-
纳米材料通常被定义为外部尺寸或内部结构单元至少在一维尺度上小于100 nm的材料,包括纳米颗粒(nanoparticles,NPs)、纳米纤维、纳米管、纳米复合材料和纳米结构材料等[1-2]. 其中纳米颗粒是至少在二维尺度上直径小于100 nm的颗粒,因其纳米尺寸而具有较大的比表面积和其它一些独特的物理化学性质[3]. 金属纳米颗粒(metallic nanoparticles,MNPs)是含有金属元素的纳米颗粒[4],主要包括单质金属纳米颗粒、金属氧化物纳米颗粒、量子点(Quantum dots, QD)等,如纳米银(Ag NPs)[1, 5]、纳米金(Au NPs)[5-6]、纳米零价铁(nZVI)[5]、纳米氧化锌(ZnO NPs)、纳米二氧化钛(TiO2 NPs)[5]、CdSe/ZnS QD[7]等.
随着纳米技术的飞速发展,金属纳米颗粒在汽车制造、生物医学、化妆品生产、国防安全和能源制备等领域的应用日益广泛[2]. 如TiO2 NPs作为一种光催化剂,已被用在太阳能电池、油漆和涂料中[5];具有较强紫外线阻断能力的ZnO NPs、TiO2 NPs常被添加在防晒剂、化妆品中[5, 8],而Fe2O3 NPs被广泛应用于水处理、气体传感、医学研究和催化领域[9-10];CeO2 NPs可用于眼科疾病的分子治疗[11],还可加入到生物柴油中起到催化、提高燃烧效率的作用,从而改善整体燃烧质量、降低制动比油耗、减少有害气体排放[12];Au NPs生物相容性较好,在生物医学方面可以用于成像、肿瘤的诊断以及作为靶向治疗的药物运输载体[6];贵金属纳米催化剂在氧化还原反应、析氧反应、析氢反应等方面都表现出了优异的性能[13];一些贵金属纳米颗粒(钯、银等)可用于改善固体储氢材料的性能[14]. 另外,有的纳米材料表面覆盖了具有不同功能的分子[6],或通过纳米颗粒形状的控制和核-壳形态的结构设计进一步调节纳米催化剂的表面能,从而使纳米颗粒具有独特的催化活性和选择性[15-16].
近年来,纳米技术产品及应用的全球市场价值不断攀升,纳米材料的产量不断增长[2, 17]. MNPs在生产及使用过程中可通过多种途径进入水环境,在其中迁移、积累. 譬如工业生产过程产生的含MNPs废水会排放到河流、湖泊、河口、沿海水域等[18-20];在水处理及环境修复过程中使用的纳米颗粒也可能会排放到水环境中. 这会导致水环境中纳米颗粒的暴露风险增加. 目前,许多文献都阐述了MNPs在水环境中的行为[5, 17, 21-26]、生物累积[26-28]和毒性效应[2, 20, 26, 29-30]等. 以TiO2 NPs为例,Nam等[27]的研究表明TiO2 NPs在水环境中会因发生一定程度的团聚而沉降到沉积物中,其团聚过程受到溶解性有机质、离子强度、zeta电位及pH等因素的影响,而沉积物中的TiO2 NPs主要聚集在底栖生物膜中,悬浮在水体中的TiO2 NPs则主要附着于浮游型微生物上,TiO2 NPs还会通过食物链在不同营养级之间传递,也可能发生生物放大;此外,Chen等[31]的研究发现同为锐钛矿结构的TiO2 NPs对蛋白核小球藻(Chlorella pyrenoidosa)的急性毒性作用随TiO2 NPs粒径的增加而降低;Zhu等[32]的研究表明粒径相同(50—60 nm)时,晶体结构为锐钛矿结构的TiO2 NPs能够比金红石结构的TiO2 NPs引起更多的氧化损伤.
藻类作为水生生态系统中的初级生产者,是水生食物链的关键环节,亦是自然界营养循环的重要参与者[33]. 因此,了解MNPs在水环境中的行为及其与藻类间的相互作用机制对厘清MNPs的生态风险至关重要. 本文关注的藻类既包括原核藻类(如蓝藻门的藻类),也包括真核藻类(如绿藻门、硅藻门的藻类);既有浮游生活的藻类,也有固着在岩石等生长基质上的藻类(如红藻门的藻类).
本文的主要内容包括:(1)MNPs在水中的环境行为;(2)MNPs与藻类之间的相互作用,包括MNPs在藻类表面的吸附、在藻类细胞中的累积、对藻类的毒性效应、与水中其它污染物及天然有机质(natural organic matter,NOM)对藻类的共同作用以及MNPs胁迫下藻类的自我防御机制, 如图1所示.
-
MNPs在水环境中可能发生的转化过程有[22]:(1)在水环境中发生团聚、沉降及在多孔介质中的沉积[22, 27, 34];(2)溶解、硫化、光化学反应、蛋白冠的形成,但这些过程并非简单并列关系,且往往包含氧化还原反应[5, 22, 34];(3)生物介导的过程:包括生物降解和生物修饰,许多是由微生物介导的[22]. 由于本文侧重于MNPs与浮游藻类的相互作用,本部分将重点介绍MNPs可能影响该作用的行为,即在水中的聚集和沉降、溶解、硫化反应和光化学反应.
-
纳米颗粒因比表面积大而具有较高的反应活性,容易在环境中发生团聚和沉降[35]. 根据DLVO理论[36],胶体颗粒被双电层(electrostatic double layer, EDL)包围,范德华引力和静电斥力之间的平衡决定了胶体的稳定性. 与胶体颗粒类似,MNPs在水中进行布朗运动,相互碰撞、吸附后即可能导致聚集(可以是同质聚集也可以是异质聚集)并伴随着沉降[37].
影响MNPs在水环境中稳定性的因素有:
(1) 纳米颗粒自身的理化性质. 譬如,大小、晶体结构会影响颗粒的表面能,进而影响它们之间的相互作用力,且小颗粒由于具有更高的表面能而比大颗粒更容易发生团聚,因为这一行为能够降低纳米颗粒的自由能[21, 38];此外,对于具有相同原子组成的材料,其晶体结构能够在很大程度上决定其表面电荷[39]. 例如TiO2 NPs具有3种不同的晶体结构(金红石、锐钛矿、板钛矿),当锐钛矿和板钛矿为主要结构时,TiO2 NPs的zeta电位约为20 mV[40]; 当金红石结构为主要结构时,TiO2 NPs的zeta电位约为35 mV(pH=7.5)[41]. 由于具有不同的表面电荷和表面能,不同结构的TiO2 NPs在水溶液中的团聚和沉积速率也不同[42-43].
(2) 环境因素. 根据DLVO理论,双电层对于MNPs的稳定性十分重要,而EDL内部电荷的大小和厚度则与溶液性质直接相关[44]. 水环境中影响MNPs团聚和沉降行为的因素有:
(a) pH:纳米颗粒表面电荷会随介质pH变化,如向溶液中加入带正电荷的质子时,表面带负电荷的Ag NPs的能垒会降低,当能垒小于布朗运动的动能时,颗粒之间就会发生团聚[35, 45]. pH对纳米颗粒稳定性的影响也与颗粒的表面修饰物有关,如pH的变化对非离子型聚乙烯吡咯烷酮(polyvinylpyrrolidone,PVP)修饰的Ag NPs的稳定性影响不大,而不同pH下聚乙烯亚胺(branched polyethyleneimine,BPEI)修饰的Ag NPs之间的静电斥力不同,因而Ag NPs的稳定性会受到影响[35].
(b) 离子强度:纳米颗粒在水溶液中的稳定性很大程度上取决于离子强度. 当向介质中添加盐(如NaCl、KCl等),即增大离子强度时,双电层会被压缩,纳米颗粒之间的距离随之减小,进而影响颗粒之间的范德华力、疏水作用力及由于电荷不均一产生的静电吸引力,使得纳米颗粒更容易发生团聚和沉降[35, 37]. Bian等[22]利用NaCl(0.00—0.08 mol·L−1 NaCl)调节离子强度来研究中性(pH=7.0)条件下离子强度对ZnO NPs稳定性的影响,发现离子强度越高ZnO NPs越不稳定、容易团聚. 然而,对于以PVP作为空间稳定剂修饰的Ag NPs,一定范围内离子强度的变化并未对其在水中的zeta电位及水动力学直径产生显著影响[35].
(c) 阳离子价态:有研究表明,溶液中存在的阳离子价态也会影响MNPs在溶液中的团聚. French等[40]发现,在pH和离子强度相同的情况下,TiO2 NPs在含二价阳离子(如Ca2+)的溶液中比含一价阳离子(如Na+)的溶液中团聚得更快. 对于这一现象,作者给出的解释是:由于TiO2 NPs表面整体带正电荷,故Ca2+不太可能在TiO2 NPs之间起静电桥接作用;同时,由于离子的德拜长度(LD)的倒数(1/LD)与离子的价电荷成正比(式1),且离子间静电斥力随德拜长度的减小呈指数衰减(式2),电解质离子价态越高(如Ca2+相比于Na+),离子间的静电斥力越小,越容易团聚. 此外,Wang等[46]的研究认为根据库仑定律,相比于低价态金属离子(K+、Na+),高价态金属离子(Ca2+、Mg2+)更能与H+竞争吸附于ZnO NPs表面,因而能够在离子强度低于10 mmol·L−1时提高ZnO NPs的zeta电位和稳定性,且各种金属离子对ZnO NPs的团聚速率的影响大小顺序为Ca2+>Mg2+>K+>Na+. 然而,目前文献报道的结果中所用MNPs大多属于不同种类,且pH、离子强度等其它条件各不相同. 因此,还需要控制其它变量来研究阳离子价态对具有不同表面电荷MNPs团聚的影响.
其中,e为电子电荷,ni为整个溶液中离子i的个数,zi为离子i的价态.
其中VESsphere-sphere为离子间的静电斥力,h为分散距离.
(d) NOM[47]:NOM也是影响MNPs在水溶液中稳定性的重要因素. NOM在自然环境中普遍存在,它是由不同分子量和化学性质的组分组成的高度非均相混合物[48],如富里酸(fulvic acid,FA)、胡敏酸(humic acid,HA)、多糖、脂类以及一些肽和蛋白质等生物大分子[49-50]. 它们具有较小的尺寸,单位质量表面积较大[5],其物理结构和分子量会随pH、离子强度和电解质类型等溶液化学性质的变化而变化[51]. NOM可以吸附到纳米颗粒的表面,影响纳米颗粒在水中的稳定性[52-54],这种影响不仅与NOM组分的结构、分子量、浓度有关,还与溶液化学性质(pH、离子强度等)有关. 如芳香度更高的NOM组分对Au NPs团聚的抑制作用更强[51];具有不同重均分子量(691000 g·mol−1和12800 g·mol−1)的两种NOM组分对于14 nm的Au NPs在溶液中稳定性的影响也有所不同[51];腐殖酸能够通过电荷中和作用降低TiO2 NPs的zeta电位,且只有当离子强度低于TiO2 NPs的临界聚沉浓度(critical coagulation concentration,CCC)、pH低于TiO2 NPs的零电荷点时,HA的加入才能促进TiO2 NPs的团聚,而当离子强度高于CCC时HA的加入会因产生空间位阻而抑制TiO2 NPs的团聚[55];此外,水环境中的生物大分子能够通过改变纳米颗粒的表面电荷影响其稳定性[56],如胞外聚合物(extracellular polymetric substance,EPS)[55]、细胞色素c[56]、胱氨酸[57]等.
(e) 温度:温度是影响纳米颗粒稳定性的重要环境因素,但目前关于温度如何影响MNPs在水中团聚行为的研究较少[21]. 胶体颗粒在水中与MNPs做类似的布朗运动,温度越高,胶体颗粒在水中的布朗运动速度越大,碰撞频率也越高. 但并非温度越高,胶体颗粒一定越容易团聚,如Sandra等[58]的研究发现温度对蒙脱土胶体颗粒稳定性的影响与pH值和离子强度有关,在中等pH范围内,除最高离子强度组颗粒在水中的聚合速率常数随温度的升高而增大外,其余均随温度的升高而减小. 这一结果说明MNPs在水中的稳定性可能受到自身性质和多种环境因素的综合影响,还需要更多的相关研究来阐明其具体机制.
(f) 溶解氧:Zou等[59]的研究表明,Ag NPs的稳定性在贫氧水溶液(含氧量为0.25 mg·L−1)中显著增加了. 这是因为缺氧条件下Ag NPs溶解减少,且释放出的Ag+能够被水中的还原性物质(如柠檬酸、NOM)还原形成再生Ag NPs. 这表明Ag NPs在贫氧水体中可长时间稳定存在,其环境危害比在富氧水体中更甚.
-
MNPs在环境中不仅以颗粒形式存在,还可能会发生溶解. MNPs是否溶解、溶解到什么程度是决定其吸收途径、毒性机制的一个重要特性[60],也是对纳米颗粒可能产生的环境健康风险进行评估的重要依据. 目前,许多研究发现MNPs会发生溶解,释放出金属离子,如Ag NPs[24, 34, 61-63]、CuO NPs[64-65]、ZnO NPs[64, 66-68]、nZVI[69]等. 这一过程主要受到以下几方面因素的影响:
(1) 纳米颗粒自身的性质,如颗粒的大小和形状、组成金属、表面化学性质、表面修饰物等[34, 70]. 根据DLVO理论,粒子间的相互作用能垒随粒子尺寸的减小而减小,颗粒尺寸的减小导致颗粒表面原子比例的增加,改变了颗粒的电子结构、表面电荷和表面反应活性[38],所以不同大小和形状的纳米颗粒在相同的表面积和起始浓度下溶解速度不同[71];表面具有凸结构的纳米颗粒因为具有较薄的扩散层而溶解更快[72]. 此外,表面修饰物不同时纳米颗粒的溶解情况也可能不同,如Kittler等[73]的研究表明,在25 ℃的水中,PVP修饰的Ag NPs有约50%溶解释放出了Ag+,而柠檬酸钠修饰的Ag NPs只有约14%溶解,作者推测这可能是因为柠檬酸钠作为化学屏障能够减少银离子的释放.
(2) 溶液的性质. 如pH值、离子强度、离子种类、温度、是否存在可与释放的离子形成复合物的物质(如NOM等)[45, 60, 74]. 在自然水体中,由于水体理化性质复杂,纳米颗粒在不同水体中的溶解情况往往不同. Zhang等[75]的研究表明, ZnO NPs在海水水样中的溶解程度远大于其他水样,且与水样pH值和离子强度均呈正相关;Ag NPs在远离城市区域的淡水中溶出量最大,这可能是因为Ag NPs释放的Ag+可与水中Cl−反应生成AgCl沉淀,而该水样中Cl−含量在所有水样中最少,故使其中的Ag+浓度高于其它水样.
(3) 光照、温度、溶解氧等其它因素. Niksa等[76]2017年发表的一项研究称,可见光能够促进纳米银在自然水体中的溶解,而紫外光在照射8 d后能够减少Ag+的释放;Kittler等[73]发现在37 ℃的水中,PVP和柠檬酸钠修饰的Ag NPs的溶解度比25 ℃时均有所增加,说明Ag NPs的溶解也会受到温度的影响;当水中存在溶解氧时,Ag NPs会发生缓慢氧化,随后溶解并释放出Ag+(式(3))[77-78].
不过,纳米颗粒的溶解过程并非取决于单一因素,而是多种因素共同作用的结果[75]. 例如,Bian等[53]提到,当体系中存在NOM时,金属氧化物纳米颗粒的溶解会同时受到NOM和pH的影响. 他们的研究表明:当pH≤6时,HA的羧基和酚基被质子化,对ZnO NPs的吸附活性降低;而当pH≥9时,尽管ZnO NPs的溶解较少,但当颗粒表面发生溶解、暴露出不同的晶面并产生更多的表面缺陷时,HA就会吸附到亲和力较高的吸附位点,随后促进溶解.
如前所述,MNPs在水环境中发生溶解会释放出金属离子,这一过程带来的毒性常常能与MNPs本身的毒性相提并论,甚至有的纳米颗粒对水生生物的毒性主要来源于其溶解释放的离子. 有研究表明ZnO NPs对于藻类如月牙藻(Pseudokirchneriella subcapitata)[79-80]、拟微型海链藻(Thalassiosira pseudonana)、纤细角毛藻(Chaetoceros gracilis)[81]的毒性与锌盐相当. Sotiriou等[77]的研究表明当氧气存在时,Ag NPs在水中会发生缓慢氧化、溶解并释放出银离子;Xiu等[82]发现在厌氧条件下Ag NPs不具有任何抗菌作用,并且Ag NPs的形态特性(如大小、形状、表面修饰物)主要通过影响银离子的释放而间接影响其抗菌作用;另外,一些有氧条件下的研究发现Ag NPs的Ag+溶出速率与其对莱茵衣藻(Chlamydomonas reinhardtii)[83]、近头状尖胞藻(Raphidocelis subcapitata)[84]的毒性呈正相关. 上述研究都表明MNPs的溶解在很大程度上影响其对浮游藻类的毒性作用并改变其生物可利用性.
-
硫化反应是许多MNPs在环境中发生的一类重要反应,特别是在污水处理厂或缺氧、厌氧的沉积物中[85-87]. 对于部分纳米颗粒(如ZnO NPs[88]、Ag NPs[85-86]、CuO NPs[89]),硫化反应能够在很大程度上影响其在环境中的行为归趋[88]. 以ZnO NPs为例,Ma等[88]通过同步辐射(X射线吸收和衍射光谱)分析观察到当Na2S存在时,ZnO NPs可通过溶解和再沉淀的机制转变成直径为2.5—5 nm的ZnS NPs,且硫化程度与硫化物浓度有关,硫化物过量时,5 d内ZnO NPs转化率可达将近100%;硫化反应还会导致ZnO NPs的团聚,通过减少ZnO NPs在环境中的迁移而降低ZnO NPs的生物可利用性.
纳米颗粒表面常常结合修饰物以赋予特定功能,如提高其稳定性和分散性[90-91]、用于靶向治疗[92-95]等. 这些物质在光照下可能发生变化,进而影响纳米颗粒的环境行为和归趋. Louie等[96]的一项研究证明,在紫外线照射下,Au NPs表面的硫代甲氧基聚二乙醇(mPEGSH)涂层在24 h内迅速降解,导致其稳定性下降;其后他们观察到紫外光照射能够诱导PVP涂层在Au NPs上发生显著氧化,进一步导致涂层坍塌,形成更薄、水合程度更低的涂层,且残余转化产物的存在能够使PVP-Au NPs与溶液中Ca2+的相互作用发生改变;另外,MNPs表面涂层经光化学反应后其表面物质可能为原始涂层聚合物、聚合物转化产物和NOM的混合物,因此,还需要通过更多的研究了解MNPs其它环境行为与相关生态风险[97].
-
近十多年来,人们关注到了MNPs的环境风险,并且进行了大量的研究工作以了解其对水生生物的潜在毒性及机制. 在水生态系统中,藻类作为初级生产者,暴露于MNPs后可能会通过食物链给更高营养级的生物带来风险[33]. 目前,许多生态毒理学方面的研究多用绿藻[98-102]、硅藻[103-105]、蓝藻[98, 102, 106-110]等单细胞藻类作为实验生物,且不同研究所用MNPs的大小、浓度、暴露时间及表征方法都有所不同,因此很难进行系统比较. 本部分从以下五个方面对MNPs与藻类之间的相互作用进行总结:(1)MNPs在藻类表面的吸附;(2)在藻类中的吸收和累积(3)对藻类的毒性效应;(4)与其它物质对藻类的共同作用;(5)MNPs胁迫下藻类的自我防御机制.
-
MNPs与藻细胞表面的直接接触和吸附是它们发生相互作用的第一步,也是后续MNPs进入细胞、产生毒性效应的先决条件,如MNPs在水环境中聚集后吸附到藻细胞表面可能会对藻类细胞壁和细胞膜造成损伤,Chen等[111]观察到相比于对照组,经0.81 mg·L−1 ZnO NPs暴露24 h后,小球藻(Chlorella sp.)表面更加粗糙,并发生了不规则形变,表明ZnO NPs聚集体对小球藻细胞造成了严重损伤. 许多藻类是研究MNPs生物毒性的模式生物,因此对藻类吸附MNPs过程的研究至关重要. 吸附过程中涉及的作用力有范德华力、疏水力、静电引力、氢键和配体-受体相互作用[21, 112]. 在藻类吸附动力学研究中,常采用准一级模型和准二级模型来分析藻类对纳米颗粒的吸附动力学,Langmuir和Freundlich这两种等温模型也被广泛用于拟合吸附等温实验数据,且研究表明藻类对纳米颗粒的吸附最符合准二级动力学模型和Langmuir等温模型[113]. 藻类对纳米颗粒吸附的初始阶段吸附过程非常快,随后缓慢下降,最终吸附达到饱和,且吸附能力随着初始浓度的增加而增加[114].
MNPs在细胞表面的吸附受到其物理化学性质、环境因素、藻细胞形态和表面性质的综合影响[21]. 例如,研究表明具有不同表面电荷的Ag NPs因吸附差异而在普通小球藻(Chlorella vulgaris)内的生物累积动力学不同,其中聚乙烯亚胺(polyethyleneimine,PEI)修饰的PEI-Ag NPs表面带正电,由于与带负电的C. vulgaris表面存在静电引力而能够迅速吸附在C. vulgaris表面[115];P. subcapitata细胞表面能够吸附相当于其自身重量2.3倍的TiO2 NPs,且其吸附动力学高度依赖于pH值,最大吸附发生在pH=5.5时[79];另有研究表明氮限制诱导了Fe2O3 NPs在裸藻(Euglena intermedia)表面的吸附和随后的内化,其原因可能是细胞膜粗糙度的增加和细胞表面多糖的显著增加[116];Zhang等[117]发现不同形状和特性的藻类对ZnO NPs的敏感性不同,其表面对ZnO NPs的吸附也不同,其中不具有细胞壁的杜氏藻(Dunaliella salina)表面吸附ZnO NPs及其团聚体后发生了明显的损伤,中肋骨条藻(Skeletonema costatum)粗糙表面的小泡容易将ZnO NPs固定在细胞表面,而三角褐指藻(Phaeodactylum tricomutum)和、亚心型扁藻(Platymonas subcordiforus)分别由于具有细长的形状和较为光滑的表面而没有吸附ZnO NPs.
-
Luoma等[28]将水生生物经水或食物暴露后对MNPs的累积分为两大部分:一部分是纳米颗粒在生物体组织上或组织内的累积;另一部分是由于纳米颗粒在细胞外、细胞表面或细胞内发生溶解造成的金属离子累积,如Ribeiro等[118]认为,暴露于Ag NPs的R. subcapitata,其细胞中累积的Ag来源于Ag NPs溶解释放的Ag+. 已有研究表明,不同聚集状态的MNPs在细胞内的分布因其与细胞内DNA和蛋白质的不同相互作用而异[119]. 目前已有许多研究证明了Ag NPs[62, 115]、Fe2O3 NPs[116]、CuO NPs、TiO2 NPs[120]在藻类中的累积,但藻类吸收MNPs的具体机制很大程度上是未明确的,已有文献中提及的主动和被动机制多数并未得到实验证实[121-122](表1).
MNPs在进入有细胞壁的藻细胞之前首先要穿过细胞壁. 藻类细胞壁主要成分为纤维素和果胶,还含有一些糖蛋白和多糖,它能够支持细胞结构、发挥保护作用,抵抗包括NPs在内的外来潜在有害物质[112]. Worm等[123]发现,在20 nmol·L−1的CdSe/ZnS QD中暴露1 h后,无细胞壁的C. reinhardtii细胞中有约16%在CdSe/ZnS QD的发射波长处有荧光信号,而有细胞壁的野生型C. reinhardtii株系细胞中仅有约1%的细胞检测到了CdSe/ZnS QD荧光,这说明细胞壁具有一定的保护作用. 一般来说,藻类细胞壁上的微孔平均直径为5—20 nm,这使得细胞壁能够起到筛的作用. 只有孔径小于细胞壁孔径的纳米颗粒能够才穿过孔[33]. 由于不同藻类的细胞壁结构和化学组成不同,纳米颗粒通过不同藻类细胞壁的扩散速度也不同,但目前还没有研究来系统比较MNPs在不同藻类中穿过细胞壁的扩散情况[121]. 当藻细胞暴露于MNPs时,MNPs一方面可能会聚集在藻细胞表面,造成细胞壁孔的堵塞或孔数量的减少[33];另一方面可能会对细胞壁造成损伤、形成更大的孔隙,从而使得纳米颗粒更容易被细胞吸收[124-125]. 因此,需要更多的研究来测定经MNPs暴露后藻类细胞壁孔隙的变化,以明确细胞壁在藻细胞吸收MNPs中的作用.
MNPs穿过细胞壁后即与由磷脂双分子层组成的细胞膜接触并发生相互作用. MNPs在细胞膜上可能发生溶解[28],使得藻细胞表面金属离子浓度升高[121]. MNPs在环境介质中及在细胞膜上所释放的金属离子也可能通过离子通道/载体进入藻细胞[121],例如从纳米颗粒释放出的Ce3+能够通过钙离子通道进入到C. reinhardtii细胞内[132]. 然而,目前还不清楚离子载体能否直接运输纳米颗粒. 此外,一些MNPs还能够进入叶绿体、线粒体、细胞核,但具体机制尚不清楚[121]. 许多研究表明纳米颗粒能够破坏膜结构,还会通过过量活性氧自由基(reactive oxygen species,ROS)的积累等造成膜穿孔[33],这可能是MNPs进入藻细胞的途径之一[121].
另外,内吞也被认为是许多MNPs直接被藻细胞吸收的一个途径. 完整的MNPs往往较大,难以利用离子通道,故它们与自由金属离子的转运机制有所不同. 大颗粒跨膜运输机制主要包括:网格蛋白介导的内吞作用[28, 133-134]、小窝蛋白介导的内吞作用[28]、巨胞饮作用[20, 28]和不依赖于网格蛋白和小窝蛋白的内吞作用[135]. 目前藻类吸收MNPs的途径和机制并未完全明了,但已经有研究发现用NaN3和氯丙嗪分别抑制能量依赖性的内吞作用和网格蛋白介导的内吞作用后,C. pyrenoidosa对CuO NPs的吸收被显著抑制[128],Ag NPs也能够通过内吞被棕鞭藻(Ochromonas danica)吸收[62].
-
前文提到,纳米颗粒因其纳米尺寸而具有高比表面积和高反应活性,还能够在藻细胞中累积. 目前已有许多关于纳米颗粒对藻类毒性作用的研究,结果表明纳米颗粒会影响藻类的生长、叶绿素含量、蛋白质含量及酶活性等(表2). MNPs在水中环境行为的复杂性决定了其毒性效应的复杂性,其毒性作用与纳米颗粒的形态、大小、化学组成、浓度、环境行为均有关,也与被暴露的藻类的细胞结构和生理生化特性相关[113, 136-139]. 如MNPs在藻细胞表面大量聚集可能造成遮光效应、阻碍藻细胞内外营养物质传递,从而对藻细胞产生毒性[33, 124, 140];一些MNPs在水中发生溶解后释放出的金属离子对藻类具有较强的毒性[141-142]. MNPs的团聚行为将影响其细胞毒性,如MNPs团聚程度越大,对细胞的影响(包括累积、毒性等)越小[119],因此对于易团聚的MNPs,其毒性往往受团聚状态影响变化较大[143]. 不同藻类对于不同MNPs的耐受性/敏感性不同. 例如,与绿藻相比,藻红蛋白含量高的红藻对CdS-NPs和ZnS-NPs的敏感性较低,这与它们的细胞壁结构不同有关[144];据Wang等[138]的统计,P. subcapitata对ZnO NPs最敏感(72 h EC50<50 μg·L−1),Ag NPs对其也有较高的毒性(96 h EC50=9.9—190 μg·L−1),而ZnO NPs和Ag NPs对海洋藻类产生毒性需要更高的浓度(高达1 mg·L−1或更高数量级). 根据目前的文献报道,MNPs对藻类造成毒性的主要机制有以下几种:
(1) 藻细胞被大量的MNPs包裹,可能影响营养物质的吸收,还可能起到遮光作用,影响藻类的光合作用;藻类细胞会通过色素组成的变化而快速适应光照条件的变化,以优化光合作用,保护细胞免受光照胁迫[145]. 因此,MNPs在藻细胞表面吸附、聚集可能会造成物理遮蔽作用,进而影响藻类的光合作用和对营养物质的摄取、抑制其生长[33, 124]. 经过nZVI处理的蓝藻细胞表面会迅速形成一层铁和氢氧化铁的涂层,其遮光作用会影响光系统Ⅱ(PSⅡ)的捕光复合物,影响蓝藻细胞的光合作用[140]. 有研究将纳米颗粒与藻细胞进行物理分离,即将纳米颗粒放置在藻类和光源之间,通过消除纳米颗粒与藻类的直接接触和吸附以评估其遮光作用对藻类生长的影响[146],但这一方法存在其局限性:实验中藻类可能由于不断摇晃的原因并不能时刻处于遮蔽条件下,且由于纳米颗粒均匀分散时,溶液内部的光强随与光源距离的增加而指数性降低,其实际遮蔽效果与光源的位置及MNPs的分散性密切相关[145]. 由此,Hjorth等提出利用分析藻类色素组成变化的方法确定遮蔽作用对藻类的影响,叶绿素含量可能随着遮光程度的增加而增加,以补偿光照的减少[145].
(2) MNPs可诱导ROS的过量产生,造成一系列后续的损伤,如膜损伤、基因毒性等. MNPs在与生物体相互作用及受到紫外线照射时均可能产生ROS,尤其是有光催化性质的纳米颗粒[124],如TiO2 NPs[147-149]、Ag NPs[150-152]等. ROS是细胞氧化代谢的副产物,包括超氧阴离子自由基、羟基自由基、单线态氧和过氧化氢,它们的过量产生会引发氧化应激,使细胞无法维持正常的生理氧化还原调节功能[124, 153],导致膜脂过氧化和抗氧化酶的激活[33],而膜脂过氧化会增加细胞膜的通透性、影响细胞膜的完整性、流动性和选择性[101]. 膜损伤是评估纳米颗粒暴露对藻类毒性最常用的生物学指标之一[151],通常与磷脂代谢过程有关[33]. 当MNPs穿过细胞壁与细胞膜表面发生相互作用时可能破坏细胞膜的结构,也可能通过酶的激活或ROS的过量积累而造成膜损伤(细胞膜通透性的增加或细胞膜穿孔)[33],还可能导致乳酸脱氢酶的释放,这可能是诱导细胞死亡的毒性机制之一[113, 131]. 例如,三氧化二铬纳米颗粒(Cr2O3 NPs)可诱导 C. reinhardtii的酯酶活性,导致磷脂降解[154];有研究表明,CuO NPs能够引起 C. vulgaris轻微的氧化应激和膜损伤,并且使其发生叶绿素中间体的积累、膜脂重塑、谷胱甘肽代谢紊乱等代谢途径的变化[142]. 此外,ROS过量产生还会抑制光合作用相关蛋白的基因编码使其在暴露后下调,甚至对线粒体膜及DNA造成损伤[33].
(3) MNPs溶解释放出离子对藻类细胞产生毒性. 如前所述,MNPs在水环境中会因溶解释放出金属离子,这些离子往往对藻类是有害的,如Ag+、Cu2+、Zn2+等. 溶解过程既可以发生在细胞外,也可以发生在细胞内[155-156],并且离子在细胞内可能重新形成能够破坏液泡的纳米颗粒[122]. 尽管溶出的金属离子对于总累积质量的影响非常有限,但它们的毒性作用不可小视[138]. Navarro等[141]利用半胱氨酸-银络合的方法证实了Ag+会影响C. reinhardtii的光合作用,且Ag NPs对其产生的毒性作用主要是由溶解的Ag+所引起;Wang等发现CuO NPs对C. vulgaris代谢途径的影响与氧化应激和膜损伤有关,而它们主要来源于溶解的Cu2+[142];近50%的文献将ZnO NPs的毒性作用主要归因于溶解的Zn2+[138]. 目前,许多关于MNPs的毒理学研究将等当量离子暴露的毒性与MNPs的毒性进行比较[118, 141-142],但由于MNPs在水溶液中的溶解往往需要较长时间,溶液中金属离子的浓度处于动态变化中[138]. 因此,评估溶出离子毒性对MNPs毒性效应的贡献需要综合其溶解及吸收动力学,才能更好地了解MNPs的毒性机制、评估MNPs在水环境中的生态风险.
-
纳米颗粒在水环境中与共存污染物的相互影响是非常复杂的,它们可能对藻类产生协同或拮抗作用. 据报道,纳米颗粒可以通过多种不同的机制增加污染物的毒性:(1)被生物摄取后在其体内释放出所吸附的污染物[165];(2)吸附水中其它阳离子,减少其与重金属离子的竞争,从而增加重金属离子的毒性/累积[166-167];(3)促进污染物代谢生成更活泼的代谢产物而增加毒性[168];(4)破坏细胞膜,使得污染物累积和毒性增强[166]. 但纳米颗粒也可能降低其它污染物的生物有效性和毒性,如吸附污染物后发生沉降或在细胞内储存、促进污染物在体外的无毒性降解或与污染物竞争膜受体等[166]. 同时其它污染物也会通过改变生物细胞膜通透性和完整性、改变生物体的耐受性、清除或增加ROS、改变纳米颗粒的表面电荷和官能团等影响纳米颗粒的毒性[166].
MNPs进入水环境中后,不可避免地会与环境中共存的污染物及溶解性的有机物发生相互作用,还可能吸附重金属离子或有机物形成复合物,这些复合物的形成可能会改变MNPs的大小、表面电势、浓度等[169],从而影响其生物可利用性以及与藻类的相互作用. MNPs吸附污染物后不仅能够作为污染物在环境中的运输载体,还可能在与生物发生相互作用的过程中重新释放污染物,使得污染物的累积和毒性发生变化[170]:(1)与重金属离子的相互影响. MNPs能够通过静电相互作用和/或表面络合吸附重金属离子[166, 171- 172],影响重金属离子对藻类的毒性效应. 例如Al2O3 NPs与Pb2+对球等鞭金藻(Isochrysis galbana)的毒性存在协同效应,Al2O3 NPs能够显著提高I. galbana对Pb2+的累积,且细胞内Al2O3 NPs上的Pb2+比游离Pb2+具有更强的毒性[173]. (2)与有机污染物的相互作用. 已有研究表明MNPs表面能够通过疏水键、氢键等相互作用吸附有机物[53, 174-175];共存的有机物也能够影响MNPs的累积和毒性,如十六烷基三甲基氯化铵(cetyltrimethyl ammonium chloride,CTAC)能够静电吸附在ZnO NPs表面,形成一层疏水膜,阻止ZnO NPs溶解释放Zn2+,从而减少ZnO NPs在C. vulgaris中的累积、减轻其毒性[169].
MNPs与其它纳米材料的共暴露也是影响其与藻类相互作用的重要因素. 碳纳米管(carbon nanotubes,CNTs)可以缓解铜纳米颗粒(Cu NPs)对海洋硅藻S. costatum生长和光合作用的抑制,Cu2+在CNTs上的吸附和Cu NPs与CNTs在介质中团聚是其主要原因[176]. Huang等[171]报道了Ag NPs与Fe2O3 NPs或聚苯乙烯纳米颗粒共暴露对C. reinhardtii和O. danica的毒性,其中无毒的Fe2O3 NPs能够通过吸附Ag+减轻Ag NPs对C. reinhardtii的毒性,并且通过竞争性地抑制Ag NPs的摄取来降低Ag NPs对O. danica的毒性. 然而,目前MNPs与其它纳米材料的共暴露对藻类的影响机制还不够明朗,缺乏更多的实验证据,还需要更多的研究来全面了解相同或不同形态和组成的纳米材料与藻类的相互作用.
除上述污染物外,水中的NOM分子也可能通过改变MNPs的稳定性、促进或抑制MNPs溶解释放金属离子、吸附于MNPs表面使其电负性增加、阻止MNPs与细胞直接接触、增强或减少MNPs诱导的氧化应激来影响其毒性[74]. 有文献报道称,NOM的存在能够在一定程度上缓解TiO2 NPs对P. subcapitata生长的抑制[177];FA能够提高CuO NPs的分散性,促使其溶解释放出高达6倍的Cu2+,因而使CuO NPs对铜绿微囊藻(Microcystis aeruginosa)的毒性增加,但同时又能够减少藻细胞ROS的产生[178];此外,Li等[179]的研究表明Au NPs表面吸附的HA与大肠杆菌(Escherichia coli)表面的脂多糖负电荷基团之间存在静电排斥作用,从而阻碍了Au NPs与E. coli的结合、减轻细菌细胞膜损伤,降低了Au NPs的毒性. 由此可见,不同成分的NOM对不同MNPs的环境行为有着不尽相同的影响,因而对MNPs与藻类相互作用的影响也较为复杂. 目前已有研究结果并不能直接解释大规模复杂水环境中NOM对MNPs的影响,并且缺乏对该过程分子机制的解释,需要更多的模型研究和基因组学/蛋白组学/代谢组学研究阐明真实环境下NOM对MNPs与藻类相互作用的影响及其机理.
-
当藻类暴露于对其有害的纳米颗粒时会诱导产生多种防御策略以尽可能减小纳米颗粒带来的危害,如激活抗氧化防御系统以消除生成的ROS[180-181]、向胞外分泌EPS以形成保护层[182-183]、通过细胞内的局部储存或转化减少细胞内纳米颗粒的含量等[126].
(1) 抗氧化防御机制. ROS的过量积累是MNPs诱导毒性的重要机制,为了应对这一变化,藻细胞内超氧化物歧化酶(superoxide dismutase,SOD)和过氧化物酶(peroxidase,POD)将作为主要的抗氧化酶被激活[33];过氧化氢酶(catalase,CAT)活性、可溶性蛋白及糖类含量亦有所提高,这表明抗氧化酶等物质将参与藻类细胞抗氧化应激[184]. 尽管如此,超出抗氧化酶清除能力的ROS依旧会对藻细胞造成氧化损伤,如2.3所述.
(2) 藻类分泌EPS,在胞外形成一层保护层[182]. EPS主要由蛋白质、多糖、腐殖质等组成[185],通常形成动态的双层结构:可溶性EPS(soluble-EPS,S-EPS)和结合性EPS(bound-EPS,B-EPS)[186]. Zhao等观察到CuO NPs的暴露使C. pyrenoidosa外的EPS层增厚了近4倍[128]. EPS具有丰富的官能团,能够与外源性有机化合物和无机离子结合[187],可以通过静电作用和化学键作用减少MNPs在藻类表面的吸附,甚至改变MNPs的表面电荷,降低其与藻类的亲和度[33]. EPS还可以通过阻止MNPs与藻细胞直接接触而降低其累积和毒性. Liu等认为C. vulgaris能够通过调节B-EPS的分泌防止ZnO NPs进入细胞[169]. 但是,MNPs暴露导致EPS分泌增多,意味着藻类需耗费更多的能量和物质,因此其生长将受到影响[188]。此外,EPS的组成也会受到MNPs暴露的影响,如Ag NPs的暴露能使C. reinhardtii的EPS化合物从高分子量组分转变为低分子量组分[188],而高分子量组分可以促进纳米颗粒的团聚和沉降[189],其含量减少将使纳米颗粒在水环境中停留更长时间,可能增加其对包括藻类在内的水生生物的风险.
(3) 在细胞内对MNPs进行局部储存或转化. 一些MNPs在进入藻细胞后仍保留其纳米颗粒形态(如Au NPs),此时藻类可以直接将其沉积在不同的细胞位置/结构(如液泡)中,以抑制其在细胞内的转化和毒性[33]. 如Ag NPs在进入C. reinhardtii后累积于胞浆质间隙,减少了其对细胞器的破坏[126];在O. danica的液泡中发现了Ag NPs的存在[62];由CuO NPs转化形成的Cu2O NPs也被储存在C. pyrenoidosa的液泡中[128]. MNPs在藻类细胞中的暂时储存虽可在一定程度上降低其毒性,但可能会增加向更高营养级生物传递的风险;此外,MNPs在液泡内仍可能发生溶解,产生具有更高毒性的离子,值得进一步研究.
此外,一些研究结果显示, 低浓度MNPs暴露反而会刺激藻类的生长,如5 mg·L−1 CeO2 NPs和0.01—1 mg·L−1 Ag NPs暴露分别促进了三角褐指藻(Phaeodactylum tricornutum)和自养小球藻(Chlorella autotrophica)的生长[105, 151];Fe2O3 NPs在0—20 mg·L−1范围内能够促进斜生栅藻(Scenedesmus obliquus)的生长,这可能是因为较低浓度下藻细胞能够以某种方式应对氧化应激并维持生长,或MNPs发生微量溶解释放的离子(如Fe3+)能够作为微量元素来源对藻类生长产生积极影响[185].
-
综上,本文重点总结了MNPs在水环境中的行为及其与藻类的相互作用. MNPs进入水环境后将发生一系列物理、化学变化,如团聚及沉降、溶解、硫化反应、光化学反应等,以上过程受到MNPs自身的性质(大小、形状、表面电荷、晶体结构)及环境化学性质(pH、离子强度、阳离子价态、NOM和溶解氧的存在等)的影响. 目前已有许多关于MNPs与藻类相互作用的研究. MNPs与藻类表面的直接接触和吸附是它们相互作用的第一步,是后续发生累积和毒性效应的前提. MNPs可穿过藻类细胞壁,使细胞膜发生损伤或穿孔后进入细胞,也可通过离子通道或内吞的方式在细胞内累积. MNPs在藻细胞表面的大量聚集可能产生物理遮蔽,影响藻类的光合作用,同时也可诱导ROS过量积累,引发氧化应激,发生磷脂降解或膜脂过氧化,造成细胞膜损伤,甚至损伤线粒体膜及DNA;MNPs溶解释放的离子也是其对藻类造成毒性的重要原因. 此外,环境中其它污染物及NOM均可能改变MNPs在藻类中的累积和毒性效应,并因不同的物质和藻类而不同. 最后,藻类在应对MNPs胁迫时将激活抗氧化酶、增加胞外EPS的分泌或将MNPs在细胞内进行局部储存或转化.
然而,由于现阶段许多研究结果局限于简单实验室条件下,无法反映复杂环境中MNPs的综合命运,许多机制还未明确,如MNPs如何进入叶绿体、线粒体、细胞核,与其他纳米材料如何共同对藻类产生影响,水中NOM影响MNPs累积和毒性的分子机制,MNPs如何从藻细胞中排出等. 此外,在评估MNPs的生态风险时,还应重视MNPs与环境中其它污染物(如重金属离子、有机污染物、其它纳米材料等)及NOM的相互作用,以更全面、准确地评估MNPs对水中藻类的生态风险. 因此,未来需要更多关于真实环境中MNPs的环境行为及其与藻类相互作用的研究,并通过多组学技术阐明更深层次的作用机制.
金属纳米颗粒的环境行为及其与藻类的相互作用概述
Environmental behavior of metal nanoparticles and their interactions with planktonic algae:A review
-
摘要: 金属纳米颗粒在各领域的应用日益广泛,可通过多种途径进入环境,并在水生生态系统中积累. 因此关于其进入水环境后的行为及与水中初级生产者—藻类相互作用的研究至关重要. 金属纳米颗粒在水中会发生团聚、沉降、溶解、硫化反应及光化学反应等,这些行为受到自身理化性质(大小、形状、表面电荷、晶体结构、化学组成等)和环境因素(pH、离子强度、阳离子价态等)的影响,进而改变金属纳米颗粒在藻类表面的吸附聚集和可能的吸收累积. 金属纳米颗粒还可能影响藻类光合作用、引起氧化应激、甚至造成藻类的凋亡. 同时,与金属纳米颗粒共存的其它污染物及天然有机质也可能改变金属纳米颗粒的行为、生物吸附、生物累积和生物效应. 相应地,藻类在面对金属纳米颗粒胁迫时也会启动自我防御机制. 尽管如此,真实环境中金属纳米颗粒与藻类的相互作用及分子机制仍有待进一步研究.Abstract: Metallic nanoparticles (MNPs) have been widely used in various fields. They can enter the environment through a variety of ways and accumulate in aquatic ecosystems. Researches on their behavior and interactions with planktonic algae, the important primary producers in the aquatic environment, are important. Once MNPs enter aquatic environment, they will undergo aggregation, settlement, dissolution, sulfidation, and photochemical reactions. The behavior of MNPs is affected by their own physicochemical properties (e.g., size, shape, surface charge, crystal structure and chemical composition) and environmental factors (e.g., pH, ionic strength, cation valence, etc.), which in turn changes the adsorption of MNPs on algal surface, as well as possible absorption and accumulation. MNPs may also affect algal photosynthesis, induce oxidative stress, and even cause algal apoptosis. At the same time, other pollutants and natural organic matter coexisting with MNPs may change the behavior, biosorption, bioaccumulation and biological effects of MNPs. Correspondingly, algae will also activate self-defense mechanisms when facing the stress of MNPs. Nevertheless, interactions between MNPs and algae in the real environment and the molecular mechanism underlying the above interactions still need to be studied in the future.
-
Key words:
- metallic nanoparticles /
- algae /
- environmental behavior /
- bioaccumulation /
- toxicity
-
近年来,我国高铁事业迅猛发展,运营里程预计在2025年达到3.8×104 km[1-2]。高铁采用真空集便器代替传统沿线直排的如厕方式[3],集中收集和处理列车运行过程中产生的集便器污水[4]。而粪便、尿液及部分洗漱用水较常规市政污水来说具有高有机物、高氨氮、低碳氮比[5-6]等特点,极大增加了站段污水处理设施的压力。因此,在铁路段污水排放需满足《污水排入城镇下水道水质标准》 (GB/T31962-2015) B级标准[7]情况下,选择更高效的工艺对集便器污水中污染物,特别是氨氮、总氮的去除,成为铁路运输行业的水污染治理的重要内容。
集便器污水水质与黑水水质相近,有机物及悬浮固体浓度较高,且氨氮远大于有机物浓度,还可能含有病原微生物[8-9],多采用化学脱氮方法及传统硝化反硝化工艺进行处理。部分国家采用化学脱氮方法,如电解法、地面焚烧法等,但需对处理后水质及处理装置内产生的悬浮性固体进行二次分离,操作流程较复杂[10]。而传统硝化反硝化工艺脱氮往往需要足够碳氮比,在碳源不足情况下会大大影响生物脱氮处理效果,导致总氮去除效果偏低[11]。如采用单级与多级A/O-SBR工艺处理黑水,在未加碳源情况下,单级处理总氮平均去除率为51.5%;而多级处理虽有所提升,但最高仅为87.8%[12]。
厌氧氨氧化反应指在厌氧或缺氧的环境条件下厌氧氨氧化菌以氨氮为电子供体,以亚硝态氮为电子受体作用产生氮气[13]。相较于常规的生物脱氮工艺,厌氧氨氧化反应及其耦合工艺过程无需碳源消耗且可降低50%的曝气量[14-15],目前已成功应用于多种低碳氮比污水的处理。本研究采用以厌氧氨氧化反应为核心的一体式短程硝化-厌氧氨氧化耦合反硝化工艺对高铁动车段内实际集便器污水中的氨氮、总氮及耗氧有机物 (以COD计) 进行去除研究,在实验进行的不同阶段取样分析观察系统内微生物群落多样性,以了解污染物去除性能在各个阶段的变化及耦合系统的去除机制,从而为该工艺扩大研究及现场应用提供参考。
1. 材料与方法
1.1 污水水质来源及接种污泥
本研究选取武汉某动车站段作为实验地点,取站段内厌氧化粪池出口处的集便器污水作为实验用水。污水水质指标:氨氮为750~1 000 mg·L−1,总氮为800~1 100 mg·L−1,COD为800~1 800 mg·L−1,亚硝态氮及硝态氮均<1 mg·L−1,pH为7.8~8.2。装置内接种悬浮污泥为含厌氧氨氧化菌 (anaerobic ammonium oxidation bacteria,AnAOB)的颗粒污泥,取自北京高碑店污水处理厂内高氨氮废水处理反应器,MLSS为2 000~2 500 mg·L−1。生物膜填料取自实验室成功运行的厌氧氨氧化耦合反应器,该反应器脱氮性能良好。
1.2 实验装置及运行方式
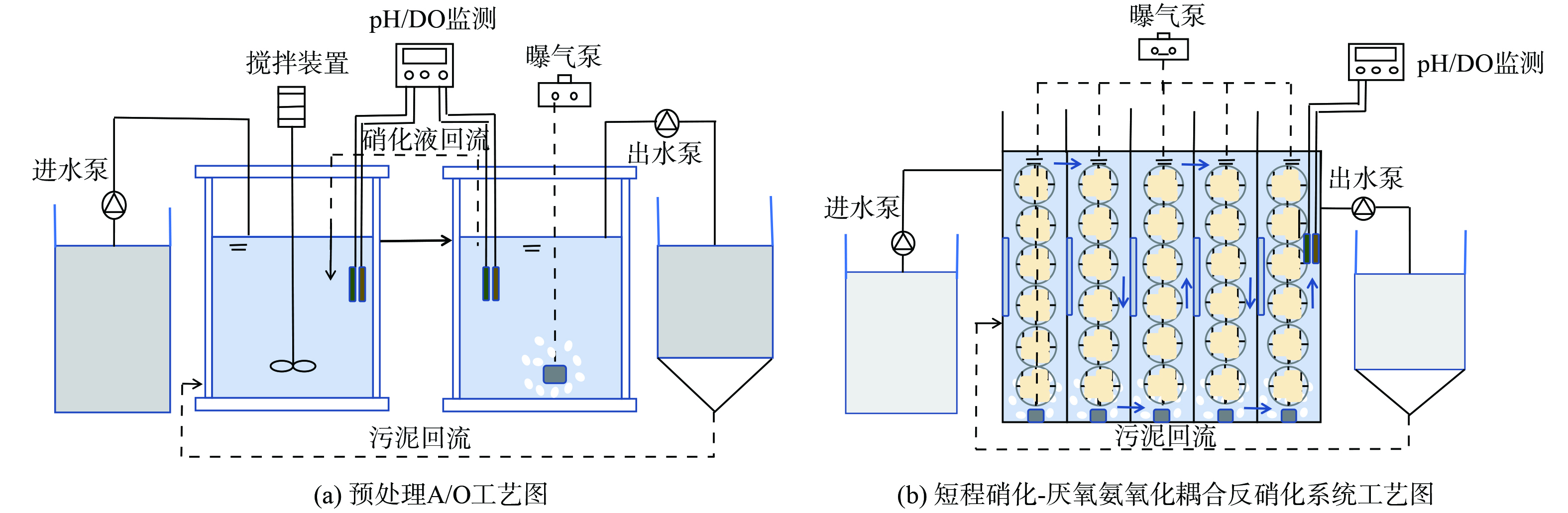
为减轻水质波动变化及高负荷水质对后续耦合反应系统所承受的冲击负荷、增强微生物活性,采用A/O工艺进行预处理。A/O工艺装置包含集便器污水进水水箱、厌氧反应池、好氧反应池及沉淀池。进水箱为塑料圆柱形水桶,容积约50 L;厌氧池为直径25 cm、高20 cm的圆柱形容器,容积为10 L、有效容积为6 L;好氧池为直径32 cm、高30 cm的圆柱形容器,容积为25 L、有效容积为18 L;沉淀池容积约20 L。其中,厌氧池设电动搅拌器,使反应池中的活性污泥能充分混合;好氧池由曝气机供气,曝气石曝气。短程硝化-厌氧氨氧化耦合反硝化实验装置由有机玻璃制成,其长、宽、高分别为50 cm、10 cm、115 cm,容积为57.5 L、有效容积为50 L。内置1 cm×1 cm×1 cm的立方体聚氨酯海绵生物膜填料,填料填充比为85%。沉淀池容积约15 L。预处理及耦合装置中分别放置加热棒以提供适宜微生物生长的温度条件。配置实验设备型号如下:蠕动泵 (YZ1515x,兰格恒流泵有限公司) 、曝气泵 (ACO-004,森森集团股份有限公司) 、加热棒(AR-4,森森集团股份有限公司)、搅拌机(DJ-120,上海予星仪器设备有限公司)及便携式pH、DO测定仪 (Multi 3620,上海世禄仪器有限公司) 。具体工艺流程如图1所示。
预处理及耦合工艺设置适宜的实验环境,使集便器污水中的氨氮及COD在氨氧化菌(ammonia oxidizing bacteria,AOB)、厌氧氨氧化菌(AnAOB)和反硝化菌的协同作用下被反应去除,具体参数如表1所示。同时,实验启动过程由人工配水与经预处理后的集便器污水混合作为实验进水,采用分阶段不同比例的进水方式,具体进水方式如表2所示。
表 1 不同工艺实验环境参数Table 1. Environmental parameters of different process test工艺类型 温度/ ℃ 溶解氧/(mg·L−1) HRT /h pH 污泥回流比 A/O工艺 25±1 A:0.01~0.04 O:1.00~2.50 A:6.00 O:28.80 7.60~8.30 150% 短程硝化-厌氧氨氧化耦合反硝化工艺 32±2 0.10~0.40 96.00 7.90~8.10 150% 表 2 短程硝化-厌氧氨氧化耦合反硝化系统运行参数Table 2. Running parameters of the denitrification system of short-cut nitrification and anammox阶段 时间/d 集便器污水所占比 氨氮/(mg·L−1) COD/(mg·L−1) Ⅰ 0~28 20% 550~600 110~160 Ⅱ 29~38 40% 490~550 210~230 Ⅲ 39~48 60% 500~550 280~300 Ⅳ 49~59 80% 500~550 340~360 Ⅴ 60~75 100% 440~500 400~450 1.3 主要检测指标及分析方法
实验过程中氨氮、亚硝态氮、硝态氮和总氮的检测均参照《水和废水监测分析方法》[16]。COD的测定采用哈希快速消解法。微生物分析样品取自系统添加集便器污水前及稳定运行后2个阶段,随机取单块海绵生物膜填料,剪碎后加入50 mL离心管中,经超纯水超声处理后得到生物膜样品。采用NEXTflexTM Rapid DNA-Seq Kit (Bioo Scientific,美国)进行建库,利用Illumina公司的Miseq PE300/NovaSeq PE250平台进行测序 (上海美吉生物医药科技有限公司) 。
1.4 计算方法
短程硝化厌氧氨氧化脱氮贡献率A采用公式 (1) ,反硝化脱氮贡献率采用公式 (2) 进行计算[17]。
A= (1+1.32-0.26) ×(NH+4-N)remTNrem (1) D=1−A (2) 式中:(NH4+-N)rem、TNrem分别为耦合反应器中氨氮和总氮去除量,mg·L−1。
2. 结果与讨论
2.1 预处理工艺的污染物去除性能
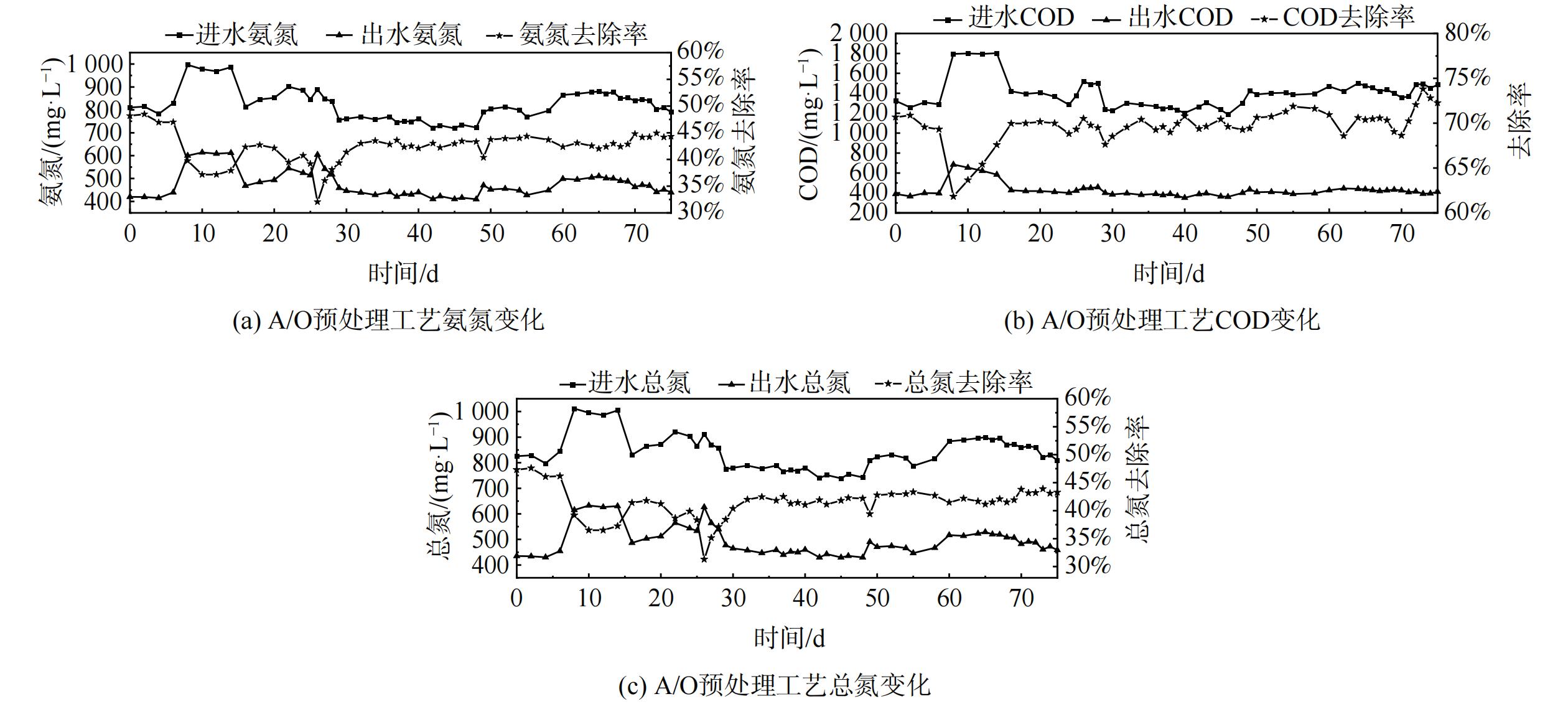
采用A/O工艺对厌氧池中的集便器污水进行初步降解,为后续一体式短程硝化-厌氧氨氧化耦合反硝化系统提供适宜的水质条件。反应器运行期间的污染物去除性能具体如图2所示。
在为期75 d的集便器污水处理过程中,出水中的氨氮、COD和总氮随着进水水质的波动存在一定差异性,但整体较为稳定。第1~51天溶解氧为1.50~2.00 mg·L−1,氨氮、COD及总氮去除率始终保持在约43%。从第52天开始,将溶解氧提升至2.00~2.50 mg·L−1,氨氮降解速率有所提升。但由于进水中碳氮比较低, O池在持续曝气下将大部分有机物消耗并产生少量亚硝态氮,A池缺乏额外基质来促进反硝化菌的生长,总氮的去除效率较之前有所降低。而在曝气提升作用下,COD进一步降低。这与林明等[18]的研究一致,采用A/O-MBBR组合工艺对生活污水进行实验研究,在未添加碳源的情况下,出现总氮处理不达标情况。最终,预处理后的氨氮在400~500 mg·L−1,去除率约为44%;COD降低的速率较快,进水COD在(1 400±50) mg·L−1的条件下出水COD约为400 mg·L−1,降低了约73%;由于集便器污水中存在少量有机氮及A/O工艺生成的亚硝态氮、硝态氮,总氮的去除效率约为43%。
污染物的降解效果表明,A/O预处理工艺的运行状况稳定且良好,为后续短程硝化厌氧氨氧化耦合反硝化工艺的运行提供了保证。因短程硝化厌氧氨氧化耦合反硝化装置内接种生物膜填料曾在低氨氮、无COD环境中培养,可能无法承受进水中过高氨氮及COD。则A/O工艺对进水预处理后出水降低部分氨氮及COD,可在一定程度上减少对耦合装置内菌种的冲击破坏作用,最大程度保证后续实验的准确性。同时,出水水质仍属低碳氮比污水,无大量硝态氮积累,仅含有少量亚硝态氮,可为后续厌氧氨氧化反应的进行提供反应基质[19]。
2.2 短程硝化-厌氧氨氧化耦合反硝化系统的污染物去除性能
2.2.1 耦合系统处理效果分析
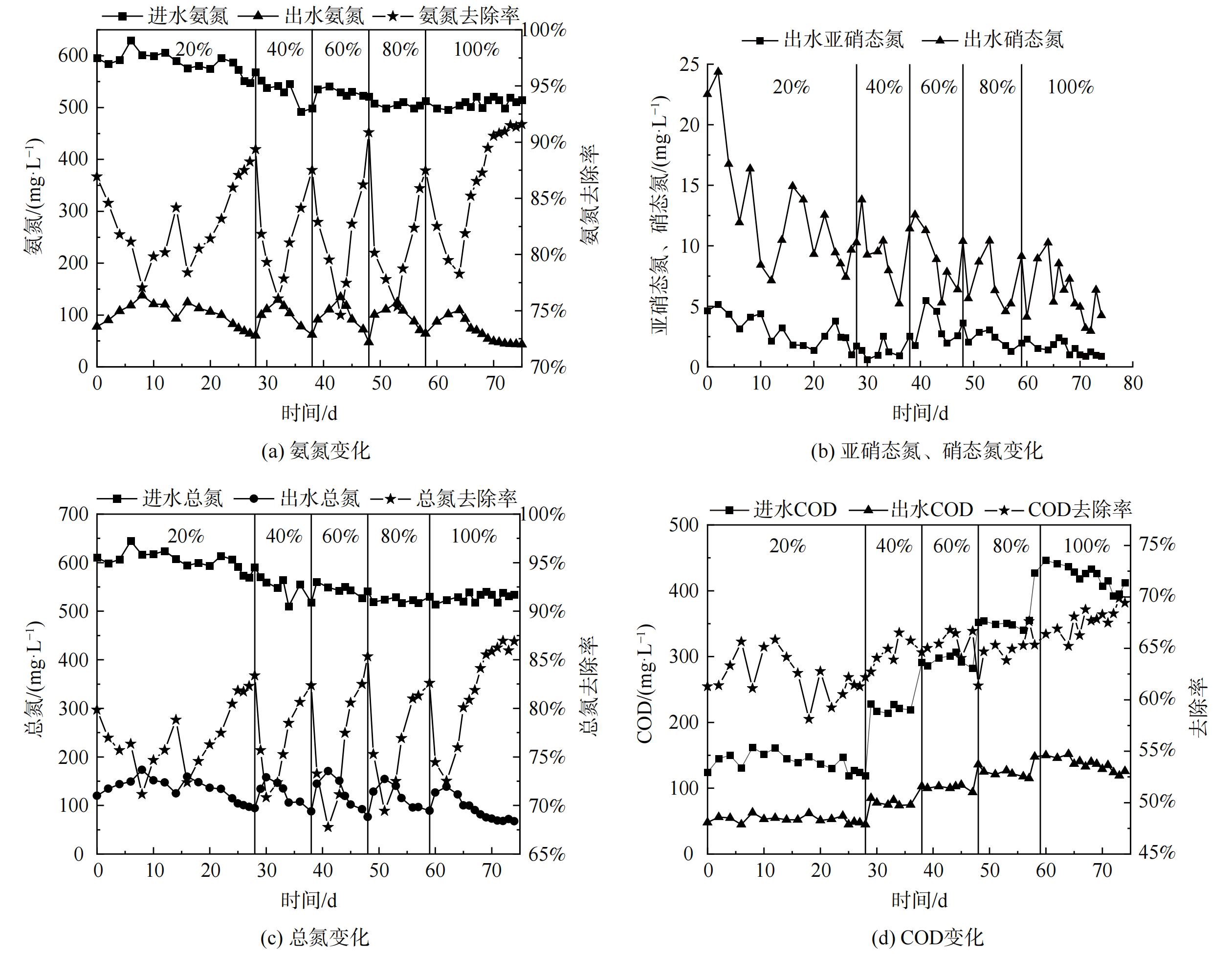
按照集便器污水所占比例分别为20%、40%、60%、80%和100%的原则进水。在75 d的运行过程中,短程硝化-厌氧氨氧化耦合反硝化工艺的进水全部替换为经预处理后的集便器污水,污染物去除效果较好,如图3所示。
第Ⅰ阶段 (第0~28天) 以20%比例集便器污水作为实验进水。在第1~7 天,出水氨氮升高,由最初的77 mg·L−1逐渐升至137 mg·L−1,总氮去除负荷由0.144 kg·(m3·d)−1降至0.129 kg·(m3·d)−1,氨氮去除率由87.03%下降为77.06%。按比例在进水中增加集便器污水会导致系统性能出现短暂的波动,且反应初期所需恢复时间较中后期长。这是由于采用配水启动反应器时系统内有机物较低,反硝化菌生长量较少。观察耦合系统的波动现象分析,这可能是由于系统中增加一定量的耗氧有机物 (以COD计) ,使短期内反硝化菌生长速度加快。因此,突增过高的COD表明过多耗氧有机物可能会加强自养反硝化和异养反硝化脱氮,促进异养微生物生长而抑制AnAOB活性,使厌氧氨氧化脱氮受到抑制[20]。由于本研究采用逐步增加进水比例方式,虽进水COD呈升高趋势,但耦合系统保持了较为平稳地增加。系统内反硝化菌增长速度及趋势也受到控制,并未保持像刚添加集便器污水时的状态。实验添加20%比例集便器污水过程中,由于AOB对氧的亲和力高于亚硝酸盐氧化细菌(nitrite-oxidizing bacteria, NOB),采用低氧曝气策略,控制溶解氧为0.10~0.25 mg·L−1。在溶解氧浓度不足的情况下,促使系统内氧气优先与AOB结合,以达到减缓NOB的好氧速率来抑制其生长的目的[21-23],同时促进耦合系统内短程硝化反应的发生。观察发现出水亚硝态氮值呈逐步降低趋势,这说明此时系统内NOB可能已完全被洗脱出,AOB菌种已成为优势菌种。进水中的氨氮在AOB的作用下利用系统提供溶解氧部分氧化为亚硝态氮,之后AnAOB利用产生的亚硝态氮及水中剩余氨氮发生厌氧氨氧化反应降解污染物,并生成硝态氮。同时,进水中COD持续增加说明耗氧有机物被反硝化菌消耗,将系统内可能存留的少量亚硝态氮、厌氧氨氧化生成的硝态氮还原生成氮气从系统中逸出。不同功能菌群在协同作用下对集便器污水中的污染物进行降解,实现短程硝化厌氧氨氧化耦合反硝化脱氮。经过为期28 d的适应运行,出水氨氮为60.2 mg·L−1,去除率为89.4%,总氮去除负荷达0.141 kg·(m3·d)−1,出水COD为45 mg·L−1,降低了约62%。
第Ⅱ阶段 (29~38 d) 添加40%比例集便器污水后,由于进水中COD进一步升高,系统波动导致氨氮去除率提升变化较小。但经一段时间水质适应后,系统很快恢复至较高处理水平,这说明采用按比例添加集便器污水可保证系统内微生物保持平衡状态。为加强短程硝化作用,同时提升厌氧氨氧化反应速率,溶解氧提升至0.25~0.35 mg·L−1。此时,出水亚硝态氮变化趋势较为平稳,这说明耦合系统内AOB活性得到增强,AnAOB获得充足的生长基质,抵消亚硝酸盐积累现象则未产生AnAOB受抑制情况[24-26],体现了AOB及AnAOB间协同生长作用优势。同时,研究表明,NOB最适宜的溶解氧为0.7~1.4 mg·L−1,此时系统溶解氧低于该范围,故NOB活性将受到抑制,这表明该阶段耦合系统内短程硝化反应稳定发生[27-29]。同时,出水硝态氮呈持续降低趋势可知其已被反硝化菌利用,但由于进水中COD较低,造成COD降低程度较小。此阶段最终出水氨氮为57.02 mg·L−1,去除率为88.5%,总氮去除负荷为 0.136 kg(m3·d)−1,出水COD为75 mg·L−1,降低了66%。
之后,第Ⅲ (第39~48天) 、Ⅳ (第40~59天) 、Ⅴ (第60~75天) 阶段,逐步添加60%、80%及100%比例集便器污水,保持耦合系统内溶解氧为0.25~0.35 mg·L−1。系统始终保持较好的抗冲击负荷能力和恢复能力,出水氨氮去除趋势与前2个阶段相似。此现象说明耦合工艺在逐步添加定比例的集便器污水后,系统对水质的适应性已逐步增强,恢复时间逐步变短,促使耦合系统的氨氮、COD及总氮处理率随之增加。因此,在第Ⅲ、Ⅳ阶段,出水氨氮分别为47.62 mg·L−1和54.21 mg·L−1,氨氮去除率分别为90.86%和89.41%,总氮去除负荷分别为0.139 kg·(m3·d)−1和0.137 kg·(m3·d)−1,出水COD分别为94 mg·L−1和115 mg·L−1,分别降低了67%、68%。直至添加进水比例至100%,出水亚硝态氮含量变化趋势基本保持一致,未出现逐步升高现象,这说明耦合系统内的短程硝化及厌氧氨氧化反应稳定协同发生。在第Ⅰ至Ⅴ阶段采用多阶段逐步采用低氧曝气(<0.4 mg·L−1)并根据运行情况调整固定至适宜范围,对调节集便器污水处理耦合系统内的氧环境、对短程硝化反应的发生和强化及促进厌氧氨氧化反应与其耦合发生均有促进作用[30-31]。另外,对应COD处于适宜水平,可实现短程硝化厌氧氨氧化作用与反硝化作用有效耦合[32],共同提升耦合系统的脱氮效能。
最终,系统稳定运行100%比例的集便器污水后,出水氨氮、总氮和COD仅为40.25 mg·L−1、67.40 mg·L−1和120 mg·L−1,去除率分别达90.84%、86.90%和70.02%,最高总氮去除负荷为0.141 kg·(m3·d)−1。同时,系统生成亚硝态氮和硝态氮随着COD的升高呈下降趋势,与反应运行初期相差20 mg·L−1以上,这表明在第Ⅰ~Ⅴ阶段中耦合系统的反硝化过程良好,且硝态氮优先被反硝化菌利用。因此,观察第Ⅰ~Ⅴ阶段发现,在进水COD逐步升高情况下,系统内反硝化菌增长速度较稳定,未像初步添加20%比例集便器污水时出现大量增长趋势。这说明耦合系统已完全适应该水质,且短程硝化、厌氧氨氧化及反硝化反应已形成平衡状态。出水氨氮、COD及总氮的降解效果可达到《污水排入城镇下水道水质标准》 (GB/T31962-2015) B级标准。这说明耦合系统的运行良好,对集便器污水的处理性能较优。
2.2.2 耦合系统去除贡献分析
在逐步添加集便器污水进水比例时,短程硝化厌氧氨氧化和反硝化作用对脱氮的贡献也发生了不同变化。当进水比例为20%,由于耦合系统内初步增加一定量COD,导致反硝化作用较未添加时有所增强。系统内短程硝化厌氧氨氧化脱氮贡献率低于反硝化脱氮贡献率,分别为31.22%和68.78%,出水氨氮及COD分别平均降低了77.06%和62.01%。耦合系统在适应进水水质变化后,增加进水比例为40%、60%时,短程硝化厌氧氨氧化的脱氮贡献率出现上升趋势,提升至45.64%和46.72%,反硝化脱氮贡献率降低至54.36%和53.28%,耦合系统平均出水氨氮及COD分别为62.31 mg·L−1、47.62 mg·L−1和75 mg·L−1、94 mg·L−1。由于亚硝酸盐可同时作为厌氧氨氧化反应和反硝化反应的电子供体[33],二者作用可共存且对亚硝酸盐存在竞争关系[34]。但进水碳氮比较小,无法为反硝化作用提供足够驱动力与厌氧氨氧化反应争夺利用亚硝酸盐,因此厌氧氨氧化反应较反硝化反应竞争力更强[35-36]。因此,在后期进水比例加大时,进水氨氮充足的条件下逐渐形成耦合系统内AnAOB生长及对亚硝酸盐竞争均大于反硝化菌的局势[37],系统内短程硝化厌氧氨氧化作用的脱氮贡献率也逐步大于反硝化脱氮作用。因此,在进水比例增加至80%及100%,短程硝化厌氧氨氧化及反硝化对脱氮的平均贡献率基本保持在77.90%和22.10%。这说明耦合系统已完全适应水质变化,实现最佳的耦合脱氮效果。
2.3 微生物群落多样性
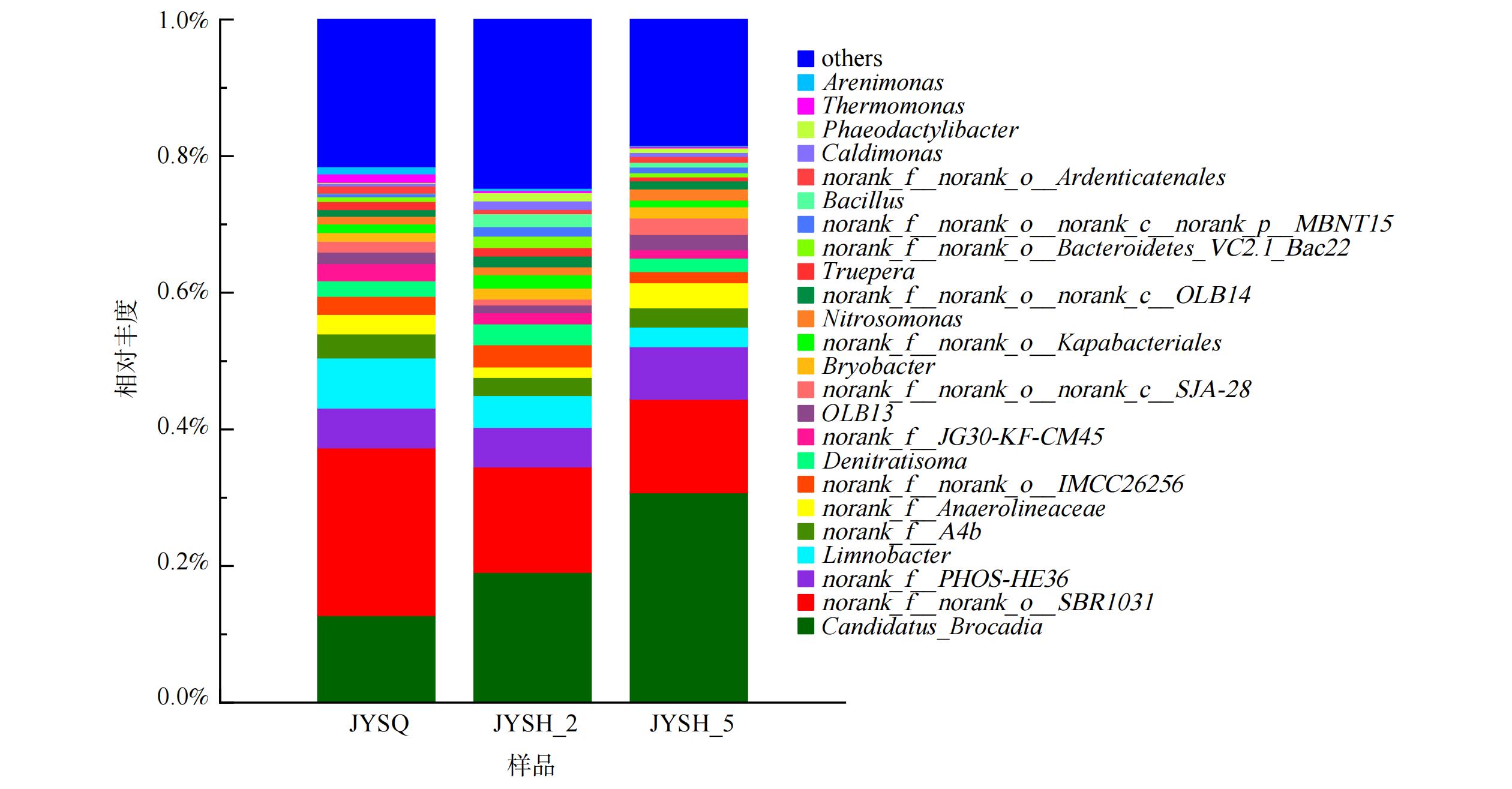
为更加清楚地了解污染物去除性能的变化与系统的去除机制,对短程硝化-厌氧氨氧化耦合反硝化工艺不同运行时期的微生物进行群落多样性分析。分别取进集便器污水前第5格 (JYSQ) 、处理实际集便器污水且运行稳定后第5格 (JYSH_2) 和第2格 (JYSH_5) 内的海绵填料进行16sRNA高通量测序,以了解工艺运行过程中属水平上的微生物群落结构变化,从而获得厌氧氨氧化耦合工艺高效运行的策略。
由图4可知,Candidatus_Brocadia始终是反应器内的AnAOB优势菌属且随工艺的运行,其相对丰度逐渐升高。第5格填料( (JYSQ) 内所占比例在运行集便器污水前为13%,耦合工艺稳定运行后升高至约30.70%。这证明AnAOB在填料内较好生长,与系统脱氮性能的逐渐优化相对应。另外, AOB菌属亚硝化单胞菌Nitrosomonas和反硝化菌属Denitratisoma均被检出,而无亚硝酸盐氧化菌(NOB)相关菌属。启动前Nitrosomonas及反硝化菌属Denitratisoma丰度分别为1.37%和2%;启动后增加至1.62%及3.2%。从微生物的角度证明了耦合系统的作用机制,即在短程硝化-厌氧氨氧化耦合反硝化协同作用下去除集便器污水中的污染物。
同时,由于反应器递进式的运行方式,第2格中 (JYSH_2) 的污染物浓度远高于第5格 (JYSH_5) 。第2格与第5格填料中差异最大的菌属为Candidatus_Brocadia,两者相差11.60%。这说明进水污染物浓度会影响该菌种的丰富度,该菌属更适合在低基质浓度中生存[38]。而norank_f__norank_o__SBR1031、Limnobacter等菌属的相对丰度差异说明不同菌属对底物浓度的耐受程度不同。
2.4 工艺应用分析
1) 可行性。短程硝化-厌氧氨氧化耦合反硝化系统采用逐步提升进水中的集便器污水比例可减少系统处理效能波动及冲击,保证系统启动及运行所需恢复时间的稳定。同时,AOB、AnAOB及反硝化菌保持协同生长作用,维持AOB及AnAOB的增殖趋势,避免异养菌过量繁殖抑制AnAOB的活性,以减少对进水水质状况变化的适应时间。因此,采用逐步增加集便器污水进水比例,虽提升了各阶段有机物浓度,但系统已具备一定量的反硝化菌和抗冲击负荷能力,减缓 AnAOB的抑制作用,系统能更快适应新的进水水质,达到稳定的去除效果。
2) 应用性。传统SBR工艺处理粪便污水,氨氮去除率基本保持在61.00%~65.40%,鲁帅等[39]对SBR工艺进行改良提升后,平均氨氮去除率提升至67.10%~72.90%。同时,需采用在线控制,对工艺中搅拌、曝气、停曝搅拌、沉淀、排水这几个阶段进行合理且精密控制[40-41]。本研究采用一体式连续流装置,仅需对系统内曝气量及温度进行控制,控制方式较为简便,同时可以达到更优的出水处理效果。另外,采用一体式部分硝化-厌氧氨氧化 (PNA) 工艺处理低碳氮比污水可改善SBR等传统工艺的缺点,其中颗粒污泥及生物膜工艺处理效果更为突出。颗粒污泥在应用方面存在一定限制性,颗粒尺寸大小及溶解氧的控制对AnAOB菌种活性及系统脱氮性能均有影响[42]。而本研究采用生物膜工艺,相较颗粒污泥提升了对菌种的保留度,来抵抗水质变化的冲击。同时,形成AOB生长在外层,AnAOB生长在内层的稳定生物结构,可为功能菌提供适宜的溶解氧浓度环境。
3) 经济性。比较传统工艺及厌氧氨氧化耦合工艺对低碳氮比污水脱氮的实际应用,传统硝化反硝化工艺在运行成本及控制成本等方面要求较高,需要较多的曝气量并外加碳源,同时生成大量污泥[43]。本研究采用短程硝化厌氧氨氧化耦合反硝化工艺,无需补充碳源、曝气量低、污泥产量低。由表3可知,不论是污泥处置和补充药耗等产生的运行成本,还是系统的控制难度及运行维护,均优于传统硝化反硝化工艺[44]。但本工艺中所需厌氧氨氧化菌种倍增时间长,需要对生长环境进行维护 (保证工艺运行的适宜水温) ,故是否需要增加对菌种的补充及维护成本需结合各因素进行考虑。
表 3 短程硝化厌氧氨氧化耦合反硝化工艺与传统硝化反硝化工艺经济性比较[44]Table 3. Economic comparison between the coupled denitrification process of short-cut nitrification anammox and the traditional anammox process工艺类别 产泥量 污泥处置费用 药耗 电耗 加热 系统控制 短程硝化厌氧氨氧化耦合反硝化工艺 0.013 kg·m−3 (5.13 kg·d−1) 污泥可重复回收利用,处理量较少 — 32 元·d−1 3.33 元·d−1 (四季维持在32 ℃) 曝气控制、温控等 传统硝化反硝化工艺 0.117 kg·m−3 (47.01 kg·d−1) 70.50 元·d−1 (污泥脱水后泥饼外运) 8.7 元·m−3 (甲醇) 36 元·d−1 2.18 元·d−1 (冬季,25 ℃) 定时运行、内外回流等PLC控制、曝气控制等 其中,干污泥处理费用以400 元·t−1计,补充药耗费用以处理每吨水量计,电耗费用以处理设施运行设备所需的电费计,加热所需费用以武汉地区提升水温所产生电加热费用的平均值计 (传统工艺所需费用需根据实际情况是否在冬季进行生物处理后进行考虑) 。
3. 结论
1) 在为期75 d的运行过程中,A/O预处理工艺的运行良好且稳定,对氨氮和COD的去除率分别达到44%和73%,为后续耦合工艺提供了水质条件。
2) 按照分阶段进水的原则向系统内添加集便器污水,工艺运行稳定后出水氨氮及总氮为40.25 mg·L−1、67.40 mg·L−1,去除率分别高达90.84%和86.90%,最高氮去除负荷达0.141 kg·(m3·d)−1。同时,反应器内亚硝态氮、硝态氮含量保持在5 mg·L−1以下,较反应初期明显减少。这证明系统在短程硝化-厌氧氨氧化耦合反硝化工艺的协同作用下对污水中的各项污染物进行降解。
3) 厌氧氨氧化菌Candidatus_Brocadia始终是系统内的厌氧氨氧化优势菌属,证明Candidatus_Brocadia对此类水质的适应性高。同时,系统内检测出氨氧化菌AOB菌属Nitrosomonas和反硝化菌属Denitratisoma存在,未检测出NOB相关菌属。这证明短程硝化-厌氧氨氧化耦合反硝化系统处理集便器污水的成功建立及运行。
4) 采用A/O工艺+短程硝化-厌氧氨氧化耦合反硝化工艺集便器处理污水,实现了系统成功启动及稳定的运行效果。但实施运行过程中是否存在其他影响厌氧氨氧化耦合工艺的因素而影响系统的处理效果,如DO、温度、COD、pH等变化,有待进一步研究。
-
表 1 一些MNPs在藻细胞中的吸收和累积
Table 1. Absorption and accumulation of some MNPs in algal cells
金属纳米颗粒MNPs 浮游藻类Toxic effects 吸收途径Absorption pathway 在细胞中的分布Intracellular distribution 存在形式Existing forms 成像/实验方法Methods 参考文献References 名称Name 直径/nmDiameter Ag NPs 11.7±1.9 Chlamydomonas reinhardtii 细胞内化 细胞浆周间隙 Ag NPs HAADF-STEM* [126] 细胞质中 Ag2S Ag NPs <100 Ochromonas danica 细胞内化 细胞质中 Ag NPs TEM*、EDX* [62] CeO2 NPs 5,7,12 Chlamydomonas reinhardtii 网格蛋白依赖介导的内吞/直接穿过膜孔 — — TEM、抑制剂实验 [127] CuO NPs 40±10 Chlorella pyrenoidosa 内吞 液泡 Cu2O NPs TEM [128] CuO NPs 30—40 Chlamydomonas reinhardtii 细胞内化 细胞质、液泡和细胞核 CuO NPs TEM [129] TiO2 NPs 192±0.8 Anabaena variabilis 尚未明确 细胞质中 TiO2 NPs 拉曼光谱TEM [130] ZnO NPs 40—44 Scenedesmus obliquus 细胞内化 细胞壁内细胞质中细胞核内 ZnO NPs TEM [131] *HAADF-STEM:high-angle annular dark field scanning transmission electron microscopy,高角度环形暗场扫描透射电子显微镜;TEM:transmission electron microscope,透射电子显微镜;EDX:energy dispersive X-ray spectrum,能量色散X射线光谱 表 2 常见MNPs对藻类的毒性效应
Table 2. Toxic effects of MNPs on algae
金属纳米颗粒MNPs 浮游藻类Planktonic algae 毒性效应Toxic effects 参考文献References TiO2 NPs Nitzschia frustulum,Desmodesmus subspicatus,Pseudokirchneriella subcapitata,Chlamydomonas reinhardtii 生长抑制;光合作用抑制(叶绿素含量变化等);诱导氧化应激(SOD*活性↑,MDA*含量↑,CAT*活性↑);在藻细胞表面聚集;使藻细胞变形;改变细胞膜通透性 [79, 104, 137, 146, 149, 157-159] Ag NPs Thalassiosira weissflogii,Raphidocelis subcapitata,Chlamydomonas reinhardtii,Dunaliella salina,Chlorella autotrophica 生长抑制(特异生长率↓,细胞密度↓);光合作用抑制(Fv/Fm*↓,叶绿素a含量↓);诱导氧化应激(ROS过量累积,SOD活性↑,CAT活性↑,POD*活性↑ [61, 83-84] ZnO NPs Pseudokirchneriella subcapitata,Thalassiosira pseudonana,Chaetoceros gracilis,Phaeodactylum tricornutum,Scenedesmus obliquus 生长抑制(特异生长率↓);在藻细胞表面吸附;进入藻细胞 [79, 81, 131] CuO NPs Pseudokirchneriella subcapitata,Chlorella vulgaris,Scenedesmus sp.,Chlamydomonas reinhardtii 生长抑制;光合作用抑制;呼吸作用抑制;破坏细胞亚结构(破坏叶绿体);诱导ROS过量累积;造成谷胱甘肽代谢紊乱;破坏细胞膜结构;诱导膜脂重塑相关代谢反应 [79, 124, 142, 160] Au NPs Scenedesmus subcapitata, Chlamydomonas reinhardtii 生长抑制;在藻细胞表面吸附聚集 [161-163] Al2O3 NPs Chlorella sp. 生长抑制 [159] CeO2 NPs Dunaliella salina,Chlorella autotrophica 生长抑制(细胞密度↓);光合作用抑制;诱导ROS过量累积 [151] Cr2O3 NPs Chlamydomonas reinhardtii 生长抑制;诱导ROS生成;影响细胞代谢(酯酶活性增加) [154] ThO2 NPs Chlorella pyrenoidosa 生长抑制;光合作用抑制(叶绿素含量降低);在藻细胞表面聚集;破坏藻类细胞壁;诱导ROS生成、损伤细胞膜 [164] *SOD:superoxide dismutase,超氧化物歧化酶;MDA:malondialdehyde,丙二醛,与脂质过氧化有关;CAT:catalase,过氧化氢酶;Fv/Fm:光系统Ⅱ的量子产率;POD:peroxidase,过氧化物酶. -
[1] TOURINHO P S, van GESTEL C A M, LOFTS S, et al. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates [J]. Environmental Toxicology and Chemistry, 2012, 31(8): 1679-1692. doi: 10.1002/etc.1880 [2] SENGUL A B, ASMATULU E. Toxicity of metal and metal oxide nanoparticles: A review [J]. Environmental Chemistry Letters, 2020, 18(5): 1659-1683. doi: 10.1007/s10311-020-01033-6 [3] AUFFAN M, ROSE J, WIESNER M R, et al. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro [J]. Environmental Pollution, 2009, 157(4): 1127-1133. doi: 10.1016/j.envpol.2008.10.002 [4] VENKATESH N. Metallic nanoparticle: A review [J]. Biomedical Journal of Scientific & Technical Research, 2018, 4(2): 3765-3775. [5] KLAINE S J, ALVAREZ P J J, BATLEY G E, et al. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects [J]. Environmental Toxicology and Chemistry, 2008, 27(9): 1825-1851. doi: 10.1897/08-090.1 [6] YANG Q Q, WEI X L, FANG Y P, et al. Nanochemoprevention with therapeutic benefits: An updated review focused on epigallocatechin gallate delivery [J]. Critical Reviews in Food Science and Nutrition, 2020, 60(8): 1243-1264. doi: 10.1080/10408398.2019.1565490 [7] KLOEPFER J A, MIELKE R E and NADEAU, J L. Uptake of CdSe and CdSe/ZnS quantum dots into bacteria via purine-dependent mechanisms [J]. Applied and Environmental Microbiology, 2005, 71(5): 2548-2557. doi: 10.1128/AEM.71.5.2548-2557.2005 [8] SCHNEIDER S L, LIM H W. A review of inorganic UV filters zinc oxide and titanium dioxide [J]. Photodermatology, Photoimmunology & Photomedicine, 2019, 35(6): 442-446. [9] HUANG B, YAN S, XIAO L, et al. Label-free imaging of nanoparticle uptake competition in single cells by hyperspectral stimulated Raman scattering [J]. Small (Weinheim an Der Bergstrasse, Germany), 2018, 14(10). [10] LIU Y Y, GUO W B, ZHAO Y T, et al. Algal foods reduce the uptake of hematite nanoparticles by downregulating water filtration in Daphnia magna [J]. Environmental Science & Technology, 2019, 53(13): 7803-7811. [11] WANG Y H, RAJALA A, RAJALA R V S. Nanoparticles as delivery vehicles for the treatment of retinal degenerative diseases [J]. Advances in Experimental Medicine and Biology, 2018, 1074: 117-123. [12] E J, ZHANG Z Q, CHEN J W, et al. Performance and emission evaluation of a marine diesel engine fueled by water biodiesel-diesel emulsion blends with a fuel additive of a cerium oxide nanoparticle [J]. Energy Conversion and Management, 2018, 169: 194-205. doi: 10.1016/j.enconman.2018.05.073 [13] PARK J, KWON T, KIM J, et al. Hollow nanoparticles as emerging electrocatalysts for renewable energy conversion reactions [J]. Chemical Society Reviews, 2018, 47(22): 8173-8202. doi: 10.1039/C8CS00336J [14] ZLOTEA C, LATROCHE M. Role of nanoconfinement on hydrogen sorption properties of metal nanoparticles hybrids [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2013, 439: 117-130. [15] TAO A R, HABAS S, YANG P D. Shape control of colloidal metal nanocrystals [J]. Small, 2008, 4(3): 310-325. doi: 10.1002/smll.200701295 [16] CHEN M, WU B H, YANG J, et al. Small adsorbate-assisted shape control of Pd and Pt nanocrystals [J]. Advanced Materials (Deerfield Beach, Fla. ), 2012, 24(7): 862-879. doi: 10.1002/adma.201104145 [17] NOWACK B, BUCHELI T D. Occurrence, behavior and effects of nanoparticles in the environment [J]. Environmental Pollution, 2007, 150(1): 5-22. doi: 10.1016/j.envpol.2007.06.006 [18] DAUGHTON C G. Non-regulated water contaminants: Emerging research [J]. Environmental Impact Assessment Review, 2004, 24(7/8): 711-732. [19] MOORE M N. Biocomplexity: The post-genome challenge in ecotoxicology [J]. Aquatic Toxicology, 2002, 59(1/2): 1-15. [20] MOORE M N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? [J]. Environment International, 2006, 32(8): 967-976. doi: 10.1016/j.envint.2006.06.014 [21] PENG C, ZHANG W, GAO H P, et al. Behavior and potential impacts of metal-based engineered nanoparticles in aquatic environments [J]. Nanomaterials (Basel, Switzerland), 2017, 7(1): 21. doi: 10.3390/nano7010021 [22] LEAD J R, BATLEY G E, ALVAREZ P J J, et al. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects-An updated review [J]. Environmental Toxicology and Chemistry, 2018, 37(8): 2029-2063. doi: 10.1002/etc.4147 [23] CHRISTIAN P, von der KAMMER F, BAALOUSHA M, et al. Nanoparticles: structure, properties, preparation and behaviour in environmental media [J]. Ecotoxicology (London, England), 2008, 17(5): 326-343. doi: 10.1007/s10646-008-0213-1 [24] ZHANG C Q, HU Z Q, DENG B L. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms [J]. Water Research, 2016, 88: 403-427. doi: 10.1016/j.watres.2015.10.025 [25] FABREGA J, LUOMA S N, TYLER C R, et al. Silver nanoparticles: Behaviour and effects in the aquatic environment [J]. Environment International, 2011, 37(2): 517-531. doi: 10.1016/j.envint.2010.10.012 [26] THWALA M, KLAINE S J, MUSEE N. Interactions of metal-based engineered nanoparticles with aquatic higher plants: A review of the state of current knowledge [J]. Environmental Toxicology and Chemistry, 2016, 35(7): 1677-1694. doi: 10.1002/etc.3364 [27] NAM D H, LEE B C, EOM I C, et al. Uptake and bioaccumulation of titanium- and silver-nanoparticles in aquatic ecosystems [J]. Molecular & Cellular Toxicology, 2014, 10(1): 9-17. [28] LUOMA S N, KHAN F R, CROTEAU M N. Bioavailability and bioaccumulation of metal-based engineered nanomaterials in aquatic environments[M]. Nanoscience and the Environment. Amsterdam: Elsevier, 2014: 157-193. [29] ROCHA T L, GOMES T, SOUSA V S, et al. Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: An overview [J]. Marine Environmental Research, 2015, 111: 74-88. doi: 10.1016/j.marenvres.2015.06.013 [30] SCOWN T M, van AERLE R, TYLER C R. Review: Do engineered nanoparticles pose a significant threat to the aquatic environment? [J]. Critical Reviews in Toxicology, 2010, 40(7): 653-670. doi: 10.3109/10408444.2010.494174 [31] CHEN X J, ZHU Y, YANG K, et al. Nanoparticle TiO2 size and rutile content impact bioconcentration and biomagnification from algae to Daphnia [J]. Environmental Pollution, 2019, 247: 421-430. doi: 10.1016/j.envpol.2019.01.022 [32] ZHU R R, WANG S L, CHAO J, et al. Bio-effects of Nano-TiO2 on DNA and cellular ultrastructure with different polymorph and size [J]. Materials Science and Engineering:C, 2009, 29(3): 691-696. doi: 10.1016/j.msec.2008.12.023 [33] CHEN F R, XIAO Z G, YUE L, et al. Algae response to engineered nanoparticles: Current understanding, mechanisms and implications [J]. Environmental Science:Nano, 2019, 6(4): 1026-1042. doi: 10.1039/C8EN01368C [34] TANGAA S R, SELCK H, WINTHER-NIELSEN M, et al. Trophic transfer of metal-based nanoparticles in aquatic environments: A review and recommendations for future research focus [J]. Environmental Science:Nano, 2016, 3(5): 966-981. doi: 10.1039/C5EN00280J [35] EL BADAWY A M, LUXTON T P, SILVA R G, et al. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions [J]. Environmental Science & Technology, 2010, 44(4): 1260-1266. [36] HERMANSSON M. The DLVO theory in microbial adhesion [J]. Colloids and Surfaces B:Biointerfaces, 1999, 14(1/2/3/4): 105-119. [37] HANDY R D, von der KAMMER F, LEAD J R, et al. The ecotoxicology and chemistry of manufactured nanoparticles [J]. Ecotoxicology (London, England), 2008, 17(4): 287-314. doi: 10.1007/s10646-008-0199-8 [38] WAYCHUNAS G A, KIM C S, BANFIELD J F. Nanoparticulate iron oxide minerals in soils and sediments: Unique properties and contaminant scavenging mechanisms [J]. Journal of Nanoparticle Research, 2005, 7(4/5): 409-433. [39] HOTZE E M, PHENRAT T, LOWRY G V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment [J]. Journal of Environmental Quality, 2010, 39(6): 1909-1924. doi: 10.2134/jeq2009.0462 [40] FRENCH R A, JACOBSON A R, KIM B, et al. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles [J]. Environmental Science & Technology, 2009, 43(5): 1354-1359. [41] LEBRETTE S, PAGNOUX C, ABÉLARD P. Stability of aqueous TiO2 suspensions: Influence of ethanol [J]. Journal of Colloid and Interface Science, 2004, 280(2): 400-408. doi: 10.1016/j.jcis.2004.07.033 [42] BUETTNER K M, RINCIOG C I, MYLON S E. Aggregation kinetics of cerium oxide nanoparticles in monovalent and divalent electrolytes [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2010, 366(1/2/3): 74-79. [43] ZHOU D X, JI Z X, JIANG X M, et al. Influence of material properties on TiO2 nanoparticle agglomeration [J]. PLoS One, 2013, 8(11): e81239. doi: 10.1371/journal.pone.0081239 [44] JIANG J K, OBERDÖRSTER G, BISWAS P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies [J]. Journal of Nanoparticle Research, 2009, 11(1): 77-89. doi: 10.1007/s11051-008-9446-4 [45] FERNANDO I, ZHOU Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles [J]. Chemosphere, 2019, 216: 297-305. doi: 10.1016/j.chemosphere.2018.10.122 [46] WANG X G, SUN T S, ZHU H, et al. Roles of pH, cation valence, and ionic strength in the stability and aggregation behavior of zinc oxide nanoparticles [J]. Journal of Environmental Management, 2020, 267: 110656. doi: 10.1016/j.jenvman.2020.110656 [47] MORELLI E, GABELLIERI E, BONOMINI A, et al. TiO2 nanoparticles in seawater: Aggregation and interactions with the green alga Dunaliella tertiolecta [J]. Ecotoxicology and Environmental Safety, 2018, 148: 184-193. doi: 10.1016/j.ecoenv.2017.10.024 [48] FILELLA M. Freshwaters: which NOM matters? [J]. Environmental Chemistry Letters, 2009, 7(1): 21-35. doi: 10.1007/s10311-008-0158-x [49] ZHAO J, WANG Z Y, GHOSH S, et al. Phenanthrene binding by humic acid-protein complexes as studied by passive dosing technique [J]. Environmental Pollution, 2014, 184: 145-153. doi: 10.1016/j.envpol.2013.08.028 [50] ERHAYEM M, SOHN M. Stability studies for titanium dioxide nanoparticles upon adsorption of Suwannee River humic and fulvic acids and natural organic matter [J]. Science of the Total Environment, 2014, 468/469: 249-257. doi: 10.1016/j.scitotenv.2013.08.038 [51] LOUIE S M, TILTON R D, LOWRY G V. Effects of molecular weight distribution and chemical properties of natural organic matter on gold nanoparticle aggregation [J]. Environmental Science & Technology, 2013, 47(9): 4245-4254. [52] ZHANG Y, CHEN Y S, WESTERHOFF P, et al. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles [J]. Water Research, 2009, 43(17): 4249-4257. doi: 10.1016/j.watres.2009.06.005 [53] BIAN S W, MUDUNKOTUWA I A, RUPASINGHE T, et al. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2011, 27(10): 6059-6068. doi: 10.1021/la200570n [54] ZHOU D X, KELLER A A. Role of morphology in the aggregation kinetics of ZnO nanoparticles [J]. Water Research, 2010, 44(9): 2948-2956. doi: 10.1016/j.watres.2010.02.025 [55] LIN D, STORY S D, WALKER S L, et al. Influence of extracellular polymeric substances on the aggregation kinetics of TiO2 nanoparticles [J]. Water Research, 2016, 104: 381-388. doi: 10.1016/j.watres.2016.08.044 [56] SHENG A X, LIU F, XIE N, et al. Impact of proteins on aggregation kinetics and adsorption ability of hematite nanoparticles in aqueous dispersions [J]. Environmental Science & Technology, 2016, 50(5): 2228-2235. [57] AFSHINNIA K, GIBSON I, MERRIFIELD R, et al. The concentration-dependent aggregation of Ag NPs induced by cystine [J]. Science of the Total Environment, 2016, 557/558: 395-403. doi: 10.1016/j.scitotenv.2016.02.212 [58] GARCÍA-GARCÍA S, WOLD S, JONSSON M. Effects of temperature on the stability of colloidal montmorillonite particles at different pH and ionic strength [J]. Applied Clay Science, 2009, 43(1): 21-26. doi: 10.1016/j.clay.2008.07.011 [59] ZOU X Y, LI P H, LOU J, et al. Stability of single dispersed silver nanoparticles in natural and synthetic freshwaters: Effects of dissolved oxygen [J]. Environmental Pollution, 2017, 230: 674-682. doi: 10.1016/j.envpol.2017.07.007 [60] SOHAL I S, O'FALLON K S, GAINES P, et al. Ingested engineered nanomaterials: State of science in nanotoxicity testing and future research needs [J]. Particle and Fibre Toxicology, 2018, 15(1): 29. doi: 10.1186/s12989-018-0265-1 [61] MIAO A J, SCHWEHR K A, XU C, et al. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances [J]. Environmental Pollution, 2009, 157(11): 3034-3041. doi: 10.1016/j.envpol.2009.05.047 [62] MIAO A J, LUO Z P, CHEN C S, et al. Intracellular uptake: A possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica [J]. PLoS One, 2010, 5(12): e15196. doi: 10.1371/journal.pone.0015196 [63] ZHANG W C, XIAO B D, FANG T. Chemical transformation of silver nanoparticles in aquatic environments: Mechanism, morphology and toxicity [J]. Chemosphere, 2018, 191: 324-334. doi: 10.1016/j.chemosphere.2017.10.016 [64] MORTIMER M, KASEMETS K, KAHRU A. Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila [J]. Toxicology, 2010, 269(2/3): 182-189. [65] IVASK A, JUGANSON K, BONDARENKO O, et al. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review [J]. Nanotoxicology, 2014, 8(sup1): 57-71. doi: 10.3109/17435390.2013.855831 [66] MCCRACKEN C, ZANE A, KNIGHT D A, et al. Minimal intestinal epithelial cell toxicity in response to short- and long-term food-relevant inorganic nanoparticle exposure [J]. Chemical Research in Toxicology, 2013, 26(10): 1514-1525. doi: 10.1021/tx400231u [67] de ANGELIS I, BARONE F, ZIJNO A, et al. Comparative study of ZnO and TiO2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells [J]. Nanotoxicology, 2013, 7(8): 1361-1372. doi: 10.3109/17435390.2012.741724 [68] MILLER R J, LENIHAN H S, MULLER E B, et al. Impacts of metal oxide nanoparticles on marine phytoplankton [J]. Environmental Science & Technology, 2010, 44(19): 7329-7334. [69] ZHANG W Y, QIAN L B, da OUYANG, et al. Effective removal of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization [J]. Chemosphere, 2019, 221: 683-692. doi: 10.1016/j.chemosphere.2019.01.070 [70] PERETYAZHKO T S, ZHANG Q B, COLVIN V L. Size-controlled dissolution of silver nanoparticles at neutral and acidic pH conditions: Kinetics and size changes [J]. Environmental Science & Technology, 2014, 48(20): 11954-11961. [71] TANG R, ORME C A, NANCOLLAS G H. Dissolution of crystallites: Surface energetic control and size effects [J]. Chemphyschem, 2004, 5(5): 688-696. doi: 10.1002/cphc.200300956 [72] BORM P, KLAESSIG F C, LANDRY T D, et al. Research strategies for safety evaluation of nanomaterials, part V: Role of dissolution in biological fate and effects of nanoscale particles [J]. Toxicological Sciences, 2006, 90(1): 23-32. doi: 10.1093/toxsci/kfj084 [73] KITTLER S, GREULICH C, DIENDORF J, et al. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions [J]. Chemistry of Materials, 2010, 22(16): 4548-4554. doi: 10.1021/cm100023p [74] WANG Z Y, ZHANG L, ZHAO J, et al. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter [J]. Environmental Science:Nano, 2016, 3(2): 240-255. doi: 10.1039/C5EN00230C [75] ZHANG L Q, LI J Y, YANG K, et al. Physicochemical transformation and algal toxicity of engineered nanoparticles in surface water samples [J]. Environmental Pollution, 2016, 211: 132-140. doi: 10.1016/j.envpol.2015.12.041 [76] ODZAK N, KISTLER D, SIGG L. Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments [J]. Environmental Pollution, 2017, 226: 1-11. doi: 10.1016/j.envpol.2017.04.006 [77] SOTIRIOU G A, MEYER A, KNIJNENBURG J T N, et al. Quantifying the origin of released Ag+ ions from nanosilver [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2012, 28(45): 15929-15936. doi: 10.1021/la303370d [78] LIU J Y, HURT R H. Ion release kinetics and particle persistence in aqueous nano-silver colloids [J]. Environmental Science & Technology, 2010, 44(6): 2169-2175. [79] ARUOJA V, DUBOURGUIER H C, KASEMETS K, et al. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata [J]. Science of the Total Environment, 2009, 407(4): 1461-1468. doi: 10.1016/j.scitotenv.2008.10.053 [80] FRANKLIN N M, ROGERS N J, APTE S C, et al. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility [J]. Environmental Science & Technology, 2007, 41(24): 8484-8490. [81] PENG X H, PALMA S, FISHER N S, et al. Effect of morphology of ZnO nanostructures on their toxicity to marine algae [J]. Aquatic Toxicology, 2011, 102(3/4): 186-196. [82] XIU Z M, ZHANG Q B, PUPPALA H L, et al. Negligible particle-specific antibacterial activity of silver nanoparticles [J]. Nano Letters, 2012, 12(8): 4271-4275. doi: 10.1021/nl301934w [83] NAVARRO E, PICCAPIETRA F, WAGNER B, et al. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii [J]. Environmental Science & Technology, 2008, 42(23): 8959-8964. [84] WANG Z, CHEN J W, LI X H, et al. Aquatic toxicity of nanosilver colloids to different trophic organisms: Contributions of particles and free silver ion [J]. Environmental Toxicology and Chemistry, 2012, 31(10): 2408-2413. doi: 10.1002/etc.1964 [85] KAEGI R, VOEGELIN A, ORT C, et al. Fate and transformation of silver nanoparticles in urban wastewater systems [J]. Water Research, 2013, 47(12): 3866-3877. doi: 10.1016/j.watres.2012.11.060 [86] KIM B, PARK C S, MURAYAMA M, et al. Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products [J]. Environmental Science & Technology, 2010, 44(19): 7509-7514. [87] LIU J Y, PENNELL K G, HURT R H. Kinetics and mechanisms of nanosilver oxysulfidation [J]. Environmental Science & Technology, 2011, 45(17): 7345-7353. [88] MA R, LEVARD C, MICHEL F M, et al. Sulfidation mechanism for zinc oxide nanoparticles and the effect of sulfidation on their solubility [J]. Environmental Science & Technology, 2013, 47(6): 2527-2534. [89] MA R, STEGEMEIER J, LEVARD C, et al. Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide [J]. Environ Sci:Nano, 2014, 1(4): 347-357. doi: 10.1039/C4EN00018H [90] DONGARGAONKAR A A, CLOGSTON J D. Quantitation of surface coating on nanoparticles using thermogravimetric analysis [J]. Methods in Molecular Biology , 2018, 1682: 57-63. [91] CARTWRIGHT A, JACKSON K, MORGAN C, et al. A review of metal and metal-oxide nanoparticle coating technologies to inhibit agglomeration and increase bioactivity for agricultural applications [J]. Agronomy, 2020, 10(7): 1018. doi: 10.3390/agronomy10071018 [92] HUA M Y, YANG H W, CHUANG C K, et al. Magnetic-nanoparticle-modified paclitaxel for targeted therapy for prostate cancer [J]. Biomaterials, 2010, 31(28): 7355-7363. doi: 10.1016/j.biomaterials.2010.05.061 [93] VIGDERMAN L, ZUBAREV E R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules [J]. Advanced Drug Delivery Reviews, 2013, 65(5): 663-676. doi: 10.1016/j.addr.2012.05.004 [94] SHARMA A, GOYAL A K, RATH G. Recent advances in metal nanoparticles in cancer therapy [J]. Journal of Drug Targeting, 2018, 26(8): 617-632. doi: 10.1080/1061186X.2017.1400553 [95] KENNEDY L C, BICKFORD L R, LEWINSKI N A, et al. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies [J]. Small (Weinheim an Der Bergstrasse, Germany), 2011, 7(2): 169-183. doi: 10.1002/smll.201000134 [96] LOUIE S M, GORHAM J M, MCGIVNEY E A, et al. Photochemical transformations of thiolated polyethylene glycol coatings on gold nanoparticles [J]. Environmental Science:Nano, 2016, 3(5): 1090-1102. doi: 10.1039/C6EN00141F [97] LOUIE S M, GORHAM J M, TAN J J, et al. Ultraviolet photo-oxidation of polyvinylpyrrolidone (PVP) coatings on gold nanoparticles[J]. Environmental Science. Nano, 2017, 4(9): 1866-1875. [98] RODEA-PALOMARES I, BOLTES K, FERNÁNDEZ-PIÑAS F, et al. Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms [J]. Toxicological Sciences, 2010, 119(1): 135-145. [99] BONDARENKO O, JUGANSON K, IVASK A, et al. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review [J]. Archives of Toxicology, 2013, 87(7): 1181-1200. doi: 10.1007/s00204-013-1079-4 [100] KÖSER J, ENGELKE M, HOPPE M, et al. Predictability of silver nanoparticle speciation and toxicity in ecotoxicological media [J]. Environmental Science:Nano, 2017, 4(7): 1470-1483. doi: 10.1039/C7EN00026J [101] LEI C, ZHANG L Q, YANG K, et al. Toxicity of iron-based nanoparticles to green algae: Effects of particle size, crystal phase, oxidation state and environmental aging [J]. Environmental Pollution, 2016, 218: 505-512. doi: 10.1016/j.envpol.2016.07.030 [102] LU T, ZHANG Q, ZHANG Z Y, et al. Pollutant toxicology with respect to microalgae and cyanobacteria [J]. Journal of Environmental Sciences, 2021, 99: 175-186. doi: 10.1016/j.jes.2020.06.033 [103] HUANG J, CHENG J P, YI J. Impact of silver nanoparticles on marine diatom Skeletonema costatum [J]. Journal of Applied Toxicology:JAT, 2016, 36(10): 1343-1354. doi: 10.1002/jat.3325 [104] JIA K, SUN C L, WANG Y L, et al. Effect of TiO2 nanoparticles and multiwall carbon nanotubes on the freshwater diatom Nitzschia frustulum: Evaluation of growth, cellular components and morphology [J]. Chemistry and Ecology, 2019, 35(1): 69-85. doi: 10.1080/02757540.2018.1528240 [105] DENG X Y, CHENG J, HU X L, et al. Biological effects of TiO2 and CeO2 nanoparticles on the growth, photosynthetic activity, and cellular components of a marine diatom Phaeodactylum tricornutum [J]. Science of the Total Environment, 2017, 575: 87-96. doi: 10.1016/j.scitotenv.2016.10.003 [106] DEDMAN C J, NEWSON G C, DAVIES G L, et al. Mechanisms of silver nanoparticle toxicity on the marine cyanobacterium Prochlorococcus under environmentally-relevant conditions [J]. Science of the Total Environment, 2020, 747: 141229. doi: 10.1016/j.scitotenv.2020.141229 [107] BRAYNER R, SICARD C, SASSI H B, et al. Design of ZnO nanostructured films: Characterization and ecotoxicological studies [J]. Thin Solid Films, 2011, 519(10): 3340-3345. doi: 10.1016/j.tsf.2011.01.257 [108] MAHFOOZ S, SHAMIM A, HUSAIN A, et al. Physicochemical characterisation and ecotoxicological assessment of nano-silver using two cyanobacteria Nostoc muscorum and Plectonema boryanum [J]. International Journal of Environmental Science and Technology, 2019, 16(8): 4407-4418. doi: 10.1007/s13762-018-1923-4 [109] SOHN E K, JOHARI S A, KIM T G, et al. Aquatic toxicity comparison of silver nanoparticles and silver nanowires [J]. BioMed Research International, 2015, 2015: 893049. [110] BRAYNER R, DAHOUMANE S A, YÉPRÉMIAN C, et al. ZnO nanoparticles: Synthesis, characterization, and ecotoxicological studies [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2010, 26(9): 6522-6528. doi: 10.1021/la100293s [111] CHEN P Y, POWELL B A, MORTIMER M, et al. Adaptive interactions between zinc oxide nanoparticles and Chlorella sp. [J]. Environmental Science & Technology, 2012, 46(21): 12178-12185. [112] MA S, LIN D H. The biophysicochemical interactions at the interfaces between nanoparticles and aquatic organisms: Adsorption and internalization [J]. Environmental Science. Processes & Impacts, 2013, 15(1): 145-160. [113] WANG F, GUAN W, XU L, et al. Effects of nanoparticles on algae: Adsorption, distribution, ecotoxicity and fate [J]. Applied Sciences, 2019, 9(8): 1534. doi: 10.3390/app9081534 [114] HARJA M, BUEMA G, BULGARIU L, et al. Removal of cadmium(II) from aqueous solution by adsorption onto modified algae and ash [J]. Korean Journal of Chemical Engineering, 2015, 32(9): 1804-1811. doi: 10.1007/s11814-015-0016-z [115] ZHANG J L, XIANG Q Q, SHEN L, et al. Surface charge-dependent bioaccumulation dynamics of silver nanoparticles in freshwater algae [J]. Chemosphere, 2020, 247: 125936. doi: 10.1016/j.chemosphere.2020.125936 [116] HUANG B, MIAO A J, XIAO L, et al. Influence of nitrogen limitation on the bioaccumulation kinetics of hematite nanoparticles in the freshwater alga Euglena intermedia [J]. Environmental Science:Nano, 2017, 4(9): 1840-1850. doi: 10.1039/C7EN00477J [117] ZHANG C, CHEN X H, WANG J T, et al. Toxicity of zinc oxide nanoparticles on marine microalgae possessing different shapes and surface structures [J]. Environmental Engineering Science, 2018, 35(8): 785-790. doi: 10.1089/ees.2017.0241 [118] RIBEIRO F, GALLEGO-URREA J A, GOODHEAD R M, et al. Uptake and elimination kinetics of silver nanoparticles and silver nitrate by Raphidocelis subcapitata: The influence of silver behaviour in solution [J]. Nanotoxicology, 2015, 9(6): 686-695. doi: 10.3109/17435390.2014.963724 [119] LANKOFF A, SANDBERG W J, WEGIEREK-CIUK A, et al. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells [J]. Toxicology Letters, 2012, 208(3): 197-213. doi: 10.1016/j.toxlet.2011.11.006 [120] von MOOS N, BOWEN P, SLAVEYKOVA V I. Bioavailability of inorganic nanoparticles to planktonic bacteria and aquatic microalgae in freshwater [J]. Environmental Science:Nano, 2014, 1(3): 214. doi: 10.1039/c3en00054k [121] MAHANA A, GULIY O I, MEHTA S K. Accumulation and cellular toxicity of engineered metallic nanoparticle in freshwater microalgae: Current status and future challenges [J]. Ecotoxicology and Environmental Safety, 2021, 208: 111662. doi: 10.1016/j.ecoenv.2020.111662 [122] NGUYEN M K, MOON J Y, LEE Y C. Microalgal ecotoxicity of nanoparticles: An updated review [J]. Ecotoxicology and Environmental Safety, 2020, 201: 110781. doi: 10.1016/j.ecoenv.2020.110781 [123] WORMS I A M, BOLTZMAN J, GARCIA M, et al. Cell-wall-dependent effect of carboxyl-CdSe/ZnS quantum dots on lead and copper availability to green microalgae [J]. Environmental Pollution, 2012, 167: 27-33. doi: 10.1016/j.envpol.2012.03.030 [124] NAVARRO E, BAUN A, BEHRA R, et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi [J]. Ecotoxicology (London, England), 2008, 17(5): 372-386. doi: 10.1007/s10646-008-0214-0 [125] TRIPATHI D K, TRIPATHI A, Shweta, et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review [J]. Frontiers in Microbiology, 2017, 8: 07. [126] WANG S S, LV J T, MA J Y, et al. Cellular internalization and intracellular biotransformation of silver nanoparticles in Chlamydomonas reinhardtii [J]. Nanotoxicology, 2016, 10(8): 1129-1135. doi: 10.1080/17435390.2016.1179809 [127] PULIDO-REYES G, BRIFFA S M, HURTADO-GALLEGO J, et al. Internalization and toxicological mechanisms of uncoated and PVP-coated cerium oxide nanoparticles in the freshwater alga Chlamydomonas reinhardtii [J]. Environmental Science:Nano, 2019, 6(6): 1959-1972. doi: 10.1039/C9EN00363K [128] ZHAO J, CAO X S, LIU X Y, et al. Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: Adhesion, uptake, and toxicity [J]. Nanotoxicology, 2016, 10(9): 1297-1305. doi: 10.1080/17435390.2016.1206149 [129] MELEGARI S P, PERREAULT F, COSTA R H R, et al. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii [J]. Aquatic Toxicology, 2013, 142/143: 431-440. doi: 10.1016/j.aquatox.2013.09.015 [130] CHERCHI C, CHERNENKO T, DIEM M, et al. Impact of nano titanium dioxide exposure on cellular structure of Anabaena variabilis and evidence of internalization [J]. Environmental Toxicology and Chemistry, 2011, 30(4): 861-869. doi: 10.1002/etc.445 [131] BHUVANESHWARI M, ISWARYA V, ARCHANAA S, et al. Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions [J]. Aquatic Toxicology, 2015, 162: 29-38. doi: 10.1016/j.aquatox.2015.03.004 [132] KOSAK NÉE RÖHDER L A, BRANDT T, SIGG L, et al. Uptake and effects of cerium(III) and cerium oxide nanoparticles to Chlamydomonas reinhardtii [J]. Aquatic Toxicology, 2018, 197: 41-46. doi: 10.1016/j.aquatox.2018.02.004 [133] NAM H Y, KWON S M, CHUNG H, et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles [J]. Journal of Controlled Release, 2009, 135(3): 259-267. doi: 10.1016/j.jconrel.2009.01.018 [134] LIMBACH L K, WICK P, MANSER P, et al. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress [J]. Environmental Science & Technology, 2007, 41(11): 4158-4163. [135] DAMM E M, PELKMANS L, KARTENBECK J, et al. Clathrin- and caveolin-1-independent endocytosis: Entry of Simian virus 40 into cells devoid of caveolae [J]. The Journal of Cell Biology, 2005, 168(3): 477-488. doi: 10.1083/jcb.200407113 [136] BUNDSCHUH M, SEITZ F, ROSENFELDT R R, et al. Effects of nanoparticles in fresh waters: Risks, mechanisms and interactions [J]. Freshwater Biology, 2016, 61(12): 2185-2196. doi: 10.1111/fwb.12701 [137] ROY R, PARASHAR A, BHUVANESHWARI M, et al. Differential effects of P25 TiO2 nanoparticles on freshwater green microalgae: Chlorella and Scenedesmus species [J]. Aquatic Toxicology, 2016, 176: 161-171. doi: 10.1016/j.aquatox.2016.04.021 [138] WANG J, WANG W X. Significance of physicochemical and uptake kinetics in controlling the toxicity of metallic nanomaterials to aquatic organisms [J]. Journal of Zhejiang University Science A, 2014, 15(8): 573-592. doi: 10.1631/jzus.A1400109 [139] MORENO-GARRIDO I, PÉREZ S, BLASCO J. Toxicity of silver and gold nanoparticles on marine microalgae [J]. Marine Environmental Research, 2015, 111: 60-73. doi: 10.1016/j.marenvres.2015.05.008 [140] MARSALEK B, JANCULA D, MARSALKOVA E, et al. Multimodal action and selective toxicity of zerovalent iron nanoparticles against cyanobacteria [J]. Environmental Science & Technology, 2012, 46(4): 2316-2323. [141] NAVARRO E, WAGNER B, ODZAK N, et al. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii [J]. Environmental Science & Technology, 2015, 49(13): 8041-8047. [142] WANG L, HUANG X L, SUN W L, et al. A global metabolomic insight into the oxidative stress and membrane damage of copper oxide nanoparticles and microparticles on microalga Chlorella vulgaris [J]. Environmental Pollution, 2020, 258: 113647. doi: 10.1016/j.envpol.2019.113647 [143] AKTER M, SIKDER M T, RAHMAN M M, et al. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives [J]. Journal of Advanced Research, 2018, 9: 1-16. doi: 10.1016/j.jare.2017.10.008 [144] PIKULA K, MINTCHEVA N, KULINICH S A, et al. Aquatic toxicity and mode of action of CdS and ZnS nanoparticles in four microalgae species [J]. Environmental Research, 2020, 186: 109513. doi: 10.1016/j.envres.2020.109513 [145] HJORTH R, SØRENSEN S N, OLSSON M E, et al. A certain shade of green: Can algal pigments reveal shading effects of nanoparticles? [J]. Integrated Environmental Assessment and Management, 2016, 12(1): 200-202. doi: 10.1002/ieam.1728 [146] HARTMANN N B, der KAMMER F V, HOFMANN T, et al. Algal testing of titanium dioxide nanoparticles—Testing considerations, inhibitory effects and modification of cadmium bioavailability [J]. Toxicology, 2010, 269(2/3): 190-197. [147] WU D, YANG S X, DU W C, et al. Effects of titanium dioxide nanoparticles on Microcystis aeruginosa and microcystins production and release [J]. Journal of Hazardous Materials, 2019, 377: 1-7. doi: 10.1016/j.jhazmat.2019.05.013 [148] FU L, HAMZEH M, DODARD S, et al. Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation [J]. Environmental Toxicology and Pharmacology, 2015, 39(3): 1074-1080. doi: 10.1016/j.etap.2015.03.015 [149] DALAI S, PAKRASHI S, BHUVANESHWARI M, et al. Toxic effect of Cr(VI) in presence of n-TiO2 and n-Al2O3 particles towards freshwater microalgae [J]. Aquatic Toxicology, 2014, 146: 28-37. doi: 10.1016/j.aquatox.2013.10.029 [150] ZHAO Z L, XU L M, WANG Y, et al. Toxicity mechanism of silver nanoparticles to Chlamydomonas reinhardtii: Photosynthesis, oxidative stress, membrane permeability, and ultrastructure analysis [J]. Environmental Science and Pollution Research International, 2021, 28(12): 15032-15042. doi: 10.1007/s11356-020-11714-y [151] SENDRA M, BLASCO J, ARAÚJO C V M. Is the cell wall of marine phytoplankton a protective barrier or a nanoparticle interaction site? Toxicological responses of Chlorella autotrophica and Dunaliella salina to Ag and CeO2 nanoparticles [J]. Ecological Indicators, 2018, 95: 1053-1067. doi: 10.1016/j.ecolind.2017.08.050 [152] HAZANI A A, IBRAHIM M M, ARIF I A, et al. Ecotoxicity of Ag-nanoparticles to microalgae[J]. Journal of Pure and Applied Microbiology, 2013, 7: 233-241. [153] FU P P, XIA Q S, HWANG H M, et al. Mechanisms of nanotoxicity: Generation of reactive oxygen species [J]. Journal of Food and Drug Analysis, 2014, 22(1): 64-75. doi: 10.1016/j.jfda.2014.01.005 [154] da COSTA C H, PERREAULT F, OUKARROUM A, et al. Effect of chromium oxide (III) nanoparticles on the production of reactive oxygen species and photosystem II activity in the green alga Chlamydomonas reinhardtii [J]. Science of the Total Environment, 2016, 565: 951-960. doi: 10.1016/j.scitotenv.2016.01.028 [155] MISRA S K, DYBOWSKA A, BERHANU D, et al. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies [J]. Science of the Total Environment, 2012, 438: 225-232. doi: 10.1016/j.scitotenv.2012.08.066 [156] YU S J, LAI Y J, DONG L J, et al. Intracellular dissolution of silver nanoparticles: Evidence from double stable isotope tracing [J]. Environmental Science & Technology, 2019, 53(17): 10218-10226. [157] HUND-RINKE K, SIMON M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids [J]. Environmental Science and Pollution Research International, 2006, 13(4): 225-232. doi: 10.1065/espr2006.06.311 [158] CHEN L Z, ZHOU L N, LIU Y D, et al. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii [J]. Ecotoxicology and Environmental Safety, 2012, 84: 155-162. doi: 10.1016/j.ecoenv.2012.07.019 [159] JI J, LONG Z F, LIN D H. Toxicity of oxide nanoparticles to the green algae Chlorella sp. [J]. Chemical Engineering Journal, 2011, 170(2/3): 525-530. [160] CHE X K, DING R R, LI Y T, et al. Mechanism of long-term toxicity of CuO NPs to microalgae [J]. Nanotoxicology, 2018, 12(8): 923-939. doi: 10.1080/17435390.2018.1498928 [161] van HOECKE K, de SCHAMPHELAERE K A C, ALI Z, et al. Ecotoxicity and uptake of polymer coated gold nanoparticles [J]. Nanotoxicology, 2013, 7(1): 37-47. doi: 10.3109/17435390.2011.626566 [162] RENAULT S, BAUDRIMONT M, MESMER-DUDONS N, et al. Impacts of gold nanoparticle exposure on two freshwater species: A phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea) [J]. Gold Bulletin, 2008, 41(2): 116-126. doi: 10.1007/BF03216589 [163] PERREAULT F, BOGDAN N, MORIN M, et al. Interaction of gold nanoglycodendrimers with algal cells (Chlamydomonas reinhardtii) and their effect on physiological processes [J]. Nanotoxicology, 2012, 6(2): 109-120. doi: 10.3109/17435390.2011.562325 [164] HE X X, XIE C J, MA Y H, et al. Size-dependent toxicity of ThO2 nanoparticles to green algae Chlorella pyrenoidosa [J]. Aquatic Toxicology, 2019, 209: 113-120. doi: 10.1016/j.aquatox.2019.02.003 [165] YANG K, XING B S. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water [J]. Environmental Pollution, 2007, 145(2): 529-537. doi: 10.1016/j.envpol.2006.04.020 [166] DENG R, LIN D H, ZHU L Z, et al. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk [J]. Nanotoxicology, 2017, 11(5): 591-612. doi: 10.1080/17435390.2017.1343404 [167] KIM I, LEE B T, KIM H A, et al. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna [J]. Chemosphere, 2016, 143: 99-105. doi: 10.1016/j.chemosphere.2015.06.046 [168] FANG Q, SHI X J, ZHANG L P, et al. Effect of titanium dioxide nanoparticles on the bioavailability, metabolism, and toxicity of pentachlorophenol in zebrafish larvae [J]. Journal of Hazardous Materials, 2015, 283: 897-904. doi: 10.1016/j.jhazmat.2014.10.039 [169] LIU N, WANG Y P, GE F, et al. Antagonistic effect of nano-ZnO and cetyltrimethyl ammonium chloride on the growth of Chlorella vulgaris: Dissolution and accumulation of nano-ZnO [J]. Chemosphere, 2018, 196: 566-574. doi: 10.1016/j.chemosphere.2017.12.184 [170] NADDAFI K, ZARE M R, NAZMARA S. Investigating potential toxicity of phenanthrene adsorbed to nano-ZnO using Daphnia magna [J]. Toxicological & Environmental Chemistry, 2011, 93(4): 729-737. [171] HUANG B, WEI Z B, YANG L Y, et al. Combined toxicity of silver nanoparticles with hematite or plastic nanoparticles toward two freshwater algae [J]. Environmental Science & Technology, 2019, 53(7): 3871-3879. [172] LING L L, LIU W J, ZHANG S, et al. Magnesium oxide embedded nitrogen self-doped biochar composites: Fast and high-efficiency adsorption of heavy metals in an aqueous solution [J]. Environmental Science & Technology, 2017, 51(17): 10081-10089. [173] HU J, ZHANG Z C, ZHANG C, et al. Al2O3 nanoparticle impact on the toxic effect of Pb on the marine microalga Isochrysis galbana [J]. Ecotoxicology and Environmental Safety, 2018, 161: 92-98. doi: 10.1016/j.ecoenv.2018.05.090 [174] SAHLE-DEMESSIE E, HAN C, ZHAO A, et al. Interaction of engineered nanomaterials with hydrophobic organic pollutants [J]. Nanotechnology, 2016, 27(28): 284003. doi: 10.1088/0957-4484/27/28/284003 [175] 何莹, 刘洋, 陈治廷, 等. 溶解性有机质的表面吸附行为及其对金属基纳米颗粒环境行为的影响 [J]. 环境化学, 2019, 38(8): 1757-1767. HE Y, LIU Y, CHEN Z T, et al. Surface adsorption of dissolved organic matters and their effects on environmental behaviors of metal-based nanoparticles [J]. Environmental Chemistry, 2019, 38(8): 1757-1767(in Chinese).
[176] ZHANG C, CHEN X H, TAN L J, et al. Combined toxicities of copper nanoparticles with carbon nanotubes on marine microalgae Skeletonema costatum [J]. Environmental Science and Pollution Research International, 2018, 25(13): 13127-13133. doi: 10.1007/s11356-018-1580-7 [177] NEALE P A, JÄMTING Å K, O'MALLEY E, et al. Behaviour of titanium dioxide and zinc oxide nanoparticles in the presence of wastewater-derived organic matter and implications for algal toxicity [J]. Environmental Science:Nano, 2015, 2(1): 86-93. doi: 10.1039/C4EN00161C [178] WANG Z Y, LI J, ZHAO J, et al. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter [J]. Environmental Science & Technology, 2011, 45(14): 6032-6040. [179] LI S X, WANG S Q, YAN B, et al. Surface properties of nanoparticles dictate their toxicity by regulating adsorption of humic acid molecules [J]. ACS Sustainable Chemistry & Engineering, 2021, 9(41): 13705-13716. [180] QIAN H F, ZHU K, LU H P, et al. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses [J]. Science of the Total Environment, 2016, 572: 1213-1221. doi: 10.1016/j.scitotenv.2016.08.039 [181] DU S T, ZHANG P, ZHANG R R, et al. Reduced graphene oxide induces cytotoxicity and inhibits photosynthetic performance of the green alga Scenedesmus obliquus [J]. Chemosphere, 2016, 164: 499-507. doi: 10.1016/j.chemosphere.2016.08.138 [182] ZHOU K, HU Y, ZHANG L, et al. The role of exopolymeric substances in the bioaccumulation and toxicity of Ag nanoparticles to algae [J]. Scientific Reports, 2016, 6: 32998. doi: 10.1038/srep32998 [183] CHIU M H, KHAN Z A, GARCIA S G, et al. Effect of engineered nanoparticles on exopolymeric substances release from marine phytoplankton [J]. Nanoscale Research Letters, 2017, 12(1): 620. doi: 10.1186/s11671-017-2397-x [184] HE M, YAN Y, PEI F, et al. Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles [J]. Scientific Reports, 2017, 7(1): 15526. doi: 10.1038/s41598-017-15667-0 [185] CHEN B, LI F, LIU N, et al. Role of extracellular polymeric substances from Chlorella vulgaris in the removal of ammonium and orthophosphate under the stress of cadmium [J]. Bioresource Technology, 2015, 190: 299-306. doi: 10.1016/j.biortech.2015.04.080 [186] HENDERSON R K, BAKER A, PARSONS S A, et al. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms [J]. Water Research, 2008, 42(13): 3435-3445. doi: 10.1016/j.watres.2007.10.032 [187] WINGENDER J, NEU T R, FLEMMING H C. What are bacterial extracellular polymeric substances?[M]. Microbial Extracellular Polymeric Substances. Berlin, Heidelberg: Springer Berlin Heidelberg, 1999: 1-19. [188] TAYLOR C, MATZKE M, KROLL A, et al. Toxic interactions of different silver forms with freshwater green algae and cyanobacteria and their effects on mechanistic endpoints and the production of extracellular polymeric substances [J]. Environmental Science:Nano, 2016, 3(2): 396-408. doi: 10.1039/C5EN00183H [189] QUIGG A, CHIN W C, CHEN C S, et al. Direct and indirect toxic effects of engineered nanoparticles on algae: Role of natural organic matter [J]. ACS Sustainable Chemistry & Engineering, 2013, 1(7): 686-702. -




 下载:
下载: