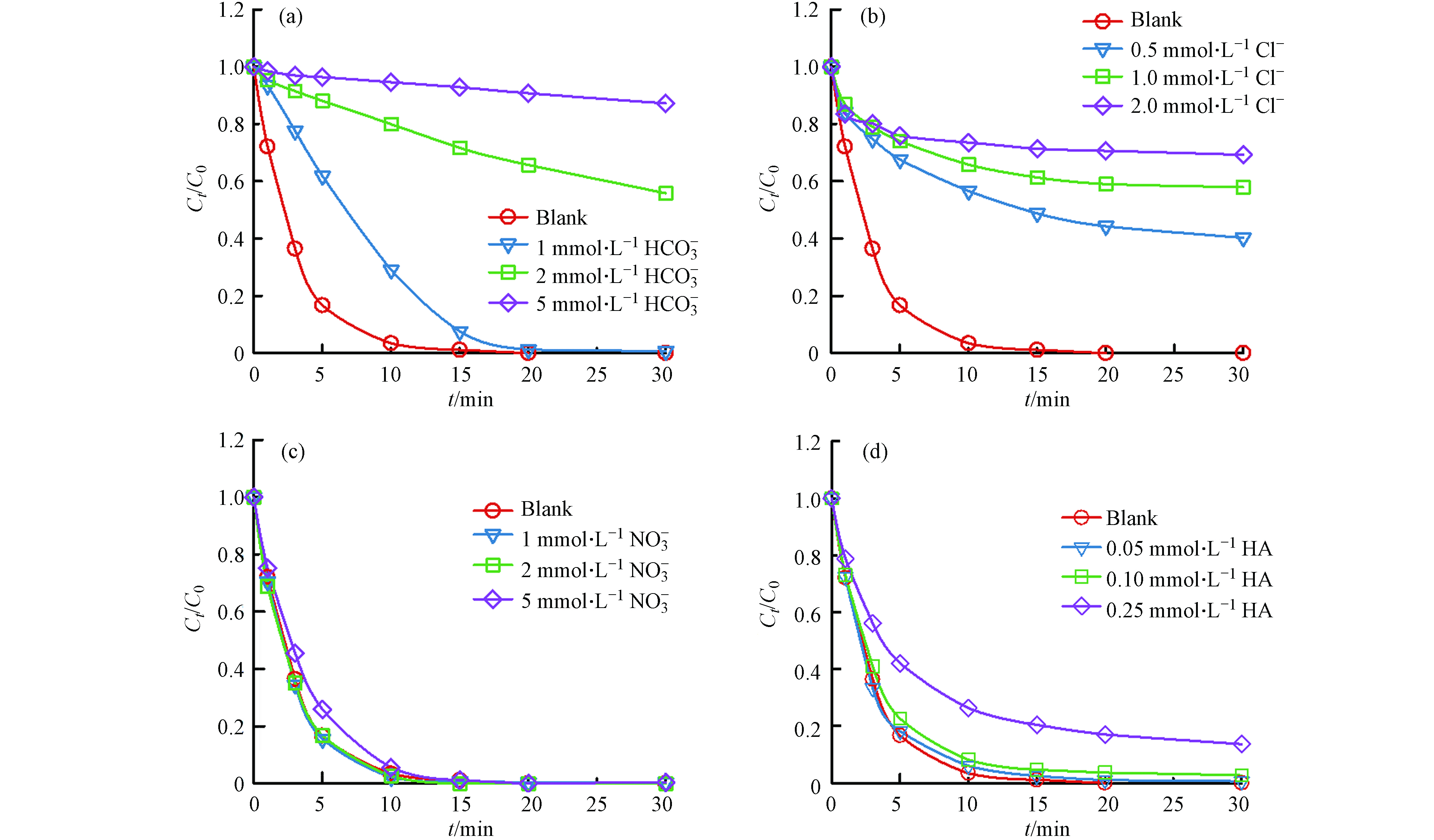
如图5(a)所示,研究了不同体系下2,4-D的降解效率. 在2,4-D浓度为0.1 mmol·L−1,催化剂的投加量为0.1 g·L−1,PMS浓度为1 mmol·L−1的反应条件下,单独加入PMS时,2,4-D几乎不降解. 表明PMS的自分解性差. 同时,在没有PMS的情况下,NPF-FeCo2O4对2,4-D的吸附作用也可以忽略不计. 相比之下,当反应体系达到吸附饱和后加入PMS,20 min内2,4-D的降解率可达到100%. 而由图5(b)NPF-FeCo2O4/PMS体系中2,4-D的浓度和PMS浓度变化对比可知,在该体系中,PMS浓度的变化与2,4-D的降解趋势高度吻合,结果表明NPF-FeCo2O4可有效活化PMS产生活性氧物种促进2,4-D的降解.
-
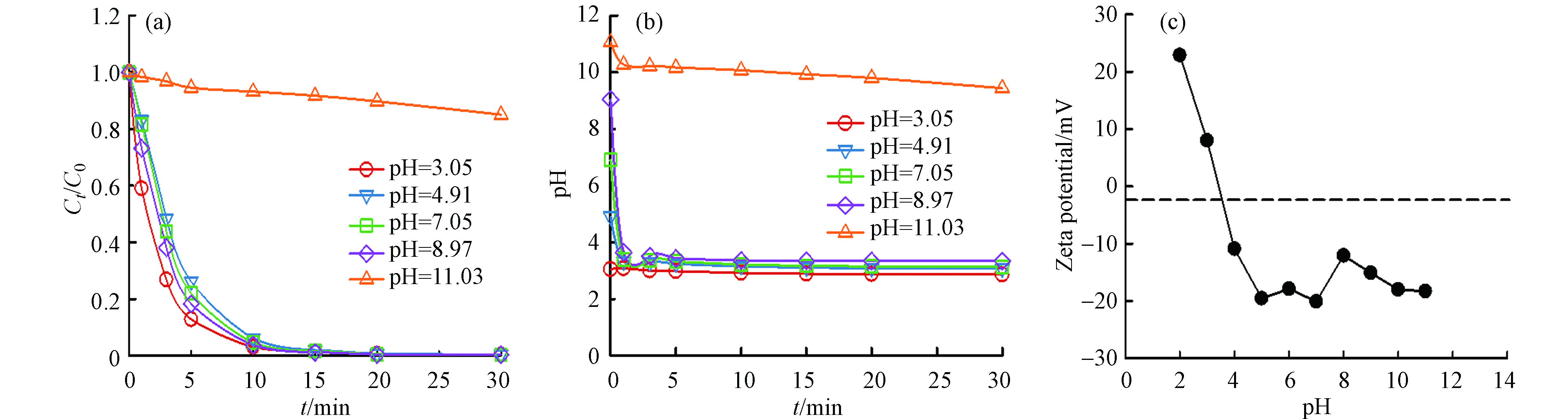
在2,4-D浓度为0.1 mmol·L−1,催化剂的投加量为0.1 g·L−1,PMS浓度为1 mmol·L−1的反应条件下,考察初始pH对2,4-D降解效率的影响,由于常用的缓冲盐如
${\rm{PO}}_4^{3-} $ 、${\rm{HCO}}_3^{-} $ 会淬灭自由基从而抑制污染物的降解[13-15],因此,未使用缓冲盐来控制溶液pH. 如图6(a)所示,在反应前测得0.1 mmol·L−1浓度的2,4-D初始pH 3.82,当pH值在3—9之间时,反应20 min内2,4-D的去除率均能达到100%,但是当反应的初始pH11时,2,4-D的去除率只有15%. 进一步考察在不同初始pH的反应体系过程中pH的变化,如图6(b)所示,可以看出初始pH值在3—9之间的反应体系在投加PMS之后体系中的pH会骤降到3左右,而当初始pH11时,添加PMS后反应体系的pH只有轻微的下降,体系依然处于碱性条件. 图7(c)为NPF-FeCo2O4的Zeta电位,催化剂的零点电荷位于pH=3左右,因此当反应体系为碱性时,催化剂表面所带负电荷增多,导致催化剂与氧化剂之间的静电斥力增强,较强的静电斥力抑制了PMS的活化从而降低了2,4-D的降解效率. -
在实际水体中,无机阴离子能够通过改变pH、捕获自由基、抑制PMS分解等途径来影响污染物的降解[16]. 因此选取了水体中常见的几种无机阴离子(Cl−、
${\rm{NO}}_3^{-} $ 、$ {\rm{HCO}}_3^{-}$ )来研究对体系中2,4-D降解效率的影响. 如图7(a)−(d)所示,加入不同浓度梯度的${\rm{HCO}}_3^{-} $ 和Cl−对2,4-D的去除率有明显的抑制作用,这是因为Cl−能够与溶液中的∙OH和${\rm{SO}}_4^{\cdot -} $ 发生弱反应生成氧化还原电位较低的氯自由基Cl∙[17],而碳酸氢盐具有淬灭效应,能与溶液中的∙OH和${\rm{SO}}_4^{\cdot -} $ 反应生成氧化能力较弱的碳酸氢盐自由基${\rm{CO}}_3^{\cdot -} $ ,从而抑制了2,4-D的降解效率[18]. 而加入不同浓度的${\rm{NO}}_3^{-} $ 对2,4-D的降解效率没有明显影响,说明体系中${\rm{NO}}_3^{-} $ 的存在不会对自由基产生捕获作用,表明体系对${\rm{NO}}_3^{-} $ 具有较强的抗性.水中的天然有机物 (NOM) 是一种普遍存在的含有羧基和酚羟基的物质. NOM 可以通过淬灭溶液中的自由基来抑制底物降解,并阻断催化剂表面的活性位点[19]. 如图8(d)所示,5 mg腐殖酸(HA)的加入使2,4-D的去除率下降到87%,这是因为HA作为一种有机物,与2,4-D存在着竞争∙OH和
${\rm{SO}}_4^{\cdot -} $ 的关系,因此导致了2,4-D降解效率的降低[20]. -
为了验证NPF-FeCo2O4催化剂的可重复使用性,进行了5个循环的实验. 如图8(a)所示,在循环反应中催化剂活性逐渐降低,在第5次反应中2,4-D的去除率为68%. 采用原子发射光谱测定了反应30 min溶液中的金属离子析出量,如图8(b)所示,在反应30 min后的溶液里检测到Co离子析出量为15 mg·L−1,Fe离子析出量为0.5 mg·L−1. 进一步测量浸出的金属离子对反应体系中降解2,4-D的贡献,如图8(c)所示,在NPF-FeCo2O4/PMS体系中反应15 min后2,4-D降解率即高于99%,而溶液中Co离子的贡献仅为40%,表明溶液中2,4-D的去除主要是催化剂NPF-FeCo2O4的作用. 对比了反应5次前后NPF-FeCo2O4催化剂的XRD图,如图8(d)所示,催化剂反应前后的特征峰强度有轻微的减小,催化剂的形态结构有所消耗,表明反应过程中Co离子的浸出和催化剂结构的消耗损失是催化剂失活的原因.
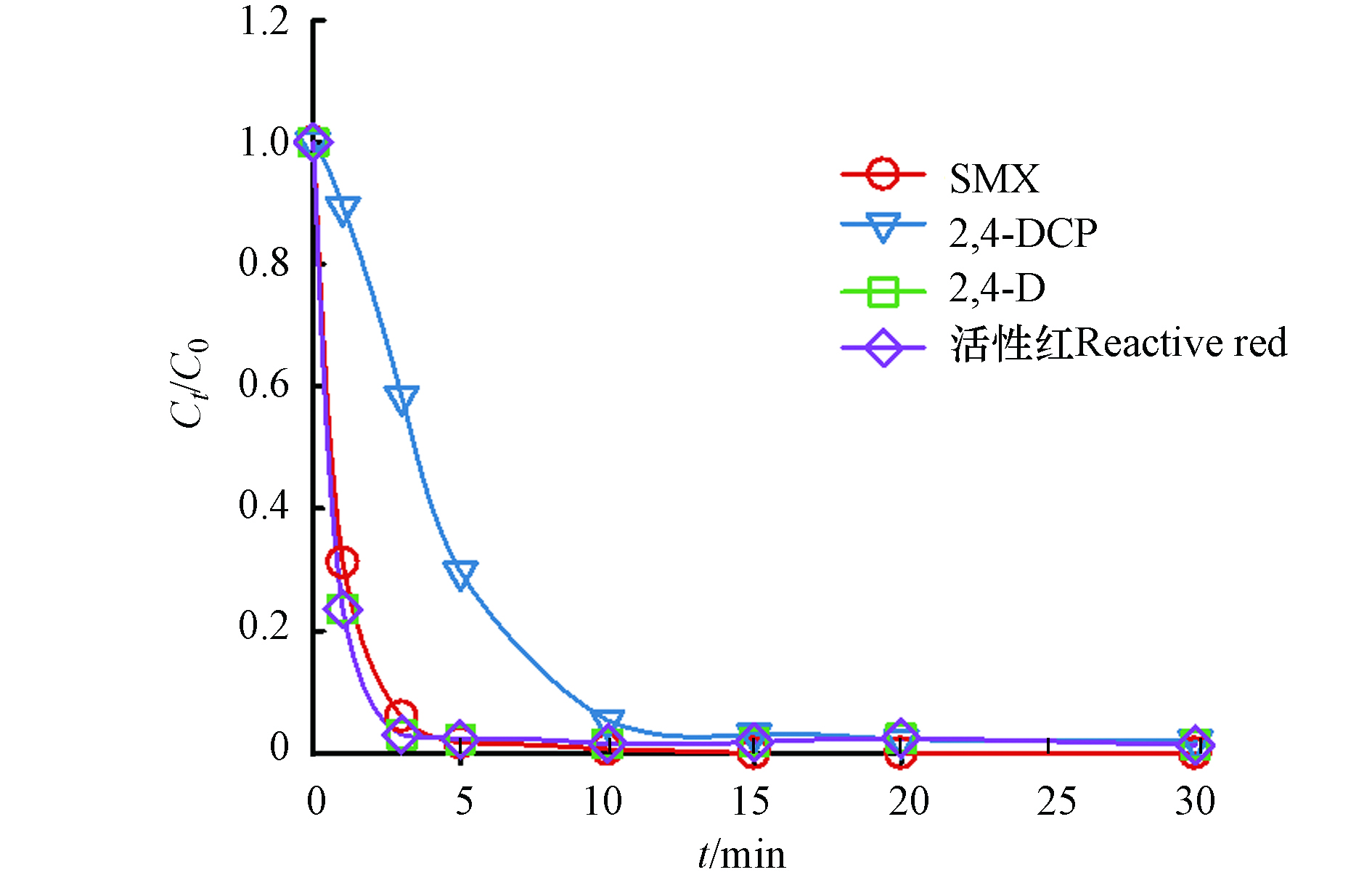
为了探究NPF-FeCo2O4/PMS体系对不同污染物的去除效率,选择了2,4-二氯苯氧乙酸(2,4-D),磺胺甲恶唑(SMX)、2,4-二氯苯酚(2,4-DCP)和活性红为代表的不同官能团污染物. 如图9所示,在反应30 min后,对4种污染物的去除率均能达到100%. 表明 NPF-FeCo2O4/PMS体系对不同种类污染物降解的广泛适用性.
-
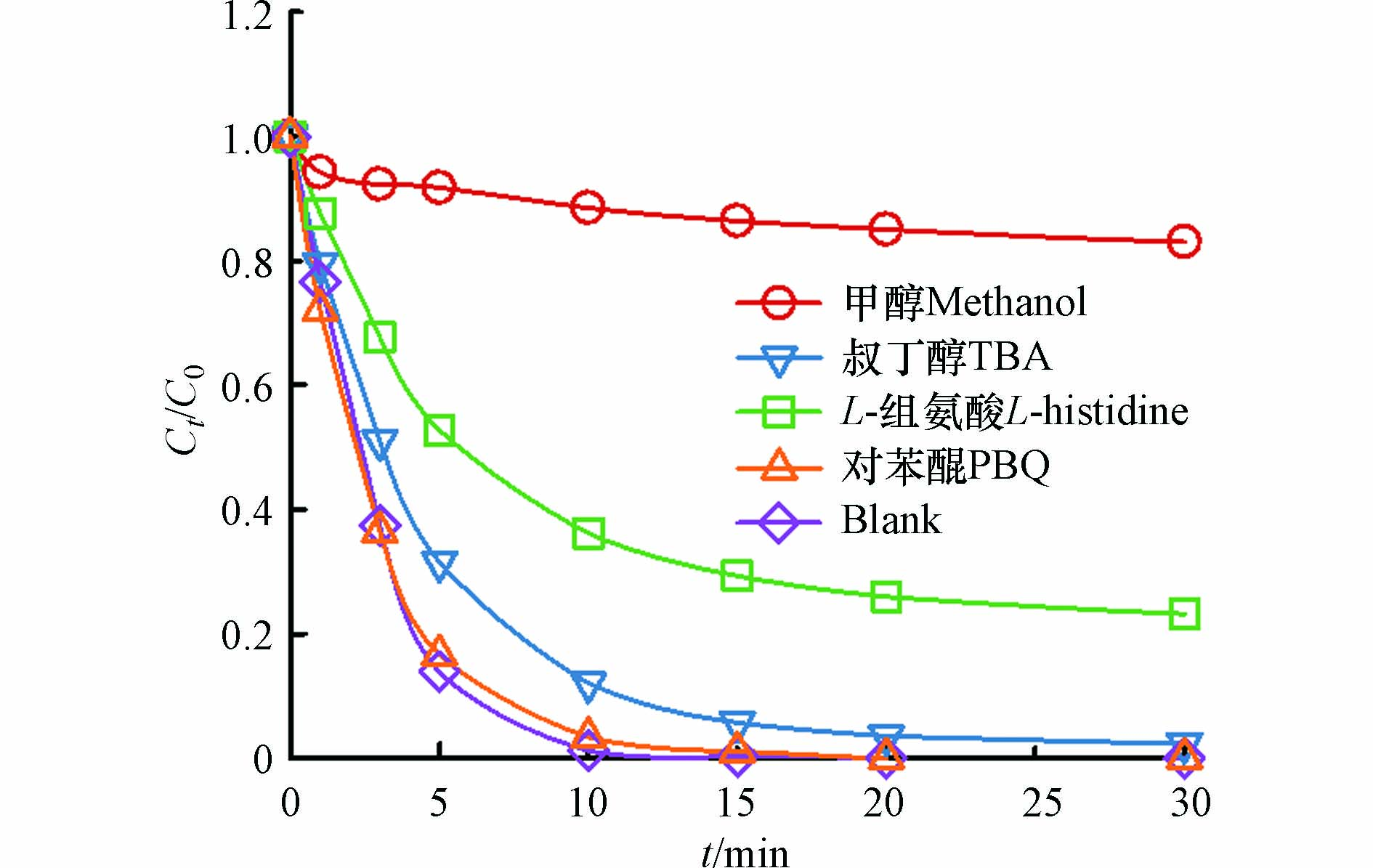
应用EPR技术原位捕获反应体系中产生的活性氧物种,使用DMPO作为∙OH、
${\rm{SO}}_4^{\cdot -} $ 和${\rm{O}}_2^{\cdot -} $ 的捕获剂,TEMPO作为1O2的捕获剂,探究了体系中产生的自由基种类. 如图10所示,单独PMS时,没有出现特征峰信号,而在3个体系中都能观察到DMPO-·OH 加合物和 DMPO-${\rm{SO}}_4^{\cdot -} $ 加合物的的特征峰,表明在3个体系中都产生了∙OH、${\rm{SO}}_4^{\cdot -} $ 和1O2,但是可以明显看出NPF-FeCo2O4/PMS体系的信号最强,FeCo2O4/PMS体系的信号最弱. 由NPF-FeCo2O4/PMS体系的EPR测试图中的特征峰可以看出NPF-FeCo2O4/PMS体系产生了∙OH、${\rm{SO}}_4^{\cdot -} $ 和1O2和${\rm{O}}_2^{\cdot -} $ .为了进一步确定NPF-FeCo2O4/PMS体系中主要的活性氧物种,进行了自由基淬灭实验. 如图10所示,分别选择了甲醇、叔丁醇、L-组氨酸、对苯醌作为自由基淬灭剂. 其中,甲醇能够同时淬灭反应体系中的∙OH和SO4∙-,叔丁醇则用于淬灭体系中的∙OH,对苯醌和L-组氨酸能够有效的淬灭体系中的
$ {\mathrm{O}}_{2}^{\cdot -} $ 和1O2. 如图11所示,50 mmol·L−1的甲醇使2,4-D的去除率降到19%,0.1 mmol·L−1的L-组氨酸使2,4-D的去除率降到80%. 而对苯醌和叔丁醇对反应体系没有明显的抑制. 因此,淬灭试验和EPR结果共同证实,SO4∙-是NPF-FeCo2O4/PMS体系中降解2,4-D的主要活性氧物种. -
(1)通过简便的一步水热法利用离子液体合成了N、P、F 3种元素掺杂的NPF-FeCo2O4催化剂,该催化剂能够高效活化PMS去除水中的2,4-D. 在催化剂投加量为0.1 g·L−1,2,4-D浓度为0.1 mmol·L−1,PMS投加量为0.3 g·L−1时,30 min内体系能去除100%的2,4-D.
(2)相较于未掺杂离子液体的FeCo2O4和只掺杂了N、P两种元素的NP/ FeCo2O4催化剂,掺杂了N、P、F的3种元素使得催化剂能够有更多氧空位、Co—N、Fe—N、P—O和吡啶N等表面活性位点,从而提高了催化性能.
(3)NPF-FeCo2O4/PMS体系可在pH值为3—9的范围内对2,4-D有高效的去除效果,同时该体系对不同官能团的污染物都能达到一定的去除率,水中低浓度的阴离子Cl-、HCO3-和腐殖酸对反应有轻微的抑制,通过EPR测试和淬灭试验共同结果可以得知NPF-FeCo2O4/PMS体系中SO4∙-是降解2,4-D的主要活性氧物种.
(4)本文为双金属催化剂在活化PMS降解水中有机污染物的应用提供了参考,但是在实际处理中还应考虑到Co离子的浸出问题. 今后的研究重点,需提高催化剂中金属Co的利用率如制备成单原子催化剂等,可显著减少催化剂中金属Co的含量,同时保持较高的催化效率. 另外,还可将Co基催化剂与碳材料结合,利用碳基材料稳定金属纳米颗粒,减少金属离子浸出对环境造成的潜在危害.
改性FeCo2O4活化PMS降解水中2,4-二氯苯氧乙酸
Modified FeCo2O4 to activate PMS to degrade 2,4-dichlorophenoxyacetic acid in water
-
摘要: 以离子液体为氮源、磷源和氟源,采用一步水热法将N、P、F的3种元素掺杂到FeCo2O4中合成了NPF-FeCo2O4催化剂,用于活化过一硫酸氢钾(PMS)降解水中2,4-二氯苯氧乙酸(2,4-D). 分别采用透射电子显微镜(TEM)、X射线光电子能谱(XPS)、傅立叶红外光谱(FTIR)、X射线衍射(XRD)对催化剂进行表征. 实验结果表明,当催化剂投加量为0.1 g·L−1、PMS浓度为1 mmol·L−1时,反应30 min后2,4-D的去除率可达100%;初始pH值在3到9的范围内,体系对2,4-D的去除效果不受影响,水中低浓度的Cl−、
${\rm{HCO}}_3^{-} $ 和腐殖酸对反应有轻微的抑制. 通过电子自旋共振波谱(EPR)测试结果显示,相较于FeCo2O4和NP-FeCo2O4催化剂,NPF-FeCo2O4/PMS体系能产生更多的∙OH、${\rm{SO}}_4^{\cdot- } $ 和1O2. 淬灭试验结果显示${\rm{SO}}_4^{\cdot- } $ 为2, 4-D降解过程中的主要活性氧物种.Abstract: The N, P and F co-doped FeCo2O4 (NPF-FeCo2O4) catalyst was prepared via the one-step hydrothermal method using ionic liquid as nitrogen, phosphorus and fluorine sources. The catalyst was applied to activate potassium hydrogen persulfate (PMS) for the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) in water. Transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction analysis (XRD) were employed to characterize the catalyst. The results showed that the removal rate of 2,4-D reached 100% after reaction for 30 min with 0.1 g·L−1 of catalyst and 1 mmol·L−1 of PMS. The removal of 2,4-D was not affected within pH 3—9, and the reaction was slightly inhibited by low concentrations of Cl−,${\rm{HCO}}_3^{-} $ and humic acid. Electron paramagnetic resonance (EPR) results exhibited that the NPF-FeCo2O4/PMS system produced more ∙OH,${\rm{SO}}_4^{\cdot- } $ and 1O2 than the FeCo2O4 and NP-FeCo2O4 counterparts, and the predomination of${\rm{SO}}_4^{\cdot- } $ was clarified by the quenching test during the 2,4-D degradation.-
Key words:
- ferric cobaltate catalyst /
- PMS /
- advanced oxidation /
- sulfate radical /
- 2,4-D
-
2,4-二氯苯氧乙酸(2,4-D)是一种含有苯氧乙酸基团的选择性除草剂,由于其具有廉价易得、高选择性等优点广泛用于去除农业和林业中的阔叶杂草[1]. 但是由于其具有急性神经毒性和慢性毒性,对皮肤和眼睛有刺激作用;慢性毒性表现为对血液、肝、肾的毒性及抑制某些酶的活力,抑制某些蛋白质的合成[2]. 并且其在环境中难以降解、具有生物累积性,会对人体和自然环境造成潜在危害.
因此2,4-D及其衍生物被世界卫生组织(WHO)定义为中度毒物,并且规定饮用水当中的2,4-D浓度不得超过100 mg·L−1 [3],因此探究高效安全的技术去除环境中残留的2,4-D是十分必要的.
高级氧化技术是一种使用强氧化物质与水中溶解性污染物反应的方法,目前高级氧化技术主要包括芬顿法和类芬顿法、臭氧类高级氧化法、光催化氧化技术、电化学氧化技术和基于硫酸根自由基(
${\rm{SO}}_4^{\cdot- } $ 由于传统的芬顿技术存在Fe2+利用率低、反应pH要求严格、易产生二次污染等缺点,基于硫酸根自由基的高级氧化技术能够解决传统芬顿技术的诸多问题[5]. 氧化剂过一硫酸盐(PMS)具有不对称结构,更容易被活化产生更多的活性物质,同时其标准氧化电位E0=2.5—3.1V,高于常用氧化剂的氧化性,在反应中pH适用范围更广并且硫酸根自由基比羟基自由基稳定时间更长[6],因此基于硫酸根自由基的高级氧化技术成为近年来处理有机污染物的研究热点.
本文采用一步水热法合成了NPF-FeCo2O4催化剂,该催化剂能够高效活化PMS产生硫酸根自由基降解2,4-D. 此外,分别采用了TEM、XPS等对材料进行表征分析,同时研究了不同pH、不同阴离子等条件下对2,4-D的去除效率,最后通过淬灭试验和EPR测试对反应机理进行探究.
1. 材料与方法(Materials and methods)
1.1 试验材料
2,4-二氯苯氧乙酸(2,4-D)、2,4-二氯苯酚(2,4-DCP)、活性红、磺胺甲恶唑(SMX)、过硫酸氢钾(PMS)、六水合硝酸钴(Co(NO3)2·6H2O)、九水合硝酸铁(Fe(NO3)3·9H2O)、柠檬酸钾、尿素、聚丙烯酰胺(PAM)、1-丁基-3-甲基咪唑磷酸二丁酯盐、1-丁基-3-甲基咪唑六氟磷酸盐、L-组氨酸、对苯醌、无水乙醇、甲醇、叔丁醇,试验用水为去离子水.
1.2 催化剂的制备
NPF三掺杂的FeCo2O4由一步水热法合成[7]. 首先,将0.45 g 1-丁基-3-甲基咪唑六氟磷酸盐溶解在含有0.6549 g CO(NO3)2·6H2O,0.4539 g Fe(NO3)3·9H2O,2.064 g柠檬酸钾,0.6 g尿素,0.525 g PAM的60 mL去离子水中,充分搅拌后将得到的混合物转移至100 mL高压釜,在200 ℃下保持24 h,冷却至室温后,用蒸馏水和乙醇洗涤产物,最终在真空条件下80 ℃干燥12 h,得到N、P、F三掺杂的催化剂NPF-FeCo2O4,简称NPF-FCO,用同样的方法制作了不加离子液体和加1-丁基-3-甲基咪唑磷酸二丁酯盐作为杂原子掺杂剂的催化剂FeCo2O4和NP-FeCo2O4,分别简称FCO和NP-FCO.
1.3 催化剂表征
通过透射电子显微镜(TEM,JEM-2100,日本JEOL公司)对 NPF-FeCo2O4催化剂进行形貌表征,通过X射线光电子能谱仪(美国ThermoFischer,ESCALAB Xi+)对催化剂的元素组成以及价态进行分析,通过X射线衍射仪(日本理学rigaku Ultima IV)对催化剂反应前后晶型变化进行测试,采用傅立叶红外光谱(赛默飞IN10)确定样品中的官能团,通过电子自旋共振波谱仪(Bruker EMXPLUS)测定体系中产生的自由基.
1.4 试验及分析方法
在室温条件下,取100 mL的浓度为0.1 mmol·L−1的2,4-D溶液于150 mL的烧杯中,加入一定量的催化剂,磁力搅拌30 min,加入一定量的PMS启动反应. 在一定时间间隔取样,取样时通过0.45 μm滤膜过滤,并提前加入100 μL甲醇淬灭样品中剩余的自由基.
采用配有285 nm紫外检测器的高效液相色谱仪(Thermofisher,Ultimate 3000)测定2,4-D的浓度,流动相种类及比例为水(含有0.5%的乙酸):甲醇 = 40:60,检测波长为 285 nm,流速为 1 mL·min−1.
2. 结果与讨论(Results and discussion)
2.1 催化剂的表征
2.1.1 TEM分析
图1(a)为不掺杂离子液体的FeCo2O4 TEM图像,可以看出原始的FeCo2O4 催化剂呈现出均匀的球形形态. 图1(b)−(c)表示随着离子液体的掺杂,催化剂的平均尺寸逐渐减小,并且NP-FeCo2O4和NPF-FeCo2O4催化剂开始形成表面粗糙的不规则球形. 表明在水热过程中离子液体的掺杂影响了FeCo2O4的形态和尺寸. 图1(d)为循环5次使用后的NPF-FeCo2O4催化剂,可以看出经过5次循环后催化剂仍然呈现较规则的圆球状.
2.1.2 傅里叶红外光谱分析
为了确定金属和氧之间的键合状态以及样品中的官能团,对NPF-FeCo2O4催化剂进行了傅里叶红外光谱表征. 如图2所示,傅里叶红外光谱证明材料中存在尖晶石结构. 红外光谱在400—700 cm−1区域的两个吸收带对应于尖晶石FeCo2O4结构. 500 cm−1附近的吸收峰对应尖晶石结构四面体体系中金属-氧键的伸缩振动,即Fe—O. 600—700 cm−1间的吸收峰对应于八面体系统中金属键的拉伸[8],1610 cm−1和3400 cm−1处的拉伸和弯曲模式是羟基(OH)引起的[9].
2.1.3 XPS分析
如图3所示,从拟合的XPS光谱图可以看出,合成的催化剂含有Co、Fe、O、C、N、P和F元素. 位于781.3 eV和783.5 eV的峰属于Co 2p3/2,797.4 eV处的峰属于Co 2p1/2,同时也产生了787.7 eV和803.0 eV的卫星峰. 结合能为781.3 eV和783.5 eV的峰说明Co2+氧化物物种的存在,而797.4 eV的峰属于Co3+氧化物种[10]. 715.7 eV和724.2 eV的峰分别属于Fe3+的2p3/2和2p1/2,表明存在Fe3+阳离子,712.1 eV的峰属于Fe2+的2p3/2[11].
由NPF-FeCo2O4的O1s谱图可以看出有3个峰,530.7 eV的峰值属于晶格氧(O1),结合能为 532.3 eV的峰属于表面羟基(O2),531.3 eV处的峰对应于氧空位(O3)[7]. 可以看出,相较于FeCo2O4和NP-FeCo2O4,NPF-FeCo2O4催化剂的氧空位含量最高,而表面羟基含量最低. 相较于NP-FeCo2O4,NPF-FeCo2O4在P 2p谱图中出现了在133.1 eV处的P—O3(氧空位缺陷)新峰[12],表明N/P/F的共掺杂能够通过表面羟基的损失将氧空位结合到NPF-FeCo2O4中. 同时,在N 1s谱图中,相较于NP-FeCo2O4催化剂,NPF-FeCo2O4出现了峰值为398.4 eV的吡啶N,Co—N的含量高于NP-FeCo2O4,在Fe的2p谱图中,Fe3+/Fe2+的相对面积比随着掺杂元素的增加在逐渐增加,表明Co—N和Fe—N物种的形成能够促进Co/Fe向N原子的电荷转移[11].
因此,由XPS图可以得出,加入含有N、P、F元素的离子液体,能够增加Fe3+/Fe2+氧化还原对、催化剂的氧空位和Co—N、Fe—N、P—O、吡啶N等表面活性位点,他们之间的相互作用有利于提高催化剂的催化性能[7].
2.2 改性FeCo2O4催化PMS降解2,4-D的效率
分别对Mn、Fe、Co的3种过渡金属采用同样的方法合成了不同的催化剂,通过催化PMS降解2,4-D的效率来判断催化活性.
如图4所示,在相同条件下,FeCo2O4作为催化剂活化PMS降解2,4-D的效果最好,30 min内去除率能达到90%,但是不能完全降解2,4-D. 因此选择FeCo2O4进行后续的改性研究,如图4(b)中,改性后的NPF-FeCo2O4催化剂在20 min内活化PMS对2,4-D的去除率可达到100%,表明离子液体的加入能够提高FeCo2O4催化性能.
如图5(a)所示,研究了不同体系下2,4-D的降解效率. 在2,4-D浓度为0.1 mmol·L−1,催化剂的投加量为0.1 g·L−1,PMS浓度为1 mmol·L−1的反应条件下,单独加入PMS时,2,4-D几乎不降解. 表明PMS的自分解性差. 同时,在没有PMS的情况下,NPF-FeCo2O4对2,4-D的吸附作用也可以忽略不计. 相比之下,当反应体系达到吸附饱和后加入PMS,20 min内2,4-D的降解率可达到100%. 而由图5(b)NPF-FeCo2O4/PMS体系中2,4-D的浓度和PMS浓度变化对比可知,在该体系中,PMS浓度的变化与2,4-D的降解趋势高度吻合,结果表明NPF-FeCo2O4可有效活化PMS产生活性氧物种促进2,4-D的降解.
2.3 反应pH的影响
在2,4-D浓度为0.1 mmol·L−1,催化剂的投加量为0.1 g·L−1,PMS浓度为1 mmol·L−1的反应条件下,考察初始pH对2,4-D降解效率的影响,由于常用的缓冲盐如
${\rm{PO}}_4^{3-} $ ${\rm{HCO}}_3^{-} $ 2.4 水中共存物质的影响
在实际水体中,无机阴离子能够通过改变pH、捕获自由基、抑制PMS分解等途径来影响污染物的降解[16]. 因此选取了水体中常见的几种无机阴离子(Cl−、
${\rm{NO}}_3^{-} $ $ {\rm{HCO}}_3^{-}$ ${\rm{HCO}}_3^{-} $ ${\rm{SO}}_4^{\cdot -} $ ${\rm{SO}}_4^{\cdot -} $ ${\rm{CO}}_3^{\cdot -} $ ${\rm{NO}}_3^{-} $ ${\rm{NO}}_3^{-} $ ${\rm{NO}}_3^{-} $ $ \cdot \mathrm{O}\mathrm{H}+\mathrm{C}{\mathrm{l}}^-\to \mathrm{C}\mathrm{l}\mathrm{O}{\mathrm{H}}^- $ $ (1) $ $ \mathrm{C}\mathrm{l}\mathrm{O}{\mathrm{H}}^-+{\mathrm{H}}^+\to \mathrm{C}\mathrm{l}\cdot +{\mathrm{H}}_{2}\mathrm{O} $ $ (2) $ $ \mathrm{S}{\mathrm{O}}_{4}^{\cdot -}+\mathrm{C}{\mathrm{l}}^-\to \mathrm{C}\mathrm{l}\cdot +\mathrm{S}{\mathrm{O}}_{4}^{2-} $ $ (3) $ 水中的天然有机物 (NOM) 是一种普遍存在的含有羧基和酚羟基的物质. NOM 可以通过淬灭溶液中的自由基来抑制底物降解,并阻断催化剂表面的活性位点[19]. 如图8(d)所示,5 mg腐殖酸(HA)的加入使2,4-D的去除率下降到87%,这是因为HA作为一种有机物,与2,4-D存在着竞争∙OH和
${\rm{SO}}_4^{\cdot -} $ 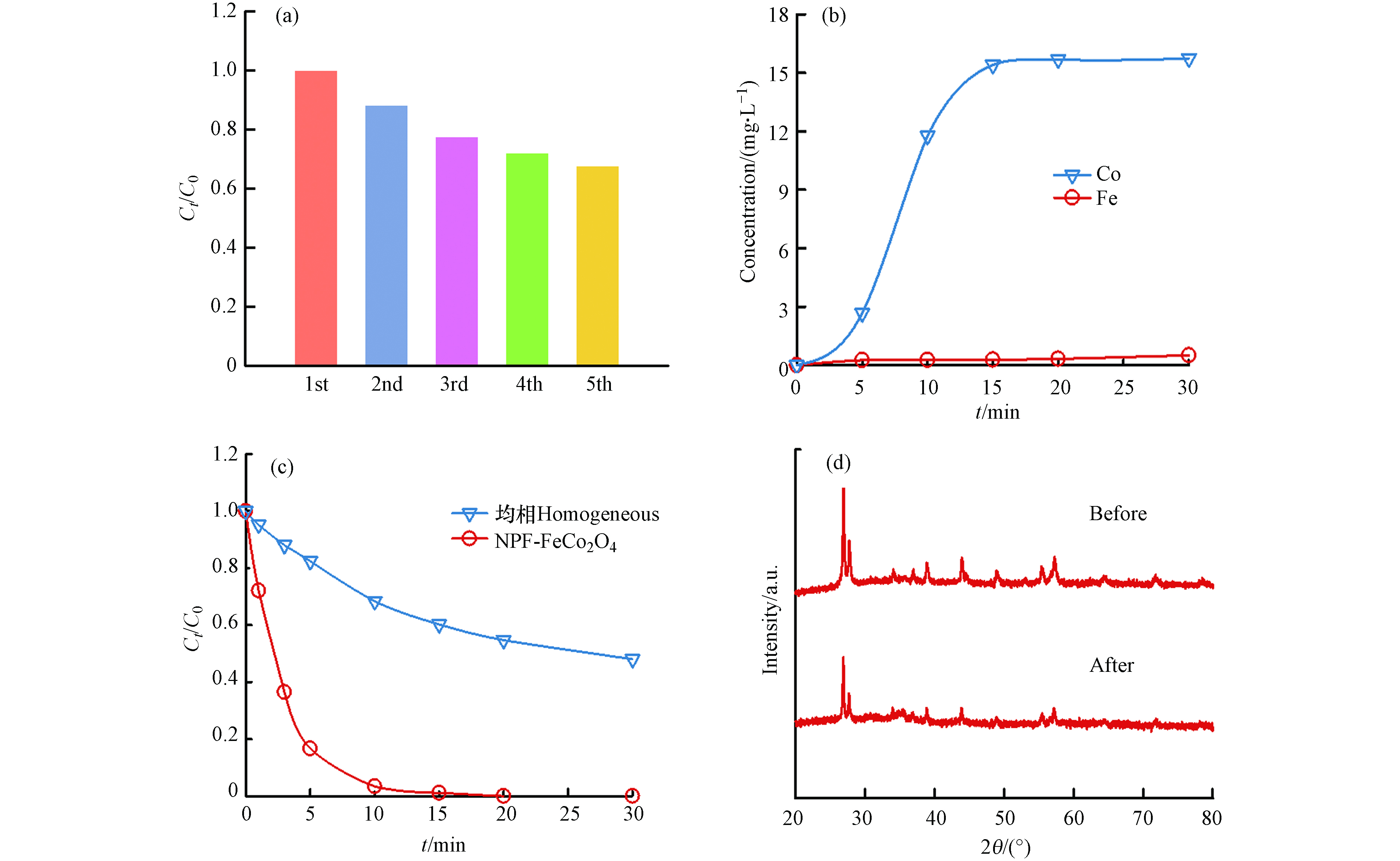 图 8 催化剂的稳定性实验Figure 8. Stability experiment of catalyst(a)NPF-FeCo2O4催化剂的循环试验,(b)体系中金属离子的析出量,(c)均相与非均相体系降解2,4-D,(d)反应前后催化剂的XRD图(a) the cycle test of NPF-FeCo2O4 catalyst, (b) the dissolved amount of metal ions in the system, (c) degradation of 2,4-D in homogeneous and heterogeneous systems, (d) XRD diagram of the catalyst before and after the reaction.
图 8 催化剂的稳定性实验Figure 8. Stability experiment of catalyst(a)NPF-FeCo2O4催化剂的循环试验,(b)体系中金属离子的析出量,(c)均相与非均相体系降解2,4-D,(d)反应前后催化剂的XRD图(a) the cycle test of NPF-FeCo2O4 catalyst, (b) the dissolved amount of metal ions in the system, (c) degradation of 2,4-D in homogeneous and heterogeneous systems, (d) XRD diagram of the catalyst before and after the reaction.$ \cdot \mathrm{O}\mathrm{H}+\mathrm{H}\mathrm{C}{\mathrm{O}}_{3}^-\to \mathrm{C}{\mathrm{O}}_{3}^{\cdot -}+{\mathrm{H}}_{2}\mathrm{O} $ $ (4) $ $ \mathrm{S}{\mathrm{O}}_{4}^{\cdot -}+\mathrm{H}\mathrm{C}{\mathrm{O}}_{3}^-\to \mathrm{S}{\mathrm{O}}_{4}^{2-}+\mathrm{H}\mathrm{C}{\mathrm{O}}_{3}^{\cdot } $ $ (5) $ $ \mathrm{H}\mathrm{C}{\mathrm{O}}_{3}^{\cdot }\to {\mathrm{H}}^++\mathrm{C}{\mathrm{O}}_{3}^{\cdot -} $ $ (6) $ 2.5 催化剂的稳定性
为了验证NPF-FeCo2O4催化剂的可重复使用性,进行了5个循环的实验. 如图8(a)所示,在循环反应中催化剂活性逐渐降低,在第5次反应中2,4-D的去除率为68%. 采用原子发射光谱测定了反应30 min溶液中的金属离子析出量,如图8(b)所示,在反应30 min后的溶液里检测到Co离子析出量为15 mg·L−1,Fe离子析出量为0.5 mg·L−1. 进一步测量浸出的金属离子对反应体系中降解2,4-D的贡献,如图8(c)所示,在NPF-FeCo2O4/PMS体系中反应15 min后2,4-D降解率即高于99%,而溶液中Co离子的贡献仅为40%,表明溶液中2,4-D的去除主要是催化剂NPF-FeCo2O4的作用. 对比了反应5次前后NPF-FeCo2O4催化剂的XRD图,如图8(d)所示,催化剂反应前后的特征峰强度有轻微的减小,催化剂的形态结构有所消耗,表明反应过程中Co离子的浸出和催化剂结构的消耗损失是催化剂失活的原因.
为了探究NPF-FeCo2O4/PMS体系对不同污染物的去除效率,选择了2,4-二氯苯氧乙酸(2,4-D),磺胺甲恶唑(SMX)、2,4-二氯苯酚(2,4-DCP)和活性红为代表的不同官能团污染物. 如图9所示,在反应30 min后,对4种污染物的去除率均能达到100%. 表明 NPF-FeCo2O4/PMS体系对不同种类污染物降解的广泛适用性.
2.6 催化机理探究
应用EPR技术原位捕获反应体系中产生的活性氧物种,使用DMPO作为∙OH、
${\rm{SO}}_4^{\cdot -} $ ${\rm{O}}_2^{\cdot -} $ ${\rm{SO}}_4^{\cdot -} $ ${\rm{SO}}_4^{\cdot -} $ ${\rm{SO}}_4^{\cdot -} $ ${\rm{O}}_2^{\cdot -} $ 为了进一步确定NPF-FeCo2O4/PMS体系中主要的活性氧物种,进行了自由基淬灭实验. 如图10所示,分别选择了甲醇、叔丁醇、L-组氨酸、对苯醌作为自由基淬灭剂. 其中,甲醇能够同时淬灭反应体系中的∙OH和SO4∙-,叔丁醇则用于淬灭体系中的∙OH,对苯醌和L-组氨酸能够有效的淬灭体系中的
$ {\mathrm{O}}_{2}^{\cdot -} $ 3. 结论(Conclusion)
(1)通过简便的一步水热法利用离子液体合成了N、P、F 3种元素掺杂的NPF-FeCo2O4催化剂,该催化剂能够高效活化PMS去除水中的2,4-D. 在催化剂投加量为0.1 g·L−1,2,4-D浓度为0.1 mmol·L−1,PMS投加量为0.3 g·L−1时,30 min内体系能去除100%的2,4-D.
(2)相较于未掺杂离子液体的FeCo2O4和只掺杂了N、P两种元素的NP/ FeCo2O4催化剂,掺杂了N、P、F的3种元素使得催化剂能够有更多氧空位、Co—N、Fe—N、P—O和吡啶N等表面活性位点,从而提高了催化性能.
(3)NPF-FeCo2O4/PMS体系可在pH值为3—9的范围内对2,4-D有高效的去除效果,同时该体系对不同官能团的污染物都能达到一定的去除率,水中低浓度的阴离子Cl-、HCO3-和腐殖酸对反应有轻微的抑制,通过EPR测试和淬灭试验共同结果可以得知NPF-FeCo2O4/PMS体系中SO4∙-是降解2,4-D的主要活性氧物种.
(4)本文为双金属催化剂在活化PMS降解水中有机污染物的应用提供了参考,但是在实际处理中还应考虑到Co离子的浸出问题. 今后的研究重点,需提高催化剂中金属Co的利用率如制备成单原子催化剂等,可显著减少催化剂中金属Co的含量,同时保持较高的催化效率. 另外,还可将Co基催化剂与碳材料结合,利用碳基材料稳定金属纳米颗粒,减少金属离子浸出对环境造成的潜在危害.
-
-
[1] 金党琴, 龚爱琴, 王元有, 等. 石墨烯-钙钛矿纳米复合材料分子印迹光电化学传感器的构建及测定蔬果中2, 4-D残留 [J]. 化学研究与应用, 2021, 33(8): 1433-1439. JIN D Q, GONG A Q, WANG Y Y, et al. Construction of Gr/CH3NH3PbI3 nanocomposite molecularly imprinted photoelectrochemical sensor and determination of 2, 4-D in vegetables and fruits [J]. Chemical Research and Application, 2021, 33(8): 1433-1439(in Chinese).
[2] 徐爱东. 我国蔬菜中常用植物生长调节剂的毒性及残留问题研究进展 [J]. 中国蔬菜, 2009(8): 1-6. XU A D. Research advance in the toxicity and residue of plant growth regulator in vegetables in China [J]. China Vegetables, 2009(8): 1-6(in Chinese).
[3] SUN C, BAIG S A, LOU Z M, et al. Electrocatalytic dechlorination of 2, 4-dichlorophenoxyacetic acid using nanosized titanium nitride doped palladium/nickel foam electrodes in aqueous solutions [J]. Applied Catalysis B:Environmental, 2014, 158/159: 38-47. doi: 10.1016/j.apcatb.2014.04.004 [4] 杨梖, 刘颢, 俞映倞, 等. 高级氧化技术去除水体中抗性基因污染的研究进展 [J]. 环境化学, 2021, 40(4): 1263-1273. YANG B, LIU H, YU Y L, et al. A review: Elimination of antibiotic resistance genes in water by advanced oxidation progress [J]. Environmental Chemistry, 2021, 40(4): 1263-1273(in Chinese).
[5] 刘萌, 胡莉敏, 张广山, 等. Co/Zn双金属氧化物活化过一硫酸盐降解双酚A的性能研究 [J]. 环境化学, 2018, 37(4): 753-760. LIU M, HU L M, ZHANG G S, et al. Activation of peroxymonosulfate by the Co/Zn bimetallic oxide for the degradation of bisphenol A [J]. Environmental Chemistry, 2018, 37(4): 753-760(in Chinese).
[6] 韩爽, 肖鹏飞. 过硫酸盐活化技术在四环素类抗生素降解中的应用进展 [J]. 环境化学, 2021, 40(9): 2873-2883. HAN S, XIAO P F. Application progress of persulfate activation technology in degradation of tetracycline antibiotics [J]. Environmental Chemistry, 2021, 40(9): 2873-2883(in Chinese).
[7] SUN J, GUO N K, SHAO Z Y, et al. Electrocatalysts: A facile strategy to construct amorphous spinel-based electrocatalysts with massive oxygen vacancies using ionic liquid dopant [J]. Advanced Energy Materials, 2018, 8(27): 1870121. doi: 10.1002/aenm.201870121 [8] LOBO L S, KALAINATHAN S, KUMAR A R. Investigation of electrical studies of spinel FeCo2O4 synthesized by Sol-gel method [J]. Superlattices and Microstructures, 2015, 88: 116-126. doi: 10.1016/j.spmi.2015.09.010 [9] SHAHEEN N, AADIL M, ZULFIQAR S, et al. Fabrication of different conductive matrix supported binary metal oxides for supercapacitors applications [J]. Ceramics International, 2021, 47(4): 5273-5285. doi: 10.1016/j.ceramint.2020.10.108 [10] XU M J, LI J, YAN Y, et al. Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles [J]. Chemical Engineering Journal, 2019, 369: 403-413. doi: 10.1016/j.cej.2019.03.075 [11] HU M Z, ZHU J Y, ZHOU W J. Synthesis of oxygen vacancy-enriched N/P co-doped CoFe2O4 for high-efficient degradation of organic pollutant: Mechanistic insight into radical and nonradical evolution [J]. Environmental Pollution, 2021, 270: 116092. doi: 10.1016/j.envpol.2020.116092 [12] YAN Y, XIA B Y, GE X M, et al. A flexible electrode based on iron phosphide nanotubes for overall water splitting [J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2015, 21(50): 18062-18067. [13] WANG J L, WANG S Z. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants [J]. Chemical Engineering Journal, 2021, 411: 128392. doi: 10.1016/j.cej.2020.128392 [14] WANG J L, WANG S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants [J]. Chemical Engineering Journal, 2018, 334: 1502-1517. doi: 10.1016/j.cej.2017.11.059 [15] ZHOU H Y, LAI L D, WAN Y J, et al. Molybdenum disulfide (MoS2): A versatile activator of both peroxymonosulfate and persulfate for the degradation of carbamazepine [J]. Chemical Engineering Journal, 2020, 384: 123264. doi: 10.1016/j.cej.2019.123264 [16] MA W J, WANG N, FAN Y N, et al. Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate [J]. Chemical Engineering Journal, 2018, 336: 721-731. doi: 10.1016/j.cej.2017.11.164 [17] FANG G D, DIONYSIOU D D, WANG Y, et al. Sulfate radical-based degradation of polychlorinated biphenyls: Effects of chloride ion and reaction kinetics [J]. Journal of Hazardous Materials, 2012, 227/228: 394-401. doi: 10.1016/j.jhazmat.2012.05.074 [18] CUI X L, LIU X T, LIN C Y, et al. Activation of peroxymonosulfate using drinking water treatment residuals modified by hydrothermal treatment for imidacloprid degradation [J]. Chemosphere, 2020, 254: 126820. doi: 10.1016/j.chemosphere.2020.126820 [19] ZHOU X Q, LUO M Y, XIE C Y, et al. Tunable S doping from Co3O4 to Co9S8 for peroxymonosulfate activation: Distinguished Radical/Nonradical species and generation pathways [J]. Applied Catalysis B:Environmental, 2021, 282: 119605. doi: 10.1016/j.apcatb.2020.119605 [20] LATIFOGLU A, GUROL M D. The effect of humic acids on nitrobenzene oxidation by ozonation and O3/UV processes [J]. Water Research, 2003, 37(8): 1879-1889. doi: 10.1016/S0043-1354(02)00583-3 期刊类型引用(1)
1. 彭建彪,常宇,吴佳琪,郭欣婷,司宁,马霖轩,刘海津,曹治国,高士祥. Co-Cu双金属氧化物活化过一硫酸盐去除水中磺胺甲噁唑. 环境化学. 2024(02): 642-649 .  本站查看
本站查看
其他类型引用(1)
-














 下载:
下载: